Documentation of Phytotoxic Compounds Existing in Parthenium hysterophorus L. Leaf and Their Phytotoxicity on Eleusine indica (L.) Gaertn. and Digitaria sanguinalis (L.) Scop
Abstract
:1. Introduction
2. Results
2.1. Identified Compounds from P. hysterophorus Leaf Methanol Extract through LC-MS Analysis
2.2. Documentation of Phytotoxic Compounds from P. hysterophorus Leaf Methanol Extract through LC–MS Analysis
2.3. Allelopathic Effects of the Phytochemicals on D. sanguinalis and E. indica
2.3.1. Effects on Germination and Early Growth of D. sanguinalis
2.3.2. Comparison between Phytochemicals in Their Effects on Growth Parameters
2.3.3. Germination and Early Growth of E. indica Treated with Detected Allelochemicals
2.3.4. Comparison of Phytochemicals in Their Effects on Examined Initial Growth Parameters
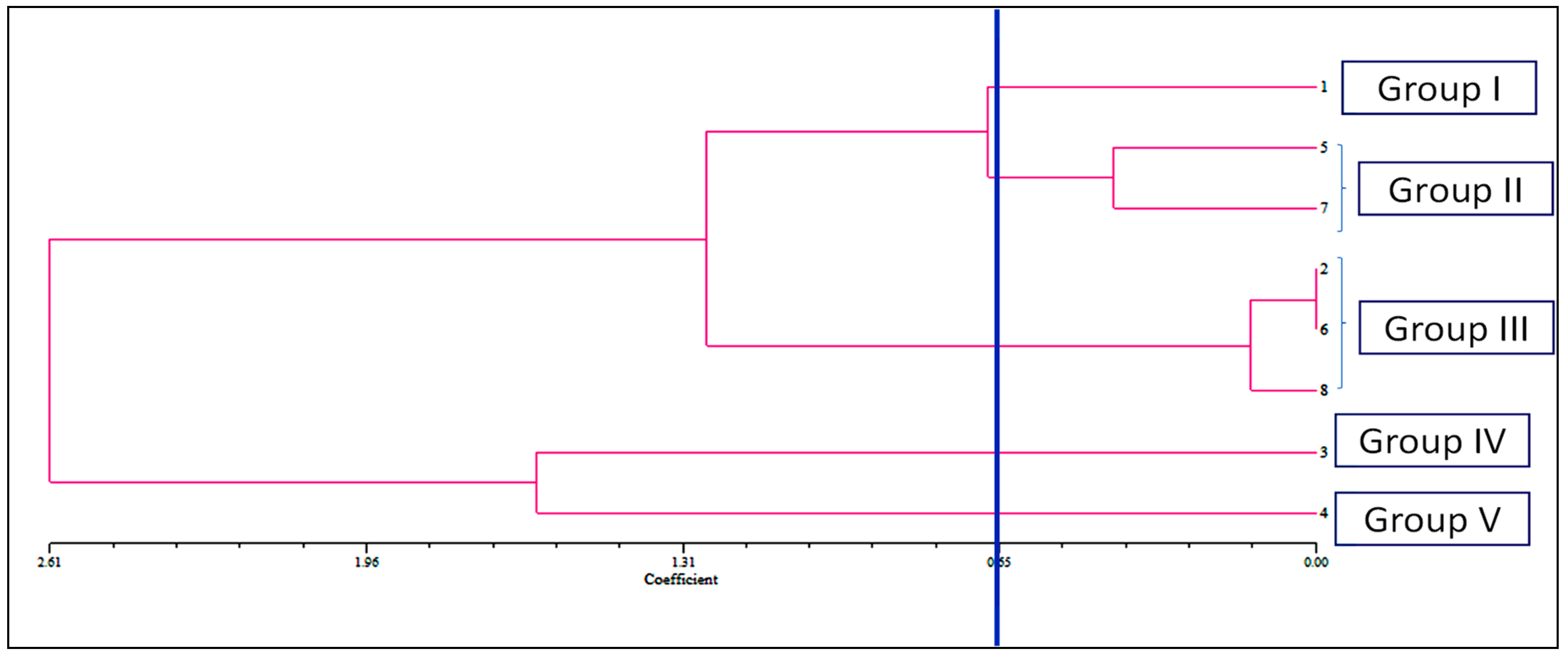
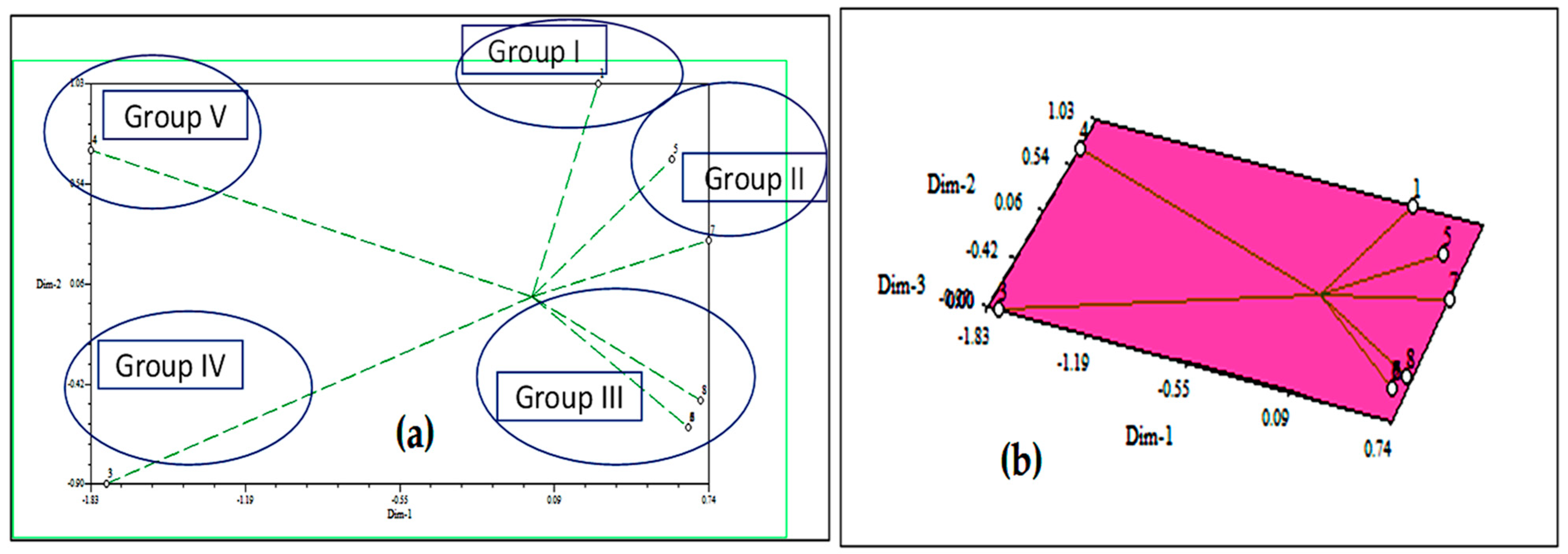
2.3.5. Cluster Analysis and Assessment of Principal Component Analysis
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Site Description
5.2. Extract Preparation
5.3. Identification of Phytotoxic Compounds from P. hysterophorus Leaf Methanol Extract
5.4. Experimental Treatments and Layout
5.5. Plant Materials and Compounds
5.6. Bioassay
5.7. Data Measurement
5.8. Identified Compounds from P. hysterophorus Leaf Extract
5.9. Details of the Phytotoxic Compounds
5.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kwinda, M. Weed Profiling Fields of Herbicide Tolerant Maize in the Mthatha Region, Eastern Cape Province. Ph.D. Thesis, North-West University, Potchefstroom, South Africa, 2021; pp. 1–146. [Google Scholar]
- Chuah, T.S.; Lim, W.K. Combination Ratio Affects Synergistic Activity of Oil Palm Frond Residue and S-Metolachlor on Goosegrass (Eleusine indica). Pakistan J. Bot. 2021, 53, 1473–1477. [Google Scholar] [CrossRef]
- Chuah, T.S.; Lim, W.K. Assessment of Phytotoxic Potential of Oil Palm Leaflet, Rachis and Frond Extracts and Powders on Goosegrass (Eleusine indica (L.) Gaertn) Germination, Emergence and Seedling Growth. Malaysian Appl. Biol. 2015, 44, 75–84. [Google Scholar]
- Chauhan, B.S.; Johnson, D.E. Germination Ecology of Goosegrass (Eleusine indica): An Important Grass Weed of Rainfed Rice. Weed Sci. 2008, 56, 699–706. [Google Scholar] [CrossRef]
- Shrestha, A.; Anwar, M.P.; Islam, A.; Gurung, T.; Dhakal, S.; Tanveer, A.; Javaid, M.M.; Nadeem, M.; Ikram, N.A. Weed Science as a New Discipline and Its Status in Some South Asian Universities and Colleges: Examples from Bangladesh, Bhutan, Nepal and Pakistan. CAB Rev. 2021, 16, 1–14. [Google Scholar] [CrossRef]
- Sunohara, Y.; Shirai, S.; Wongkantrakorn, N.; Matsumoto, H. Sensitivity and Physiological Responses of Eleusine indica and Digitaria adscendens to Herbicide Quinclorac and 2,4-D. Environ. Exp. Bot. 2010, 68, 157–164. [Google Scholar] [CrossRef]
- Khaket, T.P.; Aggarwal, H.; Jodha, D.; Dhanda, S.; Singh, J. Parthenium hysterophorus in current scenario: A toxic weed with industrial, agricultural and medicinal applications. J. Plant Sci. 2015, 10, 42–53. [Google Scholar] [CrossRef]
- Scavo, A.; Mauromicale, G. Crop Allelopathy for Sustainable Weed Management in Agroecosystems: Knowing the Present with a View to the Future. Agronomy 2021, 11, 2104. [Google Scholar] [CrossRef]
- Motmainna, M.; Juraimi, A.S.; Uddin, M.K.; Asib, N.B.; Islam, A.K.M.M.; Hasan, M. Bioherbicidal Properties of Parthenium hysterophorus, Cleome rutidosperma and Borreria alata Extracts on Selected Crop and Weed Species. Agronomy 2021, 11, 643. [Google Scholar] [CrossRef]
- Ambika, S.R. Multifaceted Attributes of Allelochemicals and Mechanism of Allelopathy. In Allelopathy; Springer: Berlin/Heidelberg, Germany, 2013; pp. 389–405. [Google Scholar]
- Aslani, F.; Juraimi, A.S.; Ahmad-Hamdani, M.S.; Hashemi, F.S.G.; Alam, M.A.; Hakim, M.A.; Uddin, M.K. Effects of Tinospora tuberculata Leaf Methanol Extract on Seedling Growth of Rice and Associated Weed Species in Hydroponic Culture. J. Integr. Agric. 2016, 15, 1521–1531. [Google Scholar] [CrossRef]
- Hao, W.; Ren, L.; Ran, W.; Shen, Q. Allelopathic Effects of Root Exudates from Watermelon and Rice Plants on Fusarium oxysporum f. Sp. Niveum. Plant Soil 2010, 336, 485–497. [Google Scholar] [CrossRef]
- Belz, R.G.; Reinhardt, C.F.; Foxcroft, L.C.; Hurle, K.; Singh, I. Residue Allelopathy in Parthenium hysterophorus L.-Does Parthenin Play a Leading Role? Crop. Prot. 2007, 26, 237–245. [Google Scholar] [CrossRef]
- Hasan, M.; Ahmad-Hamdani, M.S.; Rosli, A.M.; Hamdan, H. Bioherbicides: An eco-friendly tool for sustainable weed management. Plants 2021, 10, 1212. [Google Scholar] [CrossRef]
- Holt, J.S.; Welles, S.R.; Silvera, K.; Heap, I.M.; Heredia, S.M.; Martinez-Berdeja, A.; Palenscar, K.T.; Sweet, L.C.; Ellstrand, N.C. Taxonomic and Life History Bias in Herbicide Resistant Weeds: Implications for Deployment of Resistant Crops. PLoS ONE 2013, 8, 71916. [Google Scholar] [CrossRef] [PubMed]
- Dilipkumar, M.; Chuah, T.S.; Goh, S.S.; Sahid, I. Weed Management Issues, Challenges, and Opportunities in Malaysia. Crop. Prot. 2020, 134, 104347. [Google Scholar] [CrossRef]
- Saini, A.; Aggarwal, N.K.; Sharma, A.; Kaur, M.; Yadav, A. Utility Potential of Parthenium hysterophorus for Its Strategic Management. Adv. Agric. 2014, 381859, 381859. [Google Scholar] [CrossRef]
- Hossen, K.; Ozaki, K.; Teruya, T.; Kato-noguchi, H. Three Active Phytotoxic Compounds from the Leaves of Albizia richardiana (Voigt.) King and Prain for the Development of Bioherbicides to Control Weeds. Cells 2021, 10, 2385. [Google Scholar] [CrossRef] [PubMed]
- De Mastro, G.; El Mahdi, J.; Ruta, C. Bioherbicidal Potential of the Essential Oils from Mediterranean lamiaceae for Weed Control in Organic Farming. Plants 2021, 10, 818. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Shukla, A.; Attri, K.; Kumar, M.; Kumar, P.; Suttee, A.; Singh, G.; Barnwal, R.P.; Singla, N. Global Trends in Pesticides: A Looming Threat and Viable Alternatives. Ecotoxicol. Environ. Saf. 2020, 201, 110812. [Google Scholar] [CrossRef]
- Thien, B.N.; Ba, V.N.; Man, M.T.; Loan, T.T.H. Analysis of the Soil to Food Crops Transfer Factor and Risk Assessment of Multi-Elements at the Suburban Area of Ho Chi Minh City, Vietnam Using Instrumental Neutron Activation Analysis (INAA). J. Environ. Manag. 2021, 291, 112637. [Google Scholar] [CrossRef]
- Patel, S. Harmful and beneficial aspects of Parthenium hysterophorus: An update. 3 Biotech 2011, 1, 1–9. [Google Scholar] [CrossRef]
- Xie, G.; Zhou, J.; Yan, X. Encyclopedia of Traditional Chinese Medicines: Molecular Structures, Pharmacological Activities, Natural Sources and Applications, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2011; ISBN 3642167381. [Google Scholar]
- Pareek, A.; Suthar, M.; Rathore, G.S.; Bansal, V. Feverfew (Tanacetum parthenium L.): A Systematic Review. Pharmacogn. Rev. 2011, 5, 103. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, J.; Khan, A.A.; Aziz, T.; Ali, W.; Ahmad, S.; Rahman, S.U.; Iqbal, Z.; Dablool, A.S.; Alruways, M.W.; Almalki, A.A.; et al. Phytochemical Investigation, Antioxidant Properties and In Vivo Evaluation of the Toxic Effects of Parthenium hysterophorus. Molecules 2022, 27, 4189. [Google Scholar] [CrossRef] [PubMed]
- NTSYS-pc, N.T.; Taxonomy, N. Multivariate Analysis System; Version 2.2; Exet. Softw.: Setauket, NY, USA, 2005. [Google Scholar]
- Tarinezhad, A.; Sabouri, A.; Mohammadi, S.A. Statistical Software NTSYS PC Application in Plant Breeding. The 7th Conference of Iran Statistics. Allame Tabatabaei University, Tehran, Iran, September 2005. Available online: http://irstat.ir/files/site1/files/conference/7thconference_(English).pdf (accessed on 24 June 2022).
- Parsons, W.T.; Parsons, W.T.; Cuthbertson, E.G. Noxious Weeds of Australia, 2nd ed.; CSIRO Publishing: Canberra, Australia; Inkata Press: Melbourne, Australia, 2001; ISBN 0643065148. [Google Scholar]
- Petersen, J.; Belz, R.; Walker, F.; Hurle, K. Weed Suppression by Release of Isothiocyanates from Turnip-rape Mulch. Agron. J. 2001, 93, 37–43. [Google Scholar] [CrossRef]
- Bezuneh, T.T. Phytochemistry and antimicrobial activity of Parthenium hysterophorus L.: A review. Sci. J. Anal. Chem. 2015, 3, 30–38. [Google Scholar] [CrossRef]
- Pandey, R.A.; Gole, A.R.; Sankpal, R.V.; Jadav, P.V.; Waghmode, M.S.; Patil, N.N. Bioactive Potential of Parthenium hysterophorus and Cytotoxicity Assay of Parthenin. Int. J. Pharm. Biol. Sci. 2019, 9, 296–313. [Google Scholar]
- Marwat, S.K.; Fazal-ur-Rehman; Khan, I.U. Ethnobotanical Importance and Phytochemical Constituents of Parthenium Weed (Parthenium hysterophorus L.)—A Review. Plant Sci. Today 2015, 2, 77–81. [Google Scholar] [CrossRef]
- Roy, D.C.; Shaik, M. Journal of Medicinal Plants Studies Toxicology, Phytochemistry, Bioactive Compounds and Pharmacology of Parthenium hysterophorus. J. Med. Plants Stud. 2013, 1, 126–141. [Google Scholar]
- Verdeguer, M.; Blázquez, M.A.; Boira, H. Phytotoxic Effects of Lantana camara, Eucalyptus camaldulensis and Eriocephalus africanus Essential Oils in Weeds of Mediterranean Summer Crops. Biochem. Syst. Ecol. 2009, 37, 362–369. [Google Scholar] [CrossRef]
- Javaid, A.; Anjum, T. Control of Parthenium hysterophorus L., by Aqueous Extracts of Allelopathic Grasses. Pakistan J. Bot. 2006, 38, 139. [Google Scholar]
- Verma, A.K.; Maurya, S.K.; Kumar, A.; Barik, M.; Yadav, V.; Umar, B.; Lawal, M.; Usman, Z.A.; Adam, M.A.; Awal, B. Inhibition of Multidrug Resistance Property of Candida Albicans by Natural Compounds of Parthenium hysterophorus L. An In-Silico Approach. J. Pharmacogn. Phytochem. 2020, 9, 55–64. [Google Scholar] [CrossRef]
- Asaduzzaman, M.; Asao, T. Autotoxicity in Strawberry under Recycled Hydroponics and Its Mitigation Methods. Hortic. J. 2020, 89, 124–137. [Google Scholar] [CrossRef]
- Batish, D.R.; Singh, H.P.; Kaur, S.; Kohli, R.K.; Yadav, S.S. Caffeic Acid Affects Early Growth, and Morphogenetic Response of Hypocotyl Cuttings of Mung Bean (Phaseolus aureus). J. Plant Physiol. 2008, 165, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Singh, H.P.; Mittal, S.; Batish, D.R.; Kohli, R.K. Phytotoxic Effects of Volatile Oil from Artemisia scoparia against Weeds and Its Possible Use as a Bioherbicide. Ind. Crops Prod. 2010, 32, 54–61. [Google Scholar] [CrossRef]
- Sharma, A.; Singh, H.P.; Batish, D.R.; Kohli, R.K. Chemical Profiling, Cytotoxicity and Phytotoxicity of Foliar Volatiles of Hyptis suaveolens. Ecotoxicol. Environ. Saf. 2019, 171, 863–870. [Google Scholar] [CrossRef]
- Reigosa, M.J.; Pazos-Malvido, E. Phytotoxic Effects of 21 Plant Secondary Metabolites on Arabidopsis thaliana Germination and Root Growth. J. Chem. Ecol. 2007, 33, 1456–1466. [Google Scholar] [CrossRef]
- Bajwa, A.A.; Weston, P.A.; Gurusinghe, S.; Latif, S.; Adkins, S.W.; Weston, L.A. Toxic Potential and Metabolic Profiling of Two Australian Biotypes of the Invasive Plant Parthenium Weed (Parthenium hysterophorus L.). Toxins 2020, 12, 447. [Google Scholar] [CrossRef]
- Guo, Y.; Kim, K.-U.; Yoder, J.I.; Shin, D. Parasitic Plants as a New Target Plant for Screening Rice Allelopathic Potential. J. Life Sci. 2011, 5, 201. [Google Scholar]
- Rasouli, H.; Farzaei, M.H.; Mansouri, K.; Mohammadzadeh, S.; Khodarahmi, R. Plant Cell Cancer: May Natural Phenolic Compounds Prevent Onset and Development of Plant Cell Malignancy? A Literature Review. Molecules 2016, 21, 1104. [Google Scholar] [CrossRef]
- Macías, F.A.; Mejías, F.J.R.; Molinillo, J.M.G. Recent Advances in Allelopathy for Weed Control: From Knowledge to Applications. Pest Manag. Sci. 2019, 75, 2413–2436. [Google Scholar] [CrossRef]
- Latif, S.; Chiapusio, G.; Weston, L.A. Allelopathy and the Role of Allelochemicals in Plant Defence. In How Plants Communicate with Their Biotic Environment; Becard, G., Ed.; Academic Press: Cambridge, MA, USA, 2017; Volume 82, pp. 19–54. ISBN 0065-2296. [Google Scholar]
- Aslam, F.; Khaliq, A.; Matloob, A.; Tanveer, A.; Hussain, S.; Zahir, Z.A. Allelopathy in Agro-Ecosystems: A Critical Review of Wheat Allelopathy-Concepts and Implications. Chemoecology 2017, 27, 1–24. [Google Scholar] [CrossRef]
- Amarowicz, R.; Cwalina-Ambroziak, B.; Janiak, M.A.; Bogucka, B. Effect of N Fertilization on the Content of Phenolic Compounds in Jerusalem Artichoke (Helianthus tuberosus L.) Tubers and Their Antioxidant Capacity. Agronomy 2020, 10, 1215. [Google Scholar] [CrossRef]
- Braga, T.M.; Rocha, L.; Chung, T.Y.; Oliveira, R.F.; Pinho, C.; Oliveira, A.I.; Morgado, J.; Cruz, A. Biological Activities of Gedunin—A Limonoid from the Meliaceae Family. Molecules 2020, 25, 493. [Google Scholar] [CrossRef] [PubMed]
- Abbas, T.; Tanveer, A.; Khaliq, A.; Safdar, M.E.; Nadeem, M.A. Allelopathic Effects of Aquatic Weeds on Germination and Seedling Growth of Wheat. Herbologia 2014, 14, 11–25. [Google Scholar] [CrossRef]
- Scognamiglio, M.; Esposito, A.; D’Abrosca, B.; Pacifico, S.; Fiumano, V.; Tsafantakis, N.; Monaco, P.; Fiorentino, A. Isolation, Distribution and Allelopathic Effect of Caffeic Acid Derivatives from Bellis perennis L. Biochem. Syst. Ecol. 2012, 43, 108–113. [Google Scholar] [CrossRef]
- Krumsri, R.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Assessment of Allelopathic Potential of Senna garrettiana Leaves and Identification of Potent Phytotoxic Substances. Agronomy 2022, 12, 139. [Google Scholar] [CrossRef]
- Scognamiglio, M.; D’Abrosca, B.; Esposito, A.; Pacifico, S.; Monaco, P.; Fiorentino, A. Plant Growth Inhibitors: Allelopathic Role or Phytotoxic Effects? Focus on Mediterranean Biomes. Phytochem. Rev. 2013, 12, 803–830. [Google Scholar] [CrossRef]
- Safdar, M.E.; Aslam, A.; Qamar, R.; Ali, A.; Javaid, M.M.; Hayyat, M.S.; Raza, A. Allelopathic Effect of Prickly Chaff Flower (Achyranthes aspera L.) Used as a Tool for Managing Noxious Weeds. Asian J. Agric. Biol. 2021, 2021. [Google Scholar] [CrossRef]
- Ahn, J.K.; Chung, I.M. Allelopathic Potential of Rice Hulls on Germination and Seedling Growth of Barnyardgrass. Agron. J. 2000, 92, 1162–1167. [Google Scholar] [CrossRef]
- Salam, M.A.; Kato-Noguchi, H. Allelopathic potential of methanol extract of Bangladesh rice seedlings. Asian J. Crop Sci. 2010, 2, 70–77. [Google Scholar] [CrossRef]
- Mao, R.; Shabbir, A.; Adkins, S. Parthenium hysterophorus: A Tale of Global Invasion over Two Centuries, Spread and Prevention Measures. J. Environ. Manag. 2021, 279, 111751. [Google Scholar] [CrossRef]
- Schymanski, E.L.; Jeon, J.; Gulde, R.; Fenner, K.; Ruff, M.; Singer, H.P.; Hollender, J. Identifying Small Molecules via High Resolution Mass Spectrometry: Communicating Confidence. Environ. Sci. Technol. 2014, 48, 2097–2098. [Google Scholar] [CrossRef] [PubMed]
- Aslani, F.; Juraimi, A.S.; Ahmad-Hamdani, M.S.; Omar, D.; Alam, M.A.; Hashemi, F.S.G.; Hakim, M.A.; Uddin, M.K. Allelopathic Effect of Methanol Extracts from Tinospora Tuberculata on Selected Crops and Rice Weeds. Acta Agric. Scand. Sect. B Soil Plant Sci. 2014, 64, 165–177. [Google Scholar] [CrossRef]
- Mirmostafaee, S.; Azizi, M.; Fujii, Y. Study of Allelopathic Interaction of Essential Oils from Medicinal and Aromatic Plants on Seed Germination and Seedling Growth of Lettuce. Agronomy 2020, 10, 163. [Google Scholar] [CrossRef]
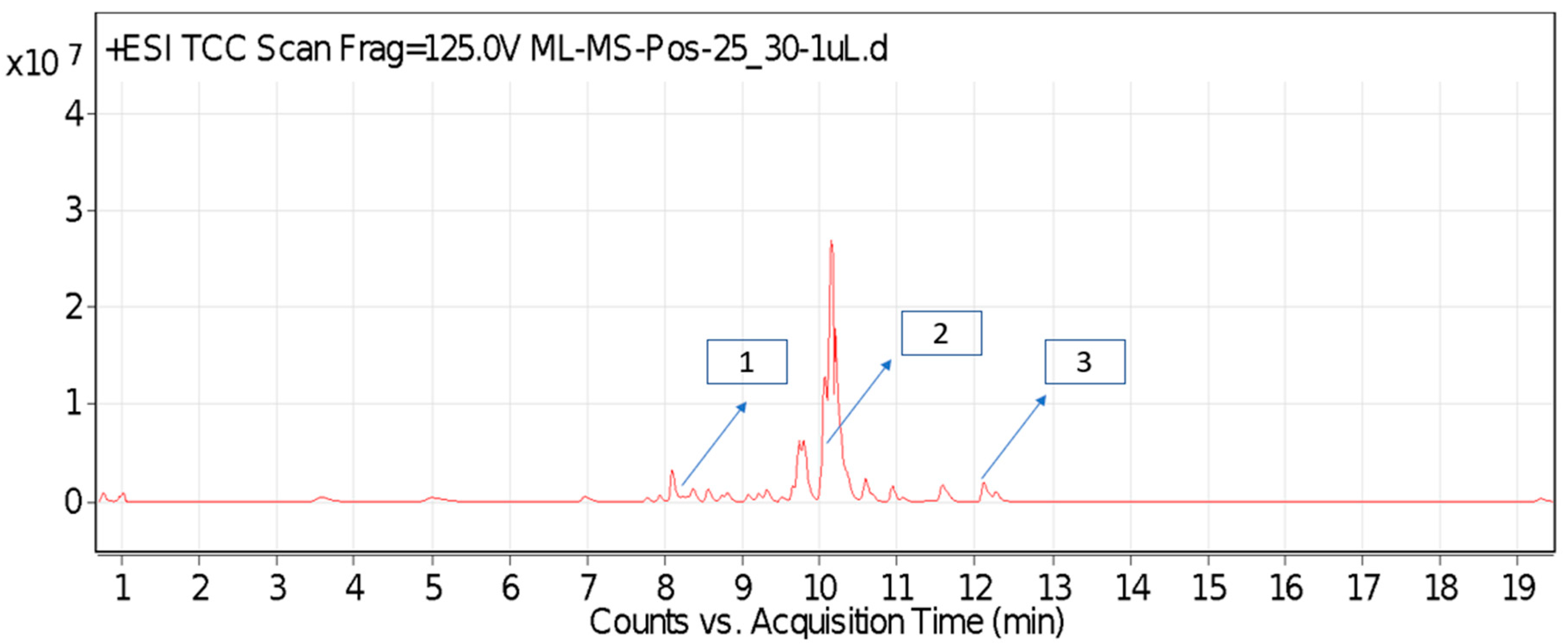
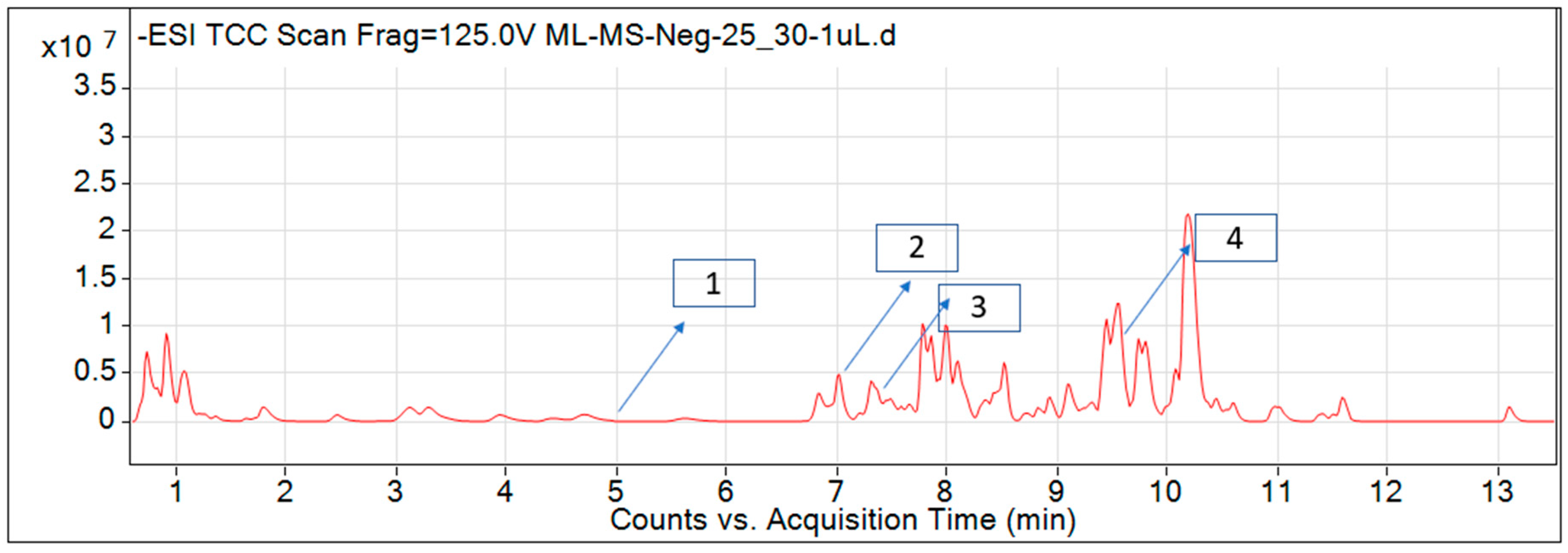
| Compounds | Retention Time | m/z | Mass | Group | References |
|---|---|---|---|---|---|
| 1-Methyl-1,3-cyclohexadiene | 0.742 | 112.1123 | 94.0784 | Oils | [22] |
| Octylamine | 5.022 | 130.1591 | 129.1518 | Unknown | |
| 3-(1-Pyrrolidinyl)-2-butanone | 0.779 | 142.1227 | 141.1154 | Unknown | |
| Quinic acid | 12.116 | 181.12 | 180.1129 | Phenolics | [22,23] |
| 3,5-dimethyl-Phenol | 9.084 | 197.1164 | 196.1091 | Volatile oils | [24] |
| Flossonol | 10.013 | 221.1164 | 220.1091 | Unknown | |
| 3,4,5-Trimethoxyphenyl acetate | 3.583 | 227.0923 | 226.085 | Unknown | |
| Undecenyl acetate | 11.071 | 235.1675 | 212.1782 | Unknown | |
| Lumichrome | 9.802 | 243.0879 | 242.0807 | Unknown | |
| D-Biotin | 10.156 | 245.0963 | 244.089 | Unknown | |
| L-beta-aspartyl-L-leucine | 10.065 | 247.1293 | 246.1221 | Amino acids | [22,25] |
| Histidylproline diketopiperazine | 10.117 | 249.1346 | 248.1275 | Amino acids | |
| 4,5-Dihydrovomifoliol | 10.356 | 249.1455 | 226.1563 | Volatile oils | [24] |
| Carbamic Acid tert-butyl ester | 9.522 | 249.1468 | 231.113 | Unknown | |
| Grosshemin (Parthenin) | 10.004 | 263.1267 | 262.1194 | Terpenoids, Phenolics | [22,25,26] |
| Sudan Brown RR | 9.658 | 280.1556 | 262.1217 | Unknown | |
| 16-hydroxy hexadecanoic acid | 12.274 | 290.2681 | 272.2342 | Amino acids | [25] |
| Artecanin | 8.592 | 296.1484 | 278.1145 | Terpenoids | [25] |
| Autumnolide | 8.738 | 298.1637 | 280.1298 | Unknown | |
| Hymenoflorin | 9.323 | 298.1655 | 280.1316 | Terpenoids | [25] |
| N-Histidyl-2-Aminonaphthalene | 8.372 | 298.1658 | 280.132 | Unknown | |
| EHNA | 8.18 | 300.1794 | 277.1901 | Unknown | |
| Artemisinin | 9.229 | 300.1795 | 282.1456 | Terpenoids | [25] |
| Dihydroartemisinin | 8.24 | 302.1951 | 284.1604 | Terpenoids | [25] |
| Ligulatin B | 11.653 | 324.1801 | 306.1464 | Terpenoids | [25] |
| Tetraneurin A | 9.918 | 340.176 | 322.1421 | Pseuguaianolids | [22] |
| Chlorogenic acid | 8.09 | 300.183 | 282.1482 | Phenolics | [22,26] |
| 4-O-Demethyl-13-dihydroadriamycinone | 8.555 | 403.1035 | 402.0961 | Unknown | |
| Cynaroside A | 7.774 | 462.2336 | 444.1996 | Flavonoids | |
| Maltotriitol | 10.154 | 507.1936 | 506.1861 | Unknown | |
| Hexafluoro-25-hydroxycholecalciferol | 10.109 | 531.2666 | 508.2779 | Unknown | |
| p-benzamidophenyl ester | 10.099 | 548.2992 | 547.292 | Unknown | |
| 7-Deacetoxy-7-Oxokhivorin | 10.69 | 560.2848 | 542.2514 | Unknown |
| Compounds | Retention Time | m/z | Mass | Group | References |
|---|---|---|---|---|---|
| (-)-12-hydroxy-9,10-dihydrojasmonic acid | 11.517 | 227.12948 | 228.13676 | Volatile oils | [24] |
| ®-3-®-3-Hydroxybutanoyloxy) butanoate | 8.163 | 189.07773 | 190.08498 | Unknown | |
| 1,3,7-Trimethyluric acid | 0.682 | 209.06864 | 210.07593 | Unknown | |
| 1,3,8-Trihydroxy-4-methyl-2,7-diprenylxanthone | 6.831 | 393.1689 | 394.17594 | Unknown | |
| 16-bromo-9E-hexadecanoic acid | 8.268 | 367.10596 | 332.13723 | Amino acids | [27] |
| 17-α, 1-Dihydroxy-11,20-dioxo-5-β-pregnan-3-α-yl-β-d-glucuronide | 10.169 | 539.24843 | 540.24875 | Unknown | |
| 1 alpha,5alpha-Epidithio-17a-oxa-D-homoandrostan-3,17-dione | 9.818 | 365.12623 | 366.13357 | Unknown | |
| 1-Methylhypoxanthine | 0.725 | 149.04721 | 150.05456 | Unknown | |
| 2,2,4,4-Tetramethyl-6-(1-oxobutyl)-1,3,5-cyclohexanetrione | 9.778 | 251.13009 | 252.13678 | Unknown | |
| 2,3-dimethyl-3-hydroxy-glutaric acid | 3.108 | 175.06173 | 176.06896 | Carbohydrate | [22] |
| 2,3-dinor Thromboxane B1 | 10.89 | 343.21388 | 344.22105 | Unknown | |
| 2,4,6-Triethyl-1,3,5-oxadithiane | 3.668 | 205.07267 | 206.08007 | Unknown | |
| 2,4-Diamino-6,7-dimethoxyquinazoline | 7.794 | 219.08808 | 220.09536 | Unknown | |
| 3-(4-Hydroxy-3-methoxyphenyl)-1,2-propanediol 2-O-(galloyl-glucoside) | 7.299 | 511.14783 | 512.15661 | Carbohydrate | [22] |
| 3,4-Dihydroxybenzoic acid (Vanillic acid) | 7.367 | 153.01983 | 154.02711 | Phenolics | [22,25,26] |
| 3-Amino-3-(4-hydroxyphenyl) propanoate | 0.998 | 180.06692 | 181.07393 | Amino acids | |
| 3-carboxy-4-methyl-5-propyl-2-furanpropanoic acid | 8.307 | 239.09312 | 240.10037 | Amino acids | |
| 3H-1,2,4-Triazol-3-one, 5-ethyl-2,4-dihydro-2-(3-hydroxypropyl)-4-(2-phenoxyethyl)- | 10.076 | 326.12684 | 291.15819 | Unknown | |
| 3-Hydroxy-3-methyl-2-oxo-Butyric acid | 1.633 | 131.03516 | 132.04241 | Oils | [22] |
| 3-Hydroxylidocaine | 9.798 | 285.13672 | 250.16761 | Amino acids | |
| 3-Methoxy-4-hydroxyphenylglycol glucuronide | 4.138 | 359.09968 | 360.10747 | Carbohydrate | [22] |
| 3-propylmalic acid | 3.918 | 175.06159 | 176.0688 | Unknown | |
| 4-(3-Methylbut-2-enyl)-L-tryptophan | 10.178 | 307.12149 | 272.152 | Amino acids | [27] |
| 4,4′-Stilbenedicarboxamidine | 10.027 | 263.13026 | 264.13744 | Unknown | |
| 4-Cyano-4-(3,4-dimethoxyphenyl)-5-methylhexylamine | 10.128 | 311.15235 | 276.18326 | Unknown | |
| 4-Hydroxyphenylpyruvic acid | 7.635 | 179.03577 | 180.04303 | Amino acids | [27] |
| 5,8,12-trihydroxy-9-octadecenoic acid | 11.435 | 329.23494 | 330.24207 | Amino acids | [27] |
| 7-beta-D-Glucopyranosyloxybutylidenephthalide | 9.821 | 365.12586 | 266.1332 | Unknown | |
| Abruquinone C | 10.18 | 375.10912 | 376.11641 | Flavonoids | [25] |
| Absindiol | 10.36 | 301.12166 | 266.15233 | Terpenoids | [24] |
| AFMK | 6.439 | 299.07923 | 264.10991 | Unknown | |
| Ala Tyr Pro | 9.747 | 384.1318 | 349.1631 | Unknown | |
| alpha-Carboxy-delta-decalactone | 10.168 | 213.11385 | 214.12108 | Unknown | |
| Amlodipine | 8.963 | 407.137 | 408.14433 | Flavonoids | [25] |
| Apodine | 8.371 | 401.12848 | 366.15935 | Flavonoids | |
| Apuleidin | 0.966 | 359.07565 | 360.08359 | Flavonoids | |
| Asparagoside F | 11.384 | 516.259 | 1034.5319 | Flavonoids | |
| Austalide C | 9.327 | 573.23642 | 574.24438 | Flavonoids | [25] |
| Baccatin III | 10.962 | 585.23644 | 586.24356 | Unknown | |
| Benzocaine | 1.797 | 164.07181 | 165.07903 | Unknown | |
| Benzyl O-[arabinofuranosyl-(1->6)-glucoside] | 9.634 | 401.14728 | 402.15456 | Carbohydrate | [22] |
| beta-Snyderol | 0.718 | 299.10119 | 300.10852 | Unknown | |
| Caffeic acid | 7.183 | 341.0894 | 342.09698 | Phenolics | [22,25,26] |
| Cardiogenol C | 8.864 | 259.12027 | 260.12766 | Flavonoides | [25] |
| Carteolol | 9.245 | 327.1469 | 292.17758 | Unknown | |
| Cys Arg Asn | 8.928 | 390.1574 | 391.16503 | Amino acids | [27] |
| Cys Asp Trp | 7.819 | 421.1202 | 422.12913 | Amino acids | [27] |
| Delavirdine | 9.697 | 491.16194 | 456.19328 | Unknown | |
| Diethyl (2R,3R)-2-methyl-3-hydroxysuccinate | 9.422 | 203.09338 | 204.10062 | Unknown | |
| Dihydroartemisinin | 7.893 | 283.15577 | 284.16289 | Flavonoids | [25] |
| Diphenylcarbazide | 10.381 | 241.10929 | 242.11671 | Unknown | |
| Enoxacin | 9.817 | 355.09854 | 320.12917 | Flavonoids | |
| Ent-afzelechin-7-O-beta-D-glucopyranoside | 9.752 | 435.1295 | 436.13611 | Cabohydrate | |
| Eremopetasitenin B2 | 9.553 | 463.17991 | 464.18582 | Terpenoids | |
| Ethotoin | 4.401 | 203.08308 | 204.09031 | Flavonoids | |
| Ethyl (S)-3-hydroxybutyrate glucoside | 6.87 | 293.12519 | 294.13239 | Carbohydrate | [22] |
| Ethyl 3-hydroxybutyrate | 7.386 | 131.07191 | 132.07915 | Unknown | |
| Ethyl Oxalacetate | 3.317 | 187.06185 | 188.06916 | Unknown | |
| Fenspiride | 9.779 | 295.12102 | 260.15156 | Unknown | |
| Ferulic acid | 9.84 | 193.05129 | 194.05855 | Phenolics | [22,25,26] |
| Florilenalin | 10.259 | 299.10472 | 264.13681 | Terpenoids | [25] |
| Fluvoxamine acid | 4.695 | 353.0896 | 318.12038 | Terpenoids | [25] |
| Formononetin 7-O-glucoside-6”-O-malonate | 9.121 | 515.12161 | 516.12902 | Unknown | [25] |
| Ganglioside GT1b (d18:1/22:1(13Z)) | 10.45 | 1089.5495 | 2181.1127 | Unknown | [25] |
| Gingerol | 13.113 | 293.1772 | 294.18435 | Terpenoids | [25] |
| Gitonin | 10.546 | 524.25681 | 1050.5274 | Unknown | [25] |
| Gly Val | 0.692 | 209.06916 | 174.09982 | Unknown | [25] |
| Glycobismine A | 8.04 | 601.23429 | 602.24125 | Terpenoids | [25] |
| Granisetron metabolite 4 glucuronide | 7.266 | 489.19925 | 490.20683 | Terpenoids | |
| Guanosine | 1.22 | 282.08553 | 283.09279 | Unknown | |
| Hinokitiol glucoside | 8.384 | 325.13062 | 326.13781 | Carbohydrate | [24] |
| Imazethapyr | 10.179 | 324.11121 | 289.14209 | Unknown | |
| Isobavachalcone | 7.011 | 323.12875 | 324.13481 | Terpenoids | [25] |
| Isoetin 4′-glucuronide | 8.936 | 477.06972 | 478.07703 | Terpenoids | [25] |
| Isopropyl β-D-Thiogalacto Pyranoside | 3.041 | 237.08105 | 238.08826 | Unknown | |
| Isoxaben | 9.746 | 367.14195 | 332.17282 | Phenolics | [22,25,26] |
| LPA(18:2(9Z,12Z)/0:0) | 9.989 | 469.21108 | 434.24192 | Unknown | |
| Leukotriene F4 | 6.989 | 603.25077 | 568.28124 | Unknown | |
| Levoglucosan | 1.872 | 161.04586 | 162.05315 | Unknown | |
| Licoagrone | 10.094 | 370.13067 | 742.28883 | Flavonoids | [25] |
| Maltopentaose | 9.11 | 863.24564 | 828.2779 | Unknown | |
| Manumycin A | 10.612 | 585.23644 | 550.26916 | Unknown | |
| Melleolide L | 8.696 | 485.11475 | 450.14606 | Unknown | |
| Mepiprazole | 8.597 | 303.13857 | 304.14582 | Unknown | |
| Methitural | 1.936 | 287.09005 | 288.0975 | Unknown | |
| Methyl ®-9-hydroxy-10-undecene-5,7-diynoate glucoside | 9.062 | 367.14164 | 368.14881 | Carbohydrate | [22] |
| Methyl 2-(4-isopropyl-4-methyl-5-oxo-2-imidazolin-2-yl)-p-toluate | 6.102 | 323.11808 | 288.14871 | Unknown | |
| Methyl 6-O-digalloyl-beta-D-glucopyranoside- | 10.076 | 309.13704 | 310.14433 | Unknown | |
| Methylthiomethyl 2-methylbutanethiolate | 0.925 | 177.04192 | 178.0498 | Unknown | |
| Mitoxantrone | 7.792 | 479.17082 | 444.20125 | Unknown | |
| Monodeallydihydroxyalmitrine | 7.792 | 506.18981 | 471.22059 | Unknown | |
| Metofluthrin | 7.452 | 395.10599 | 360.13672 | Terpenoids | [25] |
| N-Ac-Tyr-Val-Ala-Asp-CHO | 6.791 | 491.21465 | 492.22186 | Unknown | |
| Nomilinic acid 17-glucoside | 7.361 | 747.2638 | 712.2946 | Carbohydrate | [22] |
| Novobiocin | 8.00 | 611.22246 | 612.22878 | Flavonoids | |
| N-Benzoylaspartic acid | 7.193 | 236.0572 | 237.06511 | Unknown | |
| N-Carboxytocainide glucuronide | 4.809 | 447.11614 | 412.14707 | Terpenoids | |
| N-Feruloylglycine | 7.739 | 250.07333 | 251.08065 | Unknown | |
| N-Histidyl-2-Aminonaphthalene (βNA) | 8.382 | 279.12482 | 280.13203 | Unknown | |
| N-isovalerylglycine | 4.074 | 158.08237 | 159.08961 | Amino acids | |
| O-b-D-Gal-(1->3)-O-2-(acetylamino)-2-deoxy-D-Galactose | 7.361 | 747.26473 | 748.27167 | Carbohydrate | |
| Octadecanoic acid-1,2,2,2-tetrafluoro-1-(trifluoromethyl)ethyl ester | 9.992 | 487.22004 | 452.25135 | Unknown | |
| Osmanthuside A | 1.275 | 445.14833 | 446.15547 | Unknown | |
| p-Anisic acid | 5.121 | 151.04047 | 152.04764 | Phenolics | [23,26,28,29] |
| Pantothenic Acid | 2.325 | 218.10344 | 219.11059 | Carbohydrate | [22] |
| Phe Gln Cys | 10.04 | 395.14093 | 396.14923 | Unknown | |
| Phe-Phe-OH | 8.36 | 455.1035 | 420.13444 | Unknown | |
| Phomopsin A | 9.462 | 823.25172 | 788.28311 | Unknown | |
| Pirenzepine | 7.02 | 350.16201 | 351.16907 | Flavonoids | [24] |
| Podolactone B | 8.382 | 393.11983 | 394.12758 | Unknown | |
| Polyethylene | 9.984 | 243.12483 | 244.13204 | Unknown | |
| Prasugrel | 0.94 | 372.10699 | 373.11437 | Unknown | |
| Procaterol | 9.339 | 325.13113 | 290.16163 | Unknown | |
| Prostaglandin M | 6.886 | 327.1464 | 328.15356 | Unknown | |
| Pumilaisoflavone B | 9.54 | 463.17725 | 464.18226 | Unknown | |
| p-Salicylic acid | 9.691 | 137.0249 | 138.03214 | Flavonoids | |
| Pseudomonine | 7.478 | 329.12565 | 330.13297 | Unknown | |
| Pymetrozine | 4.305 | 216.08865 | 217.09607 | Unknown | |
| Pyrimidifen | 7.763 | 753.36276 | 377.18236 | Unknown | |
| Quinic acid | 1.733 | 191.05593 | 192.06314 | Phenolics | [24] |
| Sandoricin | 10.709 | 587.25136 | 588.25887 | Unknown | |
| Schizonepetoside C | 8.254 | 329.15925 | 330.16633 | Unknown | |
| Scopolin | 7.497 | 353.08966 | 354.09698 | Unknown | |
| Scutellarein 5-glucuronide | 9.323 | 461.07476 | 462.08215 | Unknown | |
| Semilepidinoside A | 8.2 | 371.10076 | 336.13199 | Unknown | |
| Senkirkine | 7.206 | 364.17768 | 365.18464 | Unknown | |
| Septentriodine | 9.546 | 735.32993 | 700.36121 | Unknown | |
| Sesamex | 8.316 | 297.1338 | 298.13951 | Unknown | |
| Sulfometuron | 7.517 | 349.06195 | 350.06935 | Unknown | |
| Sudan Brown RR | 10.168 | 523.23569 | 262.12103 | Unknown | |
| Taraxacolide 1-O-b-D-glucopyranoside | 8.53 | 855.40326 | 428.20581 | Unknown | |
| Tetraneurin A | 9.749 | 357.11227 | 322.14331 | Pseudo guaianolides | [22] |
| Tetranor-PGEM | 8.757 | 325.12836 | 326.13554 | Unknown | |
| Tolbutamide | 7.664 | 305.07242 | 270.10384 | Unknown | |
| Torasemide | 9.091 | 347.11884 | 348.12624 | Flavonoids | [25] |
| Toyocamycin | 0.844 | 290.09081 | 291.09822 | Unknown | |
| Trans-trismethoxy Resveratrol-d4 | 1.263 | 309.12077 | 274.15123 | Unknown | |
| Trimethylolpropane triacrylate | 9.781 | 295.11991 | 296.12733 | Unknown | |
| Trp Glu Leu | 7.86 | 891.42472 | 446.21425 | Unknown | |
| Trp Ser Pro | 7.828 | 387.16782 | 388.17496 | Unknown | |
| Trp Thr Ile | 9.228 | 417.21434 | 418.22135 | Unknown | |
| Tutin | 9.327 | 293.10437 | 294.11161 | Unknown | |
| Ustiloxin D | 9.905 | 493.23059 | 494.23751 | Unknown | |
| Val Trp Glu | 7.589 | 431.194 | 432.20106 | Unknown | |
| Vanilloloside | 7.238 | 315.10961 | 316.117 | Unknown | |
| Vinylacetylglycine | 1.368 | 142.05147 | 143.0587 | Unknown |
| Sl No. | Compounds | Retention Time | m/z | Mass | Polarity | Synonyms | Chemical Formula | Chemical Structure | Biological Activity | References |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. | Caffeic acid | 7.183 | 341.0894 | 342.09698 | Negative | 3-4-Dihydroxy cinnamic acid | C9H8O4 |  | Antifungal, dermatitis, autotoxic, inhibitory effect to other plants | [22,23,24,25,26,27,28,29,30,31,32] |
| 3-(3,4-dihydroxy phenyl) acrylic acid | ||||||||||
| 2. | Ferulic acid | 9.84 | 193.05129 | 194.05855 | Negative | Trans-ferulic acid | C10H10O4 | 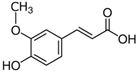 | ||
| 4-hydroxy-3-methoxy cinnamic acid | ||||||||||
| Coniferic acid | ||||||||||
| 2 Propenoic acid, 3-(4-hydroxy-3-methoxy phenyl) | ||||||||||
| 3. | Vanillic acid | 7.367 | 153.01983 | 154.02711 | Negative | 4-hydroxy-3-methoxybenzoic acid | C8H8O4 |  | ||
| Benzoic acid, 4-hydroxy-3-methoxy | ||||||||||
| 4. | Quinic acid | 12.116 | 181.12 | 180.1129 | Positive | D-(-)-Quinic acid | C7H12O6 | 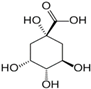 | ||
| Chinic acid | ||||||||||
| Quinate | ||||||||||
| 1,3,4,5-tetrahydroxy cyclohexanecarboxylic acid | ||||||||||
| 5. | Parthenin | 10.004 | 263.1267 | 262.1194 | Positive | 10-alpha-H-Ambrosa-2,11(13)-1,6-beta di-hydroxy-4-oxo-,gamma –lactone | C15H18O4 |  | ||
| Grosshemin | ||||||||||
| Helenalin | ||||||||||
| 6. | Chlorogenic acid | 8.09 | 300.183 | 282.1421 | Positive | 3, O -caffeoylquinic acid | C16H18O9 | 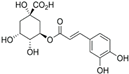 | ||
| 3-(3,4-dihydroxy cinnamoyl) quinic acid | ||||||||||
| 3-caffeoylquinic acid | ||||||||||
| 1,3,4,5-tetrahydroxy cyclohexanecarboxylic acid | ||||||||||
| 7. | p-Anisic acid | 5.121 | 151.04047 | 152.04764 | Negative | 4-methoxy benzoic acid | C8H8O3 | 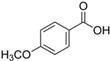 | ||
| p-anisic acid | ||||||||||
| p-methoxybenzoic acid |
| Compounds | Concentration (μM) | |||||
|---|---|---|---|---|---|---|
| 0.00 | 100 | 200 | 400 | 800 | 1600 | |
| Caffeic acid | 83.33 ± 3.33 aA (0) | 71.11 ± 1.92 bB (14.66) | 54.44 ± 1.92 cC (34.66) | 45.55 ± 1.92 dB (45.33) | 33.31 ± 3.30 eC (60.02) | 0.00 fE (100.00) |
| Vanillic acid | 83.33 ± 3.33 aA (0) | 75.55 ± 1.92 bA (9.33) | 69.99 ± 3.33 cA (16.00) | 64.44 ± 1.92 dA (22.66) | 58.88 ± 1.92 eA (29.34) | 53.33 ± 3.33 fAB (36.00) |
| Ferulic acid | 83.33 ± 3.33 aA (0) | 73.33 ± 3.33 bAB (12.00) | 66.66 ± 3.33 cAB (20.00) | 61.11 ± 1.92 dA (26.66) | 55.55 ± 1.92 eAB (33.33) | 52.22 ± 1.92 eAB (37.33) |
| Chlorogenic acid | 83.33 ± 3.33 aA (0) | 73.33 ± 3.33 bAB (12.00) | 67.77 ± 1.92 bcAB (18.67) | 63.33 ± 3.33 cA (24.00) | 53.33 ± 3.33 dB (36.00) | 43.33 ± 3.33 eC (48.00) |
| Quinic acid | 83.33 ± 3.33 aA (0) | 72.22 ± 1.92 bAB (13.33) | 66.66 ± 3.33 bcAB (20.00) | 64.44 ± 5.09 cA (22.66) | 59.99 ± 3.33 cA (28.00) | 52.21 ± 5.09 dAB (37.34) |
| p-Anisic acid | 83.33 ± 3.33 aA (0) | 73.33 ± 3.33 bAB (12.00) | 64.44 ± 5.09 cB (22.66) | 61.10 ± 5.09 cdA (26.67) | 59.99 ± 3.33 cdA (28.00) | 56.66 ± 3.33 dA (32.00) |
| Parthenin | 83.33 ± 3.33 aA (0) | 74.44 ± 1.92 bAB (10.66) | 64.44 ± 1.92 cB (22.66) | 62.22 ± 1.92 cA (25.33) | 53.33 ± 3.33 dB (36.00) | 33.33 ± 3.33 eD (60.00) |
| Mixture (all compounds) | 83.33 ± 3.33 aA (0) | 72.22 ± 1.92 bAB (13.33) | 68.88 ± 1.92 bAB (17.34) | 63.33 ± 3.33 cA (24.00) | 59.99 ± 3.33 cA (28.00) | 48.88 ± 1.92 dB (41.34) |
| CV (%) | 3.99 | 3.47 | 4.65 | 5.48 | 5.59 | 7.33 |
| LSD (0.05) | 5.76 | 4.40 | 5.26 | 5.76 | 5.25 | 5.39 |
| Compounds | Concentration (μM) | |||||
|---|---|---|---|---|---|---|
| 0.00 | 100 | 200 | 400 | 800 | 1600 | |
| Caffeic acid | 2.68 ± 0.03 aA (0) | 1.46 ± 0.02 bG (45.52) | 1.24 ± 0.02 cF (53.73) | 1.12 ± 0.01 dF (58.20) | 0.63 ± 0.02 eE (76.49) | 0.00 fG (100.00) |
| Vanillic acid | 2.68 ± 0.03 aA (0) | 2.46 ± 0.02 bA (8.20) | 2.36 ± 0.03 cA (11.94) | 2.06 ± 0.03 dA (23.13) | 1.65 ± 0.01 eA (38.43) | 1.13 ± 0.02 fCD (57.83) |
| Ferulic acid | 2.68 ± 0.03 aA (0) | 1.88 ± 0.02 bC (29.85) | 1.79 ± 0.01 cB (33.20) | 1.66 ± 0.02 dB (38.05) | 1.56 ± 0.01 eB (41.79) | 1.42 ± 0.01 fA (47.01) |
| Chlorogenic acid | 2.68 ± 0.03 aA (0) | 1.78 ± 0.02 bDE (33.58) | 1.68 ± 0.02 bcC (37.31) | 1.55 ± 0.01 dD (42.16) | 1.42 ± 0.02 eC (47.01) | 1.23 ± 0.02 fB (54.10) |
| Quinic acid | 2.68 ± 0.03 aA (0) | 1.75 ± 0.01 bE (34.70) | 1.60 ± 0.01 bcD (40.29) | 1.51 ± 0.02 dD (43.65) | 1.44 ± 0.02 eC (46.26) | 0.87 ± 0.01 fE (67.53) |
| p-Anisic acid | 2.68 ± 0.03 aA (0) | 1.94 ± 0.02 bB (27.61) | 1.70 ± 0.02 cC (36.56) | 1.63 ± 0.02 dBC (39.17) | 1.51 ± 0.02 eB (43.65) | 1.10 ± 0.01 fD (58.95) |
| Parthenin | 2.68 ± 0.03 aA (0) | 1.59 ± 0.03 bF (40.67) | 1.40 ± 0.03 cE (47.76) | 1.25 ± 0.02 dE (53.35) | 1.07 ± 0.07 eD (60.07) | 0.56 ± 0.01 fF (79.10) |
| Mixture (all compounds) | 2.68 ± 0.03 aA (0) | 1.82 ± 0.02 bD (32.08) | 1.68 ± 0.03 cC (37.31) | 1.61 ± 0.02 dC (39.92) | 1.42 ± 0.01 eC (47.01) | 1.14 ± 0.02 fC (57.46) |
| CV (%) | 1.13 | 1.27 | 1.49 | 1.46 | 2.50 | 1.78 |
| LSD (0.05) | 0.05 | 0.04 | 0.04 | 0.03 | 0.05 | 0.02 |
| Compounds | Concentration (μM) | |||||
|---|---|---|---|---|---|---|
| 0.00 | 100 | 200 | 400 | 800 | 1600 | |
| Caffeic acid | 2.98 ± 0.03 aA (0) | 2.58 ± 0.02 bC (13.42) | 2.12 ± 0.02 cF (28.85) | 1.56 ± 0.02 dF (47.65) | 0.86 ± 0.01 eF (71.14) | 0.00 fE (100) |
| Vanillic acid | 2.98 ± 0.03 aA (0) | 2.89 ± 0.04 bA (3.02) | 2.68 ± 0.02 cB (10.06) | 2.41 ± 0.02 dC (19.12) | 1.82 ± 0.03 eDE (38.92) | 1.41 ± 0.02 fC (52.68) |
| Ferulic acid | 2.98 ± 0.03 aA (0) | 2.73 ± 0.03 bB (8.38) | 2.64 ± 0.02 cC (11.40) | 2.52 ± 0.01 dB (15.43) | 2.02 ± 0.03 eC (32.21) | 1.67 ± 0.01 fA (43.95) |
| Chlorogenic acid | 2.98 ± 0.03 aA (0) | 2.73 ± 0.02 bB (8.38) | 2.66 ± 0.01 cBC (10.73) | 2.43 ± 0.02 dC (18.45) | 2.22 ± 0.02 eB (25.50) | 1.69 ± 0.02 fA (43.28) |
| Quinic acid | 2.98 ± 0.03 aA (0) | 2.62 ± 0.02 bC (12.08) | 2.33 ± 0.02 cE (21.81) | 2.19 ± 0.02 dE (26.51) | 1.83 ± 0.02 eD (38.59) | 1.63 ± 0.02 fB (45.30) |
| p-Anisic acid | 2.98 ± 0.03 aA (0) | 2.85 ± 0.02 bA (4.36) | 2.76 ± 0.02 cA (7.38) | 2.70 ± 0.01 dA (9.39) | 2.53 ± 0.02 eA (15.10) | 1.67 ± 0.02 fA (43.95) |
| Parthenin | 2.98 ± 0.03 aA (0) | 2.61 ± 0.02 bC (12.41) | 2.31 ± 0.03 cE (22.48) | 2.21 ± 0.01 dE (25.83) | 1.81 ± 0.02 eDE (39.26) | 1.11 ± 0.01 fD (62.75) |
| Mixture (all compounds) | 2.98 ± 0.03 aA (0) | 2.71 ± 0.02 bB (9.06) | 2.53 ± 0.02 cD (15.10) | 2.32 ± 0.02 dD (22.14) | 1.78 ± 0.02 eE (40.26) | 1.43 ± 0.03 fC (52.01) |
| CV (%) | 1.17 | 0.95 | 0.92 | 0.94 | 1.27 | 1.58 |
| LSD (0.05) | 0.06 | 0.04 | 0.03 | 0.03 | 0.04 | 0.03 |
| Allelopathic Compounds | ECg50 | ECr50 | ECs50 | Rank |
|---|---|---|---|---|
| Values in μM (Lower–Upper) | ||||
| Caffeic acid | 379.1(124.7–1054.7) | 168.5 | 361.9 (66.8–1471.1) | 909.5 |
| Vanillic acid | 4228.9(2024.1–23321.2) | 1251.0 | 1349.7 | 6829.6 |
| Ferulic acid | 3893.5(1797.5–27099.9) | 2650.6 | 2334.7 | 8878.8 |
| Chlorogenic acid | 1927.9(1217.9–4441.5) | 1060.6 | 2792.6 | 5781.1 |
| Quinic acid | 6081.9(2284.1–112731.5) | 562.3 | 2003.2 | 8647.4 |
| p-Anisic acid | 10972.3(2881.5–3224192.2) | 956.3 | 2917.2 | 14845.8 |
| Parthenin | 1234.1(895.3–2009.4) | 245.2 | 1090.1 | 2569.4 |
| Mixture (all compounds) | 3880.2(1843.9–23076.2) | 916.7 | 1442.3 | 6239.2 |
| Rank | 32,597.9 | 7811.2 | 14,291.7 | 54,700.8 |
| Compounds | Concentration (μM) | |||||
|---|---|---|---|---|---|---|
| 0.00 | 100 | 200 | 400 | 800 | 1600 | |
| Caffeic acid | 94.44 ± 1.92 aA (0) | 73.33 ± 3.33 bC (22.35) | 55.55 ± 1.92 cC (41.17) | 47.77 ± 1.92 dE (49.41) | 0.00 eE (100) | 0.00eE (100) |
| Vanillic acid | 94.44 ± 1.92 aA (0) | 92.21 ± 5.09 aA (2.36) | 79.99 ± 3.33 bB (15.30) | 71.11 ± 1.92 cCD (24.70) | 63.33 ± 3.33 dC (32.94) | 47.77 ± 1.92 eB (49.41) |
| Ferulic acid | 94.44 ± 1.92 aA (0) | 89.99 ± 3.33 bA (4.71) | 81.11 ± 1.92 cB (14.11) | 76.66 ± 3.33 dB (18.82) | 71.11 ± 1.92 eB (24.70) | 51.11 ± 1.92 fB (45.88) |
| Chlorogenic acid | 94.44 ± 1.92 aA (0) | 79.99 ± 3.33 bB (15.30) | 78.88 ± 1.92 bB (16.47) | 67.77 ± 1.92 cD (28.24) | 53.33 ± 3.33 dD (43.53) | 33.33 ± 3.33 eC (64.70) |
| Quinic acid | 94.44 ± 1.92 aA (0) | 92.22 ± 1.92 aA (2.35) | 91.11 ± 1.92 aA (3.52) | 84.44 ± 1.92 bA (10.58) | 71.11 ± 1.92 cB (24.70) | 49.99 ± 3.33 dB (47.06) |
| p-Anisic acid | 94.44 ± 1.92 aA (0) | 93.33 ± 3.84 aA (2.35) | 92.22 ± 3.33 aA (1.17) | 83.33 ± 3.33 bA (11.76) | 83.33 ± 3.33 bA (11.76) | 79.99 ± 3.33 bA (15.30) |
| Parthenin | 94.44 ± 1.92 aA (0) | 87.77 ± 5.09 bA (7.06) | 79.99 ± 3.33 cB (15.30) | 71.11 ± 1.92 dCD (24.70) | 53.33 ± 3.33 eD (43.53) | 21.11 ± 1.92 dD (77.64) |
| Mixture (all compounds) | 94.44 ± 1.92 aA (0) | 79.99 ± 3.33 bB (15.30) | 76.66 ± 3.33 bcB (18.82) | 73.33 ± 3.33 cdBC (22.35) | 68.88 ± 1.92 dB (27.06) | 49.99 ± 3.33 eB (47.06) |
| CV (%) | 2.03 | 4.40 | 3.42 | 3.53 | 4.53 | 6.32 |
| LSD (0.05) | 3.32 | 6.55 | 4.71 | 4.40 | 4.55 | 4.56 |
| Compounds | Concentration (μM) | |||||
|---|---|---|---|---|---|---|
| 0.00 | 100 | 200 | 400 | 800 | 1600 | |
| Caffeic acid | 2.74 ± 0.035 aA (0) | 1.49 ± 0.015 bG (45.62) | 1.28 ± 0.02 CG (53.28) | 0.403 ± 0.005 dG (85.29) | 0.00 eF (100) | 0.00eG (100) |
| Vanillic acid | 2.74 ± 0.035 aA (0) | 2.54 ± 0.020 bA (7.29) | 2.45 ± 0.025 cA (10.58) | 2.33 ± 0.025 dA (14.96) | 1.55 ± 0.010 eB (43.43) | 0.97 ± 0.010 fD (64.59) |
| Ferulic acid | 2.74 ± 0.035 aA (0) | 1.93 ± 0.025 bC (29.56) | 1.90 ± 0.015 bB (30.65) | 1.79 ± 0.01 CB (34.67) | 1.77 ± 0.01 CA (35.40) | 1.40 ± 0.005 dA (48.90) |
| Chlorogenic acid | 2.74 ± 0.035 aA (0) | 1.83 ± 0.032 bDE (33.57) | 1.82 ± 0.020 bC (33.21) | 1.74 ± 0.015 cC (36.49) | 1.50 ± 0.015 dC (45.25) | 1.40 ± 0.020 eA (48.90) |
| Quinic acid | 2.74 ± 0.035 aA (0) | 1.80 ± 0.025 bE (34.30) | 1.79 ± 0.020 bCD (34.67) | 1.60 ± 0.015 cE (41.60) | 1.55 ± 0.010 dB (43.43) | 0.67 ± 0.010 eE (75.54) |
| p-Anisic acid | 2.74 ± 0.035 aA (0) | 2.02 ± 0.025 bB (26.27) | 1.73 ± 0.025 cE (36.86) | 1.70 ± 0.025 cD (37.95) | 1.45 ± 0.015 dD (47.08) | 1.10 ± 0.011 eC (59.85) |
| Parthenin | 2.74 ± 0.035 aA (0) | 1.62 ± 0.025 bF (40.87) | 1.60 ± 0.026 bF (41.60) | 0.96 ± 0.015 cF (65.32) | 0.95 ± 0.01 CE (64.96) | 0.56 ± 0.015 dF (79.56) |
| Mixture (all compounds) | 2.74 ± 0.035 aA (0) | 1.86 ± 0.025 bD (32.11) | 1.79 ± 0.026 cD (34.67) | 1.68 ± 0.030 dD (38.68) | 1.51 ± 0.020 eC (44.89) | 1.16 ± 0.020 fB (57.66) |
| CV (%) | 1.28 | 1.30 | 1.26 | 1.26 | 0.98 | 1.48 |
| LSD (0.05) | 0.06 | 0.04 | 0.03 | 0.03 | 0.02 | 0.02 |
| Compounds | Concentration (μM) | |||||
|---|---|---|---|---|---|---|
| 0.00 | 100 | 200 | 400 | 800 | 1600 | |
| Caffeic acid | 3.09 ± 0.068 aA (0) | 2.71 ± 0.011 bE (12.29) | 2.23 ± 0.03 CF (27.83) | 1.66 ± 0.015 dH (46.27) | 0.00 eG (100) | 0.00 eF (100) |
| Vanillic acid | 3.09 ± 0.068 aA (0) | 3.03 ± 0.020 bA (1.94) | 2.88 ± 0.02 CB (6.79) | 2.09 ± 0.025 dG (32.36) | 1.77 ± 0.010 eF (42.71) | 1.42 ± 0.015 fD (54.04) |
| Ferulic acid | 3.09 ± 0.068 aA (0) | 2.86 ± 0.010 bBC (7.44) | 2.73 ± 0.025 cC (11.65) | 2.72 ± 0.023 cB (11.97) | 2.04 ± 0.02 C (33.98) | 1.69 ± 0.010 eA (45.30) |
| Chlorogenic acid | 3.09 ± 0.068 aA (0) | 2.86 ± 0.015 bB (7.44) | 2.76 ± 0.025 cC (10.67) | 2.44 ± 0.020 dD (21.03) | 2.24 ± 0.025 eB (27.50) | 1.67 ± 0.010 fA (45.95) |
| Quinic acid | 3.09 ± 0.068 aA (0) | 2.75 ± 0.015 bD (11.00) | 2.42 ± 0.015 cE (21.68) | 2.36 ± 0.010 dF (23.62) | 1.94 ± 0.015 eD (37.21) | 1.61 ± 0.020 fB (47.89) |
| p-Anisic acid | 3.09 ± 0.068 aA (0) | 3.01 ± 0.035 bA (2.58) | 2.98 ± 0.025 bA (3.55) | 2.89 ± 0.011 cA (6.47) | 2.75 ± 0.010 dA (11.00) | 1.67 ± 0.011 eA (45.95) |
| Parthenin | 3.09 ± 0.068 aA (0) | 2.75 ± 0.020 bD (11.00) | 2.43 ± 0.02 CE (21.35) | 2.39 ± 0.015 cE (22.65) | 1.86 ± 0.020 dE (39.80) | 0.20 ± 0.010 eE (93.52) |
| Mixture (all compounds) | 3.09 ± 0.068 aA (0) | 2.83 ± 0.020 bC (8.41) | 2.65 ± 0.02 CD (14.23) | 2.51 ± 0.025 dC (18.77) | 1.86 ± 0.025 eE (39.80) | 1.46 ± 0.025 fC (52.75) |
| CV (%) | 2.19 | 0.73 | 0.89 | 0.80 | 0.98 | 1.19 |
| LSD (0.05) | 0.11 | 0.03 | 0.04 | 0.03 | 0.03 | 0.02 |
| Allelopathic Compounds | ECg50 | ECr50 | ECs50 | Rank |
|---|---|---|---|---|
| Values in μM (Lower–Upper) | ||||
| Caffeic acid | 246.18 (30.74–672.05) | 138.00 | 300.52 (96.31–764.42) | 684.7 |
| Vanillic acid | 1558.74 (1158.61–2415.20) | 1074.88 | 1125.16 | 3758.78 |
| Ferulic acid | 2298.80 (1536.33–4482.93) | 3549.40 | 2121.82 | 7970.02 |
| Chlorogenic acid | 976.58 (755.64–1384.74) | 2149.42 | 2221.36 | 5347.36 |
| Quinic acid | 1870.23 (1438.35–2748.53) | 545.58 | 1865.92 | 4281.73 |
| p-Anisic acid | 16271.87 (5369.83–315463.09) | 849.02 | 2432.36 | 19553.25 |
| Parthenin | 795.38 (670.38–973.10) | 221.41 | 620.87 | 1637.66 |
| Mixture (all compounds) | 3131.83 (1662.03–s12079.69) | 1029.80 | 1452.17 | 5613.8 |
| Rank | 27,149.61 | 9557.51 | 12,140.18 | 48,847.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bashar, H.K.; Juraimi, A.S.; Ahmad-Hamdani, M.S.; Uddin, M.K.; Asib, N.; Anwar, M.P.; Karim, S.R.; Rahaman, F.; Haque, M.A.; Hossain, A. Documentation of Phytotoxic Compounds Existing in Parthenium hysterophorus L. Leaf and Their Phytotoxicity on Eleusine indica (L.) Gaertn. and Digitaria sanguinalis (L.) Scop. Toxins 2022, 14, 561. https://doi.org/10.3390/toxins14080561
Bashar HK, Juraimi AS, Ahmad-Hamdani MS, Uddin MK, Asib N, Anwar MP, Karim SR, Rahaman F, Haque MA, Hossain A. Documentation of Phytotoxic Compounds Existing in Parthenium hysterophorus L. Leaf and Their Phytotoxicity on Eleusine indica (L.) Gaertn. and Digitaria sanguinalis (L.) Scop. Toxins. 2022; 14(8):561. https://doi.org/10.3390/toxins14080561
Chicago/Turabian StyleBashar, HM Khairul, Abdul Shukor Juraimi, Muhammad Saiful Ahmad-Hamdani, Md Kamal Uddin, Norhayu Asib, Md. Parvez Anwar, SM Rezaul Karim, Ferdoushi Rahaman, Mohammad Amdadul Haque, and Akbar Hossain. 2022. "Documentation of Phytotoxic Compounds Existing in Parthenium hysterophorus L. Leaf and Their Phytotoxicity on Eleusine indica (L.) Gaertn. and Digitaria sanguinalis (L.) Scop" Toxins 14, no. 8: 561. https://doi.org/10.3390/toxins14080561
APA StyleBashar, H. K., Juraimi, A. S., Ahmad-Hamdani, M. S., Uddin, M. K., Asib, N., Anwar, M. P., Karim, S. R., Rahaman, F., Haque, M. A., & Hossain, A. (2022). Documentation of Phytotoxic Compounds Existing in Parthenium hysterophorus L. Leaf and Their Phytotoxicity on Eleusine indica (L.) Gaertn. and Digitaria sanguinalis (L.) Scop. Toxins, 14(8), 561. https://doi.org/10.3390/toxins14080561







