Abstract
Background/Objectives: Lifestyle modification is a cornerstone of obesity and metabolic syndrome (MetS) management, yet pharmacological agents are increasingly prescribed in the treatment of these highly prevalent issues. This meta-analysis compared the effects of diet-only, exercise-only, combined diet + exercise, and pharmacological interventions on MetS severity (MetS z-score) and related cardiometabolic outcomes in adults with overweight or obesity. Methods: A systematic search of relevant databases identified eligible studies published up to October 2025. Random-effects meta-analyses were conducted for pooled pre–post changes within intervention types and, where available, intervention-versus-control and head-to-head comparisons. Results: Forty-six studies comprising 85 intervention arms and 12,128 participants were included. Significant reductions in pooled MetS z-scores were observed following diet-only (−0.72 units; 17 arms), exercise-only (−0.63 units; 40 arms), diet + exercise (−0.68 units; 23 arms), and pharmacological interventions (−0.30 units; 5 arms) (all p < 0.001). Compared with controls, exercise-only reduced MetS z-score by −0.68 units (21 arms), and diet + exercise by −0.45 units (6 arms) (both p < 0.001), whereas pharmacotherapy showed no significant effect (−0.07 units; 5 arms; p = 0.134). Direct comparisons demonstrated that combined diet + exercise achieved greater MetS z-score reductions than diet-only (−0.75 units; 10 arms, p < 0.001). Moreover, lifestyle interventions consistently improved fasting glucose, triglycerides, HDL cholesterol, waist circumference, and blood pressure. Subgroup analyses identified caloric restriction as a key dietary moderator for cardiometabolic improvements, and meta-regression revealed exercise volume as a predictor of MetS z-score decrease. Conclusions: All intervention types improved cardiometabolic risk, but lifestyle strategies—particularly diet + exercise—demonstrated the most consistent and robust effects. While contemporary pharmacological therapies are known to induce substantial weight loss, their effects on overall cardiometabolic risk assessed by composite measures such as the MetS z-score remain insufficiently characterized, as most medication trials report single outcomes and frequently include concurrent lifestyle interventions. Therefore, there is a need for further trials evaluating the impact of anti-obesity medications on overall cardiometabolic health.
1. Introduction
The prevalence of overweight and obesity has reached epidemic proportions, representing a major global public health challenge [1]. Excess adipose tissue accumulation significantly increases risk of several serious health conditions, including type 2 diabetes mellitus (T2DM), cardiovascular disease and certain types of cancer [2]. The clustering of obesity-related cardiometabolic risk factors is conceptualized as the metabolic syndrome (MetS), which substantially amplifies the risk of major diseases and premature mortality [3]. Traditionally, MetS is diagnosed by the presence of at least three of the following risk factors: abdominal obesity, hypertension, dyslipidemia and hyperglycemia [4]. However, this dichotomous classification has been criticized for failing to capture gradations of cardiometabolic risk and for overlooking meaningful improvements that do not cross diagnostic thresholds [5,6]. Thus, continuous indices such as the MetS severity score (MetS z-score) have been developed to quantify global cardiometabolic burden in a sex- and age-specific manner, demonstrating strong validity and clinical relevance [5,6,7,8]. Importantly, the MetS z-score integrates information across all core MetS components into a single continuous metric, allowing for greater sensitivity to intervention-induced changes and facilitating the assessment of overall cardiometabolic risk beyond binary diagnostic categories.
Lifestyle interventions, principally dietary modifications and physical exercise, are first-line recommendations in obesity and MetS management guidelines [9,10,11]. Caloric restriction and targeted exercise programs have consistently shown favorable effects on a wide range of cardiometabolic outcomes [11,12,13,14,15]. Pharmacotherapy is generally regarded as a second-line option in the treatment of obesity and related cardiometabolic disorders, typically considered when lifestyle modification alone appears to be insufficient to achieve meaningful clinical improvements [11]. Metformin, for example, is an established insulin receptor-sensitizing antihyperglycemic agent for the treatment of T2DM. Although not a dedicated “weight-loss drug”, it has also been shown to provide modest effects on weight reduction [16,17]. Dipeptidyl peptidase-4 inhibitors (DPP-4i), another type of anti-diabetic medication, have also been reported to improve lipid levels or blood pressure [18]. More recently, the introduction of glucagon-like peptide-1 receptor agonists (GLP-1RA) has ushered in a new era of pharmacological treatment for obesity. The application of semaglutide and tirzepatide has demonstrated unprecedented weight loss in obese adults in large randomized trials, alongside improvements in several cardiometabolic risk factors, including waist circumference, blood pressure, glycemic markers, and lipid parameters [19,20,21,22,23]. These findings have fueled debate as to whether pharmacological treatment might reduce the necessity of diet and exercise. However, growing evidence indicates that pharmacotherapy often achieves its greatest and most durable benefits when implemented in conjunction with lifestyle interventions rather than as a stand-alone strategy [24]. A recent meta-analysis has indicated that lifestyle modification remains equally—if not more—effective for the prevention of T2DM compared to metformin treatment [25].
Despite the rapid expansion of pharmacological obesity research, important knowledge gaps remain. First, the majority of previous studies—across both lifestyle and medication-based interventions—have focused on weight loss and/or isolated cardiometabolic endpoints, whereas far fewer trials have assessed integrated cardiometabolic risk using composite measures such as the MetS z-score. Second, direct comparative evaluations of diet-only, exercise-only, combined diet + exercise, and pharmacological interventions on overall cardiometabolic health are scarce. Third, the frequent inclusion of concurrent lifestyle modification in medication studies, including landmark trials [12,20,22], complicates the interpretation of effects attributable specifically to pharmacotherapy on cardiometabolic risk. Therefore, whether the remarkable weight-loss effects observed with newer agents translate into proportionally larger improvements in cardiometabolic health remains insufficiently characterized. Addressing these limitations, the present systematic review and meta-analysis aimed to comprehensively compare the effects of diet, exercise, combined diet + exercise, and pharmacological interventions on MetS severity and its individual cardiometabolic components in adults with overweight and obesity.
2. Materials and Methods
This systematic review and meta-analysis was conducted following the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [26] and the Cochrane Handbook of Systematic Reviews of Interventions [27]. The study protocol, including detailed methodology and predefined eligibility criteria, was prospectively registered in the International Prospective Register of Systematic Reviews (PROSPERO; registration number: CRD420251112621).
2.1. Search Strategy
A comprehensive literature search was performed across the electronic databases PubMed, SPORT Discus, Science Direct, and Google Scholar, aiming to identify studies that investigated the impact of diet, exercise, combined diet + exercise or medication on MetS z-score in overweight/obese cohorts. The searches covered all original articles published from journal inception through October 2025 using logical combinations of keywords and Boolean operators related to MetS severity, cardiometabolic outcomes, diet, exercise, and pharmacotherapy. The complete search strategies for each database are provided in Supplementary Table S1. All duplicate records were removed prior to screening. Titles and abstracts were independently screened by two reviewers (V.V.W. and J.S.), followed by full-text evaluation of potentially eligible articles. Disagreements were resolved through discussion or, when necessary, consultation with a third reviewer (D.R.).
2.2. Study Selection and Inclusion Criteria
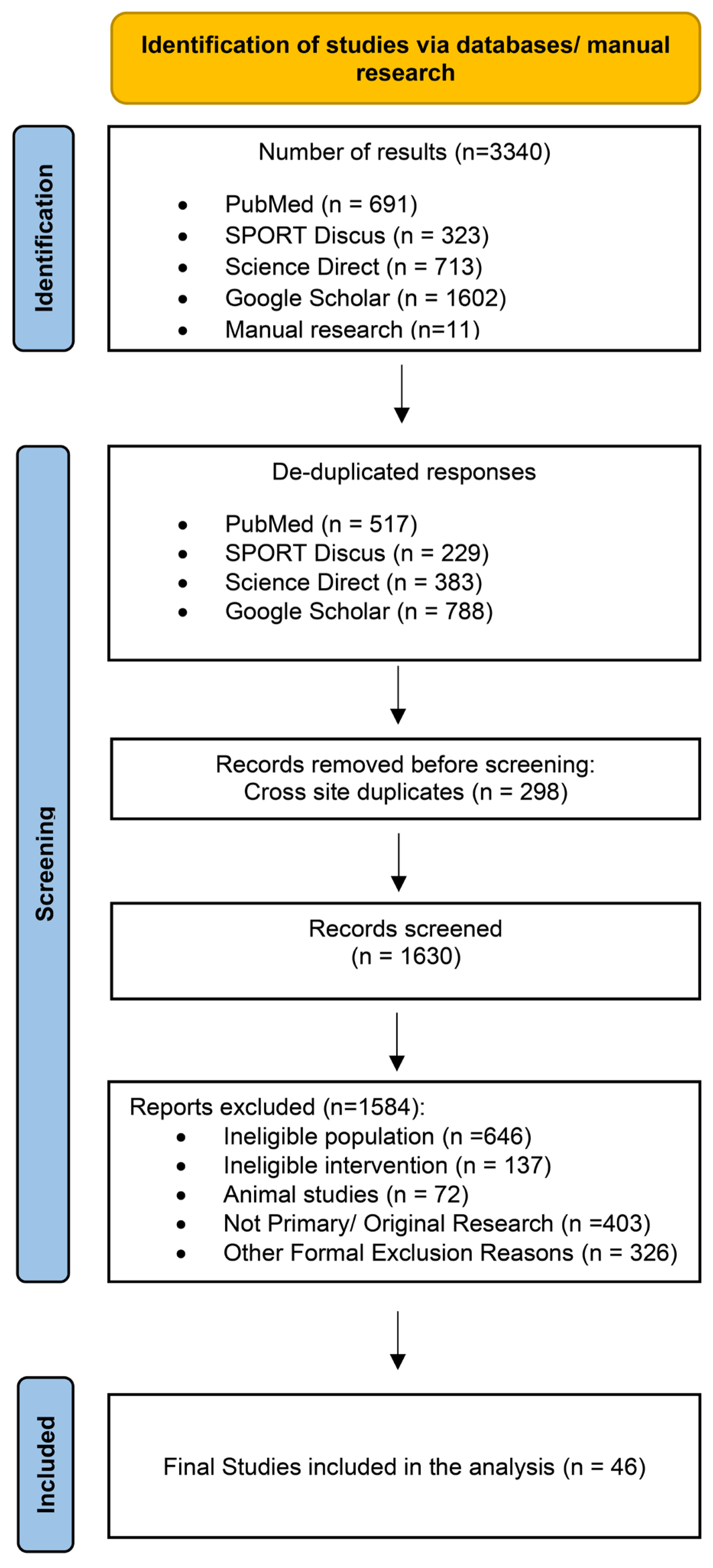
Studies were eligible if they (1) were published in English; (2) included adults (≥18 years) with overweight or obesity; (3) evaluated dietary, exercise, combined diet + exercise, or pharmacological interventions; (4) reported changes in the MetS z-score as the primary outcome; and (5) had an intervention duration of at least two weeks. The MetS z-score was defined as a continuous composite index integrating waist circumference, mean arterial blood pressure (MAB), fasting glucose, triglycerides, and HDL cholesterol (HDL) into a single sex- and age-specific measure of overall cardiometabolic risk. Studies were eligible regardless of the specific MetS z-score equation applied, provided that the score was derived from established MetS components and reported as a continuous outcome. Fasting glucose, MAB, waist circumference, triglycerides, HDL, total cholesterol, LDL cholesterol (LDL), systolic and diastolic blood pressure and homeostasis model assessment—insulin resistance (HOMA-IR) were recorded as secondary outcomes when available. Randomized, quasi-randomized, and non-controlled intervention studies were considered for inclusion. Observational research, narrative or systematic reviews, book chapters, conference abstracts, editorials, letters, and symposium proceedings were excluded. To minimize heterogeneity related to advanced disease states, studies conducted exclusively in cohorts with T2DM, cardiovascular disease, cancer, or other major chronic conditions were excluded. When studies did not report complete MetS z-score data (e.g., missing post-intervention values or standard deviations), corresponding authors were contacted to request the required information. If the missing data could not be obtained after an initial contact and a follow-up reminder within 2–4 weeks, the study was excluded from the quantitative synthesis. The study selection process is shown in Figure 1.
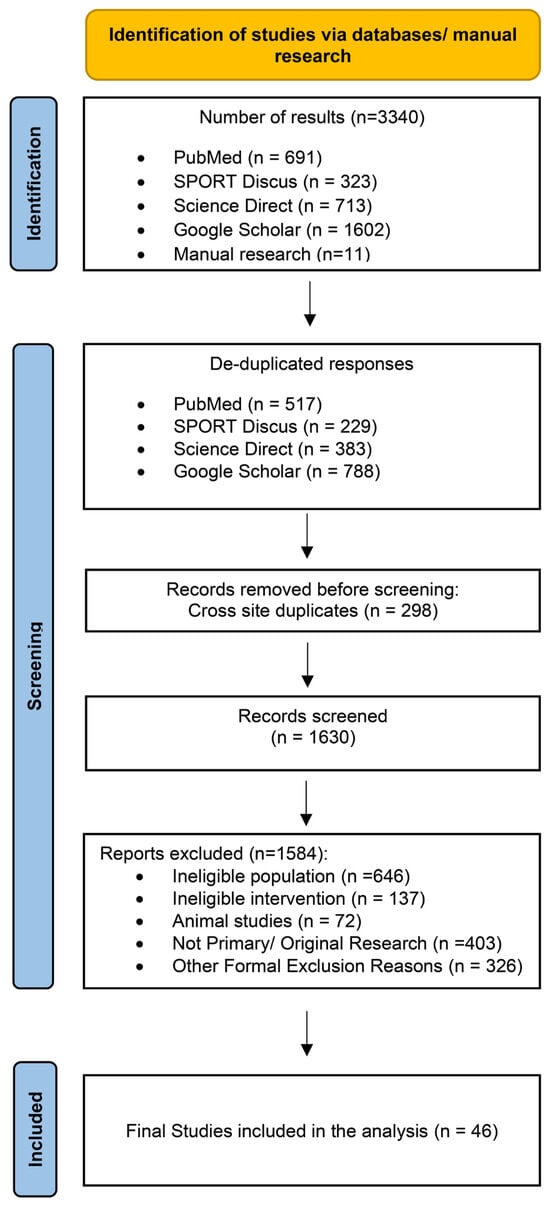
Figure 1.
Flow diagram of the systematic literature search.
2.3. Data Extraction
Data extraction was conducted by one reviewer (V.V.W.) and independently verified by a second reviewer (J.S.) using a predefined standardized template. Extracted data included study design, sample size, participant characteristics, intervention details, comparator conditions where applicable, and mean values with standard deviations for all outcomes at baseline and post-intervention. In studies with multiple intervention arms, data were extracted separately for each arm. When a single control group was shared across multiple interventions, comparisons were conducted independently, and control group sample sizes were proportionally adjusted to avoid double-counting. Further, information on study retention was extracted where reported, including completion rates and/or dropout proportions. These metrics were collected as pragmatic indicators of intervention feasibility and adherence.
2.4. Quality Assessment and Sensitivity Analyses
Risk of bias was assessed independently by two reviewers (V.V.W. and J.S.) using the Physiotherapy Evidence Database (PEDro) scale [28], which evaluates key methodological domains including randomization, allocation concealment, baseline comparability, completeness of follow-up, and reporting of between-group comparisons. PEDro scores range from 0 to 10, with higher scores indicating greater methodological rigor and lower risk of bias. Although originally developed for physiotherapy and exercise trials, the PEDro scale captures core quality criteria that are applicable across intervention modalities. Importantly, the majority of included studies involved exercise-based or combined lifestyle interventions, for which PEDro is widely accepted. Applying a single quality assessment instrument across all intervention types was preferred over using multiple tools, as this ensured consistency, comparability, and avoided introducing additional methodological heterogeneity into the risk-of-bias evaluation. Discrepancies in ratings were resolved through discussion, and when necessary, by consultation with a third reviewer (D.R.). The overall risk-of-bias assessments were summarized and considered in the interpretation of the results. Additionally, sensitivity analyses were performed for all outcomes using a leave-one-out approach, in which the meta-analysis was repeated after sequentially removing each individual study. This procedure allowed us to evaluate whether any single trial exerted an undue influence on the overall pooled estimates.
2.5. Statistical Analysis
Given that many studies lacked control groups or direct comparisons between intervention types, three complementary meta-analytic approaches were applied: (1) pooled pre–post analyses were calculated separately for each intervention type; (2) intervention versus control comparisons where available; and (3) direct head-to-head comparisons between intervention types where available. Weighted mean differences with 95% confidence intervals (CIs) were calculated using random-effects models to account for expected clinical and methodological heterogeneity across study populations, intervention characteristics, and study designs. Random-effects models were retained even in the presence of very high heterogeneity, as the primary objective was to estimate average intervention effects across diverse populations and intervention designs rather than to infer a single common effect. High I2 values were therefore interpreted as reflecting between-study variability in effect magnitude rather than grounds for excluding studies or abandoning quantitative synthesis.
Heterogeneity was assessed using the I2 statistic and Cochran’s Q test, with thresholds interpreted according to Cochrane recommendations [27]. Given the anticipated diversity across interventions and populations, heterogeneity was further explored through predefined subgroup analyses and meta-regressions rather than by excluding studies based on I2 values. Pharmacological interventions were pooled in a single meta-analysis to estimate the overall magnitude of medication-associated changes in cardiometabolic risk. This approach was chosen because the number of eligible studies per medication class was small, precluding adequately powered class-specific meta-analyses. Pooling was therefore performed to provide an exploratory estimate of the average effect of pharmacotherapy on MetS severity, while acknowledging underlying mechanistic heterogeneity. To partially address this, medication class was used as a subgroup variable and explored where sufficient data were available.
Prespecified subgroup analyses were conducted based on intervention characteristics (e.g., diet type, exercise modality and intensity, medication class), provided that at least three independent study arms were available per subgroup. Random-effects meta-regression analyses were conducted to examine the potential moderating effects of intervention duration, baseline age, baseline BMI, and weekly exercise volume. All moderators were entered as continuous variables in separate univariable models, with regression coefficients representing the change in effect size per unit increase in the respective moderator. In accordance with Cochrane recommendations, meta-regressions were performed only when ≥10 independent effect sizes were available for a given outcome to ensure adequate statistical power and reliability [27]. Potential publication bias was examined visually by inspecting funnel plots for asymmetry. The Egger’s regression test was additionally applied as a quantitative measure of possible publication bias [29].
Because MetS z-scores were derived using population-specific equations, all analyses were additionally replicated using standardized mean differences to ensure robustness against differences in score scaling. As these analyses produced equivalent significance patterns, mean differences were retained for primary reporting to enhance clinical interpretability. All statistical analyses were performed using Comprehensive Meta-Analysis software (version 4.0, Biostat Inc., Englewood, NJ, USA). A two-sided p < 0.05 was considered statistically significant unless otherwise specified.
3. Results
3.1. Included Studies
The systematic search identified 3340 records (PubMed: 691; SPORT Discus: 323; Science Direct: 713; Google Scholar: 1602; manual search: 11). After removal of duplicates, 1630 unique records remained for screening. Following title, abstract, and full-text evaluation, 46 studies comprising 85 intervention arms met the inclusion criteria and were included in the quantitative synthesis (Figure 1). Diet-only interventions were examined in 12 studies (17 arms) [30,31,32,33,34,35,36,37,38,39,40,41], exercise-only interventions in 26 studies (40 arms) [42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67], combined diet + exercise interventions in 15 studies (23 arms) [31,36,37,38,40,41,45,48,67,68,69,70,71,72,73], and pharmacological interventions in three studies (five arms) [69,74,75]. Intervention-versus-control comparisons were available in 24 studies, which included either exercise-only (21 arms), combined diet + exercise (six arms) or pharmacological treatment (five arms) contrasted against non-intervention or standard-care controls. Direct head-to-head comparisons between active interventions were only available for combined diet + exercise versus diet-only interventions (six studies, 10 arms).
3.2. Participant and Intervention Characteristics
Across all analyses, 12,128 adults with overweight or obesity were included. Sample sizes ranged from 8 to 4963 participants per intervention arm. Most studies enrolled mixed-sex cohorts, while nine included only women and two included only men. Mean participant age was 48.7 years (range: 21 to 77 years), and mean baseline BMI was 30.5 kg/m2 (range: 24.1–40.4 kg/m2). In all intervention types, participants’ mean BMI values decreased from pre- to post-intervention (diet: −2.6 kg/m2; exercise: −0.6 kg/m2; diet + exercise: −1.3 kg/m2; medication: −0.6 kg/m2).
The mean intervention duration was 28 weeks (range: 2–416 weeks), with 12 weeks being the most common duration. Dietary interventions consisted primarily of caloric restriction (10 arms) or dietary modification without explicit energy restriction (e.g., Mediterranean diet or specific macronutrient adjustments) (7 arms). Exercise interventions used predominantly cardiovascular training (32 arms), including continuous endurance training, walking programs, and interval training protocols, performed across a wide spectrum of exercise intensities. Resistance training was implemented in six arms, using free weights, weight-machine–based protocols or whole-body electromyostimulation (WB-EMS) exercise. Two arms combined aerobic and resistance components. The frequency of exercise sessions ranged from two to five sessions per week, with three sessions per week being the most common. Combined diet + exercise interventions universally included caloric restriction, with some protocols additionally incorporating increased protein intake. Exercise modalities included cardiovascular training (11 arms), resistance training (5 arms), combined aerobic–resistance training (6 arms), and yoga-based exercise (1 arm). Exercise frequency ranged from two to six sessions per week, with two weekly sessions being most frequently reported. Pharmacological interventions comprised metformin (two arms), GLP-1RAs (two arms), and one DPP-4i arm, administered at varying dosages.
Across intervention types, reporting of retention or dropout rates was heterogeneous but available for the majority of included trials. Where reported, mean completion rates were highest for diet-only and exercise-only interventions (both 86%), followed by pharmacological interventions (81%) and combined diet + exercise programs (79%).
3.3. Quality of Included Studies
The overall methodological quality of the included studies was rated as moderate to good, with a mean PEDro score of 6.1. Among the 46 publications evaluated, two studies achieved an “excellent” quality rating (9), 31 studies were classified as “good” (scores 6–8), 10 studies were rated as “fair” (scores 4–5), and three studies scored “low quality” (scores ≤ 3). Detailed PEDro quality ratings for each study are presented in Table 1.

Table 1.
Assessment of methodological quality and risk of bias with Pedro Score of all included studies.
3.4. Meta-Analyses on Dietary Interventions
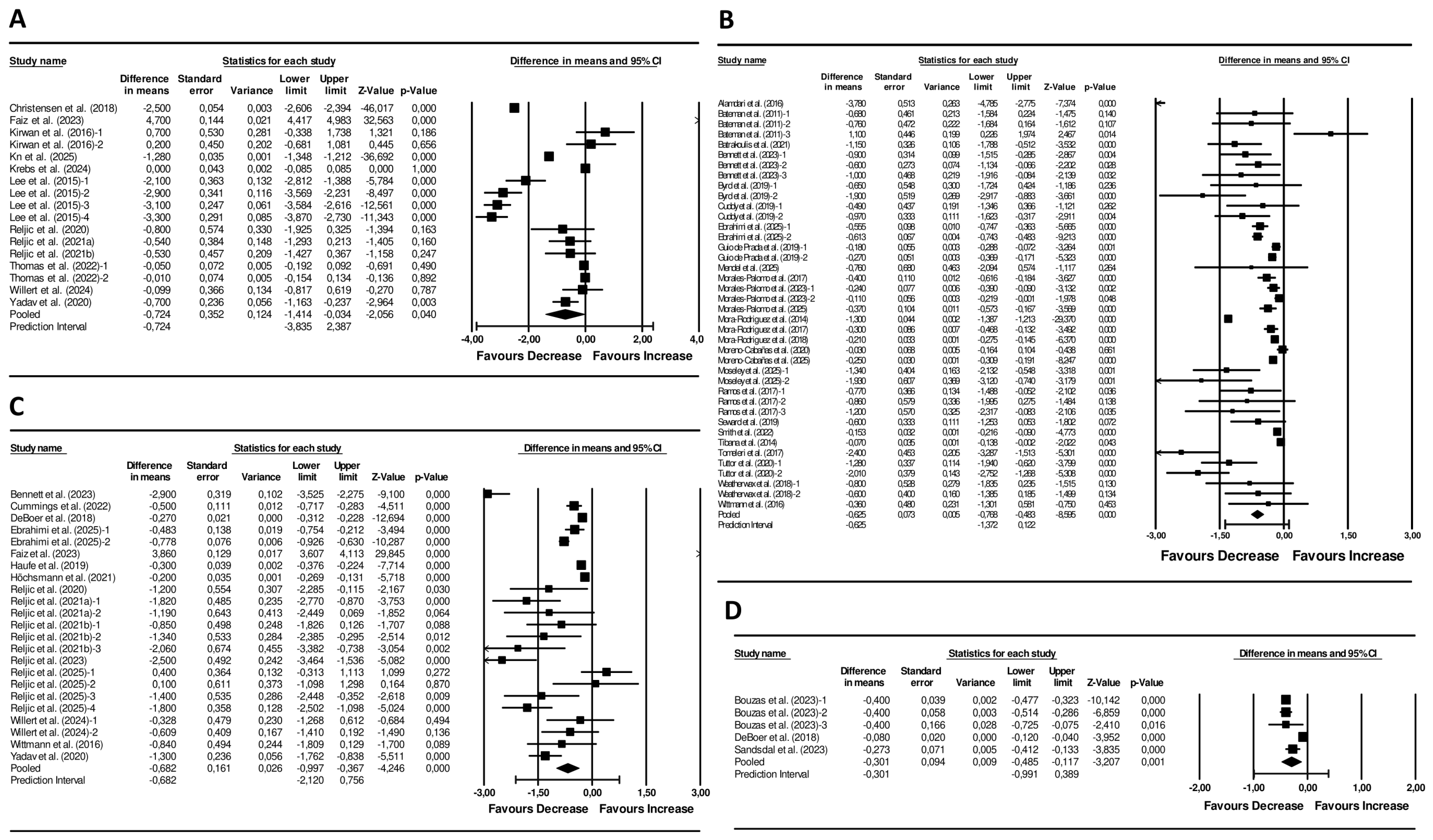
Dietary interventions showed a significant pooled reduction in the MetS z-score (pooled mean change: −0.72 units; 95% CI −1.41 to 0.03; p = 0.040; I2 = 99%, p < 0.001; 17 arms) (Figure 2). Visual inspection of the funnel plot displayed asymmetry (Figure S1), although Egger’s test was not significant (p = 0.698). Sensitivity analyses showed no change in the significance or direction of the pooled effect sizes (Figure S2).
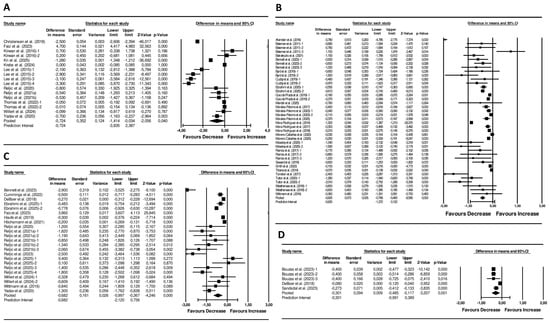
Figure 2.
Forest plot of the effects of (A) Diet Interventions [30,31,32,33,34,35,36,37,38,39,40,41], (B) Exercise Interventions [42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67], (C) Diet + Exercise Interventions [31,36,37,38,40,41,45,48,67,68,69,70,71,72,73], and (D) Pharmacological Interventions [69,74,75] on the MetS z-score. Mean differences and 95% CI confidence intervals. A negative value indicates an improvement in cardiometabolic health status.
Significant reductions were also observed for fasting glucose (−5 mg/dL; 95% CI −7 to −4; p < 0.001; I2 = 91%, p < 0.001; 16 arms), triglycerides (−21 mg/dL; 95% CI −30 to −11; p < 0.001; I2 = 91%, p < 0.001; 17 arms), MAB (−5 mmHg; 95% CI −7 to −3; p < 0.001; I2 = 89%, p < 0.001; 11 arms), and waist circumference (−3.6 cm; 95% CI −6.0 to −1.3; p = 0.002; I2 = 96%, p < 0.001; 17 arms). In addition, significant decreases were found for total cholesterol (−13 mg/dL; 95% CI −24 to −3; p = 0.014; I2 = 97%, p < 0.001; 12 arms), LDL (−9 mg/dL; 95% CI −16 to −4; p = 0.002; I2 = 93%, p < 0.001; 14 arms), HOMA-IR (−0.8 units; 95% CI −1.2 to −0.4; p < 0.001; I2 = 91%, p < 0.001; 12 arms), systolic (−7 mmHg; 95% CI −9 to −4; p < 0.001; I2 = 91%, p < 0.001; 13 arms) and diastolic blood pressure (−3 mmHg; 95% CI −5 to −2; p < 0.001; I2 = 85%, p < 0.001; 13 arms). Table 2 summarizes the included dietary interventions.

Table 2.
Diet Interventions.
3.4.1. Subgroup Analyses for Diet Interventions
Subgroup analyses demonstrated that diet type moderated intervention effects for systolic blood pressure (Q = 10.7, df = 1, p = 0.001), diastolic blood pressure (Q = 6.4, df = 1, p = 0.012), and HOMA-IR (Q = 9.2, df = 1, p = 0.002). Specifically, caloric-restriction diets were associated with larger pooled reductions in systolic blood pressure, (−8 mmHg; 95% CI −11 to −5; p < 0.001), diastolic blood pressure (−4 mmHg; 95% CI −6 to −2; p < 0.001), and HOMA-IR (−1.1 units; 95% CI −1.5 to −0.7; p < 0.001) compared with dietary modification approaches without an explicit energy deficit (systolic blood pressure: −3 mmHg; 95% CI −4 to −1; p < 0.001; diastolic blood pressure: −1 mmHg; 95% CI −3 to 0; p = 0.026; HOMA-IR: −0.2 units; 95% CI −0.6 to 0.2; p = 0.245).
3.4.2. Meta Regressions for Diet Interventions
Meta-regressions showed no significant associations between intervention duration, participant age, or baseline BMI and changes in any outcome (all p-values > 0.05).
3.5. Meta-Analyses on Exercise Interventions
Exercise interventions resulted in a significant pooled reduction in the MetS z-score of −0.63 units (95% CI −0.77 to −0.48; p < 0.001; I2 = 95%, p < 0.001; 40 arms) (Figure 2). Funnel plot asymmetry was observed (Figure S1) and the Egger’s test result was significant (p = 0.035). Sensitivity analyses revealed no alteration in the significance or direction of the pooled effect sizes (Figure S2).
Pooled decreases were also observed in fasting glucose (−3 mg/dL; 95% CI −5 to −2; p < 0.001; I2 = 92%, p < 0.001; 38 arms), triglycerides (−10 mg/dL; 95% CI −14 to −6; p < 0.001; I2 = 77%, p < 0.001; 40 arms), waist circumference (−2.3 cm; 95% CI −2.8 to −1.8; p < 0.001; I2 = 61%, p < 0.001; 38 arms), MAB (4 mmHg; 95% CI −6 to −2; p < 0.001; I2 = 97%, p < 0.001; 23 arms), and a significant increase was found for HDL levels (2 mg/dL; 95% CI 3 to 1; p < 0.001; I2 = 84%, p < 0.001; 40 arms). Moreover, significant reductions were evident for systolic (−6 mmHg; 95% CI −9 to −3; p < 0.001; I2 = 99%, p < 0.001; 29 arms), and diastolic blood pressure (−4 mmHg; 95% CI −6 to −2; p < 0.001; I2 = 98%, p < 0.001; 29 arms), total cholesterol (−2 mg/dL; 95% CI −3 to −1; p < 0.001; I2 = 6%, p = 0.379; 8 arms), LDL (−5 mg/dL; 95% CI −7 to −2; p < 0.001; I2 = 79%, p < 0.001; 8 arms), and HOMA-IR (−0.4 units; 95% CI −0.5 to −0.3; p < 0.001; I2 = 33%, p = 0.128; 11 arms). Table 3 provides an overview of the included exercise interventions.

Table 3.
Exercise Interventions.
3.5.1. Subgroup Analyses for Exercise Interventions
No significant moderating effects of exercise modality were observed for any outcome. However, exercise intensity moderated effects on waist circumference (Q = 8.6, df = 1, p = 0.013) and triglyceride concentrations (Q = 7.5, df = 1, p = 0.024). Interventions employing higher-intensity exercise produced more pronounced reductions in waist circumference (−2.7 cm; 95% CI −3.4 to −2.2; p < 0.001) compared with moderate-intensity protocols (−2.2 cm; 95% CI −2.9 to −1.6; p < 0.001). Conversely, moderate-intensity exercise was associated with larger decreases in triglyceride levels (−16 mg/dL; 95% CI −22 to −10; p < 0.001) than higher-intensity exercise (−7 mg/dL; 95% CI −13 to −1; p = 0.020).
3.5.2. Meta Regressions for Exercise Interventions
Meta-regression analyses found no evidence that intervention length, weekly exercise volume, average participant age, or baseline BMI significantly modified effects on the MetS z-score or any secondary outcomes (all p > 0.05).
3.5.3. Meta-Analyses on Exercise Interventions Versus Controls
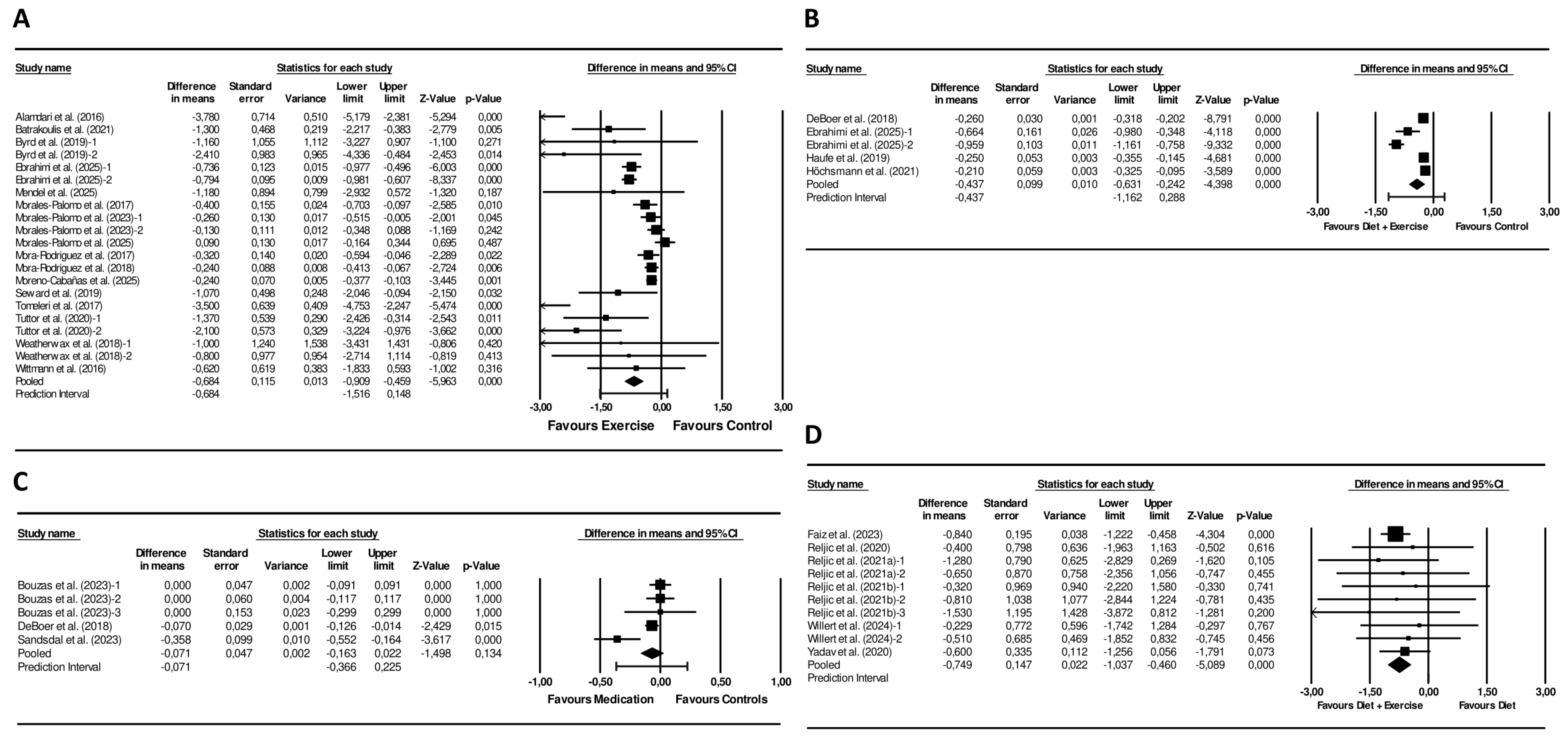
Exercise interventions led to a significantly larger reduction in the MetS z-score compared with controls (mean difference = −0.68 units; 95% CI −0.91 to −0.46; p < 0.001; I2 = 83%, p < 0.001; 21 arms) (Figure 3). Funnel plot asymmetry was present (Figure S3) and this observation was supported by a significant Egger’s test (p = 0.006). Sensitivity analyses did not affect the significance or direction of the pooled differences (Figure S4).

Figure 3.
Forest plot of the effects of (A) Exercise Interventions versus Control Groups [42,44,46,48,50,51,52,53,54,56,57,61,64,65,66,67], (B) Diet + Exercise Interventions versus Control Groups [48,69,70,71], (C) Pharmacological Interventions versus Control Groups [69,74,75], (D) Diet + Exercise Interventions versus Diet Interventions [31,36,37,38,40,41] on the MetS z-score Mean differences and 95% CI confidence intervals.
In addition, exercise caused larger pooled reductions in fasting glucose (−5 mg/dL; 95% CI −8 to −1; p = 0.013; I2 = 82%, p < 0.001; 19 arms), triglycerides (−11 mg/dL; 95% CI −18 to −4; p = 0.002; I2 = 58%, p < 0.001; 21 arms), MAB (−5 mmHg, 95% CI −7 to −2; p = 0.001; I2 = 95%, p < 0.001; 15 arms), and waist circumference (−2.7 cm; 95% CI −3.2 to −2.1; p < 0.001; I2 = 0%, p = 0.743; 18 arms), and a greater increase in HDL (3 mg/dL, 95% CI 2 to 5; p < 0.001; I2 = 43%, p = 0.020; 21 arms) compared to controls. Significantly larger decreases were also observed across the exercise interventions for HOMA-IR (−0.42 units; 95% CI −0.63 to −0.20; p < 0.001; I2 = 0%, p = 0.874; 7 arms), systolic (−6 mmHg, 95% CI −7 to −4; p < 0.001; I2 = 66%, p < 0.001; 13 arms), and diastolic blood pressure (−5 mmHg, 95% CI −6 to −4; p < 0.001; I2 = 25%, p = 0.188; 13 arms) versus controls.
3.6. Meta-Analysis on Combined Diet and Exercise Interventions
Combined diet + exercise interventions resulted in a significant decline of the MetS z-score (−0.68 units; 95% CI −1.00 to −0.34; p < 0.001; I2 = 98%, p < 0.001; 23 arms) (Figure 2). Funnel plot asymmetry was observed (Figure S1), but Egger’s test was not significant (p = 0.609). In sensitivity analyses, the significance and direction of the pooled effect sizes remained unchanged (Figure S2).
Further, significant decreases were detected for fasting glucose (−2 mg/dL, 95% CI −3 to −1; p = 0.002; I2 = 78%, p < 0.001; 22 arms), triglycerides (−15 mg/dL, 95% CI −22 to −9; p < 0.001; I2 = 82%, p < 0.001; 22 arms), MAB (−5 mg/dL, 95% CI −6 to −4; p < 0.001; I2 = 92%, p < 0.001; 18 arms), waist circumference (−3.5 mg/dL, 95% CI −4.6 to −2.3; p < 0.001; I2 = 68%, p = 0.001; 19 arms), as well as an increase in HDL (1 mg/dL, 95% CI 0 to 2; p = 0.048; I2 = 60%, p < 0.001; 22 arms). Furthermore, HOMA-IR (−0.4 units, 95% CI −0.7 to −0.1; p = 0.012; I2 = 0%, p = 0.449; 8 arms), systolic (−6 mg/dL, 95% CI −8 to −4; p < 0.001; I2 = 91%, p < 0.001; 20 arms), and diastolic blood pressure (−4 mg/dL, 95% CI −5 to −3; p < 0.001; I2 = 85%, p < 0.001; 19 arms) significantly decreased following diet + exercise interventions. Table 4 outlines the included diet + exercise interventions.

Table 4.
Diet + Exercise Interventions.
3.6.1. Subgroup Analyses for Combined Diet and Exercise Interventions
Subgroup analyses based on exercise type revealed moderating effects for the MetS z-score (Q-value: 16.723, df = 3, p < 0.001), fasting glucose (Q-value: 21.928, df = 3, p < 0.001), triglycerides (Q-value: 9.313, df = 3, p < 0.025), and total cholesterol (Q-value: 17.530, df = 3, p < 0.001). Specifically, resistance training (−1.17 units, 95% CI −1.65 to −0.70, p < 0.001), cardiovascular training (−0.47 units, 95% CI −0.91 to −0.03, p = 0.036) and combined cardiovascular + resistance training (−0.64 units, 95% CI −1.23 to −0.05, p = 0.034) produced significant reductions in MetS z-scores, while yoga-based exercise showed no significant change (−0.20 units, 95% CI −0.66 to 0.26, p = 0.396). For fasting glucose and triglycerides, significantly lower post-intervention values were only observed in studies emphasizing cardiovascular training (fasting glucose: −3 mg/dL, 95% CI −5 to −2, p < 0.001; triglycerides: −22 mg/dL, 95% CI −30 to −13, p < 0.001), while the other exercise modalities did not cause significant reductions. In contrast, reductions in total cholesterol were only found in studies using resistance training protocols (−9 mg/dL, 95% CI −15 to −3, p = 0.006).
3.6.2. Meta Regressions for Combined Diet and Exercise Interventions
Meta-regression analyses indicated significant associations between weekly exercise volume and changes in the MetS z-score (β = −0.014, 95% CI −0.017 to −0.011; p < 0.001), triglyceride levels (β = −0.097, 95% CI −0.171 to −0.023; p = 0.010), MAB (β = −0.036, 95% CI −0.060 to −0.010; p = 0.007), diastolic blood pressure (β = −0.018, 95% CI −0.031 to −0.001; p = 0.005), and total cholesterol (β = −0.065, 95% CI −0.100 to −0.030; p < 0.001), with higher exercise volumes predicting larger reductions. Longer intervention duration predicted larger decreases in total cholesterol (β = −0.106, 95% CI −0.160 to −0.052; p < 0.001) and larger increases in HDL concentrations (β = 0.044, 95% CI 0.030 to 0.058; p < 0.001).
3.6.3. Meta-Analyses on Combined Diet and Exercise Versus Control Interventions
Combined diet + exercise interventions produced a greater decrease in the MetS z-score compared with control conditions (mean difference −0.45 units; 95% CI −0.65 to −0.26; p < 0.001; I2 = 90%, p < 0.001; 6 arms) (Figure 3). The funnel plot revealed pronounced asymmetry (Figure S3), while Egger’s test was not statistically significant (p = 0.174). Sensitivity analyses showed consistent results (Figure S4).
Furthermore, combined diet + exercise interventions produced greater reductions in fasting glucose (−3 mg/dL; 95% CI −5 to −1; p = 0.027; I2 = 43%, p < 0.118; 6 arms), triglycerides (−20 mg/dL; 95% CI −31 to −10; p < 0.001; I2 = 72%, p = 0.003; 6 arms), systolic blood pressure (−7 mmHg, 95% CI −11 to −3; p = 0.001; I2 = 69%, p = 0.010; 5 arms), and larger increases in HDL (3 mg/dL, 95% CI 0 to 4; p = 0.040; I2 = 59%, p = 0.032; 6 arms) compared to controls. For the remaining outcomes, the number of available studies was insufficient to permit quantitative meta-analysis.
3.7. Meta-Analysis on Combined Diet and Exercise Versus Diet Interventions
Combined interventions resulted in a larger reduction in the MetS z-score than diet-only interventions (mean difference = −0.75 units; 95% CI −1.04 to −0.46; p < 0.001; I2 = 0%, p = 0.986; 10 arms) (Figure 3). The funnel plot showed a symmetrical distribution of studies (Figure S3). Additionally, the Egger’s test was not statistically significant (p = 0.547). Sensitivity analyses showed that the significance and direction of the pooled effect sizes were stable (Figure S4).
Combined interventions also yielded greater reductions in triglycerides (−12 mg/dL; 95% CI −19 to −5; p < 0.001; I2 = 0%, p = 0.956; 10 arms), waist circumference (−3 mg/dL; 95% CI −4 to −1; p = 0.014; I2 = 0%, p = 0.972; 10 arms), MAB (−6 mmHg; 95% CI −9 to −3; p < 0.001; I2 = 32%, p = 0.192; 6 arms), and systolic blood pressure (−6 mmHg; 95% CI −10 to −1; p = 0.017; I2 = 80%, p < 0.001; 8 arms), and larger increases in HDL concentrations (1 mg/dL; 95% CI 0 to 2; p = 0.009; I2 = 0%, p = 0.551; 10 arms), compared to diet-only interventions.
3.8. Meta-Analysis on Pharmacological Interventions
The analysis demonstrated a significant reduction in the MetS z-score (mean difference = −0.30 units; 95% CI −0.49 to −0.12; p = 0.001; I2 = 95%, p < 0.001; 5 arms) (Figure 2). The funnel plot displayed an asymmetrical spread of studies (Figure S1), but the Egger’s test was not statistically significant (p = 0.188). Sensitivity analyses did not change the significance or direction of effect sizes (Figure S2).
Significant reductions were also observed in fasting glucose (−2 mg/dL; 95% CI −4 to −1; p = 0.010; I2 = 72%, p = 0.014; 4 arms), HDL (1 mg/dL; 95% CI 0 to 2; p = 0.011; I2 = 62%, p = 0.047; 4 arms), triglycerides (−5 mg/dL; 95% CI −8 to −2; p = 0.001; I2 = 0%, p = 0.720; 4 arms), and waist circumference (−2.1 cm; 95% CI −2.7 to −1.6; p < 0.001; I2 = 51%, p = 0.107; 4 arms). Furthermore, total cholesterol (−2 mg/dL; 95% CI −4 to 0; p = 0.025; I2 = 0%, p = 0.376; 3 arms), diastolic (−2 mmHg; 95% CI −3 to −1; p < 0.001; I2 = 55%, p = 0.107; 3 arms), and systolic blood pressure (−2 mmHg; 95% CI −4 to −1; p = 0.002; I2 = 83%, p = 0.001, 4 arms) decreased following pharmacological interventions. No studies reported MAB or HOMA-IR data suitable for pooling. Table 5 presents a summary of the included pharmacological interventions.

Table 5.
Pharmacological Interventions.
3.8.1. Subgroup Analyses for Pharmacological Interventions
Subgroup analysis did not reveal any significant differences between medication types for any outcome.
3.8.2. Meta Regressions for Pharmacological Interventions
Due to the small number of available studies, no meaningful meta-regressions could be conducted to explore moderators such as treatment duration, baseline BMI, or participant age.
3.8.3. Meta-Analyses on Pharmacological Interventions Versus Controls
The pooled effect on the MetS z-score did not reach statistical significance (mean difference = −0.07; 95% CI −0.16 to 0.02; p = 0.134; I2 = 67%; p = 0.017; 5 arms) when pharmacological interventions were compared with controls. (Figure 3). The funnel plot suggested marginal asymmetry (Figure S3), and the Egger’s test was not significant (p = 0.707). Sensitivity analyses did not affect the significance or direction of the pooled differences (Figure S4).
Moreover, pharmacological treatments yielded larger reductions in fasting glucose levels (−3 mg/dL; 95% CI −4 to −1; p = 0.002; I2 = 70%, p = 0.019; 4 arms) versus controls. The remaining outcomes did not show statistically significant differences compared with controls.
3.9. Characteristics of Higher-Quality Interventions
A descriptive synthesis of intervention characteristics among studies rated as methodologically good or excellent (PEDro score ≥ 6) identified some common design and implementation features across higher-quality trials. Briefly, the majority of higher-quality interventions had a minimum duration of 12 weeks, with many extending to 12–26 weeks. Exercise-based protocols frequently employed either combined aerobic and resistance training or HIIT protocols, typically prescribed at vigorous intensities (~65–85% VO2peak or ≥80% HRmax). Dietary interventions in these studies predominantly applied structured caloric restriction, typically in the range of 500–800 kcal/day, with some incorporating higher protein intake. Implementation strategies commonly included progressive increases in exercise volume, supervised or coached delivery by qualified professionals, and either individualized monitoring (e.g., heart-rate–based prescriptions) or structured group-based formats. Due to the limited number of pharmacological studies meeting higher methodological quality thresholds, a comparable descriptive synthesis was not feasible for medication-based interventions.
4. Discussion
To our knowledge, this systematic review and meta-analysis provides one of the most comprehensive comparative syntheses to date examining the effects of diet-only, exercise-only, combined diet and exercise, and pharmacological interventions on overall cardiometabolic health, assessed using the MetS z-score, in adults with overweight or obesity. Across 46 studies and 85 intervention arms encompassing more than 12,100 participants, lifestyle-based interventions consistently led to significant improvements in the MetS z-score and individual cardiometabolic risk markers, while preliminary evidence from a small number of pharmacological studies demonstrated smaller and more variable effects.
Importantly, interpretation of the present findings provides insights that extend beyond those obtainable from individual cardiometabolic markers alone. Because the MetS z-score integrates multiple interrelated risk factors into a single continuous construct, reductions in this score are more likely to reflect coordinated improvements across metabolic systems rather than isolated changes in single parameters. In this context, the consistent reductions observed for lifestyle-based interventions suggest an attenuation of the underlying cardiometabolic risk burden, even when changes in individual components may appear modest when considered in isolation. Significant changes in MetS z-scores are also informative in the presence of heterogeneous intervention responses. While substantial between-study variability was observed for many single outcomes, the direction of change in MetS severity was largely consistent across diet-only, exercise-only, and combined diet + exercise interventions. This indicates that, despite variability in magnitude, lifestyle interventions reliably shift global metabolic risk in a favorable direction. The graded nature of the MetS z-score further facilitates interpretation of comparative effectiveness. Although absolute differences between lifestyle strategies were not large in independent meta-analyses, head-to-head comparisons indicated additional reductions in MetS severity with combined diet and exercise, supporting additive benefits at the level of global risk. In contrast, the smaller and less consistent MetS z-score changes observed for pharmacological interventions underscore a conceptual distinction, as medications often target specific pathways, whereas lifestyle interventions tend to induce broader multisystem adaptations.
Although the MetS z-score is a continuous, unitless measure and no universally accepted minimal clinically important difference currently exists, prior evidence suggests that relatively small reductions may correspond to clinically meaningful improvements. Based on component-level modeling, a MetS z-score reduction of ~0.2 units is linked to a 5–10% improvement in a single MetS component (e.g., blood pressure, glucose, or waist circumference) and has been proposed as a threshold for clinically meaningful change [71]. Moreover, DeBoer et al. [69] demonstrated that a reduction of ~0.6 units following lifestyle intervention was associated with a significant reduction in cardiometabolic disease risk in the follow-up. Thus, while pooled estimates should not be interpreted as fixed clinical cut-offs, the magnitude of change observed in the present analyses—particularly for lifestyle interventions—supports the clinical relevance of the present findings.
Specifically, dietary, exercise, and combined diet + exercise interventions produced marked improvements in the MetS z-score (ranging between 0.63 and 0.72 units) and its underlying components. These findings are consistent with established beneficial effects of caloric restriction [76,77,78], increased physical activity [79,80,81,82] and combined diet + exercise approaches [83,84] on a broad range of cardiometabolic risk outcomes in adults with overweight or obesity, including glycemic regulation, lipid profiles, blood pressure, and central adiposity. However, substantial variability across studies indicates that the magnitude of benefit is highly dependent on intervention characteristics and population context, and pooled estimates should therefore be interpreted as reflecting overall potential rather than uniform treatment effects.
The interpretation of the present findings for pharmacological interventions must be contextualized within the existing evidence base. Importantly, contemporary GLP-1 RA can achieve substantial—and typically greater—weight loss than traditional lifestyle programs and are often accompanied by favorable changes in specific individual cardiometabolic risk factors [19,20,22]. Moreover, semaglutide, in particular, has been shown to reduce major adverse cardiovascular events in adults with overweight or obesity and established cardiovascular disease, underscoring clinically important cardiometabolic benefits beyond weight loss alone [85,86,87]. However, it is important to note that pivotal, large-scale anti-obesity medication trials have implemented pharmacotherapy as an adjunct to structured lifestyle modification, rather than as a stand-alone intervention. Moreover, the majority of medication studies have primarily focused on investigating weight loss or specific single cardiometabolic endpoints (e.g., glucose concentration). As a result, although these trials demonstrate large weight loss effects and favorable changes in selected cardiometabolic risk markers, they provide limited insight into the independent effects of pharmacotherapy on global cardiometabolic risk.
Against this background, the findings from the present meta-analysis on pharmacological interventions suggest modest and variable improvements in overall cardiometabolic health among adults with overweight and obesity. The pooled reduction in the MetS z-score of −0.30 units indicates that medications may contribute to lowering overall metabolic risk, although effects were less prominent and consistent than those observed for lifestyle-based strategies. This pattern is consistent with prior evidence reporting that pharmacotherapy typically exerts target-specific effects (e.g., glucose metabolism or appetite regulation) rather than broad, system-wide improvements across all components of MetS [88]. However, given the limited number of available studies and substantial heterogeneity, these findings should be considered exploratory. Therefore, rather than implying that pharmacotherapy is categorically less effective, our findings highlight an important reporting and evidence gap. Recent anti-obesity medication trials provide robust evidence for highly effective weight loss and improvements in individual risk markers, but integrated global cardiometabolic health outcomes (e.g., MetS severity) are not yet routinely reported. Consequently, more pharmacotherapy trials reporting composite cardiometabolic risk measures are needed to determine whether observed improvements are broadly distributed across metabolic systems or concentrated within specific domains.
Controlled comparisons provide additional context for interpreting the pooled findings. Exercise-only and combined diet + exercise interventions were associated with a significantly larger reduction in the MetS z-score compared with control conditions, whereas pharmacological interventions did not achieve greater reductions than controls. However, substantial heterogeneity indicates variable intervention effects across studies. Notably, the only available direct head-to-head comparison—combined diet + exercise versus diet-only—showed a substantially greater mean reduction in the MetS z-score (−0.75 units) favoring the combined approach. This difference suggests additive or synergistic benefits of incorporating exercise into dietary interventions on body composition, lipid metabolism, insulin signaling and vascular function [89]. Mechanistically, the combination of diet and exercise simultaneously addresses energy balance, insulin-mediated glucose uptake, adipokine secretion, endothelial function and sympathetic regulation—providing a plausible explanation why combined interventions outperform isolated dietary changes as consistently reported in previous research [13,88,90,91]. Thus, the most robust available evidence supports the diet + exercise intervention, suggesting that the combination of caloric restriction and structured exercise provides superior metabolic benefit compared with either strategy in isolation. However, these conclusions remain limited by the small number of comparative trials and should be interpreted cautiously.
Subgroup and moderator analyses refined these findings. Notably, across all intervention types, meta-regression analyses of treatment duration, baseline age, and baseline BMI did not identify significant moderating effects, suggesting that these factors exerted limited influence within the available evidence base. For dietary interventions, an explicit energy deficit emerged as the critical determinant of effect magnitude, whereas modifications in macronutrient composition alone had limited impact on individual metabolic outcomes. Subgroup analysis for combined diet + exercise programs indicated that exercise type mattered, favoring a combination of both cardiovascular and resistance training for holistic health benefits.
Contrary to expectations, exercise intensity did not emerge as a significant moderator of metabolic outcomes. This finding contrasts with prior evidence suggesting that higher-intensity exercise is often associated with greater improvements in cardiometabolic risk markers [92,93]. A plausible explanation may relate to methodological variability across studies. Specifically, exercise intensity was assessed and reported using heterogeneous metrics, which necessitated a pragmatic classification into “moderate” and “vigorous” categories based on established threshold recommendations [94]. This categorical approach may have limited sensitivity to detect dose–response relationships and obscured more nuanced effects of exercise intensity when treated as a continuous variable. Second, only a few trials provided information on whether participants actually achieved the prescribed intensity, which potentially introduced measurement error. Additionally, in obese or metabolically compromised populations, low-to-moderate intensity exercise can already produce meaningful improvements, potentially attenuating between-group differences.
The exercise modality-specific effects observed within combined diet and exercise interventions are physiologically plausible and consistent with established mechanisms of metabolic adaptation. Cardiovascular-based training has been shown to robustly improve fasting glucose and triglyceride levels, likely through enhanced skeletal muscle glucose uptake and increased mitochondrial oxidative capacity, as supported by a recent meta-analysis in prediabetic and MetS populations [95]. In contrast, resistance training appears particularly effective in lowering total cholesterol, potentially mediated by increases in lean mass, resting metabolic rate, and muscle-driven improvements in insulin sensitivity [96]. The absence of significant effects in yoga-based interventions likely reflects a comparatively lower metabolic stimulus. Taken together, these findings indicate that different exercise modalities confer complementary metabolic benefits, supporting the use of multimodal or target-specific exercise prescriptions within combined lifestyle interventions.
Furthermore, meta-regressions revealed that greater exercise volume per week was associated with larger decreases in the MetS z-score, triglycerides, blood pressure, and total cholesterol in diet + exercise interventions, indicating a dose–response relationship between exercise and cardiometabolic adaptations as previously reported in the literature [97]. However, it is to note that recent evidence has demonstrated that low-volume exercise programs (e.g., low-volume high-intensity interval training) can induce similar improvements in key cardiometabolic health markers when compared to higher-volume exercise programs [98,99]. Therefore, while a higher weekly total exercise volume is generally expected to yield greater health benefits, exercise recommendations should be tailored to individual patient needs and preferences to maximize long-term adherence.
The observed associations between longer intervention duration and greater reductions in total cholesterol, as well as larger increases in HDL following combined diet + exercise interventions, are also physiologically plausible and likely reflect the cumulative effects of sustained lifestyle modification on lipid metabolism. Prolonged adjustments in diet and physical activity may promote progressive improvements in hepatic lipid handling, enhanced reverse cholesterol transport, and favorable shifts in lipoprotein turnover that require extended exposure to behavioral change to fully manifest [100]. However, these findings should be interpreted cautiously given the observational nature of meta-regression and the potential influence of unmeasured confounding factors.
Notably, visual inspection of funnel plots suggested asymmetry for several analyses, particularly for exercise-only interventions and exercise versus control comparisons, where Egger’s tests were statistically significant. This pattern indicates the potential presence of small-study effects, whereby smaller trials may report larger beneficial effects than larger studies. Such effects are common in behavioral and lifestyle intervention research and may reflect selective publication, stronger supervision in small trials, or random variation. In contrast, for dietary interventions, combined diet + exercise interventions, and pharmacological interventions, funnel plot asymmetry was not consistently supported by Egger’s tests, likely reflecting limited statistical power due to the smaller number of included studies rather than true bias. Importantly, sensitivity analyses across all intervention types demonstrated that pooled effect sizes remained robust in direction and statistical significance after sequential exclusion of individual studies, suggesting that the main conclusions are not driven by single influential trials. Thus, while the presence of small-study effects—particularly in exercise-based analyses—warrants cautious interpretation of effect magnitude, the consistency of findings across analytic approaches supports the overall robustness of the observed intervention effects on cardiometabolic outcomes.
The overall methodological quality of the included studies was moderate to good, with the majority rated as “good” or “excellent” according to the PEDro scale. This suggests that most trials adhered to key methodological standards, including appropriate randomization, baseline comparability, and reporting of outcome data. However, blinding of participants and intervention providers was generally limited, reflecting inherent challenges in dietary and exercise-based interventions and potentially contributing to performance bias. A smaller proportion of studies were rated as fair or low quality, underscoring some variability in study rigor across the evidence base. While these limitations may have influenced effect size estimates, sensitivity analyses indicated that no single study disproportionately affected the pooled results, supporting the robustness of the overall findings. Nevertheless, future trials would benefit from improved methodological reporting, clearer specification and monitoring of intervention fidelity and, where feasible, enhanced blinding strategies to strengthen internal validity.
To strengthen clinical interpretability, we additionally examined characteristics of higher-quality trials. This qualitative synthesis suggests that interventions yielding the most consistent cardiometabolic improvements shared several implementation features, including intervention durations of at least 12 weeks, structured caloric restriction, and exercise programs delivered at vigorous intensities using either combined aerobic–resistance training or HIIT protocols. Notably, higher-quality studies typically emphasized supervised or coached delivery and progressive overload principles, supporting the importance of professional guidance and gradual intensification for achieving meaningful metabolic adaptations. These findings align with current exercise and lifestyle guidelines and help explain some of the between-study variability observed in the pooled analyses. While these descriptive observations cannot establish causality, they provide practical insight into how effective interventions were operationalized in rigorously conducted trials.
Beyond efficacy, feasibility and participant retention are central to the real-world impact of cardiometabolic health interventions. In the present analysis, mean completion rates were relatively high across all intervention types, and within or above the range typically reported in lifestyle and obesity intervention trials [101,102,103]. Combined diet + exercise programs showed slightly lower retention than single-modality interventions, likely reflecting higher time and behavioral demands, but completion remained acceptable overall. These findings suggest that all intervention strategies evaluated are feasible in controlled settings, while reinforcing the importance of tailoring intervention complexity to individual capacity and long-term adherence potential.
5. Practical Implications
The findings of this meta-analysis offer several practical implications for clinicians, exercise specialists, and public-health practitioners involved in the management and treatment of obesity-related metabolic risk. Foremost, the present evidence reinforces previous research [9,10,11] suggesting that comprehensive lifestyle modification—particularly combining dietary energy restriction with structured physical exercise—should be prioritized as the first-line therapeutic strategy for improving MetS severity and its individual components. Lifestyle-based programs produced the most consistent and most wide-ranging benefits across glycemic control, lipid metabolism, abdominal adiposity, and blood pressure. Within lifestyle programs, exercise prescriptions should emphasize sufficient weekly training volume and, where feasible, incorporate both aerobic and resistance modalities, as these in combination likely produce the most robust metabolic improvements. Time-efficient formats such as low-volume high-intensity interval training may serve as effective alternatives for individuals with limited time or lower adherence to long-duration programs.
However, it is important to note that pharmacological therapies need to be regarded an essential component of obesity care, particularly for individuals who do not achieve adequate metabolic control through lifestyle measures alone, who have specific comorbidities, or who require targeted glucose or lipid lowering. Although the average effects of medications on global metabolic risk were modest and more variable than those of lifestyle interventions, they can provide meaningful improvements for selected outcomes, and their use should be considered within a broader, stepped-care or adjunctive treatment framework. Ultimately, intervention planning should be individualized, taking into account comorbidities, physical capabilities, and likelihood of long-term adherence. Regular monitoring of metabolic markers and ongoing behavioral support are essential to sustain improvements and to adapt treatment intensity over time. In clinical practice, the most effective strategy will, in certain scenarios, consist of integrating lifestyle modification with adjunctive pharmacotherapy as part of a structured, patient-centered treatment pathway.
6. Limitations and Strengths
Several limitations must be acknowledged when interpreting the findings of our analyses. First, the evidence base for pharmacological interventions was limited, with only a small number of eligible studies contributing data for each outcome. Owing to this scarcity, a pooled analysis was conducted to provide an exploratory estimate of the overall impact of medication-based strategies on MetS severity. Although the included agents differed in their mechanisms of action, metabolic targets, and expected effects, separate analyses by drug class were not feasible due to insufficient statistical power. Accordingly, the pooled estimates should not be interpreted as reflecting a uniform pharmacological class effect but rather as an average indication of medication-associated changes in global cardiometabolic risk. Subgroup analyses did not reveal consistent differences between medication classes. However, these findings are constrained by the small number of available trials. Moreover, funnel plot asymmetry suggested the possibility of small-study or publication bias, although Egger’s tests were not statistically significant, likely due to limited power. Therefore, the pharmacological findings should be interpreted cautiously as low-certainty, exploratory evidence indicating modest, outcome-specific benefits rather than comprehensive improvements in MetS severity. The limited number of eligible pharmacotherapy studies reflects broader gaps in the literature. Many trials in obese populations focus on isolated metabolic endpoints (e.g., fasting glucose or body weight) rather than integrated cardiometabolic risk, and a substantial proportion of studies typically combine pharmacological treatment with lifestyle interventions, precluding isolation of drug-specific effects. These limitations highlight the need for rigorously designed trials that evaluate pharmacological therapies both independently and in combination with lifestyle strategies, with transparent reporting of their effects on global cardiometabolic health using composite measures such as the MetS z-score.
Second, substantial heterogeneity was observed across many outcomes, irrespective of intervention type, with I2 values exceeding 90% in several analyses. This variability likely reflects differences in intervention design (e.g., dietary composition, amount of caloric deficit, exercise modality, intensity, and volume), participant characteristics, adherence and study context. Importantly, the heterogeneity primarily reflects variation in the magnitude of effects rather than inconsistency in their direction, as the vast majority of pooled estimates favored improvements in cardiometabolic outcomes. Although predefined subgroup and meta-regression analyses identified several meaningful contributors to heterogeneity, particularly caloric restriction in dietary interventions and exercise volume in combined diet + exercise interventions, most variability remained unexplained, suggesting additional influences such as intervention quality or behavioral support. From a clinical perspective, these findings underscore that pooled estimates represent average effects across diverse real-world scenarios rather than uniform treatment responses. Thus, the observed pooled effects—especially those associated with very high heterogeneity—should be interpreted as indicative of overall direction and approximate magnitude, with clinical applicability dependent on contextual similarity to the included studies.
Third, despite the advantages of the MetS z-score as an integrated measure of cardiometabolic health, many studies did not report sufficient data to compute this composite metric. These trials—often reporting only some individual outcomes such as glucose or lipid levels—could not be included in the analyses. As a result, the evidence base may be biased toward research groups or study designs that routinely quantify composite metabolic risk. More widespread adoption of standardized metabolic risk scoring systems would substantially enhance comparability across studies. Furthermore, it is to note that the analyzed MetS z-scores were not derived from a single universally standardized equation. Included studies applied sex- and age-specific MetS z-score formulations developed in different populations, which may have contributed to between-study variability. To address this, primary analyses were conducted using differences in means, facilitating direct clinical interpretation of changes in MetS severity. In parallel, all analyses were repeated using standardized mean differences to account for potential variation in score derivation across studies. These additional analyses yielded similar directions and levels of statistical significance (Figures S5 and S6).
Fourth, many included interventions assessed short- to medium-term effects, with follow-up durations typically ranging from 8 to 24 weeks. Consequently, little can be inferred about long-term sustainability, maintenance of metabolic improvements, real-world adherence, or the durability of changes in MetS severity. Fifth, the majority of included studies were conducted in high-income countries, primarily in Europe and North America, which may limit the applicability of the findings to populations in low- and middle-income regions with different healthcare systems, dietary patterns, physical activity behaviors, and sociocultural determinants of health. Further, it is to note that most study samples consisted of middle-aged adults, while older adults and younger populations were underrepresented. Additionally, participants were typically recruited from clinical or research settings and may therefore represent more health-conscious or motivated individuals, potentially limiting extrapolation to broader, real-world populations. Thus, caution is warranted when generalizing the results to other demographic or geographic contexts.
Finally, adverse events were inconsistently reported, limiting the ability to evaluate risk–benefit ratios for all interventions—although the consensus of previous research is that lifestyle modifications generally carry a significantly lower risk of adverse events compared to pharmaceutical treatments [104,105,106,107]. Future research should incorporate extended follow-up periods and systematically assess adherence, tolerability, and potential compensatory behaviors to better understand the long-term clinical relevance of these interventions.
Despite these limitations, this systematic review and meta-analysis has several notable strengths that enhance the validity and clinical relevance of its findings. First, it comprehensively synthesizes the effects of diet-only, exercise-only, combined diet-plus-exercise, and pharmacological interventions on a broad spectrum of cardiometabolic outcomes in adults with overweight and obesity. By simultaneously evaluating these four major intervention categories, the study provides an integrated perspective on their relative and absolute effectiveness—an approach rarely undertaken in previous reviews that mostly focus on a single intervention type.
Second, the use of the MetS z-score as the primary outcome allowed for a holistic assessment of global cardiometabolic risk, capturing changes across multiple interrelated metabolic domains rather than relying on isolated biomarkers. This composite approach improves clinical interpretability and aligns with contemporary conceptualizations of MetS as a clustered, systemic condition [7,8]. Third, the study applied rigorous and transparent methodology, including random-effects modeling, sensitivity analyses, formal assessments of publication bias, and a comprehensive suite of subgroup and meta-regression analyses to explore potential moderators. These procedures enhance confidence in the stability and robustness of the observed effects.
Finally, the review incorporated a large sample (over 12,100 participants) spanning a broad range of ages, BMI categories, and clinical backgrounds. This diversity increases the generalizability of the findings to real-world populations and supports the translational value of the conclusions for clinical practice and public-health guidance.
7. Conclusions
In summary, lifestyle interventions—particularly energy-restricted dietary strategies combined with structured exercise—were associated with the most consistent and robust improvements in overall cardiometabolic health, as reflected by reductions in the MetS z-score, alongside favorable changes in its individual cardiometabolic components. While contemporary anti-obesity pharmacotherapies are known to induce substantial weight loss and improve selected metabolic risk markers, the present analysis indicates that evidence for their impact on integrated cardiometabolic risk, as captured by composite MetS severity scores, remains limited. Notably, MetS z-scores are infrequently reported in pharmacotherapy trials, and many pivotal studies have evaluated medications as adjuncts to lifestyle modification, which restricts inference on stand-alone medication effects on global metabolic health. Accordingly, the current evidence most strongly supports comprehensive lifestyle modification as the cornerstone strategy for improving overall cardiometabolic risk, with pharmacotherapy best considered as a complementary approach. Future trials should systematically incorporate standardized composite measures of MetS severity to clarify how modern pharmacological agents influence integrated cardiometabolic risk and how these effects compare with, or add to, lifestyle-based interventions.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu18030473/s1, Table S1. Detailed search strategy. Figure S1. Funnel plots for the MetS z-scores in (A) Diet Interventions, (B) Exercise Interventions, (C) Diet + Exercise Interventions, and (D) Pharmacological Interventions. Figure S2. Forest Plot of Sensitivity Analyses for the MetS z-Score of (A) Diet Interventions, (B) Exercise Interventions, (C) Diet + Exercise Interventions, and (D) Pharmacological Interventions. Mean differences and 95% CI confidence intervals. A negative value indicates an improvement in cardiometabolic health status. Figure S3. Funnel plots for the MetS z-scores in (A) Exercise Interventions versus Control Groups, (B) Diet + Exercise Interventions versus Control Groups, (C) Pharmacological Interventions versus Control Groups, (D) Diet + Exercise Interventions versus Diet Interventions. Figure S4. Forest Plot of Sensitivity Analyses for the MetS z-Score of (A) Exercise Interventions versus Control Groups, (B) Diet + Exercise Interventions versus Control Groups, (C) Pharmacological Interventions versus Control Groups, (D) Diet + Exercise Interventions versus Diet Interventions. Mean differences and 95% CI confidence intervals. Figure S5. Forest Plot for the MetS z-Score of (A) Diet Interventions, (B) Exercise Interventions, (C) Diet + Exercise Interventions, and (D) Pharmacological Interventions. Standardized differences in means and 95% CI confidence intervals. A negative value indicates an improvement in cardiometabolic health status. Figure S6. Forest Plot for the MetS z-Score of (A) Exercise Interventions versus Control Groups, (B) Diet + Exercise Interventions versus Control Groups, (C) Pharmacological Interventions versus Control Groups, (D) Diet + Exercise Interventions versus Diet Interventions. Standardized differences in means and 95% CI confidence intervals.
Author Contributions
Conceptualization, D.R.; methodology, D.R.; validation, V.V.W., J.S. and D.R.; data collection, V.V.W. and J.S.; formal analysis, V.V.W. and D.R.; writing—original draft preparation, V.V.W.; writing—review and editing, J.S. and D.R.; supervision, D.R.; project administration, D.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
The present work was performed by Valentina Victoria Werndle in (partial) fulfillment of the requirements for obtaining the degree, Dr. med. dent. from the Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- GBD 2021 Adult BMI Collaborators. Global, regional, and national prevalence of adult overweight and obesity, 1990–2021, with forecasts to 2050: A forecasting study for the Global Burden of Disease Study 2021. Lancet 2025, 405, 813–838. [Google Scholar] [CrossRef]
- Jin, X.; Qiu, T.; Li, L.; Yu, R.; Chen, X.; Li, C.; Proud, C.G.; Jiang, T. Pathophysiology of obesity and its associated diseases. Acta Pharm. Sin. B 2023, 13, 2403–2424. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Qiu, X.; Ma, H.; Geng, Q. Incidence and long-term specific mortality trends of metabolic syndrome in the United States. Front. Endocrinol. 2023, 13, 1029736. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.L. A comprehensive definition for metabolic syndrome. Dis. Models Mech. 2009, 2, 231–237. [Google Scholar] [CrossRef] [PubMed]
- DeBoer, M.D.; Gurka, M.J.; Jones, M.K.; Smolkin, M.E. Using metabolic syndrome severity in the “real world”: Associations with diabetes and cardiovascular disease in all of us vs. NHANES. Metab. Open 2025, 28, 100415. [Google Scholar] [CrossRef]
- Hsu, P.W.; Chen, Y.R.; Sheu, W.H. MetS-Z: A gender- and age-specific scoring system for predicting type 2 diabetes. J. Diabetes Investig. 2025, 16, 907–916. [Google Scholar] [CrossRef]
- DeBoer, M.D.; Gurka, M.J. Clinical utility of metabolic syndrome severity scores: Considerations for practitioners. Diabetes Metab. Syndr. Obes. 2017, 10, 65–72. [Google Scholar] [CrossRef]
- Khazdouz, M.; Hasani, M.; Mehranfar, S.; Ejtahed, H.S.; Djalalinia, S.; Mahdavi Gorabi, A.; Esmaeili-Abdar, M.; Karbalahi Saleh, S.; Arzaghi, S.M.; Zahedi, H.; et al. Validity of continuous metabolic syndrome score for predicting metabolic syndrome; a systematic review and meta-analysis. J. Diabetes Metab. Disord. 2021, 20, 497–510. [Google Scholar] [CrossRef]
- Castro-Barquero, S.; Ruiz-León, A.M.; Sierra-Pérez, M.; Estruch, R.; Casas, R. Dietary strategies for metabolic syndrome: A comprehensive review. Nutrients 2020, 12, 2983. [Google Scholar] [CrossRef]
- Joseph, M.S.; Tincopa, M.A.; Walden, P.; Jackson, E.; Conte, M.L.; Rubenfire, M. The impact of structured exercise programs on metabolic syndrome and its components: A systematic review. Diabetes Metab. Syndr. Obes. 2019, 12, 2395–2404. [Google Scholar] [CrossRef]
- Peri, K.; Eisenberg, M. Review on obesity management: Diet, exercise and pharmacotherapy. BMJ Public Health 2024, 2, e000246. [Google Scholar] [CrossRef] [PubMed]
- Olateju, I.V.; Opaleye-Enakhimion, T.; Udeogu, J.E.; Asuquo, J.; Olaleye, K.T.; Osa, E.; Oladunjoye, A.F. A systematic review on the effectiveness of diet and exercise in the management of obesity. Diabetes Metab. Syndr. 2023, 17, 102759. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Gu, Y.; Li, Z.; He, B.; Zhang, L. Effects of different exercises combined with different dietary interventions on body composition: A systematic review and network meta-analysis. Nutrients 2024, 16, 3007. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liu, Y.; Li, Y.; Luo, B.; Lin, Z.; Chen, K.; Liu, Y. Dietary patterns and cardiometabolic health: Clinical evidence and mechanism. MedComm 2023, 4, e212. [Google Scholar] [CrossRef]
- Belanger, M.J.; Rao, P.; Robbins, J.M. Exercise, physical activity, and cardiometabolic health: Pathophysiologic insights. Cardiol. Rev. 2022, 30, 134–144. [Google Scholar] [CrossRef]
- Amer, B.E.; Abdelgalil, M.S.; Hamad, A.A.; Abdelsayed, K.; Elaraby, A.; Abozaid, A.M.; Abd-ElGawad, M. Metformin plus lifestyle interventions versus lifestyle interventions alone for the delay or prevention of type 2 diabetes in individuals with prediabetes: A meta-analysis of randomized controlled trials. Diabetol. Metab. Syndr. 2024, 16, 273. [Google Scholar] [CrossRef]
- Pu, R.; Shi, D.; Gan, T.; Ren, X.; Ba, Y.; Huo, Y.; Bai, Y.; Zheng, T.; Cheng, N. Effects of metformin in obesity treatment in different populations: A meta-analysis. Ther. Adv. Endocrinol. Metab. 2020, 11, 2042018820926000. [Google Scholar] [CrossRef]
- Velija-Asimi, Z.; Izetbegovic, S.; Karamehic, J.; Coric, J.; Panjeta, M.; Macic-Dzankovic, A.; Djelilovic-Vranic, J.; Dizdarevic-Bostandzic, A. The effects of dipeptidyl peptidase-4 inhibitors in treatment of obese patients with type 2 diabetes. Med. Arch. 2013, 67, 365–367. [Google Scholar] [CrossRef]
- Jastreboff, A.M.; Aronne, L.J.; Ahmad, N.N.; Wharton, S.; Connery, L.; Alves, B.; Kiyosue, A.; Zhang, S.; Liu, B.; Bunck, M.C.; et al. Tirzepatide once weekly for the treatment of obesity. N. Engl. J. Med. 2022, 387, 205–216. [Google Scholar] [CrossRef]
- Kosiborod, M.N.; Bhatta, M.; Davies, M.; Deanfield, J.E.; Garvey, W.T.; Khalid, U.; Kushner, R.; Rubino, D.M.; Zeuthen, N.; Verma, S. Semaglutide improves cardiometabolic risk factors in adults with overweight or obesity: STEP 1 and 4 exploratory analyses. Diabetes Obes. Metab. 2023, 25, 468–478. [Google Scholar] [CrossRef]
- Rizzo, M.; Nikolic, D.; Patti, A.M.; Mannina, C.; Montalto, G.; McAdams, B.S.; Rizvi, A.A.; Cosentino, F. GLP-1 receptor agonists and reduction of cardiometabolic risk: Potential underlying mechanisms. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 2814–2821. [Google Scholar] [CrossRef] [PubMed]
- Wilding, J.P.H.; Batterham, R.L.; Calanna, S.; Davies, M.; Van Gaal, L.F.; Lingvay, I.; McGowan, B.M.; Rosenstock, J.; Tran, M.T.D.; Wadden, T.A.; et al. Once-weekly semaglutide in adults with overweight or obesity. N. Engl. J. Med. 2021, 384, 989–1002. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Zhang, M.; Cao, Q.; Lin, L.; Lu, J.; Bi, Y.; Chen, Y. Efficacy of GLP-1 Receptor agonist-based therapies on cardiovascular events and cardiometabolic parameters in obese individuals without diabetes: A meta-analysis of randomized controlled trials. J. Diabetes 2025, 17, e70082. [Google Scholar] [CrossRef] [PubMed]
- Sannidhi, D.; Abeles, R.; Andrew, W.; Bonnet, J.P.; Vitale, K.; Niranjan, V.; Gulati, M.; Pauly, K.; Moran, R.; Alexander, L.; et al. Lifestyle medicine for obesity in the era of highly effective anti-obesity treatment. Nutrients 2025, 17, 2382. [Google Scholar] [CrossRef]
- Rahmanian, M.; Mollahosseini, M. Comparing the effectiveness of metformin with lifestyle modification for the primary prevention of type II diabetes: A systematic review and meta-analysis. BMC Endocr. Disord. 2023, 23, 198. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef]
- Higgins, J.P.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions: Cochrane Book Series; Wiley: Hoboken, NJ, USA, 2008. [Google Scholar]
- DeMorton, N.A. The PEDro scale is a valid measure of the methodological quality of clinical trials: A demographic study. Aust. J. Physiother. 2009, 55, 129–133. [Google Scholar] [CrossRef]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Christensen, P.; Meinert Larsen, T.; Westerterp-Plantenga, M.; Macdonald, I.; Martinez, J.A.; Handjiev, S.; Poppitt, S.; Hansen, S.; Ritz, C.; Astrup, A.; et al. Men and women respond differently to rapid weight loss: Metabolic outcomes of a multi-centre intervention study after a low-energy diet in 2500 overweight, individuals with pre-diabetes (PREVIEW). Diabetes Obes. Metab. 2018, 20, 2840–2851. [Google Scholar] [CrossRef]
- Faiz, H.; Malin, S.K. A low-calorie diet raises β-aminoisobutyric acid in relation to glucose regulation and leptin independent of exercise in women with obesity. Front. Physiol. 2023, 14, 1210567. [Google Scholar] [CrossRef]
- Kirwan, J.P.; Malin, S.K.; Scelsi, A.R.; Kullman, E.L.; Navaneethan, S.D.; Pagadala, M.R.; Haus, J.M.; Filion, J.; Godin, J.-P.; Kochhar, S.; et al. A whole-grain diet reduces cardiovascular risk factors in overweight and obese adults: A randomized controlled trial. J. Nutr. 2016, 146, 2244–2251. [Google Scholar] [CrossRef] [PubMed]
- Kn, M.; Agarwal, R.; Patange, A.; Rao, P.; Ganu, G.; Khan, K.; Sawant, S. Impact of a probiotic-fiber blend on body weight, metabolic regulation, and digestive function in obese adults: A randomized, placebo-controlled, multicentric trial. Cureus 2025, 17, e82613. [Google Scholar] [CrossRef] [PubMed]
- Krebs, J.D.; Parry-Strong, A.; Braakhuis, A.; Worthington, A.; Merry, T.L.; Gearry, R.B.; Foster, M.; Weatherall, M.; Davies, C.; Mullaney, J.; et al. A Mediterranean dietary pattern intervention does not improve cardiometabolic risk but does improve quality of life and body composition in an Aotearoa New Zealand population at increased cardiometabolic risk: A randomised controlled trial. Diabetes Obes. Metab. 2025, 27, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-C.; Cheng, W.-Y.; Hsu, Y.-H.; Su, H.-Y.; Huang, B.E.T.-G.; Lin, Y.-K.; Chan, W.P.; Su, C.-T.; Huang, S.-Y. Effects of calorie restriction with n-3 long-chain polyunsaturated fatty acids on metabolic syndrome severity in obese subjects: A randomize-controlled trial. J. Funct. Foods. 2015, 19, 929–940. [Google Scholar] [CrossRef]
- Reljic, D.; Frenk, F.; Herrmann, H.J.; Neurath, M.F.; Zopf, Y. Effects of very low volume high intensity versus moderate intensity interval training in obese metabolic syndrome patients: A randomized controlled study. Sci. Rep. 2021, 11, 2836. [Google Scholar] [CrossRef]
- Reljic, D.; Herrmann, H.J.; Neurath, M.F.; Zopf, Y. Iron beats electricity: Resistance training but not whole-body electromyostimulation improves cardiometabolic health in obese metabolic syndrome patients during caloric restriction-a randomized-controlled study. Nutrients 2021, 13, 1640. [Google Scholar] [CrossRef]
- Reljic, D.; Konturek, P.C.; Herrmann, H.J.; Neurath, M.F.; Zopf, Y. Effects of whole-body electromyostimulation exercise and caloric restriction on cardiometabolic risk profile and muscle strength in obese women with the metabolic syndrome: A pilot study. J. Physiol. Pharmacol. 2020, 71, 89–98. [Google Scholar] [CrossRef]
- Thomas, M.S.; Puglisi, M.; Malysheva, O.; Caudill, M.A.; Sholola, M.; Cooperstone, J.L.; Fernandez, M.L. Eggs improve plasma biomarkers in patients with metabolic syndrome following a plant-based diet-a randomized crossover study. Nutrients 2022, 14, 2138. [Google Scholar] [CrossRef]
- Willert, S.; von Stengel, S.; Kohl, M.; Uder, M.; Kemmler, W. Effects of whole-body electromyostimulation and lifestyle modifications on the metabolic syndrome in premenopausal overweight women. A randomized controlled trial. Ger. J. Sports Med. 2024, 75, 72–78. [Google Scholar] [CrossRef]
- Yadav, R.; Yadav, R.K.; Khadgawat, R.; Pandey, R.M.; Upadhyay, A.D.; Mehta, N. Randomized controlled trial of a 12-week yoga-based (including diet) lifestyle vs. dietary intervention on cardio-metabolic risk factors and continuous risk score in indian adults with metabolic syndrome. Behav. Med. 2020, 46, 9–20. [Google Scholar] [CrossRef]
- Alamdari, K.A.; Choobineh, S. Metabotrophic effects of aerobic training in male patients with metabolic syndrome. Adipobiology 2016, 7, 21. [Google Scholar] [CrossRef]
- Bateman, L.A.; Slentz, C.A.; Willis, L.H.; Shields, A.T.; Piner, L.W.; Bales, C.W.; Houmard, J.A.; Kraus, W.E. Comparison of aerobic versus resistance exercise training effects on metabolic syndrome (from the studies of a targeted risk reduction intervention through defined exercise—STRRIDE-AT/RT). Am. J. Cardiol. 2011, 108, 838–844. [Google Scholar] [CrossRef] [PubMed]
- Batrakoulis, A.; Jamurtas, A.Z.; Draganidis, D.; Georgakouli, K.; Tsimeas, P.; Poulios, A.; Syrou, N.; Deli, C.K.; Papanikolaou, K.; Tournis, S.; et al. Hybrid neuromuscular training improves cardiometabolic health and alters redox status in inactive overweight and obese women: A randomized controlled trial. Antioxidants 2021, 10, 1601. [Google Scholar] [CrossRef] [PubMed]
- Bennett, W.C.; Collins, K.A.; Johnson, J.L.; Slentz, C.A.; Willis, L.H.; Bales, C.W.; Huffman, K.M.; Kraus, W.E. Effects of exercise amount and intensity versus a combined exercise and lifestyle intervention on metabolic syndrome in adults with prediabetes: A STRRIDE-PD randomized trial. Front. Physiol. 2023, 14, 1199763. [Google Scholar] [CrossRef] [PubMed]
- Byrd, B.R.; Keith, J.; Keeling, S.M.; Weatherwax, R.M.; Nolan, P.B.; Ramos, J.S.; Dalleck, L.C. Personalized moderate-intensity exercise training combined with high-intensity interval training enhances training responsiveness. Int. J. Environ. Res. Public Health 2019, 16, 2088. [Google Scholar] [CrossRef]
- Cuddy, T.F.; Ramos, J.S.; Dalleck, L.C. Reduced Exertion High-intensity interval training is more effective at improving cardiorespiratory fitness and cardiometabolic health than traditional moderate-intensity continuous training. Int. J. Environ. Res. Public Health 2019, 16, 483. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Azizi, M.; Tahmasebi, W.; Hoseini, R. Combined effects of sodium alginate supplementation with HIIT and MICT on CCK and PYY levels in men with metabolic syndrome. J. Diabetes Metab. Disord. 2025, 24, 150. [Google Scholar] [CrossRef]
- Guio de Prada, V.; Ortega, J.F.; Morales-Palomo, F.; Ramirez-Jimenez, M.; Moreno-Cabañas, A.; Mora-Rodriguez, R. Women with metabolic syndrome show similar health benefits from high-intensity interval training than men. PLoS ONE 2019, 14, e0225893. [Google Scholar] [CrossRef]
- Mendel, L.; Kast, S.; von Stengel, S.; Kohl, M.; Roemer, F.W.; Uder, M.; Kemmler, W. The effect of whole-body electromyostimulation on metabolic syndrome in affected adults: A subanalysis of a randomized controlled trial. Front. Sports Act. Living 2025, 7, 1585579. [Google Scholar] [CrossRef]
- Morales-Palomo, F.; Moreno-Cabañas, A.; Alvarez-Jimenez, L.; Mora-Gonzalez, D.; Ortega, J.F.; Mora-Rodriguez, R. Efficacy of morning versus afternoon aerobic exercise training on reducing metabolic syndrome components: A randomized controlled trial. J. Physiol. 2024, 602, 6463–6477. [Google Scholar] [CrossRef]
- Morales-Palomo, F.; Moreno-Cabañas, A.; Alvarez-Jimenez, L.; Mora-Gonzalez, D.; Mora-Rodriguez, R. Long-term effects of high-intensity aerobic training on metabolic syndrome: An 8-year follow-up randomized clinical trial. J. Cachexia Sarcopenia Muscle 2025, 16, e13780. [Google Scholar] [CrossRef] [PubMed]
- Morales-Palomo, F.; Ramirez-Jimenez, M.; Ortega, J.F.; Lopez-Galindo, P.L.; Fernandez-Martin, J.; Mora-Rodriguez, R. Effects of repeated yearly exposure to exercise-training on blood pressure and metabolic syndrome evolution. J. Hypertens. 2017, 35, 1992–1999. [Google Scholar] [CrossRef] [PubMed]
- Mora-Rodriguez, R.; Fernandez-Elias, V.E.; Morales-Palomo, F.; Pallares, J.G.; Ramirez-Jimenez, M.; Ortega, J.F. Aerobic interval training reduces vascular resistances during submaximal exercise in obese metabolic syndrome individuals. Eur. J. Appl. Physiol. 2017, 117, 2065–2073. [Google Scholar] [CrossRef] [PubMed]
- Mora-Rodriguez, R.; Ortega, J.F.; Hamouti, N.; Fernandez-Elias, V.E.; Cañete Garcia-Prieto, J.; Guadalupe-Grau, A.; Saborido, A.; Martin-Garcia, M.; Guio de Prada, V.; Ara, I.; et al. Time-course effects of aerobic interval training and detraining in patients with metabolic syndrome. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 792–798. [Google Scholar] [CrossRef]
- Mora-Rodriguez, R.; Ortega, J.F.; Morales-Palomo, F.; Ramirez-Jimenez, M. Weight loss but not gains in cardiorespiratory fitness after exercise-training predicts improved health risk factors in metabolic syndrome. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 1267–1274. [Google Scholar] [CrossRef]
- Moreno-Cabañas, A.; Morales-Palomo, F.; Alvarez-Jimenez, L.; Mora-Gonzalez, D.; Garcia-Camacho, E.; Martinez-Mulero, B.; Mora-Rodriguez, R. Clinical and physiological effects of high-intensity aerobic training on metabolic syndrome: Understanding the individual exercise response variability. J. Appl. Physiol. (1985) 2025, 138, 144–156. [Google Scholar] [CrossRef]
- Moreno-Cabañas, A.; Ortega, J.F.; Morales-Palomo, F.; Ramirez-Jimenez, M.; Alvarez-Jimenez, L.; Pallares, J.G.; Mora-Rodriguez, R. The use of a graded exercise test may be insufficient to quantify true changes in VO2max following exercise training in unfit individuals with metabolic syndrome. J. Appl. Physiol. (1985) 2020, 129, 760–767. [Google Scholar] [CrossRef]
- Moseley, G.A.; Ross, L.M.; Collins-Bennett, K.A.; North, R.; Johnson, J.L.; Kraus, W.E. Cumulative effects of training at different weekly energy expenditures on cardiorespiratory fitness and markers of metabolic syndrome in STRRIDE-Extension. J. Appl. Physiol. (1985) 2025, 138, 1532–1542. [Google Scholar] [CrossRef]
- Ramos, J.S.; Dalleck, L.C.; Borrani, F.; Beetham, K.S.; Wallen, M.P.; Mallard, A.R.; Clark, B.; Gomersall, S.; Keating, S.E.; Fassett, R.G.; et al. Low-volume high-intensity interval training is sufficient to ameliorate the severity of metabolic syndrome. Metab. Syndr. Relat. Disord. 2017, 15, 319–328. [Google Scholar] [CrossRef]
- Seward, S.; Ramos, J.; Drummond, C.; Dalleck, A.; Byrd, B.; Kehmeier, M.; Dalleck, L. Inter-individual variability in metabolic syndrome severity score and VO2max changes following personalized, community-based exercise programming. Int. J. Environ. Res. Public Health 2019, 16, 4855. [Google Scholar] [CrossRef]
- Smith, B.E.; Peterman, J.E.; Harber, M.P.; Imboden, M.T.; Fleenor, B.S.; Kaminsky, L.A.; Whaley, M.H. Change in metabolic syndrome and cardiorespiratory fitness following exercise training—The Ball State adult fitness longitudinal lifestyle study (BALL ST). Diabetes Metab. Syndr. Obes. 2022, 15, 1553–1562. [Google Scholar] [CrossRef] [PubMed]
- Tibana, R.A.; Nascimento Dda, C.; de Sousa, N.M.; de Souza, V.C.; Durigan, J.; Vieira, A.; Bottaro, M.; Nóbrega Ode, T.; de Almeida, J.A.; Navalta, J.W.; et al. Enhancing of women functional status with metabolic syndrome by cardioprotective and anti-inflammatory effects of combined aerobic and resistance training. PLoS ONE 2014, 9, e110160. [Google Scholar] [CrossRef]
- Tomeleri, C.M.; Souza, M.F.; Burini, R.C.; Cavaglieri, C.R.; Ribeiro, A.S.; Antunes, M.; Nunes, J.P.; Venturini, D.; Barbosa, D.S.; Sardinha, L.B.; et al. Resistance training reduces metabolic syndrome and inflammatory markers in older women: A randomized controlled trial. J. Diabetes 2018, 10, 328–337. [Google Scholar] [CrossRef]
- Tuttor, M.; von Stengel, S.; Kohl, M.; Lell, M.; Scharf, M.; Uder, M.; Wittke, A.; Kemmler, W. High intensity resistance exercise training vs. high intensity (endurance) interval training to fight cardiometabolic risk factors in overweight men 30–50 years old. Front. Sports Act. Living 2020, 2, 68. [Google Scholar] [CrossRef] [PubMed]
- Weatherwax, R.M.; Ramos, J.S.; Harris, N.K.; Kilding, A.E.; Dalleck, L.C. Changes in metabolic syndrome severity following individualized versus standardized exercise prescription: A feasibility study. Int. J. Environ. Res. Public Health 2018, 15, 2594. [Google Scholar] [CrossRef]
- Wittmann, K.; Sieber, C.; von Stengel, S.; Kohl, M.; Freiberger, E.; Jakob, F.; Lell, M.; Engelke, K.; Kemmler, W. Impact of whole body electromyostimulation on cardiometabolic risk factors in older women with sarcopenic obesity: The randomized controlled FORMOsA-sarcopenic obesity study. Clin. Interv. Aging 2016, 11, 1697–1706. [Google Scholar] [CrossRef]
- Cummings, P.J.; Noakes, T.D.; Nichols, D.M.; Berchou, K.D.; Kreher, M.D.; Washburn, P.J. Lifestyle therapy targeting hyperinsulinemia normalizes hyperglycemia and surrogate markers of insulin resistance in a large, free-living population. AJPM Focus 2022, 1, 100034. [Google Scholar] [CrossRef]
- DeBoer, M.D.; Filipp, S.L.; Gurka, M.J. Use of a metabolic syndrome severity z score to track risk during treatment of prediabetes: An analysis of the diabetes prevention program. Diabetes Care 2018, 41, 2421–2430. [Google Scholar] [CrossRef]
- Haufe, S.; Kerling, A.; Protte, G.; Bayerle, P.; Stenner, H.T.; Rolff, S.; Sundermeier, T.; Kück, M.; Ensslen, R.; Nachbar, L.; et al. Telemonitoring-supported exercise training, metabolic syndrome severity, and work ability in company employees: A randomised controlled trial. Lancet Public Health 2019, 4, e343–e352. [Google Scholar] [CrossRef]
- Höchsmann, C.; Dorling, J.L.; Martin, C.K.; Newton, R.L., Jr.; Apolzan, J.W.; Myers, C.A.; Denstel, K.D.; Mire, E.F.; Johnson, W.D.; Zhang, D.; et al. Effects of a 2-year primary care lifestyle intervention on cardiometabolic risk factors: A cluster-randomized trial. Circulation 2021, 143, 1202–1214. [Google Scholar] [CrossRef]
- Reljic, D.; Frenk, F.; Herrmann, H.J.; Neurath, M.F.; Zopf, Y. Maximum heart rate- and lactate threshold-based low-volume high-intensity interval training prescriptions provide similar health benefits in metabolic syndrome patients. Healthcare 2023, 11, 711. [Google Scholar] [CrossRef] [PubMed]
- Reljic, D.; Herrmann, H.J.; Neurath, M.F.; Zopf, Y. Impact of different low-volume concurrent training regimens on cardiometabolic health, inflammation, and fitness in obese metabolic syndrome patients. Nutrients 2025, 17, 561. [Google Scholar] [CrossRef] [PubMed]
- Bouzas, C.; Pastor, R.; Garcia, S.; Monserrat-Mesquida, M.; Martínez-González, M.Á.; Salas-Salvadó, J.; Corella, D.; Goday, A.; Martínez, J.A.; Alonso-Gómez, Á.M.; et al. Comparative effects of glucagon-like peptide-1 receptors agonists, 4-dipeptidyl peptidase inhibitors, and metformin on metabolic syndrome. Biomed. Pharmacother. 2023, 161, 114561. [Google Scholar] [CrossRef] [PubMed]
- Sandsdal, R.M.; Juhl, C.R.; Jensen, S.B.K.; Lundgren, J.R.; Janus, C.; Blond, M.B.; Rosenkilde, M.; Bogh, A.F.; Gliemann, L.; Jensen, J.-E.B.; et al. Combination of exercise and GLP-1 receptor agonist treatment reduces severity of metabolic syndrome, abdominal obesity, and inflammation: A randomized controlled trial. Cardiovasc. Diabetol. 2023, 22, 41. [Google Scholar] [CrossRef]
- Soare, A.; Weiss, E.P.; Pozzilli, P. Benefits of caloric restriction for cardiometabolic health, including type 2 diabetes mellitus risk. Diabetes Metab. Res. Rev. 2014, 30, 41–47. [Google Scholar] [CrossRef]
- Stelmach-Mardas, M.; Walkowiak, J. Dietary interventions and changes in cardio-metabolic parameters in metabolically healthy obese subjects: A systematic review with meta-analysis. Nutrients 2016, 8, 455. [Google Scholar] [CrossRef]
- Voglhuber, J.; Ljubojevic-Holzer, S.; Abdellatif, M.; Sedej, S. Targeting cardiovascular risk factors through dietary adaptations and caloric restriction mimetics. Front. Nutr. 2021, 8, 758058. [Google Scholar] [CrossRef]
- Battista, F.; Ermolao, A.; van Baak, M.A.; Beaulieu, K.; Blundell, J.E.; Busetto, L.; Carraça, E.V.; Encantado, J.; Dicker, D.; Farpour-Lambert, N.; et al. Effect of exercise on cardiometabolic health of adults with overweight or obesity: Focus on blood pressure, insulin resistance, and intrahepatic fat-A systematic review and meta-analysis. Obes. Rev. 2021, 22, e13269. [Google Scholar] [CrossRef]
- Chen, X.; He, H.; Xie, K.; Zhang, L.; Cao, C. Effects of various exercise types on visceral adipose tissue in individuals with overweight and obesity: A systematic review and network meta-analysis of 84 randomized controlled trials. Obes. Rev. 2024, 25, e13666. [Google Scholar] [CrossRef]
- Chen, Z.; Zhou, R.; Liu, X.; Wang, J.; Wang, L.; Lv, Y.; Yu, L. Effects of aerobic exercise on blood lipids in people with overweight or obesity: A systematic review and meta-analysis of randomized controlled trials. Life 2025, 15, 166. [Google Scholar] [CrossRef]
- Cortes, M.B.; da Silva, R.S.N.; de Oliveira, P.C.; da Silva, D.S.; Irigoyen, M.C.C.; Waclawovsky, G.; Schaun, M.I. Effect of aerobic and resistance exercise training on endothelial function in individuals with overweight and obesity: A systematic review with meta-analysis of randomized clinical trials. Sci. Rep. 2023, 13, 11826. [Google Scholar] [CrossRef]
- Schmidt-Trucksäss, A.; Lichtenstein, A.H.; von Känel, R. Lifestyle factors as determinants of atherosclerotic cardiovascular health. Atherosclerosis 2024, 395, 117577. [Google Scholar] [CrossRef] [PubMed]
- Anderssen, S.A.; Carroll, S.; Urdal, P.; Holme, I. Combined diet and exercise intervention reverses the metabolic syndrome in middle-aged males: Results from the Oslo diet and exercise study. Scand. J. Med. Sci. Sports 2007, 17, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Deanfield, J.; Lincoff, A.M.; Kahn, S.E.; Emerson, S.S.; Lingvay, I.; Scirica, B.M.; Plutzky, J.; Kushner, R.F.; Colhoun, H.M.; Hovingh, G.K.; et al. Semaglutide and cardiovascular outcomes by baseline and changes in adiposity measurements: A prespecified analysis of the SELECT trial. Lancet 2025, 406, 2257–2268. [Google Scholar] [CrossRef] [PubMed]
- Lincoff, A.M.; Brown-Frandsen, K.; Colhoun, H.M.; Deanfield, J.; Emerson, S.S.; Esbjerg, S.; Hardt-Lindberg, S.; Hovingh, G.K.; Kahn, S.E.; Kushner, R.F.; et al. Semaglutide and cardiovascular outcomes in obesity without diabetes. N. Engl. J. Med. 2023, 389, 2221–2232. [Google Scholar] [CrossRef]
- Verma, S.; Emerson, S.; Plutzky, J.; Kahn, S.E.; Stensen, S.; Weeke, P.E.; Musinga, D.; Poirier, P.; Lingvay, I.; Lincoff, A.M. Semaglutide improves cardiovascular outcomes in patients with history of coronary artery bypass graft and obesity. J. Am. Coll. Cardiol. 2025, 85, 541–545. [Google Scholar] [CrossRef]
- Chan, B.S.; Yu, D.S.F.; Wong, C.W.Y.; Li, P.W.C. Multi-modal interventions outperform nutritional or exercise interventions alone in reversing metabolic syndrome: A systematic review and network meta-analysis. Eur. J. Prev. Cardiol. 2025, Epub ahead of print. [Google Scholar] [CrossRef]
- Forte, M.; Rodolico, D.; Ameri, P.; Catalucci, D.; Chimenti, C.; Crotti, L.; Schirone, L.; Pingitore, A.; Torella, D.; Iacovone, G.; et al. Molecular mechanisms underlying the beneficial effects of exercise and dietary interventions in the prevention of cardiometabolic diseases. J. Cardiovasc. Med. 2023, 24, e3–e14. [Google Scholar] [CrossRef]
- Beals, J.W.; Kayser, B.D.; Smith, G.I.; Schweitzer, G.G.; Kirbach, K.; Kearney, M.L.; Yoshino, J.; Rahman, G.; Knight, R.; Patterson, B.W.; et al. Dietary weight loss-induced improvements in metabolic function are enhanced by exercise in people with obesity and prediabetes. Nat. Metab. 2023, 5, 1221–1235. [Google Scholar] [CrossRef]
- Johns, D.J.; Hartmann-Boyce, J.; Jebb, S.A.; Aveyard, P.; Behavioural Weight Management Review Group. Diet or exercise interventions vs combined behavioral weight management programs: A systematic review and meta-analysis of direct comparisons. J. Acad. Nutr. Diet. 2014, 114, 1557–1568. [Google Scholar] [CrossRef]
- Guo, Z.; Li, M.; Cai, J.; Gong, W.; Liu, Y.; Liu, Z. Effect of high-intensity interval training vs. moderate-intensity continuous training on fat loss and cardiorespiratory fitness in the young and middle-aged a systematic review and meta-analysis. Int. J. Environ. Res. Public Health 2023, 20, 4741. [Google Scholar] [CrossRef] [PubMed]
- Sabouri, M.; Amirshaghaghi, F.; Hesari, M.M. High-intensity interval training improves the vascular endothelial function comparing moderate-intensity interval training in overweight or obese adults: A meta-analysis. Clin. Nutr. ESPEN 2023, 53, 100–106. [Google Scholar] [CrossRef]
- MacIntosh, B.R.; Murias, J.M.; Keir, D.A.; Weir, J.M. What is moderate to vigorous exercise intensity? Front. Physiol. 2021, 12, 682233. [Google Scholar] [CrossRef]
- Yan, R.; Chen, L.; Lin, G.; Shi, Y.; Huang, W.; Mai, Y.; Sun, J.; Li, D. Comparative effectiveness of different exercise modality on glycaemic control and lipid profile for prediabetes: Systematic review and network meta-analysis. Front. Endocrinol. 2025, 16, 1518871. [Google Scholar] [CrossRef] [PubMed]
- Paluch, A.E.; Boyer, W.R.; Franklin, B.A.; Laddu, D.; Lobelo, F.; Lee, D.C.; McDermott, M.M.; Swift, D.L.; Webel, A.R.; Lane, A.; et al. Resistance exercise training in individuals with and without cardiovascular disease: 2023 Update: A scientific statement from the American Heart Association. Circulation 2024, 149, e217–e231. [Google Scholar] [CrossRef]
- Rao, P.; Belanger, M.J.; Robbins, J.M. Exercise, physical activity, and cardiometabolic health: Insights into the prevention and treatment of cardiometabolic diseases. Cardiol. Rev. 2022, 30, 167–178. [Google Scholar] [CrossRef]
- Sabag, A.; Little, J.P.; Johnson, N.A. Low-volume high-intensity interval training for cardiometabolic health. J. Physiol. 2022, 600, 1013–1026. [Google Scholar] [CrossRef]
- Yin, M.; Li, H.; Bai, M.; Liu, H.; Chen, Z.; Deng, J.; Deng, S.; Meng, C.; Vollaard, N.B.J.; Little, J.P.; et al. Is low-volume high-intensity interval training a time-efficient strategy to improve cardiometabolic health and body composition? A meta-analysis. Appl. Physiol. Nutr. Metab. 2024, 49, 273–292. [Google Scholar] [CrossRef]
- Berisha, H.; Hattab, R.; Comi, L.; Giglione, C.; Migliaccio, S.; Magni, P. Nutrition and lifestyle interventions in managing dyslipidemia and cardiometabolic risk. Nutrients 2025, 17, 776. [Google Scholar] [CrossRef]
- Dombrowski, S.U.; Knittle, K.; Avenell, A.; Araújo-Soares, V.; Sniehotta, F.F. Long term maintenance of weight loss with non-surgical interventions in obese adults: Systematic review and meta-analyses of randomised controlled trials. BMJ 2014, 348, g2646. [Google Scholar] [CrossRef]
- Garvey, W.T.; Mechanick, J.I.; Brett, E.M.; Garber, A.J.; Hurley, D.L.; Jastreboff, A.M.; Nadolsky, K.; Pessah-Pollack, R.; Plodkowski, R.; Reviewers of the AACE/ACE Obesity Clinical Practice Guidelines. American Association of Clinical Endocrinologists and American College of Endocrinology Comprehensive Clinical Practice Guidelines for Medical Care of Patients with Obesity. Endocr. Pract. 2016, 22, 1–203. [Google Scholar] [CrossRef] [PubMed]
- Middleton, K.R.; Anton, S.D.; Perri, M.G. Long-term adherence to health behavior change. Am. J. Lifestyle Med. 2013, 7, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Gillies, C.L.; Abrams, K.R.; Lambert, P.C.; Cooper, N.J.; Sutton, A.J.; Hsu, R.T.; Khunti, K. Pharmacological and lifestyle interventions to prevent or delay type 2 diabetes in people with impaired glucose tolerance: Systematic review and meta-analysis. BMJ 2007, 334, 299. [Google Scholar] [CrossRef] [PubMed]
- Kolanu, N.D.; Syeda, Z.R.; Joshi, N.; Singh, P.; Erukulla, M. The differential impact of medical therapy and lifestyle modification on cardiovascular health and risk of adverse cardiovascular events: A narrative review. Cureus 2024, 16, e57742. [Google Scholar] [CrossRef]
- Marques-Vidal, P. Comparison of lifestyle changes and pharmacological treatment on cardiovascular risk factors. Heart 2020, 106, 852–862. [Google Scholar] [CrossRef]
- Price, S.; Le, Q.N.; White, N.D. Lifestyle and pharmacotherapy for weight loss in preventing or delaying diabetes. Am. J. Lifestyle Med. 2017, 12, 34–37. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.