Iron Consumption and Colorectal Cancer in Korean Adults: A Prospective Cohort Study
Abstract
1. Introduction
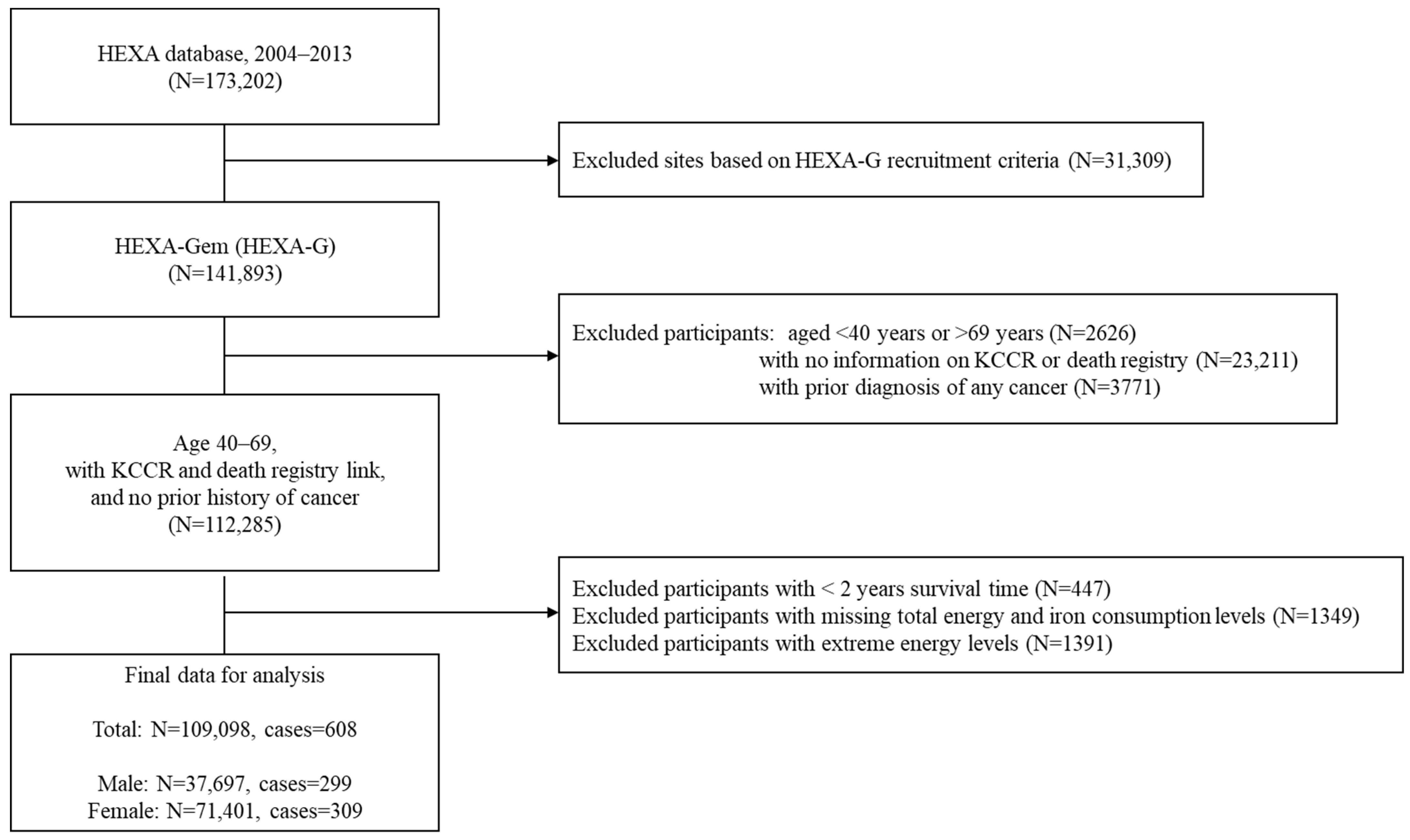
2. Materials and Methods
2.1. Study Design and Population
2.2. Exposure Assessment
2.3. Outcome Ascertainment
2.4. Covariates
2.5. Statistical Analysis
3. Results
3.1. Association Between Total Iron Consumption and Cancer Risk
3.2. Association Between Heme Iron Consumption and Cancer Risk
3.3. Association Between Non-Heme Iron Consumption and Cancer Risk
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BMI | Body mass index |
| CI | Confidence interval |
| CRC | Colorectal cancer |
| FFQ | Food frequency questionnaire |
| HR | Hazard ratio |
| IARC | International Agency for Research on Cancer |
| ICD | International classification of diseases |
| KoGES HEXA | Korean Genome and Epidemiology Study Health Examinees |
| RDA | Rural Development Administration |
| SD | Standard deviation |
| WCRF | World Cancer Research Fund |
References
- Morgan, E.; Arnold, M.; Gini, A.; Lorenzoni, V.; Cabasag, C.J.; Laversanne, M.; Vignat, J.; Ferlay, J.; Murphy, N.; Bray, F. Global burden of colorectal cancer in 2020 and 2040: Incidence and mortality estimates from GLOBOCAN. Gut 2023, 72, 338–344. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Information Center. Available online: https://cancer.go.kr/lay1/S1T639C641/contents.do (accessed on 29 May 2024).
- Chan, A.T.; Giovannucci, E.L. Primary prevention of colorectal cancer. Gastroenterology 2010, 138, 2029–2043.e10. [Google Scholar] [CrossRef]
- Onyoh, E.F.; Hsu, W.F.; Chang, L.C.; Lee, Y.C.; Wu, M.S.; Chiu, H.M. The Rise of Colorectal Cancer in Asia: Epidemiology, Screening, and Management. Curr. Gastroenterol. Rep. 2019, 21, 36. [Google Scholar] [CrossRef]
- Ionescu, V.A.; Gheorghe, G.; Bacalbasa, N.; Chiotoroiu, A.L.; Diaconu, C. Colorectal Cancer: From Risk Factors to Oncogenesis. Medicina 2023, 59, 1646. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Red Meat and Processed Meat; International Agency for Research on Cancer: Lyon, France, 2018. [Google Scholar]
- World Cancer Research Fund International. Diet, Nutrition, Physical Activity and Cancer: A Global Perspective: A Summary of the Third Expert Report, 3rd ed.; World Cancer Research Fund International: London, UK, 2018. [Google Scholar]
- Seiwert, N.; Heylmann, D.; Hasselwander, S.; Fahrer, J. Mechanism of colorectal carcinogenesis triggered by heme iron from red meat. Biochim. Biophys. Acta Rev. Cancer 2020, 1873, 188334. [Google Scholar] [CrossRef] [PubMed]
- Bastide, N.M.; Pierre, F.H.; Corpet, D.E. Heme iron from meat and risk of colorectal cancer: A meta-analysis and a review of the mechanisms involved. Cancer Prev. Res. 2011, 4, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Etemadi, A.; Abnet, C.C.; Graubard, B.I.; Beane-Freeman, L.; Freedman, N.D.; Liao, L.; Dawsey, S.M.; Sinha, R. Anatomical subsite can modify the association between meat and meat compounds and risk of colorectal adenocarcinoma: Findings from three large US cohorts. Int. J. Cancer 2018, 143, 2261–2270. [Google Scholar] [CrossRef]
- Balder, H.F.; Vogel, J.; Jansen, M.C.; Weijenberg, M.P.; van den Brandt, P.A.; Westenbrink, S.; Van Der Meer, R.; Goldbohm, R.A. Heme and chlorophyll intake and risk of colorectal cancer in the Netherlands cohort study. Cancer Epidemiol. Biomark. Prev. 2006, 15, 717–725. [Google Scholar] [CrossRef]
- Cross, A.J.; Harnly, J.M.; Ferrucci, L.M.; Risch, A.; Mayne, S.T.; Sinha, R. Developing a heme iron database for meats according to meat type, cooking method and doneness level. Food Nutr. Sci. 2012, 3, 905–913. [Google Scholar] [CrossRef]
- Kabat, G.C.; Miller, A.B.; Jain, M.; Rohan, T.E. A cohort study of dietary iron and heme iron intake and risk of colorectal cancer in women. Br. J. Cancer 2007, 97, 118–122. [Google Scholar] [CrossRef]
- Aglago, E.K.; Cross, A.J.; Riboli, E.; Fedirko, V.; Hughes, D.J.; Fournier, A.; Jakszyn, P.; Freisling, H.; Gunter, M.J.; Dahm, C.C.; et al. Dietary intake of total, heme and non-heme iron and the risk of colorectal cancer in a European prospective cohort study. Br. J. Cancer 2023, 128, 1529–1540. [Google Scholar] [CrossRef]
- Zhang, X.; Giovannucci, E.L.; Smith-Warner, S.A.; Wu, K.; Fuchs, C.S.; Pollak, M.; Willett, W.C.; Ma, J. A prospective study of intakes of zinc and heme iron and colorectal cancer risk in men and women. Cancer Causes Control. 2011, 22, 1627–1637. [Google Scholar] [CrossRef] [PubMed]
- Hara, A.; Sasazuki, S.; Inoue, M.; Iwasaki, M.; Shimazu, T.; Sawada, N.; Yamaji, T.; Takachi, R.; Tsugane, S. Zinc and heme iron intakes and risk of colorectal cancer: A population-based prospective cohort study in Japan. Am. J. Clin. Nutr. 2012, 96, 864–873. [Google Scholar] [CrossRef]
- Iso, H. Dietary Patterns and Cardiovascular Disease Risk in Asia. Nutrients 2023, 15, 2481. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; McLerran, D.F.; Rolland, B.; Chen, Y.; Grant, E.J.; Vedanthan, R.; Inoue, M.; Tsugane, S.; Gao, Y.-T.; Tsuji, I.; et al. Meat intake and cause-specific mortality: A pooled analysis of Asian prospective cohort studies. Am. J. Clin. Nutr. 2013, 98, 1032–1041. [Google Scholar] [CrossRef] [PubMed]
- Zhong, V.W.; Van Horn, L.; Greenland, P.; Carnethon, M.R.; Ning, H.; Wilkins, J.T.; Lloyd-Jones, D.M.; Allen, N.B. Associations of Processed Meat, Unprocessed Red Meat, Poultry, or Fish Intake with Incident Cardiovascular Disease and All-Cause Mortality. JAMA Intern. Med. 2020, 180, 503–512. [Google Scholar] [CrossRef]
- Health Examinees Study, G. The Health Examinees (HEXA) study: Rationale, study design and baseline characteristics. Asian Pac. J. Cancer Prev. 2015, 16, 1591–1597. [Google Scholar]
- Shin, S.; Lee, H.-W.; Kim, C.E.; Lim, J.; Lee, J.-K.; Lee, S.-A.; Kang, D. Egg Consumption and Risk of Metabolic Syndrome in Korean Adults: Results from the Health Examinees Study. Nutrients 2017, 9, 687. [Google Scholar] [CrossRef]
- Ahn, Y.; Kwon, E.; Shim, J.E.; Park, M.K.; Joo, Y.; Kimm, K.; Park, C.; Kim, D.H. Validation and reproducibility of food frequency questionnaire for Korean genome epidemiologic study. Eur. J. Clin. Nutr. 2007, 61, 1435–1441. [Google Scholar] [CrossRef]
- Rural Development Administration National Institute of Agricultural Sciences Korean Food Composition Database. Available online: https://koreanfood.rda.go.kr/kfi/fct/fctFoodSrch/list?menuId=PS03563 (accessed on 29 May 2024).
- United States Department of Agriculture Agricultural Research Service Food Data Central. Available online: https://fdc.nal.usda.gov (accessed on 29 May 2024).
- World Health Organization Regional Office for the Western Pacific. The Asia-Pacific Perspective: Redefining Obesity and Its Treatment; Health Communications Australia: Sydney, Australia, 2000. [Google Scholar]
- Park, E.H.; Jung, K.-W.; Park, N.J.; Kang, M.J.; Yun, E.H.; Kim, H.-J.; Kim, J.-E.; Kong, H.-J.; Im, J.-S.; Seo, H.G. Cancer Statistics in Korea: Incidence, Mortality, Survival, and Prevalence in 2021. Cancer Res. Treat. 2024, 56, 357–371. [Google Scholar] [CrossRef]
- Meng, Y.; Sun, J.; Yu, J.; Wang, C.; Su, J. Dietary Intakes of Calcium, Iron, Magnesium, and Potassium Elements and the Risk of Colorectal Cancer: A Meta-Analysis. Biol. Trace Elem. Res. 2019, 189, 325–335. [Google Scholar] [CrossRef]
- Korean Nutrition Society. Recommended Dietary Allowances for Koreans, 7th ed.; The Korean Nutrition Society: Seoul, Republic of Korea, 2000. [Google Scholar]
- Institute of Medicine Food and Nutrition Board. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc: A Report of the Panel on Micronutrients; National Academy Press: Washington, DC, USA, 2001. [Google Scholar]
- Aksan, A.; Farrag, K.; Aksan, S.; Schroeder, O.; Stein, J. Flipside of the Coin: Iron Deficiency and Colorectal Cancer. Front. Immunol. 2021, 12, 635899. [Google Scholar] [CrossRef] [PubMed]
- Estêvão, D.; da Cruz-Ribeiro, M.; Cardoso, A.P.; Costa, Â.M.; Oliveira, M.J.; Duarte, T.L.; da Cruz, T.B. Iron metabolism in colorectal cancer: A balancing act. Cell. Oncol. 2023, 46, 1545–1558. [Google Scholar] [CrossRef]
- Japanese Ministry of Health, Labour and Welfare. 2016 National Health and Nutrition Survey. Available online: https://www.e-stat.go.jp/stat-search/files?page=1 (accessed on 2 April 2025).
- Lee, J.; Kwon, S.O.; Yeoh, Y.; Seo, M.J.; Lee, G.H.; Kim, C. Dietary Iron Intake of Koreans Estimated using 2 Different Sources of Iron Contents are Comparable: Food & Nutrient Database and Iron Contents of Cooked Foods in the Korean Total Diet Study. Korea J. Community Nutr. 2022, 27, 245–253. [Google Scholar]
- Zhu, Z.; Wu, F.; Lu, Y.; Wu, C.; Wang, Z.; Zang, J.; Guo, C.; Jia, X.; Yao, J.; Peng, H.; et al. Total and Nonheme Dietary Iron Intake Is Associated with Metabolic Syndrome and Its Components in Chinese Men and Women. Nutrients 2018, 10, 1663. [Google Scholar] [CrossRef]
- Milman, N.T. Dietary Iron Intakes in Men in Europe Are Distinctly Above the Recommendations: A Review of 39 National Studies From 20 Countries in the Period 1995–2016. Gastroenterol. Res. 2020, 13, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Lattanzi, G.; Perillo, F.; Díaz-Basabe, A.; Caridi, B.; Amoroso, C.; Baeri, A.; Cirrincione, E.; Ghidini, M.; Galassi, B.; Cassinotti, E.; et al. Estrogen-related differences in antitumor immunity and gut microbiome contribute to sexual dimorphism of colorectal cancer. Oncoimmunology 2024, 13, 2425125. [Google Scholar] [CrossRef]
- Das, P.K.; Saha, J.; Pillai, S.; Lam, A.K.; Gopalan, V.; Islam, F. Implications of estrogen and its receptors in colorectal carcinoma. Cancer Med. 2023, 12, 4367–4379. [Google Scholar] [CrossRef]
- Wu, Z.; Huang, Y.; Zhang, R.; Zheng, C.; You, F.; Wang, M.; Xiao, C.; Li, X. Sex differences in colorectal cancer: With a focus on sex hormone-gut microbiome axis. Cell Commun. Signal. 2024, 22, 167. [Google Scholar] [CrossRef]
| Characteristic | All Sample | Total Iron Consumption (mg/Day) | p-Value 1 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Q1 (1.09–4.99) | Q2 (5.00–6.27) | Q3 (6.28–7.60) | Q4 (7.61–9.54) | Q5 (9.55–47.6) | ||||||||
| N | % | N | % | N | % | N | % | N | % | |||
| Number of individuals | 109,098 | 21,820 | 20.0 | 21,820 | 20.0 | 21,820 | 20.0 | 21,819 | 20.0 | 21,819 | 20.0 | <0.001 |
| Person-years | 367,079,182 | 201,327 | 199,804 | 199,848 | 201,291 | 203,427 | ||||||
| Follow-up year, y (median) | 9.1 | 9.2 | 9.1 | 9.1 | 9.1 | 9.2 | ||||||
| Sex | ||||||||||||
| Men | 37,697 | 5823 | 15.4 | 7132 | 18.9 | 7772 | 20.6 | 8317 | 22.1 | 8653 | 23.0 | <0.001 |
| Women | 71,401 | 15,997 | 22.4 | 14,688 | 20.6 | 14,048 | 19.7 | 13,502 | 18.9 | 13,166 | 18.4 | |
| Age, y (mean (SD)) | 52.8 (8.0) | 53.7 (7.9) | 53.0 (8.0) | 52.0 (8.0) | 52.0 (8.0) | 51.0 (7.9) | <0.001 | |||||
| BMI 2, kg/m2 (mean(SD)) | 23.9 (2.9) | 23.9 (2.9) | 23.9 (2.9) | 23.9 (2.9) | 24.0 (2.9) | 24.1 (2.9) | <0.001 | |||||
| Underweight (<18.5) | 1922 | 453 | 23.6 | 425 | 22.1 | 358 | 18.6 | 359 | 18.7 | 327 | 17.0 | <0.001 |
| Normal (≥18.5 and <23.0) | 41,613 | 8694 | 20.9 | 8580 | 20.6 | 8260 | 19.8 | 8253 | 19.8 | 7826 | 18.8 | |
| Overweight (≥23.0 and <25.0) | 30,318 | 6018 | 19.8 | 5976 | 19.7 | 6112 | 20.2 | 6057 | 20.0 | 6155 | 20.3 | |
| Obese I (≥25.0 and <30.0) | 32,208 | 6113 | 19.0 | 6277 | 19.5 | 6493 | 20.2 | 6505 | 20.2 | 6820 | 21.2 | |
| Obese II (≥30.0 kg/m2) | 3037 | 542 | 17.8 | 562 | 18.5 | 597 | 19.7 | 645 | 21.2 | 691 | 22.8 | |
| Education | ||||||||||||
| Middle school | 32,963 | 8652 | 26.2 | 7248 | 22.0 | 6492 | 19.7 | 5659 | 17.2 | 4912 | 14.9 | <0.001 |
| High school | 47,332 | 8720 | 18.4 | 9285 | 19.6 | 9439 | 19.9 | 9788 | 20.7 | 10,100 | 21.3 | |
| College degree | 28,803 | 4448 | 15.4 | 5287 | 18.4 | 5889 | 20.4 | 6372 | 22.1 | 6807 | 23.6 | |
| Smoking | ||||||||||||
| Never | 79,217 | 17,179 | 21.7 | 16,242 | 20.5 | 15,658 | 19.8 | 15,169 | 19.1 | 14,969 | 18.9 | <0.001 |
| Former | 16,301 | 2533 | 15.5 | 3104 | 19.0 | 3489 | 21.4 | 3622 | 22.2 | 3553 | 21.8 | |
| Current | 13,580 | 2108 | 15.5 | 2474 | 18.2 | 2673 | 19.7 | 3028 | 22.3 | 3297 | 24.3 | |
| Alcohol | ||||||||||||
| Never | 55,383 | 12,453 | 22.5 | 11,456 | 20.7 | 10,860 | 19.6 | 10,518 | 19.0 | 10,096 | 18.2 | <0.001 |
| Former | 3744 | 763 | 20.4 | 734 | 19.6 | 708 | 18.9 | 712 | 19.0 | 827 | 22.1 | |
| Current | 49,971 | 8604 | 17.2 | 9630 | 19.3 | 10,252 | 20.5 | 10,589 | 21.2 | 10,896 | 21.8 | |
| Physical activity | ||||||||||||
| Do not regularly exercise | 54,818 | 11,943 | 21.8 | 11,260 | 20.5 | 10,956 | 20.0 | 10,668 | 19.5 | 9991 | 18.2 | <0.001 |
| Regularly exercise, MIPA 3 < 150 | 12,956 | 2592 | 20.0 | 2634 | 20.3 | 2552 | 19.7 | 2468 | 19.0 | 2710 | 20.9 | |
| Regularly exercise, MIPA 3 ≥ 150 | 41,324 | 7285 | 17.6 | 7926 | 19.2 | 8312 | 20.1 | 8683 | 21.0 | 9118 | 22.1 | |
| History of hypertension | ||||||||||||
| Yes | 20,405 | 4442 | 21.8 | 4221 | 20.7 | 4086 | 20.0 | 3976 | 19.5 | 3680 | 18.0 | <0.001 |
| No | 88,693 | 17,378 | 19.6 | 17,599 | 19.8 | 17,734 | 20.0 | 17,843 | 20.1 | 18,139 | 20.5 | |
| History of diabetes | ||||||||||||
| Yes | 6965 | 1551 | 22.3 | 1452 | 20.8 | 1365 | 19.6 | 1258 | 18.1 | 1339 | 19.2 | <0.001 |
| No | 102,133 | 20,269 | 19.8 | 20,368 | 19.9 | 20,455 | 20.0 | 20,561 | 20.1 | 20,480 | 20.1 | |
| History of hyperlipidemia | ||||||||||||
| Yes | 10,195 | 2219 | 21.8 | 2108 | 20.7 | 2017 | 19.8 | 1937 | 19.0 | 1914 | 18.8 | <0.001 |
| No | 98,903 | 19,601 | 19.8 | 19,712 | 19.9 | 19,803 | 20.0 | 19,882 | 20.1 | 19,905 | 20.1 | |
| Family history of colorectal cancer | ||||||||||||
| Yes | 752 | 166 | 22.1 | 171 | 22.7 | 139 | 18.5 | 152 | 20.2 | 124 | 16.5 | 0.03 |
| No | 108,346 | 21,654 | 20.0 | 21,649 | 20.0 | 21,681 | 20.0 | 21,667 | 20.0 | 21,695 | 20.0 | |
| Total energy consumption, kcal/day (mean, SD) | 1694.7 (496.7) | 1261.6 (276.5) | 1536.3 (262.3) | 1712.3 (283.2) | 1913.0 (322.2) | 2280.3 (475.3) | <0.001 | |||||
| Total Iron Consumption (mg/Day) | p-Trend | |||||
|---|---|---|---|---|---|---|
| Q1 (1.09–4.99) | Q2 (5.00–6.27) | Q3 (6.28–7.60) | Q4 (7.61–9.54) | Q5 (9.55–47.6) | ||
| HR (95%CI) | HR (95%CI) | HR (95%CI) | HR (95%CI) | HR (95%CI) | ||
| Person-years | 201,327 | 199,804 | 199,848 | 201,291 | 203,427 | |
| N | 21,820 | 21,820 | 21,820 | 21,819 | 21,819 | |
| Colorectal cancer | ||||||
| Cases | 143 | 107 | 110 | 136 | 112 | |
| Model 1 1 | 1.00 (ref) | 0.75 (0.59–0.97) | 0.78 (0.61–1.00) | 0.97 (0.76–1.23) | 0.80 (0.62–1.02) | 0.07 |
| Model 2 2 | 1.00 (ref) | 0.75 (0.58–0.97) | 0.77 (0.59–1.01) | 0.96 (0.72–1.28) | 0.79 (0.55–1.13) | 0.08 |
| Colon cancer | ||||||
| Cases | 40 | 26 | 32 | 43 | 33 | |
| Model 1 1 | 1.00 (ref) | 0.71 (0.51–0.99) | 0.75 (0.54–1.03) | 1.11 (0.83–1.50) | 0.91 (0.67–1.25) | 0.03 |
| Model 2 2 | 1.00 (ref) | 0.71 (0.51–1.00) | 0.75 (0.53–1.07) | 1.12 (0.79–1.61) | 0.93 (0.59–1.46) | 0.03 |
| Rectal cancer | ||||||
| Cases | 47 | 34 | 30 | 48 | 41 | |
| Model 1 1 | 1.00 (ref) | 0.84 (0.55–1.29) | 0.89 (0.58–1.36) | 0.78 (0.50–1.20) | 0.66 (0.42–1.05) | 0.48 |
| Model 2 2 | 1.00 (ref) | 0.84 (0.54–1.32) | 0.90 (0.56–1.43) | 0.79 (0.46–1.33) | 0.68 (0.35–1.31) | 0.82 |
| Heme Iron Consumption (mg/Day) | p-Trend | ||||
|---|---|---|---|---|---|
| Q1 (0.00–0.02) | Q2 (0.03–0.04) | Q3 (0.05–0.06) | Q4 (0.07–0.68) | ||
| HR (95%CI) | HR (95%CI) | HR (95%CI) | HR (95%CI) | ||
| Person-years | 252,608 | 251,361 | 251,815 | 249,912 | |
| N | 27,274 | 27,277 | 27,273 | 27,274 | |
| Colorectal cancer | |||||
| Cases | 156 | 153 | 153 | 146 | |
| Model 1 1 | 1.00 (ref) | 1.01 (0.81–1.26) | 1.03 (0.82–1.29) | 0.98 (0.78–1.23) | 0.97 |
| Model 2 2 | 1.00 (ref) | 1.01 (0.80–1.26) | 1.04 (0.82–1.31) | 0.99 (0.77–1.29) | 0.98 |
| Colon cancer | |||||
| Cases | 98 | 93 | 85 | 98 | |
| Model 1 1 | 1.00 (ref) | 1.00 (0.75–1.33) | 0.95 (0.71–1.28) | 1.11 (0.83–1.47) | 0.78 |
| Model 2 2 | 1.00 (ref) | 0.99 (0.74–1.32) | 0.94 (0.69–1.28) | 1.09 (0.79–1.50) | 0.82 |
| Rectal cancer | |||||
| Cases | 48 | 53 | 56 | 38 | |
| Model 1 1 | 1.00 (ref) | 1.08 (0.73–1.60) | 1.12 (0.76–1.66) | 0.74 (0.48–1.14) | 0.21 |
| Model 2 2 | 1.00 (ref) | 1.11 (0.75–1.66) | 1.19 (0.79–1.79) | 0.81 (0.50–1.31) | 0.31 |
| Non-Heme Iron Consumption (mg/Day) | p-Trend | |||||
|---|---|---|---|---|---|---|
| Q1 (1.09–4.97) | Q2 (4.98–6.24) | Q3 (6.25–7.56) | Q4 (7.57–9.48) | Q5 (9.49–47.53) | ||
| HR (95%CI) | HR (95%CI) | HR (95%CI) | HR (95%CI) | HR (95%CI) | ||
| Person-years | 201,319 | 199,742 | 199,883 | 201,296 | 203,456 | |
| N | 21,819 | 21,821 | 21,819 | 21,821 | 21,818 | |
| Colorectal cancer | ||||||
| Cases | 142 | 107 | 113 | 136 | 110 | |
| Model 1 1 | 1.00 (ref) | 0.76 (0.59–0.98) | 0.81 (0.63–1.03) | 0.97 (0.77–1.23) | 0.79 (0.61–1.01) | 0.09 |
| Model 2 2 | 1.00 (ref) | 0.75 (0.58–0.98) | 0.80 (0.61–1.04) | 0.96 (0.72–1.28) | 0.77 (0.54–1.11) | 0.10 |
| Colon cancer | ||||||
| Cases | 87 | 59 | 65 | 91 | 72 | |
| Model 1 1 | 1.00 (ref) | 0.70 (0.50–0.97) | 0.78 (0.57–1.08) | 1.11 (0.83–1.50) | 0.89 (0.65–1.22) | 0.04 |
| Model 2 2 | 1.00 (ref) | 0.70 (0.49–0.98) | 0.78 (0.55–1.10) | 1.11 (0.77–1.58) | 0.88 (0.56–1.39) | 0.04 |
| Rectal cancer | ||||||
| Cases | 44 | 40 | 42 | 37 | 32 | |
| Model 1 1 | 1.00 (ref) | 0.88 (0.57–1.35) | 0.91 (0.60–1.39) | 0.79 (0.51–1.23) | 0.68 (0.43–1.07) | 0.54 |
| Model 2 2 | 1.00 (ref) | 0.89 (0.57–1.39) | 0.92 (0.58–1.47) | 0.81 (0.48–1.37) | 0.71 (0.37–1.37) | 0.87 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Min, S.; De la Torre, K.; Lee, H.; Shin, W.-K.; Kang, D. Iron Consumption and Colorectal Cancer in Korean Adults: A Prospective Cohort Study. Nutrients 2025, 17, 1309. https://doi.org/10.3390/nu17081309
Min S, De la Torre K, Lee H, Shin W-K, Kang D. Iron Consumption and Colorectal Cancer in Korean Adults: A Prospective Cohort Study. Nutrients. 2025; 17(8):1309. https://doi.org/10.3390/nu17081309
Chicago/Turabian StyleMin, Sukhong, Katherine De la Torre, Hyobin Lee, Woo-Kyoung Shin, and Daehee Kang. 2025. "Iron Consumption and Colorectal Cancer in Korean Adults: A Prospective Cohort Study" Nutrients 17, no. 8: 1309. https://doi.org/10.3390/nu17081309
APA StyleMin, S., De la Torre, K., Lee, H., Shin, W.-K., & Kang, D. (2025). Iron Consumption and Colorectal Cancer in Korean Adults: A Prospective Cohort Study. Nutrients, 17(8), 1309. https://doi.org/10.3390/nu17081309






