Abstract
Ultra-processed foods (UPFs), industrial formulations rich in refined substrates and additives, have been increasingly examined as plausible contributors to gut dysbiosis and mucosal inflammation relevant to inflammatory bowel disease (IBD). This narrative review synthesizes epidemiological, mechanistic, and interventional evidence on UPF intake and IBD based on a structured literature search from 2010 to 2025. Large-scale prospective cohorts consistently associate higher UPF intake with increased risk of Crohn’s disease (CD), whereas findings for ulcerative colitis (UC) remain weaker or inconsistent. Among individuals with established IBD, observational data suggest that greater UPF consumption correlates with higher disease activity and relapse, although potential confounding and reverse causation must be considered. Preclinical studies demonstrate that specific UPF constituents—including emulsifiers, carrageenan, maltodextrin, microparticles, and excess dietary salt—can disrupt epithelial barrier integrity, alter the gut microbiota, and activate immune pathways, providing biological plausibility while underscoring translational gaps. Interventional evidence, particularly for exclusive enteral nutrition and the Crohn’s Disease Exclusion Diet, suggests clinical benefit from reducing UPFs or selected additives, mainly in CD, though data in adults and UC remain limited. Overall, current evidence indicates that dietary strategies to limit UPF exposure may represent a promising and modifiable component of IBD management. Future research should prioritize standardized exposure assessment, mechanism-based human trials, and personalized nutrition approaches to refine clinical applicability.
1. Introduction
Inflammatory bowel disease (IBD), encompassing Crohn’s disease (CD) and ulcerative colitis (UC), represents a group of chronic, relapsing inflammatory disorders of the gastrointestinal tract whose global incidence continues to rise. Although genetic susceptibility contributes to disease risk, the rapid increase in IBD in newly industrialized countries underscores the critical role of environmental and lifestyle factors in disease pathogenesis [1]. Among these, diet has emerged as one of the most significant and modifiable determinants of disease onset and course.
Over the past few decades, the global dietary landscape has undergone a profound transition, marked by the pervasive consumption of ultra-processed foods (UPFs). According to the NOVA classification, UPFs are industrial formulations composed primarily of refined ingredients such as sugars, starches, hydrogenated oils, and protein isolates combined with cosmetic additives designed to enhance flavor, texture, and shelf life [2]. Common examples include sugar-sweetened beverages, packaged snacks, processed meats, and ready-to-eat meals. UPFs currently account for more than half of total caloric intake in many high-income nations and are rapidly expanding across low- and middle-income regions [2,3,4].
Epidemiological studies increasingly implicate high UPF intake as a risk factor for several chronic conditions, including obesity, metabolic syndrome, type 2 diabetes, cardiovascular disease, and certain cancers [5]. More recently, large-scale prospective cohorts, such as the Prospective Urban Rural Epidemiology (PURE) study, the U.S. and the UK Biobank, have examined UPF consumption in relation to IBD risk [1,6,7]. While findings vary across populations, a consistent trend has emerged linking high UPF intake with an increased risk of CD, whereas associations with UC appear weaker or absent [1,6,7]. While findings vary across populations, many studies report a positive association between higher UPF intake and increased CD risk, whereas associations with UC appear weaker or inconsistent. These observations require cautious interpretation, given potential confounding by socioeconomic status, lifestyle factors, or reverse causation (e.g., individuals with prodromal symptoms modifying their diet before diagnosis).
Beyond disease onset, UPFs may also impact disease activity and relapse rates among patients with established IBD [8,9]. Mechanistic investigations using animal models provide biological plausibility for these observations: additives such as emulsifiers (e.g., carboxymethylcellulose, polysorbate-80) and carrageenan have been shown to disrupt the intestinal barrier, alter the gut microbiota, and promote mucosal immune activation [10,11]. However, translation of these findings to humans is limited by differences in dosing, exposure duration, and species-specific physiology.
Given the growing global burden of IBD and the feasibility of dietary modification as a preventive and therapeutic approach, elucidating the relationship between UPFs and IBD is an urgent research priority.
This narrative review synthesizes current epidemiological evidence linking UPF intake with IBD risk and activity, examines potential mechanistic pathways while acknowledging translational limitations, and discusses the clinical implications of dietary strategies that reduce UPF exposure.
A conceptual overview of the proposed links between UPF components, mechanistic pathways, inflammatory responses, and clinical outcomes is presented in Figure 1.
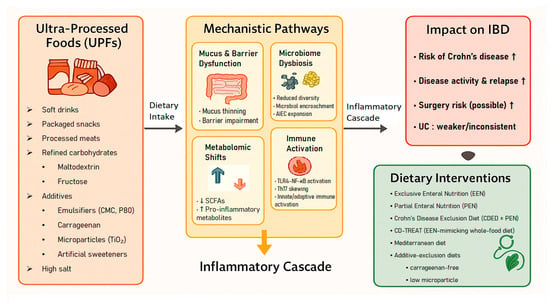
Figure 1.
Conceptual framework linking ultra-processed foods (UPFs) to mechanistic pathways, inflammatory cascade, clinical outcomes, and dietary interventions in inflammatory bowel disease (IBD).
2. Methods
2.1. Study Design
This article is a narrative review designed to synthesize epidemiological, mechanistic, and interventional evidence regarding the impact of UPFs on IBD. While this review was not registered in PROSPERO as it is not a systematic review, we followed a structured and transparent approach appropriate for narrative synthesis, ensuring a comprehensive and reproducible selection of the literature.
2.2. Search Strategy
We conducted a comprehensive literature search using the electronic databases PubMed/MEDLINE, Embase, and Scopus for articles published from 1 January 2010, to 31 March 2025. To systematically identify evidence corresponding to the review’s three main thematic areas (epidemiology, mechanisms, and therapeutics), we employed a multi-step search strategy:
- Primary Search: We broadly searched for combinations of “ultra-processed foods,” “NOVA classification,” and “inflammatory bowel disease” (including “Crohn’s disease” and “ulcerative colitis”).
- Targeted Secondary Search: Based on initial findings, we performed targeted searches for specific UPF-associated components identified in the literature, such as “food additives,” “emulsifiers” (e.g., carboxymethylcellulose), “carrageenan,” and “maltodextrin.”
- Reference Screening: We manually screened the reference lists of retrieved articles and relevant systematic reviews to identify additional pivotal studies not captured by the initial electronic search.
We did not apply automated filters for study design or outcomes during the electronic search in order to avoid inadvertently excluding mechanistically important or emerging interventional studies. Instead, relevance was assessed during manual screening as outlined in Section 2.3.
2.3. Eligibility Criteria
Studies were selected based on inclusion criteria: human studies (cohort, case–control, RCTs) and relevant mechanistic models (in vivo/in vitro) defining UPF intake or additive exposure. Studies were required to provide clearly defined exposure measures (e.g., NOVA classification or additive doses relevant to dietary intake). Exclusion criteria included studies focusing solely on single nutrients without the context of processing, or irrelevant animal models.
2.4. Study Selection and Synthesis
Data were selected through a qualitative review process. Titles and abstracts were initially screened for relevance to the core themes (epidemiology, mechanisms, therapeutics). Full texts of potentially relevant articles were then assessed based on the inclusion criteria. Consistent with narrative review methodology, study selection emphasized conceptual relevance and biological plausibility rather than quantitative pooling. Priority was given to recent high-quality cohorts, mechanistic studies demonstrating biological plausibility (e.g., specific additive effects), and interventional trials, while redundant or non-specific studies were excluded.
Finally, evidence was synthesized qualitatively and organized into three thematic domains: (1) epidemiological associations, (2) mechanistic pathways, and (3) clinical and therapeutic implications.
3. Epidemiology
The relationship between UPF consumption and IBD has been examined in multiple large-scale prospective cohorts and patient-based studies. Collectively, evidence points to a consistent association between higher UPF intake and increased risk of CD, whereas the relationship with UC remains weaker or inconsistent [6,7,12,13]. Despite differences in study design, exposure definitions, and population characteristics, the signal for CD appears robust. However, interpreting these findings requires careful consideration of methodological variations, including potential misclassification of dietary exposures and residual confounding.
Among individuals with established IBD, observational studies suggest that higher UPF intake is linked to greater disease activity and an increased risk of relapse, extending the association beyond disease onset [8,9]. Additional studies that examined Westernized or processed dietary patterns, although not explicitly using the NOVA classification, provide complementary evidence. Diets rich in fats, sweets, processed meats, and sugar-sweetened beverages are associated with an increased risk of IBD, particularly CD, whereas higher dietary fiber intake (especially from fruits) appears protective [14,15,16,17].
3.1. Prospective Cohorts
The major prospective cohort studies evaluating UPF intake and incident IBD are summarized in Table 1. The PURE study followed 116,087 adults across 21 countries for nearly 10 years. Individuals consuming ≥5 servings/day of UPFs had a significantly higher risk of IBD than those consuming <1 serving/day (HR 1.82, 95% CI 1.22–2.72), with the strongest associations for soft drinks, processed meats, and salty snacks [1].

Table 1.
Prospective cohort studies evaluating ultra-processed food (UPF) intake and incident inflammatory bowel disease (IBD).
In three large U.S. cohorts (NHS I, NHS II, and HPFS; >200,000 participants; up to 20 years of follow-up), higher UPF intake was significantly associated with CD risk in a dose—response manner, but not with UC [6]. In contrast, the NutriNet-Santé cohort (France; 105,832 adults; mean follow-up 2.3 years) found no significant association between UPF proportion and incident IBD. This null finding may reflect limited statistical power due to short follow-up duration and a small number of incident cases [12].
In the UK Biobank, participants in the highest tertile of UPF intake had approximately a twofold higher risk of CD (HR 2.00, 95% CI 1.32–3.03) but no association for UC. Notably, higher UPF consumption also predicted a fourfold increased risk of IBD-related surgery, suggesting a possible role in disease progression [7].
3.2. Patient Cohorts
Patient-based studies evaluating UPF intake among individuals with established IBD are summarized in Table 2. In Israel, a cross-sectional study of 242 patients with established IBD found that higher UPF intake was associated with active disease (OR 3.82, 95% CI 1.49–9.8), while minimally processed foods were inversely associated [8]. In a subsequent prospective cohort of 111 CD patients in remission, higher UPF intake predicted nearly a fourfold increased risk of relapse at one year (HR 3.86, 95% CI 1.30–11.47) [9]. These findings extend the epidemiologic signal beyond disease onset to disease activity and relapse. However, these observational findings warrant caution due to the possibility of reverse causation, where individuals with prodromal symptoms or active flares may preferentially consume softer, processed foods to minimize gastrointestinal discomfort, thereby complicating interpretation of temporality.

Table 2.
Patient-based studies examining UPF intake in relation to disease activity and relapse among individuals with established IBD.
3.3. Meta-Analyses
Several systematic reviews and meta-analyses have consolidated the epidemiologic evidence linking UPF intake with IBD. Narula et al. performed the first quantitative synthesis combining seven observational studies and reported a consistent positive association between UPF consumption and CD (pooled RR 1.70, 95% CI 1.24–2.35), with less consistent findings for UC [13]. Babaei et al. conducted a large-scale dose–response meta-analysis including more than four million participants, confirming that higher UPF intake was significantly associated with increased overall IBD risk (pooled RR 1.28, 95% CI 1.11–1.48) [19].
Together, these quantitative syntheses strengthen the evidence that UPFs represent a modifiable dietary risk factor for IBD, particularly for CD. In addition, a complementary umbrella review has broadly linked dietary and nutritional exposures, including processed food consumption, to the incidence and progression of IBD, thereby providing a broader context for these quantitative findings [18]. This broader evidence base underscores how UPF-rich dietary patterns, embedded within global nutritional and cultural transitions, may contribute to the rising incidence of IBD worldwide.
3.4. Complementary Evidence from Dietary Pattern Studies
Several studies that did not explicitly use the NOVA classification nevertheless support a link between processed dietary patterns and IBD. In a Canadian pediatric case–control study, imbalances in fatty acid intake and low consumption of fruits and vegetables were associated with increased CD risk [14]. A follow-up analysis in the same cohort showed that dietary patterns rich in fast foods and sugar-sweetened beverages were linked to higher pediatric CD risk [15]. Likewise, a multicenter Case–Control study in Japan (n = 579 cases, 1246 controls) reported that higher intake of sweets, fats, and oils was associated with increased IBD risk, consistent with a Westernized dietary pattern [16].
In addition, systematic and prospective evidence indicates that diets high in total fat, n-6 polyunsaturated fatty acids, processed meats, and refined sugars are associated with greater IBD risk, whereas higher dietary fiber, particularly from fruits, appears protective against CD [17,20].
3.5. Synthesis and Methodological Considerations
Taken together, evidence from multinational cohorts, meta-analyses, and patient-based studies consistently indicates that higher consumption of UPFs increases the risk of developing CD and may exacerbate disease activity or relapse in established IBD.
However, significant uncertainties regarding causality and disease specificity remain. The stronger associations observed for CD compared with UC may reflect plausible biological differences, including the greater exposure of the small intestine where CD predominantly manifests to dietary emulsifiers and additives, whereas the colon’s thicker, double-layered mucus barrier may offer relative protection [6,7,21].
Furthermore, potential confounding factors must be acknowledged. Socioeconomic status (SES) strongly influences UPF consumption, with lower SES often linked to higher intake; simultaneously, higher SES has historically been associated with increased IBD risk, complicating the direction of association [1,22]. Genetic susceptibility also plays a role, as host genetics influence microbiome composition and responses. In addition, dietary exposure misclassification remains a concern, as food frequency questionnaires may inadequately capture additive-rich foods or processing levels. Finally, reverse causation remains a critical concern, particularly in patient-based studies, as individuals with undiagnosed or active IBD may self-restrict fiber-rich whole foods in favor of processed alternatives to manage symptoms [6,8].
Overall, while the epidemiologic signal is robust for CD, future research must employ designs that better account for these confounders and utilize standardized exposure measures to clarify the causal role of UPFs.
4. Mechanistic Pathways
UPFs are distinguished by their composition of refined substrates such as added sugars and starches and a wide array of industrial additives including emulsifiers, thickeners, colorants, and non-nutritive sweeteners. These components collectively alter host–microbe interactions and disrupt intestinal homeostasis. Emerging experimental and translational evidence delineates at least four interrelated mechanisms through which UPFs may promote intestinal inflammation: (i) erosion of the mucus layer and impairment of epithelial barrier integrity; (ii) gut microbial dysbiosis characterized by bacterial “encroachment” and expansion of pathobionts; (iii) pro-inflammatory metabolomic alterations, including reductions in short-chain fatty acids (SCFAs); and (iv) activation of innate and adaptive immune pathways, particularly involving TLR–NF-κB and Th17 signaling axes.
Together, these processes create a biologically plausible, though not yet definitively causal, framework linking UPF consumption to the onset and progression of IBD. A conceptual schematic summarizing the major biological pathways through which UPFs may influence intestinal inflammation is presented in Figure 1.
4.1. Emulsifiers (Carboxymethylcellulose, Polysorbate-80)
In murine models, chronic exposure to the dietary emulsifiers carboxymethylcellulose (CMC) and polysorbate-80 (P80) leads to thinning of the protective mucus layer, microbial encroachment toward the epithelium, reduced microbial diversity, and the development of colitis in genetically susceptible hosts; even wild-type mice display low-grade inflammation and metabolic disturbances under prolonged exposure [10]. Mechanistically, these alterations are accompanied by closer bacteria–epithelium proximity and an increased pro-inflammatory potential of the gut microbiota [10].
Translational relevance has been demonstrated in humans: in a randomized controlled feeding trial in healthy adults, supplementation of CMC (15 g/day) to an additive-free diet significantly altered the gut microbiota composition, reduced fecal SCFAs, and increased postprandial abdominal discomfort, providing human proof-of-principle for emulsifier-induced dysbiosis and barrier dysfunction [23]. However, this trial was small and short-term, limiting generalizability to broader clinical populations.
4.2. Carrageenan (CGN)
CGN exposure activates the TLR4–Bcl10–NF-κB signaling axis and induces IL-8 secretion in human intestinal epithelial cells [24,25,26]. Comprehensive reviews suggest that CGN may trigger multifaceted innate and adaptive immune responses, and animal studies demonstrate CGN-associated dysbiosis, including reduced Akkermansia muciniphila [27,28]. Disruption of epithelial tight-junction proteins has been reported even at food-grade concentrations [29]. At concentrations relevant to typical dietary exposure, CGN also causes cell-cycle arrest and epithelial stress responses [30]. In murine models, carrageenan administration (e.g., 10–50 mg/kg/day via drinking water) induces colitis, with significantly reduced severity observed in Bcl10-deficient mice, underscoring the role of the Bcl10–NF-κB pathway in mediating inflammation [31]. Translationally, a double-blind randomized trial in patients with ulcerative colitis in remission demonstrated that carrageenan supplementation (200 mg/day) accelerated clinical relapse compared with placebo, suggesting that CGN restriction may be beneficial in susceptible individuals [32]. While clinically meaningful, this study was limited by small sample size, and larger trials are required to confirm the effect.
4.3. Refined Carbohydrate Additives: Maltodextrin and Added Sugars
Maltodextrin (MDX), widely used in UPFs, enhances biofilm formation and adhesion of adherent-invasive E. coli (AIEC), a CD-associated pathobiont [33], and impairs antimicrobial defenses in intestinal epithelial cells and macrophages [34]. In IL-10−/− mice, chronic MDX exposure increases colitis severity, reduces goblet cells, and promotes microbial encroachment into the mucosa, suggesting impaired mucus-mediated protection [35,36]. Additional work indicates that MDX may also interfere with autophagy pathways that are relevant to CD susceptibility genes, although these findings remain primarily experimental and require human validation.
Beyond MDX, diets enriched in added sugars—particularly fructose—have been shown to worsen colitis in murine models by disrupting epithelial tight-junction integrity and altering gut microbial composition [37,38]. These effects appear modifiable, as switching to a standard chow diet or adding psyllium restores SCFA-linked pathways and improves inflammatory outcomes. However, the translational relevance of these findings is limited by the high fructose doses commonly used in animal studies and by differences in human dietary patterns and food-matrix contexts.
4.4. Non-Nutritive Sweeteners (NNS)
NNS, widely used in UPFs, have been shown to alter the gut microbiota and modulate metabolic responses in humans. In a recent controlled trial, several commonly consumed NNS produced person-specific impairments in glycemic control, driven by individual microbiome configurations and accompanied by microbiota-dependent functional alterations [39]. Earlier foundational work demonstrated that NNS could induce glucose intolerance in mice and in subsets of healthy human volunteers, with the effect fully transferable via microbiota transplantation, confirming a causal, microbiome-mediated mechanism [40]. Although these studies do not assess IBD directly, they illustrate how UPF-associated additives can reshape host–microbe interactions in ways potentially relevant to mucosal inflammation.
4.5. Microparticles and Colorants (Titanium Dioxide, E171)
Food-grade titanium dioxide (E171) contains micro- and nanoparticles that can interact with the intestinal barrier and alter gut microbial composition in experimental models [41,42,43]. These particles have been shown to accumulate within intestinal mucus, modulate microbial biofilms, and influence epithelial and immune responses [44].
Regulatory assessments reflect ongoing uncertainty regarding the safety of E171. In 2021, the European Food Safety Authority (EFSA) concluded that titanium dioxide could no longer be considered safe as a food additive due to unresolved concerns related to potential genotoxicity, leading to its removal from the European Union food market in 2022 [45,46]. In contrast, the U.S. Food and Drug Administration (FDA) continues to permit E171 as a food colorant. This regulatory divergence is noteworthy, particularly because several U.S. food products—including candies, confectioneries, and brightly colored processed snacks marketed to children—contain higher relative amounts of titanium dioxide, resulting in proportionally greater exposure per body weight in younger populations.
However, despite these safety concerns, direct clinical evidence linking titanium dioxide to the onset or progression of IBD remains limited. Existing findings are derived largely from in vitro studies and animal models, and the doses and exposure forms used may not fully reflect real-world dietary conditions. As such, while E171 is biologically plausible as a contributor to mucosal dysfunction, its specific role in human IBD pathogenesis has not been definitively established and warrants further investigation.
4.6. High Salt as a UPF-Linked Property
Dietary salt, which is typically elevated in many UPF formulations, has been implicated as an independent modifier of intestinal inflammation. In murine models, high-salt diets exacerbate chemically induced colitis, characterized by reductions in Lactobacillus species, depletion of SCFA, and impaired barrier-related gene expression [47,48]. Additional studies demonstrate that excessive salt intake promotes Th17 differentiation and worsens 2,4,6-trinitrobenzenesulfonic acid (TNBS)-induced colitis through immune-mediated pathways [49,50]. Although these findings do not establish a direct causal link between dietary salt and IBD onset in humans, they suggest that salt-rich dietary patterns, common in UPFs, may potentiate mucosal inflammation in susceptible hosts.
4.7. Translational Gaps and Limitations
Although experimental models provide compelling evidence for the pro-inflammatory effects of individual UPF components, several important limitations constrain direct translation to human IBD. Many animal studies involve continuous, high-dose additive exposures (e.g., emulsifiers or carrageenan in drinking water) that may not mimic the intermittent, mixed-matrix consumption patterns typical of human diets. Species-specific differences such as thinner mucus layers, faster intestinal transit, and distinct microbial communities in mice further influence host responses to dietary additives.
Moreover, human evidence remains limited. Most randomized controlled trials evaluating UPF additives include small sample sizes (e.g., 16 participants in the CMC feeding study) and short exposure durations, and they rarely assess clinical IBD endpoints. Observational associations in humans may also be influenced by confounding factors, including socioeconomic status, genetic susceptibility, comorbid dietary patterns, and potential reverse causation, in which early symptoms prompt patients to shift toward softer, more processed foods.
Thus, while the mechanistic data provide biological plausibility, establishing a causal relationship between specific UPF components and human IBD will require long-term, adequately powered clinical studies using standardized exposure measurements.
4.8. Integrated Mechanistic Synthesis
Across experimental models and, increasingly, human intervention studies, UPF-characteristic ingredients and processing properties show a reproducible ability to disrupt intestinal homeostasis. These effects include: (i) erosion of the mucus layer and impairment of epithelial barrier integrity, (ii) induction of dysbiosis and microbial encroachment, including the expansion of pathobionts such as AIEC, (iii) reductions in SCFAs and broader metabolomic disturbances, and (iv) activation of innate and adaptive inflammatory pathways, including TLR4–NF-κB and Th17-mediated responses. Collectively, these mechanistic pathways provide a coherent biological framework supporting the epidemiologic signal linking UPF consumption and IBD risk, while also emphasizing the need to interpret current evidence within its translational limitations. Representative mechanistic evidence supporting these pathways is summarized in Table 3.

Table 3.
Mechanistic evidence linking UPF components and food additives to intestinal barrier dysfunction, microbiota alterations, and immune activation relevant to IBD.
5. Therapeutic Implications
Growing epidemiological and mechanistic evidence has renewed interest in dietary interventions that reduce UPFs intake or eliminate specific UPF-associated additives as potential therapeutic strategies for IBD. While nutrition counseling is increasingly recognized as an integral component of comprehensive IBD care, the quality and robustness of evidence supporting each dietary approach differ substantially. At present, the strongest and most reproducible clinical evidence exists for CD, particularly in children, whereas data for UC remain limited, heterogeneous, and often exploratory. As a result, dietary strategies must be interpreted within the context of available clinical trials, mechanistic plausibility, and patient-centered feasibility.
5.1. Exclusive Enteral Nutrition (EEN) and Partial Enteral Nutrition (PEN)
EEN, which replaces the habitual diet entirely with a nutritionally complete formula for 6~8 weeks, remains the recommended first-line induction therapy for pediatric CD according to ECCO/ESPGHAN guidelines [51]. Beyond its established clinical efficacy, EEN is hypothesized to exert benefit partly through the removal of habitual dietary exposures, including UPFs and common food additives, while providing controlled, antigen-light nutrition [52,53]. However, real-world implementation remains challenging due to palatability issues, psychosocial burden, and declining adherence over time.
PEN, which provides a proportion of calories from formula alongside whole foods, is generally more acceptable and less restrictive than EEN. Earlier studies suggested that PEN alone yields lower and more variable induction efficacy; however, recent findings suggest that higher-percentage PEN (≥50% of energy) may offer improved outcomes, although results remain inconsistent and are based on relatively small and heterogeneous cohorts. This variability highlights that the “whole food” component must be carefully structured to maintain efficacy [54,55,56].
5.2. Crohn’s Disease Exclusion Diet (CDED)
The CDED is a structured whole-food diet that excludes UPFs, emulsifiers, and other suspect components while encouraging fruits, vegetables, and unprocessed proteins. In a pivotal randomized controlled trial, CDED combined with PEN was non-inferior to EEN for induction of remission in pediatric CD, with better tolerance and sustainability [57]. Longer-term follow-up showed higher rates of sustained remission compared with PEN alone [56]. These findings positioned CDED as an evidence-based, UPF-reduction diet with both mechanistic plausibility and clinical efficacy. Nonetheless, evidence is predominantly pediatric, and adult data remain limited.
5.3. Individualized Food-Based Diet (CD-TREAT)
The CD-TREAT diet reproduces the nutritional composition of EEN using whole, ordinary foods while excluding UPFs and known dietary triggers. In a pilot randomized trial involving 25 healthy adults and 5 children with active CD, CD-TREAT demonstrated anti-inflammatory and microbiota-modulating effects comparable to EEN, including significant reductions in fecal calprotectin [58]. Interpretation is limited, however, by very small sample size, short duration, and lack of diverse populations. Although still experimental, CD-TREAT highlights the feasibility of a food-based dietary therapy that minimizes UPFs while maintaining nutritional adequacy.
5.4. Specific Carbohydrate Diet (SCD) and Mediterranean Diet (MedDiet)
The SCD, which restricts complex carbohydrates, processed foods, and food additives, has demonstrated clinical and mucosal improvements in small pediatric cohorts with CD [59]. Similarly, a MedDiet emphasizing minimally processed plant foods, olive oil, whole grains, and fish was shown in the DINE-CD randomized trial to be non-inferior to SCD for achieving symptomatic remission, while offering superior palatability and adherence [60]. Because both diets substantially reduce UPFs by design, they illustrate the potential benefits of whole-food dietary patterns; however, long-term adherence to restrictive diets such as SCD is difficult, and evidence in adults remains inconsistent.
5.5. Additive-Exclusion Approaches
Targeted elimination of specific food additives has also been explored as a therapeutic strategy. A double-blind randomized trial in patients with UC in remission showed that carrageenan supplementation accelerated clinical relapse compared with a carrageenan-free diet, suggesting a potential benefit of carrageenan exclusion in susceptible individuals [32].
In CD, small pilot trials of low-microparticle diets aimed at reducing exposure to titanium dioxide (E171) and other particulate additives reported improvements in symptoms and inflammatory markers; however, these studies were conducted more than a decade ago, involve small samples, and have not been validated in modern cohorts [44]. Collectively, these studies suggest that specific UPF components may modulate inflammation, but evidence remains preliminary.
5.6. Guideline and Consensus Perspectives
Professional society documents increasingly acknowledge the role of diet and food processing in IBD care. The 2024 AGA Clinical Practice Update advises clinicians to counsel patients to emphasize Mediterranean-style, whole-food dietary patterns and to limit UPFs, added sugars, and salt, while noting that no single diet has consistently reduced flare rates in adults [61].
The 2025 ECCO Consensus on Dietary Management of IBD frames diet as a therapeutic modality beyond nutritional support, summarizing evidence for formula-based and food-based strategies (e.g., EEN, CDED, MedDiet) and providing practical consensus statements for clinical implementation. At the same time, it highlights evidence gaps and stops short of recommending blanket avoidance of all ultra-processed foods for every patient [62,63]. At the same time, ECCO cautions that evidence gaps remain substantial, particularly for UC, and does not endorse universal UPF avoidance for all patients.
The ESPEN Clinical Nutrition Guideline provides graded recommendations across disease phases and endorses individualized assessment, enteral nutrition for induction in CD, and whole-food dietary patterns, while warning that cultural, economic, and access-related barriers may limit feasibility in routine practice [64].
5.7. Therapeutic Synthesis
Therapeutic dietary approaches in IBD increasingly converge on a common principle: minimizing exposure to UPFs and certain additives. Robust evidence supports EEN and CDED in pediatric CD, with emerging, but less definitive, data for adult CD (MedDiet, SCD). Evidence for UC is limited mainly to additive-specific exclusion trials such as carrageenan restriction. Although targeting UPFs is biologically plausible, major challenges include the small size and methodological limitations of existing trials, variation in adherence, scalability of complex diets, nutritional adequacy, and long-term sustainability.
6. Conclusions
UPFs, characterized by refined substrates and multiple food additives, are increasingly recognized as plausible environmental contributors to IBD. Large-scale prospective studies consistently demonstrate a positive association between high UPF consumption and CD, whereas findings for UC are less consistent, highlighting disease-specific heterogeneity and the need for cautious interpretation.
Mechanistic studies predominantly in animal models implicate components typical of UPFs including emulsifiers, carrageenan, maltodextrin, fructose, microparticles, and excess dietary salt in disrupting epithelial barrier integrity, altering gut microbial composition and function, reducing beneficial metabolites, and activating innate and adaptive immune pathways. However, these effects are dose-dependent and species-specific, and their direct relevance to human IBD remains to be fully established.
Clinical data from dietary interventions further support the concept that reducing UPF exposure may offer therapeutic benefit, particularly in CD. Currently, the strongest evidence supports formula-based and structured exclusion diets such as EEN and the CDED for induction of remission in pediatric CD, while evidence for whole-food patterns like the Mediterranean diet or additive-exclusion strategies in adult IBD remains emerging, with limited sample sizes and heterogeneous outcomes requiring further validation.
7. Future Directions
To advance the role of diet in IBD management and prevention, future research must address several key gaps:
- Standardization of exposure: Development of harmonized definitions of UPFs and validated tools. including AI-assisted dietary monitoring and specific biomarkers of food processing, to enable cross-study comparability.
- Mechanism-focused trials: Well-designed randomized controlled trials targeting specific additives (e.g., emulsifiers, carrageenan) at physiologically relevant doses, utilizing endoscopic, histologic, and multi-omics outcomes to bridge the translational gap between animal models and human disease.
- Personalized nutrition strategies: Integration of individual microbiome characteristics, host genetics, and baseline immune signatures to tailor dietary interventions for specific IBD phenotypes.
- Long-term feasibility and safety: Rigorous evaluation of adherence, nutritional adequacy, cost-effectiveness, and real-world barriers to implementing UPF-restricted diets in diverse real-world clinical settings.
- Policy and clinical translation: Development of public health strategies for reducing population-level UPF intake, regulatory guidance for industry reformulation to minimize harmful additives, and clear pathways for integrating dietitian-led counseling into routine IBD care.
By addressing these priorities, future research may move beyond general associations to establish evidence-based, mechanism-informed, and personalized nutrition approaches that complement pharmacologic therapy and contribute to broader food policy efforts relevant to chronic inflammatory diseases.
Author Contributions
Conceptualization, S.Y.C. and W.M.; methodology, S.Y.C. and W.M.; writing—original draft preparation, S.Y.C.; writing—review and editing, S.Y.C. and W.M.; visualization, S.Y.C.; supervision, W.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
This study is a narrative review based on previously published literature. No new data were generated, and all referenced materials are available within the cited publications.
Acknowledgments
The authors express their gratitude to their colleagues for their valuable assistance and constructive feedback. During the preparation of this manuscript, the authors used ChatGPT (model GPT-5, OpenAI) for language editing and to improve readability. The authors reviewed and edited the content as needed and take full responsibility for the content of the publication.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| IBD | Inflammatory bowel disease |
| CD | Crohn’s disease |
| UC | Ulcerative colitis |
| UPF | Ultra-processed food |
| NOVA | NOVA food classification system |
| FFQ | Food frequency questionnaire |
| HR | Hazard ratio |
| OR | Odds ratio |
| RR | Relative risk |
| SES | Socioeconomic status |
| SCFA | Short-chain fatty acid |
| AIEC | Adherent-invasive Escherichia coli |
| CMC | Carboxymethylcellulose |
| P80 | Polysorbate-80 |
| CGN | Carrageenan |
| MDX | Maltodextrin |
| NNS | Non-nutritive sweetener |
| IEC | Intestinal epithelial cell |
| TLR | Toll-like receptor |
| NF-κB | Nuclear factor kappa B |
| Th17 | T helper 17 cell |
| EFSA | European Food Safety Authority |
References
- Narula, N.; Wong, E.C.L.; Dehghan, M.; Mente, A.; Rangarajan, S.; Lanas, F.; Lopez-Jaramillo, P.; Rohatgi, P.; Lakshmi, P.V.M.; Varma, R.P.; et al. Association of ultra-processed food intake with risk of inflammatory bowel disease: Prospective cohort study. Bmj 2021, 374, n1554. [Google Scholar] [CrossRef]
- Monteiro, C.A.; Cannon, G.; Moubarac, J.C.; Levy, R.B.; Louzada, M.L.C.; Jaime, P.C. The UN Decade of Nutrition, the NOVA food classification and the trouble with ultra-processing. Public Health Nutr. 2018, 21, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Baker, P.; Machado, P.; Santos, T.; Sievert, K.; Backholer, K.; Hadjikakou, M.; Russell, C.; Huse, O.; Bell, C.; Scrinis, G.; et al. Ultra-processed foods and the nutrition transition: Global, regional and national trends, food systems transformations and political economy drivers. Obes. Rev. 2020, 21, e13126. [Google Scholar] [CrossRef]
- Elizabeth, L.; Machado, P.; Zinöcker, M.; Baker, P.; Lawrence, M. Ultra-Processed Foods and Health Outcomes: A Narrative Review. Nutrients 2020, 12, 1955. [Google Scholar] [CrossRef] [PubMed]
- Srour, B.; Fezeu, L.K.; Kesse-Guyot, E.; Allès, B.; Méjean, C.; Andrianasolo, R.M.; Chazelas, E.; Deschasaux, M.; Hercberg, S.; Galan, P.; et al. Ultra-processed food intake and risk of cardiovascular disease: Prospective cohort study (NutriNet-Santé). Bmj 2019, 365, l1451. [Google Scholar] [CrossRef]
- Lo, C.H.; Khandpur, N.; Rossato, S.L.; Lochhead, P.; Lopes, E.W.; Burke, K.E.; Richter, J.M.; Song, M.; Ardisson Korat, A.V.; Sun, Q.; et al. Ultra-processed Foods and Risk of Crohn’s Disease and Ulcerative Colitis: A Prospective Cohort Study. Clin. Gastroenterol. Hepatol. 2022, 20, e1323–e1337. [Google Scholar] [CrossRef]
- Chen, J.; Wellens, J.; Kalla, R.; Fu, T.; Deng, M.; Zhang, H.; Yuan, S.; Wang, X.; Theodoratou, E.; Li, X.; et al. Intake of Ultra-processed Foods Is Associated with an Increased Risk of Crohn’s Disease: A Cross-sectional and Prospective Analysis of 187 154 Participants in the UK Biobank. J. Crohns Colitis 2023, 17, 535–552. [Google Scholar] [CrossRef] [PubMed]
- Sarbagili-Shabat, C.; Zelber-Sagi, S.; Isakov, N.F.; Hirsch, A.; Ron, Y.; Grinshpan, L.S.; Anbar, R.; Bromberg, A.; Thurm, T.; Maharshak, N. Ultra-Processed Foods Consumption Is Positively Associated with the Clinical Activity of Inflammatory Bowel Diseases: A Cross-Sectional Single-Center Study. Inflamm. Intest. Dis. 2024, 9, 241–251. [Google Scholar] [CrossRef]
- Sarbagili-Shabat, C.; Zelber-Sagi, S.; Fliss Isakov, N.; Hirsch, A.; Ron, Y.; Sol Grinshpan, L.; Aviv Cohen, N.; Leibovitzh, H.; Thurm, T.; Maharshak, N. High Ultra-Processed Food Consumption Is Associated with Clinical Exacerbation in Patients with Crohn’s Disease in Remission: A Prospective Cohort Study. Dig. Dis. 2025, 43, 466–475. [Google Scholar] [CrossRef]
- Chassaing, B.; Koren, O.; Goodrich, J.K.; Poole, A.C.; Srinivasan, S.; Ley, R.E.; Gewirtz, A.T. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature 2015, 519, 92–96. [Google Scholar] [CrossRef]
- Bancil, A.S.; Sandall, A.M.; Rossi, M.; Chassaing, B.; Lindsay, J.O.; Whelan, K. Food Additive Emulsifiers and Their Impact on Gut Microbiome, Permeability, and Inflammation: Mechanistic Insights in Inflammatory Bowel Disease. J. Crohns Colitis 2021, 15, 1068–1079. [Google Scholar] [CrossRef] [PubMed]
- Vasseur, P.; Dugelay, E.; Benamouzig, R.; Savoye, G.; Lan, A.; Srour, B.; Hercberg, S.; Touvier, M.; Hugot, J.P.; Julia, C.; et al. Dietary Patterns, Ultra-processed Food, and the Risk of Inflammatory Bowel Diseases in the NutriNet-Santé Cohort. Inflamm. Bowel Dis. 2021, 27, 65–73. [Google Scholar] [CrossRef]
- Narula, N.; Chang, N.H.; Mohammad, D.; Wong, E.C.L.; Ananthakrishnan, A.N.; Chan, S.S.M.; Carbonnel, F.; Meyer, A. Food Processing and Risk of Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. Clin. Gastroenterol. Hepatol. 2023, 21, 2483–2495.e2481. [Google Scholar] [CrossRef]
- Amre, D.K.; D’Souza, S.; Morgan, K.; Seidman, G.; Lambrette, P.; Grimard, G.; Israel, D.; Mack, D.; Ghadirian, P.; Deslandres, C.; et al. Imbalances in dietary consumption of fatty acids, vegetables, and fruits are associated with risk for Crohn’s disease in children. Am. J. Gastroenterol. 2007, 102, 2016–2025. [Google Scholar] [CrossRef]
- D’Souza, S.; Levy, E.; Mack, D.; Israel, D.; Lambrette, P.; Ghadirian, P.; Deslandres, C.; Morgan, K.; Seidman, E.G.; Amre, D.K. Dietary patterns and risk for Crohn’s disease in children. Inflamm. Bowel Dis. 2008, 14, 367–373. [Google Scholar] [CrossRef]
- Sakamoto, N.; Kono, S.; Wakai, K.; Fukuda, Y.; Satomi, M.; Shimoyama, T.; Inaba, Y.; Miyake, Y.; Sasaki, S.; Okamoto, K.; et al. Dietary risk factors for inflammatory bowel disease: A multicenter case-control study in Japan. Inflamm. Bowel Dis. 2005, 11, 154–163. [Google Scholar] [CrossRef]
- Ananthakrishnan, A.N.; Khalili, H.; Konijeti, G.G.; Higuchi, L.M.; de Silva, P.; Korzenik, J.R.; Fuchs, C.S.; Willett, W.C.; Richter, J.M.; Chan, A.T. A prospective study of long-term intake of dietary fiber and risk of Crohn’s disease and ulcerative colitis. Gastroenterology 2013, 145, 970–977. [Google Scholar] [CrossRef]
- Christensen, C.; Knudsen, A.; Arnesen, E.K.; Hatlebakk, J.G.; Sletten, I.S.; Fadnes, L.T. Diet, Food, and Nutritional Exposures and Inflammatory Bowel Disease or Progression of Disease: An Umbrella Review. Adv. Nutr. 2024, 15, 100219. [Google Scholar] [CrossRef] [PubMed]
- Babaei, A.; Pourmotabbed, A.; Talebi, S.; Mehrabani, S.; Bagheri, R.; Ghoreishy, S.M.; Amirian, P.; Zarpoosh, M.; Mohammadi, H.; Kermani, M.A.H.; et al. The association of ultra-processed food consumption with adult inflammatory bowel disease risk: A systematic review and dose-response meta-analysis of 4,035,694 participants. Nutr. Rev. 2024, 82, 861–871. [Google Scholar] [CrossRef]
- Hou, J.K.; Abraham, B.; El-Serag, H. Dietary intake and risk of developing inflammatory bowel disease: A systematic review of the literature. Am. J. Gastroenterol. 2011, 106, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.E.; Phillipson, M.; Petersson, J.; Velcich, A.; Holm, L.; Hansson, G.C. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc. Natl. Acad. Sci. USA 2008, 105, 15064–15069. [Google Scholar] [CrossRef] [PubMed]
- Wardle, R.A.; Wardle, A.J.; Charadva, C.; Ghosh, S.; Moran, G.W. Literature review: Impacts of socioeconomic status on the risk of inflammatory bowel disease and its outcomes. Eur. J. Gastroenterol. Hepatol. 2017, 29, 879–884. [Google Scholar] [CrossRef]
- Chassaing, B.; Compher, C.; Bonhomme, B.; Liu, Q.; Tian, Y.; Walters, W.; Nessel, L.; Delaroque, C.; Hao, F.; Gershuni, V.; et al. Randomized Controlled-Feeding Study of Dietary Emulsifier Carboxymethylcellulose Reveals Detrimental Impacts on the Gut Microbiota and Metabolome. Gastroenterology 2022, 162, 743–756. [Google Scholar] [CrossRef]
- Borthakur, A.; Bhattacharyya, S.; Dudeja, P.K.; Tobacman, J.K. Carrageenan induces interleukin-8 production through distinct Bcl10 pathway in normal human colonic epithelial cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 292, G829–G838. [Google Scholar] [CrossRef]
- Tobacman, J.K. Review of harmful gastrointestinal effects of carrageenan in animal experiments. Environ. Health Perspect. 2001, 109, 983–994. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Gill, R.; Chen, M.L.; Zhang, F.; Linhardt, R.J.; Dudeja, P.K.; Tobacman, J.K. Toll-like receptor 4 mediates induction of the Bcl10-NFkappaB-interleukin-8 inflammatory pathway by carrageenan in human intestinal epithelial cells. J. Biol. Chem. 2008, 283, 10550–10558. [Google Scholar] [CrossRef]
- Borsani, B.; De Santis, R.; Perico, V.; Penagini, F.; Pendezza, E.; Dilillo, D.; Bosetti, A.; Zuccotti, G.V.; D’Auria, E. The Role of Carrageenan in Inflammatory Bowel Diseases and Allergic Reactions: Where Do We Stand? Nutrients 2021, 13, 3402. [Google Scholar] [CrossRef]
- Shang, Q.; Sun, W.; Shan, X.; Jiang, H.; Cai, C.; Hao, J.; Li, G.; Yu, G. Carrageenan-induced colitis is associated with decreased population of anti-inflammatory bacterium, Akkermansia muciniphila, in the gut microbiota of C57BL/6J mice. Toxicol. Lett. 2017, 279, 87–95. [Google Scholar] [CrossRef]
- Liu, F.; Hou, P.; Zhang, H.; Tang, Q.; Xue, C.; Li, R.W. Food-grade carrageenans and their implications in health and disease. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3918–3936. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.; Borthakur, A.; Dudeja, P.K.; Tobacman, J.K. Carrageenan induces cell cycle arrest in human intestinal epithelial cells in vitro. J. Nutr. 2008, 138, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.; Xue, L.; Devkota, S.; Chang, E.; Morris, S.; Tobacman, J.K. Carrageenan-induced colonic inflammation is reduced in Bcl10 null mice and increased in IL-10-deficient mice. Mediat. Inflamm. 2013, 2013, 397642. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Shumard, T.; Xie, H.; Dodda, A.; Varady, K.A.; Feferman, L.; Halline, A.G.; Goldstein, J.L.; Hanauer, S.B.; Tobacman, J.K. A randomized trial of the effects of the no-carrageenan diet on ulcerative colitis disease activity. Nutr. Healthy Aging 2017, 4, 181–192. [Google Scholar] [CrossRef]
- Nickerson, K.P.; McDonald, C. Crohn’s disease-associated adherent-invasive Escherichia coli adhesion is enhanced by exposure to the ubiquitous dietary polysaccharide maltodextrin. PLoS ONE 2012, 7, e52132. [Google Scholar] [CrossRef] [PubMed]
- Nickerson, K.P.; Chanin, R.; McDonald, C. Deregulation of intestinal anti-microbial defense by the dietary additive, maltodextrin. Gut Microbes 2015, 6, 78–83. [Google Scholar] [CrossRef]
- Laudisi, F.; Di Fusco, D.; Dinallo, V.; Stolfi, C.; Di Grazia, A.; Marafini, I.; Colantoni, A.; Ortenzi, A.; Alteri, C.; Guerrieri, F.; et al. The Food Additive Maltodextrin Promotes Endoplasmic Reticulum Stress-Driven Mucus Depletion and Exacerbates Intestinal Inflammation. Cell Mol. Gastroenterol. Hepatol. 2019, 7, 457–473. [Google Scholar] [CrossRef]
- Zangara, M.T.; Ponti, A.K.; Miller, N.D.; Engelhart, M.J.; Ahern, P.P.; Sangwan, N.; McDonald, C. Maltodextrin Consumption Impairs the Intestinal Mucus Barrier and Accelerates Colitis Through Direct Actions on the Epithelium. Front. Immunol. 2022, 13, 841188. [Google Scholar] [CrossRef] [PubMed]
- Montrose, D.C.; Nishiguchi, R.; Basu, S.; Staab, H.A.; Zhou, X.K.; Wang, H.; Meng, L.; Johncilla, M.; Cubillos-Ruiz, J.R.; Morales, D.K.; et al. Dietary Fructose Alters the Composition, Localization, and Metabolism of Gut Microbiota in Association With Worsening Colitis. Cell Mol. Gastroenterol. Hepatol. 2021, 11, 525–550. [Google Scholar] [CrossRef]
- Nishiguchi, R.; Basu, S.; Staab, H.A.; Ito, N.; Zhou, X.K.; Wang, H.; Ha, T.; Johncilla, M.; Yantiss, R.K.; Montrose, D.C.; et al. Dietary interventions to prevent high-fructose diet-associated worsening of colitis and colitis-associated tumorigenesis in mice. Carcinogenesis 2021, 42, 842–852. [Google Scholar] [CrossRef] [PubMed]
- Suez, J.; Cohen, Y.; Valdés-Mas, R.; Mor, U.; Dori-Bachash, M.; Federici, S.; Zmora, N.; Leshem, A.; Heinemann, M.; Linevsky, R.; et al. Personalized microbiome-driven effects of non-nutritive sweeteners on human glucose tolerance. Cell 2022, 185, 3307–3328.e3319. [Google Scholar] [CrossRef] [PubMed]
- Suez, J.; Korem, T.; Zeevi, D.; Zilberman-Schapira, G.; Thaiss, C.A.; Maza, O.; Israeli, D.; Zmora, N.; Gilad, S.; Weinberger, A.; et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature 2014, 514, 181–186. [Google Scholar] [CrossRef]
- Bettini, S.; Boutet-Robinet, E.; Cartier, C.; Coméra, C.; Gaultier, E.; Dupuy, J.; Naud, N.; Taché, S.; Grysan, P.; Reguer, S.; et al. Food-grade TiO2 impairs intestinal and systemic immune homeostasis, initiates preneoplastic lesions and promotes aberrant crypt development in the rat colon. Sci. Rep. 2017, 7, 40373. [Google Scholar] [CrossRef]
- Lomer, M.C.; Hutchinson, C.; Volkert, S.; Greenfield, S.M.; Catterall, A.; Thompson, R.P.; Powell, J.J. Dietary sources of inorganic microparticles and their intake in healthy subjects and patients with Crohn’s disease. Br. J. Nutr. 2004, 92, 947–955. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, N.S.; de Kok, T.M.; Sijm, D.; van Breda, S.G.; Briedé, J.J.; Castenmiller, J.J.M.; Opperhuizen, A.; Chirino, Y.I.; Dirven, H.; Gott, D.; et al. Possible Adverse Effects of Food Additive E171 (Titanium Dioxide) Related to Particle Specific Human Toxicity, Including the Immune System. Int. J. Mol. Sci. 2020, 22, 207. [Google Scholar] [CrossRef] [PubMed]
- Lomer, M.C.; Harvey, R.S.; Evans, S.M.; Thompson, R.P.; Powell, J.J. Efficacy and tolerability of a low microparticle diet in a double blind, randomized, pilot study in Crohn’s disease. Eur. J. Gastroenterol. Hepatol. 2001, 13, 101–106. [Google Scholar] [CrossRef]
- Younes, M.; Aquilina, G.; Castle, L.; Engel, K.H.; Fowler, P.; Frutos Fernandez, M.J.; Fürst, P.; Gundert-Remy, U.; Gürtler, R.; Husøy, T.; et al. Safety assessment of titanium dioxide (E171) as a food additive. Efsa J. 2021, 19, e06585. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commission Regulation (EU) 2022/63 of 14 January 2022 amending Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards the food additive titanium dioxide (E171). Off. J. Eur. Union 2022, L11, 1–5. Available online: https://eur-lex.europa.eu/eli/reg/2022/63/oj (accessed on 5 December 2025).
- Tubbs, A.L.; Liu, B.; Rogers, T.D.; Sartor, R.B.; Miao, E.A. Dietary Salt Exacerbates Experimental Colitis. J. Immunol. 2017, 199, 1051–1059. [Google Scholar] [CrossRef]
- Miranda, P.M.; De Palma, G.; Serkis, V.; Lu, J.; Louis-Auguste, M.P.; McCarville, J.L.; Verdu, E.F.; Collins, S.M.; Bercik, P. High salt diet exacerbates colitis in mice by decreasing Lactobacillus levels and butyrate production. Microbiome 2018, 6, 57. [Google Scholar] [CrossRef]
- Kleinewietfeld, M.; Manzel, A.; Titze, J.; Kvakan, H.; Yosef, N.; Linker, R.A.; Muller, D.N.; Hafler, D.A. Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature 2013, 496, 518–522. [Google Scholar] [CrossRef]
- Wei, Y.; Lu, C.; Chen, J.; Cui, G.; Wang, L.; Yu, T.; Yang, Y.; Wu, W.; Ding, Y.; Li, L.; et al. High salt diet stimulates gut Th17 response and exacerbates TNBS-induced colitis in mice. Oncotarget 2017, 8, 70–82. [Google Scholar] [CrossRef]
- Ruemmele, F.M.; Veres, G.; Kolho, K.L.; Griffiths, A.; Levine, A.; Escher, J.C.; Amil Dias, J.; Barabino, A.; Braegger, C.P.; Bronsky, J.; et al. Consensus guidelines of ECCO/ESPGHAN on the medical management of pediatric Crohn’s disease. J. Crohns Colitis 2014, 8, 1179–1207. [Google Scholar] [CrossRef]
- Levine, A.; Sigall Boneh, R.; Wine, E. Evolving role of diet in the pathogenesis and treatment of inflammatory bowel diseases. Gut 2018, 67, 1726–1738. [Google Scholar] [CrossRef]
- Diederen, K.; Li, J.V.; Donachie, G.E.; de Meij, T.G.; de Waart, D.R.; Hakvoort, T.B.M.; Kindermann, A.; Wagner, J.; Auyeung, V.; Te Velde, A.A.; et al. Exclusive enteral nutrition mediates gut microbial and metabolic changes that are associated with remission in children with Crohn’s disease. Sci. Rep. 2020, 10, 18879. [Google Scholar] [CrossRef] [PubMed]
- Wall, C.L.; Gearry, R.B.; Day, A.S. Treatment of Active Crohn’s Disease with Exclusive and Partial Enteral Nutrition: A Pilot Study in Adults. Inflamm. Intest. Dis. 2018, 2, 219–227. [Google Scholar] [CrossRef]
- Nardone, O.M.; Calabrese, G.; La Mantia, A.; Testa, A.; Rispo, A.; Alfonsi, L.; Pasanisi, F.; Castiglione, F. Effectiveness of Partial Enteral Nutrition as Add-On to Biologics in Patients With Refractory and Difficult-to-Treat Crohn’s Disease: A Pilot Study. Crohns Colitis 360 2024, 6, otae011. [Google Scholar] [CrossRef]
- Levine, A.; Wine, E.; Assa, A.; Sigall Boneh, R.; Shaoul, R.; Kori, M.; Cohen, S.; Peleg, S.; Shamaly, H.; On, A.; et al. Crohn’s Disease Exclusion Diet Plus Partial Enteral Nutrition Induces Sustained Remission in a Randomized Controlled Trial. Gastroenterology 2019, 157, 440–450.e448. [Google Scholar] [CrossRef]
- Sigall Boneh, R.; Sarbagili Shabat, C.; Yanai, H.; Chermesh, I.; Ben Avraham, S.; Boaz, M.; Levine, A. Dietary Therapy With the Crohn’s Disease Exclusion Diet is a Successful Strategy for Induction of Remission in Children and Adults Failing Biological Therapy. J. Crohns Colitis 2017, 11, 1205–1212. [Google Scholar] [CrossRef] [PubMed]
- Svolos, V.; Hansen, R.; Nichols, B.; Quince, C.; Ijaz, U.Z.; Papadopoulou, R.T.; Edwards, C.A.; Watson, D.; Alghamdi, A.; Brejnrod, A.; et al. Treatment of Active Crohn’s Disease With an Ordinary Food-based Diet That Replicates Exclusive Enteral Nutrition. Gastroenterology 2019, 156, 1354–1367.e1356. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.A.; Gold, B.D.; Oliva, S.; Lewis, J.; Stallworth, A.; Koch, B.; Eshee, L.; Mason, D. Clinical and mucosal improvement with specific carbohydrate diet in pediatric Crohn disease. J. Pediatr. Gastroenterol. Nutr. 2014, 59, 516–521. [Google Scholar] [CrossRef]
- Lewis, J.D.; Sandler, R.S.; Brotherton, C.; Brensinger, C.; Li, H.; Kappelman, M.D.; Daniel, S.G.; Bittinger, K.; Albenberg, L.; Valentine, J.F.; et al. A Randomized Trial Comparing the Specific Carbohydrate Diet to a Mediterranean Diet in Adults With Crohn’s Disease. Gastroenterology 2021, 161, 837–852.e839. [Google Scholar] [CrossRef]
- Hashash, J.G.; Elkins, J.; Lewis, J.D.; Binion, D.G. AGA Clinical Practice Update on Diet and Nutritional Therapies in Patients With Inflammatory Bowel Disease: Expert Review. Gastroenterology 2024, 166, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Sigall-Boneh, R.; Levine, A.; Lomer, M.; Wierdsma, N.; Allan, P.; Fiorino, G.; Gatti, S.; Jonkers, D.; Kierkus, J.; Katsanos, K.H.; et al. Research Gaps in Diet and Nutrition in Inflammatory Bowel Disease. A Topical Review by D-ECCO Working Group [Dietitians of ECCO]. J. Crohns Colitis 2017, 11, 1407–1419. [Google Scholar] [CrossRef] [PubMed]
- Svolos, V.; Gordon, H.; Lomer, M.C.E.; Aloi, M.; Bancil, A.; Day, A.S.; Day, A.S.; Fitzpatrick, J.A.; Gerasimidis, K.; Gkikas, K.; et al. European Crohn’s and Colitis Organisation consensus on dietary management of inflammatory bowel disease. J. Crohn’s Colitis 2025, 19, jjaf122. [Google Scholar] [CrossRef]
- Bischoff, S.C.; Bager, P.; Escher, J.; Forbes, A.; Hébuterne, X.; Hvas, C.L.; Joly, F.; Klek, S.; Krznaric, Z.; Ockenga, J.; et al. ESPEN guideline on Clinical Nutrition in inflammatory bowel disease. Clin. Nutr. 2023, 42, 352–379. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).