Abstract
Fecal microbiota transplantation (FMT) is a therapeutic strategy designed to modify and enrich the recipient’s gut microbiota by administering processed donor stool, with the goal of treating dysbiosis and related conditions. In 2013, the United States Food and Drug Administration (FDA) approved FMT for recurrent Clostridioides difficile infection (rCDI). Since then, its use has been proposed and investigated in several other disorders characterized by gut microbiota imbalance and altered host–microbiota interactions, including inflammatory bowel disease (IBD), immune checkpoint inhibitor-induced colitis (ICI-iC), and gastrointestinal graft-versus-host disease (GI-GVHD). This review aims to highlight the commonalities among these conditions, the pathophysiological mechanisms that support the rationale for FMT, and emerging evidence from clinical studies. Although available studies are heterogeneous, FMT is a rapidly evolving field of research with promising potential to treat IBD and improve outcomes following oncological immunotherapy and allogenic stem cell transplantation. With further validation, FMT could become an important approach in managing immune-mediated gastrointestinal diseases.
1. Introduction
Fecal microbiota transplantation (FMT) involves the collection of stool from a healthy donor, which is then processed and transferred to a recipient with a quantitative or qualitative alteration of the gut microbiota, aiming to restore microbial composition and functions. The first randomized controlled trial of FMT was conducted in 2013, in which fresh donor stool was delivered via duodenal infusion to treat recurrent Clostridioides difficile infection (rCDI) [1].
In the same year, the U.S. Food and Drug Administration (FDA) approved FMT for rCDI. Over the past decade, there has been significant progress in managing C. difficile infection, most notably through the introduction of bezlotoxumab, a fully human monoclonal antibody targeting toxin B, which has been shown to reduce recurrence rates when added to standard-of-care antibiotic therapy [2].
More recently, the FDA has approved two standardized, self-administered FMT products, Rebyota, a single-dose enema, and Vowst, an oral capsule formulation, both indicated for rCDI [3]. FMT can be delivered through multiple routes, including feeding tubes (nasoduodenal or nasojejunal), enemas, endoscopic infusion during colonoscopy or proctosigmoidoscopy, and oral capsules, each with specific advantages and limitations.
A large-scale analysis of cases from 2000 to 2020 reported an adverse event rate of 19%, mostly mild and self-limiting events (e.g., nausea, bloating, vomiting, diarrhea), with procedure-related mortality estimated at 0.02% [4].
Beyond rCDI, FMT is increasingly being investigated for other gastrointestinal conditions, including inflammatory bowel disease (IBD), immune checkpoint inhibitor-induced colitis (ICI-iC), and gastrointestinal graft-versus-host disease (GI-GVHD) [5]. These disorders share clinical, endoscopic, histological and therapeutic features, as well as a common pathogenic hallmark. It has been hypothesized that this is due to a dysregulation of tolerance between the intestinal microbiota and the host. Growing evidence suggests that dysbiosis is a key driver of their pathogenesis (Figure 1). Unlike current therapies, which mainly act on extra-luminal targets (e.g., immunomodulators), FMT acts directly within the intestinal lumen to re-establish microbial balance, thereby supporting host health and intestinal homeostasis.
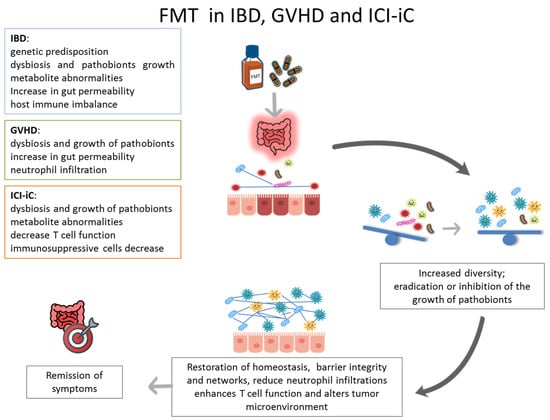
Figure 1.
The diagram illustrates the function of the FMT in the treatment of IBD, GVHD and ICI-iC, with the aim of restoring intestinal homeostasis and remission of symptoms.
2. Methods
For this narrative review, a literature search was conducted across PubMed/MEDLINE, Embase, and the Cochrane Library databases to identify relevant studies published from 2015 up to September 2025. The search strategy incorporated MeSH terms including “Inflammatory Bowel Disease”, “Crohn’s Disease”, “Ulcerative Colitis”, “Gastrointestinal Graft-Versus-Host Disease”, and “Immune Checkpoint Inhibitor–Induced Colitis”, combined with the Boolean operator AND to additional MeSH terms such as “FMT” and “Fecal Microbiota Transplantation”. This approach was designed to capture all studies investigating FMT-based interventions in intestinal immune-mediated inflammatory conditions.
Eligible studies included those enrolling patients with IBD, UC, CD, GI-GVHD, or ICI-iC, irrespective of study design (randomized, observational, or case series), number of participating centers, or age group (adult). No restrictions were applied regarding sample size, route of FMT administration, donor selection strategy, or treatment protocol. Only articles published in English were included (Table 1).

Table 1.
Summary of studies investigating the integration of fecal microbiota transplantations (FMT) with Crohn’s disease (CD), ulcerative colitis (UC), “gastrointestinal graft-versus-host disease (GI-GVHD), and immune checkpoint inhibitor-induced colitis (ICI-iC); * urothelial carcinoma and ** prostate cancer.
As an example, for CD, one of the Boolean search strings strategies employed in PubMed was (Crohn disease) AND (FMT), article type Clinical Trial or Randomized Controlled Trial, to identify articles at (https://pubmed.ncbi.nlm.nih.gov/?term=crohn+disease+and+fmt&filter=dates.2020-2025&filter=pubt.clinicaltrial&filter=pubt.randomizedcontrolledtrial&sort=date, 25 August 2025). This strategy initially retrieved 8 records published in the last five years (from 1 January 2020 to August 2025).
After the removal of duplicates, three reviewers (G.B., A.L., and G.C.) independently screened titles and abstracts according to predefined inclusion and exclusion criteria. Full texts of potentially eligible studies were retrieved and assessed for final inclusion. Any discrepancies were resolved by consensus or, when necessary, through consultation with a fourth independent and experienced reviewer (O.P.).
3. FMT in Inflammatory Bowel Disease
IBD is a group of chronic, immune-mediated disorders of the gastrointestinal tract, comprising two main entities: Crohn’s disease (CD) [31] and ulcerative colitis (UC) [32].
Crohn’s disease can affect any segment of the gastrointestinal tract, from the mouth to the anus. Inflammation is typically transmural, may follow a penetrating pattern, and is characteristically discontinuous, producing so-called “skip lesions” [31]. Ulcerative colitis predominately involves the colonic mucosa, with continuous inflammation of variable extent from the distal to the proximal colon.
IBD arises from a complex interplay of genetic susceptibility, environmental exposures, and immune dysregulation. These environmental factors facilitate the dysregulation of the immune system by triggering an abnormal response that culminates in persistent bowel inflammation [33].
The histological diagnosis of IBD is based on the evaluation of ‘minimal lesions’ present in the bowel: changes in glandular component (glandular loss or atrophy; glandular architectural changes or distortion); changes in the cellular component of the glands (mucodepletion, muciparous hyperplasia, Paneth cell metaplasia in the left colic segments, sigma and rectum, pseudopiloric metaplasia in the terminal ileum and left colic segment); and composition of the inflammatory infiltrate in the lamina propria (lymphoplasmacytic and granulocytic, with or without eosinophils) and its distribution in the segments examined (homogeneous vs. inhomogeneous). Other minimal lesions, not always present, include the presence of eversion/ulceration and non-necrotizing microgranulomas. The presence of granulocytes within the glandular epithelium (cryptitis) and/or within the lumen of the glands (cryptic pseudoabscesses) identifies disease in the active phase. Some of the minimal lesions, such as morphologic changes in the glands and lymphomonocytic infiltration of the lamina propria, require grading into mild (lesions present in <30% of biopsies), moderate (lesions present in 30% to 60% of biopsies), and severe (lesions present in >60% of biopsies) [34,35].
There is strong evidence implicating the gut microbiota in IBD pathogenesis. IBD preferentially affects sites of high microbial density (e.g., distal ileum and colon) while sparing regions with low microbial load [36]. Dysbiosis, altered composition, diversity, and function of commensal microbial communities appears central, with reductions in bacterial diversity, mainly butyrate-producing and mucin-degrading species, and expansion of taxa with pro-inflammatory potential. Whether these alterations are causal or consequential remains unclear.
Epidemiologically, IBD was once considered to be largely a disease of industrialized Western nations, following a marked North–South gradient. Incidence has since plateaued in the West but is rising in newly industrialized regions, paralleling improvements in hygiene and lifestyle. According to the “hygiene hypothesis,” reduced microbial exposure early in life impairs immune tolerance, predisposing to inflammatory disease. Supporting this, antibiotic use, particularly during childhood, has been associated with increased IBD risk [37]. From the genetic point of view, over the past 25 years, more than 200 genetic loci have been linked to IBD [38], many involved in immune regulation and host–microbe interactions.
Together, these findings underscore the multifactorial pathogenesis of IBD, where genetic susceptibility, microbial perturbations, and environmental factors converge to drive chronic intestinal inflammation [39].
3.1. FMT in Ulcerative Colitis
Most studies that used FMT in IBD focused on UC. The first randomized controlled trial (RCT), by Rossen et al. [6], investigated clinical and endoscopic outcomes in 48 patients with mild-to-moderate UC who received FMT via nasoduodenal tube. No significant differences were observed compared to placebo, although responders showed increased donor microbiota diversity.
In Moayyedi et al. [7], 75 patients with active UC without infectious diarrhea were randomized to FMT or placebo. Clinical remission occurred in 24% of FMT recipients versus 5% in the placebo group. Microbiota profiling indicated that a higher abundance of Lachnospiraceae in donor stool was associated with treatment response, raising the hypothesis of a “superdonor”.
Paramsothy et al. [8] evaluated an intensive multidonor FMT regimen in 85 patients with active UC. Delivery was via colonoscopy in 85 patients, followed by enemas, 5 days a week for 8 weeks. Clinical and endoscopic remission occurred in 27% of the FMT group versus 8% of the placebo. Microbial diversity increased post-FMT, while the presence of Fusobacterium spp. was linked to non-response.
In Costello et al. [9], 73 adults with active UC received colonoscopic FMT followed by two enemas over the first week. At eight weeks, remission was achieved in 31% of the FMT group compared with 8% of placebo; 42% of these subjects maintained remission at 12 months.
The role of FMT in maintaining remission has also been examined. In a cohort of 61 Indian UC patients in clinical remission [10], colonic infusion of FMT or placebo was administered every eight weeks for 48 weeks. Clinical remission was maintained in 87.1% of the FMT group versus 66.7% of placebo; endoscopic remission in 58.1% versus 26.7%; and histological remission in 45.2% versus 16.7%.
Crothers et al. [11] combined colonoscopic FMT with encapsulated FMT in 12 patients with mild-to-moderate UC over 12 weeks. Although statistical significance was not reached, the study highlighted the potential of combination delivery systems.
In a subsequent placebo-controlled trial, Haifer et al. [12] assessed oral lyophilized FMT in 35 patients with active UC, following a two-week antibiotic pre-treatment. After eight weeks, corticosteroid-free remission with endoscopic response was achieved in 53% of the FMT group versus 15% of placebo, supporting the feasibility of oral formulations.
To enhance engraftment, a trial tested rationally selected donors with or without budesonide pre-treatment in active UC patients [13]. Donor-dependent effects were observed, but budesonide did not significantly improve engraftment or clinical outcomes.
A recent study evaluated the repeated administration of frozen oral FMT capsules or sterile fecal filtrates from multiple donors in mild-to-moderate UC, with clinical remission at week 12 as the primary endpoint. Early data suggest that metabolites in fecal filtrates may play a role in efficacy [14].
A systematic review [40] confirmed that FMT is effective in mild-to-moderate UC without increasing adverse events, supporting its role as a potential therapeutic option in this subgroup.
Conversely, a meta-analysis of six RCTs in patients with refractory UC found that FMT did not significantly improve clinical or endoscopic remission rates, suggesting limited efficacy in this patient population despite a positive safety profile [41].
Preliminary evidence has also emerged regarding the use of FMT administered via enema in patients with refractory ulcerative proctitis (UP). In one study including 30 participants with UP, the treatment was well tolerated and demonstrated efficacy in inducing clinical remission [15].
However, the analyzed studies collectively indicate that further prospective trials are required to optimize FMT formulation and dosing, as well as to better characterize its safety profile in UC.
3.2. FMT in Crohn’s Disease
Experience of FMT for CD is more limited, although its use has shown potential as an effective treatment option. The first pilot RCT, by Sokol et al. [16], enrolled 17 patients with colonic or ileocolonic CD who received colonic FMT or sham. The primary endpoint, donor microbiota colonization at six weeks, was not achieved. However, steroid-free remission was numerically higher in the FMT group at week 10, suggesting potential benefits, particularly in colonic CD rather than penetrating disease.
Subsequent data are mainly drawn from cohort studies. A meta-analysis by Zhou et al. [42] (12 studies; 228 patients) reported remission in 57% of adults with active CD within 2–4 weeks of FMT, along with significant reductions in Crohn’s Disease Activity Index (CDAI) scores after 4–8 weeks. Subgroup analyses found no major differences between FMT methods, except that antibiotic pre-treatment appeared beneficial.
A double-blind, placebo-controlled trial conducted at three Canadian academic centers randomized 21 and 13 patients to the FMT and placebo groups, respectively. Although both groups showed statistically significant improvements in health-related quality of life, FMT was not effective in inducing clinical or endoscopic remission in these patients with CD [17].
Recently, Chukhlovin et al. [18] observed 15 patients with CD, with situations ranging from remission to high activity of disease, who received FMT standard and via oral capsules administered for two days. A complete clinical response to FMT was observed in 10 of 15 patients.
Overall, the published evidence suggests that FMT may offer short-term benefits in active CD. However, more placebo-controlled RCTs with longer follow-up are required to establish its efficacy and durability.
4. FMT in Immune Checkpoint Inhibitors Induced Colitis
In recent years, FMT has emerged as a promising therapeutic tool in oncology, both for modulating drug response and for mitigating treatment-related toxicities. ICIs, approved for several malignancies, act by enhancing lymphocyte activity against neoplastic cells and preventing tumor immune escape [43]. Their main molecular targets include CTLA-4 (ipilimumab, tremelimumab), PD-1 (nivolumab, pembrolizumab, cemiplimab, dostarlimab), PD-L1 (atezolizumab, avelumab, durvalumab) and LAG-3 (relatlimab) [44].
Although highly effective, ICI can trigger immune-related adverse events, particularly diarrhea and colitis, which often mimic IBD both endoscopically and histologically. Reported histological patterns include eosinophilic gastroenterocolitis, apoptotic colitis, IBD-like lesions, microscopic colitis (lymphocytic or collagenous), and ischemic-like changes [45].
The pathophysiology of ICI-iC remains incompletely understood, but evidence points to altered cytokine networks and gut microbial dysbiosis [46,47]. Elevated IL-6 levels have been associated with disease activity [48,49,50,51], while a higher baseline abundance of Bacteroidetes has been linked to reduced risk [52,53,54]. Conversely, antibiotic exposure appears to worsen severity [55,56]. In preclinical models, Bifidobacterium enhanced IL-10-mediated regulatory T-cell function, reducing colitis severity [56,57], whereas Lactobacillus supplementation ameliorated disease [58]. These findings support the rationale for microbiota-based therapies such as FMT.
The first use of FMT for ICI-iC was reported in 2018 [19] in patients with steroid-refractory disease, and FMT is now included in guidelines as a potential option for severe, treatment-resistant cases [59].
In 2021, Davar. et al. [20] conducted a clinical trial to evaluate the safety and efficacy of responder-derived FMT in combination with anti-PD-1 therapy in patients with PD-1-refractory melanoma. The combination was well tolerated and provided clinical benefit in 6 of 15 patients, inducing rapid and durable changes in the gut microbiota. The six responders exhibited an increased abundance of taxa previously associated with response to anti-PD-1 therapy, enhanced CD8+ T cell activation, and a reduced frequency of interleukin-8-expressing myeloid cells.
During the same year, Baruch et al. [21] administered FMT in combination with a reinduction of anti-PD-1 immunotherapy in 10 patients with anti-PD-1-refractory metastatic melanoma. Clinical responses were observed in three patients, including two partial responses and one complete response. FMT treatment was associated with favorable changes in immune cell populations and gene expression in both the gut and tumor. Together, these studies demonstrate that FMT from immunotherapy responders can help overcome resistance to anti-PD-1 therapy in patients with melanoma.
Recent studies by Halsey et al. [22] and Elkrief et al. [23] and have strengthened this evidence. In the first [22], of 12 patients with ICI-iC refractory to corticosteroids and biologics (infliximab or vedolizumab), FMT induced symptom improvement in 83%. With repeat FMT when required, 92% achieved clinical remission, and 42% achieved combined endoscopic and histological remission. Responders showed increased microbiota α-diversity with enrichment in Collinsella and Bifidobacterium. Histological responders had reduced CD8+ T-cell infiltration. In 2024, Elkrief et al. [23] treated five patients with steroid and biologic-refractory ICI-iC with FMT. Four achieved clinical improvement; two relapsed after antibiotic therapy but improved after a second FMT.
In 2024, Kim et al. [24] conducted a clinical trial combining an anti-PD-1 inhibitor with FMT derived from patients who had previously responded to anti-PD-1 therapy. The study enrolled 13 patients with advanced solid tumors refractory to anti-PD-1 treatment. FMT, administered via colonoscopy, induced sustained changes in the gut microbiota and provided clinical benefit in 6 of 13 patients, including one partial response and five cases of stable disease. These data, although preliminary, highlight the therapeutic feasibility of FMT in steroid-refractory ICI-iC.
5. FMT in Gastrointestinal Graft-Versus-Host Disease
Graft-versus-host disease is a serious and frequent complication in transplantation. GVHD occurs when the T cells in the graft recognize the recipient as foreign tissue and initiate an immune response against the host. GVHD is a major complication of allogeneic stem cell transplantation (allo-SCT), affecting up to 50% of recipients. Any part of the body can be affected. The most common targets are the skin, liver and gastrointestinal tract. GVHD can be acute (within 3 months of transplant or upon withdrawal of immunosuppression) or chronic (within the first year) [60]. When the GI tract is affected, it is referred to as GI-GVHD. Symptoms include diarrhea, abdominal pain, nausea, vomiting, dysphagia, and anemia, with the lower intestine most frequently involved. Diarrhea is the predominant manifestation. Diagnosis relies on laboratory and stool tests, imaging (ultrasound, CT, MRI), and endoscopy with biopsies [60].
Histologically, an apoptotic pattern, even if not pathognomonic, is very suggestive of acute GVHD, whereas an IBD-like pattern and apoptotic pattern are most frequently observed in chronic GVHD. Moreover, an eosinophilic-rich pattern is shared with both GVHD and mycophenolate mofetil toxicity, leading to the need for a clinical correlation for a differential diagnosis [61,62].
The pathogenesis of GI-GVHD is unclear but likely multifactorial, with growing evidence implicating the gut microbiota. Dysbiosis can occur early on due to chemotherapy, radiation, antibiotics, malnutrition, or transplantation itself. Microbial diversity correlates strongly with outcomes: in one study, the 3-year survival rates were 36%, 60%, and 67% in patients with low, medium, and high post-transplant microbial diversity, respectively [63].
The first-line treatment for GVHD relies on corticosteroids, though responses are often incomplete. Second-line therapies remain non-standardized. In this context, FMT has gained attention as a potential adjunctive therapy [64].
The first study investigating FMT in steroid-resistant acute GVHD was conducted in Japan in 2016 [25]. Four patients received FMT via nasoduodenal tube. All four responded to treatment, with three achieving complete remission and one achieving partial remission. No serious adverse events were reported. Despite the small sample size, this study represented a milestone in the management of GI-GVHD.
In 2021, Spindelboeck et al. [26] conducted a controlled study in nine patients with severe, treatment-refractory GI-GVHD. Four patients responded to FMT administered via colonoscopy, which was associated with improved survival. Responders exhibited gut microbiota features more closely resembling donor profiles, including higher alpha and beta diversity and enrichment of anaerobic butyrate-producing bacteria. Antibiotic exposure was identified as a major factor contributing to FMT failure. These findings suggest that FMT can restore microbial diversity in GI-GVHD patients.
A phase I/II clinical trial of FMT for steroid-refractory grade IV GI-GVHD included 41 patients (23 FMT, 18 control) [27]. The FMT group exhibited higher clinical remission and overall survival, with lower mortality, highlighting FMT as a promising therapeutic option.
Liu et al. [28] studied FMT combined with ruxolitinib (a Janus kinase inhibitor) in 21 patients with intestinal steroid-refractory acute GVHD. The overall response rate at day 28 was 71.4%, with responders showing improved gut microbiota composition.
A 2022 systematic review pooled data from six studies and five case reports, including 79 patients [65]. Complete remission occurred in 55.9% and partial remission in 26.5%, yielding an overall response rate of 82.4%. The analysis concluded that FMT is a safe and effective strategy for GVHD management. However, they argued that due to limited patient numbers and the absence of large, randomized trials, FMT cannot yet be considered standard care, though its low toxicity and clinical benefits justify further evaluation in randomized studies.
In 2023, a prospective, international, single-arm phase 2a study assessed pooled allogeneic fecal microbiota (MaaT013) in 52 patients with grade III–IV, steroid-refractory GI-GVHD (24 from the prospective study and 28 from compassionate use) [29]. At day 28, GI-overall response rates were 38% and 58%, respectively. The twelve-month overall survival was 25% in the prospective cohort and 38% in the compassionate cohort. Adverse events were reported, primarily bacteremia and sepsis. Responders showed increased abundance of beneficial bacteria and short-chain fatty acids, whereas non-responders had higher levels of pathogenic bacteria.
Recently, Yang et al. [30] evaluated FMT in 12 patients with refractory chronic GVHD. FMT was delivered via colonoscopy, and the response was assessed at 12 weeks. One patient achieved complete response, and five achieved partial response. Those with refractory GVHD had lower baseline diversity and higher levels of Escherichia-Shigella and Enterobacteriaceae. Post-FMT, microbial diversity increased, short-chain fatty acid levels improved, and pathogenic taxa decreased. Recently, Chukhlovin et al. [18] also observed 12 patients with experience of GVHD, after FMT via oral capsules administered for two days. Complete clinical response to FMT was observed in five patients.
Collectively, donor FMT appears to be a safe and feasible adjunctive strategy during both first- and later-line therapy, with potential utility in managing treatment-related colitis [66]. However, its efficacy and its role in preventing immune-related adverse events remain to be clarified in RCTs, as does its potential to improve gastrointestinal symptoms, overall survival, and quality of life.
6. Discussion
The gut microbiota and the immune system are intricately interconnected, and their crosstalk plays a central role in bowel diseases, including ICI-iC, GVHD, and IBD. Despite arising from distinct triggers, these conditions exhibit pathophysiological, morphological, and microscopic features that contribute to disease onset and progression.
Importantly, a shared pathogenic axis emerges from gut microbiota dysbiosis, characterized by epithelial barrier disruption, excessive innate immune overactivation, and dysregulated adaptive immune responses that are common to these conditions. These mechanisms ultimately lead to similar patterns of mucosal injury, impaired tissue repair, and sustained disease progression.
Notably, dysbiosis, often induced by chemotherapy, radiotherapy, antibiotics, or the disease itself, disrupts key microbial functions required for immune homeostasis, barrier integrity, and the regulation of intestinal inflammation.
Conversely, FMT is increasingly being explored as a therapeutic strategy across immune-mediated gastrointestinal disorders. By modulating the gut microbial community, rebalancing T-cell subsets, enhancing short-chain fatty acid (SCFA) production, and regulating systemic inflammation, FMT has the potential to restore intestinal homeostasis, attenuate mucosal injury, and improve clinical outcomes in IBD, ICI-iC, and GI-GVHD.
In IBD, and particularly UC, FMT has been shown to induce remission [6,8,9,12,18]. However, efficacy varies depending on donor microbial composition [7], route of administration, and patient characteristics. Clinical responses remain inconsistent [40], and the precise mechanisms through which FMT attenuates intestinal inflammation are still being investigated.
The expanding use of ICIs for malignancies has led to a rise in immune-mediated colitis, a potentially severe complication that can limit cancer therapy. FMT offers a potential therapeutic approach by restoring microbial balance and regulating immune responses, thereby promoting remission without compromising the antitumor efficacy of ICIs [20,21,22,23,24]. Nevertheless, available evidence is limited, and randomized controlled trials are required to confirm efficacy and safety in this setting.
Gastrointestinal involvement in GVHD is a frequent and life-threatening complication of allogeneic hematopoietic stem cell transplantation. The gut microbiota shapes donor immune responses against host tissues and contributes to disease severity. FMT has demonstrated promise in steroid-refractory GVHD by supporting microbial recolonization and reducing intestinal inflammation [65]. However, variability in treatment response suggests that efficacy depends on both donor microbiota composition and the recipient’s immune status. Importantly, the risk of opportunistic infections in immunocompromised patients necessitates close clinical monitoring.
FMT shows promise in restoring microbial balance, immune regulation, and epithelial integrity in IBD, ICI-iC, and GI-GVHD. However, several critical knowledge gaps remain. High-quality evidence is largely limited to IBD, while data for ICI-induced colitis and GI-GVHD are mostly derived from case series and open-label studies. Despite encouraging results, variability in clinical response, safety concerns, and the lack of standardized protocols highlight the need for controlled studies to optimize donor selection, dosing, and delivery strategies.
A mechanistic understanding of how FMT restores immune homeostasis, modulates T-cell subsets, or influences short-chain fatty acid production remains incomplete and debated. Optimal donor selection (whether using commercial fecal products, healthy volunteers, or patients with prior response to immunotherapy), donor characteristics (healthy volunteers, own material or patients responders to therapy), dosing (e.g., starting with ~50 g of fecal material), and route of administration (colonoscopy, enema, capsules, etc.) are not well defined. Long-term safety, particularly in immunocompromised subjects, also remains uncertain.
The interpretation of available evidence is further complicated by heterogeneity in study design (case series versus double-blind, randomized, placebo-controlled trials), primary outcomes (clinical remission, maintenance of remission, or FMT salvage therapy), microbial composition, and treatment protocols. Small sample sizes, variable clinical responses, and biases inherent to non-randomized studies limit the generalizability of findings and hinder robust conclusions regarding efficacy and safety.
Addressing these gaps will require well-designed, controlled studies to standardize FMT protocols, clarify mechanisms of action, and identify key microbial taxa responsible for therapeutic benefit. Expanding randomized trials to ICI-iC and GI-GVHD is essential, alongside an exploration of precision microbiome therapies.
Although generally well tolerated, FMT carries potential risks such as pathogen transmission and uncertain long-term effects, particularly in immunocompromised populations.
Future research should focus on clarifying the mechanisms by which FMT restores immune homeostasis, identifying key bacterial taxa responsible for therapeutic benefit, and establishing standardized treatment procedures. Well-designed randomized trials will be essential to optimize efficacy and ensure safety.
Looking forward, live biotherapeutic products (LBPs) could represent a promising and more controlled alternative or complement to FMT [67]. By using defined bacterial strains, LBPs may restore microbial balance and modulate immune and epithelial functions while potentially reducing risks associated with donor variability and pathogen transmission. However, clinical data in IBD, ICI-induced colitis, and GI-GVHD are scarce, and optimal strains, dosing regimens, and long-term safety profiles are still undefined [68]. However, there is also evidence that single- and multiple-strain probiotics and synbiotics may help with some gastrointestinal disorders. However, concerns about data quality, safety for vulnerable populations, and regulatory issues prevent widespread use.
Looking ahead, live biotherapeutic products (LBPs) may represent a controlled alternative or complement to FMT [67]. By using defined bacterial strains, LBPs can restore microbial balance and modulate immune and epithelial functions, potentially reducing risks related to donor variability and pathogen transmission. However, clinical evidence in IBD, ICI-induced colitis, and GI-GVHD are still limited, and optimal strains, dosing regimens, and long-term safety remain undefined. Similarly, single- and multi-strain probiotics or synbiotics may offer some benefit in gastrointestinal disorders [68], but concerns regarding data quality, safety in vulnerable populations, and regulatory challenges currently limit their broad application.
7. Conclusions
Overall, FMT represents a rapidly evolving field with the potential to induce remission in IBD, mitigate immune-related adverse events during cancer therapy, and improve outcomes following allogeneic stem cell transplantation. However, the collective findings emphasize the importance of conducting mechanistic studies to clarify the microbiome–host interactions that influence responsiveness to FMT in these conditions.
With further refinement and validation through controlled trials, FMT could become an important adjunctive therapy for managing immune-mediated gastrointestinal diseases.
Funding
This research was funded by the Italian Ministry of Health (Ricerca Corrente).
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| FMT | Fecal Microbiota Transplantation |
| IBD | Inflammatory Bowel Disease |
| CD | Crohn’s Disease |
| UC | Ulcerative Colitis |
| ICI-iC | Immune Checkpoint Inhibitor-Induced Colitis |
| GI-GVHD | Gastrointestinal Graft-Versus-Host Disease |
| RCT | Randomized Controlled Trial |
References
- Van Nood, E.; Vrieze, A.; Nieuwdorp, M.; Fuentes, S.; Zoetendal, E.G.; de Vos, W.M.; Visser, C.E.; Kuijper, E.J.; Bartelsman, J.F.; Tijssen, J.G.; et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N. Engl. J. Med. 2013, 368, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, M.H.; Gerding, D.N.; Poxton, I.R.; Kelly, C.; Nathan, R.; Birch, T.; Cornely, O.A.; Rahav, G.; Bouza, E.; Lee, C.; et al. MODIFY I and MODIFY II Investigators. Bezlotoxumab for Prevention of Recurrent Clostridium difficile Infection. N. Engl. J. Med. 2017, 376, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hunt, A.; Danziger, L.; Drwiega, E.N. A Comparison of Currently Available and Investigational Fecal Microbiota Transplant Products for Recurrent Clostridioides difficile Infection. Antibiotics 2024, 13, 436. [Google Scholar] [CrossRef] [PubMed]
- Marcella, C.; Cui, B.; Kelly, C.R.; Ianiro, G.; Cammarota, G.; Zhang, F. Systematic review: The global incidence of faecal microbiota transplantation-related adverse events from 2000 to 2020. Aliment Pharmacol. Ther. 2021, 53, 33–42. [Google Scholar] [CrossRef]
- Hamamah, S.; Gheorghita, R.; Lobiuc, A.; Sirbu, I.O.; Covasa, M. Fecal microbiota transplantation in non-communicable diseases: Recent advances and protocols. Front. Med. 2022, 9, 1060581. [Google Scholar] [CrossRef]
- Rossen, N.G.; Fuentes, S.; van der Spek, M.J.; Tijssen, J.G.; Hartman, J.H.; Duflou, A.; Löwenberg, M.; van den Brink, G.R.; Mathus-Vliegen, E.M.; de Vos, W.M.; et al. Findings From a Randomized Controlled Trial of Fecal Transplantation for Patients With Ulcerative Colitis. Gastroenterology 2015, 149, 110–118.e4. [Google Scholar] [CrossRef]
- Moayyedi, P.; Surette, M.G.; Kim, P.T.; Libertucci, J.; Wolfe, M.; Onischi, C.; Armstrong, D.; Marshall, J.K.; Kassam, Z.; Reinisch, W.; et al. Fecal Microbiota Transplantation Induces Remission in Patients With Active Ulcerative Colitis in a Randomized Controlled Trial. Gastroenterology 2015, 149, 102–109.e6. [Google Scholar] [CrossRef]
- Paramsothy, S.; Kamm, M.A.; Kaakoush, N.O.; Walsh, A.J.; van den Bogaerde, J.; Samuel, D.; Leong, R.W.L.; Connor, S.; Ng, W.; Paramsothy, R.; et al. Multidonor intensive faecal microbiota transplantation for active ulcerative colitis: A randomised placebo-controlled trial. Lancet 2017, 389, 1218–1228. [Google Scholar] [CrossRef]
- Costello, S.P.; Hughes, P.A.; Waters, O.; Bryant, R.V.; Vincent, A.D.; Blatchford, P.; Katsikeros, R.; Makanyanga, J.; Campaniello, M.A.; Mavrangelos, C.; et al. Effect of Fecal Microbiota Transplantation on 8-Week Remission in Patients With Ulcerative Colitis: A Randomized Clinical Trial. JAMA 2019, 321, 156–164. [Google Scholar] [CrossRef]
- Sood, A.; Mahajan, R.; Singh, A.; Midha, V.; Mehta, V.; Narang, V.; Singh, T.; Singh Pannu, A. Role of Faecal Microbiota Transplantation for Maintenance of Remission in Patients With Ulcerative Colitis: A Pilot Study. J. Crohns Colitis 2019, 13, 1311–1317. [Google Scholar] [CrossRef]
- Crothers, J.W.; Chu, N.D.; Nguyen, L.T.T.; Phillips, M.; Collins, C.; Fortner, K.; Del Rio-Guerra, R.; Lavoie, B.; Callas, P.; Velez, M.; et al. Daily, oral FMT for long-term maintenance therapy in ulcerative colitis: Results of a single-center, prospective, randomized pilot study. BMC Gastroenterol. 2021, 21, 281. [Google Scholar] [CrossRef] [PubMed]
- Haifer, C.; Paramsothy, S.; Kaakoush, N.O.; Saikal, A.; Ghaly, S.; Yang, T.; Luu, L.D.W.; Borody, T.J.; Leong, R.W. Lyophilised oral faecal microbiota transplantation for ulcerative colitis (LOTUS): A randomised, double-blind, placebo-controlled trial. Lancet Gastroenterol. Hepatol. 2022, 7, 141–151. [Google Scholar] [CrossRef] [PubMed]
- van Lingen, E.; Nooij, S.; Terveer, E.M.; Crossette, E.; Prince, A.L.; Bhattarai, S.K.; Watson, A.; Galazzo, G.; Menon, R.; Szabady, R.L.; et al. Faecal Microbiota Transplantation Engraftment After Budesonide or Placebo in Patients With Active Ulcerative Colitis Using Pre-selected Donors: A Randomized Pilot Study. J. Crohns Colitis 2024, 18, 1381–1393. [Google Scholar] [CrossRef] [PubMed]
- Stallmach, A.; Grunert, P.; Stallhofer, J.; Löffler, B.; Baier, M.; Rödel, J.; Kiehntopf, M.; Neugebauer, S.; Pieper, D.H.; Junca, H.; et al. Transfer of FRozen Encapsulated multi-donor Stool filtrate for active ulcerative Colitis (FRESCO): Study protocol for a prospective, multicenter, double-blind, randomized, controlled trial. Trials 2022, 23, 173. [Google Scholar] [CrossRef]
- Raja, S.S.; Costello, S.P.; Rayner, C.K.; Day, A.; Portmann, L.; Uylaki, W.; Wheeler, R.; Saxon, S.; Tucker, E.C.; Fon, J.; et al. Examining the role of faecal microbiota transplantation for inducing remission in resistant ulcerative proctitis and distal ulcerative colitis (up-FMT). J. Crohns Colitis 2025, 19, jjaf169. [Google Scholar] [CrossRef]
- Sokol, H.; Landman, C.; Seksik, P.; Berard, L.; Montil, M.; Nion-Larmurier, I.; Bourrier, A.; Le Gall, G.; Lalande, V.; De Rougemont, A.; et al. Fecal microbiota transplantation to maintain remission in Crohn’s disease: A pilot randomized controlled study. Microbiome 2020, 8, 12. [Google Scholar] [CrossRef]
- Kao, D.; Wong, K.; Jijon, H.; Moayyedi, P.; Franz, R.; McDougall, C.; Hotte, N.; Panaccione, R.; Semlacher, E.; Kroeker, K.I.; et al. Preliminary Results From a Multicenter, Randomized Trial Using Fecal Microbiota Transplantation to Induce Remission in Patients With Mild-to-Moderate Crohn’s Disease. Am. J. Gastroenterol. 2024, 120, 1334–1344. [Google Scholar] [CrossRef]
- Chukhlovin, A.B.; Goloshchapov, O.V.; Shchukina, O.B.; Kharitidis, A.M.; Zhloba, A.A.; Subbotina, T.F.; Kusakin, A.V.; Kosarev, O.V.; Tsai, V.V.; Kalinin, R.S.; et al. Changes in Gut Phageome and Bacteriome Following Fecal Microbiota Transfer in Patients with Intestinal Graft-Versus-Host Disease and Crohn’s Disease. Microorganisms 2025, 13, 2337. [Google Scholar] [CrossRef]
- Wang, Y.; Wiesnoski, D.H.; Helmink, B.A.; Gopalakrishnan, V.; Choi, K.; DuPont, H.L.; Jiang, Z.D.; Abu-Sbeih, H.; Sanchez, C.A.; Chang, C.C.; et al. Fecal microbiota transplantation for refractory immune checkpoint inhibitor-associated colitis. Nat. Med. 2018, 24, 1804–1808. [Google Scholar] [CrossRef]
- Davar, D.; Dzutsev, A.K.; McCulloch, J.A.; Rodrigues, R.R.; Chauvin, J.M.; Morrison, R.M.; Deblasio, R.N.; Menna, C.; Ding, Q.; Pagliano, O.; et al. Fecal microbiota transplant overcomes resistance to anti-PD-1 therapy in melanoma patients. Science 2021, 371, 595–602. [Google Scholar] [CrossRef]
- Baruch, E.N.; Youngster, I.; Ben-Betzalel, G.; Ortenberg, R.; Lahat, A.; Katz, L.; Adler, K.; Dick-Necula, D.; Raskin, S.; Bloch, N.; et al. Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science 2021, 371, 602–609. [Google Scholar] [CrossRef]
- Halsey, T.M.; Thomas, A.S.; Hayase, T.; Ma, W.; Abu-Sbeih, H.; Sun, B.; Parra, E.R.; Jiang, Z.D.; DuPont, H.L.; Sanchez, C.; et al. Microbiome alteration via fecal microbiota transplantation is effective for refractory immune checkpoint inhibitor-induced colitis. Sci. Transl. Med. 2023, 15, eabq4006. [Google Scholar] [CrossRef] [PubMed]
- Elkrief, A.; Waters, N.R.; Smith, N.; Dai, A.; Slingerland, J.; Aleynick, N.; Febles, B.; Gogia, P.; Socci, N.D.; Lumish, M.; et al. Immune-Related Colitis Is Associated with Fecal Microbial Dysbiosis and Can Be Mitigated by Fecal Microbiota Transplantation. Cancer Immunol. Res. 2024, 12, 308–321. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, G.; Kim, S.; Cho, B.; Kim, S.Y.; Do, E.J.; Bae, D.J.; Kim, S.; Kweon, M.N.; Song, J.S.; et al. Fecal microbiota transplantation improves anti-PD-1 inhibitor efficacy in unresectable or metastatic solid cancers refractory to anti-PD-1 inhibitor. Cell Host Microbe 2024, 32, 1380–1393.e9. [Google Scholar] [CrossRef]
- Kakihana, K.; Fujioka, Y.; Suda, W.; Najima, Y.; Kuwata, G.; Sasajima, S.; Mimura, I.; Morita, H.; Sugiyama, D.; Nishikawa, H.; et al. Fecal microbiota transplantation for patients with steroid-resistant acute graft-versus-host disease of the gut. Blood 2016, 128, 2083–2088. [Google Scholar] [CrossRef]
- Spindelboeck, W.; Halwachs, B.; Bayer, N.; Huber-Krassnitzer, B.; Schulz, E.; Uhl, B.; Gaksch, L.; Hatzl, S.; Bachmayr, V.; Kleissl, L.; et al. Antibiotic use and ileocolonic immune cells in patients receiving fecal microbiota transplantation for refractory intestinal GvHD: A prospective cohort study. Ther. Adv. Hematol. 2021, 12, 20406207211058333. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, X.; Zhou, Y.; Gao, J.; Jiao, Y.; Zhu, B.; Wu, D.; Qi, X. Safety and Efficacy of Fecal Microbiota Transplantation for Grade IV Steroid Refractory GI-GvHD Patients: Interim Results From FMT2017002 Trial. Front. Immunol. 2021, 12, 678476. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, Y.; Qi, J.; Ma, X.; Qi, X.; Wu, D.; Xu, Y. Fecal microbiota transplantation combined with ruxolitinib as a salvage treatment for intestinal steroid-refractory acute GVHD. Exp. Hematol. Oncol. 2022, 11, 96. [Google Scholar] [CrossRef]
- Malard, F.; Loschi, M.; Huynh, A.; Cluzeau, T.; Guenounou, S.; Legrand, F.; Magro, L.; Orvain, C.; Charbonnier, A.; Panz-Klapuch, M.; et al. Pooled allogeneic faecal microbiota MaaT013 for steroid-resistant gastrointestinal acute graft-versus-host disease: A single-arm, multicentre phase 2 trial. EClinicalMedicine 2023, 62, 102111. [Google Scholar] [CrossRef]
- Yang, K.; Du, J.; Huang, F.; Si, Y.; Gu, Y.; Xu, N.; Fan, Z.; Xue, R.; Wang, P.; Yao, X.; et al. Fecal microbiota transplantation for refractory chronic graft-versus-host disease after allogeneic hematopoietic cell transplantation: A pilot open-label, non-placebo-controlled study. BMC Med. 2025, 23, 498. [Google Scholar] [CrossRef]
- Torres, J.; Mehandru, S.; Colombel, J.F.; Peyrin-Biroulet, L. Crohn’s disease. Lancet 2017, 389, 1741–1755. [Google Scholar] [CrossRef] [PubMed]
- Ungaro, R.; Mehandru, S.; Allen, P.B.; Peyrin-Biroulet, L.; Colombel, J.F. Ulcerative colitis. Lancet 2017, 389, 1756–1770. [Google Scholar] [CrossRef] [PubMed]
- Glassner, K.L.; Abraham, B.P.; Quigley, E.M.M. The microbiome and inflammatory bowel disease. J. Allergy Clin. Immunol. 2020, 145, 16–27. [Google Scholar] [CrossRef]
- Villanacci, V.; Reggiani-Bonetti, L.; Salviato, T.; Leoncini, G.; Cadei, M.; Albarello, L.; Caputo, A.; Aquilano, M.C.; Battista, S.; Parente, P. Histopathology of IBD Colitis. A practical approach from the pathologists of the Italian Group for the study of the gastrointestinal tract (GIPAD). Pathologica 2021, 113, 39–53. [Google Scholar] [CrossRef]
- Parente, P.; Ascani, S.; Maiorano, B.A.; Zanelli, M.; Ciardiello, D. Letter: Be careful of gastrointestinal CMV infection in adverse events from ICIs therapy in solid tumours. Aliment. Pharmacol. Ther. 2023, 57, 916–917. [Google Scholar] [CrossRef]
- Sartor, R.B. Microbial influences in inflammatory bowel diseases. Gastroenterology 2008, 134, 577–594. [Google Scholar] [CrossRef]
- Kozuch, P.L.; Hanauer, S.B. Treatment of inflammatory bowel disease: A review of medical therapy. World J. Gastroenterol. 2008, 14, 354–377. [Google Scholar] [CrossRef]
- Jostins, L.; Ripke, S.; Weersma, R.K.; Duerr, R.H.; McGovern, D.P.; Hui, K.Y.; Lee, J.C.; Schumm, L.P.; Sharma, Y.; Anderson, C.A.; et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 2012, 491, 119–124. [Google Scholar] [CrossRef]
- Jauregui-Amezaga, A.; Smet, A. The Microbiome in Inflammatory Bowel Disease. J. Clin. Med. 2024, 13, 4622. [Google Scholar] [CrossRef]
- Imdad, A.; Pandit, N.G.; Zaman, M.; Minkoff, N.Z.; Tanner-Smith, E.E.; Gomez-Duarte, O.G.; Acra, S.; Nicholson, M.R. Fecal transplantation for treatment of inflammatory bowel disease. Cochrane Database Syst. Rev. 2023, 4, CD012774. [Google Scholar]
- Igbo, C.A.; Ezeano, C.; Adeniran, O.; Taha, M.; Annan, A.A.; Nriagu, V.C.; Boateng, S.; Williams, M.C.; Onyali, C. The impact of fecal microbiota transplantation on refractory ulcerative colitis: A systematic review and Meta-Analysis of randomised controlled trials. BMC Gastroenterol. 2025, 25, 654. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Cui, Y.; Zhang, Y.; Zhao, T.; Cong, J. Fecal microbiota transplantation for induction of remission in Crohn’s disease: A systematic review and meta-analysis. Int. J. Colorectal Dis. 2023, 38, 62. [Google Scholar] [CrossRef]
- Sun, Q.; Hong, Z.; Zhang, C.; Wang, L.; Han, Z.; Ma, D. Immune checkpoint therapy for solid tumours: Clinical dilemmas and future trends. Signal Transduct. Target. Ther. 2023, 8, 320. [Google Scholar] [CrossRef] [PubMed]
- Arafat Hossain, M. A comprehensive review of immune checkpoint inhibitors for cancer treatment. Int. Immunopharmacol. 2024, 143, 113365. [Google Scholar] [CrossRef] [PubMed]
- Parente, P.; Maiorano, B.A.; Ciardiello, D.; Cocomazzi, F.; Carparelli, S.; Guerra, M.; Ingravallo, G.; Cazzato, G.; Carosi, I.; Maiello, E.; et al. Clinic, Endoscopic and Histological Features in Patients Treated with ICI Developing GI Toxicity: Some News and Reappraisal from a Mono-Institutional Experience. Diagnostics 2022, 12, 685. [Google Scholar] [CrossRef]
- Morad, G.; Helmink, B.A.; Sharma, P.; Wargo, J.A. Hallmarks of response, resistance, and toxicity to immune checkpoint blockade. Cell 2021, 184, 5309–5337. [Google Scholar] [CrossRef]
- Hakozaki, T.; Richard, C.; Elkrief, A.; Hosomi, Y.; Benlaïfaoui, M.; Mimpen, I.; Terrisse, S.; Derosa, L.; Zitvogel, L.; Routy, B.; et al. The Gut Microbiome Associates with Immune Checkpoint Inhibition Outcomes in Patients with Advanced Non-Small Cell Lung Cancer. Cancer Immunol. Res. 2020, 8, 1243–1250. [Google Scholar] [CrossRef]
- Valpione, S.; Pasquali, S.; Campana, L.G.; Piccin, L.; Mocellin, S.; Pigozzo, J.; Chiarion-Sileni, V. Sex and interleukin-6 are prognostic factors for autoimmune toxicity following treatment with anti-CTLA4 blockade. J. Transl. Med. 2018, 16, 94. [Google Scholar] [CrossRef]
- Zhou, Y.; Medik, Y.B.; Patel, B.; Zamler, D.B.; Chen, S.; Chapman, T.; Schneider, S.; Park, E.M.; Babcock, R.L.; Chrisikos, T.T.; et al. Intestinal toxicity to CTLA-4 blockade driven by IL-6 and myeloid infiltration. J. Exp. Med. 2023, 220, e20221333. [Google Scholar] [CrossRef]
- Dougan, M.; Luoma, A.M.; Dougan, S.K.; Wucherpfennig, K.W. Understanding and treating the inflammatory adverse events of cancer immunotherapy. Cell 2021, 184, 1575–1588. [Google Scholar] [CrossRef]
- Luoma, A.M.; Suo, S.; Williams, H.L.; Sharova, T.; Sullivan, K.; Manos, M.; Bowling, P.; Hodi, F.S.; Rahma, O.; Sullivan, R.J.; et al. Molecular Pathways of Colon Inflammation Induced by Cancer Immunotherapy. Cell 2020, 182, 655–671.e22. [Google Scholar] [CrossRef]
- Chaput, N.; Lepage, P.; Coutzac, C.; Soularue, E.; Le Roux, K.; Monot, C.; Boselli, L.; Routier, E.; Cassard, L.; Collins, M.; et al. Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann. Oncol. 2017, 28, 1368–1379. [Google Scholar] [CrossRef]
- Dubin, K.; Callahan, M.K.; Ren, B.; Khanin, R.; Viale, A.; Ling, L.; No, D.; Gobourne, A.; Littmann, E.; Huttenhower, C.; et al. Intestinal microbiome analyses identify melanoma patients at risk for checkpoint-blockade-induced colitis. Nat. Commun. 2016, 7, 10391. [Google Scholar] [CrossRef]
- Andrews, M.C.; Duong, C.P.M.; Gopalakrishnan, V.; Iebba, V.; Chen, W.S.; Derosa, L.; Khan, M.A.W.; Cogdill, A.P.; White, M.G.; Wong, M.C.; et al. Gut microbiota signatures are associated with toxicity to combined CTLA-4 and PD-1 blockade. Nat. Med. 2021, 27, 1432–1441. [Google Scholar] [CrossRef]
- Abu-Sbeih, H.; Herrera, L.N.; Tang, T.; Altan, M.; Chaftari, A.P.; Okhuysen, P.C.; Jenq, R.R.; Wang, Y. Impact of antibiotic therapy on the development and response to treatment of immune checkpoint inhibitor-mediated diarrhea and colitis. J. Immunother. Cancer 2019, 7, 242. [Google Scholar] [CrossRef]
- Sun, S.; Luo, L.; Liang, W.; Yin, Q.; Guo, J.; Rush, A.M.; Lv, Z.; Liang, Q.; Fischbach, M.A.; Sonnenburg, J.L.; et al. Bifidobacterium alters the gut microbiota and modulates the functional metabolism of T regulatory cells in the context of immune checkpoint blockade. Proc. Natl. Acad. Sci. USA 2020, 117, 27509–27515. [Google Scholar] [CrossRef] [PubMed]
- Vétizou, M.; Pitt, J.M.; Daillère, R.; Lepage, P.; Waldschmitt, N.; Flament, C.; Rusakiewicz, S.; Routy, B.; Roberti, M.P.; Duong, C.P.; et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 2015, 350, 1079–1084. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zheng, N.; Luo, Q.; Jiang, L.; He, B.; Yuan, X.; Shen, L. Probiotics Lactobacillus reuteri Abrogates Immune Checkpoint Blockade-Associated Colitis by Inhibiting Group 3 Innate Lymphoid Cells. Front. Immunol. 2019, 10, 1235. [Google Scholar] [CrossRef] [PubMed]
- Dougan, M.; Wang, Y.; Rubio-Tapia, A.; Lim, J.K. AGA Clinical Practice Update on Diagnosis and Management of Immune Checkpoint Inhibitor Colitis and Hepatitis: Expert Review. Gastroenterology 2021, 160, 1384–1393. [Google Scholar] [CrossRef]
- Hamilton, B.K. Updates in chronic graft-versus-host disease. Hematol. Am. Soc. Hematol. Educ. Program. 2021, 2021, 648–654. [Google Scholar] [CrossRef]
- Wong, N.A. Gastrointestinal pathology in transplant patients. Histopathology 2015, 66, 467–479. [Google Scholar] [CrossRef]
- Patil, P.A.; Zhang, X. Pathologic Manifestations of Gastrointestinal and Hepatobiliary Injury in Immune Checkpoint Inhibitor Therapy. Arch. Pathol. Lab. Med. 2021, 145, 571–582. [Google Scholar] [CrossRef]
- Taur, Y.; Jenq, R.R.; Perales, M.A.; Littmann, E.R.; Morjaria, S.; Ling, L.; No, D.; Gobourne, A.; Viale, A.; Dahi, P.B.; et al. The effects of intestinal tract bacterial diversity on mortality following allogeneic hematopoietic stem cell transplantation. Blood 2014, 124, 1174–1182. [Google Scholar] [CrossRef]
- Biliński, J.; Jasiński, M.; Basak, G.W. The Role of Fecal Microbiota Transplantation in the Treatment of Acute Graft-versus-Host Disease. Biomedicines 2022, 10, 837. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Y.; Wang, Y.; Wu, D. Fecal microbiota transplantation for graft-versus-host disease: A systematic review and meta-analysis. J. Transl. Med. 2022, 20, 587. [Google Scholar]
- Wekking, D.; Ende, T.V.D.; Bijlsma, M.F.; Vidal-Itriago, A.; Nieuwdorp, M.; van Laarhoven, H.W.M. Fecal microbiota transplantation to enhance cancer treatment outcomes across different cancer types: A systematic literature review. Cancer Treat. Rev. 2025, 140, 103025. [Google Scholar] [CrossRef] [PubMed]
- Cordaillat-Simmons, M.; Rouanet, A.; Pot, B. Live biotherapeutic products: The importance of a defined regulatory framework. Exp. Mol. Med. 2020, 52, 1397–1406. [Google Scholar] [CrossRef] [PubMed]
- Kamath, S.; Bryant, R.V.; Costello, S.P.; Day, A.S.; Forbes, B.; Haifer, C.; Hold, G.; Kelly, C.R.; Li, A.; Pakuwal, E.; et al. Translational strategies for oral delivery of faecal microbiota transplantation. Gut 2025, 74, 2096–2117. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).