Glutamine Promotes Myogenesis in Myoblasts Through Glutaminolysis-Mediated Histone H3 Acetylation That Enhances Myogenin Transcription
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Cell Culture
2.3. Analysis of Myotube Diameter
2.4. Analyses of mRNA Expression by Quantitative Polymerase Chain Reaction (qPCR)
2.5. Analysis of Protein Expression by Western Blotting
2.6. Statistical Analysis
3. Results
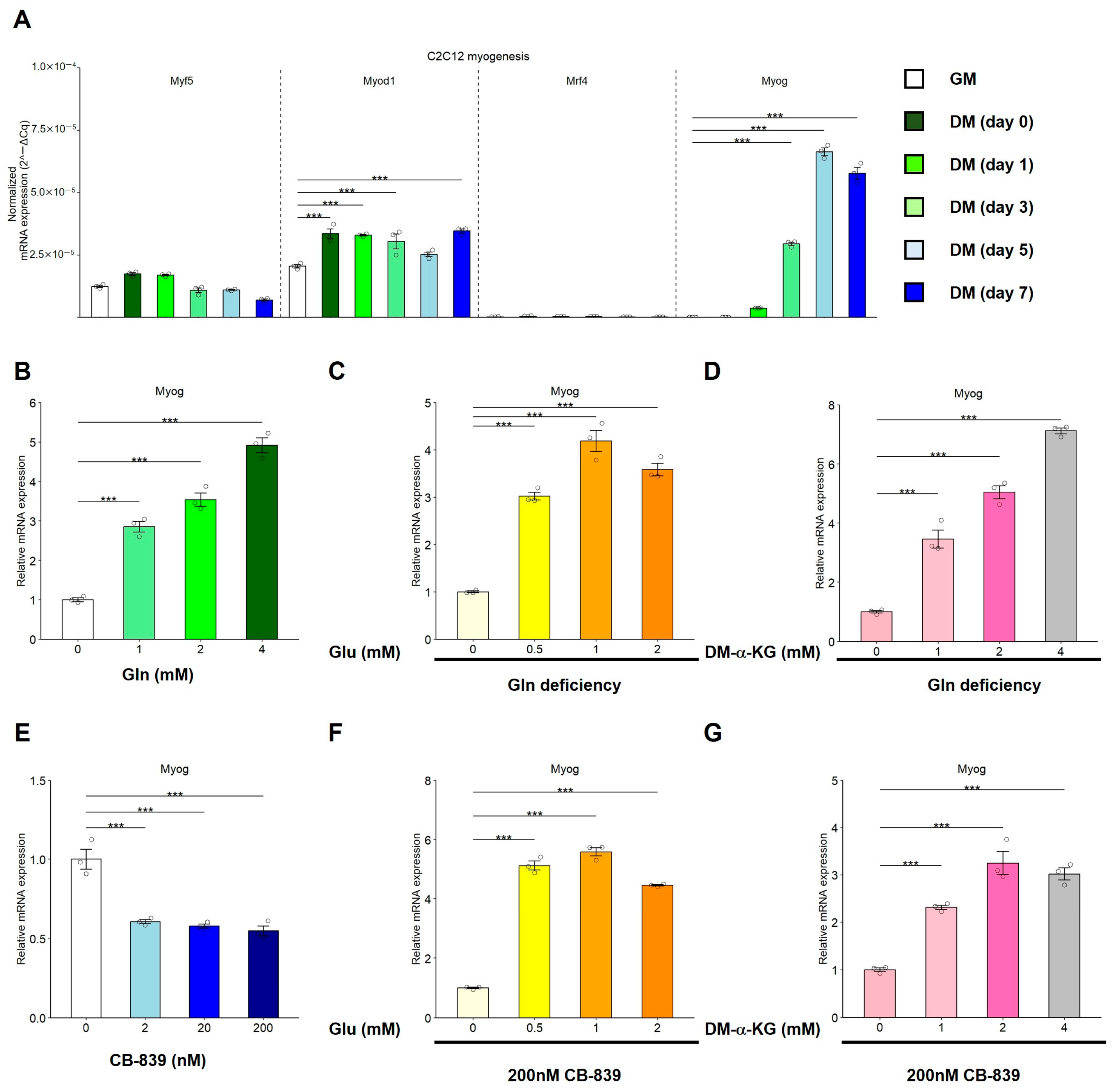
3.1. Gln Enhances the Differentiation of C2C12 Myoblasts
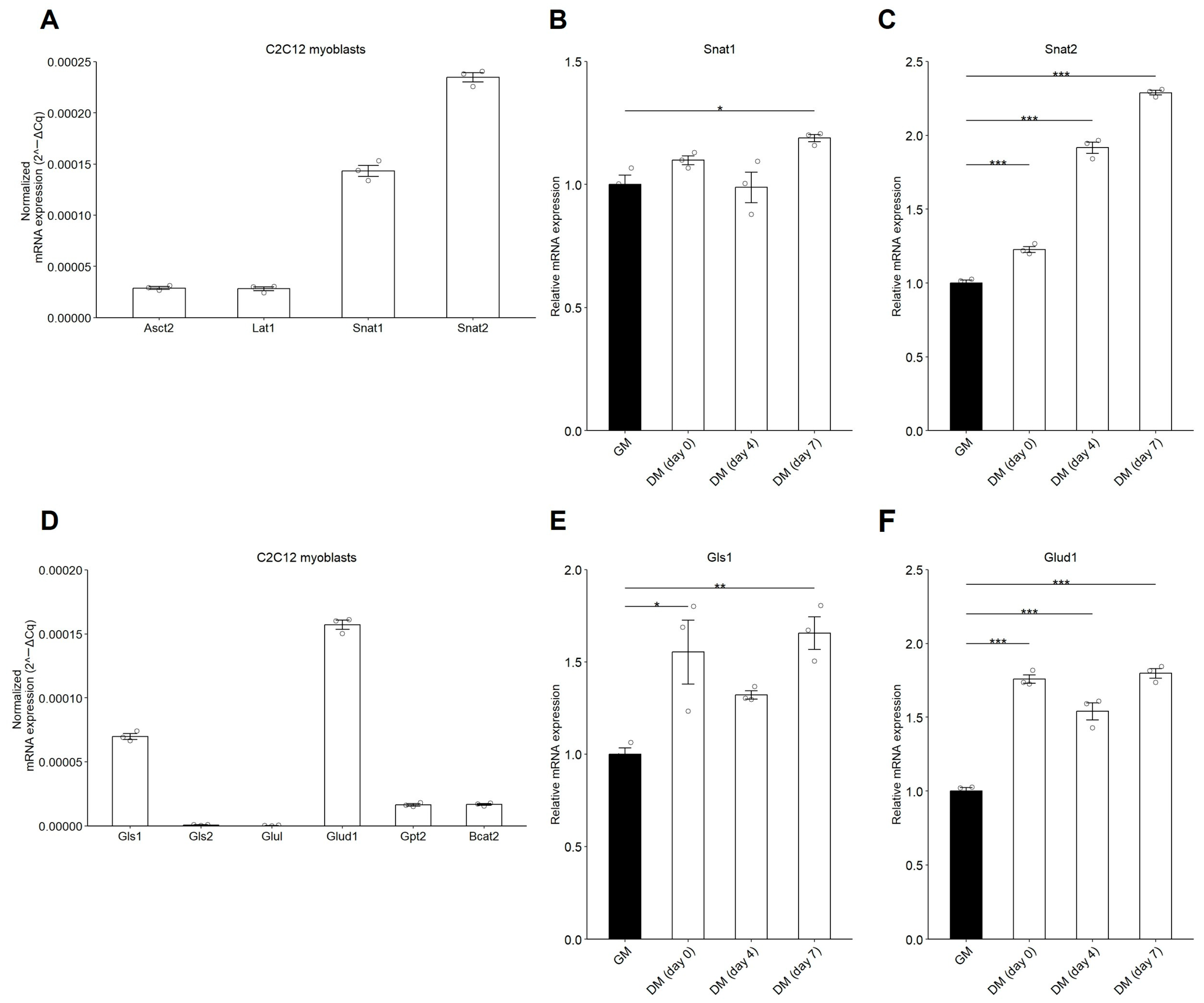
3.2. mRNA Expression of Gln Transporters and Glutaminolysis-Related Enzymes Increases During C2C12 Myogenic Differentiation
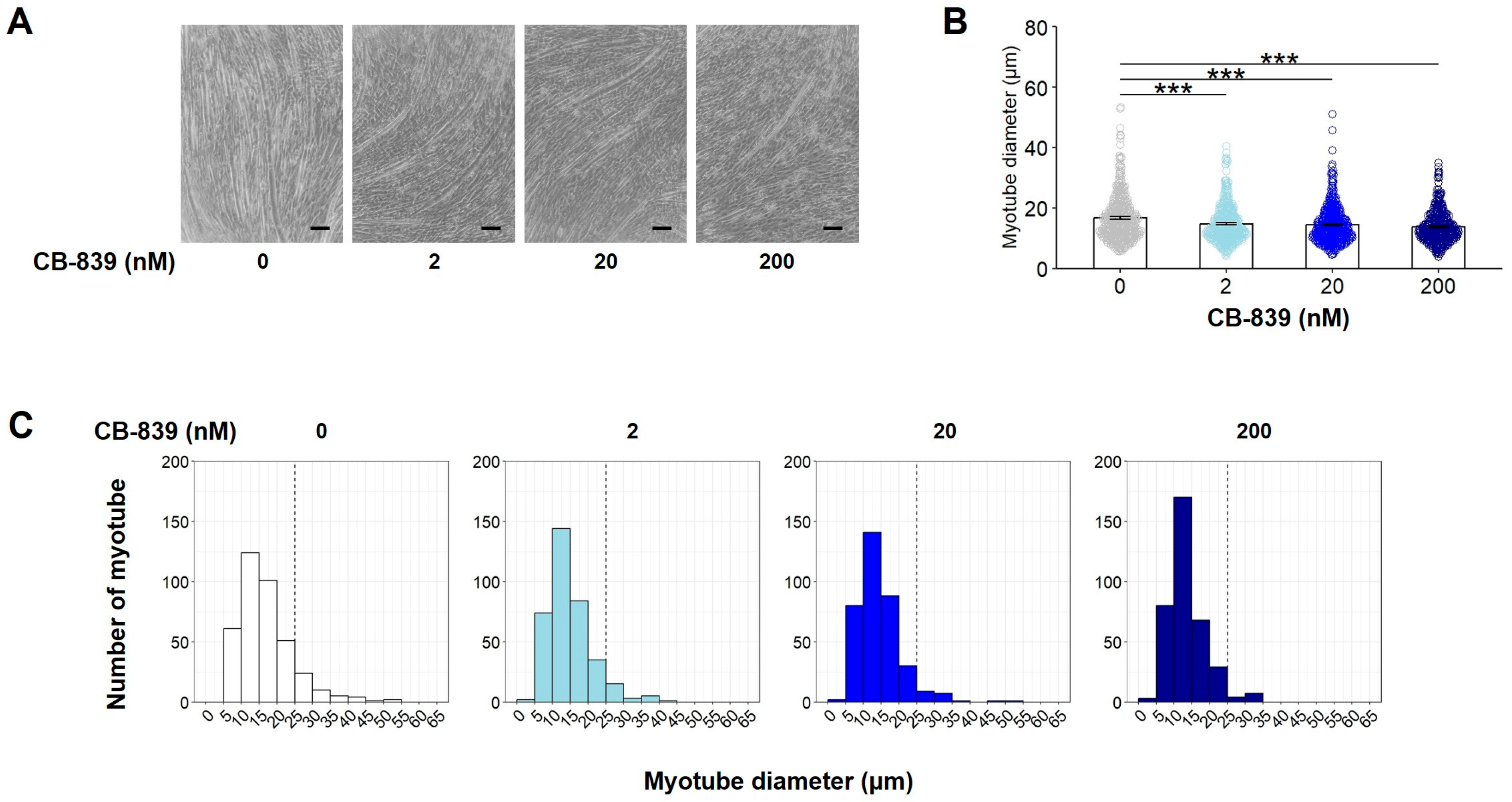
3.3. Glutaminase Inhibition by CB-839 Suppresses Gln-Dependent Myotube Formation in C2C12 Myoblasts
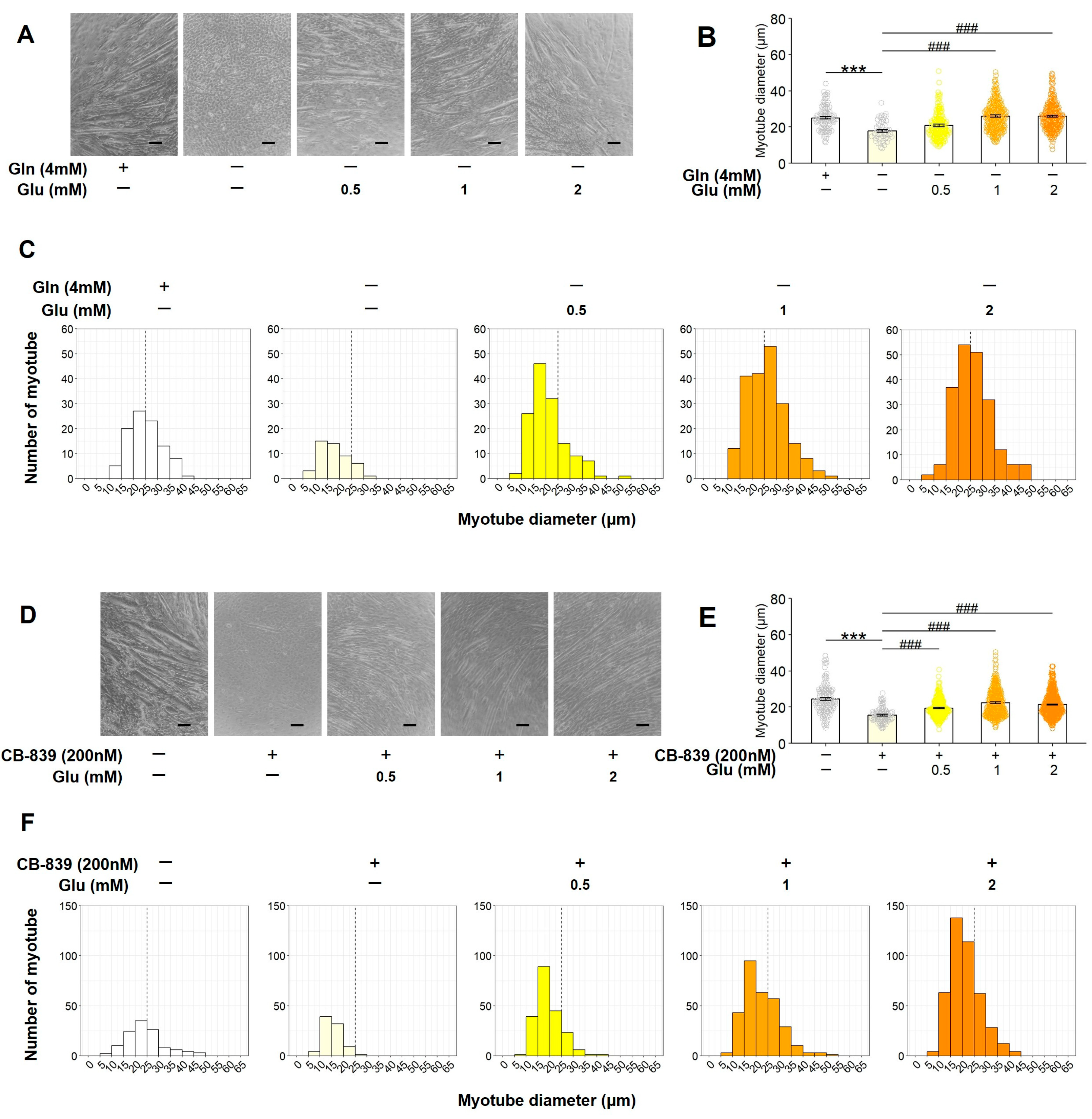
3.4. Glu Restores the Inhibition of C2C12 Myotube Formation Caused by Gln Deficiency and Gls Inactivation
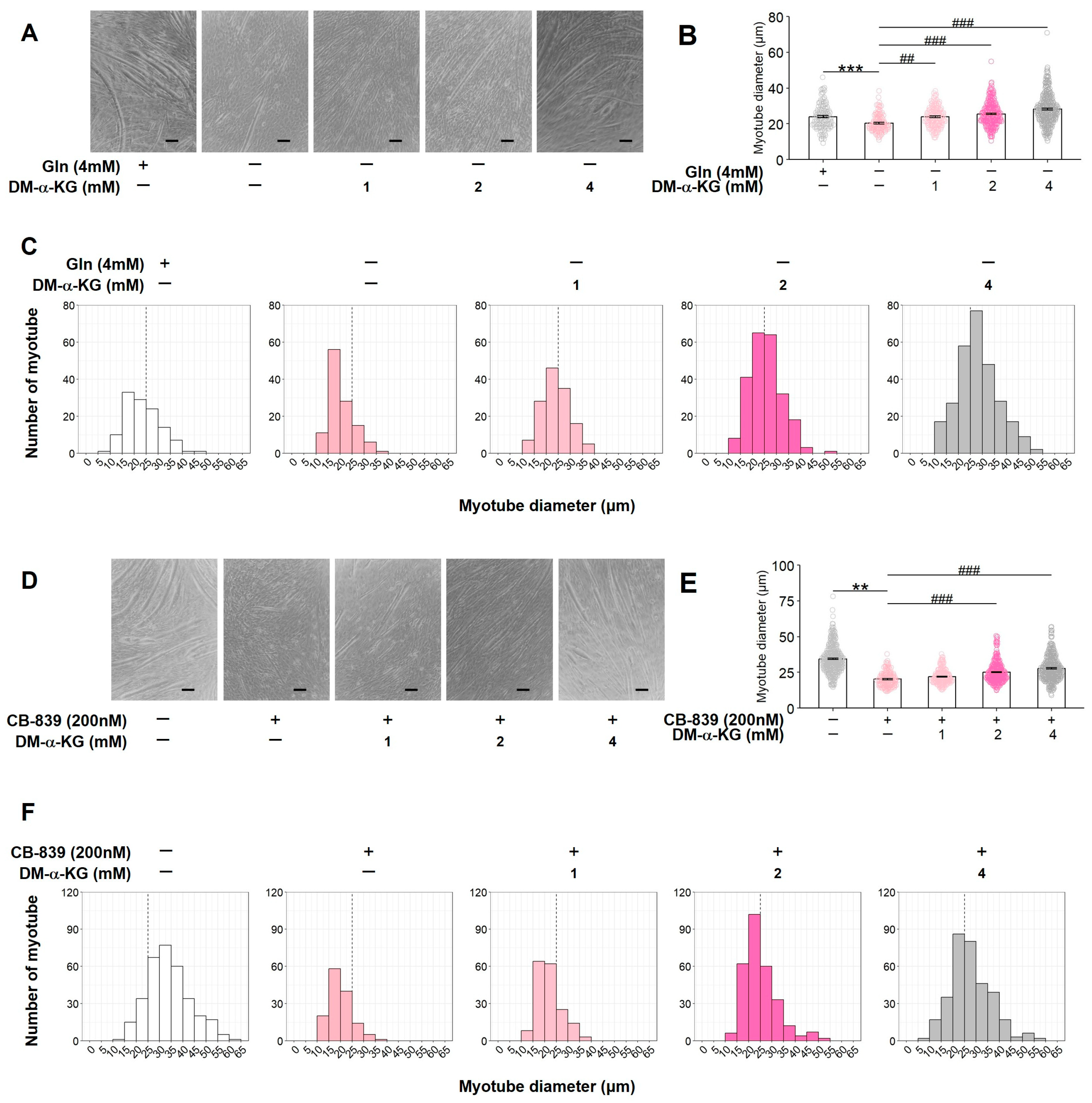
3.5. DM-α-KG Restores the Inhibition of C2C12 Myotube Formation Caused by Gln Deficiency or GLS Inactivation
3.6. Glutaminolysis Modulates the mRNA Expression of Myog During C2C12 Myogenic Differentiation
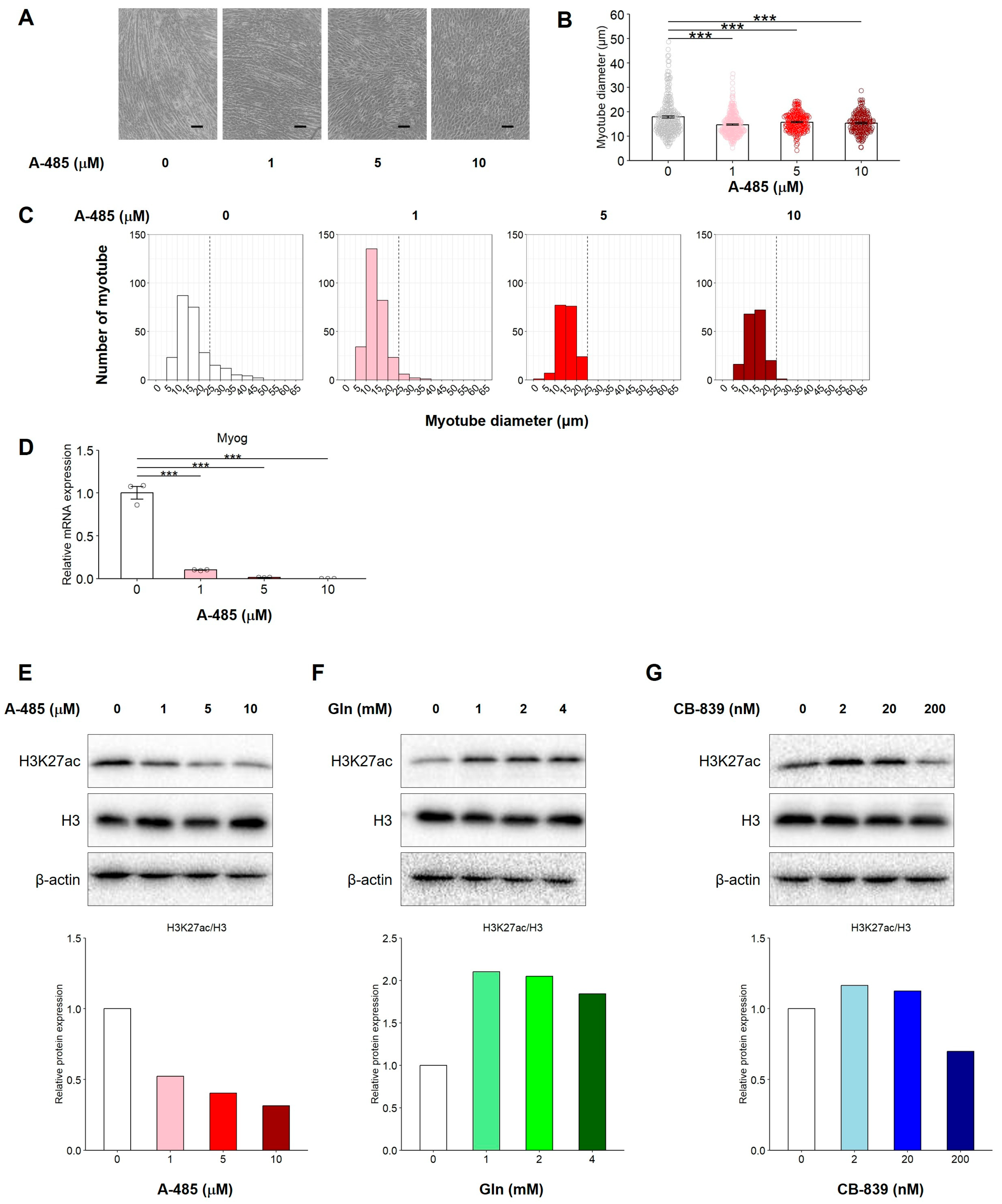
3.7. Catalytic Inhibition of CBP/p300 Suppresses C2C12 Myogenic Differentiation in the Presence of Gln Through Reduction of H3K27 Acetylation
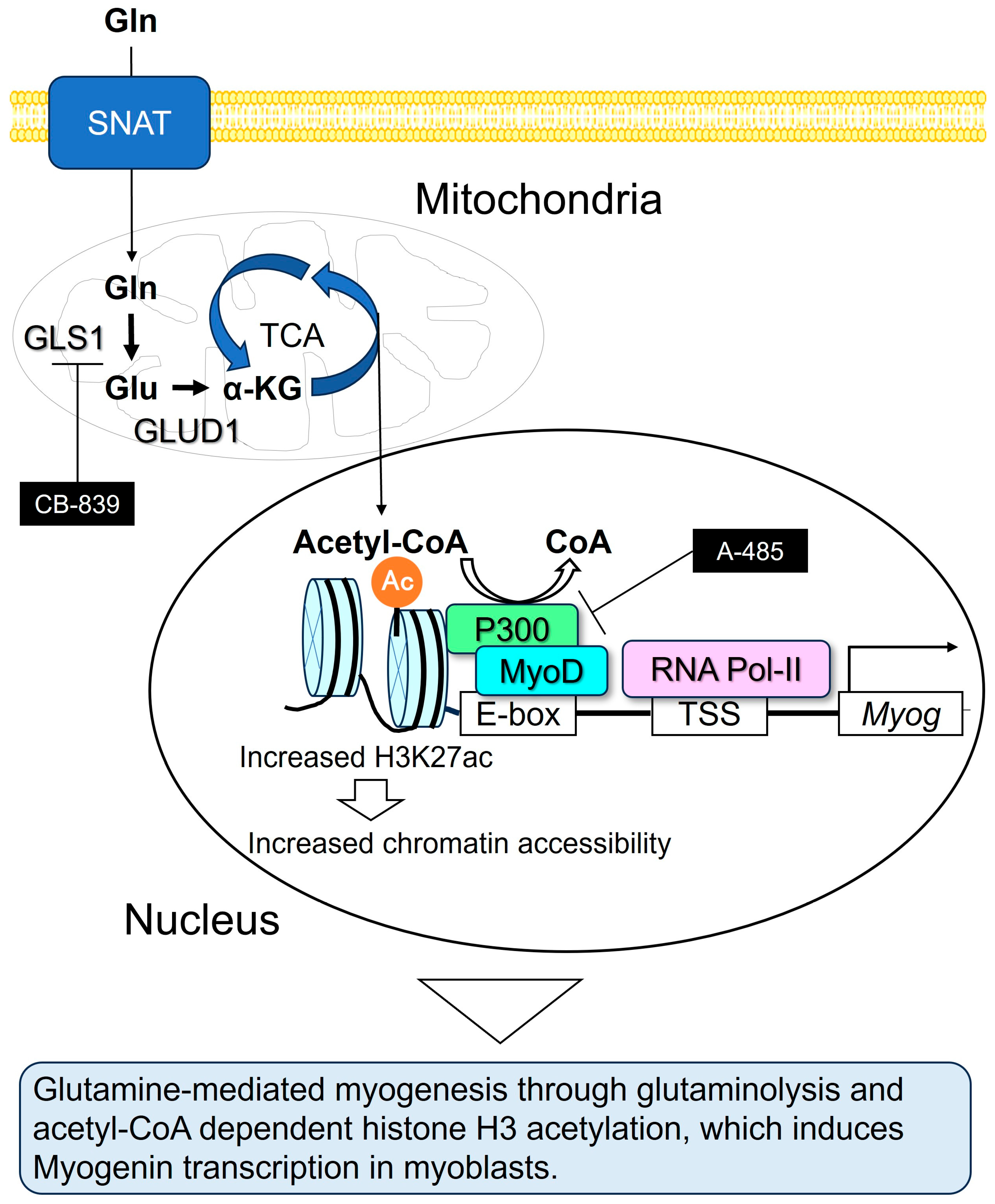
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cruzat, V.; Macedo Rogero, M.; Noel Keane, K.; Curi, R.; Newsholme, P. Glutamine: Metabolism and Immune Function, Supplementation and Clinical Translation. Nutrients 2018, 10, 1564. [Google Scholar] [CrossRef] [PubMed]
- de Vasconcelos, D.A.A.; Giesbertz, P.; Murata, G.M.; de Souza, D.R.; Fiamoncini, J.; Duque-Guimaraes, D.; Leandro, C.G.; Hirabara, S.M.; Daniel, H.; Curi, R.; et al. Myotube Protein Content Associates with Intracellular L-Glutamine Levels. Cell Physiol. Biochem. 2019, 53, 200–214. [Google Scholar] [PubMed]
- Lambertucci, A.C.; Lambertucci, R.H.; Hirabara, S.M.; Curi, R.; Moriscot, A.S.; Alba-Loureiro, T.C.; Guimaraes-Ferreira, L.; Levada-Pires, A.C.; Vasconcelos, D.A.; Sellitti, D.F.; et al. Glutamine supplementation stimulates protein-synthetic and inhibits protein-degradative signaling pathways in skeletal muscle of diabetic rats. PLoS ONE 2012, 7, e50390. [Google Scholar] [CrossRef]
- Córdova-Martínez, A.; Caballero-García, A.; Bello, H.J.; Pérez-Valdecantos, D.; Roche, E. Effect of Glutamine Supplementation on Muscular Damage Biomarkers in Professional Basketball Players. Nutrients 2021, 13, 2073. [Google Scholar] [CrossRef]
- Rowbottom, D.G.; Keast, D.; Morton, A.R. The emerging role of glutamine as an indicator of exercise stress and overtraining. Sports Med. 1996, 21, 80–97. [Google Scholar] [CrossRef]
- Yang, C.; Ko, B.; Hensley, C.T.; Jiang, L.; Wasti, A.T.; Kim, J.; Sudderth, J.; Calvaruso, M.A.; Lumata, L.; Mitsche, M.; et al. Glutamine oxidation maintains the TCA cycle and cell survival during impaired mitochondrial pyruvate transport. Mol. Cell 2014, 56, 414–424. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Sutter, B.M.; Li, B.; Tu, B.P. Acetyl-CoA induces cell growth and proliferation by promoting the acetylation of histones at growth genes. Mol. Cell 2011, 42, 426–437. [Google Scholar] [CrossRef]
- Weinert, B.T.; Iesmantavicius, V.; Moustafa, T.; Scholz, C.; Wagner, S.A.; Magnes, C.; Zechner, R.; Choudhary, C. Acetylation dynamics and stoichiometry in Saccharomyces cerevisiae. Mol. Syst. Biol. 2015, 11, 833. [Google Scholar] [CrossRef]
- Bordoli, L.; Husser, S.; Luthi, U.; Netsch, M.; Osmani, H.; Eckner, R. Functional analysis of the p300 acetyltransferase domain: The PHD finger of p300 but not of CBP is dispensable for enzymatic activity. Nucleic Acids Res. 2001, 29, 4462–4471. [Google Scholar] [CrossRef]
- Wenes, M.; Jaccard, A.; Wyss, T.; Maldonado-Perez, N.; Teoh, S.T.; Lepez, A.; Renaud, F.; Franco, F.; Waridel, P.; Yacoub Maroun, C.; et al. The mitochondrial pyruvate carrier regulates memory T cell differentiation and antitumor function. Cell Metab. 2022, 34, 731–746.e739. [Google Scholar] [CrossRef]
- Stegen, S.; Rinaldi, G.; Loopmans, S.; Stockmans, I.; Moermans, K.; Thienpont, B.; Fendt, S.M.; Carmeliet, P.; Carmeliet, G. Glutamine Metabolism Controls Chondrocyte Identity and Function. Dev. Cell 2020, 53, 530–544.e538. [Google Scholar] [CrossRef]
- Wang, S.; Lin, Y.; Gao, L.; Yang, Z.; Lin, J.; Ren, S.; Li, F.; Chen, J.; Wang, Z.; Dong, Z.; et al. PPAR-gamma integrates obesity and adipocyte clock through epigenetic regulation of Bmal1. Theranostics 2022, 12, 1589–1606. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Hernandez, J.M.; Garcia-Gonzalez, E.G.; Brun, C.E.; Rudnicki, M.A. The myogenic regulatory factors, determinants of muscle development, cell identity and regeneration. Semin. Cell Dev. Biol. 2017, 72, 10–18. [Google Scholar] [CrossRef]
- Zammit, P.S. Function of the myogenic regulatory factors Myf5, MyoD, Myogenin and MRF4 in skeletal muscle, satellite cells and regenerative myogenesis. Semin. Cell Dev. Biol. 2017, 72, 19–32. [Google Scholar] [CrossRef]
- Tajbakhsh, S.; Rocancourt, D.; Cossu, G.; Buckingham, M. Redefining the genetic hierarchies controlling skeletal myogenesis: Pax-3 and Myf-5 act upstream of MyoD. Cell 1997, 89, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, Y.; Hamed, M.; Lacroix, N.; Li, Q. Molecular Basis for the Regulation of Transcriptional Coactivator p300 in Myogenic Differentiation. Sci. Rep. 2015, 5, 13727. [Google Scholar] [CrossRef]
- Jin, X.C.; Park, J.S.; Peng, D.Q.; Kim, W.S.; Lee, J.S.; Lee, H.G. Dietary L-glutamine supplementation enhances growth performance and carcass characteristics in Hanwoo heifers via hepatic and skeletal muscle gene regulation. J. Anim. Sci. 2025, 103, skaf215. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.C.; Wu, J.M.; Chen, K.Y.; Wu, M.H.; Yang, P.J.; Lee, P.C.; Chen, P.D.; Yeh, S.L.; Lin, M.T. Glutamine and leucine administration attenuates muscle atrophy in sepsis. Life Sci. 2023, 314, 121327. [Google Scholar] [CrossRef]
- Santos, A.R.; Koike, T.E.; Santana, A.M.; Miranda, N.C.; Dell Aquila, R.A.; Silva, T.C.; Aoki, M.S.; Miyabara, E.H. Glutamine supplementation accelerates functional recovery of EDL muscles after injury by modulating the expression of S100 calcium-binding proteins. Histochem. Cell Biol. 2023, 160, 135–146. [Google Scholar] [CrossRef]
- Koike, T.E.; Dell Aquila, R.A.; Silva, K.S.; Aoki, M.S.; Miyabara, E.H. Glutamine supplementation improves contractile function of regenerating soleus muscles from rats. J. Muscle Res. Cell Motil. 2022, 43, 87–97. [Google Scholar] [CrossRef]
- Ciuffoli, V.; Feng, X.; Jiang, K.; Acevedo-Luna, N.; Ko, K.D.; Wang, A.H.J.; Riparini, G.; Khateb, M.; Glancy, B.; Dell’Orso, S.; et al. Psat1-generated α-ketoglutarate and glutamine promote muscle stem cell activation and regeneration. Genes Dev. 2024, 38, 151–167. [Google Scholar] [CrossRef]
- Legault, Z.; Bagnall, N.; Kimmerly, D.S. The Influence of Oral L-Glutamine Supplementation on Muscle Strength Recovery and Soreness Following Unilateral Knee Extension Eccentric Exercise. Int. J. Sport Nutr. Exerc. Metab. 2015, 25, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Shang, M.; Cappellesso, F.; Amorim, R.; Serneels, J.; Virga, F.; Eelen, G.; Carobbio, S.; Rincon, M.Y.; Maechler, P.; De Bock, K.; et al. Macrophage-derived glutamine boosts satellite cells and muscle regeneration. Nature 2020, 587, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Alkan, H.F.; Walter, K.E.; Luengo, A.; Madreiter-Sokolowski, C.T.; Stryeck, S.; Lau, A.N.; Al-Zoughbi, W.; Lewis, C.A.; Thomas, C.J.; Hoefler, G.; et al. Cytosolic Aspartate Availability Determines Cell Survival When Glutamine Is Limiting. Cell Metab. 2018, 28, 706–720.e6. [Google Scholar] [CrossRef]
- Reid, M.A.; Dai, Z.; Locasale, J.W. The impact of cellular metabolism on chromatin dynamics and epigenetics. Nat. Cell Biol. 2017, 19, 1298–1306. [Google Scholar] [CrossRef]
- Dohl, J.; Passos, M.E.P.; Foldi, J.; Chen, Y.; Pithon-Curi, T.; Curi, R.; Gorjao, R.; Deuster, P.A.; Yu, T. Glutamine depletion disrupts mitochondrial integrity and impairs C2C12 myoblast proliferation, differentiation, and the heat-shock response. Nutr. Res. 2020, 84, 42–52. [Google Scholar] [CrossRef]
- Liu, M.; Yue, Z.; Zhang, B.; Li, F.; Liu, L.; Li, F. mTORC1 Mediates the Processes of Lysine Regulating Satellite Cells Proliferation, Apoptosis, and Autophagy. Metabolites 2022, 12, 788. [Google Scholar] [CrossRef] [PubMed]
- Gheller, B.J.; Blum, J.E.; Lim, E.W.; Handzlik, M.K.; Hannah Fong, E.H.; Ko, A.C.; Khanna, S.; Gheller, M.E.; Bender, E.L.; Alexander, M.S.; et al. Extracellular serine and glycine are required for mouse and human skeletal muscle stem and progenitor cell function. Mol. Metab. 2021, 43, 101106. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.G.; Silva, M.T.; da Cunha, F.M.; Moriscot, A.S.; Aoki, M.S.; Miyabara, E.H. Leucine supplementation improves regeneration of skeletal muscles from old rats. Exp. Gerontol. 2015, 72, 269–277. [Google Scholar] [CrossRef]
- Pereira, M.G.; Baptista, I.L.; Carlassara, E.O.; Moriscot, A.S.; Aoki, M.S.; Miyabara, E.H. Leucine supplementation improves skeletal muscle regeneration after cryolesion in rats. PLoS ONE 2014, 9, e85283. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, X.L.; Cheng, M.B.; Zhang, Y. PRMT1 activates myogenin transcription via MyoD arginine methylation at R121. Biochim. Biophys. Acta Gene Regul. Mech. 2019, 1862, 194442. [Google Scholar] [CrossRef]
- Deato, M.D.; Marr, M.T.; Sottero, T.; Inouye, C.; Hu, P.; Tjian, R. MyoD targets TAF3/TRF3 to activate myogenin transcription. Mol. Cell 2008, 32, 96–105. [Google Scholar] [CrossRef]
- Mal, A.; Harter, M.L. MyoD is functionally linked to the silencing of a muscle-specific regulatory gene prior to skeletal myogenesis. Proc. Natl. Acad. Sci. USA 2003, 100, 1735–1739. [Google Scholar] [CrossRef] [PubMed]
- Blum, R.; Vethantham, V.; Bowman, C.; Rudnicki, M.; Dynlacht, B.D. Genome-wide identification of enhancers in skeletal muscle: The role of MyoD1. Genes Dev. 2012, 26, 2763–2779. [Google Scholar] [CrossRef]
- Jin, Q.; Yu, L.R.; Wang, L.; Zhang, Z.; Kasper, L.H.; Lee, J.E.; Wang, C.; Brindle, P.K.; Dent, S.Y.; Ge, K. Distinct roles of GCN5/PCAF-mediated H3K9ac and CBP/p300-mediated H3K18/27ac in nuclear receptor transactivation. EMBO J. 2011, 30, 249–262. [Google Scholar] [CrossRef]
- Polesskaya, A.; Naguibneva, I.; Fritsch, L.; Duquet, A.; Ait-Si-Ali, S.; Robin, P.; Vervisch, A.; Pritchard, L.L.; Cole, P.; Harel-Bellan, A. CBP/p300 and muscle differentiation: No HAT, no muscle. EMBO J. 2001, 20, 6816–6825. [Google Scholar] [CrossRef] [PubMed]
- Svensson, K.; LaBarge, S.A.; Sathe, A.; Martins, V.F.; Tahvilian, S.; Cunliffe, J.M.; Sasik, R.; Mahata, S.K.; Meyer, G.A.; Philp, A.; et al. p300 and cAMP response element-binding protein-binding protein in skeletal muscle homeostasis, contractile function, and survival. J. Cachexia Sarcopenia Muscle 2020, 11, 464–477. [Google Scholar] [CrossRef]
- Pala, F.; Di Girolamo, D.; Mella, S.; Yennek, S.; Chatre, L.; Ricchetti, M.; Tajbakhsh, S. Distinct metabolic states govern skeletal muscle stem cell fates during prenatal and postnatal myogenesis. J. Cell Sci. 2018, 131, jcs212977. [Google Scholar] [CrossRef] [PubMed]
- Jung, D.Y.; Lee, H.J.; Lee, Y.S.; Kim, M.; Lee, C.K.; Jo, C. Dynamic shifts in metabolic demand during myogenic progression in porcine skeletal muscle stem cells. npj Sci. Food 2025, 9, 115. [Google Scholar] [CrossRef]
- Xiao, M.; Wu, C.H.; Meek, G.; Kelly, B.; Castillo, D.B.; Young, L.E.A.; Martire, S.; Dhungel, S.; McCauley, E.; Saha, P.; et al. PASK links cellular energy metabolism with a mitotic self-renewal network to establish differentiation competence. eLife 2023, 12, e81717. [Google Scholar] [CrossRef]
- Li, C.; Chen, P.; Palladino, A.; Narayan, S.; Russell, L.K.; Sayed, S.; Xiong, G.; Chen, J.; Stokes, D.; Butt, Y.M.; et al. Mechanism of hyperinsulinism in short-chain 3-hydroxyacyl-CoA dehydrogenase deficiency involves activation of glutamate dehydrogenase. J. Biol. Chem. 2010, 285, 31806–31818. [Google Scholar] [CrossRef] [PubMed]
- Agostini, F.; Biolo, G. Effect of physical activity on glutamine metabolism. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Agostini, F.; Heer, M.; Guarnieri, G.; Biolo, G. Physical inactivity decreases whole body glutamine turnover independently from changes in proteolysis. J. Physiol. 2008, 586, 4775–4781. [Google Scholar] [CrossRef] [PubMed]

| Gene | Forward | Reverse |
|---|---|---|
| Rn18s | 5′-tcaagaacgaaagtcggagg-3′ | 5′-ggacatctaagggcatcac-3′ |
| Myh1 | 5′-gagggacagttcatcgatagcaa-3′ | 5′-gggccaacttgtcatctctcat-3′ |
| Asct2 | 5′-tccagcgggagatcaattcaa-3′ | 5′-gacgatagcgaagaccacca-3′ |
| Lat1 | 5′-ctggatcgagctgctcatc-3′ | 5′-gttcacagctgtgaggagc-3′ |
| Snat1 | 5′-tccatgactctcgaccagaac-3′ | 5′-cgaaggcgatggttggtaaagc-3′ |
| Snat2 | 5′-ttgctcgctgctctctttgg-3′ | 5′-cacgatctcggagtaggtatgc-3′ |
| Gls1 | 5′-caacgtcagatggtgtcatgc-3′ | 5′-cctccagactgctttttagcac-3′ |
| Gls2 | 5′-acaagatggctgggaacgaa-3′ | 5′-tgaggtaatagccgat-3′ |
| Glul | 5′-tggctggtcaacttga-3′ | 5′-tcaaaaggcccgcttt-3′ |
| Glud1 | 5′-ttggtcctggcattgatgtg-3′ | 5′-taacacaggcatgcgcattg-3′ |
| Gpt2 | 5′-aagaaggagcgcatgcaatc-3′ | 5′-atttgcttggtggctgctac-3′ |
| Bcat2 | 5′-cggacccttcattcgtcaga-3′ | 5′-ccatagttcccccccaactt-3′ |
| Myf5 | 5′-tgaatgtaacagccctgtctggtc-3′ | 5′-cgtgatagataagtccggagctgg-3′ |
| Myod1 | 5′-agcatcacagtggcgactca-3′ | 5′-ggccgctgtaatccatcat -3′ |
| Mrf4 | 5′-ggccaagtgtttcggatcattc-3′ | 5′- ttccaaatgctggctgagttacttc-3′ |
| Myog | 5′-cccatggtgcccagtgaa-3′ | 5′-gcagatgtggggcgtctgta-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takatoya, M.; Kasugai, T.; Arai, D.; Kasuga, U.; Miyaura, C.; Hirata, M.; Itoh, Y.; Tominari, T.; Aoki, Y.; Inada, M. Glutamine Promotes Myogenesis in Myoblasts Through Glutaminolysis-Mediated Histone H3 Acetylation That Enhances Myogenin Transcription. Nutrients 2025, 17, 3673. https://doi.org/10.3390/nu17233673
Takatoya M, Kasugai T, Arai D, Kasuga U, Miyaura C, Hirata M, Itoh Y, Tominari T, Aoki Y, Inada M. Glutamine Promotes Myogenesis in Myoblasts Through Glutaminolysis-Mediated Histone H3 Acetylation That Enhances Myogenin Transcription. Nutrients. 2025; 17(23):3673. https://doi.org/10.3390/nu17233673
Chicago/Turabian StyleTakatoya, Masaru, Tomoya Kasugai, Daichi Arai, Urara Kasuga, Chisato Miyaura, Michiko Hirata, Yoshifumi Itoh, Tsukasa Tominari, Yoshitsugu Aoki, and Masaki Inada. 2025. "Glutamine Promotes Myogenesis in Myoblasts Through Glutaminolysis-Mediated Histone H3 Acetylation That Enhances Myogenin Transcription" Nutrients 17, no. 23: 3673. https://doi.org/10.3390/nu17233673
APA StyleTakatoya, M., Kasugai, T., Arai, D., Kasuga, U., Miyaura, C., Hirata, M., Itoh, Y., Tominari, T., Aoki, Y., & Inada, M. (2025). Glutamine Promotes Myogenesis in Myoblasts Through Glutaminolysis-Mediated Histone H3 Acetylation That Enhances Myogenin Transcription. Nutrients, 17(23), 3673. https://doi.org/10.3390/nu17233673









