Luteolin Alleviates Vascular Senescence Through Retinoic Acid–Peroxisome Proliferator-Activated Receptor Signaling and Lipid Metabolism Remodeling Combined with Multi-Omics Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Cell Viability Assay
2.3. SA-β-Gal Activity Assay
2.4. Evaluation of Cellular Senescence Markers
2.4.1. Assessment of SASP
2.4.2. Quantification of Angiogenic Repair Factors
2.4.3. Oxidative Stress Assessment
2.5. Early Senescence Mouse Model
2.5.1. Experimental Design
2.5.2. Behavioral Test
2.5.3. Physiological Measurements
2.5.4. Serum Senescence Markers
2.5.5. Vascular Tensile Strength
2.5.6. Hematoxylin and Eosin (HE) Staining
2.6. RNA Sequencing
2.7. Untargeted Metabolomics of cVECs
2.8. Statistical Analysis
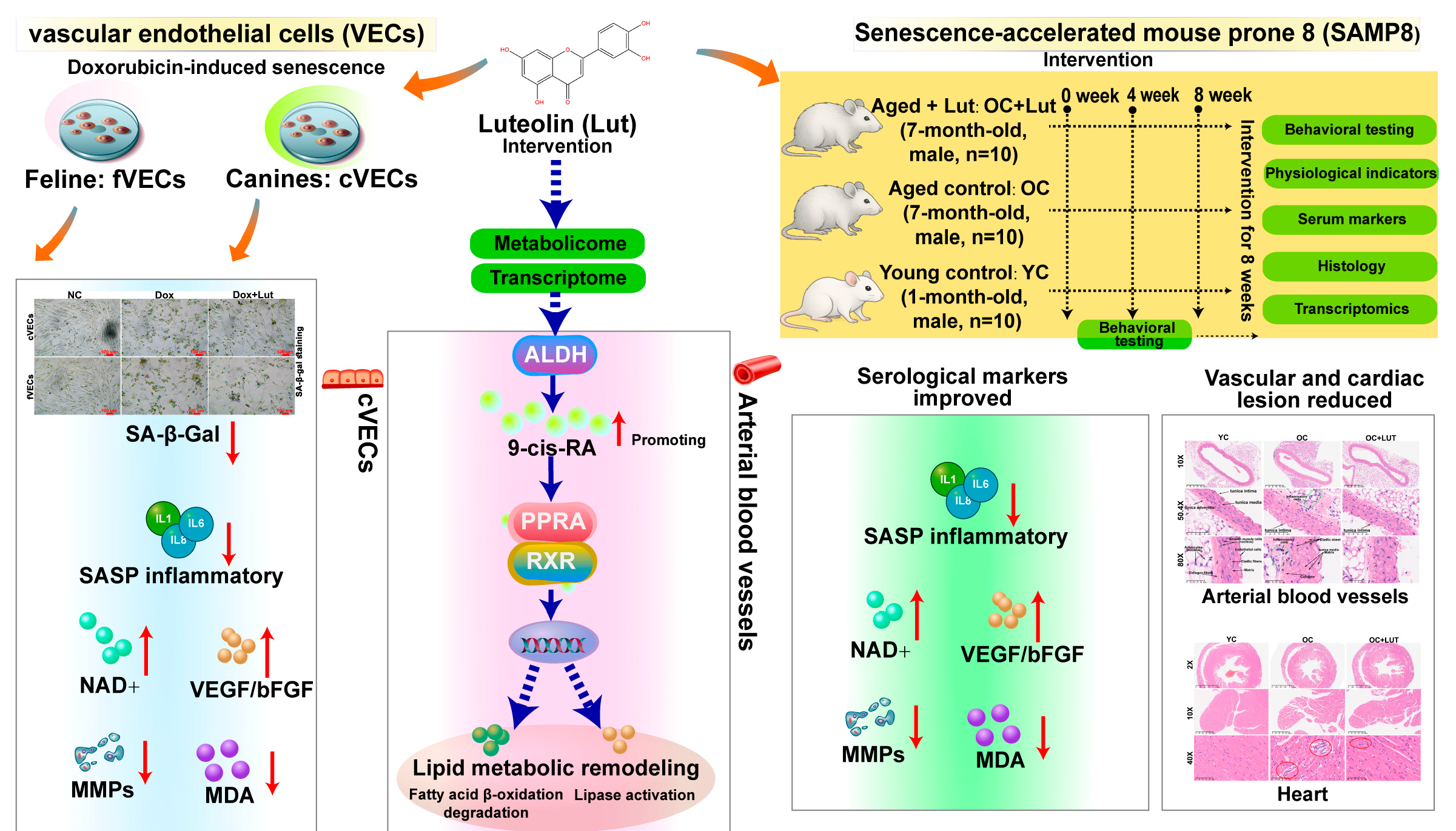
3. Results
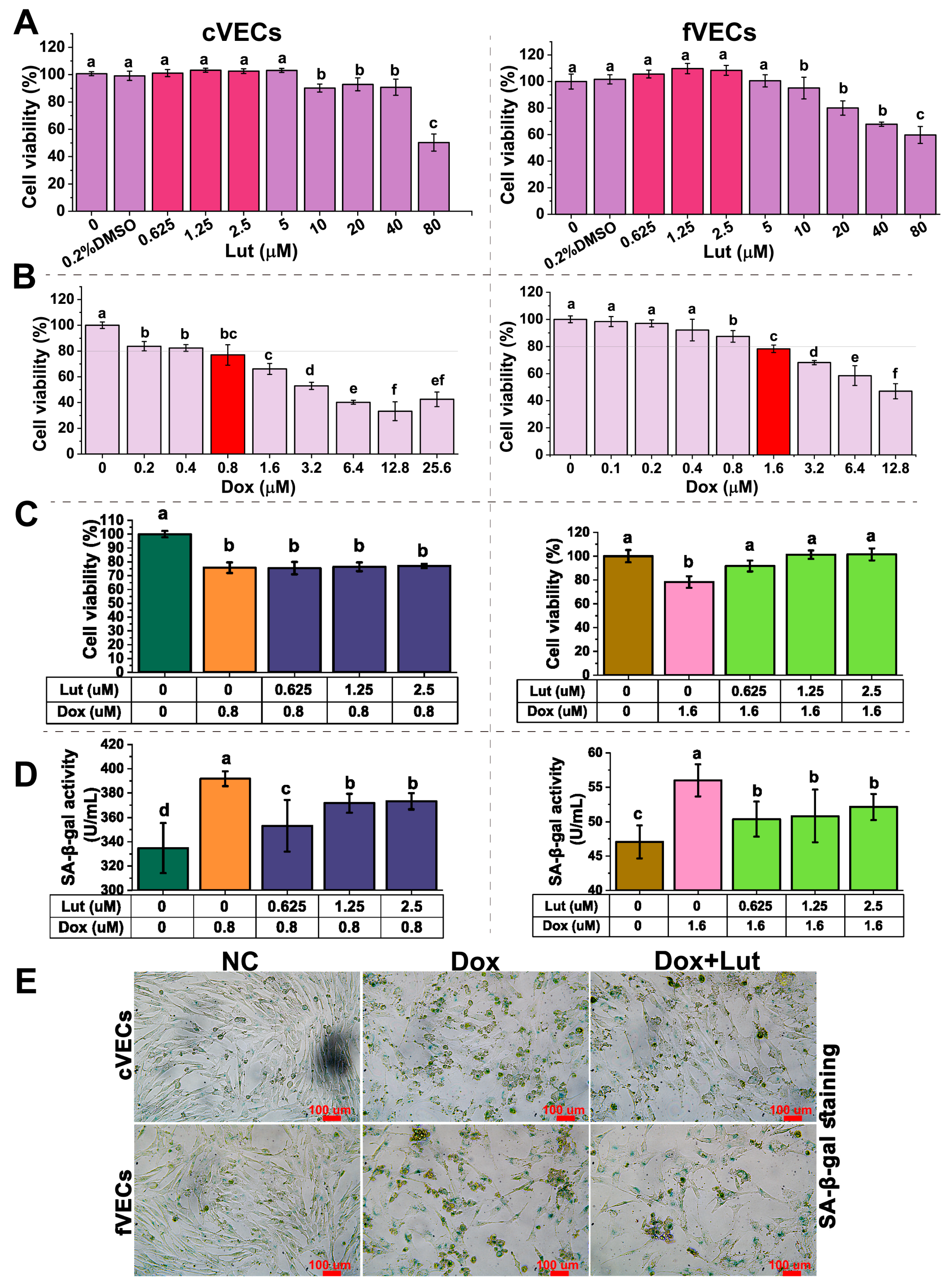
3.1. Lut’s Effect on Vascular Endothelial Cell Viability
3.2. Lut Reduced SA-β-Gal Activity
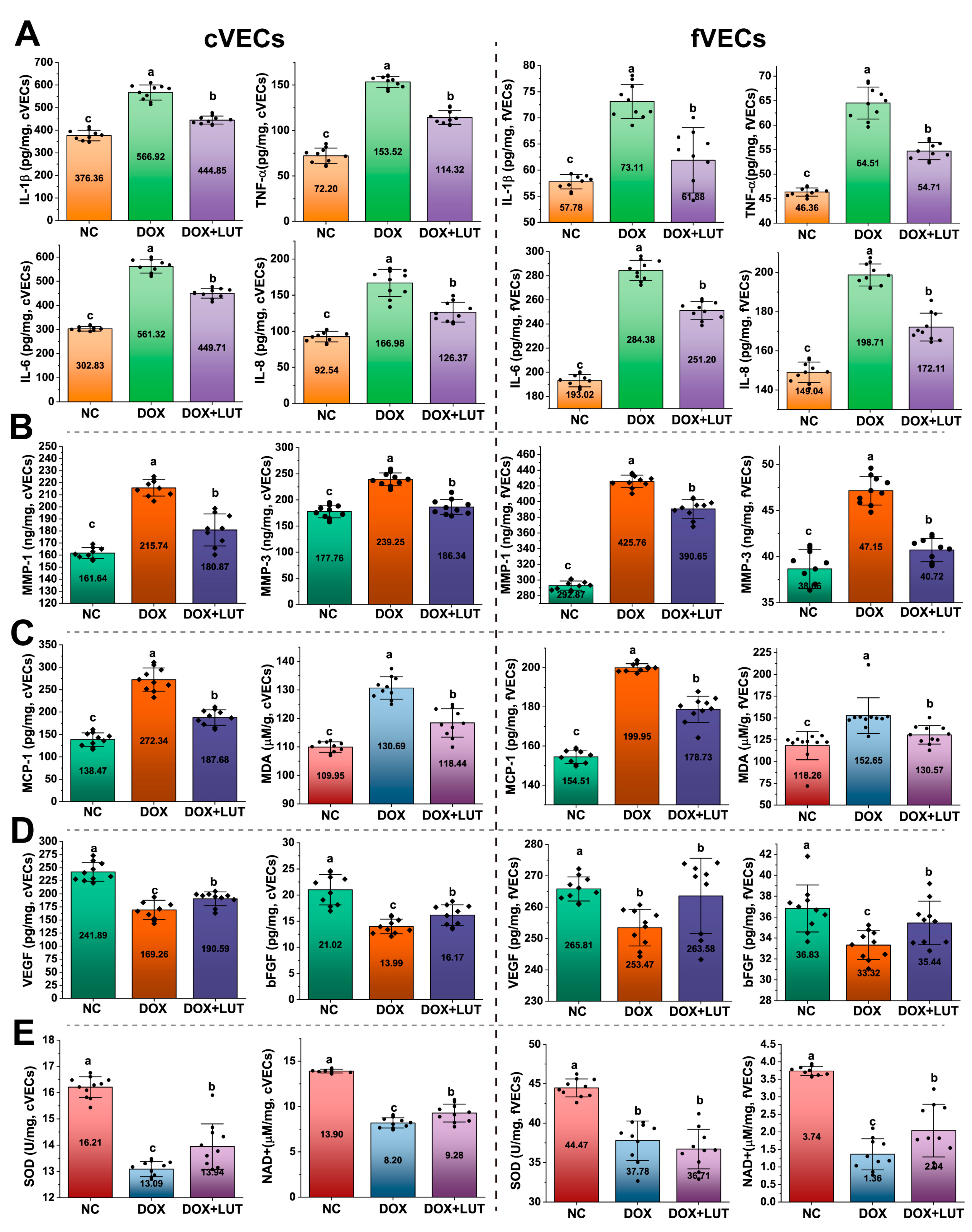
3.3. Lut Reduced Levels of SASP Markers
3.4. Lut Enhanced Angiogenic and Antioxidant Responses
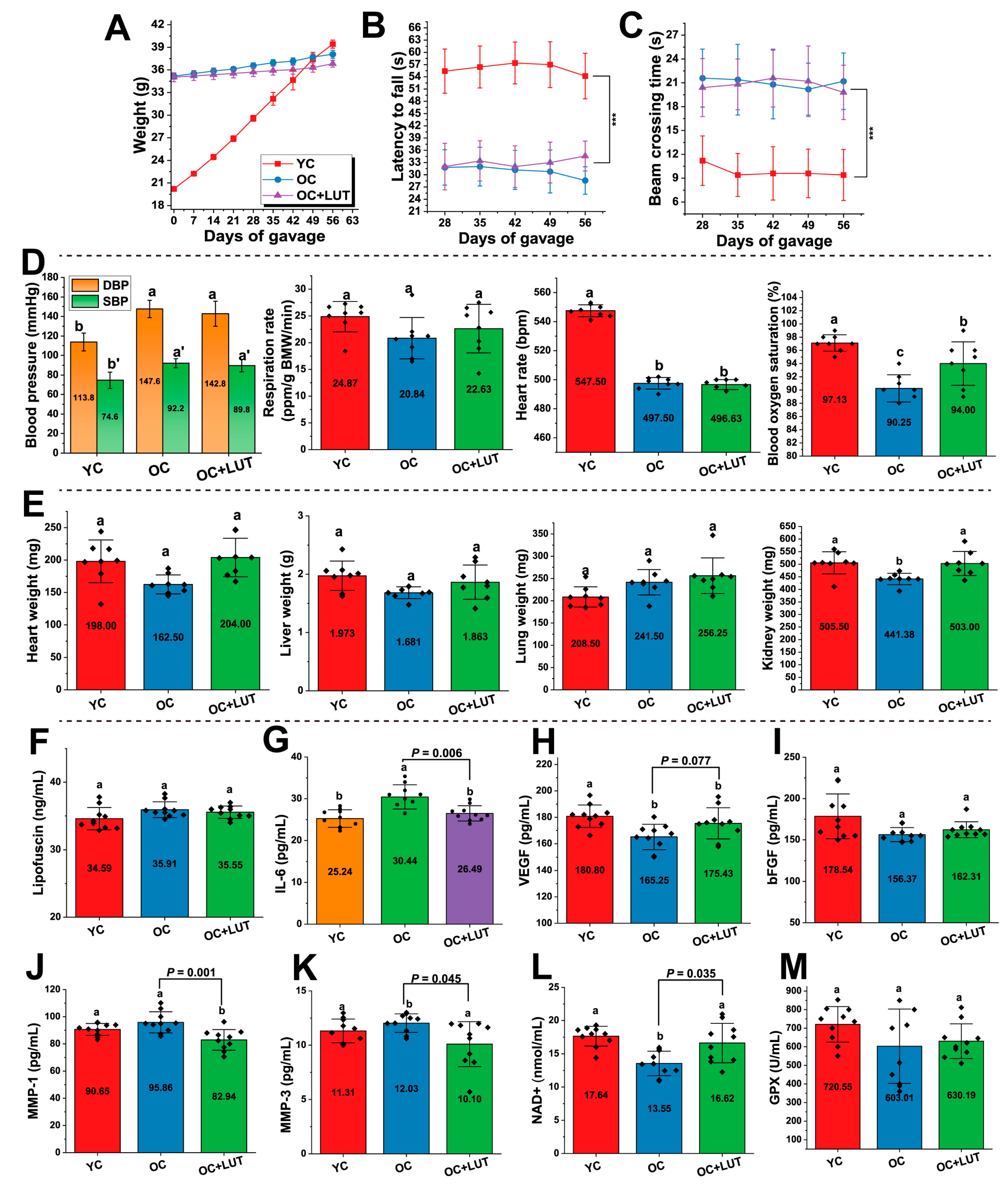
3.5. Effects of Lut on Physiological and Behavioral Parameters in Aged SAMP8 Mice
3.6. Lut Improved Serum Aging-Related Markers in Aged SAMP8 Mice
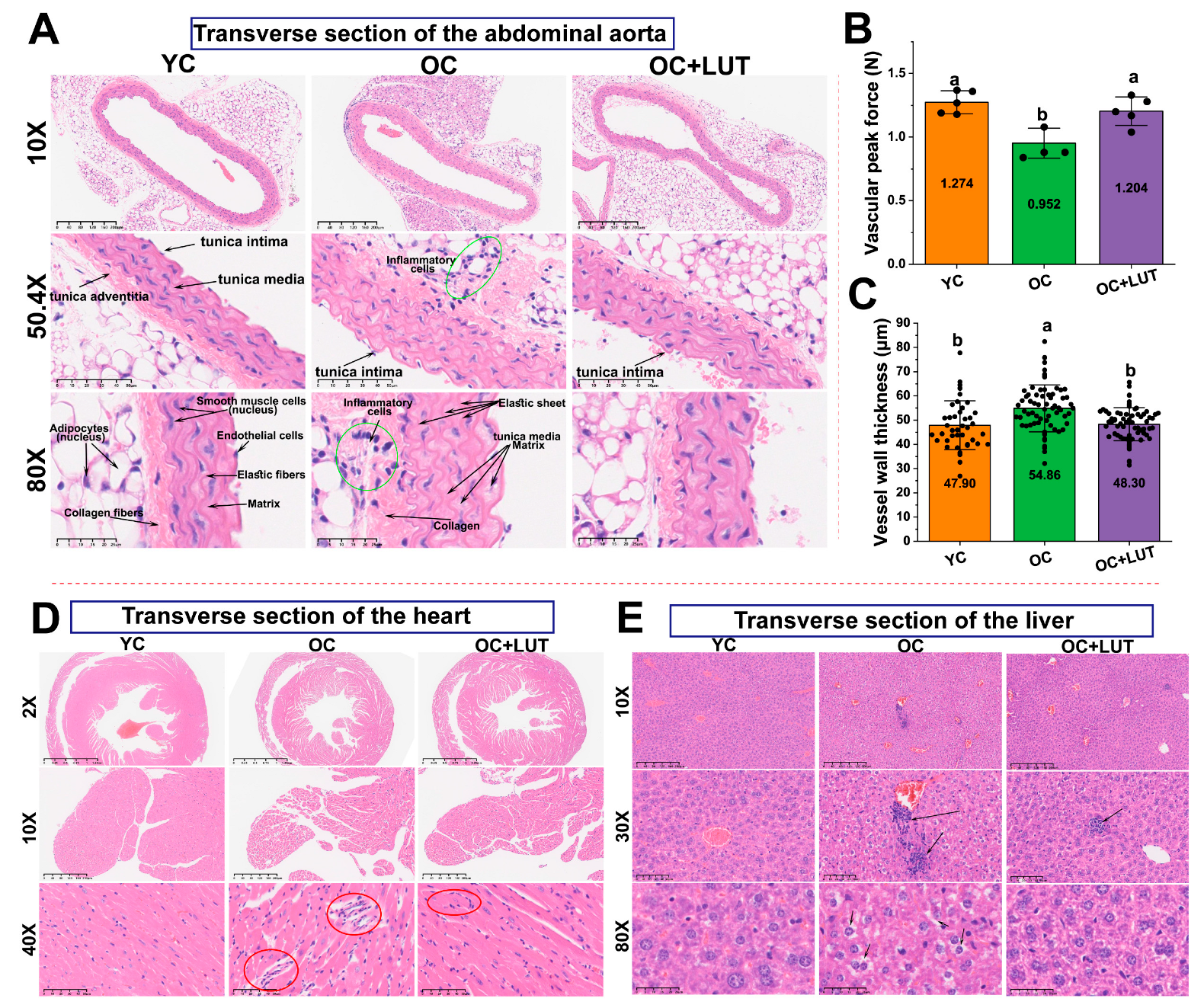
3.7. Lut Improved Vascular Structure and Mechanical Properties in Aged SAMP8 Mice
3.8. Lut Ameliorated Age-Related Cardiac and Hepatic Histological Alterations in SAMP8 Mice
3.9. Activation of the p53 Signaling Pathway in Dox-Induced cVECs
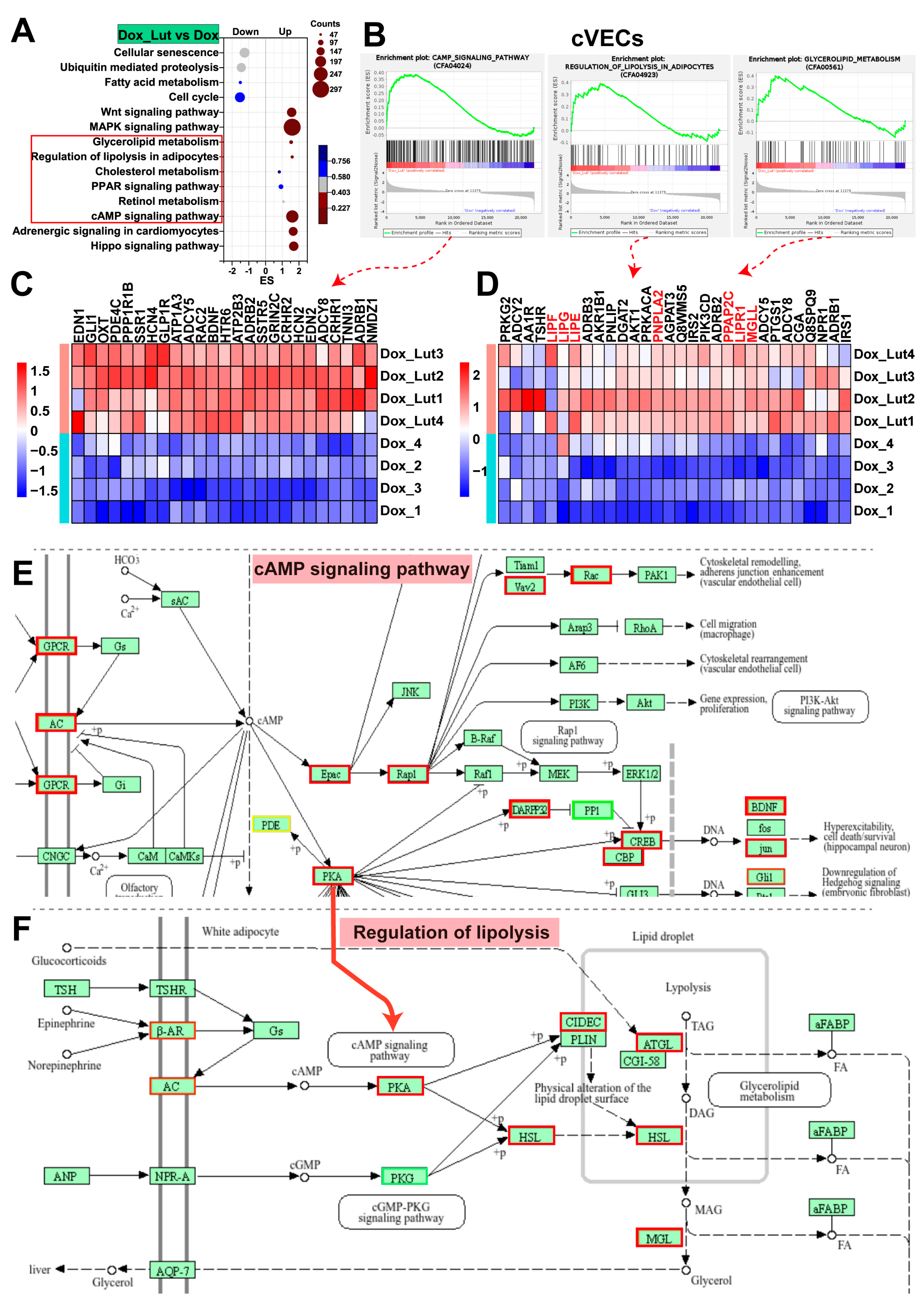
3.10. Lut Activated cAMP Signaling and Lipolytic Pathways in Dox-Induced cVECs
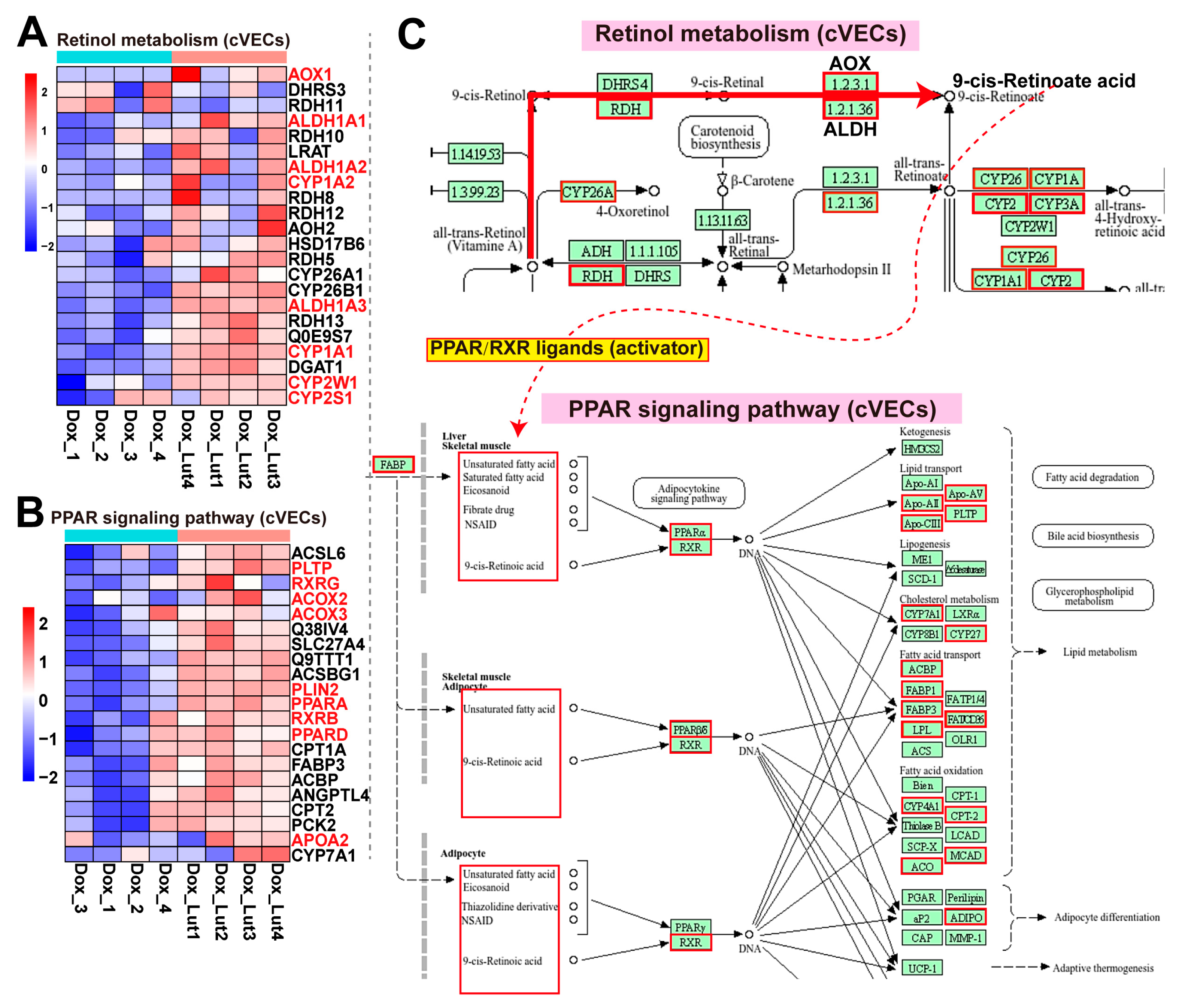
3.11. Lut Activated Retinol Metabolism and PPAR Signaling in Dox-Induced cVECs
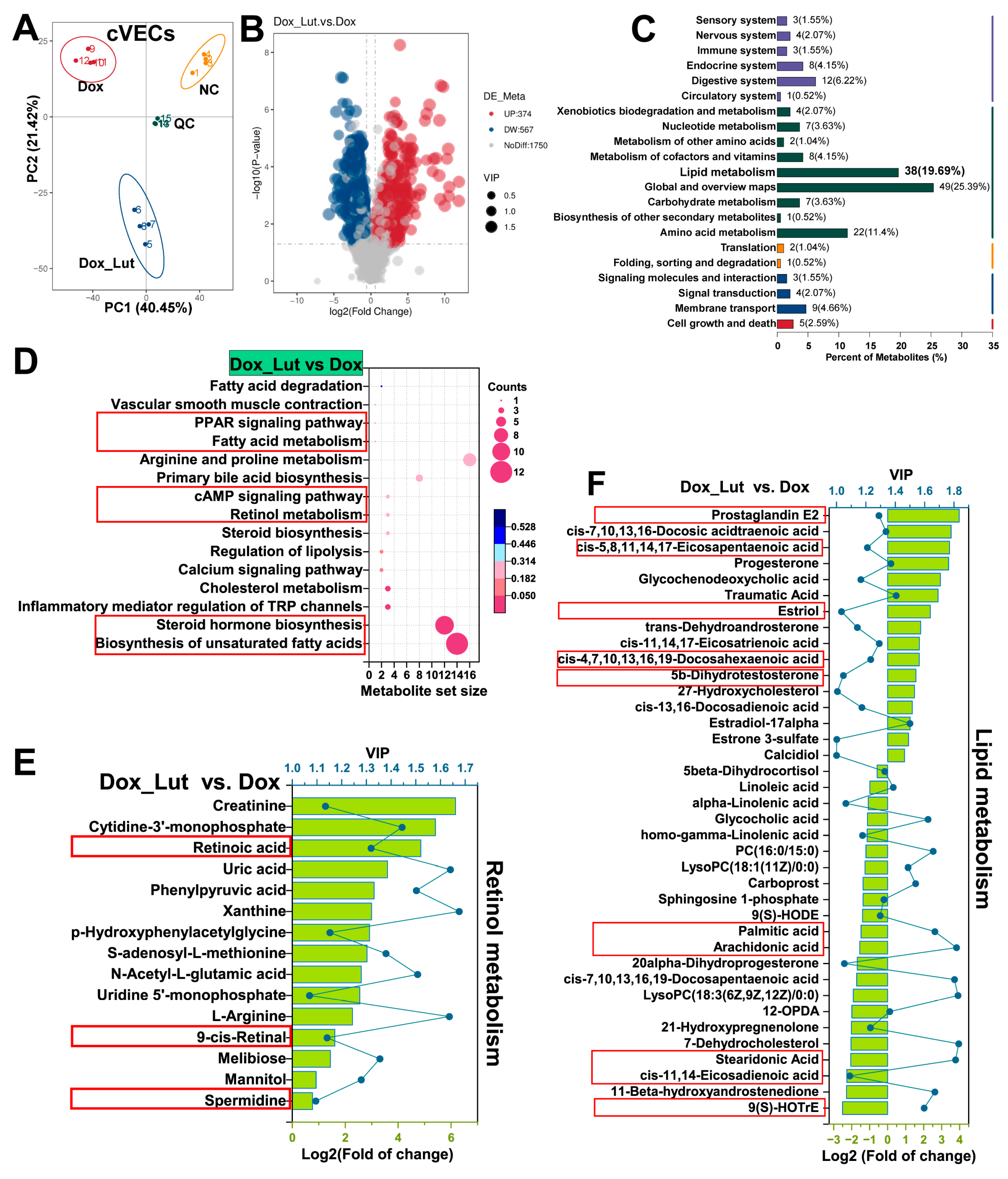
3.12. Validation of Lut-Induced Retinoid Activation and Lipid Metabolism Remodeling in cVECs via Metabolomics
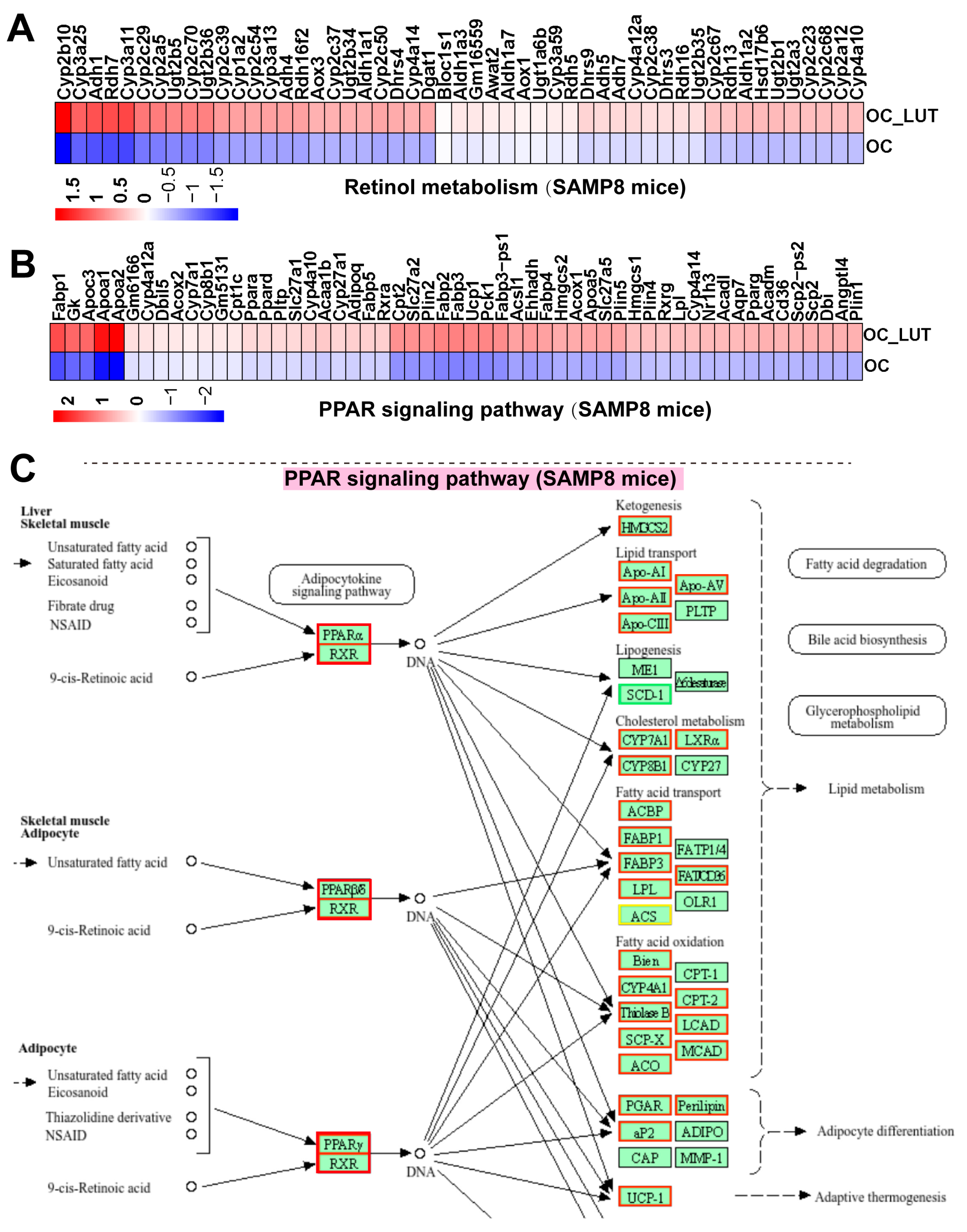
3.13. Lut Activated Retinol/PPAR Signaling and Lipid Metabolism in Vasculature of Aged SAMP8 Mice
3.14. Lut Caused Vascular Activation of the Retinol/PPAR Axis Across Models and Species
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Lut | Luteolin |
| cVECs | Canine vascular endothelial cells |
| fVECs | Feline vascular endothelial cells |
| RA | Retinoic acid |
| PPAR | Peroxisome proliferator-activated receptor |
| RXR | Retinoid X receptor |
| ALDH1 | Aldehyde dehydrogenase 1 |
| DOX | Doxorubicin |
| ROS | Reactive oxygen species |
| SOD | Superoxide dismutase |
| GPX | Glutathione peroxidase |
| CAT | Catalase |
| VEGF | Vascular endothelial growth factor |
| bFGF | Basic fibroblast growth factor |
| IL | Interleukin |
| TNF | Tumor necrosis factor |
| MMP | Matrix metalloproteinase |
| MCP | Monocyte chemoattractant protein |
| SA-β-Gal | Senescence-associated β-galactosidase |
| pNPG | p-nitrophenyl-β-D-galactopyranoside |
| SAMP8 | Senescence-accelerated mouse prone 8 |
| DEG | Differentially expressed gene |
| PCA | Principal component analysis |
| cAMP | Cyclic adenosine monophosphate |
| GPCR | G-protein coupled receptor |
| AC | Adenylyl cyclase |
| PKA | Protein kinase A |
| PD-L1 | Programmed death-ligand 1 |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| PGE2 | Prostaglandin E2 |
| AA | Arachidonic acid |
| S1P | Sphingosine-1-phosphate |
References
- Hwang, H.J.; Kim, N.; Herman, A.B.; Gorospe, M.; Lee, J.S. Factors and pathways modulating endothelial cell senescence in vascular aging. Int. J. Mol. Sci. 2022, 23, 10135. [Google Scholar] [CrossRef]
- Ahmed, B.; Rahman, A.A.; Lee, S.; Malhotra, R. The implications of aging on vascular health. Int. J. Mol. Sci. 2024, 25, 11188. [Google Scholar] [CrossRef]
- Donato, A.J.; Machin, D.R.; Lesniewski, L.A. Mechanisms of dysfunction in the aging vasculature and role in age-related disease. Circ. Res. 2018, 123, 825–848. [Google Scholar] [CrossRef]
- Hwang, H.J.; Kang, D.; Kim, J.R.; Choi, J.H.; Ryu, J.K.; Herman, A.B.; Ko, Y.G.; Park, H.J.; Gorospe, M.; Lee, J.S. FLRT2 prevents endothelial cell senescence and vascular aging by regulating the ITGB4/mTORC2/p53 signaling pathway. JCI Insight 2024, 9, e172678. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Xuan, X.; Hu, J.; Zhang, R.; Jin, H.; Dong, H. How vascular smooth muscle cell phenotype switching contributes to vascular disease. Cell Commun. Signal 2022, 20, 180. [Google Scholar] [CrossRef]
- Tylutka, A.; Morawin, B.; Wawrzyniak-Gramacka, E.; Wacka, E.; Nowicka, W.; Hiczkiewicz, J.; Zembron-Lacny, A. Immunosenescence in aging-related vascular dysfunction. Int. J. Mol. Sci. 2022, 23, 13269. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Qian, Z.; Huang, Y.; Yang, X.; Yang, J.; Xiao, N.; Liang, G.; Zhang, H.; Fu, Y.; Lin, Y.; et al. Mechanisms of endothelial senescence and vascular aging. Biogerontology 2025, 26, 128. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Gao, P.; Peng, W.; Wang, X.; Wang, Z.; Deng, W.; Yin, K.; Zhu, X. Spinster homolog 2 (SPNS2) deficiency drives endothelial cell senescence and vascular aging via promoting pyruvate metabolism mediated mitochondrial dysfunction. Cell Commun. Signal 2024, 22, 492. [Google Scholar] [CrossRef]
- Yu, Y.; Ren, Y.; Li, Z.; Li, Y.; Li, Y.; Zhang, Y.; Gui, R.; Cui, Y.; Qian, L.; Xiong, Y. Myo1b promotes premature endothelial senescence and dysfunction via suppressing autophagy: Implications for vascular aging. Oxid. Med. Cell Longev. 2023, 2023, 4654083. [Google Scholar] [CrossRef]
- Tai, S.; Sun, J.; Zhou, Y.; Zhu, Z.; He, Y.; Chen, M.; Yang, H.; Xiao, Y.; Tu, T.; Tang, L.; et al. Metformin suppresses vascular smooth muscle cell senescence by promoting autophagic flux. J. Adv. Res. 2022, 41, 205–218. [Google Scholar] [CrossRef]
- You, Y.; Chen, X.; Chen, Y.; Pang, J.; Chen, Q.; Liu, Q.; Xue, H.; Zeng, Y.; Xiao, J.; Mi, J.; et al. Epigenetic modulation of Drp1-mediated mitochondrial fission by inhibition of S-adenosylhomocysteine hydrolase promotes vascular senescence and atherosclerosis. Redox Biol. 2023, 65, 102828. [Google Scholar] [CrossRef]
- Ungvari, A.; Nyúl-Tóth, Á.; Patai, R.; Csik, B.; Gulej, R.; Nagy, D.; Shanmugarama, S.; Benyó, Z.; Kiss, T.; Ungvari, Z.; et al. Cerebromicrovascular senescence in vascular cognitive impairment: Does accelerated microvascular aging accompany atherosclerosis? Geroscience 2025, 47, 5511–5524. [Google Scholar] [CrossRef]
- Tai, G.J.; Ma, Y.J.; Feng, J.L.; Li, J.P.; Qiu, S.; Yu, Q.Q.; Liu, R.H.; Wankumbu, S.C.; Wang, X.; Li, X.X.; et al. NLRP3 inflammasome-mediated premature immunosenescence drives diabetic vascular aging dependent on the induction of perivascular adipose tissue dysfunction. Cardiovasc. Res. 2025, 121, 77–96. [Google Scholar] [CrossRef]
- Peng, Z.; Tan, X.; Xie, L.; Li, Z.; Zhou, S.; Li, Y. PKR deficiency delays vascular aging via inhibiting GSDMD-mediated endothelial cell hyperactivation. iScience 2023, 26, 105909. [Google Scholar] [CrossRef]
- Liu, S.; Wu, J.; Stolarz, A.; Zhang, H.; Boerma, M.; Byrum, S.D.; Rusch, N.J.; Ding, Z. PCSK9 attenuates efferocytosis in endothelial cells and promotes vascular aging. Theranostics 2023, 13, 2914–2929. [Google Scholar] [CrossRef]
- Li, A.; Yan, J.; Zhao, Y.; Yu, Z.; Tian, S.; Khan, A.H.; Zhu, Y.; Wu, A.; Zhang, C.; Tian, X.L. Vascular aging: Assessment and intervention. Clin. Interv. Aging 2023, 18, 1373–1395. [Google Scholar] [CrossRef] [PubMed]
- Campagna, R.; Mazzanti, L.; Pompei, V.; Alia, S.; Vignini, A.; Emanuelli, M. The multifaceted role of endothelial SIRT1 in vascular aging: An update. Cells 2024, 13, 1469. [Google Scholar] [CrossRef]
- Brandes, R.P.; Fleming, I.; Busse, R. Endothelial aging. Cardiovasc. Res. 2005, 66, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Breuss, J.M.; Atanasov, A.G.; Uhrin, P. Resveratrol and its effects on the vascular system. Int. J. Mol. Sci. 2019, 20, 1523. [Google Scholar] [CrossRef] [PubMed]
- Parsamanesh, N.; Asghari, A.; Sardari, S.; Tasbandi, A.; Jamialahmadi, T.; Xu, S.; Sahebkar, A. Resveratrol and endothelial function: A literature review. Pharmacol. Res. 2021, 170, 105725. [Google Scholar] [CrossRef]
- Ya, J.; Bayraktutan, U. Vascular ageing: Mechanisms, risk factors, and treatment strategies. Int. J. Mol. Sci. 2023, 24, 11538. [Google Scholar] [CrossRef] [PubMed]
- Pisaniello, A.D.; Psaltis, P.J.; King, P.M.; Liu, G.; Gibson, R.A.; Tan, J.T.; Duong, M.; Nguyen, T.; Bursill, C.A.; Worthley, M.I.; et al. Omega-3 fatty acids ameliorate vascular inflammation: A rationale for their atheroprotective effects. Atherosclerosis 2021, 324, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, S.A.; Venkatasubramanian, R.; Darrah, M.A.; Ludwig, K.R.; VanDongen, N.S.; Greenberg, N.T.; Longtine, A.G.; Hutton, D.A.; Brunt, V.E.; Campisi, J.; et al. Intermittent supplementation with fisetin improves arterial function in old mice by decreasing cellular senescence. Aging Cell 2024, 23, e14060. [Google Scholar] [CrossRef]
- Dagher, O.; Mury, P.; Thorin-Trescases, N.; Noly, P.E.; Thorin, E.; Carrier, M. Therapeutic potential of quercetin to alleviate endothelial dysfunction in age-related cardiovascular diseases. Front. Cardiovasc. Med. 2021, 8, 658400. [Google Scholar] [CrossRef]
- Yin, Z.; Tian, L.; Kou, W.; Cao, G.; Wang, L.; Xia, Y.; Lin, Y.; Tang, S.; Zhang, J.; Yang, H. Xiyangshen Sanqi Danshen granules attenuated D-gal-induced C57BL/6J mouse aging through the AMPK/SIRT1 signaling pathway. Phytomedicine 2025, 136, 156213. [Google Scholar] [CrossRef]
- Dong, Y.; Wu, X.; Han, L.; Bian, J.; He, C.; El-Omar, E.; Gong, L.; Wang, M. The potential roles of dietary anthocyanins in inhibiting vascular endothelial cell senescence and preventing cardiovascular diseases. Nutrients 2022, 14, 2836. [Google Scholar] [CrossRef]
- Wang, T.H.; Tseng, W.C.; Leu, Y.L.; Chen, C.Y.; Lee, W.C.; Chi, Y.C.; Cheng, S.F.; Lai, C.Y.; Kuo, C.H.; Yang, S.L.; et al. The flavonoid corylin exhibits lifespan extension properties in mouse. Nat. Commun. 2022, 13, 1238. [Google Scholar] [CrossRef] [PubMed]
- DiNicolantonio, J.J.; McCarty, M.F.; O’Keefe, J.H. Nutraceutical activation of Sirt1: A review. Open Heart 2022, 9, e002171. [Google Scholar] [CrossRef]
- Fang, Z.; Raza, U.; Song, J.; Lu, J.; Yao, S.; Liu, X.; Zhang, W.; Li, S. Systemic aging fuels heart failure: Molecular mechanisms and therapeutic avenues. ESC Heart Fail. 2025, 12, 1059–1080. [Google Scholar] [CrossRef]
- Lu, Q.Y.; Guo, L.; Zhang, Q.Y.; Yang, F.M.; Zhou, S.T.; Sun, Q.Y. Luteolin Alleviates the TNF-α-Induced Inflammatory Response of Human Microvascular Endothelial Cells via the Akt/MAPK/NF-κB Pathway. Mediators Inflamm. 2024, 2024, 6393872. [Google Scholar] [CrossRef]
- Brüser, L.; Teichmann, E.; Hinz, B. Effect of flavonoids on MCP-1 expression in human coronary artery endothelial cells and impact on MCP-1-dependent migration of human monocytes. Int. J. Mol. Sci. 2023, 24, 16047. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.I.; Hu, W.S.; Hung, M.Y.; Ou, H.C.; Huang, S.H.; Hsu, P.T.; Day, C.H.; Lin, K.H.; Viswanadha, V.P.; Kuo, W.W.; et al. Protective effects of luteolin against oxidative stress and mitochondrial dysfunction in endothelial cells. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 1032–1043. [Google Scholar] [CrossRef]
- Shimazaki, T.; Noro, N.; Hagikura, K.; Matsumoto, T.; Yoshida-Noro, C. Quantitative analysis of factors regulating angiogenesis for stem cell therapy. Biology 2021, 10, 1212. [Google Scholar] [CrossRef]
- Wu, Y.T.; Chen, L.; Tan, Z.B.; Fan, H.J.; Xie, L.P.; Zhang, W.T.; Chen, H.M.; Li, J.; Liu, B.; Zhou, Y.C. Luteolin inhibits vascular smooth muscle cell proliferation and migration by inhibiting TGFBR1 signaling. Front. Pharmacol. 2018, 9, 1059. [Google Scholar] [CrossRef]
- Su, J.; Xu, H.T.; Yu, J.J.; Gao, J.L.; Lei, J.; Yin, Q.S.; Li, B.; Pang, M.X.; Su, M.X.; Mi, W.J.; et al. Luteolin ameliorates hypertensive vascular remodeling through inhibiting the proliferation and migration of vascular smooth muscle cells. Evid. Based Complement. Alternat Med. 2015, 2015, 364876. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Zhu, H.; Li, D.; Lang, Y.; Cao, L.; Liu, Y.; Wu, W.; Chen, D. Luteolin inhibits angiotensin II-stimulated VSMC proliferation and migration through downregulation of Akt phosphorylation. Evid. Based Complement. Alternat Med. 2015, 2015, 931782. [Google Scholar] [CrossRef]
- Gentile, D.; Fornai, M.; Pellegrini, C.; Colucci, R.; Benvenuti, L.; Duranti, E.; Masi, S.; Carpi, S.; Nieri, P.; Nericcio, A.; et al. Luteolin prevents cardiometabolic alterations and vascular dysfunction in mice with HFD-induced obesity. Front. Pharmacol. 2018, 9, 1094. [Google Scholar] [CrossRef]
- Queiroz, M.; Leandro, A.; Azul, L.; Figueirinha, A.; Seiça, R.; Sena, C.M. Luteolin improves perivascular adipose tissue profile and vascular dysfunction in Goto-Kakizaki rats. Int. J. Mol. Sci. 2021, 22, 13671. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, J.; Su, S.; Xie, X.; Ji, L.; Li, Z.; Lu, D. Luteolin ameliorates hypoxic pulmonary vascular remodeling in rat via upregulating K(V)1.5 of pulmonary artery smooth muscle cells. Phytomedicine 2024, 132, 155840. [Google Scholar] [CrossRef]
- Ji, L.; Su, S.; Xin, M.; Zhang, Z.; Nan, X.; Li, Z.; Lu, D. Luteolin ameliorates hypoxia-induced pulmonary hypertension via regulating HIF-2α-Arg-NO axis and PI3K-AKT-eNOS-NO signaling pathway. Phytomedicine 2022, 104, 154329. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Xu, L.; Su, C.; Wang, C.; Wang, Z.; Wang, Y.; Lu, X.; Sun, H. Luteolin protects against vascular calcification by modulating the SIRT1/CXCR4 signaling pathway and promoting autophagy. AAPS J. 2024, 26, 111. [Google Scholar] [CrossRef]
- Wen, L.; Zhang, W.; Hu, J.; Chen, T.; Wang, Y.; Lv, C.; Li, M.; Wang, L.; Xiao, F. Luteolin target HSPB1 regulates endothelial cell ferroptosis to protect against radiation vascular injury. PLoS ONE 2024, 19, e0311922. [Google Scholar] [CrossRef]
- Ding, X.; Zheng, L.; Yang, B.; Wang, X.; Ying, Y. Luteolin attenuates atherosclerosis via modulating signal transducer and activator of transcription 3-mediated inflammatory response. Drug Des. Devel. Ther. 2019, 2019, 3899–3911. [Google Scholar] [CrossRef] [PubMed]
- Rungsung, S.; Singh, T.U.; Perumalraja, K.; Mahobiya, A.; Sharma, M.; Lingaraju, M.C.; Parida, S.; Sahoo, M.; Kumar, D. Luteolin alleviates vascular dysfunctions in CLP-induced polymicrobial sepsis in mice. Pharmacol. Rep. 2022, 74, 1054–1068. [Google Scholar] [CrossRef]
- Liu, B.; Su, H. Luteolin improves vasoconstriction function and survival of septic mice via AMPK/NF-κB pathway. Heliyon 2023, 9, e13330. [Google Scholar] [CrossRef]
- Li, L.; Pan, G.; Fan, R.; Li, D.; Guo, L.; Ma, L.; Liang, H.; Qiu, J. Luteolin alleviates inflammation and autophagy of hippocampus induced by cerebral ischemia/reperfusion by activating PPAR gamma in rats. BMC Complement. Med. Ther. 2022, 22, 176. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Li, X.; Wang, Z.; Cao, Y.; Han, S.; Li, N.; Cai, J.; Cheng, S.; Liu, Q. Protective effects of luteolin against amyloid beta-induced oxidative stress and mitochondrial impairments through peroxisome proliferator-activated receptor γ-dependent mechanism in Alzheimer’s disease. Redox Biol. 2023, 66, 102848. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gao, T.; Wang, F.; Xue, J.; Ye, H.; Xie, M. Luteolin improves myocardial cell glucolipid metabolism by inhibiting hypoxia inducible factor-1α expression in angiotensin II/hypoxia-induced hypertrophic H9c2 cells. Nutr. Res. 2019, 65, 63–70. [Google Scholar] [CrossRef]
- Shui, S.S.; Chen, J.; Lin, Y.; Yuan, J.J.; Wang, X.; Zhang, X.; Liu, J.; Zheng, L. Deficiency of circadian gene Per2 blocks luteolin-induced adipocyte browning in mice through weakening liver PPARα/RXRα/FGF21 pathway. Food Sci. Human. Wellness 2025, 14, 9250058. [Google Scholar] [CrossRef]
- Berbis, P. Retinoids: Mechanisms of action. Ann. Dermatol. Venereol. 2010, 137 (Suppl. 3), S97–S103. [Google Scholar] [CrossRef]
- Li, J.; Dong, J.Z.; Ren, Y.L.; Zhu, J.J.; Cao, J.N.; Zhang, J.; Pan, L.L. Luteolin decreases atherosclerosis in LDL receptor-deficient mice via a mechanism including decreasing AMPK-SIRT1 signaling in macrophages. Exp. Ther. Med. 2018, 16, 2593–2599. [Google Scholar] [CrossRef]
- El-Bassossy, H.M.; Abo-Warda, S.M.; Fahmy, A. Chrysin and luteolin alleviate vascular complications associated with insulin resistance mainly through PPAR-γ activation. Am. J. Chin. Med. 2014, 42, 1153–1167. [Google Scholar] [CrossRef]
- Zumerle, S.; Sarill, M.; Saponaro, M.; Colucci, M.; Contu, L.; Lazzarini, E.; Sartori, R.; Pezzini, C.; Rinaldi, A.; Scanu, A.; et al. Targeting senescence induced by age or chemotherapy with a polyphenol-rich natural extract improves longevity and healthspan in mice. Nat. Aging 2024, 4, 1231–1248. [Google Scholar] [CrossRef]
- Yoong, W.T.; Choong, S.S.; Fauzee, M.S.O.; Ahmad, Z.; Hakim, M.N. Luteolin Suppresses Endothelial Permeability and Nitric Oxide Scavenging Effects. Indones. Biomed. J. 2024, 16, 427–433. [Google Scholar] [CrossRef]
- Liu, B.; Liu, J.; Shi, J.S. SAMP8 Mice as a Model of Age-Related Cognition Decline with Underlying Mechanisms in Alzheimer’s Disease. J. Alzheimers Dis. 2020, 75, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Schiborr, C.; Eckert, G.P.; Weissenberger, J.; Müller, W.E.; Schwamm, D.; Grune, T.; Rimbach, G.; Frank, J. Cardiac Oxidative Stress and Inflammation are Similar in SAMP8 and SAMR1 Mice and Unaltered by Curcumin and Ginkgo biloba Extract Intake. Curr. Pharm. Biotechnol. 2010, 11, 861–867. [Google Scholar] [CrossRef]
- Yu, F.; Wang, G.; Chen, X.; Zhang, Y.; Yang, C.; Hu, H.; Wei, L. Luteolin alleviates cerebral ischemia/reperfusion injury by regulating cell pyroptosis. Open Med. 2024, 19, 20241063. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.F.; Chiu, S.P.; Wu, M.J.; Chen, P.Y.; Yen, J.H. Luteolin induces microRNA-132 expression and modulates neurite outgrowth in PC12 cells. PLoS ONE 2012, 7, e43304. [Google Scholar] [CrossRef]
- Tan, H.; Yuan, L.; Lu, N.; Zhang, Z.; Chang, J.; Li, Z. High-efficient cAMP biosynthesis by inhibiting xanthine oxidase activity in Arthrobacter sp. CCTCC 2013431 with quercetin. Food Ferment. Ind. 2022, 48, 22–28. [Google Scholar] [CrossRef]
- Yoon, Y.C.; Hwang, J.T.; Sung, M.J.; Wang, S.; Munkhtugs, D.; Rhyu, M.R.; Park, J.H. Inhibitory effect of luteolin on the odorant-induced cAMP level in HEK293 cells expressing the olfactory receptor. Biofactors 2012, 38, 360–364. [Google Scholar] [CrossRef] [PubMed]
- Deng, T.; Shan, S.; Li, Z.B.; Wu, Z.W.; Liao, C.Z.; Ko, B.; Lu, X.P.; Cheng, J.; Ning, Z.Q. A new retinoid-like compound that activates peroxisome proliferator-activated receptors and lowers blood glucose in diabetic mice. Biol. Pharm. Bull. 2005, 28, 1192–1196. [Google Scholar] [CrossRef]
- Ijpenberg, A.; Tan, N.S.; Gelman, L.; Kersten, S.; Seydoux, J.; Xu, J.M.; Metzger, D.; Canaple, L.; Chambon, P.; Wahli, W.; et al. In vivo activation of PPAR target genes by RXR homodimers. EMBO J. 2004, 23, 2083–2091. [Google Scholar] [CrossRef]
- Nawa, F.; Sai, M.; Vietor, J.; Schwarzenbach, R.; Biticc, A.; Wolff, S.; Ildefeld, N.; Pabel, J.; Wein, T.; Marschner, J.A.; et al. Tuning RXR Modulators for PGC1α Recruitment. J. Med. Chem. 2024, 67, 16338–16354. [Google Scholar] [CrossRef]
- Rhee, E.J.; Nallamshetty, S.; Plutzky, J. Retinoid metabolism and its effects on the vasculature. Biochim. Biophys. Acta 2012, 1821, 230–240. [Google Scholar] [CrossRef] [PubMed]
- DeRose, J.J., Jr.; Madigan, J.; Umana, J.P.; Prystowsky, J.H.; Nowygrod, R.; Oz, M.C.; Todd, G.J. Retinoic acid suppresses intimal hyperplasia and prevents vessel remodeling following arterial injury. Cardiovasc. Surg. 1999, 7, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Samuels, C.A.; Timmins, P.; Massri, N.; Chemerinski, A.; Wu, T.; Loia, R.; Cheung, E.K.; Zhang, X.; Arora, R.; et al. Signaling via retinoic acid receptors mediates decidual angiogenesis in mice and human stromal cell decidualization. FASEB J. 2025, 39, e70291. [Google Scholar] [CrossRef]
- Brtko, J.; Rock, E.; Nezbedova, P.; Krizanova, O.; Dvorcakova, M.; Minet-Quinard, R.; Farges, M.C.; Ribalta, J.; Winklhofer-Roob, B.M.; Vasson, M.P.; et al. Age-related change in the retinoid X receptor beta gene expression in peripheral blood mononuclear cells of healthy volunteers: Effect of 13-cis retinoic acid supplementation. Mech. Ageing Dev. 2007, 128, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Haas, M.J.; Naem, E.; Almdallaleh, S.; Mooradian, A.D. The effect of black seed (Nigella sativa) extract on lipid metabolism in HepG2 cells. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2022, 1867, 159155. [Google Scholar] [CrossRef]
- Hondares, E.; Rosell, M.; Díaz-Delfín, J.; Olmos, Y.; Monsalve, M.; Iglesias, R.; Villarroya, F.; Giralt, M. Peroxisome Proliferator-activated Receptor α (PPARα) Induces PPARγ Coactivator 1α (PGC-1α) Gene Expression and Contributes to Thermogenic Activation of Brown Fat INVOLVEMENT OF PRDM16. J. Biol. Chem. 2011, 286, 43112–43122. [Google Scholar] [CrossRef]
- Kersten, S.; Stienstra, R. The role and regulation of the peroxisome proliferator activated receptor alpha in human liver. Biochimie 2017, 136, 75–84. [Google Scholar] [CrossRef]
- Carracedo, M.; Artiach, G.; Arnardottir, H.; Bäck, M. The resolution of inflammation through omega-3 fatty acids in atherosclerosis, intimal hyperplasia, and vascular calcification. Semin. Immunopathol. 2019, 41, 757–766. [Google Scholar] [CrossRef]
- Sarajlic, P.; Vigor, C.; Avignon, A.; Zhou, B.Q.; Oger, C.; Galano, J.M.; Durand, T.; Sultan, A.; Baeck, M. Omega-3 to omega-6 fatty acid oxidation ratio as a novel inflammation resolution marker for metabolic complications in obesity. Nutr. Metab. Cardiovasc. Dis. 2023, 33, 1206–1213. [Google Scholar] [CrossRef]
- Jia, Z.Q.; Nallasamy, P.; Liu, D.M.; Shah, H.; Li, J.Z.; Chitrakar, R.; Si, H.W.; McCormick, J.; Zhu, H.; Zhen, W.; et al. Luteolin protects against vascular inflammation in mice and TNF-alpha-induced monocyte adhesion to endothelial cells via suppressing IKBα/NF-κB signaling pathway. J. Nutr. Biochem. 2015, 26, 293–302. [Google Scholar] [CrossRef]
- Ou, H.C.; Pandey, S.; Hung, M.Y.; Huang, S.H.; Hsu, P.T.; Day, C.H.; Pai, P.Y.; Viswanadha, V.P.; Kuo, W.W.; Huang, C.Y. Luteolin: A Natural Flavonoid Enhances the Survival of HUVECs against Oxidative Stress by Modulating AMPK/PKC Pathway. Am. J. Chin. Med. 2019, 47, 541–557. [Google Scholar] [CrossRef]
- Xia, F.; Wang, C.Y.; Jin, Y.; Liu, Q.; Meng, Q.; Liu, K.X.; Sun, H.J. Luteolin Protects HUVECs from TNF-α-induced Oxidative Stress and Inflammation via its Effects on the Nox4/ROS-NF-κB and MAPK Pathways. J. Atheroscler. Thromb. 2014, 21, 768–783. [Google Scholar] [CrossRef]
- Xiang, M.; Lin, X.D.; Chen, H.Y. Luteolin Inhibits Ferroptosis of HUVEC by Regulating the Sirt1/Nrf2 Pathway. Cell Biochem. Biophys. 2025. [Google Scholar] [CrossRef] [PubMed]
- Hsuan, C.F.; Hsu, H.F.; Tseng, W.K.; Lee, T.L.; Wei, Y.F.; Hsu, K.L.; Wu, C.C.; Houng, J.Y. Glossogyne tenuifolia Extract Inhibits TNF-α-Induced Expression of Adhesion Molecules in Human Umbilical Vein Endothelial Cells via Blocking the NF-κB Signaling Pathway. Molecules 2015, 20, 16908–16923. [Google Scholar] [CrossRef] [PubMed]
- Li, D.M.; Cui, Y.; Wang, X.J.; Liu, F.; Li, X.L. Apple Polyphenol Extract Improves High-Fat Diet-Induced Hepatic Steatosis by Regulating Bile Acid Synthesis and Gut Microbiota in C57BL/6 Male Mice. J. Agric. Food Chem. 2021, 69, 6829–6841. [Google Scholar] [CrossRef]
- Lim, M.Y.C.; Ho, H.K. Pharmacological modulation of cholesterol 7α-hydroxylase (CYP7A1) as a therapeutic strategy for hypercholesterolemia. Biochem. Pharmacol. 2024, 220, 115985. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, H.; Jin, H.; Liu, T.; Yin, Y.; Wang, H.; Ruan, S.; Li, Y.; Wang, Z. Luteolin Alleviates Vascular Senescence Through Retinoic Acid–Peroxisome Proliferator-Activated Receptor Signaling and Lipid Metabolism Remodeling Combined with Multi-Omics Analysis. Nutrients 2025, 17, 3607. https://doi.org/10.3390/nu17223607
Bai H, Jin H, Liu T, Yin Y, Wang H, Ruan S, Li Y, Wang Z. Luteolin Alleviates Vascular Senescence Through Retinoic Acid–Peroxisome Proliferator-Activated Receptor Signaling and Lipid Metabolism Remodeling Combined with Multi-Omics Analysis. Nutrients. 2025; 17(22):3607. https://doi.org/10.3390/nu17223607
Chicago/Turabian StyleBai, Huasong, Hongchen Jin, Tong Liu, Yulong Yin, Hengyan Wang, Siyu Ruan, Yunliang Li, and Zhanzhong Wang. 2025. "Luteolin Alleviates Vascular Senescence Through Retinoic Acid–Peroxisome Proliferator-Activated Receptor Signaling and Lipid Metabolism Remodeling Combined with Multi-Omics Analysis" Nutrients 17, no. 22: 3607. https://doi.org/10.3390/nu17223607
APA StyleBai, H., Jin, H., Liu, T., Yin, Y., Wang, H., Ruan, S., Li, Y., & Wang, Z. (2025). Luteolin Alleviates Vascular Senescence Through Retinoic Acid–Peroxisome Proliferator-Activated Receptor Signaling and Lipid Metabolism Remodeling Combined with Multi-Omics Analysis. Nutrients, 17(22), 3607. https://doi.org/10.3390/nu17223607






