The Influence of Berberine on Vascular Function Parameters, Among Them VEGF, in Individuals with MAFLD: A Double-Blind, Randomized, Placebo-Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
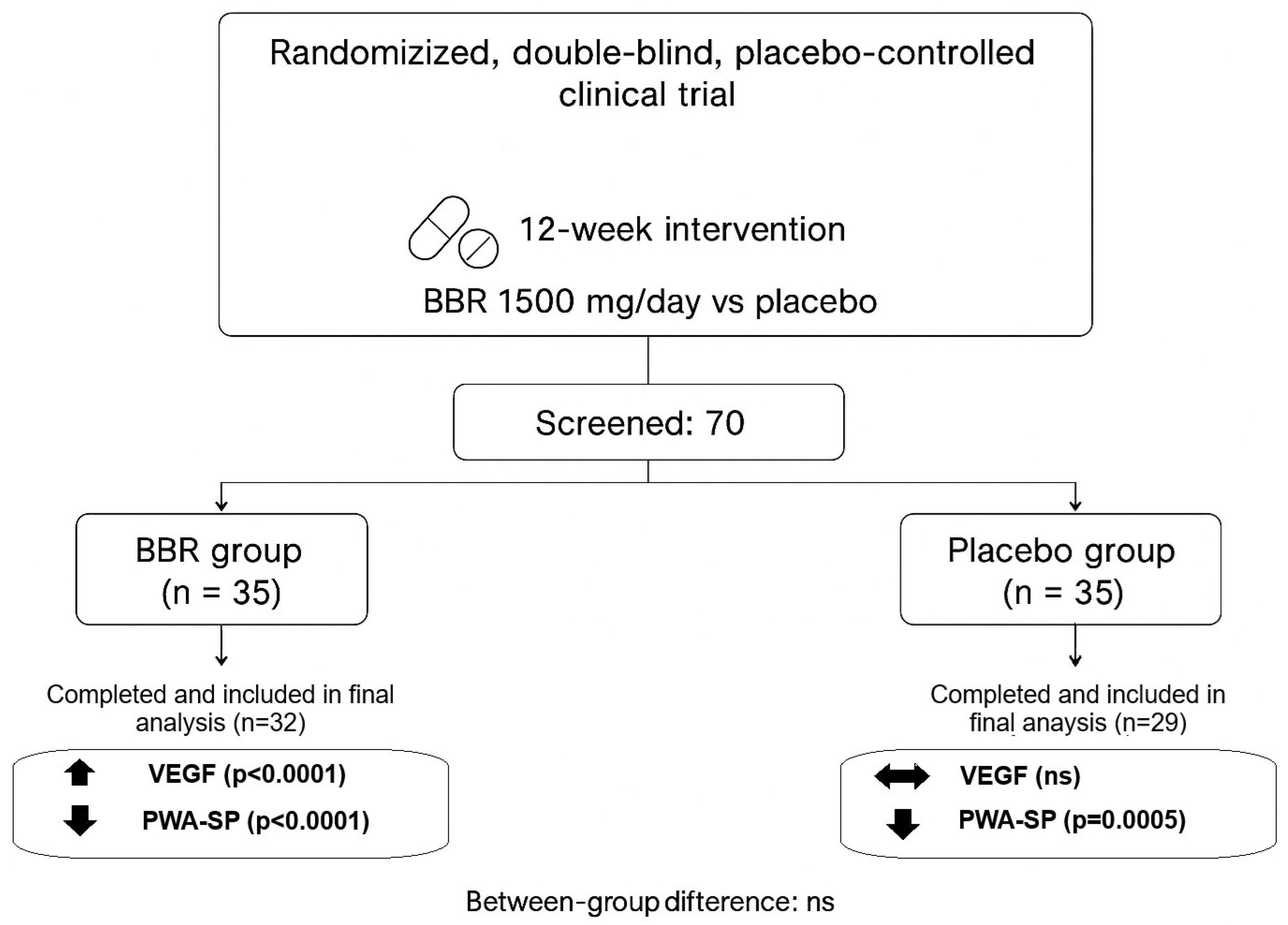
2.1. The Study Design
2.1.1. Participants
2.1.2. Inclusion Criteria
- Diagnosis of MAFLD (as per the 2020 criteria [9]), which requires hepatic steatosis plus at least one of the following: overweight/obesity or metabolic dysregulation in lean individuals. Hepatic steatosis was confirmed via ultrasound (patients were enrolled based on a prior diagnosis of hepatic steatosis, confirmed by ultrasound examination).
- Body Mass Index (BMI) between 27.0 kg/m2 and 34.9 kg/m2
- Abdominal obesity, defined by waist circumference >80 cm for women and >94 cm for men according to the International Diabetes Federation [10].
- Age between 40 and 60 years.
- Women who were at least one year post-menopause.
- Stable body weight for the three months prior to the study (within ±3 kg).
2.1.3. Exclusion Criteria
- A history of following alternative diets in the three months before the study.
- Use of dietary supplements within three months before the study.
- Recent intake (past three months) of antibiotics, probiotics, or prebiotics.
- Secondary obesity, prior bariatric surgery, or pharmacological treatment for obesity within three months of the study.
- Other liver diseases, including high risk of NASH (Fibrosis-4 (FIB-4) Index for Liver Fibrosis > 2.67), autoimmune hepatitis, hepatitis B or C, toxic hepatitis, cirrhosis, Wilson’s disease, or hemochromatosis.
- Gastrointestinal disorders such as inflammatory bowel disease (IBD), celiac disease, gastritis, duodenitis, pancreatic disorders, or symptoms indicative of irritable bowel syndrome (IBS).
- Acute inflammatory conditions with elevated high-sensitivity C-reactive protein (hsCRP).
- Impaired kidney function—Glomerular Filtration Rate (GFR) (<60 mL/min/1.73 m2).
- Type 2 diabetes mellitus (T2DM).
- Dyslipidemia or hypertension requiring the initiation or modification of pharmacological treatment within six months before the study or during the intervention. First-degree hypertension managed with a single medication and dyslipidemia treated with monotherapy (excluding statins) were acceptable.
- Chronic pharmacotherapy, including nonsteroidal anti-inflammatory drugs (NSAIDs), proton pump inhibitors, anticoagulants, or medications known to affect metabolism (e.g., second-generation antipsychotics).
- Conditions requiring specific dietary management or long-term supplementation.
- Alcohol consumption exceeding 30 g/day for men or 20 g/day for women, nicotine dependence, or substance abuse.
- Mental health disorders, including eating disorders.
- Cancer or autoimmune diseases.
- Use of hormone replacement therapy (HRT) at the time of study enrollment or during the study period.
- Any condition that could interfere with study outcomes or pose a risk to participants’ health.
2.1.4. Participant Allocation and Berberine Supplement
2.1.5. Adverse Effect, Safety, and Compliance
2.1.6. Safety Profile
2.1.7. Medical Data
2.1.8. Diet and Physical Activity Evaluation
2.1.9. Anthropometric Measurements
2.1.10. Calculated Parameters—Formulae
2.1.11. Functional Parameters
2.1.12. Laboratory Tests
2.2. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AIx | Augmentation Index |
| AMPK | AMP-Activated Protein Kinase |
| AP | Aortic Pressure |
| BBR | Berberine |
| BECs | Biliary Epithelial Cells |
| BMI | Body Mass Index |
| BSE | Bovine Spongiform Encephalopathy |
| CAS | Chemical Abstracts Service |
| CD34+ | Cluster of Differentiation 34 Positive Cells |
| CFU | Colony-Forming Unit |
| DBP | Diastolic Blood Pressure |
| FFQ-6 | Food Frequency Questionnaire |
| FIB-4 | Fibrosis-4 Index for Liver Fibrosis |
| FLI | Fatty Liver Index |
| GFR | Glomerular Filtration Rate |
| GMO | Genetically Modified Organism |
| HRT | Hormone Replacement Therapy |
| HSC | Hematopoietic Stem Cell |
| hsCRP | high sensitivity C-Reactive Protein |
| ICAM-1 | Intercellular Adhesion Molecule-1 |
| IDF | International Diabetes Federation |
| IBD | Inflammatory Bowel Disease |
| IBS | Irritable Bowel Syndrome |
| IPAQ | International Physical Activity Questionnaire |
| MAP | Mean Arterial Pressure |
| MAFLD | Metabolically Associated Fatty Liver Disease |
| MAPK | Mitogen-Activated Protein Kinase |
| MCP-1 | Monocyte Chemoattractant Protein-1 |
| MASH | Metabolic Dysfunction-Associated Steatohepatitis |
| mmHg | Millimeters of Mercury |
| m/s | Meters per Second |
| NAFLD | Non-Alcoholic Fatty Liver Disease |
| NF-κB | Nuclear Factor Kappa-Light-Chain-Enhancer of Activated B Cells |
| NASH | Non-Alcoholic Steatohepatitis |
| NO | Nitric Oxide |
| NSAIDs | Nonsteroidal Anti-Inflammatory Drugs |
| p | p-Value |
| PPARγ | Peroxisome Proliferator-Activated Receptor Gamma |
| PP | Pulse Pressure |
| PI3K/Akt | Phosphoinositide 3-kinase/Protein kinase B |
| PWA | Pulse Wave Analysis |
| PWA-DP | Pulse Wave Analysis–Diastolic Pressure |
| PWA-SP | Pulse Wave Analysis–Systolic Pressure |
| PWV | Pulse Wave Velocity |
| r | Spearman Correlation Index |
| ROS | Reactive Oxygen Species |
| SBP | Systolic Blood Pressure |
| SD | Standard Deviation |
| SIRT1 | Sirtuin 1 |
| Stem Enhance | Stem Cell Mobilizer Supplement derived from Aphanizomenon flos-aquae |
| T2DM | Type 2 Diabetes Mellitus |
| TSE | Transmissible Spongiform Encephalopathy |
| VCAM-1 | Vascular Cell Adhesion Molecule-1 |
| VEGF | Vascular Endothelial Growth Factor |
| VEGF-R1 | Vascular Endothelial Growth Factor Receptor 1 |
| VEGF-R2 | Vascular Endothelial Growth Factor Receptor 2 |
| VEGF-R3 | Vascular Endothelial Growth Factor Receptor 3 |
| WC | Waist Circumference |
References
- Koperska, A.; Moszak, M.; Seraszek-Jaros, A.; Bogdanski, P.; Szulinska, M. Does berberine impact anthropometric, hepatic, and metabolic parameters in patients with metabolic dysfunction-associated fatty liver disease? Randomized, double-blind, placebo-controlled trial. J. Physiol. Pharmacol. 2024, 75, 291–302. [Google Scholar]
- Theofilis, P.; Vordoni, A.; Nakas, N.; Kalaitzidis, R.G. Endothelial Dysfunction in Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Life 2022, 12, 718. [Google Scholar] [CrossRef] [PubMed]
- Nijhawans, P.; Behl, T.; Bhardwaj, S. Angiogenesis in obesity. Biomed. Pharmacother. 2020, 126, 110103. [Google Scholar] [CrossRef]
- Nasiri-Ansari, N.; Androutsakos, T.; Flessa, C.-M.; Kyrou, I.; Siasos, G.; Randeva, H.S.; Kassi, E.; Papavassiliou, A.G. Endothelial Cell Dysfunction and Nonalcoholic Fatty Liver Disease (NAFLD): A Concise Review. Cells 2022, 11, 2511. [Google Scholar] [CrossRef]
- Koperska, A.; Wesołek, A.; Moszak, M.; Szulińska, M. Berberine in Non-Alcoholic Fatty Liver Disease—A Review. Nutrients 2022, 14, 3459. [Google Scholar] [CrossRef]
- Bellavite, P.; Fazio, S.; Affuso, F. A Descriptive Review of the Action Mechanisms of Berberine, Quercetin and Silymarin on Insulin Resistance/Hyperinsulinemia and Cardiovascular Prevention. Molecules 2023, 28, 4491. [Google Scholar] [CrossRef]
- Xing, L.; Zhou, X.; Li, A.H.; Li, H.J.; He, C.X.; Qin, W.; Zhao, D.; Li, P.-Q.; Zhu, L.; Cao, H.-L. Atheroprotective Effects and Molecular Mechanism of Berberine. Front. Mol. Biosci. 2021, 8, 762673. [Google Scholar] [CrossRef]
- Rui, R.; Yang, H.; Liu, Y.; Zhou, Y.; Xu, X.; Li, C.; Liu, S. Effects of Berberine on Atherosclerosis. Front. Pharmacol. 2021, 12, 764175. [Google Scholar] [CrossRef]
- Eslam, M.; Newsome, P.N.; Sarin, S.K.; Anstee, Q.M.; Targher, G.; Romero-Gomez, M.; Zelber-Sagi, S.; Wong, V.W.-S.; Dufour, J.-F.; Schattenberg, J.M.; et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 2020, 73, 202–209. [Google Scholar] [CrossRef]
- Alberti, K.G.M.M.; Zimmet, P.; Shaw, J. Metabolic syndrome—A new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet. Med. 2006, 23, 469–480. [Google Scholar] [CrossRef]
- Szulińska, M.; Łoniewski, I.; Skrypnik, K.; Sobieska, M.; Korybalska, K.; Suliburska, J.; Bogdański, P. Multispecies Probiotic Supplementation Favorably Affects Vascular Function and Reduces Arterial Stiffness in Obese Postmenopausal Women—A 12-Week Placebo-Controlled and Randomized Clinical Study. Nutrients 2018, 10, 1672. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Song, M. New Insights into the Pathogenesis of Metabolic-Associated Fatty Liver Disease (MAFLD): Gut–Liver–Heart Crosstalk. Nutrients 2023, 15, 3970. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Ei Mourabit, H.; Housset, C.; Cadoret, A.; Lemoinne, S. Role of Angiogenesis in the Pathogenesis of NAFLD. J. Clin. Med. 2021, 10, 1338. [Google Scholar] [CrossRef]
- Lefere, S.; Van De Velde, F.; Devisscher, L.; Bekaert, M.; Raevens, S.; Verhelst, X.; Van Nieuwenhove, Y.; Praet, M.; Hoorens, A.; Van Steenkiste, C.; et al. Serum vascular cell adhesion molecule-1 predicts significant liver fibrosis in non-alcoholic fatty liver disease. Int. J. Obes. 2017, 41, 1207–1213. [Google Scholar] [CrossRef]
- Rizvi, F.; Lee, Y.R.; Diaz-Aragon, R.; Bawa, P.S.; So, J.; Florentino, R.M.; Wu, S.; Sarjoo, A.; Truong, E.; Smith, A.R.; et al. VEGFA mRNA-LNP promotes biliary epithelial cell-to-hepatocyte conversion in acute and chronic liver diseases and reverses steatosis and fibrosis. Cell Stem Cell 2023, 30, 1640–1657.e8. [Google Scholar] [CrossRef]
- Papageorgiou, M.V. Serum levels of vascular endothelial growth factor in non-alcoholic fatty liver disease. Ann. Gastroenterol. 2017, 30, 209–216. [Google Scholar] [CrossRef]
- Dai, L.; Zhu, L.; Ma, S.; Liu, J.; Zhang, M.; Li, J.; Luo, Y.; Zhou, X.; Chen, Q.; Wang, L.; et al. Berberine alleviates NLRP3 inflammasome induced endothelial junction dysfunction through Ca2+ signalling in inflammatory vascular injury. Phytomedicine 2022, 101, 154131. [Google Scholar] [CrossRef]
- Almowallad, S.; Al-Massabi, R. Berberine modulates cardiovascular diseases as a multitarget-mediated alkaloid with insights into its downstream signals using in silico prospective screening approaches. Saudi J. Biol. Sci. 2024, 31, 103977. [Google Scholar] [CrossRef]
- Wan, Q.; Liu, Z.; Yang, Y.; Cui, X. Suppressive effects of berberine on atherosclerosis via downregulating visfatin expression and attenuating visfatin-induced endothelial dysfunction. Int. J. Mol. Med. 2018, 41, 1939–1948. [Google Scholar] [CrossRef]
- Zhang, G.; Lin, X.; Shao, Y.; Su, C.; Tao, J.; Liu, X. Berberine reduces endothelial injury and arterial stiffness in spontaneously hypertensive rats. Clin. Exp. Hypertens. 2020, 42, 257–265. [Google Scholar] [CrossRef]
- Xu, M.G.; Wang, J.M.; Chen, L.; Wang, Y.; Yang, Z.; Tao, J. Berberine-induced mobilization of circulating endothelial progenitor cells improves human small artery elasticity. J. Hum. Hypertens. 2008, 22, 389–393. [Google Scholar] [CrossRef] [PubMed]
- El-Akabawy, G.; El-Mehi, A. Mobilization of endogenous bone marrow-derived stem cells in a thioacetamide-induced mouse model of liver fibrosis. Tissue Cell 2015, 47, 257–265. [Google Scholar] [CrossRef]
- Pugh, N.; Ross, S.A.; ElSohly, H.N.; ElSohly, M.A.; Pasco, D.S. Isolation of three high molecular weight polysaccharide preparations with potent immunostimulatory activity from Spirulina platensis, aphanizomenon flos-aquae and Chlorella pyrenoidosa. Planta Med. 2001, 67, 737–742. [Google Scholar] [CrossRef]
| Parameter | Berberine Supplement (BBR) | Placebo |
|---|---|---|
| Safety compliance | Compliant with all regulatory safety standards | Compliant with all regulatory safety standards |
| Heavy metal content | ||
| Lead | ≤3 ppm (µg/g) | ≤3 ppm (µg/g) |
| Arsenic | ≤1 ppm (µg/g) | ≤1 ppm (µg/g) |
| Cadmium | ≤1 ppm (µg/g) | ≤1 ppm (µg/g) |
| Mercury | ≤0.1 ppm (µg/g) | ≤0.1 ppm (µg/g) |
| Residual pesticides | Not applicable | Not applicable |
| Polycyclic Aromatic hydrocarbons | ||
| Benzo(a)pyrene | ≤10 ppb (µg/kg) | ≤10 ppb (µg/kg) |
| PAH4 (sum of 4 PAHs) | ≤50 ppb (µg/kg) | ≤50 ppb (µg/kg) |
| Microbiological testing | ||
| Total aerobic microbial count | ≤5000 cfu/g | ≤5000 cfu/g |
| Yeasts and molds | ≤100 cfu/g | ≤100 cfu/g |
| Escherichia coli | Negative/10 g | Negative/10 g |
| Salmonella | Negative/25 g | Negative/25 g |
| Staphylococcus aureus | Negative/10 g | Negative/10 g |
| Pseudomonas aeruginosa | Negative/10 g | Negative/10 g |
| Bile-tolerant Gram-negative bacteria | Negative/10 g | Negative/10 g |
| Coliforms | <10 cfu/g | <10 cfu/g |
| Additional Information | ||
| GMO status | Free from genetically modified organisms | Free from genetically modified organisms |
| BSE/TSE status | Free from BSE/TSE | Free from BSE/TSE |
| Dietary compatibility | Gluten-free, lactose-free, suitable for vegans | Gluten-free, lactose-free, suitable for vegans |
| Manufacturing and supply | ||
| Manufacturer | Sami Labs Ltd., Bangalore, India | Standard Sp. z o.o., Lublin, Poland |
| Supplier | Sabinsa Poland Sp. z o.o., Lubon, Poland | Not applicable |
| Substance name | Berberine extract | Potato starch |
| Chemical formula | C20H18NO4 | (C6H10O5)n |
| CAS number | 2086-83-1 | 9005-84-9 |
| Molar mass | 336.36 g/mol | Not specified (polymer) |
| Variable | BBR (n = 32) | Mean ± SD | Median | Min–Max | PLACEBO (n = 29) | Mean ± SD | Median | Min–Max | p (Between Groups) |
|---|---|---|---|---|---|---|---|---|---|
| Age | 49.09 ± 5.00 | 50.0 | 40.00–59.00 | 53 ± 6.25 | 52.5 | 42.00–60.00 | 0.0175 | ||
| BMI [kg/m2] | 32.22 ± 3.46 | 31.7 | 27.80–35.90 | 30.90 ± 3.55 | 29.8 | 27.80–34.80 | 0.0975 | ||
| VEGF [pg/mL] | 456.23 ± 307.61 | 402.26 | 110.45–1739.65 | 414.51 ± 230.48 | 359.32 | 25.25–882.96 | 0.8569 | ||
| PWA-SP [mmHg] | 134.85 ± 16.26 | 133.00 | 110.00–175.00 | 132.29 ± 12.65 | 133.00 | 105.00–154.00 | 0.4870 * | ||
| PWA-DP [mmHg] | 83.61 ± 9.17 | 83.00 | 68.00–100.00 | 80.81 ± 11.14 | 81.00 | 56.00–106.00 | 0.5970 * | ||
| Brachial SBP [mmHg] | 135.63 ± 16.11 | 134.00 | 110.00–175.00 | 133.32 ± 13.80 | 133.50 | 105.00–165.00 | 0.7213 | ||
| Brachial DBP [mmHg] | 84.00 ± 9.04 | 84.00 | 68.00–100.00 | 81.89 ± 10.80 | 82.50 | 56.00–106.00 | 0.3944 | ||
| MAP | 100.74 ± 10.34 | 101.00 | 84.00–118.00 | 99.74 ± 11.31 | 99.50 | 77.00–125.00 | 0.6887 | ||
| PP | 40.11 ± 9.68 | 39.00 | 28.00–63.00 | 39.97 ± 9.28 | 39.00 | 17.00–63.00 | 0.7880 | ||
| AP | 13.34 ± 7.37 | 12.00 | 0.00–34.00 | 15.03 ± 10.46 | 12.50 | −2.00–52.00 | 0.6487 | ||
| AIx | 31.57 ± 13.72 | 29.00 | 0.00–66.00 | 35.26 ± 22.70 | 35.00 | −10.00–131.00 | 0.3883 | ||
| PWV [m/s] | 7.31 ± 1.31 | 7.00 | 5.00–10.40 | 6.89 ± 2.01 | 6.90 | 2.30–12.30 | 0.2635 | ||
| hsCRP | 0.32 ± 0.24 | 0.27 | 0.04–1.02 | 0.30 ± 0.30 | 0.22 | 0.03–1.58 | 0.3952 | ||
| FLI | 68.49 ± 25.58 | 75.90 | 16.20–99.30 | 58.18 ± 25.24 | 55.60 | 16.00–97.90 | 0.0948 |
| Parameter | BBR (n = 32) | Mean ± SD | Median | Min–Max | Placebo (n = 29) | Mean ± SD | Median | Min–Max | p (Between Groups) |
|---|---|---|---|---|---|---|---|---|---|
| VEGF [pg/mL] | 561.22 ± 389.77 | 522.84 | 149.12–2037.87 | 421.79 ± 270.15 | 362.11 | 102.19–1352.21 | 0.0638 | ||
| PWA-SP [mmHg] | 124.46 ± 13.47 | 124.00 | 98.00–154.00 | 122.66 ± 14.82 | 119.00 | 101.00–167.00 | 0.8102 | ||
| PWA-DP [mmHg] | 85.20 ± 10.41 | 83.00 | 58.00–115.00 | 83.23 ± 10.38 | 80.00 | 64.00–116.00 | 0.1976 | ||
| Brachial SBP [mmHg] | 134.49 ± 14.79 | 133.00 | 107.00–168.00 | 132.26 ± 17.32 | 128.00 | 107.00–185.00 | 0.3618 | ||
| Brachial DBP [mmHg] | 84.31 ± 10.58 | 82.00 | 56.00–115.00 | 82.06 ± 10.11 | 80.00 | 64.00–116.00 | 0.1493 | ||
| MAP | 100.54 ± 11.50 | 100.00 | 74.00–132.00 | 98.69 ± 11.05 | 96.00 | 85.00–133.00 | 0.2714 | ||
| PP | 39.34 ± 8.90 | 39.00 | 21.00–65.00 | 39.69 ± 10.73 | 38.00 | 25.00–72.00 | 0.7974 | ||
| Ap | 13.29 ± 6.68 | 12.00 | 3.00–32.00 | 14.88 ± 12.02 | 11.00 | −2.00–65.00 | 0.9354 | ||
| AIx | 32.54 ± 11.62 | 33.00 | 10.00–56.00 | 35.56 ± 23.47 | 33.00 | −7.00–129.00 | 0.6400 | ||
| PWV [m/s] | 7.61 ± 1.09 | 7.50 | 6.10–11.20 | 6.77 ± 2.03 | 6.60 | 6.70–10.10 | 0.7801 | ||
| hsCRP | 0.39 ± 0.42 | 0.28 | 0.03–2.00 | 0.26 ± 0.21 | 0.15 | 0.03–0.71 | 0.2633 | ||
| FLI | 67.4 ± 25.68 | 78.10 | 14.20–98.60 | 61.70 ± 25.61 | 67.10 | 17.21–100.00 | 0.3681 |
| BBR | Placebo | |||||
|---|---|---|---|---|---|---|
| Mean ± SD | p-Value | Mean ± SD | p-Value | |||
| Before | After | Before | After | |||
| VEGF [pg/mL] | 456.23 ± 307.61 | 561.22 ± 389.77 | <0.0001 | 414.51 ± 230.48 | 421.79 ± 270.15 | 0.3591 |
| PWA-SP [mmHg] | 134.85 ± 16.26 | 124.46 ± 13.47 | <0.0001 * | 132.29 ± 12.65 | 122.66 ± 14.82 | 0.0005 |
| PWA-DP [mmHg] | 83.61 ± 9.17 | 85.2 ± 10.41 | 0.5866 * | 80.81 ± 11.14 | 83.23 ± 10.38 | 0.2301 |
| Brachial SBP [mmHg] | 135.63 ± 16.11 | 134.49 ± 14.79 | 0.4167 | 133.32 ± 13.80 | 132.26 ± 17.32 | 0.5071 |
| Brachial DBP [mmHg] | 84.00 ± 9.04 | 84.31 ± 10.58 | 0.9869 | 81.89 ± 10.80 | 82.06 ± 10.11 | 1 |
| MAP | 100.74 ± 10.34 | 100.54 ± 11.50 | 0.9288 * | 99.74 ± 11.31 | 98.69 ± 11.05 | 0.4082 |
| PP | 40.11 ± 9.68 | 39.34 ± 8.90 | 0.4022 | 39.97 ± 9.28 | 39.69 ± 10.73 | 0.9935 |
| AP | 13.34 ± 7.37 | 13.29 ± 6.68 | 0.7791 | 15.03 ± 10.46 | 14.88 ± 12.02 | 0.8457 |
| AIx | 31.57 ± 13.72 | 32.54 ± 11.62 | 0.7477 | 35.26 ± 22.70 | 35.56 ± 23.47 | 0.7223 |
| PWV [m/s] | 7.31 ± 1.31 | 7.61 ± 1.09 | 0.0984 | 6.89 ± 2.01 | 6.77 ± 2.03 | 0.8122 |
| hsCRP | 0.32 ± 0.24 | 0.39 ± 0.42 | 0.4237 | 0.30 ± 0.30 | 0.26 ± 0.21 | 0.4549 |
| FLI | 68.49 ± 25.58 | 67.4 ± 25.68 | 0.9766 | 58.18 ± 25.24 | 61.70 ± 25.61 | 0.6766 |
| Parameter | BBR (n = 32) | Berberine Mean ± SD | Berberine Median (Min–Max) | Placebo (n = 29) | Placebo Mean ± SD | Placebo Median (Min–Max) | p-Value (Between Groups) |
|---|---|---|---|---|---|---|---|
| ΔVEGF [pg/mL] | 104.99 ± 111.76 | 73.64 (−89.28–454.66) | 76.68 ± 125.17 | 57.27 (−62.68–548.02) | 0.1090 | ||
| ΔBrachial SBP [mmHg] | −1.14 ± 13.52 | −1.00 (−27.00–44.00) | −1.51 ± 13.44 | −1.00 (−33.00–24.00) | 0.9085 * | ||
| ΔBrachial DBP [mmHg] | 0.31 ± 9.90 | −2.00 (−19.00–27.00) | 0.09 ± 7.95 | −1.00 (−15.00–18.00) | 0.9155 * | ||
| ΔPWV [m/s] | 0.29 ± 0.88 | 0.25 (−1.30–2.00) | −0.11 ± 2.03 | −0.70 (−6.40–3.00) | 0.6121 * | ||
| ΔPWA-SP [mmHg] | −0.40 ± 11.62 | −1.00 (−23.00–38.00) | −1.06 ± 10.13 | −1.00 (−28.00–19.00) | 0.8017 * | ||
| ΔPWA-DP [mmHg] | 0.43 ± 9.64 | −1.00 (−18.00–24.00) | −0.14 ± 8.47 | 0.00 (−16.00–19.00) | 0.7930 | ||
| ΔMAP | 0.29 ± 9.52 | −1.50 (−20.00–20.00) | −1.29 ± 9.43 | −1.00 (−16.00–16.00) | 0.4910 * | ||
| ΔPP | −0.77 ± 11.05 | −2.00 (−22.00–37.00) | −0.60 ± 9.03 | −1.00 (−34.00–14.00) | 0.5912 | ||
| ΔAP | −0.06 ± 6.85 | 0.00 (−13.00–22.00) | −0.34 ± 13.76 | 0.50 (−43.00–51.00) | 0.7600 | ||
| ΔAIx | 0.97 ± 11.59 | 0.00 (−19.00–30.00) | 0.43 ± 14.79 | 2.00 (−32.00–36.00) | 0.9012 | ||
| ΔhsCRP | −0.07 ± 0.46 | 0.02 (−1.85–0.68) | −0.02 ± 0.13 | 0.00 (−0.41–0.32) | 0.2560 | ||
| ΔFLI | −0.46 ± 12.54 | −0.10 (−31.9–26.00) | −1.76 ± 11.11 | 0.25 (−30.90–18.77) | 0.9479 |
| Parameter | d (Within BBR) | d (Within Placebo) | d (Between Groups) |
|---|---|---|---|
| ΔVEGF [pg/mL] | 0.94 | 0.61 | 0.24 |
| ΔBrachial SBP [mmHg] | −0.08 | −0.11 | 0.03 |
| ΔBrachial DBP [mmHg] | 0.03 | 0.01 | 0.02 |
| ΔPWV [m/s] | 0.33 | −0.05 | 0.26 |
| ΔPWA-SP [mmHg] | −0.03 | −0.10 | 0.06 |
| ΔPWA-DP [mmHg] | 0.05 | −0.02 | 0.06 |
| ΔMAP [mmHg] | 0.03 | −0.14 | 0.17 |
| ΔPP [mmHg] | −0.07 | −0.07 | −0.02 |
| ΔAP [mmHg] | −0.01 | −0.03 | 0.03 |
| ΔAIx | 0.08 | 0.03 | 0.04 |
| ΔhsCRP | −0.15 | −0.15 | −0.15 |
| ΔFLI | −0.04 | −0.16 | 0.11 |
| Parameter | Placebo ΔVEGF (r) | Placebo ΔPWV (r) | BBR ΔVEGF (r) | BBR ΔPWV (r) |
|---|---|---|---|---|
| ΔFat mass corp [kg] | −0.34 | −0.29 | 0.18 | −0.19 |
| ΔFLI | −0.30 | −0.09 | −0.42 | 0.09 |
| ΔhsCRP | −0.43 | 0.18 | −0.01 | −0.07 |
| ΔBrachial SBP [mmHg] | 0.06 | −0.18 | −0.28 | 0.36 |
| ΔBrachial DBP [mmHg] | 0.14 | −0.09 | −0.20 | 0.51 |
| ΔNumber of waveforms | −0.07 | 0.03 | 0.05 | 0.00 |
| ΔPWA-SP [mmHg] | −0.08 | −0.33 | −0.24 | 0.42 |
| ΔPWA-DP [mmHg] | 0.09 | −0.23 | −0.20 | 0.50 |
| ΔMAP | −0.13 | −0.23 | −0.17 | 0.51 |
| ΔPP | −0.16 | −0.22 | −0.08 | −0.16 |
| ΔAp | −0.24 | −0.11 | 0.10 | −0.23 |
| ΔAIx | −0.16 | 0.02 | 0.28 | −0.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koperska, A.; Miller-Kasprzak, E.; Seraszek-Jaros, A.; Musialik, K.; Bogdański, P.; Szulińska, M. The Influence of Berberine on Vascular Function Parameters, Among Them VEGF, in Individuals with MAFLD: A Double-Blind, Randomized, Placebo-Controlled Trial. Nutrients 2025, 17, 3585. https://doi.org/10.3390/nu17223585
Koperska A, Miller-Kasprzak E, Seraszek-Jaros A, Musialik K, Bogdański P, Szulińska M. The Influence of Berberine on Vascular Function Parameters, Among Them VEGF, in Individuals with MAFLD: A Double-Blind, Randomized, Placebo-Controlled Trial. Nutrients. 2025; 17(22):3585. https://doi.org/10.3390/nu17223585
Chicago/Turabian StyleKoperska, Anna, Ewa Miller-Kasprzak, Agnieszka Seraszek-Jaros, Katarzyna Musialik, Paweł Bogdański, and Monika Szulińska. 2025. "The Influence of Berberine on Vascular Function Parameters, Among Them VEGF, in Individuals with MAFLD: A Double-Blind, Randomized, Placebo-Controlled Trial" Nutrients 17, no. 22: 3585. https://doi.org/10.3390/nu17223585
APA StyleKoperska, A., Miller-Kasprzak, E., Seraszek-Jaros, A., Musialik, K., Bogdański, P., & Szulińska, M. (2025). The Influence of Berberine on Vascular Function Parameters, Among Them VEGF, in Individuals with MAFLD: A Double-Blind, Randomized, Placebo-Controlled Trial. Nutrients, 17(22), 3585. https://doi.org/10.3390/nu17223585






