1. Introduction
Malnutrition is a critical global health concern, especially among older adults facing age-related physiological, social, and health challenges. Factors contributing to malnutrition include reduced appetite, dietary inadequacy, and social isolation, leading to poor intake of energy and key nutrients such as protein, calcium, zinc, and folate [
1]. These challenges, compounded by social determinants of health, result in increased healthcare utilization, including primary care visits, hospitalizations, and emergency consultations, further increasing the burden of malnutrition on the healthcare system [
2]. Malnutrition is a leading cause of functional decline, delayed recovery, and increased mortality among older adults, particularly those with sarcopenia and frailty. A meta-analysis found that malnutrition increased the risk of rehospitalization by 33%, mortality nearly threefold, and functional decline by 45%, while prolonging hospital stays by an average of two days [
3].
Across diverse healthcare settings globally, timely nutritional interventions, including the use of oral nutrition supplements (ONSs), have been shown to mitigate these impacts by improving clinical outcomes and reducing healthcare resource use. Specialized ONS formulations enriched with β-hydroxy-β-methylbutyrate (HMB), a leucine metabolite that promotes protein synthesis and limits muscle breakdown [
4,
5], have demonstrated efficacy in preserving muscle mass, enhancing physical function, and improving quality of life among older adults with malnutrition or at risk of malnutrition [
6,
7].
Randomized controlled trials have reported that HMB-ONS improves muscle strength, handgrip strength, and functional outcomes, as shown in the SHIELD study among community-dwelling older adults at risk of malnutrition [
8] and in the NOURISH trial among hospitalized older adults with malnutrition and COPD [
9,
10].
Observational studies from Europe have similarly highlighted the benefits of ONS and HMB-enriched supplementation, demonstrating improvements in recovery and functional outcomes in older adults following acute hospitalization and surgery [
11,
12]. Nonetheless, ONS remains underutilized globally [
13,
14], representing a missed opportunity to improve outcomes among high-risk patient populations.
Meta-analyses further support the role of HMB in improving muscle strength and physical performance among older adults and sarcopenic populations, particularly when combined with exercise [
15,
16]. However, there is limited real-world evidence on the effectiveness of HMB-ONS in hospitalized older adults globally and within Israel, where malnutrition-related healthcare challenges remain underexplored.
This study aimed to address this gap by evaluating the association between the use of HMB-ONS, hospital readmission rates, and healthcare costs among hospitalized patients with malnutrition or at risk of malnutrition in Israel. Using six years of hospitalization data from two large tertiary care hospitals, this analysis examined the potential of specialized nutritional interventions to reduce the burden of malnutrition and improve resource utilization in high-risk patient populations.
2. Methods
This study was a retrospective analysis of electronic medical records from 1 January 2015 to 31 December 2021 at two large tertiary hospitals in Israel affiliated with the Clalit Health Maintenance Organization (HMO): Rabin Medical Center–Beilinson Hospital, and Hasharon Hospital. Both hospitals are located within the same metropolitan area and adhere to uniform clinical nutrition policies, protocols, and ONS formulations. Institutional prescribing patterns are therefore highly aligned. The study population included hospitalized adult patients aged 18 and above with malnutrition or at risk of malnutrition, as identified based on clinical criteria, screening tools, and nutritional interventions.
Patients were included if they met at least one of the following criteria: received a recommendation for ONS during hospitalization; had a body mass index (BMI) of ≤20 kg/m
2 in accordance with the institution’s screening protocol and the European Society for Clinical Nutrition and Metabolism (ESPEN) guidelines [
17]; had a diagnosis of malnutrition based on International Classification of Diseases, Ninth Revision (ICD-9) codes; or had a Malnutrition Universal Screening Tool (MUST) score indicating malnutrition or risk of malnutrition.
To focus on patient populations that could tolerate ONS, exclusion criteria included patients receiving total parenteral nutrition (TPN), peripheral parenteral nutrition (PPN), or enteral nutrition (EN), and those admitted to intensive care units. Additionally, patients referred from or discharged to rehabilitation centers or nursing homes, pregnant women, and those who died during hospitalization were excluded. Of note, patients with end-stage metastatic cancer or a life expectancy of less than one year were not systematically excluded due to data limitations. Patients who were transferred within Rabin Medical Center were also excluded. Physical therapy was not part of the in-hospital nutritional intervention analyzed in this study. All included patients were hospitalized in acute-care wards, where any physiotherapy provided followed standard ward-based clinical practice rather than a predefined rehabilitation protocol. Since this analysis focused on general-purpose ONS formulations designed for malnourished polymorbid patients, patients on disease-specific ONS (e.g., diabetes- or renal-specific formulas) were excluded from the analysis to ensure a more uniform comparison.
Data extracted from the medical records included demographic, clinical, and nutritional data. Insurance coverage was defined in 3 categories: (1) Basic—provided by public health funds that included a standardized basket of services; (2) Standard—provided by the same public health funds as basic for an additional monthly fee that included an expanded choice of specialists and quicker access to surgeries; and (3) Extended—optional insurance purchased and offered full dental and optical care and wider elective surgery options. The primary objective was to compare hospital readmission rates at 1-, 3-, and 6-month post-discharge between patients receiving specialized ONS containing HMB (HMB-ONS) and those receiving standard ONS (S-ONS) during hospitalization; both groups received nutrition consultations from Registered Dietitians. Descriptive statistics were used for continuous data and categorical demographic data. Between-group comparisons were performed using the chi-squared test for categorical variables, the
t-test for continuous variables, and the Wilcoxon test for ordinal variables. Multivariate regression models—both linear and nonlinear—were employed based on the nature and distribution of the available data. To mitigate potential confounding due to baseline differences between groups, a propensity score matching (PSM) approach was utilized (
Table S1). Body mass index (BMI) was excluded from the matching process due to reliance on self-reported data. All statistical analyses were performed using SAS software (Version 9.4), with a significance threshold set at
p ≤ 0.05. The study protocol received ethical approval from the Rabin Medical Center Institutional Review Board (313-22-RMC), approved on 30 August 2022.
3. Results
From 2015 to 2021, there were a total of 391,838 hospitalizations of patients affiliated with Clalit HMO. Of those, 48,000 (12.2%) hospitalizations met the primary inclusion criteria for malnutrition. After excluding patients based on the predefined criteria, this study included a total of 16,751 patients.
3.1. Baseline Characteristics
The included patients were 50% female, with an average age of 72.0 ± 17.2 years and a median BMI of 24.5 kg/m
2 (IQR: 21.5–28). (
Table 1). The socioeconomic status (SES) of the patients varied, with the highest percentages in the middle SES brackets [
18]: SES 6 (3077/18.37%) and SES 7 (3072/18.34%). Insurance coverage among patients was categorized as basic (3160/18.86%), standard (7943/47.42%), and extended (5648/33.72%).
Most patients were admitted to internal medicine (52.8%), followed by surgery (30.4%), geriatrics (9.6%), and oncology (7.2%). Regarding primary diagnoses, the most common conditions were oncological (12.7%), respiratory (11.1%), gastrointestinal and liver diseases (9.6%), musculoskeletal disorders (7.2%), and cardiovascular diseases (6.4%). Nearly half of the patients (49.6%) were classified under the “other” category.
3.2. Comparison of Baseline and Hospitalization Characteristics Across ONS Groups
Baseline and hospitalization characteristics differ across ONS groups, especially between the ONS and non-ONS groups. Days of hospitalization were significantly higher in the ONS group compared with the non-ONS group (12.16 (±11.68) vs. 3.77 (±4.04) days,
p < 0.0001), though they were comparable between the HMB-ONS and S-ONS groups (11.06 (±9.60) vs. 11.09 (±10.49) days,
p = 0.947). The mean age was 78.25 years in the HMB-ONS group compared with 74.75 years in the S-ONS group and 72.76 years in the combined ONS group. The mean BMI was significantly lower in the HMB group (24.15 kg/m
2) compared with the non-ONS group (26.52 kg/m
2,
p < 0.0001). A higher proportion of patients in the HMB-ONS group were admitted to the geriatric department (33.5%) compared to 10.2% in the S-ONS group (
p < 0.0001). Standard and extended insurance coverages were slightly but significantly higher in the HMB-ONS group, with 83.7% of HMB-ONS patients, compared to 80.2% in the S-ONS group (
p = 0.0217) (
Table 1).
The average intake of HMB-ONS was 290.42 ± 124.21 mL/day, corresponding to an approximate Ca-HMB dose of 2 g/day. The median time from admission to first ONS provision was 3 days, reflecting early initiation of nutritional support during hospitalization. Both study products were isocaloric (330 kcal per 220 mL); however, protein content differed (HMB-ONS 20 g/220 mL ≈ 9 g/100 mL vs. S-ONS 14 g/220 mL ≈ 6.4 g/100 mL), thus volume aligned with energy intake but not with protein intake.
3.3. Readmission Rates for Patients with Malnutrition
Within six months post-discharge, among all patients with and without ONS use, 52.6% had no hospital readmissions, while 47.4% had at least one readmission. Readmission rates increased over time, with 27.09% readmitted at 1 month, 39.76% at 3 months, and 47.4% at 6 months post-discharge, highlighting the progressive risk of hospital readmission among patients with or without nutritional deficits (
Figure 1). As baseline and hospitalization characteristics differed substantially between ONS users and non-users, likely reflecting greater baseline clinical complexity and illness severity among those requiring ONS, we focused our analysis on a more comparable subset of patients who all received ONS, comparing the HMB-ONS and S-ONS groups. At 1 month, readmission rates were significantly lower in the HMB-ONS group (21.5%) compared with the S-ONS group (27.3%,
p = 0.0006). Similarly, at 3 months, 33.7% of patients in the HMB-ONS group were readmitted compared with 40.4% in the S-ONS group (
p = 0.0004). By 6 months, readmission rates in the HMB-ONS group remained lower than in the S-ONS group (41.0% vs. 48.1%,
p = 0.0002) (
Table 2).
3.4. Comparative Effectiveness
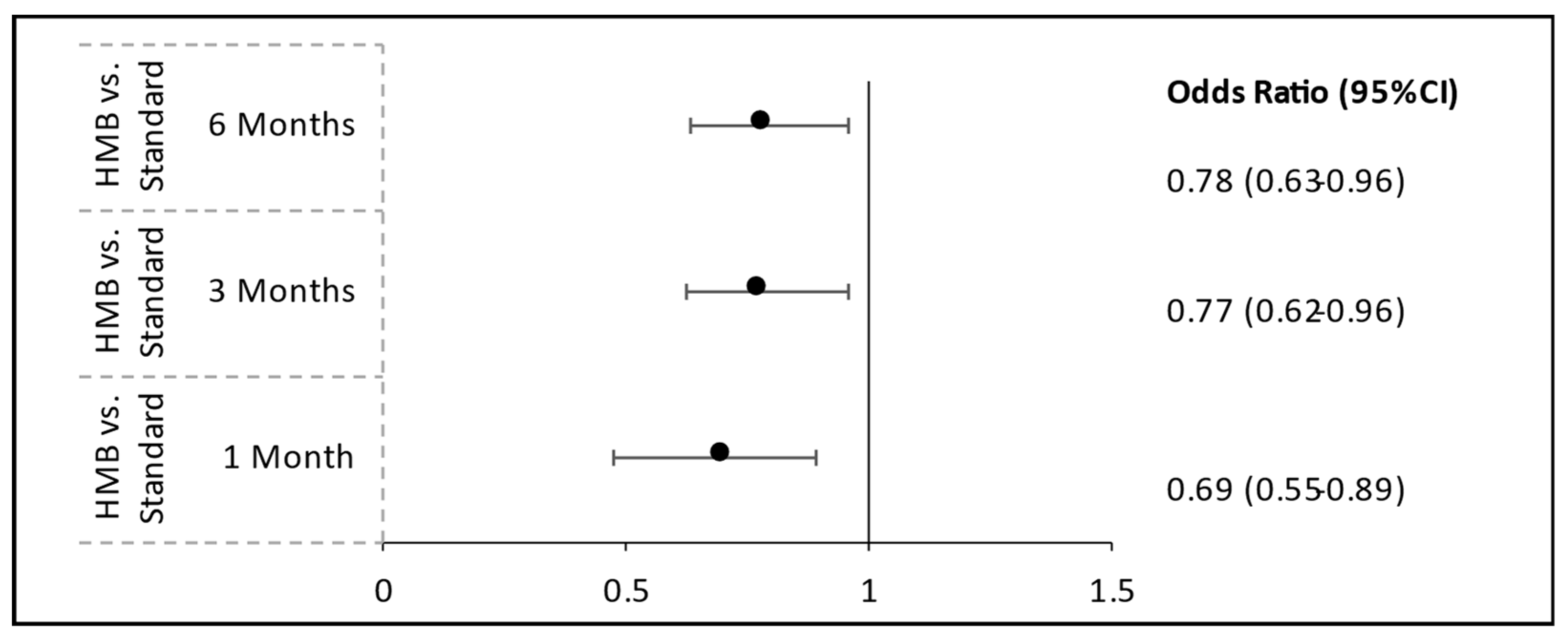
To reduce potential confounding from baseline differences between groups, we implemented PSM using key variables, including age, hospital department, volume of oral nutritional supplements administered, and length of hospital stay. A significant reduction in hospital readmission rates was observed among patients receiving HMB-ONS compared to those receiving S-ONS. At 1-month post-discharge, patients in the HMB-ONS group had significantly lower odds of readmission than those in the S-ONS group (odds ratio [OR] = 0.698; 95% confidence interval [CI]: 0.548–0.888;
p = 0.0034). This finding persisted at 3 months, where the odds of readmission were 22.8% lower in the HMB-ONS group (OR = 0.772; 95% CI: 0.623–0.958;
p = 0.0187). By 6 months, readmission rates remained significantly lower in the HMB-ONS group compared to the S-ONS group (OR = 0.780; 95% CI: 0.633–0.961;
p = 0.0195) (
Figure 2,
Table S1).
3.5. Cost Analysis
A cost analysis estimated the financial implications of using S-ONS or HMB-ONS during hospitalization. For this analysis, the S-ONS cost was EUR 2.33 (Euro) (USD 2.43 (United States Dollar)) (Ensure
® Plus, Abbott Laboratories), and the HMB-ONS cost was EUR 2.52 (USD 2.62) (Ensure
® Plus Advance, Abbott Laboratories). Assuming an average length of stay of 11 days (
Table 3), with patients consuming two bottles of ONS per day and receiving two dietitian consultation sessions, the cost of providing S-ONS and dietitian consultation to patients was EUR 327.6 (USD 340.86), while the cost of providing HMB-ONS and dietitian counseling was EUR 331.78 (USD 345.04). The difference between these amounts yielded an incremental cost of EUR 4.18 (USD 4.18) for HMB-ONS. Expected savings per patient from reduced readmissions were calculated by subtracting the expected cost of readmission using HMB-ONS from the expected cost of readmission using S-ONS. The average cost of hospitalization per day in Israel, based on Ministry of Health (MOH) tariffs, is EUR 804.88 (USD 837.08), and the average length of stay is 4.6 days. Using S-ONS, patients had a 6-month readmission rate of 48.1% (
Table 2), which resulted in an expected cost of readmission of EUR 1780.88 (USD1852.12). To calculate the expected cost of readmission using HMB-ONS, the S-ONS readmission rate was adjusted by the reduction in likelihood of readmission in the HMB-ONS group (0.78,
Figure 2), yielding an expected cost of readmission of EUR 1389.08 (USD 1444.66). Deducting the incremental cost of HMB-ONS from the expected savings due to reduced readmissions yielded net cost savings of EUR 387.61 per patient (USD 403.29).
4. Discussion
This is the first real-world study in Israel evaluating the potential clinical and economic impact of HMB-ONS in a large cohort of hospitalized patients with malnutrition or at risk of malnutrition. Using data from two tertiary care hospitals, this study highlighted that HMB-ONS supplementation was associated with reduced hospital readmission rates at 1-, 3-, and 6-month post-discharge by 30.2%, 22.8%, and 22.0%, respectively. Given that the expected cost of readmission is EUR 1780.88 (USD 1852.12), these reductions yielded an estimated per-patient cost savings of EUR 387.31 (USD 403.29).
4.1. Clinical and Functional Benefits of HMB-ONS
Malnutrition affects 3.4% of community-dwelling older adults in Israel, with ONS users representing a high-risk subgroup characterized by older age, chronic conditions, and greater healthcare utilization [
2]. This study’s findings align with prior research showing that the timely provision of ONS reduced 30-day readmissions by 38.8% among hospitalized adults with malnutrition in the United States, with oncology patients experiencing an even greater reduction of 46.1%, and earlier initiation shortening hospital length of stay by 10.2% [
13]. Similarly, a six-month nutritional therapy intervention in nutritionally at-risk older adults discharged from a geriatric unit in Iceland significantly reduced readmissions and shortened length of stay at all time points, with the largest difference observed at six months [
19].
While interpreting these associations, it is important to note that the study products were isocaloric (330 kcal/220 mL) but differed in protein content (HMB-ONS 20 g/220 mL vs. S-ONS 14 g/220 mL); therefore, volume-based comparisons align total energy intake but do not equate protein intake, and the observed associations may reflect both protein density and the HMB component.
Beyond hospital readmissions, HMB-ONS demonstrates substantial clinical benefits. In a Singapore-based study of community-dwelling older adults at risk of malnutrition, daily HMB-ONS intake improved survival without hospital readmission, muscle strength, calf circumference, and activities of daily living [
7]. Among hospitalized older adults with malnutrition and COPD in the United States, HMB-ONS use was associated with a 71% reduction in 90-day mortality, alongside significant improvements in handgrip strength and body weight [
9]. Additional studies in European and Latin American populations have confirmed gains in body weight, serum albumin levels, and handgrip strength [
11], as well as lean body mass, better physical function, and reduced mortality and hospital length of stay [
12]. HMB supplementation has also been linked to significant gains in handgrip strength (+4.7–6.2 kg) and fat-free mass, which are critical for recovery in patients with malnutrition [
20]. HMB is also known to preserve muscle mass and improve physical function in patients with cancer undergoing chemotherapy [
21,
22], with a meta-analysis demonstrating improvements in muscle strength and muscle mass across conditions like sarcopenia and cancer cachexia [
23].
4.2. Mechanistic Insights
Mechanistic studies show that HMB-ONS preserves muscle fiber cross-sectional area by activating the mTOR pathway and inhibiting proteolysis via the autophagy–lysosomal and ubiquitin–proteasome systems [
4,
5]. However, functional improvements have been modest in patients with cirrhosis, suggesting that disease-specific metabolic disruptions may limit HMB’s efficacy [
24]. HMB has also been linked to increased IGF-1 levels and reductions in inflammatory markers such as ferritin and osteopontin [
22].
4.3. Economic Implications
HMB-ONS offers a scalable strategy to reduce the substantial healthcare burden of malnutrition, which in Israel alone accounts for 60,401 annual hospital days and USD 145.6 million in costs [
25]. ONS users in Israel had significantly higher healthcare utilization, including primary care visits, dietitian consultations, and hospitalizations, reflecting the disproportionate burden among older adults with malnutrition [
2]. Economic models estimate that effective ONS use could reduce overall healthcare costs by 18.9%, primarily through fewer readmissions and hospital stays [
2].
In this study, HMB-ONS supplementation reduced per-patient costs by EUR 387.61 (USD 403.29 USD) compared with S-ONS, with slightly higher intervention cost offset by greater savings from reduced readmissions. International analyses further reinforce these findings. Malnutrition increases healthcare costs by 11–13% [
26], while early ONS interventions reduce hospital length of stay by 10.2% in patients with malnutrition and 16.6% in ICU patients [
13]. The NOURISH study reported HMB-ONS to be cost-effective, with an incremental cost of USD 398 per life-year gained and USD 524 when all costs were included. Recent 2024 models project USD 2.1 billion in annual savings post-discharge, with per-patient savings exceeding USD 1113 [
27]. Similarly, a nutrition-focused Quality Improvement Program in Colombia using mainly HMB-ONS achieved a 40% reduction in healthcare utilization, 82% fewer hospitalizations, and a 64% reduction in emergency visits, with a return on investment (ROI) of USD 1.82 per dollar spent in 2022 [
28].
4.4. Strengths and Limitations
This study provided real-world evidence from a large cohort of hospitalized patients with malnutrition or at risk of malnutrition, addressing a critical gap in nutritional research. By leveraging data from two large tertiary care hospitals in Israel, it offers insights into the potential clinical and economic impact of HMB-ONS in routine inpatient care. The use of PSM strengthened internal validity by controlling for key confounders, while an Israel-specific cost analysis enhanced the relevance of these results for local healthcare decision-making. Unlike previous trials conducted in controlled settings, this study evaluated HMB-ONS within standard hospital practice, providing pragmatic evidence that can inform clinical guidelines and policies.
Despite these strengths, several limitations should be acknowledged. The retrospective design limits causal inference, although the use of PSM mitigates some confounding. Both HMB-ONS and S-ONS were designed for polymorbid, malnourished patients and were prescribed as part of individualized dietary plans based on standardized dietitian assessments tailored to clinical condition, nutritional needs, and preferences. However, oral dietary intake beyond ONS was not systematically recorded. The ONS volume-based adjustment aligned total energy intake but not protein intake. Consequently, residual confounding by unmeasured total protein or energy intake cannot be excluded, and the observed associations may reflect a synergistic effect of higher protein density together with the HMB component rather than the isolated effect of HMB itself. Diagnostic granularity and disease-severity data were not available across all patients; thus, the admitting department was used as a proxy for clinical context in PSM. Although this approach enhanced comparability, residual confounding from unmeasured clinical factors or disease severity may persist. The relatively short in-hospital exposure to ONS limits the ability to directly attribute long-term outcomes to the intervention. Patients are usually advised to continue ONS post-discharge; however, adherence was not systematically monitored, limiting the assessment of sustained effects on 6-month readmission rates. While prior studies suggest combining HMB-ONS with resistance exercise improves functional outcomes, its feasibility in hospitalized or frail patients remains a challenge [
29]. Finally, cost analyses were based on estimated hospitalization and readmission costs, warranting further research incorporating real-world post-discharge expenditures. Taken together, further randomized controlled trials and prospective cohort studies comparing the efficacy of these therapies in stratified patient populations are needed to establish causality and expand upon these findings.
5. Conclusions
In conclusion, these findings highlight the potential clinical and economic benefits of HMB-ONS supplementation in hospitalized patients with malnutrition or at risk of malnutrition. This study indicates that HMB-ONS may serve as an effective nutritional intervention for high-risk populations. The cost analysis further suggests that HMB-ONS is associated with reduced readmission rates and optimized hospital resource utilization, leading to cost savings of EUR 387.61 (USD 403.29) per patient. These findings highlight the contribution of quality improvement programs in improving patient outcomes and reducing healthcare costs, while providing real-world insights into the integration of HMB-ONS into routine hospital practice. Further prospective cohort studies and randomized controlled trials are warranted to validate these observations, establish causality, and identify patient subgroups most likely to benefit from HMB-ONS therapy.
Author Contributions
Funding acquisition: S.F., L.G., R.D., and S.S.; conceptualization: S.F., L.G., R.D., and S.S.; methodology: S.F., R.D., S.S., and L.G.; software: R.D., L.G., and M.B.L.; formal analysis: S.F., R.D., L.G., M.B.L., A.R.S., K.W.K., and S.S.; writing—original draft preparation: S.F., R.D., A.R.S., S.S., and L.G.; writing—review and editing: S.F., R.D., M.B.L., A.R., O.W., A.R.S., K.W.K., S.S., and L.G.; visualization: R.D., M.B.L., and A.R.S. All authors have read and agreed to the published version of the manuscript.
Funding
Financial support for this study (HA56) was provided by Abbott Laboratories (Abbott Park, IL, USA).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Rabin Medical Center Institutional Review Board (313-22-RMC and date of approval 30 August 2022.
Informed Consent Statement
Patient consent was waived due to the use of deidentified, retrospectively collected electronic medical record data.
Data Availability Statement
The datasets presented in this article are not readily available due to privacy and legal reasons. Requests to access the datasets should be directed to the corresponding author.
Acknowledgments
The authors thank Amit Nachman for her collaborative contributions to this work. We also acknowledge the editorial assistance of Cecilia Hofmann (C Hofmann & Associates, Western Springs, IL, USA) and Bhavadharini Balaji, (Wunderbar Medical & Scientific Writing, ON, Canada), who provided medical writing support funded by Abbott.
Conflicts of Interest
The authors declare that this study received funding from Abbott Laboratories. Authors R.D., A.R.S., K.W.K., and S.S. were employed by Abbott Laboratories. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
BMI, Body Mass Index; CI, Confidence Interval; COPD, Chronic Obstructive Pulmonary Disease; ESPEN, European Society for Clinical Nutrition and Metabolism; HMB, β-Hydroxy-β-Methylbutyrate; HMB-ONS, HMB-Enriched Oral Nutritional Supplements; HMO, Clalit Health Maintenance Organization; ICD-9, International Classification of Diseases, Ninth Revision; IGF-1, Insulin-like Growth Factor 1; ILS, Israeli New Shekels; IQR, Interquartile Range; MD, Mean Difference; MOH, Ministry of Health; MUST, Malnutrition Universal Screening Tool; NOURISH, Nutrition effect On Unplanned Readmissions and Survival in Hospitalized patients; ONS, Oral Nutritional Supplements; OR, Odds Ratio; PPN, Peripheral Parenteral Nutrition; PSM, Propensity Score Matching; ROI, Return on Investment; RR, Relative Risk; S-ONS, Standard Oral Nutritional Supplements; SES, Socioeconomic Status; SHIELD, Strengthening Health In ELDerly through nutrition; SMD, Standard Mean Difference; TPN, Total Parenteral Nutrition.
References
- Shahar, D.; Shai, I.; Vardi, H.; Fraser, D. Dietary intake and eating patterns of elderly people in Israel: Who is at nutritional risk? Eur. J. Clin. Nutr. 2003, 57, 18–25. [Google Scholar] [CrossRef]
- Moser, S.; Doyev, R.; Cohen, B.; Kurz, R.; Suela Sulo, S.; Shalev, V.; Chodick, G. Prevalence and characteristics of malnutrition among community-dwelling older adults in Israel. Clin. Nutr. ESPEN 2018, 28, 179–185. [Google Scholar] [CrossRef]
- Wahyudi, E.R.; Ronoatmodjo, S.; Setiati, S.; Besral; Soejono, C.H.; Kuswardhani, T.; Fitriana, I.; Marsigit, J.; Putri, S.A.; Harmany, G.R.T. The risk of rehospitalization within 30 days of discharge in older adults with malnutrition: A meta-analysis. Arch. Gerontol. Geriatr. 2024, 118, 105306. [Google Scholar] [CrossRef]
- Holecek, M. Beta-hydroxy-beta-methylbutyrate supplementation and skeletal muscle in healthy and muscle-wasting conditions. J. Cachexia Sarcopenia Muscle 2017, 8, 529–541. [Google Scholar] [CrossRef]
- Giron, M.D.; Vilchez, J.D.; Shreeram, S.; Salto, R.; Manzano, M.; Cabrera, E.; Campos, N.; Edens, N.K.; Rueda, R.; Lopez-Pedrosa, J.M. beta-Hydroxy-beta-methylbutyrate (HMB) normalizes dexamethasone-induced autophagy-lysosomal pathway in skeletal muscle. PLoS ONE 2015, 10, e0117520. [Google Scholar] [CrossRef] [PubMed]
- Sierra, T.P.; Torrez, F.L.H.; Hernández, M.C.P.; Velasco, R.L.; Gómez, R.O.; Díaz, M.d.C.C.; Sánchez, A.I.H.; Ramírez, B.P.; Fernández, J.M.; Mola, S.J.; et al. A Prospective, Observational Study of the Effect of a High-Calorie, High-Protein Oral Nutritional Supplement with HMB in an Old and Malnourished or at-Risk-of-Malnutrition Population with Hip Fractures: A FracNut Study. Nutrients 2024, 16, 1223. [Google Scholar] [CrossRef]
- Chew, S.T.H.; Tan, N.C.; Cheong, M.; Oliver, J.; Baggs, G.; Choe, Y.; How, C.H.; Chow, W.L.; Tan, C.Y.L.; Kwan, S.C.; et al. Impact of specialized oral nutritional supplement on clinical, nutritional, and functional outcomes: A randomized, placebo-controlled trial in community-dwelling older adults at risk of malnutrition. Clin. Nutr. 2021, 40, 1879–1892. [Google Scholar] [CrossRef]
- Tey, S.; Huynh, D.; Kong, S.; Oliver, J.; Baggs, G.; Low, Y.; How, C.; Cheong, M.; Chow, W.; Tan, N.; et al. Effects of oral nutritional supplement with β-Hydroxy-β-methylbutyrate (HMB) on biochemical and hematological indices in community-dwelling older adults at risk of malnutrition: Findings from the SHIELD Study. Nutrients 2024, 16, 2495. [Google Scholar] [CrossRef] [PubMed]
- Deutz, N.E.; Ziegler, T.R.; Matheson, E.M.; Matarese, L.E.; Tappenden, K.A.; Baggs, G.E.; Nelson, J.L.; Luo, M.; Hegazi, R.; Jonnalagadda, S.S. Reduced mortality risk in malnourished hospitalized older adult patients with COPD treated with a specialized oral nutritional supplement: Sub-group analysis of the NOURISH study. Clin. Nutr. 2021, 40, 1388–1395. [Google Scholar] [CrossRef]
- Deutz, N.E.; Matheson, E.M.; Matarese, L.E.; Luo, M.; Baggs, G.E.; Nelson, J.L.; Hegazi, R.A.; Tappenden, K.A.; Ziegler, T.R. Readmission and mortality in malnourished, older, hospitalized adults treated with a specialized oral nutritional supplement: A randomized clinical trial. Clin. Nutr. 2016, 35, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Paris, A.; Camprubi-Robles, M.; Lopez-Pedrosa, J.M.; Pereira, S.L.; Rueda, R.; Ballesteros-Pomar, M.D.; Garcia Almeida, J.M.; Cruz-Jentoft, A.J. Role of oral nutritional supplements enriched with beta-hydroxy-beta-methylbutyrate in maintaining muscle function and improving clinical outcomes in various clinical settings. J. Nutr. Health Aging 2018, 22, 664–675. [Google Scholar] [CrossRef]
- Landi, F.; Calvani, R.; Picca, A.; Marzetti, E. Beta-hydroxy-beta-methylbutyrate and sarcopenia: From biological plausibility to clinical evidence. Curr. Opin. Clin. Nutr. Metab. Care 2019, 22, 37–43. [Google Scholar] [CrossRef]
- Mullin, G.E.; Fan, L.; Sulo, S.; Partridge, J. The association between oral nutritional supplements and 30-day hospital readmissions of malnourished patients at a US academic medical center. J. Acad. Nutr. Diet. 2019, 119, 1168–1175. [Google Scholar] [CrossRef]
- Philipson, T.J.; Snider, J.T.; Lakdawalla, D.N.; Stryckman, B.; Goldman, D.P. Impact of oral nutritional supplementation on hospital outcomes. Am. J. Manag. Care 2013, 19, 121–128. [Google Scholar] [CrossRef]
- Su, H.; Zhou, H.; Gong, Y.; Xiang, S.; Shao, W.; Zhao, X.; Ling, H.; Chen, G.; Tong, P.; Li, J. The effects of beta-hydroxy-beta-methylbutyrate or HMB-rich nutritional supplements on sarcopenia patients: A systematic review and meta-analysis. Front. Med. 2024, 11, 1348212. [Google Scholar] [CrossRef]
- Feng, Y.; Chen, P.; Li, T.; Wan, P.; Shi, R. Effects of exercise with or without β-hydroxy-β-methylbutyrate supplementation on muscle mass, muscle strength, and physical performance in patients with sarcopenia: A systematic review and meta-analysis. Front. Nutr. 2024, 11, 1460133. [Google Scholar] [CrossRef]
- Volkert, D.; Beck, A.M.; Cederholm, T.; Cruz-Jentoft, A.; Goisser, S.; Hooper, L.; Kiesswetter, E.; Maggio, M.; Raynaud-Simon, A.; Sieber, C.C.; et al. ESPEN guideline on clinical nutrition and hydration in geriatrics. Clin. Nutr. 2019, 38, 10–47. [Google Scholar] [CrossRef]
- Central Bureau of Statistics (CBS) of Isarael. Ranking of Local Authorities, According to the Socio-Economic Index 2017: Israel Bureau of Statistics. 2019. Available online: https://www.cbs.gov.il/he/publications/doclib/2021/local_authorities19_1835/t04.pdf (accessed on 1 December 2024).
- Blondal, B.S.; Geirsdottir, O.G.; Halldorsson, T.I.; Beck, A.M.; Jonsson, P.V.; Ramel, A. HOMEFOOD randomised trial—Six-month nutrition therapy in discharged older adults reduces hospital readmissions and length of stay at hospital up to 18 months of follow-up. J. Nutr. Health Aging 2023, 27, 632–640. [Google Scholar] [CrossRef]
- Cornejo-Pareja, I.; Ramirez, M.; Camprubi-Robles, M.; Rueda, R.; Vegas-Aguilar, I.M.; Garcia-Almeida, J.M. Effect on an oral nutritional supplement with beta-hydroxy-beta-methylbutyrate and vitamin D on morphofunctional aspects, body composition, and phase angle in malnourished patients. Nutrients 2021, 13, 4355. [Google Scholar] [CrossRef]
- Prado, C.M.; Orsso, C.E.; Pereira, S.L.; Atherton, P.J.; Deutz, N.E.P. Effects of β-hydroxy β-methylbutyrate (HMB) supplementation on muscle mass, function, and other outcomes in patients with cancer: A systematic review. J. Cachexia Sarcopenia Muscle 2022, 13, 1623–1641. [Google Scholar] [CrossRef]
- Pereira, S.L.; Shoemaker, M.E.; Gawel, S.; Davis, G.J.; Luo, M.; Mustad, V.A.; Cramer, J.T. Biomarker changes in response to a 12-week supplementation of an oral nutritional supplement enriched with protein, vitamin D and HMB in malnourished community dwelling older adults with sarcopenia. Nutrients 2022, 14, 1196. [Google Scholar] [CrossRef]
- Bear, D.E.; Langan, A.; Dimidi, E.; Wandrag, L.; Harridge, S.D.R.; Hart, N.; Connolly, B.; Whelan, K. Beta-hydroxy-beta-methylbutyrate and its impact on skeletal muscle mass and physical function in clinical practice: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2019, 109, 1119–1132. [Google Scholar] [CrossRef]
- Espina, S.; Sanz-Paris, A.; Gonzalez-Irazabal, Y.; Perez-Matute, P.; Andrade, F.; Garcia-Rodriguez, B.; Carpene, C.; Zakaroff, A.; Bernal-Monterde, V.; Fuentes-Olmo, J.; et al. Randomized clinical trial: Effects of beta-Hydroxy-beta-methylbutyrate (HMB)-enriched vs. HMB-free oral nutritional supplementation in malnourished cirrhotic patients. Nutrients 2022, 14, 2344. [Google Scholar] [CrossRef]
- Ginsberg, G.M.; Saday, Y. Burden of underweight in Israel. J. Parenter. Enter. Nutr. 2019, 43, 638–648. [Google Scholar] [CrossRef]
- Rodriguez-Sanchez, B.; Sulo, S.; Carnicero, J.A.; Rueda, R.; Rodriguez-Manas, L. Malnutrition prevalence and burden on healthcare resource use among Spanish community-living older adults: Results of a longitudinal analysis. Clin. Outcomes Res. 2020, 12, 355–367. [Google Scholar] [CrossRef]
- Wang, S.; Shafrin, J.; Kerr, K.W.; Schuetz, P. Health economic value of postacute oral nutritional supplementation in older adult medical patients at risk for malnutrition: A US-based modelling approach. BMJ Open 2024, 14, e086787. [Google Scholar] [CrossRef]
- Sulo, S.; Schwander, B.; Brunton, C.; Gomez, G.; Misas, J.D.; Gracia, D.A.; Chavarro-Carvajal, D.A.; Venegas-Sanabria, L.C.; Cano-Gutierrez, C. Nutrition-focused care for community-living adults: Healthcare utilization and economic benefits. Value Health Reg. Issues 2022, 32, 70–77. [Google Scholar] [CrossRef]
- Flakoll, P.; Sharp, R.; Baier, S.; Levenhagen, D.; Carr, C.; Nissen, S. Effect of beta-hydroxy-beta-methylbutyrate, arginine, and lysine supplementation on strength, functionality, body composition, and protein metabolism in elderly women. Nutrition 2004, 20, 445–451. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).