PLX3397-Induced Microglial Ablation Alters Adipose Tissue Accumulation in a Male–Female-Dependent Manner Under High-Energy-Diet Feeding
Abstract
1. Introduction
2. Materials and Methods
3. Results
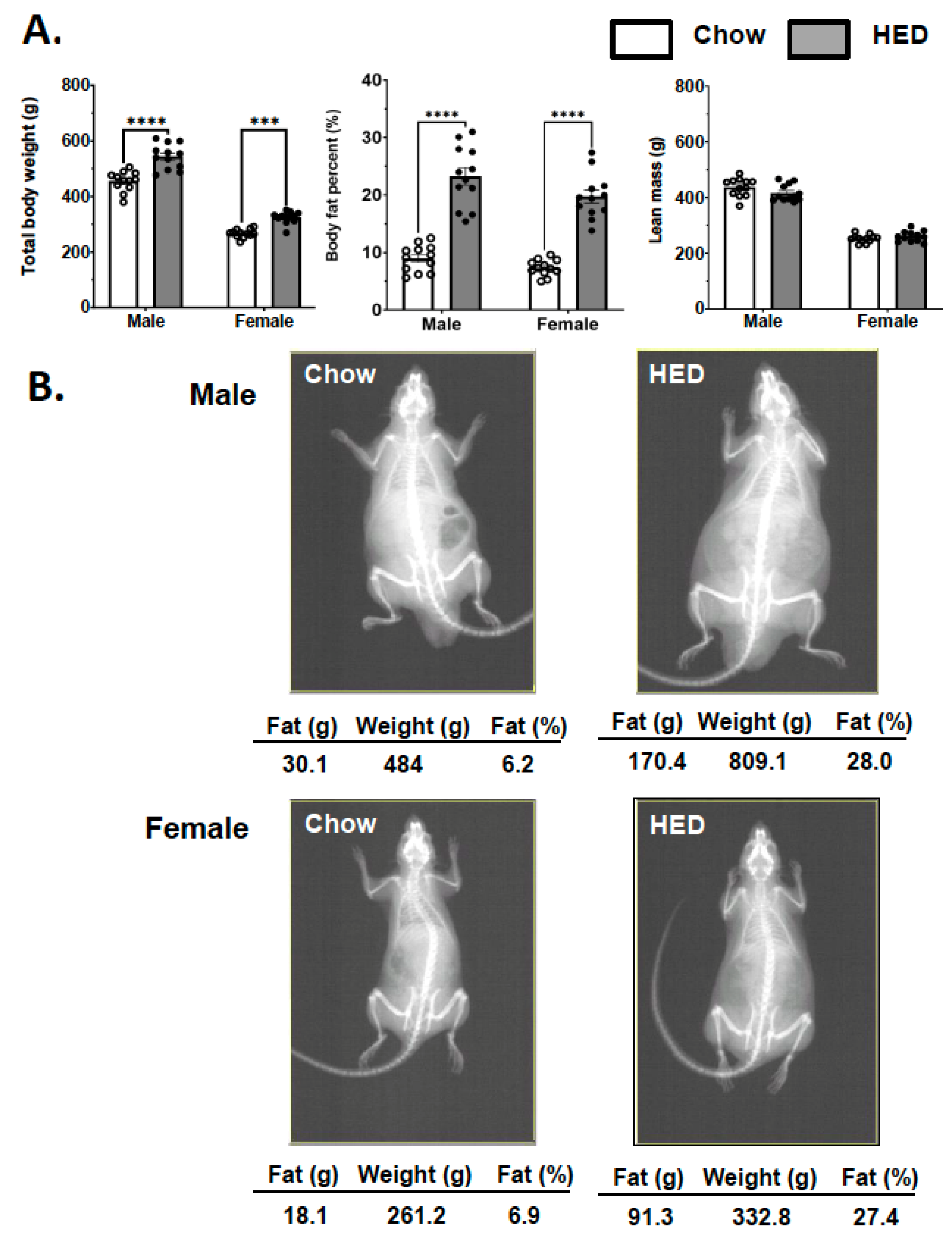
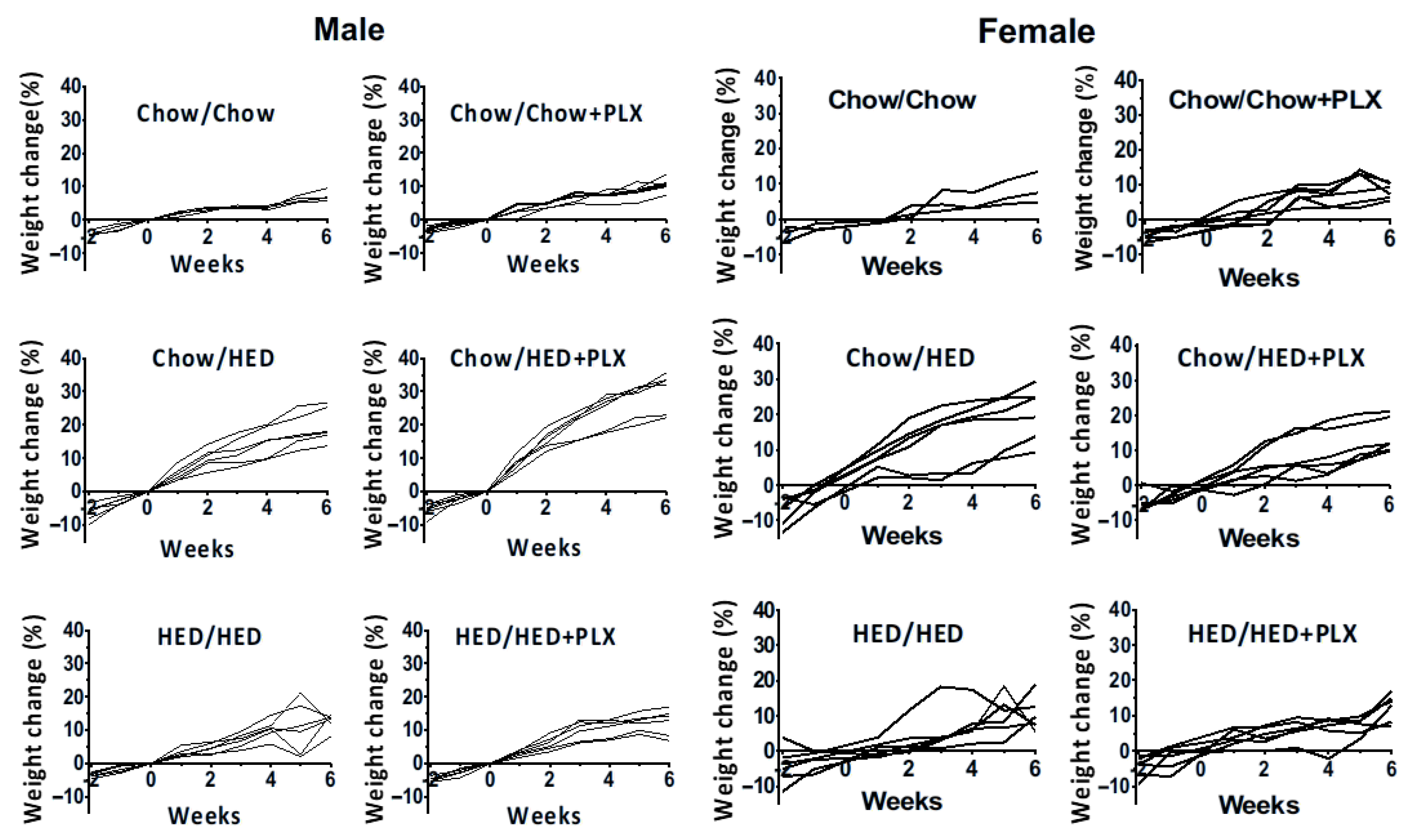
3.1. Effects of a HED on Body Composition
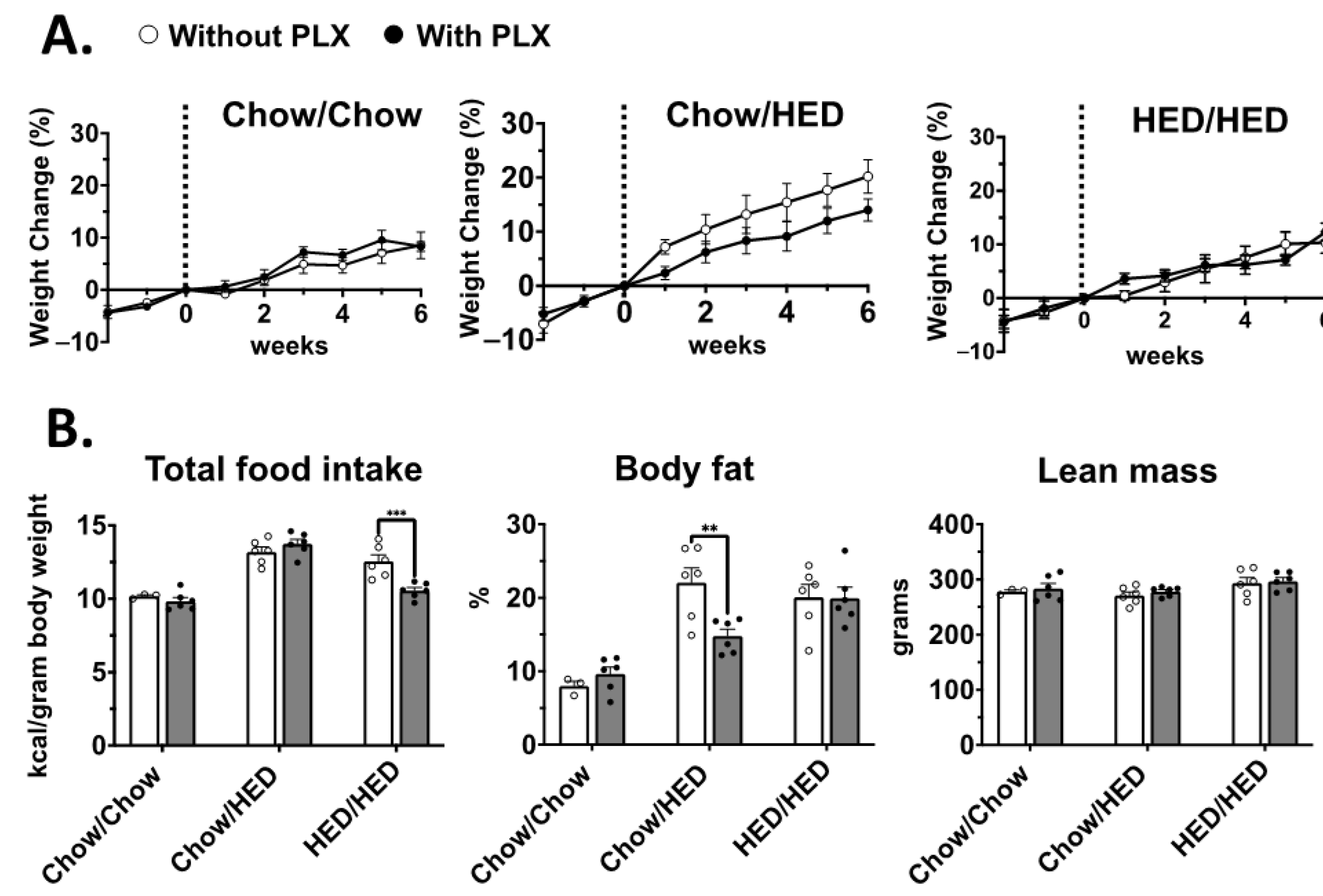
3.2. Effect of Microglia Suppression on Body Weight Was Sex- and Diet-Dependent
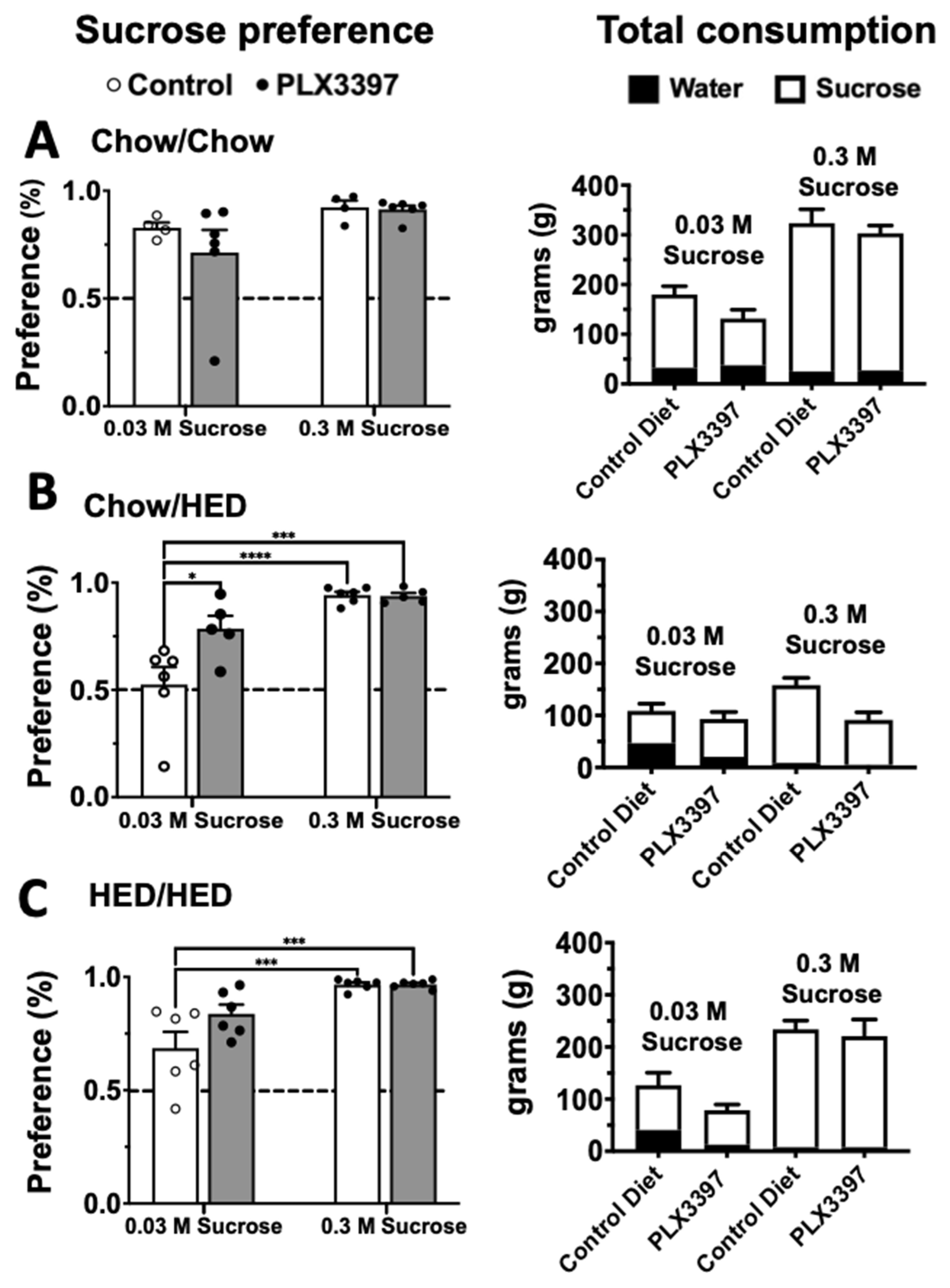
3.3. Sucrose Preference
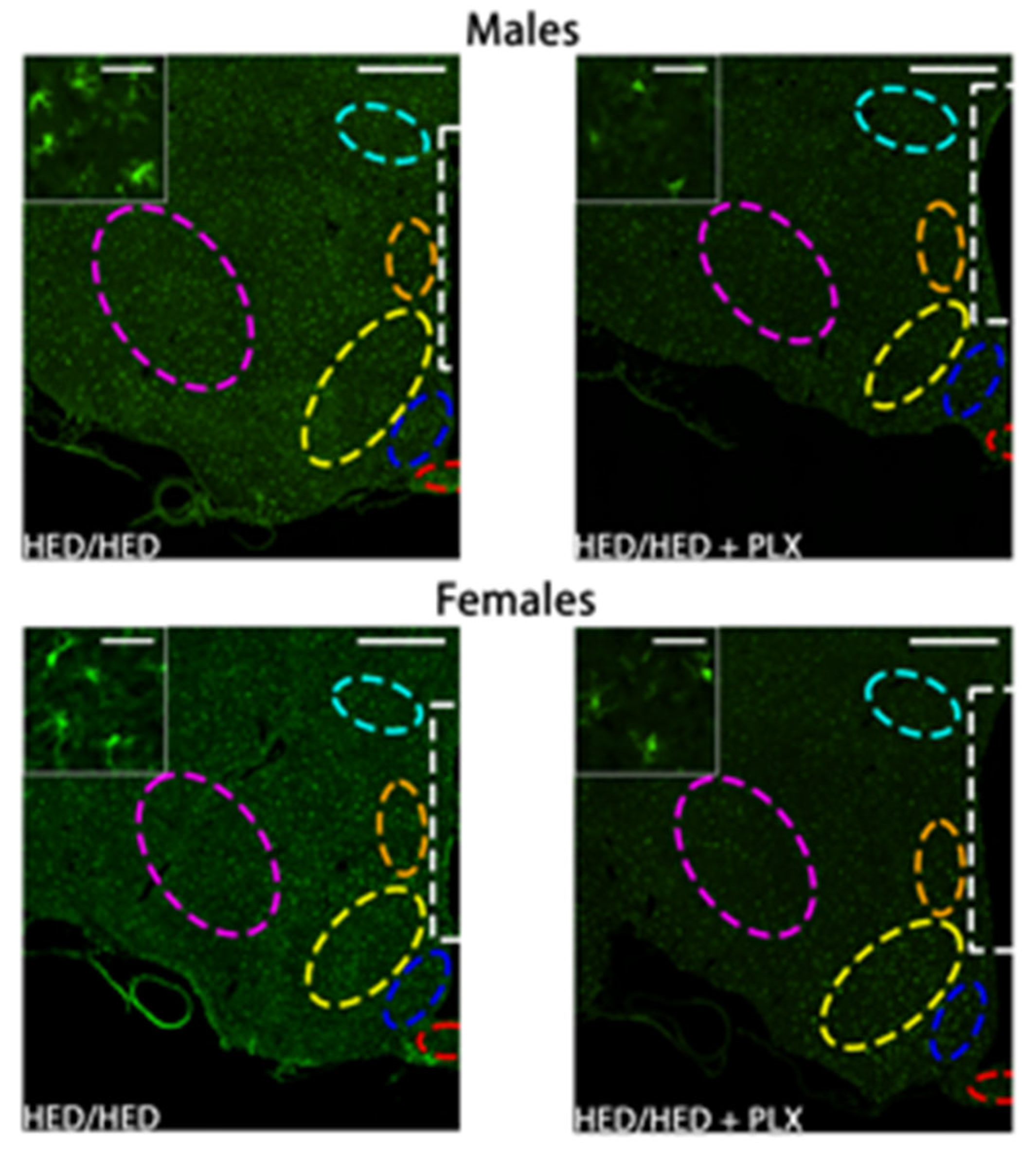
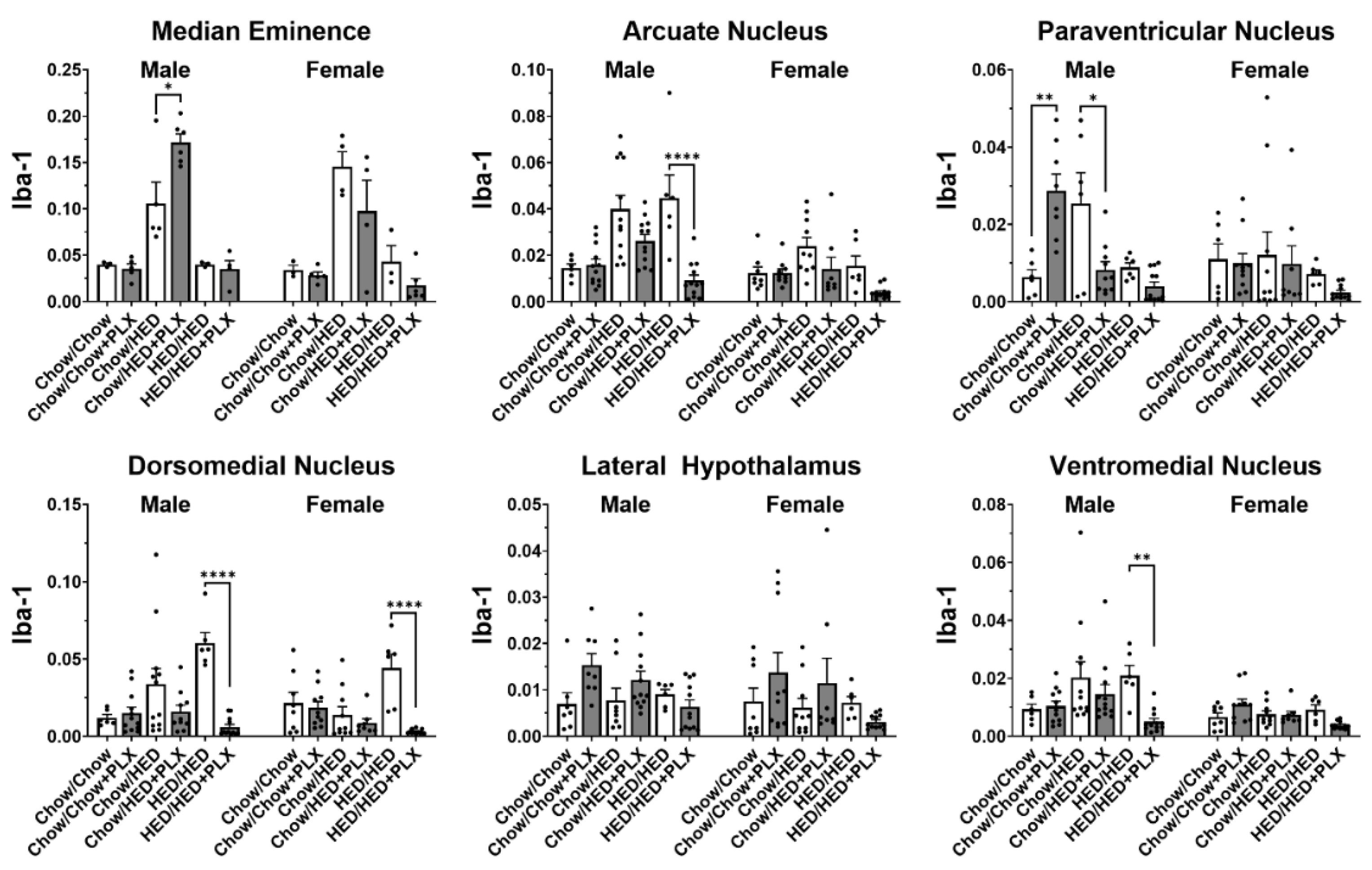
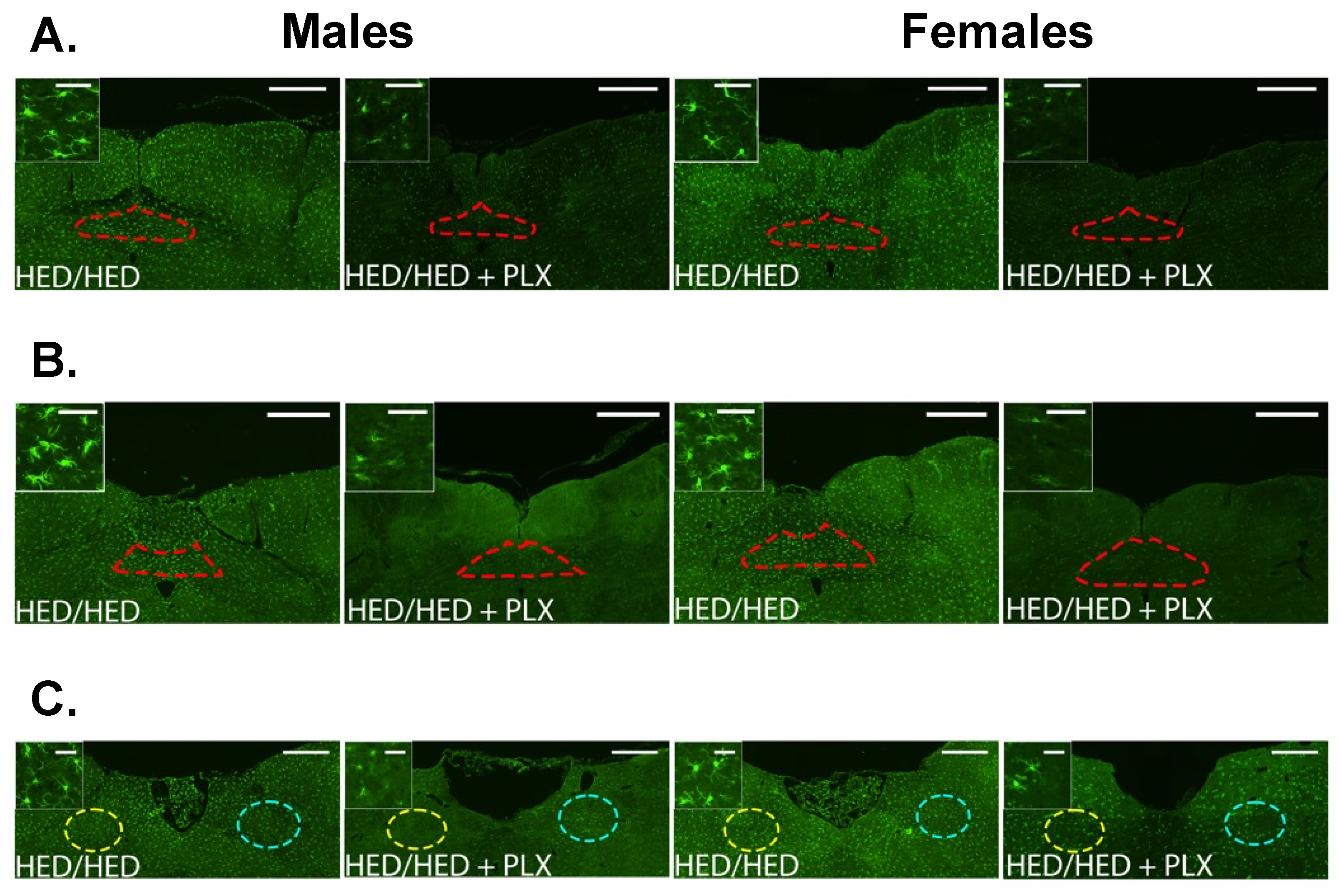
3.4. PLX Suppression of Microglia Activation in the Hypothalamus
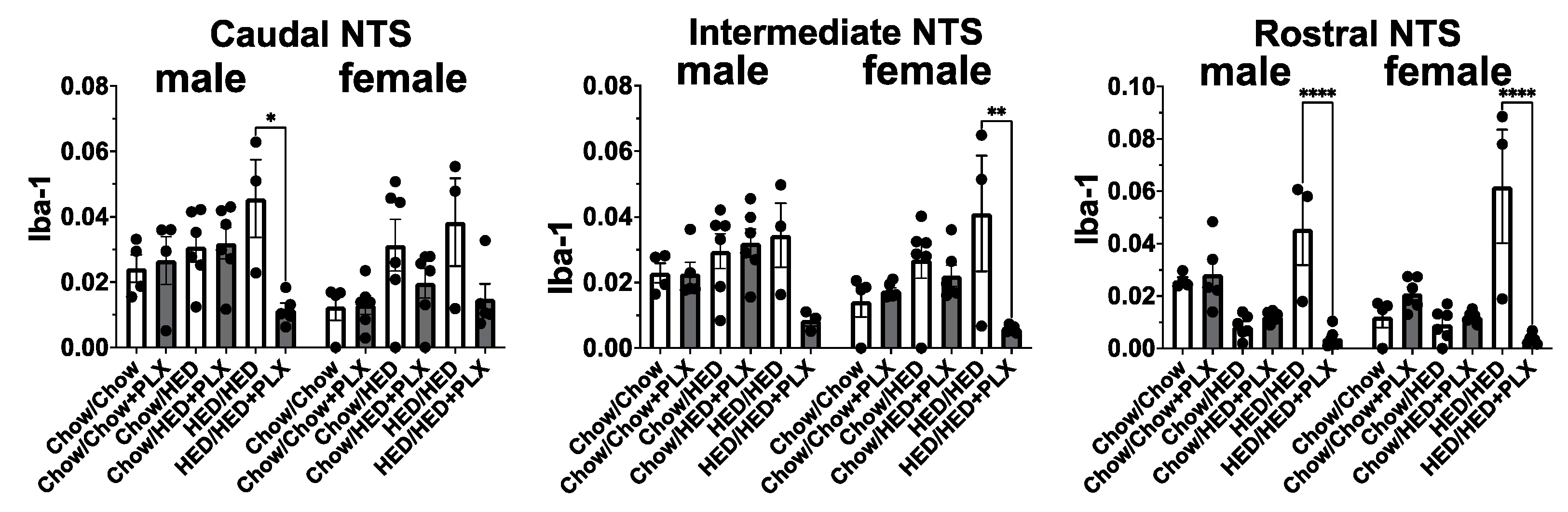
3.5. PLX Suppression of Microglia Activation in the NTS
4. Discussion
4.1. Microglia in the Brain
4.2. Sex Differences
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Arc | Arcuate nucleus |
| Atip | Atipamezole |
| CNS | Central nervous system |
| CSF-1R | Colony-stimulating factor 1 receptor |
| DIO | Diet-induced obesity |
| DMN | Dorsomedial nucleus of hypothalamus |
| DXA | Dual-energy X-ray absorptiometry |
| HED | High-energy diet |
| iNTS | Intermediate nucleus tractus solitarius |
| i.p. | Intraperitoneal |
| LH | Lateral hypothalamus |
| Med | Medetomidine hydrochloride |
| ME | Median eminence |
| NTS | Nucleus tractus solitarius |
| PaVN | Paraventricular nucleus of hypothalamus |
| PeVN | Periventricular nucleus of hypothalamus |
| PLX | Pexidartinib (PLX3397) |
| rNTS | Rostral nucleus tractus solitarius |
| s.c. | Subcutaneous |
| SD | Sprague-Dawley |
| VMH | Ventromedial hypothalamus |
References
- Briefel, R.R.; Johnson, C.L. Secular trends in dietary intake in the United States. Annu. Rev. Nutr. 2004, 24, 401–431. [Google Scholar] [CrossRef]
- Ajami, B.; Bennett, J.L.; Krieger, C.; Tetzlaff, W.; Rossi, F.M.V. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat. Neurosci. 2007, 10, 1538–1543. [Google Scholar] [CrossRef]
- Huang, Y.; Xu, Z.; Xiong, S.; Sun, F.; Qin, G.; Hu, G.; Wang, J.; Zhao, L.; Liang, Y.-X.; Wu, T.; et al. Repopulated microglia are solely derived from the proliferation of residual microglia after acute depletion. Nat. Neurosci. 2018, 21, 530–540. [Google Scholar] [CrossRef] [PubMed]
- Valdearcos, M.; Myers, M.G.; Koliwad, S.K. Hypothalamic microglia as potential regulators of metabolic physiology. Nat. Metab. 2019, 1, 314–320. [Google Scholar] [CrossRef] [PubMed]
- De Luca, S.N.; Sominsky, L.; Soch, A.; Wang, H.; Ziko, I.; Rank, M.M.; Spencer, S.J. Conditional microglial depletion in rats leads to reversible anorexia and weight loss by disrupting gustatory circuitry. Brain Behav. Immun. 2019, 77, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Douglass, J.D.; Dorfman, M.D.; Thaler, J.P. Glia: Silent partners in energy homeostasis and obesity pathogenesis. Diabetologia 2017, 60, 226–236. [Google Scholar] [CrossRef]
- Milanova, I.V.; Kalsbeek, M.J.T.; Wang, X.L.; Korpel, N.L.; Stenvers, D.J.; Wolff, S.E.C.; De Goede, P.; Heijboer, A.C.; Fliers, E.; La Fleur, S.E.; et al. Diet-induced obesity disturbs microglial immunometabolism in a time-of-day manner. Front. Endocrinol. 2019, 10, 424. [Google Scholar] [CrossRef]
- Reis, W.L.; Yi, C.X.; Gao, Y.; Tschöp, M.H.; Stern, J.E. Brain innate immunity regulates hypothalamic arcuate neuronal activity and feeding behavior. Endocrinology 2015, 156, 1303–1315. [Google Scholar] [CrossRef]
- Stifler, D.R.; Thaler, J.P.; Gerritse, I.; Dorfman, M.D.; Fasnacht, R.; Koliwad, S.K.; Robblee, M.M.; Douglass, J.D.; Valdearcos, M.; Bennett, M.L.; et al. Microglial Inflammatory Signaling Orchestrates the Hypothalamic Immune Response to Dietary Excess and Mediates Obesity Susceptibility. Cell Metab. 2017, 26, 185–197.e183. [Google Scholar] [CrossRef]
- Thaler, J.P.; Yi, C.X.; Schur, E.A.; Guyenet, S.J.; Hwang, B.H.; Dietrich, M.O.; Zhao, X.; Sarruf, D.A.; Izgur, V.; Maravilla, K.R.; et al. Obesity is associated with hypothalamic injury in rodents and humans. J. Clin. Investig. 2012, 122, 153–162. [Google Scholar] [CrossRef]
- Valdearcos, M.; Robblee, M.M.; Benjamin, D.I.; Nomura, D.K.; Xu, A.W.; Koliwad, S.K. Microglia dictate the impact of saturated fat consumption on hypothalamic inflammation and neuronal function. Cell Rep. 2014, 9, 2124–2138. [Google Scholar] [CrossRef]
- Wang, C.Y.; Liao, J.K. A mouse model of diet-induced obesity and insulin resistance. Methods Mol. Biol. 2012, 821, 421–433. [Google Scholar]
- Elmore, M.R.; Najafi, A.R.; Koike, M.A.; Dagher, N.N.; Spangenberg, E.E.; Rice, R.A.; Kitazawa, M.; Matusow, B.; Nguyen, H.; West, B.L.; et al. Colony-stimulating factor 1 receptor signaling is necessary for microglia viability, unmasking a microglia progenitor cell in the adult brain. Neuron 2014, 82, 380–397. [Google Scholar] [CrossRef] [PubMed]
- Epstein, L.H.; Temple, J.L.; Neaderhiser, B.J.; Salis, R.J.; Erbe, R.W.; Leddy, J.J. Food reinforcement, the dopamine D2 receptor genotype, and energy intake in obese and nonobese humans. Behav. Neurosci. 2007, 121, 877–886. [Google Scholar] [CrossRef] [PubMed]
- Saelens, B.E.; Epstein, L.H. Reinforcing value of food in obese and non-obese women. Appetite 1996, 27, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Tracy, A.L.; Wee, C.J.M.; Hazeltine, G.E.; Carter, R.A. Characterization of attenuated food motivation in high-fat diet-induced obesity: Critical roles for time on diet and reinforcer familiarity. Physiol. Behav. 2015, 141, 69–77. [Google Scholar] [CrossRef]
- Weiss, M.S.; Hajnal, A.; Czaja, K.; Di Lorenzo, P.M. Taste responses in the nucleus of the solitary tract of awake obese rats are blunted compared with those in lean rats. Front. Integr. Neurosci. 2019, 13, 35. [Google Scholar] [CrossRef]
- D’Agostino, G.; Lyons, D.J.; Cristiano, C.; Burke, L.K.; Madara, J.C.; Campbell, J.N.; Garcia, A.P.; Land, B.B.; Lowell, B.B.; Dileone, R.J.; et al. Appetite controlled by a cholecystokinin nucleus of the solitary tract to hypothalamus neurocircuit. eLife 2016, 5, e12225. [Google Scholar] [CrossRef]
- Grill, H.J.; Hayes, M.R. Hindbrain neurons as an essential hub in the neuroanatomically distributed control of energy balance. Cell Metab. 2012, 16, 296–309. [Google Scholar] [CrossRef]
- Rinaman, L. Hindbrain contributions to anorexia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 287, R1035–R1036. [Google Scholar] [CrossRef]
- Rinaman, L. Ascending projections from the caudal visceral nucleus of the solitary tract to brain regions involved in food intake and energy expenditure. Brain Res. 2010, 1350, 18–34. [Google Scholar] [CrossRef]
- Schwartz, G.J. Brainstem integrative function in the central nervous system control of food intake. Forum Nutr. 2010, 63, 141–151. [Google Scholar] [PubMed]
- Morton, G.J.; Cummings, D.E.; Baskin, D.G.; Barsh, G.S.; Schwartz, M.W. Central nervous system control of food intake and body weight. Nature 2006, 443, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Vaughn, A.C.; Cooper, E.M.; Di Lorenzo, P.M.; O’Loughlin, L.J.; Konkel, M.E.; Peters, J.H.; Hajnal, A.; Sen, T.; Lee, S.H.; de La Serre, C.B.; et al. Energy-dense diet triggers changes in gut microbiota, reorganization of gut brain vagal communication and increases body fat accumulation. Acta Neurobiol. Exp. 2017, 77, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Gallaher, Z.R.; Ryu, V.; Herzog, T.; Ritter, R.C.; Czaja, K. Changes in microglial activation within the hindbrain, nodose ganglia, and the spinal cord following subdiaphragmatic vagotomy. Neurosci. Lett. 2012, 513, 31–36. [Google Scholar] [CrossRef]
- Minaya, D.M.; Di Lorenzo, P.M.; Hajnal, A.; Czaja, K. Roux-en-Y gastric bypass surgery triggers rapid DNA fragmentation in vagal afferent neurons in rats. Acta Neurobiol. Exp. 2019, 79, 432–444. [Google Scholar] [CrossRef]
- Peters, J.H.; Gallaher, Z.R.; Ryu, V.; Czaja, K. Withdrawal and restoration of central vagal afferents within the dorsal vagal complex following subdiaphragmatic vagotomy. J. Comp. Neurol. 2013, 521, 3584–3599. [Google Scholar] [CrossRef]
- Milanova, I.V.; Korpel, N.L.; Correa-da-Silva, F.; Berends, E.; Osman, S.; la Fleur, S.E.; Fliers, E.; Kalsbeek, A.; Yi, C.X. Loss of microglial insulin receptor leads to sex-dependent metabolic disorders in obese mice. Int. J. Mol. Sci. 2022, 23, 2933. [Google Scholar] [CrossRef]
- Alreemi, R.M. Decoding the anti-cancer potential of Pexidartinib (PLX3397), a Fms-like tyrosine kinase 3 inhibitor, using next-generation knowledge discovery methods. Bioinformation 2024, 20, 460–472. [Google Scholar] [CrossRef]
- Hassanein, M.; Almahayni, M.H.; Ahmed, S.O.; Gaballa, S.; El Fakih, R. FLT3 Inhibitors for Treating Acute Myeloid Leukemia. Clin. Lymphoma Myeloma Leuk. 2016, 16, 543–549. [Google Scholar] [CrossRef]
- Ni, R.J.; Wang, Y.Y.; Gao, T.H.; Wang, Q.R.; Wei, J.X.; Zhao, L.S.; Ma, Y.R.; Ma, X.H.; Li, T. Depletion of microglia with PLX3397 attenuates MK-801-induced hyperactivity associated with regulating inflammation-related genes in the brain. Zool. Res. 2023, 44, 543–555. [Google Scholar] [CrossRef]
- Berthoud, H.R.; Bereiter, D.A.; Trimble, E.R.; Siegel, E.G.; Jeanrenaud, B. Cephalic phase, reflex insulin secretion. Neuroanatomical and physiological characterization. Diabetologia 1981, 20 (Suppl. 1), 393–401. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.; Graham, B.; Yakubu, F.; Lin, D.; Peters, J.C.; Hill, J.O. Metabolic differences between obesity-prone and obesity-resistant rats. Am. J. Physiol. 1990, 259 Pt 2, R1103–R1110. [Google Scholar] [CrossRef] [PubMed]
- Levin, B.E.; Dunn-Meynell, A.A.; Balkan, B.; Keesey, R.E. Selective breeding for diet-induced obesity and resistance in Sprague-Dawley rats. Am. J. Physiol. 1997, 273, R725–R730. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Kim, Y.-J.; Fillmore, J.J.; Chen, Y.; Moore, I.; Lee, J.; Yuan, M.; Li, Z.W.; Karin, M.; Perret, P.; et al. Prevention of fat-induced insulin resistance by salicylate. J. Clin. Investig. 2001, 108, 437–446. [Google Scholar] [CrossRef]
- Pahlavani, M.; Ramalho, T.; Koboziev, I.; Lemieux, M.J.; Jayarathne, S.; Ramalingam, L.; Filgueiras, L.R.; Moustaid-Moussa, N. Adipose tissue inflammation in insulin resistance: Review of mechanisms mediating anti-inflammatory effects of omega-3 polyunsaturated fatty acids. J. Investig. Med. 2017, 65, 1021–1027. [Google Scholar] [CrossRef]
- Lee, Y.S.; Li, P.; Huh, J.Y.; Hwang, I.J.; Lu, M.; Kim, J.I.; Ham, M.; Talukdar, S.; Chen, A.; Lu, W.J.; et al. Inflammation is necessary for long-term but not short-term high-fat diet-induced insulin resistance. Diabetes 2011, 60, 2474–2483. [Google Scholar] [CrossRef]
- Guillemot-Legris, O.; Masquelier, J.; Everard, A.; Cani, P.D.; Alhouayek, M.; Muccioli, G.G. High-fat diet feeding differentially affects the development of inflammation in the central nervous system. J. Neuroinflamm. 2016, 13, 206. [Google Scholar] [CrossRef]
- Tu, T.H.; Nam-Goong, I.S.; Lee, J.; Yang, S.; Kim, J.G. Visfatin Triggers Anorexia and Body Weight Loss through Regulating the Inflammatory Response in the Hypothalamic Microglia. Mediat. Inflamm. 2017, 2017, 1958947. [Google Scholar] [CrossRef]
- Minaya, D.M.; Turlej, A.; Joshi, A.; Nagy, T.; Weinstein, N.; Di Lorenzo, P.M.; Hajnal, A.; Czaja, K. Consumption of a high energy density diet triggers microbiota dysbiosis, hepatic lipidosis, and microglia activation in the nucleus of the solitary tract in rats. Nutr. Diabetes 2020, 10, 20. [Google Scholar] [CrossRef]
- Chen, J.; Cheng, M.; Wang, L.; Zhang, L.; Xu, D.; Cao, P.; Wang, F.; Herzog, H.; Song, S.; Zhan, C. A Vagal-NTS neural pathway that stimulates feeding. Curr. Biol. 2020, 30, 3986–3998.e5. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.F.; Liu, J.J.; Xia, J.; Liu, J.; Mirabella, V.; Pang, Z.P. Endogenous glucagon-like peptide-1 suppresses high-fat food intake by reducing synaptic drive onto mesolimbic dopamine neurons. Cell Rep. 2015, 12, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Clark, M.S.; Palmiter, R.D. Deciphering a neuronal circuit that mediates appetite. Nature 2012, 483, 594–597. [Google Scholar] [CrossRef] [PubMed]
- Zhan, C.; Zhou, J.; Feng, Q.; Zhang, J.E.; Lin, S.; Bao, J.; Wu, P.; Luo, M. Acute and long-term suppression of feeding behavior by POMC neurons in the brainstem and hypothalamus, respectively. J. Neurosci. 2013, 33, 3624–3632. [Google Scholar] [CrossRef]
- Hong, J.; Stubbins, R.E.; Smith, R.R.; Harvey, A.E.; Núñez, N.P. Differential susceptibility to obesity between male, female and ovariectomized female mice. Nutr. J. 2009, 8, 11. [Google Scholar] [CrossRef]
- Spence, R.D.; Wisdom, A.J.; Cao, Y.; Hill, H.M.; Mongerson, C.R.L.; Stapornkul, B.; Itoh, N.; Sofroniew, M.V.; Voskuhl, R.R. Estrogen mediates neuroprotection and anti-inflammatory effects during EAE through ER signaling on astrocytes but not through ER signaling on astrocytes or neurons. J. Neurosci. 2013, 33, 10924–10933. [Google Scholar] [CrossRef]
- Loram, L.C.; Sholar, P.W.; Taylor, F.R.; Wiesler, J.L.; Babb, J.A.; Strand, K.A.; Berkelhammer, D.; Day, H.E.; Maier, S.F.; Watkins, L.R. Sex and estradiol influence glial pro-inflammatory responses to lipopolysaccharide in rats. Psychoneuroendocrinology 2012, 37, 1688–1699. [Google Scholar] [CrossRef]
- Vegeto, E.; Belcredito, S.; Etteri, S.; Ghisletti, S.; Brusadelli, A.; Meda, C.; Krust, A.; Dupont, S.; Ciana, P.; Chambon, P.; et al. Estrogen receptor mediates the brain antiinflammatory activity of estradiol. Proc. Natl. Acad. Sci. USA 2003, 100, 9614–9619. [Google Scholar] [CrossRef]
- Musatov, S.; Chen, W.; Pfaff, D.W.; Mobbs, C.V.; Yang, X.J.; Clegg, D.J.; Kaplitt, M.G.; Ogawa, S. Silencing of estrogen receptor in the ventromedial nucleus of hypothalamus leads to metabolic syndrome. Proc. Natl. Acad. Sci. USA 2007, 104, 2501–2506. [Google Scholar] [CrossRef]
- Voskuhl, R. Sex differences in autoimmune diseases. Biol. Sex. Differ. 2011, 2, 1. [Google Scholar] [CrossRef]
- Dorfman, M.D.; Krull, J.E.; Douglass, J.D.; Fasnacht, R.; Lara-Lince, F.; Meek, T.H.; Shi, X.; Damian, V.; Nguyen, H.T.; Matsen, M.E.; et al. Sex differences in microglial CX3CR1 signalling determine obesity susceptibility in mice. Nat. Commun. 2017, 8, 14556. [Google Scholar] [CrossRef]
- Banerjee, J.; Dorfman, M.D.; Fasnacht, R.; Douglass, J.D.; Wyse-Jackson, A.C.; Barria, A.; Thaler, J.P. CX3CL1 Action on microglia protects from diet-induced obesity by restoring POMC neuronal excitability and melanocortin system activity impaired by high-fat diet feeding. Int. J. Mol. Sci. 2022, 23, 6380. [Google Scholar] [CrossRef]


| Structure | Diet Group | Sex | Interaction |
|---|---|---|---|
| Arc | F (5, 45) = 9.73, p < 0.001 *** | F (1, 45) = 16.83, p < 0.001 *** | F (5, 45) = 1.89, p = 0.011 * |
| PaVN | F (5, 39) = 3.67, p = 0.008 ** | F (1, 39) = 2.38, p = 0.131 | F (5, 39) = 1.68, p = 0.162 |
| LH | F (5, 40) = 4.82, p = 0.002 ** | F (1, 40) = 1.25, p = 0.271 | F (5, 40) = 0.35, p = 0.880 |
| VMH | F (5, 45) = 2.9, p = 0.024 * | F (1, 45) = 9.06, p = 0.004 ** | F (5, 45) = 1.47, p = 0.217 |
| DMN | F (5, 44) = 6.77, p < 0.001 *** | F (1, 44) = 1.38, p = 0.247 | F (5, 44) = 0.93, p = 0.470 |
| ME | F (5, 39) = 24.88, p < 0.001 *** | F (1, 39) = 1.44, p = 0.237 | F (5, 39) = 3.49, p = 0.011 * |
| PeVN | F (5, 45) = 1.51, p = 0.205 | F (1, 45) = 8.07, p = 0.374 | F (5, 45) = 0.46, p = 0.807 |
| cNTS | F (5, 46) = 5.05, p < 0.001 *** | F (1, 46) = 3.86, p = 0.056 | F (5, 46) = 0.81, p = 0.547 |
| iNTS | F (5, 45) = 6.15, p < 0.001 *** | F (1, 45) = 1.45, p = 0.236 | F (5, 45) = 0.51, p = 0.766 |
| rNTS | F (5, 48) = 20.94, p < 0.001 *** | F (1, 48) = 0.08, p = 0.784 | F (5, 48) = 1.57, p = 0.187 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Connell, F.P.; Hajnal, A.; Di Lorenzo, P.M.; Czaja, K. PLX3397-Induced Microglial Ablation Alters Adipose Tissue Accumulation in a Male–Female-Dependent Manner Under High-Energy-Diet Feeding. Nutrients 2025, 17, 3445. https://doi.org/10.3390/nu17213445
O’Connell FP, Hajnal A, Di Lorenzo PM, Czaja K. PLX3397-Induced Microglial Ablation Alters Adipose Tissue Accumulation in a Male–Female-Dependent Manner Under High-Energy-Diet Feeding. Nutrients. 2025; 17(21):3445. https://doi.org/10.3390/nu17213445
Chicago/Turabian StyleO’Connell, Flynn P., Andras Hajnal, Patricia M. Di Lorenzo, and Krzysztof Czaja. 2025. "PLX3397-Induced Microglial Ablation Alters Adipose Tissue Accumulation in a Male–Female-Dependent Manner Under High-Energy-Diet Feeding" Nutrients 17, no. 21: 3445. https://doi.org/10.3390/nu17213445
APA StyleO’Connell, F. P., Hajnal, A., Di Lorenzo, P. M., & Czaja, K. (2025). PLX3397-Induced Microglial Ablation Alters Adipose Tissue Accumulation in a Male–Female-Dependent Manner Under High-Energy-Diet Feeding. Nutrients, 17(21), 3445. https://doi.org/10.3390/nu17213445








