Probiotic Supplementation Enhances the Effects of a Nutritional Intervention on Quality of Life in Women with Hashimoto’s Thyroiditis—A Double-Blind Randomised Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Ethical Approval
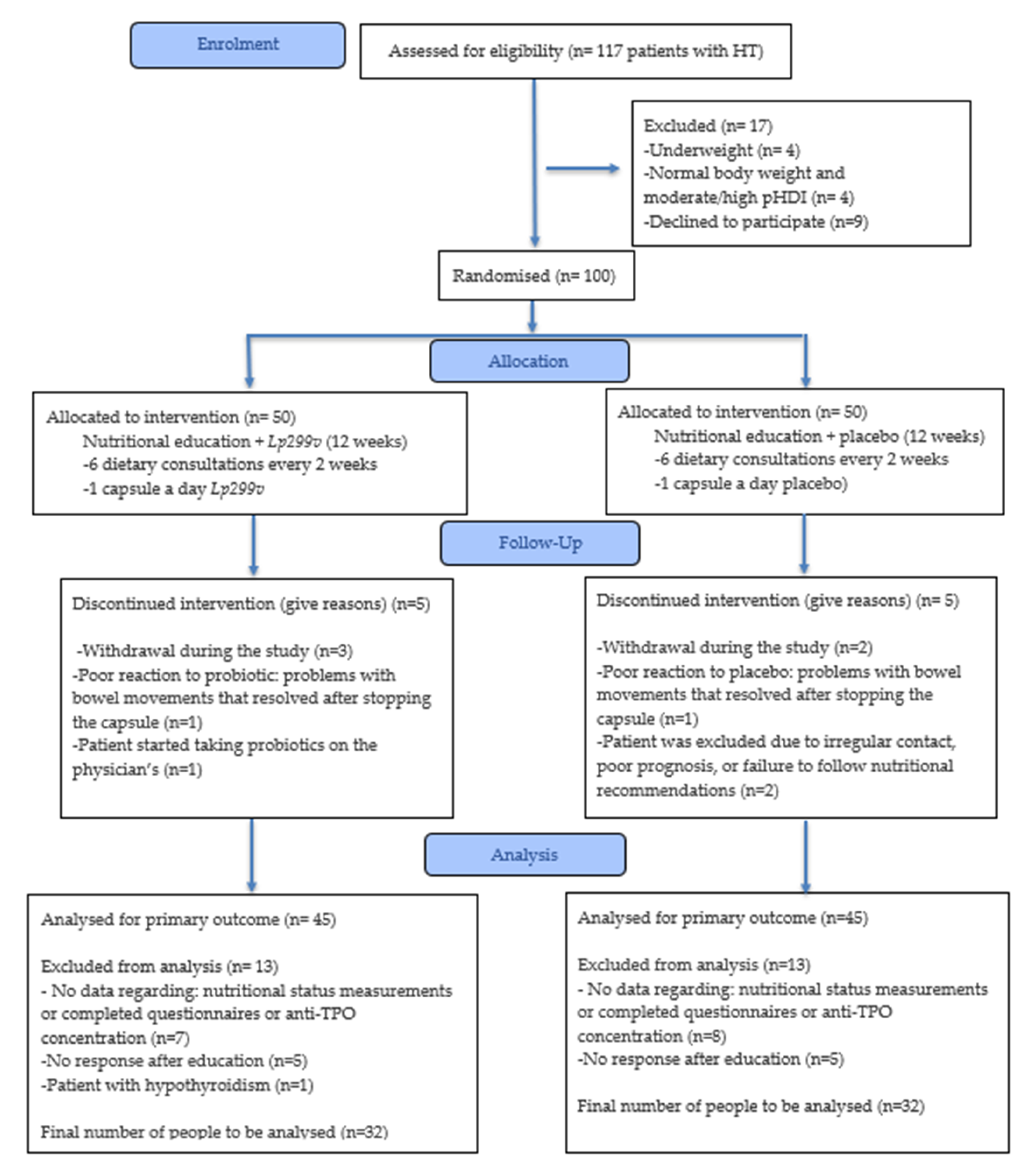
2.2. Participant Selection
2.3. Randomisation and Blinding
2.4. Intervention Procedure
2.5. Nutrient Intake and Diet Quality
2.6. Selected Lifestyle and Health Factors
2.7. Quality of Life
2.8. Nutritional and Health Status
2.9. Nutrition Education
2.10. Lactiplantibacillus plantarum 299v
2.11. Statistical Analyses
3. Results
3.1. Characteristics of the Study Group
3.2. Effect of the Intervention on Nutritional Status and Health Parameters
3.3. Effect of the Intervention on Quality of Life
3.4. Effect of the Nutritional Education on Diet Quality and Dietary Intake
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klubo-Gwiezdzinska, J.; Wartofsky, L. Hashimoto thyroiditis: An evidence-based guide to etiology, diagnosis and treatment. Pol. Arch. Intern. Med. 2022, 132, 16222. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Chen, Y.; Shen, Y.; Tian, R.; Sheng, Y.; Que, H. Global prevalence and epidemiological trends of Hashimoto’s thyroiditis in adults: A systematic review and meta-analysis. Front. Public Health 2022, 10, 1020709. [Google Scholar] [CrossRef] [PubMed]
- Ralli, M.; Angeletti, D.; Fiore, M.; D’Aguanno, V.; Lambiase, A.; Artico, M.; de Vincentiis, M.; Greco, A. Hashimoto’s thyroiditis: An update on pathogenic mechanisms, diagnostic protocols, therapeutic strategies, and potential malignant transformation. Autoimmun. Rev. 2020, 19, 102649. [Google Scholar] [CrossRef] [PubMed]
- Tywanek, E.; Michalak, A.; Świrska, J.; Zwolak, A. Autoimmunity, New Potential Biomarkers and the Thyroid Gland—The Perspective of Hashimoto’s Thyroiditis and Its Treatment. Int. J. Mol. Sci. 2024, 25, 4703. [Google Scholar] [CrossRef]
- Groenewegen, K.L.; Mooij, C.F.; van Trotsenburg, A.S.P. Persisting symptoms in patients with Hashimoto’s disease despite normal thyroid hormone levels: Does thyroid autoimmunity play a role? A systematic review. J. Transl. Autoimmun. 2021, 4, 100101. [Google Scholar] [CrossRef]
- Patti, M.; Christian, R.; Palokas, M. Association between anti-thyroid antibodies and quality of life in patients with Hashimoto thyroiditis: A systematic review and meta-analysis. JBI Evid. Synth. 2021, 19, 2307–2338. [Google Scholar] [CrossRef]
- Li, J.; Huang, Q.; Sun, S.; Zhou, K.; Wang, X.; Pan, K.; Zhang, Y.; Wang, Y.; Han, Q.; Si, C.; et al. Thyroid antibodies in Hashimoto’s thyroiditis patients are positively associated with inflammation and multiple symptoms. Sci. Rep. 2024, 14, 27902. [Google Scholar] [CrossRef]
- National Health Fund in Poland. Statistics of Reimbursed Drugs Levothyroxinum Natricum in 2023 Year. Available online: https://statystyki.nfz.gov.pl/PharmacyRefund?search=true&S.Province=&S.DateFrom=2023-01&S.DateTo=2023-12&S.MedicineProduct=&S.AggregationType=G14&S.ActiveSubstance=Levothyroxinum+natricum&S.AtcName=&S.AgeGroup=&S.RefundationType=&S.Gender=&S.PrivilegesAdditional= (accessed on 24 September 2025).
- Osowiecka, K.; Myszkowska-Ryciak, J. The Influence of Nutritional Intervention in the Treatment of Hashimoto’s Thyroiditis—A Systematic Review. Nutrients 2023, 15, 1041. [Google Scholar] [CrossRef]
- Laganà, M.; Piticchio, T.; Alibrandi, A.; Le Moli, R.; Pallotti, F.; Campennì, A.; Cannavò, S.; Frasca, F.; Ruggeri, R.M. Effects of Dietary Habits on Markers of Oxidative Stress in Subjects with Hashimoto’s Thyroiditis: Comparison Between the Mediterranean Diet and a Gluten-Free Diet. Nutrients 2025, 17, 363. [Google Scholar] [CrossRef]
- Ihnatowicz, P.; Gębski, J.; Drywień, M.E. Effects of Autoimmune Protocol (AIP) diet on changes in thyroid parameters in Hashimoto’s disease. Ann. Agric. Environ. Med. 2023, 30, 513–521. [Google Scholar] [CrossRef]
- Abbott, R.D.; Sadowski, A.; Alt, A.G. Efficacy of the Autoimmune Protocol Diet as Part of a Multi-disciplinary, Supported Lifestyle Intervention for Hashimoto’s Thyroiditis. Cureus 2019, 11, e4556. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.S.; Dai, N.; Xu, J.X.; Xiang, J.Y.; Zheng, X.Z.; Ke, T.Y.; Ma, L.Y.; Shi, Q.H.; Fan, S.F. MRI quantitative assessment of the effects of low-carbohydrate therapy on Hashimoto’s thyroiditis. Endocr. Connect. 2024, 13, e230477. [Google Scholar] [CrossRef] [PubMed]
- Ülker, M.T.; Çolak, G.A.; Baş, M.; Erdem, M.G. Evaluation of the effect of gluten-free diet and Mediterranean diet on autoimmune system in patients with Hashimoto’s thyroiditis. Food Sci. Nutr. 2023, 12, 1180–1188. [Google Scholar] [CrossRef] [PubMed]
- Kamińska, W.; Wiśniewska, K.; Okręglicka, K.; Pazura, I.; Nitsch-Osuch, A. Lifestyle intervention towards Mediterranean Diet, physical activity adherence and anthropometric parameters in normal weight women with Polycystic Ovary Syndrome or Hashimoto’s Thyroiditis—Preliminary study. Ann. Agric. Environ. Med. 2023, 30, 111–117. [Google Scholar] [CrossRef]
- Ruggeri, R.M.; Barbalace, M.C.; Croce, L.; Malaguti, M.; Campennì, A.; Rotondi, M.; Cannavò, S.; Hrelia, S. Autoimmune Thyroid Disorders: The Mediterranean Diet as a Protective Choice. Nutrients 2023, 15, 3953. [Google Scholar] [CrossRef]
- Tang, J.; Shan, S.; Li, F.; Yun, P. Effects of vitamin D supplementation on autoantibodies and thyroid function in patients with Hashimoto’s thyroiditis: A systematic review and meta-analysis. Medicine 2023, 102, e36759. [Google Scholar] [CrossRef]
- Luo, D.; Li, B.; Shan, Z.; Teng, W.; Liu, Q.; Li, J. The impacts of vitamin D supplementation on serum levels of thyroid autoantibodies in patients with autoimmune thyroid disease: A meta-analysis. PeerJ 2025, 13, e19541. [Google Scholar] [CrossRef]
- Huwiler, V.V.; Maissen-Abgottspon, S.; Stanga, Z.; Mühlebach, S.; Trepp, R.; Bally, L.; Bano, A. Selenium Supplementation in Patients with Hashimoto Thyroiditis: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Thyroid 2024, 34, 295–313. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Y.; Yang, L.; Luo, Z.; Wu, Z.; Wang, J.; Qin, S.; Ren, F.; Hu, T. Inverse association between serum iron levels and Hashimoto’s thyroiditis in United States females of reproductive age: Analysis of the NHANES 2007–2012. Front. Nutr. 2024, 11, 1410538. [Google Scholar] [CrossRef]
- Gierach, M.; Rudewicz, M.; Junik, R. Iron and ferritin deficiency in women with hypothyroidism and chronic lymphocytic thyroiditis—Systematic review. Endokrynol. Pol. 2024, 75, 253–261. [Google Scholar] [CrossRef]
- Garofalo, V.; Condorelli, R.A.; Cannarella, R.; Aversa, A.; Calogero, A.E.; La Vignera, S. Relationship between Iron Deficiency and Thyroid Function: A Systematic Review and Meta-Analysis. Nutrients 2023, 15, 4790. [Google Scholar] [CrossRef]
- Chen, L.; Mao, Y.; Chen, G. Association between total vitamin C intake and hypothyroidism among Hashimoto thyroiditis: National Health and Nutrition Examination Survey, 2007–2012. Br. J. Nutr. 2024, 132, 1575–1583. [Google Scholar] [CrossRef]
- Kravchenko, V.; Zakharchenko, T. Thyroid hormones and minerals in immunocorrection of disorders in autoimmune thyroid diseases. Front. Endocrinol. 2023, 14, 1225494. [Google Scholar] [CrossRef] [PubMed]
- Köhrle, J. Selenium, Iodine and Iron–Essential Trace Elements for Thyroid Hormone Synthesis and Metabolism. Int. J. Mol. Sci. 2023, 24, 3393. [Google Scholar] [CrossRef] [PubMed]
- Szczuko, M.; Zawadzka, K.; Szczuko, U.; Rudak, L.; Pobłocki, J. The Significance and Process of Inflammation Involving Eicosapentaenoic and Docosahexaenoic Derivatives in Hashimoto’s Disease. Nutrients 2025, 17, 1715. [Google Scholar] [CrossRef] [PubMed]
- Ramezani, M.; Reisian, M.; Sajadi Hezaveh, Z. The effect of synbiotic supplementation on hypothyroidism: A randomized double-blind placebo controlled clinical trial. PLoS ONE 2023, 18, e0277213. [Google Scholar] [CrossRef]
- AkbariRad, M.; Mazloum Khorasani, Z.; Hemmatpur, A.; Firoozi, A.; Bakhshi, A.; Ravanshad, S.; Mofatteh, A.; Mehrad-Majd, H. Effects of probiotics on thyroid function and fatigue in hypothyroid patients: A randomized placebo controlled trial. Endocrinol. Res. Pract. 2025, 29, 203–210. [Google Scholar] [CrossRef]
- Zawadzka, K.; Kałuzińska, K.; Świerz, M.J.; Sawiec, Z.; Antonowicz, E.; Leończyk-Spórna, M.; Abadi, A.K.; Trofimiuk-Müldner, M.; Bała, M.M. Are probiotics, prebiotics, and synbiotics beneficial in primary thyroid diseases? A systematic review with meta-analysis. Ann. Agric. Environ. Med. 2023, 30, 217–223. [Google Scholar] [CrossRef]
- Shu, Q.; Kang, C.; Li, J.; Hou, Z.; Xiong, M.; Wang, X.; Peng, H. Effect of probiotics or prebiotics on thyroid function: A meta-analysis of eight randomized controlled trials. PLoS ONE 2024, 19, e0296733. [Google Scholar] [CrossRef]
- Nordström, E.A.; Teixeira, C.; Montelius, C.; Jeppsson, B.; Larsson, N. Lactiplantibacillus plantarum 299v (LP299V®): Three decades of research. Benef. Microbes 2021, 12, 441–465. [Google Scholar] [CrossRef]
- Hofeld, B.C.; Puppala, V.K.; Tyagi, S.; Ahn, K.W.; Anger, A.; Jia, S.; Salzman, N.H.; Hessner, M.J.; Widlansky, M.E. Lactobacillus plantarum 299v probiotic supplementation in men with stable coronary artery disease suppresses systemic inflammation. Sci. Rep. 2021, 11, 3972. [Google Scholar] [CrossRef] [PubMed]
- Malik, M.; Suboc, T.M.; Tyagi, S.; Salzman, N.; Wang, J.; Ying, R.; Tanner, M.J.; Kakarla, M.; Baker, J.E.; Widlansky, M.E. Lactobacillus plantarum 299v Supplementation Improves Vascular Endothelial Function and Reduces Inflammatory Biomarkers in Men with Stable Coronary Artery Disease. Circ. Res. 2018, 123, 1091–1102. [Google Scholar] [CrossRef] [PubMed]
- Apte, A.; Parge, A.; Nimkar, R.; Sinha, A. Effect of probiotic and prebiotics supplementation on hemoglobin levels and iron absorption among women of reproductive age and children: A systematic review and meta-analysis. BMC Nutr. 2025, 11, 31. [Google Scholar] [CrossRef] [PubMed]
- Vonderheid, S.C.; Tussing-Humphreys, L.; Park, C.; Pauls, H.; OjiNjideka Hemphill, N.; LaBomascus, B.; McLeod, A.; Koenig, M.D. A Systematic Review and Meta-Analysis on the Effects of Probiotic Species on Iron Absorption and Iron Status. Nutrients 2019, 11, 2938. [Google Scholar] [CrossRef]
- Marlicz, W.; Skonieczna-Żydecka, K.; Krynicka, P.; Łoniewski, I.; Rydzewska, G. Probiotics in irritable bowel syndrome—Is the quest for the right strain over? Rapid review of existing guidelines and recommendations. Prz. Gastroenterol. 2021, 16, 369–382. [Google Scholar] [CrossRef]
- Rudzki, L.; Ostrowska, L.; Pawlak, D.; Małus, A.; Pawlak, K.; Waszkiewicz, N.; Szulc, A. Probiotic Lactobacillus Plantarum 299v decreases kynurenine concentration and improves cognitive functions in patients with major depression: A double-blind, randomized, placebo controlled study. Psychoneuroendocrinology 2019, 100, 213–222. [Google Scholar] [CrossRef]
- Jezewska-Zychowicz, M.; Gawecki, J.; Wadolowska, L.; Czarnocinska, J.; Galinski, G.; Kollajtis-Dolowy, A.; Roszkowski, W.; Wawrzyniak, A.; Przybylowicz, K.; Stasiewicz, B.; et al. Dietary Habits and Nutrition Beliefs Questionnaire and the Manual for Developing of Nutritional Data, 3rd ed.; Gawęcki, J., Ed.; Polish Academy of Sciences: Warsaw, Poland, 2024; Available online: https://diettools4u.uwm.edu.pl/kompan/ (accessed on 24 September 2025).
- Niedzwiedzka, E.; Wadolowska, L.; Kowalkowska, J. Reproducibility of A Non-Quantitative Food Frequency Questionnaire (62-Item FFQ-6) and PCA-Driven Dietary Pattern Identification in 13–21-Year-Old Females. Nutrients 2019, 11, 2183. [Google Scholar] [CrossRef]
- Sawicka-Gutaj, N.; Watt, T.; Sowiński, J.; Gutaj, P.; Waligórska-Stachura, J.; Ruchała, M. ThyPROpl—The Polish version of the thyroid-specific quality of life questionnaire ThyPRO. Endokrynol. Pol. 2015, 66, 367–380. [Google Scholar] [CrossRef]
- Kulich, K.R.; Reguła, J.; Stasiewicz, J.; Jasinski, B.; Carlsson, J.; Wiklund, I. Psychometric validation of the Polish translation of the Gastrointestinal Symptom Rating Scale (GSRS) and Quality of Life in Reflux and Dyspepsia (QOLRAD) Questionnaire in patients with reflux disease. Pol. Arch. Med. Wewn. 2005, 113, 241–249. [Google Scholar]
- Kostelecki, G.; Całyniuk, B.; Zajchowska, S.; Myszkowska-Ryciak, J.; Janiszewska, K.; Bronkowska, M.; Madej-Babula, M.; Lange, E.; Gajewska, D.; Pająk, R. Guidelines of the Polish Society of Dietetics and the National Consultant in the Field of Family Medicine on Providing Dietary Consultations as Part of Coordinated Care in Primary Health Care of 31/01/2023. Available online: https://www.gov.pl/web/zdrowie/wytyczne-dotyczace-udzielania-konsultacji-dietetycznych (accessed on 24 September 2025).
- Kunachowicz, H.; Przygoda, B.; Nadolna, I.; Iwanow, K. Tables of Food Composition and Nutritional Values; PZWL: Warsaw, Poland, 2017. [Google Scholar]
- United States Department of Agriculture (USDA) Nutrient Database. Available online: https://fdc.nal.usda.gov/ (accessed on 24 September 2025).
- Jarosz, M.; Rychlik, E.; Stoś, K.; Charzewska, J. (Eds.) Nutrition Standards for the Polish Population and Their Application; National Institute of Public Health—National Institute of Hygiene: Warsaw, Poland, 2020. [Google Scholar]
- Mifflin, M.D.; St Jeor, S.T.; Hill, L.A.; Scott, B.J.; Daugherty, S.A.; Koh, Y.O. A New Predictive Equation for Resting Energy Expenditure in Healthy Individuals. Am. J. Clin. Nutr. 1990, 51, 241–247. [Google Scholar] [CrossRef]
- Nutrition Division. Human Energy Requirements; Report of a Joint FAO/WHO/UNU Expert Consultation; FAO/WHO/UNU: Rome, Italy, 2004; Available online: https://openknowledge.fao.org/items/8282e44e-5197-4416-885b-250631682d14 (accessed on 24 September 2025).
- National Health and Nutrition Examination Survey (U.S.); National Center for Health Statistics (U.S.). Antropometry Procedures Manual; National Health and Nutrition Examination Survey (NHANES): Washington, DC, USA, 2017. Available online: https://stacks.cdc.gov/view/cdc/51795 (accessed on 24 September 2025).
- World Health Organization. Waist Circumference and Waist-Hip Ratio; Report of a WHO Expert Consultation; WHO: Geneva, Switzerland, 2008; Available online: https://www.who.int/publications/i/item/9789241501491 (accessed on 24 September 2025).
- Yoo, E.G. Waist-to-Height Ratio as a Screening Tool for Obesity and Cardiometabolic Risk. Korean J. Pediatr. 2016, 59, 431. [Google Scholar] [CrossRef]
- Ashwell, M.; Gibson, S. Waist-to-Height Ratio as an Indicator of “Early Health Risk”: Simpler and More Predictive than Using a “matrix” Based on BMI and Waist Circumference. BMJ Open 2016, 6, e010159. [Google Scholar] [CrossRef]
- A Healthy Lifestyle—WHO Recommendations. Available online: https://www.who.int/europe/news-room/fact-sheets/item/nutrition---maintaining-a-healthy-lifestyle (accessed on 24 September 2025).
- Osowiecka, K.; Skrypnik, D.; Myszkowska-Ryciak, J. Assessment of the Impact of Nutritional Intervention with the Probiotic Lactiplantibacillus plantarum 299v on Nutritional Status and Quality of Life of Hashimoto’s Thyroiditis Patients—A Randomized Double-Blind Study Protocol. J. Pers. Med. 2023, 13, 1659. [Google Scholar] [CrossRef]
- Shady, M.A.; Adly, N.N.; Ibrahim, S.; Aboelyazed, S. The Impact of Mediterranean Diet on Patients with Hashimoto Thyroiditis. QJM Int. J. Med. 2024, 117, hcae175.472. [Google Scholar] [CrossRef]
- Vallianou, N.G.; Kounatidis, D.; Tsilingiris, D.; Panagopoulos, F.; Christodoulatos, G.S.; Evangelopoulos, A.; Karampela, I.; Dalamaga, M. The Role of Next-Generation Probiotics in Obesity and Obesity-Associated Disorders: Current Knowledge and Future Perspectives. Int. J. Mol. Sci. 2023, 24, 6755. [Google Scholar] [CrossRef]
- Liu, E.; Ji, X.; Zhou, K. Akkermansia muciniphila for the Prevention of Type 2 Diabetes and Obesity: A Meta-Analysis of Animal Studies. Nutrients 2024, 16, 3440. [Google Scholar] [CrossRef]
- Okuka, N.; Milinkovic, N.; Velickovic, K.; Polovina, S.; Sumarac-Dumanovic, M.; Minic, R.; Korčok, D.; Djordjevic, B.; Ivanovic, N.D. Beneficial effects of a new probiotic formulation on adipocytokines, appetite-regulating hormones, and metabolic parameters in obese women. Food Funct. 2024, 15, 7658–7668. [Google Scholar] [CrossRef]
- Bakaloudi, D.R.; Chrysoula, L.; Leonida, I.; Kotzakioulafi, E.; Theodoridis, X.; Chourdakis, M. Impact of the level of adherence to the Mediterranean Diet on blood pressure: A systematic review and meta-analysis of observational studies. Clin. Nutr. 2021, 40, 5771–5780. [Google Scholar] [CrossRef] [PubMed]
- Filippou, C.D.; Thomopoulos, C.G.; Kouremeti, M.M.; Sotiropoulou, L.I.; Nihoyannopoulos, P.I.; Tousoulis, D.M.; Tsioufis, C.P. Mediterranean diet and blood pressure reduction in adults with and without hypertension: A systematic review and meta-analysis of randomized controlled trials. Clin. Nutr. 2021, 40, 3191–3200. [Google Scholar] [CrossRef] [PubMed]
- Cowell, O.R.; Mistry, N.; Deighton, K.; Matu, J.; Griffiths, A.; Minihane, A.M.; Mathers, J.C.; Shannon, O.M.; Siervo, M. Effects of a Mediterranean diet on blood pressure: A systematic review and meta-analysis of randomized controlled trials and observational studies. J. Hypertens. 2021, 39, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Naruszewicz, M.; Johansson, M.L.; Zapolska-Downar, D.; Bukowska, H. Effect of Lactobacillus plantarum 299v on cardiovascular disease risk factors in smokers. Am. J. Clin. Nutr. 2002, 76, 1249–1255. [Google Scholar] [CrossRef]
- Talebi, S.; Karimifar, M.; Heidari, Z.; Mohammadi, H.; Askari, G. The effects of synbiotic supplementation on thyroid function and inflammation in hypothyroid patients: A randomized, double-blind, placebo-controlled trial. Complement. Ther. Med. 2020, 48, 102234. [Google Scholar] [CrossRef]
- Piticchio, T.; Frasca, F.; Malandrino, P.; Trimboli, P.; Carrubba, N.; Tumminia, A.; Vinciguerra, F.; Frittitta, L. Effect of gluten-free diet on autoimmune thyroiditis progression in patients with no symptoms or histology of celiac disease: A meta-analysis. Front. Endocrinol. 2023, 14, 1200372. [Google Scholar] [CrossRef] [PubMed]
- Ostrowska, L.; Gier, D.; Zyśk, B. The Influence of Reducing Diets on Changes in Thyroid Parameters in Women Suffering from Obesity and Hashimoto’s Disease. Nutrients 2021, 13, 862. [Google Scholar] [CrossRef] [PubMed]
- Dai, N.; Shi, Q.H.; Zheng, L.W.; Huang, X.S.; Fan, S.F. Quantitative Multi-Parameter MRI Evaluation of Hashimoto’s Thyroiditis Changes After Dietary Interventions. Med. Sci. Monit. 2025, 31, e947862. [Google Scholar] [CrossRef] [PubMed]
- Danailova, Y.; Velikova, T.; Nikolaev, G.; Mitova, Z.; Shinkov, A.; Gagov, H.; Konakchieva, R. Nutritional Management of Thyroiditis of Hashimoto. Int. J. Mol. Sci. 2022, 23, 5144. [Google Scholar] [CrossRef]
- Barbalace, M.C.; Talotta, R.; Rapisarda, F.; D’Amico, V.; Laganà, M.; Malaguti, M.; Campennì, A.; Cannavò, S.; Hrelia, S.; Ruggeri, R.M. Unlocking the Power of the Mediterranean Diet: Two in One—Dual Benefits for Rheumatic and Thyroid Autoimmune Diseases. Nutrients 2025, 17, 1383. [Google Scholar] [CrossRef]
- Alijani, S.; Ghadir, M.; Gargari, B.P. The association between dietary inflammatory index and dietary total antioxidant capacity and Hashimoto’s thyroiditis: A case-control study. BMC Endocr. Disord. 2024, 24, 177. [Google Scholar] [CrossRef]
- Rostami, R.; Beiranvand, A.; Nourooz-Zadeh, S.; Rostami, M.; Mohammadi, A.; Nourooz-Zadeh, J. Association Between Essential Trace Elements and Thyroid Antibodies in the Blood of Women with Newly Diagnosed Hashimoto’s Thyroiditis. Int. J. Endocrinol. Metab. 2024, 22, e145599. [Google Scholar] [CrossRef]
- Wang, K.; Wei, H.; Zhang, W.; Li, Z.; Ding, L.; Yu, T.; Tan, L.; Liu, Y.; Liu, T.; Wang, H.; et al. Severely low serum magnesium is associated with increased risks of positive anti-thyroglobulin antibody and hypothyroidism: A cross-sectional study. Sci. Rep. 2018, 8, 9904. [Google Scholar] [CrossRef]
- Celik, E.; Celik, M.; Bulbul, B.Y.; Andac, B.; Okur, M.; Colak, S.Y.; Yekdes, A.C. Immunological harmony: The role of magnesium in the development of euthyroid Hashimoto’s thyroiditis. J. Elem. 2024, 29, 367–378. [Google Scholar] [CrossRef]
- Khan, S.Z.A.; Lungba, R.M.; Ajibawo-Aganbi, U.; Veliginti, S.; Perez Bastidas, M.V.; Saleem, S.; Cancarevic, I. Minerals: An Untapped Remedy for Autoimmune Hypothyroidism? Cureus 2020, 12, e11008. [Google Scholar] [CrossRef] [PubMed]
- An, P.; Wang, S.; Liu, L.; Li, X.; Lv, X. The association between dietary sodium density and Hashimoto’s thyroiditis in US adults. Front. Nutr. 2025, 12, 1508195. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, Y.; Li, H.; Li, H. Effects of vitamin D on thyroid autoimmunity markers in Hashimoto’s thyroiditis: Systematic review and meta-analysis. J. Int. Med. Res. 2021, 49, 3000605211060675. [Google Scholar] [CrossRef] [PubMed]
- Zivaljevic, V.R.; Bukvic Bacotic, B.R.; Sipetic, S.B.; Stanisavljevic, D.M.; Maksimovic, J.M.; Diklic, A.D.; Paunovic, I.R. Quality of life improvement in patients with Hashimoto thyroiditis and other goiters after surgery: A prospective cohort study. Int. J. Surg. 2015, 21, 150–155. [Google Scholar] [CrossRef]
- Jonklaas, J. Restoration of euthyroidism with levothyroxine: Implications of etiology of hypothyroidism and the degree of residual endogenous thyroid function. Front. Endocrinol. 2022, 13, 934003. [Google Scholar] [CrossRef]
- Borson-Chazot, F.; Terra, J.-L.; Goichot, B.; Caron, P. What Is the Quality of Life in Patients Treated with Levothyroxine for Hypothyroidism and How Are We Measuring It? A Critical, Narrative Review. J. Clin. Med. 2021, 10, 1386. [Google Scholar] [CrossRef]
- Siegmann, E.M.; Müller, H.H.O.; Luecke, C.; Philipsen, A.; Kornhuber, J.; Grömer, T.W. Association of Depression and Anxiety Disorders with Autoimmune Thyroiditis: A Systematic Review and Meta-analysis. JAMA Psychiatry 2018, 75, 577–584. [Google Scholar] [CrossRef]
- Krysiak, R.; Kowalcze, K.; Szkróbka, W.; Okopień, B. Sexual Function and Depressive Symptoms in Young Women with Euthyroid Hashimoto’s Thyroiditis Receiving Vitamin D, Selenomethionine and Myo-Inositol: A Pilot Study. Nutrients 2023, 15, 2815. [Google Scholar] [CrossRef]
- Xu, G.M.; Hu, M.X.; Li, S.Y.; Ran, X.; Zhang, H.; Ding, X.F. Thyroid disorders and gastrointestinal dysmotility: An old association. Front. Physiol. 2024, 15, 1389113. [Google Scholar] [CrossRef]
- Le Morvan de Sequeira, C.; Kaeber, M.; Cekin, S.E.; Enck, P.; Mack, I. The Effect of Probiotics on Quality of Life, Depression and Anxiety in Patients with Irritable Bowel Syndrome: A Systematic Review and Meta-Analysis. J. Clin. Med. 2021, 10, 3497. [Google Scholar] [CrossRef] [PubMed]
- Agah, S.; Akbari, A.; Heshmati, J.; Sepidarkish, M.; Morvaridzadeh, M.; Adibi, P.; Mazidi, M.; Farsi, F.; Ofori-Asenso, R.; Talley, N.J.; et al. Systematic review with meta-analysis: Effects of probiotic supplementation on symptoms in functional dyspepsia. J. Funct. Foods 2020, 68, 103902, ISSN 1756-4646. [Google Scholar] [CrossRef]
- Yang, S.-J.; Nguyen, T.T.M.; Jin, X.; Zheng, Q.; Park, S.-J.; Yi, G.-S.; Yi, T.-H. A PRISMA Systematic Review of Sexual Dysfunction and Probiotics with Pathophysiological Mechanisms. Biology 2025, 14, 286. [Google Scholar] [CrossRef] [PubMed]
- Pano, O.; Gamba, M.; Bullón-Vela, V.; Aguilera-Buenosvinos, I.; Roa-Díaz, Z.M.; Minder, B.; Kopp-Heim, D.; Laine, J.E.; Martínez-González, M.Á.; Martinez, A.; et al. Eating behaviors and health-related quality of life: A scoping review. Maturitas 2022, 165, 58–71. [Google Scholar] [CrossRef]
- Selvaraj, R.; Selvamani, T.Y.; Zahra, A.; Malla, J.; Dhanoa, R.K.; Venugopal, S.; Shoukrie, S.I.; Hamouda, R.K.; Hamid, P. Association Between Dietary Habits and Depression: A Systematic Review. Cureus 2022, 14, e32359. [Google Scholar] [CrossRef]
- Godos, J.; Guglielmetti, M.; Ferraris, C.; Frias-Toral, E.; Domínguez Azpíroz, I.; Lipari, V.; Di Mauro, A.; Furnari, F.; Castellano, S.; Galvano, F.; et al. Mediterranean Diet and Quality of Life in Adults: A Systematic Review. Nutrients 2025, 17, 577. [Google Scholar] [CrossRef]
- Schönenberger, K.A.; Schüpfer, A.-C.; Gloy, V.L.; Hasler, P.; Stanga, Z.; Kaegi-Braun, N.; Reber, E. Effect of Anti-Inflammatory Diets on Pain in Rheumatoid Arthritis: A Systematic Review and Meta-Analysis. Nutrients 2021, 13, 4221. [Google Scholar] [CrossRef]
- Nelson, J.; Sjöblom, H.; Gjertsson, I.; Ulven, S.M.; Lindqvist, H.M.; Bärebring, L. Do Interventions with Diet or Dietary Supplements Reduce the Disease Activity Score in Rheumatoid Arthritis? A Systematic Review of Randomized Controlled Trials. Nutrients 2020, 12, 2991. [Google Scholar] [CrossRef]
- Grosu, C.; Ignat, E.B.; Alexa, D.; Ciubotaru, A.; Leon, M.M.; Maștaleru, A.; Popescu, G.; Cumpăt, C.M.; Cucu, L.-E.; Smihor, M.I.; et al. The Role of Nutrition and Physical Activity in Modulating Disease Progression and Quality of Life in Multiple Sclerosis. Nutrients 2025, 17, 2713. [Google Scholar] [CrossRef]
- Garicano Vilar, E.; López Oliva, S.; Penadés, B.F.; Sánchez Niño, G.M.; Terrén Lora, A.; Sanz Rojo, S.; Mauro Martín, I.S. Mediterranean Diet Effect on the Intestinal Microbiota, Symptoms, and Markers in Patients with Functional Gastrointestinal Disorders. Microorganisms 2024, 12, 1969. [Google Scholar] [CrossRef]
- Gong, B.; Wang, C.; Meng, F.; Wang, H.; Song, B.; Yang, Y.; Shan, Z. Association Between Gut Microbiota and Autoimmune Thyroid Disease: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2021, 12, 774362. [Google Scholar] [CrossRef]
- Zang, Y.; Lai, X.; Li, C.; Ding, D.; Wang, Y.; Zhu, Y. The Role of Gut Microbiota in Various Neurological and Psychiatric Disorders-An Evidence Mapping Based on Quantified Evidence. Mediators Inflamm. 2023, 2023, 5127157. [Google Scholar] [CrossRef]
| Parameters | NE+Lp299v Group (n = 32) | NE+P Group (n = 32) | p-Value |
|---|---|---|---|
| Age [years] | 41.53 ± 11.77 | 39.28 ± 8.97 | 0.393 † |
| Mean ± SD (Me; Min–Max) | (41.00; 22–64) | (39.50; 22–54) | |
| Levothyroxine administration N (%) | 0.286 ‡ | ||
| No | 5 (15.63) | 7 (21.88) | |
| Yes | 27 (84.38) | 25 (78.12) |
| Parameters | NE+Lp299v Group (n = 32) | NE+P Group (n = 32) | ANOVA p-Value | Magnitude of Change ∆ | p-Value | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Post Intervention | Baseline | Post Intervention | Group Effect | Time Effect | Time × Group Effect | NE+Lp299v | NE+P | ||
| BMI [kg/m2] | 28.8 ± 4.69 | 28.8 ± 4.87 | 26.9 ± 4.65 | 27.0± 4.64 | 0.115 | 0.980 | 0.688 | −0.05 ± 1.20 | 0.05 ± 0.75 | 0.804 † |
| Waist circumstance [cm] | 95.1 ± 13.36 | 95.0 ± 12.98 | 90.7 ± 12.18 | 89.7 ± 11.66 | 0.124 | 0.261 | 0.292 | −0.44 ± 3.40 | −0.95 ± 2.77 | 0.509‡ |
| Hip circumstance [cm] | 109.8 ± 8.83 A | 107.5 ± 8.78 B | 106.2 ± 8.15 | 105.6 ± 8.36 | 0.192 | 0.009 | 0.089 | −2.25 ± 3.23 | −0.5 ± 4.74 | 0.167 † |
| WHR | 0.86 ± 0.08 | 0.88 ± 0.08 * | 0.85 ± 0.08 | 0.80 ± 0.06 * | 0.205 | 0.147 | 0.008 | 0.02 ± 0.03 | −0.01 ± 0.04 | 0.030 † |
| WHtR | 0.57 ± 0.08 | 0.57 ± 0.08 | 0.54 ± 0.08 | 0.50 ± 0.07 | 0.096 | 0.289 | 0.294 | 0.0 ± 0.02 | −0.01 ± 0.02 | 0.294 ‡ |
| Fat mass [kg] & | 29.7 ± 9.34 | 29.9 ± 9.78 | 26.4 ± 9.45 | 26.2 ± 9.13 | 0.156 | 0.942 | 0.332 | 0.28 ± 2.25 | −0.24 ± 1.76 | 0.331 ‡ |
| Fat-free mass [kg] & | 48.8 ± 6.06 | 48.6 ± 6.42 | 48.8 ± 4.99 | 49.1 ± 5.10 | 0.849 | 0.714 | 0.273 | −0.15 ± 1.39 | 0.31 ± 1.78 | 0.476 † |
| Muscle mass [kg] & | 27.2 ± 3.41 | 27.1 ± 3.57 | 27.2 ± 2.80 | 27.4 ± 2.83 | 0.857 | 0.746 | 0.332 | −0.08 ± 0.76 | 0.16 ± 1.02 | 0.050 † |
| TBW [Lt.] & | 35.7 ± 4.46 | 35.6 ± 4.67 | 35.7 ± 3.67 | 35.9 ± 3.68 | 0.849 | 0.760 | 0.349 | −0.10 ± 1.01 | 0.20 ± 1.34 | 0.062 † |
| Systolic pressure [mmHg] | 129.4 ± 14.05 a,A,* | 121.1 ± 14.42 B | 118.4 ± 12.13 b | 117.5 ± 14.24 * | 0.019 | 0.006 | 0.029 | −8.33 ± 13.45 | −0.95 ± 12.86 | 0.028 ‡ |
| Diastolic pressure [mmHg] | 76.2 ± 9.69 A | 71.0 ± 8.29 B | 69.8 ± 10.02 | 68.9 ± 11.26 | 0.053 | 0.015 | 0.077 | −5.22 ± 8.88 | −0.86 ± 10.44 | 0.077 ‡ |
| Pulse [bpm] | 71.59 ± 11.19 | 71.89 ± 10.49 | 70.47 ± 8.72 | 75.7 ± 14.08 | 0.573 | 0.065 | 0.099 | 0.30 ± 8.85 | 5.27 ± 14.26 | 0.124 † |
| Anti-TPO [IU/mL] | 453.1 ± 525 | 418.4 ± 467.32 | 318.9 ± 443.58 | 278.8 ± 411.01 | 0.235 | 0.065 | 0.892 | −34.64 ± 203.31 | −40.07 ± 97.14 | 0.416 † |
| Parameters | NE+Lp299v Group (n = 32) | NE+P Group (n = 32) | ANOVA p-Value | ATE | ||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Post Intervention | Baseline | Post Intervention | Group Effect | Time Effect | Time × Group Effect | ||
| ThyPROpl | ||||||||
| Goitrogen symptoms | 24.79 ± 20.22 | 18.18 ± 17.08 | 13.85 ± 13.97 | 13.99 ± 13.42 | 0.045 | 0.075 | 0.063 | −6.75 |
| Hyperthyroid symptoms | 36.23 ± 25.88 A | 26.17 ± 21.12 B | 33.20 ± 17.04 A | 21.48 ± 17.09 B | 0.416 | <0.001 | 0.692 | 1.66 |
| Hypothyroid symptoms | 48.83 ± 25.57 | 37.89 ± 23.17 | 43.95 ± 22.98 | 37.30 ± 18.61 | 0.577 | 0.004 | 0.463 | −4.30 |
| Eye symptoms | 33.89 ± 24.21 A | 25.00 ± 18.98 B | 24.32 ± 18.71 | 18.26 ± 15.74 | 0.070 | 0.001 | 0.511 | −2.83 |
| Tiredness | 67.97 ± 21.09 A | 54.13 ± 22.75 B | 71.65 ± 14.82 A | 58.37 ± 18.43 B | 0.351 | <0.001 | 0.910 | −0.56 |
| Cognitive complaints | 42.45 ± 26.04 A | 29.30 ± 22.29 B | 36.59 ± 22.34 | 30.60 ± 21.21 | 0.660 | <0.001 | 0.167 | −7.16 |
| Anxiety | 41.15 ± 23.37 A | 25.65 ± 19.32 B | 41.41 ± 18.96 | 34.64 ± 20.94 | 0.314 | <0.001 | 0.081 | −8.72 |
| Depressivity | 51.90 ± 24.19 A | 39.17 ± 21.01 B | 45.54 ± 22.28 | 41.18 ± 21.12 | 0.649 | 0.004 | 0.148 | −8.37 |
| Emotional susceptibility | 48.70 ± 23.35 A | 34.38 ± 16.68 B | 45.31 ± 21.88 | 36.72 ± 17.69 | 0.904 | <0.001 | 0.268 | −5.73 |
| Impaired social life | 23.83 ± 19.66 A | 13.28 ± 18.63 B | 20.51 ± 17.98 | 16.41 ± 18.83 | 0.981 | 0.002 | 0.149 | −6.44 |
| Impaired daily life | 32.66 ± 26.28 A | 16.72 ± 24.32 B | 24.35 ± 18.09 | 15.70 ± 19.31 | 0.338 | <0.001 | 0.192 | −7.29 |
| Impaired sex life | 36.72 ± 36.33 A | 19.92 ± 28.19 B | 35.16 ± 32.13 | 34.38 ± 34.64 | 0.387 | 0.018 | 0.031 | −16.01 |
| Cosmetic complaints | 45.18 ± 27.98 A | 26.95 ± 23.23 B | 36.20 ± 21.20 | 28.13 ± 23.50 | 0.462 | <0.001 | 0.087 | −10.16 |
| Overall quality of life | 60.94 ± 32.96 A | 35.94 ± 34.74 B | 54.69 ± 26.52 A | 39.84 ± 29.69 B | 0.867 | <0.001 | 0.146 | −10.16 |
| GSRS | ||||||||
| Diarrhoea | 2.06 ± 1.52 | 1.93 ± 1.35 | 2.49 ± 1.52 | 1.91 ± 1.21 | 0.505 | 0.049 | 0.215 | 0.45 |
| Indigestion | 3.23 ± 1.49 | 2.67 ± 1.10 | 3.32 ± 1.13 A | 2.52 ± 1.10 B | 0.907 | <0.001 | 0.426 | 0.23 |
| Constipation | 2.89 ± 1.79 A | 2.11 ± 1.29 B | 2.75 ± 1.64 | 2.06 ± 1.52 | 0.785 | <0.001 | 0.830 | −0.08 |
| Abdominal pain | 2.78 ± 1.31 A | 2.08 ± 0.92 B | 2.75 ± 0.97 A | 2.13 ± 1.13 B | 0.982 | <0.001 | 0.795 | −0.07 |
| Reflux | 2.06 ± 1.34 A | 1.45 ± 0.86 B | 2.03 ± 1.16 | 1.64 ± 0.94 | 0.730 | 0.002 | 0.482 | −0.25 |
| Parameters | NE+Lp299v Group (n = 32) | NE+P Group (n = 32) | ANOVA p-Value | Magnitude of the Change ∆ | p-Value | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Post Intervention | Baseline | Post Intervention | Group Effect | Time Effect | Group × Time Effect | NE+Lp299v Group | NE+P Group | ||
| pHDI-10 | 25.47 ± 11.49 | 28.43 ± 10.06 | 26.19 ± 8.51 | 28.07 ± 9.83 | 0.933 | 0.050 | 0.656 | 2.96 ± 8.04 | 1.88 ± 11.09 | 0.656 ‡ |
| nHDI-14 | 14.44 ± 6.74 A | 9.93 ± 5.00 B | 13.85 ± 7.71 A | 8.89 ± 5.28 B | 0.558 | <0.001 | 0.764 | −4.50 ± 5.88 | −4.96 ± 6.20 | 0.930 † |
| DQI | 11.03 ± 11.92 A | 18.50 ± 11.05 B | 12.34 ± 9.58 A | 19.18 ± 9.39 B | 0.649 | <0.001 | 0.832 | 7.47 ± 10.18 | 6.84 ± 13.28 | 0.930 † |
| Parameters | NE+Lp299v Group (n = 26) | NE+P Group (n = 23) | ANOVA p-Value | Magnitude of the Change ∆ | p-Value | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Post Intervention | Baseline | Post Intervention | Group Effect | Time Effect | Group × Time Effect | NE+Lp299v Group | NE+P Group | ||
| Energy [kcal] | 1990.2 ± 456.64 A | 1723.5 ± 322.55 B | 1964.5 ± 333.27 | 1789.6 ± 226.44 | 0.812 | <0.001 | 0.391 | −266.7 ± 413.24 | −174.9 ± 314.09 | 0.602 † |
| Protein [g] | 85.4 ± 19.70 | 87.4 ± 15.40 | 86.8 ± 17.43 | 82.8 ± 14.33 | 0.679 | 0.720 | 0.318 | 1.9 ± 20.10 | −4.0 ± 21.16 | 0.318 ‡ |
| Fat [g] | 78.7 ± 26.10 | 69.0 ± 19.18 | 79.2 ± 17.63 | 74.0 ± 16.76 | 0.596 | 0.014 | 0.451 | −9.6 ± 23.26 | −5.2 ± 16.07 | 0.451 ‡ |
| Carbohydrates [g] | 240.4 ± 62.51 A | 195.6 ± 47.46 B | 231.0 ± 61.61 | 207.4 ± 40.56 | 0.928 | <0.001 | 0.239 | −44.8 ± 64.55 | −23.6 ± 58.91 | 0.249 † |
| Dietary fibre [g] | 25.0 ± 8.39 | 22.8 ± 6.59 | 23.0 ± 6.85 | 23.0 ± 7.75 | 0.619 | 0.363 | 0.350 | −2.2 ± 9.45 | 0.0 ± 6.38 | 0.350 ‡ |
| Sucrose [g] | 34.1 ± 22.08 | 22.4 ± 16.00 | 34.1 ± 17.81 | 24.7 ± 13.17 | 0.773 | 0.001 | 0.717 | −11.7 ± 22.68 | −9.4 ± 20.51 | 0.928 † |
| SFA [g] | 26.6 ± 10.65 A | 21.5 ± 8.37 B | 26.5 ± 7.30 | 23.6 ± 7.01 | 0.632 | 0.003 | 0.394 | −3.4 ± 11.75 | −1.7 ± 7.25 | 0.552 ‡ |
| MUFA [g] | 30.5 ± 11.62 | 27.1 ± 9.81 | 30.4 ± 9.63 | 28.6 ± 8.13 | 0.783 | 0.078 | 0.552 | −3.4 ± 11.75 | −1.7 ± 7.25 | 0.568 † |
| EPA + DHA [mg] | 275.4 ± 347.58 | 607.3 ± 749.23 | 612.6 ± 852.81 | 442.6 ± 681.68 | 0.572 | 0.509 | 0.045 | 331.9 ± 763.13 | −170.0 ± 938.47 | 0.045 ‡ |
| Linoleic acid [g] | 10.2 ± 4.38 | 11.4 ± 5.02 | 10.4 ± 3.94 | 11.2 ± 4.52 | 0.974 | 0.285 | 0.777 | 1.2 ± 7.13 | 0.7 ± 5.39 | 0.992 † |
| α-linolenic acid [g] | 1.4 ± 0.80 | 1.9 ± 1.23 | 1.8 ± 0.73 | 2.3 ± 1.19 | 0.087 | 0.011 | 0.869 | 0.5 ± 1.36 | 0.5 ± 1.35 | 0.960 † |
| Total n-3 FA [g] | 1.7 ± 1.09 A | 2.6 ± 1.53 B | 2.5 ± 1.06 | 2.8 ± 1.57 | 0.125 | 0.008 | 0.258 | 0.89 ± 1.71 | 0.4 ± 1.37 | 0.258 ‡ |
| Total n-6 FA [g] | 9.2 ± 3.65 | 9.1 ± 2.93 | 10.0 ± 3.73 | 9.7 ± 3.61 | 0.352 | 0.788 | 0.879 | −0.1 ± 4.56 | −0.3 ± 4.88 | 0.879 ‡ |
| PUFA [g] | 11.9 ± 4.85 | 13.5 ± 4.04 | 13.2 ± 4.30 | 14.3 ± 6.05 | 0.344 | 0.109 | 0.772 | 1.6 ± 5.45 | 1.1 ± 6.13 | 0.772 ‡ |
| Sodium [mg] | 2599.3 ± 1091.86 | 2559.3 ± 1251.55 | 2716.4 ± 1429.6 | 2441.6 ± 510.99 | 0.999 | 0.369 | 0.502 | −40.0 ± 1039.52 | −274.8 ± 1384.45 | 0.880 † |
| Calcium [mg] | 788.8 ± 229.17 | 748.4 ± 280.84 | 820.8 ± 231.80 | 908.9 ± 397.59 | 0.148 | 0.645 | 0.218 | −40.4 ± 06.88 | 88.1 ± 411.23 | 0.258 † |
| Magnesium [mg] | 381.0 ± 91.38 | 366.1 ± 91.51 | 358.8 ± 96.06 | 364.8 ± 86.31 | 0.588 | 0.766 | 0.485 | −14.9 ± 135.14 | 6.0 ± 47.84 | 0.485 ‡ |
| Iron [mg] | 15.0 ± 3.18 | 14.0 ± 3.69 | 14.4 ± 3.38 | 13.3 ± 4.47 | 0.409 | 0.092 | 0.974 | −1.0 ± 5.12 | −1.1 ± 3.14 | 0.974 ‡ |
| Zinc [mg] | 11.3 ± 2.61 | 10.6 ± 2.50 | 10.9 ± 3.23 | 10.5 ± 1.99 | 0.687 | 0.194 | 0.639 | −0.7 ± 3.49 | −0.4 ± 2.16 | 0.465 † |
| Iodine [µg] | 68.5 ± 37.44 | 68.1 ± 28.03 | 61.7 ± 34.54 | 70.8 ± 27.39 | 0.789 | 0.418 | 0.373 | −0.4 ± 41.59 | 9.2 ± 31.71 | 0.373 ‡ |
| Vit. D (diet + supplementation) [µg] | 39.6 ± 41.65 | 52.6 ± 39.72 | 55.1 ± 51.87 | 70.4 ± 45.68 | 0.153 | 0.016 | 0.834 | 13.0 ± 31.7 | 15.3 ± 46.79 | 0.373 † |
| Folate [µg] | 382.3 ± 151.25 | 370.9 ± 94.45 | 400.5 ± 113.19 | 362.5 ± 87.89 | 0.852 | 0.217 | 0.503 | −11.3 ± 160.66 | −38.0 ± 105.79 | 0.489 † |
| Vit. B12 [µg] | 3.7 ± 1.66 | 4.3 ± 2.34 | 4.9 ± 3.27 | 4.0 ± 1.97 | 0.476 | 0.611 | 0.048 | 0.5 ± 2.51 | −0.9 ± 2.25 | 0.039 † |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osowiecka, K.; Skrypnik, D.; Myszkowska-Ryciak, J. Probiotic Supplementation Enhances the Effects of a Nutritional Intervention on Quality of Life in Women with Hashimoto’s Thyroiditis—A Double-Blind Randomised Study. Nutrients 2025, 17, 3387. https://doi.org/10.3390/nu17213387
Osowiecka K, Skrypnik D, Myszkowska-Ryciak J. Probiotic Supplementation Enhances the Effects of a Nutritional Intervention on Quality of Life in Women with Hashimoto’s Thyroiditis—A Double-Blind Randomised Study. Nutrients. 2025; 17(21):3387. https://doi.org/10.3390/nu17213387
Chicago/Turabian StyleOsowiecka, Karolina, Damian Skrypnik, and Joanna Myszkowska-Ryciak. 2025. "Probiotic Supplementation Enhances the Effects of a Nutritional Intervention on Quality of Life in Women with Hashimoto’s Thyroiditis—A Double-Blind Randomised Study" Nutrients 17, no. 21: 3387. https://doi.org/10.3390/nu17213387
APA StyleOsowiecka, K., Skrypnik, D., & Myszkowska-Ryciak, J. (2025). Probiotic Supplementation Enhances the Effects of a Nutritional Intervention on Quality of Life in Women with Hashimoto’s Thyroiditis—A Double-Blind Randomised Study. Nutrients, 17(21), 3387. https://doi.org/10.3390/nu17213387








