Gut Microbiota and Metabolites: Biomarkers and Therapeutic Targets for Diabetes Mellitus and Its Complications
Abstract
1. Introduction
2. Characteristics of Gut Microbiota in DM and Its Complications
2.1. Gut Microbiota in T1DM
2.2. Gut Microbiota in T2DM
2.3. Gut Microbiota in GDM
2.4. Gut Microbiota in DR
2.5. Gut Microbiota in DKD
2.6. Gut Microbiota in DN and DCD
2.7. The Gut Microbiome Is a Potential Target for DM Treatment
| Gut Microbiota | DM and Its Complications | Function | References | |||||
|---|---|---|---|---|---|---|---|---|
| T1DM | T2DM | GDM | DR | DKD | DN/DPN | |||
| Bacteroides | ↓ | ↓ | ↑ | ↑ | ↑ | ↓ | Facilitates carbohydrate breakdown, improving insulin sensitivity and blood glucose regulation; dual inflammatory control | [29,47,56,65,96] |
| Faecalibacterium | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | Butyrate production, enhances gut barrier integrity and insulin sensitivity | [29,32,47,57,66,96] |
| Lactobacillus | ↑ | ↑ | ↓ | ↑ | ↑ | ↑ | Probiotics; maintaining intestinal homeostasis, regulating metabolism, and immunity; SCFA production | [29,48,57,66,74] |
| Ruminococcus | ↑ | ↑ | ↓ | ↑ | ↑ | ↑ | Generate acetic acid and butyric acid, dual insulin resistance, blood glucose and inflammation regulation | [20,28,47,58,66,96] |
| Clostridium | ↓ | ↑ | ↓ | ↓ | ↑ | ↑ | Butyrate production, improves insulin sensitivity, and lowers blood glucose; dual inflammatory control | [18,29,47,57,67,74] |
| Blautia | ↓ | ↑ | ↓ | ↑ | ↑ | Generate SCFAs, reduce inflammation, and improve insulin sensitivity and glucose metabolism | [18,46,56,67,96] | |
| Roseburia | ↑ | ↓ | ↓ | ↑ | ↓ | Butyrate production, anti-inflammatory effects, and improves insulin sensitivity | [20,28,47,58,66] | |
| Romboutsia | ↓ | ↓ | ↓ | ↓ | ↑ | Modulating gut barrier, glycemia, and inflammation | [29,49,56,67] | |
| Akkermansia | ↓ | ↓ | ↓ | ↑ | Enhances gut barrier integrity and insulin sensitivity | [28,46,58,67] | ||
| Bifidobacterium | ↓ | ↓ | ↓ | ↑ | ↑ | Acetate and lactate production, improve glycemic control and insulin sensitivity | [29,46,57,73] | |
| Prevotella | ↓ | ↓ | ↓ | SCFA production, dual insulin sensitivity, and glycemic control | [29,47,67] | |||
3. Targeting the Gut Microbiota–Metabolite–Signaling Pathway Axis to Improve DM
3.1. Gut Microbiota Participates in DM by Regulating Metabolites
3.2. BCAAs, SCFAs, and IPA Regulate Signaling Pathways to Improve DM and Its Complications
3.3. Other Metabolite Regulation Pathways Improve DM and Its Complications
4. TCM Therapeutic Strategies Targeting Gut Microbiota and Metabolites
4.1. TCM Treats DM Through Gut Microbiota and Metabolites
| Types | Drug | Research Subject | Gut Microbiota | Metabolites | References | ||
|---|---|---|---|---|---|---|---|
| Increase | Decrease | Up | Down | ||||
| T1DM | Extra virgin olive oil | NOD mice | Lachnoclostridium, Ruminococcaceae UCG 005 | Lachnospira, Eubacterium | Madecassic acid, Lupeol | Ginsenoside, Oleamide | [156] |
| Astragalus polysaccharides | T1D mice | Muribaculum, Lactobacillus, Bacteroides | Corynebacterium, Brevibacterium, Brachybacterium | Acetic acid, PA, BA, SCFAs | Isobutyric acid, Isopentanoic acid, BCFAs | [158] | |
| Soluble fiber inulin and omega 3-PUFA | NOD mice | Akkermansia | Bacteroides intestinalis, Streptococcus sp. | Docosapentaenoic acid, Docesahexaenoic acid, Eicosapentaenoic acid | 2-Hydroxybutyric acid | [159] | |
| Cinnamaldehyde | T1DM model mice | Parasutterella, Odoribacter, Burkholderiales | Dorea, Mucispirillum | Myristoleic acid, 3-hydroxybutyric acid | Hydrocinnamic acid, 2-phenylpropionate | [165] | |
| Polysaccharides of D. huoshanense | T1D mice | Lactobacillus, Megasphaera | Bacteroides, Parabacteroides, Dorea, Enterocloser | Acetic acid, PA, Butyrate | / | [172] | |
| Crude polysaccharides | T1D mice | Lactobacillus | Ruminococcaceae, Lachnospiraceae, Rikenellaceae | / | / | [173] | |
| “Golden-flower” Tibetan tea | T1D mice | Lactobacillus, Lachnospiraceae NK4A136 group | Bacteroides | SCFAs, Superoxide dismutase, Catalase | / | [175] | |
| Low-methoxyl pectin | NOD mice | Firmicutes, TM7, Proteobacteria | Bacteroidetes | Cetate, Propionate, Butyrate, SCFAs | / | [176] | |
| T2DM | Ethanol extract of propolis | T2D mice | Parasutterella, Bifidobacterium, Faecalibaculum, Dubosiella, Lachnoclostridium | N-Acetyl-L-glutamic acid, D-(+)-Galactose, (R)-Lactate, L-(+) Lactic acid | Lactulose, L-Proline, O-Acetyl-L-serine, S-Adenosyl-L-homocysteine | [37] | |
| Polysaccharides from Phellinus linteus | T2D rat model | Alistipes, Prevotellaceae, Bacteroides, Parabacteroides | Faecalibaculum, Lachnospiraceae | SCFAs, Primary bile acids | Aspartate aminotransferase alanine aminotransferase, Primary bile acids | [161] | |
| Morus alba L. water extracts | T2D mice | Dubosiella | Anaerovorax, Bilophila, Blautia, Lachnoclostridium | Branched-chain ketoacid, Dehydrogenase E1α | Amino acid | [166] | |
| Jiang-Tang-San-Huang | T2D rat model | Romboutsia, Lactobacillus, Bacteroides, Bifidobacterium | Enterococcs | Primary bile acids, Chenodeoxycholic acid | Taurocholic acid | [167] | |
| Navel orange peel pectin | diabetic mouse | Dubosiella, Akkermansia, Lachnospiraceae, Atopobiaceae | Muribaculaceae, Lachnospiraceae NK4A136 group | Acetic acid, Total acid, BA | PA | [170] | |
| Polysaccharide extract | T2D mice | Akkermansia, Lactobacillus, Alistipes, Romboutsia, Faecalibaculum | Bacteroides, Alloprevotella, Escherichia-Shigella, Clostridium | Propionate, Butyrate | Triglycerides, Total cholesterol | [174] | |
| GDM | Inulin-type fructans | GDM mice | Akkermansia, Bifidobacterium | Dubosiella | BA, Acetic acid | / | [122] |
| Konjac | GDM mice | Dubosiella, Monoglobu | Bavteroides, Romboutsia, Faecalibaculum | Phenylalanine | Diamine oxidase, LPS, Valine, Leucine, Isoleucine | [164] | |
4.2. TCM Treats DM Complications Through Gut Microbiota and Metabolites
| Types | Drug | Research Subject | Gut Microbiota | Metabolites | References | ||
|---|---|---|---|---|---|---|---|
| Increase | Decrease | Up | Down | ||||
| DR | Luo Tong formula | DR rat model | Candidatus_Saccharimonas, Romboutsia, Enterorhabdus | Prevotella | / | / | [178] |
| Quercetin | Sprague Dawley mice | Turicibacter, Roseburia, Bifidobacterium | Streptococcus, Veillonella, Prevotella | Acetic acid, PA, BA | / | [179] | |
| DKD | Tangshen Formula | DKD mice | Barnesiella | Romboutsia, Akkermansia, Collinsella | Tryptophan, 5-hydroxyindoleacetate, Glutamic acid, Aspartate | Indole-3-acetic acid, Xanthurenic acid | [100] |
| Qing-Re-Xiao-Zheng formula | DKD mice | Rikenellaceae, Akkermansia | Desulfovibrio | SCFAs | LPSs | [180] | |
| Fufang-zhenzhu-tiaozhi formula | DKD mice | Bacteroidota, Actinobacteriota, Pseudonocardia | Weissella, Enterococcus, Akkermansia | PA, Methylmalonic acid, Butanoic acid | 3-hydroxybutyrylcamitine, Gamma-muricholic acid | [181] | |
| Polysaccharides | DKD rat model | Mollicutes, Bacteroidota, Ruminococcaceae_UCG-014 | Lactobacillus | Acetic acid, PA, BA | Isovaleric acid, BCFAs | [182] | |
| Salvia miltiorrhiza | DKD rat model | Akkermansia, Lactobacillus, A. musciniphila | Prevotellaceae UCG 001 | Phytosphingosine, Sphinganine | Indolyl sulfate, P-cresolsulfate, Myo-inositol | [184] | |
| DN | San-Huang-Yi-Shen capsule | DN rat model | Lactobacillus, Allobaculum, Ruminococcaceae UCG 005, Anaerovibrio, Bacteroides | Candidatus Saccharimonas | Amino sugar, Pyruvate metabolism, Nucleotide sugar metabolism | TCA cycle, Arachidonic acid, Mannose metabolism | [187] |
| Magnesium lithospermate B | diabetic mouse | Bifidobacterium, Lachnospiraceae, Aerococcus, Bacteroidales | Alistipes, Lachnospiraceae NK4A136 group | BA, Isobutyric acid, Pentanoic acid, Alanine, Threonine, Glycine, Lysine | Tyrosine | [188] | |
| DPN | Jinmaitong | DPN rats | Helicobacterae, Blautia, Escherichia-Shigella | Clostridium, Oscillibacter | / | / | [189] |
| Quercetin | DPN rats | Prevotella, Escherichia-Shigella, Bifidobacterium | Desulfovibrio, Lactobacillus | / | / | [190] | |
| Gingerol-enriched ginger | DPN rats | Lachnospiraceae | / | / | [191] | ||
| Huangqi Guizhi Wuwu Decoction | db/db mice | Lactobacillus, Alloprevotella, Bacteroides | Lachnoclostridium, Blautia, Desulfovibrio Ruminococcus, Akkermansia, Caproiciproducens | / | Sphinganine, Sphingosine 1-phosphate, Phytosphingosine | [193] | |
4.3. Future Treatment Strategies of TCM in DM
5. Diet as Medicine: Treatment Strategies for DM and Its Complications
5.1. Adjust Dietary Structure to Improve DM and Its Complications
| Type of Diet | Gut Microbiota Abundance | Function | References | |
|---|---|---|---|---|
| Increase | Decrease | |||
| MedDiet | Roseburia, Bacteroides, Faecalibacterium, Akkermansia, Bifidobacterium, Lachnospiraceae_UCG.001 | Eubacterium hallii group and Dorea, Blautia, Romboutsia, Ruminococcus, Prevotella 9 | Promotes the growth of probiotics, lowers blood glucose, and has anti-inflammatory effects | [208] |
| Japanese diet | Lachnospiracea, Gemmiger, Faecalibacterium | Alloprevotella, Bifidobacterium, Actinomyces, Parabacteroides | Reduces the risk of diabetes, improves blood glucose control | [209] |
| TRE | Faecalibacterium, Dialister | Alloprevotella, Prevotella | Enhances insulin sensitivity, reduces body fat, and optimizes metabolism | [210] |
| RF | Faecalibacterium, Roseburia, Akkermansia, Bacteroides, Allobaculum, Blautia | Prevotella 9 | Improves insulin sensitivity, promotes weight loss, and reduces inflammation | [210] |
| C. morifolium | Akkermansia, Bacteroidales, Rikenellaceae | Clostridium, Faecalibaculum | Reduces the risk of diabetes, improves blood glucose control | [211] |
| Capsaicin | Akkermansia, Anaerotruncus | Streptococcus, Alistipes, Faecalibacterium, Barnesiella intestinihominis | Improves glucose and lipid metabolism disorders | [212] |
| CDHFD | / | Muribaculum, Odoribacter | Aggravates metabolic disorders associated with diabetes | [213] |
| Eggs (selenium and/or zinc) | Blautia | Alistipes, Odoribacter | Antioxidant effects and enhances insulin sensitivity | [214] |
| HF and HS diet | Romboutsia, Lactococcus, and Enterococcus | Turicibacter, Ileibacterium, Bifidobacterium | Promotes insulin resistance, β-cell damage, and inflammation | [216] |
| HFD | Roseburia, Ruminococcus gnavus | Bacteroides, Alistipes | Promotes the proliferation of beneficial bacteria, blood glucose regulation, and anti-inflammatory effects | [217] |
| Vegetarian and vegan diets | Faecalibacterium prausnitzii | Bacteroides fragilis | Effective weight management, reduction of diabetes and metabolic syndrome risk | [220] |
| Ketogenic Diet | Akkermansia, Clostridia_UCG 014 | Bacteroides, Anaerostipes, Ruminococcus | Effective weight management and promotion of glucose metabolism | [221] |
| RS | Bifidobacterium adolescentis, Bifidobacterium longum, Ruminococcus bromii | Alisipes putredinis, Bacteroides vulgatus, Odoribacter sp. lanchnicus | Effective weight management and promotion of glucose metabolism | [222] |
5.2. Supplement Probiotics to Improve DM and Its Complications
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| DM | Diabetes mellitus |
| T2DM | Type 2 diabetes mellitus |
| T1DM | Type 1 diabetes mellitus |
| GDM | Gestational diabetes mellitus |
| β-cells | Beta cells |
| IR | Insulin resistance |
| DR | Diabetic retinopathy |
| DKD | Diabetic kidney disease |
| DN | Diabetes neuropathy |
| DCD | Diabetic cardiovascular disease |
| HFD | High-fiber diet |
| HbA1c | Glycated hemoglobin |
| LPS | Lipopolysaccharides |
| SCFAs | Short-chain fatty acids |
| BCAAs | Branched-chain amino acids |
| IPA | Indolepropionic acid |
| TCM | Traditional Chinese medicine |
| MR | Mendelian randomization |
| A. muciniphila | Akkermansia muciniphila |
| 2-HB | 2-Hydroxybutyrate |
| VEGF | Vascular endothelial growth factor |
| DPN | Diabetic peripheral neuropathy |
| Lp299v | Lactobacillus reuteri 299v |
| R. gnavus | Ruminococcus gnavus |
| TMAO | Trimethylamine N-oxide |
| B. coccoides | Blautia coccoides |
| AGEs | Advanced glycation end-products |
| RAGE | Receptor for advanced glycation end-products |
| GPR | G protein-coupled receptors |
| HF | High-fat |
| NETs | Neutrophil extracellular traps |
| EPA | Eicosapentaenoic acid |
| NOD mice | Non-obese diabetic mice |
| PA | Propionic acid |
| BA | Butyric acid. |
| MedDiet | Mediterranean diet |
| C. morifolium | Chrysanthemum morifolium flower |
| HS | High-sugar |
| CDHFD | Cold drink and high-fat diet |
| TRE | Time-restricted eating |
| RF | Ramadan fasting |
| FFA | Free fatty acids |
| GABA | Gamma aminobutyric acid |
| GLP-1 | Glucagon-like peptide-1 |
| GIP | Glucose-dependent insulin-dependent polypeptide |
| n-3 PUFA | Omega-3 polyunsaturated fatty acids |
References
- Balooch Hasankhani, M.; Mirzaei, H.; Karamoozian, A. Global trend analysis of diabetes mellitus incidence, mortality, and mortality-to-incidence ratio from 1990 to 2019. Sci. Rep. 2023, 13, 21908. [Google Scholar] [CrossRef]
- Sasidharan Pillai, S.; Has, P.; Quintos, J.B.; Serrano Gonzalez, M.; Kasper, V.L.; Topor, L.S.; Fredette, M.E. Incidence, Severity, and Presentation of Type 2 Diabetes in Youth During the First and Second Year of the COVID-19 Pandemic. Diabetes Care 2023, 46, 953–958. [Google Scholar] [CrossRef]
- Rathmann, W.; Kuss, O.; Kostev, K. Incidence of newly diagnosed diabetes after COVID-19. Diabetologia 2022, 65, 949–954. [Google Scholar] [CrossRef]
- Yang, S.; Cao, J.; Wang, Y.; Chen, Q.; Li, F.; Gao, Y.; Li, R.; Yuan, L. Small Intestinal Endocrine Cell Derived Exosomal ACE2 Protects Islet β-Cell Function by Inhibiting the Activation of NLRP3 Inflammasome and Reducing β-Cell Pyroptosis. Int. J. Nanomed. 2024, 19, 4957–4976. [Google Scholar] [CrossRef]
- Kautzky-Willer, A.; Winhofer, Y.; Kiss, H.; Falcone, V.; Berger, A.; Lechleitner, M.; Weitgasser, R.; Harreiter, J. Gestational diabetes mellitus (Update 2023). Wien. Klin. Wochenschr. 2023, 135, 115–128. [Google Scholar] [CrossRef]
- Mora, T.; Roche, D.; Rodríguez-Sánchez, B. Predicting the onset of diabetes-related complications after a diabetes diagnosis with machine learning algorithms. Diabetes Res. Clin. Pr. 2023, 204, 110910. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. Summary of Revisions: Standards of Care in Diabetes-2025. Diabetes Care 2025, 48, S6–S13. [Google Scholar] [CrossRef]
- Holt, R.I.G.; DeVries, J.H.; Hess-Fischl, A.; Hirsch, I.B.; Kirkman, M.S.; Klupa, T.; Ludwig, B.; Nørgaard, K.; Pettus, J.; Renard, E.; et al. The management of type 1 diabetes in adults. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2021, 64, 2609–2652. [Google Scholar] [CrossRef]
- Patiño-Cardona, S.; Garrido-Miguel, M.; Pascual-Morena, C.; Berlanga-Macías, C.; Lucerón-Lucas-Torres, M.; Alfaro-González, S.; Martínez-García, I. Effect of Coenzyme Q10 Supplementation on Lipid and Glycaemic Profiles: An Umbrella Review. J. Cardiovasc. Dev. Dis. 2024, 11, 377. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, B.; Chen, F.; Xia, R.; Zhu, D.; Chen, B.; Lin, A.; Zheng, C.; Hou, D.; Li, X.; et al. Fecal microbiota transplantation reverses insulin resistance in type 2 diabetes: A randomized, controlled, prospective study. Front. Cell. Infect. Microbiol. 2022, 12, 1089991. [Google Scholar] [CrossRef]
- Tan, H.; Shi, Y.; Yue, T.; Zheng, D.; Luo, S.; Weng, J.; Zheng, X. Machine learning approach reveals microbiome, metabolome, and lipidome profiles in type 1 diabetes. J. Adv. Res. 2024, 64, 213–221. [Google Scholar] [CrossRef]
- Du, E.; Wang, W.; Gan, L.; Li, Z.; Guo, S.; Guo, Y. Effects of thymol and carvacrol supplementation on intestinal integrity and immune responses of broiler chickens challenged with Clostridium perfringens. J. Anim. Sci. Biotechnol. 2016, 7, 19. [Google Scholar] [CrossRef]
- Zhao, Z.; Ning, J.; Bao, X.Q.; Shang, M.; Ma, J.; Li, G.; Zhang, D. Fecal microbiota transplantation protects rotenone-induced Parkinson’s disease mice via suppressing inflammation mediated by the lipopolysaccharide-TLR4 signaling pathway through the microbiota-gut-brain axis. Microbiome 2021, 9, 226. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhao, Y.; Wang, D.; Liu, C.; Qi, Z.; Tang, H.; Liu, Y.; Zhang, S.; Cui, Y.; Li, Y.; et al. ALK-JNK signaling promotes NLRP3 inflammasome activation and pyroptosis via NEK7 during Streptococcus pneumoniae infection. Mol. Immunol. 2023, 157, 78–90. [Google Scholar] [CrossRef]
- Rinott, E.; Meir, A.Y.; Tsaban, G.; Zelicha, H.; Kaplan, A.; Knights, D.; Tuohy, K.; Scholz, M.U.; Koren, O.; Stampfer, M.J.; et al. The effects of the Green-Mediterranean diet on cardiometabolic health are linked to gut microbiome modifications: A randomized controlled trial. Genome Med. 2022, 14, 29. [Google Scholar] [CrossRef]
- Guo, K.; Ye, J.; Li, J.; Huang, J.; Zhou, Z. Effects of gut microbiome on type 1 diabetes susceptibility and complications: A large-scale bidirectional Mendelian randomization and external validation study. Diabetes Obes. Metab. 2024, 26, 3306–3317. [Google Scholar] [CrossRef]
- Luo, M.; Sun, M.; Wang, T.; Zhang, S.; Song, X.; Liu, X.; Wei, J.; Chen, Q.; Zhong, T.; Qin, J. Gut microbiota and type 1 diabetes: A two-sample bidirectional Mendelian randomization study. Front. Cell Infect. Microbiol. 2023, 13, 1163898. [Google Scholar] [CrossRef]
- Hu, J.; Ding, J.; Li, X.; Li, J.; Zheng, T.; Xie, L.; Li, C.; Tang, Y.; Guo, K.; Huang, J.; et al. Distinct signatures of gut microbiota and metabolites in different types of diabetes: A population-based cross-sectional study. eClinicalMedicine 2023, 62, 102132. [Google Scholar] [CrossRef] [PubMed]
- Allakany, A.I.; Elbanna, A.A.; Rohoma, K.H.; Ahmed, S.M.; Ibrahim, A.E.; Fawzy, M.A.; Header, D.A. Study of the gut microbiome in Egyptian patients with type 1 diabetes mellitus. Prz. Gastroenterol. 2023, 18, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Chukhlovin, A.B.; Dudurich, V.V.; Kusakin, A.V.; Polev, D.E.; Ermachenko, E.D.; Aseev, M.V.; Zakharov, Y.A.; Eismont, Y.A.; Danilov, L.G.; Glotov, O.S. Evaluation of Gut Microbiota in Healthy Persons and Type 1 Diabetes Mellitus Patients in North-Western Russia. Microorganisms 2023, 11, 1813. [Google Scholar] [CrossRef]
- Moreira, L.A.A.; da Paz Lima, L.; de Oliveira Falcão, M.A.; Rosado, E.L. Profile of Gut Microbiota of Adults with Diabetes Mellitus Type 1: A Systematic Review. Curr. Diabetes Rev. 2023, 19, e280322202706. [Google Scholar] [CrossRef]
- Hoseini Tavassol, Z.; Ejtahed, H.S.; Atlasi, R.; Saghafian, F.; Khalagi, K.; Hasani-Ranjbar, S.; Siadat, S.D.; Nabipour, I.; Ostovar, A.; Larijani, B. Alteration in Gut Microbiota Composition of Older Adults Is Associated with Obesity and Its Indices: A Systematic Review. J. Nutr. Health Aging 2023, 27, 817–823. [Google Scholar] [CrossRef]
- Wang, L.; Gong, C.; Wang, R.; Wang, J.; Yang, Z.; Wang, X. A pilot study on the characterization and correlation of oropharyngeal and intestinal microbiota in children with type 1 diabetes mellitus. Front. Pediatr. 2024, 12, 1382466. [Google Scholar] [CrossRef]
- Zhu, B.T. Pathogenic Mechanism of Autoimmune Diabetes Mellitus in Humans: Potential Role of Streptozotocin-Induced Selective Autoimmunity against Human Islet β-Cells. Cells 2022, 11, 492. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, V.F.; Elias-Oliveira, J.; Pereira, Í.S.; Pereira, J.A.; Barbosa, S.C.; Machado, M.S.G.; Guimarães, J.B.; Pacheco, T.C.F.; Bortolucci, J.; Zaramela, L.S.; et al. Akkermansia muciniphila restrains type 1 diabetes onset by eliciting cDC2 and Treg cell differentiation in NOD and STZ-induced experimental models. Life Sci. 2025, 372, 123624. [Google Scholar] [CrossRef]
- Spanier, J.A.; Fung, V.; Wardell, C.M.; Alkhatib, M.H.; Chen, Y.; Swanson, L.A.; Dwyer, A.J.; Weno, M.E.; Silva, N.; Mitchell, J.S.; et al. Tregs with an MHC class II peptide-specific chimeric antigen receptor prevent autoimmune diabetes in mice. J. Clin. Investig. 2023, 133, e168601. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Ma, C.; Fu, W.; Xu, Y.; Wang, R.; Liu, D.; Zhang, L.; Hu, N.; Li, D.; Li, W. Changes in Type 1 Diabetes-Associated Gut Microbiota Aggravate Brain Ischemia Injury by Affecting Microglial Polarization Via the Butyrate-MyD88 Pathway in Mice. Mol. Neurobiol. 2025, 62, 3764–3780. [Google Scholar] [CrossRef]
- Fan, G.; Cao, F.; Kuang, T.; Yi, H.; Zhao, C.; Wang, L.; Peng, J.; Zhuang, Z.; Xu, T.; Luo, Y.; et al. Alterations in the gut virome are associated with type 2 diabetes and diabetic nephropathy. Gut Microbes 2023, 15, 2226925. [Google Scholar] [CrossRef]
- Yarmohammadi, H.; Soltanipur, M.; Rezaei, M.; Ejtahed, H.S.; Raei, M.; Razavi, A.; Mirhosseini, S.M.; Zangeneh, M.; Doroud, D.; Fateh, A.; et al. The Comparison of the Gut Microbiome Composition, Serum Inflammatory Markers and Faecal Short-Chain Fatty Acids Among Individuals With Type 1 and 2 Diabetes Mellitus With Healthy Controls: A Case-Control Study. Endocrinol. Diabetes Metab. 2025, 8, e70071. [Google Scholar] [CrossRef] [PubMed]
- Letchumanan, G.; Abdullah, N.; Marlini, M.; Baharom, N.; Lawley, B.; Omar, M.R.; Mohideen, F.B.S.; Addnan, F.H.; Nur Fariha, M.M.; Ismail, Z.; et al. Gut Microbiota Composition in Prediabetes and Newly Diagnosed Type 2 Diabetes: A Systematic Review of Observational Studies. Front. Cell Infect. Microbiol. 2022, 12, 943427. [Google Scholar] [CrossRef]
- Umirah, F.; Neoh, C.F.; Ramasamy, K.; Lim, S.M. Differential gut microbiota composition between type 2 diabetes mellitus patients and healthy controls: A systematic review. Diabetes Res. Clin. Pr. 2021, 173, 108689. [Google Scholar] [CrossRef]
- Alvarez-Silva, C.; Kashani, A.; Hansen, T.H.; Pinna, N.K.; Anjana, R.M.; Dutta, A.; Saxena, S.; Støy, J.; Kampmann, U.; Nielsen, T.; et al. Trans-ethnic gut microbiota signatures of type 2 diabetes in Denmark and India. Genome Med. 2021, 13, 37. [Google Scholar] [CrossRef]
- Li, H.; Li, C. Causal relationship between gut microbiota and type 2 diabetes: A two-sample Mendelian randomization study. Front. Microbiol. 2023, 14, 1184734. [Google Scholar] [CrossRef]
- Sun, K.; Gao, Y.; Wu, H.; Huang, X. The causal relationship between gut microbiota and type 2 diabetes: A two-sample Mendelian randomized study. Front. Public Health 2023, 11, 1255059. [Google Scholar] [CrossRef]
- Neri-Rosario, D.; Martínez-López, Y.E.; Esquivel-Hernández, D.A.; Sánchez-Castañeda, J.P.; Padron-Manrique, C.; Vázquez-Jiménez, A.; Giron-Villalobos, D.; Resendis-Antonio, O. Dysbiosis signatures of gut microbiota and the progression of type 2 diabetes: A machine learning approach in a Mexican cohort. Front. Endocrinol. 2023, 14, 1170459. [Google Scholar] [CrossRef]
- Wang, Y.; Xing, X.; Ma, Y.; Fan, Y.; Zhang, Y.; Nan, B.; Li, X.; Wang, Y.; Liu, J. Prevention of High-Fat-Diet-Induced Dyslipidemia by Lactobacillus plantarum LP104 through Mediating Bile Acid Enterohepatic Axis Circulation and Intestinal Flora. J. Agric. Food Chem. 2023, 71, 7334–7347. [Google Scholar] [CrossRef] [PubMed]
- Guan, R.; Ma, N.; Liu, G.; Wu, Q.; Su, S.; Wang, J.; Geng, Y. Ethanol extract of propolis regulates type 2 diabetes in mice via metabolism and gut microbiota. J. Ethnopharmacol. 2023, 310, 116385. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Yang, J.; Xia, L.; Wei, T.; Cui, X.; Wang, D.; Jin, Z.; Lin, X.; Li, F.; Yang, K.; et al. Gut Microbiota-Tryptophan Metabolism-GLP-1 Axis Participates in β-Cell Regeneration Induced by Dapagliflozin. Diabetes 2024, 73, 926–940. [Google Scholar] [CrossRef] [PubMed]
- Gofron, K.K.; Wasilewski, A.; Małgorzewicz, S. Effects of GLP-1 Analogues and Agonists on the Gut Microbiota: A Systematic Review. Nutrients 2025, 17, 1303. [Google Scholar] [CrossRef]
- Sawicki, C.M.; Pacheco, L.S.; Rivas-Tumanyan, S.; Cao, Z.; Haslam, D.E.; Liang, L.; Tucker, K.L.; Joshipura, K.; Bhupathiraju, S.N. Association of Gut Microbiota-Related Metabolites and Type 2 Diabetes in Two Puerto Rican Cohorts. Nutrients 2024, 16, 959. [Google Scholar] [CrossRef]
- Hasain, Z.; Raja Ali, R.A.; Ahmad, H.F.; Abdul Rauf, U.F.; Oon, S.F.; Mokhtar, N.M. The Roles of Probiotics in the Gut Microbiota Composition and Metabolic Outcomes in Asymptomatic Post-Gestational Diabetes Women: A Randomized Controlled Trial. Nutrients 2022, 14, 3878. [Google Scholar] [CrossRef]
- Yang, J.; Wang, J.; Wu, W.; Su, C.; Wu, Y.; Li, Q. Xylooligosaccharides ameliorate insulin resistance by increasing Akkermansia muciniphila and improving intestinal barrier dysfunction in gestational diabetes mellitus mice. Food Funct. 2024, 15, 3122–3129. [Google Scholar] [CrossRef]
- Sweeting, A.; Wong, J.; Murphy, H.R.; Ross, G.P. A Clinical Update on Gestational Diabetes Mellitus. Endocr. Rev. 2022, 43, 763–793. [Google Scholar] [CrossRef]
- Juan, J.; Sun, Y.; Wei, Y.; Wang, S.; Song, G.; Yan, J.; Zhou, P.; Yang, H. Progression to type 2 diabetes mellitus after gestational diabetes mellitus diagnosed by IADPSG criteria: Systematic review and meta-analysis. Front. Endocrinol. 2022, 13, 1012244. [Google Scholar] [CrossRef] [PubMed]
- Van, J.A.D.; Luo, Y.; Danska, J.S.; Dai, F.; Alexeeff, S.E.; Gunderson, E.P.; Rost, H.; Wheeler, M.B. Postpartum defects in inflammatory response after gestational diabetes precede progression to type 2 diabetes: A nested case-control study within the SWIFT study. Metabolism 2023, 149, 155695. [Google Scholar] [CrossRef]
- Liu, N.; Sun, Y.; Wang, Y.; Ma, L.; Zhang, S.; Lin, H. Composition of the intestinal microbiota and its variations between the second and third trimesters in women with gestational diabetes mellitus and without gestational diabetes mellitus. Front. Endocrinol. 2023, 14, 1126572. [Google Scholar] [CrossRef]
- Liang, Y.Y.; Liu, L.Y.; Jia, Y.; Li, Y.; Cai, J.N.; Shu, Y.; Tan, J.Y.; Chen, P.Y.; Li, H.W.; Cai, H.H.; et al. Correlation between gut microbiota and glucagon-like peptide-1 in patients with gestational diabetes mellitus. World J. Diabetes 2022, 13, 861–876. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xie, Z.; Chen, P.; Wang, Y.; Li, B.; Dai, F. Effect of dietary pattern on pregnant women with gestational diabetes mellitus and its clinical significance. Open Life Sci. 2022, 17, 202–207. [Google Scholar] [CrossRef]
- Mei, S.; Chen, Y.; Long, Y.; Cen, X.; Zhao, X.; Zhang, X.; Ye, J.; Gao, X.; Zhu, C. Association of gut microbiota with overweight/obesity combined with gestational diabetes mellitus. J. Med. Microbiol. 2025, 74, 002010. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Lin, D.; Li, Q.; Cai, J.; Huang, H.; Xiang, T.; Tan, H. Investigating causal associations among gut microbiota, gut microbiota-derived metabolites, and gestational diabetes mellitus: A bidirectional Mendelian randomization study. Aging 2023, 15, 8345–8366. [Google Scholar] [CrossRef]
- Liang, W.; Feng, Y.; Yang, D.; Qin, J.; Zhi, X.; Wu, W.; Jie, Q. Oral probiotics increased the proportion of Treg, Tfr, and Breg cells to inhibit the inflammatory response and impede gestational diabetes mellitus. Mol. Med. 2023, 29, 122. [Google Scholar] [CrossRef]
- Bae, G.; Berezhnoy, G.; Flores, A.; Cannet, C.; Schäfer, H.; Dahlke, M.H.; Michl, P.; Löffler, M.W.; Königsrainer, A.; Trautwein, C. Quantitative Metabolomics and Lipoprotein Analysis of PDAC Patients Suggests Serum Marker Categories for Pancreatic Function, Pancreatectomy, Cancer Metabolism, and Systemic Disturbances. J. Proteome Res. 2024, 23, 1249–1262. [Google Scholar] [CrossRef] [PubMed]
- Qin, F.; Li, J.; Mao, T.; Feng, S.; Li, J.; Lai, M. 2 Hydroxybutyric Acid-Producing Bacteria in Gut Microbiome and Fusobacterium nucleatum Regulates 2 Hydroxybutyric Acid Level In Vivo. Metabolites 2023, 13, 451. [Google Scholar] [CrossRef]
- Beldie, L.A.; Dica, C.C.; Moța, M.; Pirvu, B.F.; Burticală, M.A.; Mitrea, A.; Clenciu, D.; Efrem, I.C.; Vladu, B.E.; Timofticiuc, D.C.P.; et al. The Interactions Between Diet and Gut Microbiota in Preventing Gestational Diabetes Mellitus: A Narrative Review. Nutrients 2024, 16, 4131. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Teixidó, C.; Barrot de la Puente, J.; Miravet Jiménez, S.; Fernández-Camins, B.; Mauricio, D.; Romero Aroca, P.; Vlacho, B.; Franch-Nadal, J. Incidence of Diabetic Retinopathy in Individuals with Type 2 Diabetes: A Study Using Real-World Data. J. Clin. Med. 2024, 13, 7083. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Wan, Z.; Zhang, Y.; Wang, T.; Xue, Y.; Peng, Q. Composition and diversity of gut microbiota in diabetic retinopathy. Front. Microbiol. 2022, 13, 926926. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, Z.; Ma, H.; Ji, S.; Chen, Z.; Cui, Z.; Chen, J.; Tang, S. Dysbiosis and Implication of the Gut Microbiota in Diabetic Retinopathy. Front. Cell Infect. Microbiol. 2021, 11, 646348. [Google Scholar] [CrossRef]
- Zhou, Z.; Zheng, Z.; Xiong, X.; Chen, X.; Peng, J.; Yao, H.; Pu, J.; Chen, Q.; Zheng, M. Gut Microbiota Composition and Fecal Metabolic Profiling in Patients With Diabetic Retinopathy. Front. Cell Dev. Biol. 2021, 9, 732204. [Google Scholar] [CrossRef]
- Liu, K.; Zou, J.; Fan, H.; Hu, H.; You, Z. Causal effects of gut microbiota on diabetic retinopathy: A Mendelian randomization study. Front. Immunol. 2022, 13, 930318. [Google Scholar] [CrossRef]
- Padakandla, S.R.; Das, T.; Sai Prashanthi, G.; Angadi, K.K.; Reddy, S.S.; Reddy, G.B.; Shivaji, S. Gut mycobiome dysbiosis in rats showing retinal changes indicative of diabetic retinopathy. PLoS ONE 2022, 17, e0267080. [Google Scholar] [CrossRef]
- Ye, P.; Zhang, X.; Xu, Y.; Xu, J.; Song, X.; Yao, K. Alterations of the Gut Microbiome and Metabolome in Patients With Proliferative Diabetic Retinopathy. Front. Microbiol. 2021, 12, 667632. [Google Scholar] [CrossRef]
- Gu, X.M.; Lu, C.Y.; Pan, J.; Ye, J.Z.; Zhu, Q.H. Alteration of intestinal microbiota is associated with diabetic retinopathy and its severity: Samples collected from southeast coast Chinese. World J. Diabetes 2023, 14, 862–882. [Google Scholar] [CrossRef]
- Ai, X.; Yu, P.; Luo, L.; Sun, J.; Tao, H.; Wang, X.; Meng, X. Berberis dictyophylla F. inhibits angiogenesis and apoptosis of diabetic retinopathy via suppressing HIF-1α/VEGF/DLL-4/Notch-1 pathway. J. Ethnopharmacol. 2022, 296, 115453. [Google Scholar] [CrossRef]
- Zheng, J.; Chen, M.; Ye, C.; Sun, X.; Jiang, N.; Zou, X.; Yang, H.; Liu, H. BuZangTongLuo decoction improved hindlimb ischemia by activating angiogenesis and regulating gut microbiota in diabetic mice. J. Ethnopharmacol. 2020, 248, 112330. [Google Scholar] [CrossRef]
- He, X.; Sun, J.; Liu, C.; Yu, X.; Li, H.; Zhang, W.; Li, Y.; Geng, Y.; Wang, Z. Compositional Alterations of Gut Microbiota in Patients with Diabetic Kidney Disease and Type 2 Diabetes Mellitus. Diabetes Metab. Syndr. Obes. 2022, 15, 755–765. [Google Scholar] [CrossRef]
- Han, S.; Chen, M.; Cheng, P.; Zhang, Z.; Lu, Y.; Xu, Y.; Wang, Y. A systematic review and meta-analysis of gut microbiota in diabetic kidney disease: Comparisons with diabetes mellitus, non-diabetic kidney disease, and healthy individuals. Front. Endocrinol. 2022, 13, 1018093. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Z.; Zhang, X.; Zhao, L.; Chu, J.; Li, H.; Sun, W.; Yang, C.; Wang, H.; Dai, W.; et al. Alterations of the Gut Microbiota in Patients with Diabetic Nephropathy. Microbiol. Spectr. 2022, 10, e0032422. [Google Scholar] [CrossRef]
- Zhang, B.; Wan, Y.; Zhou, X.; Zhang, H.; Zhao, H.; Ma, L.; Dong, X.; Yan, M.; Zhao, T.; Li, P. Characteristics of Serum Metabolites and Gut Microbiota in Diabetic Kidney Disease. Front. Pharmacol. 2022, 13, 872988. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Fu, T.; Liu, W.; Du, Y.; Bu, J.; Wei, G.; Yu, M.; Lin, Y.; Min, C.; Lin, D. Jiangtang Decoction Ameliorates Diabetic Kidney Disease Through the Modulation of the Gut Microbiota. Diabetes Metab. Syndr. Obes. Targets Ther. 2023, 16, 3707–3725. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, Q.; Liu, F.; Wang, D. Lycoperoside H protects against diabetic nephropathy via alteration of gut microbiota and inflammation. J. Biochem. Mol. Toxicol. 2022, 36, e23216. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Yang, Y.; Xu, G. Empagliflozin ameliorates type 2 diabetes mellitus-related diabetic nephropathy via altering the gut microbiota. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2022, 1867, 159234. [Google Scholar] [CrossRef]
- Noureldein, M.H.; Rumora, A.E.; Teener, S.J.; Rigan, D.M.; Hayes, J.M.; Mendelson, F.E.; Carter, A.D.; Rubin, W.G.; Savelieff, M.G.; Feldman, E.L. Dietary Fatty Acid Composition Alters Gut Microbiome in Mice with Obesity-Induced Peripheral Neuropathy. Nutrients 2025, 17, 737. [Google Scholar] [CrossRef]
- Huang, W.; Lin, Z.; Sun, A.; Deng, J.; Manyande, A.; Xiang, H.; Zhao, G.F.; Hong, Q. The role of gut microbiota in diabetic peripheral neuropathy rats with cognitive dysfunction. Front. Microbiol. 2023, 14, 1156591. [Google Scholar] [CrossRef]
- Hong, J.; Fu, T.; Liu, W.; Du, Y.; Min, C.; Lin, D. Specific alterations of gut microbiota in diabetic microvascular complications: A systematic review and meta-analysis. Front. Endocrinol. 2022, 13, 1053900. [Google Scholar] [CrossRef]
- Tang, F.; Shen, L.; Gu, Z.; Zhang, L.; Fang, L.; Sun, H.; Ma, D.; Guo, Y.; Yang, Y.; Lu, B.; et al. Causal relationships between gut microbiota, gut metabolites, and diabetic neuropathy: A mendelian randomization study. Clin. Nutr. ESPEN 2024, 62, 128–136. [Google Scholar] [CrossRef]
- Wu, Y.; Bai, H.; Lu, Y.; Peng, R.; Qian, M.; Yang, X.; Cai, E.; Ruan, W.; Zhang, Q.; Zhang, J.; et al. Associations of Plasma Gut Microbiota-Derived TMAO and Precursors in Early Pregnancy with Gestational Diabetes Mellitus Risk: A Nested Case-Control Study. Nutrients 2025, 17, 810. [Google Scholar] [CrossRef]
- Jiang, S.; Shui, Y.; Cui, Y.; Tang, C.; Wang, X.; Qiu, X.; Hu, W.; Fei, L.; Li, Y.; Zhang, S.; et al. Gut microbiota dependent trimethylamine N-oxide aggravates angiotensin II-induced hypertension. Redox Biol. 2021, 46, 102115. [Google Scholar] [CrossRef]
- Yutani, M.; Matsumura, T.; Fujinaga, Y. Effects of antibiotics on the viability of and toxin production by Clostridium botulinum. Microbiol. Immunol. 2021, 65, 432–437. [Google Scholar] [CrossRef]
- Eck, A.; Rutten, N.; Singendonk, M.M.J.; Rijkers, G.T.; Savelkoul, P.H.M.; Meijssen, C.B.; Crijns, C.E.; Oudshoorn, J.H.; Budding, A.E.; Vlieger, A.M. Neonatal microbiota development and the effect of early life antibiotics are determined by two distinct settler types. PLoS ONE 2020, 15, e0228133. [Google Scholar] [CrossRef]
- Kappel, B.A.; De Angelis, L.; Heiser, M.; Ballanti, M.; Stoehr, R.; Goettsch, C.; Mavilio, M.; Artati, A.; Paoluzi, O.A.; Adamski, J.; et al. Cross-omics analysis revealed gut microbiome-related metabolic pathways underlying atherosclerosis development after antibiotics treatment. Mol. Metab. 2020, 36, 100976. [Google Scholar] [CrossRef]
- Shi, G.; Lin, Y.; Wu, Y.; Zhou, J.; Cao, L.; Chen, J.; Li, Y.; Tan, N.; Zhong, S. Bacteroides fragilis Supplementation Deteriorated Metabolic Dysfunction, Inflammation, and Aorta Atherosclerosis by Inducing Gut Microbiota Dysbiosis in Animal Model. Nutrients 2022, 14, 2199. [Google Scholar] [CrossRef]
- Malik, M.; Suboc, T.M.; Tyagi, S.; Salzman, N.; Wang, J.; Ying, R.; Tanner, M.J.; Kakarla, M.; Baker, J.E.; Widlansky, M.E. Lactobacillus plantarum 299v Supplementation Improves Vascular Endothelial Function and Reduces Inflammatory Biomarkers in Men With Stable Coronary Artery Disease. Circ. Res. 2018, 123, 1091–1102. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, Z.E.; Surana, N.K. Ruminococcus gnavus and Limosilactobacillus reuteri Regulate Reg3γ Expression through Multiple Pathways. Immunohorizons 2023, 7, 228–234. [Google Scholar] [CrossRef]
- Silverman, G.J.; Deng, J.; Azzouz, D.F. Sex-dependent Lupus Blautia (Ruminococcus) gnavus strain induction of zonulin-mediated intestinal permeability and autoimmunity. Front. Immunol. 2022, 13, 897971. [Google Scholar] [CrossRef]
- Hong, J.; Fu, T.; Liu, W.; Du, Y.; Bu, J.; Wei, G.; Yu, M.; Lin, Y.; Min, C.; Lin, D. Specific Alternation of Gut Microbiota and the Role of Ruminococcus gnavus in the Development of Diabetic Nephropathy. J. Microbiol. Biotechnol. 2024, 34, 547–561. [Google Scholar] [CrossRef]
- Shen, S.; Ren, F.; Qin, H.; Bukhari, I.; Yang, J.; Gao, D.; Ouwehand, A.C.; Lehtinen, M.J.; Zheng, P.; Mi, Y. Lactobacillus acidophilus NCFM and Lactiplantibacillus plantarum Lp-115 inhibit Helicobacter pylori colonization and gastric inflammation in a murine model. Front. Cell Infect. Microbiol. 2023, 13, 1196084. [Google Scholar] [CrossRef] [PubMed]
- Baba, Y.; Saito, Y.; Kadowaki, M.; Azuma, N.; Tsuge, D. Effect of Continuous Ingestion of Bifidobacteria and Inulin on Reducing Body Fat: A Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Comparison Study. Nutrients 2023, 15, 5025. [Google Scholar] [CrossRef]
- Magryś, A.; Pawlik, M. Postbiotic Fractions of Probiotics Lactobacillus plantarum 299v and Lactobacillus rhamnosus GG Show Immune-Modulating Effects. Cells 2023, 12, 2538. [Google Scholar] [CrossRef] [PubMed]
- Panzetta, M.E.; Valdivia, R.H. Akkermansia in the gastrointestinal tract as a modifier of human health. Gut Microbes 2024, 16, 2406379. [Google Scholar] [CrossRef]
- Parizadeh, M.; Arrieta, M.C. The global human gut microbiome: Genes, lifestyles, and diet. Trends Mol. Med. 2023, 29, 789–801. [Google Scholar] [CrossRef]
- Reis, F.; Ferreira, L.M.R.; Ortega, E.; Viana, S. Nutrition and Gut Microbiota-Immune System Interplay in Chronic Diseases. Nutrients 2025, 17, 1330. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.J.; Luo, Y.; Wang, J.B.; Chen, X.M.; Xu, Y.; Xiao, J.H. Regulated intestinal microbiota and gut immunity to ameliorate type 1 diabetes mellitus: A novel mechanism for stem cell-based therapy. Biomed. Pharmacother. 2024, 170, 116033. [Google Scholar] [CrossRef]
- Wang, Z.; Gong, M.; Fang, Y.; Yuan, H.; Zhang, C. Reconstruction characteristics of gut microbiota from patients with type 1 diabetes affect the phenotypic reproducibility of glucose metabolism in mice. Sci. China Life Sci. 2025, 68, 176–188. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, R.; Chen, Y.; Cao, Z.; Liu, C.; Bao, R.; Wang, Y.; Huang, S.; Pan, S.; Qin, L.; et al. Akkermansia muciniphila supplementation in patients with overweight/obese type 2 diabetes: Efficacy depends on its baseline levels in the gut. Cell Metab. 2025, 37, 592–605.e6. [Google Scholar] [CrossRef] [PubMed]
- Donati Zeppa, S.; Gervasi, M.; Bartolacci, A.; Ferrini, F.; Patti, A.; Sestili, P.; Stocchi, V.; Agostini, D. Targeting the Gut Microbiota for Prevention and Management of Type 2 Diabetes. Nutrients 2024, 16, 3951. [Google Scholar] [CrossRef]
- Wang, Y.; Ye, X.; Ding, D.; Lu, Y. Characteristics of the intestinal flora in patients with peripheral neuropathy associated with type 2 diabetes. J. Int. Med. Res. 2020, 48, 300060520936806. [Google Scholar] [CrossRef]
- González, A.; Fullaondo, A.; Odriozola, A. In Search of Healthy Ageing: A Microbiome-Based Precision Nutrition Approach for Type 2 Diabetes Prevention. Nutrients 2025, 17, 1877. [Google Scholar] [CrossRef]
- Han, C.; Shen, Z.; Cui, T.; Ai, S.S.; Gao, R.R.; Liu, Y.; Sui, G.Y.; Hu, H.Z.; Li, W. Yi-Shen-Hua-Shi granule ameliorates diabetic kidney disease by the “gut-kidney axis”. J. Ethnopharmacol. 2023, 307, 116257. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Gao, Z.; Zhao, L.; Wang, H.; Luo, Z.; Vandeputte, D.; He, L.; Li, M.; Di, S.; Liu, Y.; et al. Multiomics Analyses With Stool-Type Stratification in Patient Cohorts and Blautia Identification as a Potential Bacterial Modulator in Type 2 Diabetes Mellitus. Diabetes 2024, 73, 511–527. [Google Scholar] [CrossRef]
- Chen, D.Q.; Zhang, H.J.; Zhang, W.; Feng, K.; Liu, H.; Zhao, H.L.; Li, P. Tangshen Formula alleviates inflammatory injury against aged diabetic kidney disease through modulating gut microbiota composition and related amino acid metabolism. Exp. Gerontol. 2024, 188, 112393. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.Q.; Ye, S.N.; Huang, Y.H.; Ou, Y.W.; Chen, K.Y.; Chen, J.S.; Tang, S.B. Gut microbiota induced abnormal amino acids and their correlation with diabetic retinopathy. Int. J. Ophthalmol. 2024, 17, 883–895. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, A.H.; Kim, E.; Lee, S.; Yu, K.S.; Jang, I.J.; Chung, J.Y.; Cho, J.Y. Changes in the gut microbiome influence the hypoglycemic effect of metformin through the altered metabolism of branched-chain and nonessential amino acids. Diabetes Res. Clin. Pract. 2021, 178, 108985. [Google Scholar] [CrossRef]
- Chen, Y.; Song, L.; Chen, M.; Huang, Y.; Wang, Z.; Ren, Z.; Xu, J. Pediococcus pentosaceus MIANGUAN2 Alleviates Influenza Virus Infection by Modulating Gut Microbiota and Enhancing Short-Chain Fatty Acid Production. Nutrients 2024, 16, 1923. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Xu, X.; Liu, M.; Yang, J.; Yuan, M.; Sun, C.; Zhou, Q.; Chen, J.; Liu, B. Bile acid and short chain fatty acid metabolism of gut microbiota mediate high-fat diet induced intestinal barrier damage in Macrobrachium rosenbergii. Fish. Shellfish. Immunol. 2024, 146, 109376. [Google Scholar] [CrossRef]
- Li, N.; Wang, H.; Zhao, H.; Wang, M.; Cai, J.; Hao, Y.; Yu, J.; Jiang, Y.; Lü, X.; Liu, B. Cooperative interactions between Veillonella ratti and Lactobacillus acidophilus ameliorate DSS-induced ulcerative colitis in mice. Food Funct. 2023, 14, 10475–10492. [Google Scholar] [CrossRef]
- Zheng, X.X.; Li, D.X.; Li, Y.T.; Chen, Y.L.; Zhao, Y.L.; Ji, S.; Guo, M.Z.; Du, Y.; Tang, D.Q. Mulberry leaf water extract alleviates type 2 diabetes in mice via modulating gut microbiota-host co-metabolism of branched-chain amino acid. Phytother. Res. PTR 2023, 37, 3195–3210. [Google Scholar] [CrossRef]
- Yoshida, N.; Yamashita, T.; Osone, T.; Hosooka, T.; Shinohara, M.; Kitahama, S.; Sasaki, K.; Sasaki, D.; Yoneshiro, T.; Suzuki, T.; et al. Bacteroides spp. promotes branched-chain amino acid catabolism in brown fat and inhibits obesity. iScience 2021, 24, 103342. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Sun, M.; Li, A.; Gu, Q.; Kang, D.; Feng, Z.; Li, X.; Wang, X.; Chen, L.; Yang, H.; et al. Microbiota-derived IPA alleviates intestinal mucosal inflammation through upregulating Th1/Th17 cell apoptosis in inflammatory bowel disease. Gut Microbes 2025, 17, 2467235. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, S.; Liu, L.; Mao, A.; Kan, H.; Yu, F.; Ma, X.; Feng, L.; Zhou, T. The gut microbiota-derived metabolite indole-3-propionic acid enhances leptin sensitivity by targeting STAT3 against diet-induced obesity. Clin. Transl. Med. 2024, 14, e70053. [Google Scholar] [CrossRef]
- Kong, L.; Zhao, Q.; Jiang, X.; Hu, J.; Jiang, Q.; Sheng, L.; Peng, X.; Wang, S.; Chen, Y.; Wan, Y.; et al. Trimethylamine N-oxide impairs β-cell function and glucose tolerance. Nat. Commun. 2024, 15, 2526. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Lu, Y.; Yuan, S.; Cai, X.; He, Y.; Chen, J.; Wu, Q.; He, D.; Fang, A.; Bo, Y.; et al. Gut microbiota-derived metabolite trimethylamine-N-oxide and multiple health outcomes: An umbrella review and updated meta-analysis. Am. J. Clin. Nutr. 2022, 116, 230–243. [Google Scholar] [CrossRef]
- Jin, Q.; Zhang, C.; Chen, R.; Jiang, L.; Li, H.; Wu, P.; Li, L. Quinic acid regulated TMA/TMAO-related lipid metabolism and vascular endothelial function through gut microbiota to inhibit atherosclerotic. J. Transl. Med. 2024, 22, 352. [Google Scholar] [CrossRef]
- Zhen, J.; Zhang, Y.; Li, Y.; Zhou, Y.; Cai, Y.; Huang, G.; Xu, A. The gut microbiota intervenes in glucose tolerance and inflammation by regulating the biosynthesis of taurodeoxycholic acid and carnosine. Front. Cell Infect. Microbiol. 2024, 14, 1423662. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, L.; Chai, T.; Xu, H.; Du, H.Y.; Jiang, Y. Mulberry leaf multi-components exert hypoglycemic effects through regulation of the PI-3K/Akt insulin signaling pathway in type 2 diabetic rats. J. Ethnopharmacol. 2024, 319, 117307. [Google Scholar] [CrossRef] [PubMed]
- Bodur, C.; Kazyken, D.; Huang, K.; Tooley, A.S.; Cho, K.W.; Barnes, T.M.; Lumeng, C.N.; Myers, M.G.; Fingar, D.C. TBK1-mTOR Signaling Attenuates Obesity-Linked Hyperglycemia and Insulin Resistance. Diabetes 2022, 71, 2297–2312. [Google Scholar] [CrossRef]
- Wang, H.; Pan, F.; Liu, J.; Zhang, J.; Fuli, Z.; Wang, Y. Huayuwendan decoction ameliorates inflammation via IL-17/NF-κB signaling pathway in diabetic rats. J. Ethnopharmacol. 2024, 319, 117328. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zhang, F.; Sun, D.; Wang, X.; Zhang, X.; Zhang, J.; Yan, F.; Huang, C.; Xie, H.; Lin, C.; et al. Branched-Chain Amino Acids Exacerbate Obesity-Related Hepatic Glucose and Lipid Metabolic Disorders via Attenuating Akt2 Signaling. Diabetes 2020, 69, 1164–1177. [Google Scholar] [CrossRef]
- Huang, H.; Chen, H.; Yao, Y.; Lou, X. Branched-chain amino acids supplementation induces insulin resistance and pro-inflammatory macrophage polarization via INFGR1/JAK1/STAT1 signal pathway. Mol. Med. 2024, 30, 149. [Google Scholar] [CrossRef]
- Li, Y.; Xiong, Z.; Yan, W.; Gao, E.; Cheng, H.; Wu, G.; Liu, Y.; Zhang, L.; Li, C.; Wang, S.; et al. Branched chain amino acids exacerbate myocardial ischemia/reperfusion vulnerability via enhancing GCN2/ATF6/PPAR-α pathway-dependent fatty acid oxidation. Theranostics 2020, 10, 5623–5640. [Google Scholar] [CrossRef]
- Eguchi, A.; Iwasa, M.; Tamai, Y.; Tempaku, M.; Takamatsu, S.; Miyoshi, E.; Hasegawa, H.; Kobayashi, Y.; Takei, Y. Branched-chain amino acids protect the liver from cirrhotic injury via suppression of activation of lipopolysaccharide-binding protein, toll-like receptor 4, and signal transducer and activator of transcription 3, as well as Enterococcus faecalis translocation. Nutrition 2021, 86, 111194. [Google Scholar] [CrossRef]
- Zheng, H.; Zhang, X.; Li, C.; Wang, D.; Shen, Y.; Lu, J.; Zhao, L.; Li, X.; Gao, H. BCAA mediated microbiota-liver-heart crosstalk regulates diabetic cardiomyopathy via FGF21. Microbiome 2024, 12, 157. [Google Scholar] [CrossRef]
- Miao, M.; Wang, Q.; Wang, X.; Fan, C.; Luan, T.; Yan, L.; Zhang, Y.; Zeng, X.; Dai, Y.; Li, P. The Protective Effects of Inulin-Type Fructans Against High-Fat/Sucrose Diet-Induced Gestational Diabetes Mice in Association With Gut Microbiota Regulation. Front. Microbiol. 2022, 13, 832151. [Google Scholar] [CrossRef]
- Giampieri, F.; Mazzoni, L.; Cianciosi, D.; Alvarez-Suarez, J.M.; Regolo, L.; Sánchez-González, C.; Capocasa, F.; Xiao, J.; Mezzetti, B.; Battino, M. Organic vs conventional plant-based foods: A review. Food Chem. 2022, 383, 132352. [Google Scholar] [CrossRef]
- Saikachain, N.; Sungkaworn, T.; Muanprasat, C.; Asavapanumas, N. Neuroprotective effect of short-chain fatty acids against oxidative stress-induced SH-SY5Y injury via GPR43-dependent pathway. J. Neurochem. 2023, 166, 201–214. [Google Scholar] [CrossRef]
- Zhao, Z.; Tong, Y.; Kang, Y.; Qiu, Z.; Li, Q.; Xu, C.; Wu, G.; Jia, W.; Wang, P. Sodium butyrate (SB) ameliorated inflammation of COPD induced by cigarette smoke through activating the GPR43 to inhibit NF-κB/MAPKs signaling pathways. Mol. Immunol. 2023, 163, 224–234. [Google Scholar] [CrossRef]
- Yi, C.; Sun, W.; Ding, L.; Yan, M.; Sun, C.; Qiu, C.; Wang, D.; Wu, L. Short-Chain Fatty Acids Weaken Ox-LDL-Induced Cell Inflammatory Injury by Inhibiting the NLRP3/Caspase-1 Pathway and Affecting Cellular Metabolism in THP-1 Cells. Molecules 2022, 27, 8801. [Google Scholar] [CrossRef]
- Xia, T.; He, W.; Luo, Z.; Wang, K.; Tan, X. Achyranthes bidentata polysaccharide ameliorates type 2 diabetes mellitus by gut microbiota-derived short-chain fatty acids-induced activation of the GLP-1/GLP-1R/cAMP/PKA/CREB/INS pathway. Int. J. Biol. Macromol. 2024, 270, 132256. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, S.H.; Li, H.L.; Zhou, X.B.; Zhou, L.W.; Chen, C.; Mansell, T.; Novakovic, B.; Saffery, R.; Baker, P.N.; et al. The attenuation of gut microbiota-derived short-chain fatty acids elevates lipid transportation through suppression of the intestinal HDAC3-H3K27ac-PPAR-γ axis in gestational diabetes mellitus. J. Nutr. Biochem. 2024, 133, 109708. [Google Scholar] [CrossRef] [PubMed]
- Tayyeb, J.Z.; Popeijus, H.E.; Mensink, R.P.; Konings, M.; Mokhtar, F.B.A.; Plat, J. Short-Chain Fatty Acids (Except Hexanoic Acid) Lower NF-kB Transactivation, Which Rescues Inflammation-Induced Decreased Apolipoprotein A-I Transcription in HepG2 Cells. Int. J. Mol. Sci. 2020, 21, 5088. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Zeng, Y.; Tu, Q.; Jiao, Y.; Yao, S.; Chen, Y.; Sun, L.; Xia, Q.; Luo, Y.; Yuan, L.; et al. Butyrate alleviates renal fibrosis in CKD by regulating NLRP3-mediated pyroptosis via the STING/NF-κB/p65 pathway. Int. Immunopharmacol. 2023, 124, 111010. [Google Scholar] [CrossRef]
- Tillett, B.J.; Dwiyanto, J.; Secombe, K.R.; George, T.; Zhang, V.; Anderson, D.; Duggan, E.; Giri, R.; Loo, D.; Stoll, T.; et al. SCFA biotherapy delays diabetes in humanized gnotobiotic mice by remodeling mucosal homeostasis and metabolome. Nat. Commun. 2025, 16, 2893. [Google Scholar] [CrossRef]
- Liu, Z.; Dai, X.; Zhang, H.; Shi, R.; Hui, Y.; Jin, X.; Zhang, W.; Wang, L.; Wang, Q.; Wang, D.; et al. Gut microbiota mediates intermittent-fasting alleviation of diabetes-induced cognitive impairment. Nat. Commun. 2020, 11, 855. [Google Scholar] [CrossRef]
- Chen, L.; Yang, Y.; Sun, S.; Xie, Y.; Pan, C.; Li, M.; Li, C.; Liu, Y.; Xu, Z.; Liu, W.; et al. Indolepropionic acid reduces obesity-induced metabolic dysfunction through colonic barrier restoration mediated via tuft cell-derived IL-25. FEBS J. 2022, 289, 5985–6004. [Google Scholar] [CrossRef]
- Yao, W.; Huo, J.; Liu, K.; Tao, P. Exploring the beneficial effect of gut microbiota metabolites on diabetic nephropathy via network pharmacology study. Sci. Rep. 2025, 15, 11027. [Google Scholar] [CrossRef]
- Lee, H.; Park, S.; Ju, S.; Kim, S.; Yoo, J.W.; Yoon, I.S.; Min, D.S.; Jung, Y. Preparation and Evaluation of Colon-Targeted Prodrugs of the Microbial Metabolite 3-Indolepropionic Acid as an Anticolitic Agent. Mol. Pharm. 2021, 18, 1730–1741. [Google Scholar] [CrossRef]
- Zeng, Y.; Guo, M.; Wu, Q.; Tan, X.; Jiang, C.; Teng, F.; Chen, J.; Zhang, F.; Ma, X.; Li, X.; et al. Gut microbiota-derived indole-3-propionic acid alleviates diabetic kidney disease through its mitochondrial protective effect via reducing ubiquitination mediated-degradation of SIRT1. J. Adv. Res. 2025, 73, 607–630. [Google Scholar] [CrossRef]
- Rybka, M.; Mazurek, Ł.; Jurak, J.; Laskowska, A.; Zajdel, M.; Czuwara, J.; Sulejczak, D.; Szudzik, M.; Samborowska, E.; Schwartz, R.A.; et al. Keratin-TMAO dressing accelerates full-thickness skin wound healing in diabetic rats via M2-macrophage polarization and the activation of PI3K/AKT/mTOR signaling pathway. Int. J. Biol. Macromol. 2025, 310, 143313. [Google Scholar] [CrossRef]
- Lin, X.; Zhang, Y.; He, X.; Chen, Y.; Chen, N.; Liu, J.; Wang, M.; Li, Y.; Yang, H.; Fan, L.; et al. The Choline Metabolite TMAO Inhibits NETosis and Promotes Placental Development in GDM of Humans and Mice. Diabetes 2021, 70, 2250–2263. [Google Scholar] [CrossRef] [PubMed]
- Sung, M.; Lim, S.; Park, S.; Choi, Y.; Kim, S. Anti-inflammatory effects of phytosphingosine-regulated cytokines and NF-kB and MAPK mechanism. Cell. Mol. Biol. 2024, 70, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Won, J.H.; Shin, J.S.; Park, H.J.; Jung, H.J.; Koh, D.J.; Jo, B.G.; Lee, J.Y.; Yun, K.; Lee, K.T. Anti-inflammatory effects of madecassic acid via the suppression of NF-kappaB pathway in LPS-induced RAW 264.7 macrophage cells. Planta Med. 2010, 76, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Das, A.K.; Hossain, U.; Ghosh, S.; Biswas, S.; Mandal, M.; Mandal, B.; Brahmachari, G.; Bagchi, A.; Sil, P.C. Amelioration of oxidative stress mediated inflammation and apoptosis in pancreatic islets by Lupeol in STZ-induced hyperglycaemic mice. Life Sci. 2022, 305, 120769. [Google Scholar] [CrossRef]
- Liu, Y.; Deng, J.; Fan, D. Ginsenoside Rk3 ameliorates high-fat-diet/streptozocin induced type 2 diabetes mellitus in mice via the AMPK/Akt signaling pathway. Food Funct. 2019, 10, 2538–2551. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yu, H.; Ye, J.; Tong, H.; Wang, M.; Sun, G. Ginsenoside Rg3 Protects against Diabetic Cardiomyopathy and Promotes Adiponectin Signaling via Activation of PPAR-γ. Int. J. Mol. Sci. 2023, 24, 16736. [Google Scholar] [CrossRef]
- Wang, W.; Guan, F.; Sagratini, G.; Yan, J.; Xie, J.; Jin, Z.; Liu, M.; Liu, H.; Liu, J. Ginsenoside Rd attenuated hyperglycemia via Akt pathway and modulated gut microbiota in streptozotocin-induced diabetic rats. Curr. Res. Food Sci. 2023, 6, 100491. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, L.; Guo, R.; Xiao, J.; Liu, X.; Dong, M.; Luan, X.; Ji, X.; Lu, H. Ginsenoside Rb1 Ameliorates Diabetic Arterial Stiffening via AMPK Pathway. Front. Pharmacol. 2021, 12, 753881. [Google Scholar] [CrossRef]
- Zhu, Y.; Yang, H.; Deng, J.; Fan, D. Ginsenoside Rg5 Improves Insulin Resistance and Mitochondrial Biogenesis of Liver via Regulation of the Sirt1/PGC-1α Signaling Pathway in db/db Mice. J. Agric. Food Chem. 2021, 69, 8428–8439. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, J.; Guo, X.; Zhu, N.; Niu, L.; Ding, X.; Xie, Z.; Chen, X.; Yang, F. Oleic Acid and Eicosapentaenoic Acid Reverse Palmitic Acid-induced Insulin Resistance in Human HepG2 Cells via the Reactive Oxygen Species/JUN Pathway. Genom. Proteom. Bioinform. 2021, 19, 754–771. [Google Scholar] [CrossRef]
- Zang, T.; Chen, H.; Shen, S.; Xu, F.; Wang, R.; Yin, J.; Chen, X.; Guan, M.; Shen, L.; Pan, H.; et al. Highly Purified Eicosapentaenoic Acid Alleviates the Inflammatory Response and Oxidative Stress in Macrophages during Atherosclerosis via the miR-1a-3p/sFRP1/Wnt/PCP-JNK Pathway. Oxidative Med. Cell. Longev. 2022, 2022, 9451058. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Kang, M.S.; Nam, M.; Kim, S.A.; Hwang, G.S.; Kim, H.S. Eicosapentaenoic Acid (EPA) Modulates Glucose Metabolism by Targeting AMP-Activated Protein Kinase (AMPK) Pathway. Int. J. Mol. Sci. 2019, 20, 4751. [Google Scholar] [CrossRef]
- Simón, M.V.; Agnolazza, D.L.; German, O.L.; Garelli, A.; Politi, L.E.; Agbaga, M.P.; Anderson, R.E.; Rotstein, N.P. Synthesis of docosahexaenoic acid from eicosapentaenoic acid in retina neurons protects photoreceptors from oxidative stress. J. Neurochem. 2016, 136, 931–946. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wei, Y.; Jia, W.; Can, C.; Wang, R.; Yang, X.; Gu, C.; Liu, F.; Ji, C.; Ma, D. Chenodeoxycholic acid suppresses AML progression through promoting lipid peroxidation via ROS/p38 MAPK/DGAT1 pathway and inhibiting M2 macrophage polarization. Redox Biol. 2022, 56, 102452. [Google Scholar] [CrossRef]
- Song, M.; Ye, J.; Zhang, F.; Su, H.; Yang, X.; He, H.; Liu, F.; Zhu, X.; Wang, L.; Gao, P.; et al. Chenodeoxycholic Acid (CDCA) Protects against the Lipopolysaccharide-Induced Impairment of the Intestinal Epithelial Barrier Function via the FXR-MLCK Pathway. J. Agric. Food Chem. 2019, 67, 8868–8874. [Google Scholar] [CrossRef]
- Zhou, J.C.; Wu, B.; Zhang, J.J.; Zhang, W. Lupeol triggers oxidative stress, ferroptosis, apoptosis and restrains inflammation in nasopharyngeal carcinoma via AMPK/NF-κB pathway. Immunopharmacol. Immunotoxicol. 2022, 44, 621–631. [Google Scholar] [CrossRef]
- Qin, D.; Pan, P.; Lyu, B.; Chen, W.; Gao, Y. Lupeol improves bile acid metabolism and metabolic dysfunction-associated steatotic liver disease in mice via FXR signaling pathway and gut-liver axis. Biomed. Pharmacother. 2024, 177, 116942. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Jing, G.; Yin, R.; Ma, M.; Cao, W.; Zhang, M. Neuroprotective effects of traditional Chinese medicine Naofucong on diabetic cognitive impairment: Mechanisms involving insulin-degrading enzyme-mediated degradation of Amyloid-β and inhibition of ERK/JNK/p38 MAPK signaling pathway. Brain Res. 2025, 1849, 149365. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, Y.; Lu, S.; Wu, J. EVOO supplement prevents type 1 diabetes by modulating gut microbiota and serum metabolites in NOD mice. Life Sci. 2023, 335, 122274. [Google Scholar] [CrossRef]
- Ismail, H.M.; Spall, M.; Evans-Molina, C.; DiMeglio, L.A. Evaluating the effect of prebiotics on the gut microbiome profile and β cell function in youth with newly diagnosed type 1 diabetes: Protocol of a pilot randomized controlled trial. Pilot. Feasibility Stud. 2023, 9, 150. [Google Scholar] [CrossRef]
- Yang, B.; Xiong, Z.; Lin, M.; Yang, Y.; Chen, Y.; Zeng, J.; Jia, X.; Feng, L. Astragalus polysaccharides alleviate type 1 diabetes via modulating gut microbiota in mice. Int. J. Biol. Macromol. 2023, 234, 123767. [Google Scholar] [CrossRef]
- Lo Conte, M.; Antonini Cencicchio, M.; Ulaszewska, M.; Nobili, A.; Cosorich, I.; Ferrarese, R.; Massimino, L.; Andolfo, A.; Ungaro, F.; Mancini, N.; et al. A diet enriched in omega-3 PUFA and inulin prevents type 1 diabetes by restoring gut barrier integrity and immune homeostasis in NOD mice. Front. Immunol. 2022, 13, 1089987. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, Q.; Han, N.; Song, C.; Lin, Y.; Zhang, L.; Ren, D.; Zhao, Y.; Yang, X.; Li, T. Effects of Fu brick tea polysaccharides on gut microbiota and fecal metabolites of HFD/STZ-induced type 2 diabetes rats. Food Funct. 2023, 14, 10910–10923. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhao, M.; Zhang, Y.; Xu, R.; Fu, Z.; Jin, T.; Song, J.; Huang, Y.; Wang, M.; Zhao, C. Polysaccharides from Phellinus linteus attenuate type 2 diabetes mellitus in rats via modulation of gut microbiota and bile acid metabolism. Int. J. Biol. Macromol. 2024, 262, 130062. [Google Scholar] [CrossRef]
- Wang, L.; Liang, C.; Song, X.; Jia, X.; Wang, X.; Zhang, Y.; Xie, Q.; Zheng, N.; Yuan, H. Canagliflozin alters the gut, oral, and ocular surface microbiota of patients with type 2 diabetes mellitus. Front. Endocrinol. 2023, 14, 1256292. [Google Scholar] [CrossRef]
- Sun, Y.; Qu, H.; Niu, X.; Li, T.; Wang, L.; Peng, H. Carvacrol improves blood lipid and glucose in rats with type 2 diabetes mellitus by regulating short-chain fatty acids and the GPR41/43 pathway. Korean J. Physiol. Pharmacol. 2024, 28, 1–10. [Google Scholar] [CrossRef]
- Huang, S.; Chen, J.; Cui, Z.; Ma, K.; Wu, D.; Luo, J.; Li, F.; Xiong, W.; Rao, S.; Xiang, Q.; et al. Lachnospiraceae-derived butyrate mediates protection of high fermentable fiber against placental inflammation in gestational diabetes mellitus. Sci. Adv. 2023, 9, eadi7337. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wu, H.; Duan, M.; Liu, R.; Zhu, Q.; Zhang, K.; Wang, L. Cinnamaldehyde Improves Metabolic Functions in Streptozotocin-Induced Diabetic Mice by Regulating Gut Microbiota. Drug Des. Dev. Ther. 2021, 15, 2339–2355. [Google Scholar] [CrossRef]
- Du, Y.; Zhang, R.; Zheng, X.X.; Zhao, Y.L.; Chen, Y.L.; Ji, S.; Guo, M.Z.; Tang, D.Q. Mulberry (Morus alba L.) leaf water extract attenuates type 2 diabetes mellitus by regulating gut microbiota dysbiosis, lipopolysaccharide elevation and endocannabinoid system disorder. J. Ethnopharmacol. 2024, 323, 117681. [Google Scholar] [CrossRef] [PubMed]
- Tawulie, D.; Jin, L.; Shang, X.; Li, Y.; Sun, L.; Xie, H.; Zhao, J.; Liao, J.; Zhu, Z.; Cui, H.; et al. Jiang-Tang-San-Huang pill alleviates type 2 diabetes mellitus through modulating the gut microbiota and bile acids metabolism. Phytomed. Int. J. Phytother. Phytopharm. 2023, 113, 154733. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, C.; Fu, X. Dendrobium officinale Polysaccharide Alleviates Type 2 Diabetes Mellitus by Restoring Gut Microbiota and Repairing Intestinal Barrier via the LPS/TLR4/TRIF/NF-kB Axis. J. Agric. Food Chem. 2023, 71, 11929–11940. [Google Scholar] [CrossRef]
- Meng, X.; Shi, M.; Guo, G.; Xing, J.; Liu, Z.; Song, F.; Liu, S. In-depth investigation of the therapeutic effect of Tribulus terrestris L. on type 2 diabetes based on intestinal microbiota and feces metabolomics. J. Ethnopharmacol. 2024, 325, 117815. [Google Scholar] [CrossRef]
- Du, C.; Zuo, F.; Cao, Y.; Zang, Y. Anti-diabetic effects of natural and modified ‘Ganzhou’ navel orange peel pectin on type 2 diabetic mice via gut microbiota. Food Funct. 2023, 14, 10977–10990. [Google Scholar] [CrossRef]
- Pi, Y.; Fang, M.; Li, Y.; Cai, L.; Han, R.; Sun, W.; Jiang, X.; Chen, L.; Du, J.; Zhu, Z.; et al. Interactions between Gut Microbiota and Natural Bioactive Polysaccharides in Metabolic Diseases: Review. Nutrients 2024, 16, 2838. [Google Scholar] [CrossRef]
- Xu, H.; Liu, Z.; Xu, W.; Zhang, Y. Beneficial In Vitro Effects of Polysaccharide and Non-Polysaccharide Components of Dendrobium huoshanense on Gut Microbiota of Rats with Type 1 Diabetes as Opposed to Metformin. Molecules 2024, 29, 2791. [Google Scholar] [CrossRef]
- Siddiqui, N.Z.; Rehman, A.U.; Yousuf, W.; Khan, A.I.; Farooqui, N.A.; Zang, S.; Xin, Y.; Wang, L. Effect of crude polysaccharide from seaweed, Dictyopteris divaricata (CDDP) on gut microbiota restoration and anti-diabetic activity in streptozotocin (STZ)-induced T1DM mice. Gut Pathog. 2022, 14, 39. [Google Scholar] [CrossRef]
- Ye, J.; Ma, J.; Rozi, P.; Kong, L.; Zhou, J.; Luo, Y.; Yang, H. The polysaccharides from seeds of Glycyrrhiza uralensis ameliorate metabolic disorders and restructure gut microbiota in type 2 diabetic mice. Int. J. Biol. Macromol. 2024, 264, 130622. [Google Scholar] [CrossRef]
- Deng, J.; Luo, K.; Xia, C.; Zhu, Y.; Xiang, Z.; Zhu, B.; Tang, X.; Zhang, T.; Shi, L.; Lyu, X.; et al. Phytochemical composition of Tibetan tea fermented by Eurotium cristatum and its effects on type 1 diabetes mice and gut microbiota. Heliyon 2024, 10, e27145. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Pan, L.L.; Niu, W.; Fang, X.; Liang, W.; Li, J.; Li, H.; Pan, X.; Chen, W.; Zhang, H.; et al. Modulation of Gut Microbiota by Low Methoxyl Pectin Attenuates Type 1 Diabetes in Non-obese Diabetic Mice. Front. Immunol. 2019, 10, 1733. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.; Zhao, Y.; Liang, Y.; Zou, K. Flavonoids Derived from Opuntia ficus-indica Fruit Alleviate Renal Injury in Diabetic Nephropathy Mice by Altering Gut Microbiota and Promoting the Production of SCFAs. Nutrients 2025, 17, 1800. [Google Scholar] [CrossRef] [PubMed]
- Di, S.; Yao, C.; Qiao, L.; Li, X.; Pang, B.; Lin, J.; Wang, J.; Li, M.; Tong, X. Exploration of the mechanisms underlying the beneficial effect of Luo Tong formula on retinal function in diabetic rats via the “gut microbiota-inflammation-retina” axis. Chin. Med. 2022, 17, 133. [Google Scholar] [CrossRef]
- Liu, Y.; Gong, Y.; Li, M.; Li, J. Quercetin protects against hyperglycemia-induced retinopathy in Sprague Dawley rats by regulating the gut-retina axis and nuclear factor erythroid-2-related factor 2 pathway. Nutr. Res. 2024, 122, 55–67. [Google Scholar] [CrossRef]
- Gao, Y.; Yang, R.; Guo, L.; Wang, Y.; Liu, W.J.; Ai, S.; Woon, T.H.; Wang, Z.; Zhai, Y.; Wang, Z.; et al. Qing-Re-Xiao-Zheng Formula Modulates Gut Microbiota and Inhibits Inflammation in Mice With Diabetic Kidney Disease. Front. Med. 2021, 8, 719950. [Google Scholar] [CrossRef]
- Lan, T.; Tang, T.; Li, Y.; Duan, Y.; Yuan, Q.; Liu, W.; Ren, Y.; Li, N.; Liu, X.; Zhang, Y.; et al. FTZ polysaccharides ameliorate kidney injury in diabetic mice by regulating gut-kidney axis. Phytomedicine 2023, 118, 154935. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, L.; Zhu, M.; Yang, B.; Yang, Y.; Jia, X.; Feng, L. Moutan Cortex polysaccharide ameliorates diabetic kidney disease via modulating gut microbiota dynamically in rats. Int. J. Biol. Macromol. 2022, 206, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Pengrattanachot, N.; Thongnak, L.; Promsan, S.; Phengpol, N.; Sutthasupha, P.; Tocharus, J.; Lungkaphin, A. Fructooligosaccharides Ameliorate Renal Injury and Dysfunction Through the Modulation of Gut Dysbiosis, Inhibition of Renal Inflammation, Oxidative Stress, Fibrosis, and Improve Organic Anion Transporter 3 Function in an Obese Rat Model. Mol. Nutr. Food Res. 2024, 68, e2400191. [Google Scholar] [CrossRef]
- Shen, Z.; Cui, T.; Liu, Y.; Wu, S.; Han, C.; Li, J. Astragalus membranaceus and Salvia miltiorrhiza ameliorate diabetic kidney disease via the “gut-kidney axis”. Phytomed. Int. J. Phytother. Phytopharm. 2023, 121, 155129. [Google Scholar] [CrossRef]
- Luo, L.; Luo, J.; Cai, Y.; Fu, M.; Li, W.; Shi, L.; Liu, J.; Dong, R.; Xu, X.; Tu, L.; et al. Inulin-type fructans change the gut microbiota and prevent the development of diabetic nephropathy. Pharmacol. Res. 2022, 183, 106367. [Google Scholar] [CrossRef]
- Dong, W.; Zhao, Y.; Li, X.; Huo, J.; Wang, W. Corn silk polysaccharides attenuate diabetic nephropathy through restoration of the gut microbial ecosystem and metabolic homeostasis. Front. Endocrinol. 2023, 14, 1232132. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Yu, W.; Liu, A.; Wang, C.; Li, X.; Gao, J.; Liu, X.; Jiang, W.; Yang, Y.; Lv, S. San-Huang-Yi-Shen Capsule Ameliorates Diabetic Nephropathy in Rats Through Modulating the Gut Microbiota and Overall Metabolism. Front. Pharmacol. 2021, 12, 808867. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Duan, H.; Feng, Y.; Xu, W.; Shen, J.; Wang, K.; Liu, J. Magnesium lithospermate B ameliorates diabetic nephropathy by suppressing the uremic toxin formation mediated by gut microbiota. Eur. J. Pharmacol. 2023, 953, 175812. [Google Scholar] [CrossRef]
- Xie, J.; Song, W.; Liang, X.; Zhang, Q.; Shi, Y.; Liu, W.; Shi, X. Jinmaitong ameliorates diabetic peripheral neuropathy in streptozotocin-induced diabetic rats by modulating gut microbiota and neuregulin 1. Aging 2020, 12, 17436–17458. [Google Scholar] [CrossRef]
- Xie, J.; Song, W.; Liang, X.; Zhang, Q.; Shi, Y.; Liu, W.; Shi, X. Protective effect of quercetin on streptozotocin-induced diabetic peripheral neuropathy rats through modulating gut microbiota and reactive oxygen species level. Biomed. Pharmacother. 2020, 127, 110147. [Google Scholar] [CrossRef]
- Shen, C.L.; Wang, R.; Santos, J.M.; Elmassry, M.M.; Stephens, E.; Kim, N.; Neugebauer, V. Ginger alleviates mechanical hypersensitivity and anxio-depressive behavior in rats with diabetic neuropathy through beneficial actions on gut microbiome composition, mitochondria, and neuroimmune cells of colon and spinal cord. Nutr. Res. 2024, 124, 73–84. [Google Scholar] [CrossRef]
- Li, J.; Yang, S.; Liu, D.; Yan, Q.; Guo, H.; Jiang, Z. Neoagarotetraose Alleviates Atherosclerosis via Modulating Cholesterol and Bile Acid Metabolism in ApoE−/− Mice. Nutrients 2024, 16, 1502. [Google Scholar] [CrossRef]
- Zhang, K.; Peng, P.; Huang, J.; Chen, M.; Liu, F.; Zhu, C.; Lu, Q.; Wang, M.; Lin, C. Integrating plasma metabolomics and gut microbiome to reveal the mechanisms of Huangqi Guizhi Wuwu Decoction intervene diabetic peripheral neuropathy. J. Ethnopharmacol. 2024, 319, 117301. [Google Scholar] [CrossRef]
- Pan, L.; Yu, H.; Fu, J.; Hu, J.; Xu, H.; Zhang, Z.; Bu, M.; Yang, X.; Zhang, H.; Lu, J.; et al. Berberine ameliorates chronic kidney disease through inhibiting the production of gut-derived uremic toxins in the gut microbiota. Acta Pharm. Sinica. B 2023, 13, 1537–1553. [Google Scholar] [CrossRef]
- Eppig, J.T.; Blake, J.A.; Bult, C.J.; Kadin, J.A.; Richardson, J.E. The Mouse Genome Database (MGD): Facilitating mouse as a model for human biology and disease. Nucleic Acids Res. 2015, 43, D726–D736. [Google Scholar] [CrossRef] [PubMed]
- Kachapati, K.; Adams, D.; Bednar, K.; Ridgway, W.M. The non-obese diabetic (NOD) mouse as a model of human type 1 diabetes. Methods Mol. Biol. 2012, 933, 3–16. [Google Scholar] [CrossRef]
- Sharma, K.; McCue, P.; Dunn, S.R. Diabetic kidney disease in the db/db mouse. Am. J. Physiol. Ren. Physiol. 2003, 284, F1138–F1144. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Wei, Y.; He, J.; Feng, B.; Chen, Y.; Guo, R.; Griffin, M.D.; Hynes, S.O.; Shen, S.; Liu, Y.; et al. Human umbilical cord-derived mesenchymal stromal cells improve myocardial fibrosis and restore miRNA-133a expression in diabetic cardiomyopathy. Stem Cell Res. Ther. 2024, 15, 120. [Google Scholar] [CrossRef]
- Takaichi, S.; Tomimaru, Y.; Akagi, T.; Kobayashi, S.; Fukuda, Y.; Toya, K.; Asaoka, T.; Iwagami, Y.; Yamada, D.; Akita, H.; et al. Three-dimensional Vascularized β-cell Spheroid Tissue Derived From Human Induced Pluripotent Stem Cells for Subcutaneous Islet Transplantation in a Mouse Model of Type 1 Diabetes. Transplantation 2022, 106, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wu, Y.; Tian, Y.; Xu, H.; Lin, Z.X.; Xian, Y.F. Chinese herbal medicine for the treatment of intestinal cancer: Preclinical studies and potential clinical applications. Mol. Cancer 2024, 23, 217. [Google Scholar] [CrossRef]
- Dickman, K.G.; Chen, C.H.; Grollman, A.P.; Pu, Y.S. Aristolochic acid-containing Chinese herbal medicine and upper urinary tract urothelial carcinoma in Taiwan: A narrative review. World J. Urol. 2023, 41, 899–907. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.K.; Liu, T.T.; Teia, F.K.F.; Xie, M.Z. Exploring the underlying mechanisms of obesity and diabetes and the potential of Traditional Chinese Medicine: An overview of the literature. Front. Endocrinol. 2023, 14, 1218880. [Google Scholar] [CrossRef]
- Luo, W.; Zhou, J.; Yang, X.; Wu, R.; Liu, H.; Shao, H.; Huang, B.; Kang, X.; Yang, L.; Liu, D. A Chinese medical nutrition therapy diet accompanied by intermittent energy restriction alleviates type 2 diabetes by enhancing pancreatic islet function and regulating gut microbiota composition. Food Res. Int. 2022, 161, 111744. [Google Scholar] [CrossRef]
- Song, Q.; Wang, Y.; Huang, L.; Shen, M.; Yu, Y.; Yu, Q.; Chen, Y.; Xie, J. Review of the relationships among polysaccharides, gut microbiota, and human health. Food Res. Int. 2021, 140, 109858. [Google Scholar] [CrossRef] [PubMed]
- Coutiño-Hernández, D.; Sánchez-Tapia, M.; Leal-Vega, F.; Bobadilla Del Valle, M.; Ledezma, H.; Cervantes, R.; Pedraza-Chaverri, J.; Granados-Portillo, O.; Díaz, D.; Antunes-Ricardo, M.; et al. Modulation of gut microbiota by Mantequilla and Melipona honeys decrease low-grade inflammation caused by high fructose corn syrup or sucrose in rats. Food Res. Int. 2022, 151, 110856. [Google Scholar] [CrossRef] [PubMed]
- González, I.; Morales, M.A.; Rojas, A. Polyphenols and AGEs/RAGE axis. Trends and challenges. Food Res. Int. 2020, 129, 108843. [Google Scholar] [CrossRef]
- Barber, T.M.; Kabisch, S.; Pfeiffer, A.F.H.; Weickert, M.O. The Effects of the Mediterranean Diet on Health and Gut Microbiota. Nutrients 2023, 15, 2150. [Google Scholar] [CrossRef]
- García-Gavilán, J.F.; Atzeni, A.; Babio, N.; Liang, L.; Belzer, C.; Vioque, J.; Corella, D.; Fitó, M.; Vidal, J.; Moreno-Indias, I.; et al. Effect of 1-year lifestyle intervention with energy-reduced Mediterranean diet and physical activity promotion on the gut metabolome and microbiota: A randomized clinical trial. Am. J. Clin. Nutr. 2024, 119, 1143–1154. [Google Scholar] [CrossRef]
- Sato, S.; Chinda, D.; Iino, C.; Sawada, K.; Mikami, T.; Nakaji, S.; Sakuraba, H.; Fukuda, S. A Cohort Study of the Influence of the 12-Component Modified Japanese Diet Index on Oral and Gut Microbiota in the Japanese General Population. Nutrients 2024, 16, 524. [Google Scholar] [CrossRef]
- Pieczyńska-Zając, J.M.; Malinowska, A.; Łagowska, K.; Leciejewska, N.; Bajerska, J. The effects of time-restricted eating and Ramadan fasting on gut microbiota composition: A systematic review of human and animal studies. Nutr. Rev. 2024, 82, 777–793. [Google Scholar] [CrossRef]
- Liu, D.; Zhan, J.; Wang, S.; Chen, L.; Zhu, Q.; Nie, R.; Zhou, X.; Zheng, W.; Luo, X.; Wang, B.; et al. Chrysanthemum morifolium attenuates metabolic and alcohol-associated liver disease via gut microbiota and PPARα/γ activation. Phytomed. Int. J. Phytother. Phytopharm. 2024, 130, 155774. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Hu, H.; Zhang, Y.; Hu, S.; Lu, J.; Peng, W.; Luo, D. Dietary Capsaicin Exacerbates Gut Microbiota Dysbiosis and Mental Disorders in Type 1 Diabetes Mice. Nutrients 2025, 17, 593. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Fu, M.; Su, J.; Yan, M.; Yu, J.; Wang, C.; Niu, Z.; Du, Y.; Hu, X.; Zheng, J.; et al. Gut microbiota dysbiosis and intestinal barrier impairment in diarrhea caused by cold drink and high-fat diet. Toxicology 2024, 502, 153728. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wang, Y.; Wan, Y.; Liang, Y.; Tan, Y.; Wei, M.; Hou, T. Selenium- and/or Zinc-Enriched Egg Diet Improves Oxidative Damage and Regulates Gut Microbiota in D-Gal-Induced Aging Mice. Nutrients 2024, 16, 512. [Google Scholar] [CrossRef]
- Zhai, L.; Huang, C.; Ning, Z.; Zhang, Y.; Zhuang, M.; Yang, W.; Wang, X.; Wang, J.; Zhang, L.; Xiao, H.; et al. Ruminococcus gnavus plays a pathogenic role in diarrhea-predominant irritable bowel syndrome by increasing serotonin biosynthesis. Cell Host Microbe 2023, 31, 33–44.e5. [Google Scholar] [CrossRef]
- Aggarwal, H.; Gautam, J.; Kumari, D.; Gupta, S.K.; Bajpai, S.; Chaturvedi, K.; Kumar, Y.; Dikshit, M. Comparative profiling of gut microbiota and metabolome in diet-induced obese and insulin-resistant C57BL/6J mice. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2024, 1871, 119643. [Google Scholar] [CrossRef]
- Igudesman, D.; Crandell, J.L.; Corbin, K.D.; Hooper, J.; Thomas, J.M.; Bulik, C.M.; Pence, B.W.; Pratley, R.E.; Kosorok, M.R.; Maahs, D.M.; et al. Associations of Dietary Intake with the Intestinal Microbiota and Short-Chain Fatty Acids Among Young Adults with Type 1 Diabetes and Overweight or Obesity. J. Nutr. 2023, 153, 1178–1188. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, N.; Guo, X.; Fan, B.; Cheng, S.; Wang, F. Potato Resistant Starch Type 1 Promotes Obesity Linked with Modified Gut Microbiota in High-Fat Diet-Fed Mice. Molecules 2024, 29, 370. [Google Scholar] [CrossRef]
- Schwartz, L.T.; Ladouceur, J.G.; Russell, M.M.; Xie, S.Y.L.; Bu, S.; Kerver, J.M.; Comstock, S.S. The Relationship Between Fiber Intake and Gut Bacterial Diversity and Composition During the Third Trimester of Pregnancy. Nutrients 2025, 17, 773. [Google Scholar] [CrossRef]
- Kahleova, H.; Rembert, E.; Alwarith, J.; Yonas, W.N.; Tura, A.; Holubkov, R.; Agnello, M.; Chutkan, R.; Barnard, N.D. Effects of a Low-Fat Vegan Diet on Gut Microbiota in Overweight Individuals and Relationships with Body Weight, Body Composition, and Insulin Sensitivity. A Randomized Clinical Trial. Nutrients 2020, 12, 2917. [Google Scholar] [CrossRef]
- Deledda, A.; Palmas, V.; Heidrich, V.; Fosci, M.; Lombardo, M.; Cambarau, G.; Lai, A.; Melis, M.; Loi, E.; Loviselli, A.; et al. Dynamics of Gut Microbiota and Clinical Variables after Ketogenic and Mediterranean Diets in Drug-Naïve Patients with Type 2 Diabetes Mellitus and Obesity. Metabolites 2022, 12, 1092. [Google Scholar] [CrossRef]
- Li, H.; Zhang, L.; Li, J.; Wu, Q.; Qian, L.; He, J.; Ni, Y.; Kovatcheva-Datchary, P.; Yuan, R.; Liu, S.; et al. Resistant starch intake facilitates weight loss in humans by reshaping the gut microbiota. Nat. Metab. 2024, 6, 578–597. [Google Scholar] [CrossRef] [PubMed]
- Zikou, E.; Dovrolis, N.; Dimosthenopoulos, C.; Gazouli, M.; Makrilakis, K. The Effect of Probiotic Supplements on Metabolic Parameters of People with Type 2 Diabetes in Greece-A Randomized, Double-Blind, Placebo-Controlled Study. Nutrients 2023, 15, 4663. [Google Scholar] [CrossRef] [PubMed]
- Henrick, B.M.; Rodriguez, L.; Lakshmikanth, T.; Pou, C.; Henckel, E.; Arzoomand, A.; Olin, A.; Wang, J.; Mikes, J.; Tan, Z.; et al. Bifidobacteria-mediated immune system imprinting early in life. Cell 2021, 184, 3884–3898.e11. [Google Scholar] [CrossRef]
- Odenwald, M.A.; Lin, H.; Lehmann, C.; Dylla, N.P.; Cole, C.G.; Mostad, J.D.; Pappas, T.E.; Ramaswamy, R.; Moran, A.; Hutchison, A.L.; et al. Bifidobacteria metabolize lactulose to optimize gut metabolites and prevent systemic infection in patients with liver disease. Nat. Microbiol. 2023, 8, 2033–2049. [Google Scholar] [CrossRef]
- Biggio, F.; Fattuoni, C.; Mostallino, M.C.; Follesa, P. Effects of Chronic Bifidobacteria Administration in Adult Male Rats on Plasma Metabolites: A Preliminary Metabolomic Study. Metabolites 2022, 12, 762. [Google Scholar] [CrossRef]
- Li, H.; Wang, X.K.; Tang, M.; Lei, L.; Li, J.R.; Sun, H.; Jiang, J.; Dong, B.; Li, H.Y.; Jiang, J.D.; et al. Bacteroides thetaiotaomicron ameliorates mouse hepatic steatosis through regulating gut microbial composition, gut-liver folate and unsaturated fatty acids metabolism. Gut Microbes 2024, 16, 2304159. [Google Scholar] [CrossRef] [PubMed]
- Rao, Y.; Kuang, Z.; Li, C.; Guo, S.; Xu, Y.; Zhao, D.; Hu, Y.; Song, B.; Jiang, Z.; Ge, Z.; et al. Gut Akkermansia muciniphila ameliorates metabolic dysfunction-associated fatty liver disease by regulating the metabolism of L-aspartate via gut-liver axis. Gut Microbes 2021, 13, 1927633. [Google Scholar] [CrossRef]
- Lan, X.; Li, B.; Zhao, J.; Stanton, C.; Ross, R.P.; Chen, W.; Yang, B. Probiotic intervention improves metabolic outcomes in gestational diabetes mellitus: A meta-analysis of randomized controlled trials. Clin. Nutr. 2024, 43, 1683–1695. [Google Scholar] [CrossRef]
- Song, H.; Xue, H.; Zhang, Z.; Wang, J.; Li, A.; Zhang, J.; Luo, P.; Zhan, M.; Zhou, X.; Chen, L.; et al. Amelioration of Type 2 Diabetes Using Four Strains of Lactobacillus Probiotics: Effects on Gut Microbiota Reconstitution-Mediated Regulation of Glucose Homeostasis, Inflammation, and Oxidative Stress in Mice. J. Agric. Food Chem. 2023, 71, 20801–20814. [Google Scholar] [CrossRef]
- Gu, Y.; Chen, H.; Li, X.; Li, D.; Sun, Y.; Yang, L.; Ma, Y.; Chan, E.C.Y. Lactobacillus paracasei IMC 502 ameliorates type 2 diabetes by mediating gut microbiota-SCFA-hormone/inflammation pathway in mice. J. Sci. Food Agric. 2023, 103, 2949–2959. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dilidaxi, D.; Wu, Y.; Sailike, J.; Sun, X.; Nabi, X.H. Composite probiotics alleviate type 2 diabetes by regulating intestinal microbiota and inducing GLP-1 secretion in db/db mice. Biomed. Pharmacother. 2020, 125, 109914. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Zhang, Q.; Hu, R.; Yang, X.; Fang, C.; Yao, L.; Lv, J.; Wang, L.; Shi, M.; Zhang, W.; et al. Effects of Prevotella copri on insulin, gut microbiota and bile acids. Gut Microbes 2024, 16, 2340487. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Li, Y.; Li, L.; Liang, T.; Du, M.; Yang, L.; Yang, J.; Yang, R.; Zhao, H.; Chen, M.; et al. Bifidobacterium longum 070103 Fermented Milk Improve Glucose and Lipid Metabolism Disorders by Regulating Gut Microbiota in Mice. Nutrients 2022, 14, 4050. [Google Scholar] [CrossRef]
- Toshimitsu, T.; Mochizuki, J.; Ikegami, S.; Itou, H. Identification of a Lactobacillus plantarum strain that ameliorates chronic inflammation and metabolic disorders in obese and type 2 diabetic mice. J. Dairy Sci. 2016, 99, 933–946. [Google Scholar] [CrossRef]
- Dimba, N.R.; Mzimela, N.; Sosibo, A.M.; Khathi, A. Effectiveness of Prebiotics and Mediterranean and Plant-Based Diet on Gut Microbiota and Glycemic Control in Patients with Prediabetes or Type 2 Diabetes: A Systematic Review and Meta-Analysis. Nutrients 2024, 16, 3272. [Google Scholar] [CrossRef]
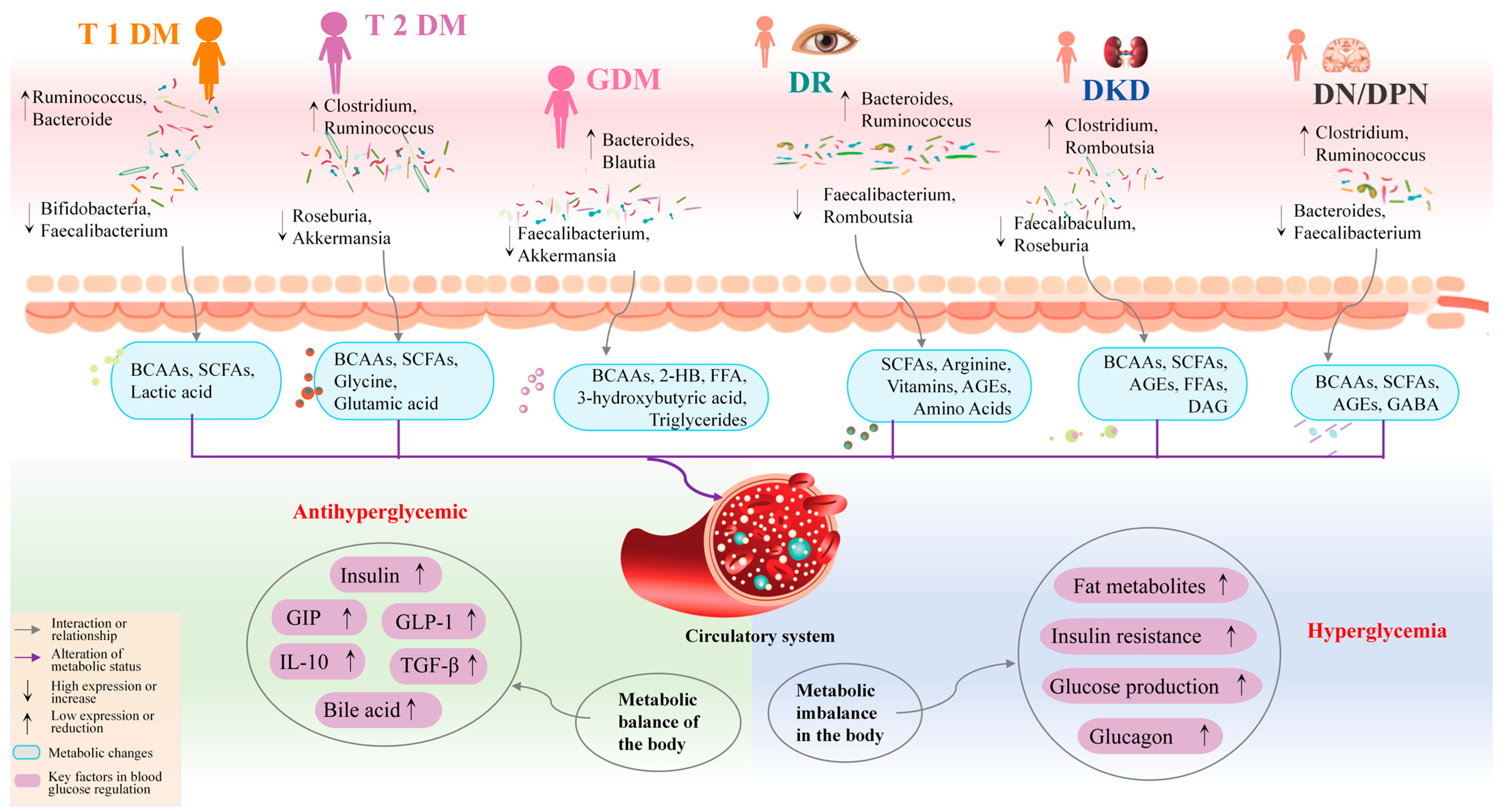

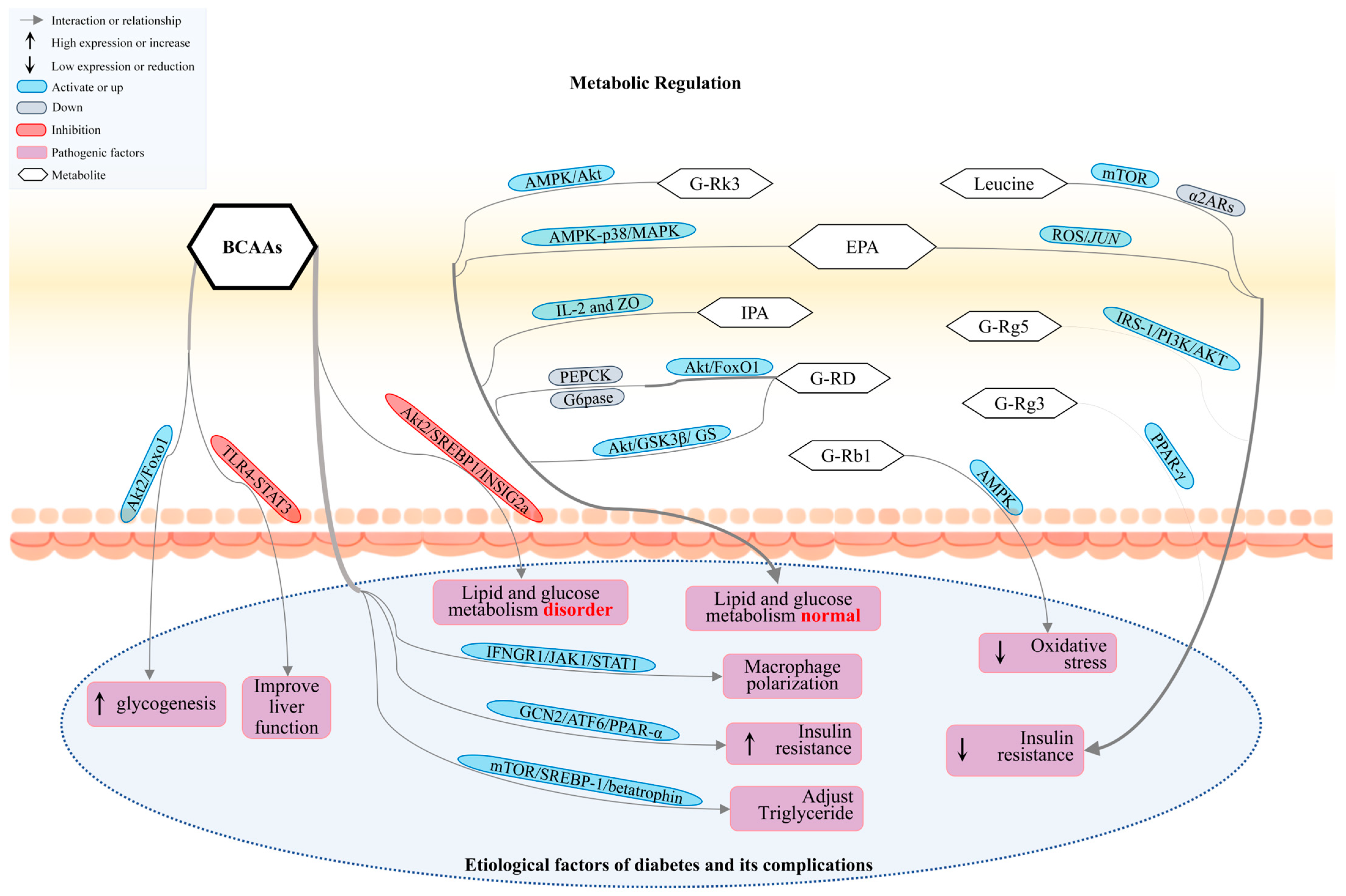
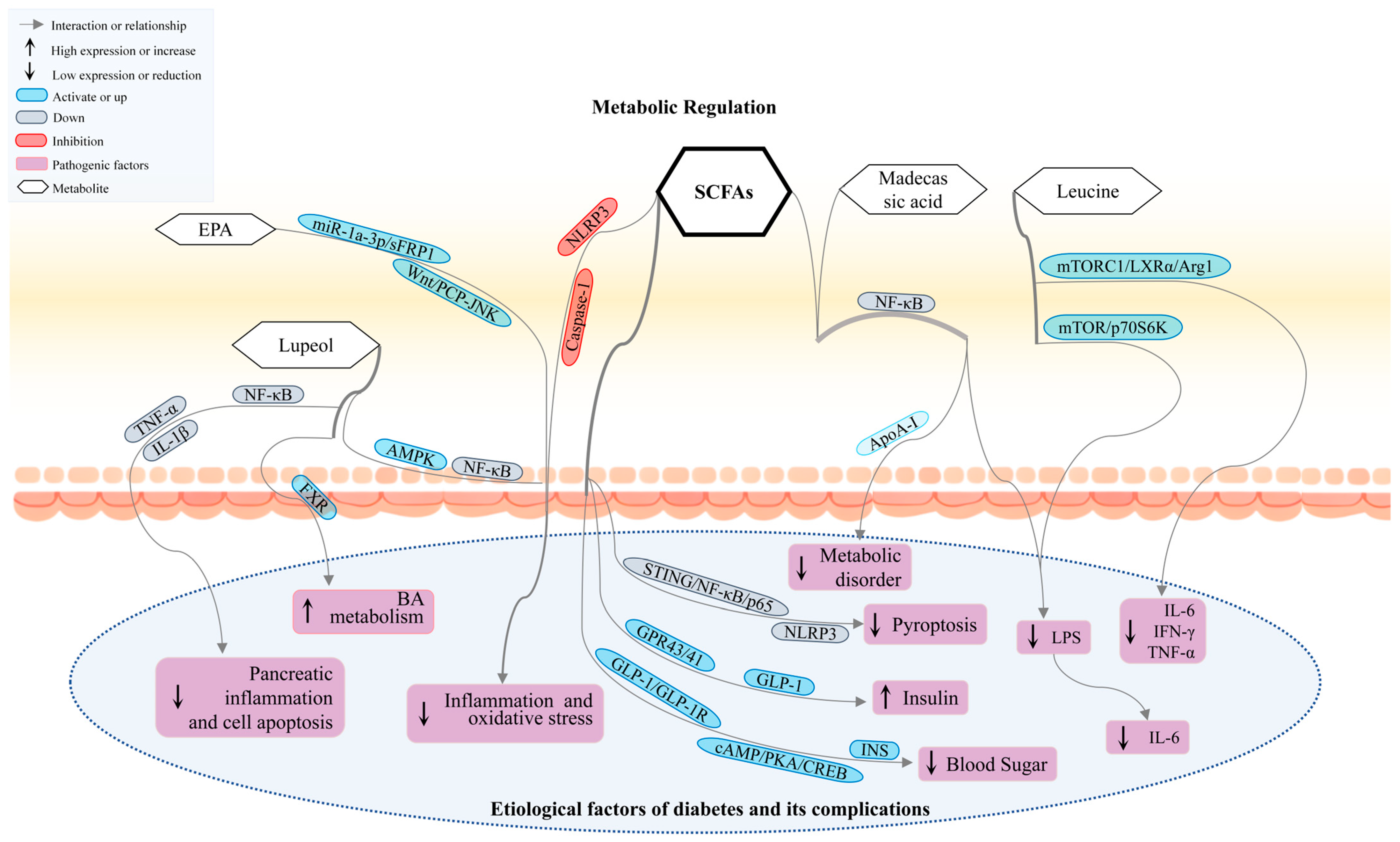
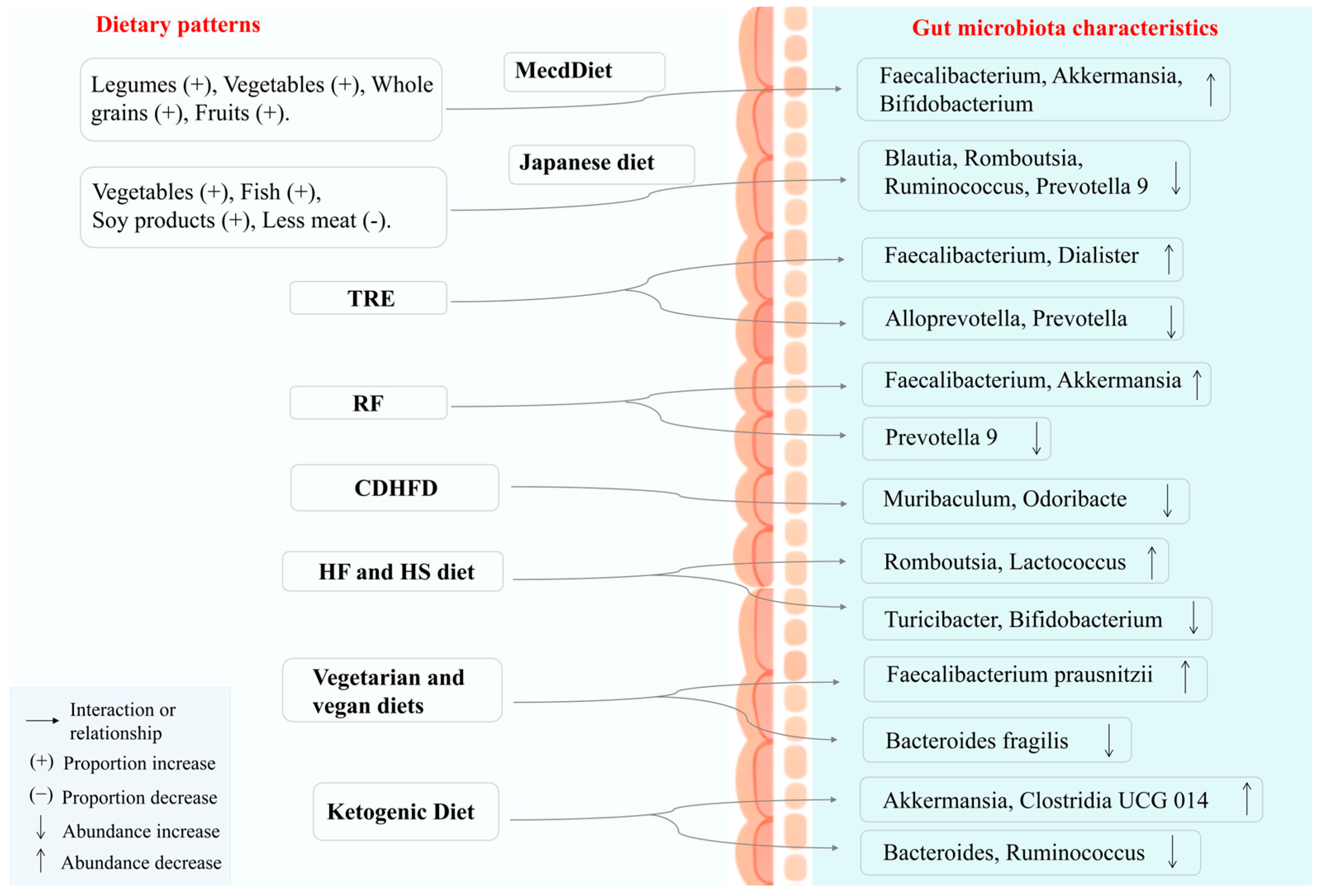
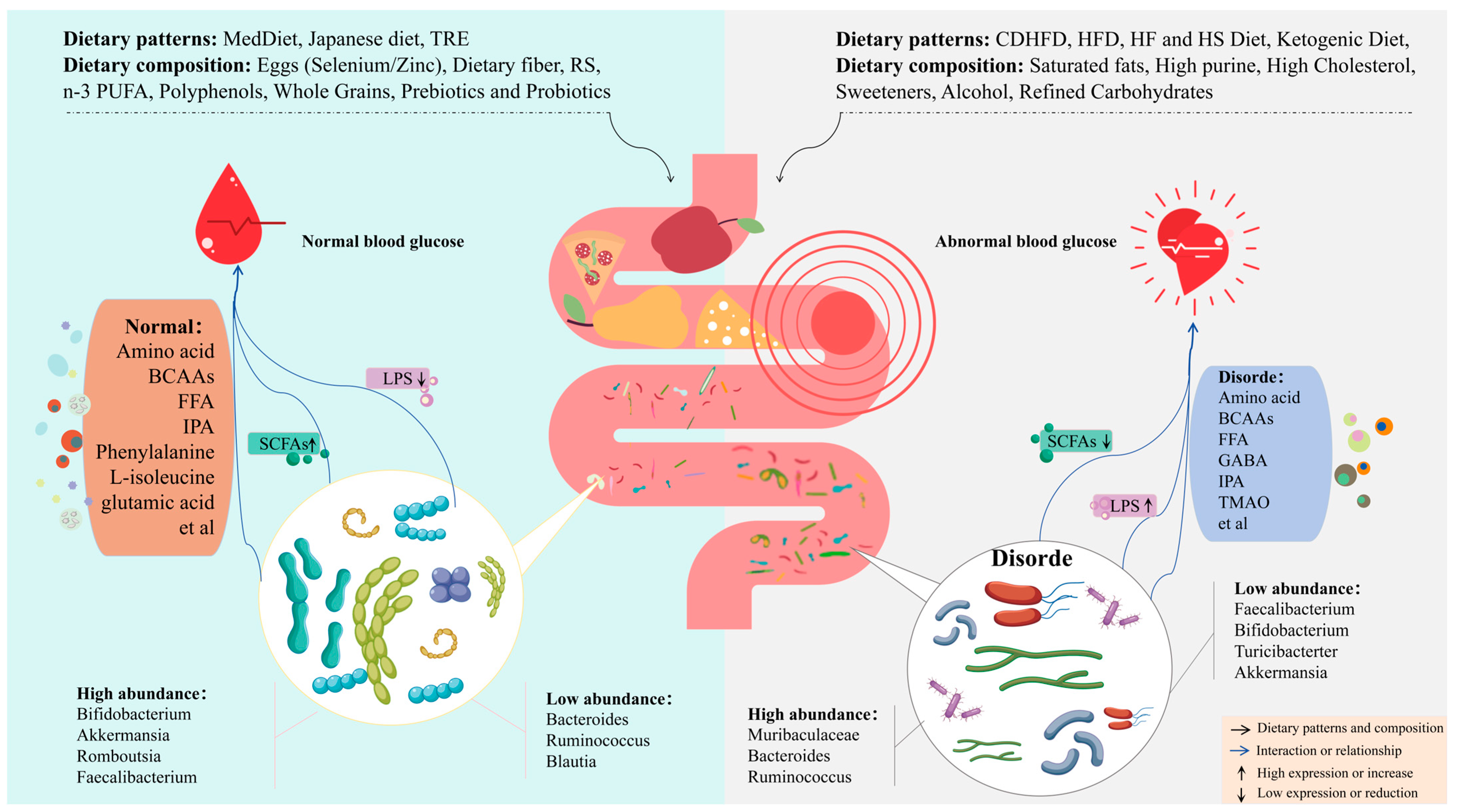
| Metabolite | Regulating Signal Pathways | Function | References |
|---|---|---|---|
| BCAAs | Akt2 | Promoting insulin secretion and β-cell dysfunction, dual inflammatory regulation | [117,118,119,121] |
| INFGR1/JAK1/STAT1 | |||
| GCN2/ATF6/PPAR-α | |||
| mTOR | |||
| SCFAs | GPR 43/NF-kappaB/MAPK | Enhancing insulin sensitivity, β-cell function, lowering blood glucose, and anti-inflammatory effects | [124,125,126,127,128,130] |
| NLRP3/Caspase-1 | |||
| STING/NF-κB/p65 | |||
| GLP-1/GLP-1R/cAMP/PKA/CREB/INS | |||
| HDAC3-H3K27ac-PPAR-γ | |||
| IPA | GPR109A | Maintain intestinal homeostasis, enhance insulin secretion, and reduce insulin resistance | [132,135,136] |
| AHR/NF-κB | |||
| SIRT1/PGC-1α | |||
| PXR | |||
| TMAO | PI3K/Akt/mTOR | Promoting inflammation, inhibiting insulin signaling suppression, and damaging β-cells | [137,138] |
| MAPK/NF-κB | |||
| PERK-FoxO1 | |||
| Phytosphingosine | MAPK | Improving metabolic disorders in diabetes and anti-inflammatory effects | [139] |
| NF-κB | |||
| Madecassic acid | NF-κB | Anti-inflammatory effects and regulating lipid metabolism | [140] |
| Ginsenosides | AMPK/Akt | Regulating hepatic glucose metabolism, alleviating inflammation and oxidative stress | [142,143,146] |
| PPAR-γ | |||
| Sirt1/PGC-1α | |||
| EPA | ROS/JUN | Increasing insulin sensitivity, promoting pancreatic β-cell function, and anti-inflammatory effects | [147,148,149] |
| miR-1a-3p/sFRP1/Wnt/PCP-JNK | |||
| AMPK | |||
| Chenodeoxycholic acid | ROS/p38 MAPK/DGAT1 | Improving glucose and lipid metabolism, protecting pancreatic β-cell function | [151,152] |
| FXR-MLCK | |||
| Lupeol | AMPK/NF-κB | Antioxidant, anti-inflammatory, and protective effects on pancreatic β-cells | [153,154] |
| FXR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, K.; Sun, X.; Wang, X.; Zheng, J.; Yu, H. Gut Microbiota and Metabolites: Biomarkers and Therapeutic Targets for Diabetes Mellitus and Its Complications. Nutrients 2025, 17, 2603. https://doi.org/10.3390/nu17162603
Yan K, Sun X, Wang X, Zheng J, Yu H. Gut Microbiota and Metabolites: Biomarkers and Therapeutic Targets for Diabetes Mellitus and Its Complications. Nutrients. 2025; 17(16):2603. https://doi.org/10.3390/nu17162603
Chicago/Turabian StyleYan, Kai, Xin Sun, Xin Wang, Jing Zheng, and Hongsong Yu. 2025. "Gut Microbiota and Metabolites: Biomarkers and Therapeutic Targets for Diabetes Mellitus and Its Complications" Nutrients 17, no. 16: 2603. https://doi.org/10.3390/nu17162603
APA StyleYan, K., Sun, X., Wang, X., Zheng, J., & Yu, H. (2025). Gut Microbiota and Metabolites: Biomarkers and Therapeutic Targets for Diabetes Mellitus and Its Complications. Nutrients, 17(16), 2603. https://doi.org/10.3390/nu17162603





