Sweetness Ratings of U.S. Infant Formulas
Abstract
1. Introduction
2. Materials and Methods
2.1. Sensory Lab Protocols
2.2. Sensory Panel Recruitment
2.3. Infant Formula Selection
2.4. Panel Formation
- Sweetness Intensity Ranking Test: Participants were presented with four different sucrose solutions, ranging from 0 g sucrose/100 mL of water to 5 g sucrose/100 mL of water. They were asked to rank the solutions in order of perceived sweetness.
- Triangle Test: Participants were given three solutions; two sucrose (2 g sucrose/100 mL of water) samples and one water solution (0 g sucrose/100 mL). They were tasked with identifying the sample that was different from the other two.
- Tetrad Test: Participants received four sucrose samples, two of one concentration (3 g sucrose/100 mL of water) and two (5 g sucrose/100 mL of water) of another concentration. They were required to identify and group samples which were the same.
2.5. Panel Descriptive Training
2.6. Sensory Evaluation
2.7. Analysis
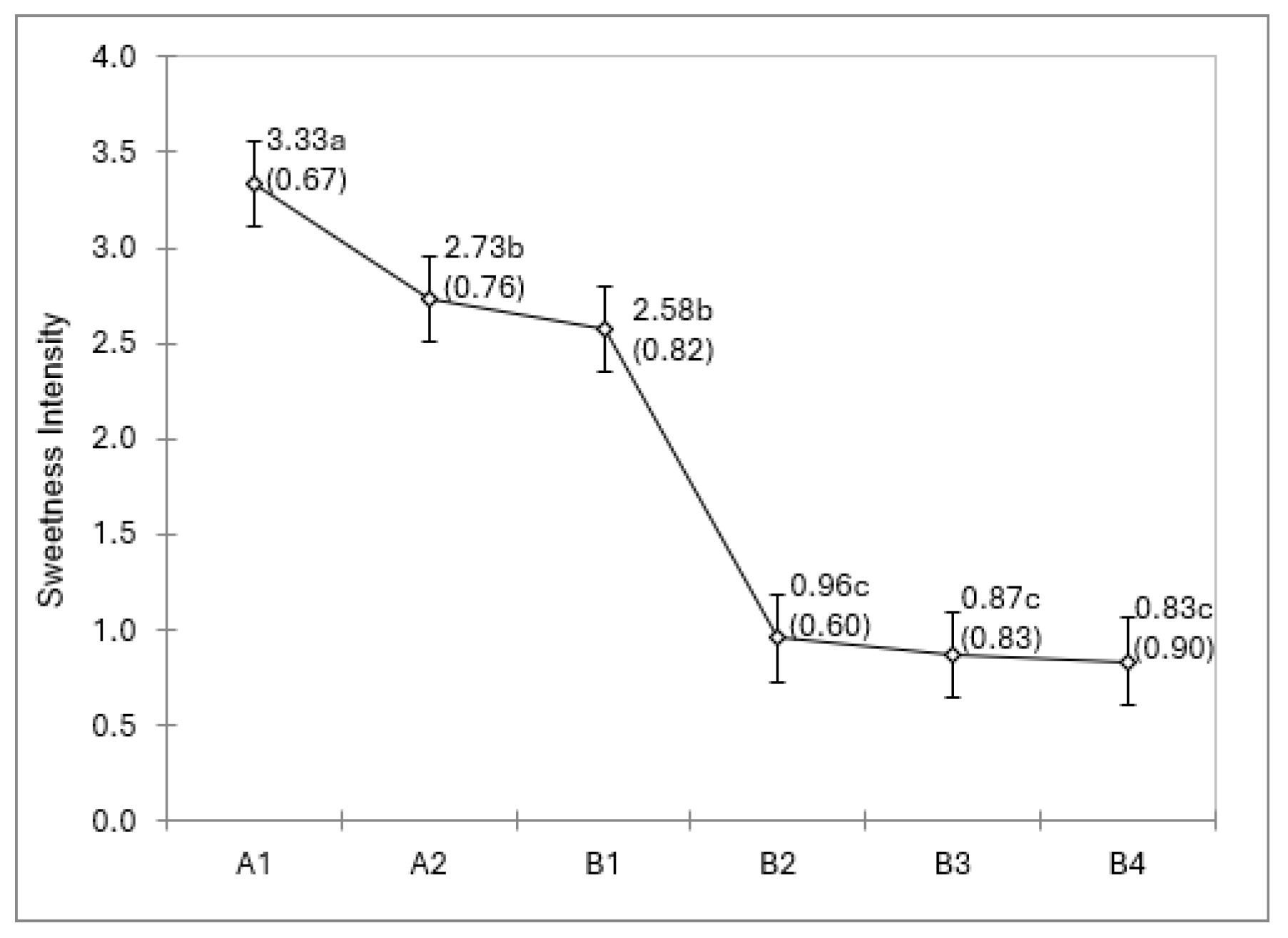
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| FDA | The U.S. Food and Drug Administration |
| WHO | World Health Organization |
| WIC | Special Supplemental Nutrition Program for Women, Infants, and Children |
| ANOVA | Analysis of variance |
| DHA | Docosahexaenoic acid |
| ARA | Arachidonic acids |
References
- Schwarzenberg, S.J.; Georgieff, M.K.; Nutrition, Committee on Nutrition. Advocacy for Improving Nutrition in the First 1000 Days to Support Childhood Development and Adult Health. Pediatrics 2018, 141, e20173716. [Google Scholar] [CrossRef]
- Stoody, E.E.; Spahn, J.M.; Casavale, K.O. The Pregnancy and Birth to 24 Months Project: A Series of Systematic Reviews on Diet and Health. Am. J. Clin. Nutr. 2019, 109, 685S–697S. [Google Scholar] [CrossRef]
- Lioret, S.; Betoko, A.; Forhan, A.; Charles, M.A.; Heude, B.; de Lauzon-Guillain, B.; Group, E.M.-C.C.S. Dietary Patterns Track from Infancy to Preschool Age: Cross-Sectional and Longitudinal Perspectives. J. Nutr. 2015, 145, 775–782. [Google Scholar] [CrossRef]
- Nicklaus, S.; Boggio, V.; Chabanet, C.; Issanchou, S. A Prospective Study of Food Variety Seeking in Childhood, Adolescence and Early Adult Life. Appetite 2005, 44, 289–297. [Google Scholar] [CrossRef]
- Demerath, E.W.; Reed, D.; Choh, A.C.; Soloway, L.; Lee, M.; Czerwinski, S.A.; Chumlea, W.C.; Siervogel, R.M.; Towne, B. Rapid Postnatal Weight Gain and Visceral Adiposity in Adulthood: The Fels Longitudinal Study. Obesity 2009, 17, 2060–2066. [Google Scholar] [CrossRef]
- Lu, Y.; Pearce, A.; Li, L. Weight Gain in Early Years and Subsequent Body Mass Index Trajectories across Birth Weight Groups: A Prospective Longitudinal Study. Eur. J. Public Health 2020, 30, 316–322. [Google Scholar] [CrossRef]
- Suri, T.M.; Bhargava, S.; Akshara, K.T.; Sinha, S.; Aggarwal, V.; Gupta, K.D.; Singh, G.; Singh, B.; Ramakrishnan, L.; Osmond, C.; et al. Postnatal Growth Trajectories and Risk of Obstructive Sleep Apnea in Middle Age: A Cohort Study. Pediatr. Pulmonol. 2024, 60, e27396. [Google Scholar] [CrossRef] [PubMed]
- Kay, M.C.; Hampton, J.; Pac, S.; Huss, L.; Eldridge, A.L. Measuring Dietary Quality among Toddlers in the Feeding Infants and Toddlers Study, 2016, Using the New Healthy Eating Index-Toddlers-2020. J. Acad. Nutr. Diet. 2024, 125, 463–471. [Google Scholar] [CrossRef]
- De Cosmi, V.; Scaglioni, S.; Agostoni, C. Early Taste Experiences and Later Food Choices. Nutrients 2017, 9, 107. [Google Scholar] [CrossRef]
- Ventura, A.K.; Worobey, J. Early Influences on the Development of Food Preferences. Curr. Biol. 2013, 23, R401–R408. [Google Scholar] [CrossRef]
- Beauchamp, G.K.; Mennella, J.A. Early Flavor Learning and Its Impact on Later Feeding Behavior. J. Pediatr. Gastroenterol. Nutr. 2009, 48, S25–S30. [Google Scholar] [CrossRef] [PubMed]
- Mennella, J.A. Ontogeny of Taste Preferences: Basic Biology and Implications for Health. Am. J. Clin. Nutr. 2014, 99, 704S–711S. [Google Scholar] [CrossRef] [PubMed]
- Savage, J.S.; Fisher, J.O.; Birch, L.L. Parental Influence on Eating Behavior: Conception to Adolescence. J. Law. Med. Ethics 2007, 35, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Ventura, A.K.; Mennella, J.A. Innate and Learned Preferences for Sweet Taste during Childhood. Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 379–384. [Google Scholar] [CrossRef]
- Ahrens, W. Sensory Taste Preferences and Taste Sensitivity and the Association of Unhealthy Food Patterns with Overweight and Obesity in Primary School Children in Europe—A Synthesis of Data from the IDEFICS Study. Flavour 2015, 4, 8. [Google Scholar] [CrossRef]
- Lanfer, A.; Knof, K.; Barba, G.; Veidebaum, T.; Papoutsou, S.; de Henauw, S.; Soós, T.; Moreno, L.A.; Ahrens, W.; Lissner, L. Taste Preferences in Association with Dietary Habits and Weight Status in European Children: Results from the IDEFICS Study. Int. J. Obes. 2012, 36, 27–34. [Google Scholar] [CrossRef]
- Sobek, G.; Łuszczki, E.; Dąbrowski, M.; Dereń, K.; Baran, J.; Weres, A.; Mazur, A. Preferences for Sweet and Fatty Taste in Children and Their Mothers in Association with Weight Status. Int. J. Environ. Res. Public Health 2020, 17, 538. [Google Scholar] [CrossRef]
- Maller, O.; Turner, R.E. Taste in Acceptance of Sugars by Human Infants. J. Comp. Physiol. Psychol. 1973, 84, 496–501. [Google Scholar] [CrossRef]
- de Macedo, I.C.; de Freitas, J.S.; da Silva Torres, I.L. The Influence of Palatable Diets in Reward System Activation: A Mini Review. Adv. Pharmacol. Pharm. Sci. 2016, 2016, 7238679. [Google Scholar] [CrossRef]
- Baik, J.H. Dopaminergic Control of the Feeding Circuit. Endocrinol. Metab. 2021, 36, 229–239. [Google Scholar] [CrossRef]
- Wagner, S.; Issanchou, S.; Chabanet, C.; Marlier, L.; Schaal, B.; Monnery-Patris, S. Infants’ Hedonic Responsiveness to Food Odors: A Longitudinal Study during and after Weaning (8, 12 and 22 Months). Flavour 2013, 2, 19. [Google Scholar] [CrossRef]
- Chiang, K.V.; Hamner, H.C.; Li, R.; Perrine, C.G. Timing of Introduction of Complementary Foods: United States, 2016-2018. MMWR Morb. Mortal. Wkly. Rep. 2023, 69, 1969–1973. [Google Scholar] [CrossRef]
- Liem, D.G.; Mennella, J.A. Sweet and Sour Preferences during Childhood: Role of Early Experiences. Dev. Psychobiol. 2002, 41, 388–395. [Google Scholar] [CrossRef]
- Mennella, J.A.; Griffin, C.E.; Beauchamp, G.K. Flavor Programming during Infancy. Pediatrics 2004, 113, 840–845. [Google Scholar] [CrossRef]
- Meek, J.Y.; Noble, L.; Breastfeeding, Section on Breastfeeding. Policy Statement: Breastfeeding and the Use of Human Milk. Pediatrics 2022, 150, e2022057988. [Google Scholar] [CrossRef]
- World Health Organization. Breast Feeding. Available online: https://www.who.int/health-topics/breastfeeding#tab=tab_2 (accessed on 30 July 2025).
- Centers for Disease Control and Prevention. Breastfeeding Report Card: United States. 2022. Available online: https://www.cdc.gov/breastfeeding-data/media/pdfs/2024/06/2022-Breastfeeding-Report-Card-508.pdf (accessed on 30 July 2025).
- U.S. Food and Drug Administration. Infant Formula. Available online: https://www.fda.gov/food/resources-you-food/infant-formula#oversee (accessed on 22 July 2025).
- Rips-Goodwin, A.R.; Jun, D.; Griebel-Thompson, A.; Kong, K.L.; Fazzino, T.L. U.S. Infant Formulas Contain Primarily Added Sugars: An Analysis of Infant Formulas on the US Market. J. Food Compos. Anal. 2025, 141, 107369. [Google Scholar] [CrossRef]
- Kleinman, R.E.; Greer, F.R. Pediatric Nutrition, 8th ed.; American Academy of Pediatrics: Itasca, IL, USA, 2020. [Google Scholar] [CrossRef]
- Strzalkowski, A.; Järvinen, K.M.; Schmidt, B.; Young, B.E. Protein and Carbohydrate Content of Infant Formula Purchased in the United States. Clin. Expl Allergy 2022, 52, 1291–1301. [Google Scholar] [CrossRef] [PubMed]
- Walker, R.W.; Goran, M.I. Laboratory Determined Sugar Content and Composition of Commercial Infant Formulas, Baby Foods and Common Grocery Items Targeted to Children. Nutrients 2015, 7, 5850–5867. [Google Scholar] [CrossRef] [PubMed]
- Young, B. Variation in Infant Formula Macronutrient Ingredients Is Associated with Infant Anthropometrics. Nutrients 2020, 12, 3465. [Google Scholar] [CrossRef]
- Kong, K.L.; Burgess, B.; Morris, K.S.; Re, T.; Hull, H.R.; Sullivan, D.K.; Paluch, R.A. Association between Added Sugars from Infant Formulas and Rapid Weight Gain in US Infants and Toddlers. J. Nutr. 2021, 151, 1572–1580. [Google Scholar] [CrossRef]
- Anderson, C.E.; Whaley, S.E.; Goran, M.I. Lactose-Reduced Infant Formula with Corn Syrup Solids and Obesity Risk among Participants in the Special Supplemental Nutrition Program for Women, Infants, and Children (WIC). Am. J. Clin. Nutr. 2022, 116, 1002–1009. [Google Scholar] [CrossRef]
- Li, Y.; Hu, X.; Li, R.; Wang, C.; Wang, H.; Liu, G.; Gao, L.; Jin, A.; Zhu, B. Variations in the Sensory Attributes of Infant Formula among Batches and Their Impact on Maternal Consumer Preferences: A Study Combining Consumer Preferences, Pivot Profile, and Quantitative Descriptive Analysis. Foods 2024, 13, 2839. [Google Scholar] [CrossRef]
- Xi, Y.; Zhao, T.; Liu, R.; Song, F.; Deng, J.; Ai, N. Assessing Sensory Attributes and Properties of Infant Formula Milk Powder Driving Consumers’ Preference. Foods 2023, 12, 997. [Google Scholar] [CrossRef]
- DuBois, G.E.; Walters, D.E.; Schiffman, S.S.; Warwick, Z.S.; Booth, B.J.; Pecore, S.D.; Gibes, K.; Carr, B.T.; Brands, L.M. Concentration–Response Relationships of Sweeteners. In Sweeteners: Discovery, Molecular Design, and Chemoreception; Walters, D.E., Orthoefer, F.T., DuBois, G.E., Eds.; ACS Publications: Washington, DC, USA, 1991; pp. 261–276. [Google Scholar]
- Trumbo, P.R.; Appleton, K.M.; de Graaf, K.; Hayes, J.E.; Baer, D.D.J.; Beauchamp, G.K.; Dwyer, J.T.; Fernstrom, J.D.; Klurfeld, D.M.; Mattes, R.D.; et al. Perspective: Measuring Sweetness in Foods, Beverages, and Diets: Toward Understanding the Role of Sweetness in Health. Adv. Nutr. 2021, 12, 343–354. [Google Scholar] [CrossRef]
- Stone, H.; Sidel, J.; Oliver, S.; Woolsey, A.; Singleton, R.C. Sensory Evaluation by Quantitative Descriptive Analysis. Food Technol. 1974, 28, 24–34. [Google Scholar]
- Meilgaard, M.; Civille, G.V.; Carr, B.T. Sensory Evaluation Techniques, 3rd ed.; CRC Press: Boca Raton, FL, USA, 1991. [Google Scholar]
- ISO 13299:2016; Sensory analysis—Methodology—General guidance for establishing a sensory profile. International Organization for Standardization: Brussels, Belgium, 2016.
- Moser, M.; Lepage, M.; Pineau, N.; Fillion, L.; Rytz, A. Replicates in Sensory Profiling: Quantification of the Impact of Moving from Two to One Assessments. Food Qual. Prefer. 2018, 65, 185–190. [Google Scholar] [CrossRef]
- Starkey, D.E.; Wang, Z.; Brunt, K.; Dreyfuss, L.; Haselberger, P.A.; Holroyd, S.E.; Janakiraman, K.; Kasturi, P.; Konings, E.J.M.; Labbe, D.; et al. The Challenge of Measuring Sweet Taste in Food Ingredients and Products for Regulatory Compliance: A Scientific Opinion. J. AOAC Int. 2022, 105, 333–345. [Google Scholar] [CrossRef]
- Bhattacharya, S. Chapter 9-Sugars, Sweeteners, Chocolates, and Sweet Snacks. In Snack Foods; Bhattacharya, S., Ed.; Academic Press: Cambridge, MA, USA, 2023; pp. 211–249. [Google Scholar] [CrossRef]
- Hofman, D.L.; van Buul, V.J.; Brouns, F.J. Nutrition, Health, and Regulatory Aspects of Digestible Maltodextrins. Crit. Rev. Food Sci. Nutr. 2016, 56, 2091–2100. [Google Scholar] [CrossRef]
- Leeder, J.G. Some Newer Ideas for Using Corn Sweeteners in Ice Cream; Extension Division, Department of Food Science and Technology, Virginia Polytechnic Institute: Blacksburg, VA, USA; Available online: https://vtechworks.lib.vt.edu/server/api/core/bitstreams/e7a35804-856f-4504-8d74-d6a6d98dff99/content (accessed on 30 July 2025).
- Lu, S. RICE: Chinese Food Uses. In Encyclopedia of Grain Science; Wrigley, C., Seetharaman, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2004; pp. 68–74. [Google Scholar] [CrossRef]
- Lasekan, J.B.; Linke, H.K.; Oliver, J.S.; Carver, J.D.; Blatter, M.M.; Kuchan, M.J.; Cramer, J.M.; Pollack, P.F. Milk Protein–Based Infant Formula Containing Rice Starch and Low Lactose Reduces Common Regurgitation in Healthy Term Infants: A Randomized, Blinded, and Prospective Trial. J. Am. Coll. Nutr. 2014, 33, 136–146. [Google Scholar] [CrossRef]
- Bao, J.; Bergman, C. Rice Flour and Starch Functionality. In Starch in Food: Structure, Function and Applications; Sjöö, M., Nilsson, L., Eds.; Woodhead Publishing: Cambridge, UK, 2018; pp. 373–419. [Google Scholar]
- Christensen, C.M. Effects of Solution Viscosity on Perceived Saltiness and Sweetness. Percept. Psychophys. 1980, 28, 347–353. [Google Scholar] [CrossRef]
- Tournier, C.; Sulmont-Rossé, C.; Sémon, E.; Vignon, A.; Issanchou, S.; Guichard, E. A Study on Texture–Taste–Aroma Interactions: Physico-Chemical and Cognitive Mechanisms. Int. Dairy J. 2009, 19, 450–458. [Google Scholar] [CrossRef]
- Aidoo, R.P.; Depypere, F.; Afoakwa, E.O.; Dewettinck, K. Industrial Manufacture of Sugar-Free Chocolates: Applicability of Alternative Sweeteners and Carbohydrate Polymers as Raw Materials in Product Development. Trends Food Sci. Technol. 2013, 32, 84–96. [Google Scholar] [CrossRef]
- Tiefenbacher, K. Technology of Main Ingredients—Water and Flours. In Water and Waffles; Acedemic Press: Cambridge, MA, USA, 2017; pp. 115–121. [Google Scholar] [CrossRef]
- Mei, Z.; Yuan, J.; Li, D. Biological Activity of Galacto-Oligosaccharides: A Review. Front. Microbiol. 2022, 13, 993052. [Google Scholar] [CrossRef]
- U.S. Department of Agriculture. Fructooligosaccharides. 2016. Available online: https://www.ams.usda.gov/sites/default/files/media/Fructooligosaccharides%20TR%202015.pdf (accessed on 30 July 2025).
- Kumar, C.; Sripada, S.; Poornachandra, Y. Status and Future Prospects of Fructo-Oligosaccharides as Nutraceuticals. In Handbook of Food Bioengineering: Role of Materials Science in Food Bioengineering; Grumezescu, A.M., Holbam, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 451–503. [Google Scholar] [CrossRef]
- Vandenplas, Y.; Ksiażyk, J.; Luna, M.S.; Migacheva, N.; Picaud, J.C.; Ramenghi, L.A.; Singhal, A.; Wabitsch, M. Partial Hydrolyzed Protein as a Protein Source for Infant Feeding: Do or Don’t? Nutrients 2022, 14, 1720. [Google Scholar] [CrossRef]
- Lee, Y.H. Food-Processing Approaches to Altering Allergenic Potential of Milk-Based Formula. J. Pediatr. 1992, 121, S47–S50. [Google Scholar] [CrossRef]
- Pedrosa, M.; Pascual, C.Y.; Larco, J.I.; Esteban, M.M. Palatability of Hydrolysates and Other Substitution Formulas for Cow’s Milk-Allergic Children: A Comparative Study of Taste, Smell, and Texture Evaluated by Healthy Volunteers. J. Investig. Allergol. Clin. Immunol. 2006, 16, 351–356. [Google Scholar]
- Alim, A.; Song, H.; Raza, A.; Hua, J. Identification of Bitter Constituents in Milk-Based Infant Formula with Hydrolyzed Milk Protein through a Sensory-Guided Technique. Int. Dairy J. 2020, 110, 104803. [Google Scholar] [CrossRef]
- Miraglia Del Giudice, M.; D’Auria, E.; Peroni, D.; Palazzo, S.; Radaelli, G.; Comberiati, P.; Galdo, F.; Maiello, N.; Riva, E. Flavor, Relative Palatability and Components of Cow’s Milk Hydrolyzed Formulas and Amino Acid-Based Formula. Ital. J. Pediatr. 2015, 41, 42. [Google Scholar] [CrossRef]
- Vandenplas, Y.; De Greef, E.; Devreker, T.; Hauser, B. Soy Infant Formula: Is It That Bad? Acta Paediatr. 2011, 100, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations. Technology of Production of Edible Flours and Protein Products from Soybeans. 1992. Available online: https://www.fao.org/4/t0532e/t0532e07.htm (accessed on 30 July 2025).
- Carrão-Panizzi, M.; Beleia, A.D.P.; Prudencio-Ferreira, S.; Oliveira, M.; Kitamura, K. Effects of Isoflavones on Beany Flavor and Astringency of Soymilk and Cooked Whole Soybean Grains. Pesqui. Agropecu. Bras. 1999, 34, 1044–1052. [Google Scholar] [CrossRef]
- Kaneko, S.; Kumazawa, K.; Nishimura, O. Studies on the Key Aroma Compounds in Soy Milk Made from Three Different Soybean Cultivars. J. Agric. Food Chem. 2011, 59, 12204–12209. [Google Scholar] [CrossRef]
- Malcolmson, L.J.; McDaniel, M.R. Magnitude Estimation of Infant Foods II. Taste, Texture and Odor of Infant Formulas. Can. Inst. Food Sci. Technol. 1980, 13, 56–63. [Google Scholar] [CrossRef]
- Zadow, J.G. Whey and Whey Powders: Production and Used. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Caballero, B., Ed.; Academic Press: Cambridge, MA, USA, 2008; pp. 6147–6152. [Google Scholar] [CrossRef]
- Drake, M.A.; Miracle, R.E.; Wright, J.M. Chapter 15-Sensory Properties of Dairy Proteins. In Milk Proteins; Thompson, A., Boland, M., Singh, H., Eds.; Food Science and Technology; Academic Press: Cambridge, MA, USA, 2008; pp. 429–448. [Google Scholar] [CrossRef]
- Carunchia-Whetstine, M.E.; Croissant, A.E.; Drake, M.A. Characterization of Dried Whey Protein Concentrate and Isolate Flavor. J. Dairy Sci. 2005, 88, 3826–3839. [Google Scholar] [CrossRef] [PubMed]
- Mazzocchi, A.; D’Oria, V.; De Cosmi, V.; Bettocchi, S.; Milani, G.P.; Silano, M.; Agostoni, C. The Role of Lipids in Human Milk and Infant Formulae. Nutrients 2018, 10, 567. [Google Scholar] [CrossRef]
- MasterClass. All About Neutral Oil: 10 Neutral Oils for Cooking. Available online: https://www.masterclass.com/articles/neutral-oil-guide (accessed on 30 July 2025).
- U.S. Soy. Cooking Benefits and Smoke Point of Soybean Oil. 2014. Available online: https://ussoy.org/cooking-benefits-and-smoke-point-of-soybean-oil/ (accessed on 30 July 2025).
- Mancini, A.; Imperlini, E.; Nigro, E.; Montagnese, C.; Daniele, A.; Orrù, S.; Buono, P. Biological and Nutritional Properties of Palm Oil and Palmitic Acid: Effects on Health. Molecules 2015, 20, 17339–17361. [Google Scholar] [CrossRef]
- Villarino, B.J.; Dy, L.M.; Lizada, M.C.C. Descriptive Sensory Evaluation of Virgin Coconut Oil and Refined, Bleached and Deodorized Coconut Oil. LWT - Food Sci. Technol. 2007, 40, 193–199. [Google Scholar] [CrossRef]
- Guéraud, F.; Atalay, M.; Bresgen, N.; Cipak, A.; Eckl, P.M.; Huc, L.; Jouanin, I.; Siems, W.; Uchida, K. Chemistry and Biochemistry of Lipid Peroxidation Products. Free Radic. Res. 2010, 44, 1098–1124. [Google Scholar] [CrossRef]
- Gibson, M.; Newsham, P. Chapter 16-Lipids, Oils, Fats, and Extracts. In Food Science and the Culinary Arts; Academic Press: Cambridge, MA, USA, 2018; pp. 323–340. [Google Scholar] [CrossRef]
- Zhang, N.; Li, Y.; Wen, S.; Sun, Y.; Chen, J.; Gao, Y.; Sagymbek, A.; Yu, X. Analytical Methods for Determining the Peroxide Value of Edible Oils: A Mini Review. Food Chem. 2021, 358, 129834. [Google Scholar] [CrossRef]
- Strzalkowski, A.; Black, G.; Young, B.E. Iron and DHA in infant formula purchased in the US fails to meet European nutrition requirements. Nutrients. 2023, 15, 1812. [Google Scholar] [CrossRef]
- Hammer, M.; Schieberle, P. Model Studies on the Key Aroma Compounds Formed by an Oxidative Degradation of ω-3 Fatty Acids Initiated by Either Copper(II) Ions or Lipoxygenase. J. Agric. Food Chem. 2013, 61, 10891–10900. [Google Scholar] [CrossRef]
- ICF International. Arachidonic Acid Single-Cell Oil (ARA): Handling/Processing; U.S. Department of Agriculture, USA, 2011. Available online: https://www.ams.usda.gov/sites/default/files/media/ARA%20TR.pdf (accessed on 30 July 2025).
- Hetherington, M.; Madrelle, C.; Nekitsing, C.; Barends, C.; de Graaf, S.; Morgan, H.; Parrott, H.; Weenen, H. Developing a Novel Tool to Assess Liking and Wanting in Infants at the Time of Complementary Feeding. Food Qual. Prefer. 2016, 48, 238–250. [Google Scholar] [CrossRef]
- Mennella, J.A.; Jagnow, C.P.; Beauchamp, G.K. Prenatal and Postnatal Flavor Learning by Human Infants. Pediatrics 2001, 107, E88. [Google Scholar] [CrossRef]
- Forestell, C.A.; Mennella, J.A. More than Just a Pretty Face. The Relationship between Infant’s Temperament, Food Acceptance, and Mothers’ Perceptions of Their Enjoyment of Food. Appetite 2012, 58, 1136–1142. [Google Scholar] [CrossRef]
- Schwartz, C.; Issanchou, S.; Nicklaus, S. Developmental Changes in the Acceptance of the Five Basic Tastes in the First Year of Life. Br. J. Nutr. 2009, 102, 1375–1385. [Google Scholar] [CrossRef]
- Mennella, J.A.; Bobowski, N.K.; Reed, D.R. The Development of Sweet Taste: From Biology to Hedonics. Rev. Endocr. Metab. Disord. 2016, 17, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Maier-Nöth, A. The Development of Healthy Eating and Food Pleasure in Infancy. Nestle Nutr. Inst. Workshop Ser. 2023, 97, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Institute for Quality and Efficiency in Health Care. In Brief: How Does Our Sense of Taste Work? Available online: https://www.ncbi.nlm.nih.gov/books/NBK279408/ (accessed on 30 July 2025).
- Zhang, G.-H.; Zhang, H.-Y.; Wang, X.-F.; Zhan, Y.-H.; Deng, S.-P.; Qin, Y.-M. The Relationship between Fungiform Papillae Density and Detection Threshold for Sucrose in the Young Males. Chem. Senses 2008, 34, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Cecati, M.; Vignini, A.; Borroni, F.; Pugnaloni, S.; Alia, S.; Sabbatinelli, J.; Nicolai, G.; Taus, M.; Santarelli, A.; Fabri, M.; et al. TAS1R3 and TAS2R38 Polymorphisms Affect Sweet Taste Perception: An Observational Study on Healthy and Obese Subjects. Nutrients 2022, 14, 1711. [Google Scholar] [CrossRef]
| Brand-Formula | Type | Carbohydrate Source |
|---|---|---|
| A-1 | Standard, milk-based | Lactose |
| A-2 | Milk-based, lactose-reduced formula | Corn syrup, sucrose |
| B-1 | Standard, milk-based | Lactose |
| B-2 | Added rice to reduce spit-up, lactose reduced | Rice starch, lactose, maltodextrin |
| B-3 | Partially hydrolyzed, milk-based, lactose reduced | Corn syrup, lactose |
| B-4 | Soy protein-based | Corn syrup |
| Panelist | Replication | A-1 | A-2 | B-1 | B-2 | B-3 | B-4 |
|---|---|---|---|---|---|---|---|
| 1 | 1 | pleasant aftertaste | pleasant taste | pleasant taste | bitter taste | mellow taste | slight aftertaste |
| 2 | strong smell | stronger smell, little aftertaste | slightly sweeter | neutral taste | slightly sour | ||
| 3 | strong smell, very little aftertaste | strong smell | very little aftertaste, slightly strong smell | foamy | neutral taste | strong smell, some aftertaste, unpleasant taste | |
| 2 | 1 | flavor seems balanced | |||||
| 2 | |||||||
| 3 | flavor and sweetness seems balanced | grass-like flavor | |||||
| 3 | 1 | thicker | sweetness not really noticeable | aftertaste, thinner consistency | lighter | no sweetener, more like milk | frothy, not sweet |
| 2 | a bit sweet | frothy, bad aftertaste | much lighter | ||||
| 3 | tasted closest to sweetened milk | smell is not bad | frothy/foamy | sour | tasted like dirt | ||
| 4 | 1 | creamy | creamy | creamy | frothy | watery, bitter | grassy odor |
| 2 | creamy | creamy | creamy | frothy | very bitter aftertaste | grainy, watery | |
| 3 | pleasant | creamy | creamy | frothy, watery | bitter, sour aftertaste | grassy taste | |
| 5 | 1 | foamy, difficult to salivate | |||||
| 2 | foamy | ||||||
| 3 | foamy texture | ||||||
| 6 | 1 | smooth texture, not gritty | |||||
| 2 | |||||||
| 3 | very thick consistency | horrible aftertaste | |||||
| 7 | 1 | creamy | nice smooth texture | a little watery | foamy | thin | |
| 2 | smooth | not tasty though sweeter, creamier | good texture | thick and frothy | frothy | not sweet | |
| 3 | milky | fine texture | thinner texture | very foamy | bitter | not tasty | |
| 8 | 1 | good consistency | unpleasant odor | liked sweetness and flavor more | thick, frothy | no aftertaste | flavor was off, very thin |
| 2 | good sweetness | perfect sweetness level | frothy | decent taste with no aftertaste | not frothy or too thick, left aftertaste | ||
| 3 | bad flavor | good sweetness, no overpowering flavors | bad odor, aftertaste | frothy, consistency overpowers taste/sweetness level | mild flavor, needs more sweetness | bland, wheat taste | |
| 9 | 1 | thin | pleasant flavor | thick | odd metal taste | earthy taste | |
| 2 | thin, creamy taste | pleasant taste | pleasant taste, sweet grass taste | thick, a bit sweet | thin with metal aftertaste, a little bitter | thick, vitamin taste | |
| 3 | pleasant taste, thin | pleasant, sweet grassy aftertaste | thick, foamy, not pleasant | bitter and taste of metal | different, not sweet, not unpleasant | ||
| 10 | 1 | smells bad | sour bitter | powdery | |||
| 2 | a little bitter | foamy | horrible | ||||
| 3 | strong aftertaste | bad aftertaste | foamy, bad taste | worst taste, bitter, acidic, spicy | horrible, spicy, bitter | ||
| 11 | 1 | ||||||
| 2 | |||||||
| 3 | |||||||
| 12 | 1 | mild aftertaste | |||||
| 2 | foamy, strong smell | ||||||
| 3 | too thick and foamy | sour, no sweetness at all | |||||
| 13 | 1 | foamy | grassy, pine nutty taste | ||||
| 2 | too foamy | sour | |||||
| 3 | bad aftertaste | smelled like a plant | |||||
| 14 | 1 | smooth, creamy | foamy | bitter aftertaste which lingers | smooth | ||
| 2 | creamy mouthfeel, smooth | bitter aftertaste | aftertaste | ||||
| 3 | creamy | foamy | little bitter | ||||
| 15 | 1 | watery, noticeable odor | watery, smells sour, tastes green | neutral flavor, watery | viscous, slight odor | slight aftertaste, sour and watery | tastes like grass, odor less desirable |
| 2 | slight vegetable taste | vegetable taste, milk like consistency | milk-like consistency, no bitterness | viscous, slight displeasing odor | neutral taste, milk-like consistency | darker, watery | |
| 3 | milk-like consistency | slight sour smell, creamy, sour aftertaste | sweet smell, no aftertaste, watery | thick consistency, no bitterness, no aftertaste, slight smell | watery, bitter | vegetable smell, creamy, slight aftertaste |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olson, C.; Kumar, R.; Talavera, M.J.; Anderson, C.E.; Hanson, J.A. Sweetness Ratings of U.S. Infant Formulas. Nutrients 2025, 17, 2602. https://doi.org/10.3390/nu17162602
Olson C, Kumar R, Talavera MJ, Anderson CE, Hanson JA. Sweetness Ratings of U.S. Infant Formulas. Nutrients. 2025; 17(16):2602. https://doi.org/10.3390/nu17162602
Chicago/Turabian StyleOlson, Chelsea, Rajesh Kumar, Martin J. Talavera, Christopher E. Anderson, and Jennifer A. Hanson. 2025. "Sweetness Ratings of U.S. Infant Formulas" Nutrients 17, no. 16: 2602. https://doi.org/10.3390/nu17162602
APA StyleOlson, C., Kumar, R., Talavera, M. J., Anderson, C. E., & Hanson, J. A. (2025). Sweetness Ratings of U.S. Infant Formulas. Nutrients, 17(16), 2602. https://doi.org/10.3390/nu17162602







