Prevalence of Nutrient Deficiencies Following Bariatric Surgery—Long-Term, Prospective Observation
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Baseline Characteristics
3.2. 10-Year General Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| aOR | Adjusted Odds Ratio |
| ASMBS | American Society for Metabolic and Bariatric Surgery |
| BMI | Body Mass Index |
| BS | Bariatric Surgery |
| CI | Confidence Interval |
| EWL | Excess Weight Loss |
| GB | Gastric Bypass |
| HbA1c | Glycated Hemoglobin |
| HDL | High-Density Lipoprotein Cholesterol |
| ID | Iron Deficiency |
| IQR | Interquartile Ranges |
| LDL | Low-Density Lipoprotein Cholesterol |
| MCV | Mean Corpuscular Volume |
| OAGB | One-Anastomosis Gastric Bypass |
| RYGB | Roux-en-Y Gastric Bypass |
| SG | Sleeve Gastrectomy |
| TIBC | Total Iron Binding Capacity |
| WHO | World Health Organization |
| 25-OHD | 25-Hydroxyvitamin D3 |
References
- Kent, S.; Green, J.; Reeves, G.; Beral, V.; Gray, A.; A Jebb, S.; Cairns, B.J.; Mihaylova, B.; Abbiss, H.; Abbott, S.; et al. Hospital costs in relation to body-mass index in 1·1 million women in England: A prospective cohort study. Lancet Public Health 2017, 2, e214–e222. [Google Scholar] [CrossRef]
- World Health Organization. Obesity and Overweight. Update 1 March 2024. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 12 May 2024).
- GBD 2019 Risk Factors Collaborators. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1223–1249. [Google Scholar] [CrossRef]
- Kozieł, P.; Jankowski, P.; Mirek-Bryniarska, E.; Nessler, J.; Podolec, P.; De Bacquer, D.; Kotseva, K.; Wood, D.; Czarnecka, D.; Kawecka-Jaszcz, K.; et al. Obesity in patients with established coronary artery disease over a 20-year period (1997–2017). Pol. Arch. Intern. Med. 2021, 131, 26–32. [Google Scholar] [CrossRef]
- Mohapatra, S.; Gangadharan, K.; Pitchumoni, C.S. Malnutrition in obesity before and after bariatric surgery. Dis Mon. 2020, 66, 100866. [Google Scholar] [CrossRef] [PubMed]
- Walker, E.; Elman, M.; Takemoto, E.E.; Fennern, E.; Mitchell, J.E.; Pories, W.J.; Ahmed, B.; Pomp, A.; Wolfe, B.M. Bariatric Surgery Among Medicare Subgroups: Short- and Long-Term Outcomes. Obesity 2019, 27, 1820–1827. [Google Scholar] [CrossRef] [PubMed]
- Sjöström, L. Review of the key results from the Swedish Obese Subjects (SOS) trial—A prospective controlled intervention study of bariatric surgery. J. Intern. Med. 2013, 273, 219–234. [Google Scholar] [CrossRef] [PubMed]
- Humięcka, M.; Sawicka, A.; Kędzierska, K.; Kotowicz, M.; Koczkodaj, M.; Jaworski, P.; Binda, A.; Tarnowski, W.; Jankowski, P. Long-term trends in cardiovascular risk factors and cardiovascular risk following bariatric surgery: A 10-year prospective cohort study. Atherosclerosis 2025, 405, 119232. [Google Scholar] [CrossRef]
- American Society for Metabolic and Bariatric Surgery. Estimate of Bariatric Surgery Numbers, 2011–2022. Available online: https://asmbs.org/resources/estimate-of-bariatric-surgery-numbers/ (accessed on 12 May 2024).
- Deledda, A.; Pintus, S.; Loviselli, A.; Fosci, M.; Fantola, G.; Velluzzi, F. Nutritional Management in Bariatric Surgery Patients. Int. J. Environ. Res. Public Health 2021, 18, 12049. [Google Scholar] [CrossRef]
- Gasmi, A.; Bjørklund, G.; Mujawdiya, P.K.; Semenova, Y.; Peana, M.; Dosa, A.; Piscopo, S.; Benahmed, A.G.; Costea, D.O. Micronutrients deficiences in patients after bariatric surgery. Eur. J. Nutr. 2021, 61, 55–67. [Google Scholar] [CrossRef]
- Ben-Porat, T.; Elazary, R.; Goldenshluger, A.; Dagan, S.S.; Mintz, Y.; Weiss, R. Nutritional deficiencies four years after laparoscopic sleeve gastrectomy—Are supplements required for a lifetime? Surg. Obes. Relat. Dis. 2017, 13, 1138–1144. [Google Scholar] [CrossRef]
- Caron, M.; Hould, F.; Lescelleur, O.; Marceau, S.; Lebel, S.; Julien, F.; Simard, S.; Biertho, L. Long-term nutritional impact of sleeve gastrectomy. Surg. Obes. Relat. Dis. 2017, 13, 1664–1673. [Google Scholar] [CrossRef] [PubMed]
- Parrott, J.; Frank, L.; Rabena, R.; Craggs-Dino, L.; Isom, K.A.; Greiman, L. American Society for Metabolic and Bariatric Surgery Integrated Health Nutritional Guidelines for the Surgical Weight Loss Patient 2016 Update: Micronutrients. Surg. Obes. Relat. Dis. 2017, 13, 727–741. [Google Scholar] [CrossRef]
- Gracia-Casa, M.N. ; WHO Steering Committee. Guideline on Haemoglobin Cutoffs to Define Anaemia in Individuals and Populations; World Health Organization: Geneva, Switzerland, 2024. [Google Scholar]
- Banach, M.; Burchardt, P.; Chlebus, K.; Dobrowolski, P.; Dudek, D.; Dyrbuś, K.; Gąsior, M.; Jankowski, P.; Jóźwiak, J.; Kłosiewicz-Latoszek, L.; et al. PoLA/CFPiP/PCS/PSLD/PSD/PSH guidelines on diagnosis and therapy of lipid disorders in Poland 2021. Arch. Med. Sci. 2021, 17, 1447–1547. [Google Scholar] [CrossRef]
- O’bRien, P.E.; Hindle, A.; Brennan, L.; Skinner, S.; Burton, P.; Smith, A.; Crosthwaite, G.; Brown, W. Long-Term Outcomes After Bariatric Surgery: A Systematic Review and Meta-analysis of Weight Loss at 10 or More Years for All Bariatric Procedures and a Single-Centre Review of 20-Year Outcomes After Adjustable Gastric Banding. Obes. Surg. 2018, 29, 3–14. [Google Scholar] [CrossRef]
- Salminen, P.; Grönroos, S.; Helmiö, M.; Hurme, S.; Juuti, A.; Juusela, R.; Peromaa-Haavisto, P.; Leivonen, M.; Nuutila, P.; Ovaska, J. Effect of Laparoscopic Sleeve Gastrectomy vs Roux-en-Y Gastric Bypass on Weight Loss, Comorbidities, and Reflux at 10 Years in Adult Patients With Obesity. JAMA Surg. 2022, 157, 656–666. [Google Scholar] [CrossRef]
- Sjöström, L.; Lindroos, A.-K.; Peltonen, M.; Torgerson, J.; Bouchard, C.; Carlsson, B.; Dahlgren, S.; Larsson, B.; Narbro, K.; Sjöström, C.D.; et al. Lifestyle, Diabetes, and Cardiovascular Risk Factors 10 Years after Bariatric Surgery. N. Engl. J. Med. 2004, 351, 2683–2693. [Google Scholar] [CrossRef]
- Gletsu-Miller, N.; Wright, B.N. Mineral Malnutrition Following Bariatric Surgery. Adv. Nutr. Int. Rev. J. 2013, 4, 506–517. [Google Scholar] [CrossRef]
- Jaworski, P.; Binda, A.; Barski, K.; Wawiernia, K.; Kudlicka, E.; Wąsowski, M.; Jankowski, P.; Tarnowski, W. OAGB with shortened excluded ileal loop as an effective treatment for type 2 diabetes mellitus in the cases of Caucasian men and women with obesity of the first degree (BMI 30–35 kg/m2). Langenbeck’s Arch. Surg. 2023, 408, 84. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, A.; Tantia, O.; Goyal, G.; Chaudhuri, T.; Khanna, S.; Poddar, A.; Gupta, S.; Majumdar, K. MGB-OAGB: Effect of Biliopancreatic Limb Length on Nutritional Deficiency, Weight Loss, and Comorbidity Resolution. Obes. Surg. 2018, 28, 3439–3445. [Google Scholar] [CrossRef] [PubMed]
- Peterson, L.A.; Cheskin, L.J.; Furtado, M.; Papas, K.; Schweitzer, M.A.; Magnuson, T.H.; Steele, K.E. Malnutrition in Bariatric Surgery Candidates: Multiple Micronutrient Deficiencies Prior to Surgery. Obes. Surg. 2015, 26, 833–838. [Google Scholar] [CrossRef]
- Zolfaghari, F.; Khorshidi, Y.; Moslehi, N.; Golzarand, M.; Asghari, G. Nutrient Deficiency After Bariatric Surgery in Adolescents: A Systematic Review and Meta-Analysis. Obes. Surg. 2023, 34, 206–217. [Google Scholar] [CrossRef]
- Arias, P.M.; Domeniconi, E.A.; García, M.; Esquivel, C.M.; Lascano, F.M.; Foscarini, J.M. Micronutrient Deficiencies After Roux-en-Y Gastric Bypass: Long-Term Results. Obes. Surg. 2019, 30, 169–173. [Google Scholar] [CrossRef]
- Bjørklund, G.; Peana, M.; Pivina, L.; Dosa, A.; Aaseth, J.; Semenova, Y.; Chirumbolo, S.; Medici, S.; Dadar, M.; Costea, D.-O. Iron Deficiency in Obesity and after Bariatric Surgery. Biomolecules 2021, 11, 613. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.M.; Shenkin, A.; Schweinlin, A.; Amrein, K.; Augsburger, M.; Biesalski, H.-K.; Bischoff, S.C.; Casaer, M.P.; Gundogan, K.; Lepp, H.-L.; et al. ESPEN micronutrient guideline. Clin. Nutr. 2022, 41, 1357–1424. [Google Scholar] [CrossRef]
- Steenackers, N.; Van der Schueren, B.; Mertens, A.; Lannoo, M.; Grauwet, T.; Augustijns, P.; Matthys, C. Iron deficiency after bariatric surgery: What is the real problem? Proc. Nutr. Soc. 2018, 77, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Hood, M.M.; Corsica, J.; Bradley, L.; Wilson, R.; Chirinos, D.A.; Vivo, A. Managing severe obesity: Understanding and improving treatment adherence in bariatric surgery. J. Behav. Med. 2016, 39, 1092–1103. [Google Scholar] [CrossRef] [PubMed]
- Broeke, R.T.; Bravenboer, B.; Smulders, F.J.F. Iron deficiency before and after bariatric surgery: The need for iron supplementation. Neth. J. Med. 2013, 71, 412–417. [Google Scholar]
- Shulpekova, Y.; Nechaev, V.; Kardasheva, S.; Sedova, A.; Kurbatova, A.; Bueverova, E.; Kopylov, A.; Malsagova, K.; Dlamini, J.C.; Ivashkin, V. The Concept of Folic Acid in Health and Disease. Molecules 2021, 26, 3731. [Google Scholar] [CrossRef]
- Xanthakos, S.A.; Khoury, J.C.; Inge, T.H.; Jenkins, T.M.; Modi, A.C.; Michalsky, M.P.; Chen, M.K.; Courcoulas, A.P.; Harmon, C.M.; Brandt, M.L.; et al. Nutritional Risks in Adolescents After Bariatric Surgery. Clin. Gastroenterol. Hepatol. 2020, 18, 1070–1081.e5. [Google Scholar] [CrossRef]
- Nunes, R.; Santos-Sousa, H.; Vieira, S.; Nogueiro, J.; Bouça-Machado, R.; Pereira, A.; Carneiro, S.; Costa-Pinho, A.; Lima-Da-Costa, E.; Preto, J.; et al. Vitamin B Complex Deficiency After Roux-en-Y Gastric Bypass and Sleeve Gastrectomy—A Systematic Review and Meta-Analysis. Obes. Surg. 2022, 32, 873–891. [Google Scholar] [CrossRef]
- Krzizek, E.-C.; Brix, J.M.; Stöckl, A.; Parzer, V.; Ludvik, B. Prevalence of Micronutrient Deficiency after Bariatric Surgery. Obes. Facts 2021, 14, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Gowanlock, Z.; Lezhanska, A.; Conroy, M.; Crowther, M.; Tiboni, M.; Mbuagbaw, L.; Siegal, D.M. Iron deficiency following bariatric surgery: A retrospective cohort study. Blood Adv. 2020, 4, 3639–3647. [Google Scholar] [CrossRef] [PubMed]
- von Drygalski, A.; Andris, D.A. Anemia After Bariatric Surgery: More Than Just Iron Deficiency. Nutr. Clin. Pract. 2009, 24, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Kędzierska, K.; Dymkowski, M.; Niegowska, W.; Humięcka, M.; Sawicka, A.; Walczak, I.; Jędral, Z.M.; Wąsowski, M.; Bogołowska-Stieblich, A.; Binda, A.; et al. Iron Deficiency Anemia Following Bariatric Surgery: A 10-Year Prospective Observational Study. Nutrients 2025, 17, 339. [Google Scholar] [CrossRef]
- Amtco, G.; Ferreira, M.J.S.; Salazar, D.A.; Neves, J.S.; Pedro, J.M.P.; Guerreiro, V.A.; Viana, S.e.S.; Mendonça, F.; Silva, M.M.; Belo, S.P.; et al. Which Factors Are Associated with a Higher Prevalence of Anemia Following Bariatric Surgery? Results from a Retrospective Study Involving 1999 Patients. Obes. Surg. 2020, 30, 3496–3502. [Google Scholar] [CrossRef]
- Bailly, L.; Schiavo, L.; Sebastianelli, L.; Fabre, R.; Pradier, C.; Iannelli, A. Anemia and Bariatric Surgery: Results of a National French Survey on Administrative Data of 306,298 Consecutive Patients Between 2008 and 2016. Obes. Surg. 2018, 28, 2313–2320. [Google Scholar] [CrossRef]
- Lespessailles, E.; Toumi, H. Vitamin D alteration associated with obesity and bariatric surgery. Exp. Biol. Med. 2017, 242, 1086–1094. [Google Scholar] [CrossRef]
- Welbourn, R.; Hollyman, M.; Kinsman, R.; Dixon, J.; Liem, R.; Ottosson, J.; Ramos, A.; Våge, V.; Al-Sabah, S.; Brown, W.; et al. Bariatric Surgery Worldwide: Baseline Demographic Description and One-Year Outcomes from the Fourth IFSO Global Registry Report 2018. Obes. Surg. 2018, 29, 782–795. [Google Scholar] [CrossRef]
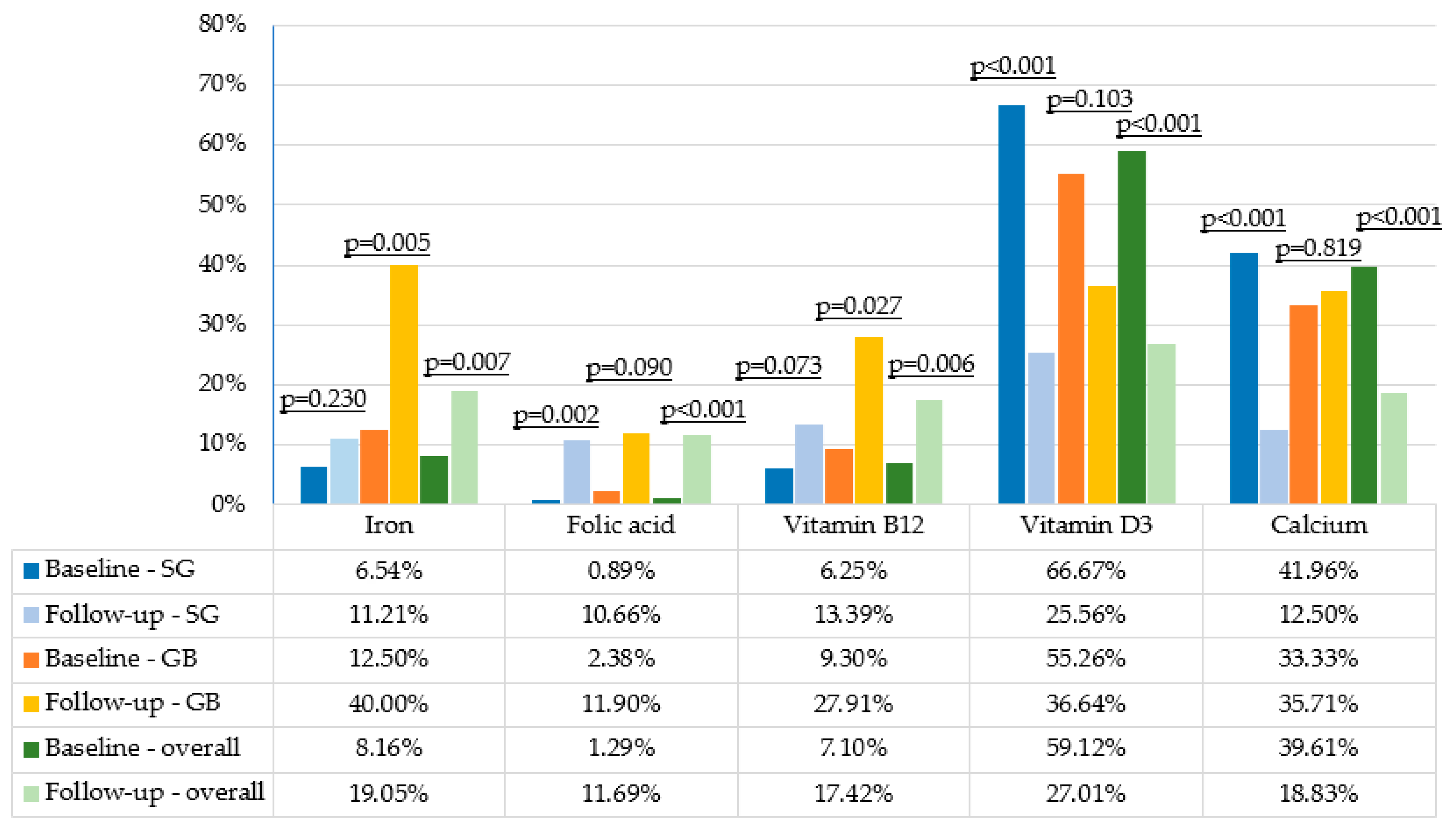
| Variable | Baseline Sleeve Gastrectomy | Follow-Up Sleeve Gastrectomy | p-Value | Baseline Gastric Bypass | Follow-Up Gastric Bypass | p-Value | Baseline Overall | Follow-Up Overall | p-Value |
|---|---|---|---|---|---|---|---|---|---|
| n = 112 | n = 112 | n = 43 | n = 43 | n = 155 | n = 155 | ||||
| Weight, kg | 119.0 (107.0–129.9) | 100.2 (86.8–114.0) | <0.001 | 121.0 (102.5–135.0) | 89.0 (75.0–100.0) | <0.001 | 119.0 (106.2–130.1) | 95.0 (85.0–112.0) | <0.001 |
| BMI 1, kg/m2 | 42.0 (38.6–46.6) | 36.2 (31.4–40.3) | <0.001 | 41.5 (37.1–46.8) | 30.8 (27.7–34.8) | <0.001 | 41.9 (38.4–46.6) | 34.5 (29.8–39.6) | <0.001 |
| HDL 2, mmol/L | 1.1 (1.0–1.3) | 1.6 (1.3–1.9) | <0.001 | 1.1 (0.9–1.3) | 1.6 (1.2–1.7) | <0.001 | 1.1 (1.0–1.3) | 1.6 (1.3–1.7) | <0.001 |
| LDL 3, mmol/L | 3.0 (2.4–3.5) | 3.0 (2.5–3.6) | 0.148 | 3.0 (2.3–3.4) | 2.3 (1.9–2.9) | 0.003 | 3.0 (2.3–3.5) | 2.8 (2.3–3.5) | 0.626 |
| Triglycerides, mmol/L | 1.6 (1.2–2.0) | 1.1 (0.8–1.6) | <0.001 | 1.4 (1.0–2.0) | 1.0 (0.8–1.4) | <0.001 | 1.5 (1.1–2.0) | 1.0 (0.8–1.5) | <0.001 |
| HbA1c 4, % | 6.0 (5.4–6.4) | 5.8 (5.5–6.0) | 0.139 | 6.4 (5.6–7.9) | 5.9 (5.5–6.7) | 0.045 | 6.0 (5.4–6.7) | 5.8 (5.5–6.1) | 0.020 |
| Hemoglobin, g/L | 140.0 (133.0–147.0) | 133.0 (124.0–145.0) | <0.001 | 142.0 (131.0–149.0) | 125.0 (97.0–138.0) | <0.001 | 140.0 (133.0–148.0) | 131.0 (120.0–143.0) | <0.001 |
| MCV 5, fL | 87.8 (85.5–90.7) | 89.6 (86.7–94.2) | 0.002 | 89.0 (85.5–92.7) | 88.6 (81.0–92.5) | 0.654 | 87.9 (85.3–90.8) | 89.2 (84.9–93.6) | 0.003 |
| Variable | Baseline Sleeve Gastrectomy | Follow-Up Sleeve Gastrectomy | p-Value | Baseline Gastric Bypass | Follow-Up Gastric Bypass | p-Value | Baseline Overall | Follow-Up Overall | p-Value |
|---|---|---|---|---|---|---|---|---|---|
| Potassium, mmol/L | 4.3 (4.1–4.5) | 4.3 (4.1–4.5) | 0.176 | 4.3 (4.2–4.6) | 4.2 (4.0–4.5) | 0.502 | 4.3 (4.1–4.5) | 4.2 (4.1–4.5) | 0.132 |
| Sodium, mmol/L | 140.0 (139.0–142.0) | 140.0 (139.0–141.0) | 0.284 | 139.0 (138.0–141.0) | 140.0 (139.0–141.0) | 0.147 | 140.0 (139.0–141.0) | 140.0 (139.0–141.0) | 0.861 |
| Iron, μmol/L | 15.9 (12.9–19.7) | 16.3 (12.4–21.5) | 0.807 | 15.0 (11.1–20.8) | 11.3 (5.9–18.6) | 0.026 | 15.8 (12.5–20.1) | 15.9 (10.7–20.1) | 0.223 |
| TIBC 1, μmol/L | 62.3 (57.7–68.6) | 61.8 (56.1–69.0) | 0.018 | 67.2 (59.1–76.1) | 69.5 (64.0–80.2) | 0.172 | 64.4 (59.1–71.7) | 64.2 (57.6–71.9) | 0.272 |
| Folic acid, nmol//L | 16.5 (13.1–22.9) | 13.4 (10.4–18.1) | <0.001 | 18.4 (14.5–24.9) | 19.7 (12.0–30.4) | 0.821 | 17.0 (13.6–23.1) | 13.6 (10.6–21.5) | 0.009 |
| Vitamin B12, pmol/L | 238.3 (197.7–323.2) | 242.0 (188.9–306.2) | 0.274 | 230.9 (180.8–290.7) | 222.8 (132.8–323.9) | 0.583 | 234.6 (194.8–301.8) | 236.1 (177.1–306.2) | 0.234 |
| Calcium, mmol/L | 2.27 (2.20–2.34) | 2.35 (2.27–2.40) | <0.001 | 2.25 (2.21–2.35) | 2.28 (2.22–2.37) | 0.125 | 2.26 (2.20–2.35) | 2.32 (2.25–2.40) | <0.001 |
| Phosphorus, mmol/L | 1.03 (0.97–1.16) | 1.13 (1.03–1.26) | <0.001 | 1.10 (1.00–1.19) | 1.19 (1.10–1.32) | 0.003 | 1.06 (0.97–1.16) | 1.16 (1.03–1.29) | <0.001 |
| 25-OHD 2, nmol/L | 46.3 (36.5–24.9) | 71.25 (50.8–96.0) | <0.001 | 47.8 (40.8–62.5) | 58.0 (35.8–88.8) | <0.001 | 46.5 (37.5–62.5) | 69.0 (46.8–67.3) | <0.001 |
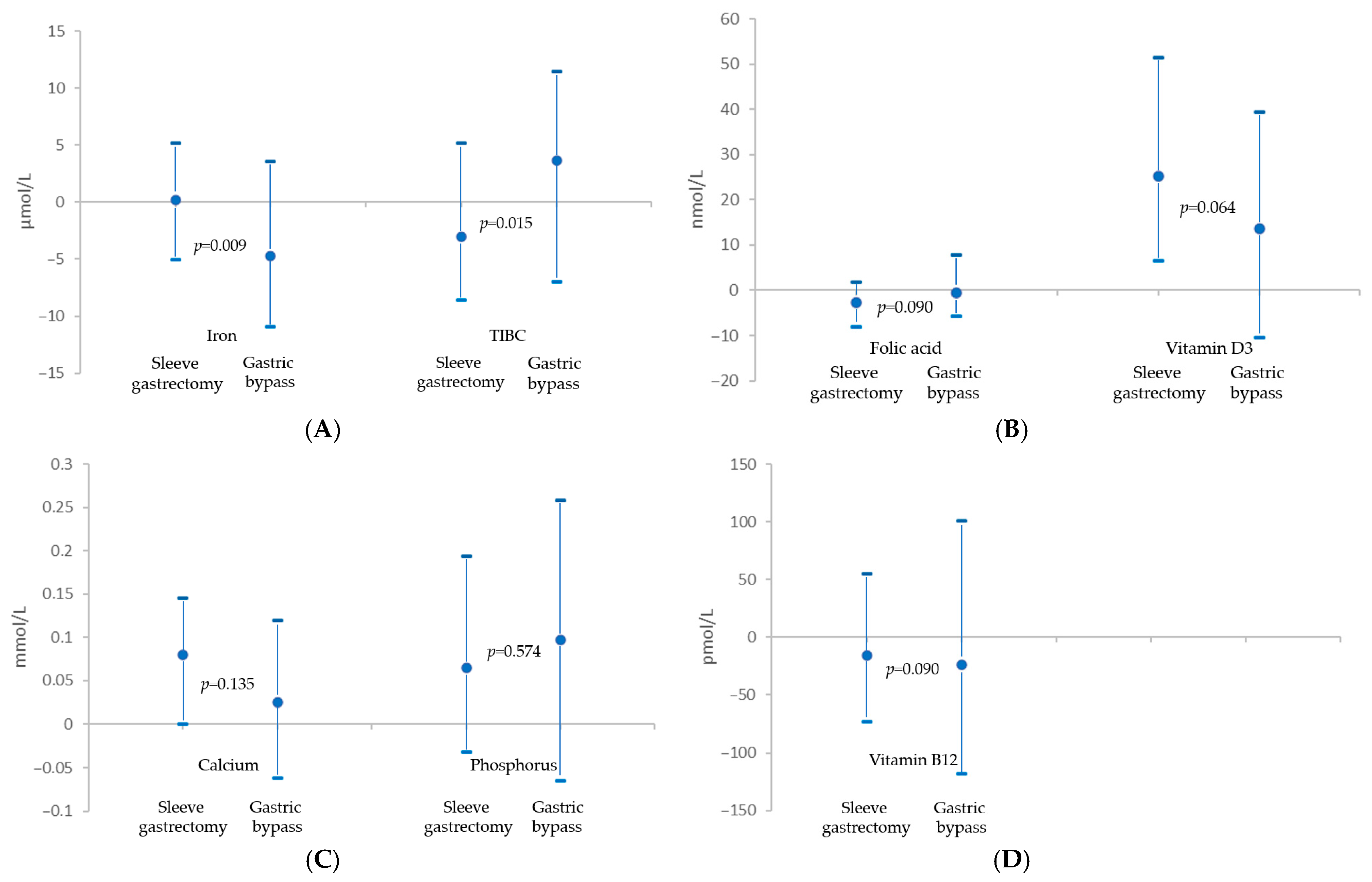
| Variable | Sleeve Gastrectomy | Gastric Bypass | p-Value |
|---|---|---|---|
| Iron, umol/L | 0.18 (−5.01–5.19) | −4.75 (−10.9–3.58) | 0.009 |
| TIBC 1, umol/L | −3.04 (−8.6–5.19) | 3.67 (−6.98–11.46) | 0.015 |
| Calcium, mmol/L | 0.080 (0.000–0.145) | 0.025 (−0.062–0.120) | 0.135 |
| Phosphorus, mmol/L | 0.065 (−0.032–0.194) | 0.097 (−0.065–0.258) | 0.574 |
| Folic acid, nmol/L | −2.60 (−8.16–1.93) | −0.63 (−5.67–7.93) | 0.090 |
| Vitamin D3, nmol/L | 25.21 (6.49–51.42) | 13.73 (−10.48–39.43) | 0.064 |
| Vitamin B12, pmol/L | −16.2 (−72.7–55.0) | −23.6 (−118.0–101.1) | 0.090 |
| Variable | Microcytic Anemia | Non-Microcytic Anemia | p-Value |
|---|---|---|---|
| Female | 87.5% | 81.8% | 0.634 |
| Age, years | 46.9 ± 8.9 | 53.6 ± 8.5 | 0.024 |
| After GB 1 | 62.5% | 40.9% | 0.189 |
| EWL 2 | 47.5 ± 46.1% | 54.5 ± 31.9% | 0.581 |
| Hemoglobin, g/dL | 96.5 (84.0–10.95) | 112.5 (109.0–117.0) | <0.001 |
| Iron, mmol/L | 4.5 (2.9–6.3) | 8.6 (6.1–15.6) | <0.001 |
| Vitamin B12, pmol/L | 194.8 (108.5–249.4) | 266.3 (193.3–374.1) | 0.020 |
| Folic acid, nmol/L | 11.6 (7.7–20.6) | 17.9 (12.7–24.9) | 0.086 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Humięcka, M.; Sawicka, A.; Kędzierska, K.; Binda, A.; Jaworski, P.; Tarnowski, W.; Jankowski, P. Prevalence of Nutrient Deficiencies Following Bariatric Surgery—Long-Term, Prospective Observation. Nutrients 2025, 17, 2599. https://doi.org/10.3390/nu17162599
Humięcka M, Sawicka A, Kędzierska K, Binda A, Jaworski P, Tarnowski W, Jankowski P. Prevalence of Nutrient Deficiencies Following Bariatric Surgery—Long-Term, Prospective Observation. Nutrients. 2025; 17(16):2599. https://doi.org/10.3390/nu17162599
Chicago/Turabian StyleHumięcka, Maria, Ada Sawicka, Kinga Kędzierska, Artur Binda, Paweł Jaworski, Wiesław Tarnowski, and Piotr Jankowski. 2025. "Prevalence of Nutrient Deficiencies Following Bariatric Surgery—Long-Term, Prospective Observation" Nutrients 17, no. 16: 2599. https://doi.org/10.3390/nu17162599
APA StyleHumięcka, M., Sawicka, A., Kędzierska, K., Binda, A., Jaworski, P., Tarnowski, W., & Jankowski, P. (2025). Prevalence of Nutrient Deficiencies Following Bariatric Surgery—Long-Term, Prospective Observation. Nutrients, 17(16), 2599. https://doi.org/10.3390/nu17162599







