Abstract
Background: Dietary assessment in inflammatory bowel disease (IBD) is moving away from individual food and nutrient analyses and towards dietary patterns (e.g., Mediterranean diet, Western diet) and diet quality assessment that are increasingly implicated in IBD onset and course. However, an IBD-specific diet quality index (DQI) does not exist. This review aimed to identify current DQIs and assess their suitability for an IBD population. Methods: MEDLINE and EmCare databases were systematically searched for a-priori, food-based DQI reflecting current dietary guidelines and/or nutrition science. Data extracted were adapted from optimal DQI criteria, including quality measures of adequacy, moderation, variety and balance and DQI evaluation. Results: Twenty-four DQI were identified. No DQI included all optimal DQI criteria. The Dietary Guideline Index 2013 (DGI-2013) most closely met the criteria, followed by the Dutch Healthy Diet Index-2015 (DHD-Index 2015), Planetary Health Diet Index (PHDI) and Healthy Eating Index for Australian Adults-2013 (HEIFA-2013). Most DQI assessed adequacy (22/24, 92%) and moderation (21/24, 88%), half assessed balance (12/24) while few assessed variety (8/24, 33%). Application of other optimal DQI criteria varied. Food frequency questionnaire (13/24) and 24 h diet recall (12/24) were the most common dietary assessment methods used. Most DQI (17/24, 71%) were validated; however, not for an IBD population. Few were evaluated for reliability (6/24) or reproducibility (1/24). Conclusions: No DQI meets all optimal criteria for an IBD-specific DQI. The DGI-2013 met the most criteria, followed by the DHD Index-2015, PHDI and HEIFA-2013 and may be most appropriate for an IBD population. An IBD-specific DQI is lacking and needed.
1. Introduction
Dietary research in inflammatory bowel disease (IBD) remains a complex and emerging area. Many large prospective cohort studies have investigated associations between specific nutrient and food components with onset and incidence of Crohn’s disease and ulcerative colitis [1,2]. These studies typically describe diet in macro- and micronutrient and/or food group composition, usually gathered from food frequency questionnaires (FFQ). This aligns with current nutrient-focused dietary recommendations for IBD, with none providing specific food-based recommendations and typically encourage dietary intakes in line with healthy eating dietary guidelines globally [3,4,5].
However, emerging evidence suggests specific dietary patterns such as the Western diet or Mediterranean diet (MED) may exert unfavourable or protective effects on IBD pathogenesis and disease course rather than individual nutrients or dietary components alone [6,7,8,9]. Dietary patterns of higher diet quality have even been associated with reduced gastrointestinal inflammatory markers in a healthy population [10]. Another approach of interest involves exploring degrees of food processing, specifically classifying ultra-processed food (UPF), such as application of the NOVA classification system [11]. This method is commonly used as a pseudo-marker for general food quality but is fraught with inaccuracies, with carefully designed diets for therapeutic trials based on healthy eating dietary guidelines still able to comprise proportions of UPF that account for up to 70% of energy intake [12].
Hence, dietary pattern assessment that can comprehensively consider diet quality and the complexity of interactions between foods and nutrients may better predict health outcomes [13]. A diet quality index (DQI) is an a-priori scoring tool that holistically evaluates dietary patterns across four dimensions of adequacy, moderation, variety and balance, defined by national dietary guidelines or commonly described dietary patterns (e.g., MED) [14,15]. Burggraf et al. has further defined optimal construction criteria for a DQI including recommendations for its theoretical framework, component structure, scoring systems and evaluation processes [14].
While appropriate DQIs to apply to healthy populations have been identified [16,17], an IBD-specific DQI does not exist. It is currently unclear which DQI is most appropriate to use in an IBD population to assess disease association or predict response to therapeutic diets or clinical outcomes. To our knowledge, only one systematic review has assessed habitual diet using DQIs (e.g., Mediterranean Diet Score, Dietary Inflammatory Index) and IBD risk, progression and disease activity in longitudinal cohort and observational case–control studies but did not evaluate the applicability or appropriateness of DQIs used [18].
To advance dietary research and gain deeper understanding of IBD development and prevention beyond nutrient intake, it is important to find an accurate and suitable DQI that can be applied to therapeutic diets for IBD to help understand their effectiveness, informing development of successful therapeutic dietary strategies for IBD. This narrative literature review aims to identify current DQIs and assess their suitability for use on an IBD population.
2. Materials and Methods
2.1. Search Strategy
Electronic databases, MEDLINE and EmCare were searched until 16 May 2025 using search terms including “diet*”, “healthy eating pattern”, “nutri*”, “food”, “index”, “indic*”, “score”, “tool”, “metric*”, “quality”, “inflammat*” (see Supplementary Table S1 for full search strategy). Articles were limited to those published between 2013 and 2025, reflecting the publishing dates of current national dietary guidelines [19,20,21,22,23], in humans and available in English.
2.2. Eligibility Criteria
Included studies were original articles of the most current developed or updated version of an a priori, food-based DQI that reflected current national dietary guidelines and/or latest nutritional science. Articles were excluded if DQI were posteriori, nutrient-only, developed for specific non-IBD population groups (e.g., children, pregnant women, athletes) or diseases (e.g., cardiovascular disease, diabetes), or not applied to the individual level (e.g., collective household intake, food industry). See Supplementary Table S2 for full criteria.
2.3. Screening and Data Synthesis
After duplicate removal, article titles and abstracts were screened by a single reviewer (LJP). Eligible articles were full text screened using inclusion and exclusion criteria by a single reviewer (LJP). If eligibility was unclear, the paper was discussed with a second reviewer (ASD) to reach consensus. Where an article was not the primary article for a DQI, the primary reference was obtained and screened for eligibility. Reference lists of similar existing DQI literature reviews were screened for further eligible articles [16,17,18]. Figure 1 outlines the PRISMA flow diagram for article inclusion and exclusion in this review. For all included articles, individual searches using Google Scholar and the articles’ citations were conducted to determine if a DQI had undergone additional validation studies, and/or had been used to evaluate diet quality in an IBD population.
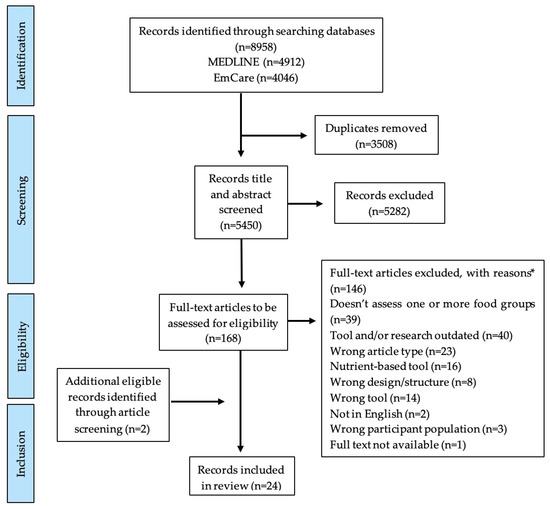
Figure 1.
PRISMA flow diagram which details review selection processes for articles eligible for this narrative review assessing current diet quality indices and their applicability to an inflammatory bowel disease population. Footnotes. * categories of exclusion reasons as listed.
2.4. Data Extraction and Quality Assessment
Data were extracted from included articles using a tool designed specifically for this review, adapted from optimal DQI criteria described by Burggraf et al. [14]. These defined criteria have not been validated or formalised into a specific assessment or quality appraisal tool; however, they were developed from expert review of international DQI and guided by the Organisation for Economic Cooperation and Development Handbook on Constructing Composite Indicators [24]. Adaptations included assessment against current dietary recommendations for IBD, being inclusion of at minimum all five key food groups (fruits, vegetables, grains, dairy products and animal- and plant-based proteins) comprising population dietary guidelines. Information extracted included the DQI’s development framework, completeness of diet quality measures of adequacy, moderation, variety and balance assessed for food components, its scoring framework and any evaluation of the DQI, including overall and gut-specific health outcomes it has been applied to assess.
3. Results
Twenty-four articles describing 24 DQIs applicable to adults with IBD were identified [25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48] (Figure 1). Table 1 outlines the study details and theoretical framework of the identified DQIs. Included articles were published between 2014 and 2025 with the majority from Australia (n = 7) [26,31,33,34,35,42,44], North America (n = 5) [27,36,38,41,43], Asia (n = 5) [39,45,46,47,48] and Europe (n = 4) [30,32,37,40], as well as one developed from an international cohort [28].

Table 1.
Diet quality indices applicable to adults with inflammatory bowel disease, published between 2014–2025.
Sixteen DQIs were adapted from existing DQI or referenced other dietary scoring tools for development [25,26,27,28,30,31,35,36,37,38,39,40,43,44,47,48] (Table 1). Population-based dietary guidelines were used to develop 19/24 (79%) DQIs [25,26,27,30,31,32,33,34,35,38,39,40,41,42,43,44,46,47,48]. Of these, seven also used existing literature on defined dietary patterns (e.g., MED, World Health Organisation recommendations) [26,27,32,34,41,43,47]. The remaining five DQIs were developed from defined dietary patterns alone [28,29,36,37,45].
3.1. Optimal Criteria Components for an IBD-Specific DQI
In Table 2, the key construct components for included DQIs are outlined.

Table 2.
Diet quality indices meeting adapted optimal criteria recommendations for an inflammatory bowel disease-specific diet quality index.
3.2. Dimensions
As Table 2 depicts, across DQI, assessment of the four key dimensions varied. None sufficiently assessed all four dimensions. Most DQIs assessed adequacy (22/24, 92%) [25,26,28,29,30,31,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48] and moderation (21/24, 88%) [25,26,28,29,30,31,33,34,36,37,38,39,40,41,42,43,44,45,46,47,48]. Half (12/24) [26,27,29,30,33,38,39,40,41,42,43,44] at least partially assessed balance, while eight (33%) [28,31,32,35,38,42,44,47] at least partially assessed variety.
All DQIs assessed whole food groups reflective of population-based dietary guidelines (e.g., fruits, vegetables, grains, dairy products and animal- and plant-based proteins); however, there was heterogeneity for food and nutrient components that were assessed. For example, Roy et al. [42] assessed “total vegetable” intake whereas Bromage et al. [28] assessed vegetables as “dark leafy green vegetables”, “cruciferous vegetables”, “deep orange vegetables” and “other vegetables” categories. Supplementary Table S3 details the food group and nutrient components included within each DQI. Ultra-processed foods were assessed in 16/24 (67%) DQI [25,26,28,30,33,34,35,36,39,40,41,42,43,44,46,48].
3.2.1. Adequacy and Moderation
While most DQIs assessed adequacy (92%), referring to encouraged dietary components perceived as beneficial to health and moderation (88%), referring to dietary components recommended to limit perceived as adverse to health, there was variation in whether food group, food and nutrient components were considered in adequacy and/or moderation dimensions (see Supplementary Table S3). For example, 11/24 (46%) DQIs [25,28,33,35,38,39,42,43,44,46,48] included red meats within the adequacy dimension; conversely, 10/24 (42%) [26,29,30,31,36,37,40,41,45,47] included red meats in the moderation dimension and one (4%) [34] included red meats in both adequacy and moderation dimensions.
3.2.2. Variety
Of eight (33%) DQIs [28,31,32,35,38,42,44,47] assessing the variety dimension, five [28,38,42,44,47] only partially assessed this dimension, measuring variety only within one or two food groups (e.g., only types of fruit and vegetables) (see Supplementary Table S3). The Dietary Diversity Score (DDS) was the only DQI that solely assessed the variety dimension [32].
3.2.3. Balance
Of twelve (50%) DQIs [26,27,29,30,33,38,39,40,41,42,43,44] assessing the balance dimension, eight only partially assessed this dimension, comparing the balance of only one or two foods or nutrients. Common components assessed included ratio of saturated, unsaturated and/or total fats to total energy (n = 8) [27,30,33,38,39,41,42,43], whole grains to total grains (n = 4) [27,38,40,44], added sugars to total energy (n = 5) [27,30,41,42,43] and water to total beverages (n = 4) [26,27,42,44] (see Supplementary Table S3). The Healthy Eating Food Index 2019 (HEFI-2019) [27] was the only DQI which solely assessed the balance dimension across all food groups.
3.3. Other DQI Construction Components
Shown in Table 2, most DQIs (23/24, 96%) [25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,44,45,46,47,48] were applied to retrospective methods of dietary assessment, specifically FFQ (n = 13) [26,28,29,31,32,33,35,37,39,40,42,44,48], 24 h diet recalls (n = 12) [25,27,28,30,34,36,38,40,41,45,46,47] and/or dietitian-collected diet history (n = 1) [34]. Only one DQI was applied to prospective methods (weighed food records) [42] and one did not specify [43]. Five DQIs [27,31,32,33,35] were developed specifically for the respective diet assessment method they were applied to.
Scoring structures and methods varied across DQIs. Two-thirds of (16/24, 67%) DQIs [25,27,29,35,36,37,38,39,40,41,43,44,45,46,47,48] used the recommended metric scoring system and all except one [31] had scoring cut-off points. Most DQIs (21/24, 88%) [25,26,27,29,30,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48] used recommended normative cut-offs, that reflect evidence-derived recommendations (e.g., nutrient reference values, guideline serving quantities) rather than percentile cut-offs derived from study populations (e.g., quartile intakes). However, only 10/21 (48%) DQIs [26,29,33,35,39,40,41,42,43,44] using normative cut-offs were group-specific, accounting for age and gender dietary intake recommendation differences.
Aggregation and Evaluation of DQIs
Diet quality indices utilised differing valuation approaches. No DQI used only non-linear valuation, referring to minimum and maximum intake thresholds for scoring, as recommended to account for foods and nutrients with both associated health benefits and risks rather than linear, one directional scoring [14]. A total of 11 of 24 (46%) DQIs [25,28,29,30,33,34,38,40,45,46,48] used a combination of linear and non-linear valuation for various components based on existing diet-disease relationship knowledge. However, non-linear valuation was not used consistently across a particular food, food group or nutrient. For example, ten DQIs [26,31,35,36,39,41,42,43,44,47] valuated the dairy food group a positive linear score where increasing intake increases points allocated, one [37] valuated dairy a negative linear score where increasing intake decreases points allocated and nine [25,29,30,33,38,40,45,46,48] valuated dairy a non-linear score, where intakes both below or above a certain range decreased points allocated (see Supplementary Table S3).
Only 10/24 (42%) DQIs [27,28,29,30,33,39,41,42,47,48] used unequal weighting across included DQI components as recommended to account for different weighted contributions of foods and nutrients to established health and disease outcomes, where intake of one particular food, food group or nutrient may have more impact on health than another (Table 2). For example, the HEFI-2019 assigns a maximum 20 points to total fruits and vegetables, but only a maximum 5 points to total protein foods [27].
Broadly, 17/24 (71%) DQIs [25,26,27,28,29,30,31,34,35,38,40,42,43,44,45,47,48] have been evaluated to some extent, with only two DQIs [40,44] having previously been applied to an IBD cohort. This is detailed further in Table 3.

Table 3.
Evaluation status of current identified diet quality indices applicable to adults with inflammatory bowel disease and health outcomes diet quality indices have been used to assess.
3.4. DQI Meeting OptimaL Criteria for an IBD Population
No DQI included all optimal criteria for an IBD-specific DQI. The Dietary Guideline Index 2013 (DGI-2013) [44] most closely met recommendations, however, it only partially assessed the variety domain and did not use nonlinear scoring. This was followed by the Dutch Healthy Diet Index-2015 (DHD Index-2015) [40], the Planetary Health Diet Index (PHDI) [29] and the Healthy Eating Index for Australian Adults (HEIFA-2013) [42], that lacked in areas of variety and balance domain assessment, scoring structure, valuation and/or weighting.
3.5. Evaluation of DQIs
As detailed in Table 3, overall, 71% (17/24) of DQIs had undergone evaluation assessment, including validation, either by its original article or a separate evaluation study. Common validation methods performed included construct validity (n = 13) [25,26,28,29,30,31,34,38,40,48,57,63,64,70], criterion validity (n = 7) [28,29,30,38,48,55,64,68] and content validity (n = 3) [30,34,43]. Few DQIs were evaluated for reliability (n = 6) [25,29,42,57,60,70] or reproducibility (n = 1) [31].
Various adult populations were utilised for evaluation, with sample sizes ranging from 96–149,975 participants. Of the four DQIs most closely meeting the adapted optimal DQI criteria, all were validated in Western populations, including Australia [42,44], the Netherlands [40] and Brazil [29]. In addition to diet quality measurement, common health outcomes that were assessed against DQI scores included anthropometry (e.g., BMI, waist circumference, weight change) (n = 12) [26,28,30,34,40,41,44,48,63,64,67,72], biomarkers (e.g., lipid studies, blood glucose, plasma carotenoid concentration) (n = 7) [30,48,55,58,64,67,69], non-communicable disease risk, incidence and/or prevalence (e.g., type 2 diabetes, cardiovascular disease) (n = 6) [28,32,33,38,59,67,75] and mortality (n = 4) [36,38,73,75].
No DQI was validated to assess an IBD population. Two DQI were applied to FFQ from IBD participants, the DGI-2013 was applied to UC participants with ileoanal pouch [65] while the DHD Index-2015 was applied to participants with IBD or irritable bowel syndrome [66]. Another two DQI, the DDS and Australian Recommended Food Score (ARFS) were used with concurrent assessment of gut microbiota composition [56,62].
4. Discussion
This narrative review identified 24 current DQIs [25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47] and assessed their suitability for use with an IBD population against adapted optimal DQI criteria [14]. No DQI included all optimal criteria for an IBD-specific DQI. Most included adequacy and moderation dimension assessment, however, none sufficiently assessed all four dimensions (adequacy, moderation, variety, balance). The DQIs were heterogeneous across all other optimal criteria and had undergone varying extent of evaluation. No DQI was developed specifically for or validated in an IBD population. The DGI-2013 best met the criteria recommendations, followed by the DHD Index-2015, PHDI and HEIFA-2013. The DGI-2013 and DHD Index-2015 have been previously applied to an IBD population.
From an Australian general population context, two systematic reviews similarly found that no DQI met all optimal DQI criteria, with similarities and differences in construction, scoring and evaluation across all DQIs [16,17]. Acknowledging differences in eligibility criteria, consistent with our review, the DGI-2013 and HEIFA-2013 were identified by these systematic reviews as top performing against their own DQI criteria [16,17].
This review observed heterogeneity in all construction components of included DQIs. Diet quality indices outlined differing criteria for measuring foods, food groups and nutrient intake, for example, measuring “total vegetable” vs. “green vegetable” and “orange vegetable” intakes separately, and placed different valuation and weighting methods on these components based on the dietary guidelines and nutrition literature used to build the DQI. In addition, DQIs were built for and applied to differing dietary assessment methods (e.g., FFQ vs. 24 h Recall), with six developed for the specific assessment method they were applied to, such as the DDS developed for the EPIC-Norfolk FFQ. Further, there is increasing interest in use of digital assessment tools and artificial intelligence for dietary assessment. This requires consideration of how this data is captured (e.g., weighed food record vs. FFQ vs. food images) and analysed to determine how and if DQIs could be integrated into these existing platforms and tools [76,77,78]. Hence, for practicality, considering what and how dietary components are measured in a DQI (e.g., serve sizes, grams/day or % of energy) and whether a chosen dietary assessment method adequately captures these data is important for accurate application of a chosen DQI.
Acknowledging that no current DQI meets all optimal criteria for an IBD-specific DQI, it is ideal to select a DQI that meets the most optimal criteria that is appropriate for a chosen study design and reflects current population-based dietary guidelines or dietary pattern literature. For example, an Australian-based feeding trial where food provided is based upon the 2013 Australian Guide to Healthy Eating principles and diet is assessed according to Australian Dietary Guideline serving sizes should use the DGI-2013. This is particularly important when assessing change in dietary intervention trials as it has been demonstrated that DQIs are responsive to measuring dietary change over time where the DQI reflects the dietary pattern implemented in the intervention trial [79].
Further, it is important that the chosen DQI has been evaluated for use in assessing health outcomes of interest [80]. While 71% of DQIs in this review had undergone some form of evaluation, each assessed against different health outcomes (e.g., anthropometry, biomarkers, disease development risk) and none were validated to assess gut-specific health outcomes or outcomes relevant to an IBD population such as clinical and endoscopic disease activity. However, two DQI (ARFS and DDS) have been used with concurrent assessment of gut microbiota and another two DQI (DGI-2013 and DHD Index-2015) had been applied to an IBD population. Assessment of diet quality in IBD is increasingly emergent. In a systematic review assessing diet quality and IBD in adults [18], only one study utilised the Healthy Eating Index-2015 (HEI-2015), the previous version of the Healthy Eating Index-2020 (HEI-2020) included in our review. Of note, there are no scoring or component changes between the HEI-2015 and HEI-2020; instead, it was reviewed to reflect the current 2020–2025 Dietary Guidelines for Americans. With rapidly evolving diet research, food systems and population-based dietary guidelines, it is important to consider the currentness of a DQI to ensure it remains accurate and relevant. The remaining studies in the systematic review utilised DQI that were MED-based, lacking assessment of all food groups or nutrient-focused [18]. No evaluation of any DQI’s suitability or validity for an IBD population was undertaken. Other emerging works have utilised various DQI to assess the inflammatory potential of diet and risk of IBD. Most were primarily nutrient-based, assessing nutrients and random food (e.g., onion, rosemary, pizza) intakes, and were therefore limited in their ability to assess actual diet quality [81,82,83]. Others were DQIs developed for scoring food product nutrition labels [84] or for other diseases such as the cardioprotective diet score [85]. Recently, the dietary index for gut microbiota (DI-GM) has been developed specifically to assess dietary composition that aligns with gut microbiota diversity [86]. The DI-GM was excluded in this review as it does not capture assessment of all food groups, assessing 14 select foods (e.g., chickpeas, cranberries, red meat) chosen for their association with α-diversity and β-diversity indices and changes in specific defined bacteria; however, has been evaluated for construct validity, with the authors postulating the need for further evaluation of its utility for practice [86]. Few DQIs have been applied to therapeutic intervention diets for IBD. Lewis et al. [87] and Haskey et al. [88] used the HEI-2015 to assess diet quality of participants with IBD randomised to follow the Specific Carbohydrate Diet or MED, or Canadian Habitual Diet or MED, respectively, for 12 weeks. The HEI-2015, as previously discussed, is identical to the HEI-2020, which performed reasonably in our review but meets fewer optimal DQI criteria than other globally-applicable DQIs. Further investigation on the validity of current, relevant DQIs to appropriately assess diet quality when investigating gut-specific health outcomes is needed, including consideration of the appropriateness of markers used for microbial diversity and IBD.
In clinical practice, use of a DQI when assessing dietary intake could be considered. If used across multiple timepoints of clinical review, a DQI could provide insight into change in diet quality over time and may measure the impact of and adherence to dietary counselling [79]. However, practically, in these time-limited settings, an optimal DQI meeting recommendations for an IBD-specific DQI may be too time-intensive and with detailed scoring systems. Therefore, it would be important to consider the utility of DQI that meet fewer optimal criteria for an IBD-specific DQI but are more time efficient, or alternative dietary screeners and short tools not included in this review, such as the short Diet Quality Screener [89], REAP-S Dietary Screener Version 2 [90] or Eetscore [91], that may be a more appropriate methods for diet quality assessment in this setting.
This is the first known review to investigate if a suitable DQI for the adult IBD population exists, further strengthened by adaptation of known defined optimal criteria for DQI construction to an IBD population. This provides guidance for IBD diet researchers in selecting appropriate DQI to assess therapeutic IBD diets. Known limitations of a narrative review were minimised by applying systematic literature search strategies including screening of reference lists for further eligible articles. Without specific, defined dietary recommendations for IBD, this review cannot conclusively determine the appropriateness of a DQI for an IBD population. DQIs meeting most optimal criteria for an IBD-specific DQI assessed at minimum all five key food groups as current IBD dietary guidelines suggest. However, assessment for evolving dietary components postulated as important in IBD was not undertaken. Regardless of this lack of dietary recommendations, no DQI has been validated for an IBD population, posing opportunity for development of a validated, IBD-specific DQI.
5. Conclusions
Despite the emerging importance of assessing diet quality in IBD, this review identified that no existing DQI meets all recommended optimal criteria for an IBD-specific DQI. The DGI-2013 meets the most criteria, lacking in variety domain assessment and nonlinear scoring; this is followed by the DHD Index-2015, PHDI and HEIFA-2013, which to differing extents lack in variety and balance domain assessment, scoring structure, valuation and/or weighting. Hence, these DQIs, depending on country, study design and dietary assessment method, may be most appropriate for an IBD population. With no existing DQI validated for an IBD population, further research is required to ascertain an appropriate, validated IBD-specific DQI.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu17142343/s1, Table S1: Full search strategy performed in MEDLINE and EmCare databases on 16 May 2025; Table S2: Full inclusion and exclusion criteria used to determine suitability of articles for literature review during title and abstract, and full-text screening; Table S3: Broad outline of food groups and nutrients included within four dimensions of diet quality for included diet quality indices.
Author Contributions
Conceptualization, A.S.D., E.P.H. and J.A.F.; methodology, A.S.D., E.P.H., J.A.F. and L.J.P.; investigation, L.J.P.; analysis; L.J.P.; writing—original draft preparation, L.J.P.; writing—review and editing, L.J.P., J.A.F., E.P.H., R.V.B. and A.S.D.; supervision, J.A.F., E.P.H., R.V.B. and A.S.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No new data were created for this review. Literature search results and data extracted from articles by the authors are presented in this article and Supplementary Materials.
Acknowledgments
Graphical abstract was created using BioRender, https://www.biorender.com/, (accessed on 15 July 2025).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Halmos, E.P.; Godny, L.; Vanderstappen, J.; Sarbagili-Shabat, C.; Svolos, V. Role of diet in prevention versus treatment of Crohn’s disease and ulcerative colitis. Frontline Gastroenterol. 2024, 15, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Gkikas, K.; Svolos, V.; Hansen, R.; Russell, R.K.; Gerasimidis, K. Take-Home Messages from 20 Years of Progress in Dietary Therapy of Inflammatory Bowel Disease. Ann. Nutr. Metab. 2024, 79, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, S.C.; Bager, P.; Escher, J.; Forbes, A.; Hébuterne, X.; Hvas, C.L.; Joly, F.; Klek, S.; Krznaric, Z.; Ockenga, J.; et al. ESPEN guideline on Clinical Nutrition in Inflammatory Bowel Disease. Clin. Nutr. 2023, 42, 352–379. [Google Scholar] [CrossRef] [PubMed]
- Levine, A.; Rhodes, J.M.; Lindsay, J.O.; Abreu, M.T.; Kamm, M.A.; Gibson, P.R.; Gasche, C.; Silverberg, M.S.; Mahadevan, U.; Boneh, R.S.; et al. Dietary guidance from the international organization for the study of inflammatory bowel diseases. Clin. Gastroenterol. Hepatol. 2020, 18, 1381–1392. [Google Scholar] [CrossRef] [PubMed]
- Hashash, J.G.; Elkins, J.; Lewis, J.D.; Binion, D.G. AGA Clinical Practice Update on Diet and Nutritional Therapies in Patients with Inflammatory Bowel Disease: Expert Review. Gastroenterology 2024, 166, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Qiu, Y.; Yang, H.S.; Li, M.Y.; Zhuang, X.J.; Zhang, S.H.; Feng, R.; Chen, B.L.; He, Y.; Zeng, Z.R.; et al. Systematic review and meta-analysis: Association of a pre-illness Western dietary pattern with the risk of developing inflammatory bowel disease. J. Dig. Dis. 2020, 21, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Peters, V.; Bolte, L.; Schuttert, E.; Andreu-Sánchez, S.; Dijkstra, G.; Weersma, R.; Campmans-Kuijpers, M. Western and carnivorous dietary patterns are associated with greater likelihood of IBD development in a large prospective population-based cohort. J. Crohn’s Colitis 2022, 16, 931–939. [Google Scholar] [CrossRef] [PubMed]
- Narula, N.; Wong, E.C.; Dehghan, M.; Mente, A.; Rangarajan, S.; Lanas, F.; Lopez-Jaramillo, P.; Rohatgi, P.; Lakshmi, P.; Varma, R.P.; et al. Association of ultra-processed food intake with risk of inflammatory bowel disease: Prospective cohort study. Bmj 2021, 374, n1554. [Google Scholar] [CrossRef] [PubMed]
- Godny, L.; Dotan, I. Is the Mediterranean Diet in Inflammatory Bowel Diseases Ready for Prime Time? J. Can. Assoc. Gastroenterol. 2024, 7, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Bouzid, Y.Y.; Wilson, S.M.; Alkan, Z.; Stephensen, C.B.; Lemay, D.G. Lower diet quality associated with subclinical gastrointestinal inflammation in healthy United States adults. J. Nutr. 2024, 154, 1449–1460. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, C.A.; Cannon, G.; Lawrence, M.; Costa Louzada, M.d.; Pereira Machado, P. Ultra-Processed Foods, Diet Quality, and Health Using the NOVA Classification System; FAO: Rome, Italy, 2019; Volume 48. [Google Scholar]
- Fitzpatrick, J.A.; Gibson, P.R.; Taylor, K.M.; Halmos, E.P. Development of Novel High and Low Emulsifier Diets Based upon Emulsifier Distribution in the Australian Food Supply for Intervention Studies in Crohn’s Disease. Nutrients 2024, 16, 1922. [Google Scholar] [CrossRef] [PubMed]
- Alkerwi, A. Diet quality concept. Nutrition 2014, 30, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Burggraf, C.; Teuber, R.; Brosig, S.; Meier, T. Review of a priori dietary quality indices in relation to their construction criteria. Nutr. Rev. 2018, 76, 747–764. [Google Scholar] [CrossRef] [PubMed]
- Australian Institute of Health and Welfare. Australian Diet Quality Index Project; AIHW Cat. No. PHE 85; AIHW: Canberra, ACT, Australia, 2007. [Google Scholar]
- Hlaing-Hlaing, H.; Pezdirc, K.; Tavener, M.; James, E.L.; Hure, A. Diet Quality Indices Used in Australian and New Zealand Adults: A Systematic Review and Critical Appraisal. Nutrients 2020, 12, 3777. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.S.; Cheung, H.C.; McAuley, E.; Ross, L.J.; MacLaughlin, H.L. Quality and validity of diet quality indices for use in Australian contexts: A systematic review. Br. J. Nutr. 2022, 128, 2021–2045. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Zhuang, X.; Zhao, M.; Zhuo, S.; Li, X.; Ma, R.; Li, N.; Liu, C.; Zhu, Y.; Tang, C.; et al. Index-based dietary patterns and inflammatory bowel disease: A systematic review of observational studies. Adv. Nutr. 2021, 12, 2288–2300. [Google Scholar] [CrossRef] [PubMed]
- National Health and Medical Research Council. Australian Dietary Guidelines; National Health and Medical Research Council: Canberra, ACT, Australia, 2013.
- Health Canada. Canada’s Dietary Guidelines; Publication No. 170463; Health Canada: Ottawa, ON, Canada, 2019; Available online: https://food-guide.canada.ca/en/?utm_source=canada-ca-foodguide-en&utm_medium=vurl&utm_campaign=foodguide-2021 (accessed on 15 July 2025).
- U.S. Department of Agriculture; U.S. Department of Human Services. Dietary Guidelines for Americans, 2020–2025, 9th ed.; Dietary Guidelines for Americans: Rockville, MD, USA, 2020. Available online: https://www.dietaryguidelines.gov/ (accessed on 15 July 2025).
- Health Council of the Netherlands. Dutch Dietary Guidelines 2015; Publication No. 2015/24E; Health Council of the Netherlands: The Hague, the Netherlands, 2015. [Google Scholar]
- Public Health England. The Eatwell Guide; Public Health England: London, UK, 2016. Available online: https://www.gov.uk/government/publications/the-eatwell-guide (accessed on 15 July 2025).
- Organisation for Economic Co-Operation and Development; European Union-Joint Research Centre. Handbook on Constructing Composite Indicators: Methodology and User Guide; OECD Publishing: Paris, France, 2008. [Google Scholar] [CrossRef]
- Bekele, T.H.; de Vries, J.H.M.; Feskens, E.J.M.; de Weijer, A.; Brouwer, I.D.; Covic, N.; Trijsburg, L. Development of the Ethiopian Healthy Eating Index (Et-HEI) and evaluation in women of reproductive age. J. Nutr. Sci. 2023, 12, e9. [Google Scholar] [CrossRef] [PubMed]
- Bivoltsis, A.; Trapp, G.S.A.; Knuiman, M.; Hooper, P.; Ambrosini, G.L. Can a Simple Dietary Index Derived from a Sub-Set of Questionnaire Items Assess Diet Quality in a Sample of Australian Adults? Nutrients 2018, 10, 486. [Google Scholar] [CrossRef] [PubMed]
- Brassard, D.; Elvidge Munene, L.-A.; St-Pierre, S.; Guenther, P.M.; Kirkpatrick, S.I.; Slater, J.; Lemieux, S.; Jessri, M.; Haines, J.; Prowse, R.; et al. Development of the Healthy Eating Food Index (HEFI)-2019 measuring adherence to Canada’s Food Guide 2019 recommendations on healthy food choices. Appl. Physiol. Nutr. Metab. 2022, 47, 595–610. [Google Scholar] [CrossRef] [PubMed]
- Bromage, S.; Batis, C.; Bhupathiraju, S.N.; Fawzi, W.W.; Fung, T.T.; Li, Y.; Deitchler, M.; Angulo, E.; Birk, N.; Castellanos-Gutierrez, A.; et al. Development and Validation of a Novel Food-Based Global Diet Quality Score (GDQS). J. Nutr. 2021, 151, 75S–92S. [Google Scholar] [CrossRef] [PubMed]
- Cacau, L.T.; De Carli, E.; de Carvalho, A.M.; Lotufo, P.A.; Moreno, L.A.; Bensenor, I.M.; Marchioni, D.M. Development and Validation of an Index Based on EAT-Lancet Recommendations: The Planetary Health Diet Index. Nutrients 2021, 13, 1698. [Google Scholar] [CrossRef] [PubMed]
- Chaltiel, D.; Adjibade, M.; Deschamps, V.; Touvier, M.; Hercberg, S.; Julia, C.; Kesse-Guyot, E. Programme National Nutrition Sante—Guidelines score 2 (PNNS-GS2): Development and validation of a diet quality score reflecting the 2017 French dietary guidelines. Br. J. Nutr. 2019, 122, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Collins, C.E.; Burrows, T.L.; Rollo, M.E.; Boggess, M.M.; Watson, J.F.; Guest, M.; Duncanson, K.; Pezdirc, K.; Hutchesson, M.J. The comparative validity and reproducibility of a diet quality index for adults: The Australian Recommended Food Score. Nutrients 2015, 7, 785–798. [Google Scholar] [CrossRef] [PubMed]
- Conklin, A.I.; Monsivais, P.; Khaw, K.-T.; Wareham, N.J.; Forouhi, N.G. Dietary diversity, diet cost, and incidence of type 2 diabetes in the United Kingdom: A prospective cohort study. PLoS Med. 2016, 13, e1002085. [Google Scholar] [CrossRef] [PubMed]
- Froud, A.; Murphy, J.; Cribb, L.; Ng, C.H.; Sarris, J. The relationship between dietary quality, serum brain-derived neurotrophic factor (BDNF) level, and the Val66met polymorphism in predicting depression. Nutr. Neurosci. 2019, 22, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Grafenauer, S.J.; Tapsell, L.C.; Beck, E.J.; Batterham, M.J. Development and validation of a Food Choices Score for use in weight-loss interventions. Br. J. Nutr. 2014, 111, 1862–1870. [Google Scholar] [CrossRef] [PubMed]
- Hendrie, G.A.; Baird, D.; Golley, R.K.; Noakes, M. The CSIRO Healthy Diet Score: An Online Survey to Estimate Compliance with the Australian Dietary Guidelines. Nutrients 2017, 9, 47. [Google Scholar] [CrossRef] [PubMed]
- Keaver, L.; Ruan, M.; Chen, F.; Du, M.; Ding, C.; Wang, J.; Shan, Z.; Liu, J.; Zhang, F.F. Plant- and animal-based diet quality and mortality among US adults: A cohort study. Br. J. Nutr. 2021, 125, 1405–1415. [Google Scholar] [CrossRef] [PubMed]
- Kesse-Guyot, E.; Rebouillat, P.; Brunin, J.; Langevin, B.; Allès, B.; Touvier, M.; Hercberg, S.; Fouillet, H.; Huneau, J.-F.; Mariotti, F.; et al. Environmental and nutritional analysis of the EAT-Lancet diet at the individual level: Insights from the NutriNet-Santé study. J. Clean. Prod. 2021, 296, 126555. [Google Scholar] [CrossRef]
- Lazarova, S.V.; Sutherland, J.M.; Jessri, M. Adherence to emerging plant-based dietary patterns and its association with cardiovascular disease risk in a nationally representative sample of Canadian adults. Am. J. Clin. Nutr. 2022, 116, 57–73. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.-Y.; Chen, I.C.; Chan, Y.-C.; Cheong, I.-F.; Wang, Y.-Y.; Jian, Z.-R.; Lee, S.-D.; Chou, C.-C.; Yang, F.L. Novel Healthy Eating Index to Examine Daily Food Guides Adherence and Frailty in Older Taiwanese. Nutrients 2021, 13, 4210. [Google Scholar] [CrossRef] [PubMed]
- Looman, M.; Feskens, E.J.; de Rijk, M.; Meijboom, S.; Biesbroek, S.; Temme, E.H.; de Vries, J.; Geelen, A. Development and evaluation of the Dutch Healthy Diet index 2015. Public Health Nutr. 2017, 20, 2289–2299. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Olmedo, N.; Popkin, B.M.; Taillie, L.S. Association between socioeconomic status and diet quality in Mexican men and women: A cross-sectional study. PLoS ONE 2019, 14, e0224385. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.; Hebden, L.; Rangan, A.; Allman-Farinelli, M. The development, application, and validation of a Healthy eating index for Australian Adults (HEIFA-2013). Nutrition 2016, 32, 432–440. [Google Scholar] [CrossRef] [PubMed]
- Shams-White, M.M.; Pannucci, T.E.; Lerman, J.L.; Herrick, K.A.; Zimmer, M.; Meyers Mathieu, K.; Stoody, E.E.; Reedy, J. Healthy Eating Index-2020: Review and Update Process to Reflect the Dietary Guidelines for Americans, 2020–2025. J. Acad. Nutr. Diet. 2023, 123, 1280–1288. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, M.G.; Milte, C.M.; Crawford, D.; McNaughton, S.A. A Revised Australian Dietary Guideline Index and Its Association with Key Sociodemographic Factors, Health Behaviors and Body Mass Index in Peri-Retirement Aged Adults. Nutrients 2016, 8, 160. [Google Scholar] [CrossRef] [PubMed]
- Trijsburg, L.; Talsma, E.F.; Crispim, S.P.; Garrett, J.; Kennedy, G.; de Vries, J.H.M.; Brouwer, I.D. Method for the Development of WISH, a Globally Applicable Index for Healthy Diets from Sustainable Food Systems. Nutrients 2020, 13, 93. [Google Scholar] [CrossRef] [PubMed]
- Van, D.T.T.; Trijsburg, L.; Do, H.T.P.; Kurotani, K.; Feskens, E.J.M.; Talsma, E.F. Development of the Vietnamese Healthy Eating Index. J. Nutr. Sci. 2022, 11, e45. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.-Q.; Li, F.; Dong, R.-H.; Chen, J.-S.; He, G.-S.; Li, S.-G.; Chen, B. The Development of a Chinese Healthy Eating Index and Its Application in the General Population. Nutrients 2017, 9, 977. [Google Scholar] [CrossRef] [PubMed]
- Susetyowati, S.; Huriyati, E.; Faza, F.; Sanubari, N.D.m.G.; Syauqy, A. Development of a quality eating index and its relationship with nutritional status in adults. J. Public Health Res. 2025, 14, 22799036251329420. [Google Scholar] [CrossRef] [PubMed]
- World Cancer Research Fund; American Institute for Cancer Research. Diet, Nutrition, Physical Activity and Cancer: A Global Perspective; World Cancer Research Fund: London, UK, 2018; Available online: https://www.wcrf.org/wp-content/uploads/2024/11/Summary-of-Third-Expert-Report-2018.pdf (accessed on 15 July 2025).
- Micha, R.; Shulkin, M.L.; Penalvo, J.L.; Khatibzadeh, S.; Singh, G.M.; Rao, M.; Fahimi, S.; Powles, J.; Mozaffarian, D. Etiologic effects and optimal intakes of foods and nutrients for risk of cardiovascular diseases and diabetes: Systematic reviews and meta-analyses from the Nutrition and Chronic Diseases Expert Group (NutriCoDE). PLoS ONE 2017, 12, e0175149. [Google Scholar] [CrossRef] [PubMed]
- Willett, W.; Rockström, J.; Loken, B.; Springmann, M.; Lang, T.; Vermeulen, S.; Garnett, T.; Tilman, D.; DeClerck, F.; Wood, A.; et al. Food in the Anthropocene: The EAT–Lancet Commission on healthy diets from sustainable food systems. Lancet 2019, 393, 447–492. [Google Scholar] [CrossRef] [PubMed]
- Grafenauer, S.; Tapsell, L.; Beck, E.; Batterham, M. Baseline dietary patterns are a significant consideration in correcting dietary exposure for weight loss. Eur. J. Clin. Nutr. 2013, 67, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Afshin, A.; Sur, P.J.; Fay, K.A.; Cornaby, L.; Ferrara, G.; Salama, J.S.; Mullany, E.C.; Abate, K.H.; Abbafati, C.; Abebe, Z. Health effects of dietary risks in 195 countries, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019, 393, 1958–1972. [Google Scholar] [CrossRef] [PubMed]
- Clark, M.A.; Springmann, M.; Hill, J.; Tilman, D. Multiple health and environmental impacts of foods. Proc. Natl. Acad. Sci. USA 2019, 116, 23357–23362. [Google Scholar] [CrossRef] [PubMed]
- Ashton, L.; Williams, R.; Wood, L.; Schumacher, T.; Burrows, T.; Rollo, M.; Pezdirc, K.; Callister, R.; Collins, C. Comparison of Australian Recommended Food Score (ARFS) and Plasma Carotenoid Concentrations: A Validation Study in Adults. Nutrients 2017, 9, 888. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.A.; Collier, F.; Mohebbi, M.; Pasco, J.A.; Shivappa, N.; Hébert, J.R.; Jacka, F.N.; Loughman, A. The associations of butyrate-producing bacteria of the gut microbiome with diet quality and muscle health. Gut Microbiome 2021, 2, e2. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.-Q.; Li, F.; Wu, H.; Wang, Y.-C.; Chen, J.-S.; He, G.-S.; Li, S.-G.; Chen, B. Evaluation of the Validity and Reliability of the Chinese Healthy Eating Index. Nutrients 2018, 10, 114. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Wang, Y.; Zhang, H.; Li, Z.; Zhang, Y.; Nie, J. Higher Chinese Healthy Eating Index Scores are Associated with a Lower Risk of Hyperuricemia: A Large Cross-Sectional Survey Based in China. Am. J. Lifestyle Med. 2023. [Google Scholar] [CrossRef]
- Cui, N.; Ouyang, Y.; Li, Y.; Yang, Y.; Liu, S.; Li, J.; Zhang, C.; Ge, Y.; Huang, S.; Yang, X.; et al. Better adherence to the Chinese Healthy Eating Index is associated with a lower prevalence of metabolic syndrome and its components. Nutr. Res. 2022, 104, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Hendrie, G.A.; Rebuli, M.A.; Golley, R.K. Reliability and relative validity of a diet index score for adults derived from a self-reported short food survey. Nutr. Diet. 2017, 74, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Hendrie, G.A.; Rebuli, M.A.; Golley, R.K.; Noakes, M. Adjustment factors can improve estimates of food group intake assessed using a short dietary assessment instrument. J. Acad. Nutr. Diet. 2018, 118, 1864–1873. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Wang, J.-T.; Su, C.; Miao, Z.; Tang, J.; Ouyang, Y.; Yan, Y.; Jiang, Z.; Fu, Y.; Shuai, M.; et al. Associations of dietary diversity with the gut microbiome, fecal metabolites, and host metabolism: Results from 2 prospective Chinese cohorts. Am. J. Clin. Nutr. 2022, 116, 1049–1058. [Google Scholar] [CrossRef] [PubMed]
- Ward, S.J.; Coates, A.M.; Hill, A.M. Application of an Australian Dietary Guideline Index to Weighed Food Records. Nutrients 2019, 11, 1286. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.E.; Blizzard, L.; Gall, S.L.; Magnussen, C.G.; Oddy, W.H.; Dwyer, T.; Venn, A.J.; Smith, K.J. An age- and sex-specific dietary guidelines index is a valid measure of diet quality in an Australian cohort during youth and adulthood. Nutr. Res. 2019, 65, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Ardalan, Z.S.; Livingstone, K.M.; Polzella, L.; Avakian, J.; Rohani, F.; Sparrow, M.P.; Gibson, P.R.; Yao, C.K. Perceived dietary intolerances, habitual intake and diet quality of patients with an ileoanal pouch: Associations with pouch phenotype (and behaviour). Clin. Nutr. 2023, 42, 2095–2108. [Google Scholar] [CrossRef] [PubMed]
- de Graaf, M.C.G.; Spooren, C.E.G.M.; Hendrix, E.M.B.; Hesselink, M.A.M.; Feskens, E.J.M.; Smolinska, A.; Keszthelyi, D.; Pierik, M.J.; Mujagic, Z.; Jonkers, D.M.A.E. Diet Quality and Dietary Inflammatory Index in Dutch Inflammatory Bowel Disease and Irritable Bowel Syndrome Patients. Nutrients 2022, 14, 1945. [Google Scholar] [CrossRef] [PubMed]
- Castellanos-Gutierrez, A.; Rodriguez-Ramirez, S.; Bromage, S.; Fung, T.T.; Li, Y.; Bhupathiraju, S.N.; Deitchler, M.; Willett, W.; Batis, C. Performance of the Global Diet Quality Score with Nutrition and Health Outcomes in Mexico with 24-h Recall and FFQ Data. J. Nutr. 2021, 151, 143S–151S. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, M.; Birk, N.; Bromage, S.; Bowen, L.; Batis, C.; Fung, T.T.; Li, Y.; Stampfer, M.J.; Deitchler, M.; Willett, W.C.; et al. Validation of global diet quality score among nonpregnant women of reproductive age in India: Findings from the Andhra Pradesh children and parents Study (APCAPS) and the Indian migration Study (IMS). J. Nutr. 2021, 151, 101S–109S. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Liu, Y.; Zhan, B.; Huang, Z.; Deng, G. Association of healthy eating index 2020 and its components with systemic inflammatory biomarkers among US general adults: A large nationwide cross-sectional study. Food Sci. Nutr. 2023, 12, 7212–7222. [Google Scholar] [CrossRef] [PubMed]
- Brassard, D.; Elvidge Munene, L.-A.; St-Pierre, S.; Gonzalez, A.; Guenther, P.M.; Jessri, M.; Vena, J.; Olstad, D.L.; Vatanparast, H.; Prowse, R.; et al. Evaluation of the Healthy Eating Food Index (HEFI)-2019 measuring adherence to Canada’s Food Guide 2019 recommendations on healthy food choices. Appl. Physiol. Nutr. Metab. 2022, 47, 582–594. [Google Scholar] [CrossRef] [PubMed]
- Grech, A.; Rangan, A.; Allman-Farinelli, M. Social determinants and poor diet quality of energy-dense diets of Australian young adults. Healthcare 2017, 5, 70. [Google Scholar] [CrossRef] [PubMed]
- Cacau, L.T.; Benseñor, I.M.; Goulart, A.C.; Cardoso, L.O.; Lotufo, P.A.; Moreno, L.A.; Marchioni, D.M. Adherence to the planetary health diet index and obesity indicators in the Brazilian longitudinal study of adult health (ELSA-Brasil). Nutrients 2021, 13, 3691. [Google Scholar] [CrossRef] [PubMed]
- Bui, L.; Pham, T.; Wang, F.; Guasch-Ferre, M.; Willett, W. P14-090-23 Planetary Health Diet Index and Risk of Total and Cause-Specific Mortality in Two Prospective Cohort Studies. Curr. Dev. Nutr. 2023, 7, 100704. [Google Scholar] [CrossRef]
- Chaltiel, D.; Julia, C.; Adjibade, M.; Touvier, M.; Hercberg, S.; Kesse-Guyot, E. Adherence to the 2017 French dietary guidelines and adult weight gain: A cohort study. PLoS Med. 2019, 16, e1003007. [Google Scholar] [CrossRef] [PubMed]
- Chaltiel, D.; Julia, C.; Chaltiel, R.; Baudry, J.; Touvier, M.; Deschamps, V.; Latino-Martel, P.; Fezeu, L.; Hercberg, S.; Kesse-Guyot, E. Prospective association between adherence to the 2017 French dietary guidelines and risk of death, CVD and cancer in the NutriNet-Santé cohort. Br. J. Nutr. 2022, 127, 619–629. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Lieffers, J.; Bauman, A.; Hanning, R.; Allman-Farinelli, M. The use of smartphone health apps and other mobile health (mHealth) technologies in dietetic practice: A three country study. J. Hum. Nutr. Diet. 2017, 30, 439–452. [Google Scholar] [CrossRef] [PubMed]
- Murai, U.; Tajima, R.; Matsumoto, M.; Sato, Y.; Horie, S.; Fujiwara, A.; Koshida, E.; Okada, E.; Sumikura, T.; Yokoyama, T.; et al. Validation of Dietary Intake Estimated by Web-Based Dietary Assessment Methods and Usability Using Dietary Records or 24-h Dietary Recalls: A Scoping Review. Nutrients 2023, 15, 1816. [Google Scholar] [CrossRef] [PubMed]
- Chotwanvirat, P.; Prachansuwan, A.; Sridonpai, P.; Kriengsinyos, W. Advancements in Using AI for Dietary Assessment Based on Food Images: Scoping Review. J. Med. Internet Res. 2024, 26, e51432. [Google Scholar] [CrossRef] [PubMed]
- McAuley, E.A.; MacLaughlin, H.L.; Hannan-Jones, M.T.; King, N.; Ross, L.J. Effectiveness of diet quality indices in measuring a change in diet quality over time: A systematic review and meta-analysis of randomized controlled trials. Nutr. Rev. 2023, 81, 361–383. [Google Scholar] [CrossRef] [PubMed]
- Wirt, A.; Collins, C.E. Diet quality—What is it and does it matter? Public Health Nutr. 2009, 12, 2473–2492. [Google Scholar] [CrossRef] [PubMed]
- Guevara, M.; Salamanca-Fernández, E.; Miqueleiz, E.; Gavrila, D.; Amiano, P.; Bonet, C.; Rodríguez-Barranco, M.; Huerta, J.M.; Bujanda, L.; Sánchez, M.J.; et al. Inflammatory potential of the diet and incidence of Crohn’s disease and ulcerative colitis in the EPIC-Spain Cohort. Nutrients 2021, 13, 2201. [Google Scholar] [CrossRef] [PubMed]
- Shivappa, N.; Hébert, J.R.; Rashvand, S.; Rashidkhani, B.; Hekmatdoost, A. Inflammatory potential of diet and risk of ulcerative colitis in a case—Control study from Iran. Nutr. Cancer 2016, 68, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Yin, T.; Tu, W.; Li, Y.; Huang, L.; Bai, Y.; Xu, G. Nutrients, diet quality, and dietary patterns in patients with inflammatory bowel disease: A comparative analysis. Nutrients 2024, 16, 3093. [Google Scholar] [CrossRef] [PubMed]
- Meyer, A.; Dong, C.; Chan, S.S.; Touvier, M.; Julia, C.; Huybrechts, I.; Nicolas, G.; Oldenburg, B.; Heath, A.K.; Tong, T.Y.; et al. Dietary index based on the Food Standards Agency nutrient profiling system and risk of Crohn’s disease and ulcerative colitis. Aliment. Pharmacol. Ther. 2024, 59, 558–568. [Google Scholar] [CrossRef] [PubMed]
- Fu, T.; Ye, S.; Sun, Y.; Dan, L.; Wang, X.; Chen, J. Greater adherence to cardioprotective diet can reduce inflammatory bowel disease risk: A longitudinal cohort study. Nutrients 2022, 14, 4058. [Google Scholar] [CrossRef] [PubMed]
- Kase, B.E.; Liese, A.D.; Zhang, J.; Murphy, E.A.; Zhao, L.; Steck, S.E. The Development and Evaluation of a Literature-Based Dietary Index for Gut Microbiota. Nutrients 2024, 16, 1045. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.D.; Sandler, R.S.; Brotherton, C.; Brensinger, C.; Li, H.; Kappelman, M.D.; Daniel, S.G.; Bittinger, K.; Albenberg, L.; Valentine, J.F.; et al. A randomized trial comparing the specific carbohydrate diet to a Mediterranean diet in adults with Crohn’s disease. Gastroenterology 2021, 161, 837–852.e9. [Google Scholar] [CrossRef] [PubMed]
- Haskey, N.; Shim, R.C.; Davidson-Hunt, A.; Ye, J.; Singh, S.; Dieleman, L.A.; Jacobson, K.; Ghosh, S.; Gibson, D.L. Dietary adherence to the Mediterranean diet pattern in a randomized clinical trial of patients with quiescent ulcerative colitis. Front. Nutr. 2022, 9, 1080156. [Google Scholar] [CrossRef] [PubMed]
- Schröder, H.; Arciniega, A.B.; Soler, C.; Covas, M.-I.; Baena-Díez, J.M.; Marrugat, J. Validity of two short screeners for diet quality in time-limited settings. Public Health Nutr. 2012, 15, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Shankar, V.; Thompson, K.H.; Wylie-Rosett, J.; Segal-Isaacson, C. Validation and reliability for the updated REAP-S dietary screener, (Rapid Eating Assessment of Participants, Short Version, v. 2). BMC Nutr. 2023, 9, 88. [Google Scholar] [CrossRef] [PubMed]
- De Rijk, M.G.; Slotegraaf, A.I.; Brouwer-Brolsma, E.M.; Perenboom, C.W.; Feskens, E.J.; De Vries, J.H. Development and evaluation of a diet quality screener to assess adherence to the Dutch food-based dietary guidelines. Br. J. Nutr. 2022, 128, 1615–1625. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).