Serum Magnesium, Prescribed Magnesium Replacement and Cardiovascular Events in Adults with Type 2 Diabetes: A National Cohort Study in U.S. Veterans
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
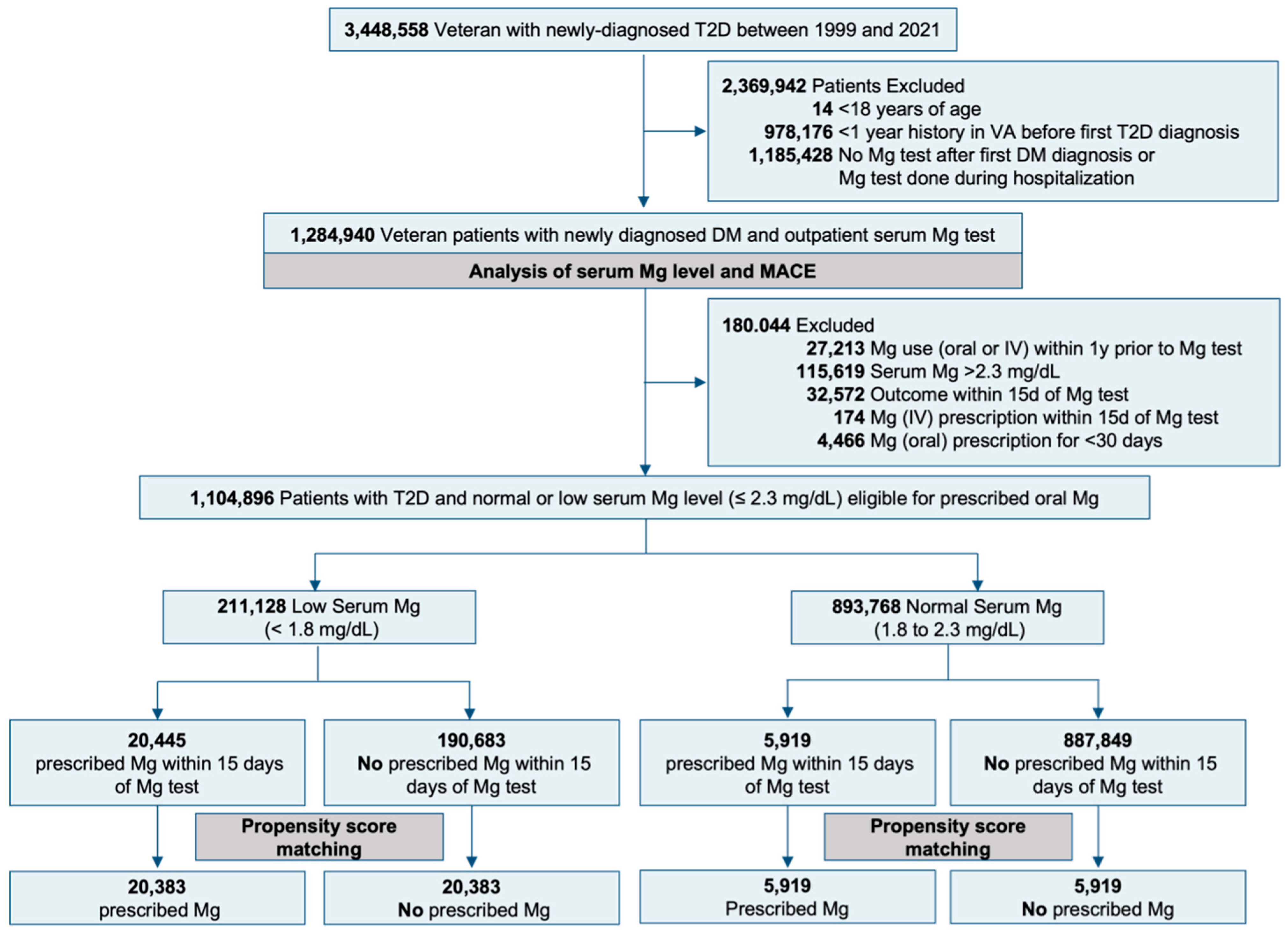
2.2. Eligibility Criteria
2.3. Treatment Strategies
2.4. Outcomes
2.5. Covariates
2.6. Statistical Analysis
2.6.1. Serum Magnesium Level and MACE Outcome
2.6.2. Propensity Score Matching
2.6.3. Sensitivity Analysis
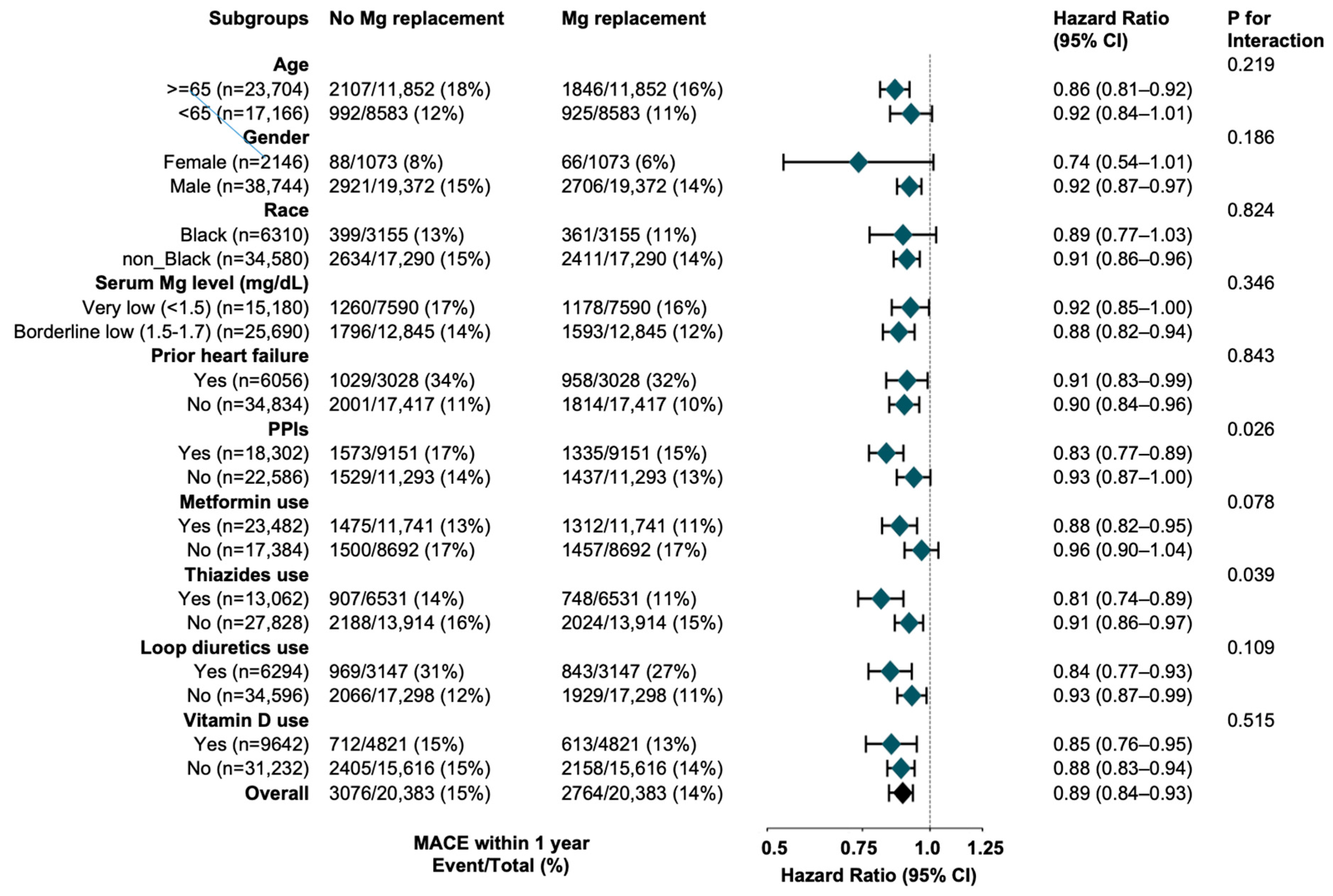
2.6.4. Subgroup Analyses
2.6.5. Software
3. Results
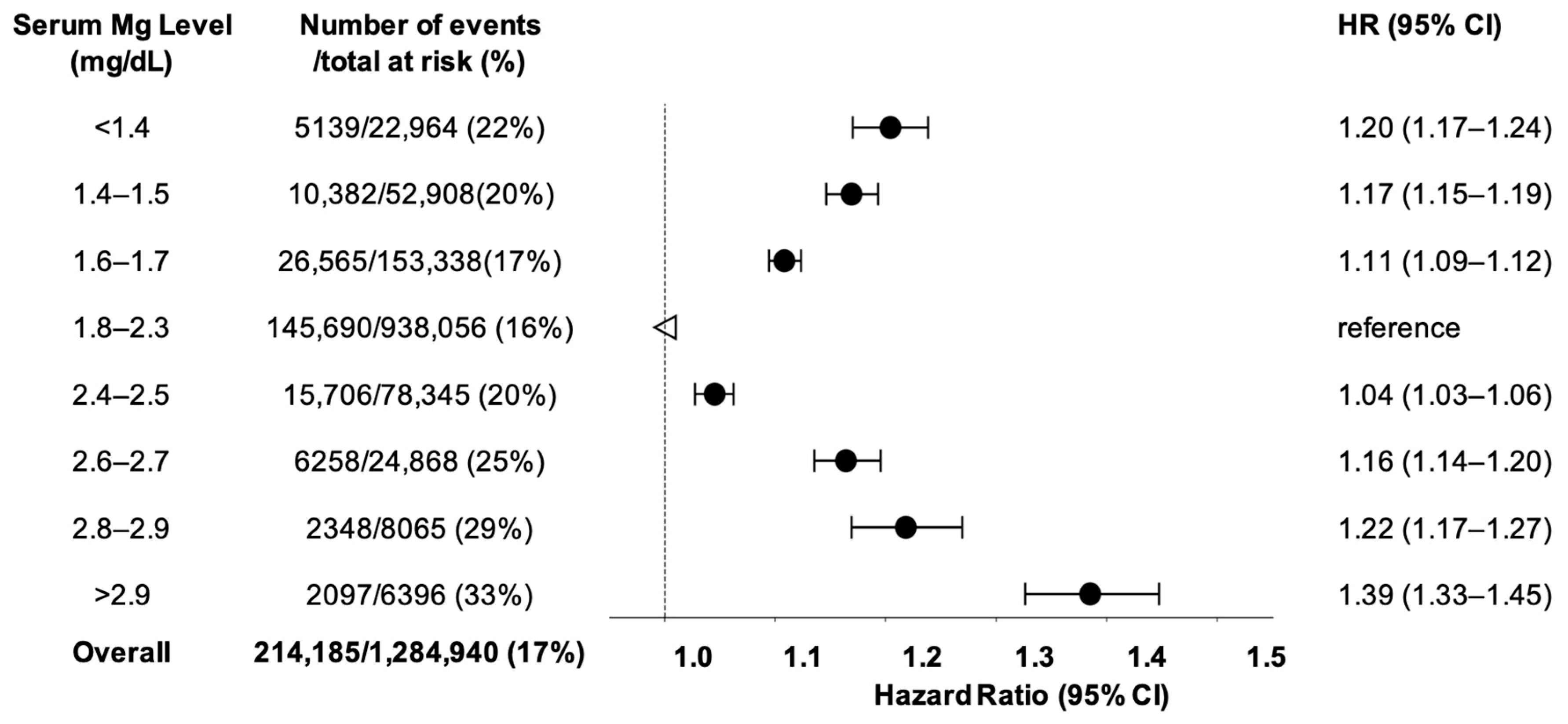
3.1. Serum Magnesium and MACE in Type-2 Diabetes
3.2. Prescribed Oral Magnesium and Outcomes
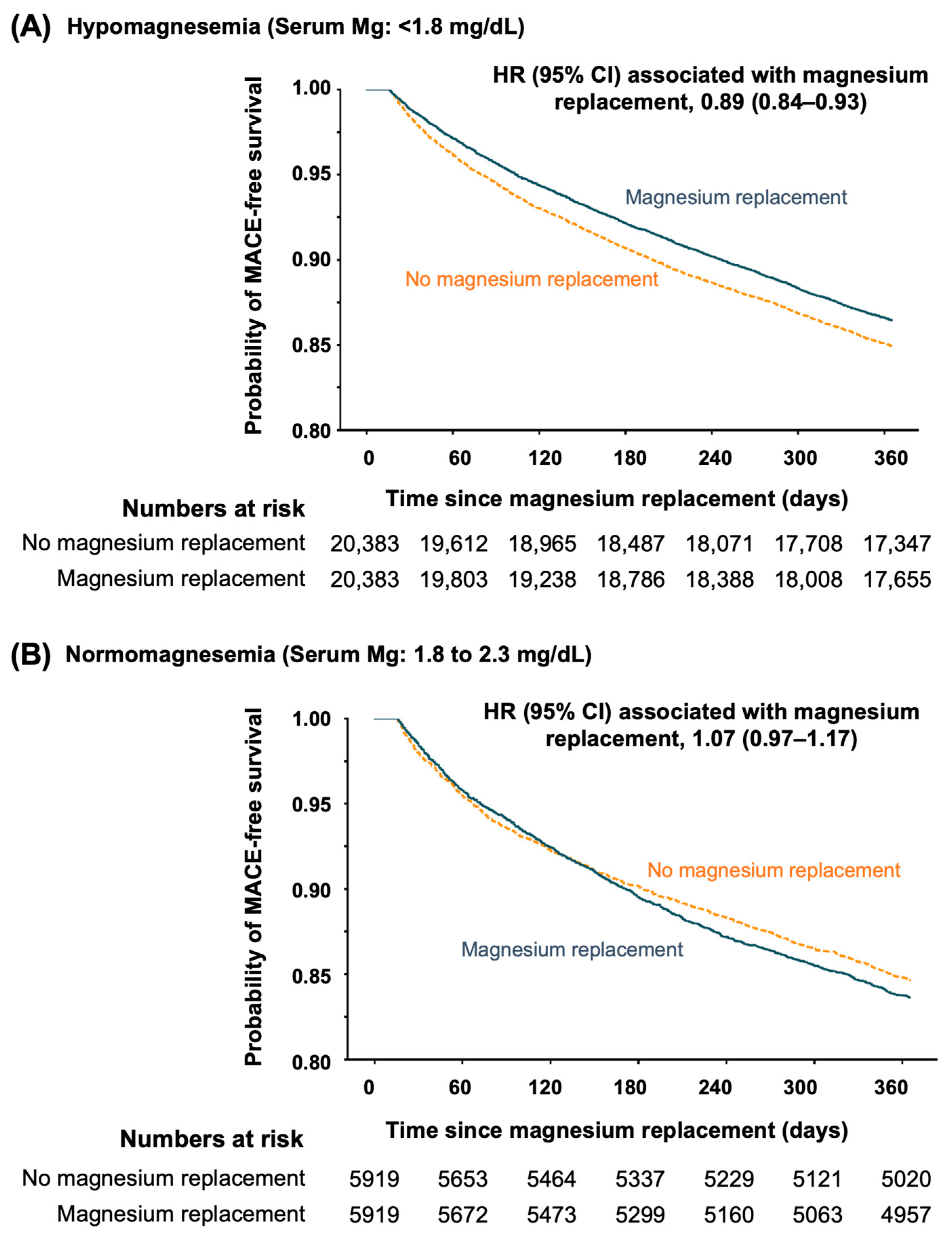
3.2.1. Oral Magnesium in Hypomagnesemia
3.2.2. Oral Magnesium in Normomagnesemia
4. Discussion
4.1. Hypomagnesemia and Cardiovascular Outcomes
4.2. Hypermagnesemia and Cardiovascular Outcomes
4.3. Clinical Implications
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACEI | Angiotensin-Converting Enzyme Inhibitor |
| AMI | Acute Myocardial Infarction |
| ARBs | Angiotensin II Receptor Blockers |
| ASD | Absolute Standardized Differences |
| CDW | Corporate Data Warehouse |
| CI | Confidence Interval |
| COPD | Chronic Obstructive Pulmonary Disease |
| CVD | Cardiovascular Disease |
| EMR | Electronic Medical Record |
| GLP1 | Glucagon-Like Peptide-1 |
| ICD | International Classification of Diseases |
| MACE | Major Adverse Cardiovascular Events |
| MRAs | Mineralocorticoid Receptor Antagonists |
| PCP | Primary Care Physician |
| PPIs | Proton Pump Inhibitors |
| PS | Propensity Scores |
| SGLT2 | Sodium-Glucose Cotransporter-2 |
| VHA | Veterans Health Administration |
| VISN | Veterans Integrated Services Network |
References
- Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride; The National Academies Press: Washington, DC, USA, 1997. [Google Scholar]
- Pham, P.C.; Pham, P.M.; Pham, S.V.; Miller, J.M.; Pham, P.T. Hypomagnesemia in patients with type 2 diabetes. Clin. J. Am. Soc. Nephrol. 2007, 2, 366–373. [Google Scholar] [CrossRef]
- Kandeel, F.R.; Balon, E.; Scott, S.; Nadler, J.L. Magnesium deficiency and glucose metabolism in rat adipocytes. Metabolism 1996, 45, 838–843. [Google Scholar] [CrossRef]
- Suarez, A.; Pulido, N.; Casla, A.; Casanova, B.; Arrieta, F.J.; Rovira, A. Impaired tyrosine-kinase activity of muscle insulin receptors from hypomagnesaemic rats. Diabetologia 1995, 38, 1262–1270. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.Y.; Xun, P.; He, K.; Qin, L.Q. Magnesium intake and risk of type 2 diabetes: Meta-analysis of prospective cohort studies. Diabetes Care 2011, 34, 2116–2122. [Google Scholar] [CrossRef]
- Paolisso, G.; Scheen, A.; D’Onofrio, F.; Lefebvre, P. Magnesium and glucose homeostasis. Diabetologia 1990, 33, 511–514. [Google Scholar] [CrossRef] [PubMed]
- Bosman, W.; Hoenderop, J.G.J.; de Baaij, J.H.F. Genetic and drug-induced hypomagnesemia: Different cause, same mechanism. Proc. Nutr. Soc. 2021, 80, 327–338. [Google Scholar] [CrossRef]
- Barbagallo, M.; Dominguez, L.J.; Galioto, A.; Ferlisi, A.; Cani, C.; Malfa, L.; Pineo, A.; Busardo, A.; Paolisso, G. Role of magnesium in insulin action, diabetes and cardio-metabolic syndrome X. Mol. Asp. Med. 2003, 24, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Chacko, S.A.; Song, Y.; Nathan, L.; Tinker, L.; de Boer, I.H.; Tylavsky, F.; Wallace, R.; Liu, S. Relations of dietary magnesium intake to biomarkers of inflammation and endothelial dysfunction in an ethnically diverse cohort of postmenopausal women. Diabetes Care 2010, 33, 304–310. [Google Scholar] [CrossRef]
- Kostov, K.; Halacheva, L. Role of Magnesium Deficiency in Promoting Atherosclerosis, Endothelial Dysfunction, and Arterial Stiffening as Risk Factors for Hypertension. Int. J. Mol. Sci. 2018, 19, 1724. [Google Scholar] [CrossRef]
- Angkananard, T.; Anothaisintawee, T.; Eursiriwan, S.; Gorelik, O.; McEvoy, M.; Attia, J.; Thakkinstian, A. The association of serum magnesium and mortality outcomes in heart failure patients: A systematic review and meta-analysis. Medicine 2016, 95, e5406. [Google Scholar] [CrossRef]
- Oost, L.J.; van der Heijden, A.; Vermeulen, E.A.; Bos, C.; Elders, P.J.M.; Slieker, R.C.; Kurstjens, S.; van Berkel, M.; Hoenderop, J.G.J.; Tack, C.J.; et al. Serum Magnesium Is Inversely Associated with Heart Failure, Atrial Fibrillation, and Microvascular Complications in Type 2 Diabetes. Diabetes Care 2021, 44, 1757–1765. [Google Scholar] [CrossRef]
- Peters, K.E.; Chubb, S.A.; Davis, W.A.; Davis, T.M. The relationship between hypomagnesemia, metformin therapy and cardiovascular disease complicating type 2 diabetes: The Fremantle Diabetes Study. PLoS ONE 2013, 8, e74355. [Google Scholar] [CrossRef]
- Pikosky, M.; Cifelli, C.; Agarwal, S.; Fulgoni, V.L. Do Americans Get Enough Nutrients from Food? Assessing Nutrient Adequacy with NHANES 2013–2016. Curr. Dev. Nutr. 2019, 3, 1585. [Google Scholar] [CrossRef]
- Asbaghi, O.; Hosseini, R.; Boozari, B.; Ghaedi, E.; Kashkooli, S.; Moradi, S. The Effects of Magnesium Supplementation on Blood Pressure and Obesity Measure Among Type 2 Diabetes Patient: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Biol. Trace Elem. Res. 2021, 199, 413–424. [Google Scholar] [CrossRef] [PubMed]
- An, P.; Wan, S.; Luo, Y.; Luo, J.; Zhang, X.; Zhou, S.; Xu, T.; He, J.; Mechanick, J.I.; Wu, W.C.; et al. Micronutrient Supplementation to Reduce Cardiovascular Risk. J. Am. Coll. Cardiol. 2022, 80, 2269–2285. [Google Scholar] [CrossRef]
- Verma, H.; Garg, R. Effect of magnesium supplementation on type 2 diabetes associated cardiovascular risk factors: A systematic review and meta-analysis. J. Hum. Nutr. Diet. 2017, 30, 621–633. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.C.; Orsini, N.; Wolk, A. Dietary magnesium intake and risk of stroke: A meta-analysis of prospective studies. Am. J. Clin. Nutr. 2012, 95, 362–366. [Google Scholar] [CrossRef]
- Zhao, B.; Zeng, L.; Zhao, J.; Wu, Q.; Dong, Y.; Zou, F.; Gan, L.; Wei, Y.; Zhang, W. Association of magnesium intake with type 2 diabetes and total stroke: An updated systematic review and meta-analysis. BMJ Open 2020, 10, e032240. [Google Scholar] [CrossRef]
- Taveira, T.H.; Ouellette, D.; Gulum, A.; Choudhary, G.; Eaton, C.B.; Liu, S.; Wu, W.C. Relation of Magnesium Intake With Cardiac Function and Heart Failure Hospitalizations in Black Adults: The Jackson Heart Study. Circ. Heart Fail. 2016, 9, e002698. [Google Scholar] [CrossRef]
- Wu, W.C.; Huang, M.; Taveira, T.H.; Roberts, M.B.; Martin, L.W.; Wellenius, G.A.; Johnson, K.C.; Manson, J.E.; Liu, S.; Eaton, C.B. Relationship Between Dietary Magnesium Intake and Incident Heart Failure Among Older Women: The WHI. J. Am. Heart Assoc. 2020, 9, e013570. [Google Scholar] [CrossRef]
- Miller, D.R.; Safford, M.M.; Pogach, L.M. Who has diabetes? Best estimates of diabetes prevalence in the Department of Veterans Affairs based on computerized patient data. Diabetes Care 2004, 27 (Suppl. S2), B10–B21. [Google Scholar] [CrossRef]
- de Lordes Lima, M.; Cruz, T.; Pousada, J.C.; Rodrigues, L.E.; Barbosa, K.; Cangucu, V. The effect of magnesium supplementation in increasing doses on the control of type 2 diabetes. Diabetes Care 1998, 21, 682–686. [Google Scholar] [CrossRef]
- Gagne, J.J.; Glynn, R.J.; Avorn, J.; Levin, R.; Schneeweiss, S. A combined comorbidity score predicted mortality in elderly patients better than existing scores. J. Clin. Epidemiol. 2011, 64, 749–759. [Google Scholar] [CrossRef] [PubMed]
- Rubin, D.B. On principles for modeling propensity scores in medical research. Pharmacoepidemiol. Drug Saf. 2004, 13, 855–857. [Google Scholar] [CrossRef] [PubMed]
- Rubin, D.B. Using propensity score to help design observational studies: Application to the tobacco litigation. Health Serv. Outcomes Res. Methodol. 2001, 2, 169–188. [Google Scholar] [CrossRef]
- Austin, P.C. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat. Med. 2009, 28, 3083–3107. [Google Scholar] [CrossRef]
- Buzkova, P. Competing risk of mortality in association studies of non-fatal events. PLoS ONE 2021, 16, e0255313. [Google Scholar] [CrossRef] [PubMed]
- Austin, P.C.; Lee, D.S.; Fine, J.P. Introduction to the Analysis of Survival Data in the Presence of Competing Risks. Circulation 2016, 133, 601–609. [Google Scholar] [CrossRef]
- Wang, S.V.; Jin, Y.; Fireman, B.; Gruber, S.; He, M.; Wyss, R.; Shin, H.; Ma, Y.; Keeton, S.; Karami, S.; et al. Relative Performance of Propensity Score Matching Strategies for Subgroup Analyses. Am. J. Epidemiol. 2018, 187, 1799–1807. [Google Scholar] [CrossRef]
- Al Alawi, A.M.; Al Shukri, Z.; Al-Busaidi, S.; Al-Maamari, Q.; Al Thihli, M.; Sharji, A.A.; Balushi, R.A.; Al Amri, D.; Falhammar, H.; Al-Maqbali, J.S. Prevalence, clinical characteristics, and health outcomes of dysmagnesemia measured by ionized and total body concentrations among medically hospitalized patients. Sci. Rep. 2024, 14, 23668. [Google Scholar] [CrossRef]
- Rubeiz, G.J.; Thill-Baharozian, M.; Hardie, D.; Carlson, R.W. Association of hypomagnesemia and mortality in acutely ill medical patients. Crit. Care Med. 1993, 21, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Safavi, M.; Honarmand, A. Admission hypomagnesemia--impact on mortality or morbidity in critically ill patients. Middle East J. Anaesthesiol. 2007, 19, 645–660. [Google Scholar] [PubMed]
- Sakaguchi, Y.; Fujii, N.; Shoji, T.; Hayashi, T.; Rakugi, H.; Isaka, Y. Hypomagnesemia is a significant predictor of cardiovascular and non-cardiovascular mortality in patients undergoing hemodialysis. Kidney Int. 2014, 85, 174–181. [Google Scholar] [CrossRef]
- Li, L.; Streja, E.; Rhee, C.M.; Mehrotra, R.; Soohoo, M.; Brunelli, S.M.; Kovesdy, C.P. Hypomagnesemia and Mortality in Incident Hemodialysis Patients. Am. J. Kidney Dis. 2015, 66, 1047–1055. [Google Scholar] [CrossRef] [PubMed]
- Cai, K.; Luo, Q.; Dai, Z.; Zhu, B.; Fei, J.; Xue, C.; Wu, D. Hypomagnesemia Is Associated with Increased Mortality among Peritoneal Dialysis Patients. PLoS ONE 2016, 11, e0152488. [Google Scholar] [CrossRef]
- Gant, C.M.; Soedamah-Muthu, S.S.; Binnenmars, S.H.; Bakker, S.J.L.; Navis, G.; Laverman, G.D. Higher Dietary Magnesium Intake and Higher Magnesium Status Are Associated with Lower Prevalence of Coronary Heart Disease in Patients with Type 2 Diabetes. Nutrients 2018, 10, 307. [Google Scholar] [CrossRef]
- Kostov, K. Effects of Magnesium Deficiency on Mechanisms of Insulin Resistance in Type 2 Diabetes: Focusing on the Processes of Insulin Secretion and Signaling. Int. J. Mol. Sci. 2019, 20, 1351. [Google Scholar] [CrossRef]
- Barbagallo, M.; Dominguez, L.J. Magnesium and type 2 diabetes. World J. Diabetes 2015, 6, 1152–1157. [Google Scholar] [CrossRef]
- Oost, L.J.; Tack, C.J.; de Baaij, J.H.F. Hypomagnesemia and Cardiovascular Risk in Type 2 Diabetes. Endocr. Rev. 2023, 44, 357–378. [Google Scholar] [CrossRef]
- Song, Y.; He, K.; Levitan, E.B.; Manson, J.E.; Liu, S. Effects of oral magnesium supplementation on glycaemic control in Type 2 diabetes: A meta-analysis of randomized double-blind controlled trials. Diabet Med. 2006, 23, 1050–1056. [Google Scholar] [CrossRef]
- Chacko, S.A.; Sul, J.; Song, Y.; Li, X.; LeBlanc, J.; You, Y.; Butch, A.; Liu, S. Magnesium supplementation, metabolic and inflammatory markers, and global genomic and proteomic profiling: A randomized, double-blind, controlled, crossover trial in overweight individuals. Am. J. Clin. Nutr. 2011, 93, 463–473. [Google Scholar] [CrossRef]
- Dickinson, H.O.; Nicolson, D.J.; Campbell, F.; Cook, J.V.; Beyer, F.R.; Ford, G.A.; Mason, J. Magnesium supplementation for the management of essential hypertension in adults. Cochrane Database Syst. Rev. 2006, 3, CD004640. [Google Scholar]
- Sueta, C.A.; Patterson, J.H.; Adams, K.F., Jr. Antiarrhythmic action of pharmacological administration of magnesium in heart failure: A critical review of new data. Magnes. Res. 1995, 8, 389–401. [Google Scholar]
- Sueta, C.A.; Clarke, S.W.; Dunlap, S.H.; Jensen, L.; Blauwet, M.B.; Koch, G.; Patterson, J.H.; Adams, K.F., Jr. Effect of acute magnesium administration on the frequency of ventricular arrhythmia in patients with heart failure. Circulation 1994, 89, 660–666. [Google Scholar] [CrossRef]
- Zehender, M.; Meinertz, T.; Faber, T.; Caspary, A.; Jeron, A.; Bremm, K.; Just, H. Antiarrhythmic effects of increasing the daily intake of magnesium and potassium in patients with frequent ventricular arrhythmias. Magnesium in Cardiac Arrhythmias (MAGICA) Investigators. J. Am. Coll. Cardiol. 1997, 29, 1028–1034. [Google Scholar] [CrossRef]
- Cheungpasitporn, W.; Thongprayoon, C.; Chewcharat, A.; Petnak, T.; Mao, M.A.; Davis, P.W.; Bathini, T.; Vallabha-josyula, S.; Qureshi, F.; Erickson, S.B. Hospital-Acquired Dysmagnesemia and In-Hospital Mortality. Med. Sci. 2020, 8, 37. [Google Scholar] [CrossRef] [PubMed]
- Al Shukri, Z.; Al-Maqbali, J.S.; Al Alawi, A.M.; Al Riyami, N.; Al Riyami, S.; Al Alawi, H.; Al Farai, Q.; Falhammar, H. Incidence of Dysmagnesemia among Medically Hospitalized Patients and Associated Clinical Characteristics: A Prospective Cohort Study. Int. J. Endocrinol. 2023, 2023, 6650620. [Google Scholar] [CrossRef] [PubMed]
- Al Harasi, S.; Al-Maqbali, J.S.; Falhammar, H.; Al-Mamari, A.; Al Futisi, A.; Al-Farqani, A.; Kumar, S.; Osman, A.; Al Riyami, S.; Al Riyami, N.; et al. Prevalence of Dysmagnesemia among Patients with Diabetes Mellitus and the Associated Health Outcomes: A Cross-Sectional Study. Biomedicines 2024, 12, 1068. [Google Scholar] [CrossRef]
- Kothari, M.; Wanjari, A.; Shaikh, S.M.; Tantia, P.; Waghmare, B.V.; Parepalli, A.; Hamdulay, K.F.; Nelakuditi, M. A Comprehensive Review on Understanding Magnesium Disorders: Pathophysiology, Clinical Manifestations, and Management Strategies. Cureus 2024, 16, e68385. [Google Scholar] [CrossRef]
- Vormann, J. Magnesium: Nutrition and metabolism. Mol. Asp. Med. 2003, 24, 27–37. [Google Scholar] [CrossRef]
| Serum Magnesium Level, mg/dL | |||
|---|---|---|---|
| Characteristic, n (%) | Hypomagnesemia (<1.8) | Normomagnesemia (1.8 to 2.3) | Hypermagnesemia (>2.3) |
| (n = 229,210) | (n = 938,056) | (n = 117,674) | |
| Age, Mean (SD), y | 65.7 (10.9) | 65.6 (11.8) | 67.0 (12.0) |
| Sex | |||
| Male | 217,505 (95) | 893,416 (95) | 113,747 (97) |
| Female | 11,705 (5) | 44,640 (5) | 3927 (3) |
| Diabetes Duration, Mean (SD), y | 4.6 (4.5) e | 3.6 (4.0) | 2.9 (3.6) e |
| Serum Magnesium, Mean (SD), mg/dL | 1.6 (0.2) e | 2.0 (0.2) | 2.6 (0.3) |
| Race | |||
| Hispanic | 12,198 (5) | 55,066 (6) | 5750 (5) |
| non-Hispanic White | 154,197 (67) | 608,270 (65) | 75,214 (64) |
| non-Hispanic Black | 41,876 (18) | 179,829 (19) | 23,179 (20) |
| Other/Unknown | 20,939 (9) | 94,891 (10) | 13,531 (11) |
| Marital Status | |||
| Single | 22,028 (10) | 91,298 (10) | 11,332 (10) |
| Divorced/Separated | 66,173 (29) | 258,158 (28) | 31,781 (27) |
| Married | 121,614 (53) | 506,738 (54) | 61,898 (53) |
| Widowed | 19,395 (8) | 81,862 (9) | 12,663 (11) |
| Cormobidities a | |||
| Heart Failure | 35,282 (15) | 143,957 (15) | 25,978 (22) e |
| AMI | 26,147 (11) | 107,176 (11) | 16,347 (14) |
| Ischemic stroke | 22,202 (10) | 88,633 (9) | 12,939 (11) |
| Hemorrhagic stroke | 2126 (1) | 8184 (1) | 1123 (1) |
| Atrial Fibrillation | 30,526 (13) | 117,157 (12) | 18,580 (16) |
| Hyperlipidemia | 183,775 (80) | 745,272 (79) | 92,170 (78) |
| Hypertension | 205,182 (90) e | 803,045 (86) | 101,232 (86) |
| Anemia | 67,440 (29) e | 220,724 (24) | 32,201 (27) |
| Alcohol abuse | 50,968 (22)e | 170,155 (18) | 20,419 (17) |
| Liver Disease | 34,260 (15) e | 100,288 (11) | 10,710 (9) |
| Respiratory Failure | 10,803 (5) | 35,305 (4) | 7128 (6) e |
| COPD | 62,578 (27) | 244,926 (26) | 34,745 (30) |
| Cancer | 75,523 (33) | 287,773 (31) | 36,914 (31) |
| Neuro Disorders | 82,647 (36) | 333,242 (36) | 36,471 (31) |
| Weight Loss | 20,619 (9) | 68,358 (7) | 10,111 (9) |
| Arthritis | 107,185 (47) | 436,185 (46) | 52,159 (44) |
| Gagne Comorbidity Score b | 2.3 (2.5) e | 2.0 (2.4) | 2.4 (2.6) e |
| Comedications c | |||
| Diabetes Medications | |||
| Insulin | 45,470 (20) e | 115,937 (12) | 12,233 (10) |
| Metformin | 108,915 (48) e | 298,678 (32) | 23,799 (20) e |
| GLP1 | 2818 (1) | 6136 (1) | 405 (0) |
| SGLT2 inhibitors | 1857 (1) | 9109 (1) | 971 (1) |
| Other Diabetes Medication | 67,608 (29) e | 196,048 (21) | 22,621 (19) |
| Other Medications | |||
| Thiazides | 64,467 (28) | 233,432 (25) | 30,027 (26) |
| Loop Diuretics | 31,269 (14) | 131,606 (14) | 25,321 (22) e |
| PPIs | 85,687 (37) e | 284,807 (30) | 33,744 (29) |
| Vitamin D | 42,932 (19) | 151,372 (16) | 14,697 (12) e |
| ACEIs | 110,178 (48) e | 398,710 (43) | 51,173 (43) |
| ARBs | 29,014 (13) | 103,785 (11) | 11,864 (10) |
| Other anti-hypertension | 55,274 (24) | 210,594 (22) | 31,094 (26) |
| Selected beta blockers | 76,746 (33) | 276,890 (30) | 39,030 (33) |
| Non-selected beta blockers | 24,755 (11) | 90,112 (10) | 12,629 (11) |
| Digoxin or Other Inotropes | 9124 (4) | 40,934 (4) | 7256 (6) |
| Aspirin | 49,509 (22) | 195,517 (21) | 27,703 (24) |
| Anti-platelet | 18,415 (8) | 72,019 (8) | 10,707 (9) |
| Glucocorticoids | 24,240 (11) | 93,739 (10) | 12,834 (11) |
| Statins | 136,168 (59) | 525,964 (56) | 66,209 (56) |
| Other non-statin lipid lowering | 23,527 (10) | 90,287 (10) | 13,781 (12) |
| Calcium Channel Blocker | 63,174 (28) | 242,155 (26) | 33,705 (29) |
| MRAs | 9951 (4) | 31,537 (3) | 5490 (5) |
| Health examination data d | |||
| HbA1c, Mean (SD), % | 7.4 (1.8) e | 7.0 (1.6) | 6.9 (1.6) e |
| BMI, Mean (SD), kg/m2 | 30.7 (6.8) | 30.8 (6.6) | 29.9 (6.4) e |
| Systolic BP, Mean (SD), mmHg | 134.5 (19.8) | 134.6 (19.1) | 132.4 (20.2) e |
| Diastolic BP, Mean (SD), mmHg) | 76.0 (11.8) | 76.4 (11.8) | 74.2 (12.5) e |
| eGFR, Mean (SD), mL/min/1.73 m2 | 75.1 (24.3) | 74.6 (23.4) | 62.9 (27.1) e |
| Serum Vitamin D, Mean (SD), ng/mL | |||
| <20 | 14,650 (6) | 57,252 (6) | 5535 (5) |
| 20–30 | 21,273 (9) | 88,333 (9) | 8251 (7) |
| 30–100 | 35,754 (16) | 134,419 (14) | 12,611 (11) e |
| Unknown | 157,533 (69) | 658,052 (70) | 91,277 (78) e |
| Serum Sodium, Mean (SD), mEq/L | 137.7 (3.8) e | 138.4 (3.4) | 138.6 (4.5) |
| Serum Potassium, Mean (SD), mEq/L | 4.1 (0.5) e | 4.2 (0.5) | 4.3 (0.6) e |
| Serum Calcium, Mean (SD), mg/dL | 9.1 (0.8) e | 9.2 (0.6) | 9.1 (0.7) |
| LDL cholesterol, Mean (SD), mg/dL | 86.4 (35.3) e | 94.9 (38.8) | 97.0 (37.0) |
| Triglycerides, Mean (SD), mg/dL | 177.0 (119.8) | 170.2 (114.5) | 171.8 (117.3) |
| Cholesterol, Mean (SD), mg/dL | 160.5 (46.0) e | 168.0 (45.3) | 171.4 (50.8) |
| HDL cholesterol, mg/dL | 41.6 (14.1) | 41.7 (12.9) | 41.2 (13.0) |
| Patient Residence | |||
| Rural | 31,967 (14) | 124,048 (13) | 15,866 (13) |
| Urban | 113,071 (49) | 448,385 (48) | 54,226 (46) |
| Unknown | 84,172 (37) | 365,623 (39) | 47,582 (40) |
| PCP visits in the past five year | |||
| 0 | 7511 (3) | 32,228 (3) | 4251 (4) |
| 1–9 | 46,709 (20) | 205,534 (22) | 27,622 (23) |
| 10–19 | 57,848 (25) | 259,443 (28) | 34,360 (29) |
| 20–29 | 47,808 (21) | 191,427 (20) | 23,551 (20) |
| ≥30 | 69,334 (30) | 249,424 (27) | 27,890 (24) |
| Homeless in past year | 10,950 (5) | 44,951 (5) | 4936 (4) |
| Long-Term Care in past two year | 3866 (2) | 14,828 (2) | 2752 (2) |
| Characteristic, n (%) | Before Matching (n = 211,128) | After Matching (n = 40,766) | ||
|---|---|---|---|---|
| Prescribed Magnesium | Prescribed Magnesium | |||
| No | Yes | No | Yes | |
| (n = 190,683) | (n = 20,445) | (n = 20,383) | (n = 20,383) | |
| Age, Mean (SD), y | 65.6 (10.9) | 66.5 (10.1) | 66.6 (10.3) | 66.5 (10.1) |
| Sex | ||||
| Male | 180,965 (95) | 19,372 (95) | 19,339 (95) | 19,311 (95) |
| Female | 9718 (5) | 1073 (5) | 1044 (5) | 1072 (5) |
| Diabetes Duration, Mean (SD), y | 4.5 (4.5) | 5.5 (4.8) e | 5.6 (4.8) | 5.5 (4.8) |
| Serum Magnesium, Mean (SD), mg/dL | 1.6 (0.2) | 1.5 (0.2) e | 1.5 (0.2) | 1.5 (0.2) |
| Race | ||||
| Hispanic | 10,559 (6) | 772 (4) | 713 (3) | 770 (4) |
| non-Hispanic White | 127,468 (67) | 14,812 (72) e | 14,866 (73) | 14,760 (72) |
| non-Hispanic Black | 35,208 (18) | 3155 (15) | 3059 (15) | 3150 (15) |
| Other/Unknown | 17,448 (9) | 1706 (8) | 1745 (9) | 1703 (8) |
| Marital Status | ||||
| Single | 18,498 (10) | 1701 (8) | 1701 (8) | 1697 (8) |
| Divorced/Separated | 55,079 (29) | 5578 (27) | 5509 (27) | 5561 (27) |
| Married | 101,041 (53) | 11,525 (56) | 11,504 (56) | 11,492 (56) |
| Widowed | 16,065 (8) | 1641 (8) | 1669 (8) | 1633 (8) |
| Cormobidities a | ||||
| Heart Failure | 26,785 (14) | 3028 (15) | 3074 (15) | 3019 (15) |
| AMI | 20,489 (11) | 2091 (10) | 2079 (10) | 2084 (10) |
| Ischemic stroke | 17,691 (9) | 1734 (8) | 1756 (9) | 1730 (8) |
| Hemorrhagic stroke | 1659 (1) | 145 (1) | 149 (1) | 145 (1) |
| Atrial Fibrillation | 23,787 (12) | 2888 (14) | 2925 (14) | 2873 (14) |
| Hyperlipidemia | 152,162 (80) | 17,385 (85) e | 17,286 (85) | 17,327 (85) |
| Hypertension | 169,799 (89) | 18,882 (92) e | 18,752 (92) | 18,823 (92) |
| Anemia | 54,195 (28) | 6087 (30) | 6069 (30) | 6063 (30) |
| Alcohol abuse | 41,360 (22) | 4497 (22) | 4516 (22) | 4477 (22) |
| Liver Disease | 27,556 (14) | 3029 (15) | 2888 (14) | 3023 (15) |
| Respiratory Failure | 8394 (4) | 590 (3) | 600 (3) | 590 (3) |
| COPD | 50,423 (26) | 5599 (27) | 5539 (27) | 5579 (27) |
| Cancer | 62,495 (33) | 6359 (31) | 6280 (31) | 6339 (31) |
| Neuro Disorders | 67,338 (35) | 7935 (39) | 7833 (38) | 7909 (39) |
| Weight Loss | 16,505 (9) | 1562 (8) | 1603 (8) | 1557 (8) |
| Arthritis | 87,972 (46) | 10,249 (50) | 10,144 (50) | 10,208 (50) |
| Gagne Comorbidity Score b | 2.2 (2.5) | 2.2 (2.4) | 2.2 (2.4) | 2.2 (2.4) |
| Comedications c | ||||
| Diabetes Medications | ||||
| Insulin | 36,989 (19) | 4719 (23) | 4648 (23) | 4713 (23) |
| Metformin | 89,716 (47) | 11,753 (57) e | 11,704 (57) | 11,712 (57) |
| GLP1 | 2287 (1) | 327 (2) | 322 (2) | 327 (2) |
| SGLT2 inhibitors | 1504 (1) | 207 (1) | 194 (1) | 206 (1) |
| Other Diabetes Medication | 55,719 (29) | 7065 (35) e | 7051 (35) | 7045 (35) |
| Other Medications | ||||
| Thiazides | 53,071 (28) | 6531 (32) | 6459 (32) | 6502 (32) |
| Loop Diuretics | 23,632 (12) | 3147 (15) | 3174 (16) | 3136 (15) |
| PPIs | 68,107 (36) | 9151 (45) e | 8945 (44) | 9100 (45) |
| Vitamin D | 33,566 (18) | 4828 (24) e | 4796 (24) | 4811 (24) |
| ACEIs | 90,817 (48) | 10,466 (51) | 10,397 (51) | 10,433 (51) |
| ARBs | 23,504 (12) | 3134 (15) | 3125 (15) | 3117 (15) |
| Other anti-hypertension | 44,324 (23) | 5310 (26) | 5231 (26) | 5289 (26) |
| Selected beta blockers | 62,014 (33) | 7251 (35) | 7226 (35) | 7231 (35) |
| Non-selected beta blockers | 19,443 (10) | 2458 (12) | 2429 (12) | 2445 (12) |
| Digoxin or Other Inotropes | 7016 (4) | 884 (4) | 920 (5) | 879 (4) |
| Aspirin | 40,048 (21) | 4405 (22) | 4351 (21) | 4386 (22) |
| Anti-platelet | 14,750 (8) | 1684 (8) | 1642 (8) | 1676 (8) |
| Glucocorticoids | 19,131 (10) | 1992 (10) | 1917 (9) | 1985 (10) |
| Statins | 111,767 (59) | 13,462 (66) e | 13,448 (66) | 13,411 (66) |
| Other non-statin lipid lowering | 19,304 (10) | 2292 (11) | 2267 (11) | 2279 (11) |
| Calcium Channel Blocker | 51,103 (27) | 6198 (30) | 6115 (30) | 6172 (30) |
| MRAs | 7251 (4) | 1086 (5) | 1068 (5) | 1081 (5) |
| Health examination data d | ||||
| HbA1c, Mean (SD), % | 7.4 (1.8) | 7.4 (1.6) | 7.3 (1.6) | 7.4 (1.6) |
| BMI, Mean (SD), kg/m2 | 30.7 (6.8) | 31.3 (6.7) | 31.3 (6.9) | 31.3 (6.8) |
| Systolic BP, Mean (SD), mmHg | 134.8 (19.8) | 133.4 (18.8) | 133.3 (19.1) | 133.4 (18.8) |
| Diastolic BP, Mean (SD), mmHg) | 76.0 (11.8) | 75.7 (11.3) | 75.5 (11.3) | 75.7 (11.3) |
| eGFR, Mean (SD), mL/min/1.73 m2 | 75.5 (24.4) | 74.2 (22.2) | 74.0 (23.3) | 74.2 (22.2) |
| Serum Vitamin D, Mean (SD), ng/mL | ||||
| <20 | 11,780 (6) | 1788 (9) e | 1804 (9) | 1784 (9) |
| 20–30 | 17,328 (9) | 2499 (12) e | 2494 (12) | 2492 (12) |
| 30–100 | 28,700 (15) | 4413 (22) e | 4453 (22) | 4389 (22) |
| Unknown | 132,875 (70) | 11,745 (57) e | 11,632 (57) | 11,718 (57) |
| Serum Sodium, Mean (SD), mEq/L | 137.7 (3.7) | 138.1 (3.5) e | 138.1 (3.6) | 138.1 (3.5) |
| Serum Potassium, Mean (SD), mEq/L | 4.1 (0.5) | 4.1 (0.5) | 4.1 (0.5) | 4.1 (0.5) |
| Serum Calcium, Mean (SD), mg/dL | 9.1 (0.8) | 9.2 (0.7) e | 9.2 (0.8) | 9.2 (0.7) |
| LDL cholesterol, Mean (SD), mg/dL | 87.0 (35.3) | 82.2 (34.0) e | 81.8 (33.9) | 82.2 (34.1) |
| Triglycerides, Mean (SD), mg/dL | 177.6 (120.1) | 178.8 (121.3) | 177.9 (119.5) | 178.8 (121.3) |
| Cholesterol, Mean (SD), mg/dL | 161.2 (46.0) | 155.6 (43.7) e | 154.9 (41.1) | 155.6 (43.7) |
| HDL cholesterol, mg/dL | 41.5 (13.8) | 42.3 (15.2) | 42.5 (15.0) | 42.3 (15.2) |
| Patient Residence | ||||
| Rural | 26,004 (14) | 3283 (16) | 3291 (16) | 3264 (16) |
| Urban | 93,229 (49) | 10,263 (50) | 10,284 (50) | 10,232 (50) |
| Unknown | 71,450 (37) | 6899 (34) | 6808 (33) | 6887 (34) |
| PCP visits in the past five year | ||||
| 0 | 6542 (3) | 542 (3) | 545 (3) | 540 (3) |
| 1–9 | 40,173 (21) | 3394 (17) e | 3384 (17) | 3388 (17) |
| 10–19 | 48,662 (26) | 4946 (24) | 5105 (25) | 4932 (24) |
| 20–29 | 39,645 (21) | 4481 (22) | 4341 (21) | 4462 (22) |
| ≥30 | 55,661 (29) | 7082 (35) e | 7008 (34) | 7061 (35) |
| Homeless in past year | 9112 (5) | 807 (4) | 831 (4) | 806 (4) |
| Long-Term Care in past two year | 3080 (2) | 172 (1) | 181 (1) | 172 (1) |
| Before Propensity Score Matching | After Propensity Score Matching | |||||
|---|---|---|---|---|---|---|
| Events (%) by prescribed magnesium in patient with hypomagnesemia | Adjusted HR a (95% CI) | Events (%) by prescribed magnesium in patient with hypomagnesemia | HR b (95% CI) | |||
| No (n = 190,683) | Yes (n = 20,445) | No (n = 20,383) | Yes (n = 20,383) | |||
| MACE | 29,846 (15.7%) | 2772 (13.6%) | 0.93 (0.89–0.96) | 3076 (15.1%) | 2764 (13.6%) | 0.89 (0.84–0.93) |
| All-cause mortality | 14,410 (7.6%) | 1267 (6.2%) | 0.93 (0.88–0.99) | 1452 (7.1%) | 1263 (6.2%) | 0.86 (0.80–0.93) |
| Events (%) by prescribed magnesium in patient with normomagnesemia | Adjusted HR a (95% CI) | Events (%) by prescribed magnesium in patient with normomagnesemia | HR b (95% CI) | |||
| No (n = 887,849) | Yes (n = 5919) | No (n = 5919) | Yes (n = 5919) | |||
| MACE | 115,632 (13.0%) | 970 (16.4%) | 1.14 (1.07–1.21) | 912 (15.4%) | 970 (16.4%) | 1.07 (0.97–1.17) |
| All-cause mortality | 48,537 (5.5%) | 391 (6.6%) | 1.11 (1.01–1.23) | 389 (6.6%) | 391 (6.6%) | 1.00 (0.87–1.15) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, Y.; Cheng, Y.; Zullo, A.R.; Shao, Y.; Sheriff, H.M.; Faselis, C.; Liu, S.; Ahmed, A.; Zeng-Treitler, Q.; Wu, W.-C. Serum Magnesium, Prescribed Magnesium Replacement and Cardiovascular Events in Adults with Type 2 Diabetes: A National Cohort Study in U.S. Veterans. Nutrients 2025, 17, 2067. https://doi.org/10.3390/nu17132067
Yin Y, Cheng Y, Zullo AR, Shao Y, Sheriff HM, Faselis C, Liu S, Ahmed A, Zeng-Treitler Q, Wu W-C. Serum Magnesium, Prescribed Magnesium Replacement and Cardiovascular Events in Adults with Type 2 Diabetes: A National Cohort Study in U.S. Veterans. Nutrients. 2025; 17(13):2067. https://doi.org/10.3390/nu17132067
Chicago/Turabian StyleYin, Ying, Yan Cheng, Andrew R. Zullo, Yijun Shao, Helen M. Sheriff, Charles Faselis, Simin Liu, Ali Ahmed, Qing Zeng-Treitler, and Wen-Chih Wu. 2025. "Serum Magnesium, Prescribed Magnesium Replacement and Cardiovascular Events in Adults with Type 2 Diabetes: A National Cohort Study in U.S. Veterans" Nutrients 17, no. 13: 2067. https://doi.org/10.3390/nu17132067
APA StyleYin, Y., Cheng, Y., Zullo, A. R., Shao, Y., Sheriff, H. M., Faselis, C., Liu, S., Ahmed, A., Zeng-Treitler, Q., & Wu, W.-C. (2025). Serum Magnesium, Prescribed Magnesium Replacement and Cardiovascular Events in Adults with Type 2 Diabetes: A National Cohort Study in U.S. Veterans. Nutrients, 17(13), 2067. https://doi.org/10.3390/nu17132067






