Role of Mediterranean Diet and Ultra-Processed Foods on Sperm Parameters: Data from a Cross-Sectional Study
Highlights
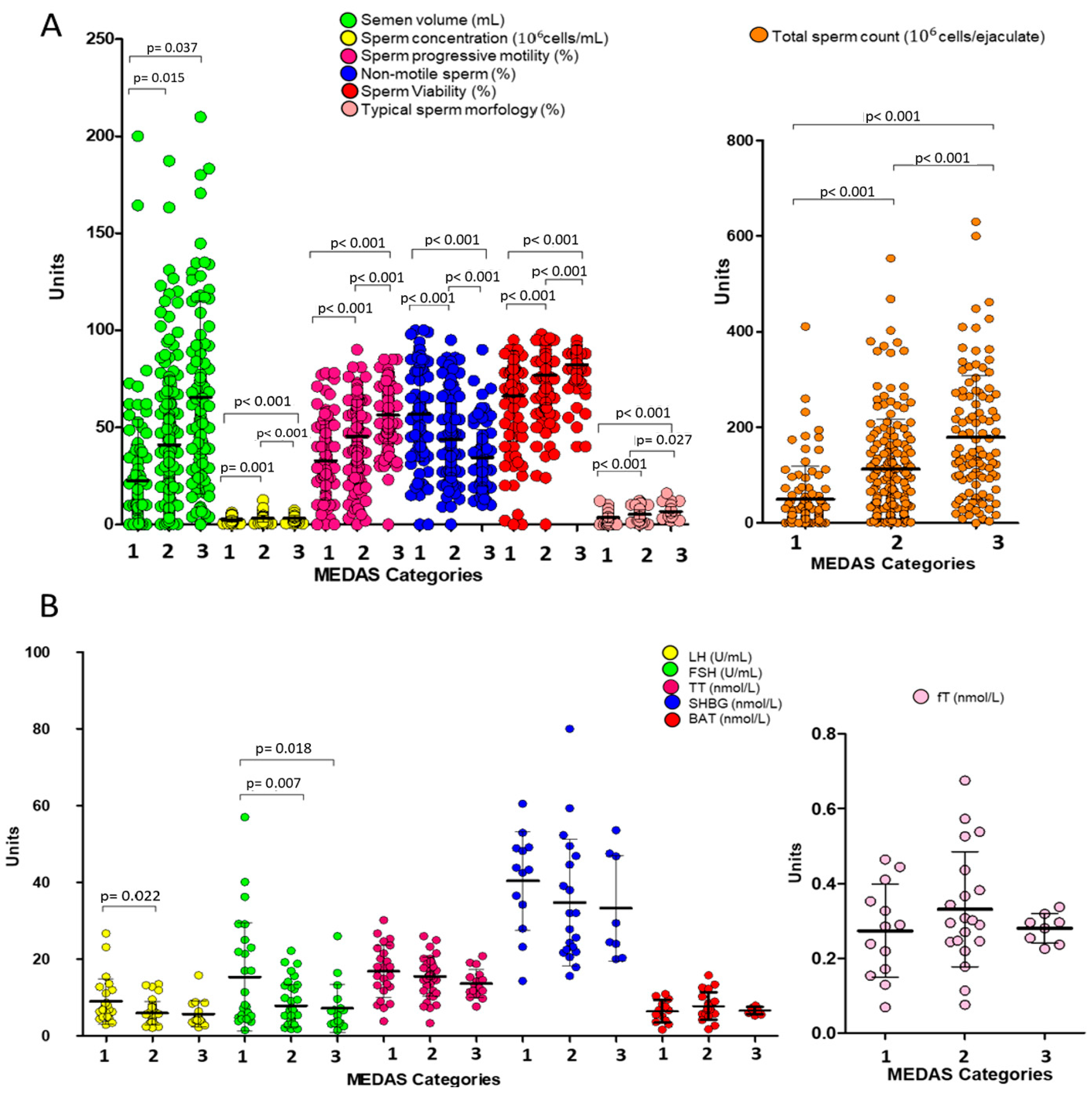
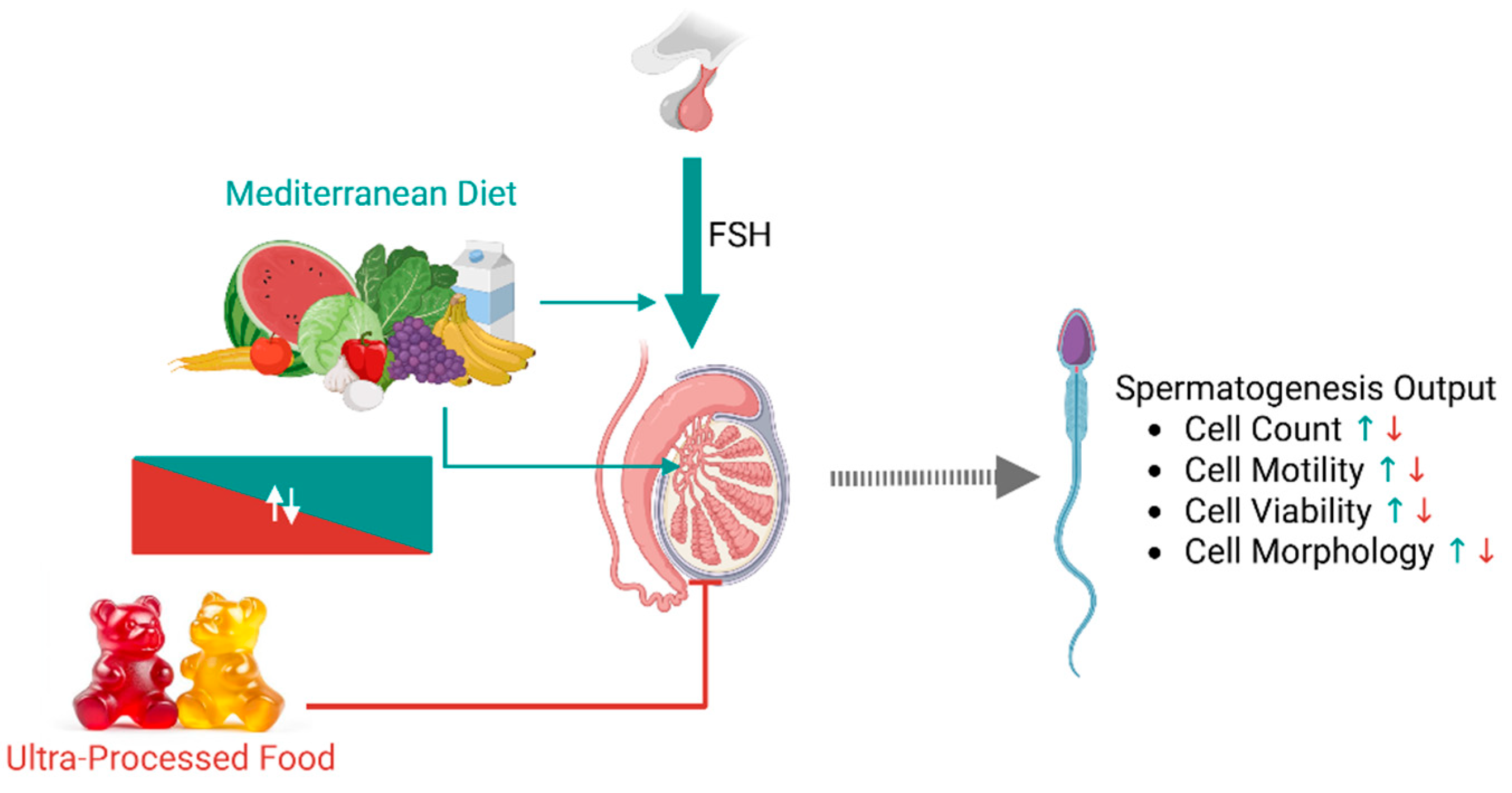
- Greater adherence to the Mediterranean diet (MEDAS value ≥ 6) is positively associated with all key sperm parameters—including concentration, total sperm count, progressive motility, viability, and morphology—even after adjusting for age and BMI.
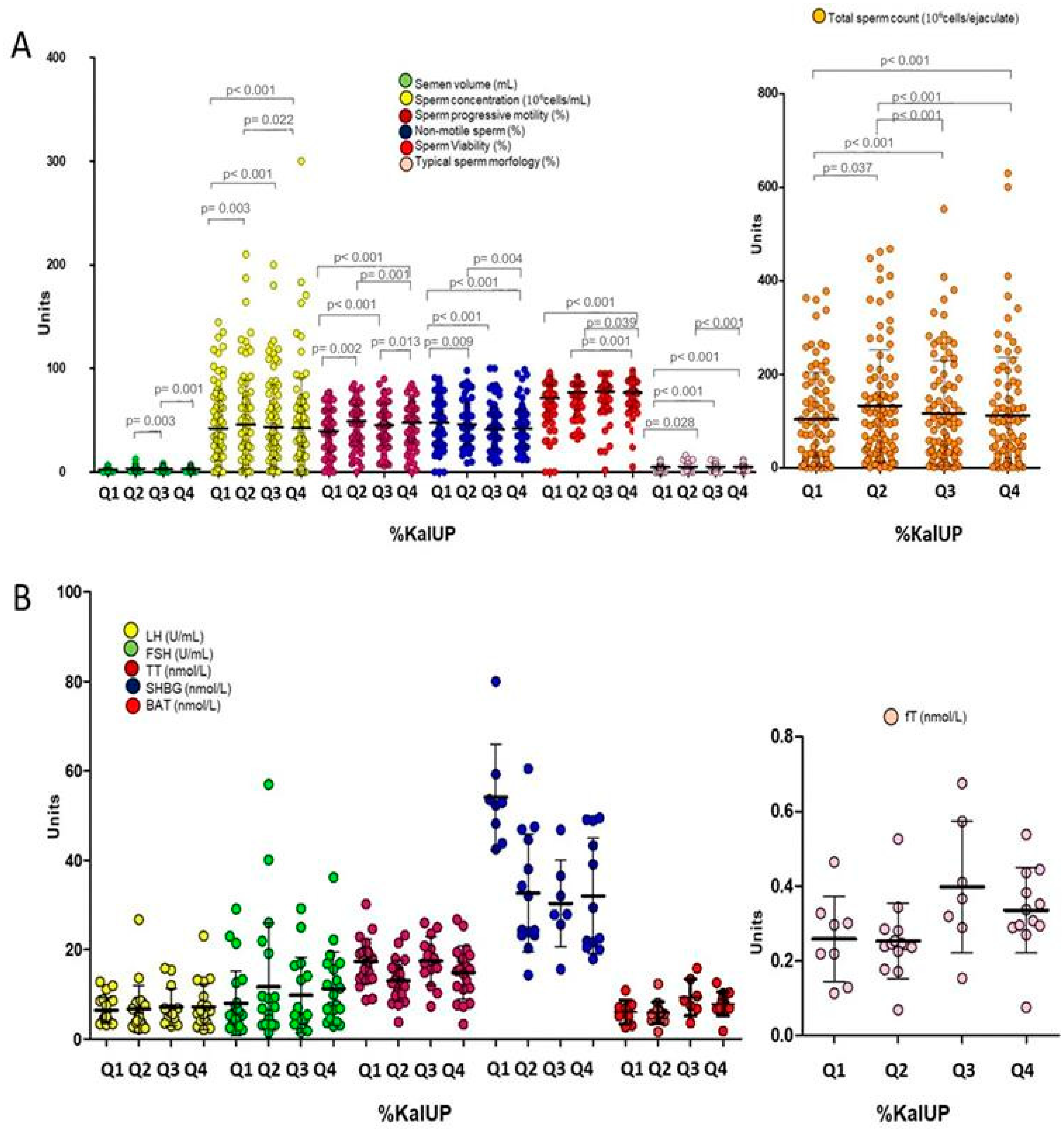
- A higher intake of ultra-processed foods (UPFs) correlates inversely with sperm quality metrics, with the greatest reductions observed in those consuming the largest proportion of calories from UPFs.
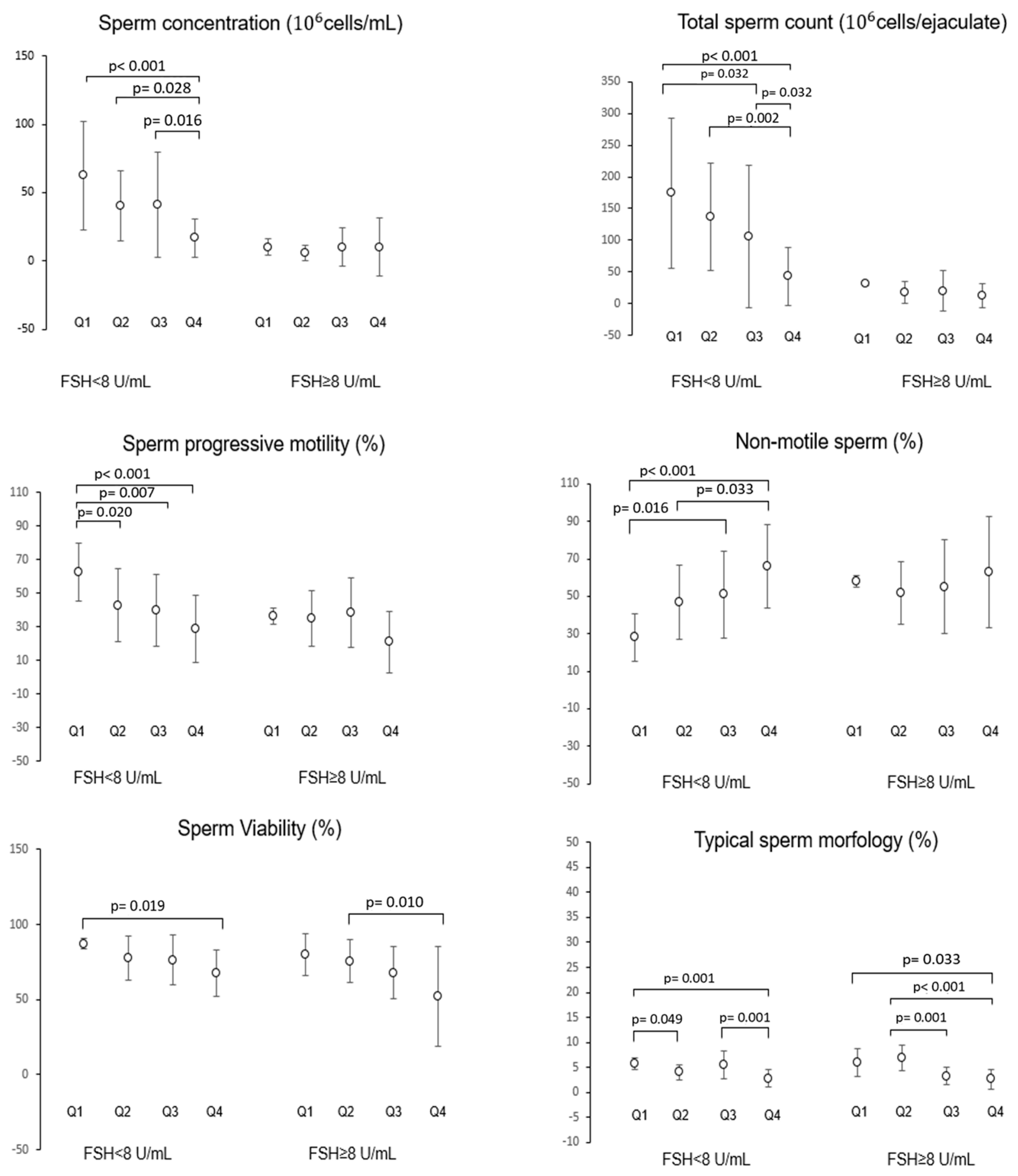
- The impact of diet on semen parameters is modulated by testicular function: men with FSH < 8 IU/mL exhibit stronger associations between their diet and sperm quality (showing a positive impact from Mediterranean diet adherence and a negative effect due to UPF consumption) than those with elevated FSH serum levels.
- Multivariate logistic regression reveals that medium and high adherence to the Mediterranean diet reduces the risk of low total sperm count by 69% and 75%, respectively, whereas UPF intake increases the risk by 249% and 349%, respectively, for medium–low and medium–high consumption.
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Nutritional Habits
2.2. Statistics
3. Results
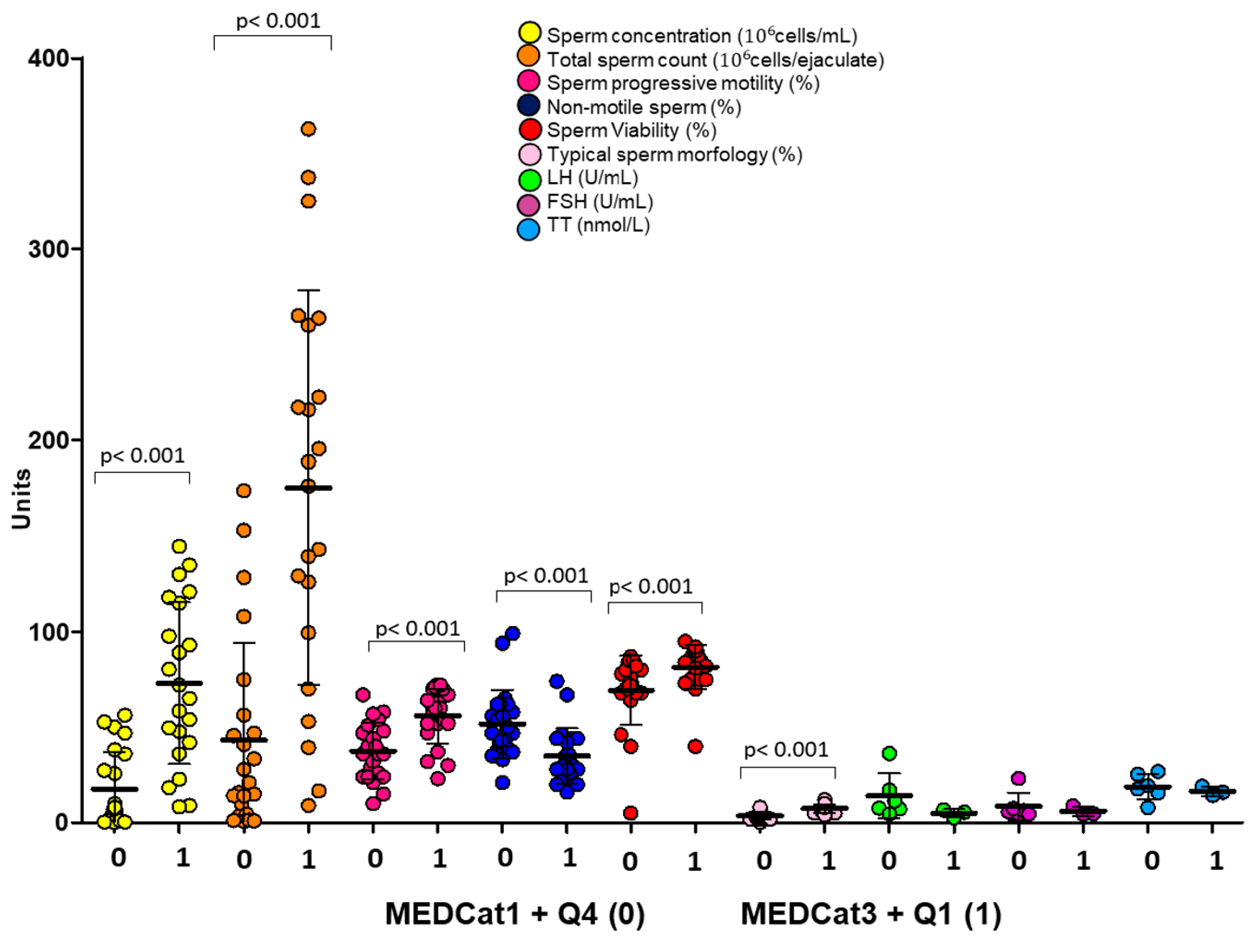
Evaluation of the Possible Effect of the Interaction Between Dietary Regimen and Hormonal Parameters on Semen Parameters by Multivariate Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Jonge, C.J.; Barratt, C.L.R.; Aitken, R.J.; Anderson, R.A.; Baker, P.; Chan, D.Y.L.; Connolly, M.P.; Eisenberg, M.L.; Garrido, N.; Jørgensen, N.; et al. Current Global Status of Male Reproductive Health. Hum. Reprod. Open 2024, 2024, hoae017. [Google Scholar] [CrossRef] [PubMed]
- Levine, H.; Jørgensen, N.; Martino-Andrade, A.; Mendiola, J.; Weksler-Derri, D.; Jolles, M.; Pinotti, R.; Swan, S.H. Temporal Trends in Sperm Count: A Systematic Review and Meta-Regression Analysis of Samples Collected Globally in the 20th and 21st Centuries. Hum. Reprod. Update 2023, 29, 157–176. [Google Scholar] [CrossRef] [PubMed]
- Alesi, S.; Villani, A.; Mantzioris, E.; Takele, W.W.; Cowan, S.; Moran, L.J.; Mousa, A. Anti-Inflammatory Diets in Fertility: An Evidence Review. Nutrients 2022, 14, 3914. [Google Scholar] [CrossRef] [PubMed]
- Garolla, A.; Petre, G.C.; Francini-Pesenti, F.; De Toni, L.; Vitagliano, A.; Di Nisio, A.; Grande, G.; Foresta, C. Systematic Review and Critical Analysis on Dietary Supplements for Male Infertility: From a Blend of Ingredients to a Rationale Strategy. Front. Endocrinol. 2022, 12, 824078. [Google Scholar] [CrossRef]
- Humaidan, P.; Haahr, T.; Povlsen, B.B.; Kofod, L.; Laursen, R.J.; Alsbjerg, B.; Elbaek, H.O.; Esteves, S.C. The Combined Effect of Lifestyle Intervention and Antioxidant Therapy on Sperm DNA Fragmentation and Seminal Oxidative Stress in IVF Patients: A pilot study. Int. Braz. J. Urol. 2022, 48, 131–156. [Google Scholar] [CrossRef]
- De Toni, L.; De Rocco Ponce, M.; Petre, G.C.; Rtibi, K.; Di Nisio, A.; Foresta, C. Bisphenols and Male Reproductive Health: From Toxicological Models to Therapeutic Hypotheses. Front. Endocrinol. 2020, 11, 301. [Google Scholar] [CrossRef]
- Salas-Huetos, A.; Bulló, M.; Salas-Salvadó, J. Dietary Patterns, Foods and Nutrients in Male Fertility Parameters and Fecundability: A Systematic Review of Observational Studies. Hum. Reprod. Update 2017, 23, 371–389. [Google Scholar] [CrossRef]
- Arab, A.; Rafie, N.; Mansourian, M.; Miraghajani, M.; Hajianfar, H. Dietary Patterns and Semen Quality: A Systematic Review and Meta-analysis of Observational Studies. Andrology 2018, 6, 20–28. [Google Scholar] [CrossRef]
- Leisegang, K.; Bouic, P.J.; Menkveld, R.; Henkel, R.R. Obesity Is Associated with Increased Seminal Insulin and Leptin alongside Reduced Fertility Parameters in a Controlled Male Cohort. Reprod. Biol. Endocrinol. 2014, 12, 34. [Google Scholar] [CrossRef]
- Björndahl, L.; Esteves, S.C.; Ferlin, A.; Jørgensen, N.; O’Flaherty, C. Improving Standard Practices in Studies Using Results from Basic Human Semen Examination. Andrology 2023, 11, 1225–1231. [Google Scholar] [CrossRef]
- Petre, G.C.; Francini-Pesenti, F.; Di Nisio, A.; De Toni, L.; Grande, G.; Mingardi, A.; Cusmano, A.; Spinella, P.; Ferlin, A.; Garolla, A. Observational Cross-Sectional Study on Mediterranean Diet and Sperm Parameters. Nutrients 2023, 15, 4989. [Google Scholar] [CrossRef] [PubMed]
- Pecora, G.; Sciarra, F.; Gangitano, E.; Venneri, M.A. How Food Choices Impact on Male Fertility. Curr. Nutr. Rep. 2023, 12, 864–876. [Google Scholar] [CrossRef] [PubMed]
- Oteri, V.; Galeano, F.; Panebianco, S.; Piticchio, T.; Le Moli, R.; Frittitta, L.; Vella, V.; Baratta, R.; Gullo, D.; Frasca, F.; et al. Influence of Mediterranean Diet on Sexual Function in People with Metabolic Syndrome: A Narrative Review. Nutrients 2024, 16, 3397. [Google Scholar] [CrossRef] [PubMed]
- Valle-Hita, C.; Salas-Huetos, A.; Fernández de la Puente, M.; Martínez, M.Á.; Canudas, S.; Palau-Galindo, A.; Mestres, C.; Manzanares, J.M.; Murphy, M.M.; Marquès, M.; et al. Ultra-Processed Food Consumption and Semen Quality Parameters in the Led-Fertyl Study. Hum. Reprod. Open 2024, 2024, hoae001. [Google Scholar] [CrossRef]
- Salvaleda-Mateu, M.; Rodríguez-Varela, C.; Labarta, E. Do Popular Diets Impact Fertility? Nutrients 2024, 16, 1726. [Google Scholar] [CrossRef]
- Ferramosca, A.; Zara, V. Diet and male fertility: The Impact of Nutrients and Antioxidants on Sperm Energetic Metabolism. Int. J. Mol. Sci. 2022, 23, 2542. [Google Scholar] [CrossRef]
- Nätt, D.; Kugelberg, U.; Casas, E.; Nedstrand, E.; Zalavary, S.; Henriksson, P.; Nijm, C.; Jäderquist, J.; Sandborg, J.; Flinke, E.; et al. Human Sperm Displays Rapid Responses to Diet. PLoS Biol. 2019, 17, e3000559. [Google Scholar] [CrossRef]
- Macartney, E.L.; Crean, A.J.; Nakagawa, S.; Bonduriansky, R. Effects of Nutrient Limitation on Sperm and Seminal Fluid: A Systematic Review and Meta-analysis. Biol. Rev. 2019, 94, 1722–1739. [Google Scholar] [CrossRef]
- Caruso, P.; Caputo, M.; Cirillo, P.; Scappaticcio, L.; Longo, M.; Maiorino, M.I.; Bellastella, G.; Esposito, K. Effects of Mediterranean Diet on Semen Parameters in Healthy Young Adults: A Randomized Controlled Trial. Minerva Endocrinol. 2021, 45, 280–287. [Google Scholar] [CrossRef]
- Ventriglio, A.; Sancassiani, F.; Contu, M.P.; Latorre, M.; Di Slavatore, M.; Fornaro, M.; Bhugra, D. Mediterranean Diet and Its Benefits on Health and Mental health: A Literature Review. Clin. Pract. Epidemiol. Ment. Health 2020, 16, 156–164. [Google Scholar] [CrossRef]
- Lane, M.M.; Gamage, E.; Du, S.; Ashtree, D.N.; McGuinness, A.J.; Gauci, S.; Baker, P.; Lawrence, M.; Rebholz, C.M.; Srour, B.; et al. Ultra-Processed Food Exposure and Adverse Health Outcomes: Umbrella Review of Epidemiological Meta-Analyses. BMJ 2024, 384, e077310. [Google Scholar] [CrossRef]
- Zhang, Y.; Giovannucci, E.L. Ultra-Processed Foods and Health: A Comprehensive Review. Crit. Rev. Food Sci. Nutr. 2023, 63, 10836–10848. [Google Scholar] [CrossRef]
- Pagliai, G.; Dinu, M.; Madarena, M.P.; Bonaccio, M.; Iacoviello, L.; Sofi, F. Consumption of Ultra-Processed Foods and Health Status: A Systematic Review and Meta-Analysis. Br. J. Nutr. 2021, 125, 308–318. [Google Scholar] [CrossRef]
- Belladelli, F.; Basran, S.; Eisenberg, M.L. Male Fertility and Physical Exercise. World J. Mens. Health 2023, 41, 482. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen, 6th ed.; World Health Organization, HRP: Geneva, Switzerland, 2021; ISBN 978 92 4 0030787.
- Facondo, P.; Di Lodovico, E.; Pezzaioli, L.C.; Cappelli, C.; Ferlin, A.; Delbarba, A. Usefulness of Routine Assessment of Free Testosterone for the Diagnosis of Functional Male Hypogonadism. Aging Male 2022, 25, 65–71. [Google Scholar] [CrossRef] [PubMed]
- García-Conesa, M.-T.; Philippou, E.; Pafilas, C.; Massaro, M.; Quarta, S.; Andrade, V.; Jorge, R.; Chervenkov, M.; Ivanova, T.; Dimitrova, D.; et al. Exploring the Validity of the 14-Item Mediterranean Diet Adherence Screener (MEDAS): A cross-national study in seven european countries around the mediterranean region. Nutrients 2020, 12, 2960. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, C.A.; Cannon, G.; Levy, R.B.; Moubarac, J.-C.; Louzada, M.L.; Rauber, F.; Khandpur, N.; Cediel, G.; Neri, D.; Martinez-Steele, E.; et al. Ultra-processed foods: What They Are and How to Identify Them. Public Health Nutr. 2019, 22, 936–941. [Google Scholar] [CrossRef]
- Ferlin, A.; Calogero, A.E.; Krausz, C.; Lombardo, F.; Paoli, D.; Rago, R.; Scarica, C.; Simoni, M.; Foresta, C.; Rochira, V.; et al. Management of Male Factor Infertility: Position Statement from the Italian Society of Andrology and Sexual Medicine (SIAMS): Endorsing Organization: Italian Society of Embryology, Reproduction, and Research (SIERR). J. Endocrinol. Investig. 2022, 45, 1085–1113. [Google Scholar] [CrossRef]
- Ferlin, A.; Garolla, A.; Ghezzi, M.; Selice, R.; Palego, P.; Caretta, N.; Di Mambro, A.; Valente, U.; De Rocco Ponce, M.; Dipresa, S.; et al. Sperm Count and Hypogonadism as Markers of General Male Health. Eur. Urol. Focus 2021, 7, 205–213. [Google Scholar] [CrossRef]
- Gianfrilli, D.; Ferlin, A.; Isidori, A.M.; Garolla, A.; Maggi, M.; Pivonello, R.; Santi, D.; Sansone, A.; Balercia, G.; Granata, A.R.M.; et al. Risk Behaviours and Alcohol in Adolescence are Negatively Associated with Testicular Volume: Results from the Amico-andrologo Survey. Andrology 2019, 7, 769–777. [Google Scholar] [CrossRef]
- Service, C.A.; Puri, D.; Al Azzawi, S.; Hsieh, T.-C.; Patel, D.P. The Impact of Obesity and Metabolic Health on Male Fertility: A Systematic Review. Fertil. Steril. 2023, 120, 1098–1111. [Google Scholar] [CrossRef]
- Ricci, E.; Bravi, F.; Noli, S.; Ferrari, S.; De Cosmi, V.; La Vecchia, I.; Cavadini, M.; La Vecchia, C.; Parazzini, F. Mediterranean Diet and the Risk of Poor Semen Quality: Cross-sectional Analysis of Men Referring to an Italian Fertility Clinic. Andrology 2019, 7, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Muffone, A.R.M.C.; de Oliveira Lübke, P.D.P.; Rabito, E.I. Mediterranean Diet and Infertility: A Systematic Review with Meta-Analysis of Cohort Studies. Nutr. Rev. 2023, 81, 775–789. [Google Scholar] [CrossRef]
- Reza Safarinejad, M.; Safarinejad, S. The Roles of Omega-3 and Omega-6 Fatty Acids in Idiopathic Male Infertility. Asian J. Androl. 2012, 14, 514–515. [Google Scholar] [CrossRef] [PubMed]
- Collodel, G.; Castellini, C.; Lee, J.C.-Y.; Signorini, C. Relevance of Fatty Acids to Sperm Maturation and Quality. Oxid. Med. Cell. Longev. 2020, 2020, 1–14. [Google Scholar] [CrossRef]
- Mititelu, M.; Lupuliasa, D.; Neacșu, S.M.; Olteanu, G.; Busnatu Ștefan, S.; Mihai, A.; Popovici, V.; Măru, N.; Boroghină, S.C.; Mihai, S.; et al. Polyunsaturated Fatty Acids and Human Health: A Key to Modern Nutritional Balance in Association with Polyphenolic Compounds from Food Sources. Foods 2024, 14, 46. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, B.; Nourmohamadi, M.; Hajipour, S.; Taghizadeh, M.; Asemi, Z.; Keshavarz, S.A.; Jafarnejad, S. The Effect of Omega-3 Fatty Acids, EPA, and/or DHA on Male Infertility: A Systematic Review and Meta-Analysis. J. Diet. Suppl. 2019, 16, 245–256. [Google Scholar] [CrossRef]
- La Vignera, S.; Basile, L. Diet and Prostate Health: An Underrated Tool? Aging Male 2022, 25, 67–71. [Google Scholar] [CrossRef]
- Gupta, N.; Patel, H.D.; Taylor, J.; Borin, J.F.; Jacobsohn, K.; Kenfield, S.A.; Eggener, S.E.; Price, C.; Davuluri, M.; Byrne, N.; et al. Systematic Review of the Impact of a Plant-Based Diet on Prostate Cancer Incidence and Outcomes. Prostate Cancer Prostatic Dis. 2022, 25, 444–452. [Google Scholar] [CrossRef]
- Vanderhout, S.M.; Panah, M.R.; Garcia-Bailo, B.; Grace-Farfaglia, P.; Samsel, K.; Dockray, J.; Jarvi, K.; El-Sohemy, A. Nutrition, Genetic Variation and Male Fertility. Transl. Androl. Urol. 2021, 10, 1410–1431. [Google Scholar] [CrossRef]
- Shukla, S.; Shrivastava, D. Nutritional deficiencies and subfertility: A Comprehensive Review of Current Evidence. Cureus 2024, 16, e66477. [Google Scholar] [CrossRef]
- Wong, W.Y.; Thomas, C.M.G.; Merkus, J.M.W.M.; Zielhuis, G.A.; Steegers-Theunissen, R.P.M. Male Factor Subfertility: Possible Causes and the Impact of Nutritional Factors. Fertil. Steril. 2000, 73, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Karayiannis, D.; Kontogianni, M.D.; Mendorou, C.; Douka, L.; Mastrominas, M.; Yiannakouris, N. Association between Adherence to the Mediterranean Diet and Semen Quality Parameters in Male Partners of Couples Attempting Fertility. Hum. Reprod. 2016, 32, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Peng, Y.; Jiang, Z.; Quan, Z. Effectiveness of Exercise Interventions on Sperm Quality: A Systematic Review and Network Meta-Analysis. Front. Endocrinol. 2025, 16, 1537271. [Google Scholar] [CrossRef] [PubMed]
- Bingham, S.A. Limitations of the Various Methods for Collecting Dietary Intake Data. Ann. Nutr. Metab. 1991, 35, 117–127. [Google Scholar] [CrossRef]
- Lim, E.J.K.; Ong, C.; Said Abdul Rashid, N.S.; Lee, J.J.-M.; Chew, J.; Chua, M.C. Food Accessibility and Nutritional Outcomes Among Food-Insecure Pregnant Women in Singapore. Nutrients 2025, 17, 835. [Google Scholar] [CrossRef]
- Tu, S.J.; Gallagher, C.; Elliott, A.D.; Bradbury, K.E.; Marcus, G.M.; Linz, D.; Pitman, B.M.; Middeldorp, M.E.; Hendriks, J.M.; Lau, D.H.; et al. Associations of dietary patterns, ultra-processed food and nutrient intake with incident atrial fibrillation. Heart 2023, 109, 1683. [Google Scholar] [CrossRef]
- Greenberg, I.; Stampfer, M.J.; Schwarzfuchs, D.; Shai, I.; DIRECT Group. Adherence and success in long-term weight loss diets: The dietary intervention randomized controlled trial (DIRECT). J. Am. Coll. Nutr. 2009, 28, 159. [Google Scholar] [CrossRef]

| Parameter (Unit; Reference Values) | N | Mean ± SD | Min–Max |
|---|---|---|---|
| BMI (Kg/m2; 18.5–25 kg/m2) | 358 | 24.4 ± 4.2 | 18.4–46.0 |
| Age (years; N/A) | 358 | 34.6 ± 9.3 | 18–67 |
| MEDAS (N/A) | 358 | 7.5 ± 2.8 | 2.5–13 |
| Total Calories (Kcals; N/A) | 358 | 1923.8 ± 344.9 | 1372–3965 |
| Kcals from UPFs (%; N/A) | 358 | 29.1 ± 21.9 | 0.64–96.6 |
| Proteins Kcals from UPFs (%; N/A) | 358 | 3.8 ± 2.7 | 0.1–9.7 |
| Lipids Kcals from UPFs (%; N/A) | 358 | 8.1 ± 17.4 | 0.16–28.9 |
| Carbohydrates Kcals from UPFs (%; N/A) | 358 | 17.4 ± 13.0 | 0.3–57.9 |
| Sperm Volume (mL; >1.4 mL) | 358 | 2.9 ± 1.5 | 0.2–12.5 |
| Sperm pH (>7.2 and <8.0) | 358 | 7.6 ± 0.2 | 6.4–8.0 |
| Sperm Concentration (106 cells/mL; >16 × 106 cells/mL) | 358 | 43.5 ± 42.3 | n.d.–300.0 |
| Sperm Count (106 cells; >39 × 106 cells) | 358 | 116.6 ± 114.9 | n.d.–629.8 |
| Progressive Motility (%; >30%) | 358 | 45.5 ± 20.7 | 0.0–90.0 |
| Non-motile Sperm (%; <20%) | 358 | 44.2 ± 20.5 | 9.0–100.0 |
| Sperm Viability (%; >54%) | 358 | 75.8 ± 17.5 | 2.0–98.0 |
| Sperm Typical Morphology (%; >4%) | 358 | 5.2 ± 2.7 | 0.0–16.0 |
| FSH (U/mL; 1.5–9.4 U/mL) | 80 | 10.2 ± 9.9 | 1.4–57 |
| LH (U/mL; 1.5–9.4 U/mL) | 76 | 6.8 ± 4.4 | 2.1–26.7 |
| SHBG (nmol/L; 10–57 nmol/L) | 41 | 36.2 ± 14.9 | 14.3–80.0 |
| TT (nmoL/L; >10.4 nmol/L) | 78 | 15.5 ± 5.5 | 3.3–30.2 |
| BAT (nmoL/L; >2.4 nmol/L) | 45 | 7.1 ± 3.1 | 1.6–19.42 |
| fT (pmol/L; 229–1072 pmol/L) | 41 | 302.7 ± 130.4 | 70.0–680.0 |
| MEDAS | %Kal-UP | FSH | MEDAS | MEDAS | MEDAS | |
|---|---|---|---|---|---|---|
| Controlled for FSH | Controlled for %Kal-UP | Controlled for BMI | ||||
| Demographic/Anthropometric Data | ||||||
| BMI | r = −0.088 p = 0.096 | r = 0.018 p = 0.736 | r = 0.191 p = 0.090 | r = 0.091 p = 0.863 | r = −0.498 p = 0.315 | // |
| Age | r = −0.009 p = 0.864 | r = −0.075 p = 0.158 | r = 0.262 p = 0.019 | r = −0.138 p = 0.795 | r = −0.454 p = 0.366 | r = 0.337 p = 0.414 |
| Nutritional Data | ||||||
| MEDAS | // | r = −0.813 p < 0.001 | r = −0.301 p = 0.007 | // | r = −0.483 p = 0.331 | // |
| Tot Kcals | r = −0.146 p = 0.006 | r = 0.026 p = 0.622 | r = 0.155 p = 0.169 | r = −0.170 p = 0.747 | // | r = −0.102 p = 0.530 |
| %Kal-UP | r = −0.813 p < 0.000 | // | r = 0.217 p = 0.053 | r = −0.270 p = 0.605 | // | r = −0.770 p < 0.001 |
| %Prot Kal-UP | r = −0.803 p < 0.001 | r = 0.960 p < 0.001 | r = 0.253 p = 0.023 | r = −0.218 p = 0.677 | r = 0.207 p = 0.694 | r = −0.810 p < 0.001 |
| %Lip Kal-UP | r = −0.800 p < 0.001 | r = 0.998 p < 0.001 | r = 0.212 p = 0.059 | r = −0.256 p = 0.625 | r = 0.215 p = 0.682 | r = −0.751 p < 0.001 |
| %Cho Kal-UP | r = −0.814 p < 0.001 | r = 0.999 p < 0.001 | r = 0.211 p = 0.060 | r = −0.291 p = 0.576 | r = 0.242 p = 0.644 | r = −0.764 p < 0.001 |
| Semen Parameters | ||||||
| Semen volume | r = 0.141 p = 0.008 | r = −0.158 p = 0.003 | r = 0.000 p = 1.000 | r = 0.776 p = 0.070 | r = 0.493 p = 0.320 | r = 0.137 p = 0.009 |
| Semen pH | r = −0.059 p = 0.008 | r = 0.084 p = 0.113 | r = −0.003 p = 0.982 | r = 0.594 p = 0.214 | r = −0.069 p = 0.897 | r = −0.057 p = 0.286 |
| Sperm concentration | r = 0.416 p < 0.001 | r = −0.302 p < 0.001 | r = −0.360 p = 0.001 | r = −0.772 p = 0.072 | r = 0.090 p = 0.865 | r = 0.725 p = 0.042 |
| Sperm count | r = 0.456 p = 0.000 | r = −0.361 p < 0.001 | r = −0.394 p < 0.001 | r = −0.325 p = 0.530 | r = 0.460 p = 0.359 | r = 0.835 p = 0.010 |
| Progressive motility | r = 0.431 p < 0.001 | r = −0.365 p < 0.001 | r = −0.308 p = 0.005 | r = −0.085 p = 0.873 | r = 0.432 p = 0.392 | r = 0.721 p = 0.043 |
| Non motile sperm | r = −0.390 p < 0.001 | r = 0.322 p < 0.001 | r = 0.276 p = 0.013 | r = −0.024 p = 0.965 | r = −0.506 p = 0.306 | r = −0.768 p = 0.021 |
| Sperm viability | r = 0.349 p < 0.001 | r = −0.248 p < 0.001 | r = −0.430 p < 0.001 | r = 0.150 p = 0.777 | r = 0.753 p = 0.084 | r = 0.781 p = 0.022 |
| Sperm morphology | r = 0.468 p < 0.001 | r = −0.346 p < 0.001 | r = −0.260 p = 0.020 | r = −0.308 p = 0.552 | r = 0.284 p = 0.585 | r = 0.342 p = 0.010 |
| Hormonal Parameters | ||||||
| FSH | r = −0.301 p = 0.007 | r = 0.217 p = 0.053 | // | // | r = −0.811 p = 0.050 | r = −0.745 p = 0.034 |
| LH | r = −0.275 p = 0.16 | r = 0.164 p = 0.158 | r = 0.838 p < 0.001 | r = −0.008 p = 0.988 | r = −0.660 p = 0.153 | r = −0.739 p = 0.036 |
| SHBG | r = −0.116 p = 0.472 | r = 0.166 p = 0.299 | r = 0.241 p = 0.134 | r = 0.163 p = 0.758 | r = 0.380 p = 0.457 | r = 0.635 p = 0.090 |
| TT | r = −0.171 p = 0.135 | r = 0.158 p = 0.166 | r = −0.071 p = 0.543 | r = −0.352 p = 0.493 | r = 0.384 p = 0.452 | r = 0.711 p = 0.048 |
| BAT | r = 0.062 p = 0.702 | r = −0.141 p = 0.379 | r = −0.316 p = 0.047 | r = −0.037 p = 0.822 | r = −0.069 p = 0.670 | r = 0.014 p = 0.933 |
| fT | r = 0.062 p = 0.699 | r = −0.141 p = 0.378 | r = −0.316 p = 0.047 | r = −0.036 p = 0.826 | r = −0.069 p = 0.674 | r = 0.013 p = 0.936 |
| Model | Variables | Odd Ratio | (95% C.I) | p Value |
|---|---|---|---|---|
| Model 1 Excluding FSH | MEDCat 1 | Reference | // | 0.003 |
| MEDCat 2 | 0.312 | (0.158–0.618) | 0.001 | |
| MEDCat 3 | 0.250 | (0.073–0.856) | 0.027 | |
| %Kal-UP Q1 | Reference | // | 0.045 | |
| %Kal-UP Q2 | 1.833 | (0.592–5.674) | 0.293 | |
| %Kal-UP Q3 | 4.469 | (1.269–15.740) | 0.020 | |
| %Kal-UP Q4 | 3.940 | (1.050–14.786) | 0.042 | |
| Constant | 0.435 | 0.220 | ||
| Model 2 Including FSH ≥ 8 U/mL | MEDCat 1 | Reference | // | 0.131 |
| MEDCat 2 | 0.512 | (0.070–3.761) | 0.510 | |
| MEDCat 3 | 0.043 | (0.002–1.100) | 0.057 | |
| %Kal-UP Q1 | Reference | // | 0.626 | |
| % Kal-UP Q2 | 0.232 | (0.017–3.136) | 0.272 | |
| % Kal-UP Q3 | 0.482 | (0.029–8.053) | 0.612 | |
| % Kal-UP Q4 | 0.428 | (0.017–11.107) | 0.610 | |
| FSH ≥ 8 U/mL | 9.761 | (2.612–36.482) | 0.001 | |
| Constant | 3.494 | 0.457 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petre, G.C.; Francini-Pesenti, F.; De Toni, L.; Di Nisio, A.; Mingardi, A.; Cosci, I.; Passerin, N.; Ferlin, A.; Garolla, A. Role of Mediterranean Diet and Ultra-Processed Foods on Sperm Parameters: Data from a Cross-Sectional Study. Nutrients 2025, 17, 2066. https://doi.org/10.3390/nu17132066
Petre GC, Francini-Pesenti F, De Toni L, Di Nisio A, Mingardi A, Cosci I, Passerin N, Ferlin A, Garolla A. Role of Mediterranean Diet and Ultra-Processed Foods on Sperm Parameters: Data from a Cross-Sectional Study. Nutrients. 2025; 17(13):2066. https://doi.org/10.3390/nu17132066
Chicago/Turabian StylePetre, Gabriel Cosmin, Francesco Francini-Pesenti, Luca De Toni, Andrea Di Nisio, Asia Mingardi, Ilaria Cosci, Nicola Passerin, Alberto Ferlin, and Andrea Garolla. 2025. "Role of Mediterranean Diet and Ultra-Processed Foods on Sperm Parameters: Data from a Cross-Sectional Study" Nutrients 17, no. 13: 2066. https://doi.org/10.3390/nu17132066
APA StylePetre, G. C., Francini-Pesenti, F., De Toni, L., Di Nisio, A., Mingardi, A., Cosci, I., Passerin, N., Ferlin, A., & Garolla, A. (2025). Role of Mediterranean Diet and Ultra-Processed Foods on Sperm Parameters: Data from a Cross-Sectional Study. Nutrients, 17(13), 2066. https://doi.org/10.3390/nu17132066






