Bacopa monnieri: Preclinical and Clinical Evidence of Neuroactive Effects, Safety of Use and the Search for Improved Bioavailability
Abstract
1. Introduction
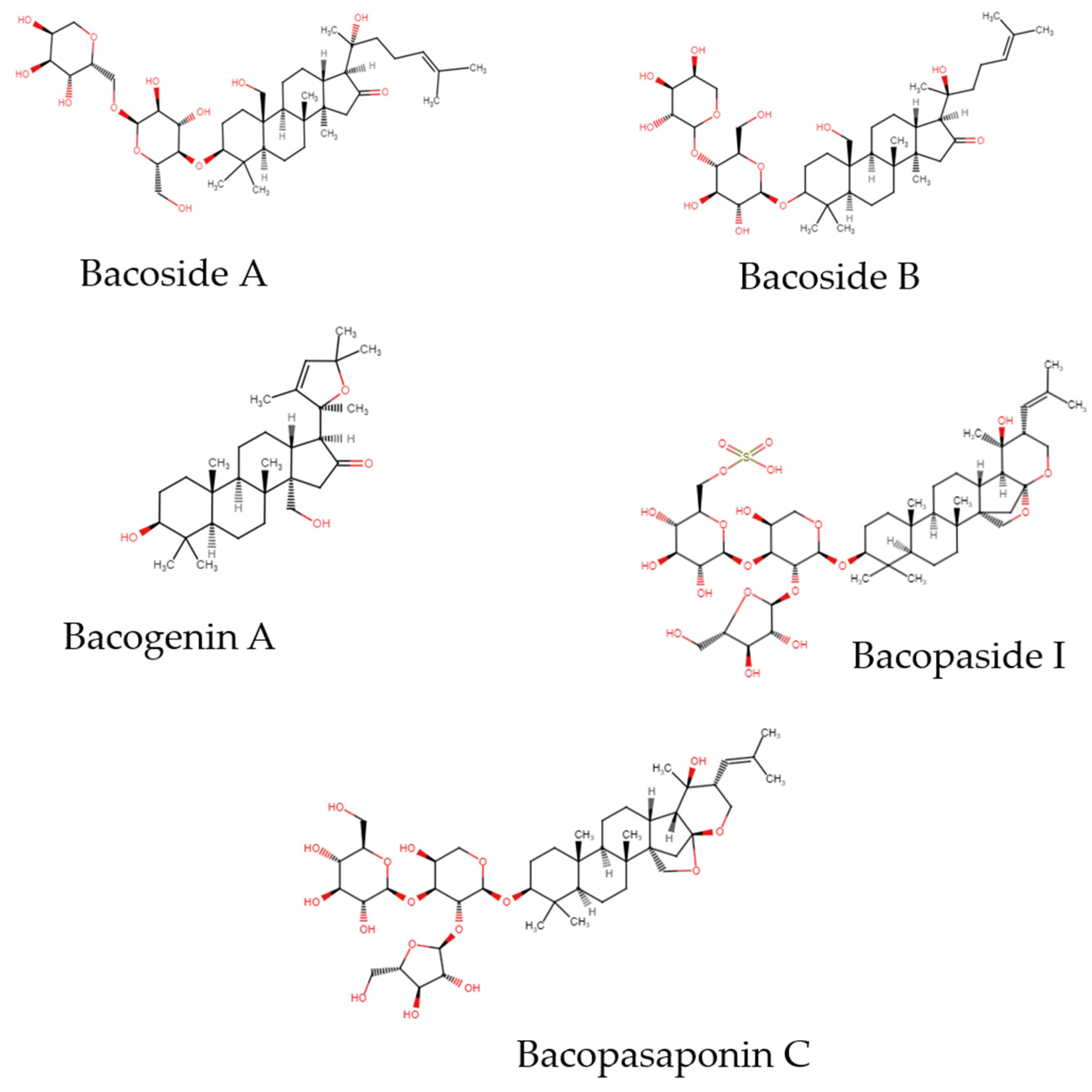
2. Active Compounds
2.1. Saponins
2.2. Alkaloids
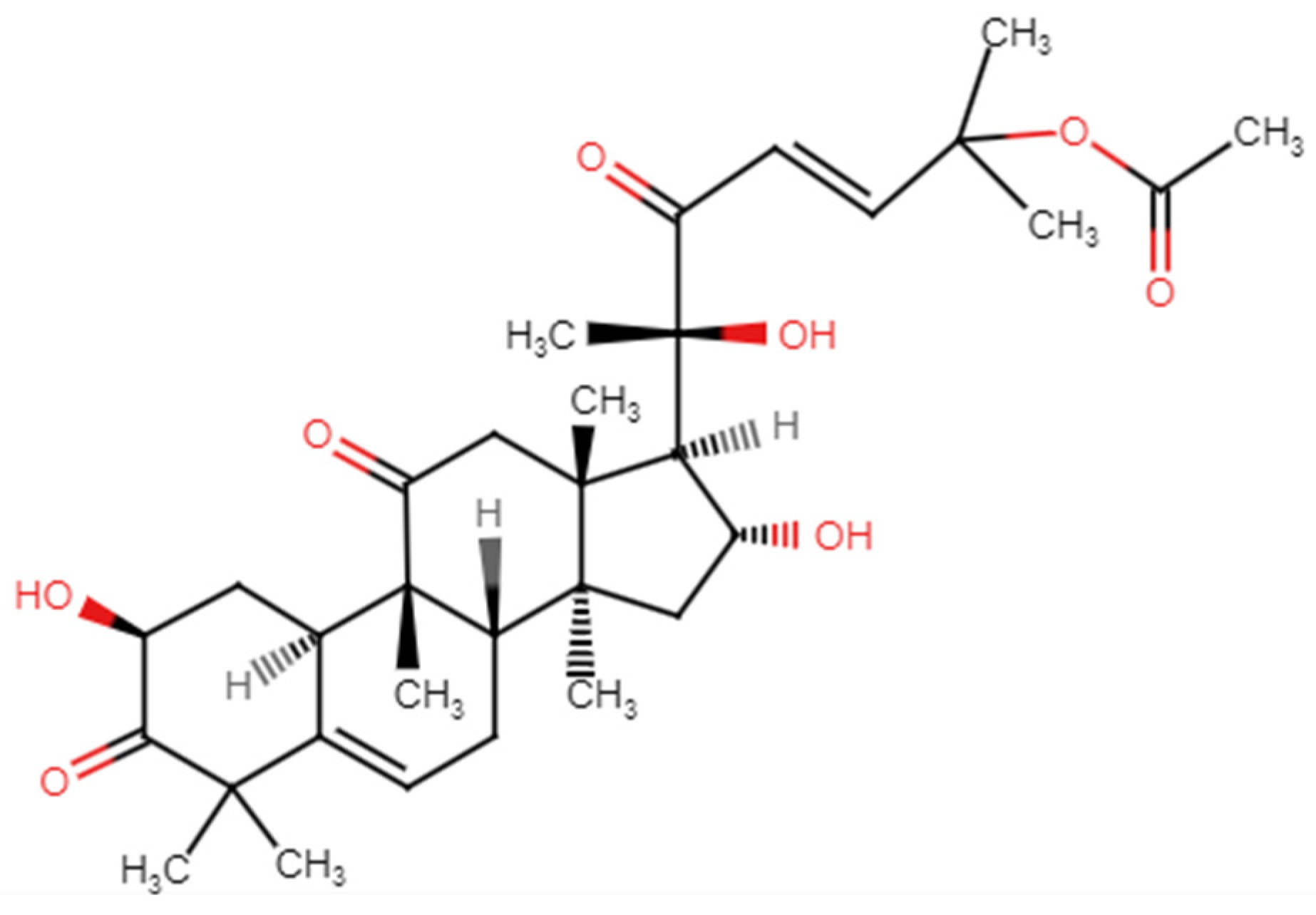
2.3. Cucurbitacins
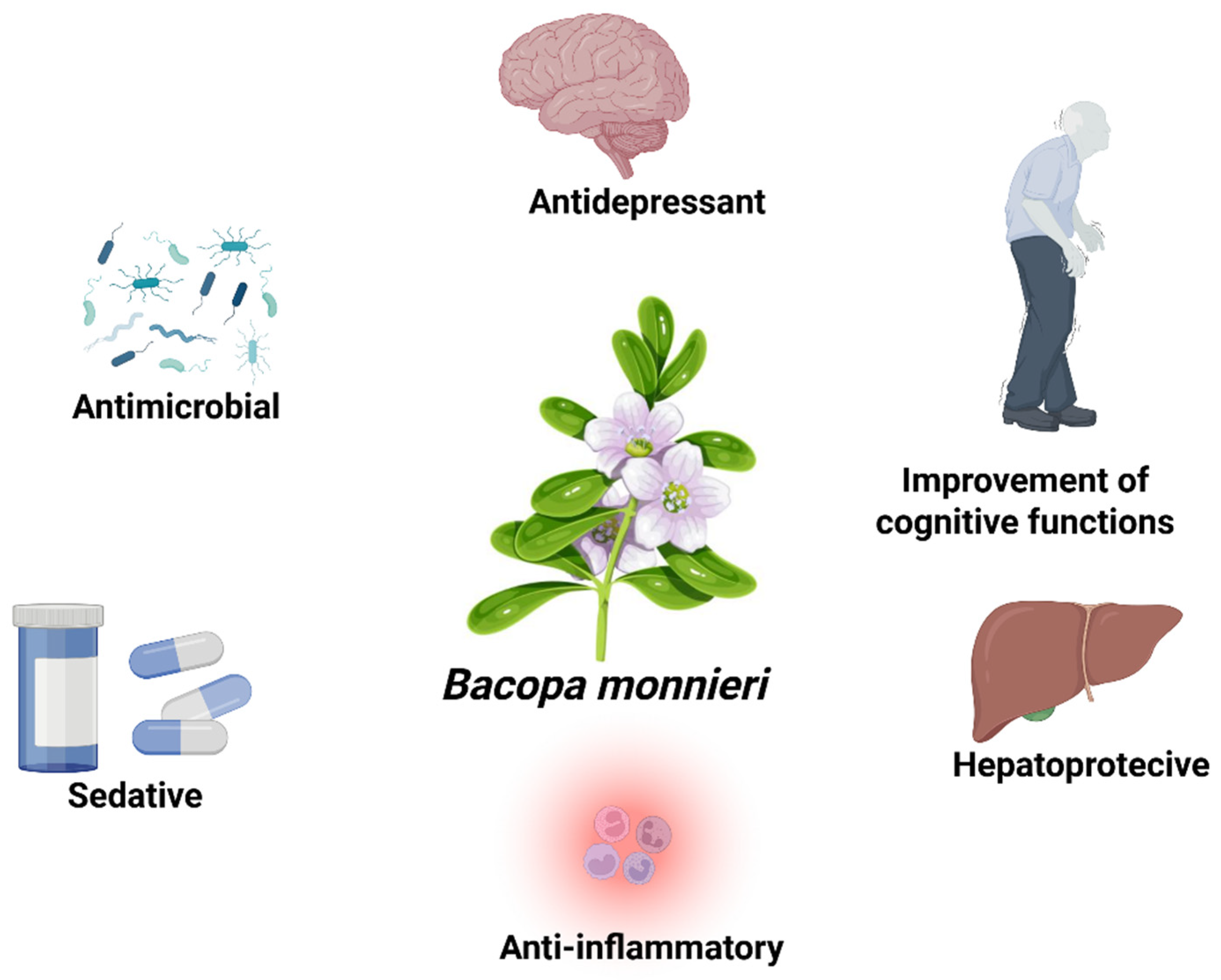
3. Biological Activity
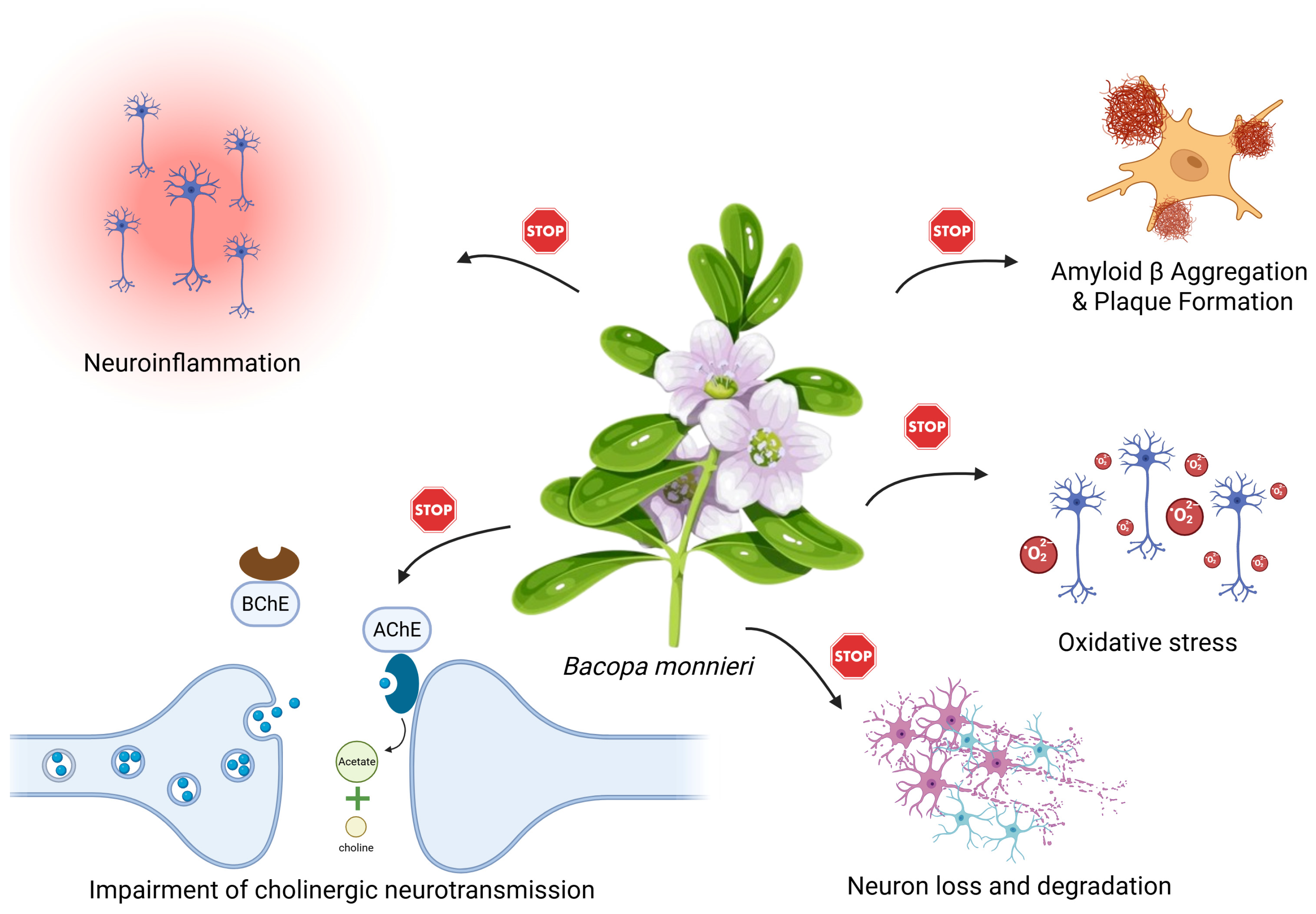
3.1. Alzheimer’s Disease
3.2. Parkinson’s Disease
3.3. Attention Deficit Hyperactivity Disorder (ADHD)
3.4. Depression
3.5. Anxiety
3.6. Other Bioactive Properties
3.7. Limitations of Studies
4. Safety of Use
4.1. Doses Used
4.2. Toxicity In Vitro
4.3. Possible Side Effects
4.4. Contraindications
4.5. Bacopa Extract in At-Risk and Sensitive Groups
5. Bioavailability
6. Modern Methods of Administration
6.1. Bilosomes
6.2. Ethosomes
6.3. Nanoparticles
6.4. Limitations of Modern Delivery Systems
7. Legal Aspects
8. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADHD | Attention deficit hyperactivity disorder |
| FDA | Food and Drug Administration |
| AChE-I | Acetylcholinesterase inhibitors |
| T4 | Thyroxine |
| KEAP1 | Kelch-like ECH-associated protein 1 |
| MPTP | 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine |
| DJ-1 | Parkinson disease protein 7 |
| DSHEA | Dietary Supplement Health and Education Act |
| CGMP | Current Good Manufacturing Practice |
References
- Ghosh, S.; Kumar, V.; Mukherjee, H.; Saini, S.; Gupta, S.; Chauhan, S.; Kushwaha, K.; Lahiri, D.; Sircar, D.; Roy, P. Assessment of the Mechanistic Role of an Indian Traditionally Used Ayurvedic Herb Bacopa monnieri (L.) Wettst. for Ameliorating Oxidative Stress in Neuronal Cells. J. Ethnopharmacol. 2024, 328, 117899. [Google Scholar] [CrossRef] [PubMed]
- Dash, V.B.; Kashyap, V.L. Materia Medica of Ayurveda; Concept Publishing Company: New Delhi, India, 1980. [Google Scholar]
- Brimson, J.M.; Brimson, S.; Prasanth, M.I.; Thitilertdecha, P.; Malar, D.S.; Tencomnao, T. The Effectiveness of Bacopa monnieri (Linn.) Wettst. as a Nootropic, Neuroprotective, or Antidepressant Supplement: Analysis of the Available Clinical Data. Sci. Rep. 2021, 11, 596. [Google Scholar] [CrossRef] [PubMed]
- Fatima, U.; Roy, S.; Ahmad, S.; Ali, S.; Elkady, W.M.; Khan, I.; Alsaffar, R.M.; Adnan, M.; Islam, A.; Hassan, M.I. Pharmacological Attributes of Bacopa monnieri Extract: Current Updates and Clinical Manifestation. Front. Nutr. 2022, 9, 972379. [Google Scholar] [CrossRef]
- Pandey, A.; Madan, S.; Sandhiya, K.; Sharma, R.; Raturi, A.; Bhatt, A.; Gaurav, N. Comparison of Antioxidant, Phytochemical Profiling of Bacopa monnieri (Brahmi). Scientifictemper 2022, 13, 286–293. [Google Scholar] [CrossRef]
- Vo, T.P.; Nguyen, T.H.P.; Nguyen, V.K.; Dang, T.C.T.; Nguyen, L.G.K.; Chung, T.Q.; Vo, T.T.H.; Nguyen, D.Q. Extracting Bioactive Compounds and Proteins from Bacopa monnieri Using Natural Deep Eutectic Solvents. PLoS ONE 2024, 19, e0300969. [Google Scholar] [CrossRef]
- Murthy, P.B.S.; Raju, V.R.; Ramakrisana, T.; Chakravarthy, M.S.; Kumar, K.V.; Kannababu, S.; Subbaraju, G.V. Estimation of Twelve Bacopa Saponins in Bacopa monnieri Extracts and Formulations by High-Performance Liquid Chromatography. Chem. Pharm. Bull. 2006, 54, 907–911. [Google Scholar] [CrossRef] [PubMed]
- Sairam, K.; Dorababu, M.; Goel, R.K.; Bhattacharya, S.K. Antidepressant Activity of Standardized Extract of Bacopa monniera in Experimental Models of Depression in Rats. Phytomedicine 2002, 9, 207–211. [Google Scholar] [CrossRef]
- Prakash, N.S.; Sundaram, R.; Mitra, S.K. In Vitro and In Vivo Anticancer Activity of Bacoside A from Whole Plant of Bacopa monnieiri (Linn). Am. J. Pharmacol. Toxicol. 2011, 6, 11–19. [Google Scholar] [CrossRef]
- Dubey, T.; Chinnathambi, S. Brahmi (Bacopa monnieri): An Ayurvedic Herb against the Alzheimer’s Disease. Arch. Biochem. Biophys. 2019, 676, 108153. [Google Scholar] [CrossRef]
- Bhandari, P.; Kumar, N.; Singh, B.; Kaul, V.K. Cucurbitacins from Bacopa monnieri. Phytochemistry 2007, 68, 1248–1254. [Google Scholar] [CrossRef]
- Deepak, M.; Sangli, G.K.; Arun, P.C.; Amit, A. Quantitative Determination of the Major Saponin Mixture Bacoside A in Bacopa monnieri by HPLC. Phytochem. Anal. 2005, 16, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Lan, T.; Wang, L.; Xu, Q.; Liu, W.; Jin, H.; Mao, W.; Wang, X.; Wang, X. Growth Inhibitory Effect of Cucurbitacin E on Breast Cancer Cells. Int. J. Clin. Exp. Pathol. 2013, 6, 1799–1805. [Google Scholar] [PubMed]
- Jayaprakasam, B.; Seeram, N.P.; Nair, M.G. Anticancer and Antiinflammatory Activities of Cucurbitacins from Cucurbita Andreana. Cancer Lett. 2003, 189, 11–16. [Google Scholar] [CrossRef]
- Martínez-García, M.; Garduño-Solórzano, G.; Lopes, G.; Sanchez, B.A.; Urbatzka, R.; Hentschke, G.S.; Campos, J.E.; Vasconcelos, V.M.O. Antioxidant, Anti-Inflammatory and Anti-Obesity Potential of Extracts Containing Phenols, Chlorophyll and Carotenoids from Mexican Wild Populations of Bacopa monnieri (L.) Wettst. Biology 2023, 12, 620. [Google Scholar] [CrossRef]
- Meena, U.K.; Maurya, A.K. Bacopa monnieri Extract Diminish Hypoxia-Induced Anxiety by Regulating HIF-1α Signaling and Enhancing the Antioxidant Defense System in Hippocampus. Neuromolecular Med. 2025, 27, 11. [Google Scholar] [CrossRef]
- Russo, A.; Izzo, A.A.; Borrelli, F.; Renis, M.; Vanella, A. Free Radical Scavenging Capacity and Protective Effect of Bacopa monniera L. on DNA Damage. Phytother. Res. 2003, 17, 870–875. [Google Scholar] [CrossRef] [PubMed]
- Malishev, R.; Shaham-Niv, S.; Nandi, S.; Kolusheva, S.; Gazit, E.; Jelinek, R. Bacoside-A, an Indian Traditional-Medicine Substance, Inhibits β-Amyloid Cytotoxicity, Fibrillation, and Membrane Interactions. ACS Chem. Neurosci. 2017, 8, 884–891. [Google Scholar] [CrossRef]
- Shoukat, S.; Zia, M.A.; Uzair, M.; Attia, K.A.; Abushady, A.M.; Fiaz, S.; Ali, S.; Yang, S.H.; Ali, G.M. Bacopa monnieri: A Promising Herbal Approach for Neurodegenerative Disease Treatment Supported by in Silico and in Vitro Research. Heliyon 2023, 9, e21161. [Google Scholar] [CrossRef]
- Deolankar, S.C.; Najar, M.A.; Ramesh, P.; Kanichery, A.; Kudva, A.K.; Raghu, S.V.; Prasad, T.S.K. Discovery of Molecular Networks of Neuroprotection Conferred by Brahmi Extract in Aβ42-Induced Toxicity Model of Drosophila Melanogaster Using a Quantitative Proteomic Approach. Mol. Neurobiol. 2023, 60, 303–316. [Google Scholar] [CrossRef]
- Tiwari, S.; Atluri, V.; Kaushik, A.; Yndart, A.; Nair, M. Alzheimer’s Disease: Pathogenesis, Diagnostics, and Therapeutics. Int. J. Nanomed. 2019, 14, 5541–5554. [Google Scholar] [CrossRef]
- Chen, Z.-R.; Huang, J.-B.; Yang, S.-L.; Hong, F.-F. Role of Cholinergic Signaling in Alzheimer’s Disease. Molecules 2022, 27, 1816. [Google Scholar] [CrossRef] [PubMed]
- Canu, N.; Amadoro, G.; Triaca, V.; Latina, V.; Sposato, V.; Corsetti, V.; Severini, C.; Ciotti, M.T.; Calissano, P. The Intersection of NGF/TrkA Signaling and Amyloid Precursor Protein Processing in Alzheimer’s Disease Neuropathology. Int. J. Mol. Sci. 2017, 18, 1319. [Google Scholar] [CrossRef]
- Roy, S.; Chakravarty, S.; Talukdar, P.; Soumendra; Talapatra, N. Identification of Bioactive Compounds Present in Bacopa monnieri Linn. Against Caspase-3 and Tau Protein Kinase I to Prevent Alzheimer’s Disease: An in Silico Study. Pharma Innov. J. 2019, 8, 855–861. [Google Scholar]
- Mathew, M.; Subramanian, S. Evaluation of the anti-amyloidogenic potential of nootropic herbal extracts in vitro. Int. J. Pharm. Sci. Res. 2012, 3, 4276–4280. [Google Scholar]
- Neuroprotective Effect of Bacopa monnieri on Beta-Amyloid-Induced Cell Death in Primary Cortical Culture-PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/18755259/ (accessed on 5 February 2025).
- Peera, D.K. Neuroprotective Effect of Bacopa monniera on Memory Deficits and ATPase System in Alzheimer’s Disease (AD) Induced Mice. J. Sci. Innov. Res. 2013, 2, 719–735. [Google Scholar]
- Peth-Nui, T.; Wattanathorn, J.; Muchimapura, S.; Tong-Un, T.; Piyavhatkul, N.; Rangseekajee, P.; Ingkaninan, K.; Vittaya-Areekul, S. Effects of 12-Week Bacopa monnieri Consumption on Attention, Cognitive Processing, Working Memory, and Functions of Both Cholinergic and Monoaminergic Systems in Healthy Elderly Volunteers. Evid. Based Complement. Altern. Med. 2012, 2012, 606424. [Google Scholar] [CrossRef]
- Modi, M.; Prabhakar, S.; Vishnu, V.; Mohanty, M.; Sharma, A.; Medhi, B.; Mittal, B.; Khandelwal, N.; Goyal, M.; Lal, V.; et al. Efficacy of Bacopa monnieri (Brahmi) and Donepezil in Alzheimer’s Disease and Mild Cognitive Impairment: A Randomized Double-Blind Parallel Phase 2b Study. Ann. Indian Acad. Neurol. 2024, 23, 767–773. [Google Scholar] [CrossRef]
- Vázquez-Vélez, G.E.; Zoghbi, H.Y. Parkinson’s Disease Genetics and Pathophysiology. Annu. Rev. Neurosci. 2021, 44, 87–108. [Google Scholar] [CrossRef]
- Chandrasekar, S.; Thangarajan, S.; Loganathan, S.; Sundaram, S.; Kumarasamy, P. Molecular Docking Studies of Bacoside-A, an Active Component of Bacopa monniera with DJ1 for Anti-Parkinson’s Drug Design. Biomirror 2013, 4, 67. [Google Scholar]
- Singh, B.; Pandey, S.; Rumman, M.; Kumar, S.; Kushwaha, P.P.; Verma, R.; Mahdi, A.A. Neuroprotective and Neurorescue Mode of Action of Bacopa monnieri (L.) Wettst in 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine-Induced Parkinson’s Disease: An In Silico and In Vivo Study. Front. Pharmacol. 2021, 12, 616413. [Google Scholar] [CrossRef]
- Jadiya, P.; Khan, A.; Sammi, S.R.; Kaur, S.; Mir, S.S.; Nazir, A. Anti-Parkinsonian Effects of Bacopa monnieri: Insights from Transgenic and Pharmacological Caenorhabditis Elegans Models of Parkinson’s Disease. Biochem. Biophys. Res. Commun. 2011, 413, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Siddique, Y.H.; Mujtaba, S.F.; Faisal, M.; Jyoti, S.; Naz, F. The Effect of Bacopa monnieri Leaf Extract on Dietary Supplementation in Transgenic Drosophila Model of Parkinson’s Disease. Eur. J. Integr. Med. 2014, 6, 571–580. [Google Scholar] [CrossRef]
- Swathi, G.; Rajendra, W. Protective role of Bacopa monnieri on induced parkinson’s disease with particular reference to catecholamine system. Int. J. Pharm. Pharm. Sci. 2014, 6, 379–382. [Google Scholar]
- Santos, A.F.D.; Souza, M.M.Q.; Amaral, E.C.; Albuquerque, E.R.; Bortoloti, D.S.; Gasparotto Junior, A.; Lourenço, E.L.B.; Lovato, E.C.W.; Lívero, F.A.D.R. Bacopa monnieri in Patients with Parkinson’s Disease: A Pilot Study. J. Med. Food 2023, 26, 2. [Google Scholar]
- Sharma, A.; Couture, J. A Review of the Pathophysiology, Etiology, and Treatment of Attention-Deficit Hyperactivity Disorder (ADHD). Ann. Pharmacother. 2014, 48, 209–225. [Google Scholar] [CrossRef] [PubMed]
- Dave, U.P.; Dingankar, S.R.; Saxena, V.S.; Joseph, J.A.; Bethapudi, B.; Agarwal, A.; Kudiganti, V. An Open-Label Study to Elucidate the Effects of Standardized Bacopa monnieri Extract in the Management of Symptoms of Attention-Deficit Hyperactivity Disorder in Children. Adv. Mind Body Med. 2014, 28, 10–15. [Google Scholar]
- Schechter, L.E.; Ring, R.H.; Beyer, C.E.; Hughes, Z.A.; Khawaja, X.; Malberg, J.E.; Rosenzweig-Lipson, S. Innovative Approaches for the Development of Antidepressant Drugs: Current and Future Strategies. NeuroRx 2005, 2, 590–611. [Google Scholar] [CrossRef]
- Chaachouay, N.; Zidane, L. Plant-Derived Natural Products: A Source for Drug Discovery and Development. Drugs Drug Candidates 2024, 3, 184–207. [Google Scholar] [CrossRef]
- Mannan, A.; Abir, A.B.; Rahman, R. Antidepressant-like Effects of Methanolic Extract of Bacopa monniera in Mice. BMC Complement. Altern. Med. 2015, 15, 337. [Google Scholar] [CrossRef]
- Yankelevitch-Yahav, R.; Franko, M.; Huly, A.; Doron, R. The Forced Swim Test as a Model of Depressive-like Behavior. J. Vis. Exp. 2015, 97, e52587. [Google Scholar] [CrossRef]
- Seibenhener, M.L.; Wooten, M.C. Use of the Open Field Maze to Measure Locomotor and Anxiety-like Behavior in Mice. J. Vis. Exp. 2015, 96, e52434. [Google Scholar] [CrossRef]
- Psychiatry.Org-What Are Anxiety Disorders? Available online: https://www.psychiatry.org/patients-families/anxiety-disorders/what-are-anxiety-disorders (accessed on 25 June 2024).
- Kumar, N.; Abichandani, L.G.; Thawani, V.; Gharpure, K.J.; Naidu, M.U.R.; Venkat Ramana, G. Efficacy of Standardized Extract of Bacopa monnieri (Bacognize®) on Cognitive Functions of Medical Students: A Six-Week, Randomized Placebo-Controlled Trial. Evid. Based Complement. Altern. Med. 2016, 2016, 4103423. [Google Scholar] [CrossRef] [PubMed]
- Benson, S.; Downey, L.A.; Stough, C.; Wetherell, M.; Zangara, A.; Scholey, A. An Acute, Double-Blind, Placebo-Controlled Cross-over Study of 320 Mg and 640 Mg Doses of Bacopa monnieri (CDRI 08) on Multitasking Stress Reactivity and Mood. Phytother. Res. 2014, 28, 551–559. [Google Scholar] [CrossRef]
- Bacopa monnieri . In LiverTox: Clinical and Research Information on Drug-Induced Liver Injury; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2012.
- Sireeratawong, S.; Jaijoy, K.; Khonsung, P.; Lertprasertsuk, N.; Ingkaninan, K. Acute and Chronic Toxicities of Bacopa monnieri Extract in Sprague-Dawley Rats. BMC Complement. Altern. Med. 2016, 16, 249. [Google Scholar] [CrossRef]
- Pravina, K.; Ravindra, K.R.; Goudar, K.S.; Vinod, D.R.; Joshua, A.J.; Wasim, P.; Venkateshwarlu, K.; Saxena, V.S.; Amit, A. Safety Evaluation of BacoMind in Healthy Volunteers: A Phase I Study. Phytomedicine 2007, 14, 301–308. [Google Scholar] [CrossRef]
- Morgan, A.; Stevens, J. Does Bacopa monnieri Improve Memory Performance in Older Persons? Results of a Randomized, Placebo-Controlled, Double-Blind Trial. J. Altern. Complement. Med. 2010, 16, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Singh, S.K. Evaluation of Antifertility Potential of Brahmi in Male Mouse. Contraception 2009, 79, 71–79. [Google Scholar] [CrossRef]
- Saraf, M.K.; Prabhakar, S.; Pandhi, P.; Anand, A. Bacopa monniera Ameliorates Amnesic Effects of Diazepam Qualifying Behavioral-Molecular Partitioning. Neuroscience 2008, 155, 476–484. [Google Scholar] [CrossRef]
- Siwek, M.; Woroń, J.; Wrzosek, A.; Gupało, J.; Chrobak, A.A. Harder, Better, Faster, Stronger? Retrospective Chart Review of Adverse Events of Interactions between Adaptogens and Antidepressant Drugs. Front. Pharmacol. 2023, 14, 1271776. [Google Scholar] [CrossRef]
- Kar, A.; Panda, S.; Bharti, S. Relative Efficacy of Three Medicinal Plant Extracts in the Alteration of Thyroid Hormone Concentrations in Male Mice. J. Ethnopharmacol. 2002, 81, 281–285. [Google Scholar] [CrossRef]
- Levenstein, S.; Rosenstock, S.; Jacobsen, R.K.; Jorgensen, T. Psychological Stress Increases Risk for Peptic Ulcer, Regardless of Helicobacter Pylori Infection or Use of Nonsteroidal Anti-Inflammatory Drugs. Clin. Gastroenterol. Hepatol. 2015, 13, 498–506.e1. [Google Scholar] [CrossRef] [PubMed]
- Evaluation of Antiulcerogenic Activity of Bacopa monnieri (LINN.) on Ethanol-Induced Gastric Injury in Mice. Available online: https://www.researchgate.net/publication/326032975_Evaluation_of_Antiulcerogenic_Activity_of_Bacopa_Monnieri_LINN_on_Ethanol-Induced_Gastric_Injury_In_Mice (accessed on 5 February 2025).
- Calabrese, C.; Gregory, W.L.; Leo, M.; Kraemer, D.; Bone, K.; Oken, B. Effects of a Standardized Bacopa monnieri Extract on Cognitive Performance, Anxiety, and Depression in the Elderly: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Altern. Complement. Med. 2008, 14, 707–713. [Google Scholar] [CrossRef] [PubMed]
- Kean, J.D.; Downey, L.A.; Sarris, J.; Kaufman, J.; Zangara, A.; Stough, C. Effects of Bacopa monnieri (CDRI 08®) in a Population of Males Exhibiting Inattention and Hyperactivity Aged 6 to 14 Years: A Randomized, Double-Blind, Placebo-Controlled Trial. Phytother. Res. 2022, 36, 996–1012. [Google Scholar] [CrossRef] [PubMed]
- Kean, J.D.; Kaufman, J.; Lomas, J.; Goh, A.; White, D.; Simpson, D.; Scholey, A.; Singh, H.; Sarris, J.; Zangara, A.; et al. A Randomized Controlled Trial Investigating the Effects of a Special Extract of Bacopa monnieri (CDRI 08) on Hyperactivity and Inattention in Male Children and Adolescents: BACHI Study Protocol (ANZCTRN12612000827831). Nutrients 2015, 7, 9931–9945. [Google Scholar] [CrossRef]
- Usha, P.D.; Wasim, P.; Joshua, J.A.; Geetharani, P.; Murali, B.; Mayachari, A.S.; Venkateshwarlu, K.; Saxena, V.S.; Deepak, M.; Amit, A. BacoMind®: A Cognitive Enhancer in Children Requiring Individual Education Programme. J. Pharmacol. Toxicol. 2008, 3, 302–310. [Google Scholar] [CrossRef]
- Acquarulo, B.; Tandon, P.; Macica, C.M. Suspected cholinergic toxicity due to cevimeline hydrochloride and Bacopa monnieri interaction: A case report. J. Med. Case Rep. 2022, 16, 253. [Google Scholar] [CrossRef]
- Best, T.; Miller, J.; Teo, W.-P. Neurocognitive Effects a Combined Polyphenolic-Rich Herbal Extract in Healthy Middle-Aged Adults-a Randomised, Double-Blind, Placebo-Controlled Study. Nutr. Neurosci. 2024, 27, 1293–1305. [Google Scholar] [CrossRef]
- Roodenrys, S.; Booth, D.; Bulzomi, S.; Phipps, A.; Micallef, C.; Smoker, J. Chronic Effects of Brahmi (Bacopa monnieri) on Human Memory. Neuropsychopharmacology 2002, 27, 279–281. [Google Scholar] [CrossRef]
- Stough, C.K.; Pase, M.P.; Cropley, V.; Myers, S.; Nolidin, K.; King, R.; Camfield, D.; Wesnes, K.; Pipingas, A.; Croft, K.; et al. A Randomized Controlled Trial Investigating the Effect of Pycnogenol and Bacopa CDRI08 Herbal Medicines on Cognitive, Cardiovascular, and Biochemical Functioning in Cognitively Healthy Elderly People: The Australian Research Council Longevity Intervention (ARCLI) Study Protocol (ANZCTR12611000487910). Nutr. J. 2012, 11, 11. [Google Scholar] [CrossRef]
- Abhishek, M.; Rubal, S.; Rohit, K.; Rupa, J.; Phulen, S.; Gurjeet, K.; Raj, S.A.; Manisha, P.; Alka, B.; Ramprasad, P.; et al. Neuroprotective Effect of the Standardised Extract of Bacopa monnieri (BacoMind) in Valproic Acid Model of Autism Spectrum Disorder in Rats. J. Ethnopharmacol. 2022, 293, 115199. [Google Scholar] [CrossRef]
- Sudhishma; Devadasa Acharya, S.; Ullal, S.D.; Blossom, V.; Parida, A.; Noushida, N. Levetiracetam Exposure during Prenatal and Postnatal Period Induces Cognitive Decline in Rat Offsprings, Not Completely Prevented by Bacopa monnieri. J. Complement. Integr. Med. 2022, 19, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, K.S.; Tiwari, N.R.; Tiwari, R.R.; Sharma, R.S. Neurocognitive Effect of Nootropic Drug Brahmi (Bacopa monnieri) in Alzheimer’s Disease. Ann. Neurosci. 2017, 24, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Habbu, P.; Madagundi, S.; Kulkarni, R.; Jadav, S.; Vanakudri, R.; Kulkarni, V. Preparation and Evaluation of Bacopa–Phospholipid Complex for Antiamnesic Activity in Rodents. Drug Invent. Today 2013, 5, 13–21. [Google Scholar] [CrossRef]
- Narayanan, V.A.; Sharma, A.; Rajesh, K.S.; Arunraj, T.R.; Gururaj, M.P.; Parasuraman, S.; John, A. Bilosomes as a Potential Carrier to Enhance Cognitive Effects of Bacopa monnieri Extract on Oral Administration. J. Health Allied Sci. NU 2023, 13, 421–430. [Google Scholar] [CrossRef]
- Dwivedi, D.; Nimisha; Mishra, N.; Singh, S.P.; Shukla, A.K. Performance Evaluation of Bacopa monneri-Loaded Ethosomes for Topical Delivery.|EBSCOhost. Available online: https://openurl.ebsco.com/contentitem/doi:10.31018%2Fjans.v16i3.5435?sid=ebsco:plink:crawler&id=ebsco:doi:10.31018%2Fjans.v16i3.5435 (accessed on 17 February 2025).
- Sekhar, V.C.; Viswanathan, G.; Baby, S. Bacopaside II Nanoparticles Inhibit Proliferation of C6 Glioma Cells. Phytomedicine Plus 2021, 1, 100040. [Google Scholar] [CrossRef]
- Kumar, R.; Garg, R.; Khurana, N. A Comparative in Vivo Evaluation of Anti-Alzheimer Activity of Bacopa Extract and Its Solid Lipid Nanoparticles. Curr. Bioact. Compd. 2021, 17, 6–16. [Google Scholar] [CrossRef]
- Sekhar, V.C.; Gulia, K.K.; Deepti, A.; Chakrapani, P.S.B.; Baby, S.; Viswanathan, G. Protection by Nano-Encapsulated Bacoside A and Bacopaside I in Seizure Alleviation and Improvement in Sleep-In Vitro and In Vivo Evidences. Mol. Neurobiol. 2024, 61, 3296–3313. [Google Scholar] [CrossRef]
- Ma, X.; Tian, Y.; Yang, R.; Wang, H.; Allahou, L.W.; Chang, J.; Williams, G.; Knowles, J.C.; Poma, A. Nanotechnology in Healthcare, and Its Safety and Environmental Risks. J. Nanobiotechnol. 2024, 22, 715. [Google Scholar] [CrossRef]
- Farjadian, F.; Ghasemi, A.; Gohari, O.; Roointan, A.; Karimi, M.; Hamblin, M.R. Nanopharmaceuticals and Nanomedicines Currently on the Market: Challenges and Opportunities. Nanomedicine 2019, 14, 93–126. [Google Scholar] [CrossRef]
- Ezike, T.C.; Okpala, U.S.; Onoja, U.L.; Nwike, C.P.; Ezeako, E.C.; Okpara, O.J.; Okoroafor, C.C.; Eze, S.C.; Kalu, O.L.; Odoh, E.C.; et al. Advances in Drug Delivery Systems, Challenges and Future Directions. Heliyon 2023, 9, e17488. [Google Scholar] [CrossRef]
- Hauber, B.; Hand, M.V.; Hancock, B.C.; Zarrella, J.; Harding, L.; Ogden-Barker, M.; Antipas, A.S.; Watt, S.J. Patient Acceptability and Preferences for Solid Oral Dosage Form Drug Product Attributes: A Scoping Review. Patient Prefer. Adherence 2024, 18, 1281–1297. [Google Scholar] [CrossRef] [PubMed]
- Food and Feed Information Portal Database|FIP. Available online: https://ec.europa.eu/food/food-feed-portal/screen/novel-food-catalogue/search (accessed on 16 February 2025).
- Center for Food Safety and Applied Nutrition. Peak Nootropics LLC Aka Advanced Nootropics-557887-02/05/2019. Available online: https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/warning-letters/peak-nootropics-llc-aka-advanced-nootropics-557887-02052019 (accessed on 16 February 2025).
- Health Canada. Natural Health Product Regulation in Canada: Overview. Available online: https://www.canada.ca/en/health-canada/services/drugs-health-products/natural-non-prescription/regulation.html (accessed on 16 February 2025).
- Branch, L.S. Consolidated Federal Laws of Canada, Natural Health Products Regulations. Available online: https://laws-lois.justice.gc.ca/eng/regulations/sor-2003-196/page-1.html#h-700387 (accessed on 16 February 2025).

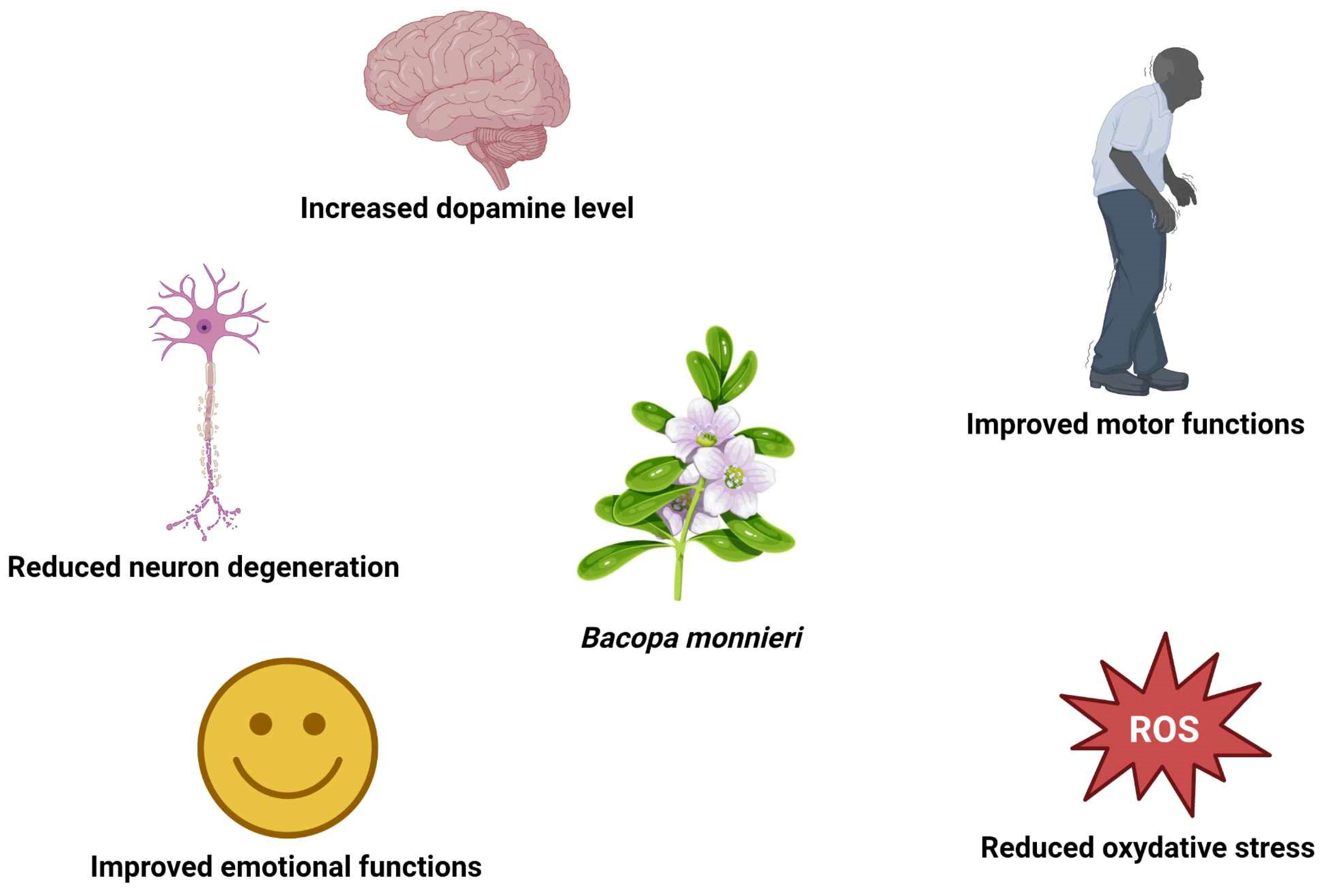

| Disease | Type of Study | Study Group | Duration of the Study | Dose | Conclusions | Reference |
|---|---|---|---|---|---|---|
| Alzheimer’s disease | In silico | - | - | - | Inhibitory effects on the CASP-3 and TPK I | Roy et al. [3] |
| In silico | - | - | - | The anti-amyloidogenic effect from dissociation of amyloid protein aggregates | Subramanian et al. [4] | |
| In vitro | Cortical cell cultures were prepared from 18-day-old rat fetuses | 72 h | 10–100 μg/μL (extract: 18 kg DW/triple percolation with 95% ethanol, 8 h) | The anti-amyloidogenic effect, limiting deposition beta-amyloid in brain | Limpeanchob et al. [6] | |
| In vivo | 72 healthy and AD mice | 180 day | 100 mg/kg (methanolic extract) | Memory and learning deficits restored to normal levels; | Kunte and Kuna [27] | |
| Randomized, double-blind clinical trial | 60 healthy volunteers | 12 weeks | 300 mg, 600 mg (methanolic extract) | memory enhancement and reduction in AChE activity | Peth-Nui et al. [28] | |
| Randomized, double-blind clinical trial | 48 patients with AD and mild cognitive impairment | 45 months | 300 mg (standardized extract) | Effect comparable to donepezil | Prabhakar et al. [29] | |
| Parkinson’s disease | In silico | - | - | - | Interaction of Basocide-A with active site of DJ-1 | Chandrasekar et al. [21] |
| In silico | - | - | - | Inhibition of KEAP1 by Bacopaside-XII | Singh et al. [32] | |
| In vivo | Caenorhabditis elegans | 48 h | B. monnieri tincture (100 g DW/650 mL 96% ethanol), diluted ten times in E. coli OP50 liquid culture | Reduction in α-synuclein accumulation, degeneration of dopaminergic neurons, and lipoperoxidation | Jadiya et al. [22] | |
| In vivo | Transgenic PD Drosophila melanogaster | 24 days | 0.25; 0.5; 1.0 µL/mL (extract: 30 g DW/300 mL acetone) | Delayed motor impairment, reduction in oxidative stress, and brain apoptosis | Siddique et al. [24] | |
| In vivo | 24 male albino mice | 30 days | 40 mg/kg (extract: 500 g DW/1000 mL ethanol) | Better results in motor tests, reduced lipid peroxidation, conjugated dienes, caspase-3, Bax protein, dopaminergic neuron degeneration, increased Bcl-2 protein, antioxidant activity, nitrate level in nigrostriatal system | Singh et al. [25] | |
| In vivo | 32 Swiss albino mice | 3 weeks | 40 mg/kg (commercial extract) | Better results in motor tests, reduced lipid peroxidation, increased GSH, dopamine, and nitrate level in the nigrostriatal system | Singh et al. [23] | |
| In vivo | 24 male Wistar rats | 80 days | 180 mg/kg (ethanolic extract) | Increased noradrenaline, adrenaline, dopamine, serotonin, reduced MAO in brain tissue | Shinomol et al. [26] | |
| Double-blind placebo-controlled, parallel trial | 20 volunteers with Parkinson’s disease | 90 days | 225; 400 mg a day (commercial extract) | Improvement in quality of life, emotional and motor functions | Fuentes dos santos et al. [36] | |
| ADHD | Open-label trial | 27 children meeting the criteria for ADHD | 6 months | 225 mg a day (commercial extract) | Significant reduction in ADHD symptoms except for social problems | Dave et al. [38] |
| Anxiety | Double-blind placebo-controlled, parallel trial | 60 students aged 19–22 having basic computer literacy | 6 weeks | 150 mg two times a day (standardized extract, 15.57 of bacopasaponins (%w/w) | Significant effect on cognitive functions | Kumar et al. [45] |
| Depression | Inbred Charles Foster albino rats | 5 days | 20 and 40 mg/kg, once a day (methanolic extract) | Decreased escape failures and increased avoidance responses | Sairam et al. [8] | |
| In vivo | Swiss albino mice | 5 min | 50, 100 and 200 mg/kg (sample extract) 30 mg/kg (standard drug) | Confirmed antidepressant-like effect of methanolic extract of B. monniera | Mannan et al. [41] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gościniak, A.; Stasiłowicz-Krzemień, A.; Szeląg, M.; Pawlak, J.; Skiera, I.; Kwiatkowska, H.; Nowak, N.; Bernady, K.; Trzaskoma, P.; Zimak-Krótkopad, O.; et al. Bacopa monnieri: Preclinical and Clinical Evidence of Neuroactive Effects, Safety of Use and the Search for Improved Bioavailability. Nutrients 2025, 17, 1939. https://doi.org/10.3390/nu17111939
Gościniak A, Stasiłowicz-Krzemień A, Szeląg M, Pawlak J, Skiera I, Kwiatkowska H, Nowak N, Bernady K, Trzaskoma P, Zimak-Krótkopad O, et al. Bacopa monnieri: Preclinical and Clinical Evidence of Neuroactive Effects, Safety of Use and the Search for Improved Bioavailability. Nutrients. 2025; 17(11):1939. https://doi.org/10.3390/nu17111939
Chicago/Turabian StyleGościniak, Anna, Anna Stasiłowicz-Krzemień, Marta Szeląg, Jakub Pawlak, Izabela Skiera, Hanna Kwiatkowska, Natasza Nowak, Krzysztof Bernady, Piotr Trzaskoma, Oskar Zimak-Krótkopad, and et al. 2025. "Bacopa monnieri: Preclinical and Clinical Evidence of Neuroactive Effects, Safety of Use and the Search for Improved Bioavailability" Nutrients 17, no. 11: 1939. https://doi.org/10.3390/nu17111939
APA StyleGościniak, A., Stasiłowicz-Krzemień, A., Szeląg, M., Pawlak, J., Skiera, I., Kwiatkowska, H., Nowak, N., Bernady, K., Trzaskoma, P., Zimak-Krótkopad, O., & Cielecka-Piontek, J. (2025). Bacopa monnieri: Preclinical and Clinical Evidence of Neuroactive Effects, Safety of Use and the Search for Improved Bioavailability. Nutrients, 17(11), 1939. https://doi.org/10.3390/nu17111939









