Abstract
Background: Childhood obesity is a growing global health concern. Metabolomics, the comprehensive study of metabolites within biological systems, offers a powerful approach to better define the phenotype and understand the complex biochemical alterations associated with obesity. The aim of this systematic review was to summarize current knowledge in the field of metabolomics in childhood obesity and to identify metabolic signatures or biomarkers associated with overweight/obesity (Ov/Ob) and Metabolically Unhealthy Obesity (MUO) in children and adolescents. Methods: We performed a systematic search of Medline and Scopus databases according to PRISMA guidelines. We included only longitudinal prospective studies or randomized controlled trials with ≥12 months of follow-up, as well as meta-analyses of the above that assessed the relation between metabolic signatures related to obesity and Body Mass Index (BMI) or other measures of adiposity in children and adolescents aged 2–19 years with overweight or obesity. Initially, 595 records were identified from PubMed and 1565 from Scopus. After removing duplicates and screening for relevance, 157 reports were assessed for eligibility. From the additional search, 75 new records were retrieved, of which none were eligible for our study. Finally, 7 reports were included in the present systematic review (4 reporting on Ov/Ob and 4 on MUO). Results: The presented studies suggest that the metabolism of amino acids and lipids is primarily affected by childhood obesity. Metabolites like glycoprotein acetyls, the Apolipoprotein B/Apolipoprotein A-1 ratio, and lactate have emerged as potential biomarkers for insulin resistance and metabolic syndrome, highlighting their potential value in clinical applications. Conclusions: There is a need for future longitudinal studies to assess metabolic changes over time, interventional studies to evaluate the efficacy of therapeutic strategies, and large-scale population studies to explore metabolic diversity across different demographics. Our findings reveal specific biomarkers in the amino acid and lipid pathway that may serve as early indicators of childhood obesity and its associated cardiometabolic complications.
1. Introduction
Obesity has emerged as a significant global health issue, with its prevalence having nearly tripled from 1975 to 2016 [1]. According to the World Health Organization (WHO), approximately 60% of adults in Europe will be overweight or obesity in 2022 [2]. This alarming trend not only poses immediate health risks but also predisposes affected subjects to long-term health complications, such as hypertension, left ventricular hypertrophy, insulin resistance and diabetes mellitus type 2 (DM2), metabolic dysfunction-associated steatotic liver disease (MASLD), as well as mental health issues and cancer [3]. Furthermore, during the last decade, obesity with or without metabolic aberrations, commonly termed Metabolically Unhealthy Obesity (MUO) or Metabolically Healthy Obesity (MHO), respectively, has been extensively investigated [4]. Metabolically Unhealthy Obesity (MUO) refers to subjects with obesity who exhibit metabolic abnormalities, such as insulin resistance, elevated blood pressure, dyslipidemia (elevated triglycerides and low HDL cholesterol concentrations), and chronic inflammation. Unlike metabolically healthy obesity (MHO), where subjects have excess body fat but normal metabolic profiles, MUO is strongly associated with an increased risk of DM2, cardiovascular disease, and non-alcoholic fatty liver disease (NAFLD).
The intricate interplay of genetic, epigenetic, environmental, and lifestyle factors contributes to the multifaceted nature of this epidemic [3]. In the quest for a better understanding of the underlying mechanisms and potential interventions, metabolomics has emerged as a powerful tool, offering insights into the metabolic alterations associated with childhood obesity [5,6].
Metabolomics is a field of study within the broader discipline of systems biology, which focuses on the comprehensive analysis of small molecules or metabolites (<1500 KDa) in a biological sample [7,8]. Metabolites are the end products of cellular processes, and their concentrations can provide insights into the biochemical pathways and physiological status of an organism at a specific point in time. It is particularly useful in understanding the dynamic responses of biological systems to various alterations, including genetic, epigenetic, or protein-level modifications, exposure to environmental factors (physical exercise, diet, and microbiome) and diseases, and helps bridge the gap between genotype and phenotype.
The primary goal of metabolomics is to profile and quantify the complete set of metabolites present in a biological sample, such as blood, urine, or tissues. This profiling involves the use of advanced analytical techniques, such as mass spectrometry (MS) and nuclear magnetic resonance (NMR) spectroscopy, coupled with various chromatographic separations to identify and quantify the diverse array of metabolites [9]. This way, the systematic study of small molecules within biological systems may help us gain insight into the metabolic changes associated with obesity, such as adipocyte-related inflammation and insulin resistance [10]. The in-depth study of the unique metabolic fingerprints associated with obesity may help us identify potential biomarkers and altered metabolic pathways, and discover novel therapeutic targets. Numerous studies have underscored the utility of metabolomics in elucidating the complex interplay between genetic predisposition, dietetic habits, gut microbiome, and environmental factors in the pathogenesis of obesity in adults [11]. However, to the best of our knowledge, few studies have been conducted in children and adolescents [12]. Metabolic signatures may differ in early life, given that children do not usually receive medical treatment for obesity. Therefore, metabolomic profiling in children and adolescents will not only facilitate the identification of potential biomarkers for the prevention and management of childhood obesity and its associated complications, but it will also unravel novel therapeutic targets.
The aim of this systematic review was to summarize the current knowledge on metabolomics, childhood obesity, and MUO, and to identify metabolic signatures or biomarkers associated with obesity in children and adolescents, thereby offering a comprehensive analysis of studies that employ metabolomic approaches. Through critical examination of the literature, this review aims to gain a better understanding of the metabolic intricacies associated with childhood obesity and inform the direction of future research and therapeutic strategies.
2. Materials and Methods
2.1. Study Design
This systematic literature review (SLR) was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) protocol [13]. The objectives were formulated using the PICO/PECO (Population, Interventions/Exposure, Comparators, Outcomes) framework (Table 1). The review was registered in the International Prospective Register of Ongoing Systematic Reviews (PROSPERO 2023 CRD42023494461; https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42023494461, accessed on 29 December 2023).

Table 1.
PICO/PECO framework for study selection on metabolomic biomarkers and childhood obesity risk.
2.2. Eligibility Criteria
The review included longitudinal prospective studies and randomized controlled trials (RCTs), with a minimum of 12 months of follow-up, and meta-analyses of the above in order to ensure a better quality of methodological design, which would also allow etiological assumptions. The studies examined the metabolic signatures related to obesity, Body Mass Index (BMI), or/and other measures of adiposity and MUO in children and adolescents aged 2–19 years with overweight or obesity. The language was restricted to English and the geographic location included only Western countries (Europe, USA, Canada, and Oceania) that share similar socioeconomic, physical, and dietary environments. The inclusion and exclusion criteria are shown in Table 2.

Table 2.
Inclusion and exclusion criteria.
2.3. Literature Search
A comprehensive literature search was conducted using PubMed and Scopus databases for studies published from 1 May 2023 to 16 September 2023. An additional data search was performed on 3 July 2024 to update the results, retrieving studies published after 16 September 2023. The search strategy included a complex string of keywords related to metabolic biomarkers, obesity, adiposity, and associated metabolic and endocrine disorders in children and adolescents. The detailed search strings used for MEDLINE (PubMed) and Scopus are presented in the Supplementary Materials, File S1.
2.4. Study Selection
Two independent researchers (GS and DK) screened the records identified from the databases. In instances of disagreement, a third researcher (EC) conducted a final review.
2.5. Data Extraction, Outcomes, and Data Synthesis
Relevant data from eligible studies were extracted, including publication details, study design, sample size, participant characteristics, metabolic signatures, biomarkers assessed, and outcomes related to obesity and metabolic disorders. The primary outcomes assessed were obesity and MUO in pediatric populations.
2.6. Validity Assessment
All included studies were assessed for risk of bias using the Risk Of Bias In Non-randomized Studies—of Exposures (ROBINS-E) tool [14]. The risk of bias for each study was evaluated across seven domains: confounding (D1), measurement of exposure (D2), selection of participants (D3), post-exposure interventions (D4), missing data (D5), measurement of outcomes (D6), and selection of reported results (D7). Three of the included studies were post hoc analyses of participants who underwent a lifestyle intervention (“Obeldicks”) within a non-randomized controlled trial [15,16] or a double-blind, randomized intervention trial [17]. Since the exposure of interest (metabolites) was not actively assigned, ROBINS-E was deemed the most appropriate tool for assessing the risk of bias in these studies.
2.7. Data Management and Synthesis
Data were managed using Mendeley and Excel. Data extraction forms were piloted and refined to ensure consistency and accuracy. Discrepancies between reviewers were resolved through discussion and consensus. The extracted data were synthesized to provide a comprehensive analysis of the metabolic signatures associated with childhood obesity and MUO. The synthesis involved qualitative analyses to summarize the findings and identify potential biomarkers and therapeutic targets. The characteristics of the included studies, e.g., study design, country, sample size, age, follow-up period, methodology, key metabolites identified, and reported outcome are presented in Table 3.

Table 3.
Characteristics of the included studies.
2.8. Ethical Considerations
Since this study is a systematic review, ethical approval was not required. However, ethical standards were maintained throughout the review process, ensuring the integrity and accuracy of the findings.
3. Results
3.1. Characteristics of Included Studies
Initially, 595 records were identified from PubMed and 1565 from Scopus. After removing duplicates and screening for relevance, 175 reports were assessed for eligibility. Ultimately, 7 (4 longitudinal and 3 post hoc analyses of interventional studies) reports were included in the review. From the additional search, 124 new records were retrieved, from which none were eligible for our study. The flow diagram is presented in Figure 1.
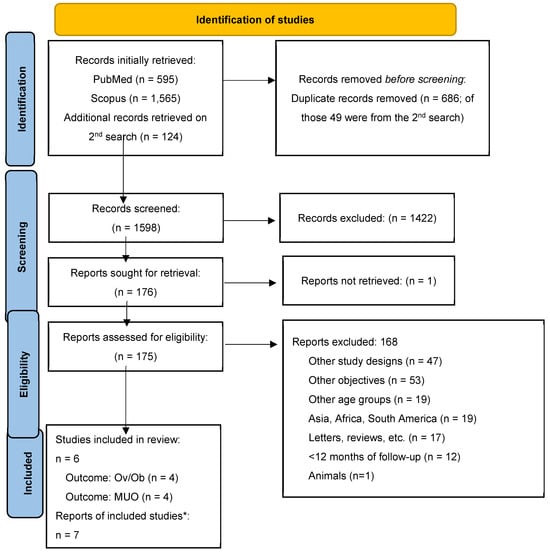
Figure 1.
PRISMA flow diagram. Notes: Ov/Ob = overweight/obesity; MUO = Metabolically Unhealthy Obesity. * Two reports were referred to a single study.
Seven reports, which were derived from six studies, met the inclusion criteria and were included in this systematic review. These studies were conducted in various countries, including the USA [18], Germany [15,16,17], Belgium [17], Italy [17], Poland [17], Spain [17], Australia [19], UK [21], Switzerland [21] and Finland [20]. The included studies were primarily longitudinal cohort studies [18,19,20,21] and post hoc analyses of intervention studies [15,16,17], with follow-up periods ranging from 1 year [15,16,18] to 11 years [21]. The intervention part included lifestyle recommendations regarding physical activity, nutrition, and behavioral therapy for the children and their families. The sample size of these studies varied significantly, from 40 to 396 participants, and the age at baseline ranged from 2 to 19 years. Serum and urine samples were used for the assessment of metabolomic biomarkers.
The included studies examined the association between metabolic profiles and obesity-related outcomes using various approaches (Table 4). Singh et al. (2023) explored the metabolic features associated with increased BMI at one-year follow-up [18]. Mansell et al. (2022) explored the association between changes in BMI and metabolomic profiles over a 5.5-year follow-up period [19]. Reinehr et al. (2014) assessed the metabolite changes in children with obesity who underwent a lifestyle intervention and compared those with substantial weight loss to those without substantial weight loss [15]. Hellmuth et al. (2019) used metabolite concentrations at 5.5 years to predict BMI z-scores at age 8 in the CHOP study [17]. Ojanen et al. (2021) developed a standardized risk score for metabolic syndrome (MetS), having incorporated metabolic and cardiovascular parameters that confer cardiometabolic risk [20]. Hellmuth et al. (2016) investigated the association between metabolite changes and HOMA-IR over a one-year lifestyle intervention (physical activity, nutrition education, and behavior therapy) [16]. Hosking et al. (2019) examined the relation between individual metabolites and insulin resistance (HOMA-IR) in healthy children, taking into account the effects of age, BMI, growth, puberty, adiposity, and physical activity [21]. This study included a pilot phase to identify metabolically distinct profiles related to insulin resistance and a follow-up phase extending the analysis to the age of 16 years to validate the findings.

Table 4.
Outcomes of interest, statistical analysis, and results of included studies.
3.2. Obesity Outcomes and Their Association with Metabolites
Our findings suggest that weight gain and weight loss influence distinct metabolic pathways, particularly in amino acids, lipids, and glycolysis-related metabolites.
3.2.1. Amino Acids and Obesity Outcomes
Changes in amino acid metabolism were consistently observed across studies. Singh et al. (2023) reported increases in Citrulline, 4-hydroxyproline, and Inosine with BMI gain [18]. Mansell et al. (2022) found that BMI reduction was associated with decreases in alanine, phenylalanine, and tyrosine [19]. In contrast, Reinehr et al. (2014) identified higher levels of glutamine and methionine in children who experienced significant weight loss [15]. These findings suggest that weight loss is associated with increased levels of certain amino acids (e.g., glutamine, methionine), while weight gain may correspond to increases in other amino acids (e.g., Citrulline, hydroxyproline).
3.2.2. Lipids and Fatty Acids and Obesity Outcomes
Metabolomic shifts in lipid profiles were evident. Mansell et al. (2022) observed that BMI reduction led to decreases in VLDL cholesterol and monounsaturated fatty acids (MUFAs), alongside increases in polyunsaturated fatty acids (PUFAs), Omega-6, and HDL cholesterol [19]. Similarly, Reinehr et al. (2014) found significant changes in phospholipids (PCaeC34:1, PCaeC34:2, etc.) among children who lost weight [15]. In contrast, Hellmuth et al. (2019) reported a positive association between sphingomyelins (SM 32:2, SM 34:2) and free carnitine with BMI at the age of 8 years, although this association was not as strong after adjusting for BMI at the age of 5.5 years [17]. These results indicate that weight gain is associated with sphingomyelins and free carnitine, whereas weight loss is associated with phospholipid alterations and shifts in fatty acid composition (e.g., increased PUFAs and Omega-6 levels).
3.2.3. Glycolysis-Related and Energy Metabolites and Obesity Outcomes
Metabolites involved in energy metabolism were also affected by weight changes. Mansell et al. (2022) demonstrated that BMI reduction was associated with lower pyruvate levels, suggesting altered glycolysis [19]. In addition, Singh et al. (2023) found that 3’-Sialyllactose increased with increases in BMI, potentially indicating metabolic shifts related to energy balance [18]. These findings suggest that an increase or decrease in BMI influences glycolysis and energy-related pathways, with weight reduction linked to decreased glycolysis activity (e.g., lower pyruvate) and weight gain associated with increased sialyllactose levels.
3.3. Metabolically Unhealthy Obesity Outcomes and Their Association with Metabolites
3.3.1. Apolipoproteins, Lipids, and Fatty Acids in Relation to Risk for Metabolic Syndrome
Lipid-related biomarkers have been consistently associated with metabolic dysfunction. Ojanen et al. (2021) found that higher ApoB/ApoA ratios and glycoprotein acetyls (GlycAs) were strong predictors of metabolic syndrome, whereas higher large high-density lipoprotein phospholipids (L-HDL-PLs) were protective, showing a negative association with MetS [20]. Similarly, findings from the CHOP study (Hellmuth et al., 2019) demonstrated that higher levels of non-esterified fatty acids (NEFAs 26:1, 26:2, 26:3) were positively associated with HOMA-IR, even after adjusting for BMI, indicating their potential role in insulin resistance [17]. These findings suggest that increased ApoB/ApoA, GlycAs, and NEFAs contribute towards a greater metabolic risk, while HDL phospholipids may play a protective role.
3.3.2. Amino Acids and Insulin Resistance
Amino acid metabolism has also been associated with insulin sensitivity and metabolic health. Hellmuth et al. (2019, CHOP study) found that higher glutamine levels were associated with lower HOMA-IR, suggesting a protective effect against insulin resistance [17]. In contrast, findings from the EarlyBird study (Hosking et al., 2019) indicated that multiple amino acids, including leucine, valine, alanine, and glutamine, were associated with insulin resistance [21]. In addition, Hellmuth et al. (2016) reported that weight loss resulted in increases in proline, tyrosine, and valine, along with acylcarnitine shifts, but decreases in Carn C6:1-DC and Carn C6-oxo, suggesting improved insulin sensitivity [16]. Taken together, these findings indicate that higher glutamine levels may be beneficial, whereas BCAAs (leucine, valine) are associated with metabolic dysfunction.
3.3.3. Energy Metabolism and Insulin Sensitivity
Metabolic markers related to energy metabolism, particularly glycolysis byproducts and mitochondrial metabolites, further illustrate the metabolic shifts associated with MUO. The EarlyBird study (Hosking et al., 2019) identified a strong positive association between lactate and insulin resistance, reinforcing its role as a key metabolic indicator of metabolic health [21]. Furthermore, Hellmuth et al. (2016) demonstrated that acylcarnitine profiles shifted in response to weight loss, with increases in Carn C0, Carn C3, and Carn C5, and decreases in Carn C6:1-DC and Carn C6-oxo levels, reflecting changes in mitochondrial fatty acid oxidation associated with improved insulin sensitivity [16]. Collectively, these findings may suggest that higher lactate levels are strongly associated with insulin resistance, while shifts in acylcarnitine metabolism may reflect improved mitochondrial function following weight loss.
The above results, as well as the statistical analysis performed, are presented in Table 4.
3.4. Risk of Bias Assessment
The studies by Singh et al., 2023 [13], and Reinehr et al., 2015 [15], have a high overall risk of bias mainly due to concerns in domains D3 and D1, respectively. The study by Hellmuth et al., 2019 [16], has a high overall risk of bias mainly due to concerns in domain D1. The other studies were of some concern regarding the overall risk of bias, mostly due to issues in domains D3 or D5. The overall risk of bias for each study is summarized in Table 5.

Table 5.
Risk of bias of the included studies.
4. Discussion
Obesity is a significant and escalating health problem globally, impacting both adults and children. Despite its prevalence, the precise mechanisms driving the development of obesity in children remain unclear. Therefore, in our systematic review, we aimed to identify candidate metabolites or profiles of obesity in children and adolescents who are relatively free of its metabolic complications. To the best of our knowledge, this is one of the first systematic reviews summarizing all available longitudinal studies focusing on metabolomics in childhood obesity.
Our thorough investigation of currently available longitudinal studies demonstrated that childhood obesity is associated with unique alterations in the metabolome, especially in lipid and amino acid metabolism. From a longitudinal perspective, our results strengthen the conclusions from other reviews that included cross-sectional data [11,12,22]. It is well known that obesity affects lipid levels through various lipid metabolism processes, including fatty acid oxidation, lipolysis, and lipogenesis [23]. Furthermore, numerous lipids act as signaling molecules in inflammation pathways or insulin resistance, contributing to obesity-related complications, such as DM2 and cardiovascular disease. Acylcarnitines are the byproducts of noncomplete fatty acid oxidation [24]. The majority of the included studies stated an association of lipids with changes in BMI, insulin resistance, and the risk of metabolic syndrome [15,17,19,20]. More specifically, these included certain lipoproteins (XL-VLDL-L, L-VLDL-L, and S-VLDL-L); Apolipoproteins (ApoB/ApoA1); cholesterols (VLDL-C); fatty acids (MUFAs, MUFAs%); glycerides and phospholipids (total triglycerides, VLDL-TGs, and TG/PG); and increases in certain cholesterols (HDL-C, HDL2-C), fatty acids (unsaturation, LA%, Omega-6%, and PUFAs), ketone bodies (Acetoacetate, 3-hydroxybutyrate), the lyso-phosphatidylcholines LPCaC18:1, LPCaC18:2, and LPCa20:4, the acyl–alkyl phosphatidylcholines PCaeC36:2, NEFAs 26:1, 26:2, and 26:6 l; and L-HDL-PLs. The study by Mansell et al. [19] provides a detailed overview of how weight loss can affect a wide array of lipoproteins, fatty acids, and amino acids. For instance, increases in unsaturated fatty acids (LA%, PUFAs%) and HDL cholesterol are indicative of an improvement in lipid metabolism, which is generally associated with better insulin sensitivity. Conversely, certain lipoproteins (like VLDL-C) and saturated fatty acids (MUFAs%) may reflect adverse metabolic changes. These results are consistent with findings from Ojanen et al. [20], who identified the Apolipoprotein B/Apolipoprotein A-1 ratio and glycoprotein acetyls as predictors of metabolic syndrome, reinforcing the concept that changes in lipoproteins and fatty acid composition may serve as useful biomarkers for cardiometabolic risk.
Amino acids play a crucial role in various physiological processes, including protein synthesis, intracellular metabolism, and immune response [25]. Six out of seven of the included reports documented an association of amino acids with changes in BMI and insulin resistance [15,16,18,19,21]. Among the overarching class of amino acids, peptides, and analogs included were glycylproline, Citrulline, formiminoglutamic acid, 4-hydroxyproline, alanine, phenylalanine, tyrosine, glutamine, methionine, serine, and alanine. In 2016, Zhao et al. reviewed insulin resistance in childhood obesity using blood metabolomics studies [26]. The authors concluded that amino acid and lipid metabolism were the most impacted. Specifically, branched-chain amino acids (BCAAs), aromatic amino acids (AAAs), and acylcarnitines were identified as being closely associated with insulin resistance and potential future cardiometabolic risk. Analysis of the cord blood metabolome of 399 newborns from four European cohorts showed that lower levels of BCAAs, valine, and leucine predicted overweight in childhood [27]. Furthermore, Hellmuth et al. [16] showed that changes in acylcarnitines and amino acids following weight loss owing to the implementation of lifestyle interventions were associated with improved insulin sensitivity, supporting the notion that these metabolites may serve as indicators of metabolic improvements. These findings suggest that metabolic shifts resulting from weight loss could improve overall health by addressing imbalances in these biomarkers.
One of the most critical metabolic disturbances associated with obesity in children is insulin resistance and DM2. The relation between obesity and insulin resistance is complex, especially in children. Understanding the mechanisms underlying insulin resistance in childhood obesity is crucial. Metabolomic profiling is an emerging approach to investigate the molecular origins of insulin resistance in children. Various cross-sectional studies in animals and adults have shown associations between insulin resistance or DM2 and BCAAs, as well as aromatic amino acids (AAAs), sulfur-containing amino acids, other amino acids, and short-chain acylcarnitines (Carns) [28,29,30]. The study by Hellmuth et al. [16] demonstrated that tyrosine and AAAs are the only metabolites significantly associated with HOMA-IR at baseline and after one year of the implementation of a lifestyle intervention program inducing substantial weight loss > 0.5 BMI standard deviation (SD) scores in children with obesity. Similarly, in a meta-analysis of 8000 adults, there was a 36% higher risk of DM2 per study-specific SD difference for isoleucine, 35% for valine, 36% for tyrosine, and 26% for phenylalanine [31]. The authors explained that tyrosine is an aromatic amino acid metabolically linked to BCAAs and may act as a primary alteration or a more significant marker in the cascade of metabolic changes, even more consistently than the BCAAs, in some cases. Furthermore, Hellmuth et al. [16] used different ratios as biomarkers for insulin resistance, such as C5/C6-oxo, C4/C5-oxo, C6-oxo/xLeu, and C5-OH/C5:1 in different subgroups. All of these indicated that incomplete fatty acid oxidation was related to a higher score of HOMA-IR. It is likely that reduced complete fatty acid oxidation results in the stimulation of proinflammatory pathways, impaired insulin action in skeletal muscle, enhanced mitochondrial stress, and finally impaired glucose metabolism in humans and rodents. Furthermore, given that C3 and C5 acylcarnitines were the byproducts of BCAAs, reduced complete fatty acid oxidation seemed to be influenced by BCAA metabolism, indicating a close interaction of amino acid metabolism and lipid metabolism. In the study of Hosking et al. [21], insulin resistance was associated with a characteristic molecular phenotype, including lower levels of BCAAs and 2-ketobutyrate. This association was mainly attributed to the role of the mitochondrial enzyme Branched-Chain Keto acid Dehydrogenase (BCKD) in the generation of elevated BCAAs and branched-chain keto acids in insulin resistance and obesity [32]. The Krebs cycle through citrate and 3-hydroxybutyrate intermediates reduced ketogenesis, while elevated lactate and alanine concentrations were found to precede insulin resistance. The association between lactate and insulin resistance was robust even after adjusting for confounders, suggesting that lactate may serve as a reliable biomarker for metabolic health. Lactate, which is traditionally considered a byproduct of anaerobic metabolism, has emerged as an important molecule in metabolic disorders, and its elevated levels may reflect a shift toward anaerobic glycolysis, which is commonly seen in conditions of insulin resistance and metabolic stress. However, these findings are derived from a longitudinal study of a cohort of healthy children and may be different in children with obesity.
Obesity also represents a cardiovascular risk factor. One study evaluated childhood metabolic predictors of adult cardiovascular disease risk (MetS score) in a cohort of 396 females [20]. The authors suggested that exposure to atherogenic Apolipoprotein profile and low HDL concentrations in childhood, as well as a proinflammatory response that includes GlycAs, may lead to changes in the arteries that contribute to the development of atherosclerosis and coronary heart disease in adulthood. The ApoB/ApoA1 ratio indicates the balance between atherogenic ApoB and antiatherogenic ApoA1 cholesterol particles and is strongly and positively correlated with cardiovascular risk in adults [33]. In a pediatric population, the ratio of ApoB/ApoA1 ratio was also strongly correlated with increased waist circumference, BMI, fat percentage, diastolic blood pressure, and incidence of MetS [34]. Furthermore, the ApoB/ApoA1 ratio in young Finns predicted carotid intima-media thickness and brachial endothelial function in adulthood [35]. The association of low HDL phospholipids with coronary artery disease has been documented in adult subjects too [36]. Finally, GlycAs might also serve as biomarkers of subclinical vascular inflammation. In the study by Mansell et al. [19], decreases in BMI were associated with decreases in glycoprotein acetyls. In a cross-sectional study of 9842 adults, GlycAs correlated with adiposity, insulin resistance, and other markers of metabolic syndrome and all-cause mortality [37,38].
The present study has notable strengths. One of the key strengths of this systematic review is its comprehensive approach to literature search and inclusion criteria, which ensured a broad capture of relevant studies. An additional strength is the diversity of the included populations, spanning multiple countries and age groups. Moreover, the focus on longitudinal studies and randomized controlled trials with long follow-up periods allows for a more in-depth understanding of causal relations and the progression of metabolic changes associated with obesity.
Despite its strengths, the review has several limitations. The potential for publication bias is significant, given that studies with positive findings are more likely to be published. This bias may overestimate the association between certain metabolites and obesity outcomes. In addition, the exclusion of non-English studies may limit the comprehensiveness of the review, as relevant research published in other languages was not considered. The included studies varied widely in their design, sample size, population characteristics, and methodologies. This heterogeneity introduces variability in the results, making direct comparisons between studies challenging. There is a lack of standardization in the metabolomic techniques and analytical platforms used across the included studies. Differences in sample preparation, metabolite extraction, and data analysis can lead to inconsistencies in the identification and quantification of metabolites. This variability may impact the reproducibility of the findings. The wide age range of participants, from early childhood to adolescence, may introduce age-related metabolic variations that are not fully accounted for in the analyses. Metabolic processes can differ significantly between younger children and adolescents, affecting the generalizability of the results. The studies included in this review were conducted in various geographic regions, including Europe, the USA, and Australia. While this diversity is a strength, it also introduces potential geographic and socioeconomic variations that may influence metabolic profiles. Differences in diet, lifestyle, and access to healthcare can impact the generalizability of the findings.
The results from our systematic review suggest that changes in BMI in children and adolescents with obesity are associated with specific metabolic changes in the metabolome of individuals, especially in amino acids and lipid metabolism, which may serve as powerful diagnostic tools for disease monitoring and risk assessment. Understanding the pathways and mechanisms driving these associations is crucial for developing targeted interventions. Further research should focus on elucidating these mechanisms to provide a more comprehensive understanding of the metabolic alterations associated with obesity. In the future, longitudinal studies that track metabolic changes over time, intervention studies that evaluate the efficacy of therapeutic strategies, and large-scale population studies that explore metabolic diversity across different demographics will be important.
5. Conclusions
Metabolomics enhances our comprehension of disease progression and metabolic pathways in an obesogenic environment. This systematic review offers valuable insights into specific metabolite patterns characteristic of childhood obesity, including metabolically healthy and unhealthy phenotypes, and potential metabolomic profiles associated with their complications. However, available longitudinal studies in children and adolescents are few, and further studies are required to confirm the proposed metabolic signature.
Weight loss appears to improve several metabolic parameters, including lipid metabolism, amino acid profiles, and inflammatory markers, which may contribute to better insulin sensitivity and reduced risk for metabolic diseases. Notably, certain metabolites such as glycoprotein acetyls, ApoB/ApoA1 ratio, and lactate emerge as promising biomarkers for insulin resistance and metabolic syndrome, suggesting their potential utility in clinical settings. The metabolic fingerprints gleaned from these studies not only shed light on the current state of metabolic health in children with obesity but also have the potential to guide preventive and therapeutic strategies. This knowledge can be particularly important in the pediatric population, where early intervention might have long-lasting effects. Given the findings, the high risk of bias in some studies does not undermine the overall value of metabolomics in childhood obesity research but emphasizes the need for stringent methodological approaches in future studies to enhance the reliability of the conclusions drawn. As such, continued exploration of metabolomic profiles in childhood obesity is warranted, particularly in pediatrics, to develop targeted interventions and prevent the long-term consequences of this condition.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/nu17111833/s1. File S1. Search strings used for MEDLINE (PubMed) and Scopus.
Author Contributions
Conceptualization, E.C.; methodology, D.K., G.S., A.K., M.D.K., P.K. and E.C.; software, G.S., validation, P.K. and E.C.; formal analysis, D.K., G.S., P.K. and E.C.; investigation, D.K., G.S. and P.K.; resources, P.K. and E.C.; data curation, D.K., G.S. and P.K.; writing—original draft preparation, D.K., G.S. and P.K.; writing—review and editing, D.K., G.S., S.-M.G., E.R., A.K., M.D.K., E.K., E.G., P.K. and E.C.; visualization, E.C.; supervision, P.K. and E.C.; project administration, P.K. and E.C.; funding acquisition, E.C. All authors have read and agreed to the published version of the manuscript.
Funding
The work leading to these results has received funding from the HORIZON European Research and Innovation Action project under Grant Agreement No. 101080718. The project is entitled “Multi-Pillar Framework for children Anti-Obesity Behavior building on an EU biobank, Micro Moments and Mobile Recommendation Systems”, Acronym: BIO-STREAMS, https://www.bio-streams.eu/.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- World Health Organization. Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 5 February 2024).
- WHO. European Regional Obesity Report. 2022. Available online: https://www.who.int/europe/publications/i/item/9789289057738 (accessed on 5 February 2024).
- Kumar, S.; Kelly, A.S. Review of Childhood Obesity: From Epidemiology, Etiology, and Comorbidities to Clinical Assessment and Treatment. Mayo Clin. Proc. 2017, 92, 251–265. [Google Scholar] [CrossRef] [PubMed]
- Mathew, H.; Farr, O.M.; Mantzoros, C.S. Metabolic Health and Weight: Understanding metabolically unhealthy normal weight or metabolically healthy obese patients. Metabolism 2016, 65, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Hivert, M.F.; Perng, W.; Watkins, S.M.; Newgard, C.S.; Kenny, L.C.; Kristal, B.S.; Patti, M.E.; Isganaitis, E.; DeMeo, D.L.; Oken, E.; et al. Metabolomics in the developmental origins of obesity and its cardiometabolic consequences. J. Dev. Orig. Health Dis. 2015, 6, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Regan, J.A.; Shah, S.H. Obesity Genomics and Metabolomics: A Nexus of Cardiometabolic Risk. Curr. Cardiol. Rep. 2020, 22, 174. [Google Scholar] [CrossRef]
- Bujak, R.; Struck-Lewicka, W.; Markuszewski, M.J.; Kaliszan, R. Metabolomics for laboratory diagnostics. J. Pharm. Biomed. Anal. 2015, 113, 108–120. [Google Scholar] [CrossRef]
- Schrimpe-Rutledge, A.C.; Codreanu, S.G.; Sherrod, S.D.; McLean, J.A. Untargeted Metabolomics Strategies-Challenges and Emerging Directions. J. Am. Soc. Mass. Spectrom. 2016, 27, 1897–1905. [Google Scholar] [CrossRef]
- Wang, R.; Li, B.; Lam, S.M.; Shui, G. Integration of lipidomics and metabolomics for in-depth understanding of cellular mechanism and disease progression. J. Genet. Genom. 2020, 47, 69–83. [Google Scholar] [CrossRef]
- Ouchi, N.; Parker, J.L.; Lugus, J.J.; Walsh, K. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 2011, 11, 85–97. [Google Scholar] [CrossRef]
- Rangel-Huerta, O.D.; Pastor-Villaescusa, B.; Gil, A. Are we close to defining a metabolomic signature of human obesity? A systematic review of metabolomics studies. Metabolomics Off. J. Metabolomic Soc. 2019, 15, 93. [Google Scholar] [CrossRef]
- Handakas, E.; Lau, C.H.; Alfano, R.; Chatzi, V.L.; Plusquin, M.; Vineis, P.; Robinson, O. A systematic review of metabolomic studies of childhood obesity: State of the evidence for metabolic determinants and consequences. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2022, 23 (Suppl. S1), e13384. [Google Scholar] [CrossRef]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; The PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ 2015, 350, g7647. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Morgan, R.L.; Rooney, A.A.; Taylor, K.W.; Thayer, K.A.; Silva, R.A.; Lemeris, C.; Akl, E.A.; Bateson, T.F.; Berkman, N.D.; et al. A tool to assess risk of bias in non-randomized follow-up studies of exposure effects (ROBINS-E). Environ. Int. 2024, 186, 108602. [Google Scholar] [CrossRef] [PubMed]
- Reinehr, T.; Wolters, B.; Knop, C.; Lass, N.; Hellmuth, C.; Harder, U.; Peissner, W.; Wahl, S.; Grallert, H.; Adamski, J.; et al. Changes in the serum metabolite profile in obese children with weight loss. Eur. J. Nutr. 2015, 54, 173–181. [Google Scholar] [CrossRef]
- Hellmuth, C.; Kirchberg, F.F.; Lass, N.; Harder, U.; Peissner, W.; Koletzko, B.; Reinehr, T. Tyrosine Is Associated with Insulin Resistance in Longitudinal Metabolomic Profiling of Obese Children. J. Diabetes Res. 2016, 2016, 2108909. [Google Scholar] [CrossRef]
- Hellmuth, C.; Kirchberg, F.F.; Brandt, S.; Moß, A.; Walter, V.; Rothenbacher, D.; Brenner, H.; Grote, V.; Gruszfeld, D.; Socha, P.; et al. An individual participant data meta-analysis on metabolomics profiles for obesity and insulin resistance in European children. Sci. Rep. 2019, 9, 5053. [Google Scholar] [CrossRef]
- Singh, A.; Kinnebrew, G.; Hsu, P.-C.; Weng, D.Y.; Song, M.-A.; Reisinger, S.A.; McElroy, J.P.; Keller-Hamilton, B.; Ferketich, A.K.; Freudenheim, J.L.; et al. Untargeted Metabolomics and Body Mass in Adolescents: A Cross-Sectional and Longitudinal Analysis. Metabolites 2023, 13, 899. [Google Scholar] [CrossRef]
- Mansell, T.; Magnussen, C.G.; Nuotio, J.; Laitinen, T.T.; Harcourt, B.E.; Bekkering, S.; McCallum, Z.; Kao, K.-T.; Sabin, M.A.; Juonala, M.; et al. Decreasing severity of obesity from early to late adolescence and young adulthood associates with longitudinal metabolomic changes implicated in lower cardiometabolic disease risk. Int. J. Obes. 2022, 46, 646–654. [Google Scholar] [CrossRef]
- Ojanen, X.; Cheng, R.; Törmäkangas, T.; Rappaport, N.; Wilmanski, T.; Wu, N.; Fung, E.; Nedelec, R.; Sebert, S.; Vlachopoulos, D.; et al. Towards early risk biomarkers: Serum metabolic signature in childhood predicts cardio-metabolic risk in adulthood. EBioMedicine 2021, 72, 1–10. [Google Scholar] [CrossRef]
- Hosking, J.; Pinkney, J.; Jeffery, A.; Cominetti, O.; Da Silva, L.; Collino, S.; Kussmann, M.; Hager, J.; Martin, F. Insulin Resistance during normal child growth and development is associated with a distinct blood metabolic phenotype (Earlybird 72). Pediatr. Diabetes 2019, 20, 832–841. [Google Scholar] [CrossRef]
- Monnerie, S.; Comte, B.; Ziegler, D.; Morais, J.A.; Pujos-Guillot, E.; Gaudreau, P. Metabolomic and Lipidomic Signatures of Metabolic Syndrome and its Physiological Components in Adults: A Systematic Review. Sci. Rep. 2020, 10, 669. [Google Scholar] [CrossRef]
- Aguilera, C.M.; Gil-Campos, M.; Cañete, R.; Gil, A. Alterations in plasma and tissue lipids associated with obesity and metabolic syndrome. Clin. Sci. 2008, 114, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Ritter, O.; Jelenik, T.; Roden, M. Lipid-mediated muscle insulin resistance: Different fat, different pathways? J. Mol. Med. Berl. Ger. 2015, 93, 831–843. [Google Scholar] [CrossRef]
- Kelly, B.; Pearce, E.L. Amino Assets: How Amino Acids Support Immunity. Cell Metab. 2020, 32, 154–175. [Google Scholar] [CrossRef]
- Zhao, X.; Gang, X.; Liu, Y.; Sun, C.; Han, Q.; Wang, G. Using Metabolomic Profiles as Biomarkers for Insulin Resistance in Childhood Obesity: A Systematic Review. J. Diabetes Res. 2016, 2016, 8160545. [Google Scholar] [CrossRef]
- Handakas, E.; Keski-Rahkonen, P.; Chatzi, L.; Alfano, R.; Roumeliotaki, T.; Plusquin, M.; Maitre, L.; Richiardi, L.; Brescianini, S.; Scalbert, A.; et al. Cord blood metabolic signatures predictive of childhood overweight and rapid growth. Int. J. Obes. 2021, 45, 2252–2260. [Google Scholar] [CrossRef]
- Würtz, P.; Mäkinen, V.P.; Soininen, P.; Kangas, A.J.; Tukiainen, T.; Kettunen, J.; Savolainen, M.J.; Tammelin, T.; Viikari, J.S.; Rönnemaa, T.; et al. Metabolic Signatures of Insulin Resistance in 7,098 Young Adults. Diabetes 2012, 61, 1372–1380. [Google Scholar] [CrossRef]
- Mohorko, N.; Petelin, A.; Jurdana, M.; Biolo, G.; Jenko-Pražnikar, Z. Elevated serum levels of cysteine and tyrosine: Early biomarkers in asymptomatic adults at increased risk of developing metabolic syndrome. BioMed Res. Int. 2015, 2015, 418681. [Google Scholar] [CrossRef]
- Zhao, X.; Han, Q.; Liu, Y.; Sun, C.; Gang, X.; Wang, G. The Relationship between Branched-Chain Amino Acid Related Metabolomic Signature and Insulin Resistance: A Systematic Review. J. Diabetes Res. 2016, 2016, 2794591. [Google Scholar] [CrossRef]
- Guasch-Ferré, M.; Hruby, A.; Toledo, E.; Clish, C.B.; Martínez-González, M.A.; Salas-Salvadó, J.; Hu, F.B. Metabolomics in Prediabetes and Diabetes: A Systematic Review and Meta-analysis. Diabetes Care. 2016, 39, 833–846. [Google Scholar] [CrossRef]
- Adams, S.H. Emerging perspectives on essential amino acid metabolism in obesity and the insulin-resistant state. Adv. Nutr. Bethesda Md. 2011, 2, 445–456. [Google Scholar] [CrossRef]
- Walldius, G.; Jungner, I.; Aastveit, A.H.; Holme, I.; Furberg, C.D.; Sniderman, A.D. The apoB/apoA-I ratio is better than the cholesterol ratios to estimate the balance between plasma proatherogenic and antiatherogenic lipoproteins and to predict coronary risk. Clin. Chem. Lab. Med. 2004, 42, 1355–1363. [Google Scholar] [CrossRef]
- Sellers, E.A.C.; Singh, G.R.; Sayers, S.M. Apo-B/AI ratio identifies cardiovascular risk in childhood: The Australian Aboriginal Birth Cohort study. Diabetes Vasc. Dis. Res. 2009, 6, 94–99. [Google Scholar] [CrossRef]
- Juonala, M.; Viikari, J.S.; Kähönen, M.; Solakivi, T.; Helenius, H.; Jula, A.; Marniemi, J.; Taittonen, L.; Laitinen, T.; Nikkari, T.; et al. Childhood levels of serum apolipoproteins B and A-I predict carotid intima-media thickness and brachial endothelial function in adulthood: The cardiovascular risk in young Finns study. J. Am. Coll. Cardiol. 2008, 52, 293–299. [Google Scholar] [CrossRef]
- Piperi, C.; Kalofoutis, C.; Papaevaggeliou, D.; Papapanagiotou, A.; Lekakis, J.; Kalofoutis, A. The significance of serum HDL phospholipid levels in angiographically defined coronary artery disease. Clin. Biochem. 2004, 37, 377–381. [Google Scholar] [CrossRef]
- Fischer, K.; Kettunen, J.; Würtz, P.; Haller, T.; Havulinna, A.S.; Kangas, A.J.; Soininen, P.; Esko, T.; Tammesoo, M.-L.; Mägi, R.; et al. Biomarker profiling by nuclear magnetic resonance spectroscopy for the prediction of all-cause mortality: An observational study of 17,345 persons. PLoS Med. 2014, 11, e1001606. [Google Scholar] [CrossRef]
- Lawler, P.R.; Akinkuolie, A.O.; Chandler, P.D.; Moorthy, M.V.; Vandenburgh, M.J.; Schaumberg, D.A.; Lee, I.-M.; Glynn, R.J.; Ridker, P.M.; Buring, J.E.; et al. Circulating N-Linked Glycoprotein Acetyls and Longitudinal Mortality Risk. Circ. Res. 2016, 118, 1106–1115. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).