The Preventive Effect of Exercise and Oral Branched-Chain Amino Acid Supplementation on Obesity-Induced Brain Changes in Ldlr−/−.Leiden Mice
Abstract
1. Introduction
2. Materials and Methods
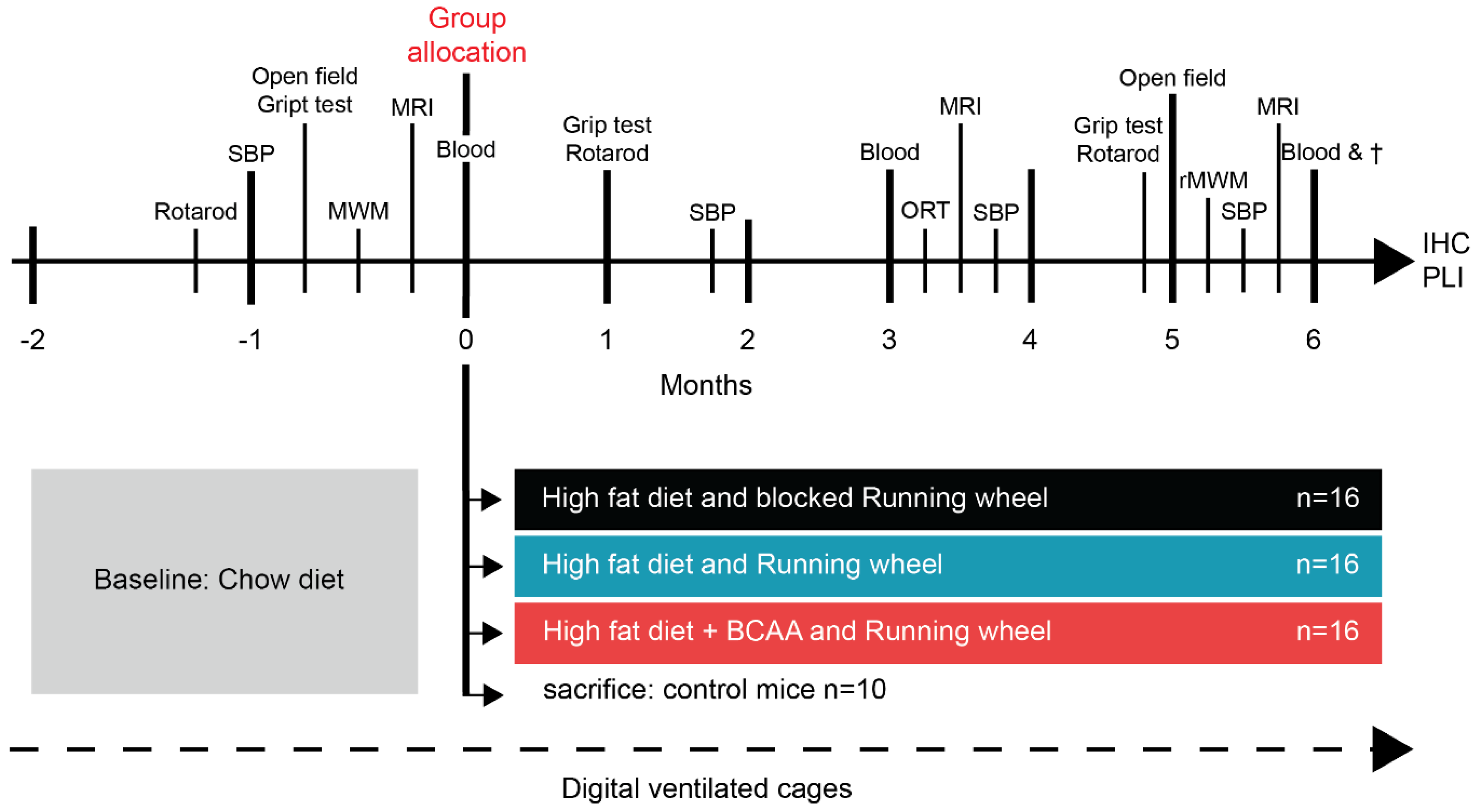
2.1. Animals, Diets, and Study Design
2.2. Plasma Analysis
2.3. Systolic Blood Pressure
2.4. Home-Cage Activity
2.5. Running Wheel
2.6. Open Field Test
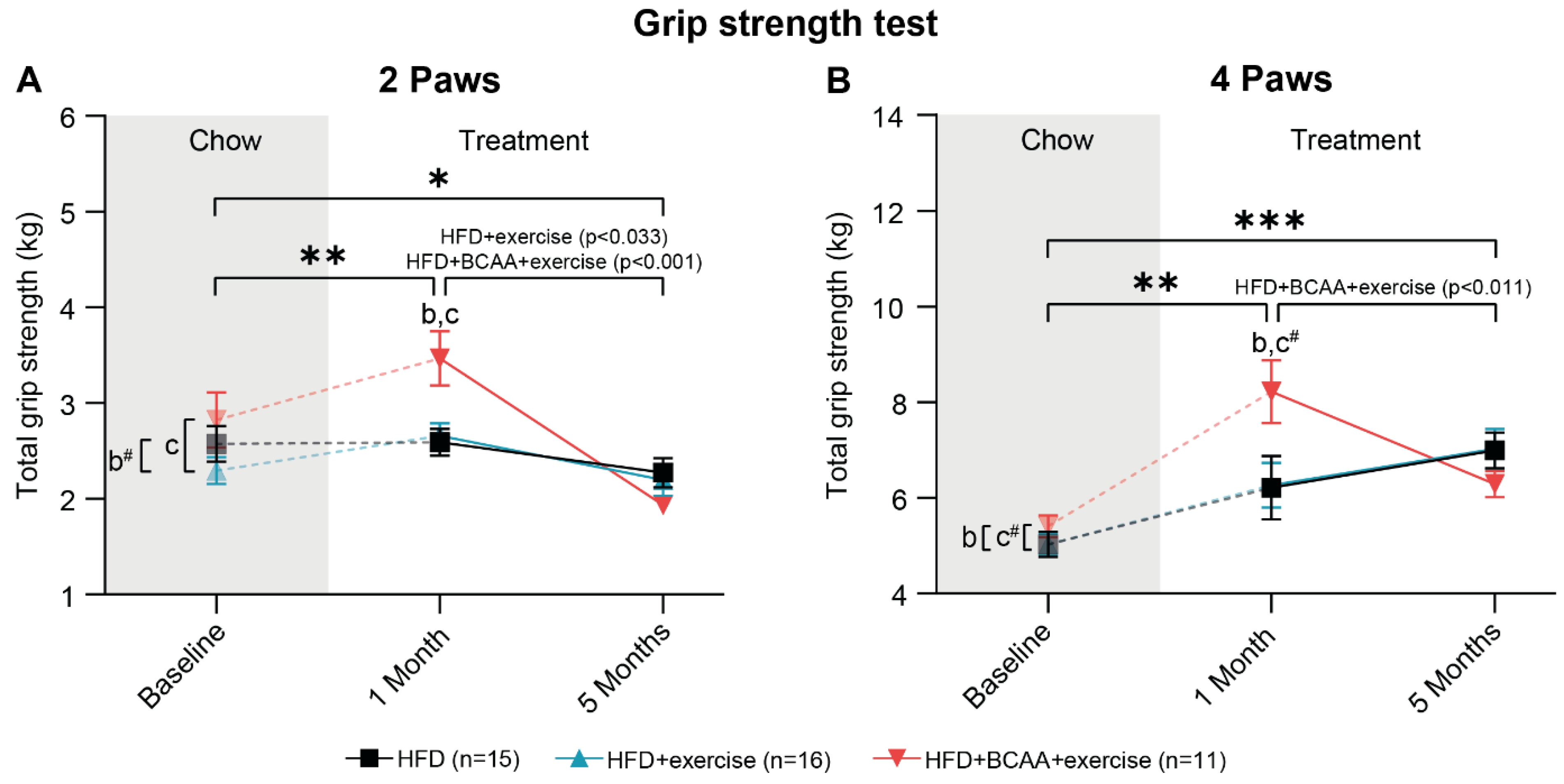
2.7. Grip Strength Test
2.8. Rotarod
2.9. Novel Object Recognition Test
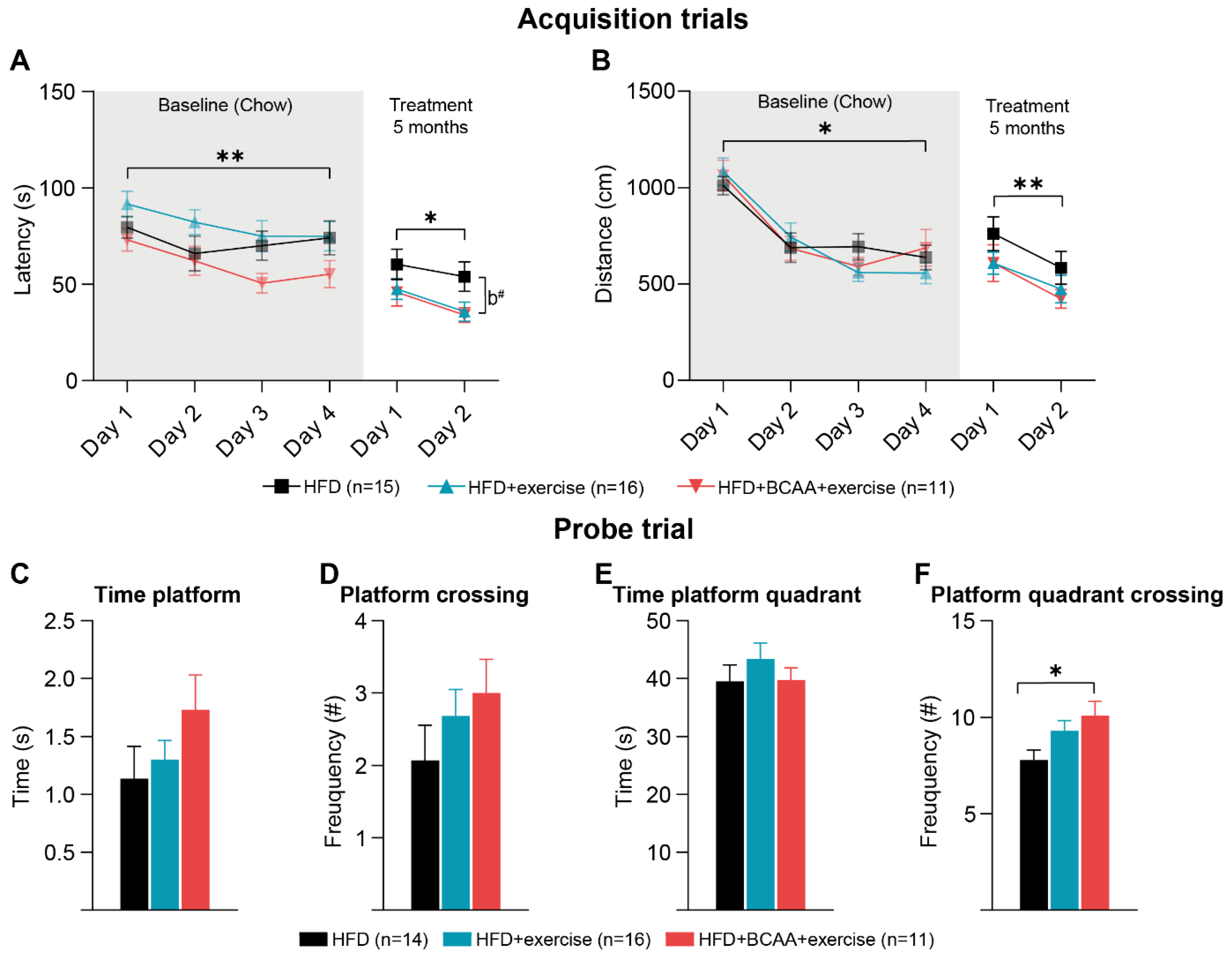
2.10. Morris Water Maze
Reverse Trial
2.11. MRI Protocol: Cortical Thickness, Hippocampal Volume, ASL, DTI, and rsfMRI
2.11.1. Hippocampal Volume and Cortical Thickness
2.11.2. Resting-State fMRI
2.11.3. Cerebral Blood Flow
2.11.4. Diffusion Tensor Imaging
2.12. Tissue Preparation
2.13. Immunohistochemical Stainings
2.14. Quantification
2.15. Polarized Light Imaging
2.16. Statistics
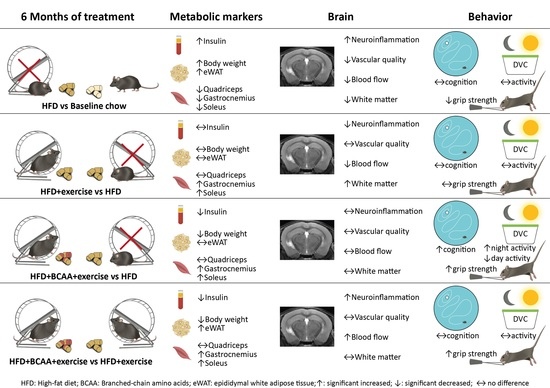
3. Results
3.1. Body Weight, Caloric Intake, Fat Depots, and Muscle Mass
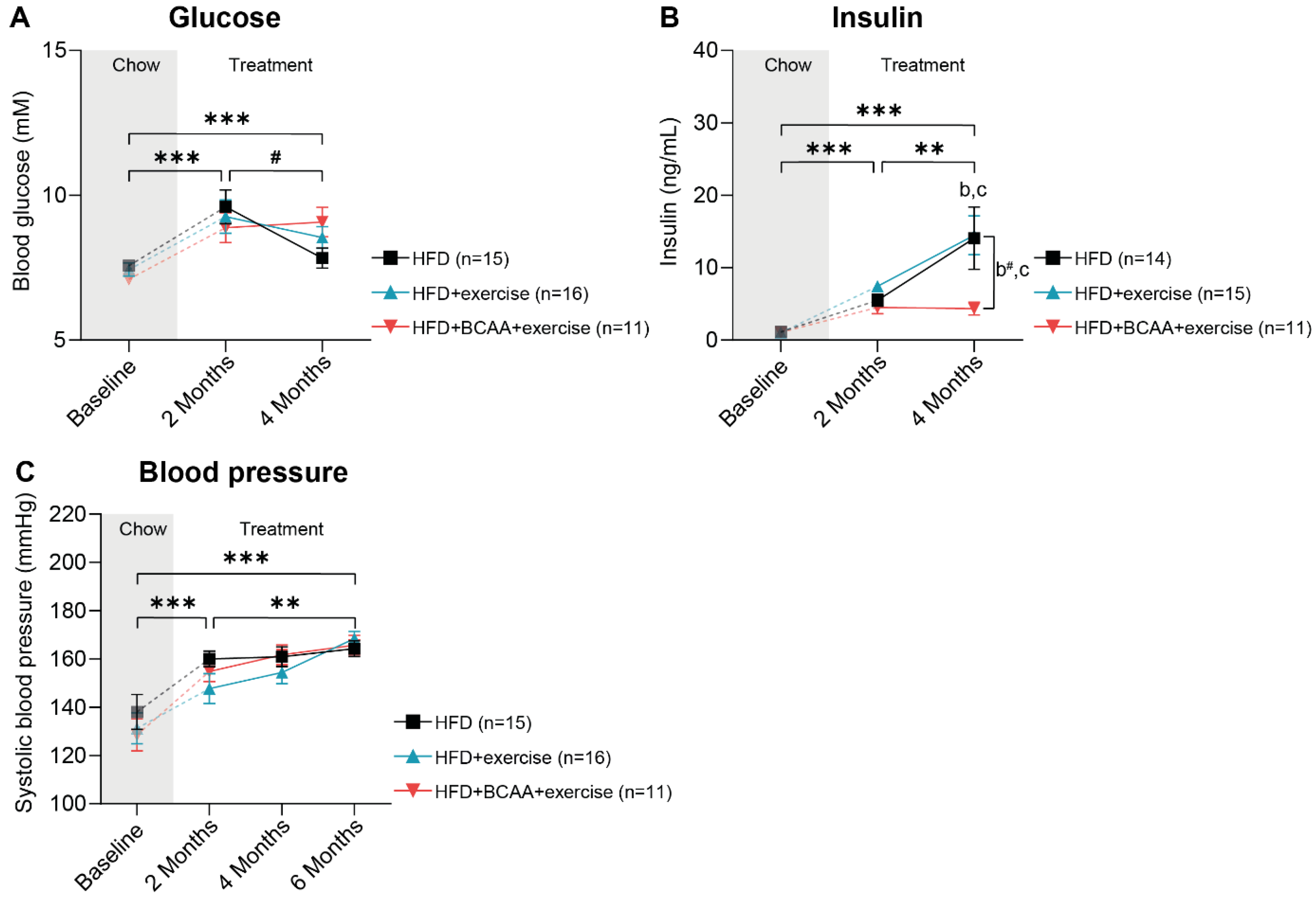
3.2. Metabolic Factors and Systolic Blood Pressure
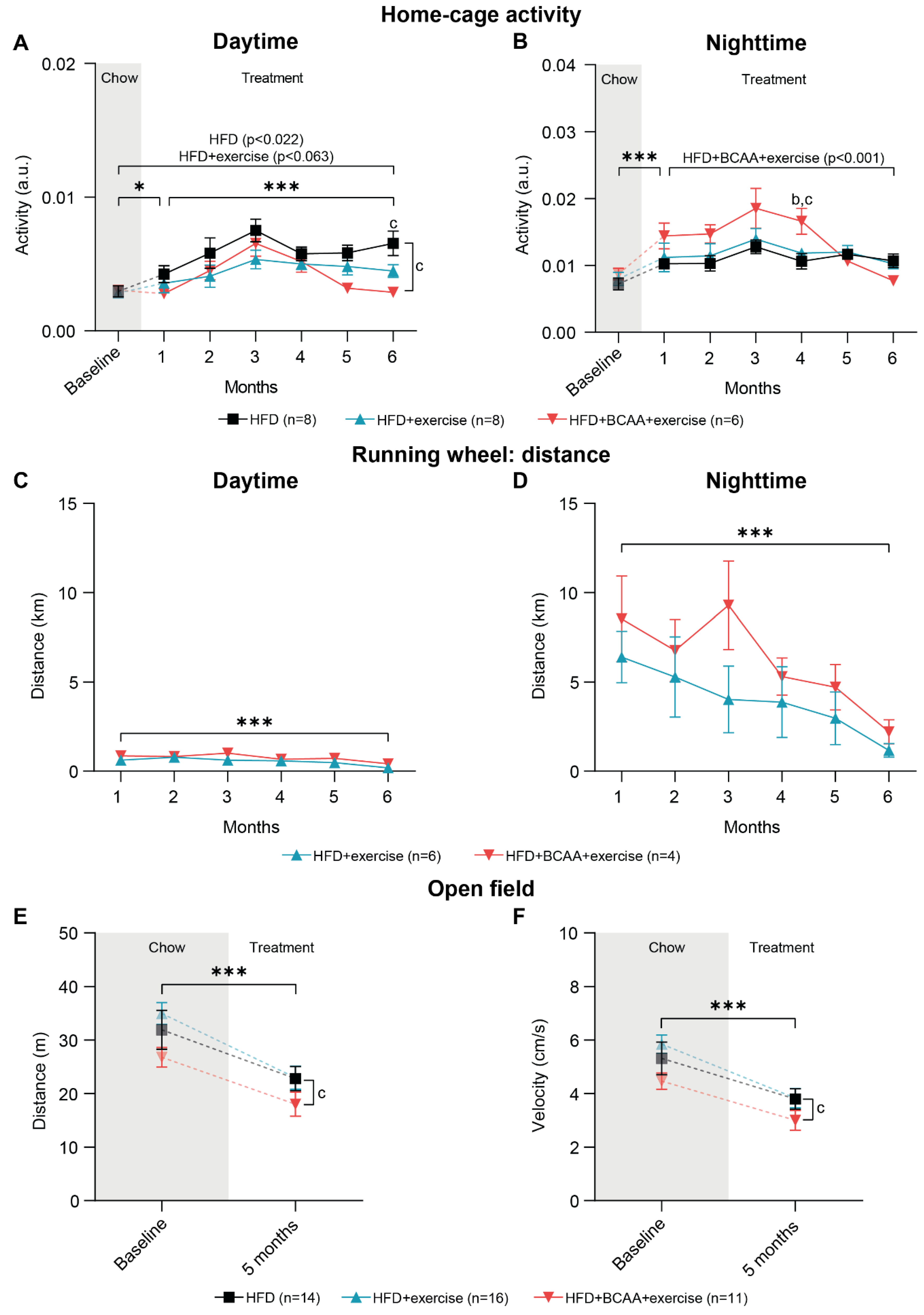
3.3. Voluntary Exercise, Home-Cage Activity, and Locomotive Behavior
3.3.1. Home-Cage Activity
3.3.2. Running Wheel Distance
3.3.3. Open Field Test
3.4. Muscle Strength
3.5. Cognition: Morris Water Maze
3.5.1. Morris Water Maze
3.5.2. Reverse Morris Water Maze
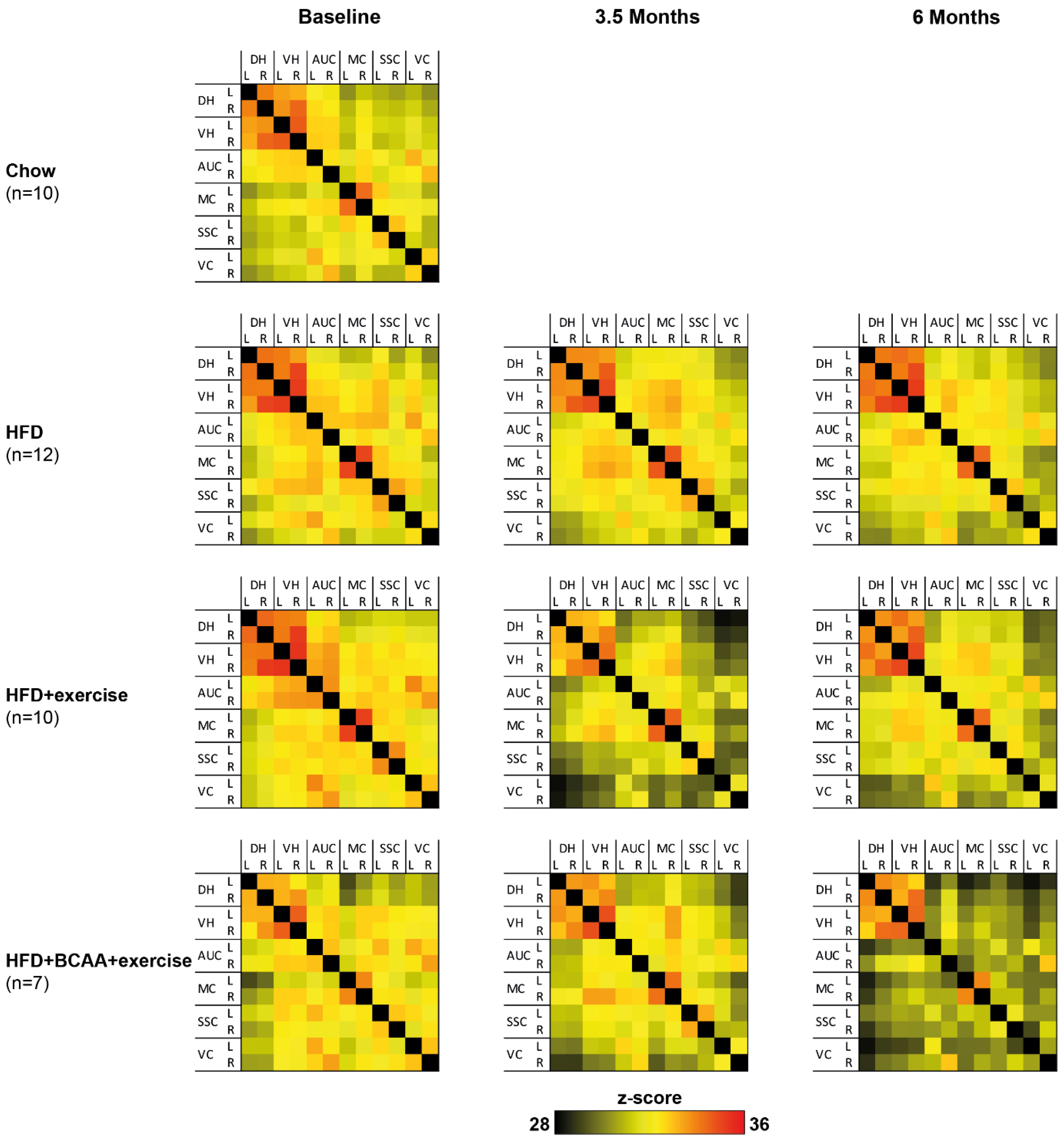
3.6. Functional Connectivity
3.6.1. Total Correlation
3.6.2. Partial Correlations
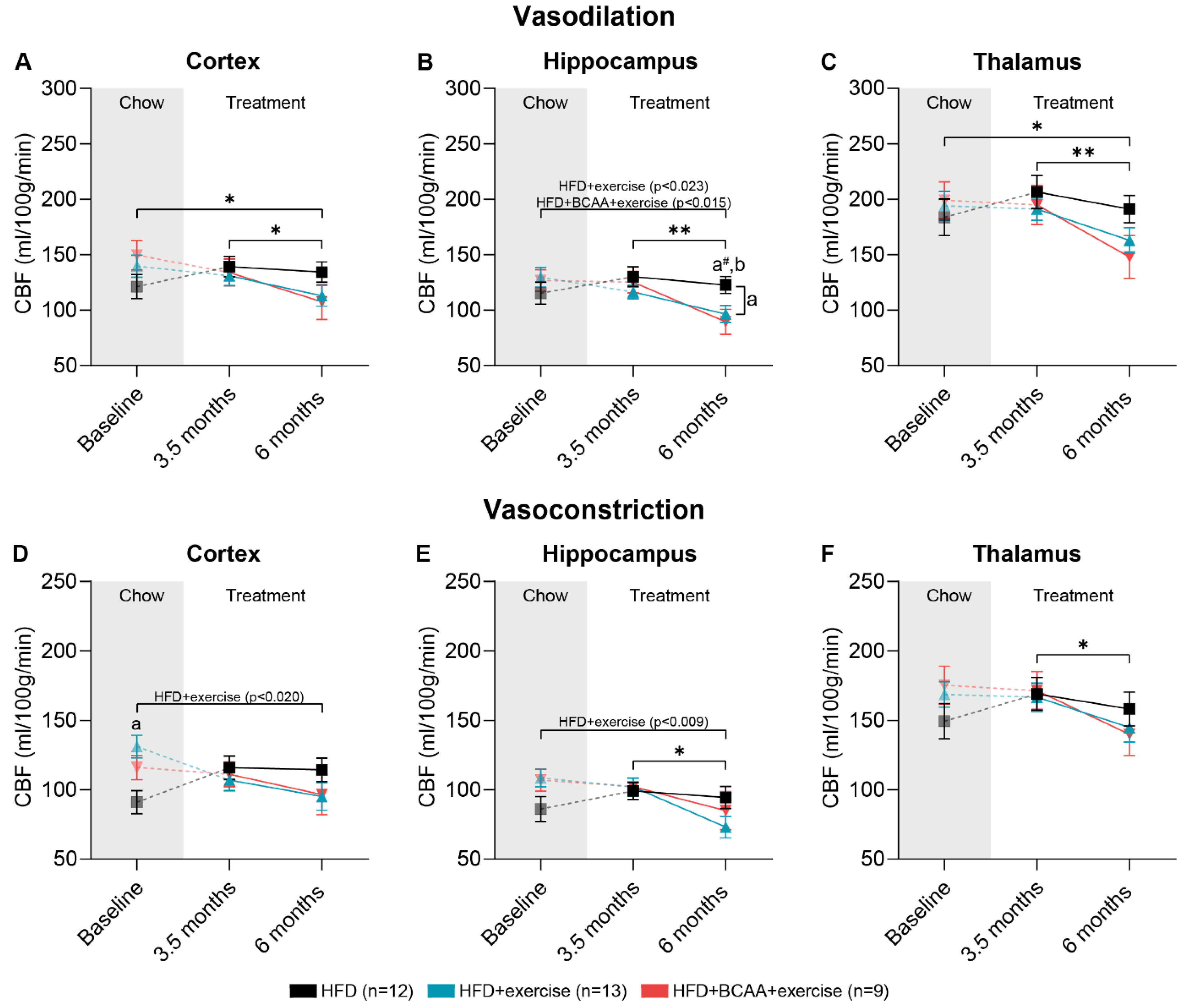
3.7. Vascular Integrity and Quality
3.7.1. CBF: Vasodilative Conditions
3.7.2. CBF: Vasoconstrictive Conditions
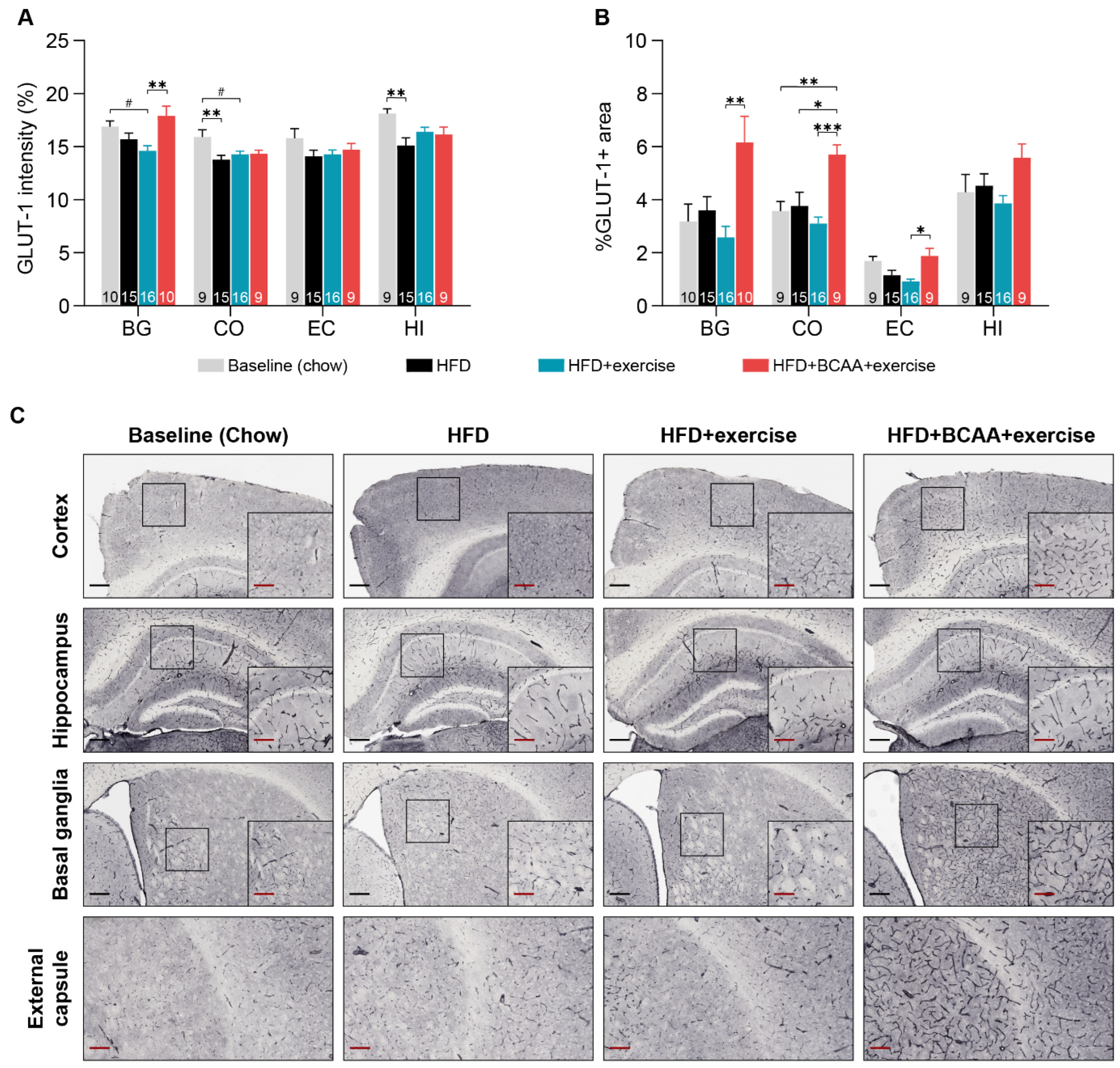
3.7.3. Glucose Transporter-1
3.8. Microstructural Changes in Grey and White Matter
3.8.1. Diffusion Tensor Imaging
3.8.2. Polarized Light Imaging
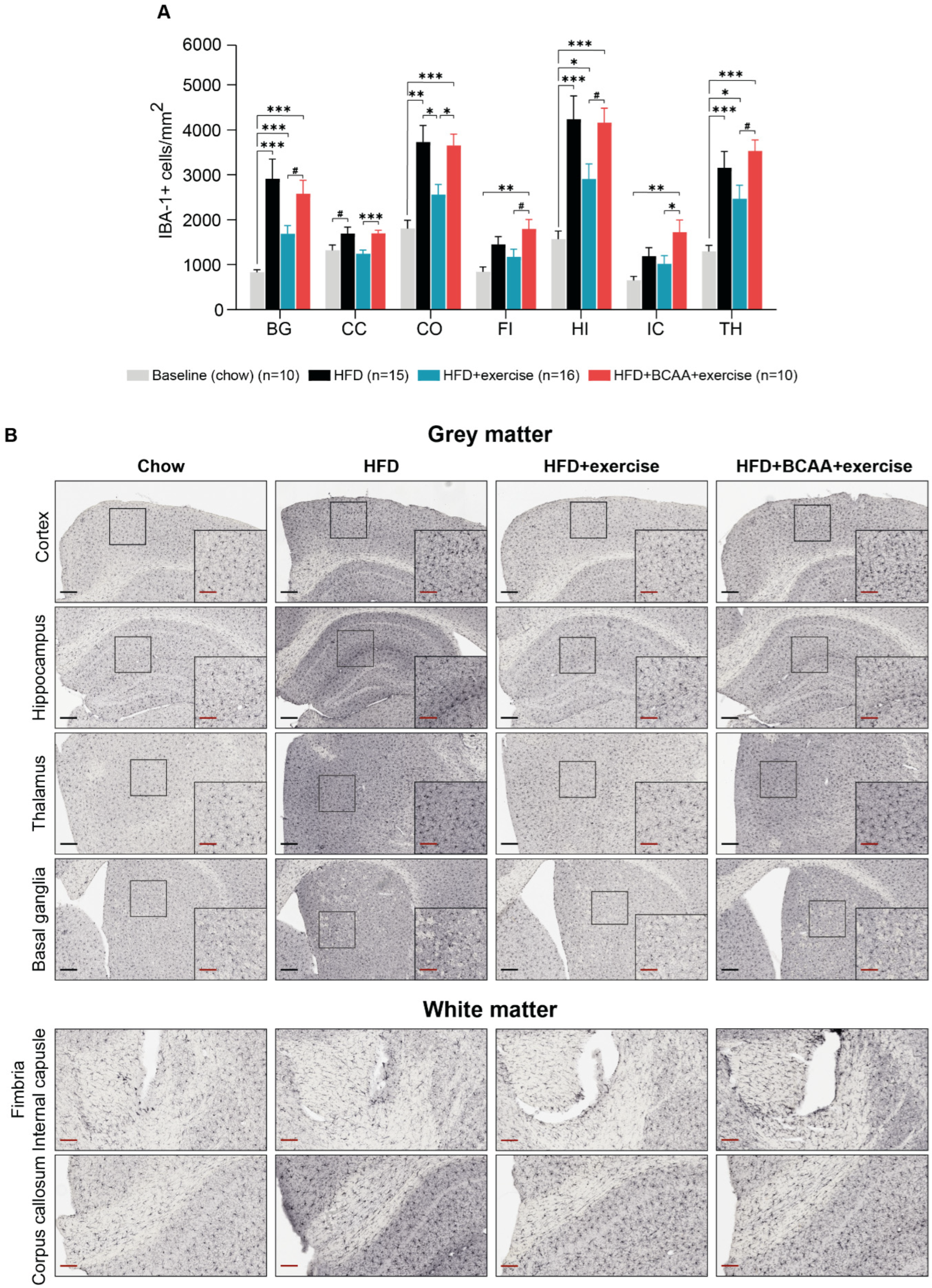
3.9. Neuroinflammation
4. Discussion
4.1. Metabolism and Physical Activity
4.2. Cerebrovasculature
4.3. White and Grey Matter Integrity
4.4. Neuroinflammation
4.5. Cognition
4.6. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chooi, Y.C.; Ding, C.; Magkos, F. The epidemiology of obesity. Metabolism 2019, 92, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Apo, E.; Mondragón-Maya, A.; Ferrari-Díaz, M.; Silva-Pereyra, J. Structural brain changes associated with overweight and obesity. J. Obes. 2021, 2021, 6613385. [Google Scholar] [CrossRef] [PubMed]
- Beydoun, M.A.; Kivimaki, M. Midlife Obesity, Related Behavioral Factors, and the Risk of Dementia in Later Life; AAN Enterprises: Uxbridge, UK, 2020; Volume 94, pp. 53–54. [Google Scholar]
- Herrmann, M.J.; Tesar, A.K.; Beier, J.; Berg, M.; Warrings, B. Grey matter alterations in obesity: A meta-analysis of whole-brain studies. Obes. Rev. 2019, 20, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Repple, J.; Opel, N.; Meinert, S.; Redlich, R.; Hahn, T.; Winter, N.R.; Kaehler, C.; Emden, D.; Leenings, R.; Grotegerd, D.; et al. Elevated body-mass index is associated with reduced white matter integrity in two large independent cohorts. Psychoneuroendocrinology 2018, 91, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Willeumier, K.C.; Taylor, D.V.; Amen, D.G. Elevated BMI is associated with decreased blood flow in the prefrontal cortex using SPECT imaging in healthy adults. Obesity 2011, 19, 1095–1097. [Google Scholar] [CrossRef] [PubMed]
- Daoust, J.; Schaffer, J.; Zeighami, Y.; Dagher, A.; García-García, I.; Michaud, A. White matter integrity differences in obesity: A meta-analysis of diffusion tensor imaging studies. Neurosci. Biobehav. Rev. 2021, 129, 133–141. [Google Scholar] [CrossRef]
- Romieu, I.; Dossus, L.; Barquera, S.; Blottière, H.M.; Franks, P.W.; Gunter, M.; Hwalla, N.; Hursting, S.D.; Leitzmann, M.; Margetts, B.; et al. Energy balance and obesity: What are the main drivers? Cancer Causes Control 2017, 28, 247–258. [Google Scholar] [CrossRef]
- Nguyen, J.C.; Killcross, A.S.; Jenkins, T.A. Obesity and cognitive decline: Role of inflammation and vascular changes. Front. Neurosci. 2014, 8, 375. [Google Scholar] [CrossRef]
- Iadecola, C.; Davisson, R.L. Hypertension and cerebrovascular dysfunction. Cell Metab. 2008, 7, 476–484. [Google Scholar] [CrossRef]
- Sarma, S.; Sockalingam, S.; Dash, S. Obesity as a multisystem disease: Trends in obesity rates and obesity-related complications. Diabetes Obes. Metab. 2021, 23, 3–16. [Google Scholar] [CrossRef]
- Solé-Guardia, G.; Custers, E.; de Lange, A.; Clijncke, E.; Geenen, B.; Gutierrez, J.; Küsters, B.; Claassen, J.A.; de Leeuw, F.-E.; Wiesmann, M.; et al. Association between hypertension and neurovascular inflammation in both normal-appearing white matter and white matter hyperintensities. Acta Neuropathol. Commun. 2023, 11, 2. [Google Scholar] [CrossRef] [PubMed]
- Grammas, P.; Samany, P.G.; Thirumangalakudi, L. Thrombin and inflammatory proteins are elevated in Alzheimer’s disease microvessels: Implications for disease pathogenesis. J. Alzheimer’s Dis. 2006, 9, 51–58. [Google Scholar] [CrossRef]
- Vreeken, D.; Seidel, F.; de La Roij, G.; Vening, W.; den Hengst, W.A.; Verschuren, L.; Özsezen, S.; Kessels, R.P.; Duering, M.; Mutsaerts, H.J.; et al. Impact of white adipose tissue on brain structure, perfusion and cognitive function in patients with severe obesity: The BARICO study. Neurology 2022, 100, e703–e718. [Google Scholar] [CrossRef] [PubMed]
- Jais, A.; Solas, M.; Backes, H.; Chaurasia, B.; Kleinridders, A.; Theurich, S.; Mauer, J.; Steculorum, S.M.; Hampel, B.; Goldau, J.; et al. Myeloid-cell-derived VEGF maintains brain glucose uptake and limits cognitive impairment in obesity. Cell 2016, 165, 882–895. [Google Scholar] [CrossRef]
- Tanaka, H.; Gourley, D.D.; Dekhtyar, M.; Haley, A.P. Cognition, brain structure, and brain function in individuals with obesity and related disorders. Curr. Obes. Rep. 2020, 9, 544–549. [Google Scholar] [CrossRef] [PubMed]
- Cotman, C.W.; Berchtold, N.C.; Christie, L.-A. Exercise builds brain health: Key roles of growth factor cascades and inflammation. Trends Neurosci. 2007, 30, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wei, Y.; Fang, C.; Liu, S.; Zhou, F.; Zhao, G.; Li, Y.; Luo, Y.; Guo, Z.; Lin, W.; et al. Exercise retards ongoing adipose tissue fibrosis in diet-induced obese mice. Endocr. Connect. 2021, 10, 325–335. [Google Scholar] [CrossRef]
- Gaesser, G.A.; Angadi, S.S.; Sawyer, B.J. Exercise and diet, independent of weight loss, improve cardiometabolic risk profile in overweight and obese individuals. Physician Sportsmed. 2011, 39, 87–97. [Google Scholar] [CrossRef]
- Bradley, R.L.; Jeon, J.Y.; Liu, F.-F.; Maratos-Flier, E. Voluntary exercise improves insulin sensitivity and adipose tissue inflammation in diet-induced obese mice. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E586–E594. [Google Scholar] [CrossRef]
- Duncan, G.E.; Perri, M.G.; Theriaque, D.W.; Hutson, A.D.; Eckel, R.H.; Stacpoole, P.W. Exercise training, without weight loss, increases insulin sensitivity and postheparin plasma lipase activity in previously sedentary adults. Diabetes Care 2003, 26, 557–562. [Google Scholar] [CrossRef]
- Lucas, S.J.; Ainslie, P.N.; Murrell, C.J.; Thomas, K.N.; Franz, E.A.; Cotter, J.D. Effect of age on exercise-induced alterations in cognitive executive function: Relationship to cerebral perfusion. Exp. Gerontol. 2012, 47, 541–551. [Google Scholar] [CrossRef]
- Erickson, K.I.; Voss, M.W.; Prakash, R.S.; Basak, C.; Szabo, A.; Chaddock, L.; Kim, J.S.; Heo, S.; Alves, H.; White, S.M.; et al. Exercise training increases size of hippocampus and improves memory. Proc. Natl. Acad. Sci. USA 2011, 108, 3017–3022. [Google Scholar] [CrossRef]
- Lim, G.; Lee, H.; Lim, Y. Potential Effects of Resistant Exercise on Cognitive and Muscle Functions Mediated by Myokines in Sarcopenic Obese Mice. Biomedicines 2022, 10, 2529. [Google Scholar] [CrossRef]
- Liang, J.; Wang, C.; Zhang, H.; Huang, J.; Xie, J.; Chen, N. Exercise-induced benefits for Alzheimer’s disease by stimulating mitophagy and improving mitochondrial function. Front. Aging Neurosci. 2021, 13, 755665. [Google Scholar] [CrossRef] [PubMed]
- Schaeffer, D.J.; Krafft, C.E.; Schwarz, N.F.; Chi, L.; Rodrigue, A.L.; Pierce, J.E.; Allison, J.D.; Yanasak, N.E.; Liu, T.; Davis, C.L.; et al. An 8-month exercise intervention alters frontotemporal white matter integrity in overweight children. Psychophysiology 2014, 51, 728–733. [Google Scholar] [CrossRef] [PubMed]
- Krafft, C.E.; Pierce, J.E.; Schwarz, N.F.; Chi, L.; Weinberger, A.L.; Schaeffer, D.J.; Rodrigue, A.L.; Camchong, J.; Allison, J.D.; Yanasak, N.E.; et al. An eight month randomized controlled exercise intervention alters resting state synchrony in overweight children. Neuroscience 2014, 256, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-S.; Park, S.-S.; Kim, C.-J.; Shin, M.-S.; Kim, T.-W. Exercise alleviates cognitive functions by enhancing hippocampal insulin signaling and neuroplasticity in high-fat diet-induced obesity. Nutrients 2019, 11, 1603. [Google Scholar] [CrossRef]
- Graham, L.C.; Grabowska, W.A.; Chun, Y.; Risacher, S.L.; Philip, V.M.; Saykin, A.J.; Rizzo, S.J.S.; Howell, G.R.; Initiative, A.s.D.N. Exercise prevents obesity-induced cognitive decline and white matter damage in mice. Neurobiol. Aging 2019, 80, 154–172. [Google Scholar] [CrossRef]
- Neinast, M.; Murashige, D.; Arany, Z. Branched chain amino acids. Annu. Rev. Physiol. 2019, 81, 139. [Google Scholar] [CrossRef]
- Rahimi, M.H.; Shab-Bidar, S.; Mollahosseini, M.; Djafarian, K. Branched-chain amino acid supplementation and exercise-induced muscle damage in exercise recovery: A meta-analysis of randomized clinical trials. Nutrition 2017, 42, 30–36. [Google Scholar] [CrossRef]
- Estrada-Alcalde, I.; Tenorio-Guzman, M.R.; Tovar, A.R.; Salinas-Rubio, D.; Torre-Villalvazo, I.; Torres, N.; Noriega, L.G. Metabolic fate of branched-chain amino acids during adipogenesis, in adipocytes from obese mice and C2C12 myotubes. J. Cell. Biochem. 2017, 118, 808–818. [Google Scholar] [CrossRef] [PubMed]
- De Simone, R.; Vissicchio, F.; Mingarelli, C.; De Nuccio, C.; Visentin, S.; Ajmone-Cat, M.A.; Minghetti, L. Branched-chain amino acids influence the immune properties of microglial cells and their responsiveness to pro-inflammatory signals. Biochim. Et Biophys. Acta BBA Mol. Basis Dis. 2013, 1832, 650–659. [Google Scholar] [CrossRef]
- Xiao, F.; Yu, J.; Guo, Y.; Deng, J.; Li, K.; Du, Y.; Chen, S.; Zhu, J.; Sheng, H.; Guo, F.; et al. Effects of individual branched-chain amino acids deprivation on insulin sensitivity and glucose metabolism in mice. Metabolism 2014, 63, 841–850. [Google Scholar] [CrossRef]
- Macotela, Y.; Emanuelli, B.; Bång, A.M.; Espinoza, D.O.; Boucher, J.; Beebe, K.; Gall, W.; Kahn, C.R. Dietary leucine-an environmental modifier of insulin resistance acting on multiple levels of metabolism. PLoS ONE 2011, 6, e21187. [Google Scholar] [CrossRef]
- Lueders, B.; Kanney, B.C.; Krone, M.J.; Gannon, N.P.; Vaughan, R.A. Effect of branched-chain amino acids on food intake and indicators of hunger and satiety-a narrative summary. Hum. Nutr. Metab. 2022, 30, 200168. [Google Scholar] [CrossRef]
- Blomstrand, E. Branched-chain amino acids and central fatigue: Implications for diet and behavior. In Handbook of Behavior, Food and Nutrition; Springer: Berlin/Heidelberg, Germany, 2011; pp. 865–877. [Google Scholar]
- Siddik, M.A.B.; Shin, A.C. Recent progress on branched-chain amino acids in obesity, diabetes, and beyond. Endocrinol. Metab. 2019, 34, 234–246. [Google Scholar] [CrossRef] [PubMed]
- van den Hoek, A.M.; Verschuren, L.; Worms, N.; van Nieuwkoop, A.; de Ruiter, C.; Attema, J.; Menke, A.L.; Caspers, M.P.; Radhakrishnan, S.; Salic, K.; et al. A translational mouse model for NASH with advanced fibrosis and atherosclerosis expressing key pathways of human pathology. Cells 2020, 9, 2014. [Google Scholar] [CrossRef]
- Arnoldussen, I.; Wiesmann, M.; Pelgrim, C.; Wielemaker, E.; Van Duyvenvoorde, W.; Amaral-Santos, P.; Verschuren, L.; Keijser, B.; Heerschap, A.; Kleemann, R.; et al. Butyrate restores HFD-induced adaptations in brain function and metabolism in mid-adult obese mice. Int. J. Obes. 2017, 41, 935–944. [Google Scholar] [CrossRef] [PubMed]
- Tengeler, A.C.; Gart, E.; Wiesmann, M.; Arnoldussen, I.A.; van Duyvenvoorde, W.; Hoogstad, M.; Dederen, P.J.; Verweij, V.; Geenen, B.; Kozicz, T.; et al. Propionic acid and not caproic acid, attenuates nonalcoholic steatohepatitis and improves (cerebro) vascular functions in obese Ldlr−/−. Leiden mice. FASEB J. 2020, 34, 9575–9593. [Google Scholar] [CrossRef]
- Arnoldussen, I.A.; Morrison, M.C.; Wiesmann, M.; van Diepen, J.A.; Worms, N.; Voskuilen, M.; Verweij, V.; Geenen, B.; Gualdo, N.P.; van der Logt, L.; et al. Milk fat globule membrane attenuates high fat diet-induced neuropathological changes in obese Ldlr−/−. Leiden mice. Int. J. Obes. 2022, 46, 342–349. [Google Scholar] [CrossRef]
- Martínez-Arranz, I.; Bruzzone, C.; Noureddin, M.; Gil-Redondo, R.; Mincholé, I.; Bizkarguenaga, M.; Arretxe, E.; Iruarrizaga-Lejarreta, M.; Fernández-Ramos, D.; Lopitz-Otsoa, F.; et al. Metabolic subtypes of patients with NAFLD exhibit distinctive cardiovascular risk profiles. Hepatology 2022, 76, 1121–1134. [Google Scholar] [CrossRef] [PubMed]
- Morrison, M.C.; Kleemann, R.; Van Koppen, A.; Hanemaaijer, R.; Verschuren, L. Key inflammatory processes in human NASH are reflected in Ldlr−/−. Leiden mice: A translational gene profiling study. Front. Physiol. 2018, 9, 132. [Google Scholar] [CrossRef]
- Morrison, M.C.; Verschuren, L.; Salic, K.; Verheij, J.; Menke, A.; Wielinga, P.Y.; Iruarrizaga-Lejarreta, M.; Gole, L.; Yu, W.M.; Turner, S. Obeticholic acid modulates serum metabolites and gene signatures characteristic of human NASH and attenuates inflammation and fibrosis progression in Ldlr−/−. leiden mice. Hepatol. Commun. 2018, 2, 1513–1532. [Google Scholar] [CrossRef] [PubMed]
- van Koppen, A.; Verschuren, L.; van den Hoek, A.M.; Verheij, J.; Morrison, M.C.; Li, K.; Nagabukuro, H.; Costessi, A.; Caspers, M.P.; van den Broek, T.J.; et al. Uncovering a predictive molecular signature for the onset of NASH-related fibrosis in a translational NASH mouse model. Cell. Mol. Gastroenterol. Hepatol. 2018, 5, 83–98. [Google Scholar] [CrossRef]
- Getz, G.S.; Reardon, C.A. Animal models of atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1104–1115. [Google Scholar] [CrossRef]
- Arnoldussen, I.A.; Zerbi, V.; Wiesmann, M.; Noordman, R.H.; Bolijn, S.; Mutsaers, M.P.; Dederen, P.J.; Kleemann, R.; Kooistra, T.; van Tol, E.A.; et al. Early intake of long-chain polyunsaturated fatty acids preserves brain structure and function in diet-induced obesity. J. Nutr. Biochem. 2016, 30, 177–188. [Google Scholar] [CrossRef]
- Rabelo, L.A.; Cortes, S.F.; Alvarez-Leite, J.I.; Lemos, V.S. Endothelium dysfunction in LDL receptor knockout mice: A role for H2O2. Br. J. Pharmacol. 2003, 138, 1215–1220. [Google Scholar] [CrossRef]
- Kraft, P.; Schuhmann, M.K.; Garz, C.; Jandke, S.; Urlaub, D.; Mencl, S.; Zernecke, A.; Heinze, H.-J.; Carare, R.O.; Kleinschnitz, C.; et al. Hypercholesterolemia induced cerebral small vessel disease. PLoS ONE 2017, 12, e0182822. [Google Scholar] [CrossRef]
- Martin, S.S.; Qasim, A.; Reilly, M.P. Leptin resistance: A possible interface of inflammation and metabolism in obesity-related cardiovascular disease. J. Am. Coll. Cardiol. 2008, 52, 1201–1210. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, J.; Engel, D.F.; de Paula, G.C.; Dos Santos, D.B.; Lopes, J.B.; Farina, M.; Moreira, E.L.; de Bem, A.F. High cholesterol diet exacerbates blood-brain barrier disruption in LDLr–/–mice: Impact on cognitive function. J. Alzheimer’s Dis. 2020, 78, 97–115. [Google Scholar] [CrossRef]
- Franciosi, S.; Gama Sosa, M.A.; English, D.F.; Oler, E.; Oung, T.; Janssen, W.G.; De Gasperi, R.; Schmeidler, J.; Dickstein, D.L.; Schmitz, C.; et al. Novel cerebrovascular pathology in mice fed a high cholesterol diet. Mol. Neurodegener. 2009, 4, 42. [Google Scholar] [CrossRef]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. J. Cereb. Blood Flow Metab. 2020, 40, 1769–1777. [Google Scholar] [CrossRef]
- Wiesmann, M.; Capone, C.; Zerbi, V.; Mellendijk, L.; Heerschap, A.; AHR Claassen, J.; Amanda, J.K. Hypertension impairs cerebral blood flow in a mouse model for Alzheimer’s disease. Curr. Alzheimer Res. 2015, 12, 914–922. [Google Scholar] [CrossRef]
- Voikar, V.; Gaburro, S. Three pillars of automated home-cage phenotyping of mice: Novel findings, refinement, and reproducibility based on literature and experience. Front. Behav. Neurosci. 2020, 14, 575434. [Google Scholar] [CrossRef] [PubMed]
- Iannello, F. Non-intrusive high throughput automated data collection from the home cage. Heliyon 2019, 5, e01454. [Google Scholar] [CrossRef] [PubMed]
- Munier, J.J.; Pank, J.T.; Severino, A.; Wang, H.; Zhang, P.; Vergnes, L.; Reue, K. Simultaneous monitoring of mouse grip strength, force profile, and cumulative force profile distinguishes muscle physiology following surgical, pharmacologic and diet interventions. Sci. Rep. 2022, 12, 16428. [Google Scholar] [CrossRef] [PubMed]
- Calahorra, J.; Shenk, J.; Wielenga, V.H.; Verweij, V.; Geenen, B.; Dederen, P.J.; Peinado Herreros, M.Á.; Siles, E.; Wiesmann, M.; Kiliaan, A.J.; et al. Hydroxytyrosol, the major phenolic compound of olive oil, as an acute therapeutic strategy after ischemic stroke. Nutrients 2019, 11, 2430. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Franklin, K.B. The Mouse Brain in Stereotaxic Coordinates, 3rd ed.; Academic Press: Cambridge, MA, USA, 2008. [Google Scholar]
- Franklin, K.B.; Paxinos, G. Paxinos and Franklin’s the Mouse Brain in Stereotaxic Coordinates, Compact: The Coronal Plates and Diagrams; Academic Press: Cambridge, MA, USA, 2019. [Google Scholar]
- Zerbi, V.; Wiesmann, M.; Emmerzaal, T.L.; Jansen, D.; Van Beek, M.; Mutsaers, M.P.; Beckmann, C.F.; Heerschap, A.; Kiliaan, A.J. Resting-state functional connectivity changes in aging apoE4 and apoE-KO mice. J. Neurosci. 2014, 34, 13963–13975. [Google Scholar] [CrossRef] [PubMed]
- Zerbi, V.; Jansen, D.; Wiesmann, M.; Fang, X.; Broersen, L.M.; Veltien, A.; Heerschap, A.; Kiliaan, A.J. Multinutrient diets improve cerebral perfusion and neuroprotection in a murine model of Alzheimer’s disease. Neurobiol. Aging 2014, 35, 600–613. [Google Scholar] [CrossRef]
- Wiesmann, M.; Zinnhardt, B.; Reinhardt, D.; Eligehausen, S.; Wachsmuth, L.; Hermann, S.; Dederen, P.J.; Hellwich, M.; Kuhlmann, M.T.; Broersen, L.M.; et al. A specific dietary intervention to restore brain structure and function after ischemic stroke. Theranostics 2017, 7, 493. [Google Scholar] [CrossRef]
- Zerbi, V.; Kleinnijenhuis, M.; Fang, X.; Jansen, D.; Veltien, A.; Van Asten, J.; Timmer, N.; Dederen, P.J.; Kiliaan, A.J.; Heerschap, A.; et al. Gray and white matter degeneration revealed by diffusion in an Alzheimer mouse model. Neurobiol. Aging 2013, 34, 1440–1450. [Google Scholar] [CrossRef]
- Le Bihan, D.; Mangin, J.F.; Poupon, C.; Clark, C.A.; Pappata, S.; Molko, N.; Chabriat, H. Diffusion tensor imaging: Concepts and applications. J. Magn. Reson. Imaging Off. J. Int. Soc. Magn. Reson. Med. 2001, 13, 534–546. [Google Scholar] [CrossRef] [PubMed]
- Feldman, H.M.; Yeatman, J.D.; Lee, E.S.; Barde, L.H.; Gaman-Bean, S. Diffusion tensor imaging: A review for pediatric researchers and clinicians. J. Dev. Behav. Pediatr. JDBP 2010, 31, 346. [Google Scholar] [CrossRef] [PubMed]
- Alexander, A.L.; Hurley, S.A.; Samsonov, A.A.; Adluru, N.; Hosseinbor, A.P.; Mossahebi, P.; Tromp, D.P.; Zakszewski, E.; Field, A.S. Characterization of cerebral white matter properties using quantitative magnetic resonance imaging stains. Brain Connect. 2011, 1, 423–446. [Google Scholar] [CrossRef]
- Janssen, C.I.; Jansen, D.; Mutsaers, M.P.; Dederen, P.J.; Geenen, B.; Mulder, M.T.; Kiliaan, A.J. The effect of a high-fat diet on brain plasticity, inflammation and cognition in female ApoE4-knockin and ApoE-knockout mice. PLoS ONE 2016, 11, e0155307. [Google Scholar] [CrossRef] [PubMed]
- Hooijmans, C.R.; Graven, C.; Dederen, P.J.; Tanila, H.; van Groen, T.; Kiliaan, A.J. Amyloid beta deposition is related to decreased glucose transporter-1 levels and hippocampal atrophy in brains of aged APP/PS1 mice. Brain Res. 2007, 1181, 93–103. [Google Scholar] [CrossRef]
- Mollink, J.; Hiemstra, M.; Miller, K.; Huszar, I.; Jenkinson, M.; Raaphorst, J.; Wiesmann, M.; Ansorge, O.; Pallebage-Gamarallage, M.; Van Cappellen Van Walsum, A.; et al. White matter changes in the perforant path area in patients with amyotrophic lateral sclerosis. Neuropathol. Appl. Neurobiol. 2019, 45, 570–585. [Google Scholar] [CrossRef]
- Mollink, J.; Kleinnijenhuis, M.; van Walsum, A.-M.v.C.; Sotiropoulos, S.N.; Cottaar, M.; Mirfin, C.; Heinrich, M.P.; Jenkinson, M.; Pallebage-Gamarallage, M.; Ansorge, O.; et al. Evaluating fibre orientation dispersion in white matter: Comparison of diffusion MRI, histology and polarized light imaging. Neuroimage 2017, 157, 561–574. [Google Scholar] [CrossRef]
- Axer, M.; Amunts, K.; Grässel, D.; Palm, C.; Dammers, J.; Axer, H.; Pietrzyk, U.; Zilles, K. A novel approach to the human connectome: Ultra-high resolution mapping of fiber tracts in the brain. Neuroimage 2011, 54, 1091–1101. [Google Scholar] [CrossRef]
- Larsen, L.; Griffin, L.D.; GRäßel, D.; Witte, O.W.; Axer, H. Polarized light imaging of white matter architecture. Microsc. Res. Tech. 2007, 70, 851–863. [Google Scholar] [CrossRef]
- Dammers, J.; Axer, M.; Gräßel, D.; Palm, C.; Zilles, K.; Amunts, K.; Pietrzyk, U. Signal enhancement in polarized light imaging by means of independent component analysis. Neuroimage 2010, 49, 1241–1248. [Google Scholar] [CrossRef]
- Axer, M.; Grässel, D.; Kleiner, M.; Dammers, J.; Dickscheid, T.; Reckfort, J.; Hütz, T.; Eiben, B.; Pietrzyk, U.; Zilles, K.; et al. High-resolution fiber tract reconstruction in the human brain by means of three-dimensional polarized light imaging. Front. Neuroinformatics 2011, 5, 34. [Google Scholar] [CrossRef]
- Morrison, M.; Mulder, P.; Salic, K.; Verheij, J.; Liang, W.; Van Duyvenvoorde, W.; Menke, A.; Kooistra, T.; Kleemann, R.; Wielinga, P.; et al. Intervention with a caspase-1 inhibitor reduces obesity-associated hyperinsulinemia, non-alcoholic steatohepatitis and hepatic fibrosis in LDLR−/−. Leiden mice. Int. J. Obes. 2016, 40, 1416–1423. [Google Scholar] [CrossRef]
- Luque-Sierra, A.; Alvarez-Amor, L.; Kleemann, R.; Martín, F.; Varela, L.M. Extra-virgin olive oil with natural phenolic content exerts an anti-inflammatory effect in adipose tissue and attenuates the severity of atherosclerotic lesions in Ldlr−/−. Leiden mice. Mol. Nutr. Food Res. 2018, 62, 1800295. [Google Scholar] [CrossRef]
- Schoemaker, M.H.; Kleemann, R.; Morrison, M.C.; Verheij, J.; Salic, K.; van Tol, E.A.; Kooistra, T.; Wielinga, P.Y. A casein hydrolysate based formulation attenuates obesity and associated non-alcoholic fatty liver disease and atherosclerosis in Ldlr−/−. Leiden mice. PLoS ONE 2017, 12, e0180648. [Google Scholar] [CrossRef] [PubMed]
- Gart, E.; van Duyvenvoorde, W.; Caspers, M.P.; van Trigt, N.; Snabel, J.; Menke, A.; Keijer, J.; Salic, K.; Morrison, M.C.; Kleemann, R.; et al. Intervention with isoleucine or valine corrects hyperinsulinemia and reduces intrahepatic diacylglycerols, liver steatosis, and inflammation in Ldlr−/−. Leiden mice with manifest obesity-associated NASH. FASEB J. 2022, 36, e22435. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhao, S.; Yan, W.; Xia, Y.; Chen, X.; Wang, W.; Zhang, J.; Gao, C.; Peng, C.; Yan, F.; et al. Branched chain amino acids cause liver injury in obese/diabetic mice by promoting adipocyte lipolysis and inhibiting hepatic autophagy. EBioMedicine 2016, 13, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Zhou, X.; Hu, L.; Chen, J.; Zhu, J.; Shan, A. Leucine and isoleucine have similar effects on reducing lipid accumulation, improving insulin sensitivity and increasing the browning of WAT in high-fat diet-induced obese mice. Food Funct. 2020, 11, 2279–2290. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Guo, K.; LeBlanc, R.E.; Loh, D.; Schwartz, G.J.; Yu, Y.-H. Increasing dietary leucine intake reduces diet-induced obesity and improves glucose and cholesterol metabolism in mice via multimechanisms. Diabetes 2007, 56, 1647–1654. [Google Scholar] [CrossRef]
- Xu, M.; Kitaura, Y.; Shindo, D.; Shimomura, Y. Branched-chain amino acid (BCAA) supplementation enhances adaptability to exercise training of mice with a muscle-specific defect in the control of BCAA catabolism. Biosci. Biotechnol. Biochem. 2018, 82, 896–899. [Google Scholar] [CrossRef]
- Crowe, M.J.; Weatherson, J.N.; Bowden, B.F. Effects of dietary leucine supplementation on exercise performance. Eur. J. Appl. Physiol. 2006, 97, 664–672. [Google Scholar] [CrossRef]
- Calders, P.; Matthys, D.; Derave, W.; Pannier, J.-L. Effect of branched-chain amino acids (BCAA), glucose, and glucose plus BCAA on endurance performance in rats. Med. Sci. Sport. Exerc. 1999, 31, 583–587. [Google Scholar] [CrossRef] [PubMed]
- Hormoznejad, R.; Zare Javid, A.; Mansoori, A. Effect of BCAA supplementation on central fatigue, energy metabolism substrate and muscle damage to the exercise: A systematic review with meta-analysis. Sport Sci. Health 2019, 15, 265–279. [Google Scholar] [CrossRef]
- Moraska, A.; Deak, T.; Spencer, R.L.; Roth, D.; Fleshner, M. Treadmill running produces both positive and negative physiological adaptations in Sprague-Dawley rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000, 279, R1321–R1329. [Google Scholar] [CrossRef]
- Svensson, M.; Rosvall, P.; Boza-Serrano, A.; Andersson, E.; Lexell, J.; Deierborg, T. Forced treadmill exercise can induce stress and increase neuronal damage in a mouse model of global cerebral ischemia. Neurobiol. Stress 2016, 5, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Kohsaka, A.; Laposky, A.D.; Ramsey, K.M.; Estrada, C.; Joshu, C.; Kobayashi, Y.; Turek, F.W.; Bass, J. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab. 2007, 6, 414–421. [Google Scholar] [CrossRef]
- Plotkin, D.L.; Delcastillo, K.; Van Every, D.W.; Tipton, K.D.; Aragon, A.A.; Schoenfeld, B.J. Isolated leucine and branched-chain amino acid supplementation for enhancing muscular strength and hypertrophy: A narrative review. Int. J. Sport Nutr. Exerc. Metab. 2021, 31, 292–301. [Google Scholar] [CrossRef]
- Iwai, S.; Hasegawa, T.; Ikeda, H.O.; Tsujikawa, A. Branched Chain Amino Acids Promote ATP Production Via Translocation of Glucose Transporters. Investig. Ophthalmol. Vis. Sci. 2022, 63, 7. [Google Scholar] [CrossRef]
- Ebadi, M.; Bhanji, R.A.; Mazurak, V.C.; Montano-Loza, A.J. Sarcopenia in cirrhosis: From pathogenesis to interventions. J. Gastroenterol. 2019, 54, 845–859. [Google Scholar] [CrossRef]
- Hiraoka, A.; Aibiki, T.; Okudaira, T.; Toshimori, A.; Kawamura, T.; Nakahara, H.; Suga, Y.; Azemoto, N.; Miyata, H.; Miyamoto, Y.; et al. Muscle atrophy as pre-sarcopenia in Japanese patients with chronic liver disease: Computed tomography is useful for evaluation. J. Gastroenterol. 2015, 50, 1206–1213. [Google Scholar] [CrossRef]
- Uojima, H.; Sakurai, S.; Hidaka, H.; Kinbara, T.; Sung, J.H.; Ichita, C.; Tokoro, S.; Masuda, S.; Sasaki, A.; Koizumi, K.; et al. Effect of branched-chain amino acid supplements on muscle strength and muscle mass in patients with liver cirrhosis. Eur. J. Gastroenterol. Hepatol. 2017, 29, 1402–1407. [Google Scholar] [CrossRef]
- Holeček, M. The role of skeletal muscle in the pathogenesis of altered concentrations of branched-chain amino acids (valine, leucine, and isoleucine) in liver cirrhosis, diabetes, and other diseases. Physiol. Res. 2021, 70, 293. [Google Scholar] [CrossRef]
- Claassen, J.A.; Thijssen, D.H.; Panerai, R.B.; Faraci, F.M. Regulation of cerebral blood flow in humans: Physiology and clinical implications of autoregulation. Physiol. Rev. 2021, 101, 1487–1559. [Google Scholar] [CrossRef] [PubMed]
- Cahill, L.S.; Bishop, J.; Gazdzinski, L.M.; Dorr, A.; Stefanovic, B.; Sled, J.G. Altered cerebral blood flow and cerebrovascular function after voluntary exercise in adult mice. Brain Struct. Funct. 2017, 222, 3395–3405. [Google Scholar] [CrossRef] [PubMed]
- Fazlollahi, A.; Calamante, F.; Liang, X.; Bourgeat, P.; Raniga, P.; Dore, V.; Fripp, J.; Ames, D.; Masters, C.L.; Rowe, C.C.; et al. Increased cerebral blood flow with increased amyloid burden in the preclinical phase of alzheimer’s disease. J. Magn. Reson. Imaging 2020, 51, 505–513. [Google Scholar] [CrossRef]
- Thomas, K.R.; Osuna, J.R.; Weigand, A.J.; Edmonds, E.C.; Clark, A.L.; Holmqvist, S.; Cota, I.H.; Wierenga, C.E.; Bondi, M.W.; Bangen, K.J.; et al. Regional hyperperfusion in older adults with objectively-defined subtle cognitive decline. J. Cereb. Blood Flow Metab. 2021, 41, 1001–1012. [Google Scholar] [CrossRef]
- Balasubramanian, P.; Kiss, T.; Tarantini, S.; Nyúl-Tóth, Á.; Ahire, C.; Yabluchanskiy, A.; Csipo, T.; Lipecz, A.; Tabak, A.; Institoris, A.; et al. Obesity-induced cognitive impairment in older adults: A microvascular perspective. Am. J. Physiol. Heart Circ. Physiol. 2021, 320, H740–H761. [Google Scholar] [CrossRef]
- Takimoto, M.; Hamada, T. Acute exercise increases brain region-specific expression of MCT1, MCT2, MCT4, GLUT1, and COX IV proteins. J. Appl. Physiol. 2014, 116, 1238–1250. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.Y.; Koo, G.H.; Park, S.C.; Shin, K.O. Effects of branched-chain amino acid and glutamine supplementation on angiogenic factors and pro-inflammatory cytokines after acute exercise in adolescence athletes. Asian J. Kinesiol. 2019, 21, 51–58. [Google Scholar] [CrossRef]
- Vogel, J.; Gehrig, M.; Kuschinsky, W.; Marti, H.H. Massive inborn angiogenesis in the brain scarcely raises cerebral blood flow. J. Cereb. Blood Flow Metab. 2004, 24, 849–859. [Google Scholar] [CrossRef]
- Tak, S.; Polimeni, J.R.; Wang, D.J.; Yan, L.; Chen, J.J. Associations of resting-state fMRI functional connectivity with flow-BOLD coupling and regional vasculature. Brain Connect. 2015, 5, 137–146. [Google Scholar] [CrossRef]
- Ou, X.; Andres, A.; Pivik, R.; Cleves, M.A.; Badger, T.M. Brain gray and white matter differences in healthy normal weight and obese children. J. Magn. Reson. Imaging 2015, 42, 1205–1213. [Google Scholar] [CrossRef] [PubMed]
- Ronan, L.; Alexander-Bloch, A.F.; Wagstyl, K.; Farooqi, S.; Brayne, C.; Tyler, L.K.; Fletcher, P.C. Obesity associated with increased brain age from midlife. Neurobiol. Aging 2016, 47, 63–70. [Google Scholar] [CrossRef]
- Dutta, S.; Sengupta, P. Men and mice: Relating their ages. Life Sci. 2016, 152, 244–248. [Google Scholar] [CrossRef]
- Posey, K.A.; Clegg, D.J.; Printz, R.L.; Byun, J.; Morton, G.J.; Vivekanandan-Giri, A.; Pennathur, S.; Baskin, D.G.; Heinecke, J.W.; Woods, S.C.; et al. Hypothalamic proinflammatory lipid accumulation, inflammation, and insulin resistance in rats fed a high-fat diet. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E1003–E1012. [Google Scholar] [CrossRef] [PubMed]
- Guillemot-Legris, O.; Muccioli, G.G. Obesity-induced neuroinflammation: Beyond the hypothalamus. Trends Neurosci. 2017, 40, 237–253. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Norman, J.E.; Srinivasan, V.J.; Rutledge, J.C. Metabolic, inflammatory, and microvascular determinants of white matter disease and cognitive decline. Am. J. Neurodegener. Dis. 2016, 5, 171. [Google Scholar]
- Kang, E.B.; Koo, J.H.; Jang, Y.C.; Yang, C.H.; Lee, Y.; Cosio-Lima, L.; Cho, J.Y. Neuroprotective effects of endurance exercise against high-fat diet-induced hippocampal neuroinflammation. J. Neuroendocrinol. 2016, 28, 5. [Google Scholar] [CrossRef] [PubMed]
- Calcaterra, V.; Vandoni, M.; Rossi, V.; Berardo, C.; Grazi, R.; Cordaro, E.; Tranfaglia, V.; Carnevale Pellino, V.; Cereda, C.; Zuccotti, G.; et al. Use of physical activity and exercise to reduce inflammation in children and adolescents with obesity. Int. J. Environ. Res. Public Health 2022, 19, 6908. [Google Scholar] [CrossRef]
- Zhenyukh, O.; González-Amor, M.; Rodrigues-Diez, R.R.; Esteban, V.; Ruiz-Ortega, M.; Salaices, M.; Mas, S.; Briones, A.M.; Egido, J. Branched-chain amino acids promote endothelial dysfunction through increased reactive oxygen species generation and inflammation. J. Cell. Mol. Med. 2018, 22, 4948–4962. [Google Scholar] [CrossRef]
- Adegoke, O.A.; Bates, H.E.; Kiraly, M.A.; Vranic, M.; Riddell, M.C.; Marliss, E.B. Exercise in ZDF rats does not attenuate weight gain, but prevents hyperglycemia concurrent with modulation of amino acid metabolism and AKT/mTOR activation in skeletal muscle. Eur. J. Nutr. 2015, 54, 751–759. [Google Scholar] [CrossRef]
- Glynn, E.L.; Piner, L.W.; Huffman, K.M.; Slentz, C.A.; Elliot-Penry, L.; AbouAssi, H.; White, P.J.; Bain, J.R.; Muehlbauer, M.J.; Ilkayeva, O.R.; et al. Impact of combined resistance and aerobic exercise training on branched-chain amino acid turnover, glycine metabolism and insulin sensitivity in overweight humans. Diabetologia 2015, 58, 2324–2335. [Google Scholar] [CrossRef] [PubMed]
- Cassilhas, R.C.; Tufik, S.; de Mello, M.T. Physical exercise, neuroplasticity, spatial learning and memory. Cell. Mol. Life Sci. 2016, 73, 975–983. [Google Scholar] [CrossRef]
- Liśkiewicz, A.D.; Liśkiewicz, D.; Marczak, Ł.; Przybyła, M.; Grabowska, K.; Student, S.; Dębiec, M.; Sługocka, A.; Lewin-Kowalik, J. Obesity-associated deterioration of the hippocampus is partially restored after weight loss. Brain Behav. Immun. 2021, 96, 212–226. [Google Scholar] [CrossRef] [PubMed]
- Cole, J.T.; Mitala, C.M.; Kundu, S.; Verma, A.; Elkind, J.A.; Nissim, I.; Cohen, A.S. Dietary branched chain amino acids ameliorate injury-induced cognitive impairment. Proc. Natl. Acad. Sci. USA 2010, 107, 366–371. [Google Scholar] [CrossRef]
- Good, M. Spatial memory and hippocampal function: Where are we now? Psicológica 2002, 23, 109–138. [Google Scholar]
- Zhao, W.-Q.; Chen, H.; Quon, M.J.; Alkon, D.L. Insulin and the insulin receptor in experimental models of learning and memory. Eur. J. Pharmacol. 2004, 490, 71–81. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lohkamp, K.J.; van den Hoek, A.M.; Solé-Guardia, G.; Lisovets, M.; Alves Hoffmann, T.; Velanaki, K.; Geenen, B.; Verweij, V.; Morrison, M.C.; Kleemann, R.; et al. The Preventive Effect of Exercise and Oral Branched-Chain Amino Acid Supplementation on Obesity-Induced Brain Changes in Ldlr−/−.Leiden Mice. Nutrients 2023, 15, 1716. https://doi.org/10.3390/nu15071716
Lohkamp KJ, van den Hoek AM, Solé-Guardia G, Lisovets M, Alves Hoffmann T, Velanaki K, Geenen B, Verweij V, Morrison MC, Kleemann R, et al. The Preventive Effect of Exercise and Oral Branched-Chain Amino Acid Supplementation on Obesity-Induced Brain Changes in Ldlr−/−.Leiden Mice. Nutrients. 2023; 15(7):1716. https://doi.org/10.3390/nu15071716
Chicago/Turabian StyleLohkamp, Klara J., Anita M. van den Hoek, Gemma Solé-Guardia, Maria Lisovets, Talissa Alves Hoffmann, Konstantina Velanaki, Bram Geenen, Vivienne Verweij, Martine C. Morrison, Robert Kleemann, and et al. 2023. "The Preventive Effect of Exercise and Oral Branched-Chain Amino Acid Supplementation on Obesity-Induced Brain Changes in Ldlr−/−.Leiden Mice" Nutrients 15, no. 7: 1716. https://doi.org/10.3390/nu15071716
APA StyleLohkamp, K. J., van den Hoek, A. M., Solé-Guardia, G., Lisovets, M., Alves Hoffmann, T., Velanaki, K., Geenen, B., Verweij, V., Morrison, M. C., Kleemann, R., Wiesmann, M., & Kiliaan, A. J. (2023). The Preventive Effect of Exercise and Oral Branched-Chain Amino Acid Supplementation on Obesity-Induced Brain Changes in Ldlr−/−.Leiden Mice. Nutrients, 15(7), 1716. https://doi.org/10.3390/nu15071716