Something to Snack on: Can Dietary Modulators Boost Mind and Body?
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Feeding Schedule and Preparation of Dietary Modulators
2.3. Compositions
2.4. Amounts
2.5. Cognitive Assays
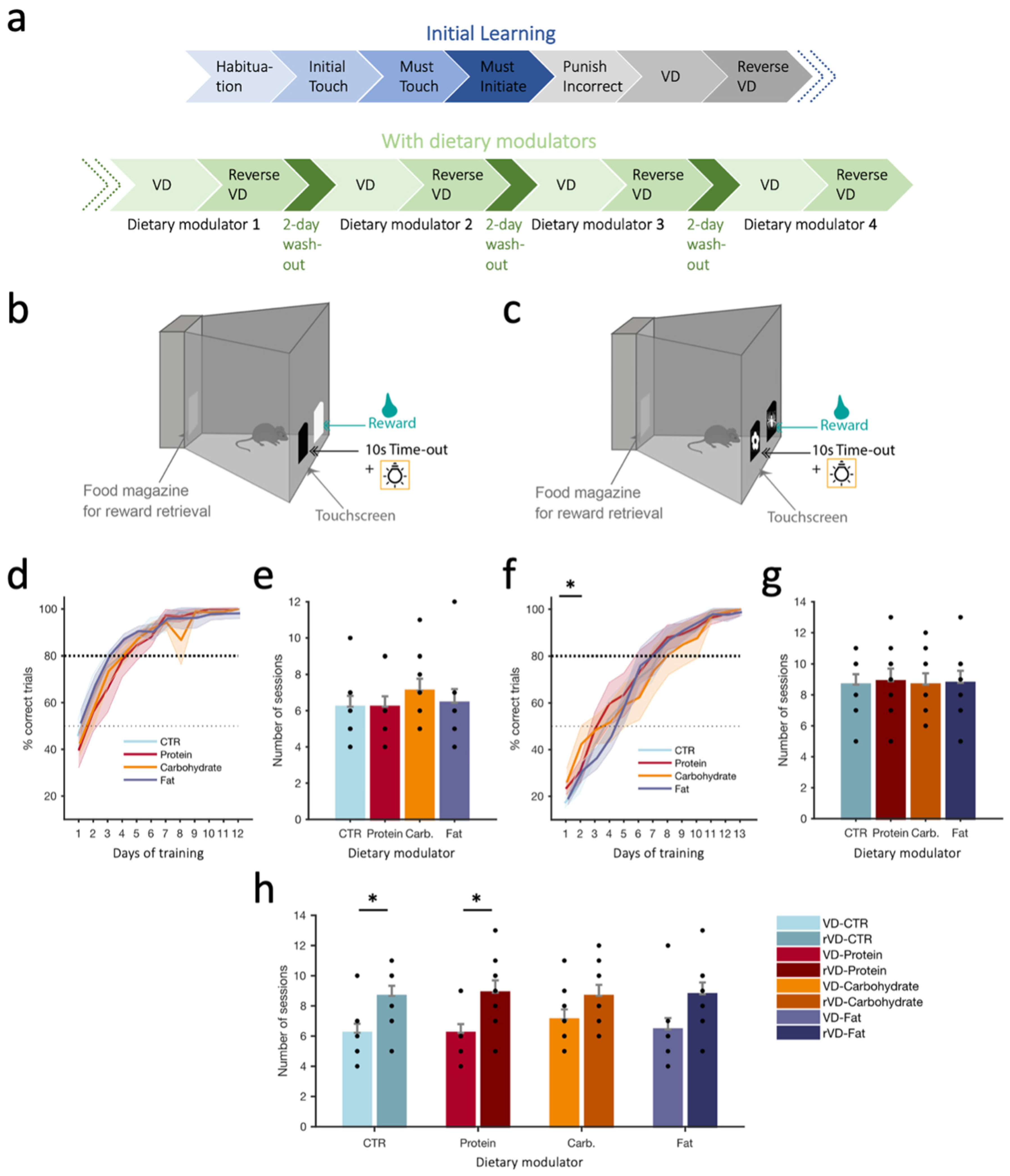
2.6. Visual Discrimination (VD) Task
2.6.1. Training Phase
2.6.2. Initial Acquisition Phase (VD/Reverse VD in the Absence of Dietary Modulators)
2.6.3. VD and rVD under Dietary Modulators
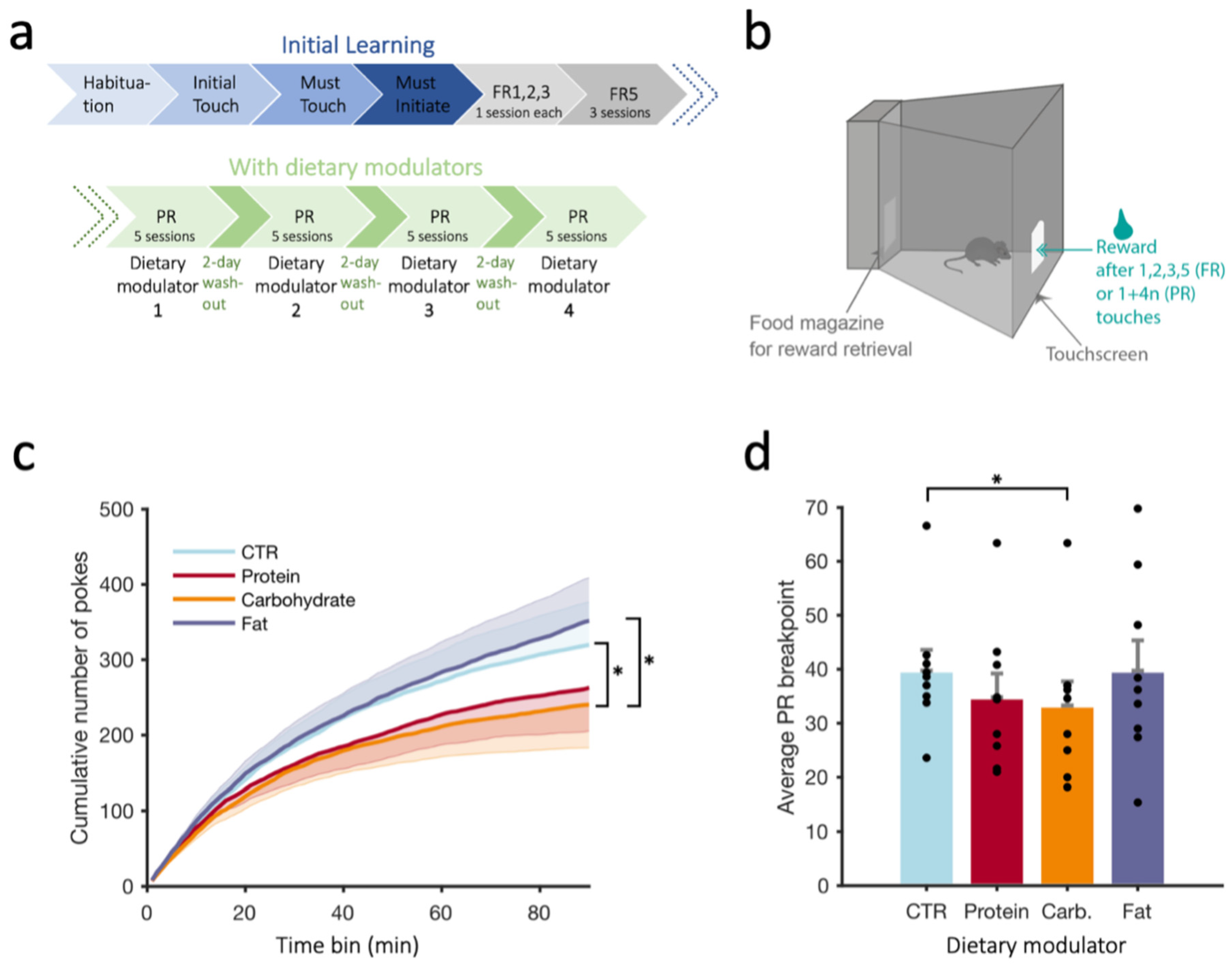
2.7. Progressive Ratio (PR) Task
2.7.1. Training Phase
2.7.2. Fixed Ratio Schedule (FR)
2.7.3. Progressive Ratio Schedule (PR)
2.8. Exercise Running Wheel Activity
2.9. Data Analysis
3. Results
3.1. Visual Discrimination (VD) Task
3.2. Progressive Ratio Schedule (PR)
3.3. Exercise Running Wheel Activity
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Schulze, M.B.; Martínez-González, M.A.; Fung, T.T.; Lichtenstein, A.H.; Forouhi, N.G. Food based dietary patterns and chronic disease prevention. BMJ 2018, 361, k2396. [Google Scholar] [CrossRef]
- Bidwell, L.C.; McClernon, F.J.; Kollins, S.H. Cognitive enhancers for the treatment of ADHD. Pharmacol. Biochem. Behav. 2011, 99, 262–274. [Google Scholar] [CrossRef]
- Hasler, C.M. Functional foods: The western perspective. Nutr. Rev. 1996, 54, S6–S10. [Google Scholar] [CrossRef]
- Froiland, K.; Koszewski, W.; Hingst, J.; Kopecky, L. Nutritional supplement use among college athletes and their sources of information. Int. J. Sport Nutr. Exerc. Metab. 2004, 14, 104–120. [Google Scholar] [CrossRef] [PubMed]
- Kårlund, A.; Gómez-Gallego, C.; Turpeinen, A.M.; Palo-Oja, O.-M.; El-Nezami, H.; Kolehmainen, M. Protein Supplements and Their Relation with Nutrition, Microbiota Composition and Health: Is More Protein Always Better for Sportspeople? Nutrients 2019, 11, 829. [Google Scholar] [CrossRef]
- Marinangeli, C.P.F.; Jones, P.J.H. Gazing into the crystal ball: Future considerations for ensuring sustained growth of the functional food and nutraceutical marketplace. Nutr. Res. Rev. 2013, 26, 12–21. [Google Scholar] [CrossRef]
- Vergari, F.; Tibuzzi, A.; Basile, G. An overview of the functional food market: From marketing issues and commercial players to future demand from life in space. Adv. Exp. Med. Biol. 2010, 698, 308–321. [Google Scholar] [CrossRef] [PubMed]
- Romberg, C.; Horner, A.E.; Bussey, T.J.; Saksida, L.M. A touch screen-automated cognitive test battery reveals impaired attention, memory abnormalities, and increased response inhibition in the TgCRND8 mouse model of Alzheimer’s disease. Neurobiol. Aging 2013, 34, 731–744. [Google Scholar] [CrossRef]
- Urai, A.E.; Aguillon-Rodriguez, V.; Laranjeira, I.C.; Cazettes, F.; Mainen, Z.F.; Churchland, A.K. Citric Acid Water as an Alternative to Water Restriction for High-Yield Mouse Behavior. Eneuro 2021, 8, ENEURO.0230-20.2020. [Google Scholar] [CrossRef]
- Reinagel, P. Training Rats Using Water Rewards without Water Restriction. Front. Behav. Neurosci. 2018, 12, 84. [Google Scholar] [CrossRef] [PubMed]
- Watson, P.; Beatey, S.; Wagner, F.; Stahl, T. Water adulteration with citric acid: Effects on drinking and responsivity to regulatory challenges. Physiol. Behav. 1986, 36, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Fischer, K.; Colombani, P.C.; Langhans, W.; Wenk, C. Carbohydrate to protein ratio in food and cognitive performance in the morning. Physiol. Behav. 2002, 75, 411–423. [Google Scholar] [CrossRef]
- Romberg, C.; Yang, S.; Melani, R.; Andrews, M.R.; Horner, A.E.; Spillantini, M.G.; Bussey, T.J.; Fawcett, J.W.; Pizzorusso, T.; Saksida, L.M. Depletion of perineuronal nets enhances recognition memory and long-term depression in the perirhinal cortex. J. Neurosci. 2013, 33, 7057–7065. [Google Scholar] [CrossRef] [PubMed]
- Cardinal, R.N.; Aitken, M.R.F. Whisker: A client–server high-performance multimedia research control system. Behav. Res. Methods 2010, 42, 1059–1071. [Google Scholar] [CrossRef]
- Markou, A.; Salamone, J.D.; Bussey, T.J.; Mar, A.C.; Brunner, D.; Gilmour, G.; Balsam, P. Measuring reinforcement learning and motivation constructs in experimental animals: Relevance to the negative symptoms of schizophrenia. Neurosci. Biobehav. Rev. 2013, 37, 2149–2165. [Google Scholar] [CrossRef]
- Eagle, D.M.; Humby, T.; Dunnett, S.B.; Robbins, T.W. Effects of regional striatal lesions on motor, motivational, and executive aspects of progressive-ratio performance in rats. Behav. Neurosci. 1999, 113, 718–731. [Google Scholar] [CrossRef]
- Hutsell, B.A.; Newland, M.C. A quantitative analysis of the effects of qualitatively different reinforcers on fixed ratio responding in inbred strains of mice. Neurobiol. Learn. Mem. 2013, 101, 85–93. [Google Scholar] [CrossRef]
- van Schaik, C.P.; Burkart, J.M. Social learning and evolution: The cultural intelligence hypothesis. Philos. Trans. R. Soc. B Biol. Sci. 2011, 366, 1008–1016. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, A.; Brigman, J.; Radke, A.; Rudebeck, P.; Holmes, A. The neural basis of reversal learning: An updated perspective. Neuroscience 2017, 345, 12–26. [Google Scholar] [CrossRef]
- Jakobsen, M.U.; Overvad, K. Macronutrient advice for ischemic heart disease prevention. Curr. Opin. Lipidol. 2011, 22, 33–36. [Google Scholar] [CrossRef]
- Alhazmi, A.; Stojanovski, E.; McEvoy, M.; Garg, M.L. Macronutrient intake and type 2 diabetes risk in middle-aged Australian women. Results from the Australian Longitudinal Study on Women’s Health. Public Health Nutr. 2014, 17, 1587–1594. [Google Scholar] [CrossRef]
- Sarris, J.; Logan, A.C.; Akbaraly, T.N.; Amminger, G.P.; Balanzá-Martínez, V.; Freeman, M.P.; Hibbeln, J.; Matsuoka, Y.; Mischoulon, D.; Mizoue, T.; et al. Nutritional medicine as mainstream in psychiatry. Lancet Psychiatry 2015, 2, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Solfrizzi, V.; Custodero, C.; Lozupone, M.; Imbimbo, B.P.; Valiani, V.; Agosti, P.; Schilardi, A.; D’Introno, A.; La Montagna, M.; Calvani, M.; et al. Relationships of dietary patterns, foods, and micro-and macronutrients with Alzheimer’s disease and late-life cognitive disorders: A systematic review. J. Alzheimer’s Dis. 2017, 59, 815–849. [Google Scholar] [CrossRef]
- Roberts, R.O.; Roberts, L.A.; Geda, Y.E.; Cha, R.H.; Pankratz, V.S.; O’Connor, H.M.; Knopman, D.S.; Petersen, R.C. Relative intake of macronutrients impacts risk of mild cognitive impairment or dementia. J. Alzheimer’s Dis. 2012, 32, 329–339. [Google Scholar] [CrossRef]
- Dye, L.; Lluch, A.; Blundell, J.E. Macronutrients and mental performance. Nutrition 2000, 16, 1021–1034. [Google Scholar] [CrossRef]
- Brigman, J.L.; Graybeal, C.; Holmes, A. Predictably irrational: Assaying cognitive inflexibility in mouse models of schizophrenia. Front. Neurosci. 2010, 4. [Google Scholar] [CrossRef] [PubMed]
- McGonigle, P.; Ruggeri, B. Animal models of human disease: Challenges in enabling translation. Biochem. Pharmacol. 2014, 87, 162–171. [Google Scholar] [CrossRef]
- Nithianantharajah, J.; McKechanie, A.G.; Stewart, T.J.; Johnstone, M.; Blackwood, D.H.; Clair, D.S.; Grant, S.G.N.; Bussey, T.J.; Saksida, L.M. Bridging the translational divide: Identical cognitive touchscreen testing in mice and humans carrying mutations in a disease-relevant homologous gene. Sci. Rep. 2015, 5, 14613. [Google Scholar] [CrossRef] [PubMed]
- Bussey, T.; Holmes, A.; Lyon, L.; Mar, A.; McAllister, K.; Nithianantharajah, J.; Oomen, C.; Saksida, L. New translational assays for preclinical modelling of cognition in schizophrenia: The touchscreen testing method for mice and rats. Neuropharmacology 2012, 62, 1191–1203. [Google Scholar] [CrossRef]
- Horner, A.E.; Heath, C.J.; Hvoslef-Eide, M.; Kent, B.A.; Kim, C.H.; Nilsson, S.R.; Alsiö, J.; Oomen, C.A.; Holmes, A.; Saksida, L.M.; et al. The touchscreen operant platform for testing learning and memory in rats and mice. Nat. Protoc. 2013, 8, 1961–1984. [Google Scholar] [CrossRef]
- Robbins, T.; James, M.; Owen, A.; Sahakian, B.; McInnes, L.; Rabbitt, P. Cambridge Neuropsychological Test Automated Battery (CANTAB): A factor analytic study of a large sample of normal elderly volunteers. Dementia 1994, 5, 266–281. [Google Scholar] [CrossRef]
- Auersperg, A.M.; Gajdon, G.K.; von Bayern, A.M. A new approach to comparing problem solving, flexibility and innovation. Commun. Integr. Biol. 2012, 5, 140–145. [Google Scholar] [CrossRef]
- Haier, R.J.; Siegel, B.V.; MacLachlan, A.; Soderling, E.; Lottenberg, S.; Buchsbaum, M.S. Regional glucose metabolic changes after learning a complex visuospatial/motor task: A positron emission tomographic study. Brain Res. 1992, 570, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Jonides, J.; Schumacher, E.H.; Smith, E.E.; Lauber, E.J.; Awh, E.; Minoshima, S.; Koeppe, R.A. Verbal working memory load affects regional brain activation as measured by PET. J. Cogn. Neurosci. 1997, 9, 462–475. [Google Scholar] [CrossRef]
- Haier, R.J.; Siegel, B.; Tang, C.; Abel, L.; Buchsbaum, M.S. Intelligence and changes in regional cerebral glucose metabolic rate following learning. Intelligence 1992, 16, 415–426. [Google Scholar] [CrossRef]
- Kennedy, D.; Scholey, A. Glucose administration, heart rate and cognitive performance: Effects of increasing mental effort. Psychopharmacology 2000, 149, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.; Ying, Z.; Gomez-Pinilla, F. Dietary omega-3 fatty acids normalize BDNF levels, reduce oxidative damage, and counteract learning disability after traumatic brain injury in rats. J. Neurotrauma 2004, 21, 1457–1467. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.; Molteni, R.; Ying, Z.; Gomez-Pinilla, F. A saturated-fat diet aggravates the outcome of traumatic brain injury on hippocampal plasticity and cognitive function by reducing brain-derived neurotrophic factor. Neuroscience 2003, 119, 365–375. [Google Scholar] [CrossRef]
- McCann, J.C.; Ames, B.N. Is docosahexaenoic acid, an n-3 long-chain polyunsaturated fatty acid, required for development of normal brain function? An overview of evidence from cognitive and behavioral tests in humans and animals. Am. J. Clin. Nutr. 2005, 82, 281–295. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Park, S.J.; Tamura, M.; Ando, S. Effect of the long-term feeding of dietary lipids on the learning ability, fatty acid composition of brain stem phospholipids and synaptic membrane fluidity in adult mice: A comparison of sardine oil diet with palm oil diet. Mech. Ageing Dev. 1998, 101, 119–128. [Google Scholar] [CrossRef]
- Wu, A.; Ying, Z.; Gomez-Pinilla, F.; Guley, N.H.; Rogers, J.T.; Del Mar, N.A.; Deng, Y.; Islam, R.M.; D’Surney, L.; Ferrell, J.; et al. Omega-3 fatty acids supplementation restores mechanisms that maintain brain homeostasis in traumatic brain injury. J. Neurotrauma 2007, 24, 1587–1595. [Google Scholar] [CrossRef]
- Winocur, G.; Greenwood, C.E. Studies of the effects of high fat diets on cognitive function in a rat model. Neurobiol. Aging 2005, 26, 46–49. [Google Scholar] [CrossRef] [PubMed]
- Molteni, R.; Barnard, R.; Ying, Z.; Roberts, C.; Gómez-Pinilla, F. A high-fat, refined sugar diet reduces hippocampal brain-derived neurotrophic factor, neuronal plasticity, and learning. Neuroscience 2002, 112, 803–814. [Google Scholar] [CrossRef]
- Calvo-Ochoa, E.; Hernández-Ortega, K.; Ferrera, P.; Morimoto, S.; Arias, C. Short-term high-fat-and-fructose feeding produces insulin signaling alterations accompanied by neurite and synaptic reduction and astroglial activation in the rat hippocampus. J. Cereb. Blood Flow Metab. 2014, 34, 1001–1008. [Google Scholar] [CrossRef] [PubMed]
- Parrott, M.D.; Greenwood, C.E. Dietary influences on cognitive function with aging: From high-fat diets to healthful eating. Ann. New York Acad. Sci. 2007, 1114, 389–397. [Google Scholar] [CrossRef]
- Tran, D.M.; Westbrook, R.F. A high-fat high-sugar diet-induced impairment in place-recognition memory is reversible and training-dependent. Appetite 2017, 110, 61–71. [Google Scholar] [CrossRef]
- Beilharz, J.; Maniam, J.; Morris, M. Short-term exposure to a diet high in fat and sugar, or liquid sugar, selectively impairs hippocampal-dependent memory, with differential impacts on inflammation. Behav. Brain Res. 2016, 306, 1–7. [Google Scholar] [CrossRef]
- Boitard, C.; Cavaroc, A.; Sauvant, J.; Aubert, A.; Castanon, N.; Layé, S.; Ferreira, G. Impairment of hippocampal-dependent memory induced by juvenile high-fat diet intake is associated with enhanced hippocampal inflammation in rats. Brain Behav. Immun. 2014, 40, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Holloway, C.J.; Cochlin, L.E.; Emmanuel, Y.; Murray, A.; Codreanu, I.; Edwards, L.M.; Szmigielski, C.; Tyler, D.J.; Knight, N.S.; Saxby, B.K.; et al. A high-fat diet impairs cardiac high-energy phosphate metabolism and cognitive function in healthy human subjects. Am. J. Clin. Nutr. 2011, 93, 748–755. [Google Scholar] [CrossRef]
- Forouhi, N.G.; Krauss, R.M.; Taubes, G.; Willett, W. Dietary fat and cardiometabolic health: Evidence, controversies, and consensus for guidance. BMJ 2018, 361, k2139. [Google Scholar] [CrossRef]
- Lutas, A.; Yellen, G. The ketogenic diet: Metabolic influences on brain excitability and epilepsy. Trends Neurosci. 2013, 36, 32–40. [Google Scholar] [CrossRef]
- Hallböök, T.; Ji, S.; Maudsley, S.; Martin, B. The effects of the ketogenic diet on behavior and cognition. Epilepsy Res. 2011, 100, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Betz, A.L.; Goldstein, G.; Katzman, R. Blood-brain-cerebrospinal fluid barriers. In Basic Neurochemistry: Molecular, Cellular, and Medical Aspects, 5th ed.; Siegel, G.J., Ed.; Raven Press: New York, NY, USA, 1994; pp. 681–698. [Google Scholar]
- Institute of Medicine (US) Committee on Military Nutrition Research. 14. Amino Acid and Protein Requirements: Cognitive Performance, Stress, and Brain Function. In The Role of Protein and Amino Acids in Sustaining and Enhancing Performance; National Academies Press (US): Washington, DC, USA, 1999. [Google Scholar]
- Institute of Medicine (US) Committee on Military Nutrition Research. 15. Tyrosine and Stress: Human and Animal Studies. In Food Components to Enhance Performance: An Evaluation of Potential Performance-Enhancing Food Components for Operational Rations; Marriott, B.M., Ed.; National Academies Press (US): Washington, DC, USA, 1994. [Google Scholar]
- Klein, M.O.; Battagello, D.S.; Cardoso, A.R.; Hauser, D.N.; Bittencourt, J.C.; Correa, R.G. Dopamine: Functions, Signaling, and Association with Neurological Diseases. Cell. Mol. Neurobiol. 2018, 39, 31–59. [Google Scholar] [CrossRef]
- Fernstrom, M.H.; Fernstrom, J.D. Brain tryptophan concentrations and serotonin synthesis remain responsive to food consumption after the ingestion of sequential meals. Am. J. Clin. Nutr. 1995, 61, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, H.R.; Caballero, B.; Finer, N. The composition of lunch determines afternoon plasma tryptophan ratios in humans. J. Neural. Transm. 1986, 65, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Onaolapo, A.Y.; Onaolapo, O.J. Dietary glutamate and the brain: In the footprints of a Jekyll and Hyde molecule. Neurotoxicology 2020, 80, 93–104. [Google Scholar] [CrossRef]
- Schwarz, R.; Kaspar, A.; Seelig, J.; Künnecke, B. Gastrointestinal transit times in mice and humans measured with 27Al and 19F nuclear magnetic resonance. Magn. Reson. Med. 2002, 48, 255–261. [Google Scholar] [CrossRef]
- Carreiro, A.L.; Dhillon, J.; Gordon, S.; Higgins, K.A.; Jacobs, A.G.; McArthur, B.M.; Redan, B.W.; Rivera, R.L.; Schmidt, L.R.; Mattes, R.D. The Macronutrients, Appetite, and Energy Intake. Annu. Rev. Nutr. 2016, 36, 73–103. [Google Scholar] [CrossRef] [PubMed]
- Marciani, L.; Cox, E.F.; Pritchard, S.E.; Major, G.; Hoad, C.L.; Mellows, M.; Hussein, M.O.; Costigan, C.; Fox, M.; Gowland, P.A.; et al. Additive effects of gastric volumes and macronutrient composition on the sensation of postprandial fullness in humans. Eur. J. Clin. Nutr. 2015, 69, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Ormsbee, M.J.; Bach, C.W.; Baur, D.A. Pre-Exercise Nutrition: The Role of Macronutrients, Modified Starches and Supplements on Metabolism and Endurance Performance. Nutrients 2014, 6, 1782–1808. [Google Scholar] [CrossRef]
- Yang, J.; Park, H.J.; Hwang, W.; Kim, T.H.; Kim, H.; Oh, J.; Cho, M.S. Changes in the glucose and insulin responses according to high-protein snacks for diabetic patients. Nutr. Res. Pract. 2021, 15, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Imamura, F.; Micha, R.; Wu, J.H.Y.; de Oliveira Otto, M.C.; Otite, F.O.; Abioye, A.I.; Mozaffarian, D. Effects of Saturated Fat, Polyunsaturated Fat, Monounsaturated Fat, and Carbohydrate on Glucose-Insulin Homeostasis: A Systematic Review and Meta-analysis of Randomised Controlled Feeding Trials. PLoS Med. 2016, 13, e1002087. [Google Scholar] [CrossRef] [PubMed]

| Control Diet | Carbohydrate Diet | |||||||
| Amount (g) | Composition | Amount (g) | Composition | |||||
| Protein | 18.5 | Egg protein (0.93 g) | Milk protein (17.57 g) | 7 | Egg protein (0.35 g) | Milk protein (6.65 g) | ||
| Fat | 4.5 | Butter (2.25 g) | Vegetable oil (2.25 g) | 2.6 | Butter (1.30 g) | Vegetable oil (1.30 g) | ||
| Carbohydrate | 54.2 | Glucose (8.67 g) | Starch (8.67 g) | Maltodextrin (36.85 g) | 69.7 | Glucose (15.47 g) | Starch (15.47 g) | Maltodextrin (38.76 g) |
| Fiber | 4.5 | Apple fiber | 4.5 | Apple fiber | ||||
| Minerals (Ash) | 6.3 | Ca, Fe, P, Mn, Mg, K | Vitamin D3 | 6.3 | Ca, Fe, P, Mn, Mg, K | Vitamin D3 | ||
| Water | 36 | 30 | ||||||
| Protein Diet | Fat Diet | |||||||
| Amount (g) | Composition | Amount (g) | Composition | |||||
| Protein | 43.6 | Egg protein (2.18 g) | Milk protein (41.42 g) | 21.8 | Egg protein (1.10 g) | Milk protein (20.70 g) | ||
| Fat | 4.5 | Butter (2.25 g) | Vegetable oil (2.25 g) | 23.2 | Butter (11.60 g) | Vegetable oil (11.60 g) | ||
| Carbohydrate | 28.9 | Glucose (4.62 g) | Starch (4.62 g) | Maltodextrin (19.65 g) | 9 | Glucose (1.44 g) | Starch (1.44 g) | Maltodextrin (6.12 g) |
| Fiber | 4.5 | Apple fiber | 4.5 | Apple fiber | ||||
| Minerals (Ash) | 6.3 | Ca, Fe, P, Mn, Mg, K | Vitamin D3 | 6.3 | Ca, Fe, P, Mn, Mg, K | Vitamin D3 | ||
| Water | 35.4 | 35.2 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guillaumin, M.C.C.; Syarov, B.; Burdakov, D.; Peleg-Raibstein, D. Something to Snack on: Can Dietary Modulators Boost Mind and Body? Nutrients 2023, 15, 1356. https://doi.org/10.3390/nu15061356
Guillaumin MCC, Syarov B, Burdakov D, Peleg-Raibstein D. Something to Snack on: Can Dietary Modulators Boost Mind and Body? Nutrients. 2023; 15(6):1356. https://doi.org/10.3390/nu15061356
Chicago/Turabian StyleGuillaumin, Mathilde C. C., Boris Syarov, Denis Burdakov, and Daria Peleg-Raibstein. 2023. "Something to Snack on: Can Dietary Modulators Boost Mind and Body?" Nutrients 15, no. 6: 1356. https://doi.org/10.3390/nu15061356
APA StyleGuillaumin, M. C. C., Syarov, B., Burdakov, D., & Peleg-Raibstein, D. (2023). Something to Snack on: Can Dietary Modulators Boost Mind and Body? Nutrients, 15(6), 1356. https://doi.org/10.3390/nu15061356







