ECHOPvir: A Mixture of Echinacea and Hop Extracts Endowed with Cytoprotective, Immunomodulatory and Antiviral Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Herbal Extracts and Mixture Preparation
2.3. Phytochemical Analysis
2.3.1. Total Polyphenols, Tannins and Flavonoids
2.3.2. Chromatographic Analysis of the Phenolic Compounds
2.4. Cell Cultures
2.5. Treatment Schedules for the Antioxidant, Cytoprotective and Immunomodulatory Activities
2.6. Trypan Blue Exclusion Assay
2.7. Cytotoxicity Assay
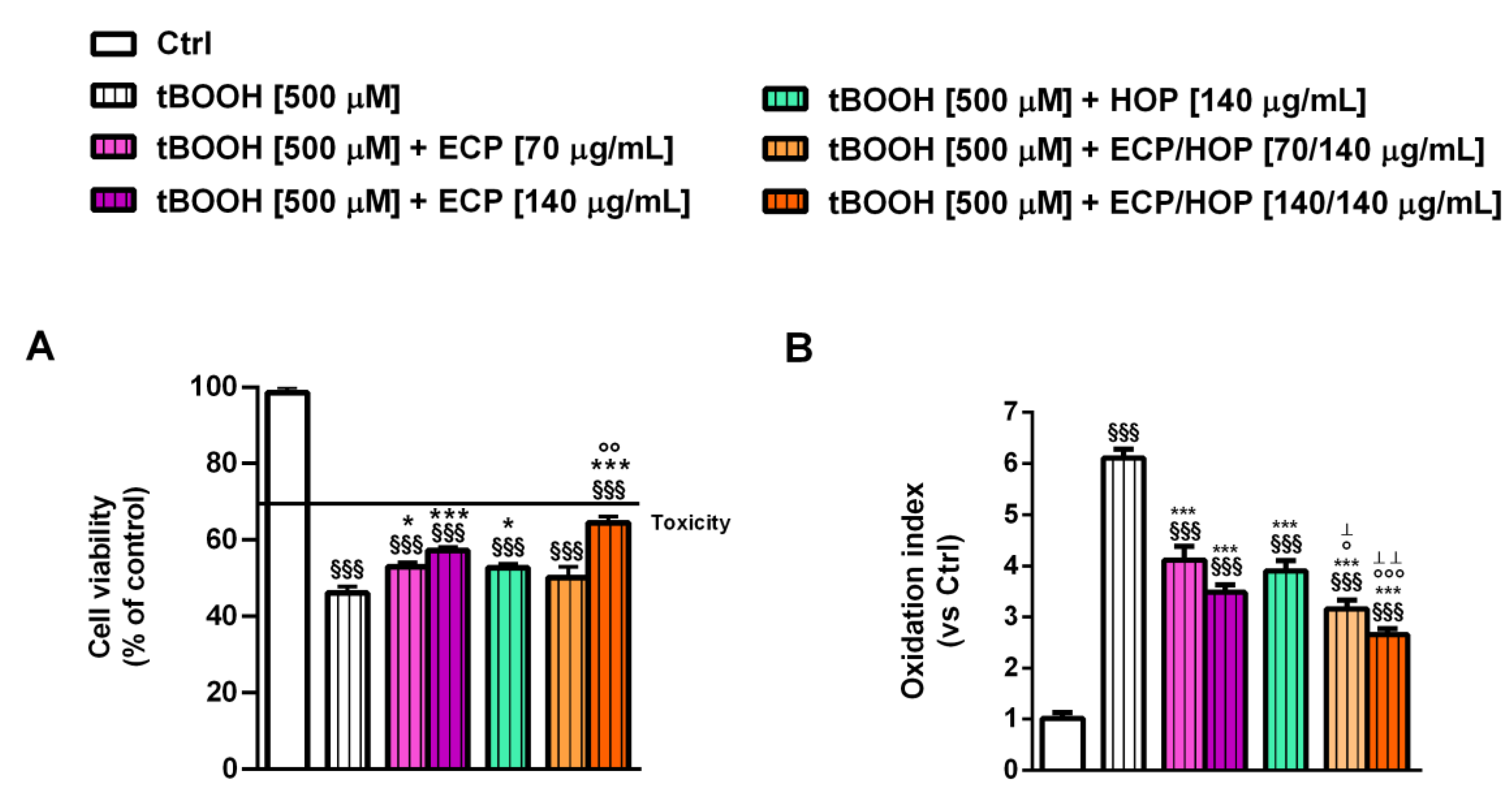
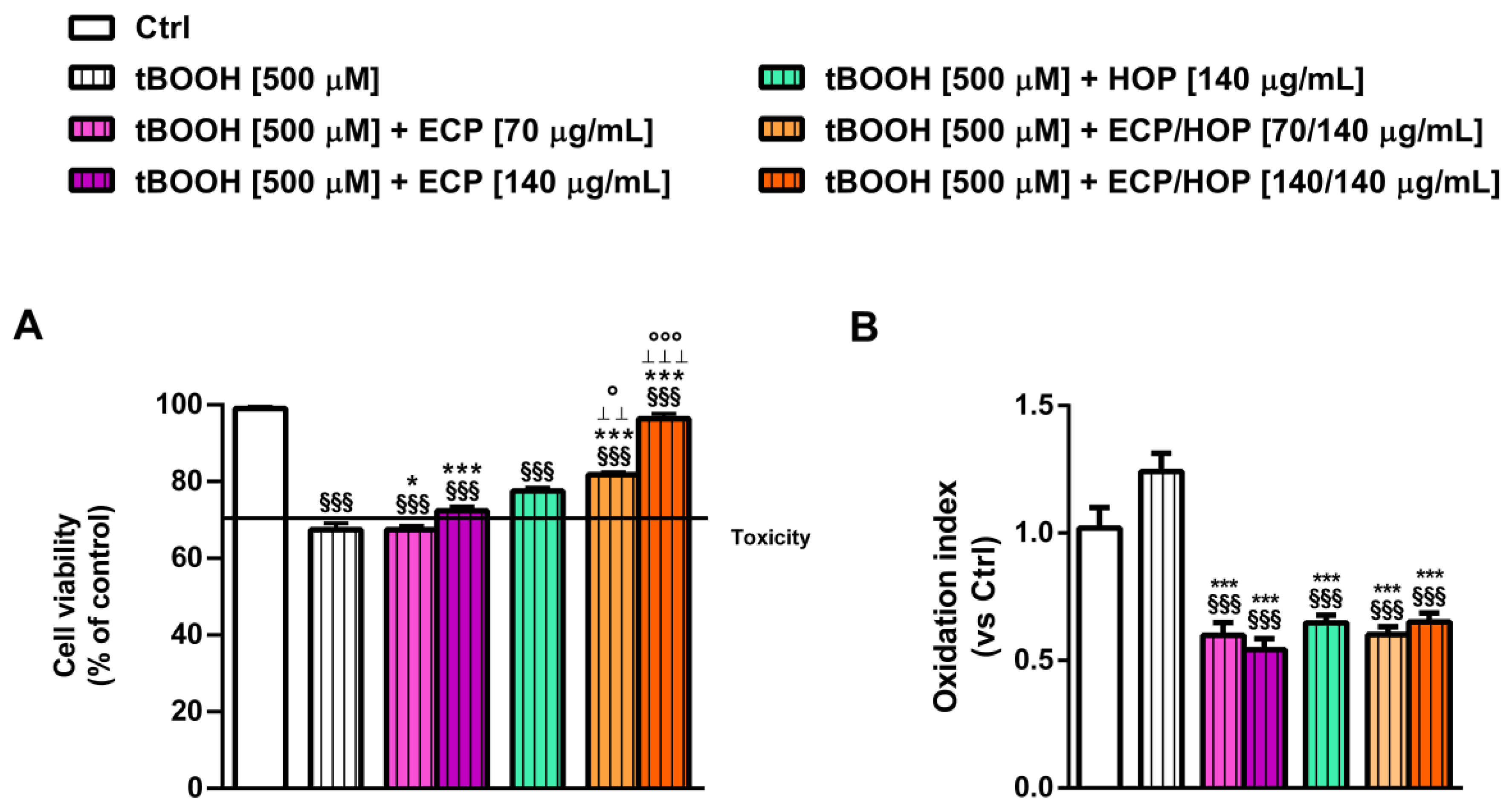
2.8. Cytoprotection towards the Oxidative Damage Induced by Tert-Butyl Hydroperoxide (tBOOH)
2.9. Intracellular Reactive Oxygen Species (ROS) Determination
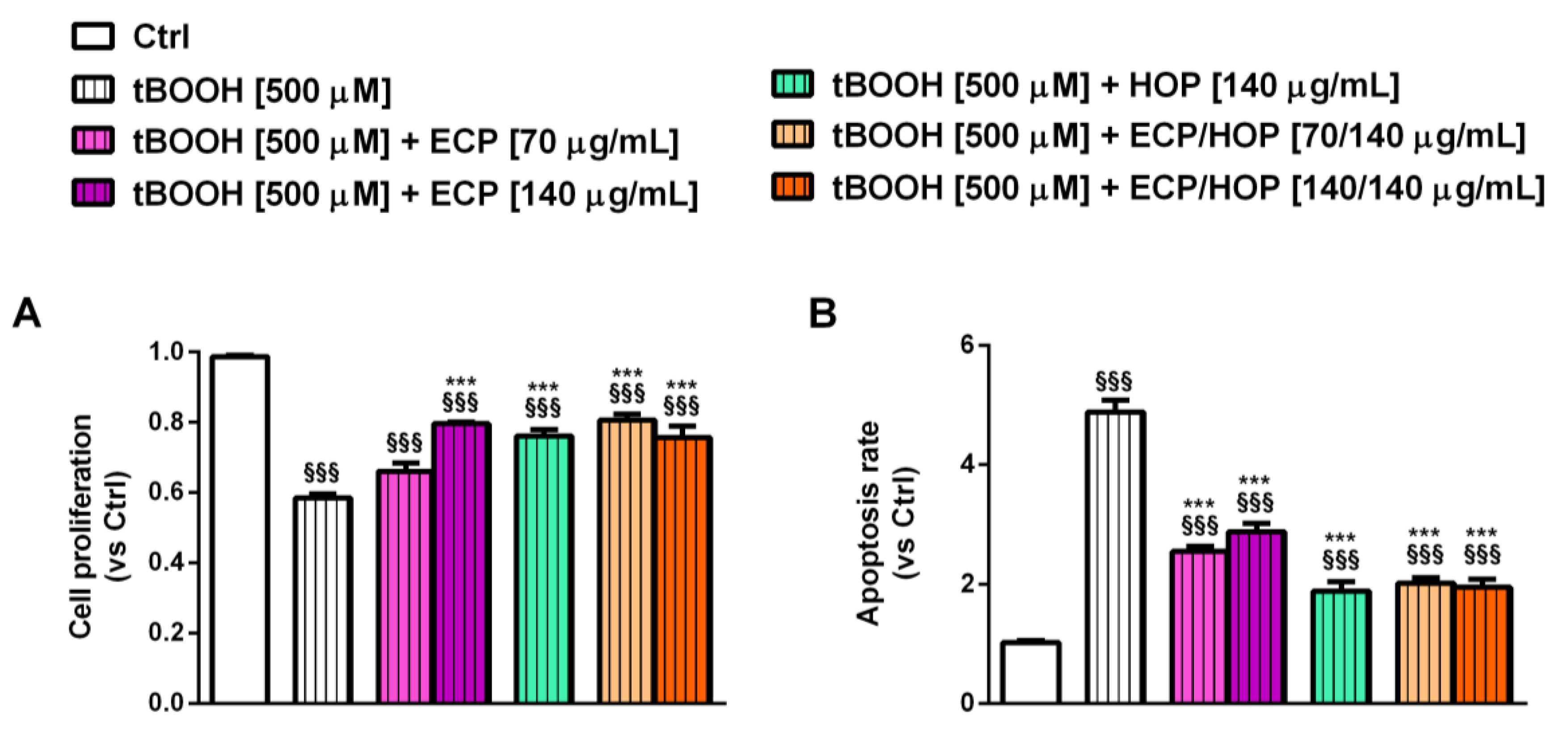
2.10. Apoptosis Detection
2.11. Neutral Red Uptake Assay
2.12. Determination of the Secreted Nitric Oxide (NO) Levels in the Cell-Free Supernatant
2.13. Viral Infection and Titration
2.13.1. Antiviral Activity
2.13.2. Immunoblotting Analysis
2.13.3. In-Cell Western (ICW) Assay
2.14. Statistical Analysis
3. Results
3.1. Phytochemical Analysis
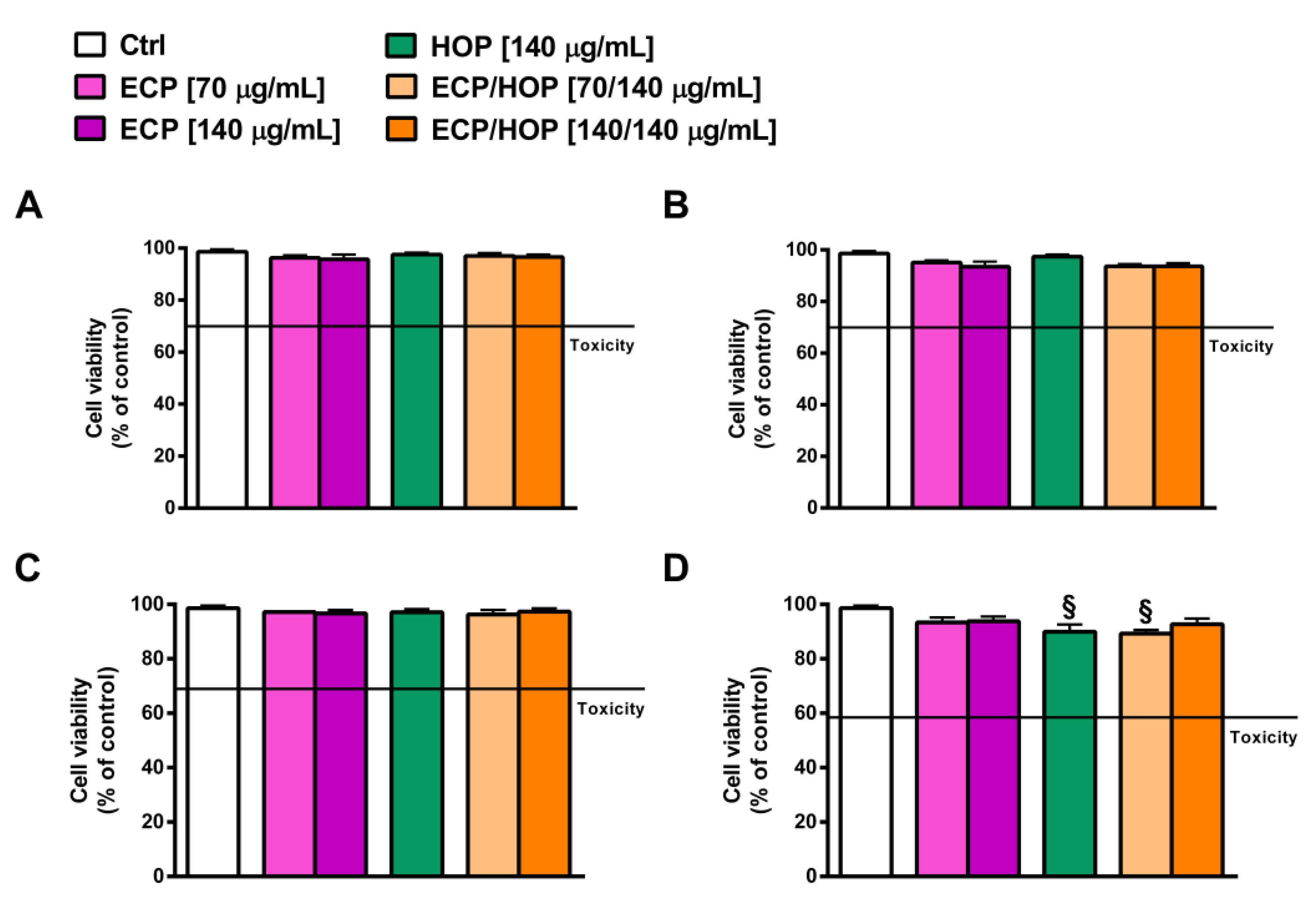
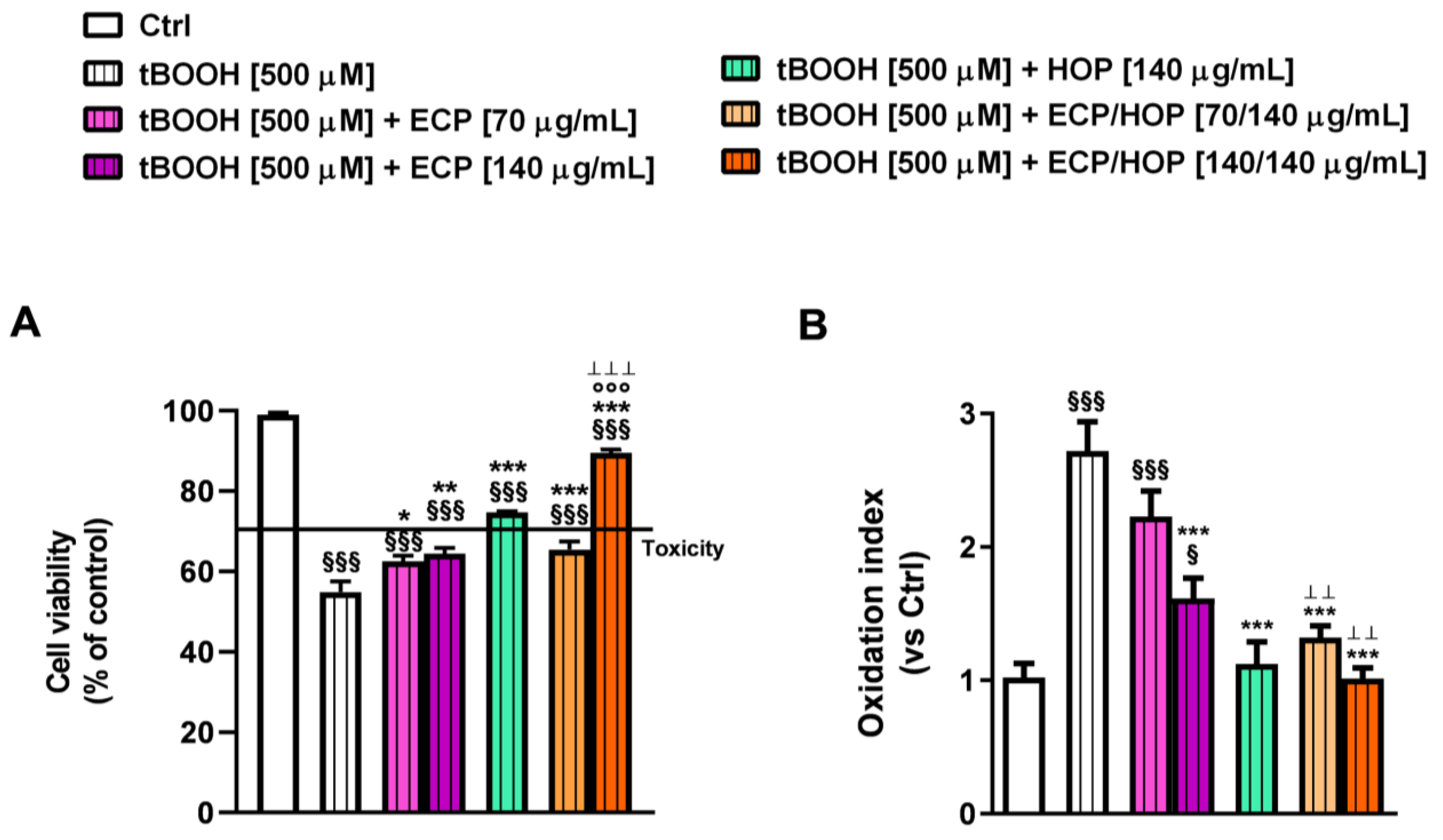
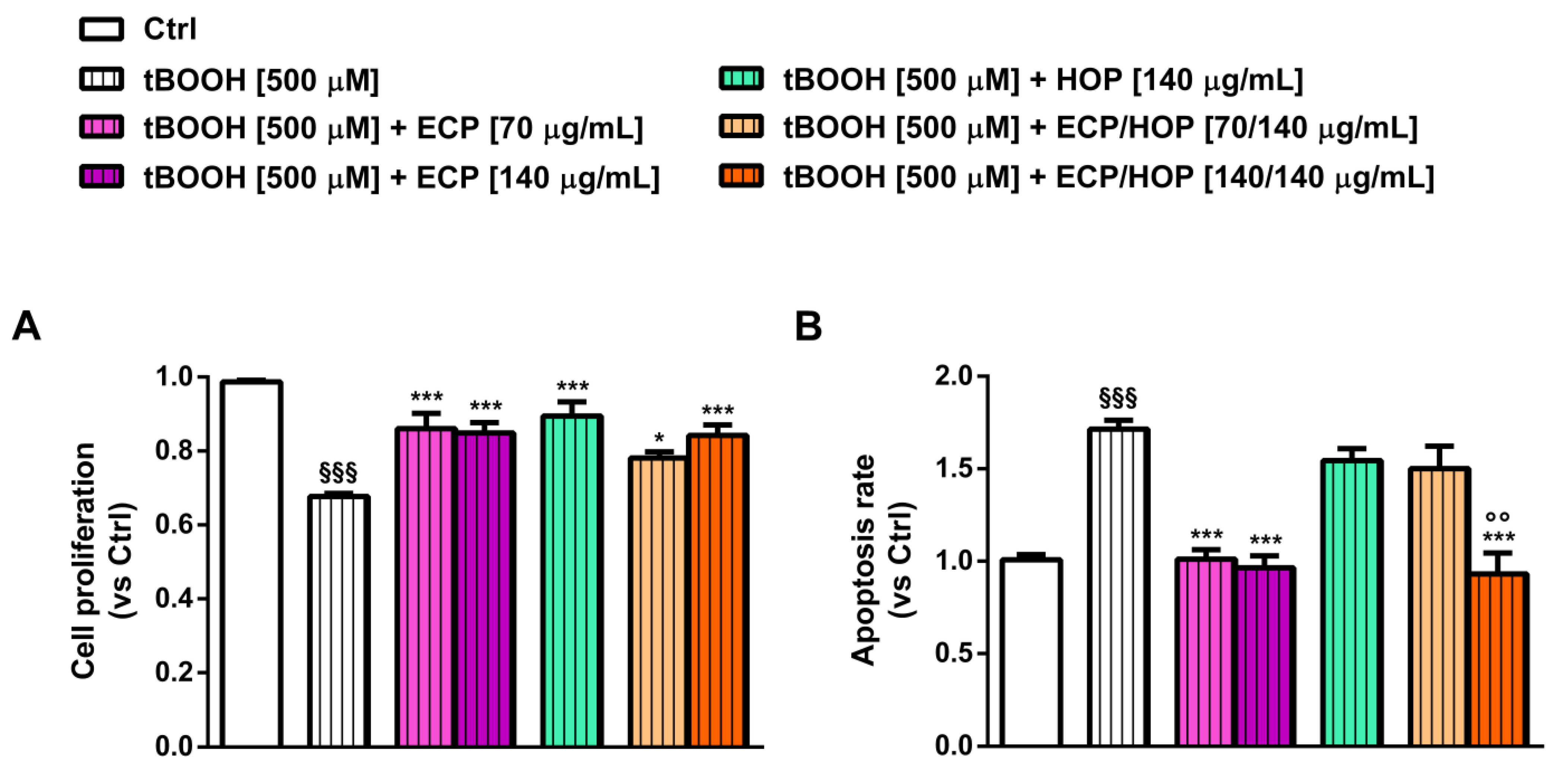
3.2. Cytotoxicity of HOP and ECP Extracts and Their Mixtures in Bronchial Epithelial BEAS-2B Cells and in Lung Adenocarcinoma A549 Cells
3.3. Effect of the Treatments on the Apoptosis Rate in Bronchial Epithelial BEAS-2B Cells
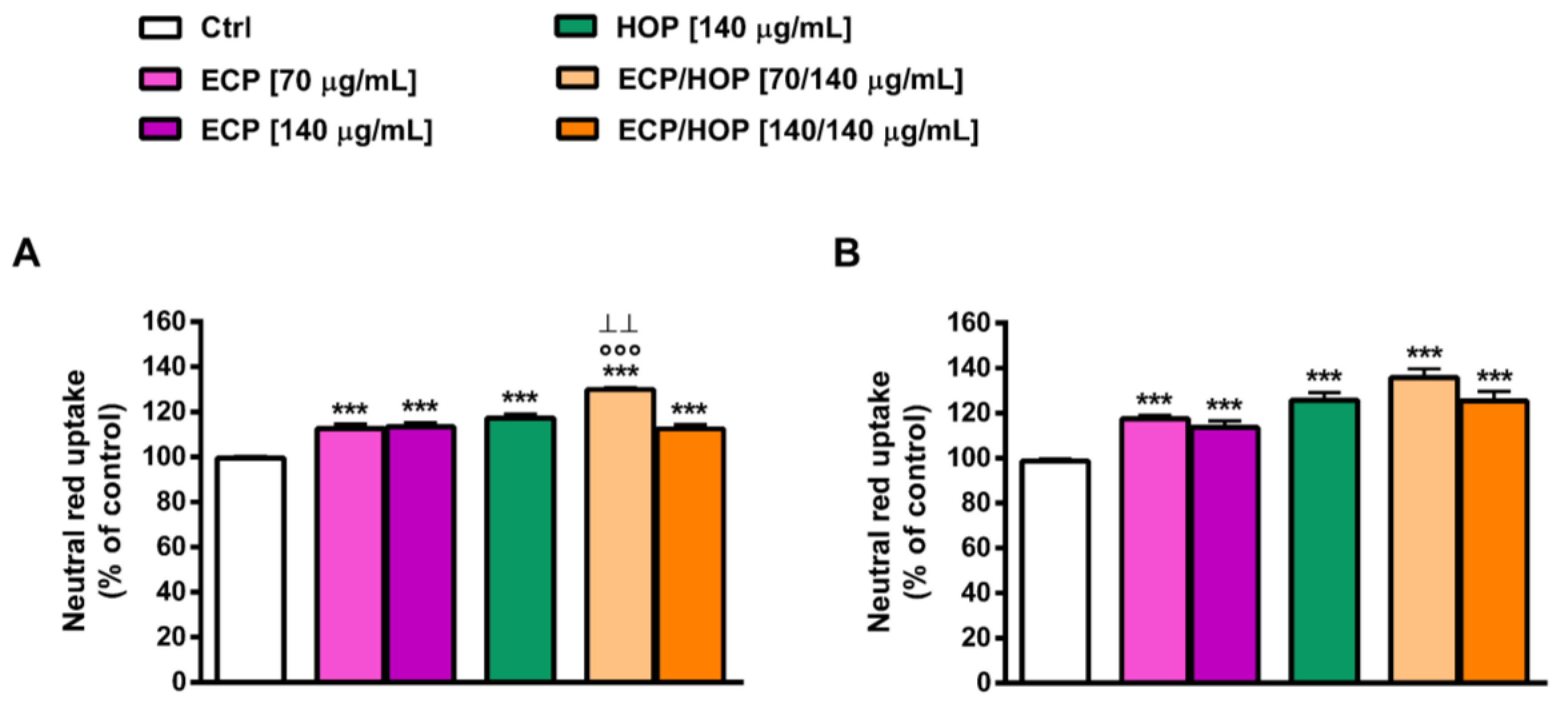
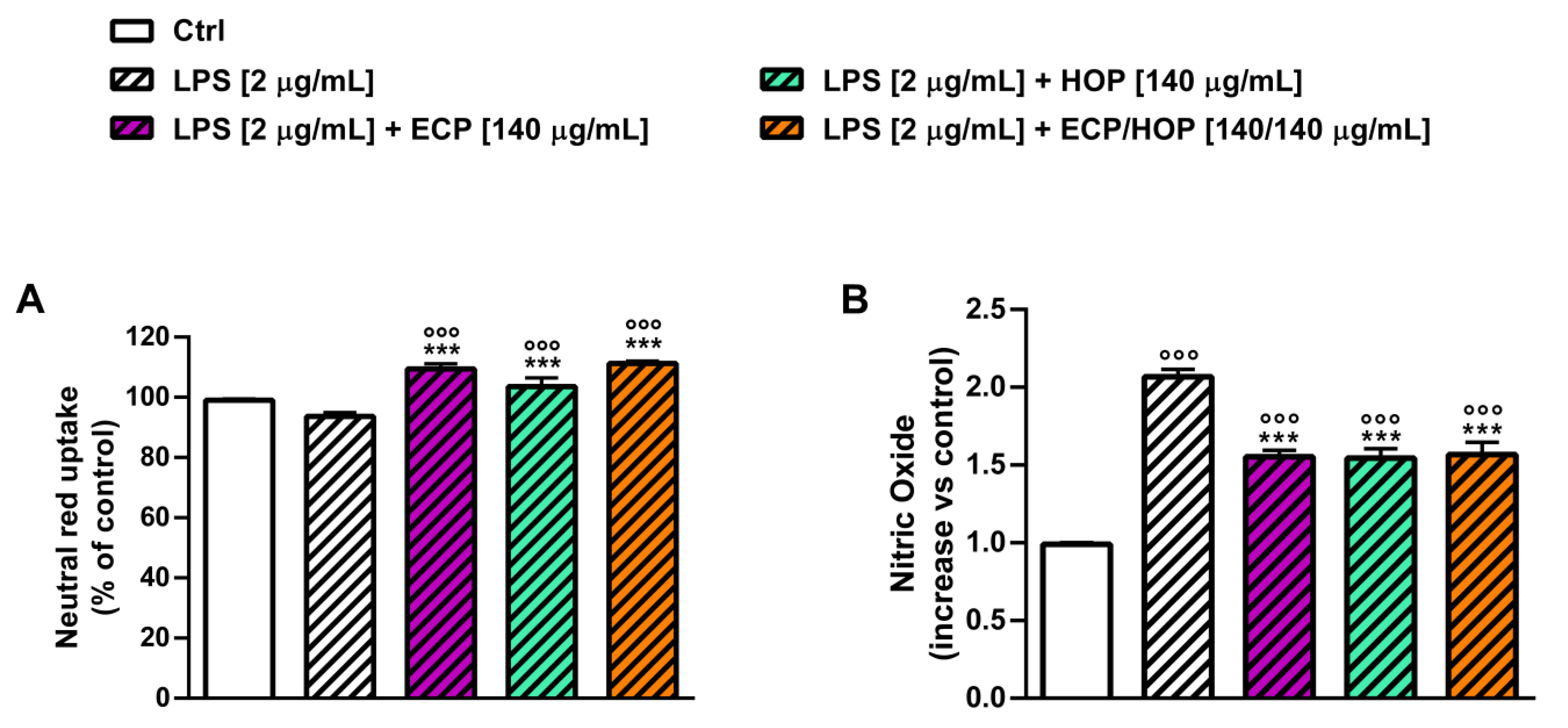
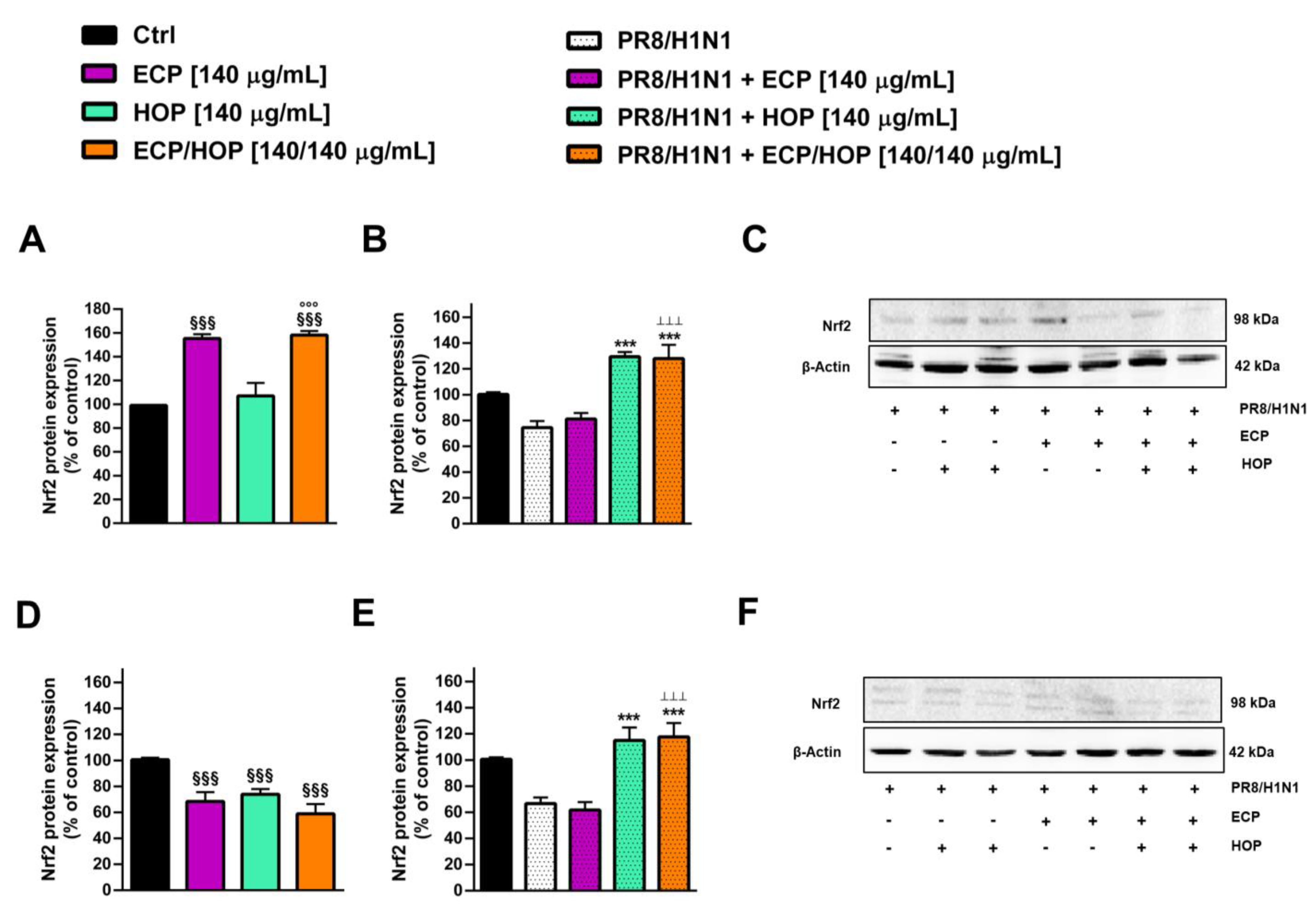
3.4. Immunomodulatory Activity in Murine Macrophages RAW 264.7
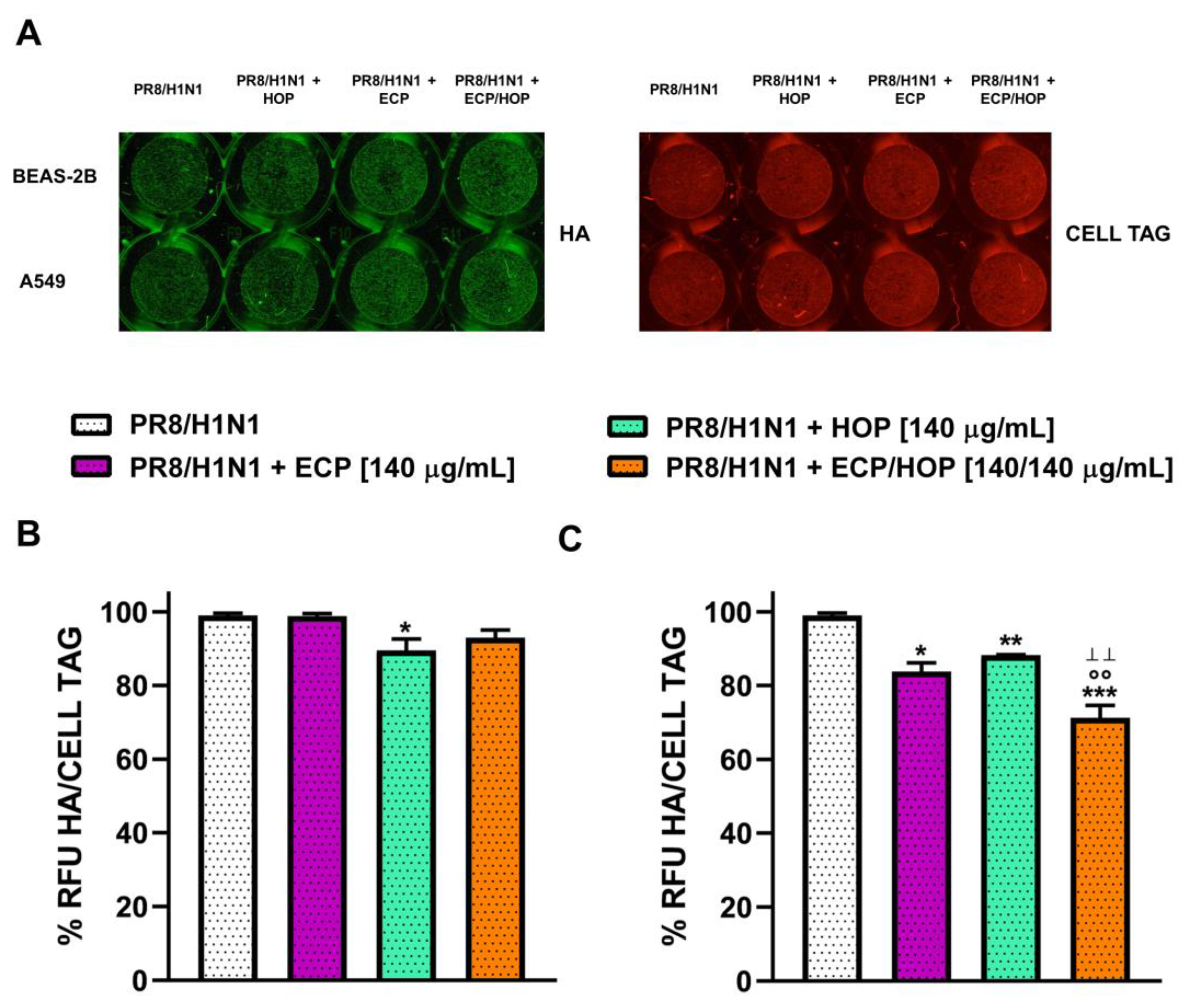
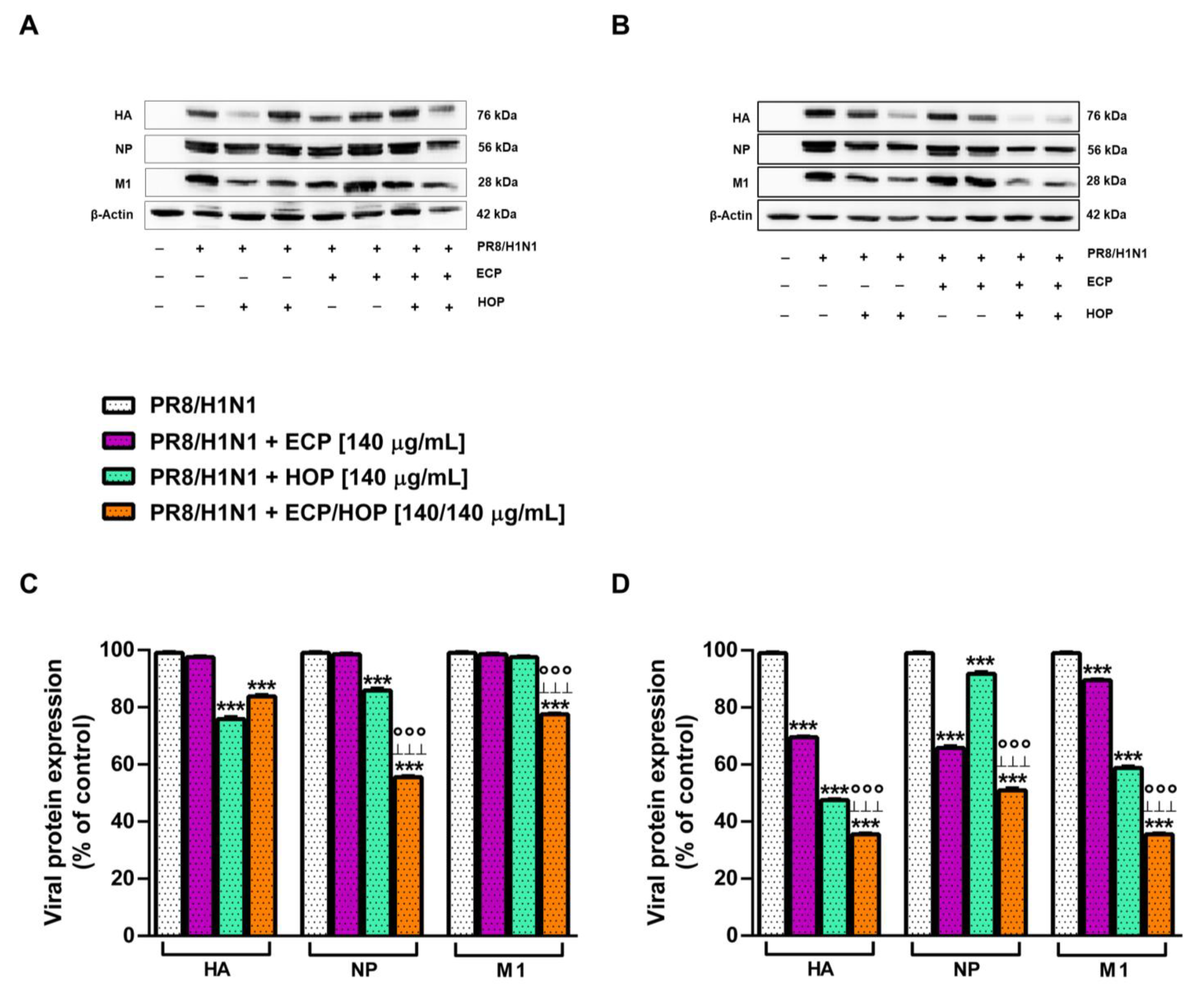
3.5. Antiviral Activity against Influenza Virus PR8/H1N1 Strain in Bronchial Epithelial BEAS-2B Cells and in Lung Adenocarcinoma A549 Cells
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Malosh, R.E.; Martin, E.T.; Ortiz, J.R.; Monto, A.S. The risk of lower respiratory tract infection following influenza virus infection: A systematic and narrative review. Vaccine 2018, 36, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Egilmezer, E.; Walker, G.J.; Bakthavathsalam, P.; Peterson, J.R.; Gooding, J.J.; Rawlinson, W.; Stelzer-Braid, S. Systematic review of the impact of point-of-care testing for influenza on the outcomes of patients with acute respiratory tract infection. Rev. Med. Virol. 2018, 28, e1995. [Google Scholar] [CrossRef] [PubMed]
- Lampejo, T. Influenza and antiviral resistance: An overview. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 1201–1208. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Perez, J.; Schafer, A.; Cheng, H.; Peet, N.; Rong, L.; Manicassamy, B. Influenza Virus: Small Molecule Therapeutics and Mechanisms of Antiviral Resistance. Curr. Med. Chem. 2018, 25, 5115–5127. [Google Scholar] [CrossRef] [PubMed]
- CDC. Influenza Antiviral Medications: Summary for Clinicians. Available online: https://www.cdc.gov/flu/professionals/antivirals/summary-clinicians.htm (accessed on 31 July 2023).
- Fraternale, A.; Zara, C.; De Angelis, M.; Nencioni, L.; Palamara, A.T.; Retini, M.; Di Mambro, T.; Magnani, M.; Crinelli, R. Intracellular Redox-Modulated Pathways as Targets for Effective Approaches in the Treatment of Viral Infection. Int. J. Mol. Sci. 2021, 22, 3603. [Google Scholar] [CrossRef]
- Amatore, D.; Sgarbanti, R.; Aquilano, K.; Baldelli, S.; Limongi, D.; Civitelli, L.; Nencioni, L.; Garaci, E.; Ciriolo, M.R.; Palamara, A.T. Influenza virus replication in lung epithelial cells depends on redox-sensitive pathways activated by NOX4-derived ROS. Cell Microbiol. 2015, 17, 131–145. [Google Scholar] [CrossRef]
- Nencioni, L.; Iuvara, A.; Aquilano, K.; Ciriolo, M.R.; Cozzolino, F.; Rotilio, G.; Garaci, E.; Palamara, A.T. Influenza A virus replication is dependent on an antioxidant pathway that involves GSH and Bcl-2. FASEB J. 2003, 17, 758–760. [Google Scholar] [CrossRef]
- De Angelis, M.; Amatore, D.; Checconi, P.; Zevini, A.; Fraternale, A.; Magnani, M.; Hiscott, J.; De Chiara, G.; Palamara, A.T.; Nencioni, L. Influenza Virus Down-Modulates G6PD Expression and Activity to Induce Oxidative Stress and Promote Its Replication. Front. Cell Infect. Microbiol. 2021, 11, 804976. [Google Scholar] [CrossRef]
- Newton, A.H.; Cardani, A.; Braciale, T.J. The host immune response in respiratory virus infection: Balancing virus clearance and immunopathology. Semin. Immunopathol. 2016, 38, 471–482. [Google Scholar] [CrossRef]
- Vareille, M.; Kieninger, E.; Edwards, M.R.; Regamey, N. The airway epithelium: Soldier in the fight against respiratory viruses. Clin. Microbiol. Rev. 2011, 24, 210–229. [Google Scholar] [CrossRef]
- Checconi, P.; De Angelis, M.; Marcocci, M.E.; Fraternale, A.; Magnani, M.; Palamara, A.T.; Nencioni, L. Redox-Modulating Agents in the Treatment of Viral Infections. Int. J. Mol. Sci. 2020, 21, 4084. [Google Scholar] [CrossRef]
- Dhochak, N.; Singhal, T.; Kabra, S.K.; Lodha, R. Pathophysiology of COVID-19: Why Children Fare Better than Adults? Indian J. Pediatr. 2020, 87, 537–546. [Google Scholar] [CrossRef]
- Jia, L.; Xie, J.; Zhao, J.; Cao, D.; Liang, Y.; Hou, X.; Wang, L.; Li, Z. Mechanisms of Severe Mortality-Associated Bacterial Co-infections Following Influenza Virus Infection. Front. Cell Infect. Microbiol. 2017, 7, 338. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Shahid, I.; Anker, S.D.; Solomon, S.D.; Vardeny, O.; Michos, E.D.; Fonarow, G.C.; Butler, J. Cardiovascular implications of COVID-19 versus influenza infection: A review. BMC Med. 2020, 18, 403. [Google Scholar] [CrossRef] [PubMed]
- Sen, T.; Samanta, S.K. Medicinal plants, human health and biodiversity: A broad review. Adv. Biochem. Eng. Biotechnol. 2015, 147, 59–110. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, B.; Marasini, B.P.; Rayamajhee, B.; Bhattarai, B.R.; Lamichhane, G.; Khadayat, K.; Adhikari, A.; Khanal, S.; Parajuli, N. Potential roles of medicinal plants for the treatment of viral diseases focusing on COVID-19: A review. Phytother. Res. 2021, 35, 1298–1312. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, M.; Della-Morte, D.; Buttinelli, G.; Di Martino, A.; Pacifici, F.; Checconi, P.; Ambrosio, L.; Stefanelli, P.; Palamara, A.T.; Garaci, E.; et al. Protective Role of Combined Polyphenols and Micronutrients against Influenza A Virus and SARS-CoV-2 Infection In Vitro. Biomedicines 2021, 9, 1721. [Google Scholar] [CrossRef]
- Van Cleemput, M.; Cattoor, K.; De Bosscher, K.; Haegeman, G.; De Keukeleire, D.; Heyerick, A. Hop (Humulus lupulus)-derived bitter acids as multipotent bioactive compounds. J. Nat. Prod. 2009, 72, 1220–1230. [Google Scholar] [CrossRef]
- Korpelainen, H.; Pietiläinen, M. Hop (Humulus lupulus L.): Traditional and Present Use, and Future Potential. Econ. Bot. 2021, 75, 302–322. [Google Scholar] [CrossRef]
- Kowalska, G.; Bouchentouf, S.; Kowalski, R.; Wyrostek, J.; Pankiewicz, U.; Mazurek, A.; Sujka, M.; Włodarczyk-Stasiak, M. The hop cones (Humulus lupulus L.) Chemical composition, antioxidant properties and molecular docking simulations. J. Herb. Med. 2022, 33, 100566. [Google Scholar] [CrossRef]
- Zugravu, C.-A.; Bohiltea, R.-E.; Salmen, T.; Pogurschi, E.; Otelea, M.R. Antioxidants in Hops: Bioavailability, Health Effects and Perspectives for New Products. Antioxidants 2022, 11, 241. [Google Scholar] [CrossRef]
- Liu, M.; Hansen, P.E.; Wang, G.; Qiu, L.; Dong, J.; Yin, H.; Qian, Z.; Yang, M.; Miao, J. Pharmacological profile of xanthohumol, a prenylated flavonoid from hops (Humulus lupulus). Molecules 2015, 20, 754–779. [Google Scholar] [CrossRef]
- Bolton, J.L.; Dunlap, T.L.; Hajirahimkhan, A.; Mbachu, O.; Chen, S.N.; Chadwick, L.; Nikolic, D.; van Breemen, R.B.; Pauli, G.F.; Dietz, B.M. The Multiple Biological Targets of Hops and Bioactive Compounds. Chem. Res. Toxicol. 2019, 32, 222–233. [Google Scholar] [CrossRef] [PubMed]
- Bocquet, L.; Sahpaz, S.; Hilbert, J.L.; Rambaud, C.; Rivière, C. Humulus lupulus L., a very popular beer ingredient and medicinal plant: Overview of its phytochemistry, its bioactivity, and its biotechnology. Phytochem. Rev. 2018, 17, 1047–1090. [Google Scholar] [CrossRef]
- Di Sotto, A.; Checconi, P.; Celestino, I.; Locatelli, M.; Carissimi, S.; De Angelis, M.; Rossi, V.; Limongi, D.; Toniolo, C.; Martinoli, L.; et al. Antiviral and Antioxidant Activity of a Hydroalcoholic Extract from Humulus lupulus L. Oxid. Med. Cell Longev. 2018, 2018, 5919237. [Google Scholar] [CrossRef] [PubMed]
- Di Sotto, A.; Vitalone, A.; Di Giacomo, S. Plant-Derived Nutraceuticals and Immune System Modulation: An Evidence-Based Overview. Vaccines 2020, 8, 468. [Google Scholar] [CrossRef] [PubMed]
- Manayi, A.; Vazirian, M.; Saeidnia, S. Echinacea purpurea: Pharmacology, phytochemistry and analysis methods. Pharmacogn. Rev. 2015, 9, 63–72. [Google Scholar] [CrossRef]
- Burlou-Nagy, C.; Bănică, F.; Jurca, T.; Vicaș, L.G.; Marian, E.; Muresan, M.E.; Bácskay, I.; Kiss, R.; Fehér, P.; Pallag, A. Echinacea purpurea (L.) Moench: Biological and Pharmacological Properties. A Review. Plants 2022, 11, 1244. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Mnayer, D.; Morais-Braga, M.F.B.; Carneiro, J.N.P.; Bezerra, C.F.; Coutinho, H.D.M.; Salehi, B.; Martorell, M.; Del Mar Contreras, M.; Soltani-Nejad, A.; et al. Echinacea plants as antioxidant and antibacterial agents: From traditional medicine to biotechnological applications. Phytother. Res. 2018, 32, 1653–1663. [Google Scholar] [CrossRef]
- Catanzaro, M.; Corsini, E.; Rosini, M.; Racchi, M.; Lanni, C. Immunomodulators Inspired by Nature: A Review on Curcumin and Echinacea. Molecules 2018, 23, 2778. [Google Scholar] [CrossRef]
- Nugraha, R.V.; Ridwansyah, H.; Ghozali, M.; Khairani, A.F.; Atik, N. Traditional Herbal Medicine Candidates as Complementary Treatments for COVID-19: A Review of Their Mechanisms, Pros and Cons. Evid. Based Complement. Alternat Med. 2020, 2020, 2560645. [Google Scholar] [CrossRef] [PubMed]
- Hensel, A.; Bauer, R.; Heinrich, M.; Spiegler, V.; Kayser, O.; Hempel, G.; Kraft, K. Challenges at the Time of COVID-19: Opportunities and Innovations in Antivirals from Nature. Planta Med. 2020, 86, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Aucoin, M.; Cooley, K.; Saunders, P.R.; Carè, J.; Anheyer, D.; Medina, D.N.; Cardozo, V.; Remy, D.; Hannan, N.; Garber, A. The effect of Echinacea spp. on the prevention or treatment of COVID-19 and other respiratory tract infections in humans: A rapid review. Adv. Integr. Med. 2020, 7, 203–217. [Google Scholar] [CrossRef] [PubMed]
- Perry, N.B.; Burgess, E.J.; Glennie, V.L. Echinacea standardization: Analytical methods for phenolic compounds and typical levels in medicinal species. J. Agric. Food Chem. 2001, 49, 1702–1706. [Google Scholar] [CrossRef] [PubMed]
- Perry, N.B.; van Klink, J.W.; Burgess, E.J.; Parmenter, G.A. Alkamide levels in Echinacea purpurea: A rapid analytical method revealing differences among roots, rhizomes, stems, leaves and flowers. Planta Med. 1997, 63, 58–62. [Google Scholar] [CrossRef]
- Di Sotto, A.; Locatelli, M.; Macone, A.; Toniolo, C.; Cesa, S.; Carradori, S.; Eufemi, M.; Mazzanti, G.; Di Giacomo, S. Hypoglycemic, Antiglycation, and Cytoprotective Properties of a Phenol-Rich Extract From Waste Peel of Punica granatum L. var. Dente di Cavallo DC2. Molecules 2019, 24, 3103. [Google Scholar] [CrossRef]
- Sissi, S.; Di Giacomo, S.; Ferrante, C.; Angelini, P.; Macone, A.; Giusti, A.M.; Toniolo, C.; Vitalone, A.; Abdellah, A.; Larhsini, M.; et al. Characterization of the Phytochemical Composition and Bioactivities of Anacyclus maroccanus Ball. and Anacyclus radiatus Loisel Aerial Parts: Preliminary Evidence for the Possible Development of Moroccan Plants. Molecules 2022, 27, 692. [Google Scholar] [CrossRef]
- Di Giacomo, S.; Di Sotto, A.; Angelis, A.; Percaccio, E.; Vitalone, A.; Gullì, M.; Macone, A.; Axiotis, E.; Skaltsounis, A.L. Phytochemical Composition and Cytoprotective Properties of the Endemic Sideritis sipylea Boiss Greek Species: A Valorization Study. Pharmaceuticals 2022, 15, 987. [Google Scholar] [CrossRef]
- Vitalone, A.; Di Giacomo, S.; Di Sotto, A.; Franchitto, A.; Mammola, C.L.; Mariani, P.; Mastrangelo, S.; Mazzanti, G. Cassia angustifolia extract is not hepatotoxic in an in vitro and in vivo study. Pharmacology 2011, 88, 252–259. [Google Scholar] [CrossRef]
- Kamiloglu, S.; Sari, G.; Ozdal, T.; Capanoglu, E. Guidelines for cell viability assays. Food Front. 2020, 1, 332–349. [Google Scholar] [CrossRef]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices Part 5: Tests for Invitro Cytotoxicity. ISO: Geneva, Switzerland, 2009.
- Di Sotto, A.; Di Giacomo, S.; Rubini, E.; Macone, A.; Gulli, M.; Mammola, C.L.; Eufemi, M.; Mancinelli, R.; Mazzanti, G. Modulation of STAT3 Signaling, Cell Redox Defenses and Cell Cycle Checkpoints by β-Caryophyllene in Cholangiocarcinoma Cells: Possible Mechanisms Accounting for Doxorubicin Chemosensitization and Chemoprevention. Cells 2020, 9, 858. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Liu, J.; Qu, C.; Wang, Y.; Zhu, Y.; Zhang, Y.; Li, H.; Zhang, B.; Sun, Y.; Zou, W. Study of immune responses in mice to oral administration of Flor·Essence. Mol. Clin. Oncol. 2020, 12, 533–540. [Google Scholar] [CrossRef] [PubMed]
- BenSaad, L.A.; Kim, K.H.; Quah, C.C.; Kim, W.R.; Shahimi, M. Anti-inflammatory potential of ellagic acid, gallic acid and punicalagin A&B isolated from Punica granatum. BMC Complement. Altern. Med. 2017, 17, 47. [Google Scholar] [CrossRef]
- Fiorentino, F.; De Angelis, M.; Menna, M.; Rovere, A.; Caccuri, A.M.; D’Acunzo, F.; Palamara, A.T.; Nencioni, L.; Rotili, D.; Mai, A. Anti-influenza A virus activity and structure-activity relationship of a series of nitrobenzoxadiazole derivatives. J. Enzyme Inhib. Med. Chem. 2021, 36, 2128–2138. [Google Scholar] [CrossRef]
- Hayes, J.D.; McMahon, M. NRF2 and KEAP1 mutations: Permanent activation of an adaptive response in cancer. Trends Biochem. Sci. 2009, 34, 176–188. [Google Scholar] [CrossRef]
- Hasret, E. The Role of Oxidative Stress in Apoptosis and Cell Proliferation of Human Bronchial Epithelial Cells. Cytol. Genet. 2021, 55, 283–289. [Google Scholar] [CrossRef]
- Jiang, L.; Chen, H.; Li, C. Advances in deciphering the interactions between viral proteins of influenza A virus and host cellular proteins. Cell Insight 2023, 2, 100079. [Google Scholar] [CrossRef]
- Dawre, S.; Maru, S. Human respiratory viral infections: Current status and future prospects of nanotechnology-based approaches for prophylaxis and treatment. Life Sci. 2021, 278, 119561. [Google Scholar] [CrossRef]
- Committee on Herbal Medicinal Products (HMPC). Assessment Report on Echinacea purpurea (L.) Moench., Herba Recens. EMA/HMPC/557979/2013. Available online: https://www.ema.europa.eu/en/documents/herbal-report/final-assessment-report-echinacea-purpurea-l-moench-herba-recens_en.pdf (accessed on 6 October 2023).
- Lyu, J.I.; Ryu, J.; Seo, K.S.; Kang, K.Y.; Park, S.H.; Ha, T.H.; Ahn, J.W.; Kang, S.Y. Comparative Study on Phenolic Compounds and Antioxidant Activities of Hop (Humulus lupulus L.) Strobile Extracts. Plants 2022, 11, 135. [Google Scholar] [CrossRef]
- Lin, X.J.; Lai, Z.S.; Luo, Q.; Kong, M.; Liang, M.J.; Wu, H.; Bai, M. Correlation between Polyphenol Contents and Antioxidant Activities in Different Echinacea Purpurea Varieties. Curr. Med. Sci. 2023, 43, 831–837. [Google Scholar] [CrossRef]
- Lee, J.; Scagel, C.F. Chicoric acid: Chemistry, distribution, and production. Front. Chem. 2013, 1, 40. [Google Scholar] [CrossRef] [PubMed]
- Dobrange, E.; Peshev, D.; Loedolff, B.; Van den Ende, W. Fructans as Immunomodulatory and Antiviral Agents: The Case of Echinacea. Biomolecules 2019, 9, 615. [Google Scholar] [CrossRef] [PubMed]
- Aarland, R.C.; Bañuelos-Hernández, A.E.; Fragoso-Serrano, M.; Sierra-Palacios, E.D.; Díaz de León-Sánchez, F.; Pérez-Flores, L.J.; Rivera-Cabrera, F.; Mendoza-Espinoza, J.A. Studies on phytochemical, antioxidant, anti-inflammatory, hypoglycaemic and antiproliferative activities of Echinacea purpurea and Echinacea angustifolia extracts. Pharm. Biol. 2017, 55, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Schönberger, C.; Kostelecky, T. 125th Anniversary Review: The Role of Hops in Brewing. J. Inst. Brew. 2011, 117, 259–267. [Google Scholar] [CrossRef]
- 58. Sun, X.-L.; Xia, T.-S.; Jiang, Y.-P.; Wang, N.-N.; Xu, L.-C.; Han, T.; Xin, H.-L. Humulus lupulus L. extract and its active constituent xanthohumol attenuate oxidative stress and nerve injury induced by iron overload via activating AKT/GSK3β and Nrf2/NQO1 pathways. J. Nat. Med. 2023, 77, 12–27. [Google Scholar] [CrossRef]
- Olas, B.; Kolodziejczyk, J.; Wachowicz, B.; Jędrejek, D.; Stochmal, A.; Oleszek, W. The extract from hop cones (Humulus lupulus) as a modulator of oxidative stress in blood platelets. Platelets 2011, 22, 345–352. [Google Scholar] [CrossRef]
- Yao, J.; Zhang, B.X.; Ge, C.P.; Peng, S.J.; Fang, J.G. Xanthohumol, a polyphenol chalcone present in hops, activating Nrf2 enzymes to confer protection against oxidative damage in PC12 cells. J. Agric. Food Chem. 2015, 63, 1521–1531. [Google Scholar] [CrossRef]
- Fernández-García, C.; Rancan, L.; Paredes, S.D.; Montero, C.; de la Fuente, M.; Vara, E.; Tresguerres, J.A.F. Xanthohumol exerts protective effects in liver alterations associated with aging. Eur. J. Nutr. 2020, 58, 653–663. [Google Scholar] [CrossRef]
- Jiao, Y.; Cao, Y.Z.; Lu, X.Y.; Wang, J.J.; Saitgareeva, A.; Kong, X.T.; Song, C.; Li, J.; Tian, K.; Zhang, S.Q.; et al. Xanthohumol protects neuron from cerebral ischemia injury in experimental stroke. Mol. Biol. Rep. 2020, 47, 2417–2425. [Google Scholar] [CrossRef]
- Sun, X.L.; Zhang, J.B.; Guo, Y.X.; Xia, T.S.; Xu, L.C.; Rahmand, K.; Wang, G.P.; Li, X.J.; Han, T.; Wang, N.N.; et al. Xanthohumol ameliorates memory impairment and reduces the deposition of β-amyloid in APP/PS1 mice via regulating the mTOR/LC3II and Bax/Bcl-2 signaling pathways. J. Pharm. Pharmacol. 2021, 73, 1230–1239. [Google Scholar] [CrossRef]
- Peng, Y.; Sun, Q.; Park, Y. The Bioactive Effects of Chicoric Acid As a Functional Food Ingredient. J. Med. Food 2019, 22, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Gonthier, M.P.; Remesy, C.; Scalbert, A.; Cheynier, V.; Souquet, J.M.; Poutanen, K.; Aura, A.M. Microbial metabolism of caffeic acid and its esters chlorogenic and caftaric acids by human faecal microbiota in vitro. Biomed. Pharmacother. 2006, 60, 536–540. [Google Scholar] [CrossRef] [PubMed]
- Saima; Anjum, I.; Najm, S.; Barkat, K.; Nafidi, H.-A.; Bin Jardan, Y.A.; Bourhia, M. Caftaric Acid Ameliorates Oxidative Stress, Inflammation, and Bladder Overactivity in Rats Having Interstitial Cystitis: An In Silico Study. ACS Omega 2023, 8, 28196–28206. [Google Scholar] [CrossRef]
- Bertelli, M.; Kiani, A.K.; Paolacci, S.; Manara, E.; Kurti, D.; Dhuli, K.; Bushati, V.; Miertus, J.; Pangallo, D.; Baglivo, M.; et al. Hydroxytyrosol: A natural compound with promising pharmacological activities. J. Biotechnol. 2020, 309, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Jayachandran, M.; Wu, Z.; Ganesan, K.; Khalid, S.; Chung, S.M.; Xu, B. Isoquercetin upregulates antioxidant genes, suppresses inflammatory cytokines and regulates AMPK pathway in streptozotocin-induced diabetic rats. Chem. Biol. Interact. 2019, 303, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Zúñiga, J.M.; Soto-Valdez, H.; Peralta, E.; Mendoza-Wilson, A.M.; Robles-Burgueño, M.R.; Auras, R.; Gámez-Meza, N. Development of an antioxidant biomaterial by promoting the deglycosylation of rutin to isoquercetin and quercetin. Food Chem. 2016, 204, 420–426. [Google Scholar] [CrossRef]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef]
- Zhao, W.; Feng, H.; Sun, W.; Liu, K.; Lu, J.J.; Chen, X. Tert-butyl hydroperoxide (t-BHP) induced apoptosis and necroptosis in endothelial cells: Roles of NOX4 and mitochondrion. Redox Biol. 2017, 11, 524–534. [Google Scholar] [CrossRef]
- Bao, Q.; Zhao, S.; Liu, Y.; Yao, Y.; You, J.; Xiong, J. Benzo[b]fluoranthene induced oxidative stress and apoptosis in human airway epithelial cells via mitochondrial disruption. J. Appl. Toxicol. 2023, 43, 1083–1094. [Google Scholar] [CrossRef]
- Lin, B.C.; Li, Q.Y.; Tian, L.; Liu, H.L.; Liu, X.H.; Shi, Y.; He, C.; Ding, S.S.; Yan, J.; Li, K.; et al. Identification of apoptosis-associated protein factors distinctly expressed in cigarette smoke condensate-exposed airway bronchial epithelial cells. J. Biochem. Mol. Toxicol. 2020, 34, e22444. [Google Scholar] [CrossRef]
- Du, H.; Sun, J.; Chen, Z.; Nie, J.; Tong, J.; Li, J. Cigarette smoke-induced failure of apoptosis resulting in enhanced neoplastic transformation in human bronchial epithelial cells. J. Toxicol. Environ. Health A 2012, 75, 707–720. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.M.; Kim, J.; Tenson, T.; Min, J.Y.; Kainov, D.E. Influenza Virus Infection, Interferon Response, Viral Counter-Response, and Apoptosis. Viruses 2017, 9, 223. [Google Scholar] [CrossRef]
- Taguchi, K.; Yamamoto, M. The KEAP1-NRF2 System in Cancer. Front. Oncol. 2017, 7, 85. [Google Scholar] [CrossRef] [PubMed]
- Mitsuishi, Y.; Taguchi, K.; Kawatani, Y.; Shibata, T.; Nukiwa, T.; Aburatani, H.; Yamamoto, M.; Motohashi, H. Nrf2 redirects glucose and glutamine into anabolic pathways in metabolic reprogramming. Cancer Cell 2012, 22, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Gururani, S.; Gairola, K.; Dubey, S.K. Antioxidants and Immunomodulation. In Immunomodulators and Human Health; Kesharwani, R.K., Keservani, R.K., Sharma, A.K., Eds.; Springer Nature Singapore Pte: Singapore, 2022. [Google Scholar] [CrossRef]
- Liu, X.W.; Song, Z.B.; Bai, J.; Nauwynck, H.; Zhao, Y.X.; Jiang, P. Xanthohumol inhibits PRRSV proliferation and alleviates oxidative stress induced by PRRSV via the Nrf2-HMOX1 axis. Vet. Res. 2019, 50, 61. [Google Scholar] [CrossRef] [PubMed]
- Dammann, I.; Keil, C.; Hardewig, I.; Skrzydlewska, E.; Biernacki, M.; Haase, H. Effects of combined cannabidiol (CBD) and hops (Humulus lupulus) terpene extract treatment on RAW 264.7 macrophage viability and inflammatory markers. Nat. Prod. Bioprospect. 2023, 13, 19. [Google Scholar] [CrossRef]
- Cho, Y.C.; Kim, H.J.; Kim, Y.J.; Lee, K.Y.; Choi, H.J.; Lee, I.S.; Kang, B.Y. Differential anti-inflammatory pathway by xanthohumol in IFN-gamma and LPS-activated macrophages. Int. Immunopharmacol. 2008, 8, 567–573. [Google Scholar] [CrossRef]
- Chen, L.; Huang, G.; Gao, M.; Shen, X.; Gong, W.; Xu, Z.; Zeng, Y.; He, F. Chicoric acid suppresses BAFF expression in B lymphocytes by inhibiting NF-κB activity. Int. Immunopharmacol. 2017, 44, 211–215. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, J.K.; Kim, H.S.; Chung, S.T.; Eom, J.H.; Kim, K.A.; Chung, S.J.; Paik, S.Y.; Oh, H.Y. Immunomodulatory effect of caffeic acid phenethyl ester in Balb/c mice. Int. Immunopharmacol. 2004, 4, 429–436. [Google Scholar] [CrossRef]
- Pahan, K. Immunomodulation of experimental allergic encephalomyelitis by cinnamon metabolite sodium benzoate. Immunopharmacol. Immunotoxicol. 2011, 33, 586–593. [Google Scholar] [CrossRef]
- Clarke, J.O.; Mullin, G.E. A review of complementary and alternative approaches to immunomodulation. Nutr. Clin. Pract. 2008, 23, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Biswas, A.; Mason, L.; Mortuza, A.; Blumenthal, E.; Mustafa, A. Stimulatory effect of Holy basil and Thai basil on mouse spleen cell proliferation. J. Immunoassay Immunochem. 2021, 42, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Rosés, R.; Risco, E.; Vila, R.; Peñalver, P.; Cañigueral, S. Effect of some essential oils on phagocytosis and complement system activity. J. Agric. Food Chem. 2015, 63, 1496–1504. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, R.; Igarashi, M.; Takada, A. Influenza A Virus M2 Protein: Roles from Ingress to Egress. Int. J. Mol. Sci. 2017, 18, 2649. [Google Scholar] [CrossRef]
- Hu, Y.; Sneyd, H.; Dekant, R.; Wang, J. Influenza A Virus Nucleoprotein: A Highly Conserved Multi-Functional Viral Protein as a Hot Antiviral Drug Target. Curr. Top. Med. Chem. 2017, 17, 2271–2285. [Google Scholar] [CrossRef]
- Nagoor Meeran, M.F.; Javed, H.; Sharma, C.; Goyal, S.N.; Kumar, S.; Jha, N.K.; Ojha, S. Can Echinacea be a potential candidate to target immunity, inflammation, and infection—The trinity of coronavirus disease 2019. Heliyon 2021, 7, e05990. [Google Scholar] [CrossRef]
- Vimalanathan, S.; Shehata, M.; Sadasivam, K.; Delbue, S.; Dolci, M.; Pariani, E.; D’Alessandro, S.; Pleschka, S. Broad Antiviral Effects of Echinacea purpurea against SARS-CoV-2 Variants of Concern and Potential Mechanism of Action. Microorganisms 2022, 10, 2145. [Google Scholar] [CrossRef]
- Signer, J.; Jonsdottir, H.R.; Albrich, W.C.; Strasser, M.; Züst, R.; Ryter, S.; Ackermann-Gäumann, R.; Lenz, N.; Siegrist, D.; Suter, A.; et al. In vitro virucidal activity of Echinaforce®, an Echinacea purpurea preparation, against coronaviruses, including common cold coronavirus 229E and SARS-CoV-2. Virol. J. 2020, 17, 136. [Google Scholar] [CrossRef]
- Jawad, M.; Schoop, R.; Suter, A.; Klein, P.; Eccles, R. Safety and Efficacy Profile of Echinacea purpurea to Prevent Common Cold Episodes: A Randomized, Double-Blind, Placebo-Controlled Trial. Evid-Based Complement. Altern. Med. 2012, 2012, 841315. [Google Scholar] [CrossRef]
- Ogal, M.; Johnston, S.L.; Klein, P.; Schoop, R. Echinacea reduces antibiotic usage in children through respiratory tract infection prevention: A randomized, blinded, controlled clinical trial. Eur. J. Med. Res. 2021, 26, 33. [Google Scholar] [CrossRef]
- Kolev, E.; Mircheva, L.; Edwards, M.R.; Johnston, S.L.; Kalinov, K.; Stange, R.; Gancitano, G.; Berghe, W.V.; Kreft, S. Echinacea purpurea For the Long-Term Prevention of Viral Respiratory Tract Infections During COVID-19 Pandemic: A Randomized, Open, Controlled, Exploratory Clinical Study. Front. Pharmacol. 2022, 13, 856410. [Google Scholar] [CrossRef]
- Nicolussi, S.; Ardjomand-Woelkart, K.; Stange, R.; Gancitano, G.; Klein, P.; Ogal, M. Echinacea as a Potential Force against Coronavirus Infections? A Mini-Review of Randomized Controlled Trials in Adults and Children. Microorganisms 2022, 10, 211. [Google Scholar] [CrossRef] [PubMed]
- Mercaldi, G.F.; Bezerra, E.H.S.; Batista, F.A.H.; Tonoli, C.C.C.; Soprano, A.S.; Shimizu, J.F.; Nagai, A.; da Silva, J.C.; Filho, H.V.R.; do Nascimento Faria, J.; et al. Discovery and structural characterization of chicoric acid as a SARS-CoV-2 nucleocapsid protein ligand and RNA binding disruptor. Sci. Rep. 2022, 12, 18500. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Aziz, N.M.; Khalifa, I.; Darwish, A.M.G.; Badr, A.N.; Aljumayi, H.; Hafez, E.S.; Shehata, M.G. Docking Analysis of Some Bioactive Compounds from Traditional Plants against SARS-CoV-2 Target Proteins. Molecules 2022, 27, 2662. [Google Scholar] [CrossRef] [PubMed]
- Schapowal, A.; Klein, P.; Johnston, S.L. Echinacea reduces the risk of recurrent respiratory tract infections and complications: A meta-analysis of randomized controlled trials. Adv. Ther. 2015, 32, 187–200. [Google Scholar] [CrossRef]
- Mandova, T.; Saivish, M.V.; La Serra, L.; Nogueira, M.L.; Da Costa, F.B. Identification of Potential Antiviral Hops Compounds against Chikungunya Virus. Int. J. Mol. Sci. 2023, 24, 3333. [Google Scholar] [CrossRef]
- Buckwold, V.E.; Wilson, R.J.; Nalca, A.; Beer, B.B.; Voss, T.G.; Turpin, J.A.; Buckheit, R.W., 3rd; Wei, J.; Wenzel-Mathers, M.; Walton, E.M.; et al. Antiviral activity of hop constituents against a series of DNA and RNA viruses. Antivir. Res. 2004, 61, 57–62. [Google Scholar] [CrossRef]
- Bouback, T.A.; Aljohani, A.M.; Albeshri, A.; Al-Talhi, H.; Moatasim, Y.; GabAllah, M.; Badierah, R.; Albiheyri, R.; Al-Sarraj, F.; Mohamed, A.A. Antiviral activity of Humulus lupulus (HOP) aqueous extract against MERS-CoV and SARS-CoV-2: In-vitro and in-silico study. Biotechnol. Biotechnol. Equip. 2023, 37, 167–179. [Google Scholar] [CrossRef]
- Wang, Q.; Ding, Z.H.; Liu, J.K.; Zheng, Y.T. Xanthohumol, a novel anti-HIV-1 agent purified from Hops Humulus lupulus. Antivir. Res. 2004, 64, 189–194. [Google Scholar] [CrossRef]
- Gerhäuser, C. Broad spectrum anti-infective potential of xanthohumol from hop (Humulus lupulus L.) in comparison with activities of other hop constituents and xanthohumol metabolites. Mol. Nutr. Food Res. 2005, 49, 827–831. [Google Scholar] [CrossRef]
- Lou, S.; Zheng, Y.M.; Liu, S.L.; Qiu, J.; Han, Q.; Li, N.; Zhu, Q.; Zhang, P.; Yang, C.; Liu, Z. Inhibition of hepatitis C virus replication in vitro by xanthohumol, a natural product present in hops. Planta Med. 2014, 80, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Bai, J.; Jiang, C.; Song, Z.; Zhao, Y.; Nauwynck, H.; Jiang, P. Therapeutic effect of Xanthohumol against highly pathogenic porcine reproductive and respiratory syndrome viruses. Vet. Microbiol. 2019, 238, 108431. [Google Scholar] [CrossRef]
- Lin, Y.; Zang, R.; Ma, Y.; Wang, Z.; Li, L.; Ding, S.; Zhang, R.; Wei, Z.; Yang, J.; Wang, X. Xanthohumol Is a Potent Pan-Inhibitor of Coronaviruses Targeting Main Protease. Int. J. Mol. Sci. 2021, 22, 12134. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.K.; Lee, M.M.; Ma, J.Y. Antiviral Effect of Isoquercitrin against Influenza A Viral Infection via Modulating Hemagglutinin and Neuraminidase. Int. J. Mol. Sci. 2022, 23, 13112. [Google Scholar] [CrossRef] [PubMed]
- Langland, J.; Jacobs, B.; Wagner, C.E.; Ruiz, G.; Cahill, T.M. Antiviral activity of metal chelates of caffeic acid and similar compounds towards herpes simplex, VSV-Ebola pseudotyped and vaccinia viruses. Antivir. Res. 2018, 160, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.L.; Wang, L.; Chen, S.J.; Yan, B.; Xun, L.Y.; Li, R.C.; Wang, P.C.; Zhao, Q.T. Design, synthesis, antiviral activities of ferulic acid derivatives. Front. Pharmacol. 2023, 14, 1133655. [Google Scholar] [CrossRef]
- Bedoya, L.M.; Beltrán, M.; Obregón-Calderón, P.; García-Pérez, J.; de la Torre, H.E.; González, N.; Pérez-Olmeda, M.; Auñón, D.; Capa, L.; Gómez-Acebo, E.; et al. Hydroxytyrosol: A new class of microbicide displaying broad anti-HIV-1 activity. Aids 2016, 30, 2767–2776. [Google Scholar] [CrossRef]
- Tonelli, C.; Chio, I.I.C.; Tuveson, D.A. Transcriptional Regulation by Nrf2. Antioxid. Redox Signal 2018, 29, 1727–1745. [Google Scholar] [CrossRef]
- Nguyen, T.; Nioi, P.; Pickett, C.B. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J. Biol. Chem. 2009, 284, 13291–13295. [Google Scholar] [CrossRef]
- Qu, Y.; Shen, Y.; Teng, L.; Huang, Y.; Yang, Y.; Jian, X.; Fan, S.; Wu, P.; Fu, Q. Chicoric acid attenuates tumor necrosis factor-α-induced inflammation and apoptosis via the Nrf2/HO-1, PI3K/AKT and NF-κB signaling pathways in C28/I2 cells and ameliorates the progression of osteoarthritis in a rat model. Int. Immunopharmacol. 2022, 111, 109129. [Google Scholar] [CrossRef]
- Zhu, L.; Fan, X.; Cao, C.; Li, K.; Hou, W.; Ci, X. Xanthohumol protect against acetaminophen-induced hepatotoxicity via Nrf2 activation through the AMPK/Akt/GSK3β pathway. Biomed. Pharmacother. 2023, 165, 115097. [Google Scholar] [CrossRef]
- Han, H.; Zhong, R.; Zhang, S.; Wang, M.; Wen, X.; Yi, B.; Zhao, Y.; Chen, L.; Zhang, H. Hydroxytyrosol attenuates diquat-induced oxidative stress by activating Nrf2 pathway and modulating colonic microbiota in mice. J. Nutr. Biochem. 2023, 113, 109256. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, M.; Kim, D.K.; Gie Kim, S.; Kang, S.C. Thymol exposure mediates pro-oxidant shift by regulating Nrf2 and apoptotic events in zebrafish (Danio rerio) embryos. Environ. Toxicol. Pharmacol. 2019, 65, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhou, D.; Yang, D.; Fu, Y.; Tao, X.; Hu, X.; Dai, Y.; Yue, H. Isoquercitrin Attenuates Osteogenic Injury in MC3T3 Osteoblastic Cells and the Zebrafish Model via the Keap1-Nrf2-ARE Pathway. Molecules 2022, 27, 3459. [Google Scholar] [CrossRef] [PubMed]
- Porwal, O.; Singh, S.K.; Patel, D.K.; Gupta, S.; Tripathi, R.; Katekhaye, S. Cultivation, collection and processing of medicinal plants. In Bioactive Phytochemicals: Drug Discovery to Product Development; Bentham Books: Singapore, 2020; Volume 17, pp. 14–30. [Google Scholar] [CrossRef]


| Sample | Total Polyphenols | Tannins | Flavonoids |
|---|---|---|---|
| µg TAE/mg of the Sample (Mean ± SE) | µg QE/mg of the Sample (Mean ± SE) | ||
| ECP | 4.89 ± 0.40 | 0.78 ± 0.01 | 3.11 ± 0.72 |
| HOP | 7.11 ± 0.35 *** | 1.72 ± 0.06 *** | 3.81 ± 0.59 |
| Compounds | ECP | HOP |
|---|---|---|
| µg/mg of the Sample (Mean ± SD) | ||
| Benzoic acid | 0.03 ± 0.01 | 0.41 ± 0.03 |
| Caffeic acid | 0.39 ± 0.02 | 0.06 ± 0.01 |
| Caftaric acid | 1.47 ± 0.03 | 0.16 ± 0.02 |
| Carvacrol | nd | nd |
| Catechin | 0.34 ± 0.03 | 0.52 ± 0.02 |
| Chicoric acid | 7.76 ± 0.23 | nd |
| Chlorogenic acid | 0.24 ± 0.01 | 0.06 ± 0.02 |
| t-Cinnamic acid | 0.01 ± 0.002 | 1.04 ± 0.06 |
| p-Coumaric acid | nd | 0.51 ± 0.03 |
| 2,3-Dimethylbenzoic acid | nd | 4.43 ± 0.23 |
| Epicatechin | 0.57 ± 0.03 | BLD |
| t-Ferulic acid | 0.25 ± 0.02 | 0.22 ± 0.03 |
| Gallic acid | 0.28 ± 0.01 | 0.25 ± 0.01 |
| Kaempferol | nd | 0.32 ± 0.06 |
| Hesperetin | 0.02 ± 0.003 | nd |
| 4-Hydroxybenzoic acid | nd | 0.08 ± 0.02 |
| 3-Hydroxytyrosol | 2.20 ± 0.11 | 0.61 ± 0.01 |
| Hyperoside | nd | nd |
| Isoquercetin | nd | 1.61 ± 0.06 |
| Loganic acid | nd | nd |
| Naringenin | nd | nd |
| Quercetin | BLD | nd |
| Resveratrol | nd | 0.06 ± 0.01 |
| Rosmarinic acid | nd | 0.12 ± 0.02 |
| Rutin | nd | nd |
| Thymol | nd | 4.09 ± 0.11 |
| Syringic acid | 0.16 ± 0.02 | nd |
| Syringaldehyde | nd | 0.07 ± 0.01 |
| Vanillic acid | nd | nd |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Percaccio, E.; De Angelis, M.; Acquaviva, A.; Nicotra, G.; Ferrante, C.; Mazzanti, G.; Di Giacomo, S.; Nencioni, L.; Di Sotto, A. ECHOPvir: A Mixture of Echinacea and Hop Extracts Endowed with Cytoprotective, Immunomodulatory and Antiviral Properties. Nutrients 2023, 15, 4380. https://doi.org/10.3390/nu15204380
Percaccio E, De Angelis M, Acquaviva A, Nicotra G, Ferrante C, Mazzanti G, Di Giacomo S, Nencioni L, Di Sotto A. ECHOPvir: A Mixture of Echinacea and Hop Extracts Endowed with Cytoprotective, Immunomodulatory and Antiviral Properties. Nutrients. 2023; 15(20):4380. https://doi.org/10.3390/nu15204380
Chicago/Turabian StylePercaccio, Ester, Marta De Angelis, Alessandra Acquaviva, Giovanna Nicotra, Claudio Ferrante, Gabriela Mazzanti, Silvia Di Giacomo, Lucia Nencioni, and Antonella Di Sotto. 2023. "ECHOPvir: A Mixture of Echinacea and Hop Extracts Endowed with Cytoprotective, Immunomodulatory and Antiviral Properties" Nutrients 15, no. 20: 4380. https://doi.org/10.3390/nu15204380
APA StylePercaccio, E., De Angelis, M., Acquaviva, A., Nicotra, G., Ferrante, C., Mazzanti, G., Di Giacomo, S., Nencioni, L., & Di Sotto, A. (2023). ECHOPvir: A Mixture of Echinacea and Hop Extracts Endowed with Cytoprotective, Immunomodulatory and Antiviral Properties. Nutrients, 15(20), 4380. https://doi.org/10.3390/nu15204380








