Botanical Mixture Containing Nitric Oxide Metabolite Enhances Neural Plasticity to Improve Cognitive Impairment in a Vascular Dementia Rat Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. BCCAO Surgery
2.3. BM Preparation and Administration
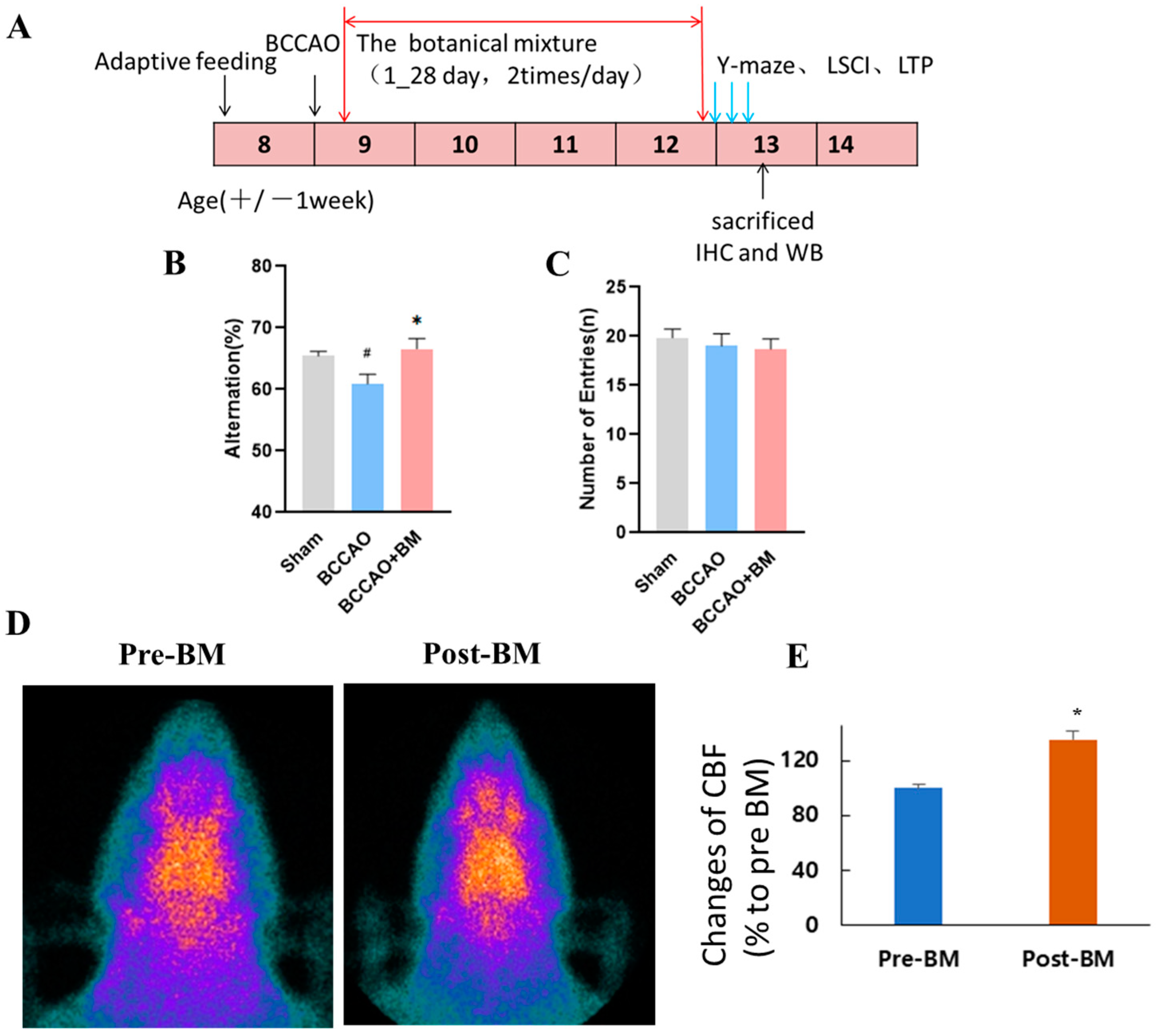
2.4. Y-Maze Test
2.5. Brain-Perfusion SPECT Imaging
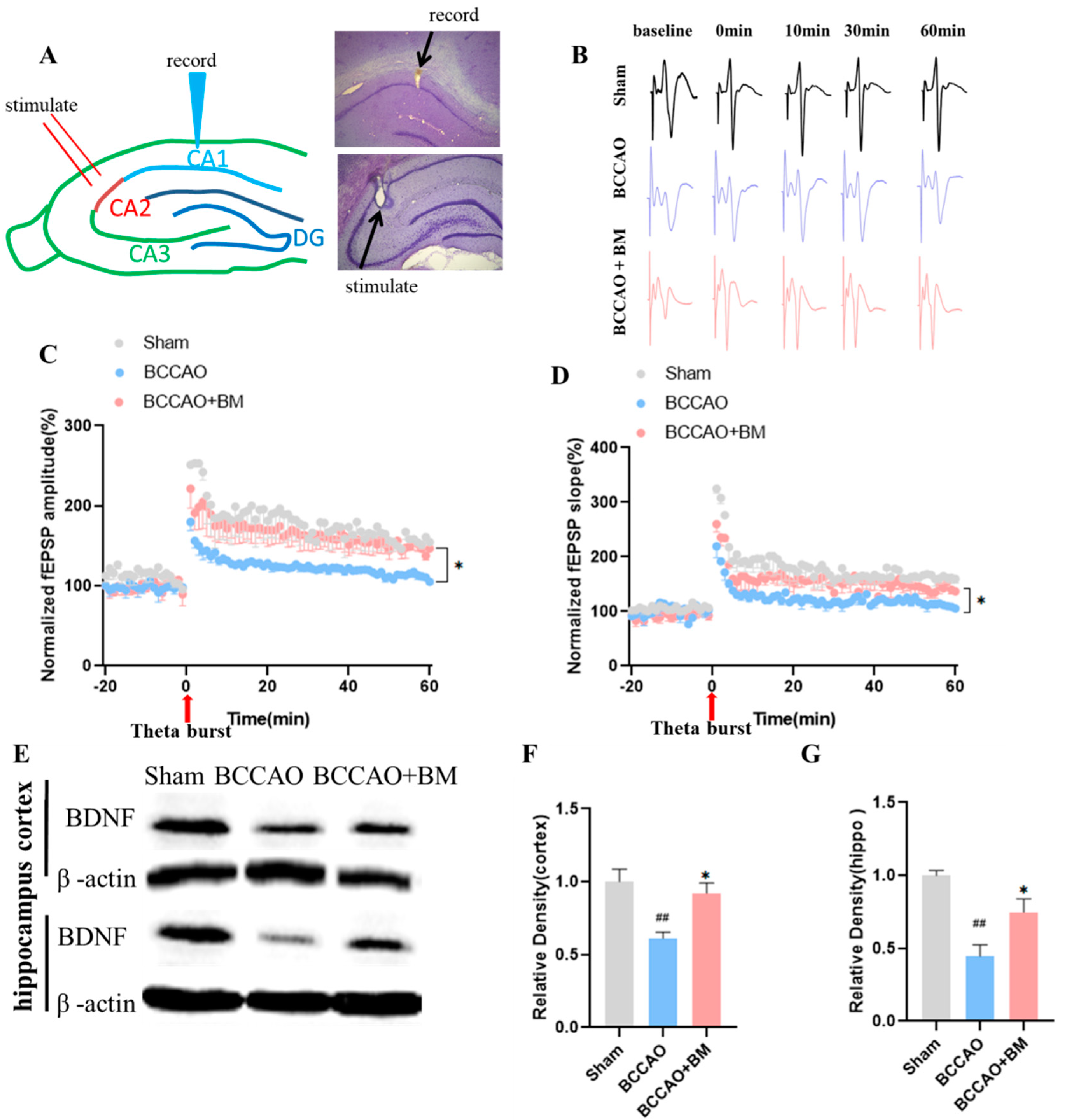
2.6. Long-Term Potentiation (LTP) in the Hippocampus
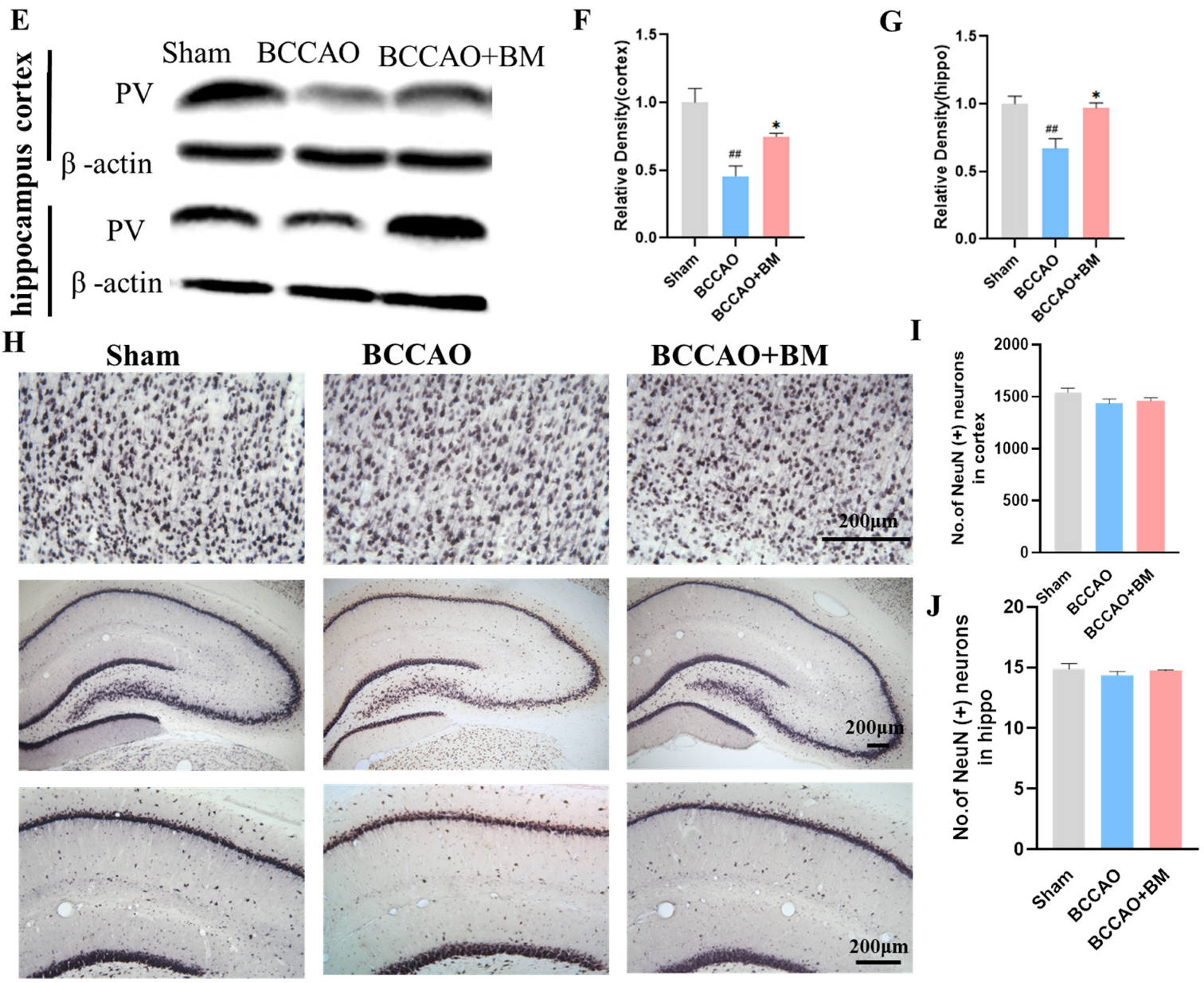
2.7. Immunohistochemistry
2.8. Cresyl Violet Staining
2.9. Western Blot Analysis
2.10. Fluoro-Jade B (FJB) Staining
2.11. Statistical Analysis
3. Results
3.1. BM Alleviates Cognitive Deficits and Increases Cerebral Blood Flow in Rats with BCCAO
3.2. BM Improves LTP and Increases BDNF Expression
3.3. BM Alleviates Damage to PV+ Neurons and Neuron Death in the Cortex and Hippocampus of BCCAO Rats
3.4. BM Reduces Oxidative Stress and GFAP Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- O’Brien, J.T.; Thomas, A. Vascular dementia. Lancet 2015, 386, 1698–1706. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.K. Potential Therapeutics for Vascular Cognitive Impairment and Dementia. Curr. Neuropharmacol. 2018, 16, 1036–1044. [Google Scholar] [CrossRef] [PubMed]
- Forstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef]
- Picon-Pages, P.; Garcia-Buendia, J.; Munoz, F.J. Functions and dysfunctions of nitric oxide in brain. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 1949–1967. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.Y.; Hong, F.F.; Yang, S.L. The Roles of Nitric Oxide Synthase/Nitric Oxide Pathway in the Pathology of Vascular Dementia and Related Therapeutic Approaches. Int. J. Mol. Sci. 2021, 22, 4540. [Google Scholar] [CrossRef] [PubMed]
- Amdahl, M.B.; DeMartino, A.W.; Gladwin, M.T. Inorganic nitrite bioactivation and role in physiological signaling and therapeutics. Biol. Chem. 2019, 401, 201–211. [Google Scholar] [CrossRef]
- Piknova, B.; Park, J.W.; Thomas, S.M.; Tunau-Spencer, K.J.; Schechter, A.N. Nitrate and Nitrite Metabolism in Aging Rats: A Comparative Study. Nutrients 2023, 15, 2490. [Google Scholar] [CrossRef] [PubMed]
- Gokalp, F. The inhibition effect of garlic-derived compounds on human immunodeficiency virus type 1 and saquinavir. J. Biochem. Mol. Toxicol. 2018, 32, e22215. [Google Scholar] [CrossRef]
- Lee, Y.J.; Lee, D.; Shin, S.M.; Lee, J.S.; Chun, H.S.; Quan, F.-S.; Shin, J.H.; Lee, G.-J. Potential protective effects of fermented garlic extract on myocardial ischemia-reperfusion injury utilizing in vitro and ex vivo models. J. Funct. Foods 2017, 33, 278–285. [Google Scholar] [CrossRef]
- Park, B.M.; Cha, S.A.; Kim, H.Y.; Kang, D.K.; Yuan, K.; Chun, H.; Chae, S.W.; Kim, S.H. Fermented garlic extract decreases blood pressure through nitrite and sGC-cGMP-PKG pathway in spontaneously hypertensive rats. J. Funct. Foods 2016, 22, 156–165. [Google Scholar] [CrossRef]
- Baik, J.S.; Min, J.H.; Ju, S.M.; Ahn, J.H.; Ko, S.H.; Chon, H.S.; Kim, M.S.; Shin, Y.I. Effects of Fermented Garlic Extract Containing Nitric Oxide Metabolites on Blood Flow in Healthy Participants: A Randomized Controlled Trial. Nutrients 2022, 14, 5238. [Google Scholar] [CrossRef]
- Choi, J.H.; Choi, A.Y.; Yoon, H.; Choe, W.; Yoon, K.S.; Ha, J.; Yeo, E.J.; Kang, I. Baicalein protects HT22 murine hippocampal neuronal cells against endoplasmic reticulum stress-induced apoptosis through inhibition of reactive oxygen species production and CHOP induction. Exp. Mol. Med. 2010, 42, 811–822. [Google Scholar] [CrossRef] [PubMed]
- Lei, K.; Shen, Y.; He, Y.; Zhang, L.; Zhang, J.; Tong, W.; Xu, Y.; Jin, L. Baicalin Represses C/EBPbeta via Its Antioxidative Effect in Parkinson’s Disease. Oxidative Med. Cell. Longev. 2020, 2020, 8951907. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.P.; Zheng, Q.; Xu, M.B.; Zhou, X.L.; Lu, L.; Li, Z.X.; Zheng, G.Q. Rhodiola rosea L. Improves Learning and Memory Function: Preclinical Evidence and Possible Mechanisms. Front. Pharmacol. 2018, 9, 1415. [Google Scholar] [CrossRef] [PubMed]
- Abidov, M.; Crendal, F.; Grachev, S.; Seifulla, R.; Ziegenfuss, T. Effect of extracts from Rhodiola rosea and Rhodiola crenulata (Crassulaceae) roots on ATP content in mitochondria of skeletal muscles. Bull. Exp. Biol. Med. 2003, 136, 585–587. [Google Scholar] [CrossRef] [PubMed]
- Panossian, A.; Wikman, G.; Sarris, J. Rosenroot (Rhodiola rosea): Traditional use, chemical composition, pharmacology and clinical efficacy. Phytomedicine 2010, 17, 481–493. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.J.; Li, X.Q.; Sun, J.W.; Jin, S.H. Nitric oxide functions as a signal in ultraviolet-B-induced baicalin accumulation in Scutellaria baicalensis suspension cultures. Int. J. Mol. Sci. 2014, 15, 4733–4746. [Google Scholar] [CrossRef] [PubMed]
- Farkas, E.; Luiten, P.G.; Bari, F. Permanent, bilateral common carotid artery occlusion in the rat: A model for chronic cerebral hypoperfusion-related neurodegenerative diseases. Brain Res. Rev. 2007, 54, 162–180. [Google Scholar] [CrossRef]
- Washida, K.; Hattori, Y.; Ihara, M. Animal Models of Chronic Cerebral Hypoperfusion: From Mouse to Primate. Int. J. Mol. Sci. 2019, 20, 6176. [Google Scholar] [CrossRef]
- Polopalli, S.; Yetukuri, A.R.; Danduga, R.; Kola, P.K. A prognostic study on the effect of post-traumatic stress disorder on cerebral ischaemia reperfusion-induced stroke. World J. Biol. Psychiatry 2022, 23, 136–150. [Google Scholar] [CrossRef] [PubMed]
- Gallo, E.F.; Iadecola, C. Neuronal nitric oxide contributes to neuroplasticity-associated protein expression through cGMP, protein kinase G, and extracellular signal-regulated kinase. J. Neurosci. 2011, 31, 6947–6955. [Google Scholar] [CrossRef] [PubMed]
- Long, Q.; Upadhya, D.; Hattiangady, B.; Kim, D.K.; An, S.Y.; Shuai, B.; Prockop, D.J.; Shetty, A.K. Intranasal MSC-derived A1-exosomes ease inflammation, and prevent abnormal neurogenesis and memory dysfunction after status epilepticus. Proc. Natl. Acad. Sci. USA 2017, 114, E3536–E3545. [Google Scholar] [CrossRef] [PubMed]
- Wilson, E.N.; Do Carmo, S.; Iulita, M.F.; Hall, H.; Austin, G.L.; Jia, D.T.; Malcolm, J.C.; Foret, M.K.; Marks, A.R.; Butterfield, D.A.; et al. Microdose Lithium NP03 Diminishes Pre-Plaque Oxidative Damage and Neuroinflammation in a Rat Model of Alzheimer’s-like Amyloidosis. Curr. Alzheimer Res. 2018, 15, 1220–1230. [Google Scholar] [CrossRef] [PubMed]
- Do Carmo, S.; Crynen, G.; Paradis, T.; Reed, J.; Iulita, M.F.; Ducatenzeiler, A.; Crawford, F.; Cuello, A.C. Hippocampal Proteomic Analysis Reveals Distinct Pathway Deregulation Profiles at Early and Late Stages in a Rat Model of Alzheimer’s-like Amyloid Pathology. Mol. Neurobiol. 2018, 55, 3451–3476. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Reed, T.; Sultana, R. Roles of 3-nitrotyrosine- and 4-hydroxynonenal-modified brain proteins in the progression and pathogenesis of Alzheimer’s disease. Free Radic. Res. 2011, 45, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Bhuiyan, M.I.H.; Liu, R.; Song, S.; Begum, G.; Young, C.B.; Foley, L.M.; Chen, F.; Hitchens, T.K.; Cao, G.; et al. Attenuating vascular stenosis-induced astrogliosis preserves white matter integrity and cognitive function. J. Neuroinflamm. 2021, 18, 187. [Google Scholar] [CrossRef]
- Otori, T.; Katsumata, T.; Muramatsu, H.; Kashiwagi, F.; Katayama, Y.; Terashi, A. Long-term measurement of cerebral blood flow and metabolism in a rat chronic hypoperfusion model. Clin. Exp. Pharmacol. Physiol. 2003, 30, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Mansour, A.; Niizuma, K.; Rashad, S.; Sumiyoshi, A.; Ryoke, R.; Endo, H.; Endo, T.; Sato, K.; Kawashima, R.; Tominaga, T. A refined model of chronic cerebral hypoperfusion resulting in cognitive impairment and a low mortality rate in rats. J. Neurosurg. 2018, 131, 892–902. [Google Scholar] [CrossRef]
- Nikonenko, A.G.; Radenovic, L.; Andjus, P.R.; Skibo, G.G. Structural features of ischemic damage in the hippocampus. Anat. Rec. 2009, 292, 1914–1921. [Google Scholar] [CrossRef]
- Thatcher, G.R.; Bennett, B.M.; Reynolds, J.N. Nitric oxide mimetic molecules as therapeutic agents in Alzheimer’s disease. Curr. Alzheimer Res. 2005, 2, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Dubey, H.; Gulati, K.; Ray, A. Amelioration by nitric oxide (NO) mimetics on neurobehavioral and biochemical changes in experimental model of Alzheimer’s disease in rats. Neurotoxicology 2018, 66, 58–65. [Google Scholar] [CrossRef]
- Ashraf, R.; Khan, R.A.; Ashraf, I.; Qureshi, A.A. Effects of Allium sativum (garlic) on systolic and diastolic blood pressure in patients with essential hypertension. Pak. J. Pharm. Sci. 2013, 26, 859–863. [Google Scholar]
- Morales-Gonzalez, J.A.; Madrigal-Bujaidar, E.; Sanchez-Gutierrez, M.; Izquierdo-Vega, J.A.; Valadez-Vega, M.D.C.; Alvarez-Gonzalez, I.; Morales-Gonzalez, A.; Madrigal-Santillan, E. Garlic (Allium sativum L.): A Brief Review of Its Antigenotoxic Effects. Foods 2019, 8, 343. [Google Scholar] [CrossRef]
- Naderi, R.; Mohaddes, G.; Mohammadi, M.; Alihemmati, A.; Badalzadeh, R.; Ghaznavi, R.; Ghyasi, R.; Mohammadi, S. Preventive effects of garlic (Allium sativum) on oxidative stress and histopathology of cardiac tissue in streptozotocin-induced diabetic rats. Acta Physiol. Hung. 2015, 102, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Colin-Gonzalez, A.L.; Santana, R.A.; Silva-Islas, C.A.; Chanez-Cardenas, M.E.; Santamaria, A.; Maldonado, P.D. The antioxidant mechanisms underlying the aged garlic extract- and S-allylcysteine-induced protection. Oxidative Med. Cell. Longev. 2012, 2012, 907162. [Google Scholar] [CrossRef]
- Farooqui, T.; Farooqui, A.A. Neuroprotective Effects of Garlic in Model Systems of Neurodegenerative Diseases. In Role of the Mediterranean Diet in the Brain and Neurodegenerative Diseases; Academic Press: Cambridge, MA, USA, 2018; pp. 253–269. [Google Scholar]
- Zhao, T.; Tang, H.; Xie, L.; Zheng, Y.; Ma, Z.; Sun, Q.; Li, X. Scutellaria baicalensis Georgi. (Lamiaceae): A review of its traditional uses, botany, phytochemistry, pharmacology and toxicology. J. Pharm. Pharmacol. 2019, 71, 1353–1369. [Google Scholar] [CrossRef]
- Tu, T.H.; Liou, D.Y.; Lin, D.Y.; Yang, H.C.; Chen, C.J.; Huang, M.C.; Huang, W.C.; Tsai, M.J.; Cheng, H. Characterizing the Neuroprotective Effects of S/B Remedy (Scutellaria baicalensis Georgi and Bupleurum scorzonerifolfium Willd) in Spinal Cord Injury. Molecules 2019, 24, 1885. [Google Scholar] [CrossRef]
- Wang, Z.L.; Wang, S.; Kuang, Y.; Hu, Z.M.; Qiao, X.; Ye, M. A comprehensive review on phytochemistry, pharmacology, and flavonoid biosynthesis of Scutellaria baicalensis. Pharm. Biol. 2018, 56, 465–484. [Google Scholar] [CrossRef]
- Gaire, B.P.; Moon, S.K.; Kim, H. Scutellaria baicalensis in stroke management: Nature’s blessing in traditional Eastern medicine. Chin. J. Integr. Med. 2014, 20, 712–720. [Google Scholar] [CrossRef]
- Kim, D.H.; Lee, Y.; Lee, H.E.; Park, S.J.; Jeon, S.J.; Jeon, S.J.; Cheong, J.H.; Shin, C.Y.; Son, K.H.; Ryu, J.H. Oroxylin A enhances memory consolidation through the brain-derived neurotrophic factor in mice. Brain Res. Bull. 2014, 108, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Fong, S.Y.; Wong, Y.C.; Zuo, Z. Development of a SPE-LC/MS/MS method for simultaneous quantification of baicalein, wogonin, oroxylin A and their glucuronides baicalin, wogonoside and oroxyloside in rats and its application to brain uptake and plasma pharmacokinetic studies. J. Pharm. Biomed. Anal. 2014, 97, 9–23. [Google Scholar] [CrossRef]
- Spasov, A.A.; Wikman, G.K.; Mandrikov, V.B.; Mironova, I.A.; Neumoin, V.V. A double-blind, placebo-controlled pilot study of the stimulating and adaptogenic effect of Rhodiola rosea SHR-5 extract on the fatigue of students caused by stress during an examination period with a repeated low-dose regimen. Phytomedicine 2000, 7, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yu, H.; Sun, Y.; Lin, X.; Chen, B.; Tan, C.; Cao, G.; Wang, Z. Protective effects of salidroside on hydrogen peroxide-induced apoptosis in SH-SY5Y human neuroblastoma cells. Eur. J. Pharmacol. 2007, 564, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Saxena, A.K.; Abdul-Majeed, S.S.; Gurtu, S.; Mohamed, W.M. Investigation of redox status in chronic cerebral hypoperfusion-induced neurodegeneration in rats. Appl. Transl. Genom. 2015, 5, 30–32. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cechetti, F.; Pagnussat, A.S.; Worm, P.V.; Elsner, V.R.; Ben, J.; da Costa, M.S.; Mestriner, R.; Weis, S.N.; Netto, C.A. Chronic brain hypoperfusion causes early glial activation and neuronal death, and subsequent long-term memory impairment. Brain Res. Bull. 2012, 87, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Jing, Z.; Shi, C.; Zhu, L.; Xiang, Y.; Chen, P.; Xiong, Z.; Li, W.; Ruan, Y.; Huang, L. Chronic cerebral hypoperfusion induces vascular plasticity and hemodynamics but also neuronal degeneration and cognitive impairment. J. Cereb. Blood Flow. Metab. 2015, 35, 1249–1259. [Google Scholar] [CrossRef] [PubMed]
- Tamano, H.; Takiguchi, M.; Shimaya, R.; Adlard, P.A.; Bush, A.I.; Takeda, A. Extracellular Zn(2+)-independently attenuated LTP by human amyloid beta1-40 and rat amyloid beta1-42. Biochem. Biophys. Res. Commun. 2019, 514, 888–892. [Google Scholar] [CrossRef]
- He, Z.; Huang, L.; Wu, Y.; Wang, J.; Wang, H.; Guo, L. DDPH: Improving cognitive deficits beyond its alpha 1-adrenoceptor antagonism in chronic cerebral hypoperfused rats. Eur. J. Pharmacol. 2008, 588, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Luo, P.; Lu, Y.; Li, C.; Zhou, M.; Chen, C.; Lu, Q.; Xu, X.; He, Z.; Guo, L. Long-lasting spatial learning and memory impairments caused by chronic cerebral hypoperfusion associate with a dynamic change of HCN1/HCN2 expression in hippocampal CA1 region. Neurobiol. Learn. Mem. 2015, 123, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Leal, G.; Afonso, P.M.; Salazar, I.L.; Duarte, C.B. Regulation of hippocampal synaptic plasticity by BDNF. Brain Res. 2015, 1621, 82–101. [Google Scholar] [CrossRef] [PubMed]
- De Vincenti, A.P.; Rios, A.S.; Paratcha, G.; Ledda, F. Mechanisms That Modulate and Diversify BDNF Functions: Implications for Hippocampal Synaptic Plasticity. Front. Cell Neurosci. 2019, 13, 135. [Google Scholar] [CrossRef] [PubMed]
- Mokhtari-Zaer, A.; Saadat, S.; Marefati, N.; Hosseini, M.; Boskabady, M.H. Treadmill exercise restores memory and hippocampal synaptic plasticity impairments in ovalbumin-sensitized juvenile rats: Involvement of brain-derived neurotrophic factor (BDNF). Neurochem. Int. 2020, 135, 104691. [Google Scholar] [CrossRef] [PubMed]
- Van Den Bosch, L.; Schwaller, B.; Vleminckx, V.; Meijers, B.; Stork, S.; Ruehlicke, T.; Van Houtte, E.; Klaassen, H.; Celio, M.R.; Missiaen, L.; et al. Protective effect of parvalbumin on excitotoxic motor neuron death. Exp. Neurol. 2002, 174, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Xia, F.; Richards, B.A.; Tran, M.M.; Josselyn, S.A.; Takehara-Nishiuchi, K.; Frankland, P.W. Parvalbumin-positive interneurons mediate neocortical-hippocampal interactions that are necessary for memory consolidation. eLife 2017, 6, e27868. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Mejias, E.; Nunez-Diaz, C.; Sanchez-Varo, R.; Gomez-Arboledas, A.; Garcia-Leon, J.A.; Fernandez-Valenzuela, J.J.; Mejias-Ortega, M.; Trujillo-Estrada, L.; Baglietto-Vargas, D.; Moreno-Gonzalez, I.; et al. Distinct disease-sensitive GABAergic neurons in the perirhinal cortex of Alzheimer’s mice and patients. Brain Pathol. 2020, 30, 345–363. [Google Scholar] [CrossRef]
- Berghuis, P.; Agerman, K.; Dobszay, M.B.; Minichiello, L.; Harkany, T.; Ernfors, P. Brain-derived neurotrophic factor selectively regulates dendritogenesis of parvalbumin-containing interneurons in the main olfactory bulb through the PLCgamma pathway. J. Neurobiol. 2006, 66, 1437–1451. [Google Scholar] [CrossRef] [PubMed]
- Albert, M.S.; DeKosky, S.T.; Dickson, D.; Dubois, B.; Feldman, H.H.; Fox, N.C.; Gamst, A.; Holtzman, D.M.; Jagust, W.J.; Petersen, R.C.; et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011, 7, 270–279. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Yang, S.-B.; Cheng, L.; Ho, K.; Kim, M.-S. Botanical Mixture Containing Nitric Oxide Metabolite Enhances Neural Plasticity to Improve Cognitive Impairment in a Vascular Dementia Rat Model. Nutrients 2023, 15, 4381. https://doi.org/10.3390/nu15204381
Zhang X, Yang S-B, Cheng L, Ho K, Kim M-S. Botanical Mixture Containing Nitric Oxide Metabolite Enhances Neural Plasticity to Improve Cognitive Impairment in a Vascular Dementia Rat Model. Nutrients. 2023; 15(20):4381. https://doi.org/10.3390/nu15204381
Chicago/Turabian StyleZhang, Xiaorong, Seung-Bum Yang, Lin Cheng, Koo Ho, and Min-Sun Kim. 2023. "Botanical Mixture Containing Nitric Oxide Metabolite Enhances Neural Plasticity to Improve Cognitive Impairment in a Vascular Dementia Rat Model" Nutrients 15, no. 20: 4381. https://doi.org/10.3390/nu15204381
APA StyleZhang, X., Yang, S.-B., Cheng, L., Ho, K., & Kim, M.-S. (2023). Botanical Mixture Containing Nitric Oxide Metabolite Enhances Neural Plasticity to Improve Cognitive Impairment in a Vascular Dementia Rat Model. Nutrients, 15(20), 4381. https://doi.org/10.3390/nu15204381





