Abstract
Aim: To determine if supplementation of infants born <33 weeks’ gestation with higher dose docosahexaenoic acid (DHA) affects growth, body composition, and blood pressure at 7 y corrected age (CA) and if treatment effects differed by infant sex at birth and birth weight strata (<1250 and ≥1250 g). Methods: Seven-year follow-up of an Australian multicenter randomized controlled trial in which 657 infants were fed high-DHA (≈1% total fatty acids) enteral feeds or standard-DHA (≈0.3% total fatty acids) from age 2–4 d until term CA. Seven-year CA outcomes were growth (weight, height), body composition (lean body mass, fat mass, waist, and hip circumference), and blood pressure. Results: There was no effect of high-DHA enteral feeds compared with standard-DHA on growth, body composition, and blood pressure at 7-year CA either overall or in subgroup analysis by sex. There was a significant interaction between high-DHA and birthweight strata on height at 7-y CA (p = 0.03). However, the post-hoc analyses by birthweight strata did not reach significance (p > 0.1). High-DHA group infants were more likely to be classified as obese (relative risk 1.6 (95% CI 1.0, 2.6); p = 0.05). Conclusions: DHA supplementation of premature infants did not affect growth, body composition, or blood pressure at 7-year CA overall by sex and birthweight strata. The finding of a higher risk of obesity in children who receive high-DHA needs to be interpreted with caution due to the small number of children classified as obese.
1. Introduction
The omega-3 (n-3) long-chain polyunsaturated fatty acid docosahexaenoic acid (DHA; 22:6 n-3) is the major fatty acid in the brain and is critical for neurogenesis and neurite growth [1,2]. DHA is transferred from the mother to the fetus during pregnancy, primarily during the last trimester [3,4]. Infants born prematurely are deprived of this in utero accretion of DHA, contributing to their higher risk of developmental delay compared to infants born at term [3,5]. Clinical trials assessing higher doses of DHA in preterm infants have been designed to improve neurodevelopmental outcomes [6,7,8,9,10,11]. However, DHA can be a potent anti-inflammatory agent and may have other actions that affect other organ systems. For this reason, follow-up of infants enrolled in these intervention trials is vital to assess effects on a broader range of outcomes and ensure long-term safety.
Growth failure is common in infants born prematurely. An early safety concern with supplementing preterm formula with n-3 fatty acids DHA and eicosapentaenoic acid (EPA) was compromised weight and length gains in early life [12,13,14], but only two studies have examined the effect of DHA supplementation on child growth and body composition beyond 24 months of age [11,15,16]. In a Scottish study, preterm infants (n = 238; born <35 weeks with birth weight < 2000 g) were randomized to receive formula supplemented with DHA (0.5%) or formula without DHA, to 9 months corrected for prematurity (corrected age [CA]) [15]. At 10 years of age, the effects on growth and blood pressure were assessed. Although there was no significant sex by treatment interaction on growth, girls who received DHA-supplemented formula were heavier and had greater skin fold thicknesses than girls who received unsupplemented formula [16]. In the largest trial of its kind, we recently reported that DHA supplementation of infants (n = 480) born before 29 weeks had no effect on weight and height z-scores at 5 years CA [11].
Preterm infants may also be at higher risk of cardiometabolic disease in later life [17]. Studies of term infants have shown that formula supplemented with n-3 fatty acids is associated with lower blood pressure. The only study in preterm infants showed that DHA supplementation in infancy was associated with higher systolic blood pressure at 10 years of age, but in girls only [16]
The DHA for the Improvement of Neurodevelopmental Outcome in preterm infants—The DINO Trial, is a large study to examine the role of DHA supplementation in premature infants <33 weeks of gestation (n = 657), and the children have been followed for up to 7 years. We previously assessed growth in these infants at multiple timepoints from birth to 18 months CA [14]. Infants fed higher DHA were 0·7 cm longer than infants fed standard DHA at 18 months CA. Higher DHA also resulted in increased length in infants born weighing ≥ 1250 g at 4 months CA and in both weight and length at 12 and 18 months CA. At 7-year CA there was no effect of higher DHA on average growth and body composition measures [18]. However, we have not reported whether sex or birthweight (<1250 and ≥1250 g) modify the effect of DHA on growth and body composition at 7-years CA. Moreover, we have not examined the effect of DHA on blood pressure in this group.
2. Materials and Methods
2.1. The DINO Trial
Full details of the DINO trial have been published elsewhere [6,14]. In summary, between 2001 and 2005, preterm infants born before 33 weeks gestation and their breastfeeding mother were enrolled from the neonatal units of five Australian hospitals within 5 days of commencing enteral feeds. Mother-infant pairs (n = 657) were randomly assigned to receive either a high-DHA diet (intervention) or standard-DHA diet (control) until 40 weeks postmenstrual age, using a computerized telephone randomization service. Randomization was stratified by hospital (center), birthweight (<1250 and ≥1250 g), and infant sex. Multiple births were considered a single randomization unit, and the randomization of twins or triplets was according to the sex and birth weight of the firstborn infant. Written informed consent was obtained from mothers. The primary outcome of the DINO trial was neurodevelopment assessed by the Bayley Scales of Infant Development (2nd edition) at 18 months CA. Approval for follow-up of secondary outcomes, specifically growth, body composition, and blood pressure, for infants enrolled in the DINO trial was granted by institutional review boards at each centre.
In the intervention group (high-DHA diet), lactating women were asked to consume six 0.5 g capsules of DHA-rich fish oil per day (900 mg of DHA and 195 mg of EPA to raise the breast milk DHA content to about 1% of total fatty acids. If infant formula was required, a matched preterm formula containing DHA as 1% of total fatty acid was provided. In the control group (standard-DHA diet), lactating women were asked to consume six 0.5 g capsules daily of soy oil (no DHA or EPA). The infants in the control group received DHA at the standard dietary level (~0.3% total fatty acids) from breast milk. If infant formula was required, a matched preterm formula containing 0.3% DHA was provided. All capsules were similar in size, shape, and color and were donated by Clover Corporation, Sydney, Australia. Ready-to-feed preterm infant formula was manufactured to trial specifications by Mead Johnson Nutritionals (Evansville, IN, USA) and packaged to match the capsule containers. Parents, outcome assessors, investigators, and clinicians were blinded to treatment allocation.
2.2. DINO 7y Follow-Up Study
All children enrolled in the DINO trial who had not withdrawn consent or died before 7-year CA were eligible for the follow-up study. Written informed consent was obtained from the parent/guardian of the child. Children attended clinic appointments at one of five centres participating in the original DINO trial.
Height and weight, waist, and hip circumference were measured by trained research personnel at each hospital using standardized techniques [14]. Sex and age-specific z-scores for children were calculated according to the WHO Child Growth Standards [19]. Bioelectrical impedance spectroscopy was used to measure lean body and fat mass [20]. The technique provides a measure of total body water used to estimate lean body and fat mass using previously validated equations in pediatric populations [20]. Systolic, diastolic, and mean arterial blood pressure was measured with the child sitting with the right arm in the horizontal position using a DINAMAP Procare V100 monitor (GE Healthcare) with an appropriately sized cuff.
2.3. Statistical Analysis
Analyses were performed on an intention-to-treat basis; all children who consented to participate in the 7-year follow-up were analyzed according to the original group to which they were allocated, whether they completed the assessments or not. The primary analysis was based on imputed data and included all participants who consented to the follow-up study. To assess missing outcome data, multiple imputation was performed separately by treatment group using chained equations to create 100 complete datasets under the assumption that data were missing at random [21]. Sensitivity analyses were performed on the available data and imputed data for n = 657 children in the original sample, excluding deaths. All analyses produced similar results, and only the results of the primary analysis are presented.
Normally distributed outcomes were analyzed using linear regression models, with the effect of treatment expressed as a mean difference with 95% confidence intervals (CI). Binary outcomes were analyzed using log-binomial models, with the effect of treatment expressed as a relative risk with 95% CI. All models used generalized estimating equations to account for the clustering of infants within mothers. Both unadjusted and adjusted analyses were performed, with adjustments made for sex and birthweight of the child and study center. Because unadjusted and adjusted analyses did not differ markedly, only adjusted analyses are presented. Secondary analyses were performed to test for evidence of effect modification by the sex and birthweight (<1250 g and ≥1250 g) of the child. Statistical significance was assessed at the two-sided p < 0.05. All analyses followed a prespecified statistical analysis plan and were performed using SAS version 9.3 (Cary, NC, USA) and Stata Release 12 (Statacorp LP, College Station, TX, USA).
3. Results
3.1. Participant Flow and Loss to Follow-Up
Six hundred fifty-seven mothers consented to the DINO trial from 2001 to 2005. From 2008 to 2013, following the exclusion of deaths (n = 18) and withdrawals (n = 13), n = 626 (95%) children and their families were approached to participate in the 7-year follow-up study. Of the 626 children, 22 were lost to follow-up leaving 604 children who participated in the study. Of these, n = 556, n = 569, n = 566, n = 560, n = 498, and n = 526 children had data for weight, height, waist circumference, hip circumference, body fat mass, and blood pressure, respectively (Figure 1).
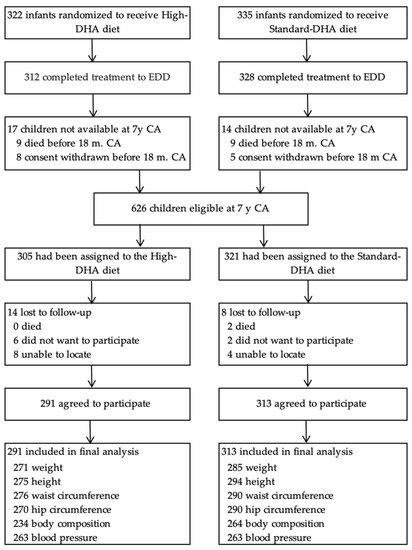
Figure 1.
Participant flow and follow-up.
3.2. Baseline and Follow-Up Characteristics
Baseline maternal and neonatal enrolment characteristics of all infants enrolled in the DINO trial have been described elsewhere. For this follow-up study, children were assessed at a mean of 7.2 y CA in both groups. As in the original trial, more mothers had multiple pregnancies in the standard-DHA group than in the high-DHA group (Table 1). The consumption of DHA-containing foods or supplements by children was similar between groups, Table 1.

Table 1.
Baseline and 7-years follow-up participant characteristics by treatment group 1.
3.3. Anthropometric Outcomes
There was no effect of high-DHA on any anthropometric measures, either raw measures or Z-scores at 7-year CA (Table 2).

Table 2.
Growth and body composition indicators at 7-year corrected age, by treatment group 1.
There was little evidence of effect modification by child sex for any anthropometric measures at 7-year CA (Table 3).

Table 3.
Comparison of growth and body composition indicators at 7-year corrected age, stratified by child sex and treatment group 1.
There were significant interactions between treatment and child birthweight strata (<1250 g and ≥1250 g) for both height and height z-score in children at 7-year CA (Table 4). In subgroup analysis, infants born <1250 g in the high-DHA group were, on average, 1.2 cm shorter than those in the standard-DHA group at 7-year CA. However, this was reversed in infants born >1250 g, with children in the high-DHA group 1.1 cm taller. These post-hoc analyses did not reach statistical significance.

Table 4.
Comparison of growth and body composition at 7-year corrected age, stratified by child birthweight strata (<1250 g and ≥1250 g) and treatment group 1.
3.4. Weight Status
At 7-year CA, there were no group differences in the percentage of children classified as underweight or overweight. (Table 5). However, children in the high-DHA group were 1.6 times more likely to be classified as obese than those in the standard-DHA group.

Table 5.
Weight status at 7-year corrected age by treatment group 1.
3.5. Blood Pressure
At 7-year CA, there was no difference in systolic, diastolic, or mean arterial blood pressure between children in the treatment groups (Table 6). There were no significant treatment differences in blood pressure in subgroup analysis by child sex at birth or birthweight strata.

Table 6.
Comparison of blood pressure measures at 7-year corrected age, by treatment group and by sex at birth and birthweight strata by treatment group 1.
4. Discussion
Since the earliest reports of supplementing preterm infants with formulas containing n-3 LCPUFA, concerns have been raised about whether growth was compromised [22]. Our DINO 7-year follow-up is the only study to evaluate the effect of supplying the estimated in utero accretion rate of DHA in infancy on growth as assessed by a broad range of anthropomorphic measures. We also examined the data according to randomization strata, including sex and birth weight. We had previously examined these children at birth, and 4, 12, and 18 months CA. There was no effect of our high-DHA intervention on weight, but infants in the high-DHA group were 0·7 (95% CI 0.1,1.4) cm (p = 0·02) longer at 18 months CA [7]. These differences had disappeared by 7 years of age. However, we found a significant interaction between birthweight strata and treatment on height at 7 years (P-interaction, 0.03). Children assigned to receive higher DHA with a birthweight < 1250 g were 1.2 cm shorter, while those with a birthweight > 1250 g were 1.1 cm taller. However, in post-hoc analyses the differences did not reach statistical significance and are not consistent with earlier findings based on birth weight strata. It may be that this is a chance effect related to the high numbers of comparisons. The slight difference in the prevalence of obesity (BMI z-score >95th percentile) in the children assigned to the high-DHA group needs to be interpreted cautiously given the small number of children classified as obese and the large number of comparisons. Bioelectrical impedance spectroscopy measurements at 7-year CA showed no effect of higher DHA on lean body mass or fat mass overall or in subgroup analysis. Our findings are consistent with our recent DHA supplementation study of infants born before 29 weeks had no effect on weight and height z-scores at 5 years CA [11]. We could not confirm the findings of the Scottish study where girls who received DHA-supplemented formula were heavier and had greater skin fold thicknesses [16]. However, the Scottish findings should be interpreted cautiously because of bias due to incomplete outcome data with 55% attrition at the 10-year follow-up.
N-3 fatty acid supplementation may improve cardiometabolic disease outcomes by lowering blood pressure [23,24]. We could detect no long-term effects of DHA treatment in infancy on blood pressure at 7-year CA. Preterm infants have been suggested to be at greater risk for cardiometabolic disease in later life than those born at term [17]. No differences were found between preterm and term-born adults for most features associated with metabolic syndrome in a recent systematic review, except blood pressure, where adults born preterm had significantly higher systolic and diastolic blood pressure [25]. A systematic review of children born at term showed that the effects of n-3 fatty acids on obesity and blood pressure were inconsistent [26]. In the Scottish study [16], DHA supplementation from birth to 9 months CA did not affect blood pressure overall in children at 10 years. However, in contrast to our study, girls responded differently to boys for systolic blood pressure (sex by treatment interaction effect p = 0.047). Girls receiving DHA-supplemented formula had significantly higher systolic blood pressure than those receiving the control formula without DHA. The effect was no longer significant once adjusted for current weight.
Our DINO 7-year CA follow-up study is the longest to evaluate the effect of supplying the estimated in utero accretion rate of DHA to preterm infants on anthropometric and cardiometabolic outcomes. The study’s strengths include the sample size, the minimal risk of bias achieved through blinding research personnel, outcome assessors, and participants, and the minimal rate of attrition. The follow-up had clinically relevant prespecified secondary outcomes and a prespecified analysis plan. Our study was a pragmatic trial involving five Australian perinatal centers, suggesting the results’ generalizability to the broader preterm infant population in high-income countries.
5. Conclusions
Overall, the DINO 7-year follow-up results show that DHA supplementation early in the life of a preterm infant is unlikely to compromise growth, body composition, or improve blood pressure later in life.
Author Contributions
M.M. and R.A.G. conceived and designed the original DINO trial; K.P.B. collected data for the original trial; M.M., R.A.G., A.W.G. and C.T.C. conceived and designed the 7-year follow-up study; K.P.B., T.R.S., R.A.G., C.T.C., J.F.G., M.M. and T.J.G. were involved in the data analysis and interpretation of the 7-year follow-up; T.J.G. and K.P.B. drafted the article. All authors were involved in critical revision and final approval of the version to be published. All authors have read and agreed to the published version of the manuscript.
Funding
Financial support for the submitted work was received from the National Health and Medical Research Council (NHMRC) Australia for the original trial (ID 250322) and 7-y follow-up study (ID 508003) and from Mead Johnson Nutrition for the 7-y follow-up study. Treatment and placebo capsules for the original trial were donated by Clover Corporation and infant formula was donated by Mead Johnson Nutritionals.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and was approved by the following Institutional Review Boards: Children, Youth and Women’s Health Service, North Adelaide, SA, Australia; Flinders Medical Centre, Bedford Park, SA, Australia; King Edward Memorial Hospital, Subiaco, WA, Australia; Royal Women’s Hospital, Parkville, VIC, Australia; and Royal Brisbane and Women’s Hospital, Herston, QLD, Australia Informed consent was obtained from mothers involved in the study.
Data Availability Statement
Deidentified data will be shared with researchers who supply a methodologically sound research proposal following review and approval by the trial steering committee and completion of a signed data access agreement.
Acknowledgments
We would like to acknowledge the input of the DINO Trial Steering Committee; coordinating center staff at the Women and Kids Theme of the South Australian Health and Medical Research Institute (formerly the Women’s and Children’s Health Research Institute), Adelaide, Australia; DINO trial collaborating centers: Women’s and Children’s Hospital, Adelaide, South Australia; King Edward Memorial Hospital, Perth, Western Australia; Royal Women’s Hospital, Victoria; Royal Brisbane and Women’s Hospital and the Perinatal Research Centre, University of Queensland Centre for Clinical Research, Queensland; Flinders Medical Centre, South Australia; This is an IMPACT (Interdisciplinary Maternal and Perinatal Australasian Clinical Trials) Network–endorsed study. We thank the DINO children and their families for their ongoing contribution to the DINO trial.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Innis, S.M. Dietary (n-3) fatty acids and brain development. J. Nutr. 2007, 137, 855–859. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.E.; Bazan, N.G. Changing fatty acid content of growth cone lipids prior to synaptogenesis. J. Neurochem. 1992, 59, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M. Tissue levels of polyunsaturated fatty acids during early human development. J. Pediatr. 1992, 120, S129–S138. [Google Scholar] [CrossRef] [PubMed]
- Auestad, N.; Innis, S.M. Dietary n-3 fatty acid restriction during gestation in rats: Neuronal cell body and growth-cone fatty acids. Am. J. Clin. Nutr. 2000, 71, 312s–314s. [Google Scholar] [CrossRef] [PubMed]
- Lapillonne, A.; Groh-Wargo, S.; Gonzalez, C.H.; Uauy, R. Lipid needs of preterm infants: Updated recommendations. J. Pediatr. 2013, 162, S37–S47. [Google Scholar] [CrossRef]
- Makrides, M.; Gibson, R.A.; McPhee, A.J.; Collins, C.T.; Davis, P.G.; Doyle, L.W.; Simmer, K.; Colditz, P.B.; Morris, S.; Smithers, L.G.; et al. Neurodevelopmental outcomes of preterm infants fed high-dose docosahexaenoic acid: A randomized controlled trial. JAMA 2009, 301, 175–182. [Google Scholar] [CrossRef]
- Collins, C.T.; Makrides, M.; McPhee, A.J.; Sullivan, T.R.; Davis, P.G.; Thio, M.; Simmer, K.; Rajadurai, V.S.; Travadi, J.; Berry, M.J.; et al. Docosahexaenoic Acid and Bronchopulmonary Dysplasia in Preterm Infants. N. Engl. J. Med. 2017, 376, 1245–1255. [Google Scholar] [CrossRef]
- Henriksen, C.; Haugholt, K.; Lindgren, M.; Aurvåg, A.K.; Rønnestad, A.; Grønn, M.; Solberg, R.; Moen, A.; Nakstad, B.; Berge, R.K.; et al. Improved cognitive development among preterm infants attributable to early supplementation of human milk with docosahexaenoic acid and arachidonic acid. Pediatrics 2008, 121, 1137–1145. [Google Scholar] [CrossRef]
- Almaas, A.N.; Tamnes, C.K.; Nakstad, B.; Henriksen, C.; Walhovd, K.B.; Fjell, A.M.; Due-Tønnessen, P.; Drevon, C.A.; Iversen, P.O. Long-chain polyunsaturated fatty acids and cognition in VLBW infants at 8 years: An RCT. Pediatrics 2015, 135, 972–980. [Google Scholar] [CrossRef]
- Guillot, M.; Synnes, A.; Pronovost, E.; Qureshi, M.; Daboval, T.; Caouette, G.; Olivier, F.; Bartholomew, J.; Mohamed, I.; Massé, E.; et al. Maternal High-Dose DHA Supplementation and Neurodevelopment at 18-22 Months of Preterm Children. Pediatrics 2022, 150, e2021055819. [Google Scholar] [CrossRef]
- Gould, J.F.; Makrides, M.; Gibson, R.A.; Sullivan, T.R.; McPhee, A.J.; Anderson, P.J.; Best, K.P.; Sharp, M.; Cheong, J.L.Y.; Opie, G.F.; et al. Neonatal Docosahexaenoic Acid in Preterm Infants and Intelligence at 5 Years. N. Engl. J. Med. 2022, 387, 1579–1588. [Google Scholar] [CrossRef]
- Carlson, S.E.; Werkman, S.H.; Tolley, E.A. Effect of long-chain n-3 fatty acid supplementation on visual acuity and growth of preterm infants with and without bronchopulmonary dysplasia. Am. J. Clin. Nutr. 1996, 63, 687–697. [Google Scholar] [CrossRef]
- Ryan, A.S.; Montalto, M.B.; Groh-Wargo, S.; Mimouni, F.; Sentipal-Walerius, J.; Doyle, J.; Siegman, J.S.; Thomas, A.J. Effect of DHA-containing formula on growth of preterm infants to 59 weeks postmenstrual age. Am. J. Hum. Biol. 1999, 11, 457–467. [Google Scholar] [CrossRef]
- Collins, C.T.; Makrides, M.; Gibson, R.A.; McPhee, A.J.; Davis, P.G.; Doyle, L.W.; Simmer, K.; Colditz, P.B.; Morris, S.; Sullivan, T.R.; et al. Pre- and post-term growth in pre-term infants supplemented with higher-dose DHA: A randomised controlled trial. Br. J. Nutr. 2011, 105, 1635–1643. [Google Scholar] [CrossRef]
- Fewtrell, M.S.; Abbott, R.A.; Kennedy, K.; Singhal, A.; Morley, R.; Caine, E.; Jamieson, C.; Cockburn, F.; Lucas, A. Randomized, Double-Blind Trial Of Long-Chain Polyunsaturated Fatty Acid Supplementation with Fish Oil And Borage Oil In Preterm Infants. J. Pediatr. 2004, 144, 471–479. [Google Scholar] [CrossRef]
- Kennedy, K.; Ross, S.; Isaacs, E.B.; Weaver, L.T.; Singhal, A.; Lucas, A.; Fewtrell, M.S. The 10-year follow-up of a randomised trial of long-chain polyunsaturated fatty acid supplementation in preterm infants: Effects on growth and blood pressure. Arch. Dis. Child 2010, 95, 588–595. [Google Scholar] [CrossRef]
- Casirati, A.; Somaschini, A.; Perrone, M.; Vandoni, G.; Sebastiani, F.; Montagna, E.; Somaschini, M.; Caccialanza, R. Preterm birth and metabolic implications on later life: A narrative review focused on body composition. Front. Nutr. 2022, 9, e978271. [Google Scholar] [CrossRef]
- Gunaratne, A.W.; Makrides, M.; Collins, C.T.; Gibson, R.A.; McPhee, A.J.; Sullivan, T.R.; Gould, J.F.; Green, T.J.; Doyle, L.W.; Davis, P.G.; et al. Docosahexaenoic acid supplementation of preterm infants and parent-reported symptoms of allergic disease at 7 years corrected age: Follow-up of a randomized controlled trial. Am. J. Clin. Nutr. 2019, 109, 1600–1610. [Google Scholar] [CrossRef]
- WHO Child Growth Standards based on length/height, weight and age. Acta Paediatr. Suppl. 2006, 450, 76–85. [CrossRef]
- Ellis, K.J.; Shypailo, R.J.; Wong, W.W. Measurement of body water by multifrequency bioelectrical impedance spectroscopy in a multiethnic pediatric population. Am. J. Clin. Nutr. 1999, 70, 847–853. [Google Scholar] [CrossRef]
- Sullivan, T.R.; White, I.R.; Salter, A.B.; Ryan, P.; Lee, K.J. Should multiple imputation be the method of choice for handling missing data in randomized trials? Stat. Methods. Med. Res. 2018, 27, 2610–2626. [Google Scholar] [CrossRef] [PubMed]
- Carlson, S.E.; Cooke, R.J.; Werkman, S.H.; Tolley, E.A. First year growth of preterm infants fed standard compared to marine oil n-3 supplemented formula. Lipids 1992, 27, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Geleijnse, J.M.; Giltay, E.J.; Grobbee, D.E.; Donders, A.R.; Kok, F.J. Blood pressure response to fish oil supplementation: Metaregression analysis of randomized trials. J. Hypertens. 2002, 20, 1493–1499. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.C.; Sacks, F.; Rosner, B. Does fish oil lower blood pressure? A meta-analysis of controlled trials. Circulation. 1993, 88, 523–533. [Google Scholar] [CrossRef]
- Markopoulou, P.; Papanikolaou, E.; Analytis, A.; Zoumakis, E.; Siahanidou, T. Preterm Birth as a Risk Factor for Metabolic Syndrome and Cardiovascular Disease in Adult Life: A Systematic Review and Meta-Analysis. J. Pediatr. 2019, 210, 69–80.e65. [Google Scholar] [CrossRef]
- Voortman, T.; van den Hooven, E.H.; Braun, K.V.E.; van den Broek, M.; Bramer, W.M.; Chowdhurry, R.; Franco, O.H. Effects of polyunsaturated fatty acid intake and status during pregnancy, lactation, and early childhood on cardiometabolic health: A systematic review. Prog. Lipid. Res. 2015, 59, 67–87. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).