Abstract
Autoimmune thyroid diseases are on the rise worldwide, and such a rapid increase is mainly driven by environmental factors related to changed lifestyles in “modern” societies. In this context, diet seems to play a crucial role. An unhealthy high-energy diet, rich in animal fat and proteins, salt and refined sugars (the so-called “Western diet”) negatively influences the risk of autoimmunity by altering the immune balance and the gut microbiota composition, enhancing oxidative stress and promoting inflammation. In contrast, the Mediterranean diet represents a unique model of healthy eating, characterized by a high intake of food from vegetable sources, a low consumption of saturated fats in favor of unsaturated fats (mainly, olive oil), a moderate consumption of fish (typically, the small oily fishes) and dairy products, as well as a moderate consumption of wine at meals, and a low intake of meat. Thanks to its nutritional components, the Mediterranean Diet positively influences immune system function, gut microbiota composition, and redox homeostasis, exerting anti-oxidants, anti-inflammatory, and immunomodulatory effects. The present review was aimed at exploring the existing knowledge on the correlations between dietary habits and thyroid autoimmunity, to evaluate the role of the Mediterranean diet as a protective model.
1. Introduction
Autoimmune thyroid diseases (AITDs) include a wide spectrum of disorders, ranging from hyperthyroidism and goiter (Graves’ disease, GD) to hypothyroidism and glandular hypo-atrophia (Hashimoto’s thyroiditis, HT) [1,2]. They are the result of a failure of immunological tolerance to self-antigens and consequent activation of immune responses against thyroid tissue. AITDs are multifactorial diseases, deriving from the interaction between genetic and environmental factors, which trigger the development and further progression of AITDs in genetically susceptible individuals [1,2,3,4]. GD is an uncommon disease, and its prevalence/incidence has remained stable over the years [5,6], whereas autoimmune thyroiditis (namely, HT), is very common, affecting about 3–5% of the general population, and its incidence has been increasing [7,8,9]. In recent decades, large epidemiological studies have reported increased prevalence and incidence of several autoimmune disorders, including rheumatic (systemic lupus erythematosus, rheumatoid arthritis, to mention a few), neurological (multiple sclerosis, myasthenia gravis), gastro-enteric (inflammatory bowel diseases, celiac disease) and endocrine (such as autoimmune thyroiditis, type 1 diabetes) diseases, mostly in developed countries of the West and North compared to developing countries of the East and South [10,11,12].
Such an increase clearly correlates with improved hygiene and health standards, as well as with socioeconomic status, suggesting that environmental factors are driving these geo-epidemiologic changes [1,10,11,12]. In particular, interest has been increasing in the “modern” lifestyle of industrialized, “westernized” societies, and to the potential environmental triggers that characterize it, including, for instance, reduced microbial exposures and improved hygiene, pollutants, psychological stress overload, deficiencies or excess of nutrients (notably, vitamin D, iodine, and selenium, to mention a few), whose role in autoimmunity is intensely debated [12,13,14,15,16,17,18,19,20]. In particular, in developed countries, excess calorie intake and frequent consumption of “unhealthy food”, associated with a sedentary lifestyle, has led to a rise in the prevalence of obesity, which in turn predisposes individuals to several chronic non-communicable diseases, including inflammatory and immune-mediated disorders [19,20,21,22]. For this reason, interest in nutritional patterns has grown in recent years and our understanding of the link between diet and thyroid function is rapidly expanding.
This narrative review was aimed at exploring the existing knowledge on the correlations between dietary habits, intake of different foods, and thyroid autoimmunity.
2. Materials and Methods
For this narrative literature review, we carried out an extensive literature search on online databases (MEDLINE via PubMed, ISI Web of Science, and Scopus), using the key terms “autoimmune thyroid disease” and “diet”. Also the MeSH terms “Hashimoto’s thyroiditis”, “autoimmune diseases”, “hypothyroidism”, “hyperthyroidism”, were included in combination with “dietary regimens”, “Western-style diet”, “vegetarianism”, “vegan diet”, “Mediterranean diet”, and “Oxidative stress”, to ensure that the majority of relevant studies have been identified. The literature search was performed up to May 2023. Reviews, meta-analyses, and original studies reporting results on the correlation between dietary regimens or specific dietary components and thyroid autoimmunity were evaluated and included in the present review according to the following criteria: English language and publication in peer-reviewed journals. Duplicates, case reports/series, and papers written in other languages apart from English were excluded. The search strategy is summarized in Table 1.

Table 1.
Narrative review searching strategy.
3. The Western Diet: Possible Links with Autoimmunity
Several studies have focused on nutrition and dietary patterns (“too much”, “too fatty”, “too salty”, “too sweet”) as risk factors for the development of autoimmune diseases in Westernized societies, and they consistently suggested these dietary traits as risk factors for rheumatoid arthritis, psoriasis, celiac, and intestinal bowel diseases, among others [21,22,23,24,25,26].
In recent decades, in Westernized countries, a high-energy diet rich in fats (mainly saturated fats, trans fatty acids, and cholesterol) and proteins (mainly meat), high in salt and refined sugars, and low in fiber has become increasingly frequent, mostly among young age groups [20,21,22]. This dietary pattern has been termed the “Western diet” (WD), and it has been associated with chronic over-nutrition and increased risk of obesity and related morbidities [20,21,22]. A major hallmark of WD is represented by excess consumption of ultra-processed food and soft drinks [27,28,29]. These are hyper-palatable and ready-to-eat products, rich in unhealthy components typical of the WD trait, such as refined carbohydrates and saturated fat, but poor in healthy nutrients, such as fiber, vitamins, and trace elements [28,29,30]. Moreover, ultra-processed food and soft drinks have a high content of additives (flavors, colors, emulsifiers, sweeteners) and other ingredients that are usually absent in “real” food (for example, hydrogenated or de-esterified oils, hydrolyzed proteins), used during industrial food processing to make the final product more appealing and palatable [27]. As consumption of ultra-processed and fast foods increases, industrial food processing and exposure to food contaminants are expanding in parallel [23,27].
The WD habits favor a condition of chronic metabolic inflammation (the so-called “meta inflammation”) both directly due to the proinflammatory effects of their components, and indirectly through increased fat mass and overweight/obesity. This condition is characterized by excess release of inflammatory cytokines (such as IL-6, IL-17, TNF-alfa), altered immune balance (CD4+ effector vs. regulatory T cells), and increased oxidative stress [20,21,31,32,33,34,35,36], and negatively influence the risk of immune-mediated disorders, including AITDs. In particular, oxidative stress, defined as an imbalance between free radical production and removal into cells, plays a crucial role in the onset and progression of several autoimmune disorders [35], by causing oxidative modifications of tissue proteins, generating novel antigenic molecules, and by further promoting inflammation and immune dysregulation [33,34,35,36]. A correlation between increased oxidative stress and the WD has been demonstrated since low consumption of fruits and vegetables causes lack of exogenous antioxidants while excess intake of animal proteins (mainly red and processed meat) and fats has been associated with increased oxidants [37].
More recently, interest has been growing in the influence of the gut microbiome in autoimmune disorders, in relation to impaired intestinal barrier functions and pro-inflammatory status. In this context, the WD pattern, which high in processed foods, refined sugar, and industrial food additives, and low in fiber from fruit and vegetables, generates an unfavorable environment in the gut and alters intestinal barrier functions and microbiota composition, leading to dysbiosis and inflammation [38,39,40,41,42]. Furthermore, altered intestinal permeability, the so-called “leaky gut” condition, allows the passage of toxins, food antigens, bacteria, and microbial products, promoting inflammation and dysregulated immune responses and triggering autoimmunity through different mechanisms [12]. On these bases, it may be speculated that a WD dietary pattern may favor the initiation and development of autoimmune disorders, including thyroid diseases, in genetically susceptible individuals [21].
4. The Mediterranean Diet as a Model of Healthy Eating
In contrast to the WD model, the Mediterranean diet, which is characterized by a high intake of food from vegetable sources, represents a model of healthy eating. The Mediterranean diet (MD) has its origins in a portion of land considered unique in its kind, the Mediterranean basin, which historians call “the cradle of society”, because within its geographical borders the whole history of the ancient world took place. The American Scientist Angel Keys coined the term in the middle of the last century with the publication of his pioneering observations in the ‘Seven Country Study’, where he demonstrated a lower mortality rate from coronary heart disease in the Mediterranean area [43]. The observed effects were ascribed to the different dietary habits of populations examined, especially for the proportions of energy from saturated fatty acids, mainly from meats and butter fat, and from monounsaturated fats, mainly olive oil. In particular, MD represents a natural experiment consisting in the combination of eating habits traditionally followed by individuals in the olive-growing areas bordering the Mediterranean Sea with a high consumption of plant foods (cereals, fruits, vegetables, legumes, nuts, seeds, and olives), fish and seafood, modest amounts of cheese, dairy products, meat and meat products, and moderate ethanol intake, mainly in the form of red wine and during meals. Extra virgin olive oil is the main source of lipids, and represents a high nutritional quality food for its richness in bioactive compounds, mostly phenols [44].
The MD is more than a simple nutritional model; it is conviviality, passion for food, a way of eating, promoting the quality and safety of foods and the link with the land of origin. In a word, it is a lifestyle. In fact, the Mediterranean diet is included in the UNESCO Intangible Cultural Heritage List as the best choice for a healthy life (UNESCO). It satisfies the criteria because “The Mediterranean diet involves a set of skills, knowledge, rituals, symbols and traditions concerning crops, harvesting, fishing, animal husbandry, conservation, processing, cooking, and particularly the sharing and consumption of food” [45].
Monounsaturated fatty acids and n-3/n-6 polyunsaturated fatty acids (PUFA) positive ratio, fibers, vitamins (like vitamin E, B, C and β-carotene), and minerals (selenium, zinc, iron, iodine) are key components of the MD, and account for most of its beneficial effects [46,47,48]. However, it is important to emphasize that the positive impact on health of the eating habits in MD derives from the synergy of various nutrients and bioactive compounds present in foods which interact with each other, resulting in increasing beneficial effects, which are not possible to mimic by isolating a single component [49,50].
The health benefits of the MD are more numerous and more significant than those evidenced in the ‘Seven Country Study’, expanding beyond coronary heart disease, to the reduction in the incidence of metabolic syndrome [51,52,53,54,55,56,57,58,59]. More recently, the knowledge about the immunomodulatory, anti-oxidant, and anti-inflammatory properties of whole dietary patterns like MD has been increased, and the protective role against autoimmunity has been emerging [60,61]. On these bases, MD should represent a healthy diet model, to be recommended to patients with autoimmune disorders, including HT, as opposed to the WD (Figure 1).
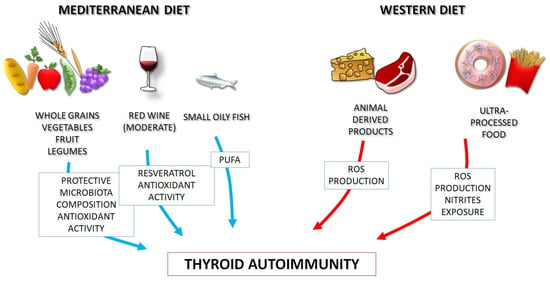
Figure 1.
Thanks to its nutritional components, the Mediterranean diet positively influences gut microbiota, redox homeostasis and immune system function, as opposite to the Western diet, and may be proposed for prevention and management of autoimmune thyroid disorders (a “precision nutrition” approach). PUFA: polyunsaturated fatty acids. ROS: reactive oxygen species.
5. Dietary Habits and Thyroid Autoimmune Diseases
5.1. Evidence from Clinical Studies
The association between diet and risk of developing autoimmune disorders was proposed more than 50 years ago by Trowell, who observed that autoimmune disorders, including thyroiditis, were extremely rare among isolated rural sub-Saharan populations following traditional near-vegan diets [62,63]. A similar low incidence of autoimmune disorders was reported in Asian societies whose diets were almost vegan [64,65]. In the following decades, evidence has been growing on the role of diet in the development of several autoimmune disorders, including rheumatoid arthritis, celiac and intestinal bowel diseases, type 1 diabetes, multiple sclerosis, and psoriasis [21,22,23,24,25,66,67,68,69,70,71].
Few studies, however, have evaluated the eating habits of subjects affected by thyroid disorders, and mainly in relation to thyroid function (Table 1) [72,73,74,75].
In 2015, Tonstad and coworkers investigated the association between incident and/or prevalent hyperthyroidism and dietary patterns among a large cohort of subjects belonging to the Seventh-day Adventist church, who exhibited a wide range of diets from vegan to omnivorous, with a high proportion of vegetarians. The authors observed that the prevalence of hyperthyroidism was significantly lower in subjects following a vegan diet compared to omnivores, while lacto-ovo and pesco-vegetarian diets were associated with intermediate protection [72]. Overall, this study suggested that diets excluding animal foods could be protective against thyroid dysfunction, likely autoimmune in etiology [72]. Even if the MD was not specifically studied in this case, and even if the MD does not completely exclude animal-derived products, these results can be considered relevant for our discussion. Indeed, it must be remembered that in MD, most of food intake is derived from whole grains and vegetables, with a much lower content in animal-derived food compared to the WD. Several recent studies focused on the effect of dietary patterns on thyroid function parameters. Zupo et al. investigated the possible relationship between adherence to the MD, assessed through the PREDIMED questionnaire, and thyroid function in 324 euthyroid overweight/obese subjects living in Southern Italy [73]. They found that a higher adherence to the MD was inversely related to serum levels of free T3 (fT3) and free T4 (fT4). When considering the single items of the PREDIMED questionnaire, it emerged that a higher consumption of extra-virgin olive oil was associated with lower levels of fT3 and fT4. A logistic regression model adjusted for gender, age, and BMI confirmed only the correlation between FT4 and adherence the MD, whereas no effect on serum TSH was observed [73]. The study by Liu and coworkers aimed at investigating the association between dietary inflammatory potential and thyroid function in a large population of adult males (2346 subjects), using data from the National Health and Nutrition Examination Survey [74]. The dietary inflammatory index was calculated based on the reported intake of several food groups that have anti-inflammatory potential (including fiber, vitamin C, flavonoids, garlic, several spices like rosemary and thyme) vs. pro-inflammatory ones (such as animal fat, carbohydrates, and animal protein). The results showed a positive association between dietary inflammatory index and total T4, and subjects adhering to a more pro-inflammatory diet appeared to have higher total T4 and total T3 levels, even within the normal range, while no consistent effect on fT3, fT4, or TSH could be observed. The relationship appeared to be stronger among obese subjects and those with urinary iodine concentration (UIC) levels indicating iodine deficiency [74]. The interpretation of these two recent studies performed on thyroid function parameters and dietary patterns can be cumbersome, due to the multiple factors that can influence circulating thyroid hormones, especially with normal TSH. The lack of a strong observed effect of the different dietary choices on TSH levels, and the fact that the variation in circulating T3 and T4 is of small entity and within the normal range seems to suggest that these subjects do not experience hypothyroidism, but rather small modifications in peripheral sensitivity to thyroid hormones and/or binding of thyroid hormones with transport proteins.
To our knowledge, only two recent studies specifically focused on the possible role of dietary patterns in increasing the risk of thyroid autoimmunity per se, apart from thyroid dysfunction.
In 2020, Kaličanin and coworkers evaluated the differences in food-group consumption between HT patients and healthy subjects as controls through a food frequency questionnaire (FFQ) [75]. A specific focus was put on fat consumption, in particular regarding the choice between vegetal and animal fats. The results showed that HT patients consumed more animal fat and processed meat than controls, who in turn consumed more non-processed red meat, non-alcoholic beverages, whole grains, and plant oils (Table 2). A subgroup analysis showed that HT patients on levothyroxine (LT4) therapy consumed more red meat than those who were not receiving substitutive therapy [76]. Moreover, from analysis of food consumption, it emerged that HT patients do not modify their dietary habits upon disease diagnosis, indicating that nutritional aspects were disregarded by both the patients and the physicians [75]. A more recent study by Ruggeri et al. evaluated the dietary habits of a cohort of euthyroid subjects from Southern Italy and found significant differences between HT patients and healthy subjects [37]. In particular, HT subjects consumed higher amounts of animal-derived food, including meat (both fresh and processed), dairy, and fish, and commercial sweetened products, while controls reported higher intake of vegetables, legumes, and nuts (Table 2). Of note, HT subjects and healthy controls did not differ in terms of either body weight or BMI, most of them being of normal weight. Adherence to the MD, as assessed by PREDIMED questionnaire, was lower in HT patients compared to controls, and in a multivariate logistic regression model, the PREDIMED score was an independent predictor of thyroid autoantibodies positivity, suggesting a protective role of the MD against thyroid autoimmunity [37].

Table 2.
Summary of studies (either clinical or experimental) evaluating the relationship between dietary habits and thyroid functional status and/or autoimmunity.
5.2. Pathophysiological Bases of the Link between Dietary Components and Thyroid Autoimmune Diseases
The pathophysiological mechanisms underlying the relationship between MD, WD, and thyroid autoimmunity are not completely understood, but some hypotheses can be made, and the possible role of the various food components can be discussed, as exemplified in Figure 2.
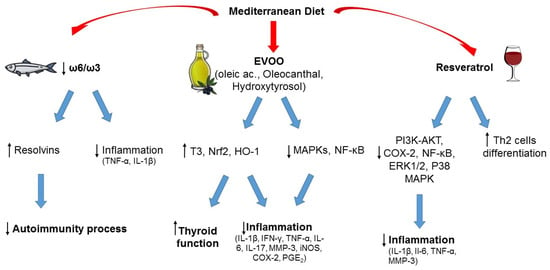
Figure 2.
Typical foods from Mediterranean diet and their possible role in the prevention of autoimmunity and inflammation. ↑ increased; ↓ decreased.
5.2.1. Animal Products
The lower consumption of animal-derived products in subjects adhering to the MD when compared to the WD appears to have a central role in protecting against thyroid autoimmunity and/or dysfunction, as suggested by pre-clinical studies [76,77,78]. Among the possible mechanisms that could be involved in this association, it could be hypothesized that high intake of animal fat could increase the risk of autoimmunity due to an increase in ROS production. Indeed, in a 2016 study by Ruggeri et al., a higher consumption of animal-derived products in patients with TH was related to a higher concentration of oxidant factors and a lower concentration of antioxidants [37]. Another reason that could explain the effect of meat consumption, especially if processed, on thyroid autoimmunity is the intake of nitrates and nitrites. Nitrites can be found in several processed foods, in particular industrial processed meat. Indeed, exposure of laboratory animals to extremely high concentrations of nitrites (~10–600 times more than the acceptable daily intake) can induce several anti-thyroid effects, including reduced levels of circulating thyroid hormones and histomorphological changes in thyroid gland. However, it should be acknowledged that no such effects have been documented in humans [79].
The role of the other main source of animal-derived food, that is fish, is more controversial in terms of effect on thyroid function and autoimmunity [37,50,80]. The MD promotes fish consumption rather than meat. Fish represents the main dietary source of omega-3 PUFAs, whose positive effects on chronic inflammatory and immune-mediated disorders are well known (see also below). On the other hand, the risk of fish contamination by environmental pollutants should be not overlooked, and a cost (contaminants) to benefits (healthy components) ratio should always be considered [50,80]. Of note, the MD is characterized by prevalent consumption of small oily fishes (the so-called “blue fish”, like anchovies, sardines, mackerels, etc.), that represent a good source of proteins and PUFAs and are associated with a low risk of contamination, whereas large, top-predator fishes (like swordfish) tend to concentrate pollutants, in particular heavy metals, persistent organic pollutants, and microplastics, due to mechanisms of bioaccumulation and biomagnification [50,80]. Several studies suggest that consumption of PUFAs, in particular omega-3, can have protective effects on the development of autoimmune thyroiditis, while heavy metal exposure was associated with an increased risk of autoimmunity [50,80]. Thus, the kind of fish consumed seems to play a role, rather than consumption of fish per se. When including fish with low content of contaminants, the MD represents a healthy nutritional model, because fish is part of a complete healthy dietary plan, and patients would benefit from both fish intake and other micronutrients [50].
5.2.2. Monounsaturated and Polyunsaturated Fatty Acids (Omega-3)
Linoleic acid and alpha-linolenic acid are essential fatty acids, as their intake totally depends on foods because they cannot be synthesized in the human body, and represent the precursors of ω-6 and ω-3 PUFA families. Besides alpha-linolenic acid, the other two main ω-3 PUFAs, directly derived from it, are eicosapentaenoic acid and docosahexaenoic acid. The main difference between the two classes of PUFA is the position of the double bonds on the carbon chain (the ω-carbon): ω-6 PUFAs have the first double bond at the sixth carbon, while ω-3 PUFAs have it at the third carbon starting from the methyl end of the carbon chain. ω-6 PUFAs can be found in vegetable oils (soybean, corn, sunflower oils) in the form of linoleic acid, and animal products, whereas green leafy vegetables are a major source of ω-3 PUFAs in the form of alpha-linolenic acid. Fish and fish oil provide a good supply of eicosapentaenoic and docosahexaenoic acid [81]. Both ω-6 and ω-3 PUFAs are precursors of inflammatory mediators but with opposite effects: ω-3 PUFAs reduce inflammation whereas ω-6 PUFAs tend to promote inflammation. For this reason, a balanced ω-6/ω-3 ratio is important to support the anti-inflammatory profile of ω-3 PUFAs, and a varied diet that includes balanced amounts of each PUFA class has prevalent anti-inflammatory effects. Different from the WD, which is characterized by an excessive ω-6 PUFA intake associated with a higher production of inflammatory cytokines [46,82], the MD ensures high consumption of ω-3-rich food promoting a better inflammatory profile [46].
Dietary ω-3 PUFAs can reduce inflammation through different mechanisms, for example by lowering the synthesis of cytokines (such as TNF-α, IL-1β), prostaglandins, and leukotrienes; by controlling leucocyte chemotaxis, adhesion molecule expression, and leucocyte–endothelial adhesive interactions [48,61]. In recentyears, new classes of lipid mediators have emerged as key players in the resolution of inflammatory process. They are endogenously produced during inflammation from PUFAs and exert potent anti-inflammatory actions, serving as specialized pro-resolving lipid mediators [83]. Bioactive metabolites from ω-3 PUFAs, such as resolvins, maresins, and protectins, demonstrated beneficial effects on global health. Among them, resolvins have a well-documented anti-inflammatory activity [84]. Increasingly, evidence has demonstrated a major role of these metabolites in the regulation of autoimmune processes [61,85].
5.2.3. Extra Virgin Olive Oil (EVOO)
Among the central foods of MD is EVOO. With more than 200 different bioactive compounds detected, including phenolics, sterols, carotenoids, and triterpenic alcohols, it represents the main fat used in the MD and is a hallmark of this dietary pattern. The phenolic composition of EVOO has been associated with its strong antioxidant activity, and it is thought to be largely responsible for the pleiotropic positive effects of the MD [44,86]. Recently, interest has grown in investigating the potential advantages of EVOO consumption in the context of autoimmunity. In several experimental models of autoimmune disease, EVOO components, namely hydroxytyrosol and oleic acid, and oleocanthal, were revealed to be efficacious in counteracting oxidative and inflammatory imbalances by enhancing Nrf2/HO-1 and inhibiting of MAPKs/NF-κB signaling pathways [87,88,89,90]. In two studies, the EVOO-diet supplementation was compared with a sunflower oil-diet supplementation, and both investigations reported greater EVOO effectiveness, probably due to its phenolic composition [89,90]. Olive derivatives showed varying effects on circulating thyroid hormone levels in animal models (both euthyroid and with experimentally induced autoimmune thyroiditis), with an increase in total T3 levels as the most frequently observed one [91]. In a review by Pang et al., the analyses of the literature demonstrated that olive oil, olive leaf extracts, and solid olive residue enhanced thyroid function in both euthyroid and hypothyroid animals, and in the latter ameliorated the oxidative status [91]. Obviously, further studies are required, and these results need to be confirmed in humans. Overall, the results from these studies are encouraging and provide preliminary evidence for the positive value of an EVOO-rich diet like MD to counteract and prevent autoimmune diseases.
5.2.4. Phenolic Compounds of Wine (Resveratrol)
A moderate wine intake, especially red wine, with meals, is considered another unique habit in the context of the MD, with beneficial health effects related to its phenolic composition [92]. Indeed, wine is rich in phenolic compounds derived from the grapes during the wine-making processes. Resveratrol, a stilbene localized in grape skin, is acknowledged as an anti-cancer, anti-inflammatory, neuroprotective, and antioxidant bioactive compound [93,94,95]. Recently, several experimental studies evidenced a potential protective effect of this compound in different autoimmune settings, through the regulation of antioxidants, inflammatory mediators, and Th cell subpopulation balance [96,97,98,99]. Interestingly, a moderate consumption of alcohol was associated with a reduced risk of HT and hypothyroidism in the study by Carlé et al. [100]. The positive effects derived from the consumption of wine are currently under debate, but we must focus on a moderate wine consumption during meals as usually done in the MD habits. Epidemiological and clinical studies, and observations on Mediterranean cohorts of subjects, have demonstrated that moderate wine consumption correlates with benefits to human health [101]. Our intent here is not to promote alcohol as a health element or to induce people to drink alcohol in the hope of gaining benefits, rather to emphasize the role of moderate alcohol consumption, particularly red wine, within the MD and how there are no results to support complete abstention, in order to reduce the risk of developing autoimmune diseases.
5.2.5. Fibers, Vitamins, and Trace Elements
The MD also stands out for the increased consumption of several food groups that can have a positive influence on thyroid autoimmunity. First, the abundant consumption of vegetables, fruits, and legumes can influence the gut microbiota composition, due to their high content in complex carbohydrates and vegetal fibers. Several studies show that patients with autoimmune thyroid disorders display alterations in gut microbiota composition and metabolism. It is well known that gut microbiota influences the host immune system via epigenetic mechanisms, and any changes in human microbiota—the so-called dysbiosis—may alter immune homeostasis and ultimately result in the development of autoimmune disorders [41]. Although the relationship between these alterations, dietary patterns, and autoimmunity has not been completely elucidated, it could be hypothesized that a favorable microbiota composition due to MD could be protective towards the development of thyroid autoimmunity [38,39,40,41,102,103]. Moreover, the MD is particularly rich in several micronutrients that have been implicated in several protective mechanisms towards thyroid dysfunction and/or autoimmunity, although in some cases with inconclusive evidence [41].
- Iodine
Iodine is an essential trace element required for the synthesis of thyroid hormones, and a daily intake of 150 μg of iodine is recommended for adults, whereas pregnant women need a greater iodine intake (250 μg/day) [104].
Severe iodine deficiency, defined as a median urinary iodine concentration (UIC) < 100 μg/L, causes impaired thyroid hormone synthesis and represents the major cause of hypothyroidism worldwide. Chronic iodine deficiency is associated with the development of goiter and thyroid nodules [105]. During pregnancy, it results in an increased risk of growth retardation and brain damage for the fetus, and both maternal and fetal goiter [105,106,107,108]. Universal salt iodization is recommended to eradicate iodine deficiency, and the use of iodized salt is considered safe in the general population [109]. On the other hand, excess (>300 mg/d) supplemental iodine, or high iodine-rich food (e.g., seaweeds) may be harmful to the thyroid gland [16]. This can happen from the intake of supplemental iodine besides iodized salt (for instance, weight loss products) and from some dietary sources, such as seaweed (i.e., nori, kelp, kombu, wakame) that are typical of traditional Asian cuisine and are now frequently consumed in Western countries [110,111]. Moreover, an increase in thyroid autoimmunity has been observed in iodine-deficient countries after the implementation of iodine supplementation programs, but this phenomenon was transient and did not result in an increased prevalence of thyroid dysfunction in the long run [16]. A U-shaped relationship exists between iodine intake and thyroid autoimmunity/dysfunction, so that both iodine excess and deficiency are harmful to the thyroid gland, and are specifically associated with increased prevalence/incidence of AITDs [16]. Thus, adequate iodine supplementation, defined as a population median UIC between 100 and 300 μg/L, appears to be safe, and the benefits largely outweigh the potential risks [16].
Dietary sources of iodine include seafood (seaweed, marine fish, and shellfish), milk and dairy products, eggs, and poultry. Iodine can also be found in plant foods, such as cereals and grains, its content depending on the amount of iodine in the soil in which food is grown, which varies widely between different countries [112]. The intake of iodine coming only from food sources for subjects following the MD is highly variable, mainly dependent on the intake of fish [113], with some reports suggesting that the MD alone could provide an insufficient intake of iodine [114]. Nevertheless, it can be suggested that a varied and balanced MD paired with regular iodized salt consumption would allow for sufficient intake of iodine without exposing subjects to the risk of an excessive iodine intake. It should also be highlighted how vegan/vegetarian diets, despite sharing with the MD the benefits of plant-derived foods, do not provide enough iodine and supplementation is often needed [115,116]. For these reasons, subjects following a vegan diet may be at risk of either deficiency (lack of animal products) or excess (consumption of vegan alternatives, such as seaweed) of iodine and related thyroid complications [116]. Changing dietary habits, with vegetarian diets and whole-salt use becoming increasingly popular among the general population, has led to the “re-emergence” of iodine deficiency in industrialized countries with deleterious effect on children’s health [117].
- Selenium
Selenium, a micronutrient contained in yeast and unprocessed cereals, has been long implicated in thyroid function and autoimmunity due to its antioxidant properties, and its involvement in immune function as well as in thyroid hormone synthesis and metabolism [118,119,120]. Indeed, selenium is an essential cofactor for several selenoproteins, including the antioxidant enzymes glutathione peroxidases (GPx) and thioredoxin reductases (TrxR), iodothyronine deiodinases (DIOs), and selenoprotein P, all of which are essential for proper thyroid function [120]. While even small amounts of selenium are sufficient for DIOs activity, a reduced selenium intake affects GPx expression and function, resulting in impaired antioxidant activity in thyrocytes [120]. For these reasons, selenium deficiency has been associated with thyroid dysfunction (mainly, in combination with ID) and increased risk of thyroid autoimmunity [121,122].
Although experimental studies have provided evidence in favor of a protective role of selenium against oxidative damage and apoptosis in thyrocytes [17,123,124], clinical trials aimed at evaluating the effects of selenium supplementation on AITDs provided sometimes conflicting and largely inconclusive results [125,126,127], suggesting that its impact on clinical outcomes is rather low, and selenium should not be routinely supplemented in individuals who are not deficient [128]. Nevertheless, an appropriate dietary intake of selenium is safe and useful to human health, the recommended need being 55 μg/day for healthy adults, 0.2 μg/kg/day for children, and 65–75 μg/day for pregnant and breastfeeding subjects [129].
Selenium is present in foods in two forms: inorganic (selenate and selenite) and organic (selenomethionine and selenocysteine) [130]. Typically, the main food sources of selenium are animal products, like seafoods, organ meats, eggs, dairy products (even fat-free or low-fat ones), and nuts. Plant-derived foods like grains and some fruits and vegetables also contain selenium, with amounts varying widely in relation to the selenium content in the soil and several other factors [130]. Once again, a varied and well balanced MD model meets the nutrient requirements of selenium since it includes grains (at least half whole grains), dairy products (mainly, low-fat ones like milk, yogurt, and some kind of cheese), a variety of protein foods (mostly fish, but also poultry, eggs, legumes), and nuts [131], even if evidence from the literature is not enough do draw conclusions [132].
- Iron and Zinc
Apart from its main function related with the transport of oxygen, iron is required for the synthesis of thyroid hormones, because the thyroid peroxidase is a heme-dependent enzyme [121,122,133]. For this reason, iron deficiency can be accompanied by a reduction in thyroid hormone synthesis and a subsequent increase in TSH levels, thereby enhancing the risk of developing thyroid disease [121,122,134]. Iron deficiency may occur as a consequence of both an inadequate dietary intake or malabsorption. Iron from food comes in two forms: heme and non-heme. Heme iron derives from hemoglobin, and it is present in animal foods, like red meat, fish, and poultry (meat, poultry, and seafood containing both heme and non-heme iron). Non-heme iron is found in plant foods, like whole grains, nuts, seeds, legumes, and leafy greens. Non-heme iron is also the form of iron added to iron-enriched and iron-fortified foods. Heme iron is better absorbed by the body than non-heme iron, and some dietary components can improve (vitamin C) or inhibit (bran fiber, phytates, and tannins) its absorption when taken in the same meal [135]. Also, iron malabsorption often occurs in patients suffering from HT due to associated autoimmune comorbidities (namely, celiac disease and atrophic gastritis) [9,121,122].
Like iron, zinc is also deeply involved in thyroid homeostasis, contributing to the synthesis and activation of thyroid hormones at several levels: by regulating deiodinases activity, TSH releasing hormone, and TSH production, as well as by modulating transcription factors involved in the synthesis of thyroid hormones [136,137]. Serum concentrations of zinc also influence serum concentrations of T3, T4, and TSH [137]. However, while specific populations (such as children with Down syndrome or patients at risk of severe malnutrition) appear to greatly benefit from zinc supplementation in terms of thyroid function, the role of zinc supplementation in the general population as a protective factor for thyroid autoimmunity has not been elucidated yet [138,139,140]. Nevertheless, an appropriate zinc nutritional status is fundamental for the maintenance of a normal thyroid function in HT patients [141,142], and an appropriate dietary intake is recommended. Dietary sources rich in zinc are meats, poultry, and seafood, but legumes and whole grains can also be good sources of this trace element, taking into account that they also contain phytates, reducing its absorption.
The MD is rich in zinc, since this micronutrient is abundant in seeds and whole-grain cereals. Moreover, animal products are not excluded in this dietary regimen, so that an appropriate intake of both zinc and iron is reached. In contrast, vegan and vegetarian diets may not provide enough iron and zinc, and the use of supplements or fortified foods may be needed [142,143].
- Vitamins
Vitamin B12 is essential for the appropriate functioning of the immune system and its deficiency is associated with an alteration in the methylation reactions leading to an increase in the levels of homocysteine in the body, which in turn favors immune dysfunction [144,145]. All foods of animal origin are good sources of vitamin B12, including meat, fish, eggs, dairy products, and poultry. As a consequence, highly restrictive plant-based regimens, like veganism and vegetarianism, are usually associated with vitamin B12 deficiency [144]. Besides dietary intake, malabsorption should be taken into account, mainly due to atrophic gastritis, celiac disease, and inflammatory bowel diseases, all autoimmune in etiology and frequently associated with AITD [9,144]. A recent study analyzed the levels of vitamin B12 in patients with AITD and found that they were significantly lower in patients than controls (p < 0.0001), and were inversely correlated with TPOAb levels. Moreover, HT patients with vitamin B12 deficit exhibited significantly higher mean values of TPOAb, suggesting that the determination of vitamin B12 status could be performed in HT patients as a diagnostic test with high sensitivity and good specificity [146].
Vitamin D is a fat-soluble steroid with multiple functions in the human body. Besides the well-known role in skeletal homeostasis, vitamin D acts as an immunomodulator in both innate and adaptive immune responses [147,148,149]. Moreover, its receptor is expressed in immune cells [147] as well as in several peripheral tissues, including the anterior pituitary [150] and the thyroid gland [151], suggesting a local action of vitamin D that may be relevant in the immune response. These findings are supported by the correlation between some polymorphisms of the vitamin D receptor gene and the occurrence of several autoimmune diseases, including HT [15,152,153]. In this light, vitamin D may act as a protective agent against autoimmunity, even if its role in autoimmune thyroiditis is not completely elucidated [154]. Clinical studies demonstrated that HT patients have lower serum levels of vitamin D than healthy controls [155], and vitamin D deficiency has been correlated with an increased risk of HT and also with higher levels of autoantibodies [156,157,158]. However, it is still unclear if this association is a pathological mechanism, a cause–effect relationship, or a mere “coincidence” due to the magnitude of hypovitaminosis D in the general health population [159]. Interestingly, different investigations proved that adequate levels of vitamin D were correlated with a reduction in the levels of TPO autoantibodies, suggesting a role of vitamin D supplementation as adjuvant of therapies [155,160,161]. Nevertheless, intervention trials showed only marginal effects on the reduction of TPO-antibody titers in treated HT subjects and failed to prove an effect of vitamin D supplementation on clinical outcomes [162]. In humans, vitamin D can be obtained from dietary sources or from UV-mediated synthesis in the epidermal layer of the skin. Few foods naturally contain significant amounts of vitamin D: cod liver oil and oily fish are rich in vitamin D, while butter, cream, and egg yolk contain only small amounts, as do human and cow’s milk [163]. Recent studies demonstrated that high adherence to the MD, mainly fish and olive oil consumption, are positively associated with serum levels of vitamin D, irrespective of anthropometric and lifestyle variables, skin phototype, and season [164,165].
6. Role of Food Contaminants
Lastly, different dietary choices could impact on the risk of thyroid autoimmunity through different exposure to environmental pollutants. Indeed, several compounds, classified as endocrine disruptors, can influence thyroid function and increase the risk of thyroid autoimmunity [166,167,168]. Xenobiotics can trigger thyroid autoimmunity via several mechanisms. They can affect factors like intestinal permeability, microbiota composition, or hormone homeostasis, all known to interact with the immune system. They can also induce epigenetic changes (such as DNA methylation, histone modifications, non-coding RNAs) in exposed cells. Finally, chemicals can act directly on thyrocyte and/or immune cells, by binding to receptors (for instance, the aryl hydrocarbon receptor AhR) or after uptake in the cells by pinocytosis, endocytosis, or diffusion. As a consequence, chemical exposures can lead to inflammation, increased oxidative stress, altered immune function (e.g., impaired Treg function, Th1/Th2 skewing, release of proinflammatory cytokine), cell apoptosis or death, and impaired function [166,167,169]. Mechanisms of molecular mimicry are also involved [169].
Food may be contaminated at several points along the way “from farm to fork” (Figure 3). Possible sources of contamination may be: use of antibiotics in livestock feed; pollutant accumulation in fishes and crops (for instance, heavy metals, pesticides, and herbicides); anthropogenic activities such as manufacturing and food processing (additives, preservatives); contaminants adsorbed into foods from packaging materials; and deposition of contaminants during cooking [170,171]. A deep analysis of these contamination sources is far from the aim of the present review. However, their understanding allows the opportunity to briefly discuss the risk of exposure to contaminants of different dietary patterns.
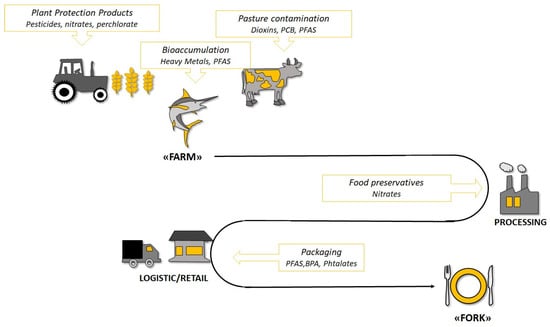
Figure 3.
Possible sources of endocrine disruptor exposure through food consumption in the “From Farm to Food” process. PFAS: Per- and polyfluoroalkyl substances; BPA: Bisphenol A; PCB: Polychlorinated biphenyl.
It could be hypothesized that the WD characterized by increased consumption of animal-derived (meat and fish) and processed foods may be associated with increased exposure to endocrine disruptors (additive, nitrites, antibiotics, phthalates, bisphenol, polyfluoroalkyl substances or PFAS, mercury, to mention a few), compared to the MD, typically characterized by reduced intake of industrial foods and meat, and by prevalent consumption of small oily fishes with few contaminants. Moreover, the MD is characterized by a high intake of fruits and vegetables rich in flavonoids that can promote the elimination of pollutants in tissues and/or counteract their harmful effects through different mechanisms, including ROS scavenging, chelation of metals, epigenetic up-regulation of detoxifying genes (codifying for receptors like AhR, enzymes like the cytochromes). On the other hand, persistent pollutants, like PFAS, polycyclic aromatic hydrocarbons (PAHs), and PCB, accumulate in water and soil and can contaminate plant foods, in addition to those released by food packaging. The same is true for pesticides and herbicides that may contaminate crops. For these reasons, data regarding the risk of exposure to pollutants through the MD are controversial. While some authors suggest that the MD could be protective toward exposure to pollutants [172], a study by Melough et al. demonstrated that different kinds of diets, even those considered healthier, like the MD, would be equally at risk for exposure to endocrine disruptors [173]. From this study, it emerged that most endocrine-disrupting chemicals had no association with the different dietary patterns, and recommended healthy diets were not more protective against pollutant exposures than other dietary models considered unhealthy [173]. Further studies are needed to identify effective strategies to reduce dietary exposure to potentially harmful pollutants.
7. Limitations
The present is a narrative review focused on the hypothesis that different dietary habits may influence the onset and progression of autoimmune thyroid disorders, and the Mediterranean diet may be protective against them. We analyzed the relevant data from the literature to provide the reader with updated information on this research question. A systematic review of the literature, as well as a metaanalysis in which the results of the studies are combined statistically, could provide a more comprehensive understanding of the state of the art and detect clinically meaningful differences between dietary habits, but studies on this topic are few and heterogeneous. Clearly, further studies are needed to better understand the role of dietary habits in AITDs.
The present review cannot evaluate in depth the complex interplay between diet, microbiota, and autoimmunity. This is a relevant topic that deserves a more in-depth and extensive discussion. There are a number of detailed, well-done reviews on this topic [41,103,104].
8. Conclusions
Even if further studies are needed to clarify the relationships between dietary habits and AITDs, current evidence from the literature suggests that a predominantly plant-based Mediterranean diet has a potentially protective effect against thyroid autoimmunity. Reducing the intake of animal proteins and fats and increasing those from vegetable sources may represent a useful lifestyle strategy for reducing the risk for thyroid autoimmunity.
Author Contributions
Conceptualization: R.M.R. and M.C.B.; Database search: R.M.R., M.C.B. and L.C.; Writing—original draft preparation, R.M.R., M.C.B. and L.C.; Writing—review and editing, R.M.R., S.H., M.M., M.R. and A.C.; Supervision, R.M.R., S.C. and S.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Caturegli, P.; De Remigis, A.; Rose, N.R. Hashimoto thyroiditis: Clinical and diagnostic criteria. Autoimmun. Rev. 2014, 13, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, R.M.; Giuffrida, G.; Campennì, A. Autoimmune endocrine diseases. Minerva Endocrinol. 2018, 43, 305–322. [Google Scholar] [CrossRef] [PubMed]
- Ajjan, R.A.; Weetman, A.P. The pathogenesis of Hashimoto’s thyroiditis: Further developments in our understanding. Horm. Metab. Res. 2015, 47, 702–710. [Google Scholar] [CrossRef] [PubMed]
- Effraimidis, G.; Wiersinga, W.M. Mechanisms in endocrinology: Autoimmune thyroid disease: Old and new players. Eur. J. Endocrinol. 2014, 170, R241–R252. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.J.; Hegedüs, L. Graves’ Disease. N. Engl. J. Med. 2016, 375, 1552–1565. [Google Scholar] [CrossRef] [PubMed]
- Bartalena, L.; Masiello, E.; Magri, F.; Veronesi, G.; Bianconi, E.; Zerbini, F.; Gaiti, M.; Spreafico, E.; Gallo, D.; Premoli, P.; et al. The phenotype of newly diagnosed Graves’ disease in Italy in recent years is milder than in the past: Results of a large observational longitudinal study. J. Endocrinol. Investig. 2016, 39, 1445–1451. [Google Scholar] [CrossRef] [PubMed]
- Vanderpump, M.P.; Tunbridge, W.M.; French, J.M.; Appleton, D.; Bates, D.; Clark, F.; Grimley Evans, J.; Hasan, D.M.; Rodgers, H.; Tunbridge, F.; et al. The incidence of thyroid disorders in the community: A twenty-year follow-up of the Whickham survey. Clin. Endocrinol. 1995, 43, 55–68. [Google Scholar] [CrossRef] [PubMed]
- McLeod, D.S.; Cooper, D.S. The incidence and prevalence of thyroid autoimmunity. Endocrine 2012, 42, 252–256. [Google Scholar] [CrossRef]
- Ruggeri, R.M.; Trimarchi, F.; Giuffrida, G.; Certo, R.; Cama, E.; Campenni, A.; Alibrandi, A.; De Luca, F.; Wasniewska, M. Autoimmune comorbidities in Hashimoto’s thyroiditis: Different patterns of association in adulthood and childhood/adolescence. Eur. J. Endocrinol. 2017, 176, 133–141. [Google Scholar] [CrossRef]
- Lerner, A.; Jeremias, P.; Matthias, T. The world incidence and prevalence of autoimmune diseases is increasing. Int. J. Celiac. Dis. 2015, 3, 151–155. [Google Scholar] [CrossRef]
- Selmi, C. The worldwide gradient of autoimmune conditions. Autoimmun. Rev. 2010, 9, A247–A250. [Google Scholar] [CrossRef] [PubMed]
- Brady, B.D.M. Autoimmune disease: A modern epidemic? Molecular mimicry, the hygiene hypothesis, stealth infections, and other examples of disconnect between medical research and the practice of clinical medicine. N. Engl. J. Med. 2014, 347, 911–920. [Google Scholar] [CrossRef]
- Okada, H.; Kuhn, C.; Feillet, H.; Bach, J.F. The ‘hygiene hypothesis’ for autoimmune and allergic diseases: An update. Clin. Exp. Immunol. 2010, 160, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Skaaby, T.; Husemoen, L.L.; Thuesen, B.H.; Linneberg, A. Prospective population-based study of the association between vitamin D status and incidence of autoimmune disease. Endocrine 2015, 50, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Giovinazzo, S.; Vicchio, T.M.; Certo, R.; Alibrandi, A.; Palmieri, O.; Campennì, A.; Cannavò, S.; Trimarchi, F.; Ruggeri, R.M. Vitamin D receptor gene poly0morphisms/haplotypes and serum 25(OH)D3 levels in Hashimoto’s thyroiditis. Endocrine 2017, 55, 599–606. [Google Scholar] [CrossRef]
- Ruggeri, R.M.; Trimarchi, F. Iodine nutrition optimization: Are there risks for thyroid autoimmunity? J. Endocrinol. Investig. 2021, 44, 1827–1835. [Google Scholar] [CrossRef]
- Ruggeri, R.M.; D’Ascola, A.; Vicchio, T.M.; Campo, S.; Gianì, F.; Giovinazzo, S.; Frasca, F.; Cannavò, S.; Campennì, A.; Trimarchi, F. Selenium exerts protective effects against oxidative stress and cell damage in human thyrocytes and fibroblasts. Endocrine 2020, 68, 151–162. [Google Scholar] [CrossRef]
- Langer, P. The impacts of organochlorines and other persistent pollutants on thyroid and metabolic health. Front. Neuroendocrinol. 2010, 31, 497–518. [Google Scholar] [CrossRef]
- Brantley, P.J.; Myers, V.H.; Roy, H.J. Environmental and lifestyle influences on obesity. J. La. State Med. Soc. 2005, 157, S19–S27. [Google Scholar]
- Procaccini, C.; Carbone, F.; Galgani, M.; La Rocca, C.; De Rosa, V.; Cassano, S.; Matarese, G. Obesity and susceptibility to autoimmune diseases. Expert Rev. Clin. Immunol. 2011, 7, 287–294. [Google Scholar] [CrossRef]
- Manzel, A.; Muller, D.N.; Hafler, D.A.; Erdman, S.E.; Linker, R.A.; Kleinewietfeld, M. Role of “Western diet” in inflammatory autoimmune diseases. Curr. Allergy Asthma Rep. 2014, 14, 404. [Google Scholar] [CrossRef] [PubMed]
- de Castro, M.M.; Pascoal, L.B.; Steigleder, K.M.; Siqueira, B.P.; Corona, L.P.; Ayrizono, M.L.S.; Milanski, M.; Leal, R.F. Role of diet and nutrition in inflammatory bowel disease. World J. Exp. Med. 2021, 11, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Philippou, E.; Nikiphorou, E. Are we really what we eat? Nutrition and its role in the onset of rheumatoid arthritis. Autoimmun. Rev. 2018, 17, 1074–1077. [Google Scholar] [CrossRef]
- Alwarith, J.; Kahleova, H.; Rembert, E.; Yonas, W.; Dort, S.; Calcagno, M.; Alwarith, J.; Kahleova, H.; Rembert, E.; Yonas, W.; et al. Nutrition interventions in rheumatoid arthritis: The potential use of plant-based diets. A review. Front. Nutr. 2019, 6, 141. [Google Scholar] [CrossRef] [PubMed]
- Gioia, C.; Lucchino, B.; Tarsitano, M.G.; Iannuccelli, C.; Di Franco, M. Dietary habits and nutrition in rheumatoid arthritis: Can diet influence disease development and clinical manifestations? Nutrients 2020, 12, 1456. [Google Scholar] [CrossRef] [PubMed]
- Ricketts, J.R.; Rothe, M.J.; Grant-Kels, J.M. Nutrition and psoriasis. Clin. Dermatol. 2010, 28, 615–626. [Google Scholar] [CrossRef]
- Monteiro, C.A.; Cannon, G.; Levy, R.B.; Moubarac, J.C.; Louzada, M.L.C.; Rauber, F.; Khandpur, N.; Cediel, G.; Neri, D.; Martinez-Steele, E.; et al. Ultra-processed foods: What they are and how to identify them. Public Health Nutr. 2019, 22, 936–941. [Google Scholar] [CrossRef]
- Martínez Steele, E.; Popkin, B.M.; Swinburn, B.; Monteiro, C.A. The share of ultra-processed foods and the overall nutritional quality of diets in the US: Evidence from a nationally representative cross-sectional study. Popul. Health Metr. 2017, 15, 6. [Google Scholar] [CrossRef]
- Monteiro, C.A.; Moubarac, J.C.; Levy, R.B.; Canella, D.S.; Da Costa Louzada, M.L.; Cannon, G. Household availability of ultra-processed foods and obesity in nineteen European countries. Public Health Nutr. 2018, 21, 18–26. [Google Scholar] [CrossRef]
- Mazzucca, C.B.; Raineri, D.; Cappellano, G.; Chiocchetti, A. How to Tackle the Relationship between Autoimmune Diseases and Diet: Well Begun Is Half-Done. Nutrients 2021, 13, 3956. [Google Scholar] [CrossRef]
- Christ, A.; Lauterbach, M.; Latz, E. Western Diet and the immune system: An inflammatory connection. Immunity 2019, 51, 794–811. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Rezaei, N. (Eds.) Nutrition and Immunity; Springer Nature: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- McCord, J.M. Human disease, free radicals, and the oxidant/antioxidant balance. Clin. Biochem. 1993, 26, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, R.M.; Vicchio, T.M.; Cristani, M.; Certo, R.; Caccamo, D.; Alibrandi, A.; Giovinazzo, S.; Saija, A.; Campennì, A.; Trimarchi, F.; et al. Oxidative stress and advanced glycation end products in Hashimoto’s thyroiditis. Thyroid 2016, 4, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, R.M.; Cristani, M.T.; Crupi, F.; Molonia, M.S.; Burduja, N.; Alibrandi, A.; Campennì, A.; Cannavò, S. Evaluation of paraoxonase activity and association with serum advanced glycation end products as reliable markers of oxidative stress in Hashimoto’s thyroiditis. Minerva Endocrinol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, R.M.; Giovinazzo, S.; Barbalace, M.C.; Cristani, M.; Alibrandi, A.; Vicchio, T.M.; Giuffrida, G.; Aguennouz, M.H.; Malaguti, M.; Angeloni, C.; et al. Influence of Dietary Habits on Oxidative Stress Markers in Hashimoto’s Thyroiditis. Thyroid 2021, 31, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Merra, G.; Noce, A.; Marrone, G.; Cintoni, M.; Tarsitano, M.G.; Capacci, A.; De Lorenzo, A. Influence of Mediterranean Diet on Human Gut Microbiota. Nutrients 2021, 13, 7. [Google Scholar] [CrossRef]
- Requena, T.; Martínez-Cuesta, M.C.; Peláez, C. Diet and microbiota linked in health and disease. Food Funct. 2018, 9, 688–704. [Google Scholar] [CrossRef]
- Rinninella, E.; Cintoni, M.; Raoul, P.; Lopetuso, L.R.; Scaldaferri, F.; Pulcini, G.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. Food Components and Dietary Habits: Keys for a Healthy Gut Microbiota Composition. Nutrients 2019, 11, 2393. [Google Scholar] [CrossRef]
- Barrea, L.; Muscogiuri, G.; Frias-Toral, E.; Laudisio, D.; Pugliese, G.; Castellucci, B.; Garcia-Velasquez, E.; Savastano, S.; Colao, A. Nutrition and immune system: From the Mediterranean diet to dietary supplementary through the microbiota. Crit. Rev. Food Sci. Nutr. 2021, 61, 3066–3090. [Google Scholar] [CrossRef]
- Lerner, A.; Matthias, T. Changes in intestinal tight junction permeability associated with industrial food additives explain the rising incidence of autoimmune disease. Autoimmun. Rev. 2015, 14, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Keys, A.; Menotti, A.; Aravanis, C.; Blackburn, H.; Djordevic, B.S.; Buzina, R.; Dontas, A.S.; Fidanza, F.; Karvonen, M.J.; Kimura, N. The seven countries study: 2289 deaths in 15 years. Prev. Med. 1984, 13, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Angeloni, C.; Malaguti, M.; Barbalace, M.C.; Hrelia, S. Bioactivity of Olive Oil Phenols in Neuroprotection. Int. J. Mol. Sci. 2017, 18, 2230. [Google Scholar] [CrossRef] [PubMed]
- UNESCO. Available online: https://ich.unesco.org/en/RL/mediterranean-diet-00884 (accessed on 3 August 2023).
- Schwingshackl, L.; Morze, J.; Hoffmann, G. Mediterranean diet and health status: Active ingredients and pharmacological mechanisms. Br. J. Pharmacol. 2020, 177, 1241–1257. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, B.; Kapoor, D.; Gautam, S.; Singh, R.; Bhardwaj, S. Dietary Polyunsaturated Fatty Acids (PUFAs): Uses and Potential Health Benefits. Curr. Nutr. Rep. 2021, 10, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Omega-3 fatty acids and inflammatory processes: From molecules to man. Biochem. Soc. Trans. 2017, 45, 1105–1115. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, D.; Gross, M.D.; Tapsell, L.C. Food synergy: An operational concept for understanding nutrition. Am. J. Clin. Nutr. 2009, 89, 1543S–1548S. [Google Scholar] [CrossRef] [PubMed]
- Hrelia, S.; Barbalace, M.C.; Cannavò, S.; Ruggeri, R.M. Commentary: Fish and the thyroid: A Janus Bifrons relationship caused by pollutants and the omega-3 polyunsaturated fatty acids. Front. Endocrinol. 2023, 14, 1138245. [Google Scholar] [CrossRef]
- Di Daniele, N.; Noce, A.; Vidiri, M.F.; Moriconi, E.; Marrone, G.; Annicchiarico-Petruzzelli, M.; D’Urso, G.; Tesauro, M.; Rovella, V.; De Lorenzo, A. Impact of Mediterranean diet on metabolic syndrome, cancer and longevity. Oncotarget 2017, 8, 8947–8979. [Google Scholar] [CrossRef]
- Kesse-Guyot, E.; Ahluwalia, N.; Lassale, C.; Hercberg, S.; Fezeu, L.; Lairon, D. Adherence to Mediterranean diet reduces the risk of metabolic syndrome: A 6-year prospective study. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 677–683. [Google Scholar] [CrossRef]
- Salas-Salvadó, J.; Fernández-Ballart, J.; Ros, E.; Martínez-González, M.A.; Fitó, M.; Estruch, R.; Corella, D.; Fiol, M.; Gómez-Gracia, E.; Arós, F.; et al. Effect of a Mediterranean diet supplemented with nuts on metabolic syndrome status: One-year results of the PREDIMED randomized trial. Arch. Intern. Med. 2008, 168, 2449–2458. [Google Scholar] [CrossRef] [PubMed]
- Romaguera, D.; Guevara, M.; Norat, T.; Langenberg, C.; Forouhi, N.G.; Sharp, S.; Slimani, N.; Schulze, M.B.; Buijsse, B.; Buckland, G.; et al. Mediterranean diet and type 2 diabetes risk in the European Prospective Investigation into Cancer and Nutrition (EPIC) study: The InterAct project. Diabetes Care 2011, 34, 1913–1918. [Google Scholar] [CrossRef]
- Salas-Salvadó, J.; Bulló, M.; Babio, N.; Martínez-González, M.; Ibarrola-Jurado, N.; Basora, J.; Estruch, R.; Covas, M.I.; Corella, D.; Arós, F.; et al. Reduction in the Incidence of Type 2 Diabetes with the Mediterranean Diet: Results of the PREDIMED-Reus nutrition intervention randomized trial. Diabetes Care 2011, 34, 14–19, Erratum in Diabetes Care 2018, 41, 2259–2260. [Google Scholar] [CrossRef] [PubMed]
- Laudisio, D.; Barrea, L.; Muscogiuri, G.; Annunziata, G.; Colao, A.; Savastano, S. Breast cancer prevention in premenopausal women: Role of the Mediterranean diet and its components. Nutr. Res. Rev. 2020, 33, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Maruca, A.; Catalano, R.; Bagetta, D.; Mesiti, F.; Ambrosio, F.A.; Romeo, I.; Moraca, F.; Rocca, R.; Ortuso, F.; Artese, A.; et al. The Mediterranean Diet as source of bioactive compounds with multi-targeting anti-cancer profile. Eur. J. Med. Chem. 2019, 181, 111579. [Google Scholar] [CrossRef] [PubMed]
- de la Rubia Ortí, J.E.; García-Pardo, M.P.; Drehmer, E.; Sancho Cantus, D.; Julián Rochina, M.; Aguilar, M.A.; Hu Yang, I. Improvement of Main Cognitive Functions in Patients with Alzheimer’s Disease after Treatment with Coconut Oil Enriched Mediterranean Diet: A Pilot Study. J. Alzheimers Dis. 2018, 65, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Gardener, H.; Caunca, M.R. Mediterranean Diet in Preventing Neurodegenerative Diseases. Curr. Nutr. Rep. 2018, 7, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Santangelo, C.; Vari, R.; Scazzocchio, B.; De Sanctis, P.; Giovannini, C.; D’Archivio, M.; Masella, R. Anti-inflammatory Activity of Extra Virgin Olive Oil Polyphenols: Which Role in the Prevention and Treatment of Immune-Mediated Inflammatory Diseases? Endocr. Metab. Immune Disord. Drug Targets 2018, 18, 36–50. [Google Scholar] [CrossRef]
- Li, X.; Bi, X.; Wang, S.; Zhang, Z.; Li, F.; Zhao, A.Z. Therapeutic Potential of ω-3 Polyunsaturated Fatty Acids in Human Autoimmune Diseases. Front. Immunol. 2019, 10, 2241. [Google Scholar] [CrossRef]
- Burkitt, D.P. Some diseases characteristic of modern Western civilization. Br. Med. J. 1973, 1, 274–278. [Google Scholar] [CrossRef]
- Trowell, H.C.; Burkitt, D.P. Western Diseases, Their Emergence and Prevention; Harvard University Press: Cambridge, UK, 1981. [Google Scholar]
- McCarty, M.F. Upregulation of lymphocyte apoptosis as a strategy for preventing and treating autoimmune disorders: A role for whole-food vegan diets, fish oil and dopamine agonists. Med. Hypotheses. 2001, 57, 258–275. [Google Scholar] [CrossRef] [PubMed]
- McCarty, M.F. GCN2 and FGF21 are likely mediators of the protection from cancer, autoimmunity, obesity, and diabetes afforded by vegan diets. Med. Hypotheses. 2014, 83, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Limketkai, B.N.; Hamideh, M.; Shah, R.; Sauk, J.S.; Jaffe, N. Dietary Patterns and Their Association with Symptoms Activity in Inflammatory Bowel Diseases. Inflamm. Bowel Dis. 2022, 28, 1627–1636. [Google Scholar] [CrossRef] [PubMed]
- Agus, A.; Denizot, J.; Thévenot, J.; Martinez-Medina, M.; Massier, S.; Sauvanet, P.; Bernalier-Donadille, A.; Denis, S.; Hofman, P.; Bonnet, R.; et al. Western diet induces a shift in microbiota composition enhancing susceptibility to Adherent-Invasive E. coli infection and intestinal inflammation. Sci. Rep. 2016, 6, 19032. [Google Scholar] [CrossRef]
- Stoiloudis, P.; Kesidou, E.; Bakirtzis, C.; Sintila, S.-A.; Konstantinidou, N.; Boziki, M.; Grigoriadis, N. The Role of Diet and Interventions on Multiple Sclerosis: A Review. Nutrients 2022, 14, 1150. [Google Scholar] [CrossRef] [PubMed]
- Kanda, N.; Hoashi, T.; Saeki, H. Nutrition and Psoriasis. Int. J. Mol. Sci. 2020, 21, 5405. [Google Scholar] [CrossRef] [PubMed]
- Virtanen, S.M. Dietary factors in the development of type 1 diabetes. Pediatr. Diabetes 2016, 22, 49–55. [Google Scholar] [CrossRef]
- Norris, J.M.; Yin, X.; Lamb, M.M.; Barriga, K.; Seifert, J.; Hoffman, M.; Orton, H.D.; Barón, A.E.; Clare-Salzler, M.; Chase, H.P.; et al. Omega-3 polyunsaturated fatty acid intake and islet autoimmunity in children at increased risk for type 1 diabetes. JAMA 2007, 298, 1420–1428. [Google Scholar] [CrossRef]
- Tonstad, S.; Nathan, E.; Oda, K.; Fraser, G. Prevalence of hyperthyroidism according to type of vegetarian diet. Public Health Nutr. 2015, 18, 1482–1487. [Google Scholar] [CrossRef]
- Zupo, R.; Castellana, F.; Panza, F.; Lampignano, L.; Murro, I.; Di Noia, C.; Triggiani, V.; Giannelli, G.; Sardone, R.; De Pergola, G. Adherence to a Mediterranean Diet and Thyroid Function in Obesity: A Cross-Sectional Apulian Survey. Nutrients 2020, 12, 3173. [Google Scholar] [CrossRef]
- Liu, N.; Ma, F.; Feng, Y.; Ma, X. The Association between the Dietary Inflammatory Index and Thyroid Function in U.S. Adult Males. Nutrients 2021, 13, 3330. [Google Scholar] [CrossRef] [PubMed]
- Kaličanin, D.; Brčić, L.; Ljubetić, K.; Barić, A.; Gračan, S.; Brekalo, M.; Torlak Lovrić, V.; Kolčić, I.; Polašek, O.; Zemunik, T.; et al. Differences in food consumption between patients with Hashimoto’s thyroiditis and healthy individuals. Sci. Rep. 2020, 10, 10670. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.S.; Zhao, Y.F.; Song, Y.F.; Xu, C.; Yang, J.M.; Xuan, S.M.; Yan, H.L.; Yu, C.X.; Zhao, M.; Xu, J.; et al. Dietary high-fat lard intake induces thyroid dysfunction and abnormal morphology in rats. Acta Pharmacol. Sin. 2014, 35, 1411–1420. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, W.; Shao, S.; Xu, G.; Song, Y.; Xu, C.; Gao, L.; Hu, C.; Zhao, J. A High-Fat Diet Rich in Saturated and Mono-Unsaturated Fatty Acids Induces Disturbance of Thyroid Lipid Profile and Hypothyroxinemia in Male Rats. Mol. Nutr. Food Res. 2018, 62, e1700599. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Kong, Y.; Zeng, L.; Wan, Q.; Hu, J.; Cai, Y. Effects of high-fat diet on thyroid autoimmunity in the female rat. BMC Endocr. Disord. 2022, 22, 179. [Google Scholar] [CrossRef] [PubMed]
- Bahadoran, Z.; Mirmiran, P.; Ghasemi, A.; Kabir, A.; Azizi, F.; Hadaegh, F. Is dietary nitrate/nitrite exposure a risk factor for development of thyroid abnormality? A systematic review and meta-analysis. Nitric Oxide 2015, 47, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Benvenga, S.; Famà, F.; Perdichizzi, L.G.; Antonelli, A.; Brenta, G.; Vermiglio, F.; Moleti, M. Fish and the Thyroid: A Janus Bifrons Relationship Caused by Pollutants and the Omega-3 Polyunsaturated Fatty Acids. Front. Endocrinol. 2022, 13, 891233. [Google Scholar] [CrossRef]
- Saini, R.K.; Keum, Y.S. Omega-3 and omega-6 polyunsaturated fatty acids: Dietary sources, metabolism, and significance—A review. Life Sci. 2018, 203, 255–267. [Google Scholar] [CrossRef]
- Marklund, M.; Wu, J.H.; Imamura, F.; Del Gobbo, L.C.; Fretts, A.; De Goede, J.; Shi, P.; Tintle, N.; Wennberg, M.; Aslibekyan, S.; et al. Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Fatty Acids and Outcomes Research Consortium (FORCE). Biomarkers of Dietary Omega-6 Fatty Acids and Incident Cardiovascular Disease and Mortality. Circulation 2019, 139, 2422–2436. [Google Scholar] [CrossRef]
- Serhan, C.N.; Petasis, N.A. Resolvins and protectins in inflammation resolution. Chem. Rev. 2011, 111, 5922–5943. [Google Scholar] [CrossRef]
- Serhan, C.N.; Chiang, N. Endogenous pro-resolving and anti-inflammatory lipid mediators: A new pharmacologic genus. Br. J. Pharmacol. 2008, 153, S200–S215. [Google Scholar] [CrossRef] [PubMed]
- Neuhofer, A.; Zeyda, M.; Mascher, D.; Itariu, B.K.; Murano, I.; Leitner, L.; Hochbrugger, E.E.; Fraisl, P.; Cinti, S.; Serhan, C.N.; et al. Impaired local production of proresolving lipid mediators in obesity and 17-HDHA as a potential treatment for obesity-associated inflammation. Diabetes 2013, 62, 1945–1956. [Google Scholar] [CrossRef] [PubMed]
- López-Miranda, J.; Pérez-Jiménez, F.; Ros, E.; De Caterina, R.; Badimón, L.; Covas, M.I.; Escrich, E.; Ordovás, J.M.; Soriguer, F.; Abiá, R.; et al. Olive oil and health: Summary of the II international conference on olive oil and health consensus report, Jaén and Córdoba (Spain) 2008. Nutr. Metab. Cardiovasc. Dis. 2010, 20, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Conde, C.; Escribano, B.M.; Luque, E.; Feijóo, M.; Caballero-Villarraso, J.; Valdelvira, M.E.; Ochoa-Sepúlveda, J.J.; Lillo, R.; Paz, E.; Santamaría, A.; et al. Extra-Virgin Olive Oil Modifies the Changes Induced in Non-Nervous Organs and Tissues by Experimental Autoimmune Encephalomyelitis Models. Nutrients 2019, 11, 2448. [Google Scholar] [CrossRef] [PubMed]
- Montoya, T.; Sánchez-Hidalgo, M.; Castejón, M.L.; Rosillo, M.Á.; González-Benjumea, A.; Alarcón-de-la-Lastra, C. Dietary Oleocanthal Supplementation Prevents Inflammation and Oxidative Stress in Collagen-Induced Arthritis in Mice. Antioxidants 2021, 10, 650. [Google Scholar] [CrossRef] [PubMed]
- Aparicio-Soto, M.; Montserrat-de la Paz, S.; Sanchez-Hidalgo, M.; Cardeno, A.; Bermudez, B.; Muriana, F.; Alarcon-de-la-Lastra, C. Virgin olive oil and its phenol fraction modulate monocyte/macrophage functionality: A potential therapeutic strategy in the treatment of systemic lupus erythematosus. Br. J. Nutr. 2018, 120, 681–692. [Google Scholar] [CrossRef] [PubMed]
- Aparicio-Soto, M.; Sanchez-Hidalgo, M.; Cardeno, A.; Rosillo, M.Á.; Sánchez-Fidalgo, S.; Utrilla, J.; Martín-Lacave, I.; Alarcon-de-la-Lastra, C. Dietary extra virgin olive oil attenuates kidney injury in pristane-induced SLE model via activation of HO-1/Nrf-2 antioxidant pathway and suppression of JAK/STAT, NF-κB and MAPK activation. J. Nutr. Biochem. 2016, 27, 278–288. [Google Scholar] [CrossRef]
- Pang, K.L.; Lumintang, J.N.; Chin, K.-Y. Thyroid-Modulating Activities of Olive and Its Polyphenols: A Systematic Review. Nutrients 2021, 13, 529. [Google Scholar] [CrossRef]
- Ditano-Vázquez, P.; Torres-Peña, J.D.; Galeano-Valle, F.; Pérez-Caballero, A.I.; Demelo-Rodríguez, P.; Lopez-Miranda, J.; Katsiki, N.; Delgado-Lista, J.; Alvarez-Sala-Walther, L.A. The fluid aspect of the Mediterranean diet in the prevention and management of cardiovascular disease and diabetes: The role of polyphenol content in moderate consumption of wine and olive oil. Nutrients 2019, 11, 2833. [Google Scholar] [CrossRef]
- Blanquer-Rosselló, M.; Hernández-López, R.; Roca, P.; Oliver, J.; Valle, A. Resveratrol induces mitochondrial respiration and apoptosis in SW620 colon cancer cells. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 431–440. [Google Scholar] [CrossRef]
- Kalantari, H.; Das, D. Physiological effects of resveratrol. BioFactors 2010, 36, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.; Kantham, S.; Rao, V.; Palanivelu, M.; Pham, H.; Shaw, P.; McGeary, R.P.; Ross, B.P. Metal chelation, radical scavenging and inhibition of Aβ42 fibrillation by food constituents in relation to Alzheimer’s disease. Food Chem. 2016, 199, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Martín, A.R.; Villegas, I.; La Casa, C.; de La Lastra, C.A. Resveratrol, a polyphenol found in grapes, suppresses oxidative damage and stimulates apoptosis during early colonic inflammation in rats. Biochem. Pharmacol. 2004, 67, 1399–1410. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.H.; Hsu, L.F.; Lee, C.W.; Chiang, Y.C.; Lee, M.H.; How, J.M.; Wu, C.M.; Huang, C.L.; Lee, I.T. Resveratrol inhibits urban particulate matter-induced COX-2/PGE2 release in human fibroblast-like synoviocytes via the inhibition of activation of NADPH oxidase/ROS/NF-κB. Int. J. Biochem. Cell Biol. 2017, 88, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Chen, J.W.; Gao, J.S.; Li, L.; Xie, X. Resveratrol inhibits TNF-a-induced IL-1b, MMP-3 production in human rheumatoid arthritis fibroblast-like synoviocytes via modulation of PI3kinase/Akt pathway. Rheumatol. Int. 2013, 33, 1829–1835. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.M.; Chien, A.; Jialal, I.; Devaraj, S. Resveratrol up-regulates SIRT1 and inhibits cellular oxidative stress in the diabetic milieu: Mechanistic insights. J. Nutr. Biochem. 2012, 23, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Carlé, A.; Pedersen, I.B.; Knudsen, N.; Perrild, H.; Ovesen, L.; Rasmussen, L.B.; Jørgensen, T.; Laurberg, P. Moderate alcohol consumption may protect against overt autoimmune hypothyroidism: A population-based case-control study. Eur. J. Endocrinol. 2012, 167, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Santos-Buelga, C.; González-Manzano, S.; González-Paramás, A.M. Wine, Polyphenols, and Mediterranean Diets. What Else Is There to Say? Molecules 2021, 26, 5537. [Google Scholar] [CrossRef] [PubMed]
- Virili, C.; Stramazzo, I.; Centanni, M. Gut microbiome and thyroid autoimmunity. Best Pract. Res. Clin. Endocrinol. Metab. 2021, 35, 101506. [Google Scholar] [CrossRef]
- García-Montero, C.; Fraile-Martínez, O.; Gómez-Lahoz, A.M.; Pekarek, L.; Castellanos, A.J.; Noguerales-Fraguas, F.; Coca, S.; Guijarro, L.G.; García-Honduvilla, N.; Asúnsolo, A.; et al. Nutritional Components in Western Diet Versus Mediterranean Diet at the Gut Microbiota–Immune System Interplay. Implications for Health and Disease. Nutrients 2021, 13, 699. [Google Scholar] [CrossRef]
- Zimmermann, M.B. The role of iodine in human growth and development. Semin. Cell Dev. Biol. 2011, 22, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.B.; Jooste, P.L.; Pandav, C.S. Iodine-deficiency disorders. Lancet 2008, 372, 1251–1262. [Google Scholar] [CrossRef] [PubMed]
- Leung, A.M.; Pearce, E.N.; Braverman, L.E. Iodine nutrition in pregnancy and lactation. Endocrinol. Metab. Clin. N. Am. 2011, 40, 765–777. [Google Scholar] [CrossRef] [PubMed]
- Bath, S.C.; Steer, C.D.; Golding, J.; Emmett, P.; Rayman, M.P. Effect of inadequate iodine status in UK pregnant women on cognitive outcomes in their children: Results from the Avon Longitudinal Study of Parents and Children (ALSPAC). Lancet 2013, 382, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Rotondi, M.; Amato, G.; Biondi, B.; Mazziotti, G.; Del Buono, A.; Rotonda Nicchio, M.; Balzano, S.; Bellastella, A.; Glinoer, D.; Carella, C. Parity as a thyroid size-determining factor in areas with moderate iodine deficiency. J. Clin. Endocrinol. Metab. 2000, 85, 4534–4537. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.B. The remarkable impact of iodisation programmes on global public health. Proc. Nutr. Soc. 2023, 82, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Farebrother, J.; Zimmermann, M.B.; Andersson, M. Excess iodine intake: Sources, assessment, and effects on thyroid function. Ann. N. Y. Acad. Sci. 2019, 1446, 44–65. [Google Scholar] [CrossRef] [PubMed]
- Murai, U.; Yamagishi, K.; Kishida, R.; Iso, H. Impact of seaweed intake on health. Eur. J. Clin. Nutr. 2021, 75, 877–889. [Google Scholar] [CrossRef]
- World Health Organization. Assessment of Iodine Deficiency Disorders and Monitoring Their Elimination: A Guide for Programme Managers, 3rd ed.; World Health Organization: Geneva, Switzerland, 2007. [Google Scholar]
- Abdelhamid, A.; Jennings, A.; Hayhoe, R.P.G.; Awuzudike, V.E.; Welch, A.A. High variability of food and nutrient intake exists across the Mediterranean Dietary Pattern—A systematic review. Food Sci. Nutr. 2020, 8, 4907–4918. [Google Scholar] [CrossRef]
- Eveleigh, E.R.; Coneyworth, L.J.; Avery, A.; Welham, S.J.M. Vegans, Vegetarians, and Omnivores: How Does Dietary Choice Influence Iodine Intake? A Systematic Review. Nutrients 2020, 12, 1606. [Google Scholar] [CrossRef]
- Bracci, E.L.; Keogh, J.B.; Milte, R.; Murphy, K.J. A comparison of dietary quality and nutritional adequacy of popular energy-restricted diets against the Australian Guide to Healthy Eating and the Mediterranean Diet. Br. J. Nutr. 2022, 128, 1357–1370. [Google Scholar] [CrossRef] [PubMed]
- Fields, C.; Borak, J. Iodine Deficiency in Vegetarian and Vegan Diets: Evidence-Based Review of the World’s Literature on Iodine Content in Vegetarian Diets. In Comprehensive Handbook of Iodine, 1st ed.; Elsevier Science Publishing Co., Ltd.: Amsterdam, The Netherlands, 2009; pp. 521–531. [Google Scholar]
- Rayman, M.P.; Bath, S.C. The new emergence of iodine deficiency in the UK: Consequences for child neurodevelopment. Ann. Clin. Biochem. 2015, 52, 705–708. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef] [PubMed]
- Avery, J.C.; Hoffmann, P.R. Selenium, Selenoproteins, and Immunity. Nutrients 2018, 10, 1203. [Google Scholar] [CrossRef] [PubMed]
- Köhrle, J. Selenium and the thyroid. Curr. Opin. Endocrinol. Diabetes Obes. 2015, 22, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Rayman, M.P. Multiple nutritional factors and the risk of Hashimoto’s thyroiditis. Thyroid 2017, 27, 597–610. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P. Multiple nutritional factors and thyroid disease, with particular reference to autoimmune thyroid disease. Proc. Nutr. Soc. 2019, 78, 34–44. [Google Scholar] [CrossRef]
- Nettore, I.C.; De Nisco, E.; Desiderio, S.; Passaro, C.; Maione, L.; Negri, M.; Albano, L.; Pivonello, R.; Pivonello, C.; Portella, G.; et al. Selenium supplementation modulates apoptotic processes in thyroid follicular cells. Biofactors 2017, 43, 415–423. [Google Scholar] [CrossRef]
- Wang, W.; Xue, H.; Li, Y.; Hou, X.; Fan, C.; Wang, H.; Zhang, H.; Shan, Z.; Teng, W. Effects of selenium supplementation on spontaneous autoimmune thyroiditis in NOD.H-2h4 mice. Thyroid 2015, 25, 1137–1144. [Google Scholar] [CrossRef]
- Negro, R.; Greco, G.; Mangieri, T.; Pezzarossa, A.; Dazzi, D.; Hassan, H. The influence of selenium supplementation on postpartum thyroid status in pregnant women with thyroid peroxidase autoantibodies. J. Clin. Endocrinol. Metab. 2007, 92, 1263–1268. [Google Scholar] [CrossRef]
- Mantovani, G.; Isidori, A.M.; Moretti, C.; Di Dato, C.; Greco, E.; Ciolli, P.; Bonomi, M.; Petrone, L.; Fumarola, A.; Campagna, G.; et al. Selenium supplementation in the management of thyroid autoimmunity during pregnancy: Results of the “SERENA study”, a randomized, double-blind, placebo-controlled trial. Endocrine 2019, 6, 542–550. [Google Scholar] [CrossRef]
- Esposito, D.; Rotondi, M.; Accardo, G.; Vallone, G.; Conzo, G.; Docimo, G.; Selvaggi, F.; Cappelli, C.; Chiovato, L.; Giugliano, D.; et al. Influence of short-term selenium supplementation on the natural course of Hashimoto’s thyroiditis: Clinical results of a blinded placebo-controlled randomized prospective trial. J. Endocrinol. Investig. 2017, 40, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-S.; Liang, S.-S.; Ren, J.-J.; Wang, Z.-Y.; Deng, X.-X.; Liu, W.-D.; Yan, Y.-L.; Song, G.-H.; Li, X.-X. The Effects of Selenium Supplementation in the Treatment of Autoimmune Thyroiditis: An Overview of Systematic Reviews. Nutrients 2023, 15, 3194. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Zhao, C.; Hu, H.; Yin, S. Food Sources of Selenium and Its Relationship with Chronic Diseases. Nutrients 2021, 13, 1739. [Google Scholar] [CrossRef] [PubMed]
- Terry, E.N.; Diamond, A.M. Selenium. In Present Knowledge in Nutrition, 10th ed.; Erdman, J.W., Macdonald, I.A., Zeisel, S.H., Eds.; Wiley-Blackwell: Washington, DC, USA, 2012; pp. 568–587. [Google Scholar]
- Simopoulos, A.P. The traditional diet of Greece and cancer. Eur. J. Cancer Prev. 2004, 13, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Jennings, A.; Tang, J.; Gillings, R.; Perfecto, A.; Dutton, J.; Speakman, J.; Fraser, W.D.; Nicoletti, C.; Berendsen, A.A.M.; de Groot, L.C.P.G.M.M.; et al. Changing from a Western to a Mediterranean-style diet does not affect iron or selenium status: Results of the New Dietary Strategies Addressing the Specific Needs of the Elderly Population for Healthy Aging in Europe (NU-AGE) 1-year randomized clinical trial in elderly Europeans. Am. J. Clin. Nutr. 2020, 111, 98–109. [Google Scholar] [CrossRef] [PubMed]
- Briguglio, M.; Hrelia, S.; Malaguti, M.; Lombardi, G.; Riso, P.; Porrini, M.; Perazzo, P.; Banfi, G. The Central Role of Iron in Human Nutrition: From Folk to Contemporary Medicine. Nutrients 2020, 12, 1761. [Google Scholar] [CrossRef] [PubMed]
- Khatiwada, S.; Gelal, B.; Baral, N.; Lamsal, M. Association between iron status and thyroid function in Nepalese children. Thyroid Res. 2016, 9, 2. [Google Scholar] [CrossRef] [PubMed]
- National Institutes of Health Office of Dietary Supplements: Iron Fact Sheet for Health Professionals. Available online: https://ods.od.nih.gov/factsheets/Iron-HealthProfessional/ (accessed on 3 August 2023).
- Beserra, J.B.; Morais, J.B.S.; Severo, J.S.; Cruz, K.J.C.; de Oliveira, A.R.S.; Henriques, G.S.; do Nascimento Marreiro, D. Relation Between Zinc and Thyroid Hormones in Humans: A Systematic Review. Biol. Trace Elem. Res. 2021, 199, 4092–4100. [Google Scholar] [CrossRef]
- Severo, J.S.; Morais, J.B.S.; de Freitas, T.E.C.; Andrade, A.L.P.; Feitosa, M.M.; Fontenelle, L.C.; de Oliveira, A.R.S.; Cruz, K.J.C.; do Nascimento Marreiro, D. The role of zinc in thyroid hormones metabolism. Int. J. Vitam. Nutr. Res. 2019, 89, 80–88. [Google Scholar] [CrossRef]
- Licastro, F.; Mocchegiani, E.; Masi, M.; Fabris, N. Modulation of the neuroendocrine system and immune functions by zinc supplementation in children with Down’s syndrome. J. Trace Elem. Electrolytes Health Dis. 1993, 7, 237–239. [Google Scholar] [PubMed]
- Bucci, I.; Napolitano, G.; Giuliani, C.; Lio, S.; Minnucci, A.; Di Giacomo, F.; Calabrese, G.; Sabatino, G.; Palka, G.; Monaco, F. Zinc sulfate supplementation improves thyroid function in hypozincemic Down children. Biol. Trace Elem. Res. 1999, 67, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Kandhro, G.A.; Kazi, T.G.; Afridi, H.I.; Kazi, N.; Baig, J.A.; Arain, M.B.; Sirajuddin; Shah, A.Q.; Sarfraz, R.A.; Jamali, M.K.; et al. Effect of zinc supplementation on the zinc level in serum and urine and their relation to thyroid hormone profile in male and female goitrous patients. Clin. Nutr. 2009, 28, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Licastro, F.; Mocchegiani, E.; Masi, M.; Fabris, N. Effects of zinc and selenium supplementation on thyroid function in overweight and obese hypothyroid female patients: A randomized double-blind controlled trial. J. Am. Coll. Nutr. 2015, 34, 391–399. [Google Scholar]
- Ihnatowicz, P.; Drywień, M.; Wątor, P.; Wojsiat, J. The importance of nutritional factors and dietary management of Hashimoto’s thyroiditis. Ann. Agric. Environ. Med. 2020, 27, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Melina, V.; Craig, W.J.; Levin, S. Position of the Academy of Nutrition and Dietetics: Vegetarian Diets. J. Acad. Nutr. Diet 2016, 116, 1266–1282, 1970–1980. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.M.; Shenkin, A.; Schweinlin, A.; Amrein, K.; Augsburger, M.; Biesalski, H.K.; Bischoff, S.C.; Casaer, M.P.; Gundogan, K.; Lepp, H.L.; et al. ESPEN micronutrient guideline. Clin. Nutr. 2022, 41, 1357–1424. [Google Scholar] [CrossRef] [PubMed]
- Mikkelsen, K.; Apostolopoulos, V. Vitamin B12, Folic Acid, and the Immune System. In Nutrition and Immunity; Mahmoudi, M., Rezaei, N., Eds.; Springer: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Kacharava, T.; Giorgadze, E.; Janjgava, S.; Lomtadze, N.; Taboridze, I. Correlation Between Vitamin B12 Deficiency and Autoimmune Thyroid Diseases. Endocr. Metab. Immune Disord. Drug Targets 2023, 23, 86–94. [Google Scholar] [CrossRef]
- Baeke, F.; Takiishi, T.; Korf, H.; Gysemans, C.; Mathieu, C. Vitamin D: Modulator of the immune system. Curr. Opin. Pharmacol. 2010, 10, 482–496. [Google Scholar] [CrossRef]
- Mele, C.; Caputo, M.; Bisceglia, A.; Samà, M.T.; Zavattaro, M.; Aimaretti, G.; Pagano, L.; Prodam, F.; Marzullo, P. Immunomodulatory Effects of Vitamin D in Thyroid Diseases. Nutrients 2020, 12, 1444. [Google Scholar] [CrossRef]
- Rotondi, M.; Chiovato, L. Vitamin D deficiency in patients with Graves’ disease: Probably something more than a casual association. Endocrine 2013, 43, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Fernandez, R.; Alonso, M.; Segura, C.; Muñoz, I.; García-Caballero, T.; Diguez, C. Vitamin D receptor gene expression in human pituitary gland. Life Sci. 1997, 60, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Berg, J.P.; Liane, K.M.; Bjørhovde, S.B.; Bjøro, T.; Torjesen, P.A.; Haug, E. Vitamin D receptor binding and biological effects of cholecalciferol analogues in rat thyroid cells. J. Steroid Biochem. Mol. Biol. 1994, 50, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Altieri, B.; Muscogiuri, G.; Barrea, L.; Mathieu, C.; Vallone, C.V.; Mascitelli, L.; Bizzaro, G.; Altieri, V.M.; Tirabassi, G.; Balercia, G.; et al. Does vitamin D play a role in autoimmune endocrine disorders? A proof of concept. Rev. Endocr. Metab. Disord. 2017, 18, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Maciejewski, A.; Kowalczyk, M.J.; Herman, W.; Czyżyk, A.; Kowalska, M.; Żaba, R.; Łącka, K. Vitamin D Receptor Gene Polymorphisms and Autoimmune Thyroiditis: Are They Associated with Disease Occurrence and Its Features? BioMed Res. Int. 2019, 2019, 8197580. [Google Scholar] [CrossRef] [PubMed]
- D’Aurizio, F.; Villalta, D.; Metus, P.; Doretto, P.; Tozzoli, R. Is vitamin D a player or not in the pathophysiology of autoimmune thyroid diseases? Autoimmun. Rev. 2015, 14, 363–369. [Google Scholar] [CrossRef]
- Wang, J.; Lv, S.; Chen, G.; Gao, C.; He, J.; Zhong, H.; Xu, Y. Meta-Analysis of the Association between Vitamin D and Autoimmune Thyroid Disease. Nutrients 2015, 7, 2485–2498. [Google Scholar] [CrossRef]
- Ma, J.; Wu, D.; Li, C.; Fan, C.; Chao, N.; Liu, J.; Li, Y.; Wang, R.; Miao, W.; Guan, H. Lower serum 25-Hydroxyvitamin D level is associated with 3 types of autoimmune thyroid diseases. Medicine 2015, 94, e1639. [Google Scholar] [CrossRef]
- Planck, T.; Shahida, B.; Malm, J.; Manjer, J. Vitamin D in Graves’ Disease: Levels, Correlation with Laboratory and Clinical Parameters, and Genetics. Eur. Thyroid J. 2018, 7, 27–33. [Google Scholar] [CrossRef]
- Taheriniya, S.; Arab, A.; Hadi, A.; Fadel, A.; Askari, G. Vitamin D and thyroid disorders: A systematic review and Meta-analysis of observational studies. BMC Endocr. Disord. 2021, 21, 171. [Google Scholar] [CrossRef]
- Cashman, K.D.; Kiely, M. Tackling inadequate vitamin D intakes within the population: Fortification of dairy products with vitamin D may not be enough. Endocrine 2016, 51, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Koehler, V.F.; Filmann, N.; Mann, W.A. Vitamin D Status and Thyroid Autoantibodies in Autoimmune Thyroiditis. Horm. Metab. Res. 2019, 51, 792–797. [Google Scholar] [CrossRef] [PubMed]
- Czarnywojtek, A.; Florek, E.; Pietrończyk, K.; Sawicka-Gutaj, N.; Ruchała, M.; Ronen, O.; Nixon, I.J.; Shaha, A.R.; Rodrigo, J.P.; Tufano, R.P.; et al. The Role of Vitamin D in Autoimmune Thyroid Diseases: A Narrative Review. J. Clin. Med. 2023, 12, 1452. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, Y.; Li, H.; Li, H. Effects of vitamin D on thyroid autoimmunity markers in Hashimoto’s thyroiditis: Systematic review and meta-analysis. J. Int. Med. Res. 2021, 49, 3000605211060675. [Google Scholar] [CrossRef] [PubMed]
- Dusso, A.S.; Brown, A.J.; Slatopolsky, E. Vitamin D. Am. J. Physiol. Renal Physiol. 2005, 289, F8–F28. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; Muscogiuri, G.; Laudisio, D.; Pugliese, G.; de Alteriis, G.; Colao, A.; Savastano, S. Influence of the Mediterranean Diet on 25-Hydroxyvitamin D Levels in Adults. Nutrients 2020, 12, 1439. [Google Scholar] [CrossRef] [PubMed]
- Dalamaga, M.; Muscogiuri, G.; Paganitsa, G.; Parvouleskou, G.; Syriou, V.; Karagkoynis, P.; Stratigou, T.; Vallianou, N.; Christodoulatos, G.S.; Karampela, I.; et al. Adherence to the Mediterranean diet is an independent predictor of circulating vitamin D levels in normal weight and non-smoker adults: An observational cross-sectional study. Int. J. Food Sci. Nutr. 2021, 72, 848–860. [Google Scholar] [CrossRef] [PubMed]
- Brent, G.A. Environmental exposures and autoimmune thyroid disease. Thyroid 2010, 20, 755–761. [Google Scholar] [CrossRef]
- Boas, M.; Feldt-Rasmussen, U.; Main, K.M. Thyroid effects of endocrine disrupting chemicals. Mol. Cell. Endocrinol. 2012, 355, 240–248. [Google Scholar] [CrossRef]
- Coperchini, F.; Croce, L.; Ricci, G.; Magri, F.; Rotondi, M.; Imbriani, M.; Chiovato, L. Thyroid Disrupting Effects of Old and New Generation PFAS. Front. Endocrinol. 2021, 11, 612320. [Google Scholar] [CrossRef]
- Bodin, J.; Stene, L.C.; Nygaard, U.C. Can exposure to environmental chemicals increase the risk of diabetes type 1 development? BioMed Res. Int. 2015, 2015, 208947. [Google Scholar] [CrossRef]
- Radford, S. Sources of Contamination in Food. In Encyclopedia of Food Security and Sustainability; Ferranti, P., Berry, E., Jock, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 518–522. [Google Scholar] [CrossRef]
- Oliveira, K.J.; Chiamolera, M.I.; Giannocco, G.; Pazos-Moura, C.C.; Ortiga-Carvalho, T.M. Thyroid function disruptors: From nature to chemicals. J. Mol. Endocrinol. 2019, 62, R1–R19. [Google Scholar] [CrossRef]
- Montano, L.; Maugeri, A.; Volpe, M.G.; Micali, S.; Mirone, V.; Mantovani, A.; Navarra, M.; Piscopo, M. Mediterranean Diet as a Shield against Male Infertility and Cancer Risk Induced by Environmental Pollutants: A Focus on Flavonoids. Int. J. Mol. Sci. 2022, 23, 1568. [Google Scholar] [CrossRef]
- Melough, M.M.; Maffini, M.V.; Otten, J.J.; Sathyanarayana, S. Diet quality and exposure to endocrine-disrupting chemicals among US adults. Environ. Res. 2022, 211, 113049. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).