Effectiveness of the Telemedical Lifestyle Intervention Program TeLIPro for Improvement of HbA1c in Type 2 Diabetes: A Randomized-Controlled Trial in a Real-Life Setting
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population
2.3. Randomization and Masking
2.4. Procedures and Interventions
2.5. Outcomes
2.6. Statistical Analysis and Power Calculation
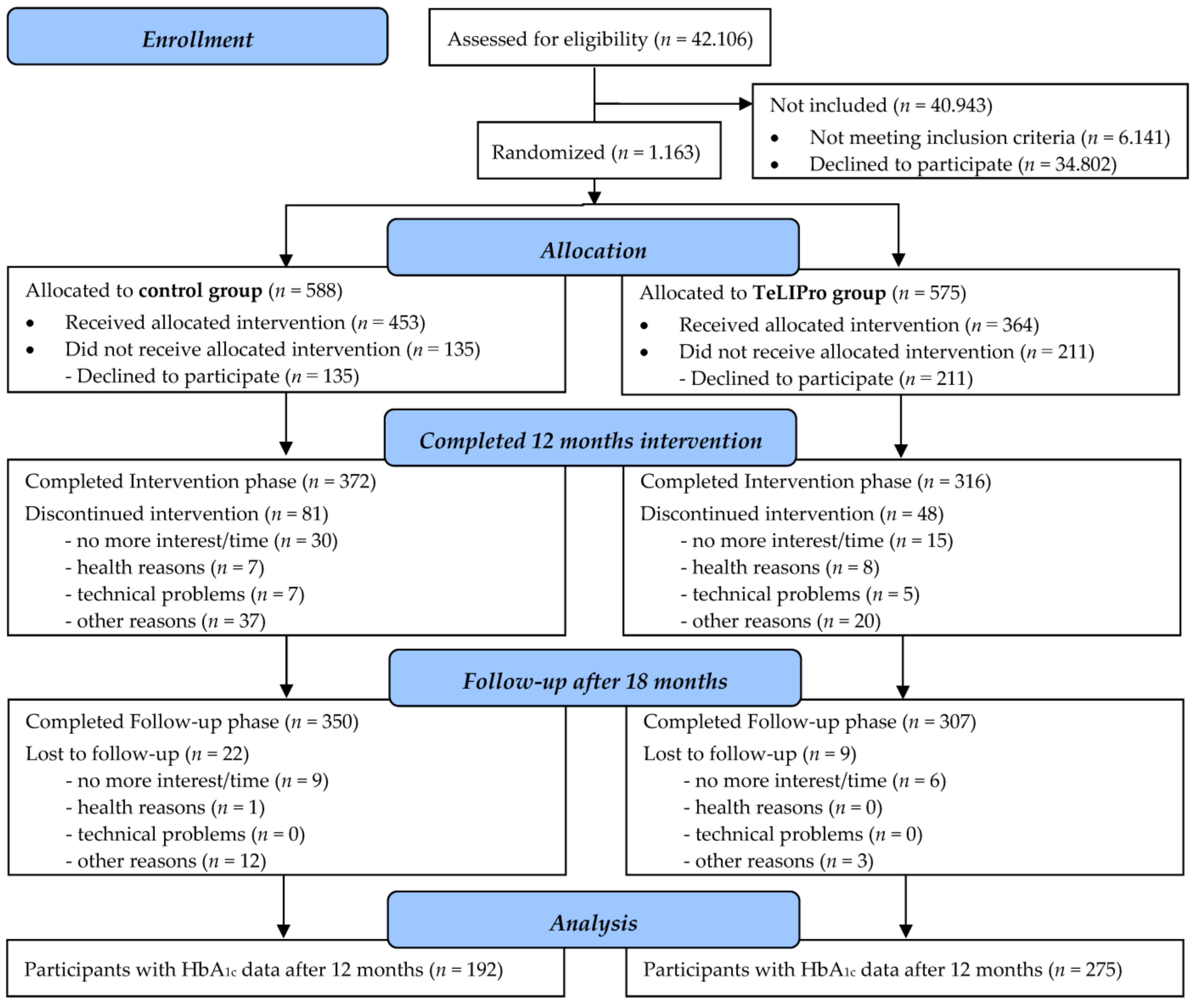
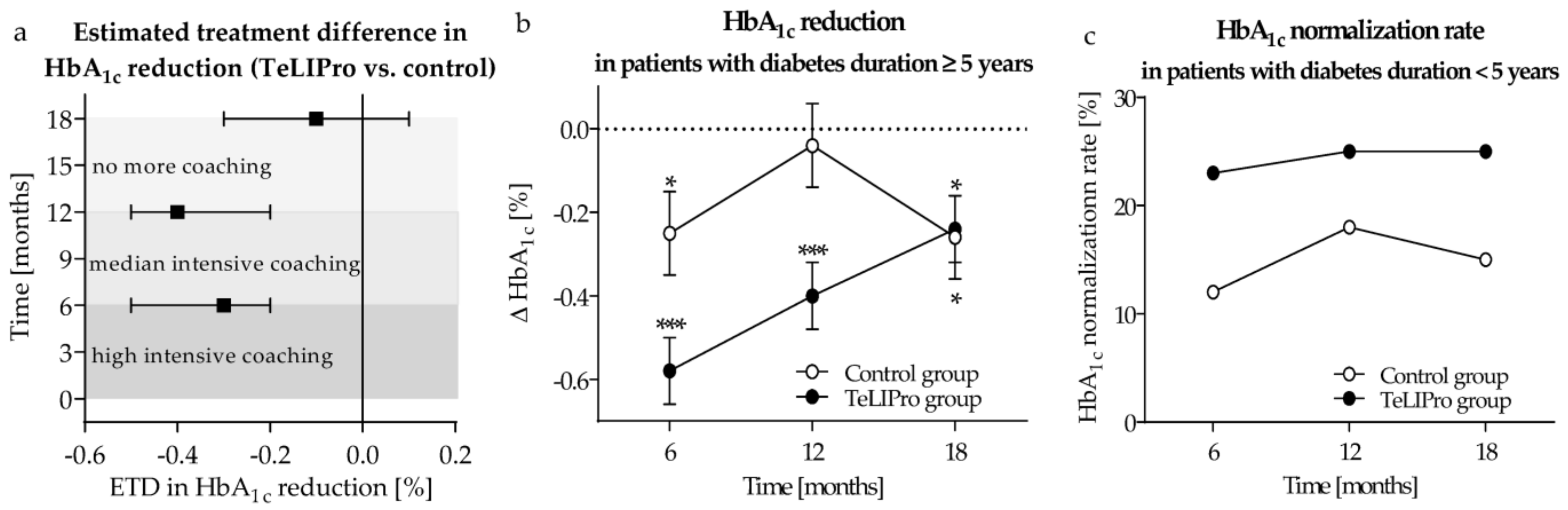
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tonnies, T.; Rockl, S.; Hoyer, A.; Heidemann, C.; Baumert, J.; Du, Y.; Scheidt-Nave, C.; Brinks, R. Projected number of people with diagnosed Type 2 diabetes in Germany in 2040. Diabet. Med. 2019, 36, 1217–1225. [Google Scholar] [CrossRef]
- Kahm, K.; Laxy, M.; Schneider, U.; Rogowski, W.H.; Lhachimi, S.K.; Holle, R. Health Care Costs Associated with Incident Complications in Patients with Type 2 Diabetes in Germany. Diabetes Care 2018, 41, 971–978. [Google Scholar] [CrossRef] [PubMed]
- Siegel, E.S.; Siegel, E.G. Versorgungsstrukturen, Berufsbilder und professionelle Diabetesorganisationen in Deutschland. In Deutscher Gesundheitsbericht Diabetes 2023; Verlag Kirchheim + Co GmbH: Mainz, Germany, 2022; pp. 201–209. [Google Scholar]
- Katula, J.A.; Dressler, E.V.; Kittel, C.A.; Harvin, L.N.; Almeida, F.A.; Wilson, K.E.; Michaud, T.L.; Porter, G.C.; Brito, F.A.; Goessl, C.L.; et al. Effects of a Digital Diabetes Prevention Program: An RCT. Am. J. Prev. Med. 2022, 62, 567–577. [Google Scholar] [CrossRef]
- Christensen, J.R.; Hesseldal, L.; Olesen, T.B.; Olsen, M.H.; Jakobsen, P.R.; Laursen, D.H.; Lauridsen, J.T.; Nielsen, J.B.; Sondergaard, J.; Brandt, C.J. Long-term weight loss in a 24-month primary care-anchored telehealth lifestyle coaching program: Randomized controlled trial. J. Telemed. Telecare 2022, 28, 764–770. [Google Scholar] [CrossRef] [PubMed]
- Toro-Ramos, T.; Michaelides, A.; Anton, M.; Karim, Z.; Kang-Oh, L.; Argyrou, C.; Loukaidou, E.; Charitou, M.M.; Sze, W.; Miller, J.D. Mobile Delivery of the Diabetes Prevention Program in People with Prediabetes: Randomized Controlled Trial. JMIR Mhealth Uhealth 2020, 8, e17842. [Google Scholar] [CrossRef]
- Christensen, J.R.; Laursen, D.H.; Lauridsen, J.T.; Hesseldal, L.; Jakobsen, P.R.; Nielsen, J.B.; Sondergaard, J.; Brandt, C.J. Reversing Type 2 Diabetes in a Primary Care-Anchored eHealth Lifestyle Coaching Programme in Denmark: A Randomised Controlled Trial. Nutrients 2022, 14, 3424. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.L.; Hollingsworth, K.G.; Aribisala, B.S.; Chen, M.J.; Mathers, J.C.; Taylor, R. Reversal of type 2 diabetes: Normalisation of beta cell function in association with decreased pancreas and liver triacylglycerol. Diabetologia 2011, 54, 2506–2514. [Google Scholar] [CrossRef]
- Lean, M.E.J.; Leslie, W.S.; Barnes, A.C.; Brosnahan, N.; Thom, G.; McCombie, L.; Peters, C.; Zhyzhneuskaya, S.; Al-Mrabeh, A.; Hollingsworth, K.G.; et al. Durability of a primary care-led weight-management intervention for remission of type 2 diabetes: 2-year results of the DiRECT open-label, cluster-randomised trial. Lancet Diabetes Endocrinol. 2019, 7, 344–355. [Google Scholar] [CrossRef]
- De, G.J.; Wu, D.; Flynn, D.; Robertson, D.; Grant, G.; Sun, J. Efficacy of telemedicine on glycaemic control in patients with type 2 diabetes: A meta-analysis. World J. Diabetes 2021, 12, 170–197. [Google Scholar]
- Fernando, M.E.; Seng, L.; Drovandi, A.; Crowley, B.J.; Golledge, J. Effectiveness of Remotely Delivered Interventions to Simultaneously Optimize Management of Hypertension, Hyperglycemia and Dyslipidemia in People with Diabetes: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front. Endocrinol. 2022, 13, 848695. [Google Scholar] [CrossRef]
- Almulhim, A.N.; Hartley, H.; Norman, P.; Caton, S.J.; Dogru, O.C.; Goyder, E. Behavioural Change Techniques in Health Coaching-Based Interventions for Type 2 Diabetes: A Systematic Review and Meta-Analysis. BMC Public Health 2023, 23, 95. [Google Scholar] [CrossRef]
- Hangaard, S.; Laursen, S.H.; Andersen, J.D.; Kronborg, T.; Vestergaard, P.; Hejlesen, O.; Udsen, F.W. The Effectiveness of Telemedicine Solutions for the Management of Type 2 Diabetes: A Systematic Review, Meta-Analysis, and Meta-Regression. J. Diabetes Sci. Technol. 2023, 17, 794–825. [Google Scholar] [CrossRef] [PubMed]
- Morris, T.; Aspinal, F.; Ledger, J.; Li, K.; Gomes, M. The Impact of Digital Health Interventions for the Management of Type 2 Diabetes on Health and Social Care Utilisation and Costs: A Systematic Review. Pharmacoecon Open 2023, 7, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Hecken, J. Typ-2-Diabetes: Etablierte Versorgungsangebote aktualisieren, innovative Modelle erproben. In Deutscher Gesundheitsbericht Diabetes 2023; Verlag Kirchheim + Co GmbH: Mainz, Germany, 2022; pp. 185–190. [Google Scholar]
- Kempf, K.; Altpeter, B.; Berger, J.; Reuss, O.; Fuchs, M.; Schneider, M.; Gartner, B.; Niedermeier, K.; Martin, S. Efficacy of the Telemedical Lifestyle intervention Program TeLiPro in Advanced Stages of Type 2 Diabetes: A Randomized Controlled Trial. Diabetes Care 2017, 40, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Gandek, B.; Ware, J.E.; Aaronson, N.K.; Apolone, G.; Bjorner, J.B.; Brazier, J.E.; Bullinger, M.; Kaasa, S.; Leplege, A.; Prieto, L.; et al. Cross-validation of item selection and scoring for the SF-12 Health Survey in nine countries: Results from the IQOLA Project. International Quality of Life Assessment. J. Clin. Epidemiol. 1998, 51, 1171–1178. [Google Scholar] [CrossRef] [PubMed]
- Hautzinger, M.; Bailer, M. ADS. Allgemeine Depressionsskala; Beltz Test GmbH: Weinheim, Germany, 1993. [Google Scholar]
- Stunkard, A.J.; Messick, S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J. Psychosom. Res. 1985, 29, 71–83. [Google Scholar] [CrossRef]
- Little, R.R.; Rohlfing, C.L. The long and winding road to optimal HbA1c measurement. Clin. Chim. Acta 2013, 418, 63–71. [Google Scholar] [CrossRef]
- Kempf, K.; Altpeter, B.; Berger, J.; Herrmann, S.; Leppert, N.; Röhling, M.; Martin, S. Das Telemedizinische Lebensstilinterventionsprogramm (TeLiPro) als Zusatzleistung zur Regelversorgung bei Personen mit Typ-2-Diabetes. Diabetol. Stoffwechs. 2019, 28, 173–179. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Lenth, R.; Singmann, H.; Love, J.; Buerkner, P.; Herve, M. Package Emmeans: Estimated Marginal Means, Aka Least-Squares means. R Package Version 1.8.3. Available online: https://CRAN.R-project.org/package=emmeans (accessed on 1 December 2022).
- van Buuren, S.; Groothuis-Oudshoorn, K. mice: Multivariate Imputation by Chaned Equitations in R. J. Stat. Softw. 2011, 45, 1–67. [Google Scholar]
- Van Walraven, C.; Austin, P.C.; Jennings, A.; Quan, H.; Forster, A.J. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med. Care 2009, 47, 626–633. [Google Scholar] [CrossRef]
- Röhling, M.; Kempf, K.; Banzer, W.; Berg, A.; Braumann, K.M.; Tan, S.; Halle, M.; McCarthy, D.; Pinget, M.; Predel, H.G.; et al. Prediabetes Conversion to Normoglycemia Is Superior Adding a Low-Carbohydrate and Energy Deficit Formula Diet to Lifestyle Intervention-A 12-Month Subanalysis of the ACOORH Trial. Nutrients 2020, 12, 2022. [Google Scholar] [CrossRef] [PubMed]
- Salas-Salvado, J.; Bullo, M.; Babio, N.; Martinez-Gonzalez, M.A.; Ibarrola-Jurado, N.; Basora, J.; Estruch, R.; Covas, M.I.; Corella, D.; Aros, F.; et al. Reduction in the incidence of type 2 diabetes with the Mediterranean diet: Results of the PREDIMED-Reus nutrition intervention randomized trial. Diabetes Care 2011, 34, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Ros, E.; Salas-Salvado, J.; Covas, M.I.; Corella, D.; Aros, F.; Gomez-Gracia, E.; Ruiz-Gutierrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef] [PubMed]
- Dehghan, M.; Mente, A.; Zhang, X.; Swaminathan, S.; Li, W.; Mohan, V.; Iqbal, R.; Kumar, R.; Wentzel-Viljoen, E.; Rosengren, A.; et al. Associations of fats and carbohydrate intake with cardiovascular disease and mortality in 18 countries from five continents (PURE): A prospective cohort study. Lancet 2017, 390, 2050–2062. [Google Scholar] [CrossRef] [PubMed]
- Kempf, K.; Röhling, M.; Niedermeier, K.; Gartner, B.; Martin, S. Individualized Meal Replacement Therapy Improves Clinically Relevant Long-Term Glycemic Control in Poorly Controlled Type 2 Diabetes Patients. Nutrients 2018, 10, 1022. [Google Scholar] [CrossRef]
- Taheri, S.; Zaghloul, H.; Chagoury, O.; Elhadad, S.; Ahmed, S.H.; El, K.N.; Amona, R.A.; El, N.K.; Suleiman, N.; Alnaama, A.; et al. Effect of intensive lifestyle intervention on bodyweight and glycaemia in early type 2 diabetes (DIADEM-I): An open-label, parallel-group, randomised controlled trial. Lancet Diabetes Endocrinol. 2020, 8, 477–489. [Google Scholar] [CrossRef]
- Durrer, C.; McKelvey, S.; Singer, J.; Batterham, A.M.; Johnson, J.D.; Gudmundson, K.; Wortman, J.; Little, J.P. A randomized controlled trial of pharmacist-led therapeutic carbohydrate and energy restriction in type 2 diabetes. Nat. Commun. 2021, 12, 5367. [Google Scholar] [CrossRef]
- Churuangsuk, C.; Hall, J.; Reynolds, A.; Griffin, S.J.; Combet, E.; Lean, M.E.J. Diets for weight management in adults with type 2 diabetes: An umbrella review of published meta-analyses and systematic review of trials of diets for diabetes remission. Diabetologia 2022, 65, 14–36. [Google Scholar] [CrossRef]
- Riddle, M.C.; Cefalu, W.T.; Evans, P.H.; Gerstein, H.C.; Nauck, M.A.; Oh, W.K.; Rothberg, A.E.; le Roux, C.W.; Rubino, F.; Schauer, P.; et al. Consensus Report: Definition and Interpretation of Remission in Type 2 Diabetes. Diabetes Care 2021, 44, 2438–2444. [Google Scholar] [CrossRef]
- ADA. 5. Lifestyle Management: Standards of Medical Care in Diabetes-2019. Diabetes Care 2019, 42, S46–S60. [Google Scholar] [CrossRef] [PubMed]
- Skurk, T.; Bosy-Westphal, A.; Grunerbel, A.; Kabisch, S.; Keuthage, W.; Kronsbein, P.; Mussig, K.; Pfeiffer, A.F.H.; Simon, M.C.; Tombek, A.; et al. Dietary recommendations for persons with type 2 diabetes mellitus. Exp. Clin. Endocrinol. Diabetes 2022, 130, S151–S184. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Molina, L.; Lewis-Mikhael, A.M.; Riquelme-Gallego, B.; Cano-Ibanez, N.; Oliveras-Lopez, M.J.; Bueno-Cavanillas, A. Improving type 2 diabetes mellitus glycaemic control through lifestyle modification implementing diet intervention: A systematic review and meta-analysis. Eur. J. Nutr. 2020, 59, 1313–1328. [Google Scholar] [CrossRef]
- Franz, M.J.; Boucher, J.L.; Rutten-Ramos, S.; VanWormer, J.J. Lifestyle weight-loss intervention outcomes in overweight and obese adults with type 2 diabetes: A systematic review and meta-analysis of randomized clinical trials. J. Acad. Nutr. Diet. 2015, 115, 1447–1463. [Google Scholar] [CrossRef]
- O’Connor, P.J.; Schmittdiel, J.A.; Pathak, R.D.; Harris, R.I.; Newton, K.M.; Ohnsorg, K.A.; Heisler, M.; Sterrett, A.T.; Xu, S.; Dyer, W.T.; et al. Randomized trial of telephone outreach to improve medication adherence and metabolic control in adults with diabetes. Diabetes Care 2014, 37, 3317–3324. [Google Scholar] [CrossRef] [PubMed]
- Kempf, K.; Röhling, M.; Banzer, W.; Braumann, K.M.; Halle, M.; Schaller, N.; McCarthy, D.; Predel, H.G.; Schenkenberger, I.; Tan, S.; et al. High-Protein, Low-Glycaemic Meal Replacement Improves Physical Health-Related Quality of Life in High-Risk Persons for Metabolic Syndrome-A Subanalysis of the Randomised-Controlled ACOORH Trial. Nutrients 2022, 14, 3161. [Google Scholar] [CrossRef]
- Dansinger, M.L.; Tatsioni, A.; Wong, J.B.; Chung, M.; Balk, E.M. Meta-analysis: The effect of dietary counseling for weight loss. Ann. Intern. Med. 2007, 147, 41–50. [Google Scholar] [CrossRef]
- Cradock, K.A.; OLaighin, G.; Finucane, F.M.; Gainforth, H.L.; Quinlan, L.R.; Ginis, K.A. Behaviour change techniques targeting both diet and physical activity in type 2 diabetes: A systematic review and meta-analysis. Int. J. Behav. Nutr. Phys. Act. 2017, 14, 18. [Google Scholar] [CrossRef]
- Look AHEAD Research Group. Effects of Intensive Lifestyle Intervention on All-Cause Mortality in Older Adults with Type 2 Diabetes and Overweight/Obesity: Results from the Look AHEAD Study. Diabetes Care 2022, 45, 1252–1259. [Google Scholar] [CrossRef]
- Röhling, M.; Kempf, K.; Altpeter, B.; Berg, A.; Herrmann, S.; Leppert, N.; Martin, S. Das Telemedizinische Lebensstilinterventionsprogramm (TeLiPro) verbessert initial wie auch bei Re-Intervention die Diabeteseinstellung—Daten zum 3-Jahres-Follow-up. Diabetol. Stoffwechs. 2019, 28, 69–75. [Google Scholar]
- Lunde, P.; Nilsson, B.B.; Bergland, A.; Kvaerner, K.J.; Bye, A. The Effectiveness of Smartphone Apps for Lifestyle Improvement in Noncommunicable Diseases: Systematic Review and Meta-Analyses. J. Med. Internet Res. 2018, 20, e162. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.; Carter, B.; Hewitt, J.; Francisa, T.; Mayor, S. Do Mobile Phone Applications Improve Glycemic Control (HbA1c) in the Self-management of Diabetes? A Systematic Review, Meta-analysis, and GRADE of 14 Randomized Trials. Diabetes Care 2016, 39, 2089–2095. [Google Scholar] [CrossRef] [PubMed]
- Bloomgarden, Z.T.; Dodis, R.; Viscoli, C.M.; Holmboe, E.S.; Inzucchi, S.E. Lower baseline glycemia reduces apparent oral agent glucose-lowering efficacy: A meta-regression analysis. Diabetes Care 2006, 29, 2137–2139. [Google Scholar] [CrossRef] [PubMed]
| Control Group | TeLIPro Group | |||||
|---|---|---|---|---|---|---|
| Parameters | All (n = 453) | Diabetes Duration < 5 Years (n = 182) | Diabetes Duration ≥ 5 Years (n = 271) | All (n = 364) | Diabetes Duration < 5 Years (n = 143) | Diabetes Duration ≥ 5 Years (n = 221) |
| Sex male/female (%) | 65/35 | 65/35 | 65/35 | 62/38 | 62/38 | 62/38 |
| Age (years) | 54 ± 9 | 51 ± 10 | 56 ± 8 | 55 ± 9 | 52 ± 10 | 57 ± 8 |
| HbA1c (%) | 7.8 ± 1.3 | 7.6 ± 1.4 | 8.0 ± 1.3 | 7.6 ± 1.2 * | 7.5 ± 1.1 | 7.7 ± 1.2 ** |
| Body weight (kg) | 103 ± 22 | 106 ± 25 | 102 ± 19 | 104 ± 23 | 108 ± 24 | 102 ± 22 |
| BMI (kg/m2) | 34.4 ± 6.5 | 35.1 ± 7.2 | 33.9 ± 6.1 | 34.8 ± 7.0 | 35.7 ± 7.8 | 34.2 ± 6.4 |
| Systolic BP (mmHg) | 133 ± 16 | 133 ± 15 | 133 ± 16 | 134 ± 17 | 132 ± 20 | 135 ± 14 * |
| Diastolic BP (mmHg) | 82 ± 9 | 82 ± 9 | 81 ± 9 | 82 ± 11 | 81 ± 11 | 82 ± 11 |
| Total cholesterol (mg/dL) | 194 ± 48 | 197 ± 46 | 193 ± 49 | 195 ± 40 | 200 ± 38 | 193 ± 42 |
| HDL cholesterol (mg/dL) | 47 ± 18 | 47 ± 19 | 47 ± 18 | 44 ± 15 | 42 ± 11 | 46 ± 17 |
| LDL cholesterol (mg/dL) | 123 ± 39 | 128 ± 41 | 120 ± 38 | 123 ± 36 | 128 ± 33 | 120 ± 38 |
| Triglycerides (mg/dL) | 211 ± 128 | 208 ± 117 | 212 ± 135 | 208 ± 121 | 202 ± 106 | 211 ± 129 |
| Physical wellbeing (au) a | 41.0 ± 10.2 | 42.2 ± 10.5 | 40.2 ± 9.9 | 41.2 ± 10.2 | 41.1 ± 10.1 | 41.2 ± 10.4 |
| Mental wellbeing (au) a | 48.4 ± 11.2 | 47.7 ± 11.4 | 48.8 ± 11.0 | 48.8 ± 10.7 | 47.9 ± 10.2 | 49.4 ± 11.0 |
| Impairment of QoL (au) b | 14.4 ± 9.8 | 15.3 ± 10.3 | 13.9 ± 9.4 | 14.5 ± 9.4 | 14.9 ± 9.0 | 14.2 ± 9.6 |
| Cognitive control (au) c | 7.5 ± 4.4 | 7.4 ± 4.4 | 7.6 ± 4.3 | 7.5 ± 4.2 | 7.5 ± 4.3 | 7.5 ± 4.1 |
| Suggestibility (au) c | 6.2 ± 3.5 | 6.0 ± 3.2 | 6.4 ± 3.6 | 6.2 ± 3.4 | 6.1 ± 3.4 | 6.2 ± 3.4 |
| Hunger (au) c | 5.5 ± 3.6 | 5.3 ± 3.4 | 5.6 ± 3.8 | 5.7 ± 3.7 | 5.5 ± 3.8 | 5.4 ± 3.7 |
| Parameters | Completers (n = 657) | Drop Outs (n = 160) |
|---|---|---|
| Control group/TeLiPro group (%) | 53/47 | 64/36 * |
| Diabetes duration < 5/≥5 years (%) | 41/59 | 34/66 * |
| Sex male/female (%) | 64/36 | 63/37 |
| Age (years) | 54 ± 9 | 55 ± 9 |
| HbA1c (%) | 7.7 ± 1.3 | 7.7 ± 1.3 |
| Body weight (kg) | 104 ± 23 | 100 ± 20 * |
| BMI (kg/m2) | 34.8 ± 7.0 | 33.5 ± 6.5 |
| Systolic BP (mmHg) | 134 ± 17 | 133 ± 14 |
| Diastolic BP (mmHg) | 82 ± 10 | 81 ± 8 |
| Total cholesterol (mg/dL) | 194 ± 46 | 198 ± 38 |
| HDL cholesterol (mg/dL) | 46 ± 16 | 46 ± 19 |
| LDL cholesterol (mg/dL) | 123 ± 39 | 124 ± 35 |
| Triglycerides (mg/dL) | 210 ± 129 | 204 ± 105 |
| Physical wellbeing (au) | 41.1 ± 10.3 | 40.9 ± 9.7 |
| Mental wellbeing (au) | 48.8 ± 11.0 | 47.7 ± 10.9 |
| Impairment of QoL (au) | 14.3 ± 9.5 | 15.0 ± 9.9 |
| Cognitive control (au) | 7.6 ± 4.3 | 7.4 ± 4.3 |
| Suggestibility (au) | 6.2 ± 3.5 | 6.2 ± 3.3 |
| Hunger (au) | 5.5 ± 3.7 | 5.4 ± 3.7 |
| Time (Months) | 6 | 12 | 18 |
|---|---|---|---|
| Estimated treatment difference (ETD) | |||
| ETD (TeLIPro vs. control) | −0.3 (−0.5; −0.2) ** | −0.4 (−0.5; −0.2) *** | −0.1 (−0.3; 0.1) |
| Model 2 | −0.3 (−0.5; −0.1) ** | −0.3 (−0.5; −0.1) ** | 0.1 (−0.1; 0.3) |
| Model 3 | −0.3 (−0.5; −0.2) ** | −0.4 (−0.6; −0.2) *** | −0.0 (−0.2; 0.2) |
| ETD (diabetes duration ≥ 5 years) | −0.4 (−0.6; −0.2) ** | −0.5 (−0.7; −0.3) *** | −0.2 (−0.4; 0.0) |
| Model 2 | −0.4 (−0.6; −0.1) ** | −0.4 (−0.6; −0.2) ** | −0.1 (−0.2; 0.3) |
| Model 3 | −0.5 (−0.7; −0.3) *** | −0.5 (−0.7; −0.3) *** | −0.1 (−0.3; 0.2) |
| ETD (diabetes duration < 5 years) | −0.2 (−0.4; 0.1) | −0.2 (−0.5; 0.1) | 0.0 (−0.3; 0.4) |
| Model 2 | −0.1 (−0.5; 0.2) | −0.2 (−0.5; 0.1) | 0.1 (−0.3; 0.5) |
| Model 3 | −0.1 (−0.4; 0.2) | −0.2 (−0.5; 0.1) | 0.1 (−0.2; 0.5) |
| Responder analysis (relative risk (RR) for clinically relevant HbA1c reduction) | |||
| RR (TeLIPro vs. control) | 1.6 (1.1; 2.0) * | 1.9 (1.3; 3.0) ** | 1.4 (0.9; 2.0) |
| RR (diabetes duration ≥ 5 years) | 1.8 (1.0; 3.0) | 1.7 (0.9; 3.0) | 1.4 (0.7; 3.0) |
| RR (diabetes duration < 5 years) | 1.4 (0.9; 2.0) | 2.1 (1.3; 4.0) ** | 1.4 (0.8; 3.0) |
| HbA1c normalisation | |||
| RR (TeLIPro vs. control) | 3.4 (1.7; 6.9) *** | 2.1 (1.1; 3.9) * | 2.8 (1.3; 6.1) ** |
| RR (diabetes duration ≥ 5 years) | 6.3 (2.9; 18.5) *** | 3.1 (1.1; 8.6) * | 5.4 (1.5; 19.3) ** |
| RR (diabetes duration < 5 years) | 2.0 (0.8; 4.8) | 1.4 (0.6; 3.1) | 1.7 (0.6; 4.7) |
| Parameters | Control Group (n = 192) | TeLIPro Group (n = 275) | ETD (TeLIPro vs. Control) | p |
|---|---|---|---|---|
| HbA1c (%) | −0.0 ± 0.1 | –0.4 ± 0.1 | −0.4 (−0.5; −0.2) | <0.001 |
| Body weight (kg) | –0.7 ± 0.4 | –2.9 ± 0.3 | −2.2 (−1.3; −3.0) | <0.001 |
| BMI (kg/m2) | –0.2 ± 0.1 | –0.9 ± 0.1 | −0.8 (−0.5; −1.0) | <0.001 |
| Systolic BP (mmHg) | –0.3 ± 1.1 | –1.5 ± 0.9 | −1.2 (1.5; −3.9) | 0.384 |
| Diastolic BP (mmHg) | –0.8 ± 0.9 | –1.1 ± 0.7 | −0.3 (1.7; −2.3) | 0.770 |
| Total cholesterol (mg/dL) | –5.3 ± 2.4 | –6.6 ± 2.5 | −1.3 (4.8; −7.5) | 0.672 |
| HDL cholesterol (mg/dL) | 0.8 ± 1.5 | 1.4 ± 1.2 | 0.6 (3.3; −2.2) | 0.675 |
| LDL cholesterol (mg/dL) | –4.9 ± 3.5 | −5.7 ± 2.9 | −0.8 (4.9; −6.5) | 0.787 |
| Triglycerides (mg/dL) | –12.8 ± 8.3 | –14.1 ± 9.3 | −1.3 (16.6; −19.1) | 0.890 |
| Physical wellbeing (au) | 1.4 ± 0.5 | 2.9 ± 0.4 | 1.4 (2.7; 0.2) | 0.023 |
| Mental wellbeing (au) | –1.6 ± 0.7 | 0.3 ± 0.5 | 1.9 (3.6; 0.2) | 0.029 |
| Impairment of QoL (au) | 1.1 ± 0.9 | –1.3 ± 0.5 | −2.3 (−0.9; −3.7) | 0.001 |
| Cognitive control (au) | 0.9 ± 0.3 | 2.7 ± 0.2 | 1.8 (2.4; 1.1) | <0.001 |
| Suggestibility (au) | –0.5 ± 0.2 | –1.1 ± 0.1 | −0.6 (−0.2; −1.0) | 0.003 |
| Hunger (au) | –0.7 ± 0.2 | –1.8 ± 0.2 | −1.1 (−0.6; −1.5) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kempf, K.; Dubois, C.; Arnold, M.; Amelung, V.; Leppert, N.; Altin, S.; Vomhof, M.; Icks, A.; Martin, S. Effectiveness of the Telemedical Lifestyle Intervention Program TeLIPro for Improvement of HbA1c in Type 2 Diabetes: A Randomized-Controlled Trial in a Real-Life Setting. Nutrients 2023, 15, 3954. https://doi.org/10.3390/nu15183954
Kempf K, Dubois C, Arnold M, Amelung V, Leppert N, Altin S, Vomhof M, Icks A, Martin S. Effectiveness of the Telemedical Lifestyle Intervention Program TeLIPro for Improvement of HbA1c in Type 2 Diabetes: A Randomized-Controlled Trial in a Real-Life Setting. Nutrients. 2023; 15(18):3954. https://doi.org/10.3390/nu15183954
Chicago/Turabian StyleKempf, Kerstin, Clara Dubois, Matthias Arnold, Volker Amelung, Nora Leppert, Sibel Altin, Markus Vomhof, Andrea Icks, and Stephan Martin. 2023. "Effectiveness of the Telemedical Lifestyle Intervention Program TeLIPro for Improvement of HbA1c in Type 2 Diabetes: A Randomized-Controlled Trial in a Real-Life Setting" Nutrients 15, no. 18: 3954. https://doi.org/10.3390/nu15183954
APA StyleKempf, K., Dubois, C., Arnold, M., Amelung, V., Leppert, N., Altin, S., Vomhof, M., Icks, A., & Martin, S. (2023). Effectiveness of the Telemedical Lifestyle Intervention Program TeLIPro for Improvement of HbA1c in Type 2 Diabetes: A Randomized-Controlled Trial in a Real-Life Setting. Nutrients, 15(18), 3954. https://doi.org/10.3390/nu15183954





