Abstract
Glutamate, the main excitatory neurotransmitter in the central nervous system, is implicated in both the initiation of migraine as well as central sensitization, which increases the frequency of migraine attacks. Excessive levels of glutamate can lead to excitotoxicity in the nervous system which can disrupt normal neurotransmission and contribute to neuronal injury or death. Glutamate-mediated excitotoxicity also leads to neuroinflammation, oxidative stress, blood-brain barrier permeability, and cerebral vasodilation, all of which are associated with migraine pathophysiology. Experimental evidence has shown the protective effects of several nutrients against excitotoxicity. The current review focuses on the mechanisms behind glutamate’s involvement in migraines as well as a discussion on how specific nutrients are able to work towards restoring glutamate homeostasis. Understanding glutamate’s role in migraine is of vital importance for understanding why migraine is commonly comorbid with widespread pain conditions and for informing future research directions.
1. Introduction
Migraine is a common neurological disorder with prevalence rates ranging from 9–16% worldwide [1]. This disorder primarily occurs during the most productive years of adulthood, from age 20 to 50 years [2]. According to the latest report from the Global Burden of Disease study, migraine is the primary cause of disability among people less than 50 years of age [3]. In 2016, the annual cost of healthcare utilization and lost productivity associated with migraine in the US was estimated at $36 billion [4]. Migraine is associated with a wide spectrum of comorbidities including gastrointestinal, psychiatric, cardiac, and cerebrovascular disorders, which can increase the physiological burden [5]. Despite the profound impact of migraine, it is still underdiagnosed and undertreated [6,7].
The pathophysiological mechanism underlying migraine is not completely understood. However, activation and sensitization of meningeal nociceptors in the trigeminovascular (TG) system are widely accepted as a key pathway in the initiation of a migraine attack [8]. The TG system is comprised of sensory neurons that originate from the trigeminal ganglion that innervate cerebral blood vessels including the dura mater, the outermost layer of the meninges [9].
Evidence supports the role of glutamate neurotransmission in both the activation and perpetuation of migraine [10,11]. Peripheral release of glutamate is involved in the generation of migraine pain through N-methyl-D-aspartate (NMDA) receptors found in the meningeal afferents of the trigeminal nerve [12]. High glutamatergic activity also leads to increased cerebral excitability and resultant cortical spreading depression (CSD) that can cause nociception in the dura mater [10,13]. Interestingly, in addition to the direct effect of glutamate in the activation of trigeminal nociceptors and the contribution to CSD development, it is also contributing to pain sensitization as well. Previous reports have indicated that the production and release of the vasodilatory neuropeptides calcitonin gene-related peptide (CGRP) and substance P (SP) can be induced by increased glutamatergic neurotransmission [14,15]. Perivascular release of these neuropeptides can eventually lead to a phenomenon called “neurogenic inflammation” which is believed to be an underlying element leading to sensitization of trigeminal meningeal nociceptors [16]. Central sensitization has been observed in migraine and can lead to contact allodynia (i.e., pain from a stimulus that does not ordinarily cause pain) [17]. Central sensitization is an augmentation of membrane excitability through upregulation of glutamatergic neurotransmission, which contributes to pain hypersensitivity in many pain conditions [18]. The NMDA glutamate receptor is pivotal for both the initiation and maintenance of central sensitization, and thus, in reverse, is also thought to be the key in stopping this process [19]. A high concentration of glutamate in the synaptic cleft can lead to excitotoxicity, which is the over-excitation of neurons which leads to apoptosis, or cell death [20]. Excitotoxicity causes oxidative stress and inflammation in the central nervous system (CNS). The reinforcing properties between excitotoxicity, oxidative stress, and neuroinflammation (the “neurotoxic triad”) have been implicated in neurologic disorders including chronic pain and migraine [21]. Thus, disorders such as migraine may benefit from interventions targeting glutamate specifically.
Therefore, in the current review, we aim to combine existing knowledge about the role of glutamate in migraine pathogenesis along with information on nutrients that are protective against glutamate excitotoxicity, and then end with a proposed dietary treatment for migraine management.
2. Glutamatergic Neurotransmission
Glutamate is the main mediator of excitatory neurotransmission in the brain. It has two types of receptors, ionotropic and metabotropic [22]. Ionotropic glutamate receptors (iGluRs) are ligand-gated channels subcategorized into NMDA, the α-amino-3-hydroxy-5-methyl-4-isoxazole propionate (AMPA), and the kainic acid (KA) receptor, which all have fast excitatory effects. Metabotropic receptors (mGluRs) are G-protein-coupled receptor channels. Eight metabotropic receptors have been characterized (mGluR1-8) and fall into three groups (group I-III) based on their similarity regarding second messenger systems and pharmacology, with group I being associated with slow excitation, and groups II and III being more associated with slow inhibition [22].
Glutamate receptors are found throughout the body; therefore, dysregulation of the glutamatergic system can impose a broad range of effects [22]. In the nervous system, glutamate is implicated in crucial aspects of normal brain function including synaptogenesis, learning, cognition, and memory [23,24]. As mentioned above, the iGluRs are involved in fast synaptic responses to glutamate, while the mGluRs play a role in slow neuromodulatory signaling [25]. Although these receptors have local and functional variability, glutamate excitotoxicity targets both families of receptors [25]. Excitotoxicity results from the excessive synaptic release of glutamate and the consequent accumulation of high concentrations of free calcium (Ca2+) in the cytosol [26] which is mediated by NMDA receptors [26]. However, AMPA and KA receptors can also contribute to Ca2+ overload through their partial permeability to Ca2+ [26]. The mGluRs function in two ways, by directly increasing cytosolic Ca2+ levels via the facilitation of Ca2+ release from the intracellular stores, and indirectly by promoting NMDA receptor migration to the cell membrane [27]. Excitotoxicity is one of the leading causes of neuronal damage and death, and this process has been implicated in a variety of neurological diseases including schizophrenia, Alzheimer’s, Parkinson’s, multiple sclerosis (MS), epilepsy, chronic pain, and migraine [28,29,30,31,32,33].
3. Physiological and Anatomical Evidence Related to the Role of Glutamate in Migraine
Figure 1 illustrates the mechanisms involving glutamate in the pathogenesis of migraine (which is reviewed in detail in this section).
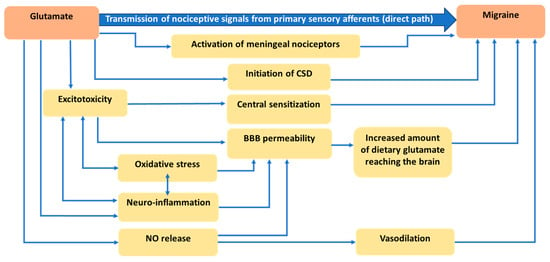
Figure 1.
Mechanisms explaining the role of glutamate in migraine pathogenesis.
3.1. Role of Glutamate in Nociception
The trigeminal system (in addition to C1 and C2 fibers) comprises all of the nociceptive neurotransmission within the head [34]. C1 and C2 fibers refer to the first and second cervical spinal nerves that, along with second-order neurons of the trigeminal nucleus caudalis, comprise the trigeminocervical complex [8]. Nociceptive signals from the meninges and cervical roots are sent to higher-order brain regions including the brainstem, hypothalamus, basal ganglia, and thalamus [8]. Projection of trigeminovascular thalamic neurons to different areas of the cortex contributes to pain perception as well as migraine-associated symptoms [8]. Glutamate has a well-known role in the transmission of nociceptive signals from primary sensory afferents to second-order neurons in the brainstem [35].
Interestingly, evidence from preclinical studies has shown that all types of glutamate receptors are characterized and found in the trigeminal system [36,37,38,39]. In line with these findings, other studies have also indicated that the administration of glutamate leads to hyperalgesia [40,41] while inhibition of glutamate blocks its nociceptive effects [42]. An interesting in vivo experiment explored the role of peripherally released glutamate in the generation of migraine pain by using trigeminal neurons from animal models [12]. They found that glutamate and aspartate (another amino acid with a similar structure that also functions as a neurotransmitter) can activate NMDA receptors on peripheral sensory trigeminal ganglion neurons in meningeal nerve terminals and that they can induce excitation of meningeal afferents implicated in the generation of migraine pain [12]. Thus, glutamate can lead to trigeminal nociception in two ways: (1) via signal transmission from primary meningeal nerves to higher brain regions, which results in cortical excitability, and (2) via activation of NMDA receptors in tissues located outside of the blood-brain barrier (BBB), which consequently leads to trigeminal nociception [12].
3.2. Role of Glutamate in Cortical Spreading Depression
Glutamate is also proposed as a key player in the initiation of CSD, which is an expanding wave of depolarization (activation of neurons) followed by a wave of hyperpolarization (inactivity of neurons while concentration gradients re-set) across the cortex [43,44]. Preclinical evidence supports the role of CSD in stimulating trigeminal neurons [45]. Local release of glutamate by neurons is assumed to trigger CSD [46]. CSD is recognized as the biological reason for migraine aura which has been further confirmed with the observation of CSD waves in migraine aura patients [47]. The latest version of the International Classification of Headache Disorders (ICHD) (Third edition) defines migraine aura as an “early symptom of an attack, believed to be the manifestations of focal cerebral dysfunction, typically lasting 20–30 min and precedes the headache” [48]. Visual aura is, by far, the most prevalent type of aura [49].
3.3. Role of Glutamate in Central Sensitization
The intensity and duration of headache attacks are attributed to the development of central sensitization [50]. Central sensitization is defined as abnormal amplification in central nociceptive processing because of increases in membrane excitability as well as reduced inhibition [50]. Central sensitization is observed in both chronic and episodic migraine [51]. Excitotoxicity is considered a major player in the onset and continuation of central sensitization [15]. This is thought to occur via the upregulation of NMDA and AMPA receptors on the primary afferent neurons, with a subsequent reduction in the threshold for neuronal activation, which contributes to the onset of central sensitization [52]. Higher levels of glutamate in plasma have been observed in both chronic and episodic migraine patients as compared to healthy controls, with no significant difference between chronic and episodic migraineurs [53]. Cutaneous allodynia, which is highly prevalent in migraine patients, is a clinical manifestation of central sensitization [54]. Interestingly, cutaneous allodynia has been associated with response to preventive treatment, with severe occurrence being associated with decreased response to treatment [55,56,57]. This evidence supports the important role of central sensitization in migraine pathophysiology.
3.4. Role of Glutamate in Disruption of the Blood-Brain Barrier (BBB)
The integrity of the BBB guarantees a unique environment for the CNS by controlling what substances can enter and leave the CNS [58]. Therefore, disruption of the BBB allows the influx of potentially toxic substances into the brain. BBB permeability can occur via the overactivation of NMDA receptors which causes excitotoxicity [59], but other events such as head trauma [60], infection [61], neurotoxic exposures [62], high stress [63], neuroinflammation [64], and oxidative stress [65] can also lead to permeability of the BBB. The contribution of neuroinflammation and oxidative stress, which are tightly tied to excitotoxicity, will be described in more detail below.
Increased glutamatergic neurotransmission in migraine leads to the sustained secretion of vasoactive substances including CGRP and SP [66]. These neuropeptides contribute to vasodilation, plasma protein extravasation, mast cell activation, and the release of proinflammatory cytokines, resulting in a phenomenon called neurogenic inflammation [16], which, as mentioned above, can lead to BBB permeability [67].
In neurons, the influx of excess Ca2+ into the cell following the activation of glutamate receptors eventually leads to the production of free radicals (atoms missing an electron) which are called reactive oxygen species (ROS) and reactive nitrogen species (RNS) [68]. Further support for this idea is provided by electron paramagnetic resonance spectroscopy showing that NMDA receptor activation results in the production of superoxide radicals [69]. Typically, ROS and RNS are counteracted by antioxidants or antioxidant enzyme systems within the cell, which have the ability to donate an electron to re-balance the free radicals. If the amount of free radicals outstrips the antioxidant defense system of the cell, oxidative stress occurs [70]. These free radicals can start a cascade of events including mitochondrial impairment, and damage to lipids, proteins, and DNA, leading to mutagenesis, and ultimately cell death [71]. Additionally, as mentioned above, oxidative stress can lead to BBB permeability [65].
Increased permeability of the BBB in migraine patients could result in the entry of blood-borne toxins, as well as increased amounts of dietary glutamate and aspartate, into the brain, which could elicit excitotoxicity. It is noteworthy that excitotoxicity, neuroinflammation, and oxidative stress have the ability to perpetuate one another, allowing this “neurotoxic triad” to be maintained over time. Thus, neurogenic inflammation and oxidative stress can also be involved in migraine initiation/sensitization through potentiating excitotoxicity.
3.5. Role of Glutamate in Nitric Oxide Release and Vasodilation
A link between glutamate and nitric oxide (NO) was initially proposed after the finding that glutamate or NMDA treatment causes the release of NO and cyclic guanosine monophosphate (cGMP) in cerebellar cultures [72]. Additionally, NMDA receptor activation results in a rise in cGMP levels in the brain, with nitric oxide synthase (NOS) inhibitors and NO scavengers preventing this rise in cGMP levels [72]. This suggests that NO has signaling functions downstream of NMDA receptor activation. In neurons, Ca2+ entry, as a result of the activation of NMDA receptors, induces NOS, which is physically coupled to NMDA receptors [73]. Glutamate can also activate NMDA receptors in the endothelial cells of capillaries, causing subsequent induction of NOS, and release of NO, which causes vasodilation [74]. The contribution of cerebral and meningeal arterial vasodilation in migraine initiation has been suspected for many decades [75,76]. Interestingly, NO can negatively affect the BBB when it combines with a superoxide radical to form peroxynitrite, a potent free radical that leads to oxidative stress and excitotoxicity [77,78,79].
4. Glutamate Concentration in Migraineurs
Increased levels of glutamate in plasma [80,81,82], cerebrospinal fluid (CSF) [14,82,83], and platelets [84,85,86] have been detected in migraine patients. This elevated level of glutamate was observed during attacks as well as during interictal periods [82,83,87], for those with and without aura [82,88,89]. Furthermore, a meta-analysis on excitatory neuro-metabolite levels across pain conditions, using data pooled from magnetic resonance spectroscopy studies, revealed a significant increase in glutamate levels in the brains of migraine patients, compared with controls [90]. This evidence could reflect cortical neuronal hyperexcitability and points to the dysfunction of glutamatergic signaling in migraine pathogenesis. In a study by Ferrari et al., prophylactic medications lowered the frequency of attacks and glutamate levels compared to baseline; however, migraine sufferers still had higher serum levels of glutamate compared to healthy controls [89]. Another case-control study among migraine patients without aura, using proton magnetic resonance spectroscopy, showed an increased level of the glutamate/glutamine ratio between attacks in both the primary occipital cortex and thalamus [91]. Table 1 represents the glutamate concentration in adult migraine patients, as compared to healthy controls, in various tissues. Significantly higher glutamate concentrations have been reported in the plasma, platelet, CSF, and brain of migraine patients, as compared to healthy controls.

Table 1.
Glutamate concentrations in migraine patients vs. healthy individuals.
5. Dietary Components Affecting Glutamate Neurotoxicity and Migraine
Dietary factors may be one of the most important modifiable lifestyle components for treating migraines. There are specific micronutrients that protect against excitotoxicity caused by excess glutamate. These same micronutrients have also shown promising efficacy in migraine reduction in clinical settings. These nutrients are reviewed below (Figure 2 illustrates the protective mechanisms for each nutrient against excitotoxicity).
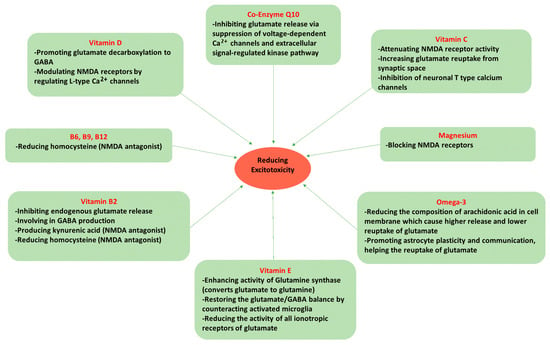
Figure 2.
Nutrients mechanism in protecting against excitotoxicity.
While outside the scope of this review, it should also be quickly noted that dietary factors can also affect the microbiome and that these important gut bacteria may also be influential in migraine. For a comprehensive review of what is known about the gut-brain axis in migraines, please refer to [97].
5.1. Omega-3 Fatty Acids
Omega-3 fatty acids are long-chain, polyunsaturated fatty acids that contribute to normal brain development and function [98]. Docosahexaenoic acid (DHA), a very long chain omega-3 fatty acid, has been identified as an important component of the lipid membrane of the CNS and an abundant phospholipid in the gray matter of the cerebral cortex [98]. Besides their structural function, omega-3s are a precursor for signaling molecules, as well as playing a role in neurotransmission and gene expression [99].
Both in vivo and in vitro evidence have shown beneficial effects of omega-3 derivations on nociception [100,101,102]. The essential omega-3 fatty acid, alpha-linolenic acid, showed a neuroprotective effect against glutamate-mediated excitotoxicity, a critical cause of neuronal injury in animal studies, including epilepsy [103], ischemia [104], stroke [105] and spinal cord injury [106]. One of the earliest studies providing evidence regarding the neuroprotective potential of omega-3 fatty acids was derived from an animal study investigating the effect of an omega-3-supplemented diet on neuronal damage, as compared to a control diet (using olive oil). The neuronal injuries were induced by middle cerebral artery occlusion and infusion of an NMDA receptor agonist, by the researchers. Rats supplemented with omega-3s had significantly reduced damage in both focal ischemia and excitotoxicity [107]. The underlying mechanism of this effect could be attributed to the change in membrane fatty acid composition. Arachidonic acid (an omega-6 fatty acid) has been reported to be associated with increased excitotoxicity by inducing a prolonged inhibition of glutamate reuptake into glial cells [108] and also increased release of glutamate into the synaptic cleft [109]. Therefore, substitution of omega-3 fatty acids for omega-6 could offer beneficial effects on excitotoxic brain damage. Additionally, eicosapentaenoic acid (EPA) and DHA (long-chain omega-3s) also showed promising benefits for protecting against monosodium glutamate (MSG) neurotoxicity in the hippocampus of prepubertal rats [110]. This neuroprotective effect of omega-3s could be attributed to their role in enhancing the plasticity, communication, and function of astrocytes [111]. Astrocytes are the major regulators of glutamate homeostasis and prevent excitotoxicity by taking glutamate up out of the synaptic cleft. This effect is supported by the finding that a lack of omega-3s can aggravate the negative impact of aging on astroglial morphology and activity [112]. In summary, omega-3 fatty acids may be effective in reducing excitotoxicity, making this an important class of nutrients for neurological protection.
Epidemiological research has shown an inverse association between dietary intake of omega-3s and the prevalence and characteristics of headache disorders including migraine [113,114]. Human clinical studies that investigated the potential effects of omega-3s on migraine suggest that omega-3 supplementation might improve migraine-related outcomes [115,116,117]. A meta-analysis indicated that omega-3s significantly reduced migraine duration; however, no significant change in terms of frequency or intensity was detected [118].
5.2. Magnesium (Mg2+)
Magnesium is an important intracellular mineral that plays vital roles in a wide range of metabolic reactions [119]. Magnesium is also critical for normal CNS function. It is involved with nerve transmission, the release of neurotransmitters, and protection against excitotoxicity [120]. Low levels of magnesium have been reported in many neurological disorders including Alzheimer’s disease [121], traumatic brain injury [122], stroke [123], epilepsy [124], Parkinson’s [125], psychiatric disorders [126], and migraine [127]. Low brain magnesium was also detected during a migraine attack using magnetic resonance spectroscopy in migraine patients [128].
There are several mechanisms underlying the anti-nociceptive effect of magnesium especially related to glutamate-mediated excitotoxicity. Magnesium blocks NMDA glutamate receptors, thereby protecting against excitotoxicity, and since NMDA receptor antagonists suppress trigeminal nociceptive transmission, this mineral could be a potential modulator of trigeminovascular nociception [34]. In a rat model of trigeminovascular activation, blocking NMDA receptors with either magnesium or memantine (an antagonist of NMDAR) inhibited nociceptive activation of the trigeminocervical complex [129]. In support of this effect on the NMDA receptor, a reduction in damage was observed in magnesium-treated mice who had induced excitotoxicity by ibotenate, a glutamate receptor agonist [130]. Moreover, in an animal model of cerebral ischemia, the extracellular level of glutamate in the cortex was reduced following magnesium administration [131]. In experimental models, magnesium also had an inhibitory effect on CSD [132,133] and deficiency in this mineral increases the sensitivity of NMDA receptors to glutamate-mediated CSD [134].
The effectiveness of magnesium has been extensively evaluated for migraine prevention. The results of a meta-analysis of randomized clinical trials indicated that oral magnesium significantly alleviated the frequency and severity of migraine, and intravenous magnesium was effective in relieving acute migraine attacks [135]. However, another meta-analysis investigating the effects of intravenous magnesium failed to show a beneficial effect in terms of pain relief [136].
5.3. Vitamin D
Vitamin D is a steroid hormone that is best known for its role in Ca2+ and phosphorus homeostasis and osteogenesis [137]. Notably, the beneficial effects of vitamin D extend well beyond mineral absorption and bone health. It is considered a neurosteroid because of its crucial role in neuronal integrity and brain development [138]. Vitamin D deficiency has been linked to neurological disorders [139]. Vitamin D receptors are broadly found in different parts of the brain including the cortex, hypothalamus, thalamus, hippocampus, and substantia nigra, supporting the potential role of vitamin D in different neurological conditions [140].
In vitro evidence has demonstrated the protective effects of vitamin D against glutamate excitotoxicity [141], which may be partially mediated by vitamin D’s role in gene transcription, affecting the production of key enzymes in the nervous system. Vitamin D deficiency can reduce glutamate decarboxylase levels in the brain. Glutamate decarboxylase is the enzyme that catalyzes the decarboxylation of glutamate to convert it into γ-aminobutyric acid (GABA), the main inhibitory neurotransmitter in the nervous system [142]. Thus, vitamin D can help prevent excitotoxicity indirectly by upregulating the production of the enzyme that increases the conversion of excitatory glutamate into inhibitory GABA. Notably, vitamin D may also reduce excitotoxicity via modulation of NMDA receptors by regulating Ca2+ influx through L-type voltage-sensitive Ca2+ channels [143].
Epidemiological studies evaluating serum levels of vitamin D in migraine patients have reported conflicting results, with some case-control studies showing no differences between migraine patients and healthy controls [144,145], and others observing significant differences [146,147]. However, a meta-analysis in 2020 summarizing the results from 8 observational studies reported overall significantly lower serum levels of 25(OH)D (the main circulating form of vitamin D) in migraine patients, as compared to healthy controls [148]. Additionally, the concentration of vitamin D in the blood has also been associated with migraine characteristics, as migraine patients with vitamin D deficiency are more likely to suffer frequent and severe attacks than migraine patients with adequate levels of vitamin D [146,149]. Vitamin D administration was found to be effective in alleviating migraine-related outcomes in a meta-analysis of five randomized controlled trials [150].
5.4. Vitamin C
Vitamin C, or ascorbic acid, is a water-soluble vitamin known mostly for its unique antioxidant properties [151]. Vitamin C has a critical role in antioxidant defense as well as many non-antioxidant activities in the CNS [151].
Ascorbic acid exerts a neuroprotective effect against excitotoxicity through attenuating NMDA receptor activity [152] and increasing glutamate reuptake from the synaptic cleft [153]. Vitamin C has also been shown to reduce oxidative stress induced by monosodium glutamate (MSG). In an experimental study on albino rats, vitamin C supplementation protected against degenerative changes in neurons and astrocytes in the cerebellar cortex induced by MSG [154]. Vitamin C also selectively inhibits T-type calcium channels in peripheral and central neurons, which are involved in the control of neuronal excitability [155]. Additionally, vitamin C neutralizes ROS, effectively addressing the oxidative stress caused by excitotoxicity. Therefore, it appears that vitamin C may possess multiple neuroprotective properties.
Despite the limited number of studies concerning the role of vitamin C in migraines, the evidence presented above supports the potential of vitamin C in fighting excitotoxicity, thereby preventing migraines. To date, the only randomized controlled trial related to this research area is a small pilot study that administered N-acetylcysteine, vitamin E, and vitamin C in migraine patients. They showed that this antioxidant combination significantly reduced the frequency, intensity, and duration of attacks, as well as the number of acute medications being used, as compared to the controlled group [156]. Clearly, more research is needed on vitamin C’s efficacy in migraine reduction.
5.5. Vitamin E
Vitamin E is a generic term for compounds called tocopherols and tocotrienols. Alpha-tocopherol is the main form (with the highest biological activity) found in human and animal tissue [157]. Vitamin E has been extensively studied for its antioxidant properties, as the dominant lipid-soluble, chain-breaking antioxidant in the body, which supports membrane integrity by preventing lipid peroxidation [157]. The brain has very high amounts of polyunsaturated fatty acids, making vitamin E essential for the antioxidant protection of these lipids [158].
In an experimental model of neuropathic pain, vitamin E had an analgesic effect by reducing central sensitization [159]. Vitamin E showed potential for fighting excitotoxicity, reducing glial cell activation, neuronal death, neuroinflammation, and oxidative stress in the hippocampus, in an epilepsy model [160,161]. A possible underlying mechanism is attributed to the regulatory effect of vitamin E on glutamine synthase activity, which is believed to be suppressed by oxidative stress [160,162]. Glutamine synthase converts glutamate to glutamine, a non-excitotoxic amino acid, to allow it to be safely shuttled from astrocytes to neurons before being recycled back to glutamate [162]. Vitamin E can also be involved in glutamate and GABA balance through counteracting microglial activation and the inflammatory cascade [163,164]. The cytokines released from microglia affect neuron excitability by modulating astrocytic glutamate receptors and transporters [165]. Substantial evidence from rodent and human studies indicates that inflammation causes downregulation of glutamate decarboxylase activity, which results in a lower conversion of glutamate into GABA, increasing the likelihood of excitotoxicity occurring [166,167,168,169]. In line with this evidence, it was shown that transgenic mice, which express increased levels of pro-inflammatory cytokines or chemokines, had lower levels of glutamate decarboxylase in the hippocampus and cerebellum [170]. Therefore, the anti-inflammatory effects of vitamin E may protect GABA production and vulnerability to more excitation. Furthermore, in vitro evidence has demonstrated that vitamin E reduces astrocytes’ permeability to Ca2+ and Na+ ions by inhibiting protein kinases and downregulating glutamate receptor genes [171].
Vitamin E, as a potential treatment option for migraine, has only been studied in regard to menstrual migraines [172]. A double-blind, placebo-controlled, crossover clinical trial indicated that vitamin E supplementation for five days during two menstrual cycles was associated with significant improvement in pain severity and functional disability [172]. A probable explanation for vitamin E efficacy as a prophylaxis of menstrual migraine is related to its inhibitory effect on phospholipase A2 and cyclooxygenase enzymes. This leads to inhibition of arachidonic acid release from cell membranes and its conversion to prostaglandin [173]. High levels of prostaglandin have been reported in the endometrium during menstruation and in the serum during the premenstrual phase [174]. The inhibitory effect of vitamin E on phospholipase A2 is of substantial value since there is evidence showing that the enzyme targets other intracellular membranes including the mitochondrial membrane as well [175]. Mitochondrial membrane damage is associated with high ROS production, oxidative stress, and ultimately cell death [176]. It is worth noting that antioxidants work together to maintain themselves in an active state, so despite their unique functions in redox balance, they can also be indirectly involved in each other’s activity as well. The benefits described in the aforementioned study looking at the combined effects of vitamin E, vitamin C, and N-acetylcysteine, may have partially been due to these interactive effects of combining antioxidants [156].
5.6. Riboflavin (Vitamin B2)
Riboflavin, also known as vitamin B2, is involved in various metabolic pathways through two coenzyme forms including flavin adenine dinucleotide (FAD) and flavin mononucleotide (FMN) [177]. In addition to riboflavin’s critical role in energy metabolism, it also has antioxidant function and plays a pivotal role in the metabolism of vitamin B6 (converting dietary pyridoxine into its active form pyridoxal L-phosphate), as well as having roles in DNA repair, and apoptosis [177]. Therefore, deficiency or any disturbance in riboflavin metabolism can contribute to broad-spectrum dysfunction including cardiovascular, neuromuscular, immune, and neurological abnormalities [177].
Riboflavin has direct and indirect ameliorating effects on glutamate excitotoxicity which is implicated in migraine pain. The reduction in voltage-gated Ca2+ channel activity by riboflavin can inhibit endogenous glutamate release by inhibiting glutamate exocytosis in synaptic clefts [178]. In addition, experimental studies demonstrated the neuroprotective effects of riboflavin and pyridoxal phosphate (PLP) on excitotoxicity [179]. As mentioned earlier, riboflavin is involved in the formation of PLP, which is required for the production of many neurotransmitters in the CNS [180]. Very importantly, the conversion of glutamate to GABA, the major inhibitory neurotransmitter in the nervous system, by glutamic acid decarboxylase, necessitates PLP as a cofactor [181]. Therefore, it is not surprising that deficiency in riboflavin and consequent reduction in PLP formation contribute to the elevation of glutamate and reduction in GABA levels, thereby resulting in excitotoxicity. These two vitamins are also crucial for the kynurenine pathway, which is considered the major pathway for the catabolism, or breakdown, of tryptophan [182]. Adequacy of riboflavin and PLP has been linked to the production of kynurenic acid which is a protective antagonist of NMDA and all ionotropic glutamate receptors [183]. Deficiency of these cofactors can lead to further metabolism down the pathway causing the production of quinolinic acid, which is an extremely neurotoxic metabolite that increases the risk of excitotoxicity in multiple ways [184,185].
Riboflavin is one of the most studied vitamins for migraine prophylaxis. In this regard, clinical studies on adult migraine patients have shown very promising results [186,187,188]. A pooled analysis of 8 randomized controlled clinical trials indicated a significant reduction in terms of migraine days, frequency, pain intensity, and duration of attacks following 400 mg/day riboflavin supplementation for three months [189]. Currently, the American Academy of Neurology (level B evidence) recommends 400 mg per day for adult migraineurs [190], as compared to the current recommended dietary allowance of 1.1–1.6 mg per day. It should be noted, that as a water-soluble vitamin, the excess is just being excreted (as can be seen by fluorescent-colored urine when you take riboflavin), and thus, such high doses are likely not needed for benefiting migraine patients. Although not all available evidence is obtained from high-quality trials, due to riboflavin’s low cost, high tolerability, and effectiveness in migraine alleviation in the majority of research, it could be considered an advantageous vitamin for migraine [191].
5.7. Vitamin B6 (Pyridoxine), Folate (Vitamin B9), and Vitamin B12 (Cobalamin)
Vitamins B6, B9, and B12 (in addition to riboflavin) play a key role in one-carbon metabolism, and their deficiency has been linked to elevated levels of homocysteine (Hcy) [192]. Hcy is another neurotoxic metabolite that has the ability to activate NMDA receptors, and vitamins B6, folate, and B12 can protect against its accumulation [192,193].
An experimental model of pain induced by acetic acid demonstrated the antinociceptive effects of B vitamins [194]. However, it seems the effectiveness of these vitamins in migraine prophylaxis could be attributed to their effect on lowering Hcy for the most part. Notably, the role of riboflavin in the production of the active form of pyridoxine makes it indirectly involved in this pathway as well [192]. The presence of high levels of Hcy in the brain might act as a trigger or amplifier in a variety of ways [195]. Hcy has a known neurotoxic effect via direct stimulation of NMDA receptors and consequent excitotoxicity [193]. Previously, it has also been shown that Hcy acts as an antagonist to GABA-A receptors, influencing the migraine pain threshold negatively [196]. Homocysteine also contributes to the breakdown of the extracellular matrix which affects BBB integrity [196]. An increase in brain microvascular permeability was also observed in mice with hyperhomocysteinemia via the activation of matrix metalloproteinases, which lead to vascular remodeling and BBB disruption [197].
Migraine, especially migraine with aura, is associated with a risk of ischemic stroke [198], and elevated levels of Hcy in migraineurs have been identified as a potential risk factor for stroke, as reported by epidemiological studies [199,200]. Evidence showing the effectiveness of B6, B9, and B12 vitamins on Hcy level reduction encouraged trials to explore the beneficial effects of Hcy-lowering vitamins. In a double-blind randomized controlled trial by Askari et al., 3 months of supplementation with folic acid plus pyridoxine in migraine patients with aura, led to significant improvement in migraine characteristics compared to placebo [201]; while in the migraine group that received folic acid alone, no significant change was detected in comparison with the placebo group [201]. One clinical trial tested pyridoxine supplementation for migraine patients with aura, and the authors reported a reduction in the severity and duration of attacks, but no effects on the frequency of attacks were noted [202].
5.8. Coenzyme Q10 (CoQ10)
CoQ10 is a fat-soluble compound mostly found in animal proteins, but also in beans, nuts, seeds, and avocado [203]. Our body can synthesize CoQ10, thus its dietary intake is not considered essential. However, evidence has shown that CoQ10 deficiency can occur secondary to several mitochondrial disorders, aging, and in those using statins (for lowering cholesterol) [204,205]. As mentioned before, migraine patients are prone to mitochondrial dysfunction as a result of excitotoxicity-mediated oxidative stress. CoQ10 plays a key role in energy production in mitochondria and also acts as an antioxidant in cell membranes [203]. Interestingly, it is involved in the restoration of the oxidized form of vitamin E, helping to restore vitamin E’s antioxidant function [206].
Preclinical evidence supports the protective effect of CoQ10 against excitotoxicity. In a mouse model of glaucoma, a diet supplemented with CoQ10 ameliorated glutamate excitotoxicity and oxidative stress compared to an un-supplemented control diet [207]. In another study, the effect of CoQ10 on the endogenous release of glutamate in the cerebral cortex was evaluated [208]. The findings suggested that CoQ10 inhibited glutamate release from cortical synaptosomes in rats via suppression of the presynaptic voltage-dependent Ca2+ channels and extracellular signal-regulated kinase pathway. Water-soluble CoQ10 (Ubisol-Q10) has also been shown to reduce glutamate-induced cell death in an in vitro model [209]. Murine hippocampal neuronal cells were exposed to glutamate, 24 h after Ubisol-Q10 treatment. The results indicated that CoQ10 protects the neuronal cells by preserving mitochondrial function and structure.
The beneficial impact of CoQ10 supplementation on migraine-related outcomes has been tested in several clinical studies [210,211,212,213]. The pooled result of the most recent meta-analysis of 6 studies supports the idea that CoQ10 supplementation can reduce the frequency and duration of migraine attacks but does not reduce severity [214].
6. Gap between Pathophysiology of Migraine and Interventions: Where Do We Stand Now?
The contribution of glutamate to neuropathological aspects of migraine has led to the development of several glutamate antagonists as migraine prophylactic drugs [215,216,217,218]. However, these drugs have limited utility and a high probability of side effects [219,220].
Clinical trials in migraineurs have provided supportive findings for all reviewed nutrients including riboflavin, folate, pyridoxine, cobalamin, vitamin D, C, E, magnesium, and omega-3 fatty acids. These nutrients have shown potential for alleviating excitotoxicity as well. Given the evidence indicating nutrient deficiency among migraineurs [148,200,221], replenishment of these nutrients seems reasonable. However, dietary nutrients are often studied one at a time, which inhibits potential synergism and cooperative effects between nutrients from being observed. Thus, applying a more comprehensive dietary approach may yield greater results.
Notably, besides being an endogenous source, glutamate is a non-essential amino acid found in the diet [222]. In a normal situation, the amount of dietary glutamate entering the brain is regulated by saturable transporters on the BBB [223]. However, considering the probability of diminished BBB integrity in migraine patients [224,225,226,227,228], it is likely that the amount of dietary glutamate entering the brain is higher than in healthy individuals. This idea is supported by multiple studies that have shown that MSG administration can induce headaches [229,230,231]. Moreover, some dietary components could have a triggering effect on a migraine attack as reported by patients in epidemiological studies [232,233]. The contribution of dietary triggers in migraines was the basis for the development of elimination diet strategies [234]. However, precisely determining random food triggers is challenging, and a diet that is overly restrictive can have a long-term negative effect on nutritional status [235,236]. Until now, no specific diet has been developed for migraine prevention, and several proposed diets have shown varying levels of efficacy [237]. Taken together, both dietary triggers and nutrient intake might be key to therapeutic benefits in migraine, which has not been possible with current interventions.
Our team previously administered a diet-based intervention called the “low glutamate diet” in patients with widespread chronic pain disorders [222,238,239]. This diet removes free forms (i.e., not bound to a protein) of glutamate and aspartate (mainly by restricting food additives with excitotoxins, in addition to a few foods that are naturally high in glutamate/aspartate such as soy sauce, fish sauce, and aged cheeses), while also emphasizing the intake of foods high in the micronutrients reviewed above [238]. This diet has shown benefits for widespread chronic pain conditions [222,240], including Gulf War illness (GWI) [238]. Interestingly, all of these studies have demonstrated widespread symptom improvement, including reduced reports of migraines. Figure 3 illustrates the significant reduction in migraine in veterans with Gulf War Illness after one month on the diet. Moreover, most subjects reported going from multiple weekly migraines to no migraines during the diet month. We believe these symptom improvements in patients suffering from widespread chronic pain disorders stem from reductions in central sensitization (from reduced excitotoxicity) and potentially corresponding improvements in the inter-related occurrence of oxidative stress and neuroinflammation. More in-depth research is warranted to further explore whether or not the low glutamate diet may be used as an effective treatment for migraine.
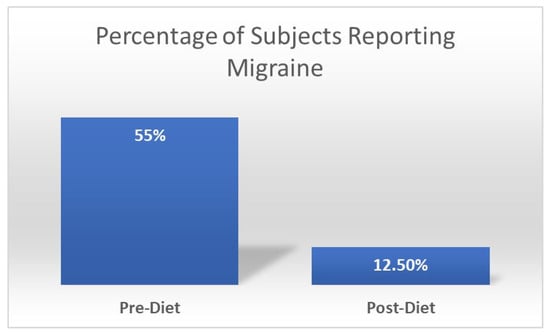
Figure 3.
Change in percentage of subjects with Gulf War Illness (n = 40) reporting migraine before and after one month on the low glutamate diet. Chi-square significance of p = 0.04.
7. Conclusions
Glutamate-mediated excitotoxicity is associated with a wide range of neurological disorders including migraine. The proposed mechanisms include the direct effect of excitotoxicity on neuronal injury or death, or its contribution to neuroinflammation, oxidative stress, blood-brain barrier permeability, and cerebral vasodilation, all of which are associated with migraine pathophysiology. Available evidence supports the role of several nutrients in protecting against excitotoxicity including riboflavin, folate, pyridoxine (vitamin B6), cobalamin (vitamin B12), vitamin D, C, E, magnesium, and omega-3 fatty acids. Additional evidence also suggests that supporting endogenous production of CoQ10 with increased dietary intake may also be protective. Interestingly, clinical data support the role of these nutrients in improving migraines as well, providing a strong rationale for designing effective interventions. There is an obvious gap between our understanding of migraines and the dietary strategies which have been administered so far, since dietary nutrients are often studied separately, and no specific diet for migraine has been developed. However, the beneficial effects of the low glutamate diet on widespread chronic pain disorders appear to have overlapping mechanistic effects, and additionally is some preliminary evidence supporting an effect on migraine. Thus, further research on this dietary strategy in migraine is warranted.
Author Contributions
F.M. was responsible for literature search and writing the first draft of the paper. K.F.H. was responsible for the design, revise and final version of the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
TG: Trigeminovascular, NMDA: N-methyl-D-aspartate, CSD: Cortical spreading depression, CGRP: Calcitonin gene-related peptide, SP: Substance P, CNS: Central nervous system, iGluRs: Ionotropic glutamate receptors, AMPA: α-amino-3-hydroxy-5-methyl-4-isoxazole propionate, KA: Kainic acid, mGluRs: Metabotropic receptors, Ca2+: Calcium, MS: Multiple sclerosis, BBB: Blood brain barrier, ICHD: International Classification of Headache Disorders, ROS: Reactive oxygen species, NO: Nitric oxide, cGMP: Cyclic guanosine monophosphate, NOS: Nitric oxide synthase, CSF: Cerebrospinal fluid, DHA: Docosahexaenoic acid, EPA: Eicosapentaenoic acid, MSG: monosodium glutamate, Mg2+: magnesium, GABA: γ-Aminobutyric acid, FAD: Flavin adenine dinucleotide, FMN: Flavin mononucleotide, PLP: Pyridoxal phosphate, Hcy: Homocysteine, GWI: Gulf war illness.
References
- Woldeamanuel, Y.W.; Cowan, R.P. Migraine affects 1 in 10 people worldwide featuring recent rise: A systematic review and meta-analysis of community-based studies involving 6 million participants. J. Neurol. Sci. 2017, 372, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Stovner, L.J.; Zwart, J.A.; Hagen, K.; Terwindt, G.; Pascual, J. Epidemiology of headache in Europe. Eur. J. Neurol. 2006, 13, 333–345. [Google Scholar] [CrossRef] [PubMed]
- Steiner, T.J.; Stovner, L.J.; Vos, T.; Jensen, R.; Katsarava, Z. Migraine is First Cause of Disability in Under 50s: Will Health Politicians Now Take Notice? Springer: Berlin/Heidelberg, Germany, 2018; Volume 19, pp. 1–4. [Google Scholar]
- Bonafede, M.; Sapra, S.; Shah, N.; Tepper, S.; Cappell, K.; Desai, P. Direct and indirect healthcare resource utilization and costs among migraine patients in the United States. Headache J. Head Face Pain 2018, 58, 700–714. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-J.; Chen, P.-K.; Fuh, J.-L. Comorbidities of migraine. Front. Neurol. 2010, 1, 16. [Google Scholar] [CrossRef]
- Katsarava, Z.; Mania, M.; Lampl, C.; Herberhold, J.; Steiner, T.J. Poor medical care for people with migraine in Europe–evidence from the Eurolight study. J. Headache Pain 2018, 19, 10. [Google Scholar] [CrossRef]
- Ashina, M.; Katsarava, Z.; Do, T.P.; Buse, D.C.; Pozo-Rosich, P.; Özge, A.; Krymchantowski, A.V.; Lebedeva, E.R.; Ravishankar, K.; Yu, S. Migraine: Epidemiology and systems of care. Lancet 2021, 397, 1485–1495. [Google Scholar] [CrossRef]
- Ashina, M.; Hansen, J.M.; Do, T.P.; Melo-Carrillo, A.; Burstein, R.; Moskowitz, M.A. Migraine and the trigeminovascular system—40 years and counting. Lancet Neurol. 2019, 18, 795–804. [Google Scholar] [CrossRef]
- May, A.; Goadsby, P.J. The Trigeminovascular System in Humans: Pathophysiologic Implications for Primary Headache Syndromes of the Neural Influences on the Cerebral Circulation. J. Cereb. Blood Flow Metab. 1999, 19, 115–127. [Google Scholar] [CrossRef]
- Ramadan, N.M. The link between glutamate and migraine. CNS Spectr. 2003, 8, 446–449. [Google Scholar] [CrossRef]
- Vikelis, M.; Mitsikostas, D.D. The role of glutamate and its receptors in migraine. CNS Neurol. Disord.-Drug Targets (Former. Curr. Drug Targets-CNS Neurol. Disord.) 2007, 6, 251–257. [Google Scholar]
- Guerrero-Toro, C.; Koroleva, K.; Ermakova, E.; Gafurov, O.; Abushik, P.; Tavi, P.; Sitdikova, G.; Giniatullin, R. Testing the Role of Glutamate NMDA Receptors in Peripheral Trigeminal Nociception Implicated in Migraine Pain. Int. J. Mol. Sci. 2022, 23, 1529. [Google Scholar] [CrossRef] [PubMed]
- Lauritzen, M.; Hansen, A.J. The effect of glutamate receptor blockade on anoxic depolarization and cortical spreading depression. J. Cereb. Blood Flow Metab. 1992, 12, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Gallai, V.; Alberti, A.; Gallai, B.; Coppola, F.; Floridi, A.; Sarchielli, P. Glutamate and nitric oxide pathway in chronic daily headache: Evidence from cerebrospinal fluid. Cephalalgia 2003, 23, 166–174. [Google Scholar] [CrossRef]
- Sarchielli, P.; Filippo, M.; Nardi, K.; Calabresi, P. Sensitization, glutamate, and the link between migraine and fibromyalgia. Curr. Pain Headache Rep. 2007, 11, 343–351. [Google Scholar] [CrossRef]
- Ramachandran, R. Neurogenic inflammation and its role in migraine. In Seminars in Immunopathology; Springer: Berlin/Heidelberg, Germany, 2018; pp. 301–314. [Google Scholar]
- Landy, S.; Rice, K.; Lobo, B. Central sensitisation and cutaneous allodynia in migraine: Implications for treatment. CNS Drugs 2004, 18, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Latremoliere, A.; Woolf, C.J. Central sensitization: A generator of pain hypersensitivity by central neural plasticity. J. Pain 2009, 10, 895–926. [Google Scholar] [CrossRef]
- Woolf, C.J.; Thompson, S.W. The induction and maintenance of central sensitization is dependent on N-methyl-D-aspartic acid receptor activation; implications for the treatment of post-injury pain hypersensitivity states. Pain 1991, 44, 293–299. [Google Scholar] [CrossRef]
- Olney, J. Excitotoxicity: An overview. Can. Dis. Wkly. Rep.=Rapp. Hebd. Mal. Can. 1990, 16, 47–57, discussion 57. [Google Scholar]
- Holton, K.F. Micronutrients May Be a Unique Weapon Against the Neurotoxic Triad of Excitotoxicity, Oxidative Stress and Neuroinflammation: A Perspective. Front. Neurosci. 2021, 15, 726457. [Google Scholar] [CrossRef]
- Willard, S.S.; Koochekpour, S. Glutamate, glutamate receptors, and downstream signaling pathways. Int. J. Biol. Sci. 2013, 9, 948. [Google Scholar] [CrossRef]
- Riedel, G.; Platt, B.; Micheau, J. Glutamate receptor function in learning and memory. Behav. Brain Res. 2003, 140, 1–47. [Google Scholar] [CrossRef] [PubMed]
- Ohgi, Y.; Futamura, T.; Hashimoto, K. Glutamate signaling in synaptogenesis and NMDA receptors as potential therapeutic targets for psychiatric disorders. Curr. Mol. Med. 2015, 15, 206–221. [Google Scholar] [CrossRef] [PubMed]
- Reiner, A.; Levitz, J. Glutamatergic signaling in the central nervous system: Ionotropic and metabotropic receptors in concert. Neuron 2018, 98, 1080–1098. [Google Scholar] [CrossRef]
- Sattler, R.; Tymianski, M. Molecular mechanisms of glutamate receptor-mediated excitotoxic neuronal cell death. Mol. Neurobiol. 2001, 24, 107–129. [Google Scholar] [CrossRef] [PubMed]
- Skeberdis, V.A.; Lan, J.-y.; Opitz, T.; Zheng, X.; Bennett, M.V.; Zukin, R.S. mGluR1-mediated potentiation of NMDA receptors involves a rise in intracellular calcium and activation of protein kinase C. Neuropharmacology 2001, 40, 856–865. [Google Scholar] [CrossRef]
- Plitman, E.; Nakajima, S.; de la Fuente-Sandoval, C.; Gerretsen, P.; Chakravarty, M.M.; Kobylianskii, J.; Chung, J.K.; Caravaggio, F.; Iwata, Y.; Remington, G. Glutamate-mediated excitotoxicity in schizophrenia: A review. Eur. Neuropsychopharmacol. 2014, 24, 1591–1605. [Google Scholar] [CrossRef]
- Dong, X.-x.; Wang, Y.; Qin, Z.-h. Molecular mechanisms of excitotoxicity and their relevance to pathogenesis of neurodegenerative diseases. Acta Pharmacol. Sin. 2009, 30, 379–387. [Google Scholar] [CrossRef]
- Pitt, D.; Werner, P.; Raine, C.S. Glutamate excitotoxicity in a model of multiple sclerosis. Nat. Med. 2000, 6, 67–70. [Google Scholar] [CrossRef]
- Olney, J.; Collins, R.; Sloviter, R. Excitotoxic mechanisms of epileptic brain damage. Adv. Neurol. 1986, 44, 857–877. [Google Scholar]
- Petrenko, A.B.; Shimoji, K. A possible role for glutamate receptor-mediated excitotoxicity in chronic pain. J. Anesth. 2001, 15, 39–48. [Google Scholar] [CrossRef]
- Longoni, M.; Ferrarese, C. Inflammation and excitotoxicity: Role in migraine pathogenesis. Neurol. Sci. 2006, 27, s107–s110. [Google Scholar] [CrossRef]
- Storer, R.; Goadsby, P. Trigeminovascular nociceptive transmission involves N-methyl-D-aspartate and non-N-methyl-D-aspartate glutamate receptors. Neuroscience 1999, 90, 1371–1376. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Montoya, J.; Avendaño, C.; Negredo, P. The Glutamatergic System in Primary Somatosensory Neurons and Its Involvement in Sensory Input-Dependent Plasticity. Int. J. Mol. Sci. 2018, 19, 69. [Google Scholar] [CrossRef] [PubMed]
- Tallaksen-Greene, S.J.; Young, A.B.; Penney, J.B.; Beitz, A.J. Excitatory amino acid binding sites in the trigeminal principal sensory and spinal trigeminal nuclei of the rat. Neurosci. Lett. 1992, 141, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Quartu, M.; Serra, M.P.; Ambu, R.; Lai, M.L.; Del Fiacco, M. AMPA-type glutamate receptor subunits 2/3 in the human trigeminal sensory ganglion and subnucleus caudalis from prenatal ages to adulthood. Mech. Ageing Dev. 2002, 123, 463–471. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, Y.; Luo, W.; Hua, X.; Wamalwa, P.; Wang, J.; Zhao, Z.; Lu, Y.; Liao, Z.; Lai, W. Trigeminal expression of N-methyl-D-aspartate receptor subunit 1 and behavior responses to experimental tooth movement in rats. Angle Orthod. 2009, 79, 951–957. [Google Scholar] [CrossRef]
- Sahara, Y.; Noro, N.; Iida, Y.; Soma, K.; Nakamura, Y. Glutamate receptor subunits GluR5 and KA-2 are coexpressed in rat trigeminal ganglion neurons. J. Neurosci. 1997, 17, 6611–6620. [Google Scholar] [CrossRef]
- Zhou, S.; Bonasera, L.; Carlton, S.M. Peripheral administration of NMDA, AMPA or KA results in pain behaviors in rats. Neuroreport 1996, 7, 895–900. [Google Scholar] [CrossRef]
- Lawand, N.B.; Willis, W.D.; Westlund, K.N. Excitatory amino acid receptor involvement in peripheral nociceptive transmission in rats. Eur. J. Pharmacol. 1997, 324, 169–177. [Google Scholar] [CrossRef]
- Raigorodsky, G.; Urca, G. Spinal antinociceptive effects of excitatory amino acid antagonists: Quisqualate modulates the action of N-methyl-D-aspartate. Eur. J. Pharmacol. 1990, 182, 37–47. [Google Scholar] [CrossRef]
- Crivellaro, G.; Tottene, A.; Vitale, M.; Melone, M.; Casari, G.; Conti, F.; Santello, M.; Pietrobon, D. Specific activation of GluN1-N2B NMDA receptors underlies facilitation of cortical spreading depression in a genetic mouse model of migraine with reduced astrocytic glutamate clearance. Neurobiol. Dis. 2021, 156, 105419. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.; Tozzi, A.; Rainero, I.; Cupini, L.M.; Calabresi, P.; Ayata, C.; Sarchielli, P. Cortical spreading depression as a target for anti-migraine agents. J. Headache Pain 2013, 14, 62. [Google Scholar] [PubMed]
- De Simone, R.; Ranieri, A.; Montella, S.; Bonavita, V. Cortical spreading depression and central pain networks in trigeminal nuclei modulation: Time for an integrated migraine pathogenesis perspective. Neurol. Sci. 2013, 34, 51–55. [Google Scholar] [CrossRef][Green Version]
- Footitt, D.R.; Newberry, N.R. Cortical spreading depression induces an LTP-like effect in rat neocortex in vitro. Brain Res. 1998, 781, 339–342. [Google Scholar] [CrossRef]
- Richter, F.; Lehmenkühler, A. Cortical spreading depression (CSD). Der Schmerz 2008, 22, 544–550. [Google Scholar] [CrossRef] [PubMed]
- Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018, 38, 1–211. [CrossRef] [PubMed]
- Cutrer, F.M.; Huerter, K. Migraine aura. Neurologist 2007, 13, 118–125. [Google Scholar] [CrossRef]
- Dodick, D.; Silberstein, S. Central sensitization theory of migraine: Clinical implications. Headache J. Head Face Pain 2006, 46, S182–S191. [Google Scholar] [CrossRef]
- Nihi, M.A.; Santos, P.S.F.; Almeida, D.B. Central sensitization in episodic and chronic migraine. Headache Med. 2020, 11, 85–89. [Google Scholar] [CrossRef]
- Nijs, J.; Meeus, M.; Versijpt, J.; Moens, M.; Bos, I.; Knaepen, K.; Meeusen, R. Brain-derived neurotrophic factor as a driving force behind neuroplasticity in neuropathic and central sensitization pain: A new therapeutic target? Expert Opin. Ther. Targets 2015, 19, 565–576. [Google Scholar] [CrossRef]
- Park, C.G.; Chu, M.K. Interictal plasma glutamate levels are elevated in individuals with episodic and chronic migraine. Sci. Rep. 2022, 12, 6921. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Suzuki, S.; Shiina, T.; Kobayashi, S.; Hirata, K. Central sensitization in migraine: A narrative review. J. Pain Res. 2022, 15, 2673–2682. [Google Scholar] [CrossRef] [PubMed]
- Delussi, M.; Vecchio, E.; Libro, G.; Quitadamo, S.; De Tommaso, M. Failure of preventive treatments in migraine: An observational retrospective study in a tertiary headache center. BMC Neurol. 2020, 20, 256. [Google Scholar] [CrossRef]
- Barbanti, P.; Aurilia, C.; Cevoli, S.; Egeo, G.; Fofi, L.; Messina, R.; Salerno, A.; Torelli, P.; Albanese, M.; Carnevale, A. Long-term (48 weeks) effectiveness, safety, and tolerability of erenumab in the prevention of high-frequency episodic and chronic migraine in a real world: Results of the EARLY 2 study. Headache J. Head Face Pain 2021, 61, 1351–1363. [Google Scholar] [CrossRef] [PubMed]
- Bono, F.; Salvino, D.; Mazza, M.; Curcio, M.; Trimboli, M.; Vescio, B.; Quattrone, A. The influence of ictal cutaneous allodynia on the response to occipital transcutaneous electrical stimulation in chronic migraine and chronic tension-type headache: A randomized, sham-controlled study. Cephalalgia 2015, 35, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Campos-Bedolla, P.; Walter, F.R.; Veszelka, S.; Deli, M.A. Role of the blood–brain barrier in the nutrition of the central nervous system. Arch. Med. Res. 2014, 45, 610–638. [Google Scholar]
- Vazana, U.; Veksler, R.; Pell, G.S.; Prager, O.; Fassler, M.; Chassidim, Y.; Roth, Y.; Shahar, H.; Zangen, A.; Raccah, R.; et al. Glutamate-Mediated Blood–Brain Barrier Opening: Implications for Neuroprotection and Drug Delivery. J. Neurosci. 2016, 36, 7727–7739. [Google Scholar] [CrossRef]
- Toklu, H.Z.; Tümer, N. Oxidative Stress, Brain Edema, Blood–Brain Barrier Permeability, and Autonomic Dysfunction from Traumatic Brain Injury; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2015. [Google Scholar]
- Galea, I. The blood–brain barrier in systemic infection and inflammation. Cell. Mol. Immunol. 2021, 18, 2489–2501. [Google Scholar] [CrossRef]
- Jones, K.H.; Dechkovskaia, A.M.; Herrick, E.A.; Abdel-Rahman, A.A.; Khan, W.A.; Abou-Donia, M.B. Subchronic effects following a single sarin exposure on blood-brain and blood-testes barrier permeability, acetylcholinesterase, and acetylcholine receptors in the central nervous system of rat: A dose-response study. J. Toxicol. Environ. Health Part A 2000, 61, 695–707. [Google Scholar] [CrossRef]
- Xu, G.; Li, Y.; Ma, C.; Wang, C.; Sun, Z.; Shen, Y.; Liu, L.; Li, S.; Zhang, X.; Cong, B. Restraint stress induced hyperpermeability and damage of the blood-brain barrier in the amygdala of adult rats. Front. Mol. Neurosci. 2019, 12, 32. [Google Scholar] [CrossRef]
- Petty, M.A.; Lo, E.H. Junctional complexes of the blood–brain barrier: Permeability changes in neuroinflammation. Prog. Neurobiol. 2002, 68, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Lochhead, J.J.; McCaffrey, G.; Quigley, C.E.; Finch, J.; DeMarco, K.M.; Nametz, N.; Davis, T.P. Oxidative stress increases blood–brain barrier permeability and induces alterations in occludin during hypoxia–reoxygenation. J. Cereb. Blood Flow Metab. 2010, 30, 1625–1636. [Google Scholar] [CrossRef]
- Rogoz, K.; Andersen, H.H.; Kullander, K.; Lagerström, M.C. Glutamate, substance P, and calcitonin gene-related peptide cooperate in inflammation-induced heat hyperalgesia. Mol. Pharmacol. 2014, 85, 322–334. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.-R.; Xu, Z.-Z.; Gao, Y.-J. Emerging targets in neuroinflammation-driven chronic pain. Nat. Rev. Drug Discov. 2014, 13, 533–548. [Google Scholar] [CrossRef]
- Rueda, C.B.; Llorente-Folch, I.; Traba, J.; Amigo, I.; Gonzalez-Sanchez, P.; Contreras, L.; Juaristi, I.; Martinez-Valero, P.; Pardo, B.; Del Arco, A. Glutamate excitotoxicity and Ca2+-regulation of respiration: Role of the Ca2+ activated mitochondrial transporters (CaMCs). Biochim. Biophys. Acta (BBA)-Bioenerg. 2016, 1857, 1158–1166. [Google Scholar] [CrossRef] [PubMed]
- Lafon-Cazal, M.; Pietri, S.; Culcasi, M.; Bockaert, J. NMDA-dependent superoxide production and neurotoxicity. Nature 1993, 364, 535–537. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial ROS-induced ROS release: An update and review. Biochim. Biophys. Acta (BBA)-Bioenerg. 2006, 1757, 509–517. [Google Scholar] [CrossRef]
- Garthwaite, J.; Charles, S.L.; Chess-Williams, R. Endothelium-derived relaxing factor release on activation of NMDA receptors suggests role as intercellular messenger in the brain. Nature 1988, 336, 385–388. [Google Scholar] [CrossRef]
- Christopherson, K.S.; Hillier, B.J.; Lim, W.A.; Bredt, D.S. PSD-95 Assembles a Ternary Complex with theN-Methyl-D-aspartic Acid Receptor and a Bivalent Neuronal NO Synthase PDZ Domain. J. Biol. Chem. 1999, 274, 27467–27473. [Google Scholar] [CrossRef]
- LeMaistre, J.L.; Sanders, S.A.; Stobart, M.J.; Lu, L.; Knox, J.D.; Anderson, H.D.; Anderson, C.M. Coactivation of NMDA receptors by glutamate and-serine induces dilation of isolated middle cerebral arteries. J. Cereb. Blood Flow Metab. 2012, 32, 537. [Google Scholar] [CrossRef] [PubMed]
- Goadsby, P.J.; Holland, P.R.; Martins-Oliveira, M.; Hoffmann, J.; Schankin, C.; Akerman, S. Pathophysiology of migraine: A disorder of sensory processing. Physiol. Rev. 2017, 97, 553–622. [Google Scholar] [CrossRef] [PubMed]
- Shevel, E. The extracranial vascular theory of migraine—A great story confirmed by the facts. Headache J. Head Face Pain 2011, 51, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.D.; Cizkova, D. The role of nitric oxide in nociception. Curr. Rev. Pain 2000, 4, 459–466. [Google Scholar] [CrossRef]
- Thiel, V.E.; Audus, K.L. Nitric oxide and blood–brain barrier integrity. Antioxid. Redox Signal. 2001, 3, 273–278. [Google Scholar] [CrossRef]
- Mayhan, W.G. Nitric oxide donor-induced increase in permeability of the blood–brain barrier. Brain Res. 2000, 866, 101–108. [Google Scholar] [CrossRef]
- Ferrari, M.D.; Odink, J.; Bos, K.; Malessy, M.; Bruyn, G. Neuroexcitatory plasma amino acids are elevated in migraine. Neurology 1990, 40, 1582. [Google Scholar] [CrossRef]
- Alam, Z.; Coombes, N.; Waring, R.H.; Williams, A.C.; Steventon, G.B. Plasma levels of neuroexcitatory amino acids in patients with migraine or tension headache. J. Neurol. Sci. 1998, 156, 102–106. [Google Scholar] [CrossRef]
- Martínez, F.; Castillo, J.; Rodríguez, J.R.; Leira, R.; Noya, M. Neuroexcitatory amino acid levels in plasma and cerebrospinal fluid during migraine attacks. Cephalalgia 1993, 13, 89–93. [Google Scholar] [CrossRef]
- Peres, M.; Zukerman, E.; Soares, C.S.; Alonso, E.; Santos, B.; Faulhaber, M. Cerebrospinal fluid glutamate levels in chronic migraine. Cephalalgia 2004, 24, 735–739. [Google Scholar] [CrossRef]
- D’andrea, G.; Cananzi, A.; Joseph, R.; Morra, M.; Zamberlan, F.; Milone, F.F.; Grunfeld, S.; Welch, K. Platelet glycine, glutamate and aspartate in primary headache. Cephalalgia 1991, 11, 197–200. [Google Scholar] [CrossRef]
- Cananzi, A.; D’andrea, G.; Perini, F.; Zamberlan, F.; Welch, K. Platelet and plasma levels of glutamate and glutamine in migraine with and without aura. Cephalalgia 1995, 15, 132–135. [Google Scholar] [CrossRef] [PubMed]
- Vaccaro, M.; Riva, C.; Tremolizzo, L.; Longoni, M.; Aliprandi, A.; Agostoni, E.; Rigamonti, A.; Leone, M.; Bussone, G.; Ferrarese, C. Platelet glutamate uptake and release in migraine with and without aura. Cephalalgia 2007, 27, 35–40. [Google Scholar] [CrossRef]
- Vieira, D.S.d.; Naffah-Mazzacoratti, M.d.G.; Zukerman, E.; Soares, C.A.S.; Cavalheiro, E.A.; Peres, M.F.P. Glutamate levels in cerebrospinal fluid and triptans overuse in chronic migraine. Headache J. Head Face Pain 2007, 47, 842–847. [Google Scholar] [CrossRef]
- Zukerman, E.; Minatti-Hannuch, S.; Mazzacoratti, M.; dos Reis Filho, J.; Cavalheiro, E. Cerebrospinal fluid neurotransmitter amino acids in migraine. Cephalalgia 1993, 13, 92. [Google Scholar]
- Ferrari, A.; Spaccalopelo, L.; Pinetti, D.; Tacchi, R.; Bertolini, A. Effective prophylactic treatments of migraine lower plasma glutamate levels. Cephalalgia 2009, 29, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Peek, A.L.; Rebbeck, T.; Puts, N.A.; Watson, J.; Aguila, M.-E.R.; Leaver, A.M. Brain GABA and glutamate levels across pain conditions: A systematic literature review and meta-analysis of 1H-MRS studies using the MRS-Q quality assessment tool. Neuroimage 2020, 210, 116532. [Google Scholar] [CrossRef]
- Bathel, A.; Schweizer, L.; Stude, P.; Glaubitz, B.; Wulms, N.; Delice, S.; Schmidt-Wilcke, T. Increased thalamic glutamate/glutamine levels in migraineurs. J. Headache Pain 2018, 19, 55. [Google Scholar] [CrossRef]
- Tripathi, G.M.; Kalita, J.; Misra, U.K. Role of glutamate and its receptors in migraine with reference to amitriptyline and transcranial magnetic stimulation therapy. Brain Res. 2018, 1696, 31–37. [Google Scholar] [CrossRef]
- Sarchielli, P.; Mancini, M.L.; Floridi, A.; Coppola, F.; Rossi, C.; Nardi, K.; Acciarresi, M.; Pini, L.A.; Calabresi, P. Increased Levels of Neurotrophins Are Not Specific for Chronic Migraine: Evidence From Primary Fibromyalgia Syndrome. J. Pain 2007, 8, 737–745. [Google Scholar] [CrossRef]
- Zielman, R.; Wijnen, J.P.; Webb, A.; Onderwater, G.L.J.; Ronen, I.; Ferrari, M.D.; Kan, H.E.; Terwindt, G.M.; Kruit, M.C. Cortical glutamate in migraine. Brain 2017, 140, 1859–1871. [Google Scholar] [CrossRef]
- Gonzalez de la Aleja, J.; Ramos, A.; Mato-Abad, V.; Martínez-Salio, A.; Hernández-Tamames, J.A.; Molina, J.A.; Hernández-Gallego, J.; Álvarez-Linera, J. Higher glutamate to glutamine ratios in occipital regions in women with migraine during the interictal state. Headache J. Head Face Pain 2013, 53, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Fayed, N.; Andrés, E.; Viguera, L.; Modrego, P.J.; Garcia-Campayo, J. Higher glutamate+ glutamine and reduction of N-acetylaspartate in posterior cingulate according to age range in patients with cognitive impairment and/or pain. Acad. Radiol. 2014, 21, 1211–1217. [Google Scholar] [CrossRef] [PubMed]
- Arzani, M.; Jahromi, S.R.; Ghorbani, Z.; Vahabizad, F.; Martelletti, P.; Ghaemi, A.; Sacco, S.; Togha, M.; School of Advanced Studies of the European Headache Federation (EHF-SAS). Gut-brain axis and migraine headache: A comprehensive review. J. Headache Pain 2020, 21, 15. [Google Scholar] [CrossRef] [PubMed]
- Czyż, K.; Bodkowski, R.; Herbinger, G.; Librowski, T. Omega-3 fatty acids and their role in central nervous system-a review. Curr. Med. Chem. 2016, 23, 816–831. [Google Scholar]
- McNamara, R.K.; Carlson, S.E. Role of omega-3 fatty acids in brain development and function: Potential implications for the pathogenesis and prevention of psychopathology. Prostaglandins Leukot. Essent. Fat. Acids 2006, 75, 329–349. [Google Scholar]
- Galán-Arriero, I.; Serrano-Muñoz, D.; Gómez-Soriano, J.; Goicoechea, C.; Taylor, J.; Velasco, A.; Ávila-Martín, G. The role of Omega-3 and Omega-9 fatty acids for the treatment of neuropathic pain after neurotrauma. Biochim. Biophys. Acta (BBA)-Biomembr. 2017, 1859, 1629–1635. [Google Scholar] [CrossRef]
- Silva, R.V.; Oliveira, J.T.; Santos, B.L.; Dias, F.C.; Martinez, A.M.; Lima, C.K.; Miranda, A.L. Long-chain omega-3 fatty acids supplementation accelerates nerve regeneration and prevents neuropathic pain behavior in mice. Front. Pharmacol. 2017, 8, 723. [Google Scholar] [CrossRef]
- Figueroa, J.D.; Cordero, K.; Serrano-Illan, M.; Almeyda, A.; Baldeosingh, K.; Almaguel, F.G.; De Leon, M. Metabolomics uncovers dietary omega-3 fatty acid-derived metabolites implicated in anti-nociceptive responses after experimental spinal cord injury. Neuroscience 2013, 255, 1–18. [Google Scholar] [CrossRef]
- Voskuyl, R.A.; Vreugdenhil, M.; Kang, J.X.; Leaf, A. Anticonvulsant effect of polyunsaturated fatty acids in rats, using the cortical stimulation model. Eur. J. Pharmacol. 1998, 341, 145–152. [Google Scholar] [CrossRef]
- Heurteaux, C.; Laigle, C.; Blondeau, N.; Jarretou, G.; Lazdunski, M. Alpha-linolenic acid and riluzole treatment confer cerebral protection and improve survival after focal brain ischemia. Neuroscience 2006, 137, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Blondeau, N. The nutraceutical potential of omega-3 alpha-linolenic acid in reducing the consequences of stroke. Biochimie 2016, 120, 49–55. [Google Scholar] [CrossRef]
- King, V.R.; Huang, W.L.; Dyall, S.C.; Curran, O.E.; Priestley, J.V.; Michael-Titus, A.T. Omega-3 fatty acids improve recovery, whereas omega-6 fatty acids worsen outcome, after spinal cord injury in the adult rat. J. Neurosci. 2006, 26, 4672–4680. [Google Scholar] [CrossRef]
- Relton, J.K.; Strijbos, P.J.L.M.; Cooper, A.L.; Rothwell, N.J. Dietary N-3 fatty acids inhibit ischaemic and excitotoxic brain damage in the rat. Brain Res. Bull. 1993, 32, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Barbour, B.; Szatkowski, M.; Ingledew, N.; Attwell, D. Arachidonic acid induces a prolonged inhibition of glutamate uptake into glial cells. Nature 1989, 342, 918–920. [Google Scholar] [CrossRef]
- Lynch, M.A.; Voss, K.L. Arachidonic Acid Increases Inositol Phospholipid Metabolism and Glutamate Release in Synaptosomes Prepared from Hippocampal Tissue. J. Neurochem. 1990, 55, 215–221. [Google Scholar] [CrossRef]
- Gürgen, S.G.; Sayın, O.; Çetïn, F.; Sarsmaz, H.Y.; Yazıcı, G.N.; Umur, N.; Yücel, A.T. The Effect of Monosodium Glutamate on Neuronal Signaling Molecules in the Hippocampus and the Neuroprotective Effects of Omega-3 Fatty Acids. ACS Chem. Neurosci. 2021, 12, 3028–3037. [Google Scholar] [CrossRef]
- Hennebelle, M.; Champeil-Potokar, G.; Lavialle, M.; Vancassel, S.; Denis, I. Omega-3 polyunsaturated fatty acids and chronic stress-induced modulations of glutamatergic neurotransmission in the hippocampus. Nutr. Rev. 2014, 72, 99–112. [Google Scholar] [CrossRef]
- Latour, A.; Grintal, B.; Champeil-Potokar, G.; Hennebelle, M.; Lavialle, M.; Dutar, P.; Potier, B.; Billard, J.M.; Vancassel, S.; Denis, I. Omega-3 fatty acids deficiency aggravates glutamatergic synapse and astroglial aging in the rat hippocampal CA 1. Aging Cell 2013, 12, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, O.; Maghsoudi, Z.; Khorvash, F.; Ghiasvand, R.; Askari, G. The relationship between different fatty acids intake and frequency of migraine attacks. Iran. J. Nurs. Midwifery Res. 2015, 20, 334. [Google Scholar]
- Evans, E.W.; Lipton, R.B.; Peterlin, B.L.; Raynor, H.A.; Thomas, J.G.; O’Leary, K.C.; Pavlovic, J.; Wing, R.R.; Bond, D.S. Dietary intake patterns and diet quality in a nationally representative sample of women with and without severe headache or migraine. Headache J. Head Face Pain 2015, 55, 550–561. [Google Scholar] [CrossRef] [PubMed]
- Tajmirriahi, M.; Sohelipour, M.; Basiri, K.; Shaygannejad, V.; Ghorbani, A.; Saadatnia, M. The effects of sodium valproate with fish oil supplementation or alone in migraine prevention: A randomized single-blind clinical trial. Iran. J. Neurol. 2012, 11, 21. [Google Scholar]
- Harel, Z.; Gascon, G.; Riggs, S.; Vaz, R.; Brown, W.; Exil, G. Supplementation with omega-3 polyunsaturated fatty acids in the management of recurrent migraines in adolescents. J. Adolesc. Health 2002, 31, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Abdolahi, M.; Jafarieh, A.; Sarraf, P.; Sedighiyan, M.; Yousefi, A.; Tafakhori, A.; Abdollahi, H.; Salehinia, F.; Djalali, M. The neuromodulatory effects of ω-3 fatty acids and nano-curcumin on the COX-2/iNOS network in migraines: A clinical trial study from gene expression to clinical symptoms. Endocr. Metab. Immune Disord.-Drug Targets (Former. Curr. Drug Targets-Immune Endocr. Metab. Disord.) 2019, 19, 874–884. [Google Scholar] [CrossRef] [PubMed]
- Maghsoumi-Norouzabad, L.; Mansoori, A.; Abed, R.; Shishehbor, F. Effects of omega-3 fatty acids on the frequency, severity, and duration of migraine attacks: A systematic review and meta-analysis of randomized controlled trials. Nutr. Neurosci. 2018, 21, 614–623. [Google Scholar] [CrossRef]
- Jahnen-Dechent, W.; Ketteler, M. Magnesium basics. Clin. Kidney J. 2012, 5, i3–i14. [Google Scholar] [CrossRef]
- Lambuk, L.; Jafri, A.J.A.; Arfuzir, N.N.N.; Iezhitsa, I.; Agarwal, R.; Rozali, K.N.B.; Agarwal, P.; Bakar, N.S.; Kutty, M.K.; Yusof, A.P.M. Neuroprotective effect of magnesium acetyltaurate against NMDA-induced excitotoxicity in rat retina. Neurotox. Res. 2017, 31, 31–45. [Google Scholar] [CrossRef]
- Veronese, N.; Zurlo, A.; Solmi, M.; Luchini, C.; Trevisan, C.; Bano, G.; Manzato, E.; Sergi, G.; Rylander, R. Magnesium status in Alzheimer’s disease: A systematic review. Am. J. Alzheimer’s Dis. Other Dement. ® 2016, 31, 208–213. [Google Scholar] [CrossRef]
- Stippler, M.; Fischer, M.R.; Puccio, A.M.; Wisniewski, S.R.; Carson-Walter, E.B.; Dixon, C.E.; Walter, K.A. Serum and cerebrospinal fluid magnesium in severe traumatic brain injury outcome. J. Neurotrauma 2007, 24, 1347–1354. [Google Scholar] [CrossRef]
- Altura, B.T.; Memon, Z.I.; Zhang, A.; Cheng, T.P.-O.; Silverman, R.; Cracco, R.Q.; Altura, B.M. Low levels of serum ionized magnesium are found in patients early after stroke which result in rapid elevation in cytosolic free calcium and spasm in cerebral vascular muscle cells. Neurosci. Lett. 1997, 230, 37–40. [Google Scholar] [CrossRef]
- Prasad, D.K.V.; Shaheen, U.; Satyanarayana, U.; Surya Prabha, T.; Jyothy, A.; Munshi, A. Association of Serum Trace Elements and Minerals with Genetic Generalized Epilepsy and Idiopathic Intractable Epilepsy. Neurochem. Res. 2014, 39, 2370–2376. [Google Scholar] [CrossRef] [PubMed]
- Oyanagi, K.; Hashimoto, T. Magnesium in Parkinson’s disease: An update in clinical and basic aspects. In Magnesium in the Central Nervous System; University of Adelaide Press: Adelaide, Australia, 2011. [Google Scholar]
- Botturi, A.; Ciappolino, V.; Delvecchio, G.; Boscutti, A.; Viscardi, B.; Brambilla, P. The role and the effect of magnesium in mental disorders: A systematic review. Nutrients 2020, 12, 1661. [Google Scholar] [CrossRef]
- Sarchielli, P.; Coata, G.; Firenze, C.; Morucci, P.; Abbritti, G.; Gallai, V. Serum and salivary magnesium levels in migraine and tension-type headache. Results in a group of adult patients. Cephalalgia 1992, 12, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, N.; Halvorson, H.; Vande-Linde, A.; Levine, S.R.; Helpern, J.; Welch, K. Low brain magnesium in migraine. Headache J. Head Face Pain 1989, 29, 590–593. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, J.; Storer, R.J.; Park, J.W.; Goadsby, P.J. N-Methyl-d-aspartate receptor open-channel blockers memantine and magnesium modulate nociceptive trigeminovascular neurotransmission in rats. Eur. J. Neurosci. 2019, 50, 2847–2859. [Google Scholar] [CrossRef]
- Manet, S.; Gressens, P.; Gadisseux, J.F.; Evrard, P. Prevention by magnesium of exototoxic neuronal death in the developing brain: An animal model for clinical intervention studies. Dev. Med. Child Neurol. 1995, 37, 473–484. [Google Scholar] [CrossRef]
- Lin, J.-Y.; Chung, S.-Y.; Lin, M.-C.; Cheng, F.-C. Effects of magnesium sulfate on energy metabolites and glutamate in the cortex during focal cerebral ischemia and reperfusion in the gerbil monitored by a dual-probe microdialysis technique. Life Sci. 2002, 71, 803–811. [Google Scholar] [CrossRef]
- van der Hel, W.S.; van den Bergh, W.M.; Nicolay, K.; Tulleken, K.A.; Dijkhuizen, R.M. Suppression of cortical spreading depressions after magnesium treatment in the rat. Neuroreport 1998, 9, 2179–2182. [Google Scholar] [CrossRef]
- Mody, I.; Lambert, J.; Heinemann, U. Low extracellular magnesium induces epileptiform activity and spreading depression in rat hippocampal slices. J. Neurophysiol. 1987, 57, 869–888. [Google Scholar] [CrossRef]
- Van Harreveld, A.; Fifková, E. Mechanisms involved in spreading depression. J. Neurobiol. 1973, 4, 375–387. [Google Scholar] [CrossRef]
- Chiu, H.-Y.; Yeh, T.-H.; Yin-Cheng, H.; Pin-Yuan, C. Effects of intravenous and oral magnesium on reducing migraine: A meta-analysis of randomized controlled trials. Pain Physician 2016, 19, E97. [Google Scholar] [PubMed]
- Choi, H.; Parmar, N. The use of intravenous magnesium sulphate for acute migraine: Meta-analysis of randomized controlled trials. Eur. J. Emerg. Med. 2014, 21, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.S.; Hewison, M. Update in Vitamin D. J. Clin. Endocrinol. Metab. 2010, 95, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Garcion, E.; Wion-Barbot, N.; Montero-Menei, C.N.; Berger, F.; Wion, D. New clues about vitamin D functions in the nervous system. Trends Endocrinol. Metab. 2002, 13, 100–105. [Google Scholar] [CrossRef]
- Mpandzou, G.; Haddou, E.A.B.; Regragui, W.; Benomar, A.; Yahyaoui, M. Vitamin D deficiency and its role in neurological conditions: A review. Rev. Neurol. 2016, 172, 109–122. [Google Scholar] [CrossRef]
- Eyles, D.W.; Smith, S.; Kinobe, R.; Hewison, M.; McGrath, J.J. Distribution of the vitamin D receptor and 1α-hydroxylase in human brain. J. Chem. Neuroanat. 2005, 29, 21–30. [Google Scholar] [CrossRef]
- Taniura, H.; Ito, M.; Sanada, N.; Kuramoto, N.; Ohno, Y.; Nakamichi, N.; Yoneda, Y. Chronic vitamin D3 treatment protects against neurotoxicity by glutamate in association with upregulation of vitamin D receptor mRNA expression in cultured rat cortical neurons. J. Neurosci. Res. 2006, 83, 1179–1189. [Google Scholar] [CrossRef]
- Groves, N.J.; Kesby, J.P.; Eyles, D.W.; McGrath, J.J.; Mackay-Sim, A.; Burne, T.H.J. Adult vitamin D deficiency leads to behavioural and brain neurochemical alterations in C57BL/6J and BALB/c mice. Behav. Brain Res. 2013, 241, 120–131. [Google Scholar] [CrossRef]
- Brewer, L.D.; Thibault, V.; Chen, K.-C.; Langub, M.C.; Landfield, P.W.; Porter, N.M. Vitamin D hormone confers neuroprotection in parallel with downregulation of L-type calcium channel expression in hippocampal neurons. J. Neurosci. 2001, 21, 98–108. [Google Scholar] [CrossRef]
- Zandifar, A.; Masjedi, S.s.; Banihashemi, M.; Asgari, F.; Manouchehri, N.; Ebrahimi, H.; Haghdoost, F.; Saadatnia, M. Vitamin D Status in Migraine Patients: A Case-Control Study. BioMed Res. Int. 2014, 2014, 514782. [Google Scholar] [CrossRef]
- Kjærgaard, M.; Eggen, A.E.; Mathiesen, E.B.; Jorde, R. Association Between Headache and Serum 25-Hydroxyvitamin D; the Tromsø Study: Tromsø 6. Headache J. Head Face Pain 2012, 52, 1499–1505. [Google Scholar] [CrossRef]
- Hussein, M.; Fathy, W.; Abd Elkareem, R.M. The potential role of serum vitamin D level in migraine headache: A case–control study. J. Pain Res. 2019, 12, 2529–2536. [Google Scholar] [CrossRef] [PubMed]
- Togha, M.; Razeghi Jahromi, S.; Ghorbani, Z.; Martami, F.; Seifishahpar, M. Serum Vitamin D Status in a Group of Migraine Patients Compared With Healthy Controls: A Case–Control Study. Headache J. Head Face Pain 2018, 58, 1530–1540. [Google Scholar] [CrossRef] [PubMed]
- Liampas, I.; Siokas, V.; Brotis, A.; Dardiotis, E. Vitamin D serum levels in patients with migraine: A meta-analysis. Rev. Neurol. 2020, 176, 560–570. [Google Scholar] [CrossRef] [PubMed]
- Song, T.-J.; Chu, M.-K.; Sohn, J.-H.; Ahn, H.-Y.; Lee, S.H.; Cho, S.-J. Effect of Vitamin D Deficiency on the Frequency of Headaches in Migraine. J. Clin. Neurol. 2018, 14, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-F.; Xu, Z.-Q.; Zhou, H.-J.; Liu, Y.-Z.; Jiang, X.-J. The Efficacy of Vitamin D supplementation for migraine: A meta-analysis of randomized controlled studies. Clin. Neuropharmacol. 2021, 44, 5–8. [Google Scholar] [CrossRef]
- May, J.M. Vitamin C Transport and Its Role in the Central Nervous System. In Water Soluble Vitamins: Clinical Research and Future Application; Stanger, O., Ed.; Springer: Dordrecht, The Netherlands, 2012; pp. 85–103. [Google Scholar] [CrossRef]
- Majewska, M.D.; Bell, J.A. Ascorbic acid protects neurons from injury induced by glutamate and NMDA. Neuroreport 1990, 1, 194–196. [Google Scholar] [CrossRef]
- Lane, D.J.; Lawen, A. The glutamate aspartate transporter (GLAST) mediates L-glutamate-stimulated ascorbate-release via swelling-activated anion channels in cultured neonatal rodent astrocytes. Cell Biochem. Biophys. 2013, 65, 107–119. [Google Scholar] [CrossRef]
- Hashem, H.E.; El-Din Safwat, M.D.; Algaidi, S. The effect of monosodium glutamate on the cerebellar cortex of male albino rats and the protective role of vitamin C (histological and immunohistochemical study). J. Mol. Histol. 2012, 43, 179–186. [Google Scholar] [CrossRef]
- Nelson, M.T.; Joksovic, P.M.; Su, P.; Kang, H.-W.; Van Deusen, A.; Baumgart, J.P.; David, L.S.; Snutch, T.P.; Barrett, P.Q.; Lee, J.-H. Molecular mechanisms of subtype-specific inhibition of neuronal T-type calcium channels by ascorbate. J. Neurosci. 2007, 27, 12577–12583. [Google Scholar] [CrossRef]
- Visser, E.J.; Drummond, P.D.; Lee-Visser, J.L.A. Reduction in Migraine and Headache Frequency and Intensity With Combined Antioxidant Prophylaxis (N-acetylcysteine, Vitamin E, and Vitamin C): A Randomized Sham-Controlled Pilot Study. Pain Pract. 2020, 20, 737–747. [Google Scholar] [CrossRef] [PubMed]
- Brigelius-Flohé, R.; Traber, M.G. Vitamin E: Function and metabolism. FASEB J. 1999, 13, 1145–1155. [Google Scholar] [CrossRef] [PubMed]
- Friedman, J. Why is the nervous system vulnerable to oxidative stress? In Oxidative Stress in Applied Basic Research and Clinical Practice; Humana Press: Totowa, NJ, USA, 2011; pp. 19–27. [Google Scholar]
- Kim, H.K.; Kim, J.H.; Gao, X.; Zhou, J.-L.; Lee, I.; Chung, K.; Chung, J.M. Analgesic effect of vitamin E is mediated by reducing central sensitization in neuropathic pain. Pain 2006, 122, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Ambrogini, P.; Minelli, A.; Galati, C.; Betti, M.; Lattanzi, D.; Ciffolilli, S.; Piroddi, M.; Galli, F.; Cuppini, R. Post-Seizure α-Tocopherol Treatment Decreases Neuroinflammation and Neuronal Degeneration Induced by Status Epilepticus in Rat Hippocampus. Mol. Neurobiol. 2014, 50, 246–256. [Google Scholar] [CrossRef]
- Betti, M.; Minelli, A.; Ambrogini, P.; Ciuffoli, S.; Viola, V.; Galli, F.; Canonico, B.; Lattanzi, D.; Colombo, E.; Sestili, P. Dietary supplementation with α-tocopherol reduces neuroinflammation and neuronal degeneration in the rat brain after kainic acid-induced status epilepticus. Free Radic. Res. 2011, 45, 1136–1142. [Google Scholar] [CrossRef]
- Martinez-Hernandez, A.; Bell, K.P.; Norenberg, M.D. Glutamine synthetase: Glial localization in brain. Science 1977, 195, 1356–1358. [Google Scholar] [CrossRef]
- Ambrogini, P.; Albertini, M.C.; Betti, M.; Galati, C.; Lattanzi, D.; Savelli, D.; Di Palma, M.; Saccomanno, S.; Bartolini, D.; Torquato, P. Neurobiological correlates of alpha-tocopherol antiepileptogenic effects and microRNA expression modulation in a rat model of kainate-induced seizures. Mol. Neurobiol. 2018, 55, 7822–7838. [Google Scholar]
- Tilleux, S.; Hermans, E. Neuroinflammation and regulation of glial glutamate uptake in neurological disorders. J. Neurosci. Res. 2007, 85, 2059–2070. [Google Scholar]
- Upaganlawar, A.B.; Wankhede, N.L.; Kale, M.B.; Umare, M.D.; Sehgal, A.; Singh, S.; Bhatia, S.; Al-Harrasi, A.; Najda, A.; Nurzyńska-Wierdak, R.; et al. Interweaving epilepsy and neurodegeneration: Vitamin E as a treatment approach. Biomed. Pharmacother. 2021, 143, 112146. [Google Scholar] [CrossRef]
- Crowley, T.; Cryan, J.F.; Downer, E.J.; O’Leary, O.F. Inhibiting neuroinflammation: The role and therapeutic potential of GABA in neuro-immune interactions. Brain Behav. Immun. 2016, 54, 260–277. [Google Scholar] [CrossRef]
- Rideau Batista Novais, A.; Crouzin, N.; Cavalier, M.; Boubal, M.; Guiramand, J.; Cohen-Solal, C.; de Jesus Ferreira, M.-C.; Cambonie, G.; Vignes, M.; Barbanel, G. Tiagabine improves hippocampal long-term depression in rat pups subjected to prenatal inflammation. PLoS ONE 2014, 9, e106302. [Google Scholar] [CrossRef] [PubMed]
- Fatemi, S.H.; Stary, J.M.; Earle, J.A.; Araghi-Niknam, M.; Eagan, E. GABAergic dysfunction in schizophrenia and mood disorders as reflected by decreased levels of glutamic acid decarboxylase 65 and 67 kDa and Reelin proteins in cerebellum. Schizophr. Res. 2005, 72, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Lanoue, A.C.; Dumitriu, A.; Myers, R.H.; Soghomonian, J.-J. Decreased glutamic acid decarboxylase mRNA expression in prefrontal cortex in Parkinson’s disease. Exp. Neurol. 2010, 226, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Gruol, D.L.; Vo, K.; Bray, J.G. Increased astrocyte expression of IL-6 or CCL2 in transgenic mice alters levels of hippocampal and cerebellar proteins. Front. Cell. Neurosci. 2014, 8, 234. [Google Scholar] [CrossRef]
- Abedi, Z.; Khaza’ai, H.; Vidyadaran, S.; Mutalib, M.S.A. The Modulation of NMDA and AMPA/Kainate Receptors by Tocotrienol-Rich Fraction and A-Tocopherol in Glutamate-Induced Injury of Primary Astrocytes. Biomedicines 2017, 5, 68. [Google Scholar] [CrossRef]
- Ziaei, S.; Kazemnejad, A.; Sedighi, A. The effect of vitamin E on the treatment of menstrual migraine. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2009, 15, CR16–CR19. [Google Scholar]
- Shaik, M.M.; Gan, S.H. Vitamin Supplementation as Possible Prophylactic Treatment against Migraine with Aura and Menstrual Migraine. BioMed Res. Int. 2015, 2015, 469529. [Google Scholar] [CrossRef]
- Granella, F.; Sances, G.; Messa, G.; De Marinis, M.; Manzoni, G. Treatment of menstrual migraine. Cephalalgia 1997, 17, 35–38. [Google Scholar] [CrossRef]
- Lee, J.C.M.; Simonyi, A.; Sun, A.Y.; Sun, G.Y. Phospholipases A2 and neural membrane dynamics: Implications for Alzheimer’s disease. J. Neurochem. 2011, 116, 813–819. [Google Scholar] [CrossRef]
- Fleury, C.; Mignotte, B.; Vayssière, J.-L. Mitochondrial reactive oxygen species in cell death signaling. Biochimie 2002, 84, 131–141. [Google Scholar] [CrossRef]
- Pinto, J.T.; Zempleni, J. Riboflavin. Adv. Nutr. 2016, 7, 973–975. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-J.; Wu, W.-M.; Yang, F.-L.; Hsu, G.-S.W.; Huang, C.-Y. Vitamin B2 inhibits glutamate release from rat cerebrocortical nerve terminals. Neuroreport 2008, 19, 1335–1338. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Desbois, A.; Jiang, S.; Hou, S.T. Group B vitamins protect murine cerebellar granule cells from glutamate/NMDA toxicity. Neuroreport 2004, 15, 2241–2244. [Google Scholar] [CrossRef] [PubMed]
- Adelekan, D.A.; Adekile, A.D.; Thurnham, D.I. Dependence of pyridoxine metabolism on riboflavin status in sickle cell patients. Am. J. Clin. Nutr. 1987, 46, 86–90. [Google Scholar] [CrossRef]
- Petroff, O.A.C. GABA and glutamate in the human brain. Neuroscientist 2002, 8, 562–573. [Google Scholar] [CrossRef]
- Theofylaktopoulou, D.; Ulvik, A.; Midttun, Ø.; Ueland, P.M.; Vollset, S.E.; Nygård, O.; Hustad, S.; Tell, G.S.; Eussen, S.J.P.M. Vitamins B2 and B6 as determinants of kynurenines and related markers of interferon-γ-mediated immune activation in the community-based Hordaland Health Study. Br. J. Nutr. 2014, 112, 1065–1072. [Google Scholar] [CrossRef]
- Zinger, A.; Barcia, C.; Herrero, M.T.; Guillemin, G.J. The Involvement of Neuroinflammation and Kynurenine Pathway in Parkinson’s Disease. Park. Dis. 2011, 2011, 716859. [Google Scholar] [CrossRef]
- Curto, M.; Lionetto, L.; Negro, A.; Capi, M.; Fazio, F.; Giamberardino, M.A.; Simmaco, M.; Nicoletti, F.; Martelletti, P. Altered kynurenine pathway metabolites in serum of chronic migraine patients. J. Headache Pain 2016, 17, 47. [Google Scholar] [CrossRef]
- Guillemin, G.J. Quinolinic acid, the inescapable neurotoxin. FEBS J. 2012, 279, 1356–1365. [Google Scholar] [CrossRef]
- Schoenen, J.; Lenaerts, M.; Bastings, E. High-dose riboflavin as a prophylactic treatment of migraine: Results of an open pilot study. Cephalalgia 1994, 14, 328–329. [Google Scholar] [CrossRef]
- Gaul, C.; Diener, H.-C.; Danesch, U.; Group, M.S. Improvement of migraine symptoms with a proprietary supplement containing riboflavin, magnesium and Q10: A randomized, placebo-controlled, double-blind, multicenter trial. J. Headache Pain 2015, 16, 21. [Google Scholar] [CrossRef] [PubMed]
- Maizels, M.; Blumenfeld, A.; Burchette, R. A combination of riboflavin, magnesium, and feverfew for migraine prophylaxis: A randomized trial. Headache J. Head Face Pain 2004, 44, 885–890. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-S.; Lee, H.-F.; Tsai, C.-H.; Hsu, Y.-Y.; Fang, C.-J.; Chen, C.-J.; Hung, Y.-H.; Hu, F.-W. Effect of Vitamin B2 supplementation on migraine prophylaxis: A systematic review and meta-analysis. Nutr. Neurosci. 2022, 25, 1801–1812. [Google Scholar] [CrossRef]
- Holland, S.; Silberstein, S.D.; Freitag, F.; Dodick, D.W.; Argoff, C.; Ashman, E. Evidence-based guideline update: NSAIDs and other complementary treatments for episodic migraine prevention in adults: [RETIRED]. Rep. Qual. Stand. Subcomm. Am. Acad. Neurol. Am. Headache Soc. 2012, 78, 1346–1353. [Google Scholar] [CrossRef] [PubMed]
- Liebman, T.N.; Crystal, S.C. What is the evidence that riboflavin can be used for migraine prophylaxis? Einstein J. Biol. Med. 2011, 27, 7–9. [Google Scholar] [CrossRef]
- Strain, J.J.; Dowey, L.; Ward, M.; Pentieva, K.; McNulty, H. B-vitamins, homocysteine metabolism and CVD. Proc. Nutr. Soc. 2004, 63, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Boldyrev, A.A. Molecular mechanisms of homocysteine toxicity. Biochemistry 2009, 74, 589–598. [Google Scholar] [CrossRef]
- Ponce-Monter, H.A.; Ortiz, M.I.; Garza-Hernández, A.F.; Monroy-Maya, R.; Soto-Ríos, M.; Carrillo-Alarcón, L.; Reyes-García, G.; Fernández-Martínez, E. Effect of diclofenac with B vitamins on the treatment of acute pain originated by lower-limb fracture and surgery. Pain Res. Treat. 2012, 2012, 104782. [Google Scholar] [CrossRef]
- Marashly, E.T.; Bohlega, S.A. Riboflavin Has Neuroprotective Potential: Focus on Parkinson’s Disease and Migraine. Front. Neurol. 2017, 8, 333. [Google Scholar] [CrossRef]
- Lominadze, D.; Tyagi, N.; Sen, U.; Ovechkin, A.; Tyagi, S.C. Homocysteine alters cerebral microvascular integrity and causes remodeling by antagonizing GABA-A receptor. Mol. Cell. Biochem. 2012, 371, 89–96. [Google Scholar] [CrossRef][Green Version]
- Lominadze, D.; Roberts, A.M.; Tyagi, N.; Moshal, K.S.; Tyagi, S.C. Homocysteine causes cerebrovascular leakage in mice. Am. J. Physiol.-Heart Circ. Physiol. 2006, 290, H1206–H1213. [Google Scholar] [CrossRef] [PubMed]
- Tietjen, G.E.; Maly, E.F. Migraine and Ischemic Stroke in Women. A Narrative Review. Headache J. Head Face Pain 2020, 60, 843–863. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Mattiuzzi, C.; Meschi, T.; Cervellin, G.; Borghi, L. Homocysteine and migraine. A narrative review. Clin. Chim. Acta 2014, 433, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Liampas, I.; Siokas, V.; Mentis, A.-F.A.; Aloizou, A.-M.; Dastamani, M.; Tsouris, Z.; Aslanidou, P.; Brotis, A.; Dardiotis, E. Serum Homocysteine, Pyridoxine, Folate, and Vitamin B12 Levels in Migraine: Systematic Review and Meta-Analysis. Headache J. Head Face Pain 2020, 60, 1508–1534. [Google Scholar] [CrossRef]
- Askari, G.; Nasiri, M.; Mozaffari-Khosravi, H.; Rezaie, M.; Bagheri-Bidakhavidi, M.; Sadeghi, O. The effects of folic acid and pyridoxine supplementation on characteristics of migraine attacks in migraine patients with aura: A double-blind, randomized placebo-controlled, clinical trial. Nutrition 2017, 38, 74–79. [Google Scholar] [CrossRef]
- Sadeghi, O.; Nasiri, M.; Maghsoudi, Z.; Pahlavani, N.; Rezaie, M.; Askari, G. Effects of pyridoxine supplementation on severity, frequency and duration of migraine attacks in migraine patients with aura: A double-blind randomized clinical trial study in Iran. Iran. J. Neurol. 2015, 14, 74–80. [Google Scholar]
- Garrido-Maraver, J.; Cordero, M.D.; Oropesa-Avila, M.; Vega, A.F.; de la Mata, M.; Pavon, A.D.; Alcocer-Gomez, E.; Calero, C.P.; Paz, M.V.; Alanis, M. Clinical applications of coenzyme Q10. Front. Biosci. 2014, 19, 619–633. [Google Scholar] [CrossRef]
- Neergheen, V.; Chalasani, A.; Wainwright, L.; Yubero, D.; Montero, R.; Artuch, R.; Hargreaves, I. Coenzyme Q 10 in the treatment of mitochondrial disease. J. Inborn Errors Metab. Screen. 2019, 5, e160063. [Google Scholar]
- Deichmann, R.; Lavie, C.; Andrews, S. Coenzyme q10 and statin-induced mitochondrial dysfunction. Ochsner J. 2010, 10, 16–21. [Google Scholar]
- Kobori, Y.; Ota, S.; Sato, R.; Yagi, H.; Soh, S.; Arai, G.; Okada, H. Antioxidant cosupplementation therapy with vitamin C, vitamin E, and coenzyme Q10 in patients with oligoasthenozoospermia. Arch. Ital. Urol. E Androl. 2014, 86, 1–4. [Google Scholar] [CrossRef]
- Lee, D.; Shim, M.S.; Kim, K.Y.; Noh, Y.H.; Kim, H.; Kim, S.Y.; Weinreb, R.N.; Ju, W.K. Coenzyme Q10 inhibits glutamate excitotoxicity and oxidative stress-mediated mitochondrial alteration in a mouse model of glaucoma. Investig. Ophthalmol. Vis. Sci. 2014, 55, 993–1005. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Huang, S.-K.; Wang, S.-J. Coenzyme Q10 Inhibits the Release of Glutamate in Rat Cerebrocortical Nerve Terminals by Suppression of Voltage-Dependent Calcium Influx and Mitogen-Activated Protein Kinase Signaling Pathway. J. Agric. Food Chem. 2012, 60, 11909–11918. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Mehta, S.L.; Milledge, G.Z.; Huang, X.; Li, H.; Li, P.A. Ubisol-Q10 Prevents Glutamate-Induced Cell Death by Blocking Mitochondrial Fragmentation and Permeability Transition Pore Opening. Int. J. Biol. Sci. 2016, 12, 688–700. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sândor, P.S.; Di Clemente, L.; Coppola, G.; Saenger, U.; Fumal, A.; Magis, D.; Seidel, L.; Agosti, R.; Schoenen, J. Efficacy of coenzyme Q10 in migraine prophylaxis: A randomized controlled trial. Neurology 2005, 64, 713–715. [Google Scholar] [CrossRef]
- Rozen, T.; Oshinsky, M.; Gebeline, C.; Bradley, K.; Young, W.; Shechter, A.; Silberstein, S. Open label trial of coenzyme Q10 as a migraine preventive. Cephalalgia 2002, 22, 137–141. [Google Scholar] [CrossRef]
- Dahri, M.; Tarighat-Esfanjani, A.; Asghari-Jafarabadi, M.; Hashemilar, M. Oral coenzyme Q10 supplementation in patients with migraine: Effects on clinical features and inflammatory markers. Nutr. Neurosci. 2019, 22, 607–615. [Google Scholar] [CrossRef]
- Hajihashemi, P.; Askari, G.; Khorvash, F.; Reza Maracy, M.; Nourian, M. The effects of concurrent Coenzyme Q10, L-carnitine supplementation in migraine prophylaxis: A randomized, placebo-controlled, double-blind trial. Cephalalgia 2019, 39, 648–654. [Google Scholar] [CrossRef]
- Sazali, S.; Badrin, S.; Norhayati, M.N.; Idris, N.S. Coenzyme Q10 supplementation for prophylaxis in adult patients with migraine—A meta-analysis. BMJ Open 2021, 11, e039358. [Google Scholar] [CrossRef]
- Shanmugam, S.; Karunaikadal, K.; Varadarajan, S.; Krishnan, M. Memantine ameliorates migraine headache. Ann. Indian Acad. Neurol. 2019, 22, 286. [Google Scholar] [CrossRef]
- Lampl, C.; Katsarava, Z.; Diener, H.; Limmroth, V. Lamotrigine reduces migraine aura and migraine attacks in patients with migraine with aura. J. Neurol. Neurosurg. Psychiatry 2005, 76, 1730–1732. [Google Scholar] [CrossRef]
- Waung, M.W.; Akerman, S.; Wakefield, M.; Keywood, C.; Goadsby, P.J. Metabotropic glutamate receptor 5: A target for migraine therapy. Ann. Clin. Transl. Neurol. 2016, 3, 560–571. [Google Scholar] [CrossRef]
- Hoffmann, J.; Charles, A. Glutamate and Its Receptors as Therapeutic Targets for Migraine. Neurotherapeutics 2018, 15, 361–370. [Google Scholar] [CrossRef]
- Muir, K.W.; Lees, K.R. Clinical experience with excitatory amino acid antagonist drugs. Stroke 1995, 26, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Watkins, J.C.; Jane, D.E. The glutamate story. Br. J. Pharmacol. 2006, 147, S100–S108. [Google Scholar] [CrossRef] [PubMed]
- Assarzadegan, F.; Asgarzadeh, S.; Hatamabadi, H.R.; Shahrami, A.; Tabatabaey, A.; Asgarzadeh, M. Serum concentration of magnesium as an independent risk factor in migraine attacks: A matched case–control study and review of the literature. Int. Clin. Psychopharmacol. 2016, 31, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Holton, K.F.; Taren, D.L.; Thomson, C.A.; Bennett, R.M.; Jones, K.D. The effect of dietary glutamate on fibromyalgia and irritable bowel symptoms. Clin. Exp. Rheumatol. 2012, 30, 10–17. [Google Scholar]
- Smith, Q.R. Transport of glutamate and other amino acids at the blood-brain barrier. J. Nutr. 2000, 130, 1016S–1022S. [Google Scholar] [CrossRef] [PubMed]
- Kuriakose, M.; Younger, D.; Ravula, A.R.; Alay, E.; Rama Rao, K.V.; Chandra, N. Synergistic role of oxidative stress and blood-brain barrier permeability as injury mechanisms in the acute pathophysiology of blast-induced neurotrauma. Sci. Rep. 2019, 9, 7717. [Google Scholar] [CrossRef]
- Huber, J.; Witt, K.; Hom, S.; Egleton, R.; Mark, K.; Davis, T. Inflammatory pain alters blood-brain barrier permeability and tight junctional protein expression. Am. J. Physiol.-Heart Circ. Physiol. 2001, 280, H1241–H1248. [Google Scholar] [CrossRef]
- Alves, J.L. Blood–brain barrier and traumatic brain injury. J. Neurosci. Res. 2014, 92, 141–147. [Google Scholar] [CrossRef]
- Borkum, J.M. Migraine triggers and oxidative stress: A narrative review and synthesis. Headache J. Head Face Pain 2016, 56, 12–35. [Google Scholar]
- Waeber, C.; Moskowitz, M.A. Migraine as an inflammatory disorder. Neurology 2005, 64, S9–S15. [Google Scholar] [CrossRef] [PubMed]
- Baad-Hansen, L.; Cairns, B.; Ernberg, M.; Svensson, P. Effect of systemic monosodium glutamate (MSG) on headache and pericranial muscle sensitivity. Cephalalgia 2009, 55, 993–1005. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.H.; Drouin, M.A.; Herbert, M.; Mao, Y.; Karsh, J. The monosodium glutamate symptom complex: Assessment in a double-blind, placebo-controlled, randomized study. J. Allergy Clin. Immunol. 1997, 99, 757–762. [Google Scholar] [CrossRef]
- Benbow, T.; Ekbatan, M.R.; Wang, G.H.Y.; Teja, F.; Exposto, F.G.; Svensson, P.; Cairns, B.E. Systemic administration of monosodium glutamate induces sexually dimorphic headache-and nausea-like behaviours in rats. Pain 2022, 163, 1838–1853. [Google Scholar] [CrossRef]
- Finocchi, C.; Sivori, G. Food as trigger and aggravating factor of migraine. Neurol. Sci. 2012, 33, 77–80. [Google Scholar] [CrossRef]
- Hindiyeh, N.A.; Zhang, N.; Farrar, M.; Banerjee, P.; Lombard, L.; Aurora, S.K. The Role of Diet and Nutrition in Migraine Triggers and Treatment: A Systematic Literature Review. Headache J. Head Face Pain 2020, 60, 1300–1316. [Google Scholar] [CrossRef]
- Alpay, K.; Ertaş, M.; Orhan, E.K.; Üstay, D.K.; Lieners, C.; Baykan, B. Diet restriction in migraine, based on IgG against foods: A clinical double-blind, randomised, cross-over trial. Cephalalgia 2010, 30, 829–837. [Google Scholar] [CrossRef]
- Martin, V.T.; Vij, B. Diet and headache: Part 1. Headache J. Head Face Pain 2016, 56, 1543–1552. [Google Scholar] [CrossRef]
- Martin, V.T.; Behbehani, M.M. Toward a rational understanding of migraine trigger factors. Med. Clin. N. Am. 2001, 85, 911–941. [Google Scholar] [CrossRef]
- Gazerani, P. Migraine and diet. Nutrients 2020, 12, 1658. [Google Scholar] [CrossRef]
- Holton, K.F.; Kirkland, A.E.; Baron, M.; Ramachandra, S.S.; Langan, M.T.; Brandley, E.T.; Baraniuk, J.N. The Low Glutamate Diet Effectively Improves Pain and Other Symptoms of Gulf War Illness. Nutrients 2020, 12, 2593. [Google Scholar] [CrossRef] [PubMed]
- Holton, K.F.; Ndege, P.K.; Clauw, D.J. Dietary correlates of chronic widespread pain in Meru, Kenya. Nutrition 2018, 53, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.D.; Terpening, C.M.; Schmidt, S.O.; Gums, J.G. Relief of fibromyalgia symptoms following discontinuation of dietary excitotoxins. Ann. Pharmacother. 2001, 35, 702–706. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).