Abstract
Excessive gestational weight gain (GWG) is associated with increased risk of maternal and neonatal complications. We investigated obesity-related polymorphisms in the FTO gene (rs9939609, rs17817449) and ADRB2 (rs1042713, rs1042714) as candidate risk factors concerning excessive GWG in pregnant women with pregestational diabetes. This nutrigenetic trial, conducted in Brazil, randomly assigned 70 pregnant women to one of the groups: traditional diet (n = 41) or DASH diet (n = 29). Excessive GWG was the total weight gain above the upper limit of the recommendation, according to the Institute of Medicine guidelines. Genotyping was performed using real-time PCR. Time-to-event analysis was performed to investigate risk factors for progression to excessive GWG. Regardless the type of diet, AT carriers of rs9939609 (FTO) and AA carriers of rs1042713 (ADRB2) had higher risk of earlier exceeding GWG compared to TT (aHR 2.44; CI 95% 1.03–5.78; p = 0.04) and GG (aHR 3.91; CI 95% 1.12–13.70; p = 0.03) genotypes, respectively, as the AG carriers for FTO haplotype rs9939609:rs17817449 compared to TT carriers (aHR 1.79; CI 95% 1.04–3.06; p = 0.02).
1. Introduction
Excessive gestational weight gain (GWG) affects half of pregnancies worldwide [1] and nearly 40% of pregnancies in Brazil [2]. In women with pregestational diabetes mellitus (DM), excessive GWG is associated with a higher risk of preterm delivery, cesarean section, large-for-gestational-age newborn, macrosomia, neonatal distress, and neonatal malformations [3,4]. In addition to increasing the immediate risk of perinatal complications, excessive GWG is associated with short- and long-term metabolic consequences for mothers and children and probably plays a key role in the metabolic programming of chronic diseases in the offspring [5].
The GWG recommendations [6,7] are based on the pre-pregnancy body mass index (BMI), but genetics, dietetics, and environmental factors appear to be involved in significant interindividual variation in weight gain during pregnancy [8,9]. The fat mass and obesity-associated (FTO) gene is located on chromosome 16, and common polymorphisms in the first intron are strongly associated with obesity. FTO encodes a protein with demethylase function and is highly expressed in the hypothalamus, particularly in the arcuate nucleus, suggesting that this gene plays an essential role in energy balance and body weight control [10].
The polymorphisms rs9939609 (T/A) and rs17817449 (T/G) in FTO are associated with body weight, BMI, and extreme obesity in the Brazilian population [11]. The A allele of the polymorphism rs9939609 is associated with higher GWG in North America [12,13] and Spanish women [14] but not with excessive GWG in Brazilian women [15]. Despite the association of rs17817449 (GG) with higher maternal BMI in pregnant Iraqi women [16], its relationship with GWG has not yet been explored in the literature.
The adrenoceptor beta 2 (ADRB2) gene is located on chromosome 5, and some polymorphisms in this gene have been consistently associated with a predisposition to obesity due to its expression in adipose tissue and its role in lipolysis and energy balance [17]. Two common polymorphisms in ADRB2 are the most studied, rs1042713 (G/A) and rs1042714 (C/G), although the results are quite divergent, with only rs1042714 (CG/GG) associated with obesity in a meta-analysis [18]. The relationship between ADRB2 polymorphisms and GWG has not yet been explored.
Interventions that focus on a healthy diet have been found to be effective in optimizing GWG [5] and should start as early as possible, even during the periconceptional period [19]. The adherence to an “Western” dietary pattern—characterized by unhealthy and energy-dense foods with high intake of red meat, fries, dipping sauces, salty snacks, and alcoholic drinks—was associated with increased GWG, especially among obese women, in a cohort of women from Southern Europe [19].
Pregnant women with DM especially benefit from nutritional guidance to prevent excessive GWG and related adverse outcomes, which are more common in high-risk pregnancies [20]. The Dietary Approach to Stop Hypertension (DASH) diet encourages the consumption of fruits, vegetables, fat-free/low-fat dairy, whole grains, nuts, and legumes as well as limits the intake of saturated fat, cholesterol, refined sugar, sodium, red, and processed meats [21]. The DASH diet was originally proposed to control hypertension; however, in recent years, it has been recommended for pregnant women with DM, obesity, and hypertension to reduce the risk of obstetric and perinatal complications [22,23].
However, the effects of diet on body physiology vary greatly among individuals, which may be partially explained by nutrigenetic interactions. The association between FTO and ADRB2 genetic polymorphisms and obesity phenotypes appears to be modified by diet composition [24,25,26]. Personalized nutrition may benefit individuals genetically predisposed to have a higher BMI using a dietary pattern that minimizes risk [27]. Studies are needed to elucidate the association between genetic variants, diet, and GWG, especially for high-risk pregnancies, such as in mothers with pregestational DM.
The aim of this study was to investigate the FTO genetic polymorphisms (rs9939609, rs17817449) and the ADRB2 genetic polymorphisms (rs1042713, rs1042714) as candidate genetic risk factors for excessive GWG in pregnant women with pregestational DM using two different types of diets, a traditional diet and the DASH diet, as well as to ascertain nutrigenetic interactions associated to the genetic make-up.
2. Materials and Methods
2.1. Subjects
The participants were pregnant women who were enrolled in the DASDIA (DASh diet for pregnant women with DIAbetes) randomized controlled clinical trial carried out at the Maternity School of the Federal University of Rio de Janeiro, Rio de Janeiro, Brazil, with the aim of evaluating the effect of the DASH diet on perinatal outcomes in pregnant women with pregestational DM (2016–2020, Brazilian Clinical Trials Registry RBR-4tbgv6). Eighty-seven pregnant women participated in the DASDIA trial, of whom 70 were included in the present study because valid data were available for performing the nutrigenetic analyses.
The participants were pregnant women with pregestational DM; 18 years or older; less than 28 weeks pregnant at the time of inclusion in the study; single fetus; no alcohol, tobacco, or drug use; no sexually transmitted disease (e.g., syphilis, genital herpes, HPV); no psychiatric diseases (e.g., anxiety, depression, eating disorders); and no DM complications (e.g., diabetic nephropathy or retinopathy). Pregnant women with treated and controlled hypothyroidism (TSH 0.1–2.5 mUI/L in the first trimester or 0.3–3.0 mUI/L in the second trimester, using levothyroxine) or chronic hypertension (systolic blood pressure <160 mmHg and diastolic blood pressure <110, using methyldopa, without SHG) were included. The eligible participants were randomly assigned to one of two parallel study groups, traditional diet or DASH diet, using a computer-generated list of random numbers prepared by the head investigator (even numbers to DASH diet group and odd numbers to traditional diet group). The participants were blinded to allocation.
The research was approved by the Ethics Committee of the Maternity School of Federal University of Rio de Janeiro (CAAE–46913115.0.0000.5275; July/2015).
2.2. Pregestational Diabetes Diagnosis and Treatment
Women with type 1 or 2 DM were included in this study, with a pre-pregnancy diagnosis or diagnosed during pregnancy, after presenting with a fasting glucose level ≥126 mg/dL [20]. Women with gestational DM were excluded from the study. Following the institutional protocol, all participants were treated with insulin therapy, which was prescribed by physicians according to individual needs.
2.3. Diet Groups and Nutritional Guidance
The participants were randomly assigned to one of two groups of nutritional guidance, a traditional diet or the DASH diet. Women in both groups received individual nutritional guidance from a registered dietitian from the date of inclusion in the study until the last prenatal appointment in six scheduled visits. The time of intervention was defined as the time between inclusion in the study and childbirth.
Both traditional and DASH diets were designed to contain 45–55% carbohydrates, 15–20% protein, and 25–30% total fat. However, the DASH diet was richer in fruits, vegetables, whole grains, and low-fat dairy products and included a serving of nuts per day. The original North American version of the DASH diet was translated and adapted for the DASDIA trial, considering the characteristics of Brazilian pregnant women with DM, as detailed elsewhere [28]. The traditional diet was a healthy diet currently prescribed for all pregnant women with DM-attending prenatal care at the maternity hospital.
The main differences in the composition of the traditional and DASH diets based on the 2100 kcal meal plan are provided in Table 1.

Table 1.
Daily composition of the diets used in the study.
The daily energy intake was calculated individually in order to formulate recommendations according to age, physical activity, pre-pregnancy BMI, and recommended GWG for each woman in both groups. All participants received a meal plan with a list of equivalents based on the traditional or DASH diet, which was explained in detail and revised at each appointment with the registered dietitian, with reinforcement of the nutritional orientations for both diets until the last visit [28].
Adherence to the diets was assessed using a 24-h dietary recall and by applying a tool with four evaluation items, which was scored from 0 to 4 points according to (1) quantity of food consumed—portions; (2) food groups consumed—variety; (3) consumed meals—number and time; and (4) gestational weight gain—adequate when no more than 20% less or above the recommended amount [29]. The adherence score was stratified into low-to-moderate adherence (<2 points) and high adherence (≥2 points). For the present analyses, we considered the adherence score obtained at the visit closest to childbirth to reflect the longest possible time of exposure to the intervention.
To improve adherence, participants in the traditional diet group received a bottle of extra virgin olive oil (500 mL) at the first visit, a can of powdered semi-skimmed milk (300 mg), and a pack of oats (250 mg) at each subsequent visit, while the participants in the DASH diet group received a bottle of extra virgin olive oil (500 mL) at the first visit, a can of powdered skimmed milk (280 mg), and a pack of nuts (150 mg) and seeds (200 mg) at each subsequent visit.
2.4. Outcome
The main outcome was excessive GWG (kg). The recommended GWG was defined according to the Institute of Medicine guidelines [6,7] considering the pre-pregnancy BMI: underweight, 12.5 to 18 kg; normal weight, 11.5 to 16 kg; overweight, 7 to 11.5 kg; and obesity, 5 to 9 kg. Excessive GWG was the total weight gain of pregnancy exceeding the maximum amount recommended by IOM [6,7] according to pre-pregnancy BMI: GWG > 18 kg, >16 kg, >11.5 kg, and >9 kg for underweight, normal weight, overweight, and obese women, respectively. The time until excessive GWG was estimated by linear interpolation, assuming a linearly increasing weight gain between different measurements.
To calculate the pre-pregnancy BMI, height was measured during the first prenatal visit, and weight was measured at each prenatal visit by nursing technicians and registered in the medical record. Pre-pregnancy weight was the self-reported weight of a woman near conception [30]. The pre-pregnancy BMI was calculated as (pre-pregnancy weight/height2) and was classified as underweight (BMI < 18.5 kg/m2), normal weight (BMI 18.5–24.9 kg/m2), overweight (BMI 25.0–29.9 kg/m2), or obesity (BMI ≥ 30.0 kg/m2) [31].
The last pregnancy weight was measured on admission for childbirth. GWG was calculated as (weight at admission for childbirth–pre-pregnancy weight), representing the total weight gain during pregnancy.
2.5. Genotyping
We collected saliva samples from each pregnant woman participating in the study, and genomic DNA was isolated from buccal epithelial cells using the Aidar and Line (2007) protocol [32]. FTO (rs9939609 and rs17817449) and ADRB2 (rs1042713 and rs1042714) polymorphisms were genotyped by real-time PCR using TaqMan® assays (Thermo Fisher Scientific, Carlsbad, CA, USA). Reactions were performed in 10-μL volumes containing DNA (2 μL), Universal Master Mix (5 μL), TaqMan Genotyping Assay specific for each polymorphism (0.25 μL), and MiliQ (2.75 μL). Amplification was carried out in a StepOne® Plus Real-Time PCR System (ThermoFisher) according to the manufacturer’s recommendations for the number of cycles and temperatures. Negative and positive controls were included in the plate.
2.6. Co-Variates
Data, such as age (years), DM type (1 or 2), education level (elementary, middle, or high school), marital status (married/single), employment (yes/no), per capita income (total family income divided by the number of persons living in the same house, in USD), housing conditions (adequate when all had regular garbage collection, tap water, and sewerage system), pre-existing chronic diseases (hypothyroidism or chronic hypertension), and parity (number of previous childbirths) were obtained from medical records and were complemented in a personal interview with the researchers using structured questionnaires. Physical activity was assessed at baseline using the short form of the International Physical Activity Questionnaire (active, irregularly active, and sedentary) [33].
Skin color (white/black/brown/yellow) and years living with DM were self-reported. Energy intake (kcal) at baseline was obtained using a 24-h dietary recall from which the reported portions of food were converted into grams to quantify the energy content using food composition tables [34,35]. Gestational age (weeks) was calculated using the first ultrasonography performed in prenatal care, which was obtained at the time of inclusion in the study (all <28 weeks of pregnancy).
2.7. Statistical Analyses
Data are presented as medians and interquartile ranges (IQR) for numeric variables, and absolute (n) and relative frequencies (%) for categorical variables. The normality of the continuous variables was assessed using histograms, kurtosis, and asymmetry measures. Mann–Whitney U and Kruskal–Wallis tests were used to compare continuous numerical variables, and chi-square or Fisher’s exact tests were used for categorical variables. Genotype and allele frequencies of each variant were determined by direct counting, and deviations from Hardy–Weinberg equilibrium (HWE) were evaluated using chi-square tests.
Paired linkage disequilibrium (LD) patterns were determined for each gene using r2 statistics (r2 cutoff ≥ 0.8). Haplotype frequencies or allelic phase determination were estimated by expectation maximization (EM algorithm), and estimation uncertainty was included in the statistical models applied for association analyses in the form of weights. The homozygous/heterozygous genotypes and lower frequency alleles (minor allele frequency, MAF) in our population, or those containing them, were compared with the higher frequency alleles or genotypes containing higher frequency alleles (reference). Haplotype analyses used the most common haplotype in our population as a reference.
The incidences of excessive GWG were analyzed based on the events and years of persons at risk based on the follow-up time from the most likely date of conception to the most probable date of the outcome. Incidences and 95% confidence intervals (95% CIs) were estimated according to asymptotic standard errors calculated from a gamma distribution. Pregnant women who did not present with the outcome were considered from the most likely date of conception and censored on the day of delivery.
The results of the time-to-event analyses were presented in the form of hazard ratios (HRs) with 95% CIs, and the risks of progression to the events described above were estimated using Cox proportional hazard models. The assumption of risk proportionality was tested using correlation analyses and χ2 tests based on Schoenfeld scaled residuals and transformed survival times. The effects of the genetic characteristics of interest were corrected for phenotypic characteristics with at least one suggested association (p-value ≤ 0.1) with the outcome of interest and the marginal effects presented in the form of aHR. Pre-gestational BMI was not included in the adjusted model because of its potential mediating effect between genotype and outcome. Each polymorphism was evaluated using the additive, dominant, and recessive models.
Statistical analyses were performed using R software (Version 4.1.1) and its “genetics” and “survival” packages. Power analysis and sample size estimates were performed using the R code available on the Power and Sample Size platform (http://powerandsamplesize.com/Calculators/Test-Time-To-Event-Data/Cox-PH-2-Sided-Equality accessed on 18 January 2022). Considering the overall prevalence of the event of 50%, the frequency of minor allele carriers of 35%, a mean hazard ratio of 2, and alpha = 0.05, the minimum sample size for Cox proportional models estimated for power (1-eta) of 0.8 was 144. Nonetheless, we had a limited sample size (n = 70) that reached 56.35% statistical power for this analysis.
3. Results
Of the 249 pregnant women with pregestational DM assessed for eligibility, 87 were included in the DASDIA clinical trial, and 70 were included in the present study because there were sufficient data for the analyses: 41 in the traditional diet group and 29 in the DASH diet group (Figure 1).
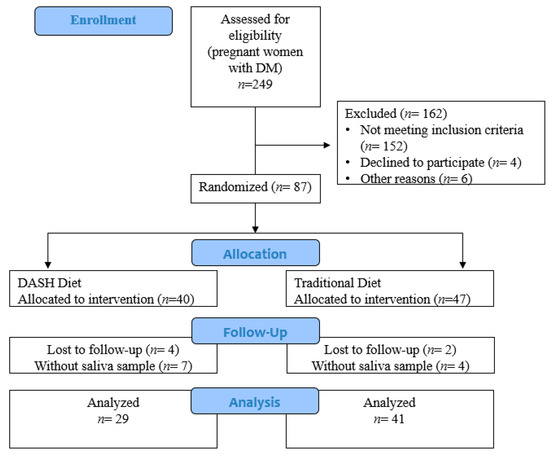
Figure 1.
Flowchart of the study (Rio de Janeiro/Brazil, 2016–2020).
The median age was 32 years (IQR 25.7–36.0), and the gestational age at randomization was 15 weeks (IQR 11.1–20.1). DM type 1 was 51.4% (n = 36) of the cases. The distribution of the variables was homogeneous among the diet groups (Table 2).

Table 2.
General characteristics of the participants at baseline (Rio de Janeiro/Brazil, 2016–2020).
The genotypic frequencies of rs9939609–FTO were TT 40%, AT 48.6%, and AA 11.4%, while for rs17817449–FTO, the values were TT 45.7%, GT 44.3%, and GG 10%, without differences among diet groups (p = 0.48 and p = 0.73, respectively; Table 3). The MAFs for rs9939609 (A) were 35.7%, while it was 32.1% for rs17817449 (G).

Table 3.
Genetic background of the participants concerning FTO and ADRB2 polymorphisms (Rio de Janeiro/Brazil, 2016–2020).
The genotypic frequencies of rs1042713–ADRB2 were GG 35.7%, AG 52.9%, and AA 11.4%, while for rs1042714–ADRB2, the values were CC 50%, CG 44.3%, and GG 5.7%, without differences among diet groups (p = 0.35 and p = 0.28, respectively; Table 3). The MAFs for rs1042713 (A) were 37.9% and 27.9% for rs1042714 (G). The genotypes of all evaluated polymorphisms were in HWE (p > 0.05).
The median time of intervention was 22.50 weeks (IQR 15.50–26.04). Most pregnant women attended six scheduled appointments (n = 38, 54.3%) or at least five of them (n = 16, 22.9%). Almost 40% of the participants had the highest adherence scores in both groups (39.5% in the traditional diet group and 40.7% in the DASH diet group).
In the overall sample (n = 70), 28.6% of the women had a normal pre-pregnancy BMI, 35.7% were overweight, and 35.7% were obese. None of the pregnant women were underweight pre-pregnancy. The median GWG was 13.7 kg (IQR 11.5–17.5), 11.8 kg (IQR 7.5–16.4), and 11.0 (IQR 5.9–14.1) for normal-weight, overweight, and obese women, respectively, without differences between diet groups (Supplementary Table S1). We found no statistically significant interaction between diet and genotype on GWG but a marginal effect for the AA genotype of rs9939609–FTO and GG genotype of rs17817449–FTO (p = 0.05 and p = 0.08, respectively) on higher GWG comparing to another genotypes, only in the traditional diet group (Supplementary Figure S1).
Thirty-seven pregnant women (52.9%) presented with excessive GWG, and the median gestational age of exceeding GWG was 31.6 weeks (IQR 26.6–35.0). Compared to the traditional diet, the DASH diet did not modify the risk of progression to excessive GWG (aHR 1.32, CI 95% 0.62–2.79; p = 0.46) in our sample. Instead, the time of living with DM ≥ 8 years (aHR 1.99, CI 95% 1.01–3.93; p = 0.04), pre-pregnancy overweight (aHR 3.15, CI 95% 1.23–8.09; p = 0.02) or obesity (aHR 2.87, CI 95% 1.11–7.42; p = 0.03) status, previous hypothyroidism (aHR 4.37, CI 95% 1.62–11.77; p = 0.00), and yellow color of the skin (aHR 74.40; CI 95% 4.25–1302.72; p = 0.00, not shown in the table) were risk factors for earlier GWG. In contrast, age ≥ 32 years was a protective factor (aHR 0.41, CI 95% 0.21–0.80; p = 0.01) (Table 4).

Table 4.
Cox proportional hazard models or time-to-event analyses (from conception to excessive gestational weight gain) of diet groups and general characteristics of the participants (Rio de Janeiro/Brazil, 2016–2020).
Adjusting for the main confounders, the A allele carriers (AT/AA) had a higher risk of earlier exceeding GWG (aHR 2.55; CI 95% 1.14–5.69; p = 0.02) than the rs9939609 TT genotype in the FTO gene, which was also found in the comparison of AT vs. TT genotypes (aHR 2.44; CI 95% 1.03–5.78; p = 0.04) (Table 5).

Table 5.
Cox proportional hazard models or time-to-event analyses (from conception to excessive gestational weight gain) stratified by the FTO polymorphisms rs9939609 and rs17817449 (Rio de Janeiro/Brazil, 2016–2020).
The A allele carriers for rs1042713 in the ADRB2 gene had a higher risk of earlier exceeding GWG than the GG genotype (aHR 2.37; CI 95% 1.01–5.52; p = 0.04), being almost four times higher for the AA carriers (aHR 3.91; CI 95% 1.12–13.70; p = 0.03). We found that the genotypes for rs17817449 (FTO) and rs1042714 (ADRB2) had no effect on the risk of excess GWG in our sample (Table 6).

Table 6.
Cox proportional hazard models or time-to-event analyses (from conception to excessive gestational weight gain) stratified by the ADRB2 polymorphisms rs1042713 and rs1042714 (Rio de Janeiro/Brazil, 2016–2020).
Although rs17817449 alone was not associated with the outcome, in the haplotype analysis of rs9939609:rs17817449 (TT/AG/AT), we found a higher risk for earlier excessive GWG among AG carriers: the A allele for rs9939609 and the G allele for rs17817449 (aHR 1.79; CI 95% 1.04–3.06; p = 0.02). We found no association between haplotype analysis of the ADRB2 gene in our sample (Table 7).

Table 7.
Cox proportional hazard models or time-to-event analyses (from conception to excessive gestational weight gain) of the haplotypes ADRB2 rs1042713:rs1042714 and FTO rs9939609:rs17817449 (Rio de Janeiro/Brazil, 2016–2020).
4. Discussion
In a sample of 70 Brazilian pregnant women with pregestational DM, we found that the A allele carriers for rs9939609 (FTO gene) and rs1042713 (ADRB2 gene) had more than twice the risk of earlier exceeding GWG compared to TT and GG genotypes, respectively. The haplotype rs9939609:rs17817449 (AG) was also a risk factor, increasing 1.8 times in terms of the progression to excessive GWG. Time of living with DM of ≥8 years, pre-pregnancy overweight or obesity, and previous hypothyroidism were risk factors for earlier excessive GWG. However, age ≥ 32 years old was a protective factor. We found no effect of the DASH diet on the risk for progression to excessive GWG, but our results of non-association need to be interpreted with caution because of our limited statistical power.
The allele frequencies in our sample were close to the frequencies in global databases (www.ncbi.nlm.nih.gov/snp (accessed on 18 January 2022)). Common polymorphisms (>5% allele frequency) are expected to be shared across different geographical regions and populations [36]. However, the Brazilian population is highly admixed and underrepresented in genomic studies as a potential source of new phenotype-associated genetic variants [37].
The A allele for the rs9939609 polymorphism in the FTO gene was previously associated with higher BMI before and after pregnancy [38,39] but not with excessive GWG [15] in Brazilian women. Studies from the USA [12,13] and Spain [14] found a higher risk of having higher GWG among A-allele carriers, contradicting the results from Turkey [40] and Mexico [41], both without association. The other evaluated polymorphisms (FTO rs17817449, ADRB2 rs1042713, and rs1042714) have not been previously investigated for GWG. The haplotype rs9939609:rs17817449 (AT) was found to increase the risk of obesity in the Brazilian adult population [11], but we identified the role of AG in the risk of progression to GWG.
The effect of the DASH diet on weight gain during pregnancy is controversial. Van Horn et al. (2018) noted that overweight and obese pregnant women (n = 280, EUA) gained less weight and had less excessive GWG using the DASH diet than by using a control diet [42], whereas Fulay et al. (2018) reported that high adherence to the DASH diet was associated with more weight gain during pregnancy in obese women (n = 1760, EUA) [43].
Genetics may partially explain the interindividual variation in body weight in response to nutritional intervention [44]; thus, it was hypothesized that the FTO and ADRB2 polymorphisms could modify the effect of diet on GWG. We found a marginal association of the FTO polymorphisms on the GWG according to the type of diet: women with the AA genotype for rs9939609 and women with the GG genotype for rs17817449 had higher GWG in the traditional diet group. This result could represent some benefit of the DASH diet on limiting GWG for women with these genotypes, but it was not confirmed in the analysis adjusted for the main confounders, as we found no effect of diet on the risk for progression to excessive GWG in our sample.
Martins et al. (2018) reported that the A allele for rs9939609 was associated with an increase in the total energy intake and increase in the percentage of energy from ultra-processed foods during pregnancy in a cohort of Brazilian women [45]. In our study, we calculated the individualized meal plan for all participants in both groups, considering the appropriate GWG, and we found similar high adherence to diet in the groups (40%). Indeed, the evaluation of dietary intake in details deserves further analyses to clarify if the genetic polymorphisms affect the GWG by dietary characteristics other than the dietary pattern (traditional or DASH), such as the level of food processing, for example.
Pregestational overweight and obesity were risk factors for progression to excessive GWG in our sample. This result agrees with the study by Brandão et al. (2021), which involved a large cohort of healthy Brazilian women and found that excessive GWG was observed in 30.1%, 30.7%, 56.4%, and 46.2% of underweight, normal weight, overweight, and obese women, respectively [2]. According to the IOM guidelines, GWG recommendations decrease when BMI increases. Thus, obese women should gain less weight than overweight and normal weight women, but the guidelines do not provide specific recommendations for women with pregestational DM [6].
In this context, Siegel et al. (2015) found no difference between BMI classes to gain less, within, or above the IOM recommendations in a sample of women with pregestational DM but noticed a higher risk for macrosomia (aOR 4.02; CI 95% 1.16–13.9) and large-for-gestational-age infants (aOR 3.08; CI 95% 1.13–8.39) in women who gained excessive weight compared to women who gained weight within the recommended amounts, even after adjusting for pregestational BMI [46]. The results from the study by Egan et al. (2014) were similar, reporting that excessive GWG in women with pregestational DM was a risk factor for macrosomia (aOR 3.58; CI 95% 1.77–7.24) and large-for-gestational-age infants (aOR 3.97; CI 95% 1.85–8.53), but they found more women with overweight or obesity presenting with excessive GWG than non-excessive GWG (44% vs. 27% for overweight and 37% vs. 25% for obesity; p < 0.01) [3].
We found that the number of years living with DM was a risk factor for progression to excessive GWG, but older age (≥32 years) was a protective factor, which appears controversial. The studies by Egan (2014) and Siegel (2015) did not find any association between age and excessive GWG [3,46], but in a cohort of 8184 healthy Brazilian women, the GWG decreased as the age increased [2].
A longer time of living with DM is expected in women with type 1 DM, who usually have lower pregestational BMI compared to those with type 2 DM [47]. Given our results for the effect of pregestational BMI on excessive GWG, it seems contradictory. However, the type of DM was not a risk factor for earlier excessive GWG in our sample. Therefore, we suggest that longer years of living with DM may impact the metabolic and behavioral factors influencing GWG not covered in the present study.
Only one participant had yellow skin. This woman was overweight before pregnancy and had the highest GWG in our sample (28.2 kg). Therefore, even though we had found the yellow color of the skin to be a risk factor for progression to excessive GWG, we considered that it cannot be properly interpreted or discussed, as it was an isolated case with a very wide confidence interval. Three women who had excessive GWG in our sample reported the color of the skin as unknown.
A meta-analysis comparing the prevalence of excessive GWG among racial/ethnic groups found that White women were more likely to exceed the IOM guidelines than their Asian and Hispanic counterparts, but White and Black women had a similar prevalence of excessive GWG [48]. Differences in the GWG regarding the color of the skin are often related to socioeconomic discrepancies [48]. We found a marginal effect of inadequate housing conditions on the risk of earlier excessive GWG, but it was not statistically significant.
Of the nine women with hypothyroidism included in our sample, eight had excessive GWG, with a risk more than four times higher than that of women without hypothyroidism (aHR 4.37, CI 95% 1.62–11.77; p = 0.00). Collares et al. (2017) found that higher maternal TSH and lower free T4 levels in early pregnancy were associated with a higher GWG [49]. Hypothyroidism is a common endocrine disorder that occurs during pregnancy [50]. In our sample, all women diagnosed with hypothyroidism were treated with oral repositioning of the T4 hormone and had adequate hormone levels at the time of inclusion in the study; however, monitoring adherence to treatment and hormone levels during pregnancy was outside the scope of this study.
Identifying risk factors for earlier exceeding GWG and implementing a dietary intervention to mitigate it may help decrease the related adverse outcomes. The sooner the excess weight is present, the more that the metabolic effects can harm the mother and fetus [51]. Of particular interest for pregnant women with DM is that an increase in maternal fat mass during early pregnancy can increase insulin resistance and thus worsen glycemic control [52]. As excess GWG does influence offspring obesity over the short- and long-term [53], faster fist and second trimester GWG but not third trimester was associated with higher mid-childhood adiposity in a cohort of 979 mother–child pairs from whom children were evaluated between 6.6 and 10.9 years of age [54].
Our study is novel in terms of several relevant characteristics. First, we investigated candidate genetic risk factors for progression to excessive GWG, and we did not find previous studies with this objective. Second, we investigated the influence of obesity-related polymorphisms in Brazilian women with pregestational diabetes since studies enrolling Brazilian pregnant women in this field are scarce and were not designed for women with DM. Additionally, women were administered two distinct types of diets, and we noticed that it had no effect on the risk of progression to excessive GWG in our sample.
However, this study had some limitations. First, we had a limited sample size to include in these analyses because the clinical trial was originally designed to analyze the effect of the two types of diets on perinatal outcomes without making use of the nutrigenetics approach. Given the increasing evidence regarding the effects of genetic characteristics and gene-diet interactions on BMI and obesity predisposition, we considered that it should gain more attention in the field of maternal and child nutrition. Therefore, we believe that this study will contribute to paving this way.
Furthermore, we had to make adaptations to maintain the study when the COVID-19 pandemic began in 2020. We used telemedicine to complete the follow-up of six women (8.6% of the sample) who were already enrolled in the study at the beginning of the pandemic quarantine. The same study protocol was used for the present visits. Prenatal visits to the physicians were maintained at the local level of the study, maintaining the measurements of weight at each visit. We also asked the participants to send a photograph of the prenatal card containing this information using a popular free smartphone application. Once the pandemic quarantine began, we did not include more participants in the study.
5. Conclusions
In this study, we investigated obesity-related polymorphisms in FTO and ADRB2 genes as candidate genetic risk factors for excessive GWG in pregnant women with pregestational DM using traditional or DASH diets.
Regardless the type of diet, the AT carriers of rs9939609 (FTO gene) had more than twice the risk of earlier exceeding GWG compared to the TT genotype, and the AA carriers of rs1042713 (ADRB2 gene) had almost four times higher risk than the GG carriers. The frequencies of these genotypes in our study population were 48.6% and 11.4%, respectively.
We found no effect of the genotypes of rs17817449 (FTO gene) and rs1042714 (ADRB2 gene) on the risk of progression to excessive GWG; however, the AG carriers for FTO haplotype rs9939609:rs17817449 had almost twice the risk of earlier exceeding GWG compared to TT carriers. Non-genetic characteristics associated with the risk of progression to GWG were time living with DM ≥ 8 years, pre-pregnancy overweight or obesity, and previous hypothyroidism. In contrast, age ≥ 32 years old was a protective factor.
Identifying women at a higher risk for exceeding GWG earlier may help improve nutritional interventions to mitigate this risk. The next step in advancing personalized nutrition is to understand which diet patterns may protect these women against excessive GWG and to investigate other genes and potential gene-diet-environment interactions with effects on GWG.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu14051050/s1, Table S1: Comparison of gestational weight gain (kg) between diet groups according to pregestational BMI (Rio de Janeiro/Brazil, 2016–2020); Figure S1: Gestational weight gain according to genotypes and dietary patterns.
Author Contributions
Conceptualization, K.d.S., E.L.R. and C.S.; data curation, K.d.S., G.P.B. and L.B.G.d.S.; formal analysis, K.d.S. and M.R.-A.; funding acquisition, E.L.R. and C.S.; investigation, K.d.S., A.C.P.d.F., G.P.B., L.B.G.d.S. and V.M.Z.; project administration, C.S.; writing—original draft, K.d.S.; writing—review and editing, E.L.R., J.A.M., and C.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received financial support from the Conselho Nacional de Desenvolvimento Científico e Tecnológico–CNPq (National Council for Scientific and Technological Development, grant number: 409032/2016-6), the Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro—FAPERJ (Carlos Chagas Filho Foundation to Research in the State of Rio de Janeiro, grant numbers: E-26/202.972/2016-6 and E-26/201.193/2021), and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior–CAPES–Brasil (Coordination for the Improvement of Higher Education Personnel). This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior-Brasil (CAPES)—Finance Code 001.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Maternidade Escola da UFRJ (CAAE–46913115.0.0000.5275; 15 July 2015). The study was registered in the Brazilian Registry of Clinical Trials (RBR-4tbgv6).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Acknowledgments
Lenita Zajdenverg, Marcus Miranda, Karina Bilda de Castro Rezende, Rita Bornia, Joffre Amin Jr., and Jorge Rezende Filho for their support during the study.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Goldstein, R.F.; Abell, S.K.; Ranasinha, S.; Misso, M.; Boyle, J.A.; Black, M.H.; Li, N.; Hu, G.; Corrado, F.; Rode, L.; et al. Association of gestational weight gain with maternal and infant outcomes: A systematic review and meta-analysis. J. Am. Med. Assoc. 2017, 317, 2207–2225. [Google Scholar] [CrossRef]
- Brandão, T.; de Carvalho Padilha, P.; de Barros, D.C.; Dos Santos, K.; Nogueira da Gama, S.G.; Leal, M.D.C.; da Silva Araújo, R.G.P.; Esteves Pereira, A.P.; Saunders, C. Gestational weight gain adequacy for favourable obstetric and neonatal outcomes: A nationwide hospital-based cohort gestational weight gain for favourable obstetric and neonatal outcomes. Clin. Nutr. ESPEN 2021, 45, 374–380. [Google Scholar] [CrossRef]
- Egan, A.M.; Dennedy, M.C.; Al-Ramli, W.; Heerey, A.; Avalos, G.; Dunne, F. ATLANTIC-DIP: Excessive gestational weight gain and pregnancy outcomes in women with gestational or pregestational diabetes mellitus. J. Clin. Endocrinol. Metab. 2014, 99, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Gualdani, E.; Di Cianni, G.; Seghieri, M.; Francesconi, P.; Seghieri, G. Pregnancy outcomes and maternal characteristics in women with pregestational and gestational diabetes: A retrospective study on 206,917 singleton live births. Acta Diabetol. 2021, 58, 1169–1176. [Google Scholar] [CrossRef] [PubMed]
- McDowell, M.; Cain, M.A.; Brumley, J. Excessive Gestational Weight Gain. J. Midwifery Women’s Heal. 2018, 64, 46–54. [Google Scholar] [CrossRef]
- Institute of Medicine. Weight Gain during Pregnancy: Reexamining the Guidelines; The National Academies Press: Washington, DC, USA, 2009. [Google Scholar]
- Institute of Medicine. Implementing Guidelines on Weight Gain and Pregnancy; The National Academies Press: Washington, DC, USA, 2013. [Google Scholar]
- Warrington, N.M.; Richmond, R.; Fenstra, B.; Myhre, R.; Gaillard, R.; Paternoster, L.; Wang, C.A.; Beaumont, R.N.; Das, S.; Murcia, M.; et al. Maternal and fetal genetic contribution to gestational weight gain. Int. J. Obes. 2018, 42, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Maugeri, A.; Magnano San Lio, R.; La Rosa, M.C.; Giunta, G.; Panella, M.; Cianci, A.; Caruso, M.A.T.; Agodi, A.; Barchitta, M. The Relationship between Telomere Length and Gestational Weight Gain: Findings from the Mamma & Bambino Cohort. Biomedicines 2021, 10, 67. [Google Scholar] [CrossRef]
- Loos, R.J.; Yeo, G.S. The bigger picture of FTO: The first GWAS-identified obesity gene. Nat. Rev. Endocrinol. 2014, 10, 51–61. [Google Scholar] [CrossRef]
- Fonseca, A.C.P.D.; Marchesini, B.; Zembrzuski, V.M.; Voigt, D.D.; Ramos, V.G.; Carneiro, J.R.I.; Nogueira Neto, J.F.; Cabello, G.M.K.; Cabello, P.H. Genetic variants in the fat mass and obesity-associated (FTO) gene confer risk for extreme obesity and modulate adiposity in a Brazilian population. Genet Mol. Biol. 2020, 43, e20180264. [Google Scholar] [CrossRef]
- Groth, S.W.; LaLonde, A.; Wu, T.; Fernandez, I.D. Obesity candidate genes, gestational weight gain, and body weight changes in pregnant women. Nutrition 2018, 48, 61–66. [Google Scholar] [CrossRef]
- Groth, S.W.; Morrison-Beedy, D. GNB3 and FTO Polymorphisms and Pregnancy Weight Gain in Black Women. Biol. Res. Nurs. 2014, 17, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Gesteiro, E.; Sánchez-Muniz, F.J.; Ortega-Azorín, C.; Guillén, M.; Corella, D.; Bastida, S. Maternal and neonatal FTO rs9939609 polymorphism affect insulin sensitivity markers and lipoprotein profile at birth in appropriate-for-gestational-age term neonates. J. Physiol. Biochem. 2016, 72, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.C.; Trujillo, J.; Farias, D.R.; Struchiner, C.J.; Kac, G. Association of the FTO (rs9939609) and MC4R (rs17782313) gene polymorphisms with maternal body weight during pregnancy. Nutrition 2016, 32, 1223–1230. [Google Scholar] [CrossRef]
- Al-Ogaidi, S.O.; Abdulsattar, S.; Al-dulaimi, M. FTO rs17817449 Gene Polymorphism as a Predictor for Maternal Obesity in Iraqi Pregnant Women. Indian J. Public Heal. Res. Dev. 2019, 10, 678–683. [Google Scholar] [CrossRef]
- Steemburgo, T.; Azevedo, M.J.; Martínez, J.A. Interação entre gene e nutriente e sua associação à obesidade e ao diabetes melito [Gene-nutrient interaction and its association with obesity and diabetes mellitus]. Arq. Bras. de Endocrinol. Metabol. 2009, 53, 497–508. (In Portuguese) [Google Scholar] [CrossRef]
- Zhang, H.; Wu, J.; Yu, L. Association of Gln27Glu and Arg16Gly polymorphisms in Beta2-adrenergic receptor gene with obesity susceptibility: A meta-analysis. PLoS ONE 2014, 9, e100489. [Google Scholar] [CrossRef]
- Maugeri, A.; Barchitta, M.; Favara, G.; La Rosa, M.C.; La Mastra, C.; Magnano San Lio, R.; Agodi, A. Maternal Dietary Patterns Are Associated with Pre-Pregnancy Body Mass Index and Gestational Weight Gain: Results from the “Mamma & Bambino”. Cohort. Nutr. 2019, 11, 1308. [Google Scholar] [CrossRef]
- American Diabetes Association. 14. Management of Diabetes in Pregnancy: Standards of Medical Care in Diabetes—2021. Diabetes Care 2020, 44, S200–S210. [Google Scholar] [CrossRef]
- Svetkey, L.P.; Sacks, F.M.; Obarzanek, E.; Vollmer, W.M.; Appel, L.J.; Lin, P.H.; Karanja, N.M.; Harsha, D.W.; Bray, G.A.; Aickin, M.; et al. The DASH Diet, Sodium Intake and Blood Pressure Trial (DASH-sodium): Rationale and design. DASH-Sodium Collaborative Research Group. J. Am. Diet. Assoc. 1999, 99, S96–S104. [Google Scholar] [CrossRef]
- Asemi, Z.; Tabassi, Z.; Samimi, M.; Fahiminejad, T.; Esmaillzadeh, A. Favourable effects of the Dietary Approaches to Stop Hypertension diet on glucose tolerance and lipid profiles in gestational diabetes: A randomised clinical trial. Br. J. Nutr. 2012, 109, 2024–2030. [Google Scholar] [CrossRef]
- Li, S.; Gan, Y.; Chen, M.; Wang, M.; Wang, X.; Santos, H.O.; Okunade, K.; Kathirgamathamby, V. Effects of the Dietary Approaches to Stop Hypertension (DASH) on Pregnancy/Neonatal Outcomes and Maternal Glycemic Control: A Systematic Review and Meta-analysis of Randomized Clinical Trials. Complement. Ther. Med. 2020, 54, 102551. [Google Scholar] [CrossRef]
- Hosseini-Esfahani, F.; Koochakpoor, G.; Daneshpour, M.S.; Sedaghati-Khayat, B.; Mirmiran, P.; Azizi, F. Mediterranean Dietary Pattern Adherence Modify the Association between FTO Genetic Variations and Obesity Phenotypes. Nutrients 2017, 9, 1064. [Google Scholar] [CrossRef]
- Hosseini-Esfahani, F.; Koochakpoor, G.; Mirmiran, P.; Daneshpour, M.S.; Azizi, F. Dietary patterns modify the association between fat mass and obesity-associated genetic variants and changes in obesity phenotypes. Br. J. Nutr. 2019, 121, 1247–1254. [Google Scholar] [CrossRef]
- Martínez, J.A.; Corbalán, M.S.; Sánchez-Villegas, A.; Forga, L.; Marti, A.; Martínez-González, M.A. Obesity risk is associated with carbohydrate intake in women carrying the Gln27Glu beta2-adrenoceptor polymorphism. J. Nutr. 2003, 133, 2549–2554. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, L.R.; De Caterina, R.; Görman, U.; Allayee, H.; Kohlmeier, M.; Prasad, C.; Choi, M.S.; Curi, R.; de Luis, D.A.; Gil, Á.; et al. Guide and Position of the International Society of Nutrigenetics/Nutrigenomics on Personalised Nutrition: Part 1-Fields of Precision Nutrition. J. Nutr. Nutr. 2016, 9, 12–27. [Google Scholar] [CrossRef]
- Saunders, C.; Moreira, T.M.; Belfort, G.P.; da Silva, C.F.M.; dos Santos, K.; da Silva, L.B.G.; Scancetti, L.B.; Fagherazzi, S.; Pereira, A.F.; Rosado, E.L.; et al. Procedimentos metodológicos para elaboração de plano alimentar adaptado baseado na dieta DASH para gestantes com diabetes mellitus. BJD 2021, 7, 116769–116788. [Google Scholar] [CrossRef]
- Della Líbera, B.; Ribeiro Baião, M.; de Souza Santos, M.M.; Padilha, P.; Dutra Alves, P.; Saunders, C. Adherence of pregnant women to dietary counseling and adequacy of total gestational weight gain. Nutr. Hosp. 2011, 26, 79–85. [Google Scholar]
- Carrilho, T.R.B.; Rasmussen, K.; Farias, D.R.; Freitas Costa, N.C.; Araújo Batalha, M.; Reichenheim, M.E.; Ohuma, E.O.; Hutcheon, J.A.; Kac, G.; Brazilian Maternal and Child Nutrition Consortium. Agreement between self-reported pre-pregnancy weight and measured first-trimester weight in Brazilian women. BMC Pregnancy Childbirth 2020, 20, 734. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Physical Status: The Use and Interpretation of Anthropometry; Report of a WHO Expert Committee; WHO: Geneva, Switzerland, 1995; Volume 452, pp. 1–542. [Google Scholar]
- Aidar, M.; Line, S.R.P. A simple and cost-effective protocol for DNA isolation from buccal epithelial cells. Braz. Dent. J. 2007, 18, 148–152. [Google Scholar] [CrossRef]
- Matsudo, S.; Araújo, T.; Matsudo, V.; Andrade, D.; Andrade, E.; Oliveira, L.C.; Braggion, G. Questionário Internacional de Atividade Física (IPAQ): Estudo de validade e reprodutibilidade no Brasil. RBAFS 2001, 6, 5–18. [Google Scholar]
- Núcleo de Estudos e Pesquisas em Alimentação (NEPA). Tabela Brasileira de Composição de Alimentos (TACO/NEPA-Unicamp). Available online: http://www.nepa.unicamp.br/taco/tabela.php?ativo=tabela (accessed on 25 February 2022).
- United States Department of Agriculture. USDA National Nutrient Database for Standard Reference. Available online: https://fdc.nal.usda.gov/ (accessed on 25 February 2022).
- Biddanda, A.; Rice, D.P.; Novembre, J. A variant-centric perspective on geographic patterns of human allele frequency variation. Elife 2020, 9, e60107. [Google Scholar] [CrossRef] [PubMed]
- Scliar, M.O.; Sant’Anna, H.P.; Santolalla, M.L.; Leal, T.P.; Araújo, N.M.; Alvim, I.; Borda, V.; Magalhães, W.C.S.; Gouveia, M.H.; Lyra, R.; et al. Admixture/fine-mapping in Brazilians reveals a West African associated potential regulatory variant (rs114066381) with a strong female-specific effect on body mass and fat mass indexes. Int. J. Obes. 2021, 45, 1017–1029. [Google Scholar] [CrossRef] [PubMed]
- Kroll, C.; Farias, D.R.; Carrilho, T.R.B.; Kac, G.; Mastroeni, M.F. Association of ADIPOQ-rs2241766 and FTO-rs9939609 genetic variants with body mass index trajectory in women of reproductive age over 6 years of follow-up: The PREDI study. Eur. J. Clin. Nutr. 2021, 76, 159–172. [Google Scholar] [CrossRef] [PubMed]
- Kroll, C.; de França, P.H.C.; Mastroeni, M.F. Association between FTO gene polymorphism and excess body weight in women from before to after pregnancy: A cohort study. Am. J. Hum. Biol. 2018, 30, e23164. [Google Scholar] [CrossRef]
- Beysel, S.; Eyerci, N.; Ulubay, M.; Caliskan, M.; Kizilgul, M.; Hafızoğlu, M.; Cakal, E. Maternal genetic contribution to pre-pregnancy obesity, gestational weight gain, and gestational diabetes mellitus. Diabetol. Metab. Syndr. 2019, 11, 37. [Google Scholar] [CrossRef] [PubMed]
- Saucedo, R.; Valencia, J.; Gutierrez, C.; Basurto, L.; Hernandez, M.; Puello, E.; Rico, G.; Vega, G.; Zarate, A. Gene variants in the FTO gene are associated with adiponectin and TNF-alpha levels in gestational diabetes mellitus. Diabetol. Metab. Syndr. 2017, 9, 32. [Google Scholar] [CrossRef]
- Van Horn, L.; Peaceman, A.; Kwasny, M.; Vincent, E.; Fought, A.; Josefson, J.; Spring, B.; Neff, L.M.; Gernhofer, N. Dietary Approaches to Stop Hypertension Diet and Activity to Limit Gestational Weight: Maternal Offspring Metabolics Family Intervention Trial, a Technology Enhanced Randomized Trial. Am. J. Prev. Med. 2018, 55, 603–614. [Google Scholar] [CrossRef]
- Fulay, A.P.; Rifas-Shiman, S.L.; Oken, E.; Perng, W. Associations of the dietary approaches to stop hypertension (DASH) diet with pregnancy complications in Project Viva. Eur. J. Clin. Nutr. 2018, 72, 1385–1395. [Google Scholar] [CrossRef]
- Camp, K.M.; Trujillo, E. Position of the Academy of Nutrition and Dietetics: Nutritional genomics. J. Acad. Nutr. Diet. 2014, 114, 299–312. [Google Scholar] [CrossRef]
- Martins, M.C.; Trujillo, J.; Freitas-Vilela, A.A.; Farias, D.R.; Rosado, E.L.; Struchiner, C.J.; Kac, G. Associations between obesity candidate gene polymorphisms (fat mass and obesity-associated (FTO), melanocortin-4 receptor (MC4R), leptin (LEP) and leptin receptor (LEPR)) and dietary intake in pregnant women. Br. J. Nutr. 2018, 120, 454–463. [Google Scholar] [CrossRef]
- Siegel, A.M.; Tita, A.; Biggio, J.R.; Harper, L.M. Evaluating gestational weight gain recommendations in pregestational diabetes. Am. J. Obstet. Gynecol. 2015, 213, 563.e1–563.e5. [Google Scholar] [CrossRef] [PubMed]
- Alessi, J.; Wiegand, D.M.; Hirakata, V.N.; Oppermann, M.L.R.; Reichelt, A.J. Temporal changes in characteristics and outcomes among pregnant women with pre-gestational diabetes. Int. J. Gynecol. Obstet. 2018, 143, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Denize, K.M.; Acharya, N.; Prince, S.A.; da Silva, D.F.; Harvey, A.L.J.; Ferraro, Z.M.; Adamo, K.B. Addressing cultural, racial and ethnic discrepancies in guideline discordant gestational weight gain: A systematic review and meta-analysis. PeerJ 2018, 6, e5407. [Google Scholar] [CrossRef] [PubMed]
- Collares, F.M.; Korevaar, T.I.M.; Hofman, A.; Steegers, E.A.P.; Peeters, R.P.; Jaddoe, V.W.V.; Gaillard, R. Maternal thyroid function, prepregnancy obesity and gestational weight gain-The Generation R Study: A prospective cohort study. Clin. Endocrinol. 2017, 87, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Alexander, E.K.; Pearce, E.N.; Brent, G.A.; Brown, R.S.; Chen, H.; Dosiou, C.; Grobman, W.A.; Laurberg, P.; Lazarus, J.H.; Mandel, S.J.; et al. 2017 Guidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease During Pregnancy and the Postpartum. Thyroid 2017, 27, 315–389. [Google Scholar] [CrossRef]
- Cheney, K.; Berkemeier, S.; Sim, K.A.; Gordon, A.; Black, K. Prevalence and predictors of early gestational weight gain associated with obesity risk in a diverse Australian antenatal population: A cross-sectional study. BMC Pregnancy Childbirth 2017, 17, 296. [Google Scholar] [CrossRef] [PubMed]
- Herring, S.J.; Oken, E.; Rifas-Shiman, S.L.; Rich-Edwards, J.W.; Stuebe, A.M.; Kleinman, K.P.; Gillman, M.W. Weight gain in pregnancy and risk of maternal hyperglycemia. Am. J. Obstet. Gynecol. 2009, 201, 61.e1–61.e7. [Google Scholar] [CrossRef]
- Mamun, A.A.; Mannan, M.; Doi, S.A. Gestational weight gain in relation to offspring obesity over the life course: A systematic review and bias-adjusted meta-analysis. Obes. Rev. 2013, 15, 338–347. [Google Scholar] [CrossRef]
- Hivert, M.F.; Rifas-Shiman, S.L.; Gillman, M.W.; Oken, E. Greater early and mid-pregnancy gestational weight gains are associated with excess adiposity in mid-childhood. Obesity 2016, 24, 1546–1553. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).