Longitudinal Associations of Plasma Phospholipid Fatty Acids in Pregnancy with Neonatal Anthropometry: Results from the NICHD Fetal Growth Studies—Singleton Cohort
Abstract
1. Introduction
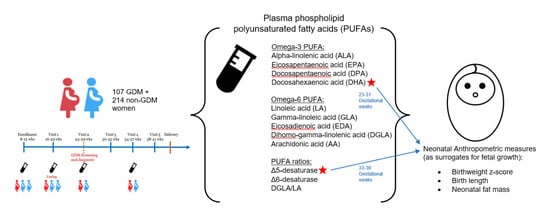
2. Materials and Methods
2.1. Study Sample
2.2. Maternal Biomarker Assessment
2.3. Neonatal Anthropometric Measures
2.4. Covariates
2.5. Statistical Methods
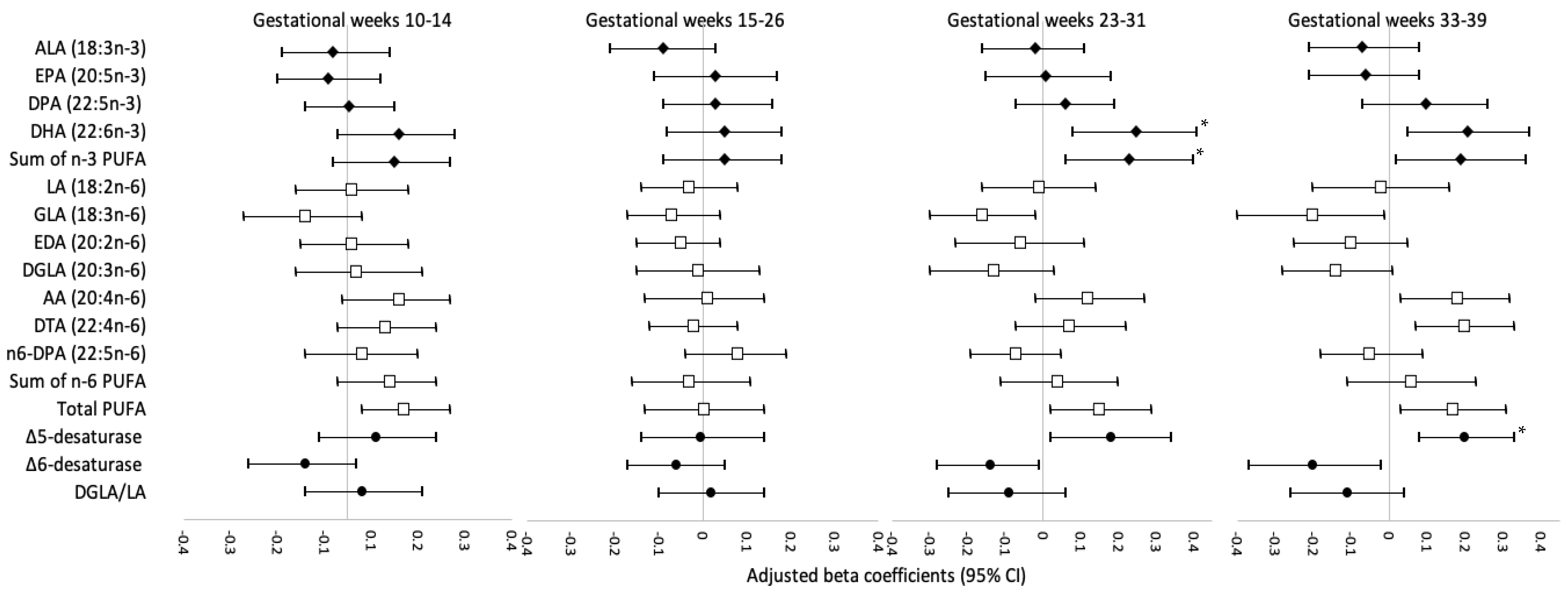
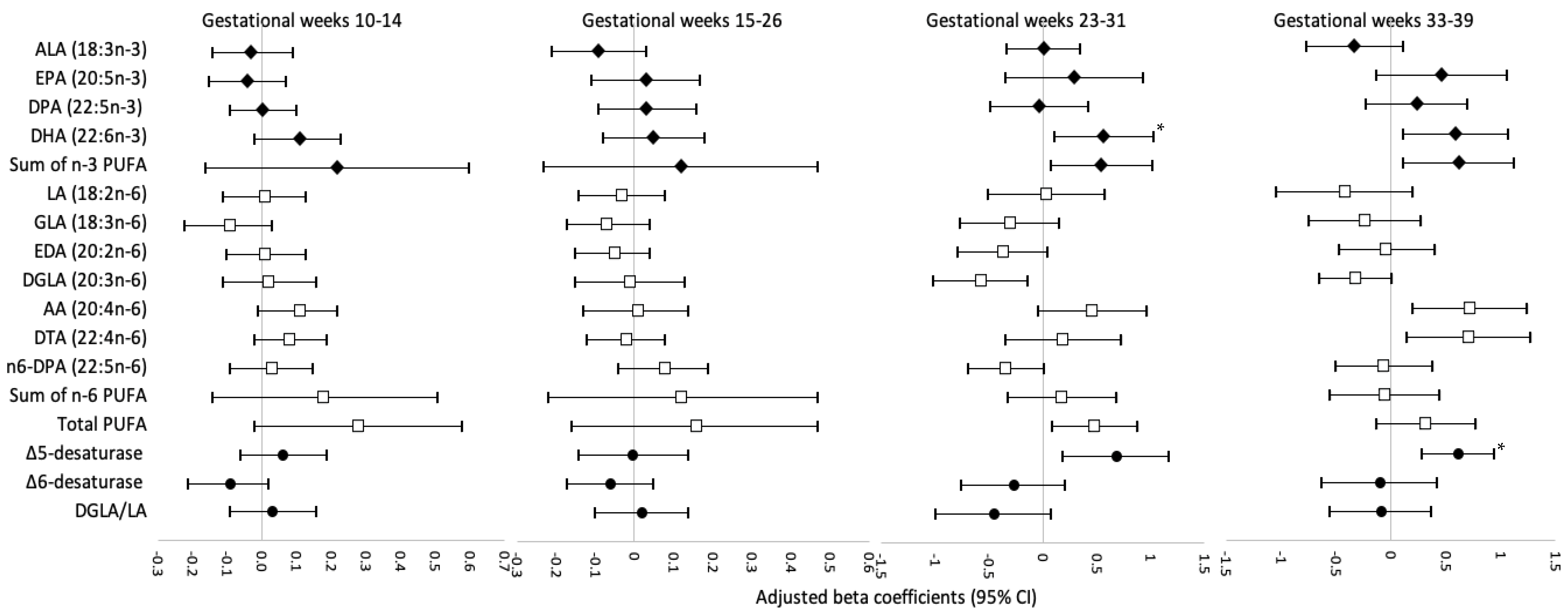
3. Results
4. Discussion
4.1. Comparison with Studies on DHA and Neonatal Anthropometry
4.2. Comparison with Studies on Estimated Δ5-Desaturase Activity and Neonatal Anthropometry
4.3. Biological Plausibility and Implications
4.4. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stettler, N. Nature and strength of epidemiological evidence for origins of childhood and adulthood obesity in the first year of life. Int. J. Obes. 2007, 31, 1035–1043. [Google Scholar] [CrossRef] [PubMed]
- Gillman, M.W.; Rifas-Shiman, S.; Berkey, C.S.; Field, A.E.; Colditz, G.A. Maternal gestational diabetes, birth weight, and adolescent obesity. Pediatrics 2003, 111, e221–e226. [Google Scholar] [CrossRef] [PubMed]
- Rogers, I. The influence of birthweight and intrauterine environment on adiposity and fat distribution in later life. Int. J. Obes. Relat. Metab. Disord. 2003, 27, 755–777. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, J. Diabetes and pregnancy; Blood sugar of newborn infants during fasting and glucose administration. Nord Med. 1952, 47, 1049. [Google Scholar] [PubMed]
- Freinkel, N. Banting Lecture 1980: Of pregnancy and progeny. Diabetes 1980, 29, 1023–1035. [Google Scholar] [CrossRef] [PubMed]
- Herrera, E.; Desoye, G. Maternal and fetal lipid metabolism under normal and gestational diabetic conditions. Horm. Mol. Biol. Clin. Investig. 2016, 26, 109–127. [Google Scholar] [CrossRef]
- Barbour, L.A.; Hernandez, T.L. Maternal Non-glycemic Contributors to Fetal Growth in Obesity and Gestational Diabetes: Spotlight on Lipids. Curr. Diab. Rep. 2018, 18, 37. [Google Scholar] [CrossRef]
- De Lorgeril, M.; Salen, P. New insights into the health effects of dietary saturated and omega-6 and omega-3 polyunsaturated fatty acids. BMC Med. 2012, 10, 50. [Google Scholar] [CrossRef]
- Forouhi, N.G.; Imamura, F.; Sharp, S.J.; Koulman, A.; Schulze, M.B.; Zheng, J.; Ye, Z.; Sluijs, I.; Guevara, M.; Huerta, J.M.; et al. Association of Plasma Phospholipid n-3 and n-6 Polyunsaturated Fatty Acids with Type 2 Diabetes: The EPIC-InterAct Case-Cohort Study. PLoS Med. 2016, 13, e1002094. [Google Scholar] [CrossRef]
- Forouhi, N.G.; Koulman, A.; Sharp, S.J.; Imamura, F.; Kröger, J.; Schulze, M.B.; Crowe, F.L.; Huerta, J.M.; Guevara, M.; Beulens, J.W.; et al. Differences in the prospective association between individual plasma phospholipid saturated fatty acids and incident type 2 diabetes: The EPIC-InterAct case-cohort study. Lancet Diabetes Endocrinol. 2014, 2, 810–818. [Google Scholar] [CrossRef]
- Zhu, Y.; Tsai, M.Y.; Sun, Q.; Hinkle, S.N.; Rawal, S.; Mendola, P.; Ferrara, A.; Albert, P.S.; Zhang, C. A prospective and longitudinal study of plasma phospholipid saturated fatty acid profile in relation to cardiometabolic biomarkers and the risk of gestational diabetes. Am. J. Clin. Nutr. 2018, 107, 1017–1026. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Li, M.; Rahman, M.L.; Hinkle, S.N.; Wu, J.; Weir, N.L.; Lin, Y.; Yang, H.; Tsai, M.Y.; Ferrara, A.; et al. Plasma phospholipid n-3 and n-6 polyunsaturated fatty acids in relation to cardiometabolic markers and gestational diabetes: A longitudinal study within the prospective NICHD Fetal Growth Studies. PLoS Med. 2019, 16, e1002910. [Google Scholar] [CrossRef] [PubMed]
- Donahue, S.M.; Rifas-Shiman, S.L.; Gold, D.R.; Jouni, Z.E.; Gillman, M.W.; Oken, E. Prenatal fatty acid status and child adiposity at age 3 y: Results from a US pregnancy cohort. Am. J. Clin. Nutr. 2011, 93, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Penfield-Cyr, A.; Monthe-Dreze, C.; Smid, M.C.; Sen, S. Maternal BMI, Mid-pregnancy Fatty Acid Concentrations, and Perinatal Outcomes. Clin. Ther. 2018, 40, 1659–1667.e1. [Google Scholar] [CrossRef]
- Grootendorst-van Mil, N.H.; Tiemeier, H.; Steenweg-de Graaff, J.; Koletzko, B.; Demmelmair, H.; Jaddoe, V.W.V.; Steegers, E.A.P.; Steegers-Theunissen, R.P.M. Maternal plasma n-3 and n-6 polyunsaturated fatty acids during pregnancy and features of fetal health: Fetal growth velocity, birth weight and duration of pregnancy. Clin. Nutr. 2018, 37, 1367–1374. [Google Scholar] [CrossRef]
- Delgado-Noguera, M.F.; Calvache, J.A.; Bonfill Cosp, X.; Kotanidou, E.P.; Galli-Tsinopoulou, A. Supplementation with long chain polyunsaturated fatty acids (LCPUFA) to breastfeeding mothers for improving child growth and development. Cochrane Database Syst. Rev. 2015, 7, CD007901. [Google Scholar] [CrossRef]
- Voortman, T.; van den Hooven, E.H.; Braun, K.V.; van den Broek, M.; Bramer, W.M.; Chowdhurry, R.; Franco, O.H. Effects of polyunsaturated fatty acid intake and status during pregnancy, lactation, and early childhood on cardiometabolic health: A systematic review. Prog. Lipid Res. 2015, 59, 67–87. [Google Scholar] [CrossRef]
- Schaefer-Graf, U.M.; Kjos, S.L.; Kilavuz, O.; Plagemann, A.; Brauer, M.; Dudenhausen, J.W.; Vetter, K. Determinants of fetal growth at different periods of pregnancies complicated by gestational diabetes mellitus or impaired glucose tolerance. Diabetes Care 2003, 26, 193–198. [Google Scholar] [CrossRef]
- Herrera, E.; Ortega-Senovilla, H. Implications of Lipids in Neonatal Body Weight and Fat Mass in Gestational Diabetic Mothers and Non-Diabetic Controls. Curr. Diab. Rep. 2018, 18, 7. [Google Scholar] [CrossRef]
- Schaefer-Graf, U.M.; Graf, K.; Kulbacka, I.; Kjos, S.L.; Dudenhausen, J.; Vetter, K.; Herrera, E. Maternal lipids as strong determinants of fetal environment and growth in pregnancies with gestational diabetes mellitus. Diabetes Care 2008, 31, 1858–1863. [Google Scholar] [CrossRef]
- Kitajima, M.; Oka, S.; Yasuhi, I.; Fukuda, M.; Rii, Y.; Ishimaru, T. Maternal serum triglyceride at 24--32 weeks’ gestation and newborn weight in nondiabetic women with positive diabetic screens. Obstet. Gynecol. 2001, 97, 776–780. [Google Scholar] [CrossRef]
- Nolan, C.J.; Riley, S.F.; Sheedy, M.T.; Walstab, J.E.; Beischer, N.A. Maternal serum triglyceride, glucose tolerance, and neonatal birth weight ratio in pregnancy. Diabetes Care 1995, 18, 1550–1556. [Google Scholar] [CrossRef] [PubMed]
- Knopp, R.H.; Magee, M.S.; Walden, C.E.; Bonet, B.; Benedetti, T.J. Prediction of infant birth weight by GDM screening tests. Importance of plasma triglyceride. Diabetes Care 1992, 15, 1605–1613. [Google Scholar] [CrossRef] [PubMed]
- Buck Louis, G.M.; Grewal, J.; Albert, P.S.; Sciscione, A.; Wing, D.A.; Grobman, W.A.; Newman, R.B.; Wapner, R.; D’Alton, M.E.; Skupski, D.; et al. Racial/ethnic standards for fetal growth: The NICHD Fetal Growth Studies. Am. J. Obstet. Gynecol. 2015, 213, 449.e1–449.e41. [Google Scholar] [CrossRef] [PubMed]
- Grewal, J.; Grantz, K.L.; Zhang, C.; Sciscione, A.; Wing, D.A.; Grobman, W.A.; Newman, R.B.; Wapner, R.; D’Alton, M.E.; Skupski, D.; et al. Cohort Profile: NICHD Fetal Growth Studies-Singletons and Twins. Int. J. Epidemiol. 2018, 47, 25–25I. [Google Scholar] [CrossRef] [PubMed]
- Practice Bulletin No. 137: Gestational Diabetes Mellitus. 2013. Available online: https://journals.lww.com/greenjournal/Fulltext/2013/08000/Practice_Bulletin_No__137__Gestational_Diabetes.46.aspx (accessed on 2 January 2022).
- Cao, J.; Schwichtenberg, K.A.; Hanson, N.Q.; Tsai, M.Y. Incorporation and clearance of omega-3 fatty acids in erythrocyte membranes and plasma phospholipids. Clin Chem. 2006, 52, 2265–2272. [Google Scholar] [CrossRef]
- Hodson, L.; Skeaff, C.M.; Fielding, B.A. Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake. Prog Lipid Res. 2008, 47, 348–380. [Google Scholar] [CrossRef]
- Merino, D.M.; Ma, D.W.; Mutch, D.M. Genetic variation in lipid desaturases and its impact on the development of human disease. Lipids Health Dis. 2010, 9, 63. [Google Scholar] [CrossRef]
- Murff, H.J.; Edwards, T.L. Endogenous Production of Long-Chain Polyunsaturated Fatty Acids and Metabolic Disease Risk. Curr. Cardiovasc. Risk Rep. 2014, 8, 418. [Google Scholar] [CrossRef]
- Guan, W.; Steffen, B.T.; Lemaitre, R.N.; Wu, J.H.Y.; Tanaka, T.; Manichaikul, A.; Foy, M.; Rich, S.S.; Wang, L.; Nettleton, J.A.; et al. Genome-wide association study of plasma N6 polyunsaturated fatty acids within the cohorts for heart and aging research in genomic epidemiology consortium. Circ. Cardiovasc. Genet. 2014, 7, 321–331. [Google Scholar] [CrossRef]
- Oken, E.; Kleinman, K.P.; Rich-Edwards, J.; Gillman, M.W. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr. 2003, 3, 6. [Google Scholar] [CrossRef] [PubMed]
- Weile, B.; Bach-Mortensen, N.; Peitersen, B. Caliper skinfold measurements in newborns: Analysis of a method. Biol. Neonate 1986, 50, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Catalano, P.M.; Thomas, A.J.; Avallone, D.A.; Amini, S.B. Anthropometric estimation of neonatal body composition. Am. J. Obstet. Gynecol. 1995, 173, 1176–1181. [Google Scholar] [CrossRef]
- Samuelsen, S.O. A psudolikelihood approach to analysis of nested case-control studies. Biometrika 1997, 84, 379–394. [Google Scholar] [CrossRef]
- Marler, J.R.; Tilley, B.C.; Lu, M.; Brott, T.G.; Lyden, P.C.; Grotta, J.C.; Broderick, J.P.; Levine, S.R.; Frankel, M.P.; Horowitz, S.H.; et al. Early stroke treatment associated with better outcome: The NINDS rt-PA stroke study. Neurology 2000, 55, 1649–1655. [Google Scholar] [CrossRef]
- The NINDS t-PA Stroke Study Group. Intracerebral hemorrhage after intravenous t-PA therapy for ischemic stroke. Stroke 1997, 28, 2109–2118. [Google Scholar] [CrossRef] [PubMed]
- NINDS t-PA Stroke Study Group. Generalized efficacy of t-PA for acute stroke: Subgroup analysis of the NINDS t-PA Stroke Trial. Stroke 1997, 28, 2119–2125. [Google Scholar] [CrossRef]
- Thiese, M.S.; Ronna, B.; Ott, U. P value interpretations and considerations. J. Thorac. Dis. 2016, 8, E928–E931. [Google Scholar] [CrossRef]
- Rump, P.; Mensink, R.P.; Kester, A.D.; Hornstra, G. Essential fatty acid composition of plasma phospholipids and birth weight: A study in term neonates. Am. J. Clin. Nutr. 2001, 73, 797–806. [Google Scholar] [CrossRef][Green Version]
- Bernard, J.Y.; Tint, M.T.; Aris, I.M.; Chen, L.W.; Quah, P.L.; Tan, K.H.; Yeo, G.S.; Fortier, M.V.; Yap, F.; Shek, L.; et al. Maternal plasma phosphatidylcholine polyunsaturated fatty acids during pregnancy and offspring growth and adiposity. Prostaglandins Leukot Essent Fat. Acids 2017, 121, 21–29. [Google Scholar] [CrossRef]
- Symonds, M.E.; Mostyn, A.; Pearce, S.; Budge, H.; Stephenson, T. Endocrine and nutritional regulation of fetal adipose tissue development. J. Endocrinol. 2003, 179, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, A.; Kawabata, T.; Kagawa, Y.; Shoji, K.; Kimura, F.; Miyazawa, T.; Tatsuta, N.; Arima, T.; Yaegashi, N.; Nakai, K. Associations of umbilical cord fatty acid profiles and desaturase enzyme indices with birth weight for gestational age in Japanese infants. Prostaglandins Leukot Essent Fat. Acids 2021, 165, 102233. [Google Scholar] [CrossRef] [PubMed]
- Van Eijsden, M.; Hornstra, G.; van der Wal, M.F.; Vrijkotte, T.G.; Bonsel, G.J. Maternal n-3, n-6, and trans fatty acid profile early in pregnancy and term birth weight: A prospective cohort study. Am. J. Clin. Nutr. 2008, 87, 887–895. [Google Scholar] [CrossRef] [PubMed]
- Meher, A.; Randhir, K.; Mehendale, S.; Wagh, G.; Joshi, S. Maternal Fatty Acids and Their Association with Birth Outcome: A Prospective Study. PLoS ONE 2016, 11, e0147359. [Google Scholar] [CrossRef] [PubMed]
- Monthé-Drèze, C.; Penfield-Cyr, A.; Smid, M.C.; Sen, S. Maternal Pre-Pregnancy Obesity Attenuates Response to Omega-3 Fatty Acids Supplementation during Pregnancy. Nutrients 2018, 10, 1908. [Google Scholar] [CrossRef]
- Vidakovic, A.J.; Jaddoe, V.W.; Gishti, O.; Felix, J.F.; Williams, M.A.; Hofman, A.; Demmelmair, H.; Koletzko, B.; Tiemeier, H.; Gaillard, R. Body mass index, gestational weight gain and fatty acid concentrations during pregnancy: The Generation R Study. Eur. J. Epidemiol. 2015, 30, 1175–1185. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am. J. Clin. Nutr. 2006, 83, 1505s–1519s. [Google Scholar] [CrossRef] [PubMed]
- Miles, E.A.; Calder, P.C. Can Early Omega-3 Fatty Acid Exposure Reduce Risk of Childhood Allergic Disease? Nutrients 2017, 9, 784. [Google Scholar] [CrossRef]
- Calabuig-Navarro, V.; Puchowicz, M.; Glazebrook, P.; Haghiac, M.; Minium, J.; Catalano, P.; Hauguel deMouzon, S.; O’Tierney-Ginn, P. Effect of ω-3 supplementation on placental lipid metabolism in overweight and obese women. Am. J. Clin. Nutr. 2016, 103, 1064–1072. [Google Scholar] [CrossRef]
- Yang, L.G.; Song, Z.X.; Yin, H.; Wang, Y.Y.; Shu, G.F.; Lu, H.X.; Wang, S.K.; Sun, G.J. Low n-6/n-3 PUFA Ratio Improves Lipid Metabolism, Inflammation, Oxidative Stress and Endothelial Function in Rats Using Plant Oils as n-3 Fatty Acid Source. Lipids 2016, 51, 49–59. [Google Scholar] [CrossRef]
- Valentine, C.J. Maternal dietary DHA supplementation to improve inflammatory outcomes in the preterm infant. Adv. Nutr. 2012, 3, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Joss-Moore, L.A.; Wang, Y.; Baack, M.L.; Yao, J.; Norris, A.W.; Yu, X.; Callaway, C.W.; McKnight, R.A.; Albertine, K.H.; Lane, R.H. IUGR decreases PPARγ and SETD8 Expression in neonatal rat lung and these effects are ameliorated by maternal DHA supplementation. Early Hum. Dev. 2010, 86, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Carlson, S.E.; Colombo, J.; Gajewski, B.J.; Gustafson, K.M.; Mundy, D.; Yeast, J.; Georgieff, M.K.; Markley, L.A.; Kerling, E.H.; Shaddy, D.J. DHA supplementation and pregnancy outcomes. Am. J. Clin. Nutr. 2013, 97, 808–815. [Google Scholar] [CrossRef] [PubMed]
- Savasan, Z.A.; Goncalves, L.F.; Bahado-Singh, R.O. Second- and third-trimester biochemical and ultrasound markers predictive of ischemic placental disease. Semin Perinatol. 2014, 38, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Coletta, J.M.; Bell, S.J.; Roman, A.S. Omega-3 Fatty acids and pregnancy. Rev. Obstet. Gynecol. 2010, 3, 163–171. [Google Scholar]

| Unweighted Overall (n = 2802) | Weighted Biomarker Sample (n = 321) 1,2,3 | |
|---|---|---|
| Age, years, median (interquartile range) | 28.0 (24.0, 32.0) | 27.6 (23.2, 31.9) |
| Race/ethnicity, % | ||
| Non-Hispanic White | 26.8 | 30.9 (23.3, 38.6) |
| Non-Hispanic Black | 27.9 | 23.3 (15.6, 31.0) |
| Hispanic | 28.7 | 27.2 (21.2, 33.2) |
| Asian/Pacific Islander | 16.7 | 18.5 (13.4, 23.7) |
| Education, % | ||
| High school or less | 29.9 | 25.1 (17.8, 32.4) |
| Some college/associate degree | 30.3 | 35.2 (27.7, 42.7) |
| College or higher | 39.7 | 39.8 (32.3, 47.3) |
| Nulliparous | 47.1 | 51.1 (43.3, 58.9) |
| Pre-pregnancy body mass index, kg/m2, % | ||
| 19.0–24.9 | 55.9 | 51.7 (43.9, 59.6) |
| 25.0–29.9 | 27.4 | 33.1 (25.3, 40.8) |
| 30.0–34.9 | 10.7 | 7.2 (3.5, 10.9) |
| 35.0–44.9 | 6.0 | 8.0 (4.2, 11.8) |
| Gestational weight gain, kg, median (interquartile range) | ||
| 10–14 weeks | 1.9 (0.2, 3.8) | 1.9 (0.4, 3.2) |
| 15–26 weeks | 4.6 (2.3, 7.3) | 4.5 (1.8, 6.8) |
| 23–31 weeks | 8.6 (5.9, 11.9) | 8.7 (5.9, 11.4) |
| 33–39 weeks | 13.5 (10.0, 16.9) | 13.6 (10.9, 17.2) |
| Total | 12.2 (8.6, 16.0) | 12.3 (8.5, 15.6) |
| Gestational diabetes, % | 3.8 | 3.9 (2.9, 4.8) |
| Preeclampsia, % | 3.5 | 2.2 (0, 4.5) |
| Preterm delivery, <37 gestational weeks, % | 5.5 | 9.1 (3.6, 14.5) |
| Gestational age at delivery, weeks, median (interquartile range) | 39.4 (38.7, 40.3) | 39.3 (38.6, 40.2) |
| Neonatal anthropometric measurements | ||
| Birthweight z-score, median (interquartile range) | −0.1 (−0.7, 0.5) | −0.1 (−0.7, 0.5) |
| Neonatal length, cm, median (interquartile range) | 50.0 (48.5, 51.8) | 50.5 (48.4, 51.8) |
| Neonatal fat mass, g, median (interquartile range) | 401.6 (300.3, 515.3) | 417.5 (329.0, 530.3) |
| 10–14 Weeks | 15–26 Weeks | 23–31 Weeks | 33–39 Weeks | p-for-Trend 1 | |
|---|---|---|---|---|---|
| n-3 PUFA | |||||
| 18:3n-3 (alpha-linolenic acid, ALA) | 0.22 (0.17, 0.26) | 0.25 (0.20, 0.29) | 0.26 (0.20, 0.30) | 0.24 (0.18, 0.29) | <0.0001 |
| 20:5n-3 (eicosapentaenoic acid, EPA) | 0.33 (0.20, 0.41) | 0.19 (0.14, 0.20) | 0.31 (0.17, 0.38) | 0.28 (0.16, 0.35) | <0.0001 |
| 22:5n-3 (docosapentaenoic acid, DPA) | 0.69 (0.55, 0.81) | 0.63 (0.50, 0.74) | 0.60 (0.48, 0.68) | 0.52 (0.41, 0.60) | <0.0001 |
| 22:6n-3 (docosahexaenoic acid, DHA) | 4.23 (3.26, 4.94) | 4.11 (3.14, 4.94) | 4.06 (3.21, 4.90) | 3.92 (3.04, 4.64) | 0.0571 |
| Sum of n-3 PUFA 2 | 5.46 (4.44, 6.30) | 5.18 (4.04, 6.13) | 5.22 (4.22, 6.12) | 4.96 (3.96, 5.69) | 0.0007 |
| n-6 PUFA | |||||
| 18:2n-6 (linoleic acid, LA) | 20.60 (18.76, 22.37) | 21.65 (19.90, 23.33) | 21.73 (19.66, 23.50) | 22.45 (20.88, 24.08) | <0.0001 |
| 18:3n-6 (gamma-linoleic acid, GLA) | 0.08 (0.06, 0.09) | 0.08 (0.05, 0.09) | 0.07 (0.05, 0.09) | 0.07 (0.05, 0.09) | 0.0514 |
| 20:2n-6 (eicosadienoic acid, EDA) | 0.51 (0.44, 0.57) | 0.52 (0.44, 0.58) | 0.50 (0.43, 0.55) | 0.44 (0.39, 0.49) | <0.0001 |
| 20:3n-6 (dihomo-gamma-linolenic acid, DGLA) | 3.58 (2.91, 4.15) | 3.56 (2.97, 4.08) | 3.59 (3.02, 4.05) | 3.29 (2.86, 3.76) | 0.0015 |
| 20:4n-6 (arachidonic acid, AA) | 11.22 (9.63, 12.53) | 10.35 (8.78, 11.85) | 9.98 (8.33, 11.26) | 9.77 (8.60, 11.04) | <0.0001 |
| 22:4n-6 (docosatetraenoic acid, DTA) | 0.49 (0.34, 0.61) | 0.29 (0.21, 0.33) | 0.48 (0.34, 0.59) | 0.45 (0.32, 0.56) | <0.0001 |
| 22:5n-6 (docosapentaenoic acid, n6-DPA) | 0.54 (0.38, 0.66) | 0.53 (0.35, 0.64) | 0.59 (0.43, 0.72) | 0.59 (0.43, 0.71) | 0.003 |
| Sum of n-6 PUFA 3 | 37.02 (35.46, 38.70) | 36.98 (35.36, 38.69) | 36.96 (35.33, 38.83) | 37.06 (35.73, 38.72) | 0.9405 |
| Total PUFA 4 | 42.48 (41.48, 43.86) | 42.15 (40.93, 43.76) | 42.16 (41.06, 43.58) | 42.02 (41.03, 43.42) | 0.0114 |
| PUFA ratios | |||||
| Δ5-desaturase (20:4n-6/20:3n-6) | 3.39 (2.47, 3.97) | 3.09 (2.31, 3.67) | 2.96 (2.17, 3.47) | 3.14 (2.46, 3.60) | 0.0001 |
| Δ6-desaturase (18:3n-6/18:2n-6) | 0.004 (0.0027, 0.005) | 0.0036 (0.0024, 0.0044) | 0.004 (0.0023, 0.004) | 0.0034 (0.0021, 0.0041) | <0.0001 |
| DGLA/LA (20:3n-6/18:2n-6) | 0.18 (0.14, 0.21) | 0.17(0.13, 0.19) | 0.17 (0.14, 0.19) | 0.15 (0.12, 0.17) | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, E.; Zhu, Y.; Chehab, R.F.; Wu, J.; Hinkle, S.N.; Weir, N.L.; Bremer, A.A.; Yang, J.; Chen, Z.; Tsai, M.Y.; et al. Longitudinal Associations of Plasma Phospholipid Fatty Acids in Pregnancy with Neonatal Anthropometry: Results from the NICHD Fetal Growth Studies—Singleton Cohort. Nutrients 2022, 14, 592. https://doi.org/10.3390/nu14030592
Wang E, Zhu Y, Chehab RF, Wu J, Hinkle SN, Weir NL, Bremer AA, Yang J, Chen Z, Tsai MY, et al. Longitudinal Associations of Plasma Phospholipid Fatty Acids in Pregnancy with Neonatal Anthropometry: Results from the NICHD Fetal Growth Studies—Singleton Cohort. Nutrients. 2022; 14(3):592. https://doi.org/10.3390/nu14030592
Chicago/Turabian StyleWang, Emily, Yeyi Zhu, Rana F. Chehab, Jing Wu, Stefanie N. Hinkle, Natalie L. Weir, Andrew A. Bremer, Jiaxi Yang, Zhen Chen, Michael Y. Tsai, and et al. 2022. "Longitudinal Associations of Plasma Phospholipid Fatty Acids in Pregnancy with Neonatal Anthropometry: Results from the NICHD Fetal Growth Studies—Singleton Cohort" Nutrients 14, no. 3: 592. https://doi.org/10.3390/nu14030592
APA StyleWang, E., Zhu, Y., Chehab, R. F., Wu, J., Hinkle, S. N., Weir, N. L., Bremer, A. A., Yang, J., Chen, Z., Tsai, M. Y., & Zhang, C. (2022). Longitudinal Associations of Plasma Phospholipid Fatty Acids in Pregnancy with Neonatal Anthropometry: Results from the NICHD Fetal Growth Studies—Singleton Cohort. Nutrients, 14(3), 592. https://doi.org/10.3390/nu14030592