Prenatal Caffeine Exposure Is Linked to Elevated Sugar Intake and BMI, Altered Reward Sensitivity, and Aberrant Insular Thickness in Adolescents: An ABCD Investigation
Abstract
1. Background
2. Methodology
2.1. Data Source
2.2. Data Selection
2.3. Prenatal Caffeine Exposure (PCE)
2.4. Block Kids Food Screener Questionnaire (BKFS) (TSI (g))
2.5. Body Mass Index (BMI)
2.6. Monetary Incentive Delay Task Functional MRI
Structural Imaging
2.7. Covariates
2.8. Statistical Analyses
2.8.1. Demographics
2.8.2. Association Analysis
2.8.3. Mediation Analysis
2.8.4. Sex Effects
3. Results
3.1. Demographic Characteristics of Children in PCE Groups
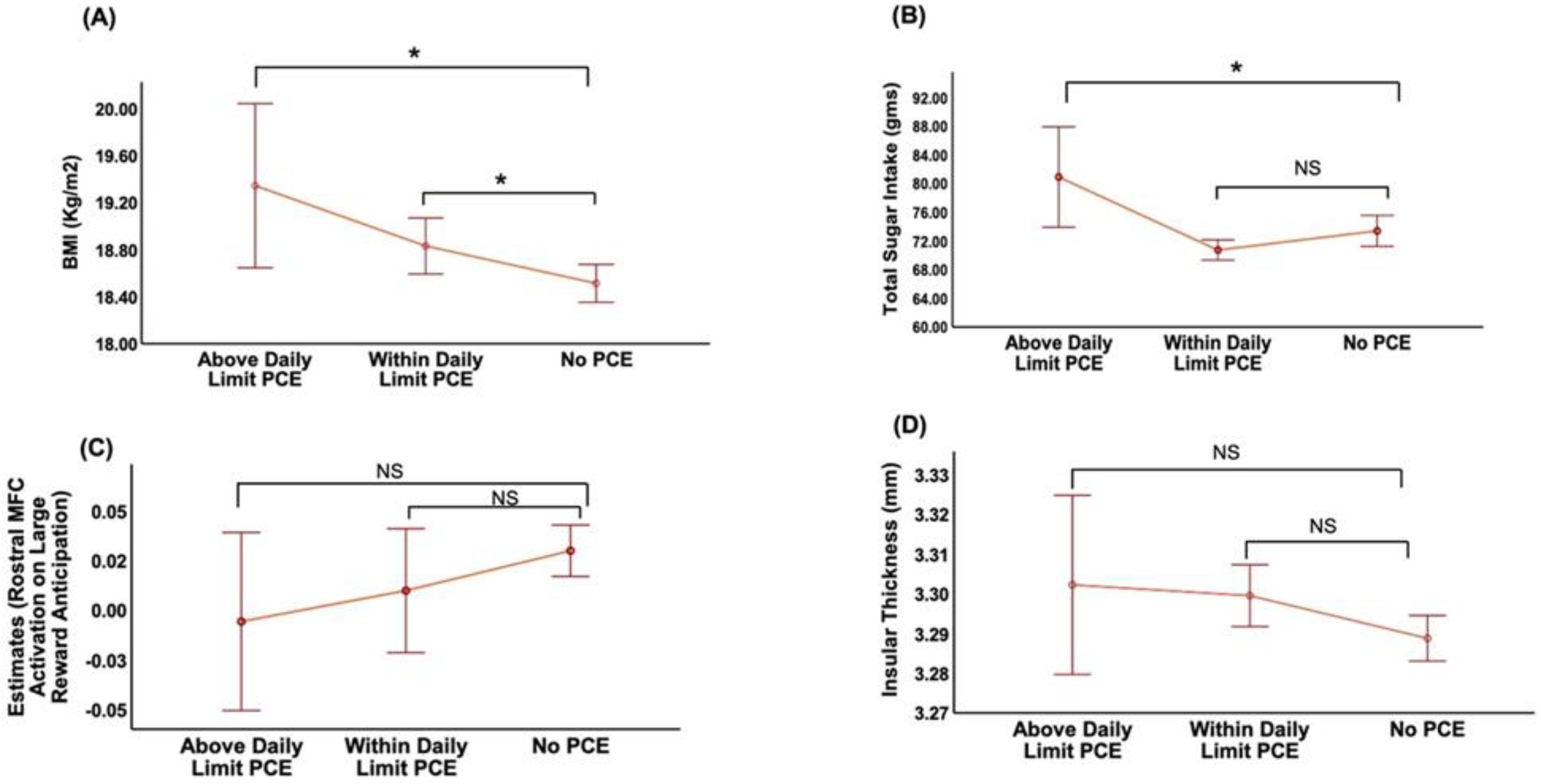
3.2. Association of PCE with BMI
3.3. Association of PCE with TSI
3.4. Association of PCE with Brain Activation during Anticipation of Large Reward and Structural Change in Taste-Processing Regions
3.5. Association of BMI with TSI, Brain Activation during Anticipation of Large Reward and Structural Change in Taste-Processing Regions
3.6. Exploratory Mediation Effects
3.7. Sex Effects
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hibbeln, J.R.; Davis, J.M.; Steer, C.; Emmett, P.; Rogers, I.; Williams, C.; Golding, J. Maternal seafood consumption in pregnancy and neurodevelopmental outcomes in childhood (ALSPAC study): An observational cohort study. Lancet 2007, 369, 578–585. [Google Scholar] [CrossRef]
- Lyall, K.; Schmidt, R.J.; Hertz-Picciotto, I. Maternal lifestyle and environmental risk factors for autism spectrum disorders. Int. J. Epidemiol. 2014, 43, 443–464. [Google Scholar] [CrossRef] [PubMed]
- Oken, E.; Wright, R.O.; Kleinman, K.P.; Bellinger, D.; Amarasiriwardena, C.J.; Hu, H.; Rich-Edwards, J.W.; Gillman, M.W. Maternal fish consumption, hair mercury, and infant cognition in a U.S. Cohort. Environ. Health Perspect. 2005, 113, 1376–1380. [Google Scholar] [CrossRef] [PubMed]
- Parsons, A.G.; Zhou, S.J.; Spurrier, N.J.; Makrides, M. Effect of iron supplementation during pregnancy on the behaviour of children at early school age: Long-term follow-up of a randomised controlled trial. Br. J. Nutr. 2008, 99, 1133–1139. [Google Scholar] [CrossRef]
- Scholl, T.O.; Johnson, W.G. Folic acid: Influence on the outcome of pregnancy. Am. J. Clin. Nutr. 2000, 71, 1295s–1303s. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies. Scientific Opinion on the safety of caffeine. EFSA J. 2015, 13, 4102. [Google Scholar]
- Ådén, U. Methylxanthines During Pregnancy and Early Postnatal Life. In Methylxanthines; Fredholm, B.B., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 373–389. [Google Scholar] [CrossRef]
- Goldstein, A.; Warren, R. Passage of caffeine into human gonadal and fetal tissue. Biochem. Pharmacol. 1962, 11, 166–168. [Google Scholar] [CrossRef]
- Kaiser, L.; Allen, L.H. Position of the American Dietetic Association: Nutrition and lifestyle for a healthy pregnancy outcome. J. Am. Diet. Assoc. 2008, 108, 553–561. [Google Scholar] [CrossRef]
- Knutti, R.; Rothweiler, H.; Schlatter, C. Effect of pregnancy on the pharmacokinetics of caffeine. Eur. J. Clin. Pharmacol 1981, 21, 121–126. [Google Scholar] [CrossRef]
- Zhang, R.; Manza, P.; Volkow, N.D. Prenatal caffeine exposure: Association with neurodevelopmental outcomes in 9- to 11-year-old children. J. Child. Psychol. Psychiatry 2022, 63, 563–578. [Google Scholar] [CrossRef]
- Bernard, J.Y.; Heude, B.; Galéra, C. Re: “Maternal Caffeine Intake During Pregnancy and Child Cognition and Behavior at 4 and 7 Years of Age”. Am. J. Epidemiol. 2016, 183, 871–872. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Berglundh, S.; Vollrath, M.; Brantsæter, A.L.; Brandlistuen, R.; Solé-Navais, P.; Jacobsson, B.; Sengpiel, V. Maternal caffeine intake during pregnancy and child neurodevelopment up to eight years of age—Results from the Norwegian Mother, Father and Child Cohort Study. Eur. J. Nutr. 2021, 60, 791–805. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou, E.; Botton, J.; Brantsæter, A.-L.; Haugen, M.; Alexander, J.; Meltzer, H.M.; Bacelis, J.; Elfvin, A.; Jacobsson, B.; Sengpiel, V. Maternal caffeine intake during pregnancy and childhood growth and overweight: Results from a large Norwegian prospective observational cohort study. BMJ Open 2018, 8, e018895. [Google Scholar] [CrossRef]
- Li, D.K.; Ferber, J.R.; Odouli, R. Maternal caffeine intake during pregnancy and risk of obesity in offspring: A prospective cohort study. Int. J. Obes. 2015, 39, 658–664. [Google Scholar] [CrossRef]
- Frayer, N.C.; Kim, Y. Caffeine Intake During Pregnancy and Risk of Childhood Obesity: A Systematic Review. Int. J. MCH AIDS 2020, 9, 364–380. [Google Scholar] [CrossRef]
- Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet 2017, 390, 2627–2642. [CrossRef]
- Keller, A.; Bucher Della Torre, S. Sugar-Sweetened Beverages and Obesity among Children and Adolescents: A Review of Systematic Literature Reviews. Child. Obes. 2015, 11, 338–346. [Google Scholar] [CrossRef]
- Cobiac, L.; Record, S.; Leppard, P.; Syrette, J.; Flight, I. Sugars in the Australian diet: Results from the 1995 National Nutrition Survey. Nutr. Diet. J. Dietit. Assoc. Aust. 2003, 60, 152–174. [Google Scholar]
- Parnell, W.; Wilson, N.; Alexander, D.; Wohlers, M.; Williden, M.; Mann, J.; Gray, A. Exploring the relationship between sugars and obesity. Public Health Nutr. 2008, 11, 860–866. [Google Scholar] [CrossRef][Green Version]
- Liberali, R.; Kupek, E.; Assis, M.A.A. Dietary Patterns and Childhood Obesity Risk: A Systematic Review. Child. Obes. 2020, 16, 70–85. [Google Scholar] [CrossRef]
- Frantsve-Hawley, J.; Bader, J.D.; Welsh, J.A.; Wright, J.T. A systematic review of the association between consumption of sugar-containing beverages and excess weight gain among children under age 12. J. Public Health Dent. 2017, 77 (Suppl. S1), S43–S66. [Google Scholar] [CrossRef]
- McGuire, S.; Ervin, R.B.; Kit, B.K.; Carroll, M.D.; Ogden, C.L. Consumption of added sugar among U.S. children and adolescents, 2005-2008. NCHS data brief no 87. Hyattsville, MD: National Center for Health Statistics. Adv. Nutr. 2012, 3, 534. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Miranda, L.M.; Briones-Villalba, R.; Ortiz-Ortiz, M.; Aburto-Corona, J.A.; Bonilla, D.A.; Pozos-Parra, P.; Espinoza-Gutiérrez, R.; Calleja-Núñez, J.J.; Moncada-Jiménez, J.; Hernández-Lepe, M.A. Association between Body Mass Index with Sugar-Sweetened and Dairy Beverages Consumption in Children from the Mexico-USA Border. Int. J. Environ. Res. Public Health 2022, 19, 6403. [Google Scholar] [CrossRef]
- Nansel, T.R.; Lipsky, L.M.; Eisenberg, M.H.; Haynie, D.L.; Liu, D.; Simons-Morton, B. Greater Food Reward Sensitivity Is Associated with More Frequent Intake of Discretionary Foods in a Nationally Representative Sample of Young Adults. Front. Nutr. 2016, 3, 33. [Google Scholar] [CrossRef] [PubMed]
- Fry Vennerød, F.F.; Nicklaus, S.; Lien, N.; Almli, V.L. The development of basic taste sensitivity and preferences in children. Appetite 2018, 127, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Kurth, F.; Zilles, K.; Fox, P.T.; Laird, A.R.; Eickhoff, S.B. A link between the systems: Functional differentiation and integration within the human insula revealed by meta-analysis. Brain Struct. Funct. 2010, 214, 519–534. [Google Scholar] [CrossRef] [PubMed]
- Small, D.M. Central gustatory processing in humans. Adv. Otorhinolaryngol. 2006, 63, 191–220. [Google Scholar] [CrossRef]
- Yamamoto, T. Central mechanisms of taste: Cognition, emotion and taste-elicited behaviors. Jpn. Dent. Sci. Rev. 2008, 44, 91–99. [Google Scholar] [CrossRef]
- Ventura, A.K.; Mennella, J.A. Innate and learned preferences for sweet taste during childhood. Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 379–384. [Google Scholar] [CrossRef]
- Casey, B.J.; Cannonier, T.; Conley, M.I.; Cohen, A.O.; Barch, D.M.; Heitzeg, M.M.; Soules, M.E.; Teslovich, T.; Dellarco, D.V.; Garavan, H.; et al. The Adolescent Brain Cognitive Development (ABCD) study: Imaging acquisition across 21 sites. Dev. Cogn. Neurosci. 2018, 32, 43–54. [Google Scholar] [CrossRef]
- Compton, W.M.; Dowling, G.J.; Garavan, H. Ensuring the Best Use of Data: The Adolescent Brain Cognitive Development Study. JAMA Pediatrics 2019, 173, 809–810. [Google Scholar] [CrossRef] [PubMed]
- Garavan, H.; Bartsch, H.; Conway, K.; Decastro, A.; Goldstein, R.Z.; Heeringa, S.; Jernigan, T.; Potter, A.; Thompson, W.; Zahs, D. Recruiting the ABCD sample: Design considerations and procedures. Dev. Cogn. Neurosci. 2018, 32, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Barch, D.M.; Albaugh, M.D.; Avenevoli, S.; Chang, L.; Clark, D.B.; Glantz, M.D.; Hudziak, J.J.; Jernigan, T.L.; Tapert, S.F.; Yurgelun-Todd, D.; et al. Demographic, physical and mental health assessments in the adolescent brain and cognitive development study: Rationale and description. Dev. Cogn. Neurosci. 2018, 32, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Hunsberger, M.; O’Malley, J.; Block, T.; Norris, J.C. Relative validation of Block Kids Food Screener for dietary assessment in children and adolescents. Matern. Child Nutr. 2015, 11, 260–270. [Google Scholar] [CrossRef]
- Breiter, H.C.; Aharon, I.; Kahneman, D.; Dale, A.; Shizgal, P. Functional Imaging of Neural Responses to Expectancy and Experience of Monetary Gains and Losses. Neuron 2001, 30, 619–639. [Google Scholar] [CrossRef]
- Kirsch, P.; Schienle, A.; Stark, R.; Sammer, G.; Blecker, C.; Walter, B.; Ott, U.; Burkart, J.; Vaitl, D. Anticipation of reward in a nonaversive differential conditioning paradigm and the brain reward system: An event-related fMRI study. Neuroimage 2003, 20, 1086–1095. [Google Scholar] [CrossRef]
- Knutson, B.; Fong, G.W.; Bennett, S.M.; Adams, C.M.; Hommer, D. A region of mesial prefrontal cortex tracks monetarily rewarding outcomes: Characterization with rapid event-related fMRI. Neuroimage 2003, 18, 263–272. [Google Scholar] [CrossRef]
- Hagler, D.J., Jr.; Hatton, S.; Cornejo, M.D.; Makowski, C.; Fair, D.A.; Dick, A.S.; Sutherland, M.T.; Casey, B.J.; Barch, D.M.; Harms, M.P.; et al. Image processing and analysis methods for the Adolescent Brain Cognitive Development Study. Neuroimage 2019, 202, 116091. [Google Scholar] [CrossRef]
- Desikan, R.S.; Ségonne, F.; Fischl, B.; Quinn, B.T.; Dickerson, B.C.; Blacker, D.; Buckner, R.L.; Dale, A.M.; Maguire, R.P.; Hyman, B.T.; et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 2006, 31, 968–980. [Google Scholar] [CrossRef]
- Rolls, E.T. Chapter 7—Taste and smell processing in the brain. In Handbook of Clinical Neurology; Doty, R.L., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 164, pp. 97–118. [Google Scholar]
- van Dam, R.M.; Hu, F.B.; Willett, W.C. Coffee, Caffeine, and Health. N. Engl. J. Med. 2020, 383, 369–378. [Google Scholar] [CrossRef]
- Shah, B.; Tombeau Cost, K.; Fuller, A.; Birken, C.S.; Anderson, L.N. Sex and gender differences in childhood obesity: Contributing to the research agenda. BMJ Nutr. Prev. Health 2020, 3, 387–390. [Google Scholar] [CrossRef] [PubMed]
- Reznick, J.S. Can Prenatal Caffeine Exposure Affect Behavioral Inhibition? Rev. Gen. Psychol. 1999, 3, 118–132. [Google Scholar] [CrossRef]
- Paquet, C.; Daniel, M.; Knäuper, B.; Gauvin, L.; Kestens, Y.; Dubé, L. Interactive effects of reward sensitivity and residential fast-food restaurant exposure on fast-food consumption. Am. J. Clin. Nutr. 2010, 91, 771–776. [Google Scholar] [CrossRef] [PubMed]
- De Decker, A.; Sioen, I.; Verbeken, S.; Braet, C.; Michels, N.; De Henauw, S. Associations of reward sensitivity with food consumption, activity pattern, and BMI in children. Appetite 2016, 100, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Christensen, Z.P.; Freedman, E.G.; Foxe, J.J. Caffeine exposure in utero is associated with structural brain alterations and deleterious neurocognitive outcomes in 9–10 year old children. Neuropharmacology 2021, 186, 108479. [Google Scholar] [CrossRef]
- Soellner, D.E.; Grandys, T.; Nuñez, J.L. Chronic prenatal caffeine exposure impairs novel object recognition and radial arm maze behaviors in adult rats. Behav. Brain Res. 2009, 205, 191–199. [Google Scholar] [CrossRef]
- Temple, J.L.; Ziegler, A.M.; Graczyk, A.M.; Crandall, A. Effects of acute and chronic caffeine on risk-taking behavior in children and adolescents. J. Psychopharmacol. 2017, 31, 561–568. [Google Scholar] [CrossRef]
- Schilling, C.; Kühn, S.; Paus, T.; Romanowski, A.; Banaschewski, T.; Barbot, A.; Barker, G.J.; Brühl, R.; Büchel, C.; Conrod, P.J.; et al. Cortical thickness of superior frontal cortex predicts impulsiveness and perceptual reasoning in adolescence. Mol. Psychiatry 2013, 18, 624–630. [Google Scholar] [CrossRef]
- Shaw, P.; Greenstein, D.; Lerch, J.; Clasen, L.; Lenroot, R.; Gogtay, N.; Evans, A.; Rapoport, J.; Giedd, J. Intellectual ability and cortical development in children and adolescents. Nature 2006, 440, 676–679. [Google Scholar] [CrossRef]
- Shaw, P.; Lerch, J.; Greenstein, D.; Sharp, W.; Clasen, L.; Evans, A.; Giedd, J.; Castellanos, F.X.; Rapoport, J. Longitudinal mapping of cortical thickness and clinical outcome in children and adolescents with attention-deficit/hyperactivity disorder. Arch. Gen. Psychiatry 2006, 63, 540–549. [Google Scholar] [CrossRef]
- Whittle, S.; Lichter, R.; Dennison, M.; Vijayakumar, N.; Schwartz, O.; Byrne, M.L.; Simmons, J.G.; Yücel, M.; Pantelis, C.; McGorry, P.; et al. Structural brain development and depression onset during adolescence: A prospective longitudinal study. Am. J. Psychiatry 2014, 171, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, J.; York, P.; Krmpotich, T.; Miller, D.; Dalwani, M.; Sakai, J.T.; Mikulich-Gilbertson, S.K.; Thompson, L.; Claus, E.; Banich, M.; et al. Insula and Orbitofrontal Cortical Morphology in Substance Dependence Is Modulated by Sex. Am. J. Neuroradiol. 2013, 34, 1150. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, A.M. An Update Overview on Brain Imaging Studies of Internet Gaming Disorder. Frontiers in Psychiatry 2017, 8, 185. [Google Scholar] [CrossRef]
- Yuan, K.; Cheng, P.; Dong, T.; Bi, Y.; Xing, L.; Yu, D.; Zhao, L.; Dong, M.; von Deneen, K.M.; Liu, Y.; et al. Cortical Thickness Abnormalities in Late Adolescence with Online Gaming Addiction. PLoS ONE 2013, 8, e53055. [Google Scholar] [CrossRef] [PubMed]
- Small, D.M. Taste representation in the human insula. Brain Struct. Funct. 2010, 214, 551–561. [Google Scholar] [CrossRef]
- Duerden, E.G.; Arsalidou, M.; Lee, M.; Taylor, M.J. Lateralization of affective processing in the insula. Neuroimage 2013, 78, 159–175. [Google Scholar] [CrossRef]
- Menon, V.; Uddin, L.Q. Saliency, switching, attention and control: A network model of insula function. Brain Struct. Funct. 2010, 214, 655–667. [Google Scholar] [CrossRef]
- Rosinger, A.; Herrick, K.; Gahche, J.; Park, S. Sugar-sweetened Beverage Consumption Among U.S. Adults, 2011–2014; National Center for Health Statistics: Hyattsville, MD, USA, 2017.
- Park, S.; Xu, F.; Town, M.; Blanck, H.M. Prevalence of Sugar-Sweetened Beverage Intake Among Adults—23 States and the District of Columbia, 2013. MMWR Morb. Mortal Wkly Rep. 2016, 65, 169–174. [Google Scholar] [CrossRef]
- Welsh, J.A.; Figueroa, J. Intake of Added Sugars During the Early Toddler Period. Nutr. Today 2017, 52, 78312001. [Google Scholar] [CrossRef]
- Lees, B.; Mewton, L.; Jacobus, J.; Valadez, E.A.; Stapinski, L.A.; Teesson, M.; Tapert, S.F.; Squeglia, L.M. Association of Prenatal Alcohol Exposure With Psychological, Behavioral, and Neurodevelopmental Outcomes in Children From the Adolescent Brain Cognitive Development Study. Am. J. Psychiatry 2020, 177, 1060–1072. [Google Scholar] [CrossRef]
- Paul, S.E.; Hatoum, A.S.; Fine, J.D.; Johnson, E.C.; Hansen, I.; Karcher, N.R.; Moreau, A.L.; Bondy, E.; Qu, Y.; Carter, E.B.; et al. Associations Between Prenatal Cannabis Exposure and Childhood Outcomes: Results From the ABCD Study. JAMA Psychiatry 2021, 78, 64–76. [Google Scholar] [CrossRef] [PubMed]

| No (n = 2222) | Less than weekly (n = 877) | Weekly (n = 1131) | Daily (n = 1304) | Chi-Square/F Statistics | p Value | |
|---|---|---|---|---|---|---|
| Age (months) a | 119.35 (7.53) | 119.71 (7.28) | 119.73 (7.35) | 119.42 (7.24) | 0.937 | 0.422 |
| Female b | 1031 (46.4) | 537 (61.2) | 410 (36.3) | 614 (47.1) | 0.394 | 0.94 |
| Race/Ethnicity b | ||||||
| Black | 333 (14.99) | 87 (9.92) | 75 (6.63) | 110 (8.44) | 123.509 | <0.001 |
| White | 1129 (50.81) | 755 (86.09) | 527 (46.59) | 783 (60.05) | ||
| Hispanic | 504 (22.68) | 168 (19.16) | 161 (14.24) | 246 (18.87) | ||
| Other | 253 (11.39) | 121 (13.79) | 114 (10.08) | 164 (12.58) | ||
| NA | 3 (0.14) | - | - | 1 (0.08) | ||
| Household Income c | 2.16 (0.83) | 2.27 (0.78) | 2.17 (0.83) | 2.26 (0.78) | 29.336 | <0.001 |
| Highest Household Education d | 2.88 (1.04) | 3.01 (0.96) | 2.84 (1.04) | 3.01 (0.97) | 44.746 | <0.001 |
| Physical Activity e | 3.63 (2.31) | 3.5 (2.31) | 3.64 (2.26) | 3.66 (2.26) | 34.716 | 0.03 |
| Daily vs. No (β; 95% CI; p) | Weekly vs. No (β; 95% CI; p) | Less than weekly vs. No (β; 95% CI; p) | |
|---|---|---|---|
| Total Sugar intake (gm) | 3.5; 1.17–5.76; 0.003 ab | 0.63; −1.76–3.02; 0.60 | 0.51; −2.07–3.09; 0.69 |
| BMI (kg/m2) | 0.45; 0.19–0.71; <0.001 ab | 0.28; 0.01–0.55; 0.03 a | −0.00; −0.29–0.29; 0.99 |
| Activation in anticipation of large reward | |||
| Rostral MFC | −0.03; −0.06–0.00; 0.02 a | 0.004; −0.02–0.03; 0.76 | 0.02; −0.01–0.04; 0.28 |
| Caudal MFC | −0.02; −0.03–0.00; 0.06 | 0.002; −0.01–0.02; 0.78 | −0.01; −0.02–0.01; 0.54 |
| Medial OFC | 0.01; −0.03–0.06; 0.58 | 0.05; 0.00–0.11; 0.04 c | 0.08; 0.02–0.14; 0.004 |
| Lateral OFC | −0.00; −0.04–0.03; 0.78 | 0.03; −0.01–0.07; 0.12 | 0.04; 0.00–0.09; 0.03 c |
| Rostral ACC | −0.02; −0.05–0.00; 0.05 | 0.00; −0.02–0.02; 0.94 | 0.003; −0.02–0.03; 0.81 |
| Caudal ACC | −0.02; −0.03–0.00; 0.02 | −0.01; −0.02–0.01; 0.52 | 0.004; −0.01–0.02; 0.69 |
| Accumbens | −0.02; −0.04–0.01; 0.34 | 0.02; −0.00–0.06; 0.10 | 0.006; −0.03–0.04; 0.72 |
| Amygdala | −0.01; −0.04–0.002; 0.34 | −0.004; −0.02–0.01; 0.68 | 0.00; −0.02–0.02; 0.99 |
| Insula | −0.01; −0.03–0.00; 0.05 | −0.006; −0.02–0.009; 0.44 | −0.003; −0.02–0.01; 0.69 |
| Thalamus | −0.01; −0.03–0.00; 0.01 c | −0.002; −0.01–0.01; 0.76 | −0.002; −0.02–0.01; 0.76 |
| Activation in anticipation of reward | |||
| Rostral MFC | −0.02; −0.04–0.00; 0.05 | 0.003; −0.02–0.03; 0.78 | 0.009; −0.01–0.03; 0.46 |
| Caudal MFC | −0.007; −0.02–0.00; 0.27 | 0.003; −0.01–0.01; 0.64 | −0.002; −0.01–0.01; 0.79 |
| Medial OFC | 0.00; −0.03–0.05; 0.80 | 0.04; 0.002–0.09; 0.03c | 0.06; 0.01–0.11; 0.01 c |
| Lateral OFC | 0.00; −0.02–0.03; 0.74 | 0.02; −0.007–0.06; 0.12 | 0.03; −0.00–0.07; 0.05 |
| Rostral ACC | −0.01; −0.03–0.00; 0.24 | 0.006; −0.01–0.03; 0.62 | 0.008; −0.01–0.03; 0.50 |
| Caudal ACC | −0.00; −0.02–0.00; 0.27 | −0.00; −0.01–0.01; 0.79 | 0.00; −0.01–0.02; 0.42 |
| Accumbens | −0.00; −0.03–0.02; 0.87 | 0.03; 0.004–0.06; 0.02 c | 0.01; −0.01–0.04; 0.35 |
| Amygdala | −0.01; −0.03–0.005; 0.15 | −0.00; −0.02–0.01; 0.92 | 0.00; −0.02–0.02; 0.95 |
| Insula | −0.00; −0.01–0.007; 0.38 | −0.00; −0.01–0.01; 0.84 | 0.00; −0.01–0.01; 0.84 |
| Thalamus | −0.00; −0.02–0.004; 0.17 | 0.00; −0.01–0.01; 0.85 | 0.00; −0.01–0.01; 0.86 |
| Thickness (mm) | |||
| Insula | 0.01; 0.00–0.01; 0.03 a | 0.01; 0.00–0.02; 0.03 a | −0.00; −0.01–0.01; 0.81 |
| Medial OFC | 0.01; −0.00–0.01; 0.25 | 0.01; 0.00–0.02; 0.03 | 0.01; −0.00–0.01; 0.22 |
| Lateral OFC | 0.00; −0.00–0.01; 0.75 | 0.002; −0.00–0.01; 0.67 | 0.00; −0.01–0.01; 0.70 |
| Rostral ACC | 0.00; −0.01–0.01; 0.33 | −0.008; −0.02–0.01; 0.23 | −0.00; −0.01–0.01; 0.99 |
| Caudal ACC | 0.00; −0.02–0.02; 0.84 | 0.009; −0.01–0.03; 0.46 | −0.01; −0.03–0.02; 0.67 |
| Group Pairwise Comparisons | Marginal Mean Difference | 95% CI | p Bonferroni-Corrected (Pairwise) |
|---|---|---|---|
| Total Sugar intake | |||
| Daily vs. No | 3.508 | 0.422–6.594 | 0.01 |
| Within limit vs. No | 3.26 | 0.13–6.40 | 0.03 |
| Above limit vs. No | 9.79 | 2.51–17.06 | 0.004 |
| BMI | |||
| Daily vs. No | 0.448 | 0.097–0.799 | 0.004 |
| Weekly vs. No | 0.285 | −0.079–0.650 | 0.03 |
| Within limit vs. No | 0.451 | 0.113–0.789 | 0.004 |
| Above limit vs. No | 0.931 | 0.149–1.713 | 0.01 |
| Rostral MFC activation in anticipation of large reward | |||
| Daily vs. less than weekly | −0.042 | −0.08–9.531 × 10−5 | 0.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agarwal, K.; Manza, P.; Tejeda, H.A.; Courville, A.B.; Volkow, N.D.; Joseph, P.V. Prenatal Caffeine Exposure Is Linked to Elevated Sugar Intake and BMI, Altered Reward Sensitivity, and Aberrant Insular Thickness in Adolescents: An ABCD Investigation. Nutrients 2022, 14, 4643. https://doi.org/10.3390/nu14214643
Agarwal K, Manza P, Tejeda HA, Courville AB, Volkow ND, Joseph PV. Prenatal Caffeine Exposure Is Linked to Elevated Sugar Intake and BMI, Altered Reward Sensitivity, and Aberrant Insular Thickness in Adolescents: An ABCD Investigation. Nutrients. 2022; 14(21):4643. https://doi.org/10.3390/nu14214643
Chicago/Turabian StyleAgarwal, Khushbu, Peter Manza, Hugo A. Tejeda, Amber B. Courville, Nora D. Volkow, and Paule V. Joseph. 2022. "Prenatal Caffeine Exposure Is Linked to Elevated Sugar Intake and BMI, Altered Reward Sensitivity, and Aberrant Insular Thickness in Adolescents: An ABCD Investigation" Nutrients 14, no. 21: 4643. https://doi.org/10.3390/nu14214643
APA StyleAgarwal, K., Manza, P., Tejeda, H. A., Courville, A. B., Volkow, N. D., & Joseph, P. V. (2022). Prenatal Caffeine Exposure Is Linked to Elevated Sugar Intake and BMI, Altered Reward Sensitivity, and Aberrant Insular Thickness in Adolescents: An ABCD Investigation. Nutrients, 14(21), 4643. https://doi.org/10.3390/nu14214643









