Anti-Inflammatory Effect of Korean Propolis on Helicobacter pylori-Infected Gastric Mucosal Injury Mice Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Propolis Solution
2.2. Bacterial Culture
2.3. Ethics
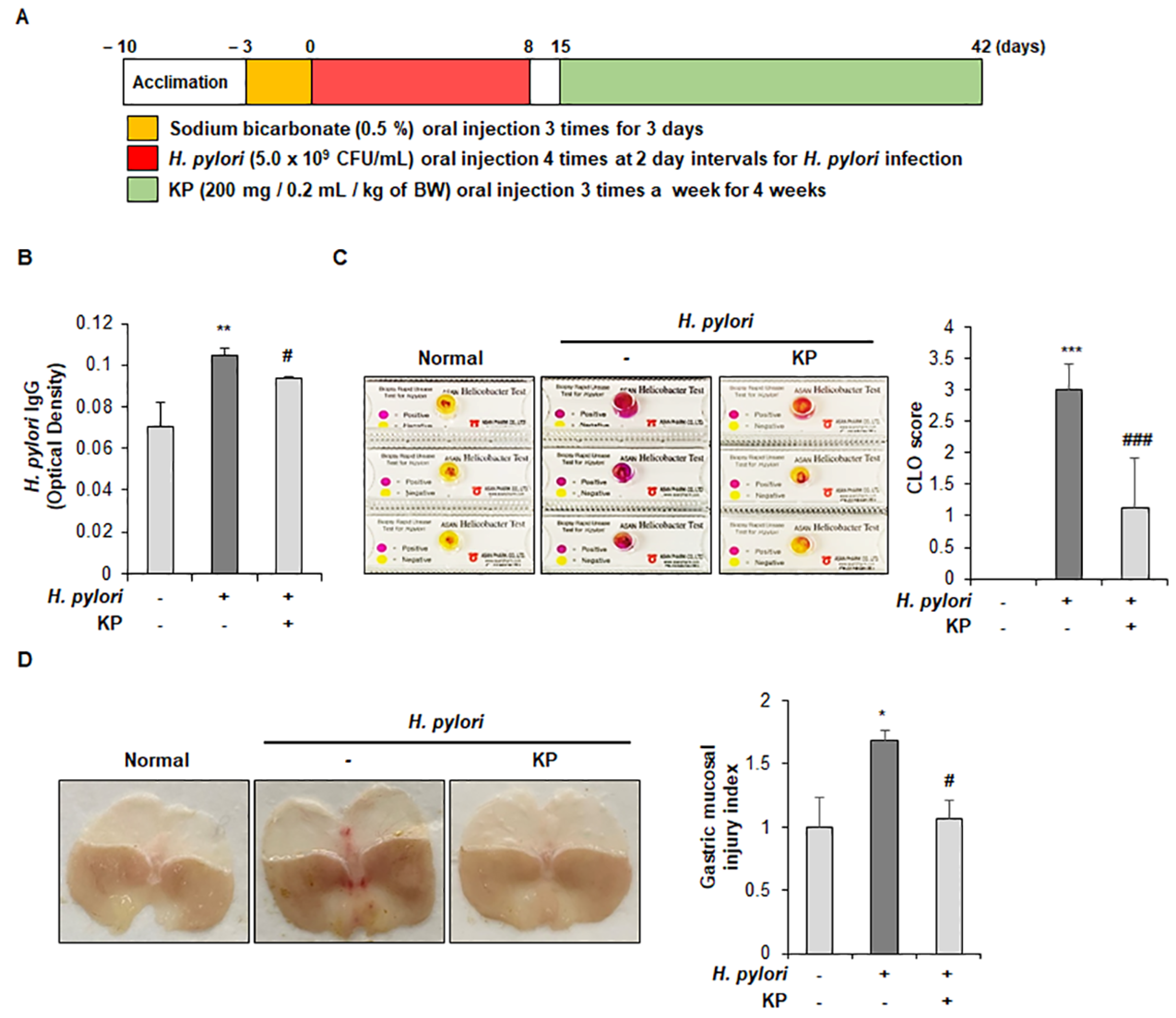
2.4. Animal Experiment
2.5. H. pylori Antigen Test in Mouse Serum
2.6. Campylobacter-Like Organism (CLO) Test
2.7. Histological Analysis
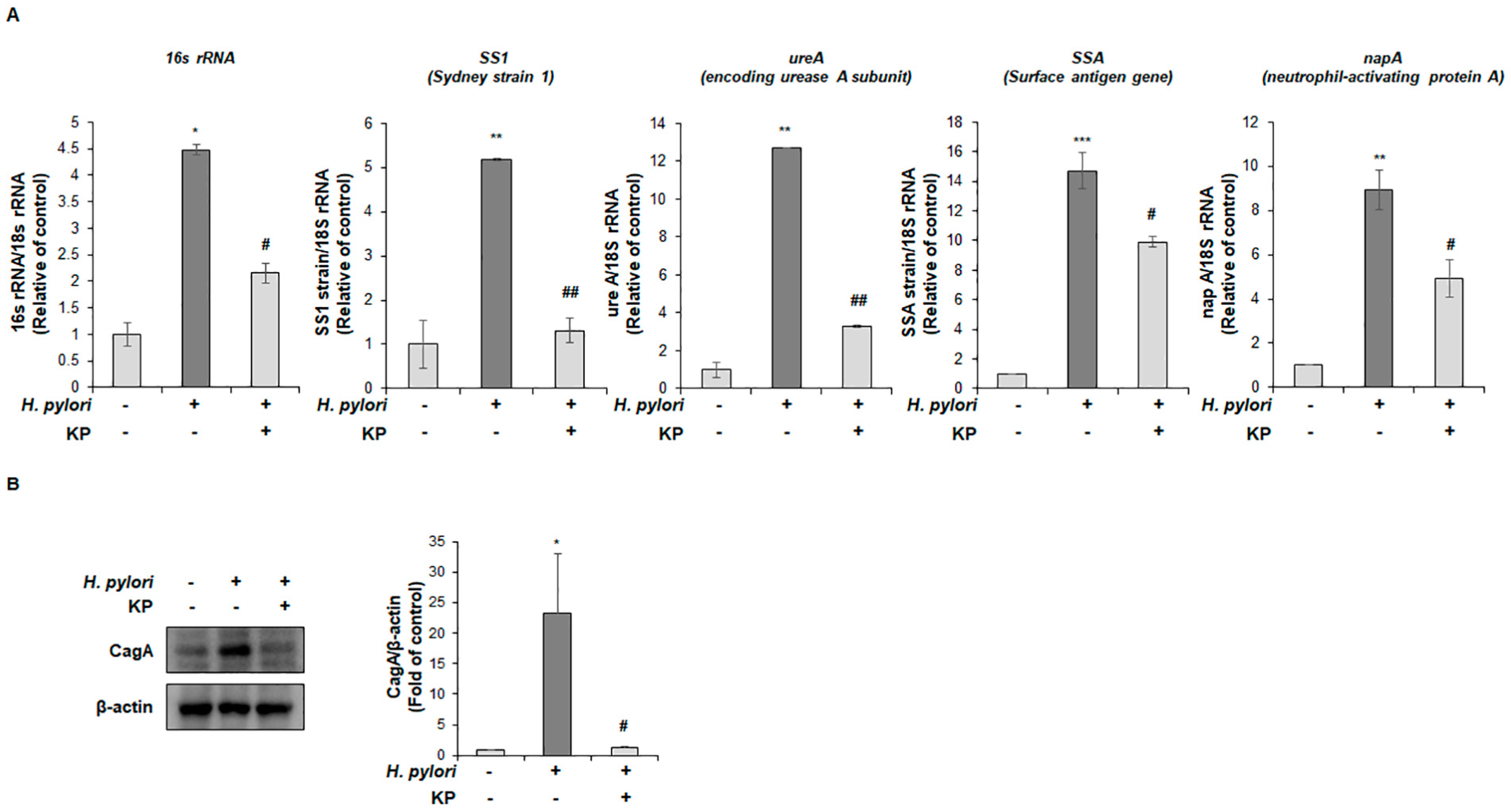
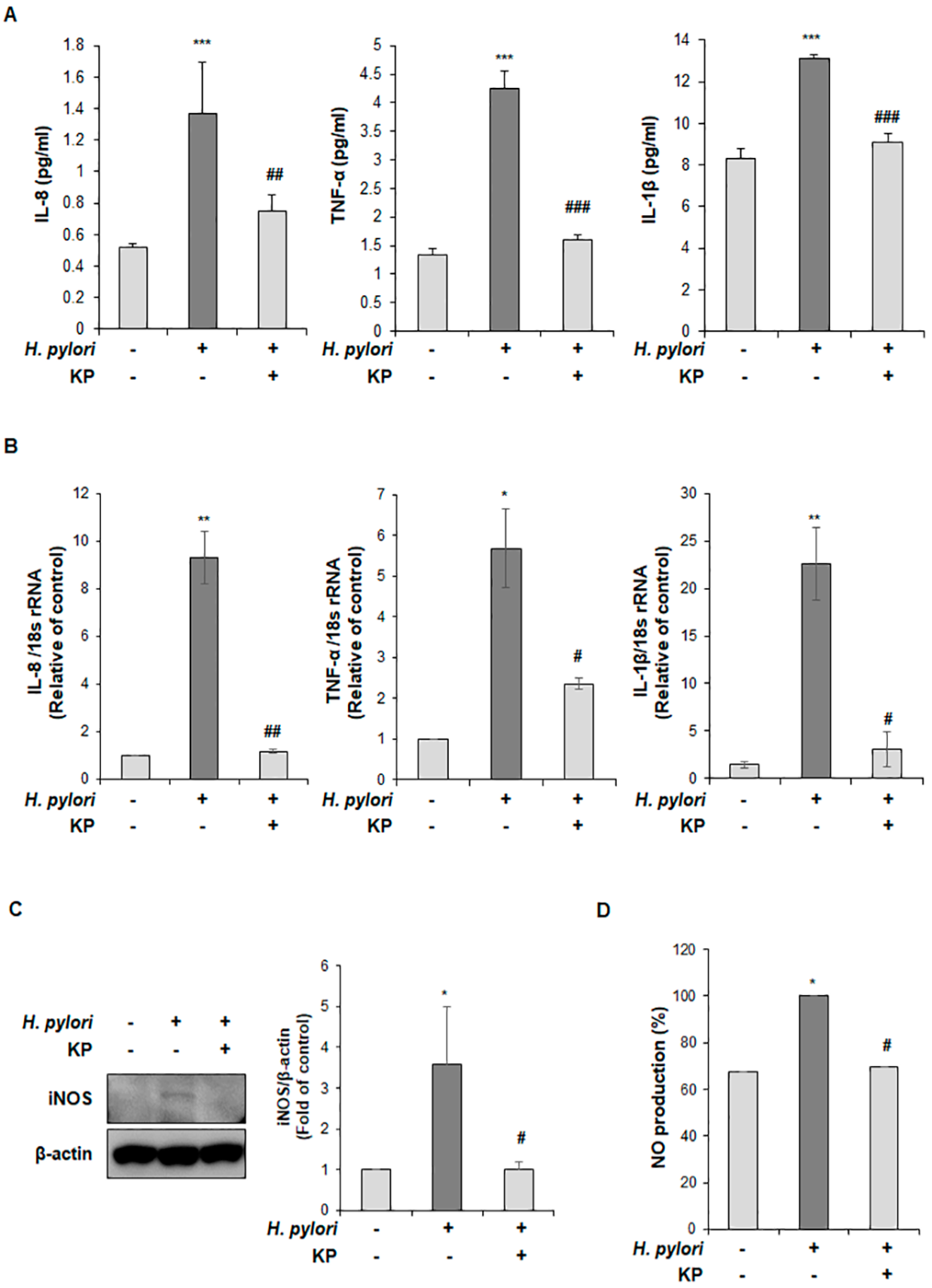
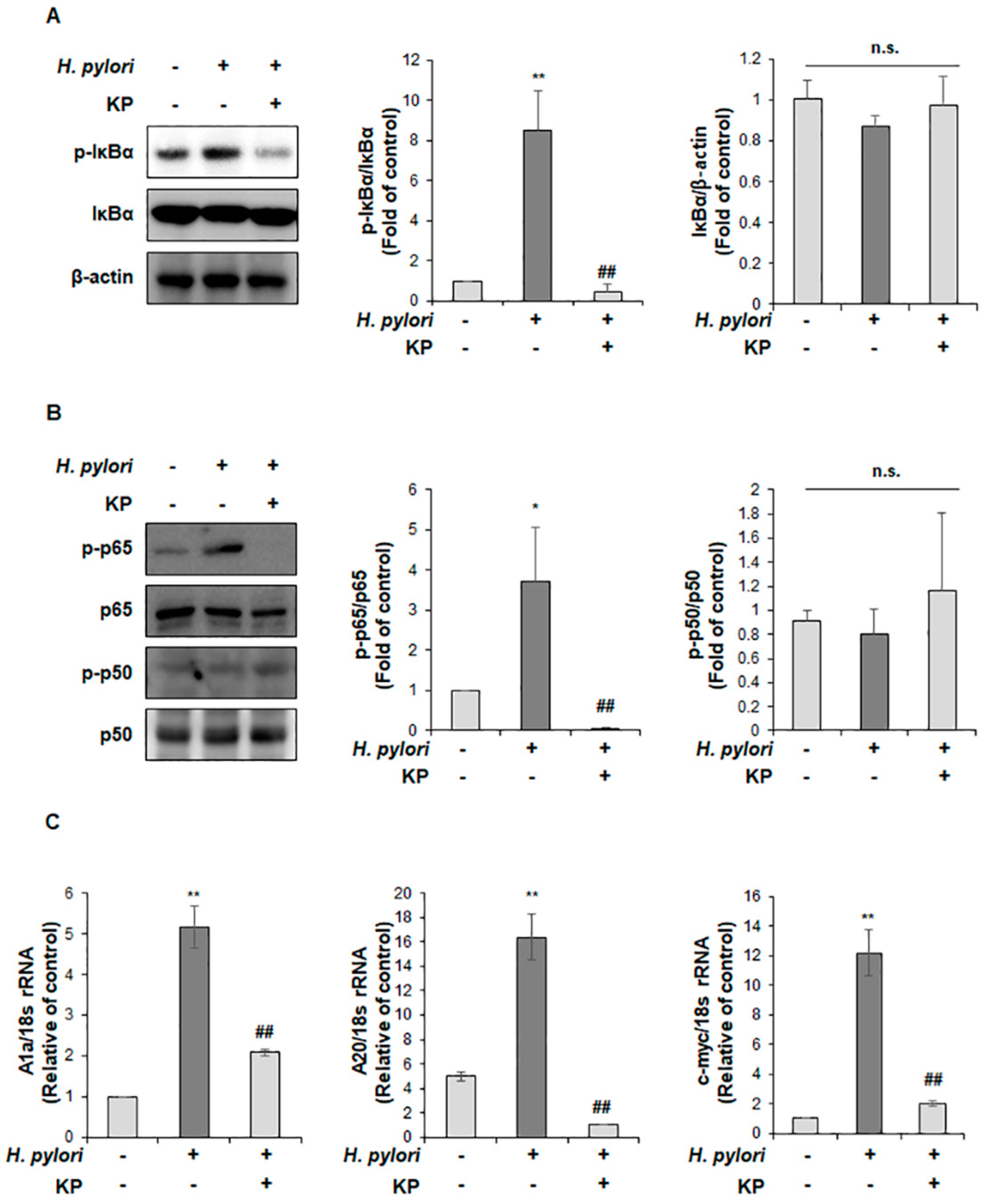
2.8. Quantitative Reverse Transcriptase Polymerase Chain Reaction (qRT-PCR) Analysis
2.9. Western Blot Analysis
2.10. Cytokine Measurement via Enzyme-Linked Iimmunoassay (ELISA)
2.11. Measurement of Nitric Oxide (NO) Production
2.12. Statistical Analysis
3. Results
3.1. Gastric Mucosal Therapeutic Effect of KP in H. pylori-Infected Mice
3.2. KP Attenuates H. pylori-Related Virulence Factors in H. pylori-Infected Mice
3.3. KP Restrains the Pro-Inflammatory Response and NO Production in H. pylori-Infected Mice
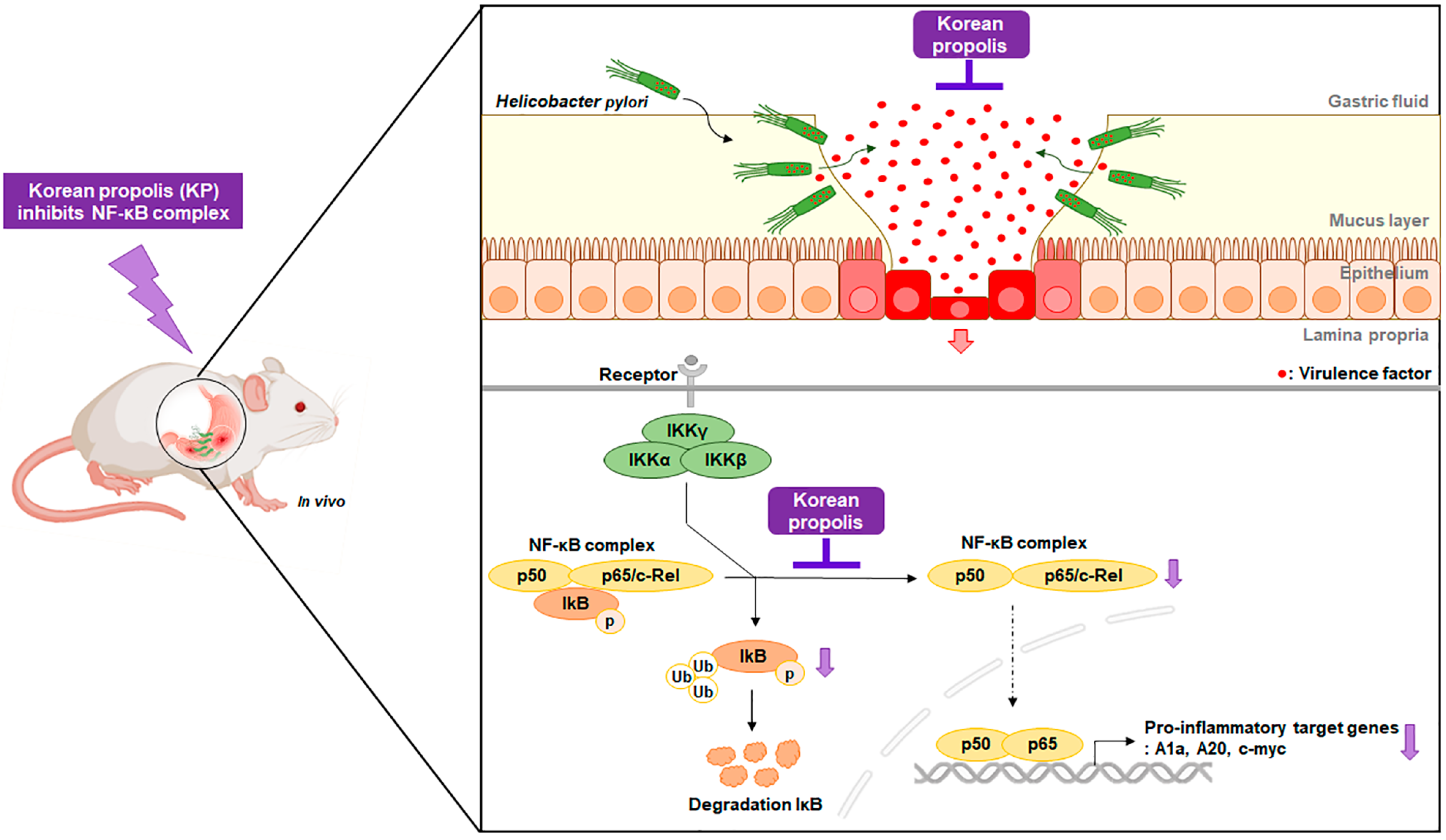
3.4. KP Regulates the NF-κB Signaling Pathway in H. pylori-Infected Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hooi, J.K.; Lai, W.Y.; Ng, W.K.; Suen, M.M.; Underwood, F.E.; Tanyingoh, D.; Malfertheiner, P.; Graham, D.Y.; Wong, V.W.; Wu, J.C. Global prevalence of Helicobacter pylori infection: Systematic review and meta-analysis. Gastroenterology 2017, 153, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Ranjbar, R.; Behzadi, P.; Farshad, S. Advances in diagnosis and treatment of Helicobacter pylori infection. Acta Microbiol. Immunol. Hung. 2017, 64, 273–292. [Google Scholar] [CrossRef] [PubMed]
- Zamani, M.; Ebrahimtabar, F.; Zamani, V.; Miller, W.; Alizadeh-Navaei, R.; Shokri-Shirvani, J.; Derakhshan, M. Systematic review with meta-analysis: The worldwide prevalence of Helicobacter pylori infection. Aliment. Pharmacol. Ther. 2018, 47, 868–876. [Google Scholar] [CrossRef] [PubMed]
- Cover, T.L.; Blaser, M.J. Helicobacter pylori in health and disease. Gastroenterology 2009, 136, 1863–1873. [Google Scholar] [CrossRef] [PubMed]
- Testerman, T.L.; Morris, J. Beyond the stomach: An updated view of Helicobacter pylori pathogenesis, diagnosis, and treatment. World J. Gastroenterol. 2014, 20, 12781. [Google Scholar] [CrossRef] [PubMed]
- Marcus, E.A.; Sachs, G.; Scott, D.R. Eradication of Helicobacter pylori infection. Curr. Gastroenterol. Rep. 2016, 18, 1–9. [Google Scholar] [CrossRef]
- Suerbaum, S.; Michetti, P. Helicobacter pylori infection. N. Engl. J. Med. 2002, 347, 1175–1186. [Google Scholar] [CrossRef]
- Roesler, B.M.; Costa, S.C.; Zeitune, J.M. Eradication treatment of Helicobacter pylori infection: Its importance and possible relationship in preventing the development of gastric cancer. Int. Sch. Res. Notices 2012, 2012, 935410. [Google Scholar] [CrossRef][Green Version]
- Sepulveda, A.R. Helicobacter, inflammation, and gastric cancer. Curr. Pathobiol. Rep. 2013, 1, 9–18. [Google Scholar] [CrossRef]
- Sharma, S.A.; Tummuru, M.K.; Blaser, M.J.; Kerr, L.D. Activation of IL-8 gene expression by Helicobacter pylori is regulated by transcription factor nuclear factor-κB in gastric epithelial cells. J. Immunol. Res. 1998, 160, 2401–2407. [Google Scholar]
- Lamb, A.; Yang, X.D.; Tsang, Y.H.N.; Li, J.D.; Higashi, H.; Hatakeyama, M.; Peek, R.M.; Blanke, S.R.; Chen, L.F. Helicobacter pylori cagA activates NF-κB by targeting TAK1 for TRAF6-mediated Lys 63 ubiquitination. EMBO Rep. 2009, 10, 1242–1249. [Google Scholar] [CrossRef] [PubMed]
- Hayden, M.S.; Ghosh, S. Signaling to NF-κB. Genes Dev. 2004, 18, 2195–2224. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B. Nuclear factor-κB: The enemy within. Cancer Cell 2004, 6, 203–208. [Google Scholar] [CrossRef]
- Crabtree, J.E.; Lindley, I. Mucosal interleukin-8 and Helicobacter pylori-associated gastroduodenal disease. Eur. J. Gastroenterol. Hepatol. 1994, 6, S33–S38. [Google Scholar] [PubMed]
- Brandt, S.; Kwok, T.; Hartig, R.; König, W.; Backert, S. NF-κB activation and potentiation of proinflammatory responses by the Helicobacter pylori CagA protein. Proc. Natl. Acad. Sci. USA 2005, 102, 9300–9305. [Google Scholar] [CrossRef]
- Veenendaal, R.A.; Götz, J.M.; Lamers, C.B. Mucosal inflammation and disease in Helicobacter pylori infection. Scand. J. Gastroenterol. 1996, 218, 86–91. [Google Scholar] [CrossRef]
- Wang, Y.-C. Medicinal plant activity on Helicobacter pylori related diseases. World J. Gastroenterol. 2014, 20, 10368. [Google Scholar] [CrossRef]
- Islam, M.; Kusumoto, Y.; Al-Mamun, M.A. Cytotoxicity and cancer (HeLa) cell killing efficacy of aqueous garlic (Allium sativum) extract. J. Sci. Res. 2011, 3, 375–382. [Google Scholar] [CrossRef]
- Kumar, D.; Kumar, M.; Saravanan, C.; Singh, S.K. Curcumin: A potential candidate for matrix metalloproteinase inhibitors. Expert Opin. Ther. Targets 2012, 16, 959–972. [Google Scholar] [CrossRef]
- Shapla, U.M.; Raihan, J.; Islam, A.; Alam, F.; Solayman, N.; Gan, S.H.; Hossen, S.; Khalil, I. Propolis: The future therapy against Helicobacter pylori-mediated gastrointestinal diseases. J. Appl. Biomed. 2018, 16, 81–99. [Google Scholar] [CrossRef]
- Cardos, I.A.; Zaha, D.C.; Sindhu, R.K.; Cavalu, S. Revisiting therapeutic strategies for H. pylori treatment in the context of antibiotic resistance: Focus on alternative and complementary therapies. Molecules 2021, 26, 6078. [Google Scholar] [CrossRef] [PubMed]
- Salatino, A.; Teixeira, É.W.; Negri, G. Origin and chemical variation of Brazilian propolis. Evid.-Based Complement. Altern. Med. 2005, 2, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; De, R.; Mukhopadhyay, A.K. Curcumin as a potential therapeutic candidate for Helicobacter pylori associated diseases. World J. Gastroenterol. 2016, 22, 2736. [Google Scholar] [CrossRef] [PubMed]
- Osés, S.M.; Pascual-Maté, A.; Fernández-Muiño, M.A.; López-Díaz, T.M.; Sancho, M.T. Bioactive properties of honey with propolis. Food Chem. 2016, 196, 1215–1223. [Google Scholar] [CrossRef]
- Pascual, C.; Gonzalez, R.; Torricella, R. Scavenging action of propolis extract against oxygen radicals. J. Ethnopharmacol. 1994, 41, 9–13. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, C.-P.; Wang, K.; Li, G.Q.; Hu, F.-L. Recent advances in the chemical composition of propolis. Molecules 2014, 19, 19610–19632. [Google Scholar] [CrossRef]
- Ahn, M.R.; Kumazawa, S.; Hamasaka, T.; Bang, K.S.; Nakayama, T. Antioxidant activity and constituents of propolis collected in various areas of Korea. J. Agric. Food Chem. 2004, 52, 7286–7292. [Google Scholar] [CrossRef]
- Kujumgiev, A.; Tsvetkova, I.; Serkedjieva, Y.; Bankova, V.; Christov, R.; Popov, S. Antibacterial, antifungal and antiviral activity of propolis of different geographic origin. J. Ethnopharmacol. 1999, 64, 235–240. [Google Scholar] [CrossRef]
- Lotfy, M. Biological activity of bee propolis in health and disease. Asian Pac. J. Cancer Prev. 2006, 7, 22–31. [Google Scholar]
- Song, M.Y.; Lee, D.Y.; Kim, E.H. Anti-inflammatory and anti-oxidative effect of Korean propolis on Helicobacter pylori-induced gastric damage in vitro. J. Microbiol. 2020, 58, 878–885. [Google Scholar] [CrossRef]
- Han, S.M.; Hong, I.P.; Woo, S.O.; Kim, S.G.; Jang, H.R.; Jang, J.S. Anti-Helicobacter pylori activity of Korean propolis. Korean J. Food Nutr. 2016, 29, 73–78. [Google Scholar] [CrossRef][Green Version]
- Sipponen, P.; Price, A.B. The Sydney system for classification of gastritis 20 years ago. J. Gastroenterol. Hepatol. 2011, 26, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Song, M.-Y.; Lee, D.-Y.; Yun, S.-M.; Kim, E.-H. GLUT3 promotes epithelial–mesenchymal transition via TGF-β/JNK/ATF2 signaling pathway in colorectal cancer cells. Biomedicines 2022, 10, 1837. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Lu, A.P.; Zhang, L.; Li, Y.D. Anti-Helicobacter pylori immunoglobulin G (IgG) and IgA antibody responses and the value of clinical presentations in diagnosis of H. pylori infection in patients with precancerous lesions. World J. Gastroenterol. 2003, 9, 755–758. [Google Scholar] [CrossRef]
- Unver, S.; Kubilay, U.; Sezen, O.S.; Coskuner, T. Investigation of Helicobacter pylori colonization in adenotonsillectomy specimens by means of the CLO Test. Laryngoscope 2001, 111, 2183–2186. [Google Scholar] [CrossRef] [PubMed]
- Allen, A.; Newton, J.; Oliver, L.; Jordan, N.; Strugala, V.; Pearson, J.P.; Dettmar, P.W. Mucus and H. pylori. J. Physiol. Pharmacol. 1997, 48, 297–305. [Google Scholar]
- Hidaka, E.; Ota, H.; Hidaka, H.; Hayama, M.; Matsuzawa, K.; Akamatsu, T.; Nakayama, J.; Katsuyama, T. Helicobacter pylori and two ultrastructurally distinct layers of gastric mucous cell mucins in the surface mucous gel layer. Gut 2001, 49, 474–480. [Google Scholar] [CrossRef]
- Chang, W.L.; Yeh, Y.C.; Sheu, B.S. The impacts of H. pylori virulence factors on the development of gastroduodenal diseases. J. Biomed. Sci. 2018, 25, 68. [Google Scholar] [CrossRef]
- Dincă, A.L.; Meliț, L.E.; Mărginean, C.O. Old and new aspects of H. pylori-associated Inflammation and gastric cancer. Children 2022, 9, 1083. [Google Scholar] [CrossRef]
- Sharma, J.; Al-Omran, A.; Parvathy, S. Role of nitric oxide in inflammatory diseases. Inflammopharmacology 2007, 15, 252–259. [Google Scholar] [CrossRef]
- Cho, K.; Lee, H.G.; Piao, J.Y.; Kim, S.J.; Na, H.K.; Surh, Y.J. Protective effects of Silibinin on Helicobacter pylori-induced gastritis: NF-κB and STAT3 as potential targets. J. Cancer Prev. 2021, 26, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Santoro, M.G.; Rossi, A.; Amici, C. NF-κB and virus infection: Who controls whom. EMBO Rep. 2003, 22, 2552–2560. [Google Scholar] [CrossRef] [PubMed]
- Catrysse, L.; Vereecke, L.; Beyaert, R.; van Loo, G. A20 in inflammation and autoimmunity. Trends Immunol. 2014, 35, 22–31. [Google Scholar] [CrossRef]
- Moser, B.; Hochreiter, B.; Basílio, J.; Gleitsmann, V.; Panhuber, A.; Pardo-Garcia, A.; Hoesel, B.; Salzmann, M.; Resch, U.; Noreen, M.; et al. The inflammatory kinase IKKα phosphorylates and stabilizes c-Myc and enhances its activity. Mol. Cancer 2021, 20, 16. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.M.; Yun, S.M.; Choi, Y.H.; Heo, J.; Kim, N.J.; Kim, S.H.; Kim, E.H. Xanthohumol prevents dextran sulfate sodium-induced colitis via inhibition of IKKβ/NF-κB signaling in mice. Oncotarget 2018, 9, 866–880. [Google Scholar] [CrossRef]
- Beales, I.L. Efficacy of Helicobacter pylori eradication therapies: A single centre observational study. BMC Gastroenterol. 2001, 1, 7. [Google Scholar] [CrossRef]
- Ford, A.C.; Yuan, Y.; Moayyedi, P. Helicobacter pylori eradication therapy to prevent gastric cancer: Systematic review and meta-analysis. Gut 2020, 69, 2113–2121. [Google Scholar] [CrossRef]
- Ghotaslou, R.; Leylabadlo, H.E.; Asl, Y.M. Prevalence of antibiotic resistance in Helicobacter pylori: A recent literature review. World J. Methodol. 2015, 5, 164. [Google Scholar] [CrossRef]
- Thung, I.; Aramin, H.; Vavinskaya, V.; Gupta, S.; Park, J.; Crowe, S.; Valasek, M. The global emergence of Helicobacter pylori antibiotic resistance. Aliment. Pharmacol. Ther. 2016, 43, 514–533. [Google Scholar] [CrossRef]
- Oršolić, N.; Landeka Jurčević, I.; Đikić, D.; Rogić, D.; Odeh, D.; Balta, V.; Perak Junaković, E.; Terzić, S.; Jutrić, D. Effect of Propolis on diet-Induced hyperlipidemia and atherogenic Indices in mice. Antioxidants 2019, 8, 156. [Google Scholar] [CrossRef]
- Tsuchiya, Y.; Sakai, H.; Hirata, A.; Yanai, T. Brazilian green propolis suppresses acetaminophen-induced hepatocellular necrosis by modulating inflammation-related factors in rats. J. Toxicol. Pathol. 2018, 31, 275–282. [Google Scholar] [CrossRef]
- Piñeros, A.R.; de Lima, M.H.F.; Rodrigues, T.; Gembre, A.F.; Bertolini, T.B.; Fonseca, M.D.; Berretta, A.A.; Ramalho, L.N.Z.; Cunha, F.Q.; Hori, J.I.; et al. Green propolis increases myeloid suppressor cells and CD4+Foxp3+ cells and reduces Th2 inflammation in the lungs after allergen exposure. J. Ethnopharmacol. 2020, 252, 112496. [Google Scholar] [CrossRef]
- Wang, K.; Jin, X.; Li, Q.; Sawaya, A.C.H.F.; Le Leu, R.K.; Conlon, M.A.; Wu, L.; Hu, F. Propolis from different geographic origins decreases intestinal inflammation and bacteroides spp. populations in a model of DSS-induced colitis. Mol. Nutr. Food Res. 2018, 62, 1800080. [Google Scholar] [CrossRef] [PubMed]
- Banskota, A.H.; Tezuka, Y.; Adnyana, I.K.; Ishii, E.; Midorikawa, K.; Matsushige, K.; Kadota, S. Hepatoprotective and anti-Helicobacter pylori activities of constituents from Brazilian propolis. Phytomedicine 2001, 8, 16–23. [Google Scholar] [CrossRef]
- Nostro, A.; Cellini, L.; Bartolomeo, S.D.; Cannatelli, M.A.; Campli, E.D.; Procopio, F.; Grande, R.; Marzio, L.; Alonzo, V. Effects of combining extracts (from propolis or Zingiber officinale) with clarithromycin on Helicobacter pylori. Phytother. Res. 2006, 20, 187–190. [Google Scholar] [CrossRef] [PubMed]
- Baltas, N.; Karaoglu, S.A.; Tarakci, C.; Kolayli, S. Effect of propolis in gastric disorders: Inhibition studies on the growth of Helicobacter pylori and production of its urease. J. Enzyme Inhib. Med. Chem. 2016, 31, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Boyanova, L.; Derejian, S.; Koumanova, R.; Katsarov, N.; Gergova, G.; Mitov, I.; Nikolov, R.; Krastev, Z. Inhibition of Helicobacter pylori growth in vitro by Bulgarian propolis: Preliminary report. J. Med. Microbiol. 2003, 52, 417–419. [Google Scholar] [CrossRef]
- Romero, M.; Freire, J.; Pastene, E.; García, A.; Aranda, M.; González, C. Propolis polyphenolic compounds affect the viability and structure of Helicobacter pylori in vitro. Rev. Bras. Farmacogn. 2019, 29, 325–332. [Google Scholar] [CrossRef]
- Roesler, B.M.; Rabelo-Gonçalves, E.M.; Zeitune, J.M. Virulence Factors of Helicobacter pylori: A Review. Clin. Med. Insights Gastroenterol. 2014, 7, 9–17. [Google Scholar] [CrossRef]
- Šterbenc, A.; Jarc, E.; Poljak, M.; Homan, M. Helicobacter pylori virulence genes. World J. Gastroenterol. 2019, 25, 4870–4884. [Google Scholar] [CrossRef]
- Lamb, A.; Chen, L.F. Role of the Helicobacter pylori-induced inflammatory response in the development of gastric cancer. J. Cell. Biochem. 2013, 114, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Nejati, S.; Karkhah, A.; Darvish, H.; Validi, M.; Ebrahimpour, S.; Nouri, H.R. Influence of Helicobacter pylori virulence factors CagA and VacA on pathogenesis of gastrointestinal disorders. Microb. Pathog. 2018, 117, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Takeshima, E.; Tomimori, K.; Takamatsu, R.; Ishikawa, C.; Kinjo, F.; Hirayama, T.; Fujita, J.; Mori, N. Helicobacter pylori vacA activates NF-κB in T Cells via the classical but not alternative pathway. Helicobacter 2009, 14, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Cover, T.L.; Lacy, D.B.; Ohi, M.D. The Helicobacter pylori cag type IV secretion system. Trends Microbiol. 2020, 28, 682–695. [Google Scholar] [CrossRef] [PubMed]
- Viala, J.; Chaput, C.; Boneca, I.G.; Cardona, A.; Girardin, S.E.; Moran, A.P.; Athman, R.; Mémet, S.; Huerre, M.R.; Coyle, A.J. Nod1 responds to peptidoglycan delivered by the Helicobacter pylori cag pathogenicity island. Nat. Immunol. 2004, 5, 1166–1174. [Google Scholar] [CrossRef] [PubMed]
- Waskito, L.A.; Salama, N.R.; Yamaoka, Y. Pathogenesis of Helicobacter pylori infection. Helicobacter 2018, 23, e12516. [Google Scholar] [CrossRef]
- McGee, D.J.; Mobley, H.L. Pathogenesis of Helicobacter pylori infection. Curr. Opin. Gastroenterol. 2000, 16, 24–31. [Google Scholar] [CrossRef]
- Pignatelli, B.; Bancel, B.; Esteve, J.; Malaveille, C.; Calmels, S.; Correa, P.; Patricot, L.; Laval, M.; Lyandrat, N.; Ohshima, H. Inducible nitric oxide synthase, anti-oxidant enzymes and Helicobacter pylori infection in gastritis and gastric precancerous lesions in humans. Eur. J. Cancer Prev. 1998, 7, 439–447. [Google Scholar] [CrossRef]
- Bothmer, C.v.; Edebo, A.; Lönroth, H.; Olbe, L.; Pettersson, A.; Fändriks, L. Helicobacter pylori infection inhibits antral mucosal nitric oxide production in humans. Scand. J. Gastroenterol. 2002, 37, 404–408. [Google Scholar] [CrossRef]
- Alderton, W.K.; Cooper, C.E.; Knowles, R.G. Nitric oxide synthases: Structure, function and inhibition. Biochem. J. 2001, 357, 593–615. [Google Scholar] [CrossRef]
- Cinelli, M.A.; Do, H.T.; Miley, G.P.; Silverman, R.B. Inducible nitric oxide synthase: Regulation, structure, and inhibition. Med. Res. Rev. 2020, 40, 158–189. [Google Scholar] [CrossRef]
- Fu, S.; Ramanujam, K.S.; Wong, A.; Fantry, G.T.; Drachenberg, C.B.; James, S.P.; Meltzer, S.J.; Wilson, K.T. Increased expression and cellular localization of inducible nitric oxide synthase and cyclooxygenase 2 in Helicobacter pylori gastritis. Gastroenterology 1999, 116, 1319–1329. [Google Scholar] [CrossRef]
- Saaed, H.K.; Chiggiato, L.; Webb, D.L.; Rehnberg, A.S.; Rubio, C.A.; Befrits, R.; Hellström, P.M. Elevated gaseous luminal nitric oxide and circulating IL-8 as features of Helicobacter pylori-induced gastric inflammation. UPS J. Med. Sci. 2021, 126, e8116. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.-T.; Oh, S.-Y.; Ahn, B.-W.; Kim, Y.; Jang, D.; Yang, K.; Hahm, K.; Kim, D. Decreased Helicobacter pylori associated gastric carcinogenesis in mice lacking inducible nitric oxide synthase. Gut 2004, 53, 1250–1255. [Google Scholar] [CrossRef] [PubMed]
- Lamb, A.; Chen, L.-F. The many roads traveled by Helicobacter pylori to NF-κB activation. Gut Microbes 2010, 1, 109–113. [Google Scholar] [CrossRef]
- Saha, K.; Sarkar, D.; Khan, U.; Karmakar, B.C.; Paul, S.; Mukhopadhyay, A.K.; Dutta, S.; Bhattacharya, S. Capsaicin inhibits inflammation and gastric damage during H pylori infection by targeting NF-kB–miRNA axis. Pathogens 2022, 11, 641. [Google Scholar] [CrossRef]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef]

| Identification | Gene | Primer Sequence (5′ to 3′) | |
|---|---|---|---|
| Specific for H. pylori | 16S rRNA | Forward | CTC ATT GCG AAG GCG ACC T |
| Reverse | TCT AAT CCT GTT TGC TCC CCA | ||
| SS1 | Forward | CTT AAC CAT AGA ACT GCA TTT GAA ACT AC | |
| Reverse | GGT CGC CTT CGC AAT GAG TA | ||
| ureA | Forward | AGG AAA CAT CGC TTC AAT ACC | |
| Reverse | AGG AAA CAT CGC TTC AAT ACC | ||
| SSA | Forward | TGG CGT GTC TAT TGA CAG CGA GC | |
| Reverse | CCT GCT GGG CAT ACT TCA CCA TG | ||
| napA | Forward | TCC TTT CAG CGA GAT CGT CA | |
| Reverse | GAA TGT GAA AGG CAC CGA TT | ||
| Specific for inflammation | IL-8 | Forward | TCC TTG TTC CAC TGT GCC TTG |
| Reverse | TGC TTC CAC ATG TCC TCA CAA | ||
| TNF-α | Forward | TCA GAG GGC CTG TAC CTC AT | |
| Reverse | GGA AGA CCC CTC CCA GAT AG | ||
| IL-1β | Forward | TTA AAG CCC GCC TGA CAG A | |
| Reverse | GCG AAT GAC AGA GGG TTT CTT | ||
| A1a | Forward | TCC ACA AGA GCA GAT TGC CCT G | |
| Reverse | GCC AGC CAG ATT TGG GTT CAA AC | ||
| A20 | Forward | AGC AAG TGC AGG AAA GCT GGC T | |
| Reverse | GCT TTC GCA GAG GCA GTA ACA G | ||
| c-myc | Forward | GCT GTT TGA AGG CTG GAT TTC | |
| Reverse | GAT GAA ATA GGG CTG TAC GGA G | ||
| Internal control | 18s rRNA | Forward | GCA ATT ATT CCC CAT GAA CG |
| Reverse | GGC CTC ACT AAA CCA TCC AA | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, M.-Y.; Lee, D.-Y.; Han, Y.-M.; Kim, E.-H. Anti-Inflammatory Effect of Korean Propolis on Helicobacter pylori-Infected Gastric Mucosal Injury Mice Model. Nutrients 2022, 14, 4644. https://doi.org/10.3390/nu14214644
Song M-Y, Lee D-Y, Han Y-M, Kim E-H. Anti-Inflammatory Effect of Korean Propolis on Helicobacter pylori-Infected Gastric Mucosal Injury Mice Model. Nutrients. 2022; 14(21):4644. https://doi.org/10.3390/nu14214644
Chicago/Turabian StyleSong, Moon-Young, Da-Young Lee, Young-Min Han, and Eun-Hee Kim. 2022. "Anti-Inflammatory Effect of Korean Propolis on Helicobacter pylori-Infected Gastric Mucosal Injury Mice Model" Nutrients 14, no. 21: 4644. https://doi.org/10.3390/nu14214644
APA StyleSong, M.-Y., Lee, D.-Y., Han, Y.-M., & Kim, E.-H. (2022). Anti-Inflammatory Effect of Korean Propolis on Helicobacter pylori-Infected Gastric Mucosal Injury Mice Model. Nutrients, 14(21), 4644. https://doi.org/10.3390/nu14214644






