Effects of Nutritional and Social Factors on Favorable Fetal Growth Conditions Using Structural Equation Modeling
Abstract
1. Introduction
2. Materials and Methods
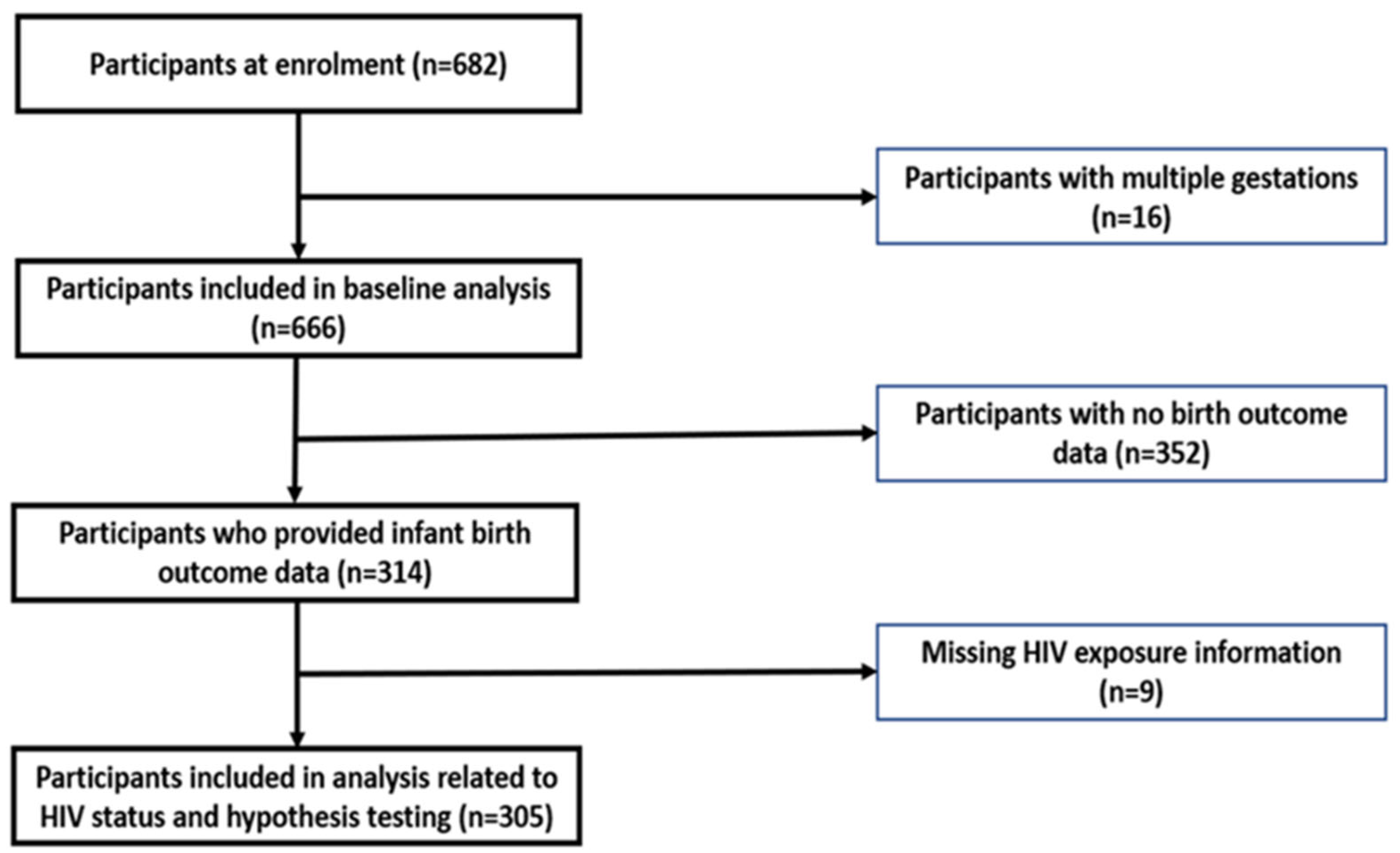
2.1. Study Design and Participants
2.2. Data Collection
2.3. Development of Latent Variables
2.4. Data Analysis
2.5. Hypothesized Diagram
3. Results
3.1. Sociodemographic Characteristics
3.2. Confirmatory Factor Analysis
3.3. Measurement Invariance
3.4. Multigroup Analysis
3.5. Structural Equation Model Findings
3.6. Final SEM Diagram
3.7. Modification Indices and Goodness-of-Fit Summary
4. Discussion
4.1. Strengths of the Study
4.2. Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chawanpaiboon, S.; Vogel, J.P.; Moller, A.-B.; Lumbiganon, P.; Petzold, M.; Hogan, D.; Landoulsi, S.; Jampathong, N.; Kongwattanakul, K.; Laopaiboon, P.M.; et al. Global, regional, and national estimates of levels of preterm birth in 2014: A systematic review and modelling analysis. Lancet Glob. Health 2019, 7, e37–e46. [Google Scholar] [CrossRef]
- UN IGME. Levels and Trends in Child Mortality Report 2018. 2018. Available online: https://www.unicef.org/media/47626/file/UN-IGME-Child-Mortality-Report-2018.pdf (accessed on 27 April 2022).
- UNICEF. Low Birthweight. UNICEF Data: Monitoring the Situation of Children and Women. 2019, pp. 1–4. Available online: https://data.unicef.org/topic/nutrition/low-birthweight/ (accessed on 4 January 2022).
- Blencowe, H.; Krasevec, J.; de Onis, M.; E Black, R.; An, X.; A Stevens, G.; Borghi, E.; Hayashi, C.; Estevez, D.; Cegolon, L.; et al. National, regional, and worldwide estimates of low birthweight in 2015, with trends from 2000: A systematic analysis. Lancet Glob. Health 2019, 7, e849–e860. Available online: https://pubmed.ncbi.nlm.nih.gov/31103470 (accessed on 7 July 2021). [CrossRef]
- Barker, D.J. Fetal origins of coronary heart disease. BMJ 1995, 311, 171–174. Available online: https://pubmed.ncbi.nlm.nih.gov/7613432 (accessed on 7 July 2021). [CrossRef] [PubMed]
- Barker, D.J.P.; Osmond, C. Infant mortality, childhood nutrition and ischemic heart disease in England and Wales. Lancet 1986, 327, 1077–1108. [Google Scholar] [CrossRef]
- Barker, D.J.P.; Osmond, C.; Winter, P.D.; Margetts, B.; Simmonds, S.J. Weight in infancy and death from ischemic heart diseases. Lancet 1989, 334, 577–580. [Google Scholar] [CrossRef]
- Li, Y.; Jaddoe, V.W.; Qi, L.; He, Y.; Wang, D.; Lai, J.; Zhang, J.; Fu, P.; Yang, X.; Hu, F.B. Exposure to the chinese famine in early life and the risk of metabolic syndrome in adulthood. Diabetes Care 2011, 34, 1014–1018. Available online: https://pubmed.ncbi.nlm.nih.gov/21310886 (accessed on 7 July 2021). [CrossRef]
- Wang, N.; Cheng, J.; Han, B.; Li, Q.; Chen, Y.; Xia, F.; Jiang, B.; Jensen, M.D.; Lu, Y. Exposure to severe famine in the prenatal or postnatal period and the development of diabetes in adulthood: An observational study. Diabetologia 2017, 60, 262–269. [Google Scholar] [CrossRef]
- Lumey, L.H.; Stein, A.D.; Susser, E. Prenatal famine and adult health. Annu. Rev. Public Health 2011, 32, 237–262. Available online: https://pubmed.ncbi.nlm.nih.gov/21219171 (accessed on 27 April 2022). [CrossRef]
- Shi, Z.; Ji, L.; Ma, R.C.W.; Zimmet, P. Early life exposure to 1959–1961 Chinese famine exacerbates association between diabetes and cardiovascular disease. J. Diabetes 2020, 12, 134–141. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, L.; Duan, W.; Meng, X.; Jia, C. Association between famine exposure in early life and type 2 diabetes mellitus and hyperglycemia in adulthood: Results from the China Health and Retirement Longitudinal Study (CHARLS). J. Diabetes 2018, 10, 724–733. [Google Scholar] [CrossRef]
- Arima, Y.; Fukuoka, H. Developmental origins of health and disease theory in cardiology. J. Cardiol. 2020, 76, 14–17. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Low-Birth-Weight Newborns (%). The Global Health Observatory: Indicator Metadata Registry List. 2021. Available online: https://www.who.int/data/gho/indicator-metadata-registry/imr-details/76 (accessed on 7 July 2021).
- INNOCENTI. The First 1000 Days of Life: The Brain’s Window of Opportunity; Unicef-Irc: Florence, Italy, 2018; Available online: https://www.unicef-irc.org/article/958-the-first-1000-days-of-life-the-brains-window-of-opportunity.html (accessed on 9 May 2020).
- Shisana, O.; Labadarios, D.; Rehle, T.; Simbayi, L.; Zuma, K.; Dhansay, A.R.P.; Parker, W.; Hoosain, E.; Naidoo, P.; Hongoro, C.; et al. South African National Health and Nutrition Examination Survey (SANHANES-1); HSRC Press: Cape Town, South Africa, 2013; Available online: http://www.hsrc.ac.za/uploads/pageNews/72/SANHANES-launch-edition-(online-version).pdf (accessed on 7 July 2021).
- Batra, K.; Pharr, J.; Olawepo, J.O.; Cruz, P. Understanding the multidimensional trajectory of psychosocial maternal risk factors causing preterm birth: A systematic review. Asian. J. Psychiatr. 2020, 54, 102436. Available online: https://www.sciencedirect.com/science/article/pii/S1876201820305499 (accessed on 27 April 2022). [CrossRef]
- Hoffman, D.J.; Powell, T.L.; Barrett, E.S.; Hardy, D.B. Developmental origins of metabolic diseases. Physiol. Rev. 2021, 101, 739–795. [Google Scholar] [CrossRef]
- Bollen, K.A.; Noble, M.D.; Adair, L.S. Are gestational age, birth weight, and birth length indicators of favorable fetal growth conditions? A structural equation analysis of Filipino infants. Stat. Med. 2013, 32, 2950–2961. [Google Scholar] [CrossRef] [PubMed]
- Statistics South Africa. South Africa Demographic and Health Survey 2016: Key Indicator Report; Statistics South Africa: Pretoria, South Africa, 2017. Available online: https://www.statssa.gov.za/publications/Report-03-00-09/Report-03-00-092016.pdf (accessed on 13 September 2019).
- Zar, H.J.; Pellowski, J.A.; Cohen, S.; Barnett, W.; Vanker, A.; Koen, N.; Stein, D. Maternal health and birth outcomes in a South African birth cohort study. PLoS ONE 2019, 14, e0222399. Available online: https://pubmed.ncbi.nlm.nih.gov/31751344 (accessed on 13 September 2019). [CrossRef] [PubMed]
- Department of Health (South Africa). Foodstuffs, Cosmetics and Disinfectants Act, 1972 (Act 54 of 1972): Regulations Related to the Labelling and Advertising of Foodstuffs. 2010. Available online: https://www.gov.za/sites/default/files/gcis_document/201409/32975146.pdf (accessed on 1 November 2022).
- National Department of Health. Cervical Cancer Prevention and Control Policy. National Policy Document. 2017. Available online: http://scielo.sld.cu/pdf/ccm/v21n1/ccm15117.pdf (accessed on 27 April 2022).
- Department of Health. South African Infant and Young Child Feeding Policy; Department of Health (DoH): Pretoria, South Africa, 2007. Available online: https://www.gov.za/sites/default/files/gcis_document/201409/infantfeed.pdf (accessed on 1 November 2022).
- Department of Health (DoH). Strategic Plan for the Prevention and Control of Non-Communicable Diseases 2020–2025; Department of Health (DoH): Pretoria, South Africa, 2019. Available online: https://www.samedical.org/file/1202 (accessed on 1 November 2022).
- Symington, E.; Norris, S.; Smuts, M. Food and nutrition security of the unborn child: The role of maternal nutrition. S. Afr. Child Gauge 2020, 72–84. Available online: http://www.ci.uct.ac.za/sites/default/files/image_tool/images/367/Child_Gauge/South_African_Child_Gauge_2020/ChildGauge_2020_screen_final.pdf (accessed on 27 April 2022).
- De Costa, A.; Moller, A.-B.; Blencowe, H.; Johansson, E.W.; Hussain-Alkhateeb, L.; Ohuma, E.O.; Okwaraji, Y.B.; Cresswell, J.; Requejo, J.H.; Bahl, R.; et al. Study protocol for WHO and UNICEF estimates of global, regional, and national preterm birth rates for 2010 to 2019. PLoS ONE 2021, 16, e0258751. [Google Scholar] [CrossRef] [PubMed]
- UNAIDS. South Africa HIV and AIDS Estimates; UNAIDS: Geneva, Switzerland, 2020; p. 1. Available online: https://www.unaids.org/en/regionscountries/countries/southafrica%0Ahttp://www.unaids.org/en/regionscountries/countries/southafrica (accessed on 9 July 2021).
- University of Witwatersrand. Birth to Twenty. 2017. Available online: https://www.wits.ac.za/health/research-entities/birth-to-20/birth-to-twenty/ (accessed on 28 January 2018).
- Stewart, A.; Marfell-Jones, M.; Olds, T.; De Ridder, H. International Standards for Anthropometric Assessment; International Society for the Advancement of Kinanthropmetry: Lower Hutt, New Zealand, 2011. [Google Scholar]
- Davies, H.; Visser, J.; Tomlinson, M.; Rotheram-Borus, M.; Gissane, C.; Harwood, J.; Leroux, I. An investigation into utilising gestational body mass index as a screening tool for adverse birth outcomes and maternal morbidities in a group of pregnant women in Khayelitsha. S. Afr. J. Clin. Nutr. 2013, 26, 116–122. Available online: https://www.ncbi.nlm.nih.gov/pubmed/25324710 (accessed on 7 July 2021). [CrossRef][Green Version]
- Cruz, M.L.S.; Harris, D.R.; Read, J.S.; Mussi-Pinhata, M.M.; Succi, R.C.M.; Group NISDI (NISDI). Association of Body Mass Index of HIV-1-Infected Pregnant Women and Infant Weight, Body Mass Index, Length, and Head Circumference: The NISDI Perinatal Study. Nutr. Res. 2007, 27, 685–691. Available online: https://pubmed.ncbi.nlm.nih.gov/19081829 (accessed on 7 March 2019). [CrossRef][Green Version]
- Symington, E.A.; Baumgartner, J.; Malan, L.; Zandberg, L.; Ricci, C.; Smuts, C.M. Nutrition during pregnancy and early development (NuPED) in urban South Africa: A study protocol for a prospective cohort. BMC Pregnancy Childbirth 2018, 18, 308. Available online: https://www.ncbi.nlm.nih.gov/pubmed/30041623 (accessed on 7 July 2021). [CrossRef]
- South African Food Data System (SAFOODS). SAMRC Food Quantities Manual for South Africa, 3rd ed.; South African Medical Research Council: Tygerberg, South Africa, 2018; Available online: http://safoods.mrc.ac.za/ (accessed on 18 July 2021).
- Wolmarans, P.; Danster, N.; Council SAMR. Condensed Food Composition Tables for South Africa; South African Medical Research Council: Tygerberg, South Africa, 2010. [Google Scholar]
- Hatløy, A.; Torheim, L.E.; Oshaug, A. Food variety—A good indicator of nutritional adequacy of the diet? A case study from an urban area in Mali, West Africa. Eur. J. Clin. Nutr. 1998, 52, 891–898. [Google Scholar] [CrossRef]
- INDDEX Project. Data4Diets: Building Blocks for Diet-Related Food Security Analysis; Tufts University: Boston, MA, USA, 2018; pp. 6–8. Available online: https://inddex.nutrition.tufts.edu/data4diets (accessed on 2 February 2021).
- Coates, J.; Swindale, A.; Bilinsky, P. Householf Food Indicator Access Scale (HFIAS) for Measurement of Food Access Indicator Guide (v. 3). Food and Nutrition Technical Assistance Project, Academy for Educational Development. 2007. Available online: http://www.fao.org/fileadmin/user_upload/eufao-fsi4dm/doc-training/hfias.pdf (accessed on 9 May 2020).
- SADHS. Road To Health Book; South Africa Department of Health: Pretoria, South Africa, 2020. Available online: https://sidebyside.co.za/resources/road-to-health-book/ (accessed on 18 July 2021).
- Institute of Medicine (IoM). Dietary Reference Intakes: A Risk Assessment Model for Establishing Upper Intake Levels for Nutrients. In What are Dietary Reference Intakes? National Academies Press (US): Washington, DC, USA, 1998. Available online: https://www.ncbi.nlm.nih.gov/books/NBK45182/ (accessed on 15 May 2020).
- Jang, W.; Kim, H.; Lee, B.-E.; Chang, N. Maternal fruit and vegetable or vitamin C consumption during pregnancy is associated with fetal growth and infant growth up to 6 months: Results from the Korean Mothers and Children’s Environmental Health (MOCEH) cohort study. Nutr. J. 2018, 17, 105. [Google Scholar] [CrossRef] [PubMed]
- WHO. Serum and Red Blood Cell Folate Concentrations for Assessing Folate Status in Populations. Vitamin and Mineral Nutrition Information System. 2015, pp. 1–7. Available online: http://apps.who.int/iris/handle/10665/162114 (accessed on 21 April 2022).
- Little, T.D. Longitudinal Structural Equation Modeling, 1st ed.; Guilford Press: New York, NY, USA, 2013; pp. 106–118. [Google Scholar]
- Hoffman, D.J.; Reynolds, R.M.; Hardy, D.B. Developmental origins of health and disease: Current knowledge and potential mechanisms. Nutr. Rev. 2017, 75, 951–970. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.T.; Nguyen, L.T.; Berde, Y.; Low, Y.L.; Tey, S.L.; Huynh, D.T.T. Maternal nutritional adequacy and gestational weight gain and their associations with birth outcomes among Vietnamese women. BMC Pregnancy Childbirth 2019, 19, 468. [Google Scholar] [CrossRef] [PubMed]
- Basel, P.L.; Singh, S. Low birth weight and its associated risk factors: Health facility-based case-control study. PLoS ONE 2020, 15, e0234907. Available online: https://pubmed.ncbi.nlm.nih.gov/32569281 (accessed on 27 April 2022).
- Chen, Y.-H.; Fu, L.; Hao, J.-H.; Wang, H.; Zhang, C.; Tao, F.-B.; Xu, D.-X. Influent factors of gestational vitamin D deficiency and its relation to an increased risk of preterm delivery in Chinese population. Sci. Rep. 2018, 8, 3608. [Google Scholar] [CrossRef] [PubMed]
- Stubert, J.; Reister, F.; Hartmann, S.; Janni, W. The Risks Associated with Obesity in Pregnancy. Dtsch. Arztebl. Int. 2018, 115, 276–283. Available online: https://pubmed.ncbi.nlm.nih.gov/29739495 (accessed on 9 December 2020). [CrossRef] [PubMed]
- Catalano, P.M.; Shankar, K. Obesity and pregnancy: Mechanisms of short term and long term adverse consequences for mother and child. BMJ 2017, 356, j1. Available online: https://pubmed.ncbi.nlm.nih.gov/28179267 (accessed on 7 July 2021). [CrossRef]
- Mariona, F.G. Perspectives in obesity and pregnancy. Women’s Health 2016, 12, 523–532. [Google Scholar] [CrossRef]
- Paredes, C.; Hsu, R.C.; Tong, A.; Johnson, J.R. Obesity and Pregnancy. Neoreviews 2021, 22, e78–e87. [Google Scholar] [CrossRef]
- Boudet-Berquier, J.; Salanave, B.; Desenclos, J.-C.; Castetbon, K. Sociodemographic factors and pregnancy outcomes associated with prepregnancy obesity: Effect modification of parity in the nationwide Epifane birth-cohort. BMC Pregnancy Childbirth 2017, 17, 273. Available online: https://pubmed.ncbi.nlm.nih.gov/28841845 (accessed on 9 May 2020). [CrossRef]
- Averett, S.L.; Fletcher, E.K. Prepregnancy Obesity and Birth Outcomes. Matern. Child. Health J. 2016, 20, 655–664. [Google Scholar] [CrossRef]
- Patel, A.; Prakash, A.A.; Das, P.K.; Gupta, S.; Pusdekar, Y.V.; Hibberd, P.L. Maternal anemia and underweight as determinants of pregnancy outcomes: Cohort study in eastern rural Maharashtra, India. BMJ Open 2018, 8, e021623. Available online: http://bmjopen.bmj.com/content/8/8/e021623.abstract (accessed on 27 April 2022). [CrossRef] [PubMed]
- Gaillard, R.; Durmuş, B.; Hofman, A.; Mackenbach, J.P.; Steegers, E.A.P.; Jaddoe, V.W.V. Risk factors and outcomes of maternal obesity and excessive weight gain during pregnancy. Obesity 2013, 21, 1046–1055. [Google Scholar] [CrossRef] [PubMed]
- SAHRC. South Africa Poverty Rates by Race; South Africa Human Rights Commission: Johannesburg, South Africa, 2018. Available online: https://www.sahrc.org.za/index.php/sahrc-media/news/item/1442-kate-wilkinson-stats-about-poverty-stricken-sa-whites-are-not-true (accessed on 16 April 2018).
- Mishra, P.S.; Sinha, D.; Kumar, P.; Srivastava, S.; Bawankule, R. Newborn low birth weight: Do socio-economic inequality still persist in India? BMC Pediatr. 2021, 21, 518. Available online: https://pubmed.ncbi.nlm.nih.gov/34798861 (accessed on 3 January 2022). [CrossRef] [PubMed]
- Curtis, D.S.; Fuller-Rowell, T.E.; Carlson, D.L.; Wen, M.; Kramer, M.R. Does a Rising Median Income Lift All Birth Weights? County Median Income Changes and Low Birth Weight Rates among Births to Black and White Mothers. Milbank Q. 2022, 100, 38–77. [Google Scholar] [CrossRef] [PubMed]
- Rosário, E.V.N.; Gomes, M.C.; Brito, M.; Costa, D. Determinants of maternal health care and birth outcome in the Dande Health and Demographic Surveillance System area, Angola. PLoS ONE 2019, 14, e0221280. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Wang, Y.; Chen, Y.; Zhou, Y.; Jiang, Q. Impact of maternal HIV infection on pregnancy outcomes in southwestern China—A hospital registrybased study. Epidemiol. Infect. 2019, 147, e124. Available online: https://pubmed.ncbi.nlm.nih.gov/30868995 (accessed on 27 April 2022). [CrossRef] [PubMed]
- Gedefaw, G.; Alemnew, B.; Demis, A. Adverse fetal outcomes and its associated factors in Ethiopia: A systematic review and meta-analysis. BMC Pediatr. 2020, 20, 269. [Google Scholar] [CrossRef]
- Zaveri, A.; Paul, P.; Saha, J.; Barman, B.; Chouhan, P. Maternal determinants of low birth weight among Indian children: Evidence from the National Family Health Survey-4, 2015–2016. PLoS ONE 2021, 15, e0244562. [Google Scholar] [CrossRef]
- Perez-Escamilla, R.; Bermudez, O.; Buccini, G.S.; Kumanyika, S.; Lutter, C.; Monsivais, P.; Victora, C. Nutrition disparities and the global burden of malnutrition. BMJ 2018, 361, k2252. Available online: http://www.bmj.com/content/361/bmj.k2252.abstract (accessed on 7 July 2021). [CrossRef]
- Arredondo, A.; Torres, C.; Orozco, E.; Pacheco, S.; Huang, F.; Zambrano, E.; Bolaños-Jiménez, F. Socio-economic indicators, dietary patterns, and physical activity as determinants of maternal obesity in middle-income countries: Evidences from a cohort study in Mexico. Int. J. Health Plann. Manag. 2019, 34, e713–e725. [Google Scholar] [CrossRef] [PubMed]
- Jebena, M.G.; Taha, M.; Nakajima, M.; Lemieux, A.; Lemessa, F.; Hoffman, R.; Tesfaye, M.; Belachew, T.; Workineh, N.; Kebede, E.; et al. Household food insecurity and mental distress among pregnant women in Southwestern Ethiopia: A cross sectional study design. BMC Pregnancy Childbirth 2015, 15, 250. Available online: https://pubmed.ncbi.nlm.nih.gov/26449375 (accessed on 27 April 2022). [CrossRef] [PubMed]
- Matare, C.; Tome, J.; Makasi, R.; Dickin, K.; Pelto, G.; Constas, M.; Chasekwa, B.; Mbuya, M.; Ntozini, R.; Prendergast, A.; et al. Maternal Decision-Making Autonomy, Mental Health, Gender Norm Attitudes, and Social Support During Pregnancy Predict Child Care-Giving and Stunting in Rural Zimbabwe. Curr. Dev. Nutr. 2020, 4 (Suppl. S2), 866. [Google Scholar] [CrossRef]
- Peng, W.; Dernini, S.; Berry, E.M. Coping With Food Insecurity Using the Sociotype Ecological Framework. Front. Nutr. 2018, 5, 107. Available online: https://www.frontiersin.org/article/10.3389/fnut.2018.00107 (accessed on 7 July 2021). [CrossRef] [PubMed]
- Larson, N.; Laska, M.N.; Neumark-Sztainer, D. Food Insecurity, Diet Quality, Home Food Availability, and Health Risk Behaviors among Emerging Adults: Findings From the EAT 2010–2018 Study. Am. J. Public Health 2020, 110, 1422–1428. Available online: https://pubmed.ncbi.nlm.nih.gov/32673120 (accessed on 2 October 2021). [CrossRef]
- Leung, C.W.; Tester, J.M. The Association between Food Insecurity and Diet Quality Varies by Race/Ethnicity: An Analysis of National Health and Nutrition Examination Survey 2011–2014 Results. J. Acad. Nutr. Diet. 2019, 119, 1676–1686. [Google Scholar] [CrossRef]
- Gonzalez-Nahm, S.; Østbye, T.; Hoyo, C.; Kravitz, R.M.; Benjamin-Neelon, S.E. Associations among Food Security, Diet Quality, and Dietary Intake During Pregnancy in a Predominantly African American Group of Women from North Carolina. J. Acad. Nutr. Diet. 2022, 122, 565–572. [Google Scholar] [CrossRef]


| Woman’s Age at Enrolment (Years) | Gestational Age at Enrolment (Weeks) | |||||
|---|---|---|---|---|---|---|
| Median | IQR | p-Value | Median | IQR | p-Value | |
| Women with HIV | 34.0 | 29–37 | 0.014 * | 32.0 | 25–36 | 0.13 |
| Women without HIV | 32.0 | 27–37 | 33.0 | 27–37 | ||
| Group 1—Women Living with HIV | Group 2—Women without HIV | ||||||
|---|---|---|---|---|---|---|---|
| Latent Variable | Unit | Loading | Standard Error | p-Value | Loading | Standard Error | p-Value |
| Dietary micronutrient quality (DMQ) | DMQ Parcel 1 | 0.661 | 0.045 | <0.001 * | 0.745 | 0.045 | <0.001 * |
| DMQ Parcel 2 | 0.709 | 0.053 | <0.001 * | 0.837 | 0.053 | <0.001 * | |
| DMQ Parcel 3 | 0.760 | 0.055 | <0.001 * | 0.771 | 0.055 | <0.001 * | |
| Stress | Stress Parcel 1 | 0.143 | 0.140 | 0.015 * | 0.208 | 0.140 | 0.015 * |
| Stress Parcel 2 | 0.377 | 0.292 | <0.001 * | 0.480 | 0.292 | <0.001 * | |
| Stress Parcel 3 | 0.427 | 0.305 | <0.001 * | 0.504 | 0.304 | <0.001 * | |
| Favorable fetal growth conditions | Birth weight | 0.857 | 0.049 | <0.001 * | 0.776 | 0.049 | <0.001 * |
| Birth length | 0.895 | 0.053 | <0.001 * | 0.827 | 0.053 | <0.001 * | |
| Gestational age | 0.695 | 0.055 | <0.001 * | 0.613 | 0.044 | <0.001 * | |
| χ2 | Df | RMSEA | SRMR | p | TLI | CFI | ΔCFI | Pass? | |
|---|---|---|---|---|---|---|---|---|---|
| Configural model | 125.46 | 96 | 0.043 | 0.054 | 0.023 | 0.940 | 0.963 | ||
| Weak invariance | 134.15 | 102 | 0.044 | 0.058 | 0.018 | 0.938 | 0.959 | 0.003 | Yes |
| Strong invariance | 139.26 | 108 | 0.043 | 0.060 | 0.023 | 0.943 | 0.960 | −0.001 | Yes |
| Model | χ2 | df | RMSEA | RMSEA 90% CI | SRMR | CFI | TLI | ANOVA | Pass? |
|---|---|---|---|---|---|---|---|---|---|
| Latent regression model | 139.26 | 108 | 0.043 | 0.017–0.062 | 0.060 | 0.960 | 0.943 | ||
| Regression constrained model | 159.57 | 129 | 0.039 | 0.011–0.057 | 0.067 | 0.961 | 0.953 | p > 0.05 | Pass |
| Path | (SE) | p |
|---|---|---|
| Stress and favorable fetal growth conditions (FFGC) | −0.032 (0.169) | 0.84 |
| Social support and FFGC | 0.022 (0.063) | 0.72 |
| Household income and FFGC | 0.141 (0.072) | 0.041 * |
| Gestational body mass index (GBMI) and FFGC | 0.223 (0.067) | <0.001 * |
| Household food security and FFGC | 0.062 (0.177) | 0.58 |
| Dietary micronutrient quality (DMQ) and FFGC | 0.075 (0.073) | 0.28 |
| Path | (SE) | p |
| Stress and dietary micronutrient quality | −0.009 (0.114) | 0.94 |
| Stress and household income | −0.313 (0.109) | 0.004 * |
| Stress and GBMI | −0.135 (0.097) | 0.17 |
| Stress and support system | −0.090 (0.098) | 0.36 |
| Stress and household food insecurity | 0.605 (0.102) | <0.001 * |
| DMQ and household income | 0.193 (0.061) | 0.002 * |
| DMQ and GBMI | 0.061 (0.062) | 0.33 |
| DMQ and support system | −0.004 (0.062) | 0.95 |
| DMQ and household food insecurity | −0.188 (0.063) | 0.061 |
| Household income and GBMI | 0.140 (0.055) | 0.012 * |
| Household income and support system | 0.028 (0.056) | 0.61 |
| Household income and household food insecurity | −0.362 (0.049) | <0.001 * |
| GBMI and support system | −0.050 (0.056) | 0.37 |
| GBMI and household food insecurity | −0.080 (0.056) | 0.15 |
| Support system and household food insecurity | −0.145 (0.055) | 0.008 * |
| Model | χ2 | df | RMSEA | RMSEA 90% CI | SRMR | CFI | TLI |
|---|---|---|---|---|---|---|---|
| SEM | 159.57 | 129 | 0.039 | 0.011–0.057 | 0.067 | 0.961 | 0.953 |
| Interpretation | Good | Acceptable | Good | Good | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moyo, G.; Stickley, Z.; Little, T.; Dawson, J.; Thomas-Jackson, S.; Ngounda, J.; Jordaan, M.; Robb, L.; Walsh, C.; Oldewage-Theron, W. Effects of Nutritional and Social Factors on Favorable Fetal Growth Conditions Using Structural Equation Modeling. Nutrients 2022, 14, 4642. https://doi.org/10.3390/nu14214642
Moyo G, Stickley Z, Little T, Dawson J, Thomas-Jackson S, Ngounda J, Jordaan M, Robb L, Walsh C, Oldewage-Theron W. Effects of Nutritional and Social Factors on Favorable Fetal Growth Conditions Using Structural Equation Modeling. Nutrients. 2022; 14(21):4642. https://doi.org/10.3390/nu14214642
Chicago/Turabian StyleMoyo, Gugulethu, Zachary Stickley, Todd Little, John Dawson, Shera Thomas-Jackson, Jennifer Ngounda, Marizeth Jordaan, Liska Robb, Corinna Walsh, and Wilna Oldewage-Theron. 2022. "Effects of Nutritional and Social Factors on Favorable Fetal Growth Conditions Using Structural Equation Modeling" Nutrients 14, no. 21: 4642. https://doi.org/10.3390/nu14214642
APA StyleMoyo, G., Stickley, Z., Little, T., Dawson, J., Thomas-Jackson, S., Ngounda, J., Jordaan, M., Robb, L., Walsh, C., & Oldewage-Theron, W. (2022). Effects of Nutritional and Social Factors on Favorable Fetal Growth Conditions Using Structural Equation Modeling. Nutrients, 14(21), 4642. https://doi.org/10.3390/nu14214642







