Do Nutrients and Nutraceuticals Play a Role in Diabetic Retinopathy? A Systematic Review
Abstract
1. Introduction
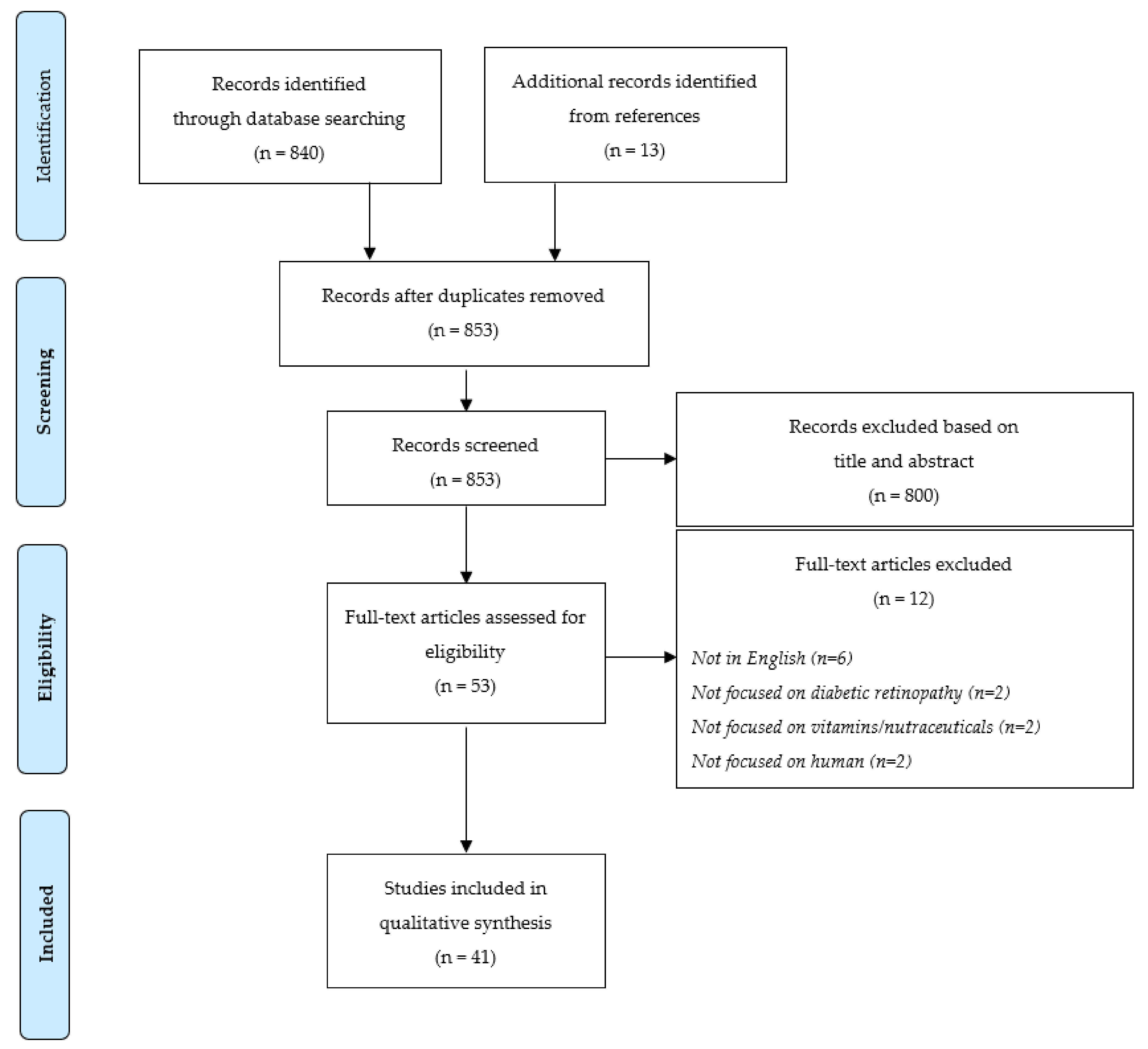
2. Materials and Methods
2.1. Literature Search
2.2. Selection Criteria
2.3. Data Extraction and Quality Assessment
3. Results
3.1. Characteristics of the Included Studies
3.2. Interventional Studies
3.3. Observational Studies
| First Author and Year | Country | Type of Diabetes | Diabetic Groups (n) | Control Group (n) | Nutraceutical | Sample | Main Results | NOS Scale |
|---|---|---|---|---|---|---|---|---|
| Zhao W. J., 2021 [50] | China | T2D | DR (235), NDR (836) | None | 25(OH)D | Serum | 25(OH)D level was not related to DR | 7 |
| Gungor A., 2015 [44] | Turkey | T2D | NPDR (100) | None | 25(OH)D | Serum | Reduced mean retinal nerve fibre layer thickness in the group with vitamin D insufficiency | 7 |
| Senyigit A., 2019 [68] | Turkey | T2D | DR (30), NDR (133) | HC (75) | 25(OH)D3 | Serum | Lower 25(OH)D in patients with DM and complications (retinopathy, nephropathy or neuropathy) than the control group and the DM + uncomplicated group | 6 |
| Herrmann M., 2015 [45] | Australia New Zealand Finland | T2D | DR (793), NDR (8731) | None | 25(OH)D3 | Serum | Increased risk of macrovascular and microvascular disease events with low blood 25(OH)D | 8 |
| Bajaj S., 2014 [46] | India | T2D | DR (54), NDR (104) | HC (130) | 25(OH)D3 | Serum | Lower levels of vitamin D in T2D and augmented risk of microvascular complication with vitamin D deficiency | 5 |
| Ahmadieh H., 2013 [47] | Lebanon | T2D | DR (32), NDR (104) | HC (74) | 25(OH)D3 | Serum | Low serum 25(OH)D level was an independent predictor of retinopathy in DM2 | 4 |
| Kaur H., 2011 [48] | Australia | T1D | DM (517) | None | 25(OH)D3 | Serum | Increased prevalence of retinopathy in T1D young people with vitamin D deficiency | 6 |
| Butler A.E., 2020 [42] | Qatar | T2D | DR (160), NDR (300) | HC (290) | 25(OH)D3, 1,25(OH)2D3, 24,25(OH)2D3, 3-epi-25(OH)D3 | Serum | Lower 25(OH)D3 and 1,25(OH)2D3 levels related to DR | 7 |
| Ahmed L.H.M., 2020 [41] | Qatar | T2D | DR (160), NDR (300) | None | 25(OH)D3, 1,25(OH)2D3, 24,25(OH)2D3, 3-epi-25(OH)D3, VMR1, VMR2, VMR3 | Plasma | VMR1 and mainly VMR2 were related to DR | 5 |
| Ahmed L.H.M., 2020 [49] | Qatar | T2D | DR (77), NDR (182) | HC (222) | 25(OH)D2, 25(OH)D3 | Serum | Lower 25(OH)D3 levels were related to DR | 5 |
| Millen A.E., 2003 [52] | USA | T2D | DR (199), NDR (799) | None | Vitamin C, Vitamin E | Serum | No significant associations between serum levels of major dietary antioxidants and DR | 7 |
| Millen A.E., 2004 [51] | USA | T2D | DR (224), NDR (1129) | None | Vitamin C, Vitamin E, Multisupplements | FFQ | Decreased odds of DR among users of vitamin C or vitamin E supplements or multisupplements | 7 |
| She C., 2021 [53] | China | T2D | DR (119), NDR (336) | None | Vitamin C, Vitamin E, Vitamin A, Vitamin B2, Selenium | FFQ | Higher vitamin E and selenium intake appeared to be the protective factors of DR | 5 |
| Fahmy R., 2021 [55] | Saudi Arabia | T2D | DR (12), NDR (12) | HC (15) | Vitamin C, TBARS, GSH, GSH S-transferase | Serum | Higher level of vit C and GSH in diabetic vs. controls were predictive of DR | 5 |
| Cinici E., 2020 [60] | Turkey | T2D | NPDR (40), PDR (20), NDR (20) | HC (20) | Thiamine Pyrophosphate (Vitamin B1) | Serum | Lower blood TPP concentrations were associated with higher risk of DR | 8 |
| Horikawa C., 2020 [62] | Japan | T2D | NDR (978) | None | Vitamin B6 | FFQ | High vitamin B6 intake was associated with lower incidence of DR | 7 |
| Malaguarnera G., 2015 [63] | Italy | T2D | NPDR (70), PDR (65), NDR (96) | HC (80) | Vitamin B9 | Serum | Severity of DR was associated with lower folic acid and red cell folate levels, especially between PDR and NPDR groups | 5 |
| Satyanarayana A., 2011 [61] | India | T2D | DR (200), NDR (100) | HC (100) | Folic Acid | Serum | Higher plasma homocysteine levels in T2D patients (especially in the DR group), lower plasma vitamin-B6 and folic acid in the NDR and DR groups than in the HC group | 4 |
| Srivastav K., 2016 [59] | India | T2D | NPDR (20), PDR (20), NDR (20) | HC (20) | Vitamin B12, Folic Acid, Homocysteine | Serum | Increased severity of DR and retinal nerve fibre layer thinning were correlated with increased serum levels of homocysteine | 6 |
| de Luis D.A., 2005 [64] | Spain | T2D | DR (65), NDR (90) | None | Homocysteine | Serum | Hyperhomocysteinemia in T2D patients was associated with higher levels of fibrinogen, lipoprotein (a), microalbuminuria, and blood pressure | 7 |
| Zhou Q., 2018 [54] | China | T1D | DR (34), NDR (103) | HC (50) | Selenium | Serum, Urine | Lower urinary Se levels in T2D patients with DR compared to uncomplicated T2D subjects | 6 |
| Yildirim Z., 2007 [56] | Turkey | DM | NPDR (25), PDR (25) | HC (25) | Copper, Zinc, Nitric Oxide, GSH, AOPP, SOD | Serum | Increased AOPP levels in PDR compared to HC | 2 |
| Ba-Ali S., 2018 [66] | Denmark | T2D | NPDR (25), NDR (29) | HC (21) | Melatonin | Saliva | Reduced nocturnal melatonin concentration and increased fragmentation of activity-rest intervals in DR group compared to NDR group | 5 |
| Hikichi T., 2011 [65] | Japan | T2D | NPDR (16), PDR (14) | HC (26) | Melatonin | Serum | Lower nighttime melatonin levels in PDR group than in the HC and NPDR groups | 5 |
| Poorabbas A., 2007 [67] | Iran | T2D | DR (20), NDR (13) | HC (18) | L-Carnitine | Serum | Almost 25% less serum-free L-carnitine levels in DM patients with complications than in those with no complications | 5 |
| Hattenbach L.O., 2000 [57] | Germany | DM | DR (14), NDR (8) | HC (20) | L-Arginine, L-Citrulline, N-hydroxy-L-arginine (HOArg) | Aqueous Humour | Higher levels of HOArg in DR and NDR patients than in HC | 3 |
| Pękala-Wojciechowska A., 2018 [58] | Poland | T1D | DR (11), NDR (10) | HC (12) | 8-Isoprostane | Serum, Exhaled breath condensate | Lower 8-isoprostane in exhaled breath condensate in T1D subjects with advanced complications than in those without advanced complications and in the HC group | 6 |
| First Author and Year | Country | Type of Diabetes | Diabetic Groups (n) | Control Group (n) | Nutraceutical | Daily Dose | Intervention Duration | Main Results | NOS Scale |
|---|---|---|---|---|---|---|---|---|---|
| Ellis J.M., 1991 [32] | USA | DM | DM (18) | None | Vitamin B6 | 50–200 mg | 8 months–28 years | No developement or mild form of DR | 5 |
| Chatziralli I.P., 2017 [34] | Greece | T2D | NPDR (188), PDR (94) | None | Vitamin E | 300 mg | 3 months | Serum MDA associated with the severity of DR, Vit. E reduces MDA levels | 5 |
| Bursell S.E., 1999 [33] | USA | T1D | NDR (36) | HC (9) | Vitamin E | 1800 IU | 4 months treatment + 4 months placebo | DM patient retinal blood flow increased and was comparable with that of HC subjects. Normalization of elevated baseline creatinine clearance in DM patients | 8 |
| Kheirouri S., 2018 [38] | Iran | DM | DR (50) | None | Zinc | 30 mg | 3 months | Levels of VEGF, BDNF and NGF were not affected by Zn supplementation | 8 |
| Britten-Jones A.C., 2021 [37] | Australia | T1D | NPDR (18), NDR (25) | None | n-3 PUFA (EPA, DHA) | 1800 mg (1080 mg, 720 mg) | 6 months | In n-3 PUFA group, improvements in central corneal nerve fibre length | 8 |
| Sepahi S., 2018 [40] | Iran | T1D, T2D | PDR (60) | None | Crocin (Saffron) | 5 or 15 mg | 3 months | Crocin 15 mg/die decreased HbA1c and central macular thickness, and improved best corrected visual acuity compared to the placebo group. No significant differences with crocin 5 mg/die. | 8 |
| Filippelli M., 2021 [39] | Italy | DM | PDR (28) | None | Curcumin, with or without Homotaurine and Vitamin D3 | 0.5 and 1 μM, 100 μM, 50 nM | Nothing (in vitro) | Improved TNF-α, IL2, and PDGF-AB with curcumin, homotaurine, and vitamin D3 treatment | 6 |
| Domanico D., 2015 [35] | Italy | T2D | NPDR (68) | None | Pycnogenol, Vitamin E, Coenzyme Q10 | 50 mg, 30 mg, 20 mg | 6 months | Reduction in ROS levels and an influence on retinal thickness | 6 |
| Parisi V., 2021 [27] | Italy | T1D | NPDR (20) | None | Citicoline 2%, Vitamin B12 0.05% (OMK2) | One drop, thrice daily | 36 months | Increased mfERG responses in the treatment group (n = 8) with functional enhancement of preganglionic elements located in the 10 central retinal degrees | 7 |
| Parravano M., 2020 [28] | Italy | T1D | NPDR (20) | None | Citicoline 2%, Vitamin B12 0.05% (OMK2) | One drop, thrice daily | 3 years | A significant reduction in terms of FDT mean sensitivity and in morphology was observed in the placebo group, while no significant changes were observed in the treated group | 6 |
| Liu z., 2021 [30] | USA | DM | NPDR (8) | HC (15) | L-methylfolate, Vitamin C, Vitamin D, Vitamin E and others * | 1 capsule for the 1st week, 2 capsules for the 2nd week, 3 capsules for the rest of the six months | 6 months | In DR MTHFR+, increase in visual acuity and vascular density, the latter particularly in subjects with both A1298C and C677T polymorphisms | 6 |
| Liu Z., 2020 [29] | USA | DM | NPDR (8) | HC (15) | L-methylfolate, Vitamin C, Vitamin D, Vitamin E and others * | 1 capsule for the 1st week, 2 capsules for the 2nd week, 3 capsules for the rest of the six months | 6 months | In DR MTHFR+, axial blood flow velocity, cross-sectional blood flow velocity, flow rate, vessel density were significantly increased compared to baseline | 6 |
| Chous A.P., 2015 [36] | USA | T1D, T2D | NPDR (30), NDR (37) | None | Alpha-Lipic Acid, Benfotiamine (Vit. B1), Vit. C, Vit. E and others ** | 2 capsules | 6 months | At 6 months, better visual function, improvements in serum lipids, hsCRP and diabetic peripheral neuropathy in subjects on active supplement compared with placebo | 6 |
| Smolek M.K., 2013 [31] | USA | T2D | NPDR (10) | None | L-Methylfolate Calcium, Pyridoxal-5′-Phosphate, Methylcobalamin (Metanx) | 2 tablets (3 mg, 35 mg, 2 mg) | 6 months | Reduced retinal edema and increased light sensitivity | 4 |
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Diabetes Association Professional Practice Committee. Retinopathy, Neuropathy, and Foot Care: Standards of Medical Care in Diabetes—2022. Diabetes Care 2022, 45, S185–S194. [Google Scholar] [CrossRef] [PubMed]
- Resnikoff, S.; Pascolini, D.; Etya’ale, D.; Kocur, I.; Pararajasegaram, R.; Pokharel, G.P.; Mariotti, S.P. Global Data on Visual Impairment in the Year 2002. Bull. World Health Organ. 2004, 82, 844–851. [Google Scholar]
- Simó-Servat, O.; Hernández, C.; Simó, R. Diabetic Retinopathy in the Context of Patients with Diabetes. Ophthalmic Res. 2019, 62, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Resnikoff, S.; Keys, T. Future Trends in Global Blindness. Indian J. Ophthalmol. 2012, 60, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, J.; Fuller, J.H. Microvascular and Acute Complications in IDDM Patients: The EURODIAB IDDM Complications Study. Diabetologia 1994, 37, 278–285. [Google Scholar] [CrossRef]
- Stratton, I.M.; Kohner, E.M.; Aldington, S.J.; Turner, R.C.; Holman, R.R.; Manley, S.E.; Matthews, D.R. UKPDS 50: Risk Factors for Incidence and Progression of Retinopathy in Type II Diabetes over 6 Years from Diagnosis. Diabetologia 2001, 44, 156–163. [Google Scholar] [CrossRef]
- Knudsen, L.L.; Lervang, H.-H.; Lundbye-Christensen, S.; Gorst-Rasmussen, A. The North Jutland County Diabetic Retinopathy Study: Population Characteristics. Br. J. Ophthalmol. 2006, 90, 1404–1409. [Google Scholar] [CrossRef]
- Milluzzo, A.; Falorni, A.; Brozzetti, A.; Pezzino, G.; Tomaselli, L.; Tumminia, A.; Frittitta, L.; Vigneri, R.; Sciacca, L. Risk for Coexistent Autoimmune Diseases in Familial and Sporadic Type 1 Diabetes Is Related to Age at Diabetes Onset. Endocr. Pract. 2021, 27, 110–117. [Google Scholar] [CrossRef]
- Wong, T.Y.; Mwamburi, M.; Klein, R.; Larsen, M.; Flynn, H.; Hernandez-Medina, M.; Ranganathan, G.; Wirostko, B.; Pleil, A.; Mitchell, P. Rates of Progression in Diabetic Retinopathy During Different Time Periods. Diabetes Care 2009, 32, 2307–2313. [Google Scholar] [CrossRef]
- Bhatwadekar, A.D.; Shughoury, A.; Belamkar, A.; Ciulla, T.A. Genetics of Diabetic Retinopathy, a Leading Cause of Irreversible Blindness in the Industrialized World. Genes 2021, 12, 1200. [Google Scholar] [CrossRef]
- Milluzzo, A.; Maugeri, A.; Barchitta, M.; Sciacca, L.; Agodi, A. Epigenetic Mechanisms in Type 2 Diabetes Retinopathy: A Systematic Review. Int. J. Mol. Sci. 2021, 22, 10502. [Google Scholar] [CrossRef] [PubMed]
- Massimino, M.; Tirrò, E.; Stella, S.; Frasca, F.; Vella, V.; Sciacca, L.; Pennisi, M.S.; Vitale, S.R.; Puma, A.; Romano, C.; et al. Effect of Combined Epigenetic Treatments and Ectopic NIS Expression on Undifferentiated Thyroid Cancer Cells. Anticancer Res. 2018, 38, 6653–6662. [Google Scholar] [CrossRef]
- Da Rosa, L.C.G.F.; Zajdenverg, L.; Souto, D.L.; Dantas, J.R.; Pinto, M.V.R.; Salles, G.F.d.C.M.d.; Rodacki, M. HbA1c Variability and Long-Term Glycemic Control Are Linked to Diabetic Retinopathy and Glomerular Filtration Rate in Patients with Type 1 Diabetes and Multiethnic Background. J. Diabetes Complicat. 2019, 33, 610–615. [Google Scholar] [CrossRef] [PubMed]
- Jampol, L.M.; Glassman, A.R.; Sun, J. Evaluation and Care of Patients with Diabetic Retinopathy. N. Engl. J. Med. 2020, 382, 1629–1637. [Google Scholar] [CrossRef] [PubMed]
- Martinell, M.; Dorkhan, M.; Stålhammar, J.; Storm, P.; Groop, L.; Gustavsson, C. Prevalence and Risk Factors for Diabetic Retinopathy at Diagnosis (DRAD) in Patients Recently Diagnosed with Type 2 Diabetes (T2D) or Latent Autoimmune Diabetes in the Adult (LADA). J. Diabetes Complicat. 2016, 30, 1456–1461. [Google Scholar] [CrossRef]
- Takao, T.; Suka, M.; Yanagisawa, H.; Matsuyama, Y.; Iwamoto, Y. Predictive Ability of Visit-to-Visit Variability in HbA1c and Systolic Blood Pressure for the Development of Microalbuminuria and Retinopathy in People with Type 2 Diabetes. Diabetes Res. Clin. Pract. 2017, 128, 15–23. [Google Scholar] [CrossRef]
- Antonetti, D.A.; Klein, R.; Gardner, T.W. Diabetic Retinopathy. N. Engl. J. Med. 2012, 366, 1227–1239. [Google Scholar] [CrossRef]
- Monami, M.; Cignarelli, A.; Pinto, S.; D’onofrio, L.; Milluzzo, A.; Miccoli, R.; Penno, G.; Mannucci, E. Alpha-Tocopherol and Contrast-Induced Nephropathy: A Meta-Analysis of Randomized Controlled Trials. Int. J. Vitam. Nutr. Res. 2021, 91, 188–196. [Google Scholar] [CrossRef]
- Milluzzo, A.; Barchitta, M.; Maugeri, A.; Agodi, A.; Sciacca, L. Body Mass Index Is Related to Short Term Retinal Worsening in Type 2 Diabetes Patients Treated with Anticancer Drugs. Minerva Endocrinol. 2022. online ahead of print. [Google Scholar] [CrossRef]
- Das, A.; McGuire, P.G.; Rangasamy, S. Diabetic Macular Edema: Pathophysiology and Novel Therapeutic Targets. Ophthalmology 2015, 122, 1375–1394. [Google Scholar] [CrossRef]
- Tyrberg, M.; Nyström, L.; Arnqvist, H.J.; Bolinder, J.; Gudbjörnsdottir, S.; Landin-Olsson, M.; Eriksson, J.W.; Svensson, M.K. Overweight, Hyperglycemia and Tobacco Use Are Modifiable Risk Factors for Onset of Retinopathy 9 and 17 Years after the Diagnosis of Diabetes—A Retrospective Observational Nation-Wide Cohort Study. Diabetes Res. Clin. Pract. 2017, 133, 21–29. [Google Scholar] [CrossRef]
- Aro, A.; Kauppinen, A.; Summanen, P.; Kivinen, N.; Selander, T.; Kinnunen, K.; Tuomilehto, J.; Keinänen-Kiukaanniemi, S.; Lindström, J.; Uusitupa, M.; et al. Life Style Intervention Improves Retinopathy Status—The Finnish Diabetes Prevention Study. Nutrients 2019, 11, 1691. [Google Scholar] [CrossRef]
- Valle, M.S.; Russo, C.; Malaguarnera, L. Protective Role of Vitamin D against Oxidative Stress in Diabetic Retinopathy. Diabetes Metab. Res. Rev. 2021, 37, e3447. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Wang, P.; Airen, S.; Brown, C.; Liu, Z.; Townsend, J.H.; Wang, J.; Jiang, H. Nutritional and Medical Food Therapies for Diabetic Retinopathy. Eye Vis. 2020, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews Interventions, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Parisi, V.; Ziccardi, L.; Barbano, L.; Giorno, P.; Varano, M.; Parravano, M. Citicoline and Vitamin B12 Eye Drops in Type 1 Diabetes: Results of a 36-Month Pilot Study Evaluating Macular Electrophysiological Changes. Adv. Ther. 2021, 38, 3924–3936. [Google Scholar] [CrossRef]
- Parravano, M.; Scarinci, F.; Parisi, V.; Giorno, P.; Giannini, D.; Oddone, F.; Varano, M. Citicoline and Vitamin B12 Eye Drops in Type 1 Diabetes: Results of a 3-Year Pilot Study Evaluating Morpho-Functional Retinal Changes. Adv. Ther. 2020, 37, 1646–1663. [Google Scholar] [CrossRef]
- Liu, Z.; Jiang, H.; Townsend, J.H.; Wang, J. Improved Conjunctival Microcirculation in Diabetic Retinopathy Patients with MTHFR Polymorphisms after OcufolinTM Administration. Microvasc. Res. 2020, 132, 104066. [Google Scholar] [CrossRef]
- Liu, Z.; Jiang, H.; Townsend, J.H.; Wang, J. Effects of Ocufolin on Retinal Microvasculature in Patients with Mild Non-Proliferative Diabetic Retinopathy Carrying Polymorphisms of the MTHFR Gene. BMJ Open Diabetes Res. Care 2021, 9, e002327. [Google Scholar] [CrossRef]
- Smolek, M.; Notaroberto, N.F.; Jaramillo, A.G.; Pradillo, L.R. Intervention with Vitamins in Patients with Nonproliferative Diabetic Retinopathy: A Pilot Study. Clin. Ophthalmol. 2013, 7, 1451–1458. [Google Scholar] [CrossRef]
- Ellis, J.M.; Folkers, K.; Minadeo, M.; VanBuskirk, R.; Xia, L.-J.; Tamagawa, H. A Deficiency of Vitamin B6 Is a Plausible Molecular Basis of the Retinopathy of Patients with Diabetes Mellitus. Biochem. Biophys. Res. Commun. 1991, 179, 615–619. [Google Scholar] [CrossRef]
- Bursell, S.E.; Clermont, A.C.; Aiello, L.P.; Aiello, L.M.; Schlossman, D.K.; Feener, E.P.; Laffel, L.; King, G.L. High-Dose Vitamin E Supplementation Normalizes Retinal Blood Flow and Creatinine Clearance in Patients with Type 1 Diabetes. Diabetes Care 1999, 22, 1245–1251. [Google Scholar] [CrossRef] [PubMed]
- Chatziralli, I.P.; Theodossiadis, G.; Dimitriadis, P.; Charalambidis, M.; Agorastos, A.; Migkos, Z.; Platogiannis, N.; Moschos, M.M.; Theodossiadis, P.; Keryttopoulos, P. The Effect of Vitamin E on Oxidative Stress Indicated by Serum Malondialdehyde in Insulin-Dependent Type 2 Diabetes Mellitus Patients with Retinopathy. Open Ophthalmol. J. 2017, 11, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Domanico, D.; Fragiotta, S.; Cutini, A.; Carnevale, C.; Zompatori, L.; Vingolo, E. Circulating Levels of Reactive Oxygen Species in Patients with Nonproliferative Diabetic Retinopathy and the Influence of Antioxidant Supplementation: 6-Month Follow-Up. Indian J. Ophthalmol. 2015, 63, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Chous, A.P.; Richer, S.P.; Gerson, J.D.; Kowluru, R.A. The Diabetes Visual Function Supplement Study (DiVFuSS). Br. J. Ophthalmol. 2016, 100, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Britten-Jones, A.C.; Kamel, J.T.; Roberts, L.J.; Braat, S.; Craig, J.P.; MacIsaac, R.J.; Downie, L.E. Investigating the Neuroprotective Effect of Oral Omega-3 Fatty Acid Supplementation in Type 1 Diabetes (NPROOFS1): A Randomized Placebo-Controlled Trial. Diabetes 2021, 70, 1794–1806. [Google Scholar] [CrossRef] [PubMed]
- Kheirouri, S.; Naghizadeh, S.; Alizadeh, M. Zinc Supplementation Does Not Influence Serum Levels of VEGF, BDNF, and NGF in Diabetic Retinopathy Patients: A Randomized Controlled Clinical Trial. Nutr. Neurosci. 2019, 22, 718–724. [Google Scholar] [CrossRef]
- Filippelli, M.; Campagna, G.; Vito, P.; Zotti, T.; Ventre, L.; Rinaldi, M.; Bartollino, S.; dell’Omo, R.; Costagliola, C. Anti-Inflammatory Effect of Curcumin, Homotaurine, and Vitamin D3 on Human Vitreous in Patients with Diabetic Retinopathy. Front. Neurol. 2021, 11, 592274. [Google Scholar] [CrossRef]
- Sepahi, S.; Mohajeri, S.A.; Hosseini, S.M.; Khodaverdi, E.; Shoeibi, N.; Namdari, M.; Tabassi, S.A.S. Effects of Crocin on Diabetic Maculopathy: A Placebo-Controlled Randomized Clinical Trial. Am. J. Ophthalmol. 2018, 190, 89–98. [Google Scholar] [CrossRef]
- Ahmed, L.H.M.; Butler, A.E.; Dargham, S.R.; Latif, A.; Chidiac, O.M.; Atkin, S.L.; Abi Khalil, C. Vitamin D3 Metabolite Ratio as an Indicator of Vitamin D Status and Its Association with Diabetes Complications. BMC Endocr. Disord. 2020, 20, 161. [Google Scholar] [CrossRef]
- Butler, A.E.; Dargham, S.R.; Latif, A.; Mokhtar, H.R.; Robay, A.; Chidiac, O.M.; Jayyousi, A.; al Suwaidi, J.; Crystal, R.G.; Abi Khalil, C.; et al. Association of Vitamin D 3 and Its Metabolites in Patients with and without Type 2 Diabetes and Their Relationship to Diabetes Complications. Ther. Adv. Chronic Dis. 2020, 11, 204062232092415. [Google Scholar] [CrossRef] [PubMed]
- Chonchol, M.; Cigolini, M.; Targher, G. Association between 25-Hydroxyvitamin D Deficiency and Cardiovascular Disease in Type 2 Diabetic Patients with Mild Kidney Dysfunction. Nephrol. Dial. Transplant. 2007, 23, 269–274. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gungor, A.; Ates, O.; Bilen, H.; Kocer, I. Retinal Nerve Fiber Layer Thickness in Early-Stage Diabetic Retinopathy with Vitamin D Deficiency. Investig. Opthalmol. Vis. Sci. 2015, 56, 6433–6437. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, M.; Sullivan, D.R.; Veillard, A.-S.; McCorquodale, T.; Straub, I.R.; Scott, R.; Laakso, M.; Topliss, D.; Jenkins, A.J.; Blankenberg, S.; et al. Serum 25-Hydroxyvitamin D: A Predictor of Macrovascular and Microvascular Complications in Patients with Type 2 Diabetes. Diabetes Care 2015, 38, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, S.; Singh, R.; Dwivedi, N.; Singh, K.; Gupta, A.; Mathur, M. Vitamin D Levels and Microvascular Complications in Type 2 Diabetes. Indian J. Endocrinol. Metab. 2014, 18, 537. [Google Scholar] [CrossRef]
- Ahmadieh, H.; Azar, S.T.; Lakkis, N.; Arabi, A. Hypovitaminosis D in Patients with Type 2 Diabetes Mellitus: A Relation to Disease Control and Complications. ISRN Endocrinol. 2013, 2013, 641098. [Google Scholar] [CrossRef]
- Kaur, H.; Donaghue, K.C.; Chan, A.K.; Benitez-Aguirre, P.; Hing, S.; Lloyd, M.; Cusumano, J.; Pryke, A.; Craig, M.E. Vitamin D Deficiency Is Associated with Retinopathy in Children and Adolescents with Type 1 Diabetes. Diabetes Care 2011, 34, 1400–1402. [Google Scholar] [CrossRef]
- Ahmed, L.H.M.; Butler, A.E.; Dargham, S.R.; Latif, A.; Robay, A.; Chidiac, O.M.; Jayyousi, A.; al Suwaidi, J.; Crystal, R.G.; Atkin, S.L.; et al. Association of Vitamin D2 and D3 with Type 2 Diabetes Complications. BMC Endocr. Disord. 2020, 20, 65. [Google Scholar] [CrossRef]
- Zhao, W.-J.; Xia, X.-Y.; Yin, J. Relationship of Serum Vitamin D Levels with Diabetic Microvascular Complications in Patients with Type 2 Diabetes Mellitus. Chin. Med. J. 2021, 134, 814–820. [Google Scholar] [CrossRef]
- Millen, A.E.; Klein, R.; Folsom, A.R.; Stevens, J.; Palta, M.; Mares, J.A. Relation between Intake of Vitamins C and E and Risk of Diabetic Retinopathy in the Atherosclerosis Risk in Communities Study. Am. J. Clin. Nutr. 2004, 79, 865–873. [Google Scholar] [CrossRef]
- Millen, A.E.; Gruber, M.; Klein, R.; Klein, B.E.K.; Palta, M.; Mares, J.A. Relations of Serum Ascorbic Acid and α-Tocopherol to Diabetic Retinopathy in the Third National Health and Nutrition Examination Survey. Am. J. Epidemiol. 2003, 158, 225–233. [Google Scholar] [CrossRef] [PubMed]
- She, C.; Shang, F.; Cui, M.; Yang, X.; Liu, N. Association between Dietary Antioxidants and Risk for Diabetic Retinopathy in a Chinese Population. Eye 2021, 35, 1977–1984. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Guo, W.; Jia, Y.; Xu, J. Serum and Urinary Selenium Status in Patients with the Pre-Diabetes and Diabetes in Northeast China. Biol. Trace Elem. Res. 2019, 191, 61–69. [Google Scholar] [CrossRef]
- Fahmy, R.; Almutairi, N.M.; Al-Muammar, M.N.; Bhat, R.S.; Moubayed, N.; El-Ansary, A. Controlled Diabetes Amends Oxidative Stress as Mechanism Related to Severity of Diabetic Retinopathy. Sci. Rep. 2021, 11, 17670. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, Z.; Ucgun, N.I.; Kilic, N.; Gursel, E.; Sepici-Dincel, A. Antioxidant Enzymes and Diabetic Retinopathy. Ann. N. Y. Acad. Sci. 2007, 1100, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Hattenbach, L.-O.; Allers, A.; Klais, C.; Koch, F.; Hecker, M. L-Arginine-Nitric Oxide Pathway-Related Metabolites in the Aqueous Humor of Diabetic Patients. Investig. Ophthalmol. Vis. Sci. 2000, 41, 213–217. [Google Scholar]
- Pękala-Wojciechowska, A.; Poznański, M.; Szyszow, K.; Antczak, A. Concentration of 8-Isoprostanes in the Exhaled Breath Condensate as a Marker of Oxidative Stress in Patients with Type 1 Diabetes. Adv. Respir. Med. 2018, 86, 3–6. [Google Scholar] [CrossRef]
- Srivastav, K.; Saxena, S.; Mahdi, A.A.; Shukla, R.K.; Meyer, C.H.; Akduman, L.; Khanna, V.K. Increased Serum Level of Homocysteine Correlates with Retinal Nerve Fiber Layer Thinning in Diabetic Retinopathy. Mol. Vis. 2016, 22, 1352–1360. [Google Scholar]
- Cinici, E.; Dilekmen, N.; Senol, O.; Arpalı, E.; Cinici, O.; Tanas, S. Blood Thiamine Pyrophosphate Concentration and Its Correlation with the Stage of Diabetic Retinopathy. Int. Ophthalmol. 2020, 40, 3279–3284. [Google Scholar] [CrossRef]
- Satyanarayana, A.; Balakrishna, N.; Pitla, S.; Reddy, P.Y.; Mudili, S.; Lopamudra, P.; Suryanarayana, P.; Viswanath, K.; Ayyagari, R.; Reddy, G.B. Status of B-Vitamins and Homocysteine in Diabetic Retinopathy: Association with Vitamin-B12 Deficiency and Hyperhomocysteinemia. PLoS ONE 2011, 6, e26747. [Google Scholar] [CrossRef]
- Horikawa, C.; Aida, R.; Kamada, C.; Fujihara, K.; Tanaka, S.; Tanaka, S.; Araki, A.; Yoshimura, Y.; Moriya, T.; Akanuma, Y.; et al. Vitamin B6 Intake and Incidence of Diabetic Retinopathy in Japanese Patients with Type 2 Diabetes: Analysis of Data from the Japan Diabetes Complications Study (JDCS). Eur. J. Nutr. 2020, 59, 1585–1594. [Google Scholar] [CrossRef] [PubMed]
- Malaguarnera, G.; Gagliano, C.; Salomone, S.; Giordano, M.; Bucolo, C.; Pappalardo, A.; Caraci, F.; Drago, F.; Avitabile, T.; Motta, M. Folate Status in Type 2 Diabetic Patients with and without Retinopathy. Clin. Ophthalmol. 2015, 9, 1437–1442. [Google Scholar] [CrossRef] [PubMed]
- De Luis, D.A.; Fernandez, N.; Arranz, M.L.; Aller, R.; Izaola, O.; Romero, E. Total Homocysteine Levels Relation with Chronic Complications of Diabetes, Body Composition, and Other Cardiovascular Risk Factors in a Population of Patients with Diabetes Mellitus Type 2. J. Diabetes Complicat. 2005, 19, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Hikichi, T.; Tateda, N.; Miura, T. Alteration of Melatonin Secretion in Patients with Type 2 Diabetes and Proliferative Diabetic Retinopathy. Clin. Ophthalmol. 2011, 5, 655–660. [Google Scholar] [CrossRef]
- Ba-Ali, S.; Brøndsted, A.E.; Andersen, H.U.; Sander, B.; Jennum, P.J.; Lund-Andersen, H. Assessment of Diurnal Melatonin, Cortisol, Activity, and Sleep–wake Cycle in Patients with and without Diabetic Retinopathy. Sleep Med. 2019, 54, 35–42. [Google Scholar] [CrossRef]
- Poorabbas, A.; Fallah, F.; Bagdadchi, J.; Mahdavi, R.; Aliasgarzadeh, A.; Asadi, Y.; Koushavar, H.; Vahed Jabbari, M. Determination of Free L-Carnitine Levels in Type II Diabetic Women with and without Complications. Eur. J. Clin. Nutr. 2007, 61, 892–895. [Google Scholar] [CrossRef]
- Senyigit, A. The Association between 25-Hydroxy Vitamin D Deficiency and Diabetic Complications in Patients with Type 2 Diabetes Mellitus. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 1381–1386. [Google Scholar] [CrossRef]
- Fung, T.H.; Patel, B.; Wilmot, E.G.; Amoaku, W.M. Diabetic Retinopathy for the Non-Ophthalmologist. Clin. Med. 2022, 22, 112–116. [Google Scholar] [CrossRef]
- Bunch, K.L.; Abdelrahman, A.A.; Caldwell, R.B.; Caldwell, R.W. Novel Therapeutics for Diabetic Retinopathy and Diabetic Macular Edema: A Pathophysiologic Perspective. Front. Physiol. 2022, 13, 831616. [Google Scholar] [CrossRef]
- Reddy, S.S.; Prabhakar, Y.K.; Kumar, C.U.; Reddy, P.Y.; Reddy, G.B. Effect of Vitamin B12 Supplementation on Retinal Lesions in Diabetic Rats. Mol. Vis. 2020, 26, 311–325. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milluzzo, A.; Barchitta, M.; Maugeri, A.; Magnano San Lio, R.; Favara, G.; Mazzone, M.G.; Sciacca, L.; Agodi, A. Do Nutrients and Nutraceuticals Play a Role in Diabetic Retinopathy? A Systematic Review. Nutrients 2022, 14, 4430. https://doi.org/10.3390/nu14204430
Milluzzo A, Barchitta M, Maugeri A, Magnano San Lio R, Favara G, Mazzone MG, Sciacca L, Agodi A. Do Nutrients and Nutraceuticals Play a Role in Diabetic Retinopathy? A Systematic Review. Nutrients. 2022; 14(20):4430. https://doi.org/10.3390/nu14204430
Chicago/Turabian StyleMilluzzo, Agostino, Martina Barchitta, Andrea Maugeri, Roberta Magnano San Lio, Giuliana Favara, Maria Grazia Mazzone, Laura Sciacca, and Antonella Agodi. 2022. "Do Nutrients and Nutraceuticals Play a Role in Diabetic Retinopathy? A Systematic Review" Nutrients 14, no. 20: 4430. https://doi.org/10.3390/nu14204430
APA StyleMilluzzo, A., Barchitta, M., Maugeri, A., Magnano San Lio, R., Favara, G., Mazzone, M. G., Sciacca, L., & Agodi, A. (2022). Do Nutrients and Nutraceuticals Play a Role in Diabetic Retinopathy? A Systematic Review. Nutrients, 14(20), 4430. https://doi.org/10.3390/nu14204430









