Targeting the Platelet-Activating Factor Receptor (PAF-R): Antithrombotic and Anti-Atherosclerotic Nutrients
Abstract
1. Introduction
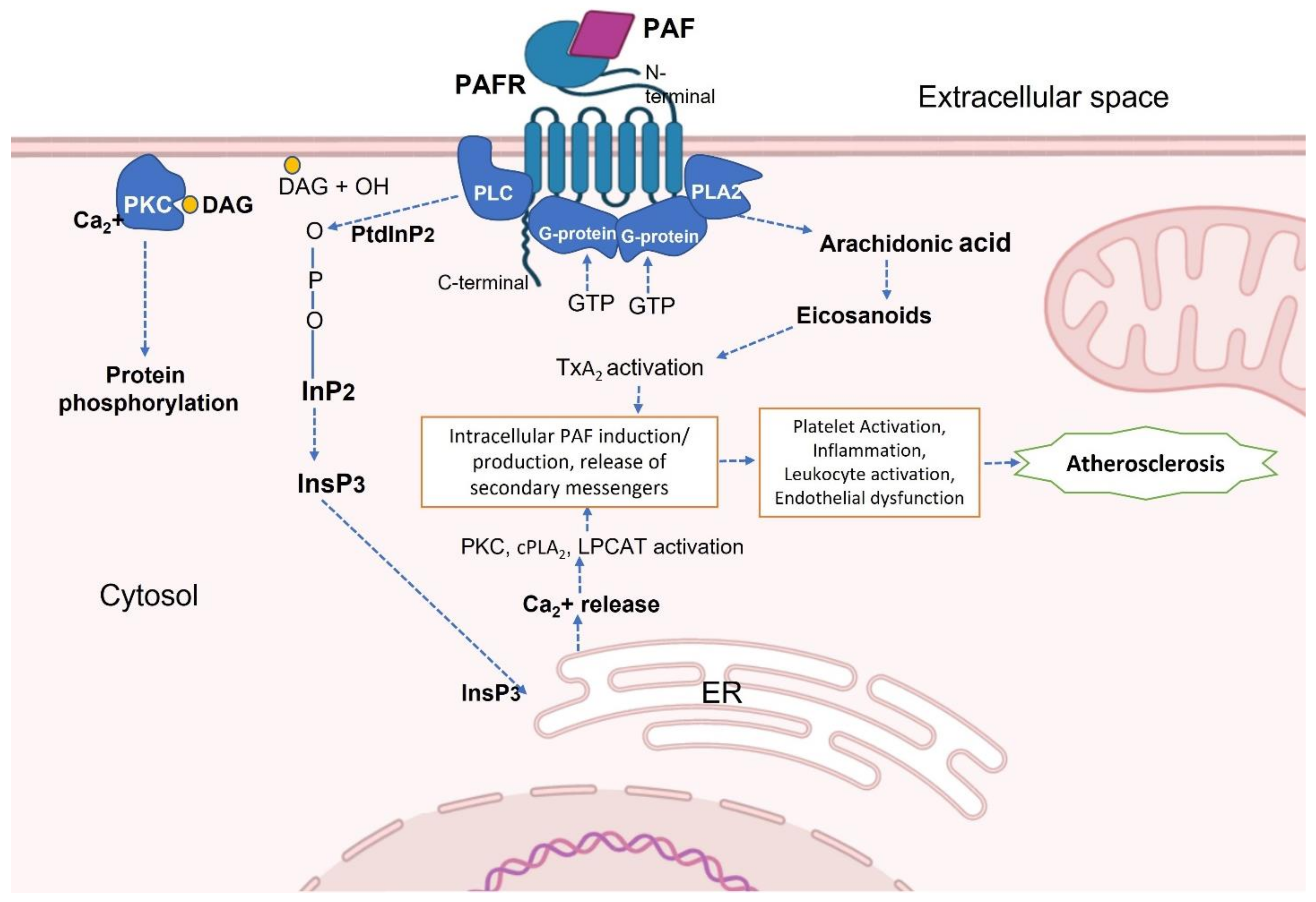
2. Platelet-Activating Factor (PAF) and PAF-Receptor (PAF-R)
PAF and PAF-R Activation in Inflammatory Diseases
3. Antiplatelet Properties of Nutrients
4. Antiplatelet Properties of Polar Lipids
4.1. In Vitro Studies of Platelet-Activating Factor Receptor (PAF-R) Antagonists
4.2. Ex Vivo and Human Studies
4.3. PAF Modulation by Micronutrients
| Micronutrient | Study Aim | Study Type | Result |
|---|---|---|---|
| Vitamin C | Effect of vitamin C on the release of PAF and PAF-like phospholipids during reperfusion injury. | In vivo | Vitamin C attenuated oxidative stress and reduced PAF and PAF-like lipid levels in rabbits [173]. |
| Vitamin D | Study the effect of vitamin D supplementation in volunteers with Type 2 diabetes in a placebo-controlled trial. | Ex vivo | Six months of vitamin D supplementation decreased platelet activation and inflammatory markers such as IL-18, TNF-α and IFN-γ [165]. |
| Vitamin D | Study the inhibitory effect of paricalcitol against PAF and thrombin-induced platelet aggregation | In vitro | Addition of paricalcitol effectively inhibited platelet aggregation as well as modulating the activity of metabolic enzymes PAF-CPT and PAF-AH in platelets and leukocytes [169]. |
| Vitamin E | Establish the role of vitamin E (alpha-tocopherol) during pregnancy in platelet function | In vivo | Vitamin E supplementation almost completely inhibited platelet aggregation in presence of PAF and ADP, with very high inhibition observed in the brush border membrane vesicles [161]. |
| Selenium (Se) | Investigate the mechanism by which selenium modulates PAF production in endothelial cells | In vitro | Selenium deficiency reduces PAF biosynthesis in bovine endothelial cells by downregulating the activity of anabolic enzymes [174]. |
| Zinc (Zn) | Consequences of abnormal Zn storage and release in mouse platelets | In vivo | Ionic Zn2+ accumulated in secretory granules is released upon platelet activation and has a procoagulant effect [175]. |
| Copper (Cu) | Role of dietary copper in platelet activation using rat models | In vivo | Platelet aggregation induced by ADP is significantly higher in copper-deficient rats compared to rats with an adequate amount of copper in their diet [176]. |
5. Importance of Essential Trace Metals on PAF-R Targets
6. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Barquera, S.; Pedroza-Tobías, A.; Medina, C.; Hernández-Barrera, L.; Bibbins-Domingo, K.; Lozano, R.; Moran, A.E. Global Overview of the Epidemiology of Atherosclerotic Cardiovascular Disease. Arch. Med. Res. 2015, 46, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Yu, E.; Malik, V.S.; Hu, F.B. Cardiovascular disease prevention by diet modification: JACC Health Promotion Series. J. Am. Coll. Cardiol. 2018, 72, 914–926. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Appel, L.J.; Van Horn, L. Components of a cardioprotective diet new insights. Circulation 2011, 123, 2870–2891. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. The changing landscape of atherosclerosis. Nature 2021, 592, 524–533. [Google Scholar] [CrossRef]
- Libby, P.; Ridker, P.M.; Maseri, A. Inflammation and atherosclerosis. Circulation 2002, 105, 1135–1143. [Google Scholar] [CrossRef]
- Jebari-Benslaiman, S.; Galicia-García, U.; Larrea-Sebal, A.; Olaetxea, J.R.; Alloza, I.; Vandenbroeck, K.; Benito-Vicente, A.; Martín, C. Pathophysiology of Atherosclerosis. Int. J. Mol. Sci. 2022, 23, 3346. [Google Scholar] [CrossRef]
- Tsoupras, A.; Lordan, R.; Zabetakis, I. Inflammation, not Cholesterol, Is a Cause of Chronic Disease. Nutrients 2018, 10, 604. [Google Scholar] [CrossRef]
- Davignon, J.; Ganz, P. Role of endothelial dysfunction in atherosclerosis. Circulation 2004, 109, III-27–III-32. [Google Scholar] [CrossRef]
- Nording, H.M.; Seizer, P.; Langer, H.F. Platelets in inflammation and atherogenesis. Front. Immunol. 2015, 6. [Google Scholar] [CrossRef]
- Lindemann, S.; KrÄMer, B.; Seizer, P.; Gawaz, M. Platelets, inflammation and atherosclerosis. J. Thromb. Haemost. 2007, 5, 203–211. [Google Scholar] [CrossRef]
- Massberg, S.; Brand, K.; Grüner, S.; Page, S.; Müller, E.; Müller, I.; Bergmeier, W.; Richter, T.; Lorenz, M.; Konrad, I.; et al. A critical role of platelet adhesion in the initiation of atherosclerotic lesion formation. J. Exp. Med. 2002, 196, 887–896. [Google Scholar] [CrossRef]
- Huo, Y.; Schober, A.; Forlow, S.B.; Smith, D.F.; Hyman, M.C.; Jung, S.; Littman, D.R.; Weber, C.; Ley, K. Circulating activated platelets exacerbate atherosclerosis in mice deficient in apolipoprotein E. Nat. Med. 2002, 9, 61. [Google Scholar] [CrossRef]
- Lordan, R.; Tsoupras, A.; Zabetakis, I. Platelet activation and prothrombotic mediators at the nexus of inflammation and atherosclerosis: Potential role of antiplatelet agents. Blood Rev. 2020, 45, 100694. [Google Scholar] [CrossRef]
- Huilcaman, R.; Venturini, W.; Fuenzalida, L.; Cayo, A.; Segovia, R.; Valenzuela, C.; Brown, N.; Moore-Carrasco, R. Platelets, a Key Cell in Inflammation and Atherosclerosis Progression. Cells 2022, 11, 1014. [Google Scholar] [CrossRef]
- Tomaiuolo, M.; Brass, L.F.; Stalker, T.J. Regulation of Platelet Activation and Coagulation and Its Role in Vascular Injury and Arterial Thrombosis. Interv. Cardiol. Clin. 2017, 6, 1–12. [Google Scholar] [CrossRef]
- Lordan, R.; Tsoupras, A.; Zabetakis, I. Investigation of Platelet Aggregation in Atherosclerosis. In Atherosclerosis: Methods and Protocols; Ramji, D., Ed.; Springer: New York, NY, USA, 2022; pp. 333–347. [Google Scholar] [CrossRef]
- Lordan, R.; Tsoupras, A.; Zabetakis, I. The Potential Role of Dietary Platelet-Activating Factor Inhibitors in Cancer Prevention and Treatment. Adv. Nutr. 2019, 10, 148–164. [Google Scholar] [CrossRef]
- Papakonstantinou, V.D.; Lagopati, N.; Tsilibary, E.C.; Demopoulos, C.A.; Philippopoulos, A.I. A Review on Platelet Activating Factor Inhibitors: Could a New Class of Potent Metal-Based Anti-Inflammatory Drugs Induce Anticancer Properties? Bioinorg. Chem. Appl. 2017, 2017, 6947034. [Google Scholar] [CrossRef]
- Nomikos, T.; Fragopoulou, E.; Antonopoulou, S.; Panagiotakos, D.B. Mediterranean diet and platelet-activating factor; a systematic review. Clin. Biochem. 2018, 60, 1–10. [Google Scholar] [CrossRef]
- English, C.J.; Mayr, H.L.; Lohning, A.E.; Reidlinger, D.P. The association between dietary patterns and the novel inflammatory markers platelet-activating factor and lipoprotein-associated phospholipase A2: A systematic review. Nutr. Rev. 2022, 80, 1371–1391. [Google Scholar] [CrossRef]
- Benveniste, J.; Henson, P.M.; Cochrane, C.G. Leukocyte-dependent histamine release from rabbit platelets: The role of IgE, basophils, and a platelet-activating factor. J. Exp. Med. 1972, 136, 1356–1377. [Google Scholar] [CrossRef]
- Demopoulos, C.A.; Antonopoulou, S. A discovery trip to compounds with PAF-like activity. In Platelet-Activating Factor and Related Lipid Mediators 2; Springer: Berlin/Heidelberg, Germany, 1996; pp. 59–63. [Google Scholar]
- Demopoulos, C.; Pinckard, R.; Hanahan, D.J. Platelet-activating factor. Evidence for 1-O-alkyl-2-acetyl-sn-glyceryl-3-phosphorylcholine as the active component (a new class of lipid chemical mediators). J. Biol. Chem. 1979, 254, 9355–9358. [Google Scholar] [CrossRef]
- Upton, J.E.M.; Grunebaum, E.; Sussman, G.; Vadas, P. Platelet Activating Factor (PAF): A Mediator of Inflammation. Biofactors 2022. [Google Scholar] [CrossRef]
- Antonopoulou, S.; Nomikos, T.; Karantonis, H.; Fragopoulou, E.; Demopoulos, C.A. PAF, a potent lipid mediator. In Bioactive Phospholipids: Role in Inflammation and Atherosclerosis; Tselepis, A.D., Ed.; Transworld Research Network: Kerala, India, 2008; pp. 85–134. [Google Scholar]
- Zimmerman, G.A.; McIntyre, T.M.; Prescott, S.M.; Stafforini, D.M. The platelet-activating factor signaling system and its regulators in syndromes of inflammation and thrombosis. Crit. Care Med. 2002, 30, S294–S301. [Google Scholar] [CrossRef]
- Prescott, S.M.; Zimmerman, G.A.; Stafforini, D.M.; McIntyre, T.M. Platelet-activating factor and related lipid mediators. Annu. Rev. Biochem. 2000, 69, 419–445. [Google Scholar] [CrossRef]
- Lordan, R.; Tsoupras, A.; Zabetakis, I.; Demopoulos, A.C. Forty years since the structural elucidation of platelet-activating factor (PAF): Historical, current, and future research perspectives. Molecules 2019, 24, 4414. [Google Scholar] [CrossRef]
- Senanayake, V.; Goodenowe, D.B. Plasmalogen deficiency and neuropathology in Alzheimer’s disease: Causation or coincidence? Alzheimer’s Dement. Transl. Res. Clin. Interv. 2019, 5, 524–532. [Google Scholar] [CrossRef]
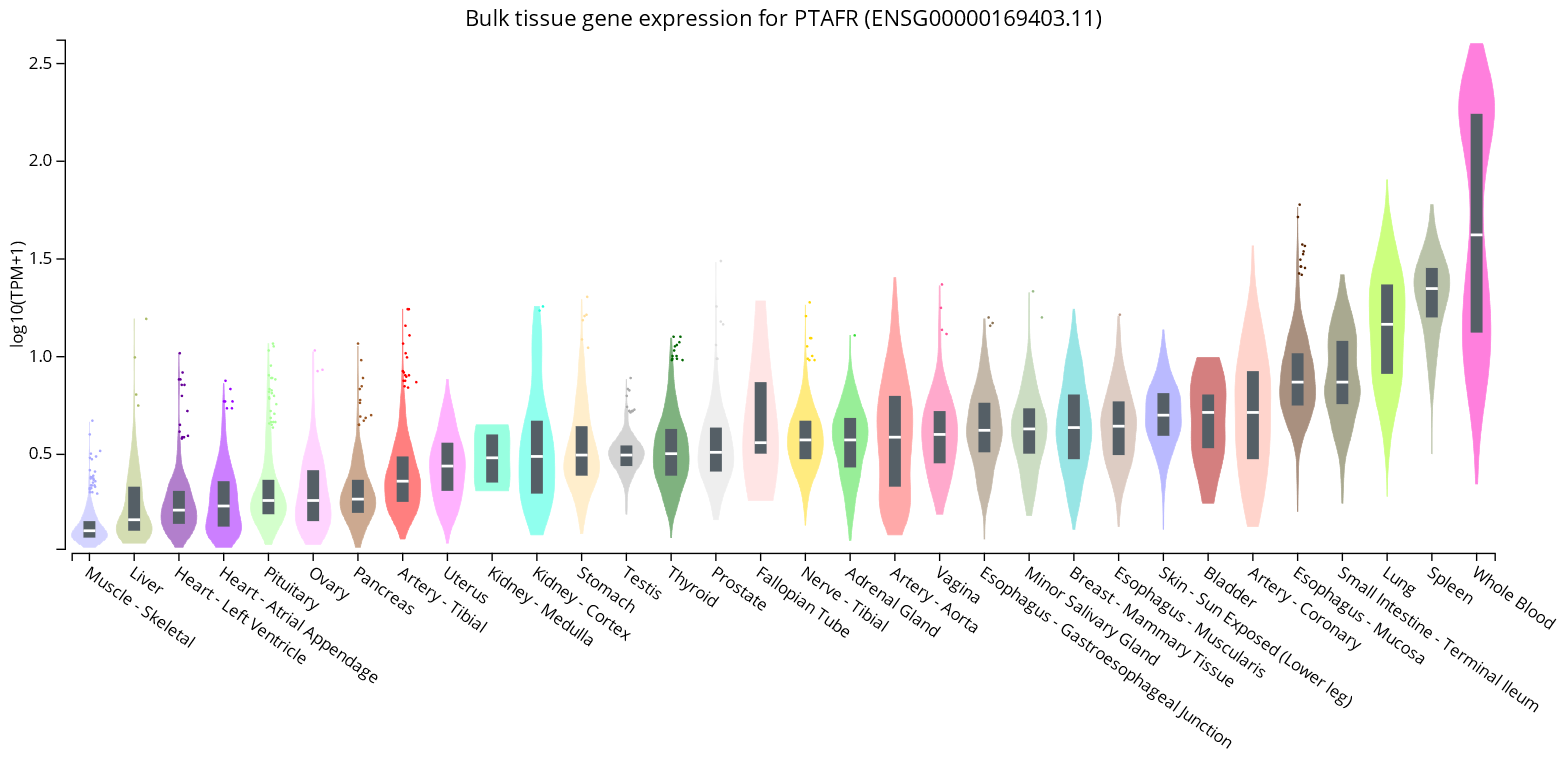
- GTExPortal. Bulk Tissue Gene Expression for PTAFR (ENSG00000169403.11). Available online: https://www.gtexportal.org/home/gene/PTAFR (accessed on 15 October 2022).
- Farooqui, F.A.F.T.; Horrocks, L.A. Roles of Platelet-Activating Factor in Brain. In Metabolism and Functions of Bioactive Ether Lipids in the Brain; Springer: New York, NY, USA, 2008; pp. 171–195. [Google Scholar] [CrossRef]
- Brailoiu, E.; Barlow, C.L.; Ramirez, S.H.; Abood, M.E.; Brailoiu, G.C. Effects of Platelet-Activating Factor on Brain Microvascular Endothelial Cells. Neuroscience 2018, 377, 105–113. [Google Scholar] [CrossRef]
- Angle, M.J.; Tom, R.; Jarvi, K.; McClure, R.D. Effect of platelet-activating factor (PAF) on human spermatozoa–oocyte interactions. Reproduction 1993, 98, 541–548. [Google Scholar] [CrossRef]
- Minhas, B.S.; Kumar, R.; Ricker, D.D.; Roudebush, W.E.; Dodson, M.G.; Fortunato, S.J. Effects of platelet activating factor on mouse oocyte fertilization in vitro. Am. J. Obstet. Gynecol. 1989, 161, 1714–1717. [Google Scholar] [CrossRef]
- Sakellariou, M.; Drakakis, P.; Antonopoulou, S.; Anagnostou, E.; Loutradis, D.; Patargias, T. Intravenous infusion of PAF affects ovulation, fertilization and preimplantation embryonic development in NZB x NZW F1 hybrid mice. Prostaglandins Other Lipid Mediat. 2008, 85, 125–133. [Google Scholar] [CrossRef]
- Lecewicz, M.; Kordan, W.; Majewska, A.; Kamiński, S.; Dziekońska, A.; Mietelska, K. Effects of the platelet-activating factor (PAF) on selected quality parameters of cryopreserved bull semen (AI) with reduced sperm motility. Pol. J. Vet. Sci. 2016, 19, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Tiemann, U. The Role of Platelet-activating Factor in the Mammalian Female Reproductive Tract. Reprod. Domest. Anim. 2008, 43, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Gao, J.; Wang, X.; Leung, T.Y.; Duan, Y.-G.; Chiu, P.C.N. Platelet-activating factor induces acrosome reaction via the activation of extracellular signal-regulated kinase in human spermatozoa. Andrologia 2020, 52, e13565. [Google Scholar] [CrossRef] [PubMed]
- Evangelou, A.M. Platelet-activating factor (PAF): Implications for coronary heart and vascular diseases. Prostaglandins Leukot. Essent. Fat. Acids 1994, 50, 1–28. [Google Scholar] [CrossRef]
- Montrucchio, G.; Alloatti, G.; Camussi, G. Role of Platelet-Activating Factor in Cardiovascular Pathophysiology. Physiol. Rev. 2000, 80, 1669–1699. [Google Scholar] [CrossRef]
- Tselepis, A.D.; Evangelou, A.; Tsoukatos, D.; Demopoulos, C.A.; Kapoulas, V.M. Electrocardiographic alterations induced by AGEPC in Wistar rats in relation to its hypotensive and hematologic effects. Comp. Biochem. Physiol. Part C Comp. Pharmacol. 1987, 87, 41–46. [Google Scholar] [CrossRef]
- Chignard, M.; Le Couedic, J.; Tence, M.; Vargaftig, B.; Benveniste, J. The role of platelet-activating factor in platelet aggregation. Nature 1979, 279, 799–800. [Google Scholar] [CrossRef]
- Chesney, C.; Pifer, D.; Byers, L.; Muirhead, E. Effect of platelet-activating factor (PAF) on human platelets. Blood 1982, 59, 582–585. [Google Scholar] [CrossRef]
- Melnikova, V.; Bar-Eli, M. Inflammation and melanoma growth and metastasis: The role of platelet-activating factor (PAF) and its receptor. Cancer Metastasis Rev. 2007, 26, 359–371. [Google Scholar] [CrossRef]
- Fan, G.J.; Kang, Y.H.; Han, Y.N.; Han, B.H. Platelet-activating factor (PAF) receptor binding antagonists from Alpinia officinarum. Bioorg. Med. Chem. Lett. 2007, 17, 6720–6722. [Google Scholar] [CrossRef]
- Souza Danielle, G.; Fagundes Caio, T.; Sousa Lirlandia, P.; Amaral Flavio, A.; Souza Rafael, S.; Souza Adriano, L.; Kroon Erna, G.; Sachs, D.; Cunha Fernando, Q.; Bukin, E.; et al. Essential role of platelet-activating factor receptor in the pathogenesis of Dengue virus infection. Proc. Natl. Acad. Sci. USA 2009, 106, 14138–14143. [Google Scholar] [CrossRef]
- Kelesidis, T.; Papakonstantinou, V.; Detopoulou, P.; Fragopoulou, E.; Chini, M.; Lazanas, M.C.; Antonopoulou, S. The role of platelet-activating factor in chronic inflammation, immune activation, and comorbidities associated with HIV infection. AIDS Rev. 2015, 17, 191. [Google Scholar]
- Theoharides, T.C.; Antonopoulou, S.; Demopoulos, C.A. Coronavirus 2019, Microthromboses, and Platelet Activating Factor. Clin. Ther. 2020, 42, 1850–1852. [Google Scholar] [CrossRef]
- Tsoupras, A.; Lordan, R.; Zabetakis, I. Thrombosis and COVID-19: The Potential role of nutrition. Front. Nutr. 2020, 7, 583080. [Google Scholar] [CrossRef]
- Honda, Z.I.; Ishii, S.; Shimizu, T. Platelet-Activating Factor Receptor. J. Biochem. 2002, 131, 773–779. [Google Scholar] [CrossRef]
- Stafforini, D.M.; McIntyre, T.M.; Zimmerman, G.A.; Prescott, S.M. Platelet-Activating Factor, a Pleiotrophic Mediator of Physiological and Pathological Processes. Crit. Rev. Clin. Lab. Sci. 2003, 40, 643–672. [Google Scholar] [CrossRef]
- Shimizu, T.; Mutoh, H.; Kato, S. Platelet-Activating Factor Receptor. In Platelet-Activating Factor and Related Lipid Mediators 2: Roles in Health and Disease; Nigam, S., Kunkel, G., Prescott, S.M., Eds.; Springer: Boston, MA, USA, 1996; pp. 79–84. [Google Scholar] [CrossRef]
- Mutoh, H.; Ishii, S.; Izumi, T.; Kato, S.; Shimizu, T. Platelet-Activating Factor (PAF) Positively Auto-Regulates the Expression of Human PAF Receptor Transcript 1 (Leukocyte-Type) Through NF-κB. Biochem. Biophys. Res. Commun. 1994, 205, 1137–1142. [Google Scholar] [CrossRef]
- Chao, W.; Olson, M.S. Platelet-activating factor: Receptors and signal transduction. Biochem. J. 1993, 292, 617–629. [Google Scholar] [CrossRef]
- Shukla, S.D. Platelet-activating factor receptor and signal transduction mechanisms 1. FASEB J. 1992, 6, 2296–2301. [Google Scholar] [CrossRef]
- Ishii, S.; Shimizu, T. Platelet-activating factor (PAF) receptor and genetically engineered PAF receptor mutant mice. Prog. Lipid Res. 2000, 39, 41–82. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera?A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Yost, C.C.; Weyrich, A.S.; Zimmerman, G.A. The platelet activating factor (PAF) signaling cascade in systemic inflammatory responses. Biochimie 2010, 92, 692–697. [Google Scholar] [CrossRef]
- Chung, K.F. LIPID MEDIATORS|Platelet-activating factors. In Encyclopedia of Respiratory Medicine; Laurent, G.J., Shapiro, S.D., Eds.; Academic Press: Oxford, UK, 2006; pp. 589–594. [Google Scholar] [CrossRef]
- Lordan, R.; Vidal, N.P.; Huong Pham, T.; Tsoupras, A.; Thomas, R.H.; Zabetakis, I. Yoghurt fermentation alters the composition and antiplatelet properties of milk polar lipids. Food Chem. 2020, 332, 127384. [Google Scholar] [CrossRef]
- Kajiwara, N.; Sasaki, T.; Bradding, P.; Cruse, G.; Sagara, H.; Ohmori, K.; Saito, H.; Ra, C.; Okayama, Y. Activation of human mast cells through the platelet-activating factor receptor. J. Allergy Clin. Immunol. 2010, 125, 1137–1145.e6. [Google Scholar] [CrossRef]
- Petersen, L.J.; Church, M.K.; Skov, P.S. Platelet-activating factor induces histamine release from human skin mast cells in vivo, which is reduced by local nerve blockade. J. Allergy Clin. Immunol. 1997, 99, 640–647. [Google Scholar] [CrossRef]
- Nilsson, G.; Metcalfe, D.D.; Taub, D.D. Demonstration that platelet-activating factor is capable of activating mast cells and inducing a chemotactic response. Immunology 2000, 99, 314–319. [Google Scholar] [CrossRef]
- Nakagome, K.; Nagata, M. Involvement and Possible Role of Eosinophils in Asthma Exacerbation. Front. Immunol. 2018, 9. [Google Scholar] [CrossRef]
- Kato, M.; Kita, H.; Tachibana, A.; Hayashi, Y.; Tsuchida, Y.; Kimura, H. Dual signaling and effector pathways mediate human eosinophil activation by platelet-activating factor. Int. Arch. Allergy Immunol. 2004, 134, 37–43. [Google Scholar] [CrossRef]
- Pałgan, K.; Bartuzi, Z. Platelet activating factor in allergies. Int. J. Immunopathol. Pharmacol. 2015, 28, 584–589. [Google Scholar] [CrossRef]
- Muñoz-Cano, R.M.; Casas-Saucedo, R.; Valero Santiago, A.; Bobolea, I.; Ribó, P.; Mullol, J. Platelet-Activating Factor (PAF) in Allergic Rhinitis: Clinical and Therapeutic Implications. J. Clin. Med. 2019, 8, 1338. [Google Scholar] [CrossRef]
- Nakagome, K.; Matsushita, S.; Nagata, M. Neutrophilic inflammation in severe asthma. Int. Arch. Allergy Immunol. 2012, 158, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Bélanger, C.; Elimam, H.; Lefebvre, J.; Borgeat, P.; Marleau, S. Involvement of endogenous leukotriene B4 and platelet-activating factor in polymorphonuclear leucocyte recruitment to dermal inflammatory sites in rats. Immunology 2008, 124, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Lotner, G.; Lynch, J.; Betz, S.; Henson, P. Human neutrophil-derived platelet activating factor. J. Immunol. 1980, 124, 676–684. [Google Scholar] [PubMed]
- Demopoulos, C.A.; Karantonis, H.C.; Antonopoulou, S. Platelet-activating factor—A molecular link between atherosclerosis theories. Eur. J. Lipid Sci. Technol. 2003, 105, 705–716. [Google Scholar] [CrossRef]
- Taggart, D.P. Neuroprotection during cardiac surgery: A randomised trial of a platelet activating factor antagonist. Heart 2003, 89, 897–900. [Google Scholar] [CrossRef]
- Kuitert, L.M.; Angus, R.M.; Barnes, N.C.; Barnes, P.J.; Bone, M.F.; Chung, K.F.; Fairfax, A.J.; Higenbotham, T.W.; O’Connor, B.J.; Piotrowska, B. Effect of a novel potent platelet-activating factor antagonist, modipafant, in clinical asthma. Am. J. Respir. Crit. Care Med. 1995, 151, 1331–1335. [Google Scholar] [CrossRef]
- Mullol, J.; Bousquet, J.; Bachert, C.; Canonica, W.G.; Gimenez-Arnau, A.; Kowalski, M.L.; Martí-Guadaño, E.; Maurer, M.; Picado, C.; Scadding, G.; et al. Rupatadine in allergic rhinitis and chronic urticaria. Allergy 2008, 63, 5–28. [Google Scholar] [CrossRef]
- Mullol, J.; Bousquet, J.; Bachert, C.; Canonica, G.W.; Giménez-Arnau, A.; Kowalski, M.L.; Simons, F.E.R.; Maurer, M.; Ryan, D.; Scadding, G. Update on rupatadine in the management of allergic disorders. Allergy 2015, 70, 1–24. [Google Scholar] [CrossRef]
- Deng, M.; Guo, H.; Tam, J.W.; Johnson, B.M.; Brickey, W.J.; New, J.S.; Lenox, A.; Shi, H.; Golenbock, D.T.; Koller, B.H.; et al. Platelet-activating factor (PAF) mediates NLRP3-NEK7 inflammasome induction independently of PAFR. J. Exp. Med. 2019, 216, 2838–2853. [Google Scholar] [CrossRef]
- Siervo, M.; Lara, J.; Chowdhury, S.; Ashor, A.; Oggioni, C.; Mathers, J.C. Effects of the Dietary Approach to Stop Hypertension (DASH) diet on cardiovascular risk factors: A systematic review and meta-analysis. Br. J. Nutr. 2015, 113, 1–15. [Google Scholar] [CrossRef]
- Widmer, R.J.; Flammer, A.J.; Lerman, L.O.; Lerman, A. The Mediterranean Diet, its Components, and Cardiovascular Disease. Am. J. Med. 2015, 128, 229–238. [Google Scholar] [CrossRef]
- Chopra, A.S.; Lordan, R.; Horbańczuk, O.K.; Atanasov, A.G.; Chopra, I.; Horbańczuk, J.O.; Jóźwik, A.; Huang, L.; Pirgozliev, V.; Banach, M.; et al. The current use and evolving landscape of nutraceuticals. Pharmacol. Res. 2022, 175, 106001. [Google Scholar] [CrossRef]
- Rabassa, M.; Hernandez Ponce, Y.; Garcia-Ribera, S.; Johnston, B.C.; Salvador Castell, G.; Manera, M.; Perez Rodrigo, C.; Aranceta-Bartrina, J.; Martínez-González, M.Á.; Alonso-Coello, P. Food-based dietary guidelines in Spain: An assessment of their methodological quality. Eur. J. Clin. Nutr. 2022, 76, 350–359. [Google Scholar] [CrossRef]
- Hassan, M.; Haq, S.M.; Majeed, M.; Umair, M.; Sahito, H.A.; Shirani, M.; Waheed, M.; Aziz, R.; Ahmad, R.; Bussmann, R.W. Traditional Food and Medicine: Ethno-Traditional Usage of Fish Fauna across the Valley of Kashmir: A Western Himalayan Region. Diversity 2022, 14, 455. [Google Scholar] [CrossRef]
- Ernst, E. The role of complementary and alternative medicine. BMJ 2000, 321, 1133. [Google Scholar] [CrossRef]
- Che, C.-T.; George, V.; Ijinu, T.; Pushpangadan, P.; Andrae-Marobela, K. Traditional medicine. In Pharmacognosy; Elsevier: Amsterdam, The Netherlands, 2017; pp. 15–30. [Google Scholar]
- Rajendran, H.; Deepika, S.; Immanuel, S.C. An Overview of Medicinal plants for Potential Cardio-Protective Activity. Res. J. Biotechnol. 2017, 4, 104–113. [Google Scholar]
- Kumar, S.; Joseph, L.; George, M.; Sharma, A. A review on anticoagulant/antithrombotic activity of natural plants used in traditional medicine. Int. J. Pharm. Sci. Rev. Res. 2011, 8, 70–74. [Google Scholar]
- Yoo, H.; Ku, S.-K.; Lee, W.; Kwak, S.; Baek, Y.-D.; Min, B.-W.; Jeong, G.-S.; Bae, J.-S. Antiplatelet, anticoagulant, and profibrinolytic activities of cudratricusxanthone A. Arch. Pharmacal Res. 2014, 37, 1069–1078. [Google Scholar] [CrossRef]
- Ku, S.-K.; Bae, J.-S. Antiplatelet, anticoagulant, and profibrinolytic activities of withaferin A. Vasc. Pharmacol. 2014, 60, 120–126. [Google Scholar] [CrossRef]
- Siritapetawee, J.; Thumanu, K.; Sojikul, P.; Thammasirirak, S. A novel serine protease with human fibrino(geno)lytic activities from Artocarpus heterophyllus latex. Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2012, 1824, 907–912. [Google Scholar] [CrossRef]
- Tsoupras, A.; O’Keeffe, E.; Lordan, R.; Redfern, S.; Zabetakis, I. Bioprospecting for Antithrombotic Polar Lipids from Salmon, Herring, and Boarfish By-Products. Foods 2019, 8, 416. [Google Scholar] [CrossRef]
- Tsoupras, A.; Lordan, R.; Shiels, K.; Saha, S.K.; Nasopoulou, C.; Zabetakis, I. In Vitro Antithrombotic Properties of Salmon (Salmo salar) Phospholipids in a Novel Food-Grade Extract. Mar. Drugs 2019, 17, 62. [Google Scholar] [CrossRef]
- Gavriil, L.; Detopoulou, M.; Petsini, F.; Antonopoulou, S.; Fragopoulou, E. Consumption of plant extract supplement reduces platelet activating factor-induced platelet aggregation and increases platelet activating factor catabolism: A randomised, double-blind and placebo-controlled trial. Br. J. Nutr. 2019, 121, 982–991. [Google Scholar] [CrossRef]
- Berge, J.P.; Debiton, E.; Dumay, J.; Durand, P.; Barthomeuf, C. In vitro anti-inflammatory and anti-proliferative activity of sulfolipids from the red alga Porphyridium cruentum. J. Agric Food Chem. 2002, 50, 6227–6232. [Google Scholar] [CrossRef]
- Shiels, K.; Tsoupras, A.; Lordan, R.; Zabetakis, I.; Murray, P.; Kumar Saha, S. Anti-inflammatory and antithrombotic properties of polar lipid extracts, rich in unsaturated fatty acids, from the Irish marine cyanobacterium Spirulina subsalsa. J. Funct. Foods 2022, 94, 105124. [Google Scholar] [CrossRef]
- Panayiotou, A.; Samartzis, D.; Nomikos, T.; Fragopoulou, E.; Karantonis, H.C.; Demopoulos, C.A.; Zabetakis, I. Lipid Fractions with Aggregatory and Antiaggregatory Activity toward Platelets in Fresh and Fried Cod (Gadus morhua): Correlation with Platelet-Activating Factor and Atherogenesis. J. Agric. Food Chem. 2000, 48, 6372–6379. [Google Scholar] [CrossRef]
- Lordan, R.; Walsh, A.; Crispie, F.; Finnegan, L.; Demuru, M.; Tsoupras, A.; Cotter, P.D.; Zabetakis, I. Caprine milk fermentation enhances the antithrombotic properties of cheese polar lipids. J. Funct. Foods 2019, 61, 103507. [Google Scholar] [CrossRef]
- Tsorotioti, S.E.; Nasopoulou, C.; Detopoulou, M.; Sioriki, E.; Demopoulos, C.A.; Zabetakis, I. In vitro anti-atherogenic properties of traditional Greek cheese lipid fractions. Dairy Sci. Technol. 2014, 94, 269–281. [Google Scholar] [CrossRef]
- Antonopoulou, S.; Detopoulou, M.; Fragopoulou, E.; Nomikos, T.; Mikellidi, A.; Yannakoulia, M.; Kyriacou, A.; Mitsou, E.; Panagiotakos, D.; Anastasiou, C. Consumption of yogurt enriched with polar lipids from olive oil by-products reduces platelet sensitivity against platelet activating factor and inflammatory indices: A randomized, double-blind clinical trial. Hum. Nutr. Metab. 2022, 28, 200145. [Google Scholar] [CrossRef]
- Shah, B.H.; Nawaz, Z.; Pertani, S.A.; Roomi, A.; Mahmood, H.; Saeed, S.A.; Gilani, A.H. Inhibitory effect of curcumin, a food spice from turmeric, on platelet-activating factor- and arachidonic acid-mediated platelet aggregation through inhibition of thromboxane formation and Ca2+ signaling. Biochem. Pharmacol. 1999, 58, 1167–1172. [Google Scholar] [CrossRef]
- Jantan, I.; Rafi, I.A.; Jalil, J. Platelet-activating factor (PAF) receptor-binding antagonist activity of Malaysian medicinal plants. Phytomedicine 2005, 12, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Sugatani, J.; Fukazawa, N.; Ujihara, K.; Yoshinari, K.; Abe, I.; Noguchi, H.; Miwa, M. Tea polyphenols inhibit acetyl-CoA:1-alkyl-sn-glycero-3-phosphocholine acetyltransferase (a key enzyme in platelet-activating factor biosynthesis) and platelet-activating factor-induced platelet aggregation. Int. Arch. Allergy Immunol. 2004, 134, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Tsoupras, A.; Lordan, R.; Harrington, J.; Pienaar, R.; Devaney, K.; Heaney, S.; Koidis, A.; Zabetakis, I. The Effects of Oxidation on the Antithrombotic Properties of Tea Lipids Against PAF, Thrombin, Collagen, and ADP. Foods 2020, 9, 385. [Google Scholar] [CrossRef] [PubMed]
- Vasange, M.; Rolfsen, W.; Bohlin, L. A sulphonoglycolipid from the fern Polypodium decumanum and its effect on the platelet activating-factor receptor in human neutrophils. J. Pharm Pharm. 1997, 49, 562–566. [Google Scholar] [CrossRef]
- Olszanecki, R.; Jawień, J.; Gajda, M.; Mateuszuk, L.; Gebska, A.; Korabiowska, M.; Chłopicki, S.; Korbut, R. Effect of curcumin on atherosclerosis in apoE/LDLR-double knockout mice. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2005, 56, 627–635. [Google Scholar]
- Lee, H. Antiplatelet property of Curcuma longa L. rhizome-derived ar-turmerone. Bioresour. Technol. 2006, 97, 1372–1376. [Google Scholar] [CrossRef]
- Maheswaraiah, A.; Jaganmohan Rao, L.; Naidu, K.A. Anti-platelet activity of water dispersible curcuminoids in rat platelets. Phytother. Res. 2015, 29, 450–458. [Google Scholar] [CrossRef]
- Cavagnaro, P.F.; Camargo, A.; Galmarini, C.R.; Simon, P.W. Effect of Cooking on Garlic (Allium sativum L.) Antiplatelet Activity and Thiosulfinates Content. J. Agric. Food Chem. 2007, 55, 1280–1288. [Google Scholar] [CrossRef]
- Cavagnaro, P.F.; Galmarini, C.R. Effect of Processing and Cooking Conditions on Onion (Allium cepa L.) Induced Antiplatelet Activity and Thiosulfinate Content. J. Agric. Food Chem. 2012, 60, 8731–8737. [Google Scholar] [CrossRef]
- Kim, S.Y.; Koo, Y.K.; Koo, J.Y.; Ngoc, T.M.; Kang, S.S.; Bae, K.; Kim, Y.S.; Yun-Choi, H.S. Platelet Anti-Aggregation Activities of Compounds from Cinnamomum cassia. J. Med. Food 2010, 13, 1069–1074. [Google Scholar] [CrossRef]
- Ahmed, S.; Gul, S.; Gul, H.; Zia-Ul-Haq, M.; Iram, S.; Jaafar, H.; Moga, M. Scientific basis for the use of Cinnamonum tamala in cardiovascular and inflammatory diseases. Exp. Clin. Card 2014, 20, 784–800. [Google Scholar]
- Li, L.-Z.; Gao, P.-Y.; Song, S.-J.; Yuan, Y.-Q.; Liu, C.-T.; Huang, X.-X.; Liu, Q.-B. Monoterpenes and flavones from the leaves of Crataegus pinnatifida with anticoagulant activities. J. Funct. Foods 2015, 12, 237–245. [Google Scholar] [CrossRef]
- Umar, A.; Zhou, W.; Abdusalam, E.; Tursun, A.; Reyim, N.; Tohti, I.; Moore, N. Effect of Ocimum basilicum L. on cyclo-oxygenase isoforms and prostaglandins involved in thrombosis. J. Ethnopharmacol. 2014, 152, 151–155. [Google Scholar] [CrossRef]
- Zaman, R.; Parvez, M.; Jakaria, M.; Sayeed, M.A.; Islam, M. In vitro clot lysis activity of different extracts of mangifera sylvatica roxb. Leaves. Res. J. Med. Plant 2015, 9, 135–140. [Google Scholar]
- Alañón, M.E.; Palomo, I.; Rodríguez, L.; Fuentes, E.; Arráez-Román, D.; Segura-Carretero, A. Antiplatelet Activity of Natural Bioactive Extracts from Mango (Mangifera Indica L.) and its By-Products. Antioxidants 2019, 8, 517. [Google Scholar] [CrossRef]
- Musfiroh, F.; Setiasih, S.; Handayani, S.; Hudiyono, S.; Ilyas, N. In Vivo antiplatelet activity aggregation assay of bromelain fractionate by ethanol from extract pineapple core (Ananas comosus [l.] merr.). In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, England, 2018; p. 012017. [Google Scholar]
- Silva-Luis, C.C.; de Brito Alves, J.L.; de Oliveira, J.C.P.L.; de Sousa Luis, J.A.; Araújo, I.G.A.; Tavares, J.F.; do Nascimento, Y.M.; Bezerra, L.S.; Araújo de Azevedo, F.d.L.A.; Sobral, M.V.; et al. Effects of Baru Almond Oil (Dipteryx alata Vog.) Treatment on Thrombotic Processes, Platelet Aggregation, and Vascular Function in Aorta Arteries. Nutrients 2022, 14, 2098. [Google Scholar] [CrossRef]
- Alarcón, M.; Fuentes, E.; Olate, N.; Navarrete, S.; Carrasco, G.; Palomo, I. Strawberry extract presents antiplatelet activity by inhibition of inflammatory mediator of atherosclerosis (sP-selectin, sCD40L, RANTES, and IL-1β) and thrombus formation. Platelets 2015, 26, 224–229. [Google Scholar] [CrossRef]
- Santhakumar, A.B.; Stanley, R.; Singh, I. The ex vivo antiplatelet activation potential of fruit phenolic metabolite hippuric acid. Food Funct. 2015, 6, 2679–2683. [Google Scholar] [CrossRef][Green Version]
- Park, B.S.; Son, D.J.; Park, Y.H.; Kim, T.W.; Lee, S.E. Antiplatelet effects of acidamides isolated from the fruits of Piper longum L. Phytomedicine 2007, 14, 853–855. [Google Scholar] [CrossRef]
- Lee, W.; Bae, J.-S. Antithrombotic and antiplatelet activities of orientin in vitro and in vivo. J. Funct. Foods 2015, 17, 388–398. [Google Scholar] [CrossRef]
- Andrikopoulos, N.K.; Antonopoulou, S.; Kaliora, A.C. Oleuropein Inhibits LDL Oxidation Induced by Cooking Oil Frying By-products and Platelet Aggregation Induced by Platelet-Activating Factor. LWT-Food Sci. Technol. 2002, 35, 479–484. [Google Scholar] [CrossRef]
- Kim, M.-G.; Lee, C.-H.; Lee, H.-S. Anti-platelet aggregation activity of lignans isolated from Schisandra chinensis fruits. J. Korean Soc. Appl. Biol. Chem. 2010, 53, 740–745. [Google Scholar] [CrossRef]
- Lee, H.-S. Anticoagulant properties of compounds derived from Fennel (Foeniculum vulgare Gaertner) fruits. Food Sci. Biotechnol. 2006, 15, 763–767. [Google Scholar]
- Jantan, I.; Raweh, S.M.; Sirat, H.M.; Jamil, S.; Mohd Yasin, Y.H.; Jalil, J.; Jamal, J.A. Inhibitory effect of compounds from Zingiberaceae species on human platelet aggregation. Phytomedicine 2008, 15, 306–309. [Google Scholar] [CrossRef]
- Tsoupras, A. The Anti-Inflammatory and Antithrombotic Properties of Bioactives from Orange, Sanguine and Clementine Juices and from Their Remaining By-Products. Beverages 2022, 8, 39. [Google Scholar] [CrossRef]
- Arabshahi-Delouee, S.; Aalami, M.; Urooj, A.; Krishnakantha, T.P. Moringa oleifera leaves as an inhibitor of human platelet aggregation. Pharm. Biol. 2009, 47, 734–739. [Google Scholar] [CrossRef]
- Choleva, M.; Boulougouri, V.; Panara, A.; Panagopoulou, E.; Chiou, A.; Thomaidis, N.S.; Antonopoulou, S.; Fragopoulou, E. Evaluation of anti-platelet activity of grape pomace extracts. Food Funct. 2019, 10, 8069–8080. [Google Scholar] [CrossRef]
- Karantonis, H.C.; Antonopoulou, S.; Demopoulos, C.A. Antithrombotic Lipid Minor Constituents from Vegetable Oils. Comparison between Olive Oils and Others. J. Agric. Food Chem. 2002, 50, 1150–1160. [Google Scholar] [CrossRef]
- Spector, A.A.; Yorek, M.A. Membrane lipid composition and cellular function. J. Lipid Res. 1985, 26, 1015–1035. [Google Scholar] [CrossRef]
- Turkish, A.R.; Sturley, S.L. The genetics of neutral lipid biosynthesis: An evolutionary perspective. Am. J. Physiol.-Endocrinol. Metab. 2009, 297, E19–E27. [Google Scholar] [CrossRef]
- Lordan, R.; Redfern, S.; Tsoupras, A.; Zabetakis, I. Inflammation and cardiovascular disease: Are marine phospholipids the answer? Food Funct. 2020, 11, 2861–2885. [Google Scholar] [CrossRef] [PubMed]
- De Lorgeril, M.; Salen, P. The Mediterranean-style diet for the prevention of cardiovascular diseases. Public Health Nutr. 2006, 9, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Lordan, R.; Walsh, A.M.; Crispie, F.; Finnegan, L.; Cotter, P.D.; Zabetakis, I. The effect of ovine milk fermentation on the antithrombotic properties of polar lipids. J. Funct. Foods 2019, 54, 289–300. [Google Scholar] [CrossRef]
- Borthakur, A.; Bhattacharyya, S.; Kumar, A.; Anbazhagan, A.N.; Tobacman, J.K.; Dudeja, P.K. Lactobacillus acidophilus alleviates platelet-activating factor-induced inflammatory responses in human intestinal epithelial cells. PLoS ONE 2013, 8, e75664. [Google Scholar] [CrossRef]
- Glenn-Davi, K.; Hurley, A.; Brennan, E.; Coughlan, J.; Shiels, K.; Moran, D.; Saha, S.K.; Zabetakis, I.; Tsoupras, A. Fermentation Enhances the Anti-Inflammatory and Anti-Platelet Properties of Both Bovine Dairy and Plant-Derived Dairy Alternatives. Fermentation 2022, 8, 292. [Google Scholar] [CrossRef]
- Shiels, K.; Tsoupras, A.; Lordan, R.; Nasopoulou, C.; Zabetakis, I.; Murray, P.; Saha, S.K. Bioactive Lipids of Marine Microalga Chlorococcum sp. SABC 012504 with Anti-Inflammatory and Anti-Thrombotic Activities. Mar. Drugs 2021, 19, 28. [Google Scholar] [CrossRef]
- Burri, L.; Hoem, N.; Banni, S.; Berge, K. Marine omega-3 phospholipids: Metabolism and biological activities. Int. J. Mol. Sci 2012, 13, 15401–15419. [Google Scholar] [CrossRef]
- Shavandi, A.; Hou, Y.; Carne, A.; McConnell, M.; Bekhit, A.E.A. Marine Waste Utilization as a Source of Functional and Health Compounds. Adv. Food Nutr. Res. 2019, 87, 187–254. [Google Scholar] [CrossRef]
- Redfern, S.; Dermiki, M.; Fox, S.; Lordan, R.; Shiels, K.; Kumar Saha, S.; Tsoupras, A.; Zabetakis, I. The effects of cooking salmon sous-vide on its antithrombotic properties, lipid profile and sensory characteristics. Food Res. Int. 2021, 139, 109976. [Google Scholar] [CrossRef]
- Lordan, R.; Tsoupras, A.; Zabetakis, I. Phospholipids of Animal and Marine Origin: Structure, Function, and Anti-Inflammatory Properties. Molecules 2017, 22, 1964. [Google Scholar] [CrossRef]
- Nasopoulou, C.; Gogaki, V.; Panagopoulou, E.; Demopoulos, C.; Zabetakis, I. Hen egg yolk lipid fractions with antiatherogenic properties. Anim Sci J. 2013, 84, 264–271. [Google Scholar] [CrossRef]
- Nowacki, D.; Martynowicz, H.; Skoczyńska, A.; Wojakowska, A.; Turczyn, B.; Bobak, Ł.; Trziszka, T.; Szuba, A. Lecithin derived from ω-3 PUFA fortified eggs decreases blood pressure in spontaneously hypertensive rats. Sci. Rep. 2017, 7, 12373. [Google Scholar] [CrossRef]
- Karantonis, H.C.; Antonopoulou, S.; Perrea, D.N.; Sokolis, D.P.; Theocharis, S.E.; Kavantzas, N.; Iliopoulos, D.G.; Demopoulos, C.A. In vivo antiatherogenic properties of olive oil and its constituent lipid classes in hyperlipidemic rabbits. Nutr. Metab. Cardiovasc. Dis. 2006, 16, 174–185. [Google Scholar] [CrossRef]
- Megalemou, K.; Sioriki, E.; Lordan, R.; Dermiki, M.; Nasopoulou, C.; Zabetakis, I. Evaluation of sensory and in vitro anti-thrombotic properties of traditional Greek yogurts derived from different types of milk. Heliyon 2017, 3, e00227. [Google Scholar] [CrossRef]
- Fragopoulou, E.; Nomikos, T.; Tsantila, N.; Mitropoulou, A.; Zabetakis, I.; Demopoulos, C.A. Biological activity of total lipids from red and white wine/must. J. Agric. Food Chem. 2001, 49, 5186–5193. [Google Scholar] [CrossRef]
- Bang, H.O.; Dyerberg, J. Plasma lipids and lipoproteins in Greenlandic west coast Eskimos. Acta Med. Scand. 1972, 192, 85–94. [Google Scholar] [CrossRef]
- Dontas, A.S.; Zerefos, N.S.; Panagiotakos, D.B.; Vlachou, C.; Valis, D.A. Mediterranean diet and prevention of coronary heart disease in the elderly. Clin. Interv. Aging 2007, 2, 109–115. [Google Scholar] [CrossRef]
- Souza, P.R.; Marques, R.M.; Gomez, E.A.; Colas, R.A.; De Matteis, R.; Zak, A.; Patel, M.; Collier, D.J.; Dalli, J. Enriched Marine Oil Supplements Increase Peripheral Blood Specialized Pro-Resolving Mediators Concentrations and Reprogram Host Immune Responses: A Randomized Double-Blind Placebo-Controlled Study. Circ. Res. 2020, 126, 75–90. [Google Scholar] [CrossRef]
- Rimm, E.B.; Williams, P.; Fosher, K.; Criqui, M.; Stampfer, M.J. Moderate alcohol intake and lower risk of coronary heart disease: Meta-analysis of effects on lipids and haemostatic factors. BMJ 1999, 319, 1523–1528. [Google Scholar] [CrossRef]
- Renaud, S.; de Lorgeril, M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet 1992, 339, 1523–1526. [Google Scholar] [CrossRef]
- Xanthopoulou, M.N.; Kalathara, K.; Melachroinou, S.; Arampatzi-Menenakou, K.; Antonopoulou, S.; Yannakoulia, M.; Fragopoulou, E. Wine consumption reduced postprandial platelet sensitivity against platelet activating factor in healthy men. Eur J. Nutr. 2017, 56, 1485–1492. [Google Scholar] [CrossRef]
- Argyrou, C.; Vlachogianni, I.; Stamatakis, G.; Demopoulos, C.A.; Antonopoulou, S.; Fragopoulou, E. Postprandial effects of wine consumption on Platelet Activating Factor metabolic enzymes. Prostaglandins Other Lipid Mediat. 2017, 130, 23–29. [Google Scholar] [CrossRef]
- Fragopoulou, E.; Choleva, M.; Antonopoulou, S.; Demopoulos, C.A. Wine and its metabolic effects. A comprehensive review of clinical trials. Metabolism 2018, 83, 102–119. [Google Scholar] [CrossRef]
- Lordan, R.; O’Keeffe, E.; Dowling, D.; Mullally, M.; Heffernan, H.; Tsoupras, A.; Zabetakis, I. The in vitro antithrombotic properties of ale, lager, and stout beers. Food Biosci. 2019, 28, 83–88. [Google Scholar] [CrossRef]
- Garcia-Carrasco, M.; Jimenez-Herrera, E.A.; Galvez-Romero, J.L.; Mendoza-Pinto, C.; Mendez-Martinez, S.; Etchegaray-Morales, I.; Munguia-Realpozo, P.; Vazquez de Lara-Cisneros, L.; Santa Cruz, F.J.; Cervera, R. The anti-thrombotic effects of vitamin D and their possible relationship with antiphospholipid syndrome. Lupus 2018, 27, 2181–2189. [Google Scholar] [CrossRef]
- Singh, U.; Devaraj, S.; Jialal, I. Vitamin E, oxidative stress, and inflammation. Annu. Rev. Nutr. 2005, 25, 151–174. [Google Scholar] [CrossRef]
- Detopoulou, P.; Demopoulos, C.A.; Antonopoulou, S. Micronutrients, Phytochemicals and Mediterranean Diet: A Potential Protective Role against COVID-19 through Modulation of PAF Actions and Metabolism. Nutrients 2021, 13, 462. [Google Scholar] [CrossRef]
- Chew, B.P.; Park, J.S. Carotenoid action on the immune response. J. Nutr. 2004, 134, 257S–261S. [Google Scholar] [CrossRef]
- Mutoh, H.; Fukuda, T.; Kitamaoto, T.; Masushige, S.; Sasaki, H.; Shimizu, T.; Kato, S. Tissue-specific response of the human platelet-activating factor receptor gene to retinoic acid and thyroid hormone by alternative promoter usage. Proc. Natl. Acad. Sci. USA 1996, 93, 774–779. [Google Scholar] [CrossRef]
- Freedman, J.E.; Farhat, J.H.; Loscalzo, J.; Keaney, J.F., Jr. Alpha-tocopherol inhibits aggregation of human platelets by a protein kinase C-dependent mechanism. Circulation 1996, 94, 2434–2440. [Google Scholar] [CrossRef]
- Fukuzawa, K.; Kurotori, Y.; Tokumura, A.; Tsukatani, H.; Vitamin, E. deficiency increases the synthesis of platelet-activating factor (PAF) in rat polymorphonuclear leucocytes. Lipids 1989, 24, 236–239. [Google Scholar] [CrossRef] [PubMed]
- Akada, S.; Iioka, H.; Moriyama, I.; Hisanaga, H.; Morimoto, K.; Ichijo, M. The role of vitamin E during pregnancy--anti-platelet aggregation activity of alpha-tocopherol. Nihon Sanka Fujinka Gakkai Zasshi 1991, 43, 523–528. [Google Scholar] [PubMed]
- Silbert, P.L.; Leong, L.L.; Sturm, M.J.; Strophair, J.; Taylor, R.R. Short term vitamin E supplementation has no effect on platelet function, plasma phospholipase A2 and lyso-PAF in male volunteers. Clin. Exp. Pharm. Physiol 1990, 17, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Charoenngam, N.; Holick, M.F. Immunologic Effects of Vitamin D on Human Health and Disease. Nutrients 2020, 12, 2097. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, S.; Mishra, A.; Ashraf, M.Z. Emerging Role of Vitamin D and its Associated Molecules in Pathways Related to Pathogenesis of Thrombosis. Biomolecules 2019, 9, 649. [Google Scholar] [CrossRef] [PubMed]
- Johny, E.; Jala, A.; Nath, B.; Alam, M.J.; Kuladhipati, I.; Das, R.; Borkar, R.M.; Adela, R. Vitamin D Supplementation Modulates Platelet-Mediated Inflammation in Subjects With Type 2 Diabetes: A Randomized, Double-Blind, Placebo-Controlled Trial. Front. Immunol. 2022, 13, 869591. [Google Scholar] [CrossRef] [PubMed]
- Verdoia, M.; Pergolini, P.; Nardin, M.; Rolla, R.; Negro, F.; Kedhi, E.; Suryapranata, H.; Marcolongo, M.; Carriero, A.; De Luca, G.; et al. Vitamin D levels and platelet reactivity in diabetic patients receiving dual antiplatelet therapy. Vasc. Pharm. 2019, 120, 106564. [Google Scholar] [CrossRef] [PubMed]
- Greiller, C.L.; Suri, R.; Jolliffe, D.A.; Kebadze, T.; Hirsman, A.G.; Griffiths, C.J.; Johnston, S.L.; Martineau, A.R. Vitamin D attenuates rhinovirus-induced expression of intercellular adhesion molecule-1 (ICAM-1) and platelet-activating factor receptor (PAFR) in respiratory epithelial cells. J. Steroid Biochem. Mol. Biol. 2019, 187, 152–159. [Google Scholar] [CrossRef]
- Lordan, R. Notable Developments for Vitamin D Amid the COVID-19 Pandemic, but Caution Warranted Overall: A Narrative Review. Nutrients 2021, 13, 740. [Google Scholar] [CrossRef]
- Verouti, S.N.; Tsoupras, A.B.; Alevizopoulou, F.; Demopoulos, C.A.; Iatrou, C. Paricalcitol effects on activities and metabolism of platelet activating factor and on inflammatory cytokines in hemodialysis patients. Int. J. Artif. Organs 2013, 36, 87–96. [Google Scholar] [CrossRef]
- Chambial, S.; Dwivedi, S.; Shukla, K.K.; John, P.J.; Sharma, P. Vitamin C in disease prevention and cure: An overview. Indian J. Clin. Biochem. 2013, 28, 314–328. [Google Scholar] [CrossRef]
- Liu, D.; Pei, D.; Hu, H.; Gu, G.; Cui, W. Effects and Mechanisms of Vitamin C Post-Conditioning on Platelet Activation after Hypoxia/Reoxygenation. Transfus Med. Hemother 2020, 47, 110–118. [Google Scholar] [CrossRef]
- Lehr, H.A.; Weyrich, A.S.; Saetzler, R.K.; Jurek, A.; Arfors, K.E.; Zimmerman, G.A.; Prescott, S.M.; McIntyre, T.M. Vitamin C blocks inflammatory platelet-activating factor mimetics created by cigarette smoking. J. Clin. Investig. 1997, 99, 2358–2364. [Google Scholar] [CrossRef]
- Lloberas, N.; Torras, J.; Herrero-Fresneda, I.; Cruzado, J.M.; Riera, M.; Hurtado, I.; Grinyo, J.M. Postischemic renal oxidative stress induces inflammatory response through PAF and oxidized phospholipids. Prevention by antioxidant treatment. FASEB J. 2002, 16, 908–910. [Google Scholar] [CrossRef]
- Cao, Y.Z.; Cohen, Z.S.; Weaver, J.A.; Sordillo, L.M. Selenium modulates 1-O-alkyl-2-acetyl-sn-glycero-3-phosphocholine (PAF) biosynthesis in bovine aortic endothelial cells. Antioxid. Redox Signal. 2001, 3, 1147–1152. [Google Scholar] [CrossRef]
- Kiran Gotru, S.; van Geffen, J.P.; Nagy, M.; Mammadova-Bach, E.; Eilenberger, J.; Volz, J.; Manukjan, G.; Schulze, H.; Wagner, L.; Eber, S.; et al. Defective Zn(2+) homeostasis in mouse and human platelets with alpha- and delta-storage pool diseases. Sci. Rep. 2019, 9, 8333. [Google Scholar] [CrossRef]
- Lominadze, D.G.; Saari, J.T.; Miller, F.N.; Catalfamo, J.L.; Justus, D.E.; Schuschke, D.A. Platelet aggregation and adhesion during dietary copper deficiency in rats. Thromb Haemost 1996, 75, 630–634. [Google Scholar] [CrossRef]
- Tako, E. Dietary Trace Minerals. Nutrients 2019, 11, 2823. [Google Scholar] [CrossRef]
- Hampel, G.; Watanabe, K.; Weksler, B.B.; Jaffe, E.A. Selenium deficiency inhibits prostacyclin release and enhances production of platelet activating factor by human endothelial cells. Biochim. Biophys. Acta 1989, 1006, 151–158. [Google Scholar] [CrossRef]
- Bao, B.; Prasad, A.S.; Beck, F.W.; Fitzgerald, J.T.; Snell, D.; Bao, G.W.; Singh, T.; Cardozo, L.J. Zinc decreases C-reactive protein, lipid peroxidation, and inflammatory cytokines in elderly subjects: A potential implication of zinc as an atheroprotective agent. Am. J. Clin. Nutr. 2010, 91, 1634–1641. [Google Scholar] [CrossRef]
- Marx, G.; Krugliak, J.; Shaklai, M. Nutritional zinc increases platelet reactivity. Am. J. Hematol 1991, 38, 161–165. [Google Scholar] [CrossRef]
- Watson, B.R.; White, N.A.; Taylor, K.A.; Howes, J.M.; Malcor, J.D.; Bihan, D.; Sage, S.O.; Farndale, R.W.; Pugh, N. Zinc is a transmembrane agonist that induces platelet activation in a tyrosine phosphorylation-dependent manner. Metallomics 2016, 8, 91–100. [Google Scholar] [CrossRef]
- Hughes, S.; Samman, S. The effect of zinc supplementation in humans on plasma lipids, antioxidant status and thrombogenesis. J. Am. Coll. Nutr. 2006, 25, 285–291. [Google Scholar] [CrossRef]
- Nunez, D.; Kumar, R.; Hanahan, D.J. Inhibition of [3H]platelet activating factor (PAF) binding by Zn2+: A possible explanation for its specific PAF antiaggregating effects in human platelets. Arch. Biochem. Biophys. 1989, 272, 466–475. [Google Scholar] [CrossRef]
- Huo, Y.; Ekholm, J.; Hanahan, D.J. A preferential inhibition by Zn2+ on platelet activating factor- and thrombin-induced serotonin secretion from washed rabbit platelets. Arch. Biochem. Biophys. 1988, 260, 841–846. [Google Scholar] [CrossRef]
- Binder, H.; Arnold, K.; Ulrich, A.S.; Zschörnig, O. Interaction of Zn2+ with phospholipid membranes. Biophys. Chem. 2001, 90, 57–74. [Google Scholar] [CrossRef]
- Vetlenyi, E.; Racz, G. The physiological function of copper, the etiological role of copper excess and deficiency. Orv. Hetil. 2020, 161, 1488–1496. [Google Scholar] [CrossRef]
- Lynch, S. Effects of a dietary copper deficiency on plasma coagulation factor activities in male and female mice. J. Nutr. Biochem. 1992, 3, 387–391. [Google Scholar] [CrossRef]
- Van Rensburg, M.J.; van Rooy, M.; Bester, M.J.; Serem, J.C.; Venter, C.; Oberholzer, H.M. Oxidative and haemostatic effects of copper, manganese and mercury, alone and in combination at physiologically relevant levels: An ex vivo study. Hum. Exp. Toxicol. 2019, 38, 419–433. [Google Scholar] [CrossRef]
- Akinloye, O.; Oyewale, O.O.; Oguntibeju, O.O. Evaluation of trace elements in pregnant women with pre-eclampsia. Afr. J. Biotechnol. 2010, 9, 5196–5202. [Google Scholar]
- Tesfa, E.; Nibret, E.; Munshea, A. Maternal Serum Zinc Level and Pre-eclampsia Risk in African Women: A Systematic Review and Meta-analysis. Biol. Trace Elem. Res. 2021, 199, 4564–4571. [Google Scholar] [CrossRef] [PubMed]
- Bassiouni, B.A.; Foda, A.I.; Rafei, A.A. Maternal and fetal plasma zinc in pre-eclampsia. Eur. J. Obstet. Gynecol. Reprod. Biol. 1979, 9, 75–80. [Google Scholar] [CrossRef]
- Jin, J.; Gao, L.; Zou, X.; Zhang, Y.; Zheng, Z.; Zhang, X.; Li, J.; Tian, Z.; Wang, X.; Gu, J.; et al. Gut Dysbiosis Promotes Preeclampsia by Regulating Macrophages and Trophoblasts. Circ. Res. 2022, 131, 492–506. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Shen, X.; Zhang, D. The Relationship between Serum Zinc Level and Preeclampsia: A Meta-Analysis. Nutrients 2015, 7, 7806–7820. [Google Scholar] [CrossRef]
- Martadiansyah, A.; Maulina, P.; Mirani, P.; Kaprianti, T. Zinc Serum Maternal Levels as a Risk Factor for Preeclampsia. Biosci. Med. J. Biomed. Transl. Res. 2021, 5, 693–701. [Google Scholar]
- Shah, N.; Sethi, R.; Shah, S.; Jafri, K.; Duran, J.; Chang, Y.; Soni, C.; Wollocko, H. The Roles of Platelet-Activating Factor and Magnesium in Pathophysiology of Hypertension, Atherogenesis, Cardiovascular Disease, Stroke and Aging. Cardiogenetics 2022, 12, 49–62. [Google Scholar] [CrossRef]

| Phytocompounds | Scientific Name (Common name) | Optimum Dose Determined or Dosage Investigated | Study Outcomes |
|---|---|---|---|
| Polyphenols such as theaflavin and its gallolyl ester, geranyl gallate, farnesyl gallate and geranylgeranyl gallate. | Camellia sinensis (Tea) | Theaflavin and its galloyl esters (IC50 = 32–77 µM), geranyl gallate, farnesyl gallate and geranylgeranyl gallate (IC50 = 6.4–7.6 µM). | Tea polyphenol such as theaflavin and its other galloyl esters showed potential antithrombotic activity against PAF and inhibited an acetyltransferase involved in its biosynthesis [100]. |
| Polar lipids | Camellia sinensis (Tea leaves) | TL (110 ± 50 µg/ µL), PL (34 ± 4 µg/µL) and NL (820 ± 460 µg/µL) from 30 min observation respectively. | Synergetic effect of the antithrombotic activity of tea polyphenol and PL were against PAF, thrombin, ADP, and collagen, due to their high unsaturated fatty acid content especially rich in omega-3 PUFA and MUFA [101]. |
| Sulphonoglycolipid | Polypodium decumanum (Fern calaguala) | IC50 = 2 μM. | Sulphoquinovosyl diacylglycerol 1,2-di-O-palmitoyl-3-O-(6-sulpho-α-d-quinovopyranosyl)-glycerol showed inhibitory activity against PAF in an in vitro model using human neutrophils [102]. |
| Curcumin | Curcuma longa (Turmeric) | Concentration: 0.3 mg/day in mice. | Oral administration of curcumin (0.3mg/day) in mice inhibited thromboxane levels and increased prostacyclin activity [103]. |
| Ar-turmerone | Curcuma longa (Turmeric) | IC50 values of 14.4 µM and 43.6 µM against collagen and arachidonic acid (AA) respectively. | In vitro study showed that aromatic (ar-)turmerone effectively inhibits platelet aggregation induced by collagen and arachidonic acid [104]. |
| Curcuminoids | Curcuma longa (Turmeric) | Concentration: 10–30 µg/mL. | The isolated PRP was exposed to various concentrations of curcuminoids (10–30 µg/mL) and showed antiplatelet activity against AA and collagen [105]. |
| Allicin and thiosulfinates | Allium sativum (Garlic) | Volume: 30 μL of garlic juice. | In vitro studies showed that allicin and thiosulfanates are the key constituents of garlic juice resulting in antiplatelet activity against collagen-induced platelet activity [106]. |
| Thiosulfinate | Allium cepa (Onion) | Volume: 220 µL of onion juice. | The study resulted that 220 μL of onion juice was enough to produce complete inhibition of platelet aggregation in vitro against AA [107]. |
| AMP48 (Serine protease) of latex | Artocarpus heterophyllus (Jack fruit) | Amount: 1, 2, 4, 8, 16, 32 μg. | Using a thrombin and CaCl2 mediated fibrin clot experiment, 4 μg of AMP48 completely hydrolyzed α-subunit of fibrinogen in 30 min. Techniques including N-terminal sequencing fibrinolysis and ATR-FTIR spectroscopy revealed this novel protein has fibrinolytic properties [88]. |
| Eugenol, amygdalactone, cinnamic alcohol, 2-hydroxycinnamaldehyde, 2-methoxycinnamaldehyde, coniferaldehyde, acetylsalicylic acid, coumarin, cinnamaldehyde, cinnamic acid, icariside DC, dihydrocinnacasside, | Cinnamomum cassia (Cinnamon bark) | IC50 values of Eugenol and coniferaldehyde obtained as 3.8 and 0.82 μM against AA; 3.51, and 0.44 μM against U46619 (thromboxane A2 mimic); 1.86 and 0.57 μM against epinephrine-induced aggregation. | Among the 13 compounds from the extract of cinnamon bark, eugenol, and coniferaldehyde were the two of the most active antiplatelet constituents against AA, U46619 (thromboxane A2 mimic) and epinephrine-induced platelet aggregation [108]. |
| Aqueous extract from the bark | Cinnamomum tamala (Indian Bay Leaf) | Various concentrations of 100, 200, 300, 400, and 500 µg. | The aqueous extract inhibited TXB2 formation through COX pathway (IC50 of 112 µg ± 16) also LP-1 by LOX pathway (IC50 of 120 µg ± 15), and 500 µg concentration showed complete inhibition of platelet aggregation [109]. |
| (6S,7Z,9R)-roseoside, Eriodectyol and 2″-O-rhamnosyl vitexin | Crataegus pinnatifida (Chinese hawberry) | Concentration: 400 µg/mL. | The isolated compounds 7, 13 and 15 exhibited potent antithrombotic activity against ADP induced platelet aggregation in vitro by 87.18, 72.92 and 75.00% respectively at 400 µg/ mL, among them the 13th compound exhibited antithrombotic activity in vivo (zebrafish) by prolonged thrombus formation (19.04 ± 3.32 min) than heparin control (17.63 ± 2.23 min) [110]. |
| Ethanolic extract | Ocimumbasilicum (Basil) | Concentrations: 0.1, 1 and 10 mg/mL of Ocimum ethanolic extract. | Overall OBL and its extracts elevated 6-keto-PGF1α production while decreasing PGE2 and TXB2 production in a dose- and time-dependent manner. This might be due to the combined inhibition of COX-2 and activation of endothelial COX-1 [111]. |
| Methanolic leaf extract | Mangifera sylvatica (Himalayan mango) | A volume of 100 µL. | Methanolic fraction showed a maximum of 46.93% clot lysis activity whereas streptokinase standard showed 80.51% [112]. |
| Mangiferin | Mangifera indica L. (Mango) | Extracts from each part of the mango such as pulp, peel, seed husk and seed with various concentrations like 0.1, 0.5, and 1 mg/mL. | Mango seed showed a 72% of inhibition against adenosine 5′-diphosphate (ADP) induced by platelet aggregation. Among the identified monogalloyl compounds and benzophenones, mangiferin showed a 31% of inhibitory effect against ADP [113]. |
| Bromelain | Ananas comosus (Pineapple) | Bromelain at various doses of 70, 140, and 210 μg/kg of body weight. | Antiplatelet aggregation tests from in vivo method exhibited that bromelain (at the dose of 210 μg/KgBW) has increased the bleeding time (515.10 ± 182.23%) on the 21st day of termination [114], indicating antiplatelet effects. |
| Baru almond oil | Dipteryx alata Vog (Baru Almond) | Ten days of Baru oil as 7.2 and 14.4 mL/kg/day. | Baru almond oil treatment has lowered about 31% of ADP-induced platelet aggregation and thrombotic processes in male Wistar rats, suggesting that it helps lower platelet activation and exert advantages in thrombotic processes [115]. |
| Aqueous extract of strawberry fruit | Fragaria ananassa (Strawberry) | Extract concentrations from 0.1–1 mg/mL. | Dose-dependent reduction against AA and ADP-induced platelet aggregation was observed as 65 ± 5% and 55 ± 4% of inhibition respectively [116]. |
| Hippuric acid | Phenol-rich fruits and plant | Concentrations: 100, 200, 500, 1 and 2 mM. | Dose-dependent inhibition against platelet surface receptor P2Y1/P2Y12 induced by ADP [117]. |
| Piperine, pipernonaline, piperoctadecalidine, piperlongumine | Piper longum L. (Black Pepper) | Concentrations: 300, 150, and 30 μM. | The most effective antiplatelet agent was piperlongumine in vitro. Piperlongumine inhibited collagen-induced platelet aggregation with inhibition rates of 100, 100, 49.8, and 19.9% at 300, 150, 30, and 10 μM, respectively. Piperlongumine had 100%, 76.4%, and 12% inhibitory activity in an AA test at 300, 150, and 30 μM, respectively. Furthermore, piperlongumine at doses of 300, 150, and 30 μM reduced PAF-induced platelet aggregation with inhibition rates of 100%, 100%, and 29.9%, respectively [118]. |
| Orientin and Iso-orientin | Vaccinium bracteatum Thunb. (Sea bilberry or Asiatic bilberry) | In vitro experiment with 5 to 50 μM and in vivo experiment with 9, 26.9 and 44.8 μg per mouse respectively. | A dose-dependent reduction in platelet aggregation was observed in vitro. In vivo experiments showed dose-dependent inhibition against thrombin was observed in mice model. From both compounds, orientin showed potent activity in both models [119]. |
| Oleuropein | Olea europaea (Olive) | IC50 = 0.41 mM. | The various concentrations ranging from 0.25 to 1.25 mM of oleuropein has shown dose-based inhibition against PAF in vitro [120]. |
| Gomisin N and pre-gomisin | Schisandra chinensis (Magnolia berry) | IC50 values of gomisin N and pre-gomisin as 96.5 and 153.3 μM against AA and 49.3 and 122.4 μM against PAF were obtained respectively. | From the various solvents extracts of S. chinensis fruit, methanol and hexane have shown higher inhibitory effects as 65.7 and 94.8% respectively against AA. When compared to all agonists such as PAF, AA, collagen and thrombin, compounds gomisin N and pre-gomisin showed higher effects against AA and PAF [121]. |
| (+)- fenchone and estragole | Foeniculum vulgare Gaertner (Fennel fruit) | Concentrations: (+)- fenchone (IC50 values 3.9μM and 27.1 μM against collagen and AA) estragole (IC50 values 4.7 μM against collagen). | From the in vitro study, (+)-fenchone’s inhibitory effect against platelet aggregation caused by AA was 1.3 times greater than that of aspirin [122]. |
| Pinocembrine, Alpinetin, Cardamonin, 2′,3′,4′,6′-Tetrahydroxychalcone, 5,6-Dehydrokawain, Flavokawain B (above all from A. mutica), Flavokawain A, Crotepoxide, 3-Deacetylcrotepoxide, Zerumbone (above all from Z. zerumbet), Xanthorrhizol (from C. xanthorrhiza), Curcumin, Xanthorrhizol epoxide, 1-Acetyl-2-methyl-5-(1′,5′-dimethylhex-4′enyl) benzene, 1-Methoxy-2-methyl-5-(1′,5′-dimethylhex-4′enyl) benzene (above all from C. aromatica) | Alpinia mutica Roxb. (Orchid Ginger) Kaempferia rotunda Linn (Blackhorm) Curcuma xanthorhiza Roxb (Javanese turmeric) Curcuma aromatica Valeton (Turmeric) Zingiber zerumbet Smith (Shampoo ginger) | Concentrations: 84 μM against AA and 45.7 μM against AA, collagen, and ADP. | Curcumin, cardamonin, pinocembrine, 5,6-dehydrokawain, and 3-deacetylcrotepoxide significantly inhibited platelet aggregation triggered by the AA with IC50 values less than 84 μM. Curcumin was the most efficient antiplatelet agent, inhibiting AA, collagen, and ADP-induced platelet aggregation with IC50 values of 37.5, 60.9, and 45.7 μM, respectively [123]. |
| Vitamin C (Ascorbic acid) and total lipids (TL) | Citrus sinensis (Sweet orange) Citrus sinensis (Blood orange) Citrus clementina (Clementine) | IC50 values against PAF with various samples are as follows, Fresh juice of Navalina oranges (23.2 µg), sanguine oranges (21.4 µg), clementines (28.6 µg), TL from navalina (14.3 µg), TL from sanguine (15.3 µg), TL from clementines (17.3 µg), TL of navalina peel (1.5 µg), TL of sanguine peel (1.2 µg), TL of clementines (1.7 µg). | In vitro antiplatelet activity of vitamin C and TL extract of three different citrus fresh and oxidized fruit juice and peels have shown possible inhibitory effects against PAF and thrombin [124]. |
| Aqueous extract of leaf | Moringa oleifera (Drumstick tree) | IC50 values against ADP-induced aggregation were 0. 48 mg and 0. 70 mg respectively. | Aqueous extract of moringa leaf (0.1 to 1mg) showed potent activity against all types of agonists used in this study such as collagen, ADP, and epinephrine. 1 mg of the extract has shown 100% inhibition against epinephrine-induced aggregation [125]. |
| Ethanolic extract of grape pomace rich in phenolics (catechin, epicatechin and quercetin) fatty acids (linoleic acid (C18:2n6), linolenic acid (C18:3n3) and palmitic acid (C16:0)) | Vitis vinifera (Grape tree) | IC50 value against PAF, ADP, and TRAP as 160.7 ± 64.2, 180.8 ± 78.8, and 158.1 ± 93.6 μg, respectively. | From the in vitro antiplatelet activity, the ethanolic extract of grape pomace was found to be rich in phenolics and fatty acids such as linoleic, linolenic, and palmitic acid. The IC50 values were calculated as 144, 176.5 and 180.5 μg of extract (healthy volunteer) and 214.2, 191.8 and 177.1 μg of extract (cardiovascular patient) against PAF, ADP and TRAP respectively [126]. |
| Olive oil rich in glycerol−glycolipid | Olea europaea (Olive) | IC50 values of Polar lipid fractions 3 showed 437.5 μL, 4 showed 162.5 μL and 5 showed 375.0 μL against PAF. | From the various olive oil fractions, it was evident that glycerol-glucolipids, phosphatidylcholine, sphingomyelin, phosphatidylinositol, and phosphatidylserine were identified and have potent antiplatelet activity against PAF [127]. |
| Lipid Source | Study Aim | Result |
|---|---|---|
| Fermented Irish ovine yoghurt milk | Comparison of in vitro inhibition against PAF-induced aggregation, among different yoghurts and unfermented ovine milk. | Fermentation enhances the antiplatelet nature of ovine milk, due to specific starter cultures, e.g., Lactobacillus (demonstrated by decreased IC50 values) [132]. |
| Fermented bovine yoghurts and coconut, almond and rice-based dairy alternative drinks | Comparison of in vitro inhibition by PL of platelet aggregation. | Fermented plant-based dairy alternatives show much higher antiplatelet activity compared to non-fermented counterparts. The PL from rice-based fermented products shows the highest platelet inhibition of all products, against aggregation induced by PAF and ADP [134]. |
| Kefalotyri and Ladotyri Greek cheeses | Investigate the in vitro inhibition of cheese PL against PAF-induced aggregation. | Lipid fractions of both kinds of cheese inhibit platelet activation, Ladotyri has stronger inhibition [96]. |
| Greek yogurts derived from cow, ewe, and goat milk | Evaluate the in vitro anti-thrombotic properties of yogurts in presence of PAF. | TPL and TL of all yogurts showed platelet inhibition, with TPL of goat and ewe yogurt demonstrated highest inhibition against PAF in WRP [143]. |
| Irish organic farmed salmon filet | Investigate the in vitro inhibition by salmon PL extract against PAF and thrombin-induced platelet aggregation. | Salmon PL, TNL and TL fractions from PE and PC showed higher inhibitory activity [90]. |
| Fresh and fried cod (Gadus morhua) | Test the PAF-like and anti-PAF properties of lipid fractions of fresh and fried cod, against PAF-induced platelet aggregation. | Lipid fractions (TPL and TNL) from fried and fresh cod showed inhibitory activity as well as slight platelet aggregation, indicating presence of both PAF agonists and inhibitors [94]. |
| Hen’s egg yolk | Comparison of the antiplatelet activity of TL, TPL and TNL of different types of hen’s egg yolk (daily fresh, organic, and cage-free hen’s eggs). | All 3 types of hen’s egg yolks displayed potent inhibition against PAF-induced aggregation, with cage-free egg yolk having the highest bioactivity of all, in washed rabbit platelets (WRP) [140]. |
| Red and white wines and musts | Assess the biological activity of lipid fraction from wines/must in vitro. | All lipid fractions of all samples exhibited inhibition against PAF-induced aggregation in washed rabbit platelets, with TPL of Ambelon (white wine) and Cabernet Sauvignon (red wine) having the most potent antiplatelet activity of all [144]. |
| Lipid Source | Study Aim | Study Type | Number of Volunteers | Control | Result |
|---|---|---|---|---|---|
| Marine oil omega-3 supplement | Establish the relationship between marine oil supplementation and specialized pro-resolving mediators (SPM) | A double-blinded, placebo-controlled crossover | 22 | Placebo | Platelet aggregates induced by PAF stimulation are reduced after consumption of marine oil supplement [147]. |
| Yoghurt enriched with olive oil pomace polar lipids | To determine the effect of the incorporation of olive oil pomace polar lipids in yoghurt and their effects on platelet function | Randomised double-blinded, placebo-controlled | 30 | Plain yoghurt | Consumption of yoghurt enriched with olive oil PL resulted in lower platelet sensitivity to PAF [97]. |
| Cabernet sauvignon red wine or Robola white wine | Assess the beneficial effects of wine intake in the postprandial state in human volunteers | Crossover study | 10 | Water and ethanol | Consumption of red or white wine along with a standardized meal resulted in reduced postprandial PAF-induced platelet aggregation in healthy male volunteers [150]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harishkumar, R.; Hans, S.; Stanton, J.E.; Grabrucker, A.M.; Lordan, R.; Zabetakis, I. Targeting the Platelet-Activating Factor Receptor (PAF-R): Antithrombotic and Anti-Atherosclerotic Nutrients. Nutrients 2022, 14, 4414. https://doi.org/10.3390/nu14204414
Harishkumar R, Hans S, Stanton JE, Grabrucker AM, Lordan R, Zabetakis I. Targeting the Platelet-Activating Factor Receptor (PAF-R): Antithrombotic and Anti-Atherosclerotic Nutrients. Nutrients. 2022; 14(20):4414. https://doi.org/10.3390/nu14204414
Chicago/Turabian StyleHarishkumar, Rajendran, Sakshi Hans, Janelle E. Stanton, Andreas M. Grabrucker, Ronan Lordan, and Ioannis Zabetakis. 2022. "Targeting the Platelet-Activating Factor Receptor (PAF-R): Antithrombotic and Anti-Atherosclerotic Nutrients" Nutrients 14, no. 20: 4414. https://doi.org/10.3390/nu14204414
APA StyleHarishkumar, R., Hans, S., Stanton, J. E., Grabrucker, A. M., Lordan, R., & Zabetakis, I. (2022). Targeting the Platelet-Activating Factor Receptor (PAF-R): Antithrombotic and Anti-Atherosclerotic Nutrients. Nutrients, 14(20), 4414. https://doi.org/10.3390/nu14204414










