An Overview of Nutritional Aspects in Juvenile Idiopathic Arthritis
Abstract
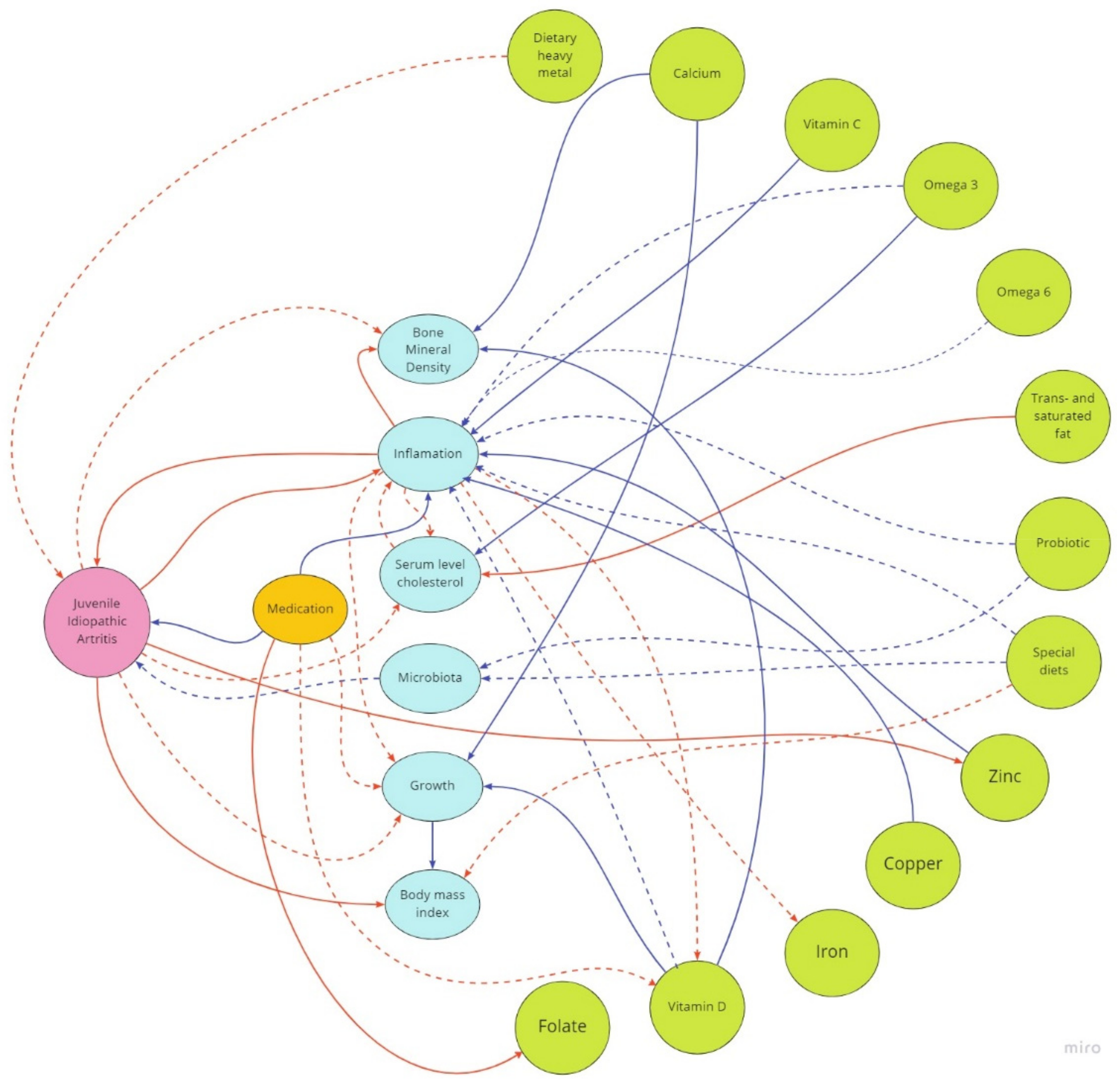
1. Introduction
2. Body Composition and Dietary Challenges in JIA
Special Dietary Patterns
3. Dietary Aspects in Juvenile Idiopathic Arthritis and Potential Benefits of Nutritional Treatment
3.1. Polyunsaturated Fatty Acids (PUFA)
3.2. Trans and Saturated Fat
3.3. Fruits and Vegetables
3.4. Vitamin D
3.5. Folate
3.6. Copper and Zinc
3.7. Calcium
3.8. Iron
3.9. Probiotics
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martini, A.; Lovell, D.J.; Albani, S.; Brunner, H.I.; Hyrich, K.L.; Thompson, S.D.; Ruperto, N. Juvenile Idiopathic Arthritis. Nat. Rev. Dis. Prim. 2022, 8, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Simon, T.A.; Priya Harikrishnan, G.; Kawabata, H.; Singhal, S.; Brunner, H.I.; Lovell, D.J. Prevalence of Co-Existing Autoimmune Disease in Juvenile Idiopathic Arthritis: A Cross-Sectional Study. Pediatr. Rheumatol. 2020, 18, 43. [Google Scholar] [CrossRef] [PubMed]
- Martini, A.; Prakken, B.; Albani, S. Arthritis 3 Juvenile Idiopathic Arthritis. Lancet 2011, 377, 2138–2187. [Google Scholar]
- McCurdy, D.; Parsa, M.F. Updates in Juvenile Idiopathic Arthritis. Adv. Pediatr. 2021, 68, 143–170. [Google Scholar] [CrossRef]
- Juvenile Idiopathic Arthritis|Pediatric Orthopaedic Society of North America (POSNA). Available online: https://posna.org/Physician-Education/Study-Guide/Juvenile-Idiopathic-Arthritis (accessed on 19 July 2022).
- Stawicki, M.K.; Abramowicz, P.; Góralczyk, A.; Młyńczyk, J.; Kondratiuk, A.; Konstantynowicz, J. Prevalence of Vitamin D Deficiency in Patients Treated for Juvenile Idiopathic Arthritis and Potential Role of Methotrexate: A Preliminary Study. Nutrients 2022, 14, 1645. [Google Scholar] [CrossRef]
- Juvenile Idiopathic Arthritis (JIA)|Arthritis Foundation. Available online: https://www.arthritis.org/diseases/juvenile-idiopathic-arthritis (accessed on 19 July 2022).
- Pelajo, C.F.; Lopez-Benitez, J.M.; Miller, L.C. Obesity and Disease Activity in Juvenile Idiopathic Arthritis. Pediatr. Rheumatol. 2012, 10, 1–5. [Google Scholar] [CrossRef]
- Caetano, M.C.; Ortiz, T.T.; Terreri, M.T.S.L.R.A.; Sarni, R.O.S.; Silva, S.G.L.; Souza, F.I.S.; Hilário, M.O.E. Inadequate Dietary Intake of Children and Adolescents with Juvenile Idiopathic Arthritis and Systemic Lupus Erythematosus. J. Pediatr. 2009, 85, 509–515. [Google Scholar] [CrossRef]
- Lofthouse, C.M.; Azad, F.; Baildam, E.M.; Akobeng, A.K. Measuring the Nutritional Status of Children with Juvenile Idiopathic Arthritis Using the Bioelectrical Impedance Method. Rheumatology 2002, 41, 1172–1177. [Google Scholar] [CrossRef]
- Cleary, A.G.; Lancaster, G.A.; Annan, F.; Sills, J.A.; Davidson, J.E. Nutritional Impairment in Juvenile Idiopathic Arthritis. Rheumatology 2004, 43, 1569–1573. [Google Scholar] [CrossRef][Green Version]
- Onel, K.B.; Horton, D.B.; Lovell, D.J.; Shenoi, S.; Cuello, C.A.; Angeles-Han, S.T.; Becker, M.L.; Cron, R.Q.; Feldman, B.M.; Ferguson, P.J.; et al. 2021 American College of Rheumatology Guideline for the Treatment of Juvenile Idiopathic Arthritis: Therapeutic Approaches for Oligoarthritis, Temporomandibular Joint Arthritis, and Systemic Juvenile Idiopathic Arthritis. Arthritis Care Res. 2022, 74, 521–537. [Google Scholar] [CrossRef]
- Little, E.M.I.; Grevich, S.; Huber, J.L.; Suskind, D.L.; Bradford, M.C.; Stevens, A.M.; Zhao, Y. Parental Perception of Dietary Intervention in Juvenile Idiopathic Arthritis. J. Altern. Complement. Med. 2019, 25, 643–647. [Google Scholar] [CrossRef]
- Kindgren, E.; Guerrero-Bosagna, C.; Ludvigsson, J. Heavy Metals in Fish and Its Association with Autoimmunity in the Development of Juvenile Idiopathic Arthritis: A Prospective Birth Cohort Study. Pediatr. Rheumatol. Online J. 2019, 17, 33. [Google Scholar] [CrossRef]
- Hari, A.; Rostom, S.; Hassani, A.; el Badri, D.; Bouaadi, I.; Barakat, A.; Chkirat, B.; Elkari, K.; Amine, B.; Hajjaj-Hassouni, N. Body Composition in Children with Juvenile Idiopathic Arthritis: Effect of Dietary Intake of Macronutrient: Results from a Cross Sectional Study. Pan Afr. Med. J. 2015, 20, 244. [Google Scholar] [CrossRef]
- Gaspari, S.; Marcovecchio, M.L.; Breda, L.; Chiarelli, F. Growth in Juvenile Idiopathic Arthritis: The Role of Inflammation. Clin. Exp. Rheumatol. 2011, 29, 104–110. [Google Scholar]
- Simon, D.; Fernando, C.; Czernichow, P.; Prieur, A.-M. Linear Growth and Final Height in Patients with Systemic Juvenile Idiopathic Arthritis Treated with Longterm Glucocorticoids. J. Rheumatol. 2002, 29, 1296–1300. [Google Scholar]
- Bacon, M.C.; White, P.H.; Raiten, D.J.; Craft, N.; Margolis, S.; Levander, O.A.; Taylor, M.L.; Lipnick, R.N.; Sami, S. Nutritional Status and Growth in Juvenile Rheumatoid Arthritis. Semin. Arthritis Rheum. 1990, 20, 97–106. [Google Scholar] [CrossRef]
- Guzman, J.; Kerr, T.; Ward, L.M.; Ma, J.; Oen, K.; Rosenberg, A.M.; Feldman, B.M.; Boire, G.; Houghton, K.; Dancey, P.; et al. Growth and Weight Gain in Children with Juvenile Idiopathic Arthritis: Results from the ReACCh-Out Cohort. Pediatr. Rheumatol. 2017, 15, 1–11. [Google Scholar] [CrossRef]
- McErlane, F.; Carrasco, R.; Kearsley-Fleet, L.; Baildam, E.M.; Wedderburn, L.R.; Foster, H.E.; Ioannou, Y.; Chieng, S.E.A.; Davidson, J.E.; Thomson, W.; et al. Growth Patterns in Early Juvenile Idiopathic Arthritis: Results from the Childhood Arthritis Prospective Study (CAPS). Semin. Arthritis Rheum. 2018, 48, 53–60. [Google Scholar] [CrossRef]
- Okumus, O.; Erguven, M.; Deveci, M.; Yilmaz, O.; Okumus, M. Growth and Bone Mineralization in Patients with Juvenile Idiopathic Arthritis. Indian J. Pediatr. 2008, 75, 239–243. [Google Scholar] [CrossRef]
- Polito, C.; Strano, C.G.; Olivieri, A.N.; Alessio, M.; Iammarrone, C.S.; Todisco, N.; Papale, M.R. Growth Retardation in Non-Steroid Treated Juvenile Rheumatoid Arthritis. Scand. J. Rheumatol. 1997, 26, 99–103. [Google Scholar] [CrossRef]
- Bechtold, S.; Simon, D. Growth Abnormalities in Children and Adolescents with Juvenile Idiopathic Arthritis. Rheumatol. Int. 2014, 34, 1483–1488. [Google Scholar] [CrossRef] [PubMed]
- Roth, J.; Bechtold, S.; Borte, G.; Dressler, F.; Girschick, H.J.; Borte, M. Osteoporosis in Juvenile Idiopathic Arthritis—A Practical Approach to Diagnosis and Therapy. Eur. J. Pediatr. 2007, 166, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Bechtold, S.; Ripperger, P.; Bonfig, W.; Schmidt, H.; Bitterling, H.; Häfner, R.; Schwarz, H.P. Bone Mass Development and Bone Metabolism in Juvenile Idiopathic Arthritis: Treatment with Growth Hormone for 4 Years. J. Rheumatol. 2004, 31, 1407–1412. [Google Scholar] [PubMed]
- Knops, N.; Wulffraat, N.; Lodder, S.; Houwen, R.; Meer, K. Resting Energy Expenditure and Nutritional Status in Children with Juvenile Rheumatoid Arthritis-PubMed. J. Rheumatol. 1999, 26, 2039–2043. [Google Scholar]
- Wiȩch, P.; Sałacińska, I.; Bazaliński, D.; Dabrowski, M. Body Composition and Phase Angle as an Indicator of Nutritional Status in Children with Juvenile Idiopathic Arthritis. Pediatr. Rheumatol. Online J. 2018, 16, 82. [Google Scholar] [CrossRef]
- Haugen, M.A.; Høeraal, H.M.; Larsed, S.; Gilboe, I.M.; Trygg, K. Nutrient Intake and Nutritional Status in Children with Juvenile Chronic Arthritis. Scand. J. Rheumatol. 2009, 21, 165–170. [Google Scholar] [CrossRef]
- Merwin, S.; Mackey, E.; Sule, S. US NHANES Data 2013–2016: Increased Risk of Severe Obesity in Individuals with History of Juvenile Idiopathic Arthritis. Pediatr. Rheumatol. 2021, 19, 1–2. [Google Scholar] [CrossRef]
- Grönlund, M.M.; Kaartoaho, M.; Putto-Laurila, A.; Laitinen, K. Juvenile Idiopathic Arthritis Patients with Low Inflammatory Activity Have Increased Adiposity. J. Clin. Med. 2014, 43, 488–492. [Google Scholar] [CrossRef]
- Weiss, J.E.; Ilowite, N.T. Juvenile Idiopathic Arthritis. Rheum. Dis. Clin. North Am. 2007, 33, 441–470. [Google Scholar] [CrossRef]
- Consolaro, A.; Giancane, G.; Alongi, A.; van Dijkhuizen, E.H.P.; Aggarwal, A.; Al-Mayouf, S.M.; Bovis, F.; de Inocencio, J.; Demirkaya, E.; Flato, B.; et al. Phenotypic Variability and Disparities in Treatment and Outcomes of Childhood Arthritis throughout the World: An Observational Cohort Study. Lancet Child Adolesc. Health 2019, 3, 255–263. [Google Scholar] [CrossRef]
- Haugen, M.A.; Kjeldsen-Kragh, J.; Skakkebæk, N.; Landaas, S.; Sjaastad, O.; Movinkel, P.; Førre, O. The Influence of Fast and Vegetarian Diet on Parameters of Nutritional Status in Patients with Rheumatoid Arthritis. Clin. Rheumatol. 1993, 12, 62–69. [Google Scholar] [CrossRef]
- Kjeldsen-Kragh, J. Rheumatoid Arthritis Treated with Vegetarian Diets. Am. J. Clin. Nutr. 1999, 70, 594s–600s. [Google Scholar] [CrossRef]
- Aalto, K.; Lahdenne, P.; Kolho, K.-L. Gluten-Free Diet in Juvenile Idiopathic Arthritis. Rheumatology 2011, 1, 1–4. [Google Scholar] [CrossRef]
- Berntson, L.; Öman, A.; Engstrand, L.; Dicksved, J. A Pilot Study Investigating Faecal Microbiota After Two Dietary Interventions in Children with Juvenile Idiopathic Arthritis. Curr. Microbiol. 2022, 79, 1–11. [Google Scholar] [CrossRef]
- Berntson, L. A Pilot Study of Possible Anti-Inflammatory Effects of the Specific Carbohydrate Diet in Children with Juvenile Idiopathic Arthritis. Pediatr. Rheumatol. 2021, 19, 1–8. [Google Scholar] [CrossRef]
- Onel, K.B.; Horton, D.B.; Lovell, D.J.; Shenoi, S.; Cuello, C.A.; Angeles-Han, S.T.; Becker, M.L.; Cron, R.Q.; Feldman, B.M.; Ferguson, P.J.; et al. 2021 American College of Rheumatology Guideline for the Treatment of Juvenile Idiopathic Arthritis: Recommendations for Nonpharmacologic Therapies, Medication Monitoring, Immunizations, and Imaging. Arthritis Rheumatol. 2022, 74, 570–585. [Google Scholar] [CrossRef]
- Gheita, T.; Kamel, S.; Helmy, N.; El-Laithy, N.; Monir, A. Omega-3 Fatty Acids in Juvenile Idiopathic Arthritis: Effect on Cytokines (IL-1 and TNF-α), Disease Activity and Response Criteria. Clin. Rheumatol. 2012, 31, 363–366. [Google Scholar] [CrossRef]
- Gorczyca, D.; Postępski, J.; Czajkowska, A.; Paściak, M.; Prescha, A.; Olesińska, E.; Gruenpeter, A.; Lachór-Motyka, I.; Szponar, B. The Profile of Polyunsaturated Fatty Acids in Juvenile Idiopathic Arthritis and Association with Disease Activity. Clin. Rheumatol. 2017, 36, 1269–1279. [Google Scholar] [CrossRef]
- Marangoni, R.G.; Hayata, A.L.; Borba, E.F.; Azevedo, P.M.; Bonfá, E.; Goldenstein-Schainberg, C. Decreased High-Density Lipoprotein Cholesterol Levels in Polyarticular Juvenile Idiopathic Arthritis. Clinics 2011, 66, 1549–1552. [Google Scholar] [CrossRef]
- Bohr, A.H.; Pedersen, F.K.; Nielsen, C.H.; Müller, K.G. Lipoprotein Cholesterol Fractions Are Related to Markers of Inflammation in Children and Adolescents with Juvenile Idiopathic Arthritis: A Cross Sectional Study. Pediatr. Rheumatol. 2016, 14, 1–9. [Google Scholar] [CrossRef]
- Douglas, W.; Rodrigues, R. Biomarkers of Lipid Metabolism in Patients with Juvenile Idiopathic Arthritis: Relationship with Subtype and Inammatory Activity. Pediatr. Rheumatol. 2021, 19, 66. [Google Scholar] [CrossRef]
- Shen, C.C.; Yao, T.C.; Yeh, K.W.; Huang, J.L. Association of Disease Activity and Anti-Rheumatic Treatment in Juvenile Idiopathic Arthritis with Serum Lipid Profiles: A Prospective Study. Semin. Arthritis Rheum. 2013, 42, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Jednacz, E.; Rutkowska-Sak, L. Assessment of the Body Composition and Parameters of the Cardiovascular Risk in Juvenile Idiopathic Arthritis. Biomed. Res. Int. 2015, 2015, 619023. [Google Scholar] [CrossRef] [PubMed]
- Rondanelli, M.; Perdoni, F.; Peroni, G.; Caporali, R.; Gasparri, C.; Riva, A.; Petrangolini, G.; Faliva, M.A.; Infantino, V.; Naso, M.; et al. Ideal Food Pyramid for Patients with Rheumatoid Arthritis: A Narrative Review. Clin. Nutr. 2021, 40, 661–689. [Google Scholar] [CrossRef]
- Perkins, A.; Sontheimer, C.; Otjen, J.P.; Shenoi, S. Scurvy Masquerading as Juvenile Idiopathic Arthritis or Vasculitis with Elevated Inflammatory Markers: A Case Series. J. Pediatr. 2020, 218, 234–237.e2. [Google Scholar] [CrossRef]
- LI, Y. Advances in Immunoregulatory Effects of Dietary Nutrition on Juvenile Idiopathic Arthritis. Int. J. Pediatr. 2019, 6, 745–748. [Google Scholar] [CrossRef]
- Zhong, Y.; Wang, Y.; Guo, J.; Chu, H.; Gao, Y.; Pang, L. Blueberry Improves the Therapeutic Effect of Etanercept on Patients with Juvenile Idiopathic Arthritis: Phase III Study. Tohoku J. Exp. Med. 2015, 237, 183–191. [Google Scholar] [CrossRef][Green Version]
- Wu, C.Y.; Yang, H.Y.; Luo, S.F.; Huang, J.L.; Lai, J.H. Vitamin D Supplementation in Patients with Juvenile Idiopathic Arthritis. Nutrients 2022, 14, 1538. [Google Scholar] [CrossRef]
- Pelajo, C.F.; Lopez-Benitez, J.M.; Miller, L.C. Vitamin D and Autoimmune Rheumatologic Disorders. Autoimmun. Rev. 2010, 9, 507–510. [Google Scholar] [CrossRef]
- Finch, S.L.; Rosenberg, A.M.; Vatanparast, H. Vitamin D and Juvenile Idiopathic Arthritis. Pediatr. Rheumatol. 2018, 16, 1–17. [Google Scholar] [CrossRef]
- Sengler, C.; Zink, J.; Klotsche, J.; Niewerth, M.; Liedmann, I.; Horneff, G.; Kessel, C.; Ganser, G.; Thon, A.; Haas, J.P.; et al. Vitamin D Deficiency Is Associated with Higher Disease Activity and the Risk for Uveitis in Juvenile Idiopathic Arthritis-Data from a German Inception Cohort. Arthritis Res. Ther. 2018, 20, 276. [Google Scholar] [CrossRef]
- Zou, J.; Thornton, C.; Chambers, E.S.; Rosser, E.C.; Ciurtin, C. Exploring the Evidence for an Immunomodulatory Role of Vitamin D in Juvenile and Adult Rheumatic Disease. Front. Immunol. 2021, 11, 616483. [Google Scholar] [CrossRef]
- Pelajo, C.F.; Lopez-Benitez, J.M.; Miller, L.C. 25-Hydroxyvitamin D Levels and Vitamin D Deficiency in Children with Rheumatologic Disorders and Controls. J. Rheumatol. 2011, 38, 2000–2004. [Google Scholar] [CrossRef]
- Sumi, S.K.; Rahman, S.A.; Islam, M.I.; Islam, M.M.; Talukder, M.K. Vitamin D Profile in Juvenile Idiopathic Arthritis Patients in a Tertiary Care Hospital in Bangladesh. Mymensingh Med. J. 2020, 29, 311–316. [Google Scholar]
- Reed, A.; Haugen, M.; Pachman, L.M.; Langman, C.B. 25-Hydroxyvitamin D Therapy in Children with Active Juvenile Rheumatoid Arthritis: Short-Term Effects on Serum Osteocalcin Levels and Bone Mineral Density. J. Pediatr. 1991, 119, 657–660. [Google Scholar] [CrossRef]
- Tang, T.; Zhang, Y.; Luo, C.; Liu, M.; Xu, L.; Tang, X. Adjunctive Vitamin D for the Treatment of Active Juvenile Idiopathic Arthritis: An Open-Label, Prospective, Randomized Controlled Trial. Exp. Ther. Med. 2019, 18, 4921–4926. [Google Scholar] [CrossRef]
- Jacobson, J.L.; Pham, J.T. Juvenile Idiopathic Arthritis: A Focus on Pharmacologic Management. J. Pediatr. Health Care 2018, 32, 515–528. [Google Scholar] [CrossRef]
- Singh, R.K.; van Haandel, L.; Kiptoo, P.; Becker, M.L.; Siahaan, T.J.; Funk, R.S. Methotrexate Disposition, Anti-Folate Activity and Efficacy in the Collagen-Induced Arthritis Mouse Model. Eur. J. Pharmacol. 2019, 853, 264–274. [Google Scholar] [CrossRef]
- Killeen, O.G.; Gardner-Medwin, J.M. In Juvenile Idiopathic Arthritis, Is Folate Supplementation Effective against Methotrexate Toxicity at the Expense of Methotrexate’s Efficacy? Arch. Dis. Child. 2006, 91, 537–538. [Google Scholar] [CrossRef][Green Version]
- Huemer, M.; Födinger, M.; Huemer, C.; Sailer-Höck, M.; Falger, J.; Rettenbacher, A.; Bernecker, M.; Artacker, G.; Kenzian, H.; Lang, T.; et al. Hyperhomocysteinemia in Children with Juvenile Idiopathic Arthritis Is Not Influenced by Methotrexate Treatment and Folic Acid Supplementation: A Pilot Study. Clin. Exp. Rheumatol. 2003, 21, 249–255. [Google Scholar]
- Ferrara, G.; Mastrangelo, G.; Barone, P.; la Torre, F.; Martino, S.; Pappagallo, G.; Ravelli, A.; Taddio, A.; Zulian, F.; Cimaz, R. Methotrexate in Juvenile Idiopathic Arthritis: Advice and Recommendations from the MARAJIA Expert Consensus Meeting. Pediatr. Rheumatol. 2018, 16, 1–14. [Google Scholar] [CrossRef]
- Yasser, S.A.; Hashaad, N.I.; Shouzan, A.M.; el Nouty, H.A. Measurement of Serum Trace Elements Levels in Patients with Juvenile Idiopathic Arthritis. Egypt. Rheumatol. Rehabil. 2016, 43, 59–66. [Google Scholar] [CrossRef]
- Lin, Z.; Li, W. The Roles of Vitamin D and Its Analogs in Inflammatory Diseases. Curr. Top. Med. Chem. 2016, 16, 1242–1261. [Google Scholar] [CrossRef]
- Stark, L.J.; Janicke, D.M.; McGrath, A.M.; Mackner, L.M.; Hommel, K.A.; Lovell, D. Prevention of Osteoporosis: A Randomized Clinical Trial to Increase Calcium Intake in Children with Juvenile Rheumatoid Arthritis. J. Pediatr. Psychol. 2005, 30, 377–386. [Google Scholar] [CrossRef]
- Rusu, T.E.; Murgu, A.; Moraru, E.; Florea, M.M.; Ioniuc, I.; Alexoaie, M.; Ruginǎ, A.; Goţia, S. [Osteopenia in Children with Juvenile Idiopathic Arthritis]. Rev. Med. Chir. Soc. Med. Nat. Iasi 2008, 112, 88–93. [Google Scholar]
- Lovell, D.J.; Glass, D.; Ranz, J.; Kramer, S.; Huang, B.; Sierra, R.I.; Henderson, C.J.; Passo, M.; Graham, B.; Bowyer, S.; et al. A Randomized Controlled Trial of Calcium Supplementation to Increase Bone Mineral Density in Children with Juvenile Rheumatoid Arthritis. Arthritis Rheum. 2006, 54, 2235–2242. [Google Scholar] [CrossRef]
- Kivivuori, S.M.; Pelkonen, P.; Ylijoki, H.; Verronen, P.; Siimes, M.A. Elevated Serum Transferrin Receptor Concentration in Children with Juvenile Chronic Arthritis as Evidence of Iron Deficiency. Rheumatology 2000, 39, 193–197. [Google Scholar] [CrossRef][Green Version]
- Wilson, A.; Yu, H.T.; Goodnough, L.T.; Nissenson, A.R. Prevalence and Outcomes of Anemia in Rheumatoid Arthritis: A Systematic Review of the Literature. Am. J. Med. 2004, 116, 50–57. [Google Scholar] [CrossRef]
- Martini, A.; Ravelli, A.; di Fuccia, G.; Rosti, V.; Cazzola, M.; Barosi, G. Intravenous Iron Therapy for Severe Anaemia in Systemic-Onset Juvenile Chronic Arthritis. Lancet 1994, 344, 1052–1054. [Google Scholar] [CrossRef]
- Ravelli, A.; Martini, A. Juvenile Idiopathic Arthritis. Lancet 2007, 369, 767–778. [Google Scholar] [CrossRef]
- Martini, A. Systemic Juvenile Idiopathic Arthritis. Autoimmun. Rev. 2012, 12, 56–59. [Google Scholar] [CrossRef] [PubMed]
- Isaacs, J.D.; Harari, O.; Kobold, U.; Lee, J.S.; Bernasconi, C. Effect of Tocilizumab on Haematological Markers Implicates Interleukin-6 Signalling in the Anaemia of Rheumatoid Arthritis. Arthritis Res. Ther. 2013, 15, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Cazzola, M.; Ponchio, L.; Benedetti, F.; Ravelli, A.; Rosti, V.; Beguin, Y.; Invernizzi, R.; Barosi, G.; Martini, A. Defective Iron Supply for Erythropoiesis and Adequate Endogenous Erythropoietin Production in the Anemia Associated with Systemic-Onset Juvenile Chronic Arthritis. Blood 1996, 87, 4824–4830. [Google Scholar] [CrossRef] [PubMed]
- De Filippo, C.; di Paola, M.; Giani, T.; Tirelli, F.; Cimaz, R. Gut Microbiota in Children and Altered Profiles in Juvenile Idiopathic Arthritis. J. Autoimmun. 2019, 98, 1–12. [Google Scholar] [CrossRef]
- Shukla, A.; Gaur, P.; Aggarwal, A. Effect of Probiotics on Clinical and Immune Parameters in Enthesitis-Related Arthritis Category of Juvenile Idiopathic Arthritis. Clin. Exp. Immunol. 2016, 185, 301–308. [Google Scholar] [CrossRef]
- Aggarwal, A.; Sarangi, A.N.; Gaur, P.; Shukla, A.; Aggarwal, R. Gut Microbiome in Children with Enthesitis-Related Arthritis in a Developing Country and the Effect of Probiotic Administration. Clin. Exp. Immunol. 2017, 187, 480–489. [Google Scholar] [CrossRef]
- Zare, N.; Mansoubi, M.; Coe, S.; Naja, A.A.; Bailey, K.; Harrison, K.; Sheehan, J.; Dawes, H.; Barker, K. An Investigation into the Relationship between Dietary Intake, Symptoms and Health-Related Quality of Life in Children and Young People with Juvenile Idiopathic Arthritis: A Systematic Review and Meta-Analysis. Res. Square 2022, 1–5. [Google Scholar] [CrossRef]
- Conigliaro, P.; Triggianese, P.; de Martino, E.; Fonti, G.L.; Chimenti, M.S.; Sunzini, F.; Viola, A.; Canofari, C.; Perricone, R. Challenges in the Treatment of Rheumatoid Arthritis. Autoimmun. Rev. 2019, 18, 706–713. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zandonadi, R.P. An Overview of Nutritional Aspects in Juvenile Idiopathic Arthritis. Nutrients 2022, 14, 4412. https://doi.org/10.3390/nu14204412
Zandonadi RP. An Overview of Nutritional Aspects in Juvenile Idiopathic Arthritis. Nutrients. 2022; 14(20):4412. https://doi.org/10.3390/nu14204412
Chicago/Turabian StyleZandonadi, Renata Puppin. 2022. "An Overview of Nutritional Aspects in Juvenile Idiopathic Arthritis" Nutrients 14, no. 20: 4412. https://doi.org/10.3390/nu14204412
APA StyleZandonadi, R. P. (2022). An Overview of Nutritional Aspects in Juvenile Idiopathic Arthritis. Nutrients, 14(20), 4412. https://doi.org/10.3390/nu14204412




