In Vitro and In Vivo Evaluation of Antidiabetic Properties and Mechanisms of Ficus tikoua Bur.
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Material
2.2.1. Extraction Procedure
2.2.2. UPLC-ESI-MS Analysis of NBF
2.3. In Vitro Study
2.3.1. α-Glucosidase Inhibition Activity
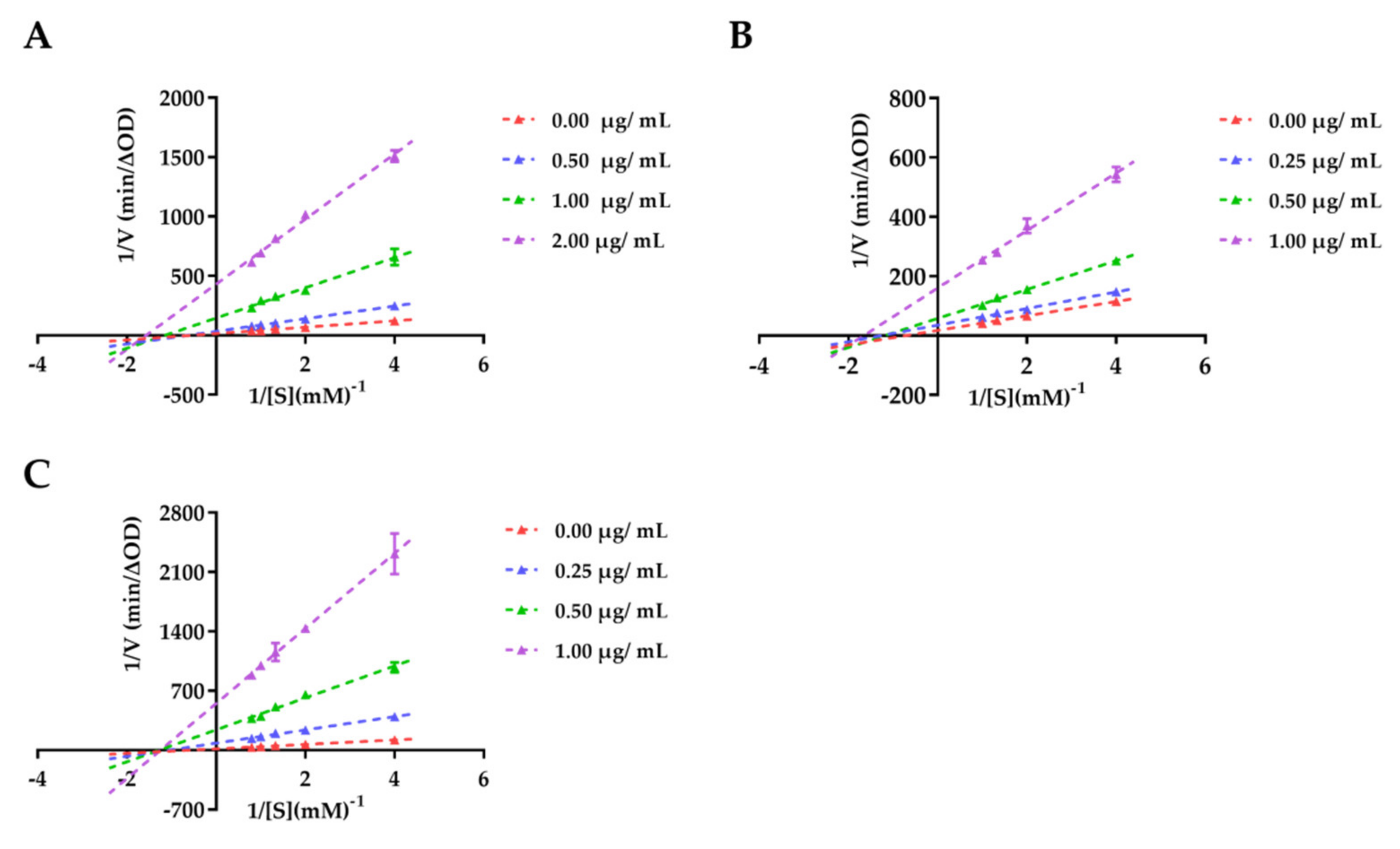
2.3.2. Enzyme Kinetic Analysis
2.3.3. 3T3-L1 Preadipocytes Culture and Differentiation
2.3.4. Glucose Uptake and Cell Viability Assay of 3T3-L1 Adipocytes
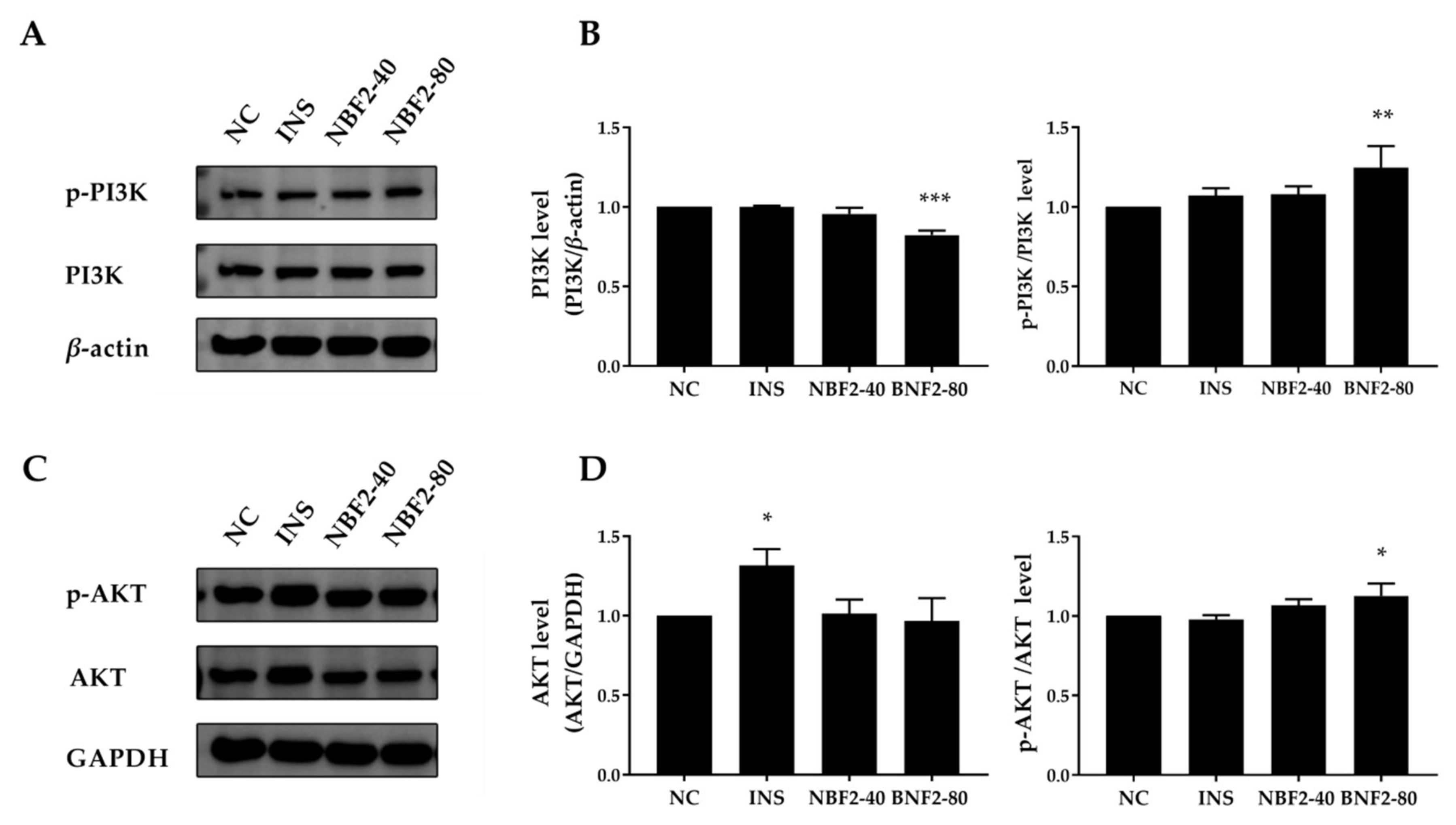
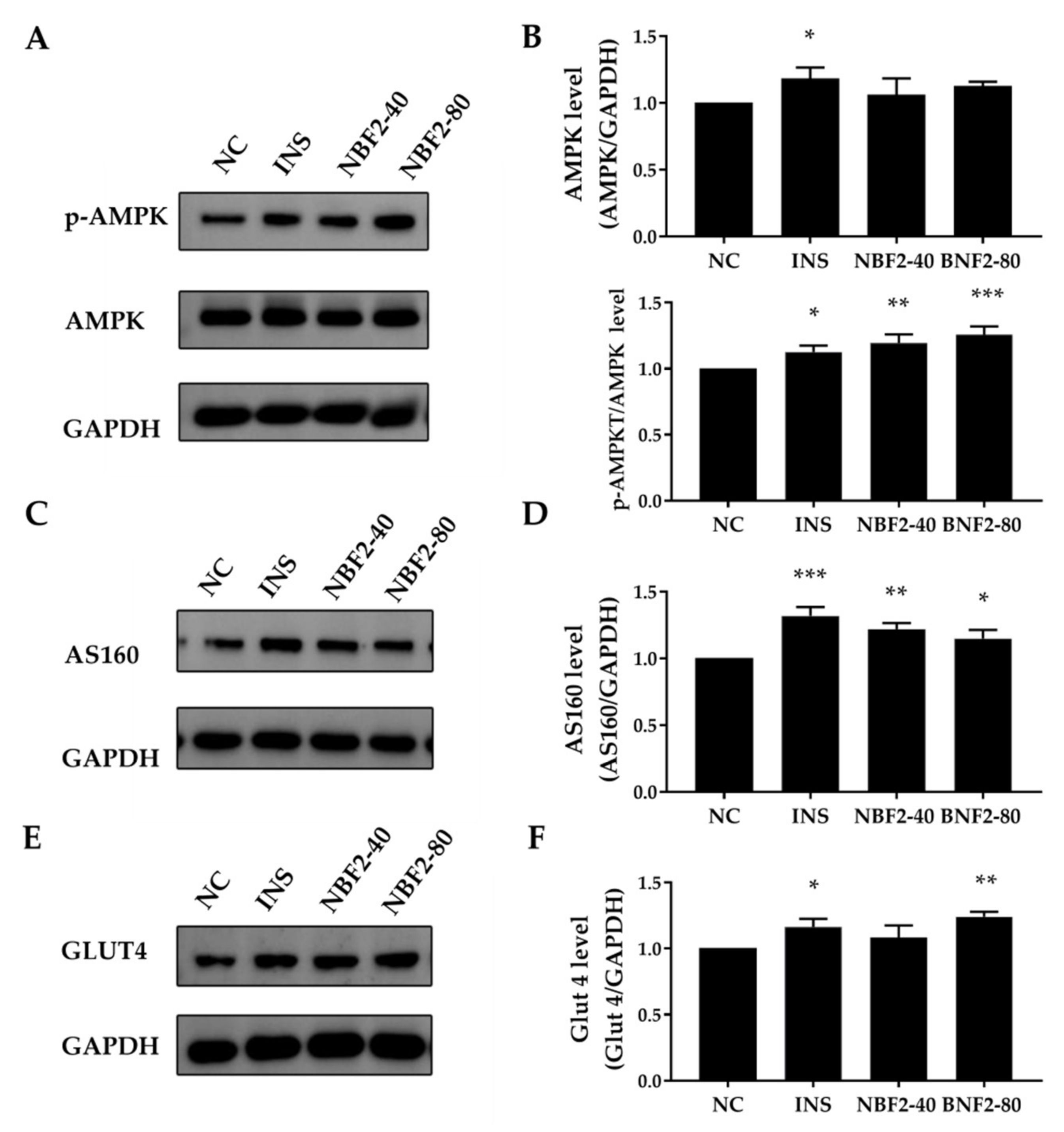
2.3.5. Western Blot Analysis
2.4. In Vivo Study
2.4.1. Experimental Animals and Ethical Statements
2.4.2. Random Blood Glucose and Fasting Blood Glucose
2.4.3. Oral Glucose Tolerance Test (OGTT)
2.4.4. Insulin Tolerance Test (ITT)
2.4.5. Biochemical Analysis
2.4.6. Histopathological Analysis
2.5. Statistical Analysis
3. Results
3.1. UPLC-ESI-MS Analysis of NBF
3.2. In Vitro Study
3.2.1. α-Glucosidase Inhibition Activity
3.2.2. Enzyme Kinetic Study
3.2.3. Glucose Uptake and Cell Viability of 3T3-L1 Adipocytes
3.2.4. Effect of NBF2 on the PI3K/Akt Pathway, AMPK Pathway and GLUT4 Expression
3.3. In Vivo Study
3.3.1. Effects of NBF on Body Weight and Food Intake
3.3.2. Effects of NBF on Random Blood Glucose and Fasting Blood Glucose
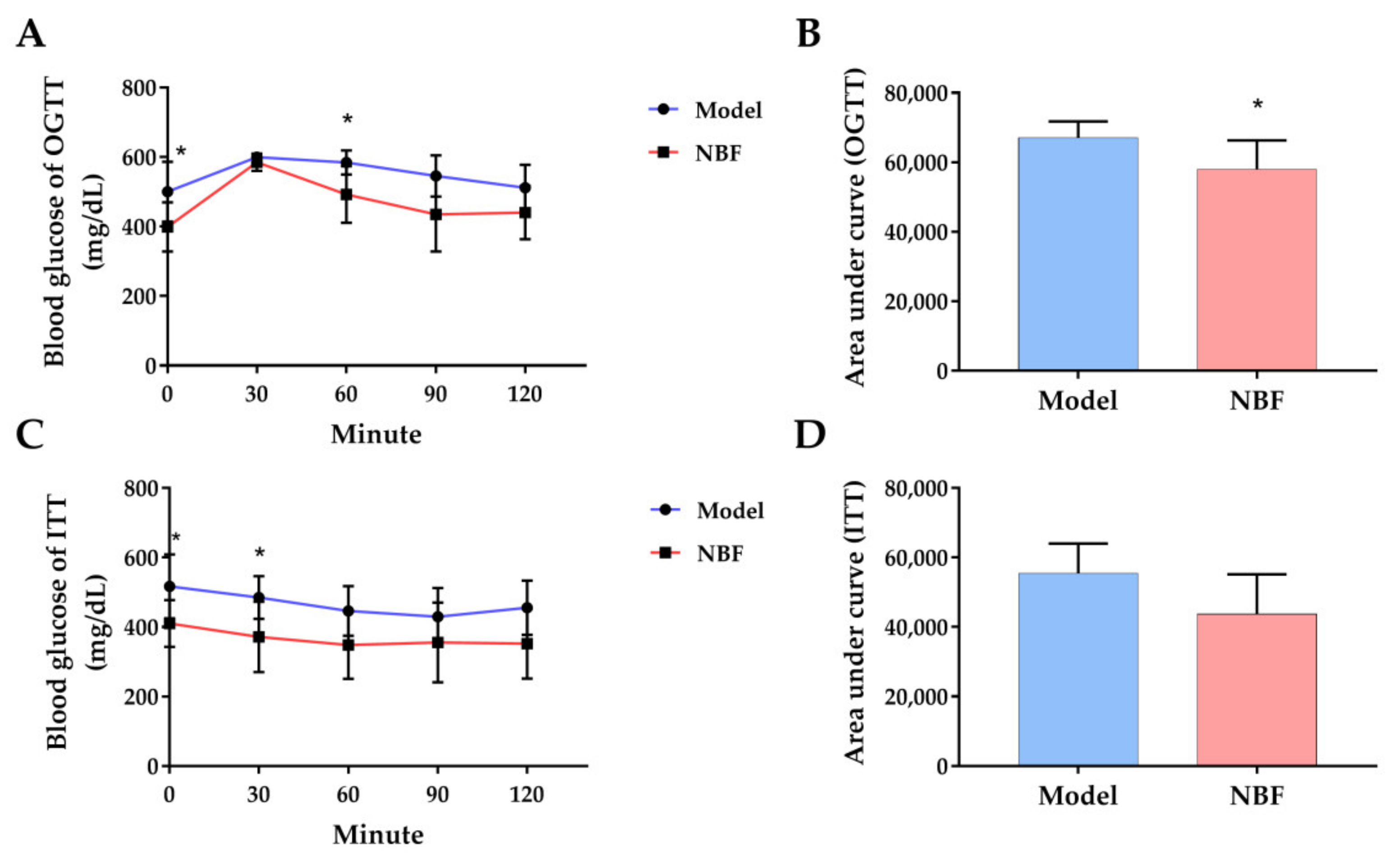
3.3.3. Effects of NBF on Oral Glucose Tolerance Test (OGTT) and Insulin Tolerance Test (ITT)
3.3.4. Effects of NBF on HbA1c, TC and TG Levels
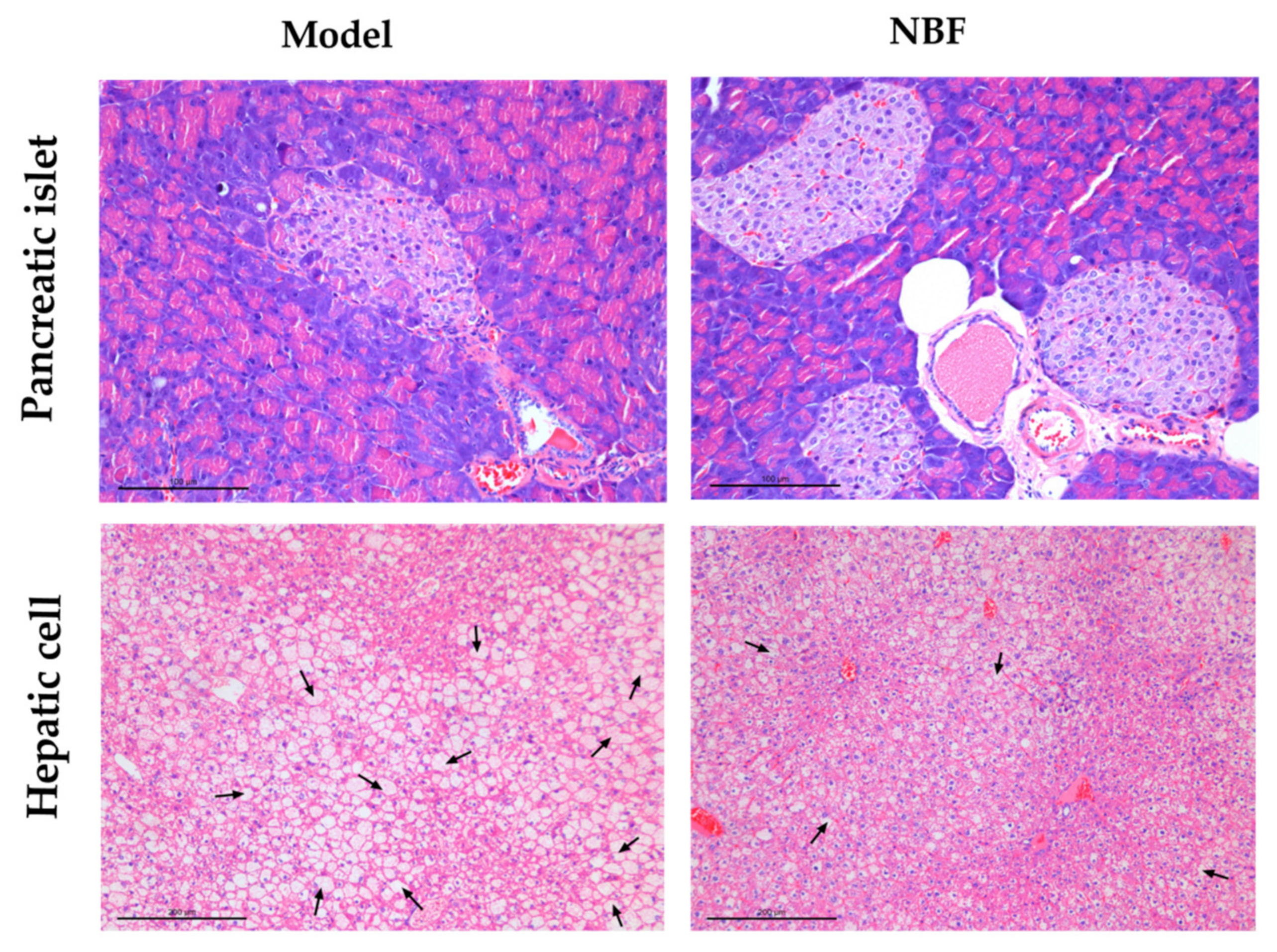
3.3.5. Effects of NBF on Micromorphology of Pancreas and Liver
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leung, P.S. Current research of the RAS in diabetes mellitus. Adv. Exp. Med. Biol. 2010, 690, 131–153. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Ding, Y.; Tanaka, Y.; Zhang, W. Risk factors contributing to type 2 diabetes and recent advances in the treatment and prevention. Int. J. Med. Sci. 2014, 11, 1185–1200. [Google Scholar] [CrossRef] [PubMed]
- Kuroe, A.; Taniuguchi, A.; Fukushima, M.; Nakai, Y.; Ohgushi, M.; Ohya, M.; Seino, Y. Early and late onset side effects of short-acting insulin analogue in seven Japanese diabetic patients. Diabetes Res. Clin. Pract. 2007, 77, 412–413. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.l.; Liu, D.; Jin, Y.; Zhao, J.; Zhao, J.; Li, H.Y.; Li, L.W.; Zhang, H.; Wang, H. In vitro and in vivo inhibitory effect of anthocyanin-rich bilberry extract on α-glucosidase and α-amylase. LWT 2021, 145, 111484. [Google Scholar] [CrossRef]
- Huang, Y.C.; Lin, C.Y.; Huang, S.F.; Lin, H.C.; Chang, W.L.; Chang, T.C. Effect and mechanism of ginsenosides CK and Rg1 on stimulation of glucose uptake in 3T3-L1 adipocytes. J. Agric. Food Chem. 2010, 58, 6039–6047. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Li, D.X.; Lu, D.Y.; Zhang, R.; Zhong, Q.Q.; Zhao, Y.Q.; Zheng, X.X.; Ji, S.; Wang, L.; Tang, D.Q. Amelioration of lipid accumulations and metabolism disorders in differentiation and development of 3T3-L1 adipocytes through mulberry leaf water extract. Phytomedicine 2022, 98, 153959. [Google Scholar] [CrossRef]
- Zheng, W.; Qian, C.; Xu, F.; Cheng, P.; Yang, C.; Li, X.; Lu, Y.; Wang, A. Fuxin Granules ameliorate diabetic nephropathy in db/db mice through TGF-β1/Smad and VEGF/VEGFR2 signaling pathways. Biomed. Pharmacother. 2021, 141, 111806. [Google Scholar] [CrossRef]
- Chang, C.L.T.; Liu, H.Y.; Kuo, T.F.; Hsu, Y.J.; Shen, M.Y.; Pan, C.Y.; Yang, W.C. Antidiabetic Effect and Mode of Action of Cytopiloyne. Evid. Based Complement. Altern. Med. 2013, 2013, 685642. [Google Scholar] [CrossRef]
- Chinese Flora Committee, C.A.S. Flora of China; Science Press: Beijing, China, 1998; Volume 23, p. 154. [Google Scholar]
- Wang, Z.K.; Zhou, M.K. AilaoMateria Medica; Shangxi Science and Technology Press: Taiyuan, China, 1991; p. 54. [Google Scholar]
- Wu, J.R.; Qiu, D.W. Colored Illustration of Commonly Used Chinese Traditional and Herbal Drugs; Guizhou Science and Technology Publishing House: Guiyang, China, 1993; p. 170. [Google Scholar]
- Guan, Y.X.; Yang, X.S.; Tong, L.H.; Yang, B.; Hao, X.J. Chemical constituents in Ficus tikoua of Miao nationality. Chin. Tradit. Herb. Drugs 2007, 38, 342–344. [Google Scholar]
- Fu, G.; Li, W.; Huang, X.; Zhang, R.; Tian, K.; Hou, S.; Li, Y. Antioxidant and alpha-glucosidase inhibitory activities of isoflavonoids from the rhizomes of Ficus tikoua Bur. Nat. Prod. Res. 2018, 32, 399–405. [Google Scholar] [CrossRef]
- Guo, L.J.; Tan, X.Q.; Zheng, W.; Kong, F.F.; Ping, L.; Ni, D.J. Chemical constituents of Ficus tikoua. Chin. Tradit. Herb Drugs 2011, 42, 1709–1711. [Google Scholar]
- Wei, S.P.; Lu, L.N.; Ji, Z.Q.; Zhang, J.W.; Wu, W.J. Chemical constituents from Ficus tikoua. Chem. Nat. Compd. 2012, 48, 484–485. [Google Scholar] [CrossRef]
- Liu, Y.L.; Ye, S.F.; Yang, Y.; Fang, L.H.; Zhang, Z.B.; Xie, G.F. Screening of main antioxidant components in n-butanol extract from the stems of Ficus tikoua Bur. Food Mach. 2020, 36, 176–181. [Google Scholar] [CrossRef]
- Wei, S.P.; Wu, W.J.; Ji, Z.Q. New Antifungal Pyranoisoflavone from Ficus tikoua Bur. Int. J. Mol. Sci. 2012, 13, 7375–7382. [Google Scholar] [CrossRef]
- Tian, M.Y.; Zhao, X.G.; Wu, X.; Hong, Y.; Zhou, Y. Chemical Composition, Antibacterial and Cytotoxic Activities of the Essential Oil from Ficus tikoua Bur. Rec. Nat. Prod. 2020, 14, 219–224. [Google Scholar] [CrossRef]
- Xi, W.; Wang, P.; Li, S.Z.; Song, Q.S. Chemical Constituents of Rhizomes of Ficus tikoua Bur. Nat. Prod. Res. Dev. 2011, 23, 270–272. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, R.; Zhang, K.; Chen, X.; Zhang, Y. Antioxidant, Hypoglycemic and Molecular Docking Studies of Methanolic Extract, Fractions and Isolated Compounds from Aerial Parts of Cymbopogon citratus (DC.) Stapf. Molecules 2022, 27, 2858. [Google Scholar] [CrossRef]
- Ding, H.; Wu, X.; Pan, J.; Hu, X.; Gong, D.; Zhang, G. New Insights into the Inhibition Mechanism of Betulinic Acid on α-Glucosidase. J. Agric. Food Chem. 2018, 66, 7065–7075. [Google Scholar] [CrossRef]
- Hu, C.M.; Luo, Y.X.; Wang, W.J.; Li, J.P.; Li, M.Y.; Zhang, Y.F.; Xiao, D.; Lu, L.; Xiong, Z.; Feng, N.; et al. Synthesis and Evaluation of Coumarin-Chalcone Derivatives as α-Glucosidase Inhibitors. Front. Chem. 2022, 10, 926543. [Google Scholar] [CrossRef]
- Zhang, K.; Chen, X.L.; Zhao, X.; Ni, J.Y.; Wang, H.L.; Han, M.; Zhang, Y.M. Antidiabetic potential of Catechu via assays for α-glucosidase, α-amylase, and glucose uptake in adipocytes. J. Ethnopharmacol. 2022, 291, 115118. [Google Scholar] [CrossRef]
- Akter, S.; Addepalli, R.; Netzel, M.E.; Tinggi, U.; Fletcher, M.T.; Sultanbawa, Y.; Osborne, S.A. Antioxidant-Rich Extracts of Terminalia ferdinandiana Interfere with Estimation of Cell Viability. Antioxidants 2019, 8, 191. [Google Scholar] [CrossRef] [PubMed]
- Vilhena, R.O.; Figueiredo, I.D.; Baviera, A.M.; Silva, D.B.; Marson, B.M.; Oliveira, J.A.; Peccinini, R.G.; Borges, I.K.; Pontarolo, R. Antidiabetic activity of Musa x paradisiaca extracts in streptozotocin-induced diabetic rats and chemical characterization by HPLC-DAD-MS. J. Ethnopharmacol. 2020, 254, 112666. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Y.F.; Li, D.; Yuan, X.P.; Li, Z.W.; Zhang, J.; Ming, X.; Shaw, P.C.; Zhang, C.b.; Kong, A.P.S.; Zuo, Z. Effects of combination treatment with metformin and berberine on hypoglycemic activity and gut microbiota modulation in db/db mice. Phytomedicine 2022, 101, 154099. [Google Scholar] [CrossRef]
- Ren, Z.; Okyere, S.K.; Zhang, M.; Zhang, X.; He, H.; Hu, Y. Glycine Nano-Selenium Enhances Immunoglobulin and Cytokine Production in Mice Immunized with H9N2 Avian Influenza Virus Vaccine. Int. J. Mol. Sci. 2022, 23, 7914. [Google Scholar] [CrossRef]
- Abbasi, S.; Moslehishad, M.; Salami, M. Antioxidant and alpha-glucosidase enzyme inhibitory properties of hydrolyzed protein and bioactive peptides of quinoa. Int. J. Biol. Macromol. 2022, 213, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Proença, C.; Freitas, M.; Ribeiro, D.; Oliveira, E.F.T.; Sousa, J.L.C.; Tomé, S.M.; Ramos, M.J.; Silva, A.M.S.; Fernandes, P.A.; Fernandes, E. α-Glucosidase inhibition by flavonoids: An in vitro and in silico structure-activity relationship study. J. Enzyme Inhib. Med. Chem. 2017, 32, 1216–1228. [Google Scholar] [CrossRef]
- Liu, X.; Zhu, L.; Tan, J.; Zhou, X.; Xiao, L.; Yang, X.; Wang, B. Glucosidase inhibitory activity and antioxidant activity of flavonoid compound and triterpenoid compound from Agrimonia Pilosa Ledeb. BMC Complementary Altern. Med. 2014, 14, 12. [Google Scholar] [CrossRef]
- Sadeghi, M.; Miroliaei, M.; Ghanadian, M. Inhibitory effect of flavonoid glycosides on digestive enzymes: In silico, in vitro, and in vivo studies. Int. J. Biol. Macromol. 2022, 217, 714–730. [Google Scholar] [CrossRef]
- Choi, E.; Baek, S.; Baek, K.; Kim, H.K. Psidium guajava L. leaf extract inhibits adipocyte differentiation and improves insulin sensitivity in 3T3-L1 cells. Nutr. Res. Pract. 2021, 15, 568–578. [Google Scholar] [CrossRef]
- Watson, R.T.; Kanzaki, M.; Pessin, J.E. Regulated membrane trafficking of the insulin-responsive glucose transporter 4 in adipocytes. Endocr. Rev. 2004, 25, 177–204. [Google Scholar] [CrossRef]
- Sinha, K.; Kumar, S.; Rawat, B.; Singh, R.; Purohit, R.; Kumar, D.; Padwad, Y. Kutkin, iridoid glycosides enriched fraction of Picrorrhiza kurroa promotes insulin sensitivity and enhances glucose uptake by activating PI3K/Akt signaling in 3T3-L1 adipocytes. Phytomedicine 2022, 103, 154204. [Google Scholar] [CrossRef]
- Hajiaghaalipour, F.; Khalilpourfarshbafi, M.; Arya, A. Modulation of glucose transporter protein by dietary flavonoids in type 2 diabetes mellitus. Int. J. Biol. Sci. 2015, 11, 508–524. [Google Scholar] [CrossRef] [PubMed]
- Treebak, J.T.; Glund, S.; Deshmukh, A.; Klein, D.K.; Long, Y.C.; Jensen, T.E.; Jørgensen, S.B.; Viollet, B.; Andersson, L.; Neumann, D.; et al. AMPK-mediated AS160 phosphorylation in skeletal muscle is dependent on AMPK catalytic and regulatory subunits. Diabetes 2006, 55, 2051–2058. [Google Scholar] [CrossRef] [PubMed]
- Wanniarachchi, K.K.; Peiris, L.D.C.; Ratnasooriya, W.D. Antihyperglycemic and hypoglycemic activities of Phyllanthus debilis aqueous plant extract in mice. Pharm. Biol. 2009, 47, 260–265. [Google Scholar] [CrossRef]
- Wu, G.J.; Liu, A.B.; Xu, Y.; Wang, Y.; Zhao, L.P.; Hara, Y.; Lam, Y.Y.; Yang, C.S. The Effects of Green Tea on Diabetes and Gut Microbiome in db/db Mice: Studies with Tea Extracts vs. Tea Powder. Nutrients 2021, 13, 3155. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.Y.; Kang, S.; Kim, D.S.; Park, S. Codonopsis lanceolata Water Extract Increases Hepatic Insulin Sensitivity in Rats with Experimentally-Induced Type 2 Diabetes. Nutrients 2017, 9, 1200. [Google Scholar] [CrossRef] [PubMed]
- Saadeldeen, F.S.A.; Niu, Y.; Wang, H.L.; Zhou, L.; Meng, L.J.; Chen, S.T.; Sun-Waterhouse, D.X.; Waterhouse, G.I.N.; Liu, Z.H.; Kang, W.Y. Natural products: Regulating glucose metabolism and improving insulin resistance. Food Sci. Hum. Wellness 2020, 9, 214–228. [Google Scholar] [CrossRef]
- Liu, J.T.; Chen, L.Y.; Shih, M.C.; Chang, Y.; Chen, W.Y. The investigation of recognition interaction between phenylboronate monolayer and glycated hemoglobin using surface plasmon resonance. Anal. Biochem. 2008, 375, 90–96. [Google Scholar] [CrossRef]
- Stratton, I.M.; Adler, A.I.; Neil, H.A.; Matthews, D.R.; Manley, S.E.; Cull, C.A.; Hadden, D.; Turner, R.C.; Holman, R.R. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): Prospective observational study. BMJ 2000, 321, 405–412. [Google Scholar] [CrossRef]
- Clark, A.; Bown, E.; King, T.; Vanhegan, R.I.; Turner, R.C. Islet changes induced by hyperglycemia in rats. Effect of insulin or chlorpropamide therapy. Diabetes 1982, 31, 319–325. [Google Scholar] [CrossRef]
- Hazlehurst, J.M.; Woods, C.; Marjot, T.; Cobbold, J.F.; Tomlinson, J.W. Non-alcoholic fatty liver disease and diabetes. Metabolism 2016, 65, 1096–1108. [Google Scholar] [CrossRef] [PubMed]
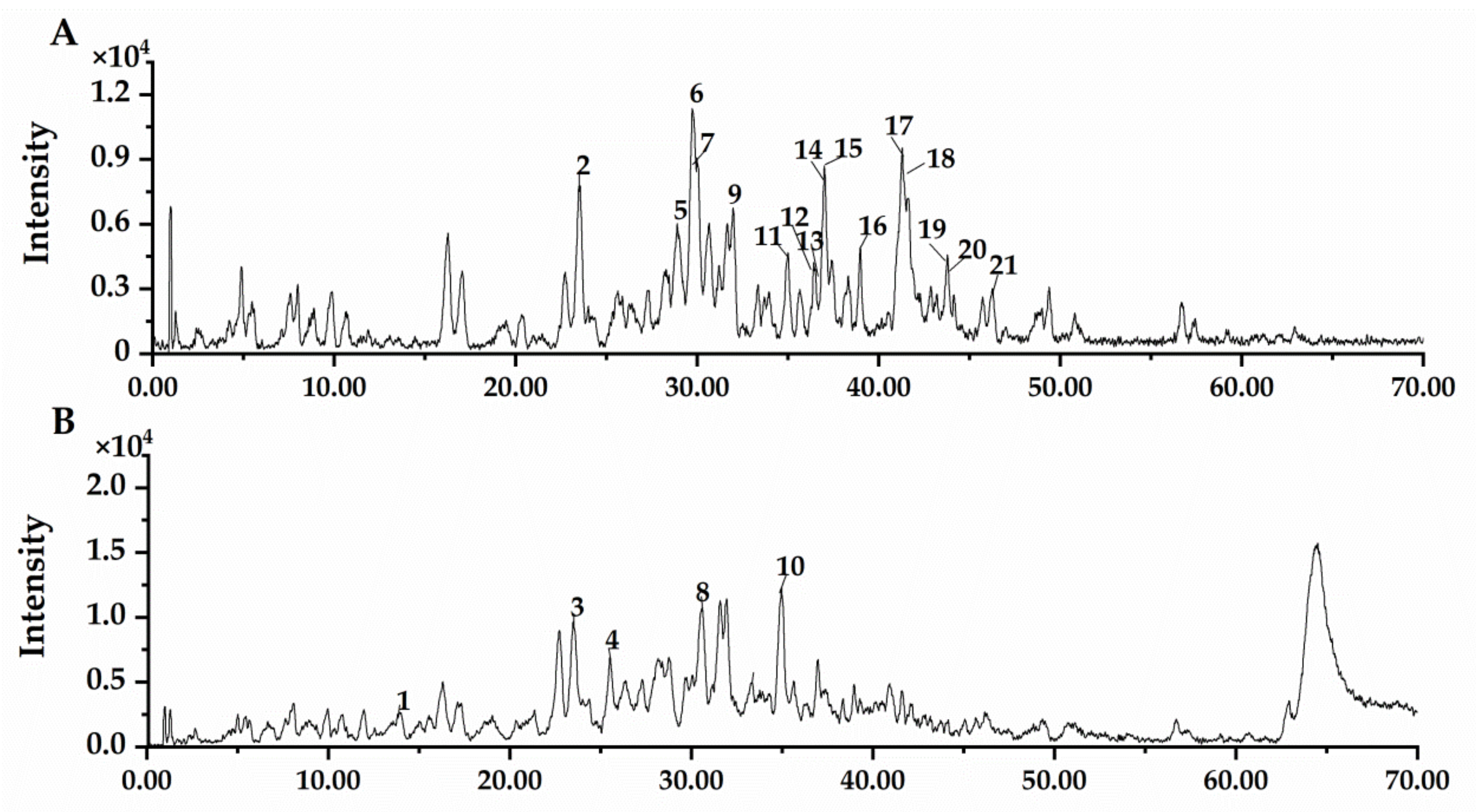


| Category | Peak | RT (min) | Identified Compounds | Ionization (ESI−/ESI+) | m/z | Molecular Formula | Error [ppm] |
|---|---|---|---|---|---|---|---|
| Flavonoids | 3 | 23.51 | 3S-(-)-Mucronulatol-7-D- glucopyranoside | [M + H]+ | 465.1739 | C23H28O10 | −3.5 |
| 4 | 25.54 | Juglanin | [M + H]+ | 419.0956 | C20H18O10 | −3.96 | |
| 5 | 29.01 | 6-Hydroxykaempferol-7-O- glucoside | [M-H]− | 463.0887 | C21H20O12 | 1.01 | |
| 6 | 29.73 | Equisetrin | [M-H]− | 609.1465 | C27H30O16 | 0.59 | |
| 12 | 36.45 | Apigenin bioside | [M + HCOO]− | 593.1513 | C26H28O13 | 0.28 | |
| 13 | 36.499 | 6,8-Bis(C-glucosyl)-apigenin | [M-H]− | 593.1513 | C27H30O15 | 0.26 | |
| 18 | 41.307 | 3,5,7,2’,6’-Pentahydroxy flavonol | [M-H]− | 301.0365 | C15H10O7 | 3.63 | |
| Terpenoids | 1 | 14.02 | Pteroside D | [M + H]+ | 411.1997 | C21H30O8 | −4.34 |
| 2 | 23.49 | Pteroside C | [M + HCOO]− | 441.1776 | C20H28O8 | 2.27 | |
| 7 | 30.07 | Zizyvoside I | [M-H]− | 531.2452 | C25H40O12 | 0.64 | |
| 10 | 34.94 | Nigakihemiacetal E | [M + H]+ | 395.2048 | C21H30O7 | −4.07 | |
| 11 | 35.04 | Icariside B9 | [M + HCOO]− | 417.2142 | C19H32O7 | 3.27 | |
| 15 | 37.088 | Dendroside F | [M + HCOO]− | 475.2193 | C21H34O9 | 1.72 | |
| 16 | 39.004 | Neohancoside A | [M + HCOO]− | 493.2296 | C21H36O10 | 0.94 | |
| 19 | 43.755 | Gibberellin A8 | [M + HCOO]− | 409.1512 | C19H24O7 | 1.65 | |
| 21 | 46.286 | Lucyoside R | [M + HCOO]− | 711.3959 | C36H58O11 | 0.12 | |
| phenylpropanoids | 8 | 30.59 | 3’-Methoxysecoisolariciresinol | [M + H]+ | 392.1835 | C21H28O7 | −3.73 |
| 9 | 31.90 | (+)-Medioresinol Di-O-β-D- glucopyranoside | [M-H]− | 711.2503 | C33H44O17 | 0.13 | |
| 20 | 43.796 | 5-O-Caffeoyl quinic acid butyl ester | [M-H]− | 409.1512 | C20H26O9 | 1.51 | |
| Steroids | 14 | 37.021 | 26-O-β-D-glucopyranosyl(25R)-5α-furostane-12-one-3β,22 α,26-triol-3-O-β-D-glucopyran | [M + HCOO]− | 979.4753 | C45H74O20 | −1.5 |
| Quinones | 17 | 41.274 | Purpurin | [M + HCOO]− | 301.0365 | C14H8O5 | 4.27 |
| Samples | α-Glucosidase Inhibitory Activity IC50 (μg/mL) |
|---|---|
| FEE | 4.46 ± 0.44 b |
| PEF | 2.15 ± 0.09 c |
| EAF | 1.60 ± 0.10 d |
| NBF | 0.89 ± 0.04 f |
| AF | >32.00 |
| NBF1 | 5.23 ± 0.37 a |
| NBF2 | 0.32 ± 0.05 g |
| NBF3 | 0.35 ± 0.03 g |
| NBF4 | 1.30 ± 0.08 e |
| NBF5 | 4.28 ± 0.16 b |
| Acarbose | 0.01 ± 0.00 h |
| Model | NBF | |
|---|---|---|
| HbA1c (%) | 8.84 ± 0.7118 | 6.52 ± 0.5463 * |
| TC (mmol/L) | 9.82 ± 0.7846 | 10.09 ± 0.6905 |
| TG (mmol/L) | 3.67 ± 0.5936 | 3.68 ± 0.2363 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Zhang, K.; Chen, X.; Han, M.; Lu, J.; Zhang, Y. In Vitro and In Vivo Evaluation of Antidiabetic Properties and Mechanisms of Ficus tikoua Bur. Nutrients 2022, 14, 4413. https://doi.org/10.3390/nu14204413
Wang H, Zhang K, Chen X, Han M, Lu J, Zhang Y. In Vitro and In Vivo Evaluation of Antidiabetic Properties and Mechanisms of Ficus tikoua Bur. Nutrients. 2022; 14(20):4413. https://doi.org/10.3390/nu14204413
Chicago/Turabian StyleWang, Hanlei, Kun Zhang, Xuelin Chen, Mei Han, Jing Lu, and Yumei Zhang. 2022. "In Vitro and In Vivo Evaluation of Antidiabetic Properties and Mechanisms of Ficus tikoua Bur." Nutrients 14, no. 20: 4413. https://doi.org/10.3390/nu14204413
APA StyleWang, H., Zhang, K., Chen, X., Han, M., Lu, J., & Zhang, Y. (2022). In Vitro and In Vivo Evaluation of Antidiabetic Properties and Mechanisms of Ficus tikoua Bur. Nutrients, 14(20), 4413. https://doi.org/10.3390/nu14204413





