Abstract
Prenatal nutrition is associated with offspring autism spectrum disorder (herein referred to as autism), yet, it remains unknown if the association is causal. Triangulation may improve causal inference by integrating the results of conventional multivariate regression with several alternative approaches that have unrelated sources of bias. We systematically reviewed the literature on the relationship between prenatal multivitamin supplements and offspring autism, and evidence for the causal approaches applied. Six databases were searched up to 8 June 2020, by which time we had screened 1309 titles/abstracts, and retained 12 articles. Quality assessment was guided using Newcastle–Ottawa in individual studies, and the Grading of Recommendations Assessment, Development and Evaluation (GRADE) for the body of evidence. The effect estimates from multivariate regression were meta-analysed in a random effects model and causal approaches were narratively synthesised. The meta-analysis of prenatal multivitamin supplements involved 904,947 children (8159 cases), and in the overall analysis showed no robust association with offspring autism; however, a reduced risk was observed in the subgroup of high-quality observational studies (RR 0.77, 95% CI (0.62, 0.96), I2 = 62.4%), early pregnancy (RR 0.76, 95% CI (0.58; 0.99), I2 = 79.8%) and prospective studies (RR 0.69, 95% CI (0.48, 1.00), I2 = 95.9%). The quality of evidence was very low, and triangulation was of limited utility because alternative methods were used infrequently and often not robustly applied.
1. Introduction
Autism spectrum disorder (hereafter “autism”) is a neurodevelopmental condition characterised by early-onset impairment in social communication and restricted and repetitive behaviour [1]. Prenatal nutrition may be a modifiable risk factor, which creates a potential target for prevention strategies and may reduce the significant public health implications of this condition. Autism in the U.K. is estimated to cost GBP 27 billion (EUR 29.8 billion) annually for health, education, and social care and in lost productivity [2] despite a modest prevalence of around 1.5% [3].
Previous reviews and meta-analysis reported a reduced risk of offspring autism associated with prenatal folic acid (FA) or multivitamin supplements [4,5], but as the conventional rhetoric states: “correlation does not imply causation”. The conditions necessary for estimating causality are greatly debated, and although randomised controlled trials are considered to be the gold-standard, their utility in nutritional epidemiology is limited because of ethical, financial, and practical barriers. For example, as the prevalence of autism is only 1.5%, a large sample size is necessary for adequate statistical power, but acquiring one is financially burdensome [6]. Conversely, a causal inference from non-experimental studies is problematic, largely due to bias [7]. For example, individuals may overreport compliance with prenatal nutritional supplements, creating a misclassification bias, or confounders, which influence both the exposure and outcome, create a spurious association.
Aetiological triangulation recognises that all approaches have bias and then exploits unrelated sources of bias to “test” the consistency of results [7]. Causal inferences are strengthened if we have consistent results across multiple approaches with different sources of bias, as the biasmay vary across different study designs, methods or analytical approaches. Some examples include conventional multivariate regression, gene-nutrient interactions, discordant sibling studies, cross-context comparisons and negative controls (see Lawlor et al. for an overview of approaches in triangulation [7]). We used the term “alternative causal approaches” to refer to the abovementioned approaches that are alternatives to conventional multivariate regression.
Evidence from alternative causal approaches is unsystematically synthesised in previous reviews, if at all, which limits transparency. As alternative causal approaches are increasingly applied within studies and may significantly alter causal reasoning, this evidence should be integrated in an explicit and scientifically rigorous process. This aligns with guidance from Cochrane [8] and a recent guideline on systematic aetiological reviews of observational studies [9]. We first reviewed the overall evidence from studies using conventional multivariate regression to investigate the association between prenatal nutritional status and autism in offspring. Second, we narratively synthesised the causal approaches. Lastly, we updated the search and addressed the limitations of previous reviews such as double-counting individual studies [5,10], or using the DerSimonian and Laird estimator, which can underestimate uncertainty [4,5].
2. Materials and Methods
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) were followed [11] (Supplementary Table S1). The review protocol was registered on the Prospective Register of Systematic Reviews, registration number: CRD42019154613. Available online: https://www.crd.york.ac.uk/prospero (accessed on 23 July 2021).
2.1. Inclusion and Exclusion Criteria
The study designs were trials, cohort, case-controls and cross-sectional studies of any duration. We focused on the use of folic acid and multivitamin supplements (hereafter simply “multivitamin supplement”) in women during preconception and prenatal periods because folic acid is generally sourced from a multivitamin supplement [4]. Comparators were high versus low or no supplement intake. The outcome was offspring autism diagnosis based on the Diagnostic and Statistical Manual of Mental Disorders, International Classification of Disease, and health registers. There were no date limitations, but non-English language and animal studies were excluded.
2.2. Study Identification and Selection
The search strategy and selection of databases were guided by an information scientist with expertise in systematic reviews. Search strategies were adapted to each database. The following databases were searched from the earliest date to 8 June 2020; MEDLINE (OVID), EMBASE (OVID), PsycINFO (EBSCO), Web of Science core collection, Open Grey and BioRix. See Table 1 for MEDLINE search strategy.

Table 1.
Search strategy for MEDLINE (OVID).
Titles and abstracts were screened, full articles reviewed, and quality assessment was completed twice independently for each study, by C.F., T.B., and M.S. Disagreements were resolved through discussion and adjudicated, by J.A. Data were extracted by C.F. using a standardised form comprised of, author, year of publication, country and cohort, study design, sample size, age of participants, nutritional supplement, measure of autism, covariates, results, and causal approach.
2.3. Quality Assessment
The Newcastle–Ottawa Scale guided the quality assessment of each observational study. Scores range from 0 to 9, where a score of 7–9 was considered high quality in the subgroup analysis and consistent with similar previous reviews [5,12]. The Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach was used to rate the body of evidence based on the degree of certainty in the result [8].
2.4. Data Synthesis and Analysis
We narratively synthesised studies that were inappropriate to meta-analysis. Additionally, we summarised the alternative approaches applied in table format.
2.5. Meta-Analysis
We conducted a meta-analysis of the fully adjusted effect estimates in a random effects model using the Hartung–Knapp–Sidik–Jonkman estimator [8]. Analyses of nutritional supplements were pooled if the exposure was categorical: no or low supplement intake as the reference category compared against supplement use. Autism is a rare outcome, so we assumed the odds ratio (OR) and hazard ratio (HR) were directly comparable to the relative risk (RR) [13]. The heterogeneity was measured with the Cochrane’s q and I2 statistics. The interpretation of heterogeneity (I2) was guided by Cochrane’s reference ranges [8]. Prediction intervals estimated the range of effect estimates that may be expected in individual settings that could improve the application of the research findings. These are distinct from the summary effect and 95% confidence intervals (CIs) that estimate the average effect of the exposure [8,14,15]. The R version 3.6.3 packages used were “meta” and “forestplots” [16]. Statistical tests of significance were 2-sided with an α of 0.05.
2.6. Sensitivity Analysis
Sources of heterogeneity were explored through the identification of outliers, leave-one-out analysis and subgroup analysis if there were ≥10 studies. A random effects model was used to estimate between and within subgroup effects. The pre-defined subgroups were study quality, study design (prospective/retrospective), region, mandatory fortification (yes/no), and stage of pregnancy, which was defined as the first trimester, compared against any point in pregnancy, if the nutritional supplement exposure period was undefined. Between-subgroup differences were measured with the q statistic and significance was indicated by p < 0.1 (2-sided). Small-study publication bias was assessed through the inspection of the funnel plot and Egger’s test [17]. The widely used DerSimonian and Laird estimator may underestimate uncertainty [8], but to facilitate comparison with previous research, we applied it in sensitivity analyses.
3. Results
3.1. Identification of Studies
A total of 1309 titles were identified with 342 duplicates, leaving 967 titles and abstracts to be screened (Figure 1). Of these, 897 were excluded based on title and abstract review, leaving 70 for a full-text review, of which 13 met the inclusion criteria. However, two reports were duplicated [18,19], and so the larger cohort was retained [19] leaving 12 studies in the final review, 10 of which were meta-analysed. The other two were narratively synthesised because the reference category was not low or no supplement use [20,21].
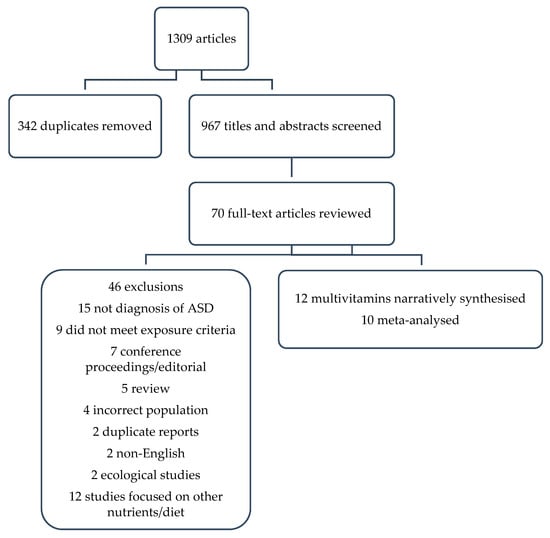
Figure 1.
PRISMA flow chart of study selection.
3.2. Quality Assessment
Based on the Newcastle–Ottawa Scale, five [20,22,23,24,25] of seven cohort studies [19,20,22,23,24,25,26] were of high quality, and two [27,28] of five case-control studies [21,27,28,29,30] were of high quality (Supplementary Tables S2 and S3). The quality of the body of evidence based on GRADE was very low (Table 2). The details for the evidence profile and rationale for GRADE rating are provided in Supplementary Tables S4 and S5. For a summary of studies and results, see Table 3.

Table 2.
Summary of GRADE evaluation.

Table 3.
Study characteristics and results from individual studies [19,20,21,22,23,24,25,26,27,28,29,30].
3.3. Meta-Analylic Results
A total of 904,947 children including 8159 cases from six countries were included in the meta-analysis. All studies measured nutritional supplements and one included fortified food. The multivitamin dose was seldom reported.
Overall, there was no robust evidence to associate taking prenatal multivitamins with autism risk compared to no/low intakes (RR 0.74, 95% CI: 0.53, 1.04) (Figure 2). The confidence intervals (CI) were wide, and there was considerable heterogeneity (I2 = 94.3%, p < 0.001). Egger’s test (p = 0.44) and inspection of the funnel plot suggests no evidence of asymmetry (Supplementary Figure S1). The precision increased when the DerSimonian and Laird estimator was applied compared to the Hartung–Knapp–Sidik–Jonkman estimator (RR 0.74, 95% CI: 0.57, 0.95, I2 = 94.3%). Upon removal of the outlier, DeSoto and Hitlan, there was a 32% reduced risk of autism (RR 0.68, 95% CI: 0.51, 0.91, I2 = 94.7%, p < 0.001); although heterogeneity remained considerable. There were no other influential studies (Supplementary Table S7). The 95% prediction interval indicated that the dispersion in the distribution of effect estimates was large and ranged from a reduced to an increased risk of autism (RR 0.21, 2.59).
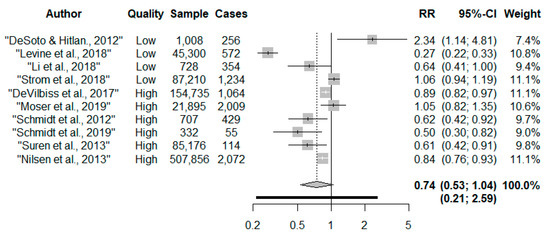
Figure 2.
Forest plot of maternal multivitamin supplements and risk of offspring autism [19,21,22,23,24,25,26,27,28,30].
Subgroup Analysis
See Supplementary Figures S2–S6. In high-quality observational studies there was a 23% reduced risk of autism associated with maternal multivitamin use, and heterogeneity was reduced from considerable to substantial (RR 0.77, 95% CI: 0.62, 0.96, I2 = 62.4%). No association was observed in low-quality studies (RR 0.78, 95% CI: 0.33, 1.86, I2 = 97.9%), and there were no between-subgroup differences detected Q = 0.00, p = 0.98). Some evidence of association was observed in prospective studies (RR 0.69, 95% CI: 0.48, 1.00, I2 = 95.9%), whilst no association was evident in the retrospective studies (RR 0.93, 95% CI: 0.41, 2.11, I2 = 81.7%). There were no differences between subgroups for study design (Q = 0.42, p = 0.51). Subgroup analysis of regions reduced heterogeneity from “considerable” in Nordic (RR 0.87, 95% CI: 0.72, 1.06, I2 = 76.8%) and American (RR 0.87, 95% CI: 0.5, 2.15, I2 = 84.5 %) studies. Asian countries had the largest effect estimate, but there was considerable heterogeneity (RR 0.56, 95% CI: 0.26, 1.23, I2 = 97.1%). No differences between subgroups were detected (Q = 1.14, p = 0.57). Subgroup analysis of the stage of pregnancy showed similar effect estimates in each group (early pregnancy: RR 0.76, 95% CI: 0.58, 0.99, I2 = 79.8%; any stage of pregnancy: RR 0.78, 95% CI: 0.40, 1.53, I2 = 96.6%). There were no between-subgroup differences (Q = 0.01, p = 0.93). Regions without mandatory fortification produced a stronger reduction in the risk of autism (RR 0.71, CI 95%: 0.50, 1.02, I2 = 96%), compared to regions with mandatory fortification (RR 0.87, CI 95%: 0.35, 2.15, I2 = 84%). There were no between-subgroup differences detected (Q = 1.14, p = 0.57).
3.4. Causal Approaches
All studies [19,22,23,24,25,26,27,28,29,30] measured the association between prenatal nutrition and autism using conventional multivariate regression. Alternative causal approaches (one discordant sibling analysis, two negative controls studies, and one genetic interaction study) were infrequently applied (Table 4) and were generally used as a secondary analysis to conventional multivariate regression. A detailed summary of the key sources of bias in each approach can be read in Supplementary Table S6.

Table 4.
Summary of causal approaches which demonstrates multivariate regression was commonly applied whilst all other approaches were infrequently used.
4. Discussion
Prenatal multivitamin supplements were not robustly associated with autism in the overall meta-analysis. However, a reduced risk of autism was observed in high-quality studies, prospective studies, early pregnancy and following the removal of an outlier. In contrast with previous meta-analyses, we did not observe any strong evidence of association in our main results (using all studies) due to the selection of the Hartung–Knapp–Sidik–Jonkman estimator, rather than DerSimonian–Laird which underestimates uncertainty and was applied in previous meta-analysis [4,5,31]. We also identified an additional two studies [21,27]. However, although some associations were identified, based on GRADE the degree of certainty was very low owing to the inherent risk of bias in observational study designs, considerable heterogeneity, and unexplained inconsistency in the direction of effect. As GRADE does not easily incorporate alternative causal approaches [32], we structured the discussion first to discuss the limitations identified through the application of GRADE. Second, we evaluated whether the alternative causal methods had been of value to the interpretation of causality.
Regional variation in baseline nutritional status and genotype [5,33] may be major contributors to both heterogeneity and inconsistency in the direction of the effect. Nutrients confer a benefit to health until physiological requirements are satisfied; thereafter, we observe a plateau effect, and toxicity or deficiency occurs when intakes are extreme [34]. To illustrate this potential U-shaped relationship, we considered baseline folate status. Studies in this review from Nordic countries generally showed effect estimates closest to the null, except Norway [24,25]. The Nordic associations correlated with rates of plasma folate deficiency/insufficiency, which were reported to be 0.7% in Denmark [35], 4% in Sweden [36], but 24.9% in a subsample of the Norwegian mother, father and child cohort [37]. However, the comparisons between supplement use and plasma folate levels were drawn from different populations, and population heterogeneity and confounding may have caused variance at the individual level. Future studies should consider the response to nutritional supplements in relation to baseline nutritional status.
Furthermore, from the reviewed studies, only the U.S. implements mandatory fortification of diet with folic acid and so has a high baseline folic acid intake. Less than 1% of its population has had deficient plasma folate levels since the introduction of mandatory fortification in 1998 [38], but this may have reduced the benefits from supplements since physiological requirements are already met. However, a plateau effect was not observed. Instead, two U.S. studies found a reduced risk of autism associated with multivitamin supplements [23,28], and two studies observed an increased risk of autism [20,29]. There is much uncertainty and debate as to whether toxicity could occur through the combined effects of mandatory fortification and supplementation with folic acid. [39]. Wiens and DeSoto (2017) argued that excessive intake saturates metabolic pathways, leading to an accumulation of unmetabolized folic acid which may cause autism [39]. Furthermore, folic acid is absorbed more readily than the folate form and is used in supplements and fortified food which the authors argued further exacerbates excessive intake. However, other research groups found inconclusive evidence to support such claims, even though limitations in available evidence were acknowledged [40].
The two studies in this review that observed a reduced risk of autism had folic acid intakes well in excess of the recommended 400 ug/day [23,28], yet they focused on early pregnancy when, it is speculated, folic acid requirements and tolerance to high doses is greater. An alternative explanation for the increased risk of autism is “birth order bias” [41]. Autism and supplement use can be positively correlated independently, as supplement use is usually greater in first pregnancies, and families affected by autism have fewer children. Indeed, an increased risk of autism in Moser et al. was attenuated to the null with restrictions to first-born boys [27].
An additional source of heterogeneity is variation in the stage of pregnancy when the multivitamin supplement was taken. In early pregnancy it was associated with a lower risk of autism and lower heterogeneity. Periconception is an established critical period in which FA can reduce the incidence of neural tube defects by up to 70% [42]. Furthermore, FA in periconception may reduce the risk of low birth weight, small gestational age size, stillbirth, neonatal mortality, preeclampsia and miscarriage [42].
Lastly, autism is a heterogeneous condition and specific features might have differential causal pathways [43], which may contribute to varied results. All studies that stratified by severity, observed a greater magnitude of association with more severe forms of autism [21,22,24,26] as defined by minimal verbal status at age 3 [24], low intelligence quotient [22,26], or high autism symptom severity [21].
4.1. Alternative Causal Approaches
4.1.1. Multivariate Regression
Alternative causal approaches were infrequently applied, and the application was often not robust; therefore, our ability to triangulate the findings was limited. All studies used multivariate regression, a key assumption of which is no residual confounding [7]. However, there was such a risk in this review as studies were adjusted for many, but seldom all, key confounders. The studies commonly adjusted formaternal age, physical or mental health, socioeconomic status, parity, planned pregnancy, pre-pregnancy BMI, and health behaviour. Due to the risk of residual confounding we attempted to triangulate the results from the multivariate regression with alternative approaches that have different sources of bias. However, only the gene-nutrient interaction analysis provided useful results.
4.1.2. Gene-Nutrient Interaction
In Schmidt et al., an association between folate use and autism risk was only observed if the mother/child had the methylenetetrahydrofolate reductase (MTHFR) 677 C > T genotype, which is unlikely owing to confounding by socioeconomic and lifestyle characteristics [28]. The MTHFR 677 C > T genotype encodes for a less-efficient enzyme to metabolise folate. Hence, larger folate doses may be necessary to overcome inefficient enzymatic function [28]. Although these findings are yet to be replicated in larger samples, there is consistency in the wider literature. A recent meta-analysis identified an 86% increased risk of autism associated with the less-efficient genotype (TT genotype verses CC genotype: OR 1.86, 95% CI: 1.12, 2.18) [44]. Furthermore, this association was not evident in countries that had a higher intake of folic acid secondary to mandatory folic acid fortification. This may imply that a genotype influences the response to supplements or mandatory folic acid fortification.
4.1.3. Discordant Sibling Analysis
Discordant sibling analyses may overcome shared unmeasured confounding despite several key methodological considerations. In DeVilbiss et al., the reduced risk of autism observed in their main analysis was attenuated by the discordant sibling analysis [22]. Sibling comparison studies are a quasi-experimental study design intended to remove shared familial confounding by matching siblings discordant for the outcome. However, this includes shared genetic risk factors [7], and siblings share, on average, 50% of their genetic material. As indicated in Schmidt et al.’s study, it may be the combination of MTHFR 677 C > T genotype and no folic acid supplements that led to an increased risk of autism. Thus, this sibling comparison may have adjusted for a causal component. Furthermore, there is a high type II error rate as only siblings discordant for the exposure and outcome contribute to the effect estimate in conditional logistic regression models [45]. DeVilbiss et al.’s discordant sibling analysis may be underpowered because the confidence intervals were wider even though the point estimate was consistent with the main analysis. Furthermore, random error can be amplified in discordant sibling analysis and bias towards the null. Conversely, time-varying confounders should be adjusted as matching on siblings does not account for this [45]. Thus, the null association observed here should be interpreted with caution as it could reflect a type II error or adjustment for a causal component, MTHFR genotype.
4.1.4. Negative Control
In two studies, negative controls were applied and acted as mock exposures that indicate the presence of bias without relying on the assumption of no unmeasured confounding [46]. Instead, they depend on the assumption that confounding, and sometimes other biases, were similar in exposure and negative control analyses. A further assumption was that the negative control had no plausible relationship with the outcome. Therefore, the exposure–outcome relationship could be distinguished from bias by comparing the strength of the association with the negative control analysis [7], but the assumptions were empirically untestable. Thus, we were limited to a subjective interpretation based on subject knowledge [46] which is presented here.
Suren et al. measured an association with multivitamin supplements, but not fish oils [24] even though they are a rich source of polyunsaturated fatty acids, especially omega-3s, and cod liver oil is a rich source of vitamin D, both of which are associated with positive neurodevelopmental outcomes [47,48]. Conversely, cod liver oil is a rich source of vitamin A which is potentially teratogenic and can harm foetal development [49]. Thus, this negative control violates the assumption of no plausible relationship with the outcome, which confused the interpretation. Similarly, Levine et al.’s negative control may have had a relationship with the exposure. The authors compared mutually exclusive groups for multivitamins: two years prior to pregnancy, two years prior to pregnancy and during pregnancy, and during pregnancy only. All groups were associated with a reduced risk of autism, yet the association was strongest and most similar for “two years prior to pregnancy” and “two years prior to pregnancy and during pregnancy”. No “wash-out” period was used, so two years prior to pregnancy may have included the potentially critical period, preconception. Furthermore, the assumption of similar bias may not be met either since women who discontinue supplements during pregnancy may have different characteristics to women who adhere to health advise and take the supplements during pregnancy. Thus, we lacked confidence in this negative control.
4.1.5. Triangulation
Collectively, triangulation as a strategy to further causal interpretation was limited, many due to infrequent use and limitations in applying alternative approaches. The multivariate regression and gene-nutrient interaction [24] findings suggest there could have been a causal association, and we feel this warrants further investigation. However, within the context of this review, the discordant sibling analysis [22] and negative control analyses [24,26] were of limited utility. The former needs to be conducted on larger samples and adjusted for a range of time-varying confounders, and the choice of the latter should be given careful consideration.
4.2. Strengths and Limitations
This review has several strengths. Numerous steps to reduce bias were taken, such as searching grey literature sources, applying GRADE guidelines, calculating prediction intervals and using the Hartung–Knapp–Sidik–Jonkman estimator. However, the strongest advance was a formal narrative synthesis of the range of causal approaches. As an explicit approach, it provided transparent evidence of the approaches applied, their findings and outlined areas for future studies. Nonetheless, there were weaknesses, mainly the heterogeneity and inconsistency observed across studies and the low study numbers. The prediction intervals indicated if there had been an effect, it could have ranged from beneficial to null or even harmful in individual settings.
5. Conclusions
At present, the evidence is inconclusive, and we are unable to confirm a causal association between prenatal multivitamin supplement use and autism in offspring. Future studies should improve the study design and data analyses through adequately powered prospective birth cohorts. The measurement of nutritional supplements could be improved through reporting their nutrient composition, dose, compliance and duration and timing of use, which are all known to affect biological responses but were rarely considered in the studies we reviewed. Furthermore, there should be greater consideration of the complexity of nutrition by modelling U-shaped relationships and considering how the response to nutrients is altered by variations in baseline requirements and whether it is affected by recent nutrient intake, changes to physiological demand in early pregnancy, or genetic variation. Lastly, we recommend the application of alternative approaches within a triangulation framework to gauge causality better.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/nu13082558/s1, Figure S1: Multivitamins: funnel plot, Figure S2: Multivitamin subgroup analysis by study quality, Figure S3: Multivitamin subgroup analysis by study design, Figure S4: Multivitamin subgroup analysis by region, Figure S5: Multivitamins subgroup analysis by stage of pregnancy, Figure S6: Multivitamins subgroup analysis by mandatory fortification. Table S1: Preferred Reporting Items for Systematic Reviews and Meta-Analyses, Table S2: Quality assessment using the Newcastle Ottawa Scale: case-control, Table S3: Quality assessment using the Newcastle Ottawa Scale: cohort, Table S4: GRADE evidence profile, Table S5: Optimal information size, Table S6: Summary of the key sources of bias for each causal approach, Table S7: Influential study analysis.
Author Contributions
C.F. was the lead author and the led all stages, including conceptualisation, methodology, formal analysis, and all stages of writing. Additionally, R.D., A.H.L. and J.J.A. provided supervision of the conceptualisation, methodology, formal analysis, and writing—review and editing. They also reviewed and critically revised the article for intellectual content. Furthermore, J.J.A. contributed to the formal data analysis by acting as adjudicator to resolve disputes over study inclusion. A.H. assisted with the methodology and formal analysis and writing—review and editing. T.B. assisted in the screening of titles and abstracts, full-text reviews and quality assessment; and writing—review and editing. M.S. assisted in the screening of titles and abstracts, full-text reviews and quality assessment; and writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Medical Research Council (MC_UU_00022/2 to R.D., C.F. and A.H.L., and 304823-02 to C.F.); the Scottish Government Chief Scientist Office (SPHSU17 to R.D., C.F. and A.H.L.); and the University of Glasgow (MC_ST_U18004 to M.S.). A.H. was supported by a career grant from the South-Eastern Norway Regional Health Authority (2018059 and 2020022).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Available on request.
Acknowledgments
We thank Valerie Wells for her expertise in information science and her support in devising the search strategy.
Conflicts of Interest
The authors declare no conflict of interest.
References
- World Health Organization. International Classification of Diseases and Related Health Problems; 11th rev., ICD-11; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Knapp, M.; Romeo, R.; Beecham, J. Economic cost of autism in the UK. Autism 2009, 13, 317–336. [Google Scholar] [CrossRef]
- Lyall, K.; Croen, L.; Daniels, J.; Fallin, M.D.; Ladd-Acosta, C.; Lee, B.K.; Park, B.Y.; Snyder, N.W.; Schendel, D.; Volk, H.; et al. The Changing Epidemiology of Autism Spectrum Disorders. Annu. Rev. Public Health 2017, 38, 81–102. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Francis, E.; Hinkle, S.N.; Ajjarapu, A.S.; Zhang, C. Preconception and Prenatal Nutrition and Neurodevelopmental Disorders: A Systematic Review and Meta-Analysis. Nutrients 2019, 11, 1628. [Google Scholar] [CrossRef] [Green Version]
- Guo, B.-Q.; Li, H.-B.; Zhai, D.-S.; Ding, S.-B. Maternal multivitamin supplementation is associated with a reduced risk of autism spectrum disorder in children: A systematic review and meta-analysis. Nutr. Res. 2019, 65, 4–16. [Google Scholar] [CrossRef]
- Kendall, J.M. Designing a research project: Randomised controlled trials and their principles. Emerg. Med. J. 2003, 20, 164–168. [Google Scholar] [CrossRef] [Green Version]
- Lawlor, D.A.; Tilling, K.; Smith, G.D. Triangulation in aetiological epidemiology. Int. J. Epidemiol. 2016, 45, 1866–1886. [Google Scholar] [CrossRef] [Green Version]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; John Wiley & Sons: Chichester, UK, 2019. [Google Scholar]
- Dekkers, O.M.; Vandenbroucke, J.P.; Cevallos, M.; Renehan, A.G.; Altman, D.G.; Egger, M. COSMOS-E: Guidance on conducting systematic reviews and meta-analyses of observational studies of etiology. PLoS Med. 2019, 16, e1002742. [Google Scholar] [CrossRef]
- Guo, B.Q.; Li, H.B.; Zhai, D.S.; Ding, S.B. Association of maternal prenatal folic acid intake with subsequent risk of autism spectrum disorder in children: A systematic review and meta-analysis. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2019, 94, 109650. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Grp, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int. J. Surg. 2010, 8, 336–341. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Li, K.; Zhao, D.; Li, L. The association between maternal use of folic acid supplements during pregnancy and risk of autism spectrum disorders in children: A meta-analysis. Mol. Autism 2017, 8, 1–4. [Google Scholar] [CrossRef] [Green Version]
- McKenzie, D.P.; Thomas, C. Relative risks and odds ratios: Simple rules on when and how to use them. Eur. J. Clin. Investig. 2020, 50, e13249. [Google Scholar] [CrossRef] [PubMed]
- Riley, R.D.; Higgins, J.P.; Deeks, J.J. Interpretation of random effects meta-analyses. BMJ 2011, 342, d549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- IntHout, J.; Ioannidis, J.P.A.; Rovers, M.M.; Goeman, J.J. Plea for routinely presenting prediction intervals in meta-analysis. BMJ Open. 2016, 6, e010247. [Google Scholar] [CrossRef] [Green Version]
- Gordon, M.; Lumley, T. Advanced Forest Plot Using ‘grid’ Graphics, December 12, 2020. R Package Version 1.10 ed.: CRAN. Available online: https://cran.r-project.org/web/packages/forestplot/forestplot.pdf (accessed on 23 July 2021).
- Duval, S.; Tweedie, R. Trim and Fill: A Simple Funnel-Plot-Based Method of Testing and Adjusting for Publication Bias in Meta-Analysis. Biometrics 2000, 56, 455–463. [Google Scholar] [CrossRef]
- Virk, J.; Liew, Z.; Olsen, J.; Nohr, E.A.; Catov, J.M.; Ritz, B. Preconceptional and prenatal supplementary folic acid and multivitamin intake and autism spectrum disorders. Autism 2016, 20, 710–718. [Google Scholar] [CrossRef] [PubMed]
- Strøm, M.; Granström, C.; Lyall, K.; Ascherio, A.; Olsen, S.F. Research letter: Folic acid supplementation and intake of folate in pregnancy in relation to offspring risk of autism spectrum disorder. Psychol. Med. 2018, 48, 1048–1054. [Google Scholar] [CrossRef] [Green Version]
- Raghavan, R.; Riley, A.W.; Volk, H.; Caruso, D.; Hironaka, L.; Sices, L.; Hong, X.; Wang, G.; Ji, Y.; Brucato, M.; et al. Maternal Multivitamin Intake, Plasma Folate and Vitamin B12 Levels and Autism Spectrum Disorder Risk in Offspring. Paediatr. Perinat. Epidemiol. 2018, 32, 100–111. [Google Scholar] [CrossRef]
- Tan, M.; Yang, T.; Zhu, J.; Li, Q.; Lai, X.; Li, Y.; Tang, T.; Chen, J.; Li, T. Maternal folic acid and micronutrient supplementation is associated with vitamin levels and symptoms in children with autism spectrum disorders. Reprod. Toxicol. 2020, 91, 109–115. [Google Scholar] [CrossRef]
- DeVilbiss, E.A.; Magnusson, C.; Gardner, R.M.; Rai, D.; Newschaffer, C.J.; Lyall, K.; Dalman, C.; Lee, B.K. Antenatal nutritional supplementation and autism spectrum disorders in the Stockholm youth cohort: Population based cohort study. Br. Med. J. 2017, 359, J4273. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, R.J.; Iosif, A.-M.; Angel, E.G.; Ozonoff, S. Association of Maternal Prenatal Vitamin Use With Risk for Autism Spectrum Disorder Recurrence in Young Siblings. JAMA Psychiatry 2019, 76, 391–398. [Google Scholar] [CrossRef]
- Suren, P.; Roth, C.; Bresnahan, M.; Haugen, M.; Hornig, M.; Hirtz, D.; Lie, K.K.; Lipkin, W.I.; Mangus, P.; Reichborn-Kjennerud, T.; et al. Association Between Maternal Use of Folic Acid Supplements and Risk of Autism Spectrum Disorders in Children. JAMA J. Am. Med Assoc. 2013, 309, 570–577. [Google Scholar] [CrossRef] [Green Version]
- Nilsen, R.M.; Gunnes, N.; Alsaker, E.R.; Bresnahan, M.; Hirtz, D.; Hornig, M.; Lie, K.K.; Lipkin, W.I.; Mangus, P.; Reichborn-Kjennerud, T.; et al. Analysis of self-selection bias in a population-based cohort study of autism spectrum disorders. Paediatr. Perinat. Epidemiol. 2013, 27, 553–563. [Google Scholar] [CrossRef] [Green Version]
- Levine, S.Z.; Kodesh, A.; Viktorin, A.; Smith, L.; Uher, R.; Reichenberg, A.; Sadin, S. Association of Maternal Use of Folic Acid and Multivitamin Supplements in the Periods Before and During Pregnancy with the Risk of Autism Spectrum Disorder in Offspring. JAMA Psychiatry 2018, 75, 176–184. [Google Scholar] [CrossRef] [Green Version]
- Moser, S.S.; Davidovitch, M.; Rotem, R.S.; Chodick, G.; Shalev, V.; Koren, G. High dose folic acid during pregnancy and the risk of autism; The birth order bias: A nested case-control study. Reprod. Toxicol. 2019, 89, 173–177. [Google Scholar] [CrossRef]
- Schmidt, R.J.; Tancredi, D.J.; Ozonoff, S.; Hansen, R.L.; Hartiala, J.; Allayee, H.; Schmidt, L.C.; Tassone, F.; Hertz-Picciotto, I. Maternal periconceptional folic acid intake and risk of autism spectrum disorders and developmental delay in the CHARGE (CHildhood Autism Risks from Genetics and Environment) case-control study. Am. J. Clin. Nutr. 2012, 96, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Desoto, M.C.; Hitlan, R.T. Synthetic folic acid supplementation during pregnancy may increase the risk of developing autism. J. Pediat. Biochem. 2012, 2, 251–261. [Google Scholar]
- Li., Y.-M.; Shen, Y.-D.; Li, Y.-J.; Xun, G.-L.; Liu, H.; Wu, R.-R.; Xia, K.; Zhao, J.-P.; Ou, J.-J. Maternal dietary patterns, supplements intake and autism spectrum disorders A preliminary case-control study. Medicine 2018, 97, e13902. [Google Scholar] [CrossRef]
- Iglesias Vazquez, L.; Canals, J.; Arija, V. Review and meta-analysis found that prenatal folic acid was associated with a 58% reduction in autism but had no effect on mental and motor development. Acta Paediatr. Int. J. Paediatr. 2019, 108, 600–610. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Oxman, A.D.; Sultan, S.; Glasziou, P.; Akl, E.A.; Alonso-Coello, P.; Atkins, D.; Kunz, R.; Brozek, J.; Montori, V.; et al. GRADE guidelines: 9. Rating up the quality of evidence. J. Clin. Epidemiol. 2011, 64, 1311–1316. [Google Scholar] [CrossRef]
- Virk, J.; Liew, Z.; Olsen, J.; Nohr, E.A.; Catov, J.M.; Ritz, B. Pre-conceptual and prenatal supplementary folic acid and multivitamin intake, behavioral problems, and hyperkinetic disorders: A study based on the Danish National Birth Cohort (DNBC). Nutr. Neurosci. 2018, 21, 352–360. [Google Scholar] [CrossRef] [Green Version]
- Scientific Committee on Food, Scientific Panel on Dietetic Products Nutrition and Allergies. Tolerable Upper Intake Levels for Vitamins and Minerals; European Food Safety Authority: Parma, Italy, 2006. [Google Scholar]
- Milman, N.; Byg, K.E.; Hvas, A.M.; Bergholt, T.; Eriksen, L. Erythrocyte folate, plasma folate and plasma homocysteine during normal pregnancy and postpartum: A longitudinal study comprising 404 Danish women. Eur. J. Haematol. 2006, 76, 200–205. [Google Scholar] [CrossRef]
- Ohrvik, V.; Lemming, E.W.; Nalsen, C.; Becker, W.; Ridefelt, P.; Lindroos, A.K. Dietary intake and biomarker status of folate in Swedish adults. Eur. J. Nutr. 2018, 57, 451–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nilsen, R.M.; Vollset, S.E.; Monsen, A.L.; Ulvik, A.; Haugen, M.; Meltzer, H.M.; Magnus, P.; Ueland, P.M. Infant birth size is not associated with maternal intake and status of folate during the second trimester in Norwegian pregnant women. J. Nutr. Nutr. Epidemiol. 2010, 140, 572–579. [Google Scholar] [CrossRef]
- Pfeiffer, C.M.; Hughes, J.P.; Lacher, D.A.; Bailey, R.L.; Berry, R.J.; Zhang, M.; Yetley, E.A.; Rader, J.I.; Sempos,, C.T.; Johnson, C.L.; et al. Estimation of trends in serum and RBC folate in the US population from pre- to postfortification using assay-adjusted data from the NHANES 1988-2010. J. Nutr. 2012, 142, 886–893. [Google Scholar] [CrossRef] [PubMed]
- Wiens, D.; DeSoto, M.C. Is High Folic Acid Intake a Risk Factor for Autism?—A Review. Brain Sci. 2017, 7, 149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scientific Advisory Committee on Nutrition (SACN). Folic Acid: Updated SACN Recommendations; Public Health England: London, UK, 2017. [Google Scholar]
- Koren, G.; Moser, S.S. Does high-dose gestational folic acid increase the risk for autism? The birth order hypothesis. Med. Hypotheses 2019, 132, 109350. [Google Scholar] [CrossRef]
- Stephenson, J.H.N.; Hall, J.; Schoenaker, D.A.J.M.; Hutchinson, J.; Cade, J.E.; Poston, L.; Barrett, G.; Crozier, S.R.; Barker, M.; Kumaran, K.; et al. Before the beginning: Nutrition and lifestyle in the preconception period and its importance for future health. Lancet 2018, 391, 1830–1841. [Google Scholar] [CrossRef]
- Whitehouse, A.J.O.; Stanley, F.J. Is autism one or multiple disorders? Med. J. Aust. 2013, 198, 3. [Google Scholar] [CrossRef] [PubMed]
- Pu, D.; Shen, Y.; Wu, J. Association between MTHFR Gene Polymorphisms and the Risk of Autism Spectrum Disorders: A Meta-Analysis. Autism Res. 2013, 6, 384–392. [Google Scholar] [CrossRef]
- Frisell, T.; Oberg, S.; Kuja-Halkola, R.; Sjolander, A. Sibling Comparison Designs Bias from Non-Shared Confounders and Measurement Error. Epidemiology 2012, 23, 713–720. [Google Scholar] [CrossRef] [Green Version]
- Shi, X.; Miao, W.; Tchetgen, E.T.A. A Selective Review of Negative Control Methods in Epidemiology. Curr. Epidemiol. Rep. 2020, 7, 190–202. [Google Scholar] [CrossRef]
- Lyall, K.; Munger, K.L.; O’Reilly, E.J.; Santangelo, S.L.; Ascherio, A. Maternal Dietary Fat Intake in Association With Autism Spectrum Disorders. Am. J. Epidemiol. 2013, 178, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.M. Relationship between Neonatal Vitamin D at Birth and Risk of Autism Spectrum Disorders: The NBSIB Study. J. Bone Miner. Res. 2018, 33, 458–466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. WHO Recommendations on Antenatal Care for a Positive Pregnancy Experience; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).