A High Protein Diet Is More Effective in Improving Insulin Resistance and Glycemic Variability Compared to a Mediterranean Diet—A Cross-Over Controlled Inpatient Dietary Study
Abstract
:1. Introduction
2. Materials and Methods
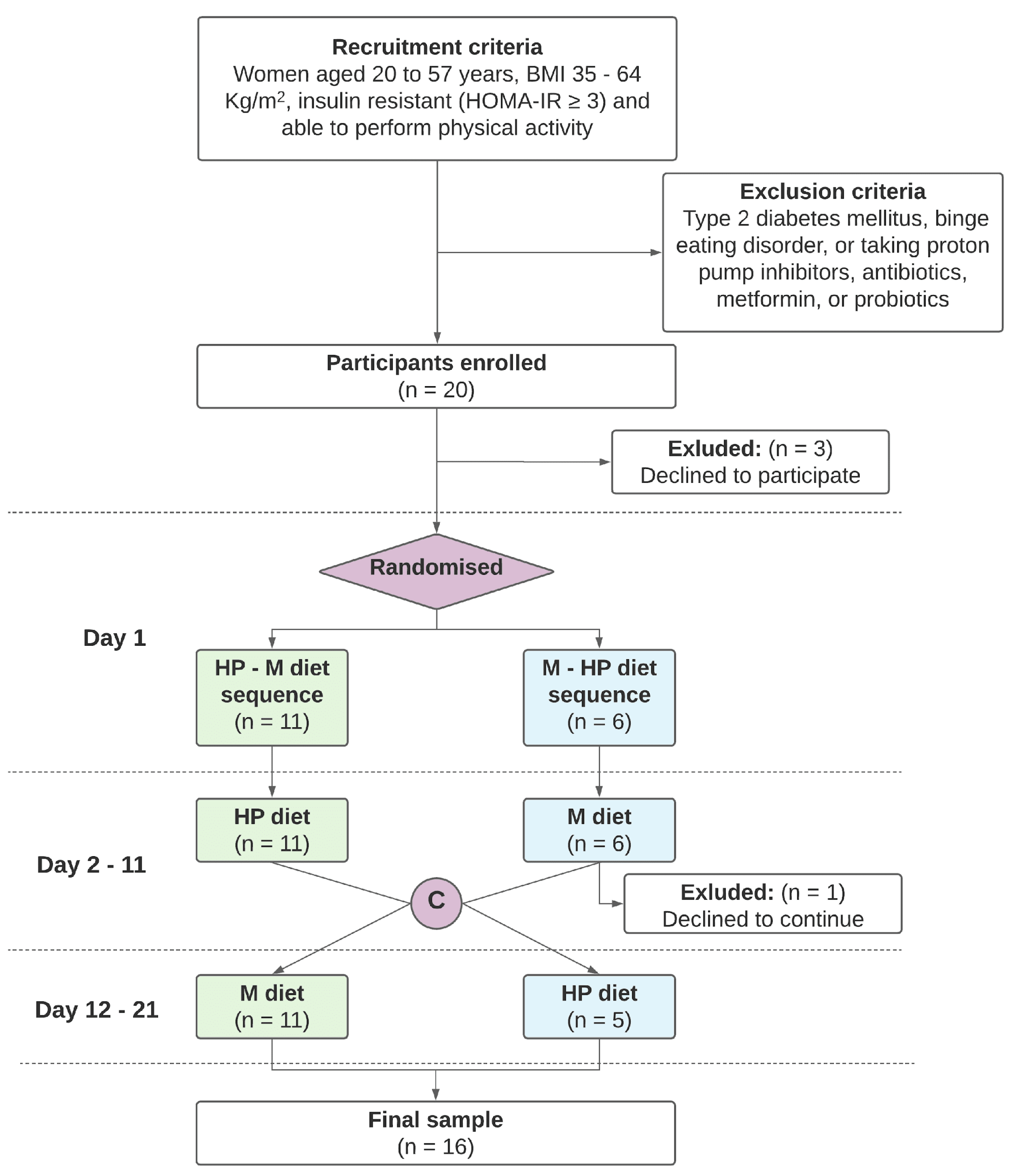
2.1. Study Design and Participants
2.2. Study Procedures
2.3. 16S rRNA Gut Microbiome
2.4. Study Outcomes
2.5. Statistical Analysis
3. Results
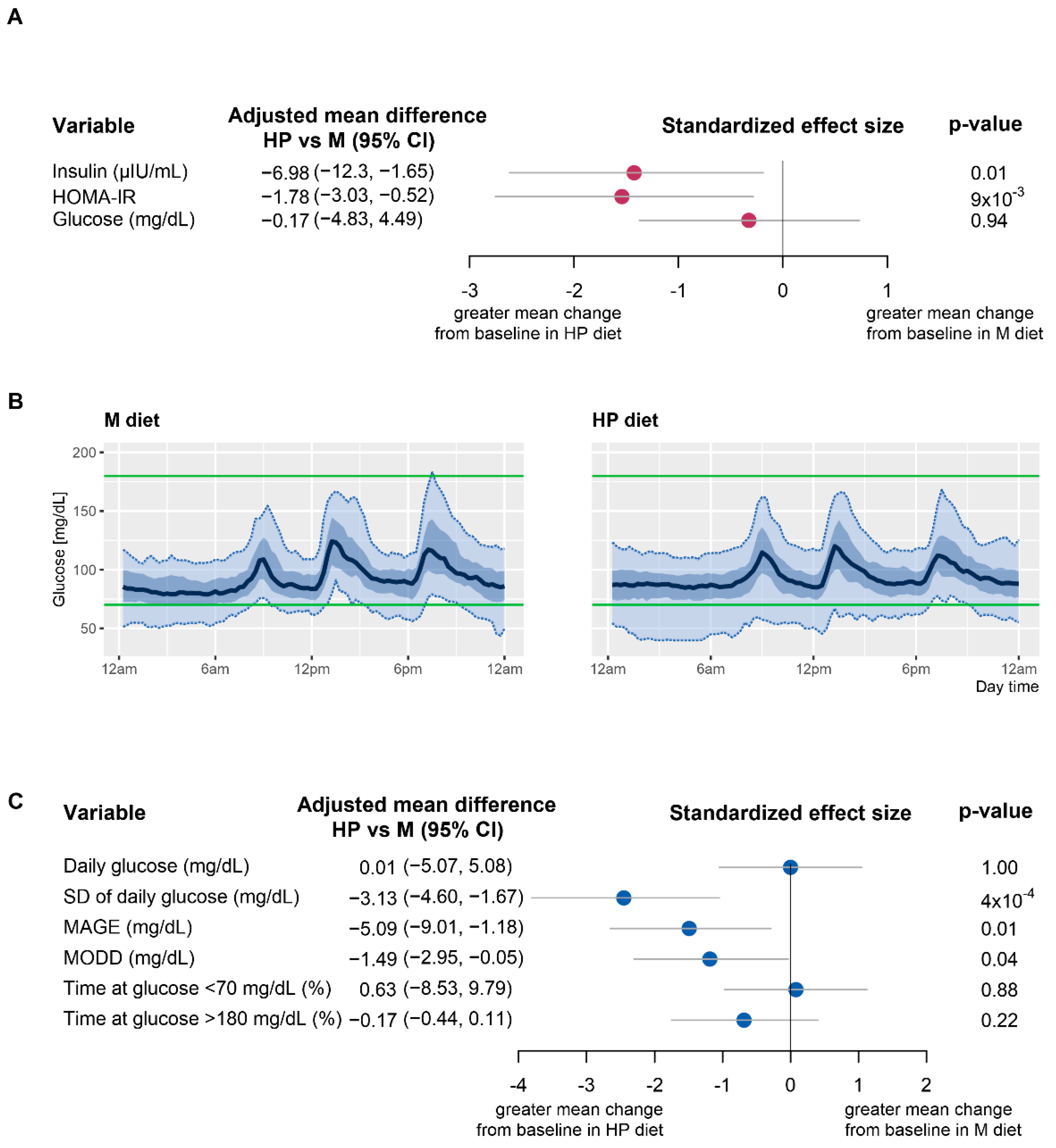
3.1. Improvement in Insulin Resistance and HOMA-IR
3.2. Effect of HP and M Diets on Glycemic Variability
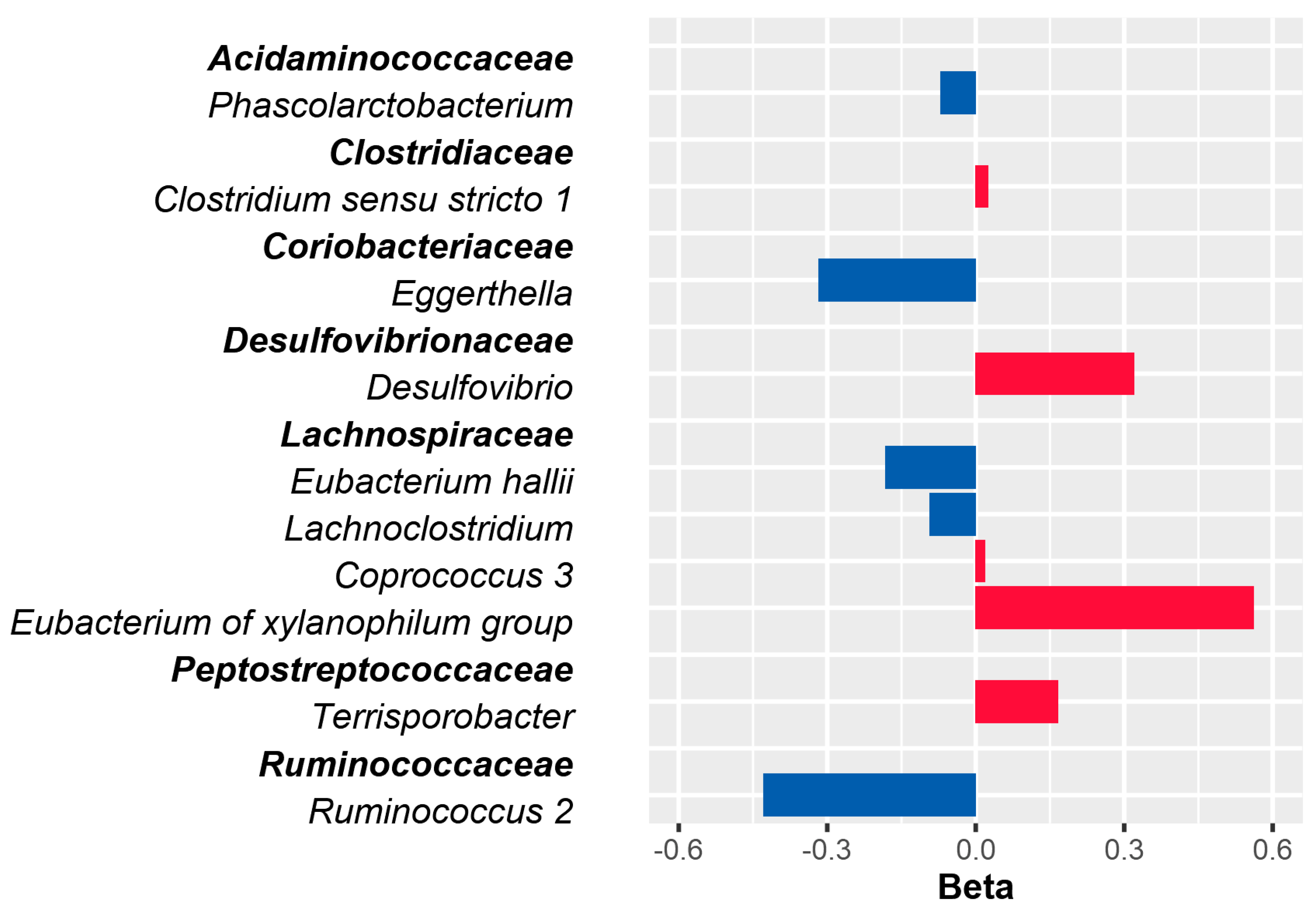
3.3. Association of Baseline Gut Microbial Composition at Genus Level with Glucose Variability
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, R.; Lee, D.H.; Subramanian, S.V. Understanding the Obesity Epidemic. BMJ 2019, 366, l4409. [Google Scholar] [CrossRef] [PubMed]
- D’Innocenzo, S.; Biagi, C.; Lanari, M. Obesity and the Mediterranean Diet: A Review of Evidence of the Role and Sustainability of the Mediterranean Diet. Nutrients 2019, 11, 1306. [Google Scholar] [CrossRef] [Green Version]
- Seidell, J.C.; Halberstadt, J. The Global Burden of Obesity and the Challenges of Prevention. Ann. Nutr. Metab. 2015, 66, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Guh, D.P.; Zhang, W.; Bansback, N.; Amarsi, Z.; Birmingham, C.L.; Anis, A.H. The Incidence of Co-Morbidities Related to Obesity and Overweight: A Systematic Review and Meta-Analysis. BMC Public Health 2009, 9, 88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poirier, P.; Giles, T.D.; Bray, G.A.; Hong, Y.; Stern, J.S.; Xavier Pi-Sunyer, F.; Eckel, R.H. Obesity and Cardiovascular Disease: Pathophysiology, Evaluation, and Effect of Weight Loss. Circulation 2006, 113, 898–918. [Google Scholar] [CrossRef] [Green Version]
- Drozdz, D.; Alvarez-Pitti, J.; Wójcik, M.; Borghi, C.; Gabbianelli, R.; Mazur, A.; Herceg-Čavrak, V.; Lopez-Valcarcel, B.G.; Brzeziński, M.; Lurbe, E.; et al. Obesity and Cardiometabolic Risk Factors: From Childhood to Adulthood. Nutrients 2021, 13, 4176. [Google Scholar] [CrossRef]
- Pergola, G.D.; De Pergola, G.; Silvestris, F. Obesity as a Major Risk Factor for Cancer. J. Obes. 2013, 2013, 291546. [Google Scholar] [CrossRef] [Green Version]
- Ba, S.; Swinburn, B.A.; Caterson, I.; Seidell, J.C.; James, W.P.T. Diet, Nutrition and the Prevention of Excess Weight Gain and Obesity. Public Health Nutr. 2004, 7, 123–146. [Google Scholar] [CrossRef]
- Widmer, R.J.; Jay Widmer, R.; Flammer, A.J.; Lerman, L.O.; Lerman, A. The Mediterranean Diet, Its Components, and Cardiovascular Disease. Am. J. Med. 2015, 128, 229–238. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Lacoba, R.; Pardo-Garcia, I.; Amo-Saus, E.; Escribano-Sotos, F. Mediterranean Diet and Health Outcomes: A Systematic Meta-Review. Eur. J. Public Health 2018, 28, 955–961. [Google Scholar] [CrossRef] [Green Version]
- Shai, I.; Schwarzfuchs, D.; Henkin, Y.; Shahar, D.R.; Witkow, S.; Greenberg, I.; Golan, R.; Fraser, D.; Bolotin, A.; Vardi, H.; et al. Weight Loss with a Low-Carbohydrate, Mediterranean, or Low-Fat Diet. N. Engl. J. Med. 2008, 359, 229–241. [Google Scholar] [CrossRef] [Green Version]
- Astrup, A.; Raben, A.; Geiker, N. The Role of Higher Protein Diets in Weight Control and Obesity-Related Comorbidities. Int. J. Obes. 2015, 39, 721–726. [Google Scholar] [CrossRef] [Green Version]
- Campos-Nonato, I.; Hernandez, L.; Barquera, S. Effect of a High-Protein Diet versus Standard-Protein Diet on Weight Loss and Biomarkers of Metabolic Syndrome: A Randomized Clinical Trial. Obes. Facts 2017, 10, 238–251. [Google Scholar] [CrossRef]
- McAuley, K.A.; Hopkins, C.M.; Smith, K.J.; McLay, R.T.; Williams, S.M.; Taylor, R.W.; Mann, J.I. Comparison of High-Fat and High-Protein Diets with a High-Carbohydrate Diet in Insulin-Resistant Obese Women. Diabetologia 2005, 48, 8–16. [Google Scholar] [CrossRef]
- Kitabchi, A.E.; McDaniel, K.A.; Wan, J.Y.; Tylavsky, F.A.; Jacovino, C.A.; Sands, C.W.; Nyenwe, E.A.; Stentz, F.B. Effects of High-Protein Versus High-Carbohydrate Diets on Markers of -Cell Function, Oxidative Stress, Lipid Peroxidation, Proinflammatory Cytokines, and Adipokines in Obese, Premenopausal Women Without Diabetes: A Randomized Controlled Trial. Diabetes Care 2013, 36, 1919–1925. [Google Scholar] [CrossRef] [Green Version]
- Moon, J.; Koh, G. Clinical Evidence and Mechanisms of High-Protein Diet-Induced Weight Loss. J. Obes. Metab. Syndr. 2020, 29, 166–173. [Google Scholar] [CrossRef]
- Yamaoka, I.; Hagi, M.; Doi, M. Circadian Changes in Core Body Temperature, Metabolic Rate and Locomotor Activity in Rats on a High-Protein, Carbohydrate-Free Diet. J. Nutr. Sci. Vitaminol. 2009, 55, 511–517. [Google Scholar] [CrossRef] [Green Version]
- Manach, C.; Milenkovic, D.; Van de Wiele, T.; Rodriguez-Mateos, A.; de Roos, B.; Garcia-Conesa, M.T.; Landberg, R.; Gibney, E.R.; Heinonen, M.; Tomás-Barberán, F.; et al. Addressing the Inter-Individual Variation in Response to Consumption of Plant Food Bioactives: Towards a Better Understanding of Their Role in Healthy Aging and Cardiometabolic Risk Reduction. Mol. Nutr. Food Res. 2017, 61, 1600557. [Google Scholar] [CrossRef] [Green Version]
- Menni, C.; Zierer, J.; Pallister, T.; Jackson, M.A.; Long, T.; Mohney, R.P.; Steves, C.J.; Spector, T.D.; Valdes, A.M. Omega-3 Fatty Acids Correlate with Gut Microbiome Diversity and Production of N-Carbamylglutamate in Middle Aged and Elderly Women. Sci. Rep. 2017, 7, 11079. [Google Scholar] [CrossRef] [Green Version]
- Mompeo, O.; Spector, T.D.; Matey Hernandez, M.; Le Roy, C.; Istas, G.; Le Sayec, M.; Mangino, M.; Jennings, A.; Rodriguez-Mateos, A.; Valdes, A.M.; et al. Consumption of Stilbenes and Flavonoids Is Linked to Reduced Risk of Obesity Independently of Fiber Intake. Nutrients 2020, 12, 1871. [Google Scholar] [CrossRef]
- Boers, S.A.; Hiltemann, S.D.; Stubbs, A.P.; Jansen, R.; Hays, J.P. Development and Evaluation of a Culture-Free Microbiota Profiling Platform (MYcrobiota) for Clinical Diagnostics. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 1081–1089. [Google Scholar] [CrossRef] [Green Version]
- Shannon, C.E.; Weaver, W. The Mathematical Theory of Communication; University of Illinois Press: Champaign, IL, USA, 1998; ISBN 9780252098031. [Google Scholar]
- Hurlbert, S.H. The Nonconcept of Species Diversity: A Critique and Alternative Parameters. Ecology 1971, 52, 577–586. [Google Scholar] [CrossRef]
- Broll, S.; Urbanek, J.; Buchanan, D.; Chun, E.; Muschelli, J.; Punjabi, N.; Gaynanova, I. Interpreting Blood GLUcose Data with R Package Iglu. PLoS ONE 2021, 16, e0248560. [Google Scholar] [CrossRef]
- Lin, W.; Shi, P.; Feng, R.; Li, H. Variable Selection in Regression with Compositional Covariates. Biometrika 2014, 101, 785–797. [Google Scholar] [CrossRef]
- Service, F.J.; Nelson, R.L. Characteristics of Glycemic Stability. Diabetes Care 1980, 3, 58–62. [Google Scholar] [CrossRef]
- Nogal, A.; Louca, P.; Zhang, X.; Wells, P.M.; Steves, C.J.; Spector, T.D.; Falchi, M.; Valdes, A.M.; Menni, C. Circulating Levels of the Short-Chain Fatty Acid Acetate Mediate the Effect of the Gut Microbiome on Visceral Fat. Front. Microbiol. 2021, 12, 711359. [Google Scholar] [CrossRef]
- Mirabelli, M.; Chiefari, E.; Arcidiacono, B.; Corigliano, D.M.; Brunetti, F.S.; Maggisano, V.; Russo, D.; Foti, D.P.; Brunetti, A. Mediterranean Diet Nutrients to Turn the Tide against Insulin Resistance and Related Diseases. Nutrients 2020, 12, 1066. [Google Scholar] [CrossRef] [Green Version]
- Feidantsis, K.; Methenitis, S.; Ketselidi, K.; Vagianou, K.; Skepastianos, P.; Hatzitolios, A.; Mourouglakis, A.; Kaprara, A.; Hassapidou, M.; Nomikos, T.; et al. Comparison of Short-Term Hypocaloric High-Protein Diets with a Hypocaloric Mediterranean Diet: Effect on Body Composition and Health-Related Blood Markers in Overweight and Sedentary Young Participants. Nutrition 2021, 91–92, 111365. [Google Scholar] [CrossRef] [PubMed]
- Tricò, D.; Moriconi, D.; Berta, R.; Baldi, S.; Quinones-Galvan, A.; Guiducci, L.; Taddei, S.; Mari, A.; Nannipieri, M. Effects of Low-Carbohydrate versus Mediterranean Diets on Weight Loss, Glucose Metabolism, Insulin Kinetics and β-Cell Function in Morbidly Obese Individuals. Nutrients 2021, 13, 1345. [Google Scholar] [CrossRef]
- Rietman, A.; Schwarz, J.; Tomé, D.; Kok, F.J.; Mensink, M. High Dietary Protein Intake, Reducing or Eliciting Insulin Resistance? Eur. J. Clin. Nutr. 2014, 68, 973–979. [Google Scholar] [CrossRef] [Green Version]
- Yılmaz, S.K.; Eskici, G.; Mertoǧlu, C.; Ayaz, A. Effect of Different Protein Diets on Weight Loss, Inflammatory Markers, and Cardiometabolic Risk Factors in Obese Women. J. Res. Med. Sci. 2021, 26, 28. [Google Scholar] [PubMed]
- Luis, D.A.; de Luis, D.A.; Izaola, O.; Aller, R.; de la Fuente, B.; Bachiller, R.; Romero, E. Effects of a High-Protein/low Carbohydrate versus a Standard Hypocaloric Diet on Adipocytokine Levels and Insulin Resistance in Obese Patients along 9 months. J. Diabetes Its Complicat. 2015, 29, 950–954. [Google Scholar] [CrossRef] [PubMed]
- Mateo-Gallego, R.; Marco-Benedí, V.; Perez-Calahorra, S.; Bea, A.M.; Baila-Rueda, L.; Lamiquiz-Moneo, I.; de Castro-Orós, I.; Cenarro, A.; Civeira, F. Energy-Restricted, High-Protein Diets More Effectively Impact Cardiometabolic Profile in Overweight and Obese Women than Lower-Protein Diets. Clin. Nutr. 2017, 36, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Nan, F.; Wang, L.Y.; Jiang, H.; Chen, W.; Jiang, Y. Effects of High-Protein Diet on Glycemic Control, Insulin Resistance and Blood Pressure in Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Clin. Nutr. 2020, 39, 1724–1734. [Google Scholar] [CrossRef]
- Stolar, M. Glycemic Control and Complications in Type 2 Diabetes Mellitus. Am. J. Med. 2010, 123, S3–S11. [Google Scholar] [CrossRef]
- Subramaniam, S.; Fletcher, C. Trimethylamine N-Oxide: Breathe New Life. Br. J. Pharmacol. 2018, 175, 1344–1353. [Google Scholar] [CrossRef]
- Chakarova, N.; Dimova, R.; Grozeva, G.; Tankova, T. Assessment of Glucose Variability in Subjects with Prediabetes. Diabetes Res. Clin. Pract. 2019, 151, 56–64. [Google Scholar] [CrossRef] [Green Version]
- Dimova, R.; Chakarova, N.; Grozeva, G.; Tankova, T. Evaluation of the Relationship between Cardiac Autonomic Function and Glucose Variability and HOMA-IR in Prediabetes. Diabetes Vasc. Dis. Res. 2020, 17, 147916412095861. [Google Scholar] [CrossRef]
- Akirov, A.; Diker-Cohen, T.; Masri-Iraqi, H.; Shimon, I. High Glucose Variability Increases Mortality Risk in Hospitalized Patients. J. Clin. Endocrinol. Metab. 2017, 102, 2230–2241. [Google Scholar] [CrossRef]
- Shohat, N.; Foltz, C.; Restrepo, C.; Goswami, K.; Tan, T.; Parvizi, J. Increased Postoperative Glucose Variability Is Associated with Adverse Outcomes Following Orthopaedic Surgery. Bone Jt. J. 2018, 100-B, 1125–1132. [Google Scholar] [CrossRef]
- Li, X.; Zhou, X.; Wei, J.; Mo, H.; Lou, H.; Gong, N.; Zhang, M. Effects of Glucose Variability on Short-Term Outcomes in Non-Diabetic Patients After Coronary Artery Bypass Grafting: A Retrospective Observational Study. Heart Lung Circ. 2019, 28, 1580–1586. [Google Scholar] [CrossRef]
- Siegelaar, S.E.; Holleman, F.; Hoekstra, J.B.L.; DeVries, J.H. Glucose Variability; Does It Matter? Endocr. Rev. 2010, 31, 171–182. [Google Scholar] [CrossRef] [Green Version]
- Zeevi, D.; Korem, T.; Zmora, N.; Israeli, D.; Rothschild, D.; Weinberger, A.; Ben-Yacov, O.; Lador, D.; Avnit-Sagi, T.; Lotan-Pompan, M.; et al. Personalized Nutrition by Prediction of Glycemic Responses. Cell 2015, 163, 1079–1094. [Google Scholar] [CrossRef] [Green Version]
- Berry, S.E.; Valdes, A.M.; Drew, D.A.; Asnicar, F.; Mazidi, M.; Wolf, J.; Capdevila, J.; Hadjigeorgiou, G.; Davies, R.; Al Khatib, H.; et al. Human Postprandial Responses to Food and Potential for Precision Nutrition. Nat. Med. 2020, 26, 964–973. [Google Scholar] [CrossRef]
- Nogal, A.; Valdes, A.M.; Menni, C. The Role of Short-Chain Fatty Acids in the Interplay between Gut Microbiota and Diet in Cardio-Metabolic Health. Gut Microbes 2021, 13, 1897212. [Google Scholar] [CrossRef]
- Souto, D.L.; Dantas, J.R.; Oliveira, M.M.D.S.; Rosado, E.L.; Luiz, R.R.; Zajdenverg, L.; Rodacki, M. Does Sucrose Affect the Glucose Variability in Patients with Type 1 Diabetes? A Pilot Crossover Clinical Study. Nutrition 2018, 55–56, 179–184. [Google Scholar] [CrossRef]
| Phenotype | HP–M | M–HP |
|---|---|---|
| N | 11 | 5 |
| Sex, n (%) | 11 (100%) | 5 (100%) |
| Impaired Fasting Glucose, n (%) | 3 (27.3%) | 1 (20%) |
| Age, years | 36.18 (12.55) | 42.40 (15.32) |
| Weight, Kg | 118.94 (17.98) | 130.86 (38.84) |
| BMI, Kg/m2 | 44.56 (4.61) | 50.40 (10.79) |
| Waist to hip ratio | 0.86 (0.07) | 0.91 (0.12) |
| Fat to lean mass ratio * | 1.06 (0.16) | 1.28 (0.25) |
| SBP, mmHg | 127.27 (11.04) | 129.00 (11.40) |
| DBP, mmHg | 79.09 (8.61) | 79.00 (8.94) |
| Heart Rate, bpm | 82.55 (11.71) | 78.40 (15.47) |
| Triglycerides, mmol/L | 1.27 (0.34) | 1.58 (0.56) |
| Total cholesterol, mmol/L | 4.12 (0.78) | 4.65 (0.86) |
| LDL cholesterol, mmol/L | 1.61 (0.43) | 1.94 (0.45) |
| HDL cholesterol *, mmol/L | 1.17 (0.11) | 1.00 (0.08) |
| Glucose, mg/dL | 93.27 (8.13) | 94.80 (6.53) |
| Insulin, µIU/mL | 19.98 (8.28) | 24.42 (11.52) |
| HOMA-IR | 4.60 (2.05) | 5.61 (2.43) |
| HbA1c, % | 5.55 (0.25) | 5.68 (0.44) |
| Shannon Index | 5.76 (0.42) | 5.58 (0.37) |
| Simpson Index | 0.96 (0.01) | 0.96 (0.01) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tettamanzi, F.; Bagnardi, V.; Louca, P.; Nogal, A.; Monti, G.S.; Mambrini, S.P.; Lucchetti, E.; Maestrini, S.; Mazza, S.; Rodriguez-Mateos, A.; et al. A High Protein Diet Is More Effective in Improving Insulin Resistance and Glycemic Variability Compared to a Mediterranean Diet—A Cross-Over Controlled Inpatient Dietary Study. Nutrients 2021, 13, 4380. https://doi.org/10.3390/nu13124380
Tettamanzi F, Bagnardi V, Louca P, Nogal A, Monti GS, Mambrini SP, Lucchetti E, Maestrini S, Mazza S, Rodriguez-Mateos A, et al. A High Protein Diet Is More Effective in Improving Insulin Resistance and Glycemic Variability Compared to a Mediterranean Diet—A Cross-Over Controlled Inpatient Dietary Study. Nutrients. 2021; 13(12):4380. https://doi.org/10.3390/nu13124380
Chicago/Turabian StyleTettamanzi, Francesca, Vincenzo Bagnardi, Panayiotis Louca, Ana Nogal, Gianna Serafina Monti, Sara P. Mambrini, Elisa Lucchetti, Sabrina Maestrini, Silvia Mazza, Ana Rodriguez-Mateos, and et al. 2021. "A High Protein Diet Is More Effective in Improving Insulin Resistance and Glycemic Variability Compared to a Mediterranean Diet—A Cross-Over Controlled Inpatient Dietary Study" Nutrients 13, no. 12: 4380. https://doi.org/10.3390/nu13124380
APA StyleTettamanzi, F., Bagnardi, V., Louca, P., Nogal, A., Monti, G. S., Mambrini, S. P., Lucchetti, E., Maestrini, S., Mazza, S., Rodriguez-Mateos, A., Scacchi, M., Valdes, A. M., Invitti, C., & Menni, C. (2021). A High Protein Diet Is More Effective in Improving Insulin Resistance and Glycemic Variability Compared to a Mediterranean Diet—A Cross-Over Controlled Inpatient Dietary Study. Nutrients, 13(12), 4380. https://doi.org/10.3390/nu13124380







