Abstract
Heavy metals causing chronic nephrotoxicity may play a key role in the pathogenesis of chronic kidney disease (CKD). This study hypothesized that plasma folate and vitamin B12 would modify the association of CKD with total urinary arsenic and blood lead and cadmium levels. We recruited 220 patients with CKD who had an estimated glomerular filtration rate of <60 mL/min/1.73 m2 for ≥3 consecutive months and 438 sex- and age-matched controls. We performed inductively coupled plasma mass spectrometry to measure blood cadmium and lead levels. The urinary arsenic level was determined using a high-performance liquid chromatography–hydride generator–atomic absorption spectrometry. Plasma vitamin B12 and folate levels were measured through the SimulTRAC-SNB radioassay. Compared with patients with plasma vitamin B12 ≤ 6.27 pg/mL, the odds ratio (OR) and 95% confidence interval of CKD for patients with plasma vitamin B12 > 9.54 pg/mL was 2.02 (1.15–3.55). However, no association was observed between plasma folate concentration and CKD. A high level of plasma vitamin B12 combined with high levels of blood lead and cadmium level and total urinary arsenic tended to increase the OR of CKD in a dose-response manner, but the interactions were nonsignificant. This is the first study to demonstrate that patients with high plasma vitamin B12 level exhibit increased OR of CKD related to high levels of blood cadmium and lead and total urinary arsenic.
1. Introduction
Chronic kidney disease (CKD) is characterized by a progressive and irreversible decline in renal function occurring gradually over a period of a few months to many years. In CKD, the kidney gradually loses its ability to filter toxins from blood. Renal impairment in CKD is diagnosed based on a decrease in the estimated glomerular filtration rate (eGFR) to <60 mL/min/1.73 m2, the presence of proteinuria, or the presence of pathological abnormalities for at least 3 months [1]. Because of the high prevalence as well as morbidity and mortality of CKD, it has become a global public health concern [2]. In Taiwan, the CKD prevalence was 11.9% in 2008 [3], and the prevalence of end-stage renal disease was the highest in the world from a 2016 report [4]; therefore, CKD is a significant public health issue in Taiwan.
Our recent study reported that the levels of blood lead and cadmium and total urinary arsenic are significantly associated with an increased odds ratio (OR) of CKD, whereas the plasma selenium level significantly reduced the OR of CKD [5]. A Thai study reported that long-term exposure to cadmium and a high urinary cadmium level were associated with a significant decrease in eGFR, resulting in CKD [6]. A Chinese follow-up study showed that the levels of plasma arsenic and lead are associated with a significant annual decline in eGFR after adjustment for demographic variables and risk factors for CKD [7]. Furthermore, an animal study demonstrated that lead causes an inflammatory response, leading to CKD [8]. A high level of lead in the blood was related to proteinuria and eGFR < 60 mL/min/1.73 m2 [9]. Exposure to arsenic, lead, and cadmium appears to be related to CKD occurrence [5,6,7,8,9].
Vitamin B9 (folate) and vitamin B12 are water-soluble vitamins involved in several normal cellar functions. Folate and vitamin B12 are vital cofactors in the remethylation pathway in humans [10]. Folate treatment was associated with a decrease in the OR of CKD progression in patients with mild-to-moderate CKD and high B12 levels [11]. A review suggested that folate and vitamin B12 can be beneficial in CKD treatment [12]. However, the levels of serum folate and vitamin B12 were not associated with increased levels of homocysteine and cysteine in patients with CKD and diabetes [13]. Thus, whether plasma folate and vitamin B12 prevent CKD remains unclear. Heavy metals with nephrotoxic effects may accumulate gradually and cause CKD [14]. Therefore, this study investigated whether the levels of plasma folate and vitamin B12 alter the OR of CKD related to total urinary arsenic and blood lead and cadmium.
2. Materials and Methods
2.1. Study Participants and Interviews
Eligible participants were nephrology outpatients and adults or elderly people participating in a health examination who had signed an informed consent form and provided blood and urine samples. In total, 220 patients with clinically confirmed CKD and 438 sex- and age-matched controls were recruited from both Taipei Medical University Hospital and Taipei Municipal Wan Fang Hospital between May 2018 and May 2019. All outpatients with CKD received the diagnosis based on biochemical criteria such as blood urea nitrogen, proteinuria, and serum creatinine at the Department of Internal Medicine/Nephrology. Patients with CKD who had an eGFR of <60 mL/min/1.73 m2 were diagnosed as having stage 3, 4, or 5 CKD for at least 3 months and did not receive hemodialysis. Those with an eGFR of ≥60 mL/min/1.73 m2 were considered healthy controls. The ratio of control participants to patients with CKD was approximately 1.5:1.
We interviewed all study participants and collected their blood and urine samples as described in a previous study [15]. The current study was approved by the Research Ethics Committee of Taipei Medical University, Taiwan (TMU Joint Institutional Review Board N201804024, 25 May 2018–24 May 2019), and was conducted in accordance with the Declaration of Helsinki.
2.2. Measurements of Urinary Arsenic Levels
The urinary arsenic level was measured as described previously [16]. The measurement method, detection limits, and standard reference material used served as the quality standard, and samples spiked with a standard solution (recovery rates) are shown in Supplementary Table S1. The total urinary arsenic level (μg/g creatinine) was calculated as the sum of the levels of inorganic arsenic (arsenite + arsenate), monomethylarsonic acid, and dimethylarsinic acid after dividing for the level of urinary creatinine, which controls hydration. The measurement of the creatinine level is shown in Supplementary Table S1.
2.3. Measurements of Blood Lead and Cadmium Levels
Because the literature indicates that the concentration of heavy metals (such as lead or cadmium) in whole blood is a valid marker for long-term exposure [17], this study used red blood cells to measure the concentration of heavy metals. Blood lead and cadmium levels were measured as described previously [18]. The validity and reliability of the measurements and detection limits are listed in Supplementary Table S1.
2.4. Measurements of Plasma Folate and Vitamin B12 Levels
The methods used for measuring plasma vitamin B12 and folate levels were described in detail in our recent study [19]. The measurement method, detection limit, and variation coefficient are presented in Supplementary Table S1.
2.5. Statistical Analysis
Continuous variables are presented as the mean ± standard deviation or median (IQR). We used the Wilcoxon rank sum test to compare differences in continuous variables between patients with CKD and controls. Furthermore, we used the chi-square test to examine the distribution of categorical variables between patients with CKD and controls. We used a multivariate linear regression model to determine the correlation between eGFR and the levels of plasma folate and vitamin B12 after adjusting for age; sex; educational level; alcohol, coffee, and tea consumption; analgesic usage; history of diabetes and hypertension; red blood cell lead and cadmium levels; and total urinary arsenic (μg/g creatinine). Subsequently, we used multiple logistic regression to assess the association between potential risk factors for CKD. The corresponding tertiles of controls were used as cutoff points for continuous variables among independent variables. This approach is generally a dose-response analysis method, which analyzes the increased CKD risk when the dose of the exposure variable increases by one-third [20]. Multivariate-adjusted ORs and 95% confidence intervals were calculated to determine CKD risk. In the significance test of the linear trend of the OR in the exposed stratification, we used categorized exposure variables as score variables, which also served as continuous variables. The respective median of controls was used as the cutoff for risk factors in the interaction analysis. Additive interactions between risk factors for CKD were evaluated in a pairwise manner by using the synergy index provided by Rothman [21]. The observed synergy index value was not equal to 1, indicating an additive interaction, and ORs and their variance–covariance matrix were used to calculate 95% confidence intervals [22]. The product term between levels of plasma vitamin B12, blood lead and cadmium, and total urinary arsenic was used pairwise to test their multiplicative interactive effect on the OR of CKD in the multiple logistic regression model. The SAS package (version 9.4; SAS Institute, Cary, NC, USA) was used for these analyses. A two-tailed p value of <0.05 indicated statistical significance.
3. Results
Table 1 lists the sociodemographic characteristics, lifestyle, and disease histories of patients with CKD and controls. CKD cases and controls were not statistically different in age, sex, and smoking status. However, CKD cases were less educated, less likely to consume alcohol, coffee, or tea, but were more likely to use analgesics and were more likely to be diabetic or hypertensive.

Table 1.
Sociodemographic characteristics, lifestyle, and disease histories of CKD cases and controls.
We analyzed the relationship of plasma nutrients, blood lead and cadmium, and urinary metals with CKD risk (Table 2). The higher the levels of plasma vitamin B12, blood lead and cadmium, and total urinary arsenic, the higher the OR of CKD. When the concentration of blood lead, cadmium, urinary total arsenic, or plasma vitamin B12 increased by a tertile, the risk of CKD increased significantly. Plasma folate levels were not related to CKD (Table 2). We also show the spread of data in Supplementary Figure S1.

Table 2.
Association of the levels of total urinary arsenic, blood cadmium and lead, and plasma vitamin B12 and folate with CKD.
The log eGFR decreased significantly with the increase of the log plasma vitamin B12 concentration. However, there was no correlation between plasma folate concentration and eGFR (Figure 1).
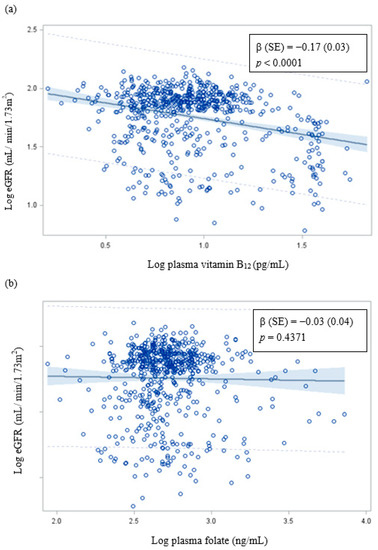
Figure 1.
Correlation of eGFR with (a) plasma vitamin B12 and (b) plasma folate. β (SE): Adjusted for age; sex; educational level; alcohol, coffee, and tea consumption; analgesic usage; diabetes; hypertension; blood lead and cadmium levels; and total urinary arsenic (μg/g creatinine).
Because plasma vitamin B12 was related to CKD, we conducted a stratified analysis to determine whether it affects the association of blood cadmium and lead or total urinary arsenic concentration with CKD risk. The effect of blood lead concentration on the OR of CKD in patients with a low plasma vitamin B12 level was higher than that in patients with a high plasma vitamin B12 level. The OR of CKD did not vary between blood cadmium and total urinary arsenic concentrations (Supplementary Table S2). Subsequently, we examined the interactive effects of plasma vitamin B12, total urinary arsenic, and blood lead and cadmium levels on CKD (Table 3). A trend analysis revealed that the OR of CKD gradually but significantly increased with exposure to no risk factors or to either one or both risk factors (a high plasma vitamin B12 level and a high blood lead level). Furthermore, the interaction of other paired risk factors exerted the same effect on CKD; however, these interactions were nonsignificant.

Table 3.
The interaction between plasma vitamin B12, urinary arsenic, and red blood cell lead and cadmium levels on CKD.
4. Discussion
The results of the present study revealed that the prevalence of hypertension and diabetes was higher in patients with CKD than in controls. Hypertension and diabetes are crucial risk factors for CKD [23]. Furthermore, the present study demonstrated that the increase in plasma vitamin B12, total urinary arsenic, and blood lead and cadmium levels gradually and significantly increased the OR of CKD. In addition, high levels of plasma vitamin B12 and blood lead and cadmium tended to increase the OR of CKD, but the interaction was nonsignificant.
Our study demonstrated a significantly positive correlation of blood cadmium and lead and total urinary arsenic levels with the OR of CKD [5,24]. In addition, this study also found that urinary total arsenic and blood lead and cadmium were related to CKD, as proposed in other studies. One study did not find an association between the blood lead level and kidney function [25]. However, a cohort study found that plasma arsenic was associated with an increased risk of kidney graft failure [26]. A Thai study showed that long-term exposure to a low cadmium level was associated with decreased renal function [27]. Another study reported that exposure to high levels of lead and cadmium reduced eGFR and increased the albumin to creatinine ratio, adversely affecting renal function [28]. Furthermore, recent studies have revealed that with an increase in plasma cadmium concentration, the risks of long-term kidney transplant failure and reduced kidney function increase [29]. These findings suggest that exposure to cadmium, lead, and arsenic is associated with CKD. Because the kidney is the main organ responsible for toxin excretion from blood, it is susceptible to the toxicity of heavy metals such as lead, cadmium, and arsenic [30,31]. Cadmium, lead, and arsenic metabolism can produce reactive oxygen species, induce oxidative stress, and cause kidney damage [32,33,34]. Lead exposure promotes lipid peroxidation and the degradation of phospholipids in kidney cells, leading to a loss of cell membrane integrity and nephrotoxicity, or a loss of mitochondrial function in proximal tubular cells [35,36].
A recent clinical trial indicated that a baseline vitamin B12 level of ≥248 pmole/L and folate treatment were associated with an increased reduction in the OR of CKD progression [11]. However, another study reported that folate, vitamin B12, homocysteine, and cysteine were not related to the CKD stage [13]. The relationship of hyperhomocysteinemia, folate, and vitamin B12 with CKD progression is controversial [12]. By contrast, a high level of plasma vitamin B12 was related to all-cause mortality after adjustment for renal function and other confounding factors [37,38]. A previous study found elevated plasma vitamin B12 concentrations in patients with liver disease, autoimmune disease, and kidney disease [39]. Why the vitamin B12 in the plasma of CKD patients is higher than that in the control group is not fully understood. The liver is the largest reservoir of vitamin B12 in the body, which may be the destruction of the absorption of vitamin B12 by the liver; alternatively, increased hepatocyte turnover/damage may cause more vitamin B12 to leak from the liver, resulting in increased levels of vitamin B12 in the plasma [34]. In addition, a high level of plasma vitamin B12 may be a response to an increased release of vitamin B12 stored in the liver, decreased clearance, upregulation of haptocorrin and transcobalamin synthesis, or decreased affinity of vitamin B12 to transporters. These conditions usually result in liver damage or CKD [38]. Furthermore, the findings of the present study suggest that the level of plasma vitamin B12 is significantly higher in patients with CKD than in controls. Thus, a high plasma vitamin B12 level, but not a high folate level, was associated with CKD. These results may have occurred by chance. Thus, at present, our knowledge regarding the association of high plasma vitamin B12 with CKD is incomplete.
In the present study, we observed that high levels of blood lead and plasma vitamin B12 tended to interact with CKD. This may be because the high levels of blood lead [5] and plasma vitamin B12 (Figure 1) significantly decrease eGFR and increase the OR of CKD or the high levels of blood lead and plasma vitamin B12 significantly increase the OR of hyperhomocysteinemia [40], which leads to increased oxidative stress and CKD risk [41]. Thus, an increase in the levels of plasma vitamin B12, blood lead or cadmium, and total urinary arsenic increase the OR of CKD.
Some limitations of this study must be considered while interpreting the results. This study is cross-sectional in nature. Patients with CKD recruited in this study were prevalent cases; therefore, the causal relationship of plasma folate and vitamin B12, blood cadmium and lead, and total urinary arsenic levels with CKD could not be confirmed. We cannot exclude the possibility of the typical reverse causality. Samples were collected only once to evaluate plasma folate and vitamin B12, blood cadmium and lead, and total urinary arsenic levels. However, if all patients maintained a stable lifestyle and had homeostatic metabolism, these measurements may be reliable. Moreover, we did not consider the homocysteine level, lipid profile, and supplement use in this study. For future research, it is necessary to determine the role of homocysteine to assess whether plasma vitamin B12 and folate concentrations could affect the metabolism of metals, and thus, affect the risk of CKD. Nevertheless, these findings are crucial to understand potential factors associated with CKD.
5. Conclusions
The findings from this study suggest that a high concentration of plasma vitamin B12 was related to the risk of CKD after adjusting for other covariates. In addition, this research indicates that there was a possible interaction between plasma vitamin B12 and blood lead or cadmium, resulting in an increased risk of CKD. However, the mechanism of this association is not fully understood, and further investigation is warranted to advance the understanding of risks associated with CKD.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/nu13113841/s1, Figure S1: The dot plots of measured variables by CKD status. (A) Plasma vitamin B12 (pg/mL) (B) Plasma folate (ng/mL) (C) Total urinary arsenic (μg/g creatinine) (D) Red blood cell lead (μg/L) (E) Red blood cell cadmium (μg/L), Table S1: Validity and reliability of measurements used for determining urinary arsenic and plasma selenium, folate, and vitamin B12 as well as red blood cell lead and cadmium concentrations, Table S2: Association of levels of total urinary arsenic and blood cadmium and lead with CKD stratified by B12 levels.
Author Contributions
Conceptualization, Y.-F.L. and H.-S.S.; Formal analysis, Y.-L.H.; Funding acquisition, Y.-M.H.; Resources, Y.-F.L. and Y.-C.L.; Writing—original draft, Y.-M.H.; Writing—review & editing, Y.-C.L. and H.-H.C.; Supervision, H.-H.C.; Editing, Y.-L.H.; Project administration, Y.-M.H. All authors have read and agreed to the published version of the manuscript.
Funding
Taipei Medical University-Wang Fang hospital: 110TMU-WFH-01; Ministry of Science and Technology, Taiwan: MOST103-2314-B-038-021-MY2 (1-2), MOST103-2314-B-038-021-MY2 (2-2), MOST105-2314-B-038-082, MOST106-2314-B-038-066, MOST107-2314-B-038-073, MOST108-2314-B-038-089, and MOST109-2314-B-038-067.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Research Ethics Committee of Taipei Medical University, Taiwan (TMU Joint Institutional Review Board N201804024).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data that support the findings of this study are available on reasonable request from the corresponding author Hsi-Hsien Chen 570713@yahoo.com.
Conflicts of Interest
No potential conflict of interest are reported by the authors.
References
- National Kidney Foundation. K/DOQI Clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am. J. Kidney Dis. 2002, 39, S1–S266. [Google Scholar]
- Levey, A.S.; Atkins, R.; Coresh, J.; Cohen, E.P.; Collins, A.J.; Eckardt, K.U.; Nahas, M.E.; Jaber, B.L.; Jadoul, M.; Levin, A.; et al. Chronic kidney disease as a global public health problem: Approaches and initiatives—A position statement from Kidney Disease Improving Global Outcomes. Kidney Int. 2007, 72, 247–259. [Google Scholar] [CrossRef] [Green Version]
- Wen, C.P.; Cheng, T.Y.; Tsai, M.K.; Chang, Y.C.; Chan, H.T.; Tsai, S.P.; Chiang, P.H.; Hsu, C.C.; Sung, P.K.; Hsu, Y.H.; et al. All-cause mortality attributable to chronic kidney disease: A prospective cohort study based on 462 293 adults in Taiwan. Lancet 2008, 371, 2173–2182. [Google Scholar] [CrossRef]
- USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States; National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Disease: Bethesda, MD, USA, 2016.
- Wu, C.Y.; Wong, C.S.; Chung, C.J.; Wu, M.Y.; Huang, Y.L.; Ao, P.L.; Lin, Y.F.; Lin, Y.C.; Shiue, H.S.; Su, C.T.; et al. The association between plasma selenium and chronic kidney disease related to lead, cadmium and arsenic exposure in a Taiwanese population. J. Hazard. Mater. 2019, 375, 224–232. [Google Scholar] [CrossRef]
- Satarug, S.; Boonprasert, K.; Gobe, G.C.; Ruenweerayut, R.; Johnson, D.W.; Na-Bangchang, K.; Vesey, D.A. Chronic exposure to cadmium is associated with a marked reduction in glomerular filtration rate. Clin. Kidney J. 2019, 12, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yuan, Y.; Xiao, Y.; Li, Y.; Yu, Y.; Mo, T.; Jiang, H.; Li, X.; Yang, H.; Xu, C.; et al. Associations of plasma metal concentrations with the decline in kidney function: A longitudinal study of Chinese adults. Ecotoxicol. Environ. Saf. 2020, 189, 110006. [Google Scholar] [CrossRef] [PubMed]
- Rana, M.N.; Tangpong, J.; Rahman, M.A. Xanthones protects lead-induced chronic kidney disease (CKD) via activating Nrf-2 and modulating NF-kB, MAPK pathway. Biochem. Biophys. Rep. 2020, 21, 100718. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.J.; Hung, C.H.; Wang, C.W.; Tu, H.P.; Li, C.H.; Tsai, C.C.; Lin, W.Y.; Chen, S.C.; Kuo, C.H. Associations among Heavy Metals and Proteinuria and Chronic Kidney Disease. Diagnostics 2021, 11, 282. [Google Scholar] [CrossRef]
- Froese, D.S.; Fowler, B.; Baumgartner, M.R. Vitamin B12, folate, and the methionine remethylation cycle-biochemistry, pathways, and regulation. J. Inherit. Metab. Dis. 2019, 42, 673–685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Spence, J.D.; Wang, X.; Huo, Y.; Xu, X.; Qin, X. Effect of Vitamin B12 Levels on the Association Between Folic Acid Treatment and CKD Progression: A Post Hoc Analysis of a Folic Acid Interventional Trial. Am. J. Kidney Dis. 2020, 75, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Capelli, I.; Cianciolo, G.; Gasperoni, L.; Zappulo, F.; Tondolo, F.; Cappuccilli, M.; La, M.G. Folic Acid and Vitamin B12 Administration in CKD, Why Not? Nutrients. 2019, 11, 383. [Google Scholar] [CrossRef] [Green Version]
- Pastore, A.; Noce, A.; Di, G.G.; De, S.A.; Calla, C.; Zenobi, R.; Dessi, M.; Di, D.N. Homocysteine, cysteine, folate and vitamin B(1)(2) status in type 2 diabetic patients with chronic kidney disease. J. Nephrol. 2015, 28, 571–576. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.L.; Lin-Tan, D.T.; Li, Y.J.; Chen, K.H.; Huang, Y.L. Low-level environmental exposure to lead and progressive chronic kidney diseases. Am. J. Med. 2006, 119, 707–709. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.J.; Huang, Y.L.; Shiue, H.S.; Chen, T.W.; Lin, Y.F.; Huang, C.Y.; Lin, Y.C.; Han, B.C.; Hsueh, Y.M. Renin-angiotensin-aldosterone system related gene polymorphisms and urinary total arsenic is related to chronic kidney disease. Toxicol. Appl. Pharmacol. 2014, 279, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, Y.M.; Huang, Y.L.; Huang, C.C.; Wu, W.L.; Chen, H.M.; Yang, M.H.; Lue, L.C.; Chen, C.J. Urinary levels of inorganic and organic arsenic metabolites among residents in an arseniasis-hyperendemic area in Taiwan. J. Toxicol. Environ. Health A 1998, 54, 431–444. [Google Scholar] [PubMed]
- Peng, L.; Wang, X.; Huo, X.; Xu, X.; Lin, K.; Zhang, J.; Huang, Y.; Wu, K. Blood cadmium burden and the risk of nasopharyngeal carcinoma: A case-control study in Chinese Chaoshan population. Environ. Sci. Pollut. Res. Int. 2015, 22, 12323–12331. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, R.L.; Huang, Y.L.; Shiue, H.S.; Huang, S.R.; Lin, M.I.; Mu, S.C.; Chung, C.J.; Hsueh, Y.M. Arsenic methylation capacity and developmental delay in preschool children in Taiwan. Int. J. Hyg. Environ. Health 2014, 217, 678–686. [Google Scholar] [CrossRef]
- Lin, Y.C.; Chung, C.J.; Huang, Y.L.; Hsieh, R.L.; Huang, P.T.; Wu, M.Y.; Ao, P.L.; Shiue, H.S.; Huang, S.R.; Su, C.T.; et al. Association of plasma folate, vitamin B12 levels, and arsenic methylation capacity with developmental delay in preschool children in Taiwan. Arch. Toxicol. 2019, 93, 2535–2544. [Google Scholar] [CrossRef]
- Szklo, M.; Nieto, F.J. Epidemiology Beyong the Basics, 4th ed.; Jones & Bartlett Publishers: Burlington, VT, USA, 2019; pp. 509–512. [Google Scholar]
- Rothman, K.J. Modern Epidemiology; Wolters Kluwer Health/Lippincott Williams & Wilkins: Philadelphia, PA, USA, 1986; pp. 322–326. [Google Scholar]
- Hosmer, D.W.; Lemeshow, S. Confidence interval estimation of interaction. Epidemiology 1992, 3, 452–456. [Google Scholar] [CrossRef]
- Kakitapalli, Y.; Ampolu, J.; Madasu, S.D.; Sai Kumar, M.L.S. Detailed Review of Chronic Kidney Disease. Kidney Dis. 2020, 6, 85–91. [Google Scholar] [CrossRef]
- Hsueh, Y.M.; Chung, C.J.; Shiue, H.S.; Chen, J.B.; Chiang, S.S.; Yang, M.H.; Tai, C.W.; Su, C.T. Urinary arsenic species and CKD in a Taiwanese population: A case-control study. Am. J. Kidney Dis. 2009, 54, 859–870. [Google Scholar] [CrossRef]
- Mujaj, B.; Yang, W.Y.; Zhang, Z.Y.; Wei, F.F.; Thijs, L.; Verhamme, P.; Staessen, J.A. Renal function in relation to low-level environmental lead exposure. Nephrol. Dial. Transplant. 2019, 34, 941–946. [Google Scholar] [CrossRef]
- Sotomayor, C.G.; Groothof, D.; Vodegel, J.J.; Gacitua, T.A.; Gomes-Neto, A.W.; Oste, M.C.J.; Pol, R.A.; Ferreccio, C.; Berger, S.P.; Chong, G.; et al. Circulating Arsenic is Associated with Long-Term Risk of Graft Failure in Kidney Transplant Recipients: A Prospective Cohort Study. J. Clin. Med. 2020, 9, 417. [Google Scholar] [CrossRef] [Green Version]
- Satarug, S.; Gobe, G.C.; Ujjin, P.; Vesey, D.A. A Comparison of the Nephrotoxicity of Low Doses of Cadmium and Lead. Toxics 2020, 8, 18. [Google Scholar] [CrossRef] [Green Version]
- Jain, R.B. Co-exposures to toxic metals cadmium, lead, and mercury and their impact on unhealthy kidney function. Environ. Sci. Pollut. Res. Int. 2019, 26, 30112–30118. [Google Scholar] [CrossRef]
- Sotomayor, C.G.; Groothof, D.; Vodegel, J.J.; Eisenga, M.F.; Knobbe, T.J.; IJmker, J.; Lammerts, R.G.M.; de Borst, M.H.; Berger, S.P.; Nolte, I.M.; et al. Plasma cadmium is associated with increased risk of long-term kidney graft failure. Kidney Int. 2021, 99, 1213–1224. [Google Scholar] [CrossRef]
- Orr, S.E.; Bridges, C.C. Chronic Kidney Disease and Exposure to Nephrotoxic Metals. Int. J. Mol. Sci. 2017, 18, 1039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rana, M.N.; Tangpong, J.; Rahman, M.M. Toxicodynamics of Lead, Cadmium, Mercury and Arsenic- induced kidney toxicity and treatment strategy: A mini review. Toxicol. Rep. 2018, 5, 704–713. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.M.; Ma, J.Q.; Sun, Y.Z. Puerarin protects rat kidney from lead-induced apoptosis by modulating the PI3K/Akt/eNOS pathway. Toxicol. Appl. Pharmacol. 2012, 258, 330–342. [Google Scholar] [CrossRef]
- Rani, A.; Kumar, A.; Lal, A.; Pant, M. Cellular mechanisms of cadmium-induced toxicity: A review. Int. J. Environ. Health Res. 2014, 24, 378–399. [Google Scholar] [CrossRef] [PubMed]
- Yen, Y.P.; Tsai, K.S.; Chen, Y.W.; Huang, C.F.; Yang, R.S.; Liu, S.H. Arsenic induces apoptosis in myoblasts through a reactive oxygen species-induced endoplasmic reticulum stress and mitochondrial dysfunction pathway. Arch. Toxicol. 2012, 86, 923–933. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Moneim, A.M.; El-Toweissy, M.Y.; Ali, A.M.; Awad Allah, A.A.; Darwish, H.S.; Sadek, I.A. Curcumin Ameliorates Lead (Pb(2+))-Induced Hemato-Biochemical Alterations and Renal Oxidative Damage in a Rat Model. Biol. Trace Elem. Res. 2015, 168, 206–220. [Google Scholar] [CrossRef]
- Liu, G.; Wang, Z.K.; Wang, Z.Y.; Yang, D.B.; Liu, Z.P.; Wang, L. Mitochondrial permeability transition and its regulatory components are implicated in apoptosis of primary cultures of rat proximal tubular cells exposed to lead. Arch. Toxicol. 2016, 90, 1193–1209. [Google Scholar] [CrossRef]
- Flores-Guerrero, J.L.; Minovic, I.; Groothof, D.; Gruppen, E.G.; Riphagen, I.J.; Kootstra-Ros, J.; Muller, K.A.; Hak, E.; Navis, G.; Gansevoort, R.T.; et al. Association of Plasma Concentration of Vitamin B12 With All-Cause Mortality in the General Population in the Netherlands. JAMA Netw. Open. 2020, 3, e1919274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendonca, N.; Jagger, C.; Granic, A.; Martin-Ruiz, C.; Mathers, J.C.; Seal, C.J.; Hill, T.R. Elevated Total Homocysteine in All Participants and Plasma Vitamin B12 Concentrations in Women Are Associated With All-Cause and Cardiovascular Mortality in the Very Old: The Newcastle 85+ Study. J. Gerontol. A Biol. Sci. Med. Sci. 2018, 73, 1258–1264. [Google Scholar] [CrossRef]
- Andres, E.; Serraj, K.; Zhu, J.; Vermorken, A.J. The pathophysiology of elevated vitamin B12 in clinical practice. QJM 2013, 106, 505–515. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.M.; Lee, M.K.; Bae, S.G.; Lee, S.H.; Kim, S.Y.; Lee, D.H. Association of homocysteine levels with blood lead levels and micronutrients in the US general population. J. Prev. Med. Public Health 2012, 45, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Nie, J. Homocysteine in Renal Injury. Kidney Dis. 2016, 2, 80–87. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).