Oral Vitamin D Therapy in Patients with Psoriasis
Abstract
1. Introduction
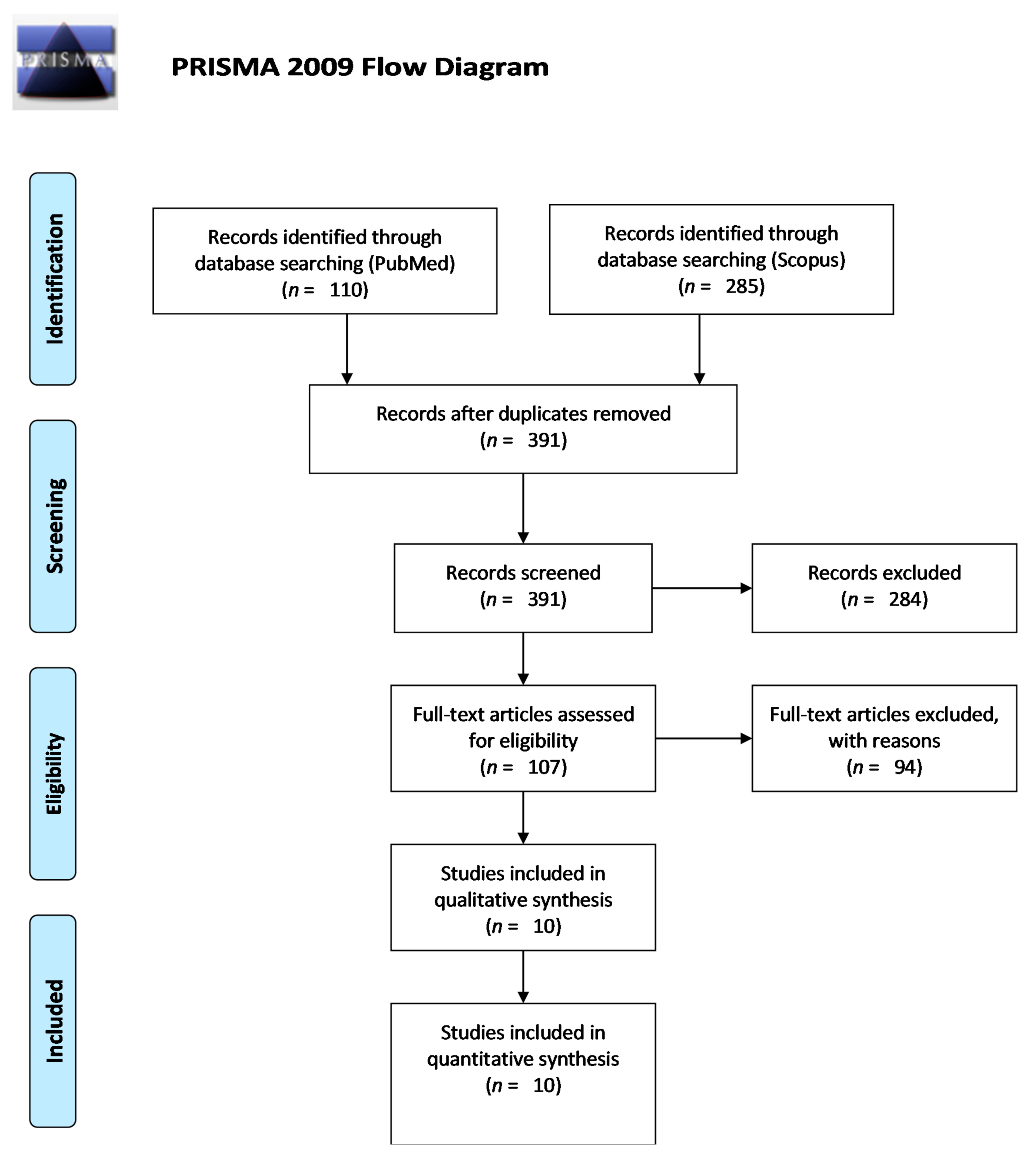
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction
3. Results
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Martens, P.J.; Gysemans, C.; Verstuyf, A.; Mathieu, A.C. Vitamin D’s effect on immune function. Nutrients 2020, 12, 1248. [Google Scholar] [CrossRef]
- Windaus, A.; Linsert, O.; Luttringhaus, A.; Weidlich, G. Über das krystallisierte Vitamin D2. Ann. Chem. Liebigs 1932, 492, 226–241. [Google Scholar] [CrossRef]
- Windaus, A.; Schenck, F.; von Werder, F. Über das antirachitisch wirksame bestrahlungs-produkt aus 7-dehydrocholesterin. Z Physiol. Chem. Hoppe Seylers 1936, 241, 100–103. [Google Scholar] [CrossRef]
- Charoenngam, N.; Holick, M.F. Immunologic effects of vitamin D on human health and disease. Nutrients 2020, 12, 2097. [Google Scholar] [CrossRef] [PubMed]
- Brandão-Lima, P.N.; Santos, B.D.C.; Aguilera, C.M.; Freire, A.R.S.; Martins-Filho, P.R.S.; Pires, L.V. Vitamin D food fortification and nutritional status in children: A systematic review of randomized controlled trials. Nutrients 2019, 11, 2766. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef]
- Misra, M.; Pacaud, D.; Petryk, A.; Collett-Solberg, P.F.; Kappy, M. Drug and therapeutics committee of the Lawson Wilkins pediatric endocrine society. Vitamin D deficiency in children and its management: Review of current knowledge and recommendations. Pediatrics 2008, 122, 398–417. [Google Scholar] [CrossRef]
- Chang, S.W.; Lee, H.C. Vitamin u8h brD and health—The missing vitamin in humans. Pediatrics Neonatol. 2019, 60, 237–244. [Google Scholar] [CrossRef]
- Rendon, A.; Schäkel, K. Psoriasis pathogenesis and treatment. Int. J. Mol. Sci. 2019, 20, 1475. [Google Scholar] [CrossRef]
- Slominski, A.; Kim, T.K.; Zmijewski, M.A.; Janjetovic, Z.; Li, W.; Chen, J.; Kusniatsova, E.I.; Semak, I.; Postlethwaite, A.; Miller, D.D.; et al. Novel vitamin D photoproducts and their precursors in the skin. Dermatoendocrinology 2013, 5, 7–19. [Google Scholar] [CrossRef]
- Stanescu, A.M.A.; Grajdeanu, I.V.; Iancu, M.A.; Pantea Stoian, A.; Bratu, O.G.; Socea, B.; Socea, L.I.; Diaconu, C.C. Correlation of oral vitamin D administration with the severity of psoriasis and the presence of metabolic syndrome. Rev. Chim. 2018, 69, 1668–1672. [Google Scholar] [CrossRef]
- Burfield, L.; Burden, A.D. Psoriasis. J. R. Coll. Physicians Edinb. 2013, 43, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.; Slatopolsky, E. Vitamin D analogs: Therapeutic applications and mechanisms for selectivity. Mol. Asp. Med. 2008, 29, 433–452. [Google Scholar] [CrossRef] [PubMed]
- Piotrowska, A.; Wierzbicka, J.; Żmijewski, M.A. Vitamin D in the skin physiology and pathology. Acta Biochim. Pol. 2016, 63, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Penna, G.; Adorini, L. 1 Alpha,25-dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. J. Immunol. 2000, 164, 2405–2411. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. The PRISMA group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, S.; Yoshikawa, K.; Kozyka, T.; Kitano, Y.; Imanaka, S.; Fukuo, K.; Koh, E.; Kumahara, Y. An open study of vitamin D3 treatment in psoriasis vulgaris. Br. J. Dermatol. 1986, 115, 421–429. [Google Scholar] [CrossRef]
- Kamangar, F.; Koo, J.; Heller, M.; Lee, E.; Bhutani, T. Oral vitamin D, still a viable treatment option for psoriasis. J. Dermatolog. Treat. 2013, 24, 261–267. [Google Scholar] [CrossRef]
- Lourencetti, M.; Abreu, M.M. Use of active metabolites of vitamin D orally for the treatment of psoriasis. Rev. Assoc. Med. Bras. 2018, 64, 643–648. [Google Scholar] [CrossRef]
- Soleymani, T.; Hung, T.; Soung, J. The role of vitamin D in psoriasis: A review. Int. J. Dermatol. 2015, 54, 383–392. [Google Scholar] [CrossRef]
- Millsop, J.W.; Bhatia, B.K.; Debbaneh, M.; Koo, J.; Liao, W. Diet and psoriasis, part III: Role of nutritional supplements. J. Am. Acad. Dermatol. 2014, 71, 561–569. [Google Scholar] [CrossRef]
- Bouillon, R.; Marcocci, C.; Carmeliet, G.; Bikle, D.; White, J.H.; Dawson-Hughes, B.; Lips, P.; Munns, C.F.; Lazaretti-Castro, M.; Giustina, A.; et al. Skeletal and extraskeletal actions of vitamin D: Current evidence and outstanding questions. Endocr. Rev. 2019, 40, 1109–1151. [Google Scholar] [CrossRef] [PubMed]
- Takamoto, S.; Onishi, T.; Morimoto, S.; Imanaka, S.; Yukawa, S.; Kozuka, T.; Kitano, Y.; Seino, Y.; Kumahara, Y. Effect of 1 alpha-hydroxycholecalciferol on psoriasis vulgaris: A pilot study. Calcif. Tissue Int. 1986, 39, 360–364. [Google Scholar] [CrossRef]
- Smith, E.L.; Pincus, S.H.; Donovan, L.; Holick, M.F. A novel approach for the evaluation and treatment of psoriasis. Oral or topical use of 1,25-dihydroxyvitamin D3 can be a safe and effective therapy for psoriasis. J. Am. Acad. Dermatol. 1988, 19, 516–528. [Google Scholar] [CrossRef]
- Hambly, R.; Kirby, B. The relevance of serum vitamin D in psoriasis: A review. Arch. Dermatol. Res. 2017, 309, 499–517. [Google Scholar] [CrossRef] [PubMed]
- Holland, D.B.; Wood, E.J.; Roberts, S.G.; West, M.R.; Cunliffe, W.J. Epidermal keratin levels during oral 1-alpha-hydroxyvitamin D3 treatment for psoriasis. Skin Pharmacol. 1989, 2, 68–76. [Google Scholar] [CrossRef]
- Huckins, D.; Felson, D.T.; Holick, M. Treatment of psoriatic arthritis with oral 1,25-dihydroxyvitamin D3: A pilot study. Arthritis Rheum. 1990, 33, 1723–172715. [Google Scholar] [CrossRef]
- Siddiqui, M.A.; Al-Khawajah, M.M. Vitamin D3 and psoriasis: A randomized double-blind placebo-controlled study. J. Dermatol. Treat. 1990, 1, 243–245. [Google Scholar] [CrossRef]
- Zuccotti, E.; Oliveri, M.; Girometta, C.; Ratto, D.; Di Iorio, C.; Occhinegro, A.; Rossi, P. Nutritional strategies for psoriasis: Current scientific evidence in clinical trials. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 8537–8551. [Google Scholar] [CrossRef]
- Lugo-Somolinos, A.; Sanchez, J.L.; Haddock, L. Efficacy of 1, alpha 25-dihydroxyvitamin D (Calcitriol) in the treatment of psoriasis vulgaris: An open study. Bol. Asoc. Med. P R 1990, 82, 450–453. [Google Scholar]
- el-Azhary, R.A.; Peters, M.S.; Pittelkow, M.R.; Kao, P.C.; Muller, S.A. Efficacy of vitamin D3 derivatives in the treatment of psoriasis vulgaris: A preliminary report. Mayo Clin. Proc. 1993, 68, 835–841. [Google Scholar] [CrossRef]
- Perez, A.; Raab, R.; Chen, T.C.; Turner, A.; Holick, M.F. Safety and efficacy of oral calcitriol (1,25-dihydroxyvitamin D3) for the treatment of psoriasis. Br. J. Dermatol. 1996, 134, 1070–1078. [Google Scholar] [CrossRef]
- Barrea, L.; Savanelli, M.C.; Di Somma, C.; Napolitano, M.; Megna, M.; Colao, A.; Savastano, S. Vitamin D and its role in psoriasis: An overview of the dermatologist and nutritionist. Rev. Endocr. Metab. Disord. 2017, 18, 195–205. [Google Scholar] [CrossRef]
- Gaál, J.; Lakos, G.; Szodoray, P.; Kiss, J.; Horváth, I.; Horkay, E.; Nagy, G.; Szegedi, A. Immunological and clinical effects of alphacalcidol in patients with psoriatic arthropathy: Results of an open, follow-up pilot study. Acta Derm. Venereol. 2009, 89, 140–144. [Google Scholar]
- Finamor, D.C.; Sinigaglia-Coimbra, R.; Neves, L.C.; Gutierrez, M.; Silva, J.J.; Torres, L.D.; Surano, F.; Neto, D.J.; Novo, N.F.; Juliano, Y.; et al. A pilot study assessing the effect of prolonged administration of high daily doses of vitamin D on the clinical course of vitiligo and psoriasis. Dermatoendocrinology 2013, 5, 222–234. [Google Scholar] [CrossRef]
- Umar, M.; Sastry, K.S.; Al Ali, F.; Al-Khulaifi, M.; Wang, E.; Chouchane, A. I: Vitamin D and the pathophysiology of inflammatory skin diseases. Skin Pharmacol. Physiol. 2018, 31, 74–86. [Google Scholar] [CrossRef]
- Hata, T.; Audish, D.; Kotol, P.; Coda, A.; Kabigting, F.; Miller, J.; Alexandrescu, D.; Boguniewicz, M.; Taylor, P.; Aertker, L.; et al. A randomized controlled double-blind investigation of the effects of vitamin D dietary supplementation in subjects with atopic dermatitis. J. Eur. Acad. Dermatol. Venereol. 2014, 28, 781–789. [Google Scholar] [CrossRef]
- Jarrett, P.; Camargo, C.A., Jr.; Coomarasamy, C.; Scragg, R. A randomized, double-blind, placebo-controlled trial of the effect of monthly vitamin D supplementation in mild psoriasis. J. Dermatolog. Treat. 2018, 29, 324–328. [Google Scholar] [CrossRef]
- Ingram, M.A.; Jones, M.B.; Stonehouse, W.; Jarrett, P.; Scragg, R.; Mugridge, O.; von Hurst, P.R. Oral vitamin D3 supplementation for chronic plaque psoriasis: A randomized, double-blind, placebo-controlled trial. J. Dermatolog. Treat. 2018, 29, 648–657. [Google Scholar] [CrossRef]
- Disphanurat, W.; Viarasilpa, W.; Chakkavittumrong, P.; Pongcharoen, P. The clinical effect of oral vitamin D2 supplementation on psoriasis: A double-blind, randomized, placebo-controlled study. Dermatol. Res. Pract. 2019, 2019, 5237642. [Google Scholar] [CrossRef]
- Marino, R.; Misra, M. Extra-sekeletal effects of vitamin D. Nutrients 2019, 11, 1460. [Google Scholar] [CrossRef]
- Fredriksson, T.; Pettersson, U. Severe psoriasis—Oral therapy with a new retinoid. Dermatologica 1978, 157, 238–244. [Google Scholar] [CrossRef]
- Heaney, R.P. Guidelines for optimizing design and analysis of clinical studies of nutrient effects. Nutr. Rev. 2014, 72, 48–54. [Google Scholar] [CrossRef]
- Le, P.; Tu, J.; Gebauer, K.; Brown, S. Serum 25-hydroxyvitamin D increases with UVB and UVA/UVB phototherapy (broadband UVB, narrowband UVB (NBUVB) and heliotherapy) in patients with psoriasis and atopic dermatitis. Australas J. Dermatol. 2016, 57, 115–121. [Google Scholar] [CrossRef]
- Tremezaygues, L.; Reichrath, J. Vitamin D analogs in the treatment of psoriasis: Where are we standing and where will we be going? Dermatoendocrinology 2011, 3, 180–186. [Google Scholar] [CrossRef]
- Orgaz-Molina, J.; Buenda-Eisman, A.; Arrabal-Polo, M.A.; Ruiz, J.C.; Arias-Santiago, S. Deficiency of serum concentration of 25-hydroxyvitamin D in psoriatic patients: A case-control study. J. Am. Acad. Dermatol. 2012, 67, 931–938. [Google Scholar] [CrossRef]
- Adams, J.S.; Hewison, M. Update in vitamin D. J. Clin. Endocrinol. Metab. 2010, 95, 471–478. [Google Scholar] [CrossRef]
- Werner de Castro, G.R.; Neves, F.S.; Pereira, I.A.; Fialho, S.C.; Ribeiro, G.; Zimmermann, A.F. Resolution of adalimumab-induced psoriasis after vitamin D deficiency treatment. Rheumatol. Int. 2011, 32, 1313–1316. [Google Scholar] [CrossRef]
- Thompson, K.G.; Kim, N. Dietary supplements in dermatology: A review of the evidence for zinc, biotin, vitamin D, nicotinamide, and Polypodium. J. Am. Acad. Dermatol. 2020. [Google Scholar] [CrossRef]
- Merola, J.F.; Han, J.; Li, T.; Qureshi, A.A. No association between vitamin D intake and incident psoriasis among US women. Arch. Dermatol. Res. 2014, 306, 305–307. [Google Scholar] [CrossRef]
- Nair, R.; Maseeh, A. Vitamin D: The “sunshine” vitamin. J. Pharmacol. Pharmacother. 2012, 3, 118–126. [Google Scholar] [CrossRef] [PubMed]
- McCullough, P.J.; Lehrer, D.S.; Amend, J.J. Daily oral dosing of vitamin D3 using 5000 TO 50,000 international units a day in long-term hospitalized patients: Insights from a seven year experience. J. Steroid Biochem. Mol. Biol. 2019, 189, 228–239. [Google Scholar] [CrossRef] [PubMed]
- McCullough, P.; Amend, J.J. Results of daily oral dosing with up to 60,000 international units (iu) of vitamin D3 for 2 to 6 years in 3 adult males. J. Steroid Biochem. Mol. Biol. 2017, 173, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Garland, C.F.; French, C.B.; Baggerly, L.L.; Heaney, R.P. Vitamin D supplement doses and serum 25-hydroxyvitamin D in the range associated with cancer prevention. Anticancer Res. 2011, 31, 617–622. [Google Scholar]
- Dawson-Hughes, B.; Staten, M.A.; Knowler, W.C.; Nelson, J.; Vickery, E.M.; LeBlanc, E.S.; Neff, L.M.; Park, J.; Pittas, A.G.; D2d Research Group. Intratrial exposure to vitamin D and new-onset diabetes among adults with prediabetes: A secondary analysis from the vitamin D and type 2 diabetes (D2d) study. Diabetes Care 2020, 43, 2916–2922. [Google Scholar] [CrossRef]
- Danik, J.S.; Manson, J.E. Vitamin d and cardiovascular disease. Curr. Treat. Options Cardiovasc. Med. 2012, 14, 414–424. [Google Scholar] [CrossRef]
- Chai, B.; Gao, F.; Wu, R.; Dong, T.; Gu, C.; Lin, Q.; Zhang, Y. Vitamin D deficiency as a risk factor for dementia and Alzheimer’s disease: An updated meta-analysis. BMC Neurol. 2019, 19, 284. [Google Scholar] [CrossRef]
- Aghajafari, F.; Letourneau, N.; Mahinpey, N.; Cosic, N.; Giesbrecht, G. Vitamin D deficiency and antenatal and postpartum depression: A systematic review. Nutrients 2018, 10, 478. [Google Scholar] [CrossRef]
- Cuomo, A.; Giordano, N.; Goracci, A.; Fagiolini, A. Depression and vitamin D deficiency: Causality, assessment, and clinical practice implications. Neuropsyvchiatry 2017, 7, 606–614. [Google Scholar] [CrossRef]
- Gruber-Bzura, B.M. Vitamin D and influenza-prevention or therapy? Int. J. Mol. Sci. 2018, 19, 2419. [Google Scholar] [CrossRef]
- American Geriatrics Society Workgroup on Vitamin D Supplementation for Older Adult. Recommendations abstracted from the American geriatrics consensus statement on Vitamin D for prevention of falls and their consequences. J. Am. Geriatr. Soc. 2014, 62, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Goulão, B.; Stewart, F.; Ford, J.A.; MacLennan, G.; Avenell, J.A. Cancer and vitamin D supplementation: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2018, 107, 652–663. [Google Scholar] [CrossRef]
- Bittenbring, J.T.; Neumann, F.; Altmann, B.; Achenbach, M.; Reichrath, J.; Ziepert, M.; Geisel, J.; Regitz, E.; Held, G.; Pfreundschuh, M. Vitamin D deficiency impairs rituximab-mediated cellular cytotoxicity and outcome of patients with diffuse large B-cell lymphoma treated with but not without rituximab. J. Clin. Oncol. 2014, 32, 3242–3248. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Tang, Z.; Wang, Y.; Ding, X.; Rinaldi, G.; Rahmani, J.; Xing, F. Relationship between vitamin D level and mortality in adults with psoriasis: A retrospective cohort study of NHANES data. Clin. Ther. 2020. [Google Scholar] [CrossRef]
| Authors and Year | Type of Study | Number of Patients | Study Location | Reviews Including the Original Study from the First Column |
|---|---|---|---|---|
| Morimoto et al., 1986 [17] | Open-design study | 21 | Japan | Kamangar et al., 2013 [18] Lourenceti et al., 2018 [19] Soleymani et al., 2015 [20] Millsop et al., 2014 [21] Bouillon et al., 2018 [22] |
| Takamoto et al., 1986 [23] | Descriptive study | 7 | Japan | Kamangar et al., 2013 [18] Lourenceti et al., 2018 [19] |
| Smith et al., 1988 [24] | Descriptive study | 14 | USA | Kamangar et al., 2013 [18] Lourenceti et al., 2018 [19] Millsop et al., 2014 [21] Bouillon et al., 2018 [22] Hambly et al., 2017 [25] |
| Holland et al., 1989 [26] | Descriptive study | 15 | UK | Hambly et al., 2017 [25] |
| Huckins et al., 1990 [27] | Open-label trial | 6 | USA | Kamangar et al., 1990 [18] Lourenceti et al., 2018 [19] |
| Siddiqui et al., 1990 [28] | Prospective randomized double-blind control study | 41 | Saudi Arabia | Millsop et al., 2014 [21] Zuccotti et al., 2018 [29] |
| Lugo-Somolinos et al., 1990 [30] | Descriptive study | 10 | Puerto Rico | Hambly et al., 2017 [25] |
| El-Alzhari et al., 1993 [31] | Descriptive study | 8 | USA | Lourenceti et al., 2018 [19] Millsop et al., 2014 [21] |
| Perez et al., 1996 [32] | Open trial | 85 | USA | Kamangar et al., 2013 [18] Lourenceti et al., 2018 [19] Soleymani et al., 2015 [20] Millsop et al., 2014 [21] Barrea et al., 2017 [33] Bouillon et al., 2018 [22] Hambly et al., 2017 [25] |
| Gaal et al., 2009 [34] | Case-control | 10 | USA | Kamangar et al., 2013 [18] Zuccotti et al., 2018 [29] |
| Finamor et al., 2013 [35] | Open-label clinical trial | 9 | Hungary | Lourenceti et al., 2018 [19] Millsop et al., 2014 [21] Umar et al., 2018 [36] Hambly et al., 2017 [25] |
| Hata et al., 2014 [37] | Randomized placebo-controlled | 16 | Brazil | Hambly et al., 2017 [25] |
| Jarret et al., 2018 [38] | Randomized double blind, placebo-controlled study | 65 | USA | Zuccotti et al., 2018 [29] |
| Ingram et al., 2018 [39] | Randomized double blind, placebo-controlled study | 101 | New Zealand | |
| Disphanurat et al., 2019 [40] | Randomized double blind, placebo-controlled study | 45 | Thailand | Marino et al., 2019 [41] |
| Individual Studies, Year | Dose | Duration of Administration | Efficacy | Type/Severity of Psoriasis | Effectiveness | Treatment Side Effects |
|---|---|---|---|---|---|---|
| Morimoto et al., 1986 [17] | 1.0 μg/day 1α-(OH)D3 (40 IU/day) | 6 months | 2.7 +/− 0.6 months | Psoriasis vulgaris | More than moderate improvement (+2) in 76% of patients | No |
| 0.5 μg/day 1,25-(OH)2-D3 (20 IU/day) | 6 months | 3 months | Psoriasis vulgaris | Moderate improvement (+2) in 25% of patients | No | |
| Takamoto et al., 1986 [23] | 1.0 μg/day 1α-(OH)D3 (40 IU/day) | 12 months | more than 8 months | Psoriasis vulgaris |
| No |
| Smith et al., 1988 [24] | 0.25 μg (10 IU) once or twice/day increased by 0.25 to 0.5 μg/day every 2 weeks to a maximum of 2.0 μg (80 IU)/day 1,25-(OH)2-D3 | 2 months | less than 2 months | moderate to severe psoriasis |
| No |
| Holland et al., 1989 [26] | 1.0 μg/day 1α-(OH)D3 (40 IU) | 6 months | 6–8 weeks | Plaque psoriasis | 46.67% of patients had complete resolution of lesions (+4), 2 within 6 weeks and the rest after 4–6 months of therapy. | No |
| Huckins et al., 1990 [27] | 1.0 μg/day 0.5 μg/day increased by 0.25 μg/day every 2 weeks to a maximum of 2.0 μg (80 IU)/day 1,25-(OH)2-D3 | 6 months | 2–3 months | Psoriatic arthritis |
| hypercalciuria in 20% of patients |
| Siddiqui et al., 1990 [28] | 1 μg/day alpha-calcidol | 12 weeks | Not specified | Psoriasis vulgaris | 45% of patients showed slight improvement (+1). | |
| Lugo-Somolinos et al., 1990 [30] | 0.5 μg/day 1α,25-(OH)2 -D3 (20 IU) | after 3 months | Moderate to severe psoriasis | 40% of patients showed moderate improvement. | No | |
| El-Alzhari et al., 1993 [31] | 0.5 μg/day increased by 0.5 μg biweekly to a maximal dosage of 2.0 μg daily. 1,25-(OH)2-D3 | 6 months | 2 months | Psoriasis vulgaris moderate to severe |
| No |
| Perez et al., 1996 [32] | 0.5 μg/day increments of 0.5 μg every 2 weeks 1,25-(OH)2-D3 | 6 months–3 years | 6 months | Psoriasisvulgaris | Global severity score for the patients’ lesions had a mean value of 7.7 ± 1.2; the mean global severity score significantly decreased to 3.2 ± 1.9. The mean baseline PASI score was 18.4 ± 1.0; at 6 and 36 months of treatment the mean PASI score was reduced to 9.7 ± 0.8 and 7.0 ± 1.3, respectively. | No |
| Gaal et al., 2009 [34] | 0.25 μg twice daily 1α-(OH)D3 | 6 months | Not specified | Psoriatic arthritis | PASI scores were 12.8 +/− 14.3 vs. 11.9 +/− 14.4. on average. | No |
| Finamor et al., 2013 [35] | 35,000 IU per day vit. D3 | 6 months | Not specified | Psoriasis vulgaris moderate to severe | The clinical condition of all patients significantly improved (+3 to +4). | - |
| Hata et al., 2014 [37] | 4000 IU/day vit. D3 | 6 months | Not specified | Mild psoriasis | No change in PASI score (0) | No |
| Jarret et al., 2018 [38] | 100,000 IU/month (3300 IU/day) vit. D3 | 4 years | Not specified | Mild psoriasis | The trial results do not support the use of monthly vitamin D3 supplementation (100,000 IU per month) as a treatment for mild psoriasis in patients over 50 years old. | |
| Ingram et al., 2018 [39] | 200,000 IU at baseline, then 100,000 IU/month vit. D3 | 11 months | 6 months | Chronic psoriasis | No benefit | Not specified |
| Individual Studies/ Year | Dose | Period of Administration | Efficacy Observed | Type/Severity of Psoriasis | Effectiveness | Treatment Side Effects |
|---|---|---|---|---|---|---|
| Disphanurat et al., 2019 [40] | 20,000 IU/every 2 weeks vit. D2 | 6 months | 3–6 months | Chronic plaque-type psoriasis—mild psoriasis | PASI score decreased at 3 and 6 months, moderate improvement | No |
| Authors | Evaluation |
|---|---|
| Morimoto et al. [17] | Clinical photographs taken at every examination Clinical score: complete remission (+4), marked improvement (+3), moderate improvement (+2), slight improvement (+1), no change (o), deterioration (−1). |
| Smith et al. [24] | Clinical examination Clinical score: no change (0), minimal improvement up to 25% improved (+1), 26% to 50% improved (+2), 51% to 75% improved (+3), >75% improved to clear (+4). |
| Takamoto et al. [23] | Clinical examination: complete remission (4) (complete flattering of plaques including borders, percentage of area improved: 95% or more); marked improvement (3) (nearly complete flattering of all plaques still palpable, area improved: 50–90%); definite improvement (2) (partial flattering of plaque, less scaling and less erythema, area improved: 20–50%), minimal improvement (1) (slightly less scaling and less erythema, area improved: 5–20%); no change (0); aggravation (−1) by the percentage of skin involvement was improved. |
| Huckins et al. [27] | Clinical photographs taken at every examination Clinical score of erythema: deterioration (−1), no change (0), mild improvement (1), moderate improvement (2), marked improvement (3) |
| Gaal et al. [34] |
|
| Perez et al. [32] | Clinical photographs taken at every examination PASI score, global severity score Global Improvement Scale: deterioration (−1), no change (0), mild improvement (1), moderate improvement (2), excellent improvement (3) |
| El-Azhary et al. [31] | Clinical evaluation of the percentage of body surface involved Grading the erythema, scale, and thickness of the lesions as worsening (−1), no improvement (0), mild improvement (+1), moderate improvement (+2), marked improvement (+3). |
| Finamor et al. [35] |
|
| Siddiqui et al. [28] | PASI score Worsening PASI score (−1), no improvement (0), slight improvement (+1), moderate improvement (+2), marked improvement (+3). |
| Holland et al. [26] |
|
| Hata et al. [37] | PASI score Punch biopsies of psoriatic skin lesion and uninvolved skin |
| Jarret et al. [38] |
|
| Ingram et al. [39] |
|
| Disphanurat et al. [40] |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stanescu, A.M.A.; Simionescu, A.A.; Diaconu, C.C. Oral Vitamin D Therapy in Patients with Psoriasis. Nutrients 2021, 13, 163. https://doi.org/10.3390/nu13010163
Stanescu AMA, Simionescu AA, Diaconu CC. Oral Vitamin D Therapy in Patients with Psoriasis. Nutrients. 2021; 13(1):163. https://doi.org/10.3390/nu13010163
Chicago/Turabian StyleStanescu, Ana Maria Alexandra, Anca Angela Simionescu, and Camelia Cristina Diaconu. 2021. "Oral Vitamin D Therapy in Patients with Psoriasis" Nutrients 13, no. 1: 163. https://doi.org/10.3390/nu13010163
APA StyleStanescu, A. M. A., Simionescu, A. A., & Diaconu, C. C. (2021). Oral Vitamin D Therapy in Patients with Psoriasis. Nutrients, 13(1), 163. https://doi.org/10.3390/nu13010163







