Silver Ions as a Tool for Understanding Different Aspects of Copper Metabolism
Abstract
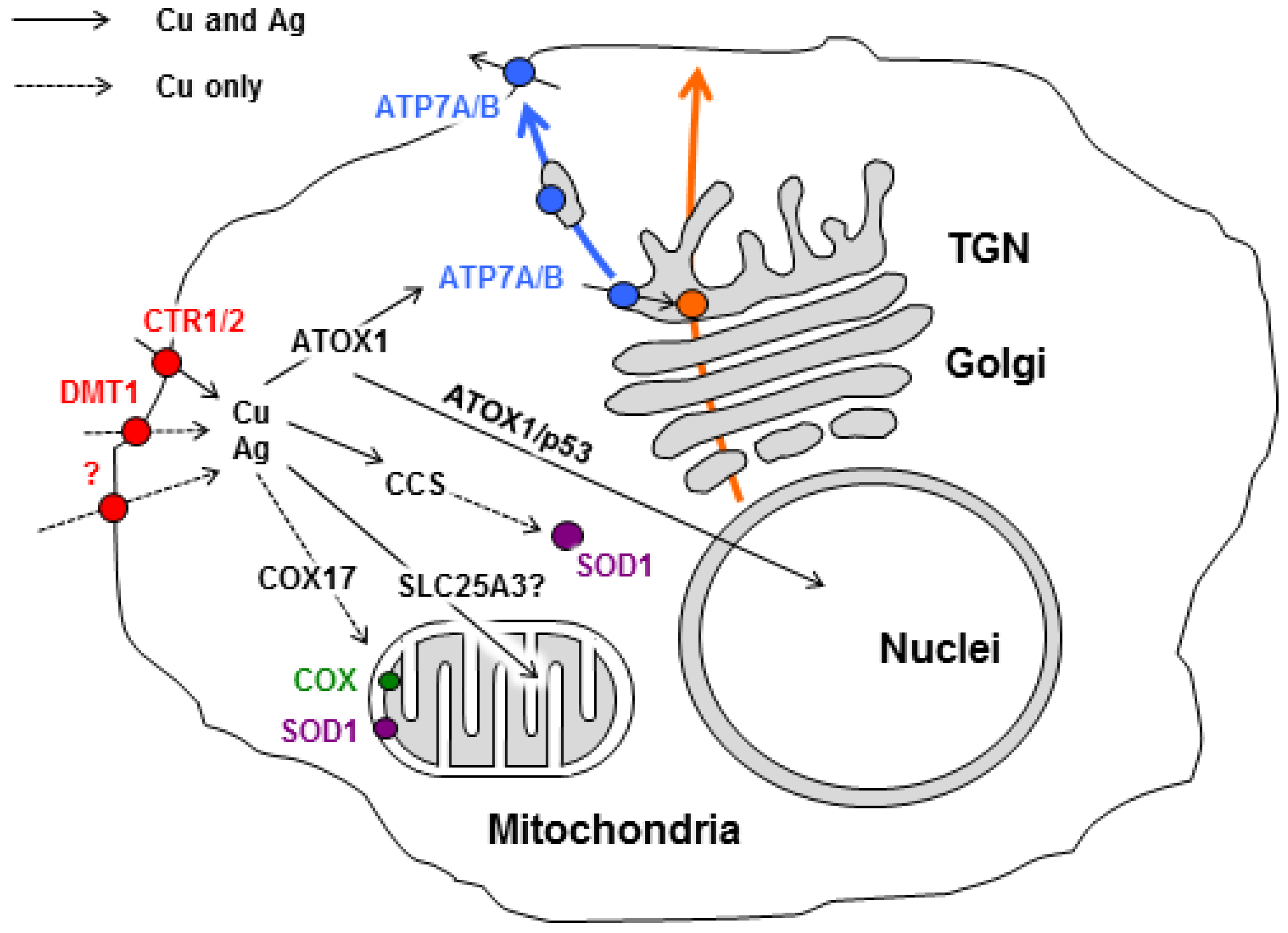
1. Introduction
2. Expedients Used to Treat Biological Objects with Silver Ions
3. Silver Transport through Extracellular Pathways
4. Pathways of Silver Import through the Plasma Membranes
4.1. CT R1
4.2. CTR2
4.3. DMT1
4.4. Other Transporters
5. Interplay between Silver and Pathways Driving Intracellular Copper Distribution
5.1. ATOX1
5.2. Copper Delivery to the Cellular Secretory Pathway
5.3. CCS
5.4. COX17
6. Interference of Silver Nanoparticles (AgNPs) in Copper Metabolism of Eukaryotes
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AgNPs | silver nanoparticles |
| ATOX1 | antioxidant protein 1 (copper chaperon for ATP7A/B) |
| ATP7A and ATP7B | copper transporting ATPases (Menkes ATPase and Wilson ATPase, respectively) |
| CCS | copper chaperone for SOD1 |
| COX | cytochrome-c-oxidase |
| COX17 | copper chaperon for cytochrome-c-oxidase |
| Cp | ceruloplasmin |
| CRD | copper related diseases |
| CTR1 | high affinity copper transporter 1 |
| CTR2 | low affinity copper transporter 2 |
| CTS | copper transporting system |
| CuL | copper ligand (complex anionic fluorescent substance with copper) |
| DMT1 | divalent metal transporter 1 |
| GIT | gastrointestinal tract |
| IMS | mitochondrial intermembrane space |
| LEC rats | Long Evans Cinnamon rats |
| MIA40 | mitochondrial intermembrane space import and assembly complex |
| OXA | oxidase assembly translocase complex |
| PIC2 | yeast phosphate carrier protein of mitochondria |
| ROS | reactive oxygen species |
| SLC25A3 | mammalian phosphate carrier protein of mitochondria |
| SOD1 | Cu,Zn-superoxide dismutase |
| SU | subunit |
| TOM | translocator outer membrane |
| TGN | trans-Golgi network |
References
- Uauy, R.; Olivares, M.; Gonzalez, M. Essentiality of copper in humans. Am. J. Clin. Nutr. 1998, 67 (Suppl. 5), 952S–959S. [Google Scholar] [CrossRef] [PubMed]
- Mason, K.E. A conspectus of research on copper metabolism and requirements of man. J. Nutr. 1979, 109, 1979–2066. [Google Scholar] [CrossRef] [PubMed]
- Wikström, M. Active site intermediates in the reduction of O(2) by cytochrome oxidase, and their derivatives. Biochim. Biophys. Acta 2012, 1817, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Mot, A.C.; Silaghi-Dumitrescu, R. Laccases: Complex architectures for one-electron oxidations. Biochemistry 2012, 77, 1395–1407. [Google Scholar] [CrossRef] [PubMed]
- Vasin, A.; Klotchenko, S.; Puchkova, L. Phylogenetic analysis of six-domain multi-copper blue proteins. PLoS Curr. 2013, 5. [Google Scholar] [CrossRef]
- Ramsden, C.A.; Riley, P.A. Tyrosinase: The four oxidation states of the active site and their relevance to enzymatic activation, oxidation and inactivation. Bioorg. Med. Chem. 2014, 22, 2388–2395. [Google Scholar] [CrossRef]
- Redinbo, M.R.; Yeates, T.O.; Merchant, S. Plastocyanin: Structural and functional analysis. J. Bioenerg. Biomembr. 1994, 26, 49–66. [Google Scholar] [CrossRef]
- Palm-Espling, M.E.; Niemiec, M.S.; Wittung-Stafshede, P. Role of metal in folding and stability of copper proteins in vitro. Biochim. Biophys. Acta 2012, 1823, 1594–1603. [Google Scholar] [CrossRef]
- Hordyjewska, A.; Popiołek, Ł.; Kocot, J. The many “faces” of copper in medicine and treatment. Biometals 2014, 27, 611–621. [Google Scholar] [CrossRef]
- Bharathi Devi, S.R.; Dhivya, M.A.; Sulochana, K.N. Copper transporters and chaperones: Their function on angiogenesis and cellular signalling. J. Biosci. 2016, 41, 487–496. [Google Scholar] [CrossRef]
- Zheng, L.; You, N.; Huang, X.; Gu, H.; Wu, K.; Mi, N.; Li, J. COMMD7 regulates NF-κB signaling pathway in hepatocellular carcinoma stem-like cells. Mol. Ther. Oncolytics 2018, 12, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.I.; Shimoda, M.; Kasai, M.; Ikeda, M.; Ishima, Y.; Kawahara, M. Involvement of SAPK/JNK signaling pathway in copper enhanced zinc-induced neuronal cell death. Toxicol. Sci. 2019, 169, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, C.M.; Chang, C.J. Copper signaling in the brain and beyond. J. Biol. Chem. 2018, 293, 4628–4635. [Google Scholar] [CrossRef] [PubMed]
- Maine, G.N.; Mao, X.; Muller, P.A.; Komarck, C.M.; Klomp, L.W.; Burstein, E. COMMD1 expression is controlled by critical residues that determine XIAP binding. Biochem. J. 2009, 417, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chang, Z.; Mehmood, K.; Abbas, R.Z.; Nabi, F.; Rehman, M.U.; Wu, X.; Tian, X.; Yuan, X.; Li, Z.; et al. Nano Copper Induces Apoptosis in PK-15 Cells via a Mitochondria-Mediated Pathway. Biol. Trace Elem. Res. 2018, 181, 62–70. [Google Scholar] [CrossRef]
- Grubman, A.; White, A.R. Copper as a key regulator of cell signalling pathways. Expert Rev. Mol. Med. 2014, 16, e11. [Google Scholar] [CrossRef] [PubMed]
- Kardos, J.; Héja, L.; Simon, Á.; Jablonkai, I.; Kovács, R.; Jemnitz, K. Copper signalling: Causes and consequences. Cell Commun. Signal. 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Chen, S.; Xi, Z.; Liu, Y. Copper-finger protein of Sp1: The molecular basis of copper sensing. Metallomics 2017, 9, 1169–1175. [Google Scholar] [CrossRef]
- Duan, X.; Block, E.; Li, Z.; Connelly, T.; Zhang, J.; Huang, Z.; Su, X.; Pan, Y.; Wu, L.; Chi, Q.; et al. Crucial role of copper in detection of metal-coordinating odorants. Proc. Natl. Acad. Sci. USA 2012, 109, 3492–3497. [Google Scholar] [CrossRef]
- Li, S.; Ahmed, L.; Zhang, R.; Pan, Y.; Matsunami, H.; Burger, J.L.; Block, E.; Batista, V.S.; Zhuang, H. Smelling sulfur: Copper and silver regulate the response of human odorant receptor OR2T11 to low-molecular-weight thiols. J. Am. Chem. Soc. 2016, 138, 13281–13288. [Google Scholar] [CrossRef]
- Linder, M.C. The relationship of copper to DNA damage and damage prevention in humans. Mutat. Res. 2012, 733, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Rubino, J.T.; Franz, K.J. Coordination chemistry of copper proteins: How nature handles a toxic cargo for essential function. J. Inorg. Biochem. 2012, 107, 129–143. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, A.; Chakraborty, K.; Shukla, A. Cellular copper homeostasis: Current concepts on its interplay with glutathione homeostasis and its implication in physiology and human diseases. Metallomics 2017, 9, 1376–1388. [Google Scholar] [CrossRef] [PubMed]
- Robinson, N.J.; Winge, D.R. Copper metallochaperones. Annu. Rev. Biochem. 2010, 79, 537–562. [Google Scholar] [CrossRef] [PubMed]
- Petzoldt, S.; Kahra, D.; Kovermann, M.; Dingeldein, A.P.; Niemiec, M.S.; Ådén, J.; Wittung-Stafshede, P. Human cytoplasmic copper chaperones Atox1 and CCS exchange copper ions in vitro. Biometals 2015, 28, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Hatori, Y.; Inouye, S.; Akagi, R. Thiol-based copper handling by the copper chaperone Atox1. IUBMB Life 2017, 69, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Hatori, Y.; Lutsenko, S. An expanding range of functions for the copper chaperone/antioxidant protein Atox1. Antioxid. Redox Signal. 2013, 19, 945–957. [Google Scholar] [CrossRef]
- Hatori, Y.; Lutsenko, S. The role of copper chaperone Atox1 in coupling redox homeostasis to intracellular copper distribution. Antioxidants 2016, 5, 25. [Google Scholar] [CrossRef]
- Matson Dzebo, M.; Ariöz, C.; Wittung-Stafshede, P. Extended functional repertoire for human copper chaperones. Biomol. Concepts 2016, 7, 29–39. [Google Scholar] [CrossRef]
- Gaggelli, E.; Kozlowski, H.; Valensin, D.; Valensin, G. Copper homeostasis and neurodegenerative disorders (Alzheimer’s, prion, and Parkinson’s diseases and amyotrophic lateral sclerosis). Chem. Rev. 2006, 106, 1995–2044. [Google Scholar] [CrossRef]
- Weber, K.T.; Weglicki, W.B.; Simpson, R.U. Macro- and micronutrient dyshomeostasis in the adverse structural remodelling of myocardium. Cardiovasc. Res. 2009, 81, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Kozlowski, H.; Kolkowska, P.; Watly, J.; Krzywoszynska, K.; Potocki, S. General aspects of metal toxicity. Curr. Med. Chem. 2014, 21, 3721–3740. [Google Scholar] [CrossRef] [PubMed]
- Giampietro, R.; Spinelli, F.; Contino, M.; Colabufo, N.A. The pivotal role of copper in neurodegeneration: A new strategy for the therapy of neurodegenerative disorders. Mol. Pharm. 2018, 15, 808–820. [Google Scholar] [CrossRef] [PubMed]
- Ojha, R.; Prasad, A.N. Menkes disease: What a multidisciplinary approach can do. J. Multidiscip. Healthc. 2016, 9, 371–385. [Google Scholar] [CrossRef]
- Członkowska, A.; Litwin, T.; Dusek, P.; Ferenci, P.; Lutsenko, S.; Medici, V.; Rybakowski, J.K.; Weiss, K.H.; Schilsky, M.L. Wilson disease. Nat. Rev. Dis. Primers 2018, 4, 21. [Google Scholar] [CrossRef] [PubMed]
- Montpetit, A.; Côté, S.; Brustein, E.; Drouin, C.A.; Lapointe, L.; Boudreau, M.; Meloche, C.; Drouin, R.; Hudson, T.J.; Drapeau, P.; et al. Disruption of AP1S1, causing a novel neurocutaneous syndrome, perturbs development of the skin and spinal cord. PLoS Genet. 2008, 4, e1000296. [Google Scholar] [CrossRef] [PubMed]
- Kaler, S.G. Inborn errors of copper metabolism. Handb. Clin. Neurol. 2013, 113, 1745–1754. [Google Scholar] [CrossRef]
- Bandmann, O.; Weiss, K.H.; Kaler, S.G. Wilson’s disease and other neurological copper disorders. Lancet Neurol. 2015, 14, 103–113. [Google Scholar] [CrossRef]
- Miyajima, H. Aceruloplasminemia. Neuropathology 2015, 35, 83–90. [Google Scholar] [CrossRef]
- Bonafede, R.; Mariotti, R. ALS pathogenesis and therapeutic approaches: The role of mesenchymal stem cells and extracellular vesicles. Front. Cell. Neurosci. 2017, 11, 80. [Google Scholar] [CrossRef]
- Bagheri, S.; Squitti, R.; Haertlé, T.; Siotto, M.; Saboury, A.A. Role of copper in the onset of Alzheimer’s disease compared to other metals. Front. Aging Neurosci. 2018, 9, 446. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Kumar, A.; Prasad, R. Predictive association of copper metabolism proteins with Alzheimer’s disease and Parkinson’s disease: A preliminary perspective. Biometals 2014, 27, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Lowe, J.; Taveira-da-Silva, R.; Hilário-Souza, E. Dissecting copper homeostasis in diabetes mellitus. IUBMB Life 2017, 69, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Mendola, D.; Giacomelli, C.; Rizzarelli, E. Intracellular bioinorganic chemistry and cross talk among different -omics. Curr. Top. Med. Chem. 2016, 16, 3103–3130. [Google Scholar] [CrossRef] [PubMed]
- Wittung-Stafshede, P. Unresolved questions in human copper pump mechanisms. Q. Rev. Biophys. 2015, 48, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Palacios, O.; Polec-Pawlak, K.; Lobinski, R.; Capdevila, M.; Gonzalez-Duarte, P. Is Ag(I) an adequate probe for Cu(I) in structural copper–metallothionein studies? The binding features of Ag(I) to mammalian metallothionein 1. J. Biol. Inorg. Chem. 2003, 8, 831–842. [Google Scholar] [CrossRef]
- Veronesi, G.; Gallon, T.; Deniaud, A.; Boff, B.; Gateau, C.; Lebrun, C.; Vidaud, C.; Rollin-Genetet, F.; Carrière, M.; Kieffer, I.; et al. XAS investigation of silver(I) coordination in copper(I) biological binding sites. Inorg. Chem. 2015, 54, 11688–11696. [Google Scholar] [CrossRef]
- Mukherjee, R.; Concepcion Gimeno, M.; Laguna, A. Comprehensive Coordination Chemistry; Wilkinson, G., Ed.; Pergamon Press: Oxford, UK, 1987; Volume 5, Chapters 53 and 54; pp. 869–909, 919–991. [Google Scholar]
- Khan, K.; Javed, S. Functionalization of inorganic nanoparticles to augment antimicrobial efficiency: A critical analysis. Curr. Pharm. Biotechnol. 2018, 19, 523–536. [Google Scholar] [CrossRef]
- Akter, M.; Sikder, M.T.; Rahman, M.M.; Ullah, A.A.; Hossain, K.F.; Banik, S.; Hosokawa, T.; Saito, T.; Kurasaki, M. A systematic review on silver nanoparticles-induced cytotoxicity: Physicochemical properties and perspectives. J. Adv. Res. 2017, 9, 1–16. [Google Scholar] [CrossRef]
- Mao, B.H.; Tsai, J.C.; Chen, C.W.; Yan, S.J.; Wang, Y.J. Mechanisms of silver nanoparticle-induced toxicity and important role of autophagy. Nanotoxicology 2016, 10, 1021–1040. [Google Scholar] [CrossRef]
- Van Campen, D.R. Effects of zinc, cadmium, silver and mercury on the absorption and distribution of copper-64 in rats. J. Nutr. 1966, 88, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Whanger, P.D.; Weswig, P.H. Effect of some copper antagonists on induction of ceruloplasmin in the rat. J. Nutr. 1970, 100, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, N.; Sugawara, C. Comparative study of effect of acute administration of cadmium and silver on ceruloplasmin and metallothionein: Involvement of disposition of copper, iron, and zinc. Environ. Res. 1984, 35, 507–515. [Google Scholar] [CrossRef]
- Pribyl, T.; Jahodová, J.; Schreiber, V. Partial inhibition of oestrogen-induced adenohypophyseal growth by silver nitrate. Horm. Res. 1980, 12, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Pribyl, T.; Monakhov, N.K.; Vasilyev, V.B.; Shavlovsky, M.M.; Gorbunova, V.N.; Aleynikova, T.D. Silver-containing ceruloplasmin without polyphenol oxidase activity in rat serum. Physiol. Bohemoslov. 1982, 31, 569–571. [Google Scholar]
- Shavlovski, M.M.; Chebotar, N.A.; Konopistseva, L.A.; Zakharova, E.T.; Kachourin, A.M.; Vassiliev, V.B.; Gaitskhoki, V.S. Embryotoxicity of silver ions is diminished by ceruloplasmin--further evidence for its role in the transport of copper. Biometals 1995, 8, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Zatulovskiy, E.A.; Skvortsov, A.N.; Rusconi, P.; Ilyechova, E.Y.; Babich, P.S.; Tsymbalenko, N.V.; Broggini, M.; Puchkova, L.V. Serum depletion of holo-ceruloplasmin induced by silver ions in vivo reduces uptake of cisplatin. J. Inorg. Biochem. 2012, 116, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Hindi, K.M.; Siciliano, T.J.; Durmus, S.; Panzner, M.J.; Medvetz, D.A.; Reddy, D.V.; Hogue, L.A.; Hovis, C.E.; Hilliard, J.K.; Mallet, R.J.; et al. Synthesis, stability, and antimicrobial studies of electronically tuned silver acetate N-heterocyclic carbenes. J. Med. Chem. 2008, 51, 1577–1583. [Google Scholar] [CrossRef]
- McCabe, J.W.; Vangala, R.; Angel, L.A. Binding selectivity of methanobactin from methylosinus trichosporium OB3b for copper(I), silver(I), zinc(II), nickel(II), cobalt(II), manganese(II), lead(II), and iron(II). J. Am. Soc. Mass Spectrom. 2017, 28, 2588–2601. [Google Scholar] [CrossRef]
- Hanson, S.R.; Donley, S.A.; Linder, M.C. Transport of silver in virgin and lactating rats and relation to copper. J. Trace Elem. Med. Biol. 2001, 15, 243–253. [Google Scholar] [CrossRef]
- Klotchenko, S.A.; Tsymbalenko, N.V.; Solov’ev, K.V.; Skvortsov, A.N.; Zatulovskii, E.A.; Babich, P.S.; Platonova, N.A.; Shavlovskii, M.M.; Puchkova, L.V.; Broggini, M. The effect of silver ions on copper metabolism and expression of genes encoding copper transport proteins in rat liver. Dokl. Biochem. Biophys. 2008, 418, 24–27. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, N.; Sugawara, C. Competition between copper and silver in Fischer rats with a normal copper metabolism and in Long-Evans Cinnamon rats with an abnormal copper metabolism. Arch. Toxicol. 2000, 74, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.H.; Starcher, B.; Matrone, G. Mercury and silver interrelationships with copper. J. Nutr. 1964, 83, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Ilyechova, E.Y.; Saveliev, A.N.; Skvortsov, A.N.; Babich, P.S.; Zatulovskaia, Y.A.; Pliss, M.G.; Korzhevskii, D.E.; Tsymbalenko, N.V.; Puchkova, L.V. The effects of silver ions on copper metabolism in rats. Metallomics 2014, 6, 1970–1987. [Google Scholar] [CrossRef] [PubMed]
- Schilsky, M.L. Wilson Disease: Diagnosis, Treatment, and Follow-up. Clin. Liver Dis. 2017, 21, 755–767. [Google Scholar] [CrossRef]
- Cousin, R.J. Absorption, transport, and hepatic metabolism of copper and zinc: Special reference to metallothionein and ceruloplasmin. Physiol. Rev. 1985, 65, 238–309. [Google Scholar] [CrossRef]
- Linder, M.C.; Moor, J.R. Plasma ceruloplasmin. Evidence for its presence in and uptake by heart and other organs of the rat. Biochim. Biophys. Acta 1977, 499, 329–336. [Google Scholar] [CrossRef]
- Ramos, D.; Mar, D.; Ishida, M.; Vargas, R.; Gaite, M.; Montgomery, A.; Linder, M.C. Mechanism of copper uptake from bood plasma ceruloplasmin by mammalian cells. PLoS ONE 2016, 11, 0149516. [Google Scholar] [CrossRef]
- Zatulovskiy, E.; Samsonov, S.; Skvortsov, A. Docking study on mammalian CTR1 copper importer motifs. BMC Syst. Biol. 2007, 1 (Suppl. 1), 54. [Google Scholar] [CrossRef]
- Broderius, M.; Mostad, E.; Wendroth, K.; Prohaska, J.R. Levels of plasma ceruloplasmin protein are markedly lower following dietary copper deficiency in rodents. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2010, 151, 473–479. [Google Scholar] [CrossRef]
- Gray, L.W.; Kidane, T.Z.; Nguyen, A.; Akagi, S.; Petrasek, K.; Chu, Y.L.; Cabrera, A.; Kantardjieff, K.; Mason, A.Z.; Linder, M.C. Copper proteins and ferroxidases in human plasma and that of wild-type and ceruloplasmin knockout mice. Biochem. J. 2009, 419, 237–245. [Google Scholar] [CrossRef] [PubMed]
- McArdle, H.J.; Danks, D.M. Secretion of copper 64 into breast milk following intravenous injection in a human subject. J. Trace Elements Exp. Med. 1991, 4, 81–84. [Google Scholar]
- Puchkova, L.V.; Babich, P.S.; Zatulovskaia, Y.A.; Ilyechova, E.Y.; Di Sole, F. Copper metabolism of newborns is adapted to milk ceruloplasmin as a nutritive source of copper: Overview of the current data. Nutrients 2018, 10, 1591. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Gitschier, J. hCTR1: A human gene for copper uptake identified by complementation in yeast. Proc. Natl. Acad. Sci. USA 1997, 94, 7481–7486. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Pena, M.M.; Nose, Y.; Thiele, D.J. Biochemical characterization of the human copper transporter Ctr1. J. Biol. Chem. 2002, 277, 4380–4387. [Google Scholar] [CrossRef] [PubMed]
- Sharp, P.A. Ctr1 and its role in body copper homeostasis. Int. J. Biochem. Cell Biol. 2003, 35, 288–291. [Google Scholar] [CrossRef]
- De Feo, C.J.; Aller, S.G.; Siluvai, G.S.; Blackburn, N.J.; Unger, V.M. Three-dimensional structure of the human copper transporter hCTR1. Proc. Natl. Acad. Sci. USA 2009, 106, 4237–4242. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Smith, K.; Lee, J.; Thiele, D.J.; Petris, M.J. Identification of methionine-rich clusters that regulate copper-stimulated endocytosis of the human Ctr1 copper transporter. J. Biol. Chem. 2004, 279, 17428–17433. [Google Scholar] [CrossRef] [PubMed]
- Puig, S.; Lee, J.; Lau, M.; Thiele, D.J. Biochemical and genetic analyses of yeast and human high affinity copper transporters suggest a conserved mechanism for copper uptake. J. Biol. Chem. 2002, 277, 26021–26030. [Google Scholar] [CrossRef]
- Jiang, J.; Nadas, I.A.; Kim, A.M.; Franz, K.J. A mets motif peptide found in copper transport proteins selectively binds Cu(I) with methionine only coordination. Inorg. Chem. 2005, 44, 9787–9794. [Google Scholar] [CrossRef]
- Parr, R.G.; Pearson, R.G. Absolute hardness: Companion parameter to absolute electronegativity. J. Am. Chem. Soc. 1983, 105, 7512–7516. [Google Scholar] [CrossRef]
- Skvortsov, A.N.; Zatulovskiĭ, E.A.; Puchkova, L.V. Structure-functional organization of eukaryotic high-affinity copper importerCTR1 determines its ability to transport copper, silver and cisplatin. Mol. Biol. 2012, 46, 335–347. [Google Scholar] [CrossRef]
- Rubino, J.T.; Riggs-Gelasco, P.; Franz, K.J. Methionine motifs of copper transport proteins provide general and flexible thioether-only binding sites for Cu(I) and Ag(I). J. Biol. Inorg. Chem. 2010, 15, 1033–1049. [Google Scholar] [CrossRef] [PubMed]
- Ren, F.; Logeman, B.L.; Zhang, X.; Liu, Y.; Thiele, D.J.; Yuan, P. X-ray structures of the high-affinity copper transporter Ctr1. Nat. Commun. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Klomp, A.E.; Tops, B.B.; Van Denberg, I.E.; Berger, R.; Klomp, L.W. Biochemical characterization and subcellular localization of human copper transporter 1 (hCTR1). Biochem. J. 2002, 364, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Nose, Y.; Kim, B.E.; Thiele, D.J. Ctr1 drives intestinal copper absorption and is essential for growth, iron metabolism, and neonatal cardiac function. Cell Metab. 2006, 4, 235–244. [Google Scholar] [CrossRef]
- Nose, Y.; Wood, L.K.; Kim, B.E.; Prohaska, J.R.; Fry, R.S.; Spears, J.W.; Thiele, D.J. Ctr1 is an apical copper transporter in mammalian intestinal epithelial cells in vivo that is controlled at the level of protein stability. J. Biol. Chem. 2010, 285, 32385–32392. [Google Scholar] [CrossRef]
- Zimnicka, A.M.; Maryon, E.B.; Kaplan, J.H. Human copper transporter hCTR1 mediates basolateral uptake of copper into enterocytes: Implications for copper homeostasis. J. Biol. Chem. 2007, 282, 26471–26480. [Google Scholar] [CrossRef]
- Logeman, B.L.; Wood, L.K.; Lee, J.; Thiele, D.J. Gene duplication and neo-functionalization in the evolutionary and functional divergence of the metazoan copper transporters Ctr1 and Ctr2. J. Biol. Chem. 2017, 292, 11531–11546. [Google Scholar] [CrossRef]
- Bertinato, J.; Swist, E.; Plouffe, L.J.; Brooks, S.P.; L’abbé, M.R. Ctr2 is partially localized to the plasma membrane and stimulates copper uptake in COS-7 cells. Biochem. J. 2008, 409, 731–740. [Google Scholar] [CrossRef]
- Van den Berghe, P.V.; Folmer, D.E.; Malingré, H.E.; Van Beurden, E.; Klomp, A.E.; Van de Sluis, B.; Merkx, M.; Berger, R.; Klomp, L.W. Human copper transporter 2 is localized in late endosomes and lysosomes and facilitates cellular copper uptake. Biochem. J. 2007, 407, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Öhrvik, H.; Thiele, D.J. The role of Ctr1 and Ctr2 in mammalian copper homeostasis and platinum-based chemotherapy. J. Trace Elem. Med. Biol. 2015, 31, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Zhang, Z.; Wang, T.; Chen, C.; Kang, Y.J. Copper uptake by DMT1: A compensatory mechanism for CTR1 deficiency in human umbilical vein endothelial cells. Metallomics 2015, 7, 1285–1289. [Google Scholar] [CrossRef] [PubMed]
- Ilyechova, E.Y.; Bonaldi, E.; Orlov, I.A.; Skomorokhova, E.; Puchkova, L.V.; Broggini, M. CRISP-R/Cas9 mediated deletion of copper transport genes cTR1 and DMT1 in NSCLC cell line H1299. Biological and pharmacological consequences. Cells 2019, 8, 322. [Google Scholar] [CrossRef]
- Öhrvik, H.; Nose, Y.; Wood, L.K.; Kim, B.E.; Gleber, S.C.; Ralle, M.; Thiele, D.J. Ctr2 regulates biogenesis of a cleaved form of mammalian Ctr1 metal transporter lacking the copper- and cisplatin-binding ecto-domain. Proc. Natl. Acad. Sci. USA 2013, 110, E4279–E4288. [Google Scholar] [CrossRef]
- Blair, B.G.; Larson, C.A.; Safaei, R.; Howell, S.B. Copper transporter 2 regulates the cellular accumulation and cytotoxicity of cisplatin and carboplatin. Clin. Cancer Res. 2009, 15, 4312–4321. [Google Scholar] [CrossRef]
- Puchkova, L.V.; Skvortsov, A.N.; Rusconi, P.; Ilyechova, E.Y.; Broggini, M. In vivo effect of copper status on cisplatin-induced nephrotoxicity. Biometals 2016, 29, 841–849. [Google Scholar] [CrossRef]
- Wang, D.; Song, Y.; Li, J.; Wang, C.; Li, F. Structure and metal ion binding of the first transmembrane domain of DMT1. Biochim. Biophys. Acta 2011, 1808, 1639–1644. [Google Scholar] [CrossRef] [PubMed]
- Arredondo, M.; Munoz, P.; Mura, C.V.; Nunez, M.T. DMT1, a physiologically relevant apical Cu1+ transporter of intestinal cells. Am. J. Physiol. Cell. Physiol. 2003, 284, C1525–C1530. [Google Scholar] [CrossRef] [PubMed]
- Coffey, R.; Knutson, M.D. The plasma membrane metal-ion transporter ZIP14 contributes to nontransferrin-bound iron uptake by human β-cells. Am. J. Physiol. Cell Physiol. 2017, 312, C169–C175. [Google Scholar] [CrossRef]
- Arredondo, M.; Mendiburo, M.J.; Flores, S.; Singleton, S.T.; Garrick, M.D. Mouse divalent metal transporter 1 is a copper transporter in HEK293 cells. BioMetals 2013, 27, 115–123. [Google Scholar] [CrossRef]
- Lee, J.; Petris, M.J.; Thiele, D.J. Characterization of mouse embryonic cells deficient in the ctr1 high affinity copper transporter. Identification of a Ctr1-independent copper transport system. J. Biol. Chem. 2002, 277, 40253–40259. [Google Scholar] [CrossRef]
- Bertinato, J.; Cheung, L.; Hoque, R.; Plouffe, L.J. Ctr1 transports silver into mammalian cells. J. Trace Elem. Med. Biol. 2010, 24, 178–184. [Google Scholar] [CrossRef]
- Zimnicka, A.M.; Ivy, K.; Kaplan, J.H. Acquisition of dietary copper: A role for anion transporters in intestinal apical copper uptake. Am. J. Physiol. Cell. Physiol. 2011, 300, C588–C599. [Google Scholar] [CrossRef]
- Pope, C.R.; De Feo, C.J.; Unger, V.M. Cellular distribution of copper to superoxide dismutase involves scaffolding by membranes. Proc. Natl. Acad. Sci. USA 2013, 110, 20491–20496. [Google Scholar] [CrossRef]
- Kahra, D.; Kovermann, M.; Wittung-Stafshede, P. The C-terminus of human copper importer Ctr1 acts as a binding site and transfers copper to Atox1. Biophys. J. 2016, 110, 95–102. [Google Scholar] [CrossRef]
- Nevitt, T.; Öhrvik, H.; Thiele, D.J. Charting the travels of copper in eukaryotes from yeast to mammals. Biochim. Biophys. Acta 2012, 1823, 1580–1593. [Google Scholar] [CrossRef]
- Palumaa, P. Copper chaperones. The concept of conformational control in the metabolism of copper. FEBS Lett. 2013, 587, 1902–1910. [Google Scholar] [CrossRef] [PubMed]
- Fetherolf, M.; Boyd, S.D.; Winkler, D.D.; Winge, D.R. Oxygen-dependent activation of Cu, Zn-superoxide dismutase-1. Metallomics 2017, 9, 1047–1059. [Google Scholar] [CrossRef] [PubMed]
- Signes, A.; Fernandez-Vizarra, E. Assembly of mammalian oxidative phosphorylation complexes I-V and supercomplexes. Essays Biochem. 2018, 62, 255–270. [Google Scholar] [CrossRef] [PubMed]
- Klomp, L.W.; Lin, S.J.; Yuan, D.S.; Klausner, R.D.; Culotta, V.C.; Gitlin, J.D. Identification and functional expression of HAH1, a novel human gene involved in copper homeostasis. J. Biol. Chem. 1997, 272, 9221–9226. [Google Scholar] [CrossRef] [PubMed]
- Wernimont, A.K.; Yatsunyk, L.A.; Rosenzweig, A.C. Binding of copper(I) by the Wilson disease protein and its copper chaperone. J. Biol. Chem. 2004, 279, 12269–12276. [Google Scholar] [CrossRef] [PubMed]
- Levy, A.R.; Turgeman, M.; Gevorkyan-Aiapetov, L.; Ruthstein, S. The structural flexibility of the human copper chaperone Atox1: Insights from combined pulsed EPR studies and computations. Protein Sci. 2017, 26, 1609–1618. [Google Scholar] [CrossRef] [PubMed]
- Rosenzweig, A.C.; Huffman, D.L.; Hou, M.Y.; Wernimont, A.K.; Pufahl, R.A.; O’Halloran, T.V. Crystal structure of the Atx1 metallochaperone protein at 1.02 A resolution. Structure 1999, 7, 605–617. [Google Scholar] [CrossRef]
- Ralle, M.; Lutsenko, S.; Blackburn, N.J. X-ray absorption spectroscopy of the copper chaperone HAH1 reveals a linear two-coordinate Cu(I) center capable of adduct formation with exogenous thiols and phosphines. J. Biol. Chem. 2003, 278, 23163–23170. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, C.J. Protein moonlighting: What is it, and why is it important? Philos. Trans. R. Soc. B 2018, 373, 20160479. [Google Scholar] [CrossRef] [PubMed]
- Beainoa, W.; Guod, Y.; Change, A.J.; Anderson, C.J. Roles of Atox1 and p53 in the trafficking of copper-64 to tumor cell nuclei: Implications for cancer therapy. J. Biol. Inorg. Chem. 2014, 19, 427–438. [Google Scholar] [CrossRef]
- Itoh, S.; Ozumi, K.; Kim, H.W.; Nakagawa, O.; McKinney, R.D.; Folz, R.J.; Zelko, I.N.; Ushio-Fukai, M.; Fukai, T. Novel mechanism for regulation of extracellular SOD transcription and activity by copper: Role of antioxidant-1. Free Radic. Biol. Med. 2009, 46, 95–104. [Google Scholar] [CrossRef]
- Lutsenko, S.; Tsivkovskii, R.; Walker, J.M. Functional properties of the human copper-transporting ATPase ATP7B (the Wilson’s disease protein) and regulation by metallochaperone Atox1. Ann. N. Y. Acad. Sci. 2003, 986, 204–211. [Google Scholar] [CrossRef]
- Blockhuys, S.; Wittung-Stafshede, P. Copper chaperone Atox1 plays role in breast cancer cell migration. Biochem. Biophys. Res. Commun. 2017, 483, 301–304. [Google Scholar] [CrossRef]
- Mercer, J.F.; Livingston, J.; Hall, B.; Paynter, J.A.; Begy, C.; Chandrasekharappa, S.; Lockhart, P.; Grimes, A.; Bhave, M.; Siemieniak, D.; et al. Isolation of a partial candidate gene for Menkes disease by positional cloning. Nat. Genet. 1993, 3, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Petrukhin, K.; Lutsenko, S.; Chernov, I.; Ross, B.M.; Kaplan, J.H.; Gilliam, T.C. Characterization of the Wilson disease gene encoding a P-type copper transporting ATPase: Genomic organization, alternative splicing, and structure/function predictions. Hum. Mol. Genet. 1994, 3, 1647–1656. [Google Scholar] [CrossRef] [PubMed]
- Lorincz, M.T. Wilson disease and related copper disorders. Handb. Clin. Neurol. 2018, 279–292. [Google Scholar] [CrossRef]
- Kaler, S.G. ATP7A-related copper transport diseases-emerging concepts and future trends. Nat. Rev. Neurol. 2011, 7, 15–29. [Google Scholar] [CrossRef]
- Lutsenko, S.; Barnes, N.L.; Bartee, M.Y.; Dmitriev, O.Y. Function and regulation of human copper-transporting ATPases. Physiol. Rev. 2007, 87, 1011–1046. [Google Scholar] [CrossRef] [PubMed]
- La Fontaine, S.; Mercer, J.F. Trafficking of the copper-ATPases, ATP7A and ATP7B: Role in copper homeostasis. Arch. Biochem. Biophys. 2007, 463, 149–167. [Google Scholar] [CrossRef]
- Lutsenko, S. Copper trafficking to the secretory pathway. Metallomics 2016, 8, 840–852. [Google Scholar] [CrossRef]
- Choi, B.S.; Zheng, W. Copper transport to the brain by the blood-brain barrier and blood-CSF barrier. Brain Res. 2009, 1248, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Barry, A.N.; Otoikhian, A.; Bhatt, S.; Shinde, U.; Tsivkovskii, R.; Blackburn, N.J.; Lutsenko, S. The luminal loop Met672-Pro707 of copper-transporting ATPase ATP7A binds metals and facilitates copper release from the intramembrane sites. J. Biol. Chem. 2011, 286, 26585–26594. [Google Scholar] [CrossRef] [PubMed]
- Tsymbalenko, N.V.; Platonova, N.A.; Puchkova, L.V.; Mokshina, S.V.; Sasina, L.K.; Skvortsova, N.N. Identification of a fragment of ceruloplasmin, interacting with copper-transporting Menkes ATPase. Bioorg. Khim. 2000, 26, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Polishchuk, E.V.; Concilli, M.; Iacobacci, S.; Chesi, G.; Pastore, N.; Piccolo, P.; Paladino, S.; Baldantoni, D.; van IJzendoorn, S.C.; Chan, J.; et al. Wilson disease protein ATP7B utilizes lysosomal exocytosis to maintain copper homeostasis. Dev. Cell. 2014, 29, 686–700. [Google Scholar] [CrossRef] [PubMed]
- Polishchuk, E.V.; Polishchuk, R.S. The emerging role of lysosomes in copper homeostasis. Metallomics 2016, 8, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Hellman, N.E.; Gitlin, J.D. Ceruloplasmin metabolism and function. Annu. Rev. Nutr. 2002, 22, 439–458. [Google Scholar] [CrossRef] [PubMed]
- Mann, K.G.; Lawler, C.M.; Vehar, G.A.; Church, W.R. Coagulation factor contains copper ion. J. Biol. Chem. 1984, 259, 12949–12951. [Google Scholar] [PubMed]
- Hollestelle, M.J.; Geertzen, H.G.; Straatsburg, I.H.; van Gulik, T.M.; van Mourik, J.A. Factor VIII expression in liver disease. Thromb. Haemost. 2004, 91, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Platonova, N.A.; Barabanova, S.V.; Povalikhin, R.G.; Tsymbalenko, N.V.; Danilovskiĭ, M.A.; Voronina, O.V.; Dorokhova, I.I.; Puchkovq, L.V. In vivo expression of copper transporting proteins in rat brain sections. Izv. Akad. Nauk Ser. Biol. 2005, 32, 108–120. [Google Scholar]
- Li, Y.W.; Li, L.; Zhao, J.Y. An inhibition of ceruloplasmin expression induced by cerebral ischemia in the cortex and hippocampus of rats. Neurosci. Bull. 2008, 24, 13–20. [Google Scholar] [CrossRef][Green Version]
- Maio, N.; Polticelli, F.; De Francesco, G.; Rizzo, G.; Bonaccorsi di Patti, M.C.; Musci, G. Role of external loops of human ceruloplasmin in copper loading by ATP7B and Ccc2p. J. Biol. Chem. 2010, 285, 20507–20513. [Google Scholar] [CrossRef]
- di Patti, M.C.; Maio, N.; Rizzo, G.; De Francesco, G.; Persichini, T.; Colasanti, M.; Polticelli, F.; Musci, G. Dominant mutants of ceruloplasmin impair the copper loading machinery in aceruloplasminemia. J. Biol. Chem. 2009, 284, 4545–4554. [Google Scholar] [CrossRef]
- Barnes, N.; Tsivkovskii, R.; Tsivkovskaia, N.; Lutsenko, S. The copper-transporting ATPases, Menkes and Wilson disease proteins, have distinct roles in adult and developing cerebellum. J. Biol. Chem. 2005, 280, 9640–9645. [Google Scholar] [CrossRef]
- Zatulovskaia, Y.A.; Ilyechova, E.Y.; Puchkova, L.V. The features of copper metabolism in the rat liver during development. PLoS ONE 2015, 10, e0140797. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.N.; Dunn, R.J.; David, S. Alternative RNA splicing generates a glycosylphosphatidylinositol-anchored form of ceruloplasmin in mammalian brain. J. Biol. Chem. 2000, 275, 4305–4310. [Google Scholar] [CrossRef]
- Platonova, N.A.; Orlov, I.A.; Klotchenko, S.A.; Babich, V.S.; Ilyechova, E.Y.; Babich, P.S.; Garmai, Y.P.; Vasin, A.V.; Tsymbalenko, N.V.; Puchkova, L.V. Ceruloplasmin gene expression profile changes in the rat mammary gland during pregnancy, lactation and involution. J. Trace Elem. Med. Biol. 2017, 43, 126–134. [Google Scholar] [CrossRef]
- Schmidt, K.; Ralle, M.; Schaffer, T.; Jayakanthan, S.; Bari, B.; Muchenditsi, A.; Lutsenko, S. ATP7A and ATP7B copper transporters have distinct functions in the regulation of neuronal dopamine-β-hydroxylase. J. Biol. Chem. 2018, 293, 20085–20098. [Google Scholar] [CrossRef] [PubMed]
- Huster, D.; Finegold, M.J.; Morgan, C.T.; Burkhead, J.L.; Nixon, R.; Vanderwerf, S.M.; Gilliam, C.T.; Lutsenko, S. Consequences of copper accumulation in the livers of the Atp7b-/- (Wilson disease gene) knockout mice. Am. J. Pathol. 2006, 168, 423–434. [Google Scholar] [CrossRef]
- Niciu, M.J.; Ma, X.M.; El Meskini, R.; Pachter, J.S.; Mains, R.E.; Eipper, B.A. Altered ATP7A expression and other compensatory responses in a murine model of Menkes disease. Neurobiol. Dis. 2007, 27, 278–291. [Google Scholar] [CrossRef]
- Gitschier, J.; Moffat, B.; Reilly, D.; Wood, W.I.; Fairbrother, W.J. Solution structure of the fourth metal-binding domain from the Menkes copper-transporting ATPase. Nat. Struct. Biol. 1998, 5, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Bernevic, B.; El-Khatib, A.H.; Jakubowski, N.; Weller, M.G. Online immunocapture ICP-MS for the determination of the metalloprotein ceruloplasmin in human serum. BMC Res. Notes 2018, 11, 213. [Google Scholar] [CrossRef] [PubMed]
- Zaitsev, V.N.; Zaitseva, I.; Papiz, M.; Lindley, P.F. An X-ray crystallographic study of the binding sites of the azide inhibitor and organic substrates to ceruloplasmin, a multi-copper oxidase in the plasma. J. Biol. Inorg. Chem. 1999, 4, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Samygina, V.R.; Sokolov, A.V.; Bourenkov, G.; Schneider, T.R.; Anashkin, V.A.; Kozlov, S.O.; Kolmakov, N.N.; Vasilyev, V.B. Rat ceruloplasmin: A new labile copper binding site and zinc/copper mosaic. Metallomics 2017, 9, 1828–1838. [Google Scholar] [CrossRef] [PubMed]
- Bielli, P.; Calabrese, L. Structure to function relationships in ceruloplasmin: A ‘moonlighting’ protein. Cell. Mol. Life Sci. 2002, 59, 1413–1427. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Sahoo, P.K. Ceruloplasmin, a moonlighting protein in fish. Fish Shellfish Immunol. 2018, 82, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Drakesmith, H.; Nemeth, E.; Ganz, T. Ironing out Ferroportin. Cell. Metab. 2015, 22, 777–787. [Google Scholar] [CrossRef] [PubMed]
- Giurgea, N.; Constantinescu, M.I.; Stanciu, R.; Suciu, S.; Muresan, A. Ceruloplasmin—Acute-phase reactant or endogenous antioxidant? The case of cardiovascular disease. Med. Sci. Monit. 2005, 11, RA48–RA51. [Google Scholar] [PubMed]
- Golenkina, E.A.; Viryasova, G.M.; Galkina, S.I.; Gaponova, T.V.; Sud’ina, G.F.; Sokolov, A.V. Fine regulation of neutrophil oxidative status and apoptosis by ceruloplasmin and its derivatives. Cells 2018, 7, 8. [Google Scholar] [CrossRef] [PubMed]
- Kostevich, V.A.; Sokolov, A.V.; Kozlov, S.O.; Vlasenko, A.Y.; Kolmakov, N.N.; Zakharova, E.T.; Vasilyev, V.B. Functional link between ferroxidase activity of ceruloplasmin and protective effect of apo-lactoferrin: Studying rats kept on a silver chloride diet. Biometals 2016, 29, 691–704. [Google Scholar] [CrossRef]
- Mukhopadhyay, B.P. Recognition dynamics of trinuclear copper cluster and associated histidine residues through conserved or semi-conserved water molecules in human ceruloplasmin: The involvement of aspartic and glutamic acid gates. J. Biomol. Struct. Dyn. 2018, 36, 3829–3842. [Google Scholar] [CrossRef] [PubMed]
- Il’icheva, E.Y.; Puchkova, L.V.; Shavlovskii, M.M.; Korzhevskii, D.E.; Petrova, E.S.; Tsymbalenko, N.V. Effect of silver ions on copper metabolism during mammalian ontogenesis. Russ. J. Dev. Biol. 2018, 49, 166–178. [Google Scholar] [CrossRef]
- Ilyechova, E.Y.; Tsymbalenko, N.V.; Puchkova, L.V. The role of subcutaneous adipose tissue in supporting the copper balance in rats with a chronic deficiency in holo-ceruloplasmin. PLoS ONE 2017, 12, e0175214. [Google Scholar] [CrossRef]
- More, S.S.; Akil, O.; Ianculescu, A.G.; Geier, E.G.; Lustig, L.R.; Giacomini, K.M. Role of the copper transporter, CTR1, in platinum-induced ototoxicity. J. Neurosci. 2010, 30, 9500–9509. [Google Scholar] [CrossRef]
- Ishida, S.; McCormick, F.; Smith-McCune, K.; Hanahan, D. Enhancing tumor-specific uptake of the anticancer drug cisplatin with a copper chelator. Cancer Cell 2010, 17, 574–583. [Google Scholar] [CrossRef] [PubMed]
- Akerfeldt, M.C.; Tran, C.M.; Shen, C.; Hambley, T.W.; New, E.J. Interactions of cisplatin and the copper transporter CTR1 in human colon cancer cells. J. Biol. Inorg. Chem. 2017, 22, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Babich, P.S.; Skvortsov, A.N.; Rusconi, P.; Tsymbalenko, N.V.; Mutanen, M.; Puchkova, L.V.; Broggini, M. Non-hepatic tumors change the activity of genes encoding copper trafficking proteins in the liver. Cancer Biol. Ther. 2013, 14, 614–624. [Google Scholar] [CrossRef] [PubMed]
- Vairo, F.P.E.; Chwal, B.C.; Perini, S.; Ferreira, M.A.P.; de Freitas Lopes, A.C.; Saute, J.A.M. A systematic review and evidence-based guideline for diagnosis and treatment of Menkes disease. Mol. Genet. Metab. 2019, 126, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Verheijen, F.V.; Beerens, C.E.M.T.; Havelaar, A.C.; Kleijer, W.J.; Mancini, G.M.S. Fibroblast silver loading for the diagnosis of Menkes disease. J. Med. Genet. 1998, 35, 849–851. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lamb, A.L.; Torres, A.S.; O’Halloran, T.V.; Rosenzweig, A.C. Heterodimeric structure of superoxide dismutase in complex with its metallochaperone. Nat. Struct. Biol. 2001, 8, 751–755. [Google Scholar] [CrossRef] [PubMed]
- Boyd, S.D.; Calvo, J.S.; Liu, L.; lrich, M.S.; Skopp, A.; Meloni, G.; Winkler, D.D. The yeast copper chaperone for copper-zinc superoxide dismutase (CCS1) is a multifunctional chaperone promoting all levels of SOD1 maturation. J. Biol. Chem. 2019, 294, 1956–1966. [Google Scholar] [CrossRef]
- Sala, F.A.; Wright, G.S.A.; Antonyuk, S.V.; Garratt, R.C.; Hasnain, S.S. Molecular recognition and maturation of SOD1 by its evolutionarily destabilised cognate chaperone hCCS. PLoS Biol. 2019, 17, e3000141. [Google Scholar] [CrossRef]
- Leitch, J.M.; Yick, P.J.; Culotta, V.C. The right to choose: Multiple pathways for activating copper,zinc superoxide dismutase. J. Biol. Chem. 2009, 284, 24679–24683. [Google Scholar] [CrossRef]
- Kawamata, H.; Manfredi, G. Import, maturation, and function of SOD1 and its copper chaperone CCS in the mitochondrial intermembrane space. Antioxid. Redox Signal. 2010, 13, 1375–1384. [Google Scholar] [CrossRef]
- Backes, S.; Herrmann, J.M. Protein translocation into the intermembrane space and matrix of mitochondria: Mechanisms and driving forces. Front. Mol. Biosci. 2017, 4, 83. [Google Scholar] [CrossRef] [PubMed]
- Zelko, I.N.; Mariani, T.J.; Folz, R.J. Superoxide dismutase multigene family: A comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radic. Biol. Med. 2002, 33, 337–349. [Google Scholar] [CrossRef]
- Takahashi, Y.; Kako, K.; Kashiwabara, S.I.; Takehara, A.; Inada, Y.; Arai, H.; Nakada, K.; Kodama, H.; Hayashi, J.I.; Baba, T.; et al. Mammalian copper chaperone Cox17p has an essential role in activation of cytochrome C oxidase and embryonic development. Mol. Cell. Biol. 2002, 22, 7614–7621. [Google Scholar] [CrossRef] [PubMed]
- Timón-Gómez, A.; Nývltová, E.; Abriata, L.A.; Vila, A.J.; Hosler, J.; Barrientos, A. Mitochondrial cytochrome c oxidase biogenesis: Recent developments. Semin. Cell Dev. Biol. 2018, 76, 163–178. [Google Scholar] [CrossRef]
- Leary, S.C. Redox regulation of SCO protein function: Controlling copper at a mitochondrial crossroad. Antioxid. Redox Signal. 2010, 13, 1403–1416. [Google Scholar] [CrossRef] [PubMed]
- Baker, Z.N.; Cobine, P.A.; Leary, S.C. The mitochondrion: A central architect of copper homeostasis. Metallomics 2017, 9, 1501–1512. [Google Scholar] [CrossRef]
- Carr, H.S.; George, G.N.; Winge, D.R. Yeast Cox11, a protein essential for cytochrome c oxidase assembly, is a Cu(I)-binding protein. J. Biol. Chem. 2002, 277, 31237–31242. [Google Scholar] [CrossRef]
- Tambosi, R.; Liotenberg, S.; Bourbon, M.L.; Steunou, A.S.; Babot, M.; Durand, A.; Kebaili, N.; Ouchane, S. Silver and copper acute effects on membrane proteins and impact on photosynthetic and respiratory complexes in bacteria. MBio 2018, 9, e01535-18. [Google Scholar] [CrossRef]
- Wolff, N.A.; Ghio, A.J.; Garrick, L.M.; Garrick, M.D.; Zhao, L.; Fenton, R.A.; Thévenod, F. Evidence for mitochondrial localization of divalent metal transporter 1 (DMT1). FASEB J. 2014, 28, 2134–2145. [Google Scholar] [CrossRef]
- Wolff, N.A.; Garrick, M.D.; Zhao, L.; Garrick, L.M.; Ghio, A.J.; Thévenod, F. A role for divalent metal transporter (DMT1) in mitochondrial uptake of iron and manganese. Sci. Rep. 2018, 8, 211. [Google Scholar] [CrossRef]
- Vest, K.E.; Leary, S.C.; Winge, D.R.; Cobine, P.A. Copper import into the mitochondrial matrix in Saccharomyces cerevisiae is mediated by Pic2, a mitochondrial carrier family protein. J. Biol. Chem. 2013, 288, 23884–23892. [Google Scholar] [CrossRef] [PubMed]
- Boulet, A.; Vest, K.E.; Maynard, M.K.; Gammon, M.G.; Russell, A.C.; Mathews, A.T.; Cole, S.E.; Zhu, X.; Phillips, C.B.; Kwong, J.Q.; et al. The mammalian phosphate carrier SLC25A3 is a mitochondrial copper transporter required for cytochrome c oxidase biogenesis. J. Biol. Chem. 2018, 293, 1887–1896. [Google Scholar] [CrossRef] [PubMed]
- Cobine, P.A.; Ojeda, L.D.; Rigby, K.M.; Winge, D.R. Yeast contain a non-proteinaceous pool of copper in the mitochondrial matrix. J. Biol. Chem. 2004, 279, 14447–14455. [Google Scholar] [CrossRef] [PubMed]
- Cobine, P.A.; Pierrel, F.; Bestwick, M.L.; Winge, D.R. Mitochondrial matrix copper complex used in metalation of cytochrome oxidase and superoxide dismutase. J. Biol. Chem. 2006, 281, 36552–36559. [Google Scholar] [CrossRef] [PubMed]
- Lindahl, P.A.; Moore, M.J. Labile low-molecular-mass metal complexes in mitochondria: Trials and tribulations of a burgeoning field. Biochemistry 2016, 55, 4140–4153. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xiao, B.; Fang, T. Chemical transformation of silver nanoparticles in aquatic environments: Mechanism, morphology and toxicity. Chemosphere 2018, 191, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Tang, J.; Xu, S.; Ge, J.; Dong, Y.; Li, H.; Jin, M. A review on silver nanoparticles-induced ecotoxicity and the underlying toxicity mechanisms. Regul. Toxicol. Pharmacol. 2018, 98, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Contreras, E.Q.; Puppala, H.L.; Escalera, G.; Zhong, W.; Colvin, V.L. Size-dependent impacts of silver nanoparticles on the lifespan, fertility, growth, and locomotion of Caenorhabditis elegans. Environ. Toxicol. Chem. 2014, 33, 2716–2723. [Google Scholar] [CrossRef] [PubMed]
- Contreras, M.; Posgai, R.; Gorey, T.J.; Nielsen, M.; Hussain, S.M.; Rowe, J.J. Silver nanoparticles induced heat shock protein 70, oxidative stress and apoptosis in Drosophila melanogaster. Toxicol. Appl. Pharmacol. 2010, 242, 263–269. [Google Scholar] [CrossRef]
- Mao, B.; Chen, Z.Y.; Wang, Y.J.; Yan, S.J. Silver nanoparticles have lethal and sublethal adverse effects on development and longevity by inducing ROS-mediated stress responses. Sci. Rep. 2018, 8, 2445. [Google Scholar] [CrossRef] [PubMed]
- Raj, A.; Shah, P.; Agrawal, N. Dose-dependent effect of silver nanoparticles (AgNPs) on fertility and survival of Drosophila: An in-vivo study. PLoS ONE 2017, 12, e0178051. [Google Scholar] [CrossRef] [PubMed]
- Maurer, L.L.; Yang, X.; Schindler, A.J.; Taggart, R.K.; Jiang, C.; Hsu-Kim, H.; Sherwood, D.R.; Meyer, J.N. Intracellular trafficking pathways in silver nanoparticle uptake and toxicity in Caenorhabditis elegans. Nanotoxicology 2016, 10, 831–835. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Alaraby, M.; Romero, S.; Hernández, A.; Marcos, R. Toxic and genotoxic effects of silver nanoparticles in Drosophila. Environ. Mol. Mutagen. 2019, 60, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Polishchuk, E.V.; Merolla, A.; Lichtmannegger, J.; Romano, A.; Indrieri, A.; Ilyechova, E.Y.; Concilli, M.; De Cegli, R.; Crispino, R.; Mariniello, M.; et al. Activation of autophagy, observed in liver tissues from patients with Wilson disease and from ATP7B-deficient animals, Protects hepatocytes from copper-induced apoptosis. Gastroenterology 2019, 156, 1173–1189. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, N.; Eom, H.J.; Choi, J. Effects of silver nanoparticles on oxidative DNA damage-repair as a function of p38 MAPK status: A comparative approach using human Jurkat T cells and the nematode Caenorhabditis elegans. Environ. Mol. Mutagen. 2014, 55, 122–133. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.; Roh, J.Y.; Eom, H.J.; Choi, J.Y.; Hyun, J.; Choi, J. Oxidative stress-related PMK-1 P38 MAPK activation as a mechanism for toxicity of silver nanoparticles to reproduction in the nematode Caenorhabditis elegans. Environ. Toxicol. Chem. 2012, 31, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Chesi, G.; Hegde, R.N.; Iacobacci, S.; Concilli, M.; Parashuraman, S.; Festa, B.P.; Polishchuk, E.V.; Di Tullio, G.; Carissimo, A.; Montefusco, S.; et al. Identification of p38 MAPK and JNK as new targets for correction of Wilson disease-causing ATP7B mutants. Hepatology 2016, 63, 1842–1859. [Google Scholar] [CrossRef]
- Armstrong, N.; Ramamoorthy, M.; Lyon, D.; Jones, K.; Duttaroy, A. Mechanism of silver nanoparticles action on insect pigmentation reveals intervention of copper homeostasis. PLoS ONE 2013, 8, e53186. [Google Scholar] [CrossRef]
- Orlov, I.A.; Sankova, T.P.; Babich, P.S.; Sosnin, I.M.; Ilyechova, E.Y.; Kirilenko, D.A.; Brunkov, P.N.; Ataev, G.L.; Romanov, A.E.; Puchkova, L.V. New silver nanoparticles induce apoptosis-like process in E. coli and interfere with mammalian copper metabolism. Int. J. Nanomed. 2016, 11, 6561–6574. [Google Scholar] [CrossRef]
- Kim, Y.; Suh, H.S.; Cha, H.J.; Kim, S.H.; Jeong, K.S.; Kim, D.H. A case of generalized argyria after ingestion of colloidal silver solution. Am. J. Ind. Med. 2009, 52, 246–250. [Google Scholar] [CrossRef]

| Class Name | Catalyzed Reaction | Electrons Transferred to Dioxygen | Cu Atoms Required |
|---|---|---|---|
| Superoxide dismutase 3, EC 1.15.1.1 | 2 superoxides + 2 H+ <=> O2 + H2O2 | 1 + 1 | 1 |
| Ferroxidase, EC 1.16.3.1 | 4 Fe2+ + 4 H+ + O2 <=> 4 Fe3+ + 2 H2O | 4 | 4 (6) |
| Peptidylglycine monooxygenase, EC 1.14.17.3 | [Peptide]-glycine + 2 ascorbates + O2 <=> [peptide]-(2S)-2-hydroxyglycine + 2 monodehydroascorbate + H2O | 2 + 2 | 2 |
| Dopamine beta-monooxygenase, EC 1.14.17.1 | 3,4-dihydroxyphenethylamine + 2 ascorbates + O2 <=> noradrenaline + 2 monodehydroascorbate + H2O | 2 + 2 | 1 |
| Diamine oxidase, EC 1.4.3.22 | Histamine + H2O + O2 <=> (imidazol-4-yl) acetaldehyde + NH3 + H2O2 | 2 | 1 |
| Primary-amine oxidase, EC 1.4.3.21 | RCH2NH2 + H2O + O2 <=> RCHO + NH3 + H2O2 | 2 | 1 |
| Protein-lysine 6-oxidase, EC 1.4.3.13 | [Protein]-L-lysine + O2 + H2O <=> [protein]-(S)-2-amino-6-oxohexanoate + NH3 + H2O2 | 2 | 1 |
| Tyrosinase, EC 1.14.18.1 | L-tyrosine + O2 <=> dopaquinone + H2O 2 L-dopa + O2 <=> 2 dopaquinone + 2 H2O | 4 | 2 |
| Enzyme | Class | Reference Structure(s), PDB ID | Copper Coordination Sphere * | Geometry * | Feasibility of Ag(I) Binding **** |
|---|---|---|---|---|---|
| COX | Cytochrome-c-oxidase; EC 1.9.3.1, | 5IY5 (cow) | CuA; Cu pair, subunit 2, C200 (bridge), C196 (bridge), H161, H204, M207, E198 amide | Distorted tetrahedral for each atom; strong Cu–Cu interaction | Low |
| CuB; subunit 1, H290, H291, H240, heme | Distorted trigonal pyramidal; Cu–heme interaction | Low | |||
| SOD1 | Superoxide dismutase, EC 1.15.1.1 | 1HL5 (human) | H46, H48, H63, H120 | Distorted tetrahedral | Low |
| SOD3 | Superoxide dismutase, EC 1.15.1.1 | 2JLP (human) | H96, H98, H113, H163 | Distorted tetrahedral/trigonal | Low |
| Cp | Ferroxidase, EC 1.16.3.1 | 1KCW, 2J5W (human) | Cu21 (blue): C319, H276, H324 | Distorted trigonal planar | Moderate |
| Cu31 **: H163, H980, H1020 (dioxygen) | Trigonal pyramidal (tetrahedral) | Low | |||
| Cu32: H103, H1061, H1022 (dioxygen) | Trigonal (distorted tetrahedral) | Low | |||
| Cu33: H101, H978, (dioxygen, water/OH), η5-bonding from H103 and H980 | Linear (square planar, with η-bonds; tetragonal distorted octahedral) | Low | |||
| Cu41 (blue): C680, H637, H685 | Distorted trigonal planar | Moderate | |||
| Cu61 (blue): C1021, H975, H1026 | Distorted trigonal planar | Moderate | |||
| Cu42 (labile): H692, D684 (water?) | Angular | Very low | |||
| Cu62 (labile): H940, D1025 (water?) | Angular | Very low | |||
| Hephaestin (HEPH) | Ferroxidase, EC 1.16.3.1 | No data | Putatively similar to Cp, the trinuclear site, Cu21 and Cu41 site are conserved, the presence of blue copper is proven | Moderate for blue sites | |
| Zyklopen (HEPH1) | Ferroxidase, EC 1.16.3.1 | No data | Putatively similar to Cp, the trinuclear site and Cu21 site are conserved | Moderate for blue sites | |
| Peptidyl-glycine alpha-amidating monooxygenase | Peptidylglycine monooxygenase, EC 1.14.17.3 | 1SDW (rat) | Cu1, H107, H108, H172 | trigonal planar | Low |
| Cu2, H242, H244, M314 (dioxygen) | Trigonal pyramidal (tetrahedral) | Low | |||
| Dopamine beta-monooxygenase | Dopamine beta-monooxygenase, EC 1.14.17.1 | 4ZEL (human) | H412, H414, M487 (substrate?) | Trigonal pyramidal (tetrahedral?) | Low |
| Amine oxidase copper-containing 1 (Dopamine oxidase) | Diamine oxidase, EC 1.4.3.22 | 3HI7 | H510, H512; H675, (substrate) | Distorted T-shaped (distorted tetrahedral) | Low |
| Amine oxidase, copper containing 3 (AOC3) | Primary-amine oxidase, EC 1.4.3.21 | 2Y73 | H520, H522, H684 (substrate, water?) | Distorted T-shaped (seesaw/octahedral?) | Low |
| Amine oxidase, copper containing 2 (AOC2) | Primary-amine oxidase, 1.4.3.21 | No data | Highly similar to AOC3, copper site conserved | Low | |
| LOX | Protein-lysine 6-oxidase, EC 1.4.3.13 | 1N9E (Pichia pastoris) | H528, H530, H694, modified Y478 (TPQ, O-donor) | Distorted tetrahedral | Low to very low |
| LOXL2 | Protein-lysine 6-oxidase, EC 1.4.3.13; putative | 5ZE3 | H626, H628, H630, Y689 (putative, Zn instead of Cu) | Distorted tetrahedral | Low to very low |
| LOXL1,3,4 | Protein-lysine 6-oxidase, EC 1.4.3.13; putative | No data | Putatively similar to LOX/LOXL2 | Low | |
| TYR | Tyrosinase, EC 1.14.18.1 | 5Z0D, 5Z0F *** (Streptomyces) | Cu1: H38, H54, H63, (η2-dioxygen) | Distorted trigonal planar (distorted tetrahedral) | Low |
| Cu2: H190, H194, H216, (η2-dioxygen) | Distorted trigonal pyramidal (distorted tetrahedral) | Low | |||
| Thiol receptor OR2T11 | No data | M115, R119, C238, H241 | Distorted tetrahedral | High | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puchkova, L.V.; Broggini, M.; Polishchuk, E.V.; Ilyechova, E.Y.; Polishchuk, R.S. Silver Ions as a Tool for Understanding Different Aspects of Copper Metabolism. Nutrients 2019, 11, 1364. https://doi.org/10.3390/nu11061364
Puchkova LV, Broggini M, Polishchuk EV, Ilyechova EY, Polishchuk RS. Silver Ions as a Tool for Understanding Different Aspects of Copper Metabolism. Nutrients. 2019; 11(6):1364. https://doi.org/10.3390/nu11061364
Chicago/Turabian StylePuchkova, Ludmila V., Massimo Broggini, Elena V. Polishchuk, Ekaterina Y. Ilyechova, and Roman S. Polishchuk. 2019. "Silver Ions as a Tool for Understanding Different Aspects of Copper Metabolism" Nutrients 11, no. 6: 1364. https://doi.org/10.3390/nu11061364
APA StylePuchkova, L. V., Broggini, M., Polishchuk, E. V., Ilyechova, E. Y., & Polishchuk, R. S. (2019). Silver Ions as a Tool for Understanding Different Aspects of Copper Metabolism. Nutrients, 11(6), 1364. https://doi.org/10.3390/nu11061364






