Protein Intake Estimated from Brief-Type Self-Administered Diet History Questionnaire and Urinary Urea Nitrogen Level in Adolescents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Urinalysis
2.3. Estimation of Daily Urea Nitrogen Excretion from Overnight Urine
2.4. Estimation of the Protein Intake from Measurement of Urinary Urea Nitrogen
2.5. Anthropometric Measurements
2.6. Questionnaire
2.7. Statistical Analysis
3. Results
4. Discussion
4.1. Protein Intake
4.2. Urinary Creatinine
4.3. Physical Activity
4.4. BDHQ Validity
4.5. Strengths and Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Mage [23] | Males with Ht <168, Males with Ht >168, Females | 1.085 × Ht × (6.265 + 0.0564 × (Ht − 168)) 1.085 × Ht × (6.265 + 0.2550 × (Ht − 168)) 1.008 × 2.045 × Ht × e0.01552 × (Ht − 90) |
| Moriyama [24] | Males Females | −221 − 0.94 × Ht + 35.01 × Wt −330 + 2.74 × Ht + 19.57 × Wt |
| Kawasaki [19] | Males Females | −12.63 × Age + 15.12 × Wt + 7.39 × Ht − 79.90 −4.72 × Age + 8.58 × Wt + 5.09 × Ht − 74.95 |
| Tanaka [20] | −2.04 × Age + 14.89 × Wt + 16.14 × Ht − 2244.45 |
References
- Moreno, L.A.; Gottrand, F.; Huybrechts, I.; Ruiz, J.R.; Gonzalez-Gross, M.; DeHenauw, S.; Helena Study Group. Nutrition and lifestyle in european adolescents: The HELENA (Healthy Lifestyle in Europe by Nutrition in Adolescence) study. Adv. Nutr. 2014, 5, 615S–623S. [Google Scholar] [CrossRef] [PubMed]
- Dahl, W.J.; Stewart, M.L. Position of the Academy of Nutrition and Dietetics: Health Implications of Dietary Fiber. J. Acad. Nutr. Diet. 2015, 115, 1861–1870. [Google Scholar] [CrossRef] [PubMed]
- Okuda, M.; Bando, N.; Terao, J.; Sasaki, S.; Sugiyama, S.; Kunitsugu, I.; Hobara, T. Association of serum carotenoids and tocopherols with atopic diseases in Japanese children and adolescents. Pediatr. Allergy Immunol. 2010, 21, e705–e710. [Google Scholar] [CrossRef] [PubMed]
- Okuda, M.; Asakura, K.; Sasaki, S. The relationship between functional constipation and dietary habits in school-age Japanese children. J. Nutr. Sci. Vitaminol. 2019, 65, 38–44. [Google Scholar]
- Bertheke Post, G.; de Vente, W.; Kemper, H.C.; Twisk, J.W. Longitudinal trends in and tracking of energy and nutrient intake over 20 years in a Dutch cohort of men and women between 13 and 33 years of age: The Amsterdam growth and health longitudinal study. Br. J. Nutr. 2001, 85, 375–385. [Google Scholar] [CrossRef]
- Lake, A.A.; Mathers, J.C.; Rugg-Gunn, A.J.; Adamson, A.J. Longitudinal change in food habits between adolescence (11–12 years) and adulthood (32–33 years): The ASH30 Study. J. Public Health 2006, 28, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Craigie, A.M.; Lake, A.A.; Kelly, S.A.; Adamson, A.J.; Mathers, J.C. Tracking of obesity-related behaviours from childhood to adulthood: A systematic review. Maturitas 2011, 70, 266–284. [Google Scholar] [CrossRef] [PubMed]
- Miki, T.; Eguchi, M.; Akter, S.; Kochi, T.; Kuwahara, K.; Kashino, I.; Hu, H.; Kabe, I.; Kawakami, N.; Nanri, A.; et al. Longitudinal adherence to a dietary pattern and risk of depressive symptoms: The Furukawa Nutrition and Health Study. Nutrition 2018, 48, 48–54. [Google Scholar] [CrossRef]
- Willet, W. Nutritional Epidemiology, 3rd ed.; Oxford University Press: New York, NY, USA, 2013; ISBN 978-0-19-975403-8. [Google Scholar]
- Kipnis, V.; Midthune, D.; Freedman, L.S.; Bingham, S.; Schatzkin, A.; Subar, A.; Carroll, R.J. Empirical evidence of correlated biases in dietary assessment instruments and its implications. Am. J. Epidemiol. 2001, 153, 394–403. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Murakami, K.; Sasaki, S.; Okubo, H.; Hirota, N.; Notsu, A.; Fukui, M.; Date, C. Comparison of relative validity for food group intake estimated by comprehensive and brief-type self-administered diet history questionnaires against 16-day dietary records in Japanese adults. Public Health Nutr. 2011, 14, 1200–1211. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Honda, S.; Murakami, K.; Sasaki, S.; Okubo, H.; Hirota, N.; Notsu, A.; Fukui, M.; Date, C. Both Comprehensive and Brief Self-Administered Diet History Questionnaires Satisfactorily Rank Nutrient intakes in Japanese Adults. J. Epidemiol. 2012, 22, 151–159. [Google Scholar] [CrossRef]
- Okuda, M.; Sasaki, S.; Bando, N.; Hashimoto, M.; Kunitsugu, I.; Sugiyama, S.; Terao, J.; Hobara, T. Carotenoid, tocopherol, and fatty acid biomarkers and dietary intake estimated by using a brief self-administered diet history questionnaire for older Japanese children and adolescents. J. Nutr. Sci. Vitaminol. 2009, 55, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Bingham, S.A.; Cummings, J.H. Urine nitrogen as an independent validatory measure of dietary intake: A study of nitrogen balance in individuals consuming their normal diet. Am. J. Clin. Nutr. 1985, 42, 1276–1289. [Google Scholar] [CrossRef]
- Momeno, L.A. Adolescence. In Pediatric Nutrition in Practice; Koletzko, B., Ed.; Krager: Basel, Swizterland, 2008; pp. 114–117. ISBN 978-3-8055-8477-7. [Google Scholar]
- World Health Organization. Expert Consultation. In Protein and Amino Acid Requirements in Human Nutrition; World Health Organization Technical Report Series 935; World Health Organization: Geneva, Switzerland, 2007. [Google Scholar]
- Cuenca-Garcia, M.; Ortega, F.B.; Ruiz, J.R.; Labayen, I.; Moreno, L.A.; Patterson, E.; Vicente-Rodriguez, G.; Gonzalez-Gross, M.; Marcos, A.; Polito, A.; et al. More physically active and leaner adolescents have higher energy intake. J. Pediatr. 2014, 164, 159–166. [Google Scholar] [CrossRef]
- Okuda, M.; Asakura, K.; Sasaki, S.; Shinozaki, K. Twenty-four-hour urinary sodium and potassium excretion and associated factors in Japanese secondary school students. Hypertens. Res. 2016, 39, 524–529. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, T.; Uezono, K.; Itoh, K.; Ueno, M. Prediction of 24-hour urinary creatinine excretion from age, body weight and height of an individual and its application. Jpn. J. Public Health 1991, 38, 567–574. [Google Scholar] [CrossRef]
- Tanaka, T.; Okamura, T.; Miura, K.; Kadowaki, T.; Ueshima, H.; Nakagawa, H.; Hashimoto, T. A simple method to estimate populational 24-h urinary sodium and potassium excretion using a casual urine specimen. J. Hum. Hypertens. 2002, 16, 97–103. [Google Scholar] [CrossRef]
- Cogswell, M.E.; Wang, C.Y.; Chen, T.C.; Pfeiffer, C.M.; Elliott, P.; Gillespie, C.D.; Carriquiry, A.L.; Sempos, C.T.; Liu, K.; Perrine, C.G.; et al. Validity of predictive equations for 24-h urinary sodium excretion in adults aged 18–39 y. Am. J. Clin. Nutr. 2013, 98, 1502–1513. [Google Scholar] [CrossRef]
- Okuda, M.; Asakura, K.; Sasaki, S. Placing Salt/Soy Sauce at Dining Tables and Out-Of-Home Behavior Are Related to Urinary Sodium Excretion in Japanese Secondary School Students. Nutrients 2017, 9, 1290. [Google Scholar] [CrossRef]
- Mage, D.T.; Allen, R.H.; Kodali, A. Creatinine corrections for estimating children’s and adult’s pesticide intake doses in equilibrium with urinary pesticide and creatinine concentrations. J. Exposure Sci. Environ. Epidemiol. 2008, 18, 360–368. [Google Scholar] [CrossRef]
- Moriyama, M.; Saito, H.; Kabuto, M. Relationship between twenty-four hour urinary creatinine excretion and weight, or weight and height of Japanese children. J. Nutr. Sci. Vitaminol. 1987, 33, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Bingham, S.A.; Williams, R.; Cole, T.J.; Price, C.P.; Cummings, J.H. Reference values for analytes of 24-h urine collections known to be complete. Ann Clin Biochem 1988, 25, 610–619. [Google Scholar] [CrossRef] [PubMed]
- Hishida, A.; Sasaki, S. Dietary Reference Intakes for Japanese, 2015; Daiichi-Shuppan Co. Ltd.: Tokyo, Japan, 2014; ISBN 978-4-8041-1312-8. [Google Scholar]
- Okuda, M.; Yoshitake, N.; Tanaka, S.; Kunitsugu, I.; Tan, N.; Uechi, H.; Sasaki, S.; Hobara, T. Validity and reliability of physical activity questionnaire for Japanese students. Pediatr. Int. 2011, 53, 956–963. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Education, Culture, Sports, Science and Technology. Science and Technology Agency Standard Tables of Food Composition in Japan, 2010; Ministry of Education, Culture, Sports, Science and Technology: Tokyo, Japan, 2010.
- Shrout, P.E.; Fleiss, J.L. Intraclass correlations: Uses in assessing rater reliability. Psychol. Bull. 1979, 86, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Passing, H.; Bablok, W. A new biometrical procedure for testing the equality of measurements from two different analytical methods. Application of linear regression procedures for method comparison studies in clinical chemistry, Part I. J. Clin. Chem. Clin. Biochem. 1983, 21, 709–720. [Google Scholar] [PubMed]
- Bland, J.M.; Altman, D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 8, 307–308. [Google Scholar] [CrossRef]
- Adolph, S.C.; Hardin, J.S. Estimating phenotypic correlations: Correcting for bias due to intraindividual variability. Funct. Ecol. 2007, 21, 178–184. [Google Scholar] [CrossRef]
- Garlick, P.J. Protein Requirements of Infants and Children; Nestec Ltd.: Vevey, Switzerland; S.Karger AG: Basel, Switzerland, 2006; pp. 39–50. [Google Scholar]
- Thivel, D.; Aucouturier, J.; Doucet, E.; Saunders, T.J.; Chaput, J.P. Daily energy balance in children and adolescents. Does energy expenditure predict subsequent energy intake? Appetite 2013, 60, 58–64. [Google Scholar] [CrossRef]
- Ocke, M.C.; Bueno-de-Mesquita, H.B.; Pols, M.A.; Smit, H.A.; van Staveren, W.A.; Kromhout, D. The Dutch EPIC food frequency questionnaire. II. Relative validity and reproducibility for nutrients. Int. J. Epidemiol. 1997, 26 (Suppl. 1), S49–S58. [Google Scholar] [CrossRef]
- Katsouyanni, K.; Rimm, E.B.; Gnardellis, C.; Trichopoulos, D.; Polychronopoulos, E.; Trichopoulou, A. Reproducibility and relative validity of an extensive semi-quantitative food frequency questionnaire using dietary records and biochemical markers among Greek schoolteachers. Int. J. Epidemiol. 1997, 26 (Suppl. 1), S118–S127. [Google Scholar] [CrossRef]
- Kroke, A.; Klipstein-Grobusch, K.; Voss, S.; Moseneder, J.; Thielecke, F.; Noack, R.; Boeing, H. Validation of a self-administered food-frequency questionnaire administered in the European Prospective Investigation into Cancer and Nutrition (EPIC) Study: Comparison of energy, protein, and macronutrient intakes estimated with the doubly labeled water, urinary nitrogen, and repeated 24-h dietary recall methods. Am. J. Clin. Nutr. 1999, 70, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Pijls, L.T.; de Vries, H.; Donker, A.J.; van Eijk, J.T. Reproducibility and biomarker-based validity and responsiveness of a food frequency questionnaire to estimate protein intake. Am. J. Epidemiol. 1999, 150, 987–995. [Google Scholar] [CrossRef] [PubMed]
- Malekshah, A.F.; Kimiagar, M.; Saadatian-Elahi, M.; Pourshams, A.; Nouraie, M.; Goglani, G.; Hoshiarrad, A.; Sadatsafavi, M.; Golestan, B.; Yoonesi, A.; et al. Validity and reliability of a new food frequency questionnaire compared to 24 h recalls and biochemical measurements: Pilot phase of Golestan cohort study of esophageal cancer. Eur. J. Clin. Nutr. 2006, 60, 971–977. [Google Scholar] [CrossRef] [PubMed]
- Sarmento, R.A.; Antonio, J.P.; Riboldi, B.P.; Montenegro, K.R.; Friedman, R.; de Azevedo, M.J.; de Almeida, J.C. Reproducibility and validity of a quantitative FFQ designed for patients with type 2 diabetes mellitus from southern Brazil. Public Health Nutr. 2014, 17, 2237–2245. [Google Scholar] [CrossRef] [PubMed]
- Timon, C.M.; Blain, R.J.; McNulty, B.; Kehoe, L.; Evans, K.; Walton, J.; Flynn, A.; Gibney, E.R. The Development, Validation, and User Evaluation of Foodbook24: A Web-Based Dietary Assessment Tool Developed for the Irish Adult Population. J. Med. Internet Res. 2017, 19, e158. [Google Scholar] [CrossRef] [PubMed]
- Tabacchi, G.; Filippi, A.R.; Amodio, E.; Jemni, M.; Bianco, A.; Firenze, A.; Mammina, C. A meta-analysis of the validity of FFQ targeted to adolescents. Public Health Nutr. 2016, 19, 1168–1183. [Google Scholar] [CrossRef] [PubMed]
- Lietz, G.; Barton, K.L.; Longbottom, P.J.; Anderson, A.S. Can the EPIC food-frequency questionnaire be used in adolescent populations? Public Health Nutr. 2002, 5, 783–789. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, M.; Asakura, K.; Masayasu, S.; Sasaki, S. Relatively severe misreporting of sodium, potassium, and protein intake among female dietitians compared with nondietitians. Nutr. Res. 2016, 36, 818–826. [Google Scholar] [CrossRef] [PubMed]
- Koster-Rasmussen, R.; Siersma, V.; Halldorsson, T.I.; de Fine Olivarius, N.; Henriksen, J.E.; Heitmann, B.L. Missing portion sizes in FFQ—Alternatives to use of standard portions. Public Health Nutr. 2015, 18, 1914–1921. [Google Scholar] [CrossRef]
- Vilela, S.; Severo, M.; Moreira, T.; Ramos, E.; Lopes, C. Evaluation of a short food frequency questionnaire for dietary intake assessment among children. Eur. J. Clin. Nutr. 2018. [Google Scholar] [CrossRef]
- Subar, A.F.; Kipnis, V.; Troiano, R.P.; Midthune, D.; Schoeller, D.A.; Bingham, S.; Sharbaugh, C.O.; Trabulsi, J.; Runswick, S.; Ballard-Barbash, R.; et al. Using intake biomarkers to evaluate the extent of dietary misreporting in a large sample of adults: The OPEN study. Am. J. Epidemiol. 2003, 158, 1–13. [Google Scholar] [CrossRef] [PubMed]
| All (N = 248) | Males (N = 118) | Females (N = 130) | p-Value | |
|---|---|---|---|---|
| Age (years) | 14.2 ± 0.9 | 14.2 ± 0.9 | 14.2 ± 0.9 | 0.973 |
| Body height (cm) | 157.8 ± 7.8 | 161.4 ± 8.2 | 154.5 ± 5.7 | 0.001 |
| Body weight (kg) | 50.6 ± 10.3 | 52.5 ± 12.1 | 48.9 ± 8.0 | 0.006 |
| Body mass index (kg/m2) | 20.4 ± 3.3 | 20.1 ± 3.6 | 20.6 ± 2.9 | 0.312 |
| Pubertal change | ||||
| No | 35 | 19 (15%) | 16 (11%) | |
| Yes | 213 | 99 (85%) | 114 (89%) | |
| MVPA (METs-min/wk) | 5455.4 ± 3638.0 | 6651.1 ± 4026.9 | 4370.1 ± 2853.0 | 0.001 |
| Overnight urine | ||||
| Urea nitrogen (mg/dL) | 1207.7 ± 296.9 | 1260.7 ± 282.2 | 1159.7 ± 302.7 | 0.007 |
| Creatinine (mg/dL) | 167.4 ± 65.0 | 172.1 ± 60.0 | 163.1 ± 69.1 | 0.276 |
| 24-h urine | N = 21 | N = 37 | ||
| Collection period (h) | 22.6 ± 0.9 | 22.6 ± 1.1 | 22.6 ± 0.8 | 0.811 |
| Urine volume (mL) | 966.3 ± 374 | 952.5 ± 320.1 | 974.2 ± 403.4 | 0.833 |
| Urea nitrogen (mg/dL) | 879.8 ± 288.6 | 941.7 ± 292.2 | 844.7 ± 284.6 | 0.222 |
| Creatinine (mg/dL) | 114.5 ± 42.2 | 121.2 ± 39.9 | 110.7 ± 43.5 | 0.367 |
| Mean ± SD | Difference | Spearman’s Correlation | Intraclass Correlation | ||
|---|---|---|---|---|---|
| (mg/d) | (mg/d) | Coefficient | p-Value | ||
| UCr24h | 1009.9 ± 229.5 | ||||
| Estimated UCr24h | |||||
| Mage’s equation [23] | 951.7 ± 190.6 | −58.2 | 0.607 | <0.001 | 0.645 |
| Moriyama’s equation [20] | 1231.1 ± 384.0 | 221.2 | 0.560 | <0.001 | 0.606 |
| Kawasaki’s equation [19] | 1321.8 ± 360.7 | 311.9 | 0.622 | <0.001 | 0.669 |
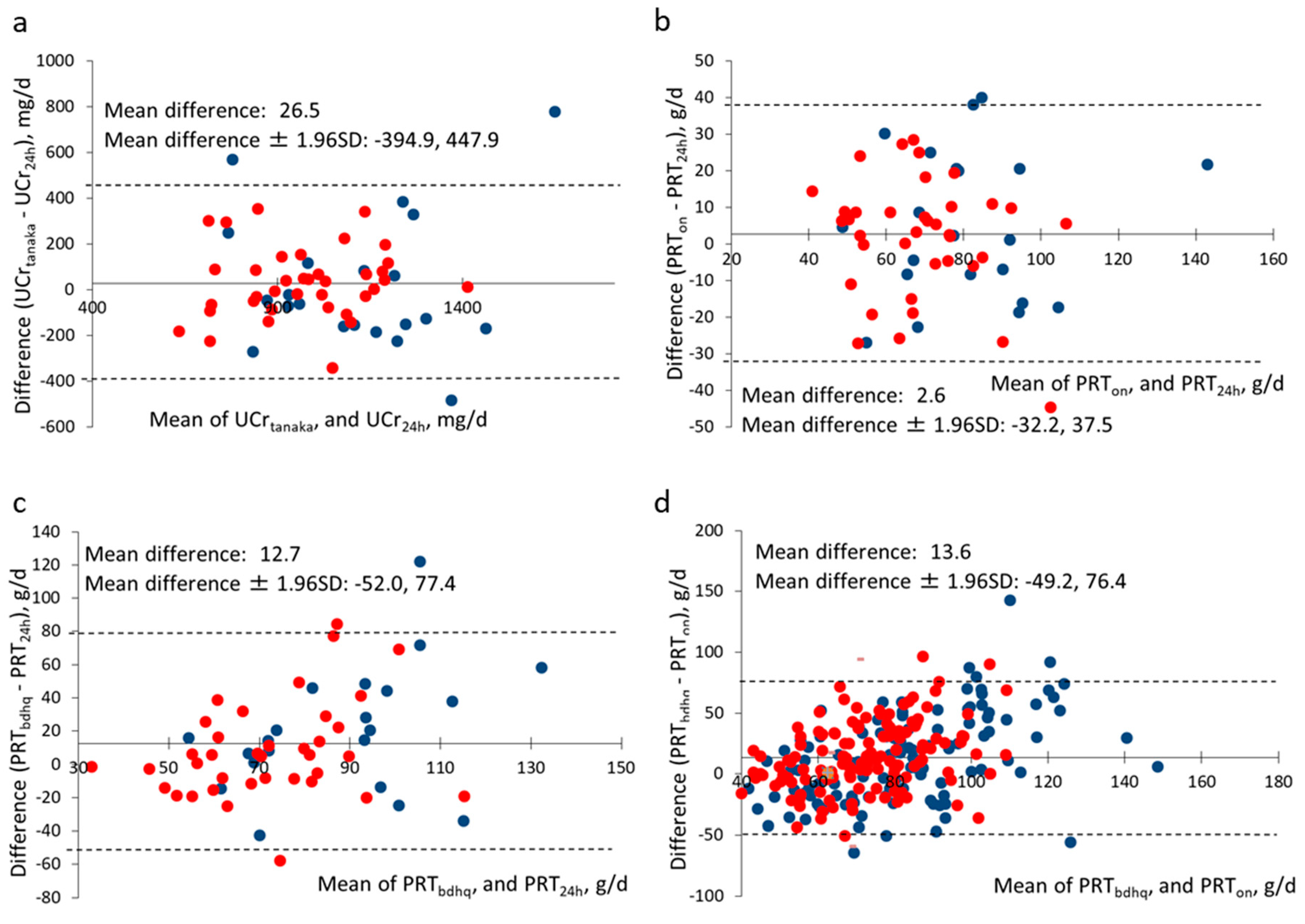
| Tanaka’s equation [24] | 1036.4 ± 241.5 | 26.5 | 0.628 | <0.001 | 0.669 |
| All | Males | Females | |
|---|---|---|---|
| Participants who provided 24-h urine | N = 58 | N = 21 | N = 37 |
| 24-h urine | |||
| Urea nitrogen (g/d) | 7.9 ± 2.3 | 8.7 ± 2.4 | 7.5 ± 2.1 |
| Protein (g/d) | 71.7 ± 20.4 | 78.5 ± 21.8 | 67.9 ± 18.8 |
| Overnight urine | |||
| Urea nitrogen (g/d) | 8.2 ± 2.2 | 9.2 ± 2.6 | 7.6 ± 1.7 |
| Protein (g/d) | 74.4 ± 20.0 | 83.4 ± 23.6 | 69.3 ± 15.9 |
| BDHQ | |||
| Protein (g/d) | 84.4 ± 30.1 | 99.0 ± 32.5 | 76.2 ± 25.5 |
| Energy (kcal/d) | 2410.7 ± 757.7 | 2899.3 ± 750.9 | 2133.4 ± 614.3 |
| Adjusted protein (%E) | 14.0 ± 2.3% | 13.6 ± 2.5% | 14.2 ± 2.2% |
| All participants | N = 248 | N = 118 | N = 130 |
| Overnight urine | |||
| Urea nitrogen (g/d) | 7.8 ± 2.1 | 8.4 ± 2.3 | 7.2 ± 1.9 |
| Protein (g/d) | 70.6 ± 19.4 | 76.4 ± 20.4 | 65.4 ± 16.9 |
| BDHQ | |||
| Protein (g/d) | 84.2 ± 29.6 | 89.3 ± 33.7 | 79.6 ± 24.6 |
| Energy (kcal/d) | 2407.6 ± 751.0 | 2663.2 ± 785.6 | 2175.7 ± 637.4 |
| Adjusted protein (%E) | 14.0 ± 2.5% | 13.3 ± 2.5% | 14.7 ± 2.4% |
| PRT24h | PRTon | |||||
|---|---|---|---|---|---|---|
| All N = 58 | Males (N = 21) | Females (N = 37) | All N = 248 | Males (N = 118) | Females (N = 130) | |
| g/day | 78.5 ± 21.8 | 67.9 ± 18.8 | 76.4 ± 20.4 | 65.4 ± 16.9 | ||
| Age | 0.027 | 0.353 | −0.113 | 0.015 | 0.220 | −0.139 |
| (0.840) | (0.116) | (0.507) | (0.811) | (0.017) | (0.115) | |
| MVPA | 0.381 | 0.343 | 0.299 | 0.143 | 0.141 | −0.014 |
| (0.003) | (0.128) | (0.072) | (0.024) | (0.127) | (0.878) | |
| BMI | 0.097 | 0.192 | 0.037 | 0.285 | 0.372 | 0.305 |
| (0.469) | (0.404) | (0.829) | (<0.001) | (<0.001) | (<0.001) | |
| Body weight | 0.179 | 0.233 | 0.062 | 0.463 | 0.449 | 0.431 |
| (0.178) | (0.310) | (0.716) | (<0.001) | (<0.001) | (<0.001) | |
| Body height | 0.215 | −0.011 | 0.231 | 0.463 | 0.339 | 0.431 |
| (0.105) | (0.962) | (0.168) | (<0.001) | (<0.001) | (<0.001) | |
| Pubertal change | ||||||
| Yes | 72.3 ± 19.4 | 78.7 ± 30.2 | 68.8 ± 10.7 | 72.6 ± 27.2 | 73.1 ± 33.2 | 71.9 ± 18.6 |
| No | 71.5 ± 20.9 | 78.5 ± 19.7 | 67.6 ± 20.9 | 70.3 ± 17.9 | 77.1 ± 17.1 | 64.4 ± 16.5 |
| (0.929) | (0.683) | (0.468) | (0.739) | (0.075) | (0.463) |
| Protein Estimated from Urinary Biomarkers (g/d) | |||
|---|---|---|---|
| All | Males | Females | |
| Protein from BDHQ | PRT24h (subjects who provided 24-h urine) | ||
| N = 58 | N = 21 | N = 37 | |
| Crude (g/d) | 0.239 | 0.325 | 0.247 |
| (0.075) | (0.164) | (0.148) | |
| Energy density (%E) | −0.028 | −0.097 | 0.189 |
| (0.833) | (0.682) | (0.229) | |
| PRTon (all subjects) | |||
| N = 248 | N = 118 | N = 130 | |
| Crude (g/d) | 0.205 | 0.247 | 0.124 |
| (0.001) | (0.008) | (0.161) | |
| Energy density (%E) | 0.204 | 0.302 | 0.109 |
| (0.001) | (0.001) | (0.218) | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okuda, M.; Asakura, K.; Sasaki, S. Protein Intake Estimated from Brief-Type Self-Administered Diet History Questionnaire and Urinary Urea Nitrogen Level in Adolescents. Nutrients 2019, 11, 319. https://doi.org/10.3390/nu11020319
Okuda M, Asakura K, Sasaki S. Protein Intake Estimated from Brief-Type Self-Administered Diet History Questionnaire and Urinary Urea Nitrogen Level in Adolescents. Nutrients. 2019; 11(2):319. https://doi.org/10.3390/nu11020319
Chicago/Turabian StyleOkuda, Masayuki, Keiko Asakura, and Satoshi Sasaki. 2019. "Protein Intake Estimated from Brief-Type Self-Administered Diet History Questionnaire and Urinary Urea Nitrogen Level in Adolescents" Nutrients 11, no. 2: 319. https://doi.org/10.3390/nu11020319
APA StyleOkuda, M., Asakura, K., & Sasaki, S. (2019). Protein Intake Estimated from Brief-Type Self-Administered Diet History Questionnaire and Urinary Urea Nitrogen Level in Adolescents. Nutrients, 11(2), 319. https://doi.org/10.3390/nu11020319





