Abstract
In recent years, sugarcane production areas in Brazil have experienced a slower evolution in productivity, and one of the reasons for this is related to the increase in phytosanitary problems, such as the presence of pests. Nevertheless, limited attention has been paid to the development of tools for simulating the spatial dynamics of pests, including their impact on production. This study aims to simulate the potential population growth and dispersal of Diatraea saccharalis (sugarcane borer) in sugarcane crop fields and its estimated impacts on this crop production and to simulate biological control strategies. We developed an agent-based model to simulate the pest population and its dispersal in a one-hectare sugarcane crop field in Pederneiras, São Paulo, Brazil, delimited with the aid of satellite imagery, considering two scenarios: the first without biological control and the second with biological control using the parasites Trichogramma galloi and Cotesia flavipes. The model was developed using the NetLogo 6.3.0 software. The results indicate that the model accurately reproduced the infestation rates reported in the literature. Additionally, it provided insights into expected pest dispersal, potential production losses, and how the use of T. galloi in association with C. flavipes could mitigate production losses. We believe that the model can be used to simulate different biological control strategies and the implementation of integrated pest management (IPM) to achieve adequate control levels and greater productivity in sugarcane production.
1. Introduction
The sugarcane industry in Brazil is of great economic importance, as it plays a critical role in the production of sugar and renewable biofuel. Brazil is growing in the sugarcane agribusiness industry and has been considered the world’s largest producer in recent decades [1]. However, various factors, including phytosanitary problems such as diseases, pests, and weeds, have decreased sugarcane’s productivity evolution [2]. To meet the growing demand for sugarcane, it is necessary to expand cultivation while simultaneously reducing the damage caused by pests.
Diatraea saccharalis (Lepidoptera: Crambidae), popularly known as sugarcane borer, is an insect from the order Lepidoptera and, according to [3,4], crop damage is caused by caterpillars, where newly hatched caterpillars feed on the parenchyma of the leaves and then penetrate into the interior of the stalks, causing the plant to lose weight and, consequently, tumble. Furthermore, they allow fungi to enter the base of the stem, causing fusarium rot and red rot, respectively, as a result of the opening of galleries. Figure 1, below, shows the life cycle of D. saccharalis.

Figure 1.
Evolutionary phases of D. saccharalis. (A) Insect eggs; (B) larva; (C) pupa; (D) pest in the adult stage, male on the left side and female on the right side [5].
The damage caused by this pest is direct and indirect (Figure 2). According to [6], direct damage results from the insect’s feeding, causing the opening of galleries inside the stems, weight loss, the drying of the tops, aerial rooting, lateral sprouting (lateral germination), the death of the apical bud, and breakage of the culm around the galleries [6,7].
With respect to indirect damages, they occur as a result from the entry of the fungi Colletotricum falcatum and Fusarium moniliforme, which cause the inversion of sucrose and an increase in the fiber content, leading to a decrease in purity due to the contamination of the broth during alcoholic fermentation and, consequently, reducing the yield (quality and quantity) of recoverable sugar [7,8].

Figure 2.
(A) Hole caused by D. saccharalis larva on sugarcane; (B) larva inside a gallery; (C) culm with a larva; (D) damaged stems. Adapted from [9].
Therefore, developing techniques and methodologies to control pests without harming nature is crucial. This involves conciliating the preservation of ecological balance with the protection of human interests, such as energy sources [10]. These best practices for pest control are part of integrated pest management (IPM) programs.
IPM is based on cost/benefit analyses of tactics and measures for pest control, taking into consideration ecological, social, economic [11], and sustainable criteria, and often results in the reduction in pesticide usage. Proper phytosanitary management not only promotes the reduction in harmful life forms detrimental to crop development, such as pests and pathogens, but also their prevention [12]. Models serve as valuable tools for testing different phytosanitary management strategies, enabling the validation of hypotheses, and providing insights without the need for immediate implementation in crop fields. Spatial dynamic models in particular are commonly employed for simulating phytosanitary management strategies.
Spatial dynamic models perform numerical simulations of time-dependent processes, serving as mathematical representations of real-world phenomena [13]. In these models, attributes (or states) of a location on the Earth’s surface change in response to variations in their driving forces [13]. Therefore, the use of spatial dynamic models, either process- or agent-based, aims to simulate diverse processes of nature occurring in space and time within a computational environment. These models focus on short-term tactical issues while considering the complex behavioral patterns of heterogeneous and autonomous entities. In the precise scope of agricultural pest management, they have been used to analyze specific pest systems within their respective environments [14,15,16].
Several spatial dynamic models were implemented to simulate different aspects of pests in crop fields [17,18,19,20,21,22]. A process-based insect population dynamics model was integrated to a process-based crop model by [17] to simulate the efficacy of pesticides against six different pests in various sites across the US. The results indicated that the integrated model realistically simulated the effects of crop management on insects and plants. An agent-based model was implemented by [18] to simulate the dispersal and strain density of Spodoptera frugiperda (Lepidoptera: Noctuidae) larvae in cotton plantations. A cellular automata model was developed by [19] to simulate the population dynamics of Aculops lycopersici in eucalyptus plantations. The authors of this study found that the model effectively depicted changes in this pest over space and time. A model using a map lattice was developed by [20] to simulate economic damage levels in crops through a theoretical case study of pest spatial dynamics using an IPM strategy. The results indicated that the spatial component was crucial for measuring economic damage levels.
Similarly, models and simulations have been developed to identify systems and subsystems analyzing the population dynamics of pest species in sugarcane crops [21,22]. These models consider sugarcane phenology, environmental dynamics, and economic factors. An example of this is found in the work of [21], where a model was developed to simulate the pest Eldana saccharina (Lepidoptera: Pyralidae). The authors demonstrated the effectiveness of this tool and its potential to incorporate the population dynamics of other pest species as well as corresponding control measures, such as biological control and habitat management, into a decision support system for IPM in sugarcane cultivation.
Another example is found in the work of [22], where a two-agent dynamic model was developed to simulate the biological control of the sugarcane borer (D. saccharalis) by the egg parasitoid Trichogramma galloi and the larval parasitoid Cotesia flavipes. The model used a verticum-type system (composition), considering that certain state variables of each subsystem influence the dynamics of the subsequent nonlinear subsystem.
The model proposed in this work differs from others found in the literature in several key aspects. Firstly, it estimates the population of D. saccharalis by its biological forms and explicitly incorporates a spatial component to simulate its dispersal in a crop field. Secondly, it also estimates the population and dispersal of the parasites T. galloi and C. flavipes, considering their biological forms. Furthermore, the model enables the simulation of various initial population sizes of D. saccharalis, as well as different biological control strategies. These strategies include determining the release location of parasites, setting the total number of parasites, and employing different release strategies.
In this context, this study aims to simulate the potential population growth and dispersal of D. saccharalis in sugarcane crop fields and its estimated impacts on this crop production and to simulate biological control strategies. An agent-based model was developed to simulate the pest population and its dispersal in a one-hectare sugarcane crop field in Pederneiras, São Paulo, Brazil. Two scenarios were simulated, one without biological control and the other with biological control using the two most effective species for controlling this pest, namely the two previously mentioned parasites.
2. Materials and Methods
2.1. Study Area
The study area corresponds to a one-hectare plot in the municipality of Pederneiras in the state of São Paulo, southeast of Brazil (Figure 3).
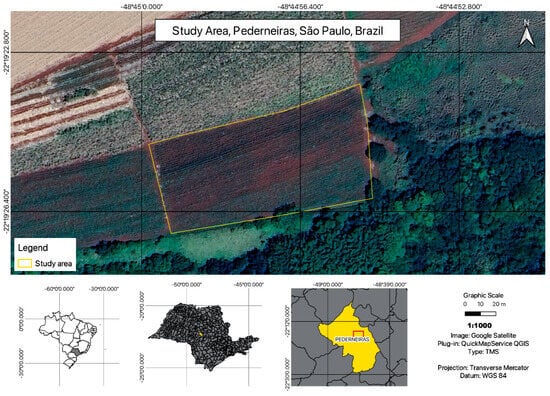
Figure 3.
Study area location map.
The municipality of Pederneiras is located at coordinates 22°21′06″S and 48°46′30″W, covering an area of 727.482 km2. The rural landscape is primarily occupied by sugarcane crops and cattle pastures, accounting for nearly 70% of the municipality surface, followed by coffee, citrus, corn, beans, and soy crops. While situated within the Cerrado and Atlantic Forest biomes, the vegetation cover is not extensive and also includes areas of pine and eucalyptus reforestation. The terrain is slightly undulating, characterized by minimal slopes and flood zones. The climate is predominantly dry, with annual temperatures ranging from 21 °C to 25 °C on average, and a dry winter [23].
2.2. The Agent-Based Model of D. saccharalis and Simulation Scenarios
To develop the agent-based model, we first imported the study area into the simulation environment. The simulation environment represents the study area as a grid of cells capable of incorporating neighborhood effects similar to the matrix format (raster) adopted in Geographic Information Systems—GIS [13]. Utilizing the QGIS 3.24.1 software, we created the simulation environment by vectorizing the boundaries of the one-hectare crop field referring to our study area using CBERS 4A satellite images and the Google Satellite plug-in. This process generated a georeferenced regular cellular grid measuring 1 m × 1 m.
The agent-based model was developed using NetLogo 6.3.0, a free and open-source software that enables multi-agent programmable modeling [24]. Two scenarios were simulated: in the first scenario, we simulated the dispersal of D. saccharalis without considering its biological control, while the second scenario incorporated the use of the parasitoids C. flavipes and T. galloi for biological control. The parameters used to simulate the life cycle and dispersal of D. saccharalis in the first scenario served as the basis for the second scenario. The complete model, including biological control, has 1167 lines, while the model without biological control has 875 lines.
The model outputs consist of two CSV files and one video. The first file contains records of the total population by biological form for each step of the simulation, while the second file includes the locations of agents throughout the simulation. The video presents the interface records of the model for each simulation step.
2.2.1. Scenario of D. saccharalis Dispersal without Biological Control
In the first scenario, we simulated the dispersal of D. saccharalis without biological control. This scenario involved modeling the complete life cycle of D. saccharalis, including its four biological forms. The flowchart of the first scenario is presented in Figure 4.
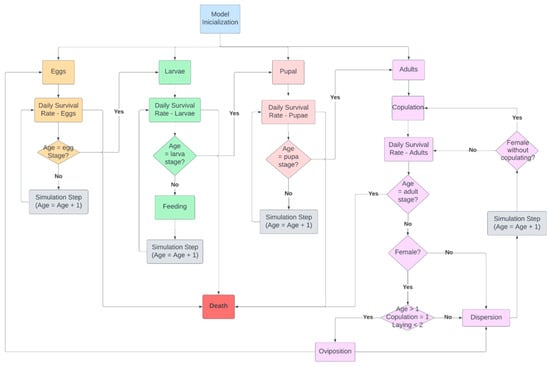
Figure 4.
Flowchart of D. saccharalis dispersal without biological control.
Before running the simulation, the model must be initialized. Model initialization consists of the following processes: setting up the simulation environment and creating the initial population of D. saccharalis.
To set up the simulation environment, the shapefile of the cellular grid is imported, and all cells designated as belonging to the crop area receive a specific cell-type attribute. For these crop cells, an attribute of 8500 g of sugarcane is assigned, considering an average productivity of 85 t/ha in the study area [25], which serves as a reference for approximate calculations of sugarcane loss resulting from D. saccharalis larvae. This attribute is also used during the adult’s dispersion stage, serving as one of the criteria for selecting new cells in the simulation environment.
The next process involved establishing step ranges for each season along with the associated population parameters. The first simulation step was set to January 1st, and since each simulation step represents a day and the model simulates a period of one year, there are a total of 365 steps. The simulation step intervals for each season and the corresponding population parameters are presented in Table 1.

Table 1.
Parameters adopted for the simulation environment and population.
Biological forms that span more than one season have their stage duration proportionally calculated and assigned. The second process of the model initialization is to create the initial population of D. saccharalis agents. The total number of agents, classified by biological form, was determined based on the work of [26], who estimated the percentage of eggs, larvae, pupae, and adults per hectare of a sugarcane crop for different months of the year. This work [26] was chosen due to the geographic proximity between the study areas, since it is important to consider the influence of climatic conditions on the life cycle of D. saccharalis. A total of 1000 biological forms of D. saccharalis per hectare, equally distributed across 10 previously selected seed cells, were defined. Following the proportions found by [28] for January, the total number of eggs, larvae, pupae, and adults were set at the simulation start as 420, 210, 150, and 220, respectively. All agents were 0 days old, with 52.4% of them being female (Table 1).
After the model initialization, the simulation begins. The initial process is to apply a daily survival rate to egg, larvae, and pupa agents (Figure 4), which represents the mortality of D. saccharalis due to environmental conditions, natural predators, and parasites. The daily survival rate varies depending on the biological form (egg, larvae, pupa, and adult) and the season (Table 1). Adult agents follow a different dynamic, engaging in copulation before the daily survival rate is applied. Female adults remain in their original position until they attract the closest male adult and copulate. After copulation, the daily survival rate is applied to all adults.
Each simulation step corresponds to one day of life. Consequently, when an egg agent is created in the summer and reaches its seventh day of life, it transforms into a larvae agent. During the larvae stage, the feeding function of the D. saccharalis is activated. Larvae older than 7 days begin to feed on sugarcane stalks (represented in the model by the sugarcane attribute). Therefore, at each simulation step, each larva consumes one unit of the “cane” attribute from the cell where it is located.
After reaching fifty days of life as a larva, it transitions into a pupal agent, which then becomes an adult agent on the eleventh day of life. Finally, it completes its life cycle and dies after four days. The duration of each biological form varies dynamically according to the season, with its range of days defined based on the simulation step (Table 1).
After copulation, the adult female of D. saccharalis undergoes a pre-oviposition period of 2 days. Consequently, the first oviposition occurs 2 days after copulation, followed by the second oviposition the next day. The number of eggs per oviposition varies according to the season, set as 195 during summer and autumn and 121 during winter and spring. Additionally, there are more males during winter. The daily survival rate is applied at each step of the simulation to all biological forms of D. saccharalis, representing deaths caused by environmental conditions and natural predators. This rate varies according to the biological form and the season.
To model the dispersal of D. saccharalis, we associated the lifespan of adults with parameters from [28]. In the work of [22], it was found that wind can influence both the distance and direction of adult D. saccharalis dispersal. Some adults fly against the wind, some with the wind, and others are indifferent to it (Table 2). Thus, when a D. saccharalis adult agent is created, it is randomly assigned to a dispersal group (with, against, or indifferent to the wind) and ascribed a total flight distance during its lifespan. This random assignment follows the distribution presented in Table 2.

Table 2.
Parameters adopted to simulate adult dispersal: flight distance, direction, and number of marked males captured over 15 days.
For example, consider an adult agent created in spring, with a lifespan of 4 days (Table 1) and programmed to fly 100 m with the prevailing winds during this period. In this case, at each step of the simulation (each day), the agent will move to a cell in the simulation environment containing sugarcane within a radius of up to 25 m. This allows the agent to cover a maximum distance of 100 m during its 4-day lifespan.
To determine the prevailing wind direction in the study area, we consulted the Wind Atlas of the São Paulo State [29]. This Atlas gathers wind direction data for one year from several municipalities in the State of São Paulo. Although Pederneiras (SP) was not included in the data collection, the municipality of Dois Córregos (SP), located 40 km from Pederneiras (SP), was part of it. Based on [29] for the municipality of Dois Corregos (SP), we determined that the prevailing winds in the study area originated from the southeast direction.
2.2.2. Scenario of D. saccharalis Dispersal with Biological Control Using the Parasites C. flavipes and T. galloi
The biological control of D. saccharalis using T. galloi and C. flavipes was based on the work of [30]. According to [30], the most effective biological control method involves three releases of T. galloi followed by one release of C. flavipes. We simulated the same biological control method, which included three releases of 200,000 adults of T. galloi distributed across 25 points, followed by the release of 6000 adults of C. flavipes distributed across 8 points of the simulation environment. Figure 5 presents the flowchart of the second scenario simulation.
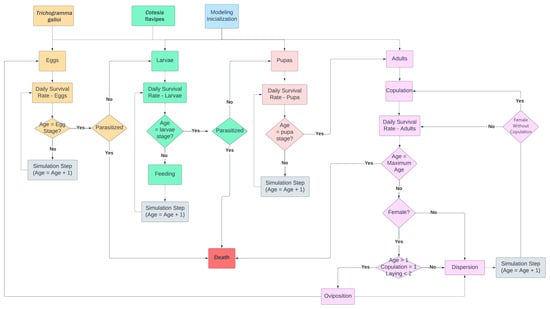
Figure 5.
Second scenario simulation flowchart.
In the second scenario, the eggs and larvae of D. saccharalis can be parasitized, leading to their death before undergoing a change in biological form, resulting in the emergence of larval parasites (Figure 5). Female adults of T. galloi and C. flavipes, after copulation and the pre-oviposition period, respectively, parasitize egg and larval agents of D. saccharalis (Figure 5). Additionally, we modeled the various biological forms and genders of the parasites, as it is crucial for simulating their copulation, dispersal, and population distribution.
The T. galloi wasp measures less than 1 mm in length and parasitizes the eggs of the sugarcane borer, laying its eggs within them. Each parasitized egg develops into 1 or 2 larvae [31]. In its life cycle, T. galloi passes through the egg, larval, prepupal, pupal, and adult stages, with all stages except the adult occurring within the parasitized egg [32]. Therefore, only two phases (egg and adult) were considered in the model. The flowchart summarizing the life cycle of T. galloi is presented in Figure 6.
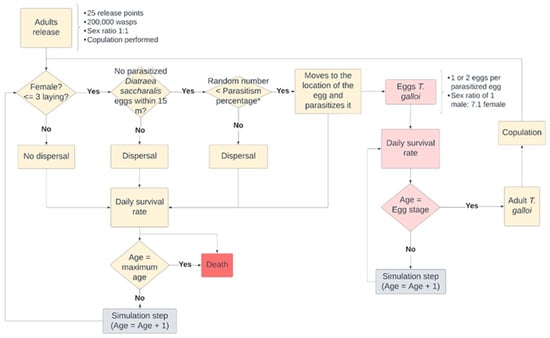
Figure 6.
Flowchart of the life cycle of the parasite T. galloi. Note: * Parasitism percentage relative to distance: up to 5 m—51%; between 5 m and 10 m—41%; and between 10 m and 15 m—21%.
The release of 200,000 T. galloi adults occurred in steps 2, 9, and 16 of the simulation, following the methods used by [30], with the three releases spaced seven days apart from each other. The 200,000 adults were evenly distributed among 25 release points, which were uniformly distributed throughout the simulation environment. The choice of 25 points was based on the observed increased effectiveness in parasitism when T. galloi wasps were released within a 15 m range of D. saccharalis eggs [33].
It was assumed that the sex ratio was one female to one male, and all adult females had copulated before release. When released, adult females will search for D. saccharalis eggs within a 15 m radius. If eggs are found, the likelihood of successful parasitism is determined based on the distance to the egg. Distances up to 5 m have a 51% probability of successful parasitism and distances from 5 to 10 m have a 41% probability, while distances from 10 to 15 m have a 21% probability [34]. A randomly generated number from 1 to 100 is compared to the percentage of successful parasitism. If the randomly generated number is lower than the percentage of successful parasitism, the adult female will move to the egg and parasitize it, laying 1 or 2 eggs with a sex ratio of 1 male to 7.1 female. An adult female that has already copulated can parasitize up to four D. saccharalis eggs. Adult females who fail the parasitism test or are more than 15 m away from the nearest D. saccharalis egg will randomly disperse within the crop area, covering distances of up to 15 m. Subsequently, a daily survival rate is applied (Table 3), the age of the adult is checked (Table 3), and a new simulation step begins.

Table 3.
Parameters adopted for modeling the life cycle of T. galloi.
For the T. galloi eggs, the daily survival rate is applied, the egg age is verified, and a new simulation step begins. This process is repeated until the egg age reaches 9 days (Table 3), at which point the egg transforms into an adult and the copulation process occurs. During copulation, the adult male seeks out the closest adult female and moves towards her. Once they are located in the cell, they copulate, resulting in a change to the female attribute. The process then repeats until the third iteration, when adult females cease parasitizing.
At simulation step 46, 6000 C. flavipes adults were released. C. flavipes originates from Asia [27] and experiences several stages of development: egg, larva, pupa, and adult. It can only complete its life cycle when associated with D. saccharalis caterpillars. Parasitism occurs through female wasp stings when many eggs are deposited inside the D. saccharalis caterpillar. The deposited eggs hatch, producing larvae that feed inside the caterpillar. Eventually, the larvae migrate out of the caterpillar’s body, causing its death. Subsequently, they enter the pupal stage, and within a few days, the adults emerge [31]. The flowchart of C. flavipes agents implemented in the model is presented in Figure 7.
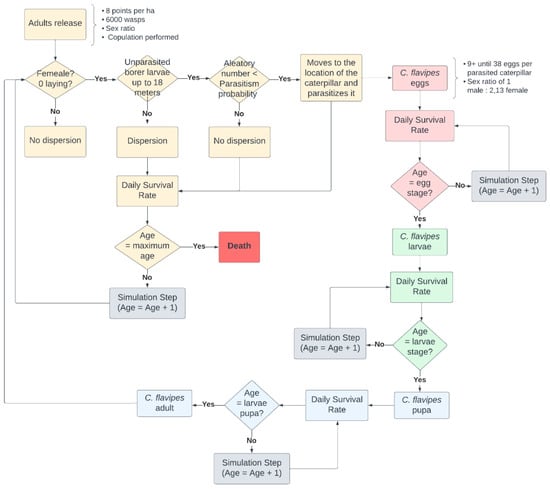
Figure 7.
Flowchart of the C. flavipes life cycle.
The 6000 C. flavipes adults were evenly distributed among the eight release points, which were uniformly distributed throughout the simulation environment. It was assumed that the sex ratio was one female to one male, and all adult females copulated before release. Upon release, adult females will search for D. saccharalis larvae within an 18 m radius. If a larva is found, the probability of parasitism is calculated (Equation (1)) based on the work of [37].
where
PP is the probability of parasitism;
E is the parasitoid efficiency constant;
D is the total number of non-parasitized caterpillars; and
C is the total number of female adults of C. flavipes that have already copulated, but have not laid eggs yet.
When a randomly generated number from 1 to 100 is lower than the probability of parasitism, the adult female of C. flavipes moves to the caterpillar and parasitizes it. It lays between 9 and 38 eggs with a sex ratio of 1 male to 2.13 females. The parasitized caterpillar ceases feeding and remains alive until it completes its life cycle phase, when, instead of progressing to the pupal stage, it dies.
Adult females who fail the parasitism probability test or are more than 18 m away from the nearest D. saccharalis caterpillar will randomly disperse within the crop area, covering distances of up to 18 m [36]. Subsequently, we applied a daily survival rate to adults (Table 4), checked their age (Table 4), and initiated a new simulation step.

Table 4.
Parameters adopted in modeling the life cycle of C. flavipes.
For the C. flavipes eggs, the daily survival rate is applied, and the egg age is verified for each simulation step. This process is repeated until the egg age reaches 4 days (Table 4), at which point the egg transforms into a larva. Then, the daily survival rate is applied to the larva, the age of the larva is checked, and a new simulation step is performed until the larva age reaches 10 days (Table 4), when the larvae transform into pupae. Finally, the C. flavipes pupae pass through the daily survival rate until the end of the pupal stage, which lasts for 5 simulation steps or 5 days (Table 4). Upon reaching the fifth day, the pupae transform into adults and the copulation process begins. During copulation, the adult female attracts the nearest adult male. Once they are located in the same cell, they copulate, resulting in a change to the female attribute, indicating that it will not copulate again until the oviposition. This process repeats until the second day of the adult’s life, when the adult dies, marking the end of its life cycle. Table 4 presents the parameters of the C. flavipes life cycle adopted in the model.
3. Results
3.1. Simulation of D. saccharalis Dispersal without Biological Control
Figure 8 illustrates the simulated population of D. saccharalis by biological form for each simulation step.
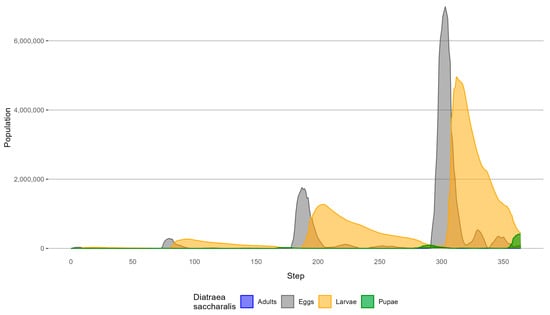
Figure 8.
D. saccharalis simulated population distribution without biological control.
The population growth throughout the simulation is evident, with eggs and larvae being the two most abundant biological forms (Figure 8). Peaks in egg population occur at steps 80 (288,765 eggs), 187 (1,760,546 eggs), and 303 (6,985,802 eggs), followed by peaks in larval population at steps 95 (268,793 larvae), 205 (1,273,561 larvae), and 312 (4,959,428 larvae).
The two least abundant biological forms are adults and pupae. Peaks in the adult population occur at steps 75 (1832 adults), 181 (11,742 adults), and 296 (47,299 adults), while peaks in the pupal population are observed at steps 176 (24,719 pupae), 291 (93,864 pupae), and 364 (416,683 pupae). The simulated dispersal of D. saccharalis and its impact on the crop field are shown in Figure 9. The simulation started with all cells in the simulation environment containing 8500 g of sugarcane (Step 1).
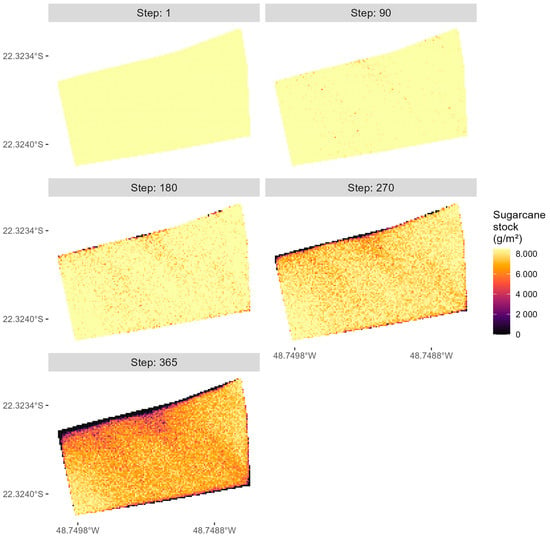
Figure 9.
Simulated sugarcane crop damage caused by D. saccharalis without biological control.
3.2. Simulation of D. saccharalis Population and Dispersal with Biological Control
Figure 10 illustrates the simulated population of D. saccharalis by biological form under biological control for each simulation step.
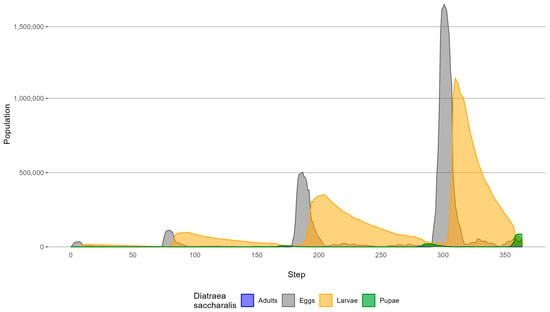
Figure 10.
D. saccharalis simulated population distribution under biological control.
Table 5 shows the simulated population of D. saccharalis without and with biological control.

Table 5.
The simulated population of D. saccharalis without and with biological control.
Figure 11 presents the parasitized population of D. saccharalis eggs, and Figure 12 shows the parasitized population of D. saccharalis larvae.
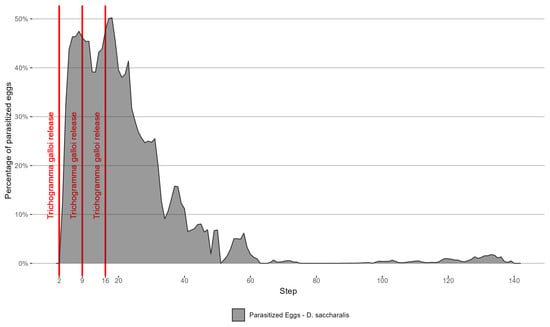
Figure 11.
The parasitized population of D. saccharalis eggs.
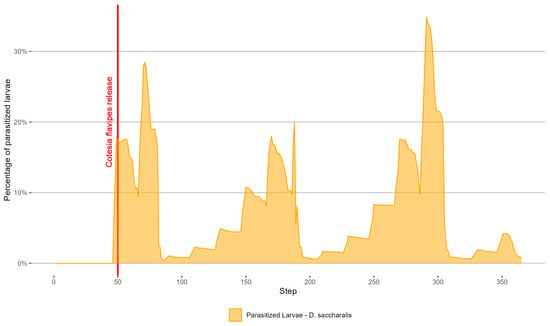
Figure 12.
The parasitized population of D. saccharalis larvae.
The simulated impact of D. saccharalis and its dispersal with biological control is presented in Figure 13.
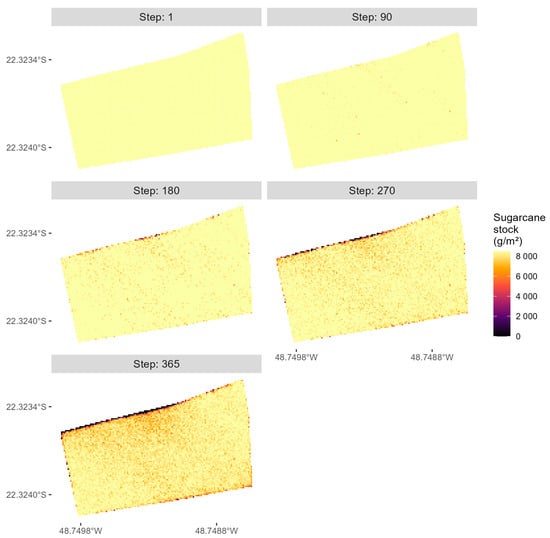
Figure 13.
Simulated sugarcane crop damage caused by D. saccharalis with biological control.
4. Discussion
4.1. Simulation of D. saccharalis Dispersal without Biological Control
The high quantity of eggs can be attributed to the two oviposition events, each resulting in 195 eggs during summer and autumn or 121 eggs during winter and spring, deposited by female adults. These eggs have a short life period (between 7 and 12 days), leading to a rapid increase in larvae numbers once the daily survival rate is applied. As shown in Figure 8, the highest peak in larvae population occurs at step 312, but during summer, the lower daily survival rate causes a quicker decline in the larval population compared to the slower reduction observed during winter, as indicated by the peak at step 205. The pupal population is less abundant because it follows the larval stage, during which many larvae die over the 50–91 days of this stage when the daily survival rate is applied. Similarly, the adult population, being the final biological form in the D. saccharalis life cycle, follows a similar pattern, as it consists of a subset of the pupal population that survived.
According to Figure 9, by step 90, a limited number of cells exhibited damage caused by larvae; however, the spatial patterns of this damage remain unclear. From step 180 onwards, it became apparent that the cells located in the northwestern region of the crop area were more damaged due to larvae originating from eggs oviposited by female adults that flew with the prevailing winds. A portion of larvae also damaged cells in the southeastern part of the simulation environment as a result of eggs oviposited by female adults that flew against the prevailing winds. Additionally, random cells were damaged due to larvae originating from eggs oviposited by female adults that flew indifferently to the prevailing winds. In the final step of the simulation, the model estimated the total damage to be 24.7% of the total sugarcane area. This result is consistent with the 25.8% infestation intensity found by [30].
Both simulations were generated using a server machine with a 2.4 GHz Xeon processor and 200 Gb of RAM. Regarding computational efficiency, the simulation with biological control took 7.3 h in total.
4.2. Simulation of D. saccharalis Population and Dispersal with Biological Control
The most numerous agents by biological form in the simulation were eggs, larvae, pupae, and adults. Population peaks occurred at the same simulation steps as in the previous simulation (Section 4.1—without biological control), except for the intermediate larvae peak. In the simulation without biological control, this peak occurred at step 205, whereas in the simulation with biological control, it occurred at step 204. The implementation of biological control resulted in a reduction of 57.9% to 79.7% in the number of D. saccharalis agents (Table 5). It is important to observe that the occurrence of the peak population with and without biological control is very similar in time because biological control does not change the life cycle of the pest; it otherwise just reduces its population.
The reduction in the population of D. saccharalis is a direct result of T. galloi and C. flavipes parasites used for biological control. Regarding the T. galloi parasite, the highest percentages of parasitized eggs were observed at steps 18 (50%), and 8 (47%), leading to a significant reduction in the population of D. saccharalis eggs (Figure 11). This result demonstrates a lower parasitism rate compared to the findings reported by [30], who observed a 71% egg parasitism after 2 days of releases. Nevertheless, our result is close to the one obtained by [33], who achieved a parasitism rate of 51% at a 5 m release distance. It is worth mentioning that [33] was the only work found in the literature containing a formulation to simulate the parasitism levels of T. galloi as a function of the release distance to the eggs of D. saccharalis. Despite the release of T. galloi adults at the beginning of the simulation, the cycle persisted, naturally generating new cycles until step 139 (Figure 11).
Regarding the parasitism of C. flavipes, the highest parasitism rates occurred at steps 291 (34.8%), 71 (28.4%), 188 (19.8%), and 49 (17.8%) (Figure 12). We observed that the simulated parasitism by C. flavipes was lower than that by T. galloi, with the maximum peak of parasitized larvae at 30.8%, compared to the maximum peak of parasitized eggs at 50%. The parasitism by C. flavipes was similar to that reported by [29], who observed a 14.6% parasitism rate after 15 days of release.
While the simulated spatial patterns of damage in the sugarcane crop persist, with the prevailing wind direction being one important factor for dispersal, the total damage is consistently reduced by biological control. In the final step of the simulation, the model estimates the total damage to be 6.2% of the total sugarcane area. This result is similar to the 10.3% infestation intensity found by [30]. Finally, in terms of computational efficiency, the simulation with biological control lasted 46.7 h.
5. Conclusions
This study proposed a model to simulate the population and dispersal of D. saccharalis in sugarcane crop fields. The simulation began with an artificial population of 1000 biological forms of D. saccharalis in a sugarcane crop field located in the municipality of Pederneiras, São Paulo, southeast of Brazil. Two scenarios were simulated to investigate the population and dispersal of D. saccharalis: the first without biological control and the second one with biological control using the parasites T. galloi and C. flavipes, following the methodology proposed by [30].
The results indicate that the model successfully replicated the total damage caused by D. saccharalis as reported by [30] for both simulation scenarios. The infestation intensity reported by [30] was 25.8% without biological control and 10.3% with biological control. The model results show losses of 24.7% and 6.2% without and with biological control, respectively.
In terms of the spatial distribution of D. saccharalis biological forms, the parameters used in the model incorporated dispersal behaviors documented in the literature, encompassing adults flying with, against, and indifferently to the wind direction. Based on prevailing wind data for the study area, the model’s output indicates that the majority of D. saccharalis are concentrated in the northwestern and southeastern portions of the sugarcane crop field. Although the dispersal parametrization was grounded in empirical studies from the literature, further information is necessary to validate the spatial patterns of dispersal.
We believe that the proposed model could provide valuable insights and support for integrated pest management plans. It enables the testing of various scenarios related to D. saccharalis infestation, prevailing wind directions, and the population of parasites for biological control. Moreover, the model is adaptable for testing different strategies of parasite releases for biological control.
This study has some limitations, the first being that the simulation environment is isolated. Consequently, the immigration or emigration of agents from the simulated environment was not considered. Another limitation is the absence of fieldwork to empirically validate the model and potentially adjust or incorporate new parameters to better simulate the behavior of D. saccharalis and/or the parasites.
For future work, our aim is to conduct fieldwork to validate the estimated population of D. saccharalis and its spatial patterns of dispersal in sugarcane crop fields. Additionally, we plan to incorporate other pests that affect the productivity of sugarcane crops, such as Sphenophorus levis, and explore additional options for biological control.
Author Contributions
Conceptualization, C.M.d.A. and D.L.R.; methodology, D.B.T.; investigations, R.B.d.O.A., D.B.T., C.M.d.A., D.L.R., L.H.P. and C.L.R.; data curation, D.L.R., D.B.T. and R.B.d.O.A.; writing—original draft preparation, R.B.d.O.A. and D.B.T.; writing—review and editing, C.M.d.A. and H.P.d.S.; supervision, C.M.d.A. and D.L.R.; project administration, C.M.d.A.; funding acquisition, C.M.d.A. All authors have read and agreed to the published version of the manuscript.
Funding
This study is funded by the Brazilian Coordination for the Upgrade of Graduate Personnel CAPES, through grant N° 88887.648762/2021-00—Finance Code 001 (R.B.d.O.A.). The authors also are grateful to the São Paulo Research Foundation (FAPESP) through research grant N° 2022/07791-2 (D.B.T.), and the Brazilian National Council for Scientific and Technological Development (CNPq) through research grant N° 311324/2021-5 (C.M.d.A.). The APC was funded by the Brazilian Space Agency–AEB (C.M.d.A.).
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- CONAB−Brazilian Supply Company. Sugar Cane Harvest Bulletin. First Survey. 2021; v. 8, n. 3, p. 56. Available online: https://www.conab.gov.br/info-agro/safras/cana/boletim-da-safra-de-cana-de-acucar (accessed on 15 April 2022).
- Fonseca, E.M.S.; Araújo, R.C. Fitossanidade: Princípios Básicos e Métodos de Controles e Doenças, 1st ed.; Saraiva: São Paulo, Brazil, 2015; Volume 1, p. 136. [Google Scholar]
- Gallo, D.; Nakano, O.; Silveira Neto, S.; Carvalho, R.L.; De Batista, G.C. Manual de Entomologia Agrícola; Agronômica Ceres: São Paulo, Brazil, 1988; p. 649. [Google Scholar]
- Rodero, D.P. Modelagem matemática da interação populacional entre Diatraea saccharalis (Fabricius, 1794) e o parasitoide Cotesia flavipes (Cameron, 1891). Master’s Thesis, Universidade de São Carlos, Araras, Brazil, 2016; p. 98. [Google Scholar]
- Cruz, I. Lepdoptera como Pragas de Milho. 2010. Available online: https://ainfo.cnptia.embrapa.br/digital/bitstream/item/31231/1/doc-111.pdf (accessed on 12 December 2022).
- Sandoval, S.S.; Senô, K.C.A. Comportamento controle da Diatraea saccharalis na cana-de-açúcar. Nucleus 2010, 7, 1–16. Available online: https://dialnet.unirioja.es/servlet/articulo?codigo=4037676 (accessed on 1 August 2022). [CrossRef]
- Portela, G.L.F.; Padua, L.E.M.; Branco, R.T.P.C.; Barbosa, O.A.; Silva, P.R.R. Flutuação populacional de Diatraea saccharalis (Fabricius, 1794) (Lepidoptera—Crambidae) em cana-de-açúcar no município de União-PI. Rev. Bras. Ciênc. Agrárias 2021, 5, 303–307. Available online: http://www.agraria.pro.br/ojs32/index.php/RBCA/article/view/v5i3a510 (accessed on 15 August 2022). [CrossRef]
- Macedo, N.; Macedo, D. As pragas de mais incidência nos canaviais e seus controles. Rev. Visão Agrícola 2004, 1, 38–46. Available online: https://www.esalq.usp.br/visaoagricola/sites/default/files/cana-producao-vegetal04.pdf (accessed on 15 August 2023).
- Bayer Agro. Broca-da-Cana: Como Identificar e Como Realizar o Manejo|Impulso Negócios EP. 58. 2023. Available online: https://www.youtube.com/watch?v=fVJergBn_3E (accessed on 5 November 2022).
- Paranhos, S.B. Cana-de-Açúcar Cultivo e Utilização; Fundação Cargil: Campinas, SP, Brazil, 1987; p. 856. [Google Scholar]
- Kogan, M. Integrated pest management: Historical, perspectives and contemporary development. Annu. Rev. Entomol. 1998, 43, 243–270. Available online: https://www.annualreviews.org/doi/abs/10.1146/annurev.ento.43.1.243?casa_token=GIwhbd6_k9YAAAAA%3Aq7N95GlmTtCLM6Qje9KJG5C8u4QJNHRlCfks7e_ylhWqKcAdsDJn_ACxqe03V-Cyzn_3n_B6qyxdu9K6 (accessed on 17 November 2022). [CrossRef] [PubMed]
- Barros, G.S.C.; Miranda, S.H.G.; Osaki, M.; Alves, L.R.A.; Adami, A.O.; Nishikawa, M.E.; Perez, F.C.; Lima, F.F.; Ribeiro, R.G. Mensuração econômica da incidência de pragas e doenças no brasil: Uma aplicação para as culturas de soja, milho e algodão. CEPEA 2019, 1, 4–12. Available online: https://www.cepea.esalq.usp.br/upload/kceditor/files/Cepea_EstudoPragaseDoencas_Parte%201.pdf (accessed on 3 October 2022).
- Burrough, P. Dynamic Modelling and Geocomputation. In Geocomputation: A Primer, 1st ed.; Longley, P., Batty, M., Mcdonnel, R., Eds.; John Wiley & Sons: London, UK, 1998; Volume 1, p. 280. [Google Scholar]
- Jones, J.W.; Luyten, J.C. Simulation of Biological Processes. In Agricultural Systems Modeling and Simulation, 1st ed.; Robert, M.P., Curry, R.B., Eds.; CRC Press: New York, NY, USA, 1998; Volume 1, pp. 19–62. [Google Scholar]
- Bonabeau, E. Agent-based modeling: Methods and techniques for simulating human systems. Proc. Natl. Acad. Sci. USA 2002, 99, 7280–7287. [Google Scholar] [CrossRef] [PubMed]
- Donatelli, A.; Magarey, R.D.; Bregaglio, S.; Willocquet, L.; Whish, P.M.; Savary, S. Modelling the impacts of pests and diseases on agricultural systems. Agric. Syst. 2017, 155, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Rasche, L.; Taylor, R.A.J. EPIC-GILSYM: Modelling crop-pest insect interactions and management with a novel coupled crop-insect model. J. Appl. Ecol. 2019, 56, 2045–2056. [Google Scholar] [CrossRef]
- Malaquias, J.B.; Godoy, W.A.C.; Garcia, A.D.; Ramalho, F.S.; Omoto, C. Larval dispersal of Spodoptera frugiperda Strains on Bt cotton: A model for understanding resistance resolution and consequences for its management. Sci. Rep. 2017, 7, 16109. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, D.; Li, L.; Yuan, Y. Design and implementation of Aculops lycopersici population dynamic model prototype based on cellular automata. In IFIP-International Federation for Information Processing, 1st ed.; IFIP: Boston, MA, USA, 2009; Volume 2, pp. 1319–1328. [Google Scholar] [CrossRef]
- Lima, E.A.B.F.; Ferreira, C.P.; Godoy, W.A.C. Ecological modeling and pest population management: A possible and necessary connection in a changing world. Neotrop. Entomol. 2009, 38, 699–707. [Google Scholar] [CrossRef] [PubMed]
- Potgieter, L.; Vuuren, J.H.V.; Conlong, D.E. Simulation modelling as a decision support in developing a sterile insect-inherited sterility release strategy for Eldana saccharina (Lepidoptera: Pyralidae). Fla. Èntomol. 2021, 99, 12–22. [Google Scholar] [CrossRef]
- Molnár, S.; López, I.; Gámez, M.; Garay, J. A two-agent model applied to the biological control of the sugarcane borer (Diatraea saccharalis) by the egg parasitoid Trichogramma galloi and the larvae parasitoid Cotesia flavipes. Biosystems 2016, 141, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Municipal Government of Pederneiras. General Aspect of Pederneiras Municipality. Available online: https://www.pederneiras.sp.gov.br/portal/servicos/67/a-nossa-cidade/aspectos-gerais#:~:text=Localiza%C3%A7%C3%A3o%3A%20O%20munic%C3%ADpio%20de%20Pederneiras,Agudos%20e%20Bauru%20a%20oeste (accessed on 18 September 2023).
- Wilensky, U. 1999. Netlogo. Available online: http://ccl.northwestern.edu/netlogo (accessed on 15 September 2022).
- IBGE−Brazilian Institute for Geography and Statistics. Municipal Agricultural Production. Available online: https://sidra.ibge.gov.br/tabela/1612 (accessed on 15 June 2023).
- Botelho, P.S.M. Tabela de Vida ecológica e Simulação da Fase Larval da Diatrea saccharalis (Fabr., 1794) (Lep: Pyralidae). Ph.D. Dissertation, São Paulo University, São Paulo, Brazil, 1985; p. 110. Available online: https://www.teses.usp.br/teses/disponiveis/11/11146/tde-20191220-132512/publico/BotelhoPauloSergioMachado.pdf (accessed on 30 September 2022).
- Sakuno, C.I.R. Efeitos da Movimentação de Diatraea saccharalis (Fabricius) (Lepidoptera: Crambidae) em Sistemas Compostos por Cana-de-Açúcar Geneticamente Modificada e Refúgio. Master’s Thesis, São Paulo University, Piracicaba, Brazil, 2021; p. 84. Available online: https://www.teses.usp.br/teses/disponiveis/11/11146/tde-04082021-134220/pt-br.php (accessed on 18 October 2022).
- Caixeta, D.F. Dispersão de Machos de Diatraea saccharalis (Fabricius) (Leipdoptera: Crambidae) em Cana-de-Açúcar. Master’s Thesis, São Paulo State University, Jaboticabal, Brazil, 2010. Available online: https://repositorio.unesp.br/server/api/core/bitstreams/779ed8b8-b20f-4fbb-b09a-5afc5ea800ae/content (accessed on 11 August 2022).
- SEMIL−Sao Paulo State Secretary for Environment, Infrastructure and Logistics. Wind Atlas of São Paulo State. Available online: https://www.infraestruturameioambiente.sp.gov.br/energias-eletrica-e-renovaveis/atlas-eolico-do-estado-de-sao-paulo/ (accessed on 3 July 2022).
- Botelho, P.S.M.; Parra, J.R.P.; Chagas Neto, J.F.; Oliveira, C.P.B. Association of the egg parasitoid Trichogramma galloi Zucchi (Hymenoptera: Trichogrammatidae) with the larval parasitoid Cotesia flavipes (Cam.) (Hymenopera: Braconidae) to control the sugarcane Borer Diatraea saccharalis (Fabr.) (Lepidoptera: Crambidae). An. Soc. Entomol. 1999, 28, 491–496. Available online: https://www.scielo.br/j/aseb/a/rX6DYzxwxp8th9JDfgYCmZf/ (accessed on 30 August 2022). [CrossRef]
- Mendonça, A.F. Distribuição de Diatraea spp. (Lep.: Pyralidae) e de seus principais parasitoides larvais no Continente Americano. In Pragas da Cana-de-Açúcar, 1st ed.; Insetos & Cia: Itabira, Brazil, 1996; Volume 1, pp. 83–121. Available online: https://ainfo.cnptia.embrapa.br/digital/bitstream/item/79313/1/documento-287.pdf (accessed on 10 September 2022).
- Moutia, L.A.; Courtois, C.M. Parasites of mothborers of sugar-cane in Mauritius. Bull. Entomol. Res. 1952, 43, 325–359. Available online: https://www.cambridge.org/core/journals/bulletin-of-entomological-research/article/abs/parasites-of-the-mothborers-of-sugarcane-in-mauritius/2EC220F628230BB6418EEF6F224552DA (accessed on 9 October 2022). [CrossRef]
- Lopes, J.R.S. Estudos Bioetológicos de Trichogramma galloi Zucchi, 1988 (Hym., Trichogrammatidae) para o Controle de Diatraea saccharalis (Fabr., 1794) (Lep., Pyralidae). Master’s Thesis, São Paulo University, Piracicaba, Brazil, 1988; p. 141. Available online: https://teses.usp.br/teses/disponiveis/11/11146/tde-20191218-104515/pt-br.php (accessed on 23 November 2022).
- Drake, V.A.; Farrow, R.A. The influence of atmospheric structure and motions on insect migration. Ann. Rev. Entomol. 1988, 33, 183–210. Available online: https://www.annualreviews.org/doi/abs/10.1146/annurev.en.33.010188.001151 (accessed on 13 December 2022). [CrossRef]
- Pereira-Barros, J.L.; Broglio-Micheletti, S.M.F.; Santos, A.J.N.D.; Carvalho, L.W.T.D.; Carvalho, L.H.T.D.; Oliveira, C.J.T.D. Biological aspects of Trichogramma galloi Zucchi, 1988 (Hymenoptera: Trichogrammatidae) reared on eggs of Diatraea saccharalis (Fabricius, 1794) (Lepidoptera: Crambidae). Ciênc. Agrotec. 2005, 29, 714–718. Available online: https://www.scielo.br/j/cagro/a/Rtz5rHpN99YKwzhPnyHSYpb/ (accessed on 22 August 2022). [CrossRef][Green Version]
- Nava, D.E.; Afonso, A.P.S.; Melo, M.; Pinto, A.S.; Anjos e Silva, S.D.; Controle Biológico da Broca da Cana-de-Açúcar. Embrapa Clima Temperado, Pelotas, Rio Grande do Sul, Brasil. 2009. Available online: https://ainfo.cnptia.embrapa.br/digital/bitstream/item/44196/1/broca-cana.pdf (accessed on 19 September 2022).
- Micheletti, S.M.F.B. Distribuição espacial e temporal de ovos de Diatraea saccharalis (Fabr., 1794) (Lep.: Pyralldae) e seu parasitismo por Trichogramma sp. (Hym., Trichogrammatidae). Master’s Thesis, São Paulo University, Piracicaba, Brazil, 1987; p. 95. Available online: https://teses.usp.br/teses/disponiveis/11/11146/tde-20181127-161021/pt-br.php (accessed on 23 September 2022).
- Souza, R.G.B.D. Otimização do Controle de Diatraea saccharalis (Fabricius, 1794) (Lepidoptera: Crambidae) por Meio do Parasitoide Cotesia flavipes (Cameron, 1891) (Hymenoptera: Braconidae) Visando a Redução de Custos. Ph.D. Dissertation, Federal University of São Carlos, São Carlos, Brazil, 2018; p. 118. Available online: https://repositorio.ufscar.br/handle/ufscar/10033 (accessed on 3 October 2022).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).