Abstract
The problems of food waste and packaging waste production currently force us to search for new solutions that are safe for people and the environment. Applying edible coatings directly onto food offers a sustainable method of maintaining shrimp freshness, eliminating the need for artificial preservatives and avoiding the high energy demands of conventional chemical or physical preservation techniques. In this study, starch materials modified with natural extracts from plants with proven health-promoting and antibacterial properties—rooibos and garlic—were obtained and tested. The structure, hydrophilicity, water vapor permeability, and thermal and mechanical properties of the obtained starch films were determined. The study further revealed that Escherichia coli was absent in all shrimp samples coated with starch-based films following seven days of refrigerated storage, in contrast to uncoated samples. For Staphylococcus aureus, coatings with rooibos extract resulted in a significant reduction in bacterial counts. Coatings with garlic extract showed a marginally reduced antibacterial effect. The effect of the coatings on the overall numbers of lactic acid bacteria and aerobic mesophilic bacteria was evaluated as well. Coatings enriched with rooibos were more effective in the early days of storage, while garlic-based coatings exhibited a less intense but more enduring antimicrobial effect.
1. Introduction
Due to the constantly growing population and the high environmental impact caused by the breeding of land animals, more and more attention is paid to seafood. One of the most commonly caught marine organisms is shrimp. These animals are particularly important for human nutrition because they are very rich in protein, amino acids, and trace elements and are considered very tasty. At the same time, however, these products are perishable, especially during transport inland, as well as during storage, e.g., in market halls or street stalls, which do not always provide conditions for safe food storage. This causes significant waste associated with the need to dispose of such spoiled shrimp but also a health risk for people associated with contamination of these products with pathogenic microorganisms. According to [], there has already been a shift in producers’ thinking from viewing pro-ecological activities as a source of additional costs to environmental management as a part of business operations. As a result, manufacturing, transport, and distribution companies are not afraid to invest and introduce new technologies that improve the quality of their products.
Shrimp preservation methods include low-temperature storage (in cold rooms or on ice), chemical preservation (including ozone), high pressure, irradiation, modified atmosphere packaging, acid electrolysis, and biological preservation []. These methods also utilize physical methods, including ultrasound, electrostatic fields, and magnetic fields. Among these methods, biological methods seem particularly promising. Yu et al. state that they include 3 types of biological preservation mechanisms: (i) delay the oxidation process and prevent discoloration by removing air and inhibiting enzyme activity; (ii) maintain food quality during storage and transportation using antibacterial, antioxidant, natural, and non-toxic substances; and (iii) protect against the growth of bacteria that cause product spoilage and against reducing water loss by creating a protective coating with natural materials []. These methods, that is, the use of edible coatings for fresh food such as shrimp, are innovative solutions that are increasingly popular due to their many advantages []. These eco-friendly solutions not only effectively increase the freshness and quality of food products but also provide alternative packaging solutions that help reduce waste and promote sustainability. In addition, preventing the growth of pathogenic microorganisms in food reduces the incidence of spoilage and related illnesses and consequently lowers expenses such as healthcare costs, worker absences, increased insurance rates, and compensation payments []. Because the coatings consist solely of natural components, they may be consumed together with the product or removed easily with water, for instance, during rinsing.
Their primary function is to protect food, ensure safety, and maintain high product quality [,]. Moreover, they can enrich a food product with additional nutritional or organoleptic values. The materials used as these coatings can also act as active packaging. Substances, such as essential oils, organic acids, or enzymes, can be added to their atrium, which can provide additional protection against microorganisms.
This type of packaging has been evaluated on various food products, including fruits and vegetables, dairy products, meat, and fish [,]. The coatings are composed of proteins, polysaccharides and their derivatives (chitin [], chitosan [,], cellulose [], alginate [,], and starch []), lipids [], as well as microalgal exopolysaccharides [] or basil seed gum []. They are often obtained from waste products from various industries, such as milk processing (whey proteins), fruit (pectin from citrus peels), and fish and seafood (shrimp shells) []. These coatings are part of a large group of biopolymer packaging products, which are gaining increasing importance in the packaging industry. The packaging industry is the main consumer of bioplastics, consuming approximately 55% of all bioplastics produced. According to data from MarketsnadmarketsTM, 1543.3 kilotons of bioplastics were used globally for packaging in 2024 []. This amount is expected to increase to approximately 5235 kilotons by 2029. These actions constitute a crucial element of a sustainable economy policy aimed at improving the natural environment.
Abidin et al. demonstrated that chitin oligosaccharides, when applied as an antimicrobial coating, effectively suppressed the growth of Listeria monocytogenes during chilled storage, while at the same time helping to preserve the overall quality of the shrimp [].
In another study, Afifi et al. [] developed an edible coating based on pullulan combined with nano-clay and watercress essential oil. Their work showed that this formulation markedly slowed both chemical and microbial deterioration in Pacific white shrimp, resulting in a noticeable extension of its storage life []. Rezaei et al. [] examined coatings prepared from carboxymethylcellulose enriched with a nano-emulsion of Shirazi thyme oil at different concentrations (10, 20, and 30 mg/mL). During a 10-day refrigerated storage period (4 ± 0.5 °C), coatings containing 10 and 20 mg/mL of the nano-oil were particularly effective: they prolonged the shrimp’s shelf life and helped preserve its quality by limiting microbial proliferation, melanosis, and undesirable chemical changes. Furthermore, these coatings enhanced the texture and sensory attributes of the breaded shrimp [].
Rooibos and garlic extracts seem to be promising materials to protect shrimp against bacteria during storage. Rooibos contains a high amount of polyphenols, particularly aspalathin and nothofagin [,]. According to Santos et al., the total content of polyphenols, estimated by spectrophotometry, ranges from 16.23 to 24.93 mg/g of dry matter in terms of gallic acid equivalent []. Garlic contains, among others, carbohydrates and sulfur compounds, such as allicin (diallyl thiosulfinate), as well as polyphenols []. The presence of polyphenols and sulfur-containing compounds in these plant extracts enables them to suppress the growth of a wide range of Gram-positive and Gram-negative bacteria [,]. Furthermore, data from the literature confirm that rooibos can effectively inhibit the growth of various microorganisms, including Escherichia coli, Staphylococcus aureus, Bacillus cereus, Listeria monocytogenes, Streptococcus mutans, and Candida albicans []. The potential of arrowroot starch and sago starch as edible coatings has already been studied [,,]. Films obtained from these starches differ in some parameters. Arrowroot starch contains the highest proportion of amylose—up to 40.86%. For comparison, the amylose levels in other starches are lower, ranging from roughly 18–20% in potato, 16–19% in cassava, about 28–33% in corn, and 30–32% in wheat starch [] and sago starch (15–30%) [], so it can be used to produce stronger and more durable films. Furthermore, arrowroot starch is valued for its excellent digestibility, has an anti-inflammatory effect and reduces chronic diseases []. Starch-based coatings derived from sago palm and arrowroot can serve as carriers of bioactive compounds naturally present in these plant species [,]. Their antimicrobial potency is highly dependent on variations in molecular structure and chemical composition, leading to substantial differences in effectiveness [,].
The aim of this study is to reduce the deterioration of shrimp quality during storage using the most environmentally and consumer-friendly methods. Sago and arrowroot starch films, modified with extracts from naturally antibacterial plant products, i.e., rooibos and garlic, were obtained. Selected structural and physicochemical properties of the obtained films were examined on the basis of ATR-FTIR, DSC, and SEM, determination of the contact angle, water vapor transmission rate, hardness, tensile strength, and elongation at break. Furthermore, the antibacterial activity of these modified starches was determined after coating fresh and blanched shrimp. The selection of these two types of starch and their individual modification with chemically diverse plant extracts was a deliberately adopted research strategy aimed at maximally differentiating the physicochemical and antimicrobial properties of the obtained materials. These starches, as mentioned above, differ in the content of amylose and amylopectin, which may affect their suitability as polymers forming edible coatings. In turn, the extracts used contain different groups of bioactive compounds—garlic extract contains mainly organosulfur compounds, while rooibos is a rich source of polyphenols. The selection of these combinations enabled the analysis of matrix–modifier interactions and the assessment of the functional efficiency of the obtained coatings in the context of their structure, mechanical properties and microbiological effectiveness.
2. Materials and Methods
2.1. Materials
Starch films were prepared using commercial sago starch (Müller’s Mühle GmbH, Gelsenkirchen-Schalke, Germany), arrowroot starch (Terrasana, Leimuiden, The Netherlands), glycerol and citric acid (Chempur, Piekary Śląskie, Poland), rooibos (William’s Manufacturing Poland Sp. z o.o., Radzymin, Poland), and regionally sourced garlic from Pomerania, all applied without further modification. Research material included raw (defrosted) (RS) and cooked (blanched) (CS) Litopenaeus vannamei shrimp, purchased from a local market (Ecuador fishing spot, area 87) immediately after delivery to the store. After purchase, the shrimp were placed in polystyrene boxes packed with ice and kept at 0–2 °C. They were delivered to the microbiology laboratory within 25 min, with the cold chain preserved throughout transport.
2.2. Preparation of Rooibos Extract
Rooibos leaves (5 g) were immersed in 100 mL of boiling distilled water for 5 min. The leaves were filtered off, and the solution was stored under refrigeration conditions and without access to light.
2.3. Preparation of Garlic Extract
To prepare the garlic extract, 100 g of garlic was peeled and then crushed in a mortar. The crushed garlic was placed in a 250 mL beaker, to which 100 mL of distilled water was added. The crushed garlic was macerated in water for 1 h at room temperature and then filtered. The solution was stored under refrigeration conditions and without access to light before being used to obtain starch films.
2.4. Starch Dispersions and Films Obtaining
Sago and arrowroot starch solutions were prepared, according to the method described previously [], by dispersing 5% w/w starch, 2% w/w glycerol as a plasticizer and 0.5% w/w citric acid as a crosslinking agent in a mixture of distilled water/plant extract (Table 1). Citric acid carboxyl groups can form hydrogen bonds with starch hydroxyl groups to prevent recrystallization and retrogradation. These solutions were then stirred at 85 °C for 45 min to induce gelatinization of starch. Some of the prepared solutions were left to cover the shrimp, and some were used to form films to test the physicochemical properties and structure by pouring them onto Teflon plates, evaporating the water at 35 °C for 24 h and conditioning in a desiccator at 50% humidity for 2 weeks.

Table 1.
Composition of gelatinized starch obtained with water and with plant extracts.
2.5. Methods
2.5.1. Fourier Transform Infrared Spectroscopy (ATR/FTIR)
Spectral data were obtained by Fourier transform infrared spectroscopy in ATR mode using a Nicolet 380 instrument (Thermo Scientific, Madison, WI, USA) fitted with a diamond ATR element. Measurements were performed at 4 cm−1 resolution across 600–4000 cm−1, with 32 scans averaged for each sample.
2.5.2. Scanning Electron Microscope (SEM)
The surface features of the samples were examined using a Quanta FEG 250 (FEI) scanning electron microscope equipped with an ET secondary electron detector. Before imaging, the samples were coated with a thin (10 nm) gold layer to prevent charging. Several areas of each specimen were imaged to ensure representative assessment of the surface. All measurements were performed at a constant accelerating voltage of 10 kV.
2.5.3. Differential Scanning Calorimetry (DSC)
The differential scanning calorimetry (DSC) measurement was performed under synthetic air atmosphere with a flow rate of 60 mL min−1 in the temperature range of 30–300 °C (with a heating rate of 10 °C min−1), and with 2–3.5 mg of sample, using a NETZSCH DSC 204 F1 Phoenix® calorimeter (NETZSCH-Gerätebau GmbH, Selb, Germany).
2.5.4. Contact Angle (CA)
Static water contact angles were measured at room temperature using a DataPhysics OCA 15EC goniometer (DataPhysics Instruments GmbH, Filderstadt, Germany). A 2 mL drop of distilled water was used for each measurement, and a photograph of the droplet was taken immediately after placing it on the sample surface. Additionally, the contact angle was determined at 1 and 3 min after droplet deposition. At least five measurements were made for each composite system.
2.5.5. Water Vapor Transmission Rate (WVTR)
Water vapor transmission was evaluated using a RADWAG MAX 60/1/NH moisture analyzer (RADWAG, Radom, Poland) equipped with a permeation probe. Each film sample was mounted between two sealing rings and positioned over a permeation cell containing a pre-weighed portion of distilled water (approximately 9 g). After assembling the cell, the unit was weighed and placed inside the analyzer chamber. The measurement chamber was maintained at 45 °C for a total of 2 h. Mass loss from the permeation cell was recorded with 0.1 mg accuracy and plotted against time. WVTR was calculated using Equation (1):
where
- WVTR—water vapor transmission rate (mg cm−2 h−1),
- W1—mass of water remaining in the permeation cell after the first hour (mg),
- W2—mass of water remaining after the second hour (mg),
- t—duration of the measurement interval (1 h),
- A—surface of evaporation (19.625 cm2).
The first hour of testing was used to stabilize the measurement chamber. Permeability values were considered reliable after one hour of conditioning at 45 °C.
2.5.6. Mechanical Properties
The thickness of each film was measured at five points using a Topex micrometer (±0.001 mm) before mechanical testing. Tensile strength and elongation at break were evaluated on a MultiTest 1-xt (Mecmesin, Slinfold, UK) tester with a 70 mm gauge length and a crosshead speed of 10 mm/min. Samples were prepared as 100 × 10 mm strips, and at least five were tested for each film formulation.
2.5.7. Sample Preparation for Microbiological Analysis
Upon delivery to the laboratory, portions of both RS and CS shrimp (approximately 60 g each, in four replicates) were prepared for microbiological analysis. The analyses were performed on day 0 (prior to storage) and after 2, 4, and 7 days of storage. The remaining shrimp samples, both raw and blanched, were subjected to a preservation method involving immersion in specific coating solutions (arrowroot starch, arrowroot starch with rooibos extract, sago starch and sago starch with garlic extract).
The solutions were distributed in sterile beakers in a laminar airflow chamber. Subsequently, the raw and blanched shrimp were immersed in the respective solutions using sterile tweezers. For each variant (RS and CS), four individual shrimp samples (approximately 60 g each) were treated in separate beakers. The shrimp were immersed for approximately 10 s to form a protective coating on their surface. Subsequently, they were placed in sterile Petri dishes and stored at 4 °C for up to 7 days.
For comparison, shrimp without treatment were also investigated as a control sample (U–Untreated).
2.5.8. Microbiological Testing
In a laminar airflow chamber, 20 g of each sample was taken and homogenized with 180 mL of Ringer’s solution using a Stomacher Lab-Blender 400 (Seward, Worthing, UK).
Microbiological analyses were performed according to international ISO standards. Total Aerobic Mesophilic bacteria (TAM) were enumerated according to PN-EN ISO 4833-1:2013/A1:2016 [] on Plate Count Agar at 30 °C for 72 h. Lactic Acid Bacteria (LAB) were determined using PN-ISO 15214:2002 [] on MRS agar incubated microaerophilically at 30 °C for 72 h. Staphylococcus aureus was counted according to PN-EN ISO 6888-2:2001/A1:2004 [] on Baird–Parker agar+RPF at 37 °C for 24–48 h. Escherichia coli was assessed following PN-ISO 16649-2:2004 [] using TBX agar incubated at 44 °C for 24 h. Results were expressed as log cfu/g.
Microbiological testing was conducted using serial dilutions up to 10−7. A 1 mL sample was pipetted into the bottom of a sterile Petri dish, followed by the addition of approximately 15 mL of molten and cooled agar medium. Microbiological analyses were performed immediately after sample delivery to the laboratory (0 day) and after 2, 4, and 7 days of storage. Each analysis was performed in two independent replicates.
Percentage reductions in bacterial counts were calculated from the logarithmic differences (Δlog cfu/g) between the control and coated samples using the following formula:
where Δlog10(N) = log10(Nc) − log10(Nt),
Reduction (%) = [1 − 10^(−Δlog10(N))] × 100
- Nc—the bacterial count in the uncoated control sample,
- Nt—the bacterial count in the coated sample.
This approach allows direct comparison between logarithmic and percentage data, commonly applied in antimicrobial efficacy studies.
2.5.9. Statistical Analysis
Statistical analyses were performed in two stages. For data on film properties, one-way analysis of variance (ANOVA) and Tukey’s post hoc test (Tukey HSD) were used to identify significant differences between starch variants and their modifications. Results are presented as means ± standard deviation, with homogeneous groups designated by index letters. Independent-samples t-tests were used to assess differences between shrimp type (RS vs. CS), coating type (U, SA, SA+R, SS, SS+G), and storage time (0, 2, 4, and 7 days). A uniform significance level of α = 0.05 was assumed for all analyses. ANOVA and Tukey’s t-test calculations were performed using the Real Statistics Resource Pack (2025) running in Microsoft Excel, while t-tests were performed in Statistica version 13 (StatSoft, Inc., Tulsa, OK, USA).
3. Results and Discussion
To determine selected structural and physicochemical properties, starches modified with rooibos and garlic solutions were formed into films. The thickness of the starch films was 0.20 and 0.19 mm for arrowroot and sago starch, respectively, which is suitable for edible films that serve as food packaging []. After starch modification with plant extracts, the thickness increased to 0.24 mm for starch film with rooibos extract and to 0.32 mm for garlic extract (Table 3). According to Hamed et al., edible films are usually thin sheets between 0.05 and 0.25 mm; however, as mentioned, this value may differ slightly from those given using other modifiers, and this is the case with the SS+G film [].
The resulting films were characterized by high transparency, which can enhance the consumer experience and the visibility of the product (Figure 1). At the same time, it also indicates the potentially low crystallinity of the obtained materials. The sago starch film was the most transparent. Raw shrimp have a slightly bluish tint, so the burgundy color of the rooibos-infused arrowroot starch film makes them look more attractive. Blanched shrimp, which are naturally pink, gain an even more intense color, further emphasizing their attractive appearance. Meanwhile, adding garlic extract to the sago starch caused the film to become slightly cloudy, but at the same time, it gave the shrimp covered with this coating a characteristic smell, pleasantly associated with the dish. The slight decrease in the transparency of this film may result from its slightly greater thickness than in the case of other films.

Figure 1.
Transparency of the arrowroot (SA) and sago (SS) starch films and their mixtures with rooibos (SA+R) and garlic extract (SS+G), respectively.
3.1. Chemical Structure
The FTIR spectra of arrowroot and sago starch plasticized with glycerol and citric acid indicate some changes in their structure after modification with plant extracts (Figure 2A).
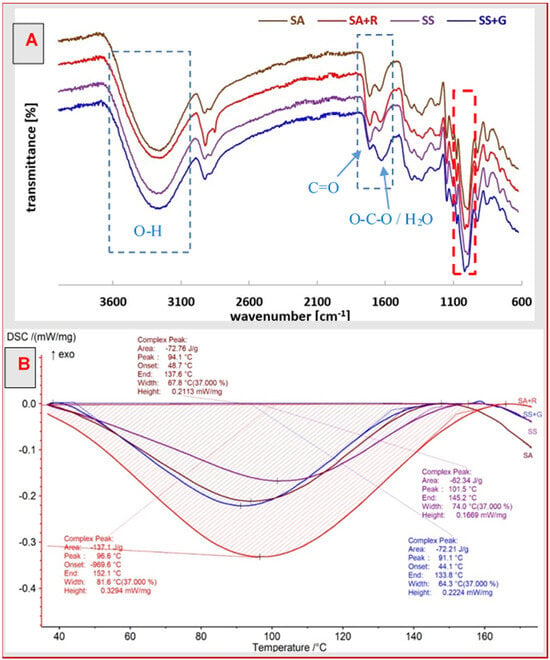
Figure 2.
FTIR spectra (A) and DSC thermograms (B) of the starch films.
The peaks with the maximum at about 2926 cm−1 and about 2888 cm−1 indicate the H-C stretching of the methyl and methylene groups, respectively, while the observed bands in the region of 1336 cm−1 are related to H-C bending vibrations []. Whereas the peak at 1716 cm−1 indicates the presence of carbonyl groups in citric acid used as a crosslinking agent for native starch. The peak at 1644 cm−1 in starch spectra corresponds to the stretching vibration of O-C-O but also to the bending vibration of the water molecule bundle in starch [,,]. After modification with plant extracts, it moves toward lower wavenumber values of 1636 cm−1 for SA+R and 1633 cm−1 for SS+G, and more importantly, its intensity increased significantly, especially in the case of SS+G. This suggests a high affinity of this film for water. The peak at 1150 cm−1 corresponds to C-O vibrations present in the C-O-H groups, while the peak at 1077 cm−1 corresponds to C-O stretching vibrations in the O-C-O groups.
According to Gu et al. [], the absorptions at 995 cm−1 and 1022 cm−1 (marked with a red frame in Figure 2A) are related to the crystalline and amorphous regions of starch, respectively. FTIR spectra indicate an increase in the intensity of the band at 995 cm−1 for films with plant extracts relative to the band at 1018 cm−1 (in our case), compared to native starch films. This suggests a reduction in starch crystallinity after modification, which may have a beneficial effect on the increase in elasticity and water vapor permeability of the samples.
In the so-called fingerprint region of starch, the band maxima at about 720 cm−1, 851 cm−1 and 923 cm−1 confirm the presence of the α-1,4-glycosidic bond (C-O-C) typical of native starch.
The large band with the maximum at approximately 3259–3282 cm−1 expressed the stretching vibrations of O-H contained in starch chains. Most of these groups are bound by hydrogen bonds formed between starch molecules. The decrease in the intensity of these bands was found by Sun et al. after destruction of the double helix structure of the crystalline regions by breaking hydrogen bonds after ball milling starch []. However, during drying in film preparation, starch retrograde occurs and new hydrogen bonds are formed, facilitating the formation of new crystalline regions. In this study, it was found that mixing the starch solution with rooibos and garlic extract in the film formation stage disturbed the entire process, which was observed by a decrease in the intensity of the peaks in the range of 3000–3700 cm−1.
3.2. Surface Structure and Hydrophilicity
The surfaces of both unmodified films exposed to air were compact, homogeneous, and had a uniform and slight roughness (Figure 3). Their appearance is comparable to the surface structure of arrowroot and sago starch films reported by other authors [,]. Modification with plant extracts resulted in the formation of additional circular protrusions (especially in the case of SA+R), which are visible in SEM images. Similarly, Wan Zullkiplee et al. observed an increase in film surface thickness, visible as spherical formations, after introducing anthocyanin solution into a sago starch matrix [].
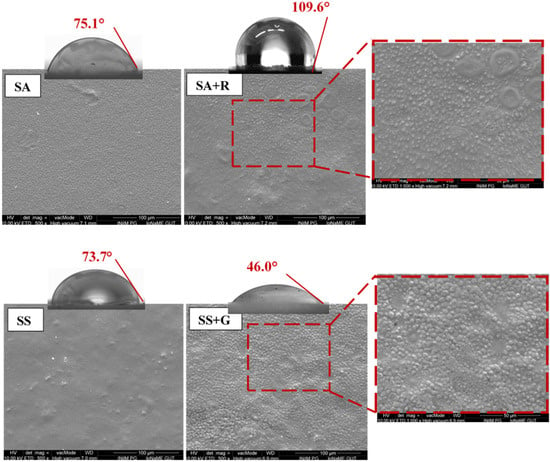
Figure 3.
SEM images of the surface of starch samples and their blends with rooibos and garlic extract, along with macroscopic images of water drops on their surface. More detailed image parameters are seen in Figure S1.
The increase in surface roughness in the case of adding rooibos extract to arrowroot starch caused an increase in contact angle. However, at the same time, it had no such effect on the wettability of the surface of the sago film modified with garlic extract. In this case, the different composition of this plant extract had a greater impact on the hydrophilicity of this material than the roughness of its surface.
The initial water contact angle of both starch films was similar and amounted to 75.1° and 73.7° for SA and SS, respectively (Table 2). It was comparable to the values obtained by other scientists []. This indicates the hydrophilic nature of the film surface. Furthermore, after 1 and 3 min from the application of the droplet to the sample surface, this angle decreased to a greater extent for the SA starch, which indicates its slightly higher wettability.

Table 2.
Contact angle and water vapor transmission rate of the starch films.
These analyses, including measurements of contact angle and water vapor transmission rate, confirm the impact of plant extracts on the properties of the film. The SS+G film exhibited a significantly lower contact angle (46.0°) compared to the SS film (73.7°), indicating increased hydrophilicity. According to the Tukey HSD post hoc test (α = 0.05), the SA and SS films did not differ significantly, forming one homogeneous group (a) (Table 2). In contrast, both modified films (SA+R and SS+G) showed statistically significant differences compared to the unmodified starch films and to each other, forming separate homogeneous groups (b and c). The results suggest that the incorporation of both plant extracts, especially garlic extract, enhances the affinity of the material for water, potentially influencing its functional applications. This is due to the better accessibility of hydroxyl groups, which is confirmed by FTIR results. The reduction in band intensity in the range of 3000–3700 cm−1 suggests less order in the starch structure. The increase in the hydrophilicity of the material also contributed to the increase in the water vapor transmission rate, which was 40.1 mg cm−2 h−1 for SS+G films, while it was lower for SS at 26.6 mg cm−2 h−1. The increased permeability suggests a more amorphous and loosely structured film, allowing water molecules to penetrate more easily. As noted by Hornung et al., phenolics derived from plant extracts may act as plasticizing agents that loosen the packing of starch chains, which enhances the diffusion of water molecules across the film matrix []. On the other hand, the increase in WVTR of the SA+R sample compared to SA indicates that the increase in contact angle does not necessarily indicate an increase in the hydrophobicity of this material but may result from an increase in surface roughness []. The surface roughness of SA+R is the highest among all films.
3.3. Mechanical Properties
Higher amylose levels in arrowroot starch resulted in films with nearly double the tensile strength of sago starch films, accompanied by a lower elongation at break, as expected (Table 3).

Table 3.
Thickness of starch film samples and their tensile strength and elongation at break.
The incorporation of plant-derived extracts (garlic or rooibos) significantly influenced the tensile strength and elongation at break of the films, which is consistent with the differences confirmed in the post hoc analysis. It is supposed that the degree of hydrogen bonding of the chains of both starches was not as high as expected, resulting in lower tensile strength values, but significantly increased the elasticity of the films obtained, compared to the film of arrowroot starch developed by Nogueira et al., which has 23.01 MPa and 1.53%, respectively []. However, this strength was comparable to the value (3.7 MPa) of the arrowroot starch film obtained by Abdillah et al. [] and 2.8 MPa of the sago starch film synthesized by [].
Paramitasai et al. noted that the introduction of hydroxypropyl groups to the starch molecules resulted in a more varied and branched structure []. This hydroxypropylation caused a shortening of the amylose chain and a disruption of its linear character, which in consequence led to a significant reduction in tensile strength while increasing the elasticity of the modified samples relative to native starches. In this study, the presence of compounds containing hydroxyl, carboxyl, sulfur groups, and other functional groups in the plant extracts used contributed to a similar effect.
3.4. Thermal Properties
Thermal analysis of starch film samples, both pure (SS and SA) and those infused with plant extracts (SS+G and SA+R), was carried out using differential scanning calorimetry (DSC), revealing notable differences in their thermal properties (Table 4).

Table 4.
The onset temperature (Tonset), the end temperature (Tend), the maximum peak temperature (Tpeak) and the enthalpy (ΔH) of phase transition of the starch samples.
The DSC curves of all the starch materials obtained have a similar pattern (Figure 2B). All of them show a wide endothermic phase transition, starting at a low temperature of about 40 °C and ending above 130 °C. The minimum of the endothermic peak is within the range of 90–101 °C, and the enthalpy of phase transformation, calculated from its surface, ranges from 62 J/g to 131 J/g. In this temperature range, both the melting of the crystalline fraction of starch and the evaporation of water trapped in its network are known to occur. Its width indicates the presence of starch crystallites of various sizes in the structure of these materials. Depending on the strength of the interaction of trapped water molecules with starch hydroxyl groups and components of both plant extracts, the water molecules gradually evaporate, which additionally induces crystallite melting [].
The SS film exhibited a peak temperature of 101.5 °C, whereas the SS+G film showed a lower peak at 91.1 °C. This slight drop in Tpeak suggests reduced thermal stability in the modified material, likely due to interactions between the bioactive compounds in garlic extract and the starch matrix. These findings are consistent with the FTIR analysis, where a change in the O-C-O band and the convulsed water band from 1644 cm−1 to 1633 cm−1 indicates structural changes. Specifically, this shift points to an increase in hydrogen bonding. Furthermore, the analysis of the transformation enthalpy showed its increase for the SS+G sample (72.21 J/g compared to 62.34 J/g for SS), which suggests the reorganization of the internal structure of the film under the influence of garlic extract, but theoretically it could also suggest an increase in the crystalline fraction. However, it should be taken into account that the enthalpy of transformation is strongly dependent on the water content in the starch network []. Generally, a higher water content corresponds to a higher enthalpy value. It should be remembered that the samples were stored under the same conditions (in a desiccator with 50% humidity), so the differences in the amount of water absorbed by the individual starch samples result from their individual predispositions and affinity for water.
A significant increase in the intensity of the peak at 1633 cm−1 in the FTIR spectrum of SS+G (Figure 2A) confirms the presence of a large amount of bound water. Since, as is shown below, this caused an increase in elasticity (the elongation at break for the SS+G film was 79.8%, while for SS it reached 56.0%) and a decrease in tensile strength, this negates the probability of the formation of a larger amount of crystalline phase. This suggests that the organic compounds contained in garlic extract disrupt the hydrogen bonds between starch molecules, stabilizing the crystallites, themselves creating new bonds with the hydroxyl groups of amylose and amylopectin. As a consequence, the number of crystallites decreased, and the temperature range of thermal transition on the DSC curves narrowed. Therefore, these compounds had a plasticizing effect on starch. This is also confirmed by the values of the contact angle and the water vapor transmission rate.
There were also changes in the thermal properties of sago starch after adding rooibos extract. The peak temperature for the SA film was recorded at 94.1 °C, while for SA+R, it shifted to 96.6 °C. This discreet rise in temperature indicates improved thermal stability in the modified film, likely due to interactions between the bioactive compounds in the rooibos extract and the starch matrix. Iaccheri et al. observed a similar effect after the introduction of pitanga leaf extract, which caused an increase in the thermal stability of the cassava starch film []. At the same time, the range of endotransition temperatures in the DSC curves has expanded significantly compared to starch itself. The significant increase in transition enthalpy for SA+R (137.1 J/g) compared to SA (72.76 J/g) cannot be clearly defined as causing an increase in crystallinity but rather suggests the evaporation of water molecules trapped in the starch matrix, as well as a structural reorganization within the film influenced by the rooibos extract. This effect is likely driven by interactions between polyphenols in the extract and hydroxyl groups of starch. This is supported by FTIR analysis, which revealed shifts in characteristic hydroxyl group bands, indicating a rearrangement of hydrogen bonds and an increased proportion of the amorphous phase in the modified film. Additionally, the transition bandwidth for SA was measured at 67.8 °C, while for SA+R, it expanded to 81.6 °C. This broader thermal transition range in the presence of rooibos extract suggests a more heterogeneous material structure. This observation aligns with SEM analysis, which revealed greater surface roughness and increased structural heterogeneity in SA+R films compared to SA. Consequently, the presence of such fine inhomogeneous crystallites, instead of larger ones with a slightly more uniform size and structure, hinders the transfer of energy during stretching, reducing the tensile strength of SA+R relative to SA (Table 3).
The results of the DSC analysis confirm that the addition of plant extracts affects the thermal properties of starch films through a plasticization effect, which was also observed in many previous studies. Nogueira et al. and Garcia et al. showed that the interactions of polyphenols with the starch matrix led to a change in the melting enthalpy and structural reorganization, increasing the amorphous nature of the material [,]. Pieros-Hernandez et al. [] noted a similar phenomenon for rosemary extract and Hornung et al. [] for yerba mate, where changes in DSC thermograms indicated a reduction in crystallinity and an increase in the mobility of polymer segments.
3.5. Microbiological Testing
After immersion in a solution of sago starch and its mixture with garlic extract, and in a solution of arrowroot starch, both raw and blanched shrimp did not change color. Whereas after immersion in a solution of arrowroot starch with rooibos extract, they acquired a slightly pink color (Figure 4A).

Figure 4.
Appearance of raw (RS) and blanched (CS) shrimp, starch coated and uncoated, before storage (A) and after 7 days of storage (B).
No significant changes in the overall color of the shrimp were observed during the 7-day storage period (Figure 4B). However, slight local darkening was noted in the head and tail regions, regardless of the type of coating applied.
The presence of E. coli was detected only in control shrimp samples, at levels of <1 log cfu/g on days 4 and 7 and 1.11 log cfu/g on day 2 of storage. And no E. coli was observed in shrimp coated with protective films.
However, significant differences in LAB and S. aureus counts were observed between raw shrimp and blanched shrimp (p < 0.001 and p = 0.007, respectively) (Table 5).

Table 5.
Mean bacterial counts (M ± SD, log cfu/g) of Lactic Acid Bacteria (LAB), Total Aerobic Mesophilic bacteria (TAM), and Staphylococcus aureus (S. aureus) in raw (RS) and blanched (CS) shrimp, uncoated and coated with different formulations (SA, SA+R, SS, and SS+G), under various storage conditions, along with statistical analysis.
The average LAB and S. aureus counts for raw shrimp were 1.41 log cfu/g and 0.27 log cfu/g, respectively, higher than in the case of blanched ones (Table 5). However, no significant differences were found in the TAM between RS and CS (p = 0.255), indicating that the overall microbial load was comparable in both types of shrimp. Regarding the effect of protective coatings, no significant differences in LAB counts were observed between the control samples and shrimp coated with SA, SA+R, SS, and SS+G (p > 0.05). However, in CS samples coated with SS+G, a 0.54 log cfu/g decrease in LAB counts was recorded compared to the control after 7 days of storage (Table S1). After 7 days, the antimicrobial activity of the coatings decreased, likely due to their gradual degradation and possible leaching of active compounds from the plant-derived additives. The protective coatings reduced LAB counts in shrimp more effectively up to day 4 of storage. At that time, the difference in LAB levels between coated and uncoated shrimp ranged from 0.12 to 0.78 log cfu/g for RS and from 0.48 to 0.84 log cfu/g for CS, depending on the type of coating. The highest reduction was observed for the SA+R coating in both RS (0.78 log cfu/g) and CS (0.84 log cfu/g). By day 7, the antimicrobial effect of the coatings diminished substantially and, in some cases, was no longer detectable. Notably, LAB levels in several coated samples (e.g., SA+R/RS: −0.02 log cfu/g; SA+R/CS: −0.34 log cfu/g) were slightly higher than in the uncoated controls, suggesting loss of antimicrobial activity over time.
For S. aureus, the SA+R coating significantly reduced its count compared to the control (p = 0.0027). After 4 days of storage, the difference in S. aureus counts between the control samples and RS and CS shrimp coated with SA+R was 0.65 log cfu/g on average, increasing to 1.1 log cfu/g after 7 days. The addition of garlic to sago starch also contributed to S. aureus reduction, regardless of whether the shrimp were raw or cooked. After 7 days of storage, the difference between the control and SS+G-coated samples averaged 0.9 log cfu/g. Similar antimicrobial effects of edible films and coatings, which protect food from spoilage and reduce the risk of pathogen growth, have been confirmed in previous studies [].
In the case of TAM, bacterial counts were comparable across all additive groups. During 7 days of storage, LAB counts remained stable (p = 0.8775), while TAM showed a significant increase, reaching 1.9 log cfu/g higher on day 7 compared to day 0 (p < 0.001; Table 5). After 4 days of shrimp storage, SS+G did not exhibit an antimicrobial effect on aerobic bacteria, whereas SA+R and SA coatings inhibited their growth. The difference between the control and shrimp coated with arrowroot starch-based film was approximately 0.4 log cfu/g. However, after 7 days, the trend changed—SS+G showed the greatest inhibition of bacterial growth (approximately 0.4 log cfu/g) relative to uncoated shrimp, while SA+R coatings reduced bacterial levels to a slightly lesser extent (about 0.26 log cfu/g). To improve the clarity of the antimicrobial evaluation, the results were additionally expressed as percentage reductions in bacterial counts and presented graphically (Figure 5).
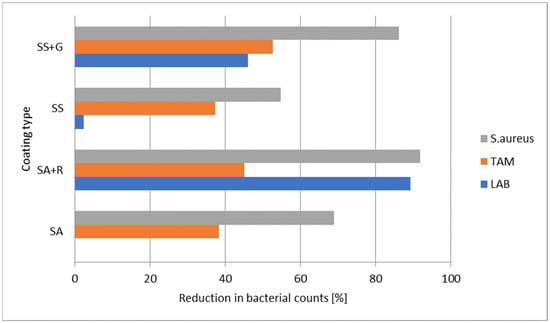
Figure 5.
Mean percentage reduction in bacterial counts (LAB, TAM, and S. aureus) after 7 days of refrigerated storage. Values represent means for raw (RS) and cooked (CS) shrimp samples compared to the uncoated control.
The percentage data provided a visual summary of coating performance, showing the average decrease in LAB, TAM, and S. aureus counts after 7 days of refrigerated storage compared to uncoated controls. Even relatively small logarithmic differences correspond to considerable percentage reductions due to the exponential nature of microbial growth. For example, 0.3 log, 0.6 log, and 1.0 log reductions are equivalent to approximately 50%, 75%, and 90% decreases in bacterial counts, respectively. Therefore, the percentage values illustrated in Figure 5 are consistent with the logarithmic data presented in Table 5. According to Endurocide [], a 1-log reduction corresponds to a 90% decrease in microbial counts, a 2-log reduction of 99%, and a 3-log reduction of 99.9%. The coatings demonstrated varying antimicrobial efficiency depending on the bacterial group. The SA+R coating (sago starch with rooibos extract) showed the highest overall reduction, with decreases of approximately 89% for LAB, 45% for TAM, and 92% for S. aureus. The SS+G coating (arrowroot starch with garlic extract) also exhibited strong antimicrobial properties, particularly against S. aureus (86% reduction). The S. aureus count was significantly lower in shrimp coated with SA+R compared to those treated with SA (p = 0.0022, t(22) = −3.47) and SS (p = 0.0043, t(22) = −3.18). This difference between the amount of S. aureus on the shrimp coated with SS+G and SA+R may be attributed to the surface characteristics of the SA+R, particularly the increased roughness observed in SEM imaging of starch mixtures with rooibos extract (Figure 3). Such surface roughness may promote microbial adhesion [] and retention of S. aureus, as reported by Bohinc et al., who demonstrated that this species adheres more readily to surfaces with increased available area []. However, it can be seen that this factor is only one of the parameters influencing the survival or growth of bacteria on starch-based coatings. It is clearly visible in the SEM images (Figure 3) that the surfaces of unmodified starch films (SS and SA) are less rough, yet bacteria developed on them to a greater extent than on shrimp coated with SA+R. Thus, the key effect here is the content of the starch coating enriched in the rooibos extract component. Although SS+G coatings showed the most hydrophilic character, which may lead to higher water activity in coated shrimp, creating more favorable conditions for bacterial growth, bacterial growth was the lowest []. This confirms the antibacterial activity of the components of the tested plant extracts.
Both starch types (sago and arrowroot) exhibited antimicrobial properties; however, the magnitude of their effects varied depending on the bacterial group, storage duration, and the plant extract incorporated. Therefore, their efficacy cannot be considered identical.
The reduction in E. coli and S. aureus counts may have been influenced not only by the properties of the coatings but also by LAB, which exhibit antagonistic activity against these pathogens. These bacteria compete with pathogenic microorganisms for nutrients and space while producing antimicrobial compounds such as bacteriocins, organic acids, and hydrogen peroxide, which inhibit pathogen growth. Furthermore, their presence may positively influence the microbiota of the product, contributing to improved safety and microbiological quality. The applied SA+R coating effectively inhibited S. aureus growth, enhancing the microbiological safety of preserved shrimp.
4. Conclusions
In this study, sago starch and arrowroot starch films modified with extracts from plants with antibacterial and health-promoting properties—garlic and rooibos—were obtained and tested. The films obtained were tested for their potential use as edible coatings for Litopenaeus vannamei shrimp to extend their shelf life. It was found that starch modification with plant extracts reduced the crystallinity of starch materials. This was determined based on the analysis of DSC, FTIR, and SEM results and the hydrophilicity of the obtained films. The decrease in the crystallinity of SA+R and SS+G compared to unmodified starches reduced their tensile strength to 1.5 MPa and 0.5 MPa, respectively, but caused an increase in elongation at break (to 67.4% and 79.9%) and increased the water vapor transmission rate (to 25.7 mg cm−2 h−1 and 40.1 mg cm−2 h−1). Most importantly, coating the shrimp with starch modified with rooibos and garlic extract reduced the development of pathogenic microorganisms, especially Staphylococcus aureus bacteria. With increasing environmental awareness and the search for methods to extend the shelf life of food, edible coatings appear to be a promising direction of development in the food industry. The findings of the study indicate that starch-based coatings enhanced with rooibos or garlic extract possess antimicrobial properties against various microorganisms, including lactic acid bacteria (LAB), Escherichia coli, Staphylococcus aureus, and total aerobic mesophilic bacteria counts (TAM). Coatings enriched with rooibos (SA+R) exhibited the most substantial decrease in microbial counts during the initial four days of storage. Conversely, garlic-based coatings (SS+G) presented a less intense but more prolonged effect, particularly in the reduction in total viable counts after seven days. These results imply that rooibos may be more efficacious for short-term microbial mitigation, while garlic might provide extended protection in refrigerated environments. Their utilization in the seafood industry has the potential to reduce product loss and decrease reliance on chemical preservatives. Considering their proven antimicrobial properties and ability to prolong shelf life, these coatings offer a promising alternative to traditional preservation methods within fish and seafood processing.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/su172310592/s1, Figure S1. SEM images of the surface of starch samples and their blends with rooibos and garlic extract, Table S1. Microbiological parameters of shrimp samples.
Author Contributions
Conceptualization, M.M. and J.B.; Methodology, M.M. and J.B.; Validation, M.M., J.B., J.K. and M.P.-W.; Investigation, M.M., J.B., A.K., J.K. and M.P.-W.; Resources, M.M.; Writing—original draft, M.M., J.B. and A.K.; Writing—review & editing, M.M., J.B., A.K., J.K. and M.P.-W.; Visualization, J.B.; Supervision, M.M. and J.B.; Funding acquisition, J.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by [The Ministry of Science under the Regional Initiative of Excellence Program] grant number [No RID/SP/0023/2024/01].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ATR-FTIR | Fourier transform infrared spectroscopy recorded with an attenuated total reflection mode |
| DSC | Differential Scanning Calorimetry |
| SEM | Scanning Electron Microscope |
| CA | Contact Angle |
| WVTR | Water Vapor Transmission Rate |
| SA | Starch Arrowroot |
| SS | Starch Sago |
| R | Rooibos |
| G | Garlic |
| CS | Cooked (blanched) Shrimp |
| RS | Raw (defrosted) Shrimp |
| U | Untreated Shrimp |
| TAM | Total Aerobic Mesophilic |
| LAB | Lactic Acid Bacteria |
References
- Ćurčić, S.; Milunović, S.; Savović, I.; Đurić, M. Logistics Information Support for Environmental Management for Organizations in the Food Chain. Int. J. Qual. Res. 2008, 2, 165–170. [Google Scholar]
- Yu, Q.; Liu, J.; Yang, J.; Lou, Y.; Li, Y.; Zhang, M. Postharvest Preservation Technologies for Marine-Capture Shrimp: A Review. Food Bioprocess Technol. 2023, 16, 2343–2358. [Google Scholar] [CrossRef]
- Aggarwal, S.; Kathuria, D.; Singh, N. Edible Films and Coatings, Its Chemical Crosslinking, Starch-Protein Interaction and Application in Food System: A Systematic Review. Int. J. Biol. Macromol. 2025, 306, 141726. [Google Scholar] [CrossRef]
- Djordjevic, M.Z.; Puskaric, H. Management of Process Safety in Food Chain. Int. J. Qual. Res. 2013, 7, 141–152. [Google Scholar]
- Valdés, A.; Ramos, M.; Beltrán, A.; Jiménez, A.; Garrigós, M. State of the Art of Antimicrobial Edible Coatings for Food Packaging Applications. Coatings 2017, 7, 56. [Google Scholar] [CrossRef]
- Kurek, M.; Pišonić, P.; Ščetar, M.; Janči, T.; Čanak, I.; Vidaček, S.F.; Benbettaieb, N.; Debeaufort, F.; Galič, K. Edible Coatings for Fish Preservation: Literature Data on Storage Temperature, Product Requirements, Antioxidant Activity, and Coating Performance—A Review. Antioxidants 2024, 13, 1417. [Google Scholar] [CrossRef] [PubMed]
- Liyanapathiranage, A.; Dassanayake, R.; Gamage, A.; Karri, R.; Manamperi, A.; Evon, P.; Merah, O. Recent Developments in Edible Films and Coatings for Fruits and Vegetables. Coatings 2023, 13, 1177. [Google Scholar] [CrossRef]
- Song, D.-H.; Hoa, V.; Kim, H.; Khang, S.; Cho, S.-H.; Ham, J.-S.; Seol, K.-H. Edible Films on Meat and Meat Products. Coatings 2021, 11, 1344. [Google Scholar] [CrossRef]
- Xie, F.; Mingzhu, M.L.; Feng, X.; He, Z.; Chen, Q.; Zhou, J. Tannic acid one-step induced quaternized chitin-based edible and easy-cleaning coatings with multifunctional preservation for perishable products. Food Hydrocoll. 2025, 159, 110636. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, C.; Liu, S.; Gao, J.; Cui, S.W.; Xia, W. Coating White Shrimp (Litopenaeus vannamei) with Edible Fully Deacetylated Chitosan Incorporated with Clove Essential Oil and Kojic Acid Improves Preservation during Cold Storage. Int. J. Biol. Macromol. 2020, 162, 1276–1282. [Google Scholar] [CrossRef]
- Wu, T.; Zhang, L.; Li, B. Chitosan-Based Edible Coatings for Food Preservation: A Review. Carbohydr. Polym. 2014, 103, 345–356. [Google Scholar] [CrossRef]
- Wang, J.; Qin, M.; Wang, W.; Xia, Y.; Wu, G.; Deng, H.; Lin, Q. Konjac Glucomannan Modification for Sustainable Functional Materials. Food Hydrocoll. 2025, 165, 111340. [Google Scholar] [CrossRef]
- Kim, J.-H.; Hong, W.-S.; Oh, S.-W. Effect of Layer-by-Layer Antimicrobial Edible Coating of Alginate and Chitosan with Grapefruit Seed Extract for Shelf-Life Extension of Shrimp (Litopenaeus vannamei) Stored at 4 °C. Int. J. Biol. Macromol. 2018, 120, 1468–1473. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.H.; Lee, S.-Y.; Oh, S.-W. Enhancing Safety and Quality of Shrimp by Nanoparticles of Sodium Alginate-Based Edible Coating Containing Grapefruit Seed Extract. Int. J. Biol. Macromol. 2021, 189, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Marta, H.; Chandra, S.; Cahyana, Y.; Sukri, N.; Pangawikan, A.D.; Yuliana, T.; Arifin, H.R. Arrowroot (Maranta arundinaceae L.) Starch-Based Edible Coating Formulation and Its Application to Shelf-Life Extension of Tomato (Solanum lycopersicum L.). Carbohydr. Polym. Technol. Appl. 2025, 9, 100674. [Google Scholar] [CrossRef]
- Devi, L.S.; Jaiswal, A.K.; Jaiswal, S. Lipid Incorporated Biopolymer-Based Edible Films and Coatings in Food Packaging: A Review. Curr. Res. Food Sci. 2024, 8, 100720. [Google Scholar] [CrossRef]
- Balti, R.; Mansour, M.B.; Zayoud, N.; Le Balc’h, R.; Brodu, N.; Arhaliass, A.; Massé, A. Active Exopolysaccharides-Based Edible Coatings Enriched with Red Seaweed (Gracilaria gracilis) Extract to Improve Shrimp Preservation during Refrigerated Storage. Food Biosci. 2020, 34, 100522. [Google Scholar] [CrossRef]
- Khazaei, N.; Esmaiili, M.; Emam-Djomeh, Z. Effect of Active Edible Coatings Made by Basil Seed Gum and Thymol on Oil Uptake and Oxidation in Shrimp during Deep-Fat Frying. Carbohydr. Polym. 2016, 137, 249–254. [Google Scholar] [CrossRef]
- Rossi-Márquez, G.; Dávalos-Saucedo, C.; Di Pierro, P. Edible Films and Coatings Applied in the Food Industry. Coatings 2023, 13, 670. [Google Scholar] [CrossRef]
- Agarwal, A.; Mirza, S.; Nimbalkar, T. Report: Sustainable Manufacturing Market—Global Forecast to 2029; MarketsandMarkets™: Delray Beach, FL, USA; London, UK, 2024. [Google Scholar]
- Abidin, M.Z.; Kourmentza, K.; Niranjan, K. Chitin Oligosaccharide N,N′-Diacetylchitobiose (GlcNAc2) as Antimicrobial Coating against Listeria monocytogenes on Ready-to-Eat Shrimp. Sustainability 2023, 15, 10099. [Google Scholar] [CrossRef]
- Afifi, M.R.; Ariaii, P.; Soltani, M.S.; Jafarian, S. The Effect of Nanocomposite Coating (Pullulan–Nano Clay) Activated with Nanoliposomes Containing the Watercress Essential Oil on the Quality of Pacific White Shrimp during Refrigerated Storage. J. Food Meas. Charact. 2023, 17, 2651–2662. [Google Scholar] [CrossRef]
- Rezaei, F.; Hosseinzadeh, S.; Basiri, S.; Golmakani, M.-T.; Gholamhosseini, A.; Shekarforoush, S.S. The Effects of Shirazi thyme (Zataria multiflora) Oil Nanoemulsion on the Quality of Shrimp (Litopenaeus vannamei) during Refrigerated Storage. J. Food Sci. Technol. 2023, 60, 710–719. [Google Scholar] [CrossRef]
- Hübsch, Z.; Van Vuuren, S.F.; Van Zyl, R.L. Can Rooibos (Aspalathus linearis) Tea Have an Effect on Conventional Antimicrobial Therapies? S. Afr. J. Bot. 2014, 93, 148–156. [Google Scholar] [CrossRef]
- Vhangani, L.N.; Favre, L.C.; Rolandelli, G.; Van Wyk, J.; del Pilar Buera, M. Optimising the Polyphenolic Content and Antioxidant Activity of Green Rooibos (Aspalathus linearis) Using Beta-Cyclodextrin Assisted Extraction. Molecules 2022, 27, 3556. [Google Scholar] [CrossRef]
- Santos, J.S.; Deolindo, C.T.P.; Esmerino, L.A.; Genovese, M.I.; Fujita, A.; Marques, M.B.; Rosso, N.D.; Daguer, H. Effects of Time and Extraction Temperature on Phenolic Composition and Functional Properties of Red Rooibos (Aspalathus linearis). Food Res. Int. 2016, 89, 476–487. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela-Gutiérrez, R.; Lago-Lestón, A.; Vargas-Albores, F.; Cicala, F.; Martínez-Porchas, M. Exploring the Garlic (Allium sativum) Properties for Fish Aquaculture. Fish Physiol. Biochem. 2021, 47, 1179–1198. [Google Scholar] [CrossRef]
- Magryś, A.; Olender, A.; Tchórzewska, D. Antibacterial Properties of Allium sativum L. against the Most Emerging Multidrug-Resistant Bacteria and Its Synergy with Antibiotics. Arch. Microbiol. 2021, 203, 2257–2268. [Google Scholar] [CrossRef] [PubMed]
- Nakamoto, M.; Kunimura, K.; Suzuki, J.-I.; Kodera, Y. Antimicrobial Properties of Hydrophobic Compounds in Garlic: Allicin, Vinyldithiin, Ajoene and Diallyl Polysulfides (Review). Exp. Ther. Med. 2020, 19, 1550–1553. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Xiao, D.; Zhu, C.; Younis, K.; Yousuf, O. Shelf-Life Extension and Quality Changes of Fresh-Cut Apple via Sago and Soy-Oil-Based Edible Coatings. Coatings 2024, 14, 1202. [Google Scholar] [CrossRef]
- Tarique, J.; Sapuan, S.M.; Khalina, A.; Ilyas, R.A.; Zainudin, E.S. Thermal, Flammability, and Antimicrobial Properties of Arrowroot (Maranta arundinacea) Fiber Reinforced Arrowroot Starch Biopolymer Composites for Food Packaging Applications. Int. J. Biol. Macromol. 2022, 213, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Paramitasari, D.; Musa, M.; Putra, O.N.; Suparman, S.; Pramana, Y.S.; Elisa, S.; Hidayat, T.; Tjahjono, A.E.; Meidiawati, D.P.; Pudjianto, K.; et al. Hydroxypropylation for Functional Enhancement of Sago Starch: The Effects of Low Propylene Oxide Concentration Using Response Surface Methodology. J. Agric. Food Res. 2024, 15, 100933. [Google Scholar] [CrossRef]
- Surendran, N.; Raju, M.V.; Chandrasekaran, M.K.; Ahalliya, R.M.; Palanisamy, C.P.; Chandrasekaran, G.; Kanniappan, G.V. Physicochemical Characterization of Starch from Maranta arundinacea L. (Arrowroot) Rhizomes and Its Inhibition of COX-2: In Vivo Validation. Bioact. Carbohydr. Diet. Fibre 2025, 33, 100465. [Google Scholar] [CrossRef]
- Duay, B.S.C.; De Leon, M.S.; Santos, A.C. Proximate Analysis of Maranta arundinacea L. Flour. Int. J. Multidiscip. Res. Dev. 2023, 2. [Google Scholar] [CrossRef]
- Taharuddin, N.H.; Jumaidin, R.; Ilyas, R.A.; Kamaruddin, Z.H.; Mansor, M.R.; Md Yusof, F.A.; Knight, V.F.; Norrrahim, M.N.F. Effect of Agar on the Mechanical, Thermal, and Moisture Absorption Properties of Thermoplastic Sago Starch Composites. Materials 2022, 15, 8954. [Google Scholar] [CrossRef]
- Nunes, N.B.; Reis, J.O.; Castro, V.S.; Machado, M.A.M.; Cunha-Neto, A.; Figueiredo, E.E. Optimizing the Antimicrobial Activity of Sodium Hypochlorite (NaClO) over Exposure Time for the Control of Salmonella spp. In Vitro. Antibiotics 2024, 13, 68. [Google Scholar] [CrossRef]
- Putri, T.R.; Adhitasari, A.; Paramita, V.; Yulianto, M.E.; Ariyanto, H.D. Effect of Different Starch on the Characteristics of Edible Film as Functional Packaging in Fresh Meat or Meat Products: A Review. Mater. Today Proc. 2023, 87, 192–199. [Google Scholar] [CrossRef]
- Morawska, M.; Kukułowicz, A.; Brzeska, J. Green Chemistry in Medical Applications: Preliminary Assessment of Kuzu Starch Films with Plant-Based Antiseptics. Sustainability 2023, 15, 16541. [Google Scholar] [CrossRef]
- PN-EN ISO 4833-1:2013/A1:2016; Microbiology of the Food Chain—Horizontal Method for the Enumeration of Microorganisms—Part 1: Colony Count at 30 °C by the Pour Plate Technique. Polish Committee for Standardization (PKN): Warsaw, Poland, 2016.
- PN-ISO 15214:2002; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Mesophilic Lactic acid Bacteria—Colony-Count Technique at 30 °C. Polish Committee for Standardization (PKN): Warsaw, Poland, 2002.
- PN-EN ISO 6888-2:2001/A1:2004; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Coagulase-Positive Staphylococci (Staphylococcus aureus and Other Species)—Part 2: Method Using Rabbit Plasma Fibrinogen Agar. Polish Committee for Standardization (PKN): Warsaw, Poland, 2004.
- PN-ISO 16649-2:2004; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of β-Glucuronidase-Positive Escherichia coli—Part 2: Colony-count technique at 44 °C Using 5-bromo-4-chloro-3-indolyl β-D-glucuronide. Polish Committee for Standardization (PKN): Warsaw, Poland, 2004.
- Abdillah, A.A.; Lee, R.-C.; Charles, A.L. Improving Physicomechanical Properties of Arrowroot Starch Films Incorporated with Kappa-Carrageenan: Sweet Cherry Coating Application. Int. J. Biol. Macromol. 2024, 277, 133938. [Google Scholar] [CrossRef] [PubMed]
- Hamed, I.; Jakobsen, A.N.; Lerfall, J. Sustainable Edible Packaging Systems Based on Active Compounds from Food Processing Byproducts: A Review. Compr. Rev. Food Sci. Food Saf. 2022, 21, 198–226. [Google Scholar] [CrossRef]
- Sondari, D.; Restu, W.K.; Septevani, A.A.; Suryaningrum, R.; Burhani, D.; Widyaningrum, B.A.; Putri, R. Effect of Catalyst and Cross-Linker Concentrations on the Functional and Chemical Properties of Sago Starch. Starch-Stärke 2022, 74, 2000266. [Google Scholar] [CrossRef]
- Shivaraju, V.K.; Appukuttan, S.V.; Kumar, S. The Influence of Bound Water on the FTIR Characteristics of Starch and Starch Nanocrystals Obtained from Selected Natural Sources. Starch-Stärke 2019, 71, 1700026. [Google Scholar] [CrossRef]
- Yin, Y.; Zhuang, Y.; Sun, L.; Gu, Y.; Zhang, G.; Fan, X.; Ding, Y. How does high hydrostatic pressure treatment improve the esterification of quinoa (Chenopodium quinoa Willd.) starch? Food Chem. 2025, 463, 141166. [Google Scholar] [CrossRef]
- Travalini, A.P.; Lamsal, B.; Magalhães, W.L.E.; Demiate, I.M. Cassava Starch Films Reinforced with Lignocellulose Nanofibers from Cassava Bagasse. Int. J. Biol. Macromol. 2019, 139, 1151–1161. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Xu, R.; McClements, D.J.; Liu, T.; Li, Q.; Su, G.; Zhao, M.; Zhao, Q. Effect of Preheating-Induced Structural Changes of Mung Bean Starch and Protein on the Phase Behavior, Physicochemical Properties, and Digestibility of Composite Hydrogels. Food Hydrocoll. 2025, 166, 111346. [Google Scholar] [CrossRef]
- Sun, C.; Du, K.; He, Z.; Zhu, Z.; Hu, Y.; Wang, C.; Mei, L.; Xie, Q.; Chen, Y.; Liu, Y.; et al. Liquid Nitrogen Ball-Milled Mechanochemical Modification of Starches with Typically Selected A, B and C Crystal Types on Multiscale Structure and Physicochemical Properties. Food Chem. 2025, 463, 141148. [Google Scholar] [CrossRef] [PubMed]
- Wan Zullkiplee, W.S.H.; Khairuddin, N.; Ramaiya, S.D.; Sarbini, S.R.; Ngaini, Z. Characterization and pH Response of Passiflora suberosa Extract as a Novel Biosensor Intended for Smart Food Packaging Film. J. Food Process Eng. 2025, 48, e70077. [Google Scholar] [CrossRef]
- Hornung, P.S.; Ávila, S.; Apea-Bah, F.B.; Liu, J.; Teixeira, G.L.; Ribani, R.H.; Beta, T. Sustainable Use of Ilex paraguariensis Waste in Improving Biodegradable Corn Starch Films’ Mechanical, Thermal and Bioactive Properties. J. Polym. Environ. 2020, 28, 1696–1709. [Google Scholar] [CrossRef]
- Brzeska, J.; Jasik, G.; Sikorska, W.; Mendrek, B.; Karczewski, K.; Kowalczuk, M.; Rutkowska, M. Susceptibility to Degradation in Soil of Branched Polyesterurethane Blends with Polylactide and Starch. Polymers 2022, 14, 2086. [Google Scholar] [CrossRef]
- Nogueira, G.F.; Fakhouri, F.M.; de Oliveira, R.A. Extraction and Characterization of Arrowroot (Maranta arundinacea L.) Starch and Its Application in Edible Films. Carbohydr. Polym. 2018, 186, 64–72. [Google Scholar] [CrossRef]
- Savita, K.; Kaur, J.; Kaur, N.; Arya, A.; Siwach, K.; Dhiman, R.; Sharma, P.K.; Sharma, A. Impact of Functionalised Silicon Carbide NPs on the Mechanical and Dielectric Performance of Sago Starch-Based Nanocomposites. Mater. Chem. Phys. 2025, 332, 130264. [Google Scholar] [CrossRef]
- Tananuwong, K.; Reid, D.S. DSC and NMR Relaxation Studies of Starch–Water Interactions during Gelatinization. Carbohydr. Polym. 2004, 58, 345–358. [Google Scholar] [CrossRef]
- Mano, J.F.; Koniarova, D.; Reis, R.L. Thermal Properties of Thermoplastic Starch/Synthetic Polymer Blends with Potential Biomedical Applicability. J. Mater. Sci. Mater. Med. 2003, 14, 127–135. [Google Scholar] [CrossRef]
- Iaccheri, E.; Siracusa, V.; Ragni, L.; De Aguiar Saldanha Pinheiro, A.C.; Romani, S.; Rocculi, P.; Rosa, M.D.; do Amaral Sobral, P.J. Studying Physical State of Films Based on Cassava Starch and/or Chitosan by Dielectric and Thermal Properties and Effects of Pitanga Leaf Hydroethanolic Extract. J. Food Eng. 2023, 339, 111280. [Google Scholar] [CrossRef]
- Nogueira, G.F.; Meneghetti, B.B.; Tagliari, I.H.B.S.; Soares, C.T.; Bevilaqua, G.; Fakhouri, F.M.; de Oliveira, R.A. Multipurpose Arrowroot Starch Films with Anthocyanin-Rich Grape Pomace Extract: Color Migration for Food Simulants and Monitoring the Freshness of Fish Meat. Int. J. Biol. Macromol. 2024, 265, 130934. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.A.V.T.; Garcia, C.F.; Gomes, F.A.A. Pharmaceutical and Biomedical Applications of Native and Modified Starch: A Review. Starch-Stärke 2020, 72, 1900270. [Google Scholar] [CrossRef]
- Piñeros-Hernandez, D.; Medina-Jaramillo, C.; López-Córdoba, A.; Goyanes, S. Edible Cassava Starch Films Carrying Rosemary Antioxidant Extracts for Potential Use as Active Food Packaging. Food Hydrocoll. 2017, 63, 488–495. [Google Scholar] [CrossRef]
- Endurocide. A Quick Guide to Log Reductions. Available online: https://endurocide.com/a-quick-guide-to-log-reductions/ (accessed on 13 November 2025).
- Guarechahi, M.; Moosavi, H.; Forghani, M. Effect of Surface Roughness and Materials Composition. J. Biomed. Nanotechnol. 2012, 3, 541. [Google Scholar] [CrossRef]
- Bohinc, K.; Dražić, G.; Fink, R.; Oder, M.; Jevšnik, M.; Nipič, D.; Godič-Torkar, K.; Raspor, P. Available Surface Dictates Microbial Adhesion Capacity. Int. J. Adhes. Adhes. 2014, 50, 265–272. [Google Scholar] [CrossRef]
- Syamaladevi, R.M.; Tang, J.; Zhong, Q.P. Water Diffusion from a Bacterial Cell in Low-Moisture Foods. J. Food Sci. 2016, 81, 2129–2134. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).