Abstract
Anaerobic digestion (AD) produces renewable energy but releases biogenic CO2 and generates digestate requiring management. This paper evaluates four emerging pathways for CO2 capture and reuse in AD systems: (1) in situ CO2 conversion to CH4 via microbial electrolysis cells (MECs), (2) hydrogenotrophic CO2 methanation using green hydrogen, (3) enzymatic CO2 capture coupled with autotrophic algae cultivation, and (4) digestate pyrolysis with syngas biomethanation. Each pathway is assessed in terms of technical feasibility, biocatalyst performance, system configuration, and key implementation challenges. Integrated scenarios demonstrate up to 98% CO2 emission reduction, substantial bioenergy yield improvements, and enhanced nutrient and biomass recovery compared to conventional AD. MEC-based and hydrogenotrophic pathways show the highest energy efficiency, while algae-based systems provide added bioproduct valorization. The remaining limitations include cost, process integration, and scale-up. The study defines development priorities to advance zero-emission AD technologies for the agri-food and waste management sectors.
1. Introduction
With growing global pressure for action on climate change, carbon capture has emerged as a crucial strategy in efforts to reduce greenhouse gas emissions and transition to a sustainable future. Carbon capture, utilization, and storage (CCUS) technologies are viewed as essential tools in achieving net-zero emissions targets and mitigating the environmental impact of industries, especially those that are hard to decarbonize, such as cement, steel, air, and marine transport and energy production. Carbon capture technologies can also be applied to biogenic carbon dioxide, such as that produced from anaerobic digestion (AD). Although biogenic CO2 is generally not counted as a net greenhouse gas emission, capturing and storing it can still help reduce atmospheric CO2 concentrations and contribute to carbon-negative outcomes []. In fact, capturing CO2 from biogenic sources is often more energy-efficient and cost-effective than from fossil sources, as it typically involves cleaner gas streams and lower temperatures. This makes point-source capture from AD facilities a promising pathway for climate mitigation, especially when compared to more energy-intensive approaches like direct air capture (DAC).
AD is a mature technology for the sustainable treatment of liquid and solid organic waste, as well as biofuel production. It has a wide range of potential feedstocks, such as high organic content wastewater, the organic fraction of solid organic waste, waste activated sludge, manure, agricultural residues, microalgae, and food waste. Furthermore, the process yields a nutrient-rich digestate, which can be valorized for nutrient recovery and reuse [,]. AD systems are highly adaptable, as they can be used locally for heating or combined heat and power production, or they can be upgraded to biomethane, which has similar properties to natural gas, allowing for direct injection into natural gas grids or use for transportation [,]. The versatility of feedstocks together with product flexibility and nutrient recycling make AD a promising technology for sustainable biofuel production, waste treatment, and circular economy []. However, digestate, an inevitable by-product of AD, requires further treatment, as its direct application to farmland is often restricted or disfavored in many regions due to a range of factors. These include regulatory constraints (notably the EU Fertilizing Products Regulation [] and the Nitrates Directive []), concerns over contaminants and pathogens (e.g., heavy metals, microplastics, and microbial risks), and practical limitations such as dilution requirements, storage logistics, and timing of application. Such limitations motivate the development of transferable and scalable digestate treatment and valorization routes to produce market-compliant bio-based fertilizers [,,]. More fundamentally, the nutrient load, especially nitrogen, is frequently excessive due to the remote origin of inputs (e.g., imported feedstock), which exacerbates issues in nitrogen-limited zones. Additionally, concerns persist over soil degradation, leaching of dissolved nutrients, and the presence of contaminants such as heavy metals, pharmaceuticals, and endocrine disruptors [,,]. Thus, despite being applied worldwide and being well-established technology with a technological readiness level (TRL) of nine, AD still faces a lot of challenges. Although the core AD process is commercially mature (TRL nine), the technology now faces second-generation challenges driven by sustainability requirements. These do not reflect inherent flaws in anaerobic digestion, but rather application-related constraints that limit its deployment in environmentally sensitive regions or high-circularity systems. In particular, biogenic CO2 emissions and digestate handling are bottlenecks in achieving negative-emission bioenergy, advanced nutrient valorization, and compliance with tightening environmental regulations.
The combination of the current geopolitical landscape and the ongoing energy crisis highlights the urgent need for stronger energy security and the adoption of climate-friendly energy sources, such as biogas and biomethane. Currently, while circularity remains a long-term goal for the AD sector, existing policies such as REPowerEU focus on rapidly increasing biomethane production, using all available feedstocks, including energy crops, rather than prioritizing a transition toward organic waste-based substrates, potentially limiting progress toward more sustainable and food-secure feedstock strategies []. Additionally, biomethane production is stimulated by biogas upgrading for grid injection and transportation fuel to reduce the dependency on natural gas. Technological advancements, such as improved digestion processes, integration with renewable systems, and the development of CCUS technologies, boost efficiency and environmental benefits. These developments align with circular economic principles, promoting effective waste management, greenhouse gas reduction, and resource recovery.
AD and carbon capture can be complementary processes that together may enhance the sustainability of waste management and bioenergy production. Capturing the CO2 from biogas upgrades its methane content. Beyond climate-mitigation benefits, the valorization of biogenic CO2 also provides an economic incentive, as CO2 of biological origin is generally recognized as climate-neutral and can generate higher-value carbon credits compared with fossil-derived CO2 under EU certification schemes. The Renewable Energy Directive (RED II and RED III revisions) explicitly promotes the displacement of fossil carbon in fuels by renewable, biogenic carbon sources and prioritizes negative-emission value chains where biogenic CO2 is permanently stored or chemically converted into long-lived products. This regulatory direction strengthens interest in CO2 capture and utilization from AD as a transferable pathway to enhance both environmental performance and revenue generation in the biogas sector.
While captured CO2 holds potential for use in various industrial applications, legal and regulatory constraints, especially concerning CO2 sourced from waste containing animal by-products [], currently limit its classification and utilization across the EU, complicating integration into circular-economy pathways [] and requiring case-by-case assessments of end-of-waste status [], product safety, and market placement []. Integrating carbon capture into AD systems can generate negative emissions by removing biogenic CO2, thereby enhancing their role in climate change mitigation. Moreover, the integration of carbon capture technologies in AD can improve economic feasibility and thereby enhance renewable energy production and waste reduction. Enhanced research and development can drive advancements in this field by addressing impurities and optimizing capture technologies, fostering a sustainable future.
In this context, this paper addresses four different technological approaches that render AD with zero or negative carbon dioxide emissions, as illustrated in Figure 1. Pathway 1 involves the integration of microbial electrolysis cell into AD system, for in situ conversion of CO2 and H2 in methane.
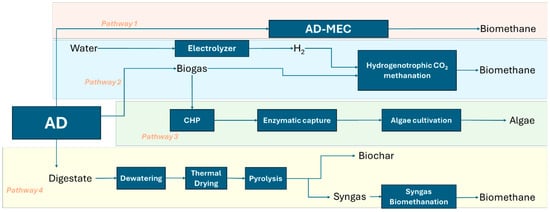
Figure 1.
Technological approaches towards sustainable anaerobic digestion.
Pathway 2 involves the upgrading of biogas by CO2 capture through biological methanation using green hydrogen, providing a sustainable conversion of electricity in biomethane.
Pathway 3 utilizes enzymatic capture of post-combustion CO2 from biogas combustion in a combined heat and power engine, subsequently using this CO2 in autotrophic algae cultivation.
Pathway 4 involves CO2 capture through digestate management by biochar production and syngas biomethanation.
The four pathways evaluated in this paper target performance bottlenecks that remain even in fully commercial TRL-9 AD installations and should be viewed as complementary system upgrades to existing AD infrastructure, aiming to improve carbon circularity and environmental performance, not as replacements for the core AD process.
The description of these four pathways will be based on a comprehensive, systematic, and critical literature review, analyzing recent advancements, applications, and challenges. Each of the pathways will be critically assessed, providing a comprehensive overview of how they can contribute to the sustainability of AD. Although preliminary techno-economic analysis studies are available in the literature [,], suggesting that intensified concepts have promising economic potential under favorable renewable electricity pricing and carbon valorization incentives, consolidated evaluation requires a separate investigation to enlighten their large-scale implementation. Detailed CAPEX, OPEX, and market-dependent revenue assessments require case-specific assumptions that extend beyond the purpose of this comparative technological evaluation. Therefore, a full techno-economic analysis is considered out of the scope of the present study and will be addressed in future work, together with the integration of updated economic indicators and policy frameworks relevant to biomethane production.
2. Technological Approaches Towards Sustainable AD
2.1. Anaerobic Digestion Combined with Microbial Electrolysis Cell Technology (Pathway 1)
2.1.1. Description
The integration of microbial electrolysis cells (MECs) into anaerobic digesters offers a promising avenue to overcome several key limitations associated with conventional AD [], as it is a technology that potentially attacks all limitations at once, i.e., it reduces the start-up times [,], enhances methane content [], stabilizes the methanogenesis phase [], and enhances AD in psychrophilic conditions [].
Microbial electrolysis cell (MEC) technology offers a potentially more energy-efficient alternative to conventional water electrolysis for hydrogen production. In MECs, electroactive microorganisms at the anode oxidize organic compounds, generating electrons that are subsequently transferred to the (bio)cathode. These electrons drive hydrogen generation through electrochemical or bio-electrochemical processes []. The integration of MECs in AD has been proposed for performance enhancement, process stabilization [], and CO2 capture [] (Figure 2).
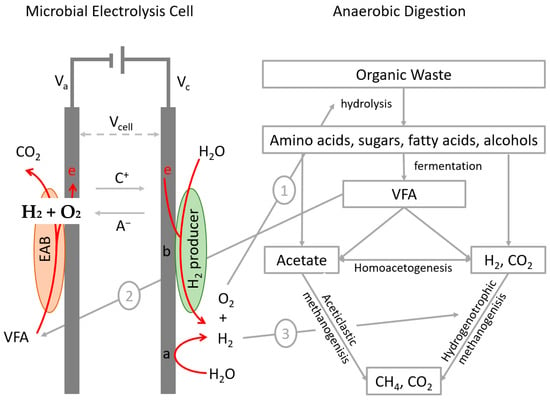
Figure 2.
Microbial electrolysis cells (MECs) with abiotic or biocathode configurations integrated into anaerobic digestion (AD). Synergies include (1) formation of micro-oxic zones enhancing hydrolysis, (2) consumption of volatile fatty acids (VFAs) by electroactive bacteria to improve AD stability, and (3) H2 production supporting hydrogenotrophic methanogenesis and increasing CH4 content.
It has been reported that the integration of MECs into AD may enhance the following critical limitations of AD:
Extended start-up time: Water electrolysis in the MEC generates oxygen [,] and therefore facilitates the presence of micro-anaerobic zones which could enhance the hydrolysis rate of organic matter and reduce start up time. Additionally, the presence of oxygen promotes the presence of facultative hydrolytic and acidogenic microorganisms which excrete extracellular enzymes such as amylase, protease, and lipase and therefore enhance the hydrolysis rate and reduce start-up time [,].
Process stability: The enrichment of hydrogenotrophic methanogens not only enhances the CH4 content but also promotes AD stability due to their faster growth compared to the other methanogens and a greater resilience towards environmental changes such as changes in pH, NH4+, and temperature [,]. In addition, hydrogenotrophic methanogens help stabilize pH levels by consuming protons, thereby counteracting the acidification effects of VFA buildup []. They also facilitate the breakdown of propionate and butyrate by lowering the partial pressure of hydrogen through its uptake, which makes these degradation pathways more thermodynamically favorable []. Moreover, the formation of micro-aerobic zones promotes the growth, metabolic activity, and diversity of rapidly proliferating facultative microorganisms capable of efficiently converting volatile fatty acids, such as propionic, butyric, valeric, and lactic acids, into acetic acid []. The enhanced degradation of these VFAs into acetic acid prevents VFA accumulation and therefore overcomes the thermodynamic constraints of syntrophic anaerobic oxidation of VFAs to acetic acid [].
Methane content: The generation of hydrogen by the MEC promotes the enrichment of hydrogenotrophic methanogens and therefore promotes methanogenesis via the hydrogenotrophic pathway which enhances the conversion of CO2 and hydrogen into CH4 []. Moreover, CH4 can be produced via alternative routes such as methanogenesis via direct electron transport by electrotrophic methanogens or via the generation of acetate by homoacetogens using the hydrogen produced by the MECs. The produced acetate is subsequently converted into methane by acetoclastic methanogens [].
Efficiency under psychrophilic conditions: Temperature plays a key role in the efficiency of AD, influencing both the rate of biogas generation and its composition. Biogas production is typically most efficient in the mesophilic range of 35–40 °C, while lower psychrophilic temperatures (below 20 °C) are known to considerably reduce or even halt production []. Despite this limitation, there is growing interest in the application of AD coupled with AD-MECs under low-temperature conditions. This interest is driven by the fact that many industrial effluents, such as those from malt production, breweries, soft drink manufacturing, and municipal wastewater, are discharged at relatively low temperatures. Furthermore, maintaining mesophilic (30–40 °C) or thermophilic (50–60 °C) conditions year-round leads to higher energy requirements, particularly in colder climates [,]. In this context, AD-MEC systems have emerged as a promising alternative, potentially reducing the need for external heating while improving digestive performance. Research has shown that such systems can enhance the specific activity of methanogenic microorganisms by a factor of five or six under psychrophilic conditions, contributing to more robust process performance [,]. A recent study by Sun et al. [] further demonstrated that AD-MEC systems operating at 17 °C achieved a 1.3-fold increase in chemical oxygen demand removal efficiency and higher methane yields compared to conventional AD processes at the same temperature. This is highly likely due to DIET (Direct Interspecies Electron Transfer) in AD-MEC systems, due to the direct contact between methane-producing archaea and electroactive microorganisms eliminating the necessity of multiple enzymatic steps in electron transfer. In addition, electroactive microorganisms are deemed less sensitive to temperature changes [].
2.1.2. Biocatalysts
The microbial consortium within an anaerobic digestion (AD) system plays a vital role in substrate breakdown and biogas generation. In microbial electrolysis cells (MECs), communities are typically dominated by Geobacter species. Recent findings have identified Geobacter as the most prevalent bacterial genus across MECs operating at varying voltages []. In one study, Geobacter sulfurreducens accounted for up to 72% of the microbial population and demonstrated the capacity to produce hydrogen []. Additionally, electrogenic microbes are known to enhance and expedite the breakdown of substrates []. The integration of AD with MECs (AD-MEC) has been shown to stimulate the proliferation of exoelectrogenic microorganisms, particularly Geobacter, thereby accelerating substrate conversion. These organisms are capable of anaerobically metabolizing diverse organic compounds, including volatile fatty acids (VFAs) and aromatic hydrocarbons []. The electrons resulting from oxidation of these compounds can be transferred either to electrodes or other microbial partners [].
Furthermore, AD-MEC systems promote the enrichment of other substrate-specific microbial groups. For instance, the genus Smithella, known for syntrophic propionate degradation in partnership with G. sulfurreducens, is significantly more prevalent in AD-MEC reactors (9 ± 2%) compared to open-circuit controls (1.2 ± 0.3%) []. Microorganisms such as Bifidobacterium and Clostridium, which facilitate the breakdown of complex organic matter into hydrogen and acetate, are also found in greater abundance in AD-MECs (38.2% and 21.1%, respectively), versus control systems (13.7% and 13.2%) []. Moreover, Clostridium species have the capability to reduce Fe(III) by transferring electrons generated from the metabolism of organic substrates to insoluble iron forms, highlighting their potential role in anodic electron donation and in further lowering chemical oxygen demand (COD) [].
The application of external voltage in AD-MEC processes therefore drives a shift in microbial structure, favoring the development of electroactive communities enriched in substrate-degrading organisms. In terms of archaeal populations, methanogenic archaea are predominant. Hydrogenotrophic methanogens such as Methanobacterium, Methanobrevibacter, and Methanospirillum, along with acetoclastic types like Methanosarcina and Methanosaeta, are commonly identified in these systems [,].
2.1.3. Configuration
The AD-MEC system typically comprises a bioanode, (bio)cathode, and AD. The MEC configuration can be either a single chamber or a dual chamber. In the single-chamber configuration, both the anode and cathode are positioned within the chamber, without physical separation, and share the same electrolyte. Conversely, the dual-chamber configuration involves the use of a membrane to physically separate the anode and cathode. The advantage of the dual chamber configuration lies in its ability to regulate microbial communities within the system and achieve more efficient conversion of electrons into H2, resulting in the production of pure H2 []. However, the dual chamber setup has associated drawbacks, primarily concerning the cost of the membrane and issues such as membrane fouling and the development of a pH gradient, which elevate the internal resistance of the system. On the other hand, single-chamber MECs have lower capital and operational costs, minimal pH gradient, and a compact structure, rendering them more promising for scalability []. Furthermore, the purity of H2 is inconsequential in an AD-MEC setup, where H2 is utilized to convert CO2 into CH4, making the single-chamber configuration well-suited for integration into AD-MEC systems.
Numerous AD-MEC integration approaches have been explored in the literature, generally classified into two main configurations: in situ and ex situ systems [,]. The in situ configuration, where the microbial electrolysis cell is embedded directly within the anaerobic digester, is widely studied for its potential in enhancing anaerobic digestion performance. In two-phase anaerobic digestion setups, where hydrolysis/acidogenesis is separated from methanogenesis, the MEC can be incorporated into either phase depending on the process objective []. The in situ AD-MEC arrangement typically promotes the activity of electroactive bacteria (EAB), electro-affinitive methanogenic archaea (EMA), and hydrogenotrophic methanogens. However, a known limitation of this approach is the simultaneous occurrence of oxidation and reduction reactions in the same vessel, which may lead to reduced energy efficiency [].
On the other hand, the ex situ setup positions the MEC downstream of the AD unit, functioning as an independent reactor. This configuration allows for further degradation of organic intermediates and improved energy recovery. For example, using pre-fermented urine as a substrate in an ex situ MEC has been shown to achieve enhanced current density, higher COD removal, and improved coulombic efficiency [].
The integration of MECs into two-phase AD systems has demonstrated synergistic improvements [,]. Zheng et al. [] reported that applying a 1.2 V voltage led to performance increases of 12.5% and 13.2% in the methanogenic and hydrolytic/acidogenic reactors, respectively. A more complex three-stage system, comprising acidogenic fermentation, methanogenesis, and MEC treatment, outperformed simpler two- and single-stage designs in terms of overall process efficiency [].
While most AD-MEC studies have been conducted in batch mode under constant voltage conditions, a few continuous-mode experiments also exist []. Interestingly, intermittent voltage application has been reported to improve energy efficiency without compromising methane production, thereby optimizing the net energy gain from the system [,,].
The arrangement of electrodes plays a critical role in determining the overall efficiency and performance of AD-MEC systems. Proper electrode configuration can lead to a reduction in internal resistance, thereby improving both methane production and energy recovery [,]. For example, minimizing the distance between electrodes has been shown to enhance methane output. Additionally, positioning the electrodes strategically, such as placing the anode at the reactor base and the cathode above, can offer hydrodynamic benefits and promote better system flow dynamics [].
System performance in AD-MECs is governed by a combination of factors including the enrichment of electrogenic microorganisms, reactor architecture, electrode spacing, and the nature of the input substrate. Nevertheless, a comprehensive understanding of how different electrode configurations impact mass transfer processes within the reactor remains an area of ongoing research and technical uncertainty [].
2.1.4. Challenges
The AD-MEC system presents a promising technology applicable to a wide range of feedstock and waste for bioenergy production. While AD-MECs show promise in bioenergy production, there are several challenges to be addressed, as presented in Table 1.

Table 1.
Key challenges and knowledge gaps for AD–MEC integration.
The current research predominantly focuses on laboratory-scale testing due to the low TRL of MEC technologies [], with limited studies conducted at a larger scale. To enhance the TRL of AD-MEC systems there are still significant knowledge gaps requiring further investigation.
Several critical questions remain unresolved, including (i) identifying the most efficient methanogenic pathway, acetoclastic versus hydrogenotrophic; (ii) understanding the mechanisms that enable the dominant pathway to outcompete the other; (iii) clarifying the exact electron transfer process between the cathode and hydrogenotrophic methanogens; (iv) quantifying the proportion of methane produced via direct cathodic electron transfer versus hydrogenotrophic routes; (v) determining the optimal anode-to-cathode specific surface area ratio; (vi) defining the most effective AD-MEC configuration to minimize internal resistance []; and (vii) exploring the utility of machine learning and artificial intelligence tools in predicting and enhancing AD-MEC performance []. Additionally, research investigating the economic feasibility of AD-MEC primarily relies on lab-scale experiments. To facilitate the practical application of AD-MEC, there is a need for experiments, life cycle assessments, and techno-economic analyses based on larger-scale implementations [,].
An additional challenge is the ionic composition and chemical characteristics of the digester broth, which may lead to metal deposition on electrodes, undesired redox reactions, or the formation of inhibitory metabolites. These phenomena could reduce electron transfer efficiency, impact electrode lifespan, and inhibit microbial activity, particularly in mixed-culture systems. Further research is needed to understand and mitigate these effects on both laboratory and pilot scales.
2.2. Hydrogenotrophic CO2 Methanation (Pathway 2)
2.2.1. Description
CO2 can be considered an attractive agent for the synthesis of high-value end products such as fuels or platform chemicals []. However, the inherent stability of the CO2 molecule poses challenges when it is used as the sole reactant, primarily due to its high energy demands. To overcome this, introducing a co-reactant with higher Gibbs free energy, such as hydrogen (H2), can improve the thermodynamic feasibility of the reaction []. A feasible approach to recycle CO2 involves biological methanation, often referred to as biomethanation []. Biological methanation relies on the activity of hydrogenotrophic methanogens, which metabolize CO2 into CH4 using H2 as an electron donor []. This reaction has negative Gibbs free energy, providing the necessary energy for the growth of hydrogenotrophic methanogens. For example, the study by Kakuk et al. [] demonstrated that when external H2 was injected into a mixed mesophilic community, the gene expression of hydrogen-metabolizing methanogens increased and the archaea involved in hydrogenotrophic methanogenesis were rapidly activated. The authors showed that provision of H2 shifts the microbial community toward hydrogenotrophic pathways by increasing the availability of the electron donor (H2), thereby favoring methanogens that specialize in H2/CO2 → CH4 over other pathways.
Alternatively, CO2 methanation can be conducted chemically (out of the scope of this paper), employing catalysts to convert CO2 to CH4 []. While chemical methanation is technically mature and widely applied, biological methanation has several notable advantages. These include operation at moderate temperatures (30–60 °C), potential flexibility with ambient or elevated pressures depending on H2 availability, tolerance to impurities in the feed gas, intermittent operation compatible with variable renewable H2 supply, and in situ production of biocatalysts, which reduces downtime and avoids costs associated with chemical catalyst replacement or deactivation (e.g., due to trace H2S or NH3). These features make biological methanation particularly suitable for integration with renewable energy systems [,]. Raw biogas is an attractive source for biological methanation due to its biogenic origin and relatively high CO2 content compared to other CO2 sources (e.g., combustion gases and ambient air). Biogas CO2 separation, however, incurs significant investment and operational operation []. To reduce these expenses, focus has shifted toward direct methanation of raw biogas without preliminary CO2 separation. While trace compounds and impurities present in biogas can inhibit microbial activity or reduce process efficiency [], some impurities, such as NH3 and H2S, can serve as substrates for ex situ methanation, partially offsetting feed requirements. This dual role can support the downsizing of purification efforts both technically and economically, particularly when green H2 is available at a reasonable cost.
The required H2 is not readily available, necessitating its generation first. When H2 is derived from water electrolysis powered by renewable sources, this process falls within the domain of the Power-to-Methane (PtM) sector []. H2 together with CO2 can be converted into CH4 through biological methanation. In this way, excess electricity can be converted into a stable form of chemical energy, i.e., CH4, taking advantage of periods of overproduction or low electricity demand []. In addition to the increase in methane content, biomethanation offers the advantage of utilizing waste heat generated during thermophilic AD or exothermic CO2 hydrogenation reactions. This recovered heat can support the maintenance of optimal operating temperatures or pre-heat incoming feedstock streams, which is particularly valuable for improving process stability and reducing heating costs in biogas plants located in colder climates.
Alternatively, H2 production can be achieved through a variety of material sources such as carbonaceous materials (e.g., biomass) and fossil fuels (fossil fuels or biofuels) by employing one or a combination of thermal, electrical, photonic, and biochemical technologies []. The most promising scheme for renewable H2 production, so-called green H2, involves utilizing surplus renewable energy to electrochemically split water into hydrogen and oxygen []. Water electrolysis can be performed using several technologies: alkaline electrolysis, proton exchange membrane (PEM) electrolysis, solid oxide electrolysis, and anion exchange membrane (AEM) electrolysis. These technologies differ in their maturity, efficiency, and operational characteristics, with AEM electrolysis representing an emerging alternative whose manufacturing is scaling up []. Table 2 summarizes the main characteristics of these technologies.

Table 2.
Summary of the main characteristics of the water electrolysis technologies [,].
2.2.2. Biocatalysts
Given the pronounced impact of CO2 and H2 concentrations on the biochemical equilibrium, the introduction of external H2 imposes strong selective pressure on the AD microbiota, resulting in a considerable rise in hydrogenotrophic species []. Previous studies suggest that the introduction of external H2 promotes the enrichment of methanogens, consequently leading to their predominance []. Nevertheless, these microbiomes typically harbor a considerable bacterial component, and further elucidation of bacterial species and their roles in both ex situ and in situ systems remains necessary. Bacterial diversity can vary strongly depending on the feeding substrate utilized and the presence of carbon sources other than CO2 [,]. Specifically, the presence of other carbon sources may serve specific metabolic reactions, e.g., catalyzing the conversion of CO2 to acetate, or may influence the production of intermediate compounds such as amino acids and formate []. Additionally, they may play an indirect role through biofilm formation, which is pertinent to the process since CO2 biomethanation is often mediated by microbiota adhered to solid supports [].
An investigation performed on CO2 biomethanation publications enabled the identification of trends regarding the presence of methanogens in terms of average relative abundance (~RA) based on operational parameters (for example, reactor type, temperature, and in situ or ex situ configuration) (only taxa above 0.5 RA were considered, Supplementary Table). The analysis focused on the ex situ configuration because, in the in situ setup, modifications induced by the feedstock composition on the microbiome are dominant and tend to obscure the effects of gas supplementation. The primary orders of hydrogenotrophic methanogens, Methanobacteriales and Methanomicrobiales, were identified as distinctive features of biomethanation systems, particularly in the ex situ configuration [,]. Unexpectedly, the Methanotrichales order, predominantly represented by acetoclastic Methanothrix species (~4% RA), was observed with a preference for mesophilic conditions (40 °C), most probably depending on homoacetogens for substrate provision []. Other taxa such as Methanolinea and Candidatus Methanosuratus were identified at low abundance (<1%) irrespective of operational parameters [].
The Methanobacteriales order, recognized for its hydrogenotrophic activity under both mesophilic (~44% RA) and thermophilic temperatures (~24% RA has seen an increasing number of its species identified and characterized in recent years [,,]. Above 50 °C, Methanothermobacter and Methanobacterium spp. are prevalent, whereas Methanobrevibacter sp. dominates under mesophilic conditions (~36% RA) [,,,]. The distribution of individual species across reactor configurations remains largely unexplored. Many individual species have been identified in specific reactor configurations; however, a comprehensive understanding of how microbial species are distributed across various systems remains limited. For example, Methanothermobacter thermautotrophicus has long been observed in ex situ biogas upgrade, demonstrating stable performance [,]. Recent studies have identified novel species of the genera Methanobacterium and Methanothermobacter as predominant in various reactor types, including TBR, CSTR, and batches, with RA ranging from 10% to 40% [,,]. Interestingly, Methanobacterium has shown no strict preference for specific reactor configurations, demonstrating resilience even in environments with elevated hydrogen sulfide concentrations or trace levels of oxygen [,].
Finally, the Methanomicrobiales order, primarily represented by Methanoculleus sp., typically maintains a lower RA (~9% RA) compared to the Methanobacteriales order, regardless of temperature []. Methanoculleus has been consistently identified across operational conditions, including high-salt and high-ammonia digestions, suggesting the adaptability to different stressors []. In particular, Methanoculleus bourgensis and Candidatus Methanoculleus thermohydrogenotrophicum are two of the most relevant species both in standard AD and in in situ biogas upgrade systems [,].
2.2.3. Configuration
CO2 biomethanation is a process that occurs naturally in an anaerobic digester as part of methanogenesis []. This step can be intensified by providing extra H2 and/or CO2 into the system, enabling the proliferation of hydrogenotrophic methanogens and thereby enhancing the conversion of CO2 to CH4, accomplishing biogas upgrading.
The bioreactor configurations in which biological hydrogen-assisted biogas upgrade occurs can be categorized into three types: in situ, ex situ, and hybrid designs [].
In situ configuration: In the in situ configuration, the H2 is directly introduced into the digester to enhance the hydrogenotrophic methanogenic community and thereby promoting the conversion of CO2 into CH4 []. This strategy can potentially be integrated into existing infrastructure as it requires only one reactor and thereby reduces investment costs []. Although the configuration can result in high methane yields reaching up to 99%, it significantly impacts the reactor’s stability, posing challenges in maintaining stable process performance []. The most important challenge is maintaining a stable pH, since CO2 consumption disrupts the carbonate equilibrium resulting in alkaline conditions with a pH that may exceed 8.5 [,]. Therefore, appropriate pH control strategies are required to ensure stable operation. Reported approaches include (i) dosing of weak acids (e.g., CO2 recirculation and organic acid addition) to partially restore the carbonate system and buffer capacity; (ii) optimization of H2 dosing rates and mass transfer to avoid excessive CO2 depletion; (iii) co-digestion with substrates generating sufficient alkalinity or VFAs to counterbalance pH rise; and (iv) process monitoring with automated control loops to enable real-time adjustment of H2 supply [,,,,]. The selection of a suitable pH regulation strategy depends on feedstock characteristics, buffer capacity, and hydraulic retention time, and remains a key aspect requiring further technological refinement.
Moreover, the introduction of H2 increases the partial H2 pressure in the digester, which makes volatile fatty acid (VFA) degradation thermodynamically less feasible, and can thereby lead to their accumulation []. The increase in VFAs has a direct impact on acidogenesis and methanogenesis, which may slow down [], affecting the overall AD process. From the above, it is evident that strict monitoring and control of the operational parameters are essential in such a setup.
Ex situ configuration: Ex situ biomethanation was introduced as a solution to the challenges posed by the in situ configuration. This process takes place in a separate bioreactor, where CO2 (obtained from sources like biogas or syngas) and H2 are introduced into its liquid phase. This reactor houses either a mixed or a pure culture of hydrogenotrophic methanogens which metabolize the gaseous substrates to CH4 []. Various types of reactors are used for ex situ biomethanation, each with its own design and operational characteristics suited to different scales and process requirements (Table 3). Ex situ technology is considered more expensive due to the need for extra equipment and materials, making it feasible primarily in full-scale plants []. Despite its configuration cost, the ex situ strategy is characterized by many important advantages such as the microbial community being simpler, reducing the apt of processing imbalances; the absence of organic matter degradation simplifies the whole process and there is flexibility in the sources from which CO2 can be obtained [,].

Table 3.
Reactor types, mass transfer coefficients, and methane productivity for gas fermentation.
Hybrid configuration: Hybrid biomethanation is a combination of in situ and ex situ biomethanation, forming an integrated system which aims to maximize the benefits of both methods. Initially, biogas generated in the in situ digester is recirculated with exogenous H2 for partial upgrading. Afterwards, the enriched biogas is introduced to the ex situ methanation reactor to further enhance its CH4 concentration to roughly 96% []. Hybrid technology provides an innovative solution by efficiently controlling pH levels throughout the in situ process while necessitating a much smaller dedicated reactor for the ex situ step [].
2.2.4. Challenges
Hydrogenotrophic CO2 methanation has several challenges that limit its widespread adoption. Challenges that need to be addressed are inter alia:
- The improvement of gas–liquid mass transfer, thereby promoting the availability of gases to microbes performing the conversion reaction.
- The intermittent availability of renewable H2.
- The establishment of a finely balanced microbial consortium within the anaerobic ecosystem.
- The increase in the competitiveness of methanogens compared to competing reactions (e.g., homoacetogenesis).
Finally, the integration of methanation with existing energy systems, such as power-to-gas or biogas upgrading, requires overcoming technical and economic barriers to ensure that the process is both efficient and cost-effective in real-world applications.
2.3. Enzymatic Capture of CO2 Coupled with Autotrophic Algae Cultivation (Pathway 3)
2.3.1. Description
Pathway 3 involves a novel approach to carbon capture and utilization (CCU) that integrates enzymatic CO2 capture with autotrophic algae cultivation. This pathway leverages the efficiency of enzymes to accelerate CO2 capture from the post-combustion flue gases of biogas, followed by the use of this captured CO2 as a carbon source for algae growth. Algae play a crucial role in capturing CO2 from the atmosphere, flue gases, or chemically bound forms such as soluble carbonates, converting it into organic matter via photosynthesis. The resulting microalgal biomass is rich in lipids, carbohydrates, proteins, and other high-value compounds, including pigments, minerals, and vitamins []. As such, it can be further utilized for various applications, such as biofuel, biomaterials, or biofertiliser production, as well as for phytopharmaceuticals for agriculture (biostimulants and biopesticides) or other active ingredients within regulatory frameworks [,].
Post-combustion capture, the most popular method removes CO2 from this gas mixture (most commonly flue gases) and is the only commercialized carbon capture technique [] due to its flexibility []. Post-combustion technologies are the only ones that can currently be integrated into existing power plants [,]. One major drawback of post-combustion CO2 capture is the low pressure and low CO2 concentration of flue gas, which is diluted with large amounts of nitrogen from air. This makes CO2 capture difficult and energy intensive []. After combustion, the flue gas discharged at atmospheric pressure (1 bar) and high temperatures (up to 200 °C) is predominantly nitrogen (up to 70% by volume), with a low percentage of CO2 (3% to 15% by volume), and high moisture. Several methods and processes for CO2 capture have been reported. These can be categorized into absorption processes (physical and chemical), gas–solid reaction processes, adsorption processes, cryogenic processes, membrane processes, and natural inclusion (biological methods) [,,].
Post-combustion CO2 capture technologies face several challenges, with one of the primary issues being the high operational cost associated with the energy-intensive desorption stage typically required to concentrate CO2 for storage or utilization. Beyond energy demands, a major obstacle to widespread adoption lies in the toxicity and corrosivity of the capture media, which can damage equipment and raise environmental concerns. These drawbacks can be mitigated by using green solvents or enzyme-based systems, which offer more sustainable and less hazardous alternatives. Importantly, desorption is not always necessary. In applications such as algae cultivation for carbon valorization, CO2 can be directly utilized in its dissolved form, eliminating the need for energy-intensive concentration steps. This makes algae-based systems an attractive and low-impact option for integrating carbon capture with resource recovery.
The overall cost of CO2 capture can be lowered by employing aqueous solvents that require less energy for desorption, particularly when combined with carbonic anhydrases (CAs), enzymes known for accelerating the absorption of CO2 [,]. The use of enzymes in carbon capture is a rapidly developing field, offering a biologically inspired and environmentally friendly solution to reduce CO2 emissions. Mimicking natural cellular CO2 processing, this enzymatic approach stands out for its high efficiency, thanks to its exceptional stereo-specificity and chemical selectivity, making it a promising method for sustainable CO2 transformation [].
CA can be utilized to accelerate two different processes: (1) CO2 capturing and concentration for industrial utilization, and (2) CO2 capturing and use for algal cultivation to enhance the efficiency of carbon capture and the productivity of algal biomass [,,]. In algae biomass production systems, CO2 availability can be a limiting factor for growth, as these systems are often designed for optimal light or nutrients utilization, rather than CO2 supply. The addition of CA into the culture enhances CO2 capture efficiency by catalyzing the hydration of CO2 at a remarkably high rate, thus improving carbon availability for photosynthesis in algae systems, resulting in increased algal growth rates and biomass productivity, particularly in high-density cultures [,] when nutrients are available in sufficient concentrations. The concept was patented in 1980s, based on the biomass yield increase from 0.25 g/L to 0.375 g/L in a Dunaliella salina pond after the addition of CA extract [].
Microalgae possess multiple CA isozymes, which can be found in various locations within and around the cell, including the chloroplast, cytosol, external periplasmic space or cell wall, and thylakoid lumen [,,]. These CAs belong to different genetic classes, including α, β, γ, δ, ε, η, and θ, and the specific types and locations can vary depending on the microalgal species []. CAs have been successfully isolated from many algae species, including easily maintained cultures of Chlorella vulgaris, Scenedesmus obliquus, Dunaliella tertiolecta, D. salina, Phaeodactylum, and many others [,,,]. Ores et al. [] investigated and characterized carbonic anhydrase (CA) production from various microalgae species. Their study included marine strains such as Tetraselmis suecica, Dunaliella tertiolecta, Isochrysis galbana, Phaeodactylum tricornutum, and Nannochloropsis oculata, as well as freshwater species like Chlorella vulgaris and Scenedesmus obliquus. Among these, C. vulgaris exhibited the highest enzyme yield, reaching an activity of 44.0 U/L. In the group of marine microalgae, P. tricornutum showed the greatest enzyme activity at 19.9 U/L. When specific activity was considered, D. tertiolecta and P. tricornutum recorded the highest values, at 44.6 U/g and 24.5 U/g, respectively.
An effective approach for extracting CA from microalgae involves the use of deep eutectic solvents (DESs). This method was successfully demonstrated on species such as Tisochrysis lutea, Chlorella vulgaris, and Spirulina sp. by Craveiro et al. []. Their findings suggest that DESs provide a suitable medium for CA extraction from microalgal biomass while maintaining enzyme activity, with specific CA activity reaching up to 0.70 mU mg−1. Additionally, the study revealed that the efficiency of CA extraction varies according to both the type of DES used and the microalgal species involved. This implies that the effectiveness of DESs in extracting CA is not uniform across all solvent–microalgae combinations. Different DES formulations may interact differently with the cell walls and intracellular components of various microalgal species, affecting the yield, purity, and activity of the extracted CA. Therefore, optimizing the choice of DES for each specific microalgal species is important to achieving high extraction efficiency while maintaining enzyme activity.
Approaches such as the immobilization of microalgae or extracted CA have been proposed to enhance enzyme stability and facilitate easier harvesting from algal cultures. Immobilization helps maintain enzyme activity over time by providing a more stable environment, reducing enzyme loss, and allowing for potential reuse in bioprocesses [].
2.3.2. Biocatalysts
Carbonic anhydrases (CAs, EC 4.2.1.1) constitute a group of metalloenzymes responsible for catalyzing the reversible hydration of carbon dioxide and the dehydration of bicarbonate. These enzymes influence only the reaction kinetics of both directions without altering the thermodynamic equilibrium []. The catalytic mechanism involves a nucleophilic attack by the Zn-bound hydroxide ion (Zn–OH−) on CO2. The process proceeds through a two-step, bidirectional mechanism as described by [,]:
EZnOH− + CO2 ⇌ EZn(OH−)CO2 ⇌ EZnHCO3− ⇌ EZnH2O + HCO3−
EZnH2O ⇌ H+ + EZnOH− + B ⇌ EZnOH− + BH+
Within the enzyme’s active site, CO2 binds to a hydrophobic region adjacent to the zinc ion, facilitating nucleophilic attack by Zn–OH−. This reaction yields bicarbonate (HCO3−), which is subsequently displaced by water molecules diffusing randomly into the active site [,]. The proton acceptor (B) is the chemical foundation for incorporating CA into CO2 separation through reactive absorption [].
CAs have attracted industrial interest as biocatalysts for the capture of carbon from the flue gases of coal-fired power plants [], and in the use of CAs to enhance algae growth for the capture of CO2 and its conversion to biofuels or other valuable products. CAs have become attractive for various industrial and medical applications due to their fast reaction kinetics (requiring smaller enzyme quantities), ease of expression, high solubility, and moderate heat resistance []. Additionally, CAs function as biomimetic CO2-sequestering agents, offering a cost-effective and reusable solution for carbon capture. As biological catalysts, they operate efficiently at ambient temperatures and mild experimental conditions, making them suitable for sustainable CO2 management [].
The stability of the enzyme depends on the enzyme source, and for CA harvesting to be economically viable, the enzyme must remain stable and functional over time [,]. Consequently, CAs exhibiting activity at elevated temperatures attract considerable attention [,,,]. Moreover, immobilizing CA in/on suitable supports or modifying the absorption process may help overcome process limitations such as enzyme instability, deactivation, loss during use, and difficult recovery, all of which can reduce the efficiency and cost effectiveness of the CO2 sequestration process [,]. The advantages of CA immobilization are increased enzyme stability, enzyme reusability, and easy enzyme recovery, leading to reduced process operation costs [,,].
The growing demand for industrial carbonic anhydrases (CAs) is reflected in the increasing number of patents and biotechnology companies focusing on this enzyme. These companies are actively developing enhanced versions of CA for diverse applications, including integration into engineered systems aimed at capturing CO2 directly from the atmosphere [,].
Despite their potential, the widespread industrial use of enzymes like CA is limited by their high cost and sensitivity to environmental conditions. To address these challenges, various strategies such as bench-scale modifications and enzyme immobilization have been pursued to reduce expenses while improving enzyme stability and activity. There is a pressing need to screen these methods and scale them for industrial implementation, alongside the development of simple, energy-efficient pathways for CO2 conversion leveraging affordable enzyme-based technologies [,].
In general, CAs exhibit compatibility with amine-based solvents and carbonates, where they can enhance CO2 absorption kinetics. However, controlling and characterizing the orientation and conformation of CA immobilized on solid surfaces remains complex due to intricate enzyme–surface interactions []. Future studies should also investigate the presence of industrial exhaust gases, including NOₓ, SOₓ, and other trace compounds, not only for their potential inhibitory effects on carbonic anhydrase activity [], but also for their impact on downstream valorization processes such as algae cultivation. These impurities may affect biomass composition and product quality, potentially constraining high-value applications, including nutritional or pharmaceutical products [,,].
2.3.3. Configurations
When designing reactors for enzymatic capture of CO2 using CA, several configurations can be employed to optimize the efficiency of CO2 capture, enzyme stability, and overall process economics. The design of reactors for this process must consider factors such as mass transfer, enzyme immobilization, and reactor scalability. Up until now, various reactor configurations have been suggested such as Packed Bed Reactor (PBR), Membrane Reactor, Stirred Tank Reactor (STR), Trickling Bed Reactor (TBR), Bubble Column Reactor, Fluidized Bed Reactor, and Monolithic Reactor. Each reactor configuration offers specific advantages and challenges depending on the application. Table 4 presents various reactor configurations for enzymatic CO2 capture using carbonic anhydrase.

Table 4.
Comparison of reactor configurations for enzymatic CO2 capture using carbonic anhydrase.
The selection of the appropriate reactor configuration depends on operational scale, desired CO2 capture performance, enzyme stability, and associated economic constraints. Current research is focused on enhancing enzyme immobilization, improving mass transfer efficiency, and reducing operational costs to enable large-scale deployment of enzymatic CO2 capture systems using carbonic anhydrase.
The CA extraction from algae and its activity in the extract depends on many factors, including the algae cell-disruption technique and extracting solvent, in combination with algae strains and conditions of operation (temperature, illumination, and CO2 concentration). Thus, each algal culture needs to be tested with a variety of extraction techniques and solvents, adjusted to the climatic conditions in the case of outdoor cultures. The final protocol of CA extraction and utilization will further depend on the energy and financial cost: for example, less energy-demanding techniques of cell disruption might be chosen in the large-scale systems, even at the cost of lower CA concentration in the extract. On the other hand, a CA solution might be preferred to immobilized enzymes to keep the efficiency of CO2 capture at a higher level, even if the enzyme cannot be recycled (but can be sustainably extracted at a low cost).
2.3.4. Challenges
Although enzyme immobilization is many times preferable because of enzyme stability and recycling possibilities, using CA in a liquid solution is preferable due to significant diffusion-related challenges. In immobilized systems, the reaction rate is often limited by the diffusion of CO2 through the boundary layer and the immobilizing matrix, such as alginate. These additional barriers slow the transport of CO2 to the enzyme, reducing the overall efficiency of the reaction. In contrast, when CA is in a liquid, it can interact directly with dissolved CO2, avoiding these diffusion bottlenecks. This leads to faster and more efficient conversion of CO2, as the enzyme continuously lowers the concentration of dissolved CO2, driving further gas absorption. Hence, using CA in liquid enhances reaction rates by minimizing diffusion limitations. Extracting sufficient amounts of enzyme from microalgae is a challenge due to variability between algal strains. As the extraction protocol should be adjusted for each strain or mixed culture, high biomass concentration significantly improves CA yield from the biomass []. This approach is especially interesting in large-scale production, where there is sufficient biomass production and at the same time the need for large amounts of the enzyme.
2.4. Digestate Management via Biochar Production and Syngas Upgrade (Pathway 4)
2.4.1. Description
Pathway 4 presents an alternative management scheme of digestate, since this is one of the key challenges of AD plants.
Prior to pyrolysis, digestate typically undergoes dewatering and thermal drying to reduce moisture content and improve process efficiency. Recent studies have demonstrated that these pretreatment steps significantly enhance the yield and quality of syngas, bio-oil, and biochar from digestate despite the energy cost [,,].
Pyrolysis of digestate has been suggested as an alternative for the valorization of digestate, apart from its use as organic fertilizer and soil conditioner. Among the products generated from pyrolysis, biochar finds use in various environmental applications; bio-oil serves as a potential fuel or additive in petroleum refining and syngas can be utilized for heat and power generation (combined heat and power, CHP) or further processed into biomethane [].
The biomethanation of syngas is an alternative carbon capture and utilization technological route that has attracted considerable attention in recent years [,]. This interest takes root in the possibility of unlocking the full conversion of recalcitrant biomass and its potential integration in current bioenergy systems. In this sense, this technology emerges as a cost-effective syngas-upgrading method, re-routing the use of biomass for conventional heat and power generation towards the synthesis of biomethane, a more versatile and energy-dense energy carrier.
The most distinctive merits of the combination of pyrolysis and the syngas biomethanation process lie in its hybrid nature as it integrates the strengths of both thermochemical and biochemical conversion technologies. On one hand, pyrolysis enables the complete conversion of the feedstock into a directly fermentable and homogeneous gas, bio-oil (which can be converted into biofuel) and biochar (which can be applied as a soil amendment that is considered carbon storage, circumventing the often slow and incomplete biological conversion of complex and recalcitrant biomass. On the other hand, the biological conversion of syngas into biomethane offers a number of advantages over its catalytic counterpart, such as mild operational pressure and temperature conditions, inexpensive biocatalysts as compared to traditional Ni-based and other catalysts, higher tolerance to syngas impurities, and very-high methane selectivity [,].
Beyond enhancing biomethane production from digestate conversion, this approach also aligns with the dynamics of the renewable electricity market. By integrating surplus renewable electricity via electrolysis-derived hydrogen, the system can optimize the electron donor/acceptor ratio (H2:CO2 = 4:1) for enhanced methane yield. This not only improves energy efficiency but also enables pyrolysis and gasification plants to act as flexible energy storage hubs, converting excess electricity into storable and transportable biomethane. Consequently, this hybrid scheme prevents grid overload and strengthens the role of biomethane as a versatile biofuel adaptable to fluctuating energy demands.
In addition to the primary components of syngas—hydrogen (H2), carbon monoxide (CO), carbon dioxide (CO2), and methane (CH4)—raw syngas generated via pyrolysis or gasification also contains various volatile compounds. These include acetylene (C2H2), ethylene (C2H4), ethane (C2H6), benzene (C6H6), hydrogen sulfide (H2S), sulfur oxides (SOx), ammonia (NH3), hydrogen cyanide (HCN), and carbonyl sulfide (COS). Certain impurities, such as tars and nitrogen-containing compounds, have been found to inhibit the activity of acetogenic bacteria, while the impact of sulfur gases varies depending on the specific bacterial and archaeal strains involved [,,,,]. More recent studies also proved a double-edged effect of tars on methanogenic microbial communities, where the addition of tars promoted methane production at low dosages and inhibited their activity as tar dosage increased []. More knowledge on the effect of raw syngas on these microbial communities is key for further advancing syngas biomethanation processes since the costs of syngas cleaning will likely have a major effect on the economic viability of the process []. To date, there is only one case study at a semi-pilot scale, where a gasification and a syngas biomethanation unit were connected online, showing encouraging results as the bioreactor presented no obvious signs of inhibition []. Nevertheless, further dedicated studies in this direction are still needed to establish the optimal syngas composition for this process.
2.4.2. Biocatalysts
Methanogenic archaea play a pivotal role in the conversion of gaseous substrates into methane in AD-MECs (pathway 1), biogas upgrading (pathway 2), and syngas biomethanation (pathway 4). The relevant methanogenic pathways used by these archaeal populations are in all cases analogous to the Wood–Ljungdahl pathway of gas-fermenting acetogens, where H2 or CO are used as electron donors for the reduction of CO2 into methane through multiple coenzyme-bound intermediates specific to methanogens such as methanofuran and tetrahydromethanopterin []. The highly diverse archaeal hydrogenotrophic microcosmos has been well documented in numerous studies on CO2 biomethanation, where Methanothermobacter spp., Methanobacterium spp., Methanobrevibacter spp., and Methanoculleus spp. were typically systematically enriched in multiple reactor settings and operational conditions. On the other hand, electrotrophic and carboxydotrophic archaea are still rather poorly characterized. To date, there are only a handful of methanogenic archaea reported to be able to utilize CO as the sole carbon and energy source, with these including Methanothermobacter marburgensis [], Methanothermobacter thermoautotrophicus [], Methanosarcina acetivorans [], and Methanosarcina barkeri [], among a few others. However, their role as carboxydotrophs in open microbial communities is typically deemed insignificant, owing to the faster activity rates of carboxydotrophic bacteria []. Similarly, acetoclastic methanogenesis constitutes an essential reaction in AD performed exclusively by the order Methanosarcinales [] that is often relegated to a marginal role in the above-mentioned biomethanation processes.
Besides the archaeal population, all these microbial communities typically exhibit a considerable bacterial segment often associated with a complex network of bacteria–archaea interactions (Figure 3A). In the case of syngas-converting microbial communities, the synthesis of methane as end-product involves a rather intricate interspecies metabolic network including the biological water–gas shift reaction, acetogenesis, hydrogenotrophic methanogenesis, syntrophic fatty acid oxidation, and acetoclastic methanogenesis (Figure 3). Pathway redundancy is a key feature of their metabolic network, which allows these communities to harness a wide range of cross-feeding, symbiotic, and mutual exclusion interactions, ultimately leading to high culture robustness and adaptive capacity []. In fact, the reactor temperature has been shown to drive drastic changes in the microbial composition and pathways used by these microbial communities, with these having major implications in the biomethane productivity of the bioreactors [,,]
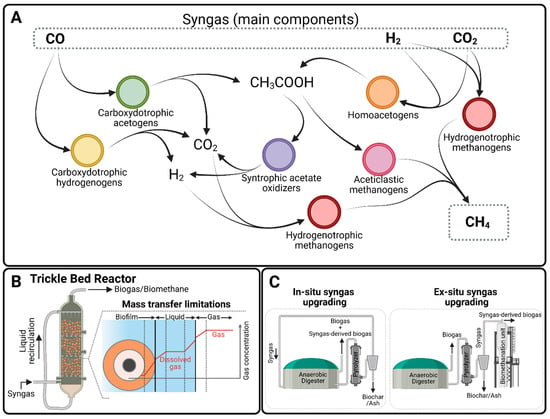
Figure 3.
Relevant features of the syngas biomethanation process. (A). Simplified interspecies metabolic network converting syngas into methane. (B) Reactor schematic of a typical trickle bed reactor used in syngas biomethanation and description of mass transfer limitations including gas–liquid and liquid–solid interphases. (C) Possible simplified process schemes for upgrading syngas into biogas or biomethane.
2.4.3. Configurations
The main challenges to achieving compact bioreactors with high volumetric productivity in gas fermentation processes are mass transfer and growth limitations. Both syngas biomethanation and biogas upgrading involve relatively insoluble gases, H2 and CO. This leaves the microbes involved in the process having to scavenge H2 and CO at very low concentrations from the liquid phase, limiting their capacity to convert them. Mass transfer from the gas phase to the liquid phase from which H2 and CO can be taken up by microbes is thus considered one of the most important bottlenecks in the process (Figure 3B). However, recent findings also indicate that diffusion through the biofilm may be the rate-limiting step in attached-growth bioreactors typically employed in syngas biomethanation (Figure 3B) []. Additionally, the strictly anaerobic nature of the microorganisms involved means that the energy available from their metabolic processes is limited. This typically leads to low biomass yields and low cell concentrations in reactors, limiting volumetric productivity.
At process-level configurations, the syngas biomethanation process is analogous to the CO2-hydrogenation process (pathway 2) and could be operated under in situ, ex situ, and hybrid production schemes when coupled with AD systems (Figure 3C) [,,]. At the bioreactor level, a variety of bioreactor configurations has been studied for gas fermentation: continuously stirred tank reactors, bubble column reactors, hollow fiber membrane reactors, and TBRs [,,,,]. The main strategies applied to overcome mass transfer limitations have included increasing the mass transfer driving force (often by increasing the operating pressure) and increasing the volumetric mass transfer coefficient. A variety of strategies have been applied to increase the concentration of cells in the reactor, including long hydraulic retention times, sludge recirculation, and the immobilization of biomass on packing material []. Table 3 shows a comparison of reactor types, mass transfer coefficients and methane productivity. It is worth noting that the CH4 production rates presented in the table are expressed per day (L CH4 L−1 d−1) and mostly represent laboratory-scale systems, which generally exhibit lower productivities (≤10 L CH4 L−1 d−1) compared to state-of-the-art H2/CO2 biomethanation systems that can achieve hourly productivities exceeding 10 NL CH4 L−1 h−1 under optimized conditions [,].
TBRs stand out from Table 3 as the most competitive reactor configuration, although bubble columns also perform well. They are columns filled with high specific surface area packing material, over which a liquid medium is trickled, but where most of the reactor volume is filled with gas (Figure 3B). Packing material breaks the fast movement of the gas and facilitates its contact with the liquid phase, increasing the volumetric mass transfer coefficient. The movement of the liquid is the other main driver of gas–liquid mass transfer. The packing material in TBRs immobilizes biomass in the form of a biofilm, making the system much less vulnerable to wash-out and allowing operation at shorter hydraulic retention times []. TBRs can be operated with gas flowing co-current or counter-current to the liquid. The co-current mode of operation is generally favored, as it prevents flooding of the bottom of the reactor and the breakthrough of unconverted gas [,]. A disadvantage particular to TBRs is a phenomenon known as channeling. Liquid medium follows preferential pathways through the packing material, resulting in unequal growth of the biofilm in the packing material []. The current proposed strategy to overcome channeling is to increase the liquid recirculation rate []. Future research efforts are required in this aspect of reactor design and operation to fulfill the need for more compact reactor designs that improve the productivity of these systems in a cost-effective manner.
2.4.4. Challenges
Syngas biomethanation faces several challenges that affect its viability and efficiency. One of the key challenges is the optimization of microbial communities to efficiently convert the raw syngas into methane. The toxicity of syngas components, particularly CO and tars, can inhibit microbial activity, especially in mixed culture setups; however, pure culture ex situ biomethanation approaches may offer improved tolerance and more stable methane yields under controlled conditions [,,]. Maintaining the stability and robustness of the microbial community under varying syngas compositions and operational conditions is another significant challenge. Mass transfer limitations also pose difficulties in syngas biomethanation. The solubility of syngas components in the liquid phase is low, which can limit the availability of these gases to the microbes and thus reduce the overall reaction rate. Reactor design plays a critical role in addressing these limitations, but optimizing reactor configurations for effective gas–liquid mass transfer while maintaining an environment conducive to microbial growth remains challenging. Furthermore, the scale-up of the syngas biomethanation processes from laboratory to industrial levels presents challenges related to maintaining consistent performance and ensuring economic feasibility. Integrating syngas biomethanation with existing industrial processes, such as waste-to-energy systems, also requires overcoming technical and economic hurdles to make the process competitive and sustainable in real-world applications.
3. Integrated Systems for Zero Emissions AD Plants
A critical challenge in large-scale anaerobic digestion (AD) facilities is reducing CO2 emissions while maintaining energy efficiency. Therefore, it is essential to develop integrated solutions that simultaneously address technical, economic, and environmental sustainability. In this regard, advancing innovative carbon capture and utilization (CCU) technologies to meet commercial viability is a key priority. Current popular strategies focus on combining bioenergy generation with the recovery of bio-based materials. Technology itself is not the sole challenge; rather, the integration of the most advanced methods into a cohesive valorization system is fundamental. Equally important is the close collaboration among all stakeholders across the supply chain, including farmers, AD operators, researchers, engineers, and policymakers.
Aligned with circular economy principles, three scenarios were explored and compared to a baseline to enhance the sustainability of AD processes. These scenarios focus on minimizing CO2 emissions and reusing them within the process to maximize both energy and material efficiency. Based on the extensive literature review and assuming the deployment of proven technologies, approximate mass balances were calculated using moderate assumptions. The proposed integrated approach aims to close the loop in AD by optimizing biomethane production and digestate management within the agri-food supply chain. Additionally, it supports the creation of new sustainable value chains that yield high-quality energy and bio-based products.
Since most anaerobic digesters in Europe are within the small-to-medium scale, with capacities between 500 to 2000 m3 and installed power ranging from 150 kW to 1 MW, a plant of 1 MW has been selected as the baseline scenario []. This plant was assumed to treat 120 tn/d organic waste producing 4500 m3/d biogas (55% CH4 and 45% CO2) and 110 tn/d digestate (60% liquid fraction and 40% solid fraction).
The three scenarios refer to the combination of the implementation of pathways 1, 2, and 3 with pathway 4, respectively.
Regarding the common digestate treatment, in all scenarios, the liquid fraction is recycled as a nutrient source within the bioprocesses. For the solid digestate fraction, its management includes pyrolysis and syngas upgrade, and the mass balances are presented in Table 5, assuming that the solids content of digestate is 25–35% []. As far as the pyrolysis of digestate is concerned, the temperature was considered as 500 °C, biochar yield 45%, pyrolysis oil yield 36%, and syngas yield 19% [,,,]. A typical syngas composition was assumed (22% H2, 17% CO, 52% CO2, 7%CH4, 1% C2H4, and 1% C2H6) [,,]. Exogenous H2 addition was assumed based on stoichiometric needs.

Table 5.
Mass balances of baseline and scenarios studied.
More specifically, scenario 1 includes an AD-MEC system where the digestate is treated via pyrolysis and syngas upgrade. The AD-MEC system could enhance biomethane production by 75% and reduce CO2 by 90% compared to the baseline scenario []. In scenario 2, the biogas produced from the conventional system is treated in a biomethanation reactor by adding green hydrogen applying Power2Gas technology and the resulting digestate from the AD is treated via pyrolysis and syngas upgrade. Assuming the stoichiometric requirements for hydrogen addition and 95% efficiency in the biomethanation process (in terms of CO2 conversion), the respective mass balances were estimated and are presented in Table 5. In scenario 3, the biogas produced is burned in a CHP engine with an overall efficiency of 83% (38% electric and 45% thermal) [,], while the digestate is managed via pyrolysis and syngas upgrade. The flue gases from the CHP engine were estimated, assuming 100% excess air input and complete CH4 combustion [] and they are led into a scrubbing system for CO2 dissolution (85% dissolution efficiency) []. The dissolved CO2 stream was used as a carbon source for algae cultivation, assuming 80% efficient carbon use []. The main mass balances were estimated and presented in Table 5 for the operation of one day.
To evaluate the sustainability and efficiency of the proposed valorization scenarios, four key indices were calculated (Table 6). The first index, CO2 emissions (S/I), quantifies the amount of carbon dioxide released into the atmosphere relative to the input feedstock, providing insight into the environmental burden of each process. The second index, CO2 captured/utilized (CC), measures the proportion of carbon dioxide that is either sequestered or converted into useful products, thereby reflecting the system’s contribution to carbon circularity and mitigation. The third index, bioenergy efficiency (%BE), evaluates how effectively the bioenergy is extracted from the feedstock, taking into account the lower heating values (LHVs) of methane, hydrogen, and organic waste as energy carriers. This index helps assess the conversion performance from a thermodynamic perspective. Finally, the biomass resource utilization (%BR) index examines how efficiently the biomass is converted into both energy products (e.g., biofuels) and material products (e.g., biochar, pyrolysis oil, and algal biomass). This broader index captures the holistic resource valorization of the system.

Table 6.
Reference values and equations for assessment of scenarios’ indicators.
Based on the mass balances, the calculated indices are presented in Table 7. Notably, the carbon sequestered in biochar is excluded from the index calculations to maintain focus on the gaseous CO2 fraction dynamics, which are central to this study.

Table 7.
Indicators for all valorization scenarios (baseline: conventional AD, scenario 1: AD-MEC system with digestate treatment via pyrolysis and syngas upgrading, scenario 2: conventional AD with Power-to-Gas biomethanation using green hydrogen and digestate treated via pyrolysis and syngas upgrading, scenario 3: conventional AD with biogas used in CHP engine; flue gas CO2 captured for algae cultivation. Digestate managed via pyrolysis and syngas upgrading).
The adoption of advanced configurations significantly reduces CO2 emissions. It should be noted that the CO2 emissions reported in Table 7 correspond to direct process emissions. Upstream emissions associated with the production of green hydrogen in scenario 2 are not included in this analysis. Incorporating such life cycle emissions would require a dedicated LCA study, which is beyond the scope of the present review. The integrated scenario analysis examines pathways to enhance bio-based CH4 production through targeted configurations that aim to achieve zero-emission biofuels. This investigation incorporates advanced resource utilization and carbon management strategies. The benchmark for this assessment is the performance of conventional AD systems, which emit 2.25 t of CO2 for every t of biofuel. By employing advanced technologies, emissions from these systems can be reduced to as low as 0.05 t of CO2 per t of biofuel produced, as evidenced in scenario 2. Scenario 1 follows closely with 0.10 t CO2 per t of biofuel, while scenario 3 attains a moderate reduction of 0.47 t CO2 per t of biofuel. These findings highlight the effectiveness of integrating hydrogenotrophic CO2 methanation (scenario 2) and AD-MECs (scenario 1) in biofuel enhancement (Figure 4).
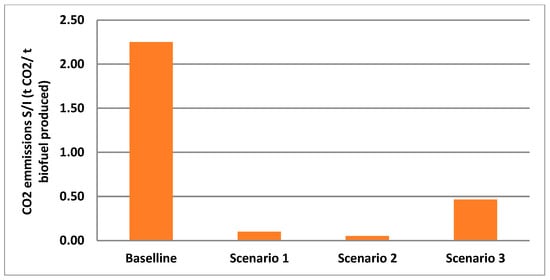
Figure 4.
Comparison of CO2 emissions per ton of biofuel from each scenario (baseline: conventional AD, scenario 1: AD-MEC system with digestate treatment via pyrolysis and syngas upgrading, scenario 2: conventional AD with Power-to-Gas biomethanation using green hydrogen and digestate treated via pyrolysis and syngas upgrading, scenario 3: conventional AD with biogas used in CHP engine; flue gas CO2 captured for algae cultivation. Digestate managed via pyrolysis and syngas upgrading).
The CO2 capture/utilization index further illustrates these improvements. While the baseline scenario does not incorporate carbon capture, scenario 1 and scenario 2 exhibit superior performance (0.03 each), indicating their potential for carbon valorization. Scenario 3 achieves a lower index of 0.02, suggesting lower carbon conversion efficiency compared to the other pathways.
The baseline scenario emits 3.98 t/d of CO2 for the production of 1.77 t/d of bio-based CH4 in the form of biogas while treating 120 t of organic waste. Using this core system, the implementation of the proposed configurations would reduce CO2 emissions from 94% to 66%, while simultaneously increasing the total energy content of the produced biofuels from 64% in scenario 3, to 139% and 142% in scenarios 1 and 2, respectively (Figure 5a).
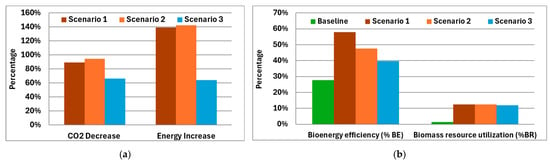
Figure 5.
Comparison of energy, emissions (a), bioenergy efficiency, and biomass resource utilization (b) for each scenario versus the baseline (baseline: conventional AD; scenario 1: AD-MEC with digestate pyrolysis and syngas upgrading; scenario 2: AD with Power-to-Gas biomethanation using green H2, digestate pyrolysis, and syngas upgrading; scenario 3: AD with biogas to CHP, CO2 capture for algae, digestate pyrolysis, and syngas upgrading).
Furthermore, the advanced scenarios can valorize the energy content of the initial raw materials up to two times more efficiently (scenario 1) than the baseline scenario, while utilizing biomass resources 12 times more effectively (Figure 5b). The low utilization factor of simple AD systems underscores the necessity for improved recovery and valorization strategies to enhance system sustainability.
An important outcome of this analysis is the potential that each scenario contributes to existing AD systems in terms of carbon capture and reduced reliance on fossil-based methane sources, while also achieving optimal valorization of the biomass in use. The integration of all advanced scenarios has significantly enhanced the initial performance of the baseline scenario. For each ton of feedstock utilized, scenarios 1 and 2 can produce more than double the energy output with nearly zero CO2 emissions. Although scenario 3 shows notable improvement over the baseline scenario, it still falls short compared to scenarios 1 and 2. This discrepancy can be attributed to the nature of this configuration, which can only produce one stream of bio-based methane, thus generating less energy compared to the other scenarios. Additionally, when considering the two primary functions of AD systems, energy production and waste treatment, it is crucial to assess the impact of integrating various CCUS technologies on CO2 reduction based on each function. In this regard, scenario 2 excels, achieving emissions as low as 3.3 kg of CO2 per MWh of energy produced and just 1.8 kg of CO2 per t of feedstock treated. This scenario outperforms alternative scenarios 1 and 3. In contrast, the baseline scenario exhibited significantly higher emissions, recording 147 kg of CO2 per MWh produced and 30 kg of CO2 per ton of waste treated. Figure 6 illustrates the correlation between these functions across the scenarios examined.
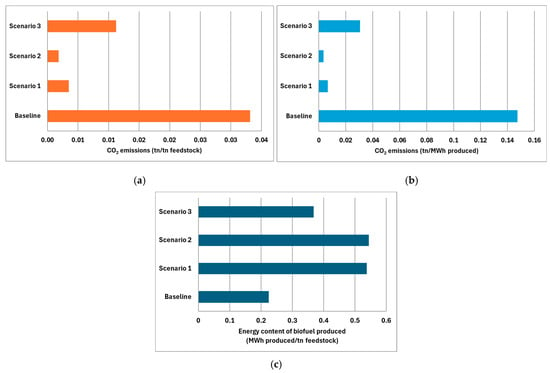
Figure 6.
(a) Comparison of CO2 emissions per organic waste treated, (b) comparison of CO2 emissions per unit of energy content from biofuels, (c) energy content of biofuel produced per ton of organic waste treated across different examined scenarios versus the baseline (baseline: conventional AD, scenario 1: AD-MEC system with digestate treatment via pyrolysis and syngas upgrading, scenario 2: conventional AD with Power-to-Gas biomethanation using green hydrogen and digestate treated via pyrolysis and syngas upgrading, scenario 3: conventional AD with biogas used in CHP engine; flue gas CO2 captured for algae cultivation. Digestate managed via pyrolysis and syngas upgrading).
To assess the broader impact of these integrated systems, a scenario-based projection was conducted for the European AD industry. Assuming a total annual organic feedstock processing capacity of 7.75 million tons [], the implementation of these technologies could lead to substantial CO2 savings, helping energy industry to meet established sustainability goals [,]. The adaptation of these scenarios for processing 10%, 20%, and 50% of the annually available organic feedstock has been calculated. According to Table 7, if only 10% of the organic waste was treated using the proposed systems, annual CO2 savings would reach approximately 23,000 t. Scaling up to 20% treatment could achieve nearly 46,000 tons in CO2 reductions, while 50% adoption would yield savings of up to 115,000 tons CO2, alongside the production of approximately 14,000 tons of biomethane.
4. Bottlenecks and Future Perspectives
Emerging bioenergy and carbon capture technologies present a promising path toward a sustainable energy transition, yet their large-scale adoption remains constrained by high capital costs, complex maintenance requirements, energy consumption, scalability limitations, and competition from established energy solutions. Addressing these challenges requires targeted innovation to reduce costs, enhance system efficiency, and integrate emerging technologies within existing infrastructure. Moreover, ensuring regulatory alignment, fostering public acceptance, and embedding circular economic principles into design and deployment strategies will be critical to achieving long-term viability.
One key priority for future research is the development of cost-effective materials and process optimizations to mitigate the high upfront capital expenditures (CapEx) associated with technologies such as AD-MEC, hydrogenotrophic CO2 methanation, enzymatic CO2 capture, and syngas biomethanation. AD-MEC systems rely on noble metals for electrodes and advanced membranes, necessitating material substitution strategies to balance cost reduction with performance stability [,]. Even when expensive noble metals in electrodes are replaced from low-cost carbon-based alternatives, these may require rare metal catalysts, presenting additional economic and environmental tradeoffs that warrant further investigation []. Similarly, electrolyzer costs for hydrogenotrophic methanation remain a significant barrier, emphasizing the need for material innovations and enhanced system efficiencies to drive down CapEx [,]. Likewise, biochar production and syngas biomethanation require costly pyrolysis reactors, gas cleaning systems, and integration infrastructure, eliminating its cost competitiveness [].
Furthermore, the capital-intensive process of enzymatic CO2 capture requires advancements in designing reactors such as packed bed or bubble columns that are less material intensive and engineering complex []. Regulating technology’s CapEx can lead to savings up to 88% of total costs. To do so, open pond systems can be suggested as less expensive compared to photobioreactors; however, the latter can be up to ten times more productive, proving that more research is needed [].
Maintenance complexities must also be addressed, particularly for electrochemical and enzymatic systems, where performance degradation and fouling significantly impact operational costs. Continuous monitoring and predictive maintenance strategies can help stabilize performance in AD-MEC systems, mitigating pH fluctuations and electrochemical inefficiencies [,]. Hydrogenotrophic CO2 methanation can reduce maintenance-related expenditures if more durable materials are used in electrolysis and gas purification systems []. Other technologies, such as enzymatic CO2 capture, encounter their own challenges, as enzyme degradation necessitates regular replenishment escalating technology costs [,]. Hence, research has been focused on enzyme immobilization techniques that balance longevity with affordability []. Developing robust impurity management strategies, particularly for hydrogen sulfide and ammonia, will be crucial for maintaining process efficiency in biochar and syngas upgrade systems [,].
Energy consumption remains a fundamental challenge affecting both the economic feasibility and environmental impact of these technologies. AD-MEC systems, for instance, require substantial external energy inputs, nearly doubling the energy demand of conventional AD to achieve economic parity []. Enzymatic CO2 capture technology is burdened by energy-intensive processes such as maintaining optimal light penetration, nutrient distribution, and CO2 delivery, which also require complex engineering solutions []. In addition to renewable-powered electrolysis, alternative hydrogen sources such as pink/red hydrogen produced from nuclear power or fossil-based hydrogen reservoirs may also support biomethanation. These sources can provide stable hydrogen supply and may offer efficiency advantages in contexts where renewable electricity is limited, expanding the applicability of biomethanation technologies beyond European conditions. Moreover, the production of high-purity hydrogen for hydrogenotrophic CO2 methanation is viable only under the condition to be powered by renewable energy sources, in order to avoid sustainability tradeoffs []. Syngas biomethanation and enzymatic CO2 capture also exhibit high energy demands, particularly for maintaining optimal reaction conditions and managing mass transfer limitations []. Future research should prioritize process integration strategies that maximize energy recovery, such as coupling biomethanation with excess renewable energy or leveraging biochar co-products for thermal energy.
Scalability represents another emerging frontier and research field towards the commercialization of emerging bioenergy solutions. For instance, AD-MEC systems must overcome embedded difficulties in reactor design and maintaining electron transfer efficiency at large scales [], while syngas biomethanation requires improvements in gas–liquid mass transfer to prevent system inefficiencies []. Enzymatic CO2 capture must resolve mass transfer bottlenecks and enzyme leaching issues that hinder industrial applicability able to regulate the cost of biomass production by 67% when scaling up from a 1 ha to a 10 ha facility []. A pressing concern in hydrogenotrophic methanation is the economic infeasibility of small-scale installations, which face disproportionately high costs for reactors, compressors, and auxiliary equipment. Future efforts should focus on optimizing process configurations that enable economies of scale while ensuring system adaptability across different facility sizes [].
Beyond technological advancements, regulatory support and policy alignment are essential for accelerating market adoption. Current frameworks primarily emphasize CCS, with limited incentives tailored to CCU applications in biofuel production. Establishing specific regulations, tax incentives, and certification schemes for CO2-derived biofuels will be crucial in fostering investment and creating a level playing field with alternative low-carbon energy solutions [,]. Additionally, integrating bioenergy with carbon capture and usage or BECCU into emission trading systems and carbon pricing mechanisms could improve the economic viability of biofuel plants that incorporate CO2 capture. Addressing inconsistencies across national and EU-level policies will further support widespread deployment, ensuring that biochar, syngas biomethanation, and enzymatic CO2 capture technologies receive appropriate recognition within sustainability policies [].
Public perception and social acceptance also play a decisive role in determining the commercial success of these technologies. Concerns regarding sustainability assurances, resource utilization, and environmental tradeoffs must be proactively managed through transparent communication and engagement strategies. The willingness of consumers to pay a premium for low-carbon biofuels underscores the need for clear sustainability certification frameworks that validate their environmental benefits []. Public skepticism toward carbon capture technologies, often stemming from perceptions of greenwashing, necessitates stronger policy safeguards and third-party verification mechanisms to build trust. Regulatory clarity on land use, soil health benefits, and contamination risks for biochar applications will be critical in fostering widespread adoption [].
Addressing material sourcing, emissions, and resource utilization presents critical research opportunities for improving the environmental sustainability of CCU technologies. The reliance of AD-MEC electrodes on rare metals necessitates research into alternative, abundant, and less environmentally damaging materials, as well as strategies for recycling and reusing electrode components [,]. Considering these alternative hydrogen sources highlights potential pathways to overcome some of the resource and energy challenges associated with hydrogenotrophic methanation, further strengthening the prospects for large-scale implementation in diverse energy systems. Similarly, hydrogenotrophic CO2 methanation requires advancements in electrolyzer materials to reduce resource intensity and enhance durability, along with innovative biogas pretreatment methods to mitigate contamination risks and improve system reliability [,]. Enzymatic CO2 capture systems require further investigation into impurity-tolerant enzyme formulations and cost-effective gas treatment processes to minimize secondary waste generation and overall energy demand []. Additionally, biochar and syngas biomethanation could benefit from emission control technologies to manage pyrolysis byproducts such as VOCs, tars, and particulate matter, thereby reducing their impact on air quality []. The water usage involved in gas cleaning processes underscores the need for water-efficient purification techniques, particularly in water-scarce regions []. Furthermore, variability in digestate-derived biochar composition highlights the necessity for studies on safe application methods and mitigation strategies for heavy metal and persistent pollutant accumulation in soils [].
Finally, integrating circular economy principles into the design and deployment of bioenergy and CCU technologies offers an opportunity to maximize resource efficiency while enhancing economic viability. This includes valorizing CO2 captured from biorefineries into valuable bio-based products, utilizing waste-derived feedstocks to mitigate land use pressures, and ensuring that biochar production aligns with soil restoration objectives rather than biomass overexploitation.
5. Conclusions
Conclusively, this paper has critically reviewed four technological pathways aimed at minimizing or even reversing carbon emissions from AD systems: MEC, hydrogenotrophic CO2 methanation, enzymatic CO2 capture coupled with algae cultivation, and digestate management via pyrolysis and syngas biomethanation. Each approach was assessed in terms of its technical maturity, integration potential, and environmental impact. While the technologies themselves are promising and, in many cases, technically mature, the primary challenge lies in their integration into cohesive, scalable systems. The combined use of these technologies can significantly enhance AD performance by increasing methane yield, promoting circular resource use, and enabling CCUS, ultimately transforming AD from a low-carbon to a potentially carbon-negative process.
The feasibility of zero-emission AD systems is not limited by technological availability but by systemic factors such as policy alignment, stakeholder collaboration, and economic viability. The proposed integrated scenarios demonstrate how synthesizing these approaches can close material and energy loops in the agri-food supply chain. However, successful implementation requires coordinated action across the value chain, including farmers, engineers, researchers, and decision makers, supported by tailored regulatory and financial frameworks. As the AD sector moves toward climate neutrality, further research and pilot-scale demonstrations are crucial to validate the synergistic effects of integration and to address outstanding bottlenecks. By leveraging these pathways, AD can evolve into a cornerstone technology for sustainable waste management, renewable energy production, and a circular bioeconomy.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/su172210385/s1. It includes a summary of recent studies on biomethanation (biogas upgrade or CO2 methanation) highlighting the dominant methanogenic taxa (average relative abundance > 0.5%) identified under different operational conditions. The table presents trends linking methanogen presence and abundance to key parameters such as temperature, pH, substrate composition, and reactor type.
Author Contributions
Conceptualization, E.M.B. and S.M.; Methodology, E.M.B. and S.M.; Formal Analysis, E.M.B., S.M., and A.T.; Investigation, D.A.B., M.G., P.K., L.T., S.C., D.H., R.A.T., M.B.Z., R.R., A.G.-A., E.M.G., I.A., V.V., A.T., E.M.B., and S.M.; Writing—Original Draft, A.T., D.A.B., M.G., L.T., R.A.T., M.B.Z., A.G.-A., E.M.G., E.M.B., and S.M.; Writing—Review and Editing, M.G., P.K., R.A.T., R.R., A.G.-A., E.M.G., I.A., E.M.B., and S.M.; Visualization, E.M.B., S.M., and A.T.; Supervision, P.K., L.T., D.H., R.R., I.A., V.V., E.M.B., S.M., and D.M.; Project Administration, D.M.; Funding Acquisition, D.M. All authors have read and agreed to the published version of the manuscript.
Funding
This project has been funded by the European Union’s Horizon 2020 research and innovation program under grant agreement No. 101084405 (CRONUS).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Dataset available on request from the authors.
Conflicts of Interest
Authors Maja Berden Zrimec and Robert Reinhardt were employed by the company Algal Technology Centre, LLC. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
The abbreviations used in this manuscript are as follows:
| %BE | Bioenergy Efficiency |
| %BR | Biomass Resource Utilization |
| ~RA | Relative Abundance |
| AD | Anaerobic Digestion |
| CA | Carbonic Anhydrase |
| CapEx | Capital Expenditures |
| CC | CO2 Captured/Utilized |
| CCU | Carbon Capture and Utilization |
| CCUS | Carbon Capture, Utilization, and Storage |
| CHP | Combined Heat and Power |
| DAC | Direct Air Capture |
| DES | Deep Eutectic Solvents |
| EAB | Electroactive Bacteria |
| EMA | Electrophilic Methanogenic Archaea |
| HC | Hydrocarbon |
| KOH | Potassium Hydroxide |
| LHV | Lower Heating Values |
| MEC | Microbial Electrolysis Cells |
| PBR | Packed Bed Reactor |
| PEM | Proton Exchange Membrane |
| PFSA | Perfluorosulfonic Acid |
| PtM | Power-To-Methane |
| S/I | CO2 Emissions |
| STR | Stirred Tank Reactor |
| TBR | Trickling Bed Reactor |
| TRL | Technological Readiness Level |
| VFAs | Volatile Fatty Acids |
| YSZ | Yttria Stabilized Zirconia |
References
- Hanson, E.; Nwakile, C.; Hammed, V.O. Carbon capture, utilization, and storage (CCUS) technologies: Evaluating the effectiveness of advanced CCUS solutions for reducing CO2 emissions. Results Surf. Interfaces 2025, 18, 100381. [Google Scholar] [CrossRef]
- Paranjpe, A.; Saxena, S.; Jain, P. A Review on Performance Improvement of Anaerobic Digestion Using Co-Digestion of Food Waste and Sewage Sludge. J. Environ. Manag. 2023, 338, 117733. [Google Scholar] [CrossRef]
- Rasapoor, M.; Young, B.; Brar, R.; Sarmah, A.; Zhuang, W.Q.; Baroutian, S. Recognizing the challenges of anaerobic digestion: Critical steps toward improving biogas generation. Fuel 2020, 261, 116497. [Google Scholar] [CrossRef]
- Angelidaki, I.; Treu, L.; Tsapekos, P.; Luo, G.; Campanaro, S.; Wenzel, H.; Kougias, P. Biogas upgrading and utilization: Current status and perspectives. Biotechnol. Adv. 2018, 36, 452–466. [Google Scholar] [CrossRef] [PubMed]
- Bacovsky, D.; Dißauer, C.; Drosg, B.; Kuba, M.; Matschegg, D.; Schmidl, C. IEA Bioenergy. 2022. Available online: https://www.ieabioenergyreview.org/wp-content/uploads/2022/12/IEA_BIOENERGY_REPORT.pdf (accessed on 7 September 2025).
- Archana, K.; Visckram, A.S.; Senthil Kumar, P.; Manikandan, S.; Saravanan, A.; Natrayan, L. A review on recent technological breakthroughs in anaerobic digestion of organic biowaste for biogas generation: Challenges towards sustainable development goals. Fuel 2024, 358, 130298. [Google Scholar] [CrossRef]
- The European Parliament and the Council of the European Union. Regulation (EU) 2019/1009 of 5 June 2019 Laying Down Rules on the Making Available on the Market of EU Fertilising Products and Amending Regulations (EC) No 1069/2009 and (EC) No 1107/2009 and Repealing Regulation (EC) No 2003/2003. Off. J. Eur. Union 2019, 62, L 170/1–L 170/132. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=OJ:L:2019:170:FULL&from=EN (accessed on 8 September 2025).
- Council of the European Union. Consolidated Version of Council Directive 91/676/EEC of 12 December 1991 Concerning the Protection of Waters Against Pollution Caused by Nitrates from Agricultural Sources (as Amended up to 11 December 2008). L 375. 2008. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:01991L0676-20081211 (accessed on 29 October 2025).
- Sobhi, M.; Elsamahy, T.; Zakaria, E.; Gaballah, M.S.; Zhu, F.; Hu, X.; Zhou, C.; Guo, J.; Huo, S.; Dong, R. Characteristics, limitations and global regulations in the use of biogas digestate as fertilizer: A comprehensive overview. Sci. Total. Environ. 2024, 957, 177855. [Google Scholar] [CrossRef]
- Porterfield, K.K.; Hobson, S.A.; Neher, D.A.; Niles, M.T.; Roy, E.D. Microplastics in composts, digestates, and food wastes: A review. J. Environ. Qual. 2023, 52, 225–240. [Google Scholar] [CrossRef]
- Lamolinara, B.; Pérez-Martínez, A.; Guardado-Yordi, E.; Guillén Fiallos, C.; Diéguez-Santana, K.; Ruiz-Mercado, G.J. Anaerobic digestate management, environmental impacts, and techno-economic challenges. Waste Manag. 2022, 140, 14–30. [Google Scholar] [CrossRef] [PubMed]
- Bondarczuk, K.; Markowicz, A.; Piotrowska-Seget, Z. The urgent need for risk assessment on the antibiotic resistance spread via sewage sludge land application. Environ. Int. 2016, 87, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Llewellyn, C.A.; Zrimec, M.B.; Reinhardt, R. Cultivation of microalgae on anaerobic digestate: Case studies. In Algal Biorefinery; Elsevier: Amsterdam, The Netherlands, 2025; pp. 147–156. Available online: https://linkinghub.elsevier.com/retrieve/pii/B9780443239670000176 (accessed on 7 September 2025).
- Andreides, D.; Pokorna, D.; Zabranska, J. Assessing the syngas biomethanation in anaerobic sludge digestion under different syngas loading rates and homogenisation. Fuel 2022, 320, 123929. [Google Scholar] [CrossRef]
- Tiara, A.R.; Tjahjono, B.; Beltran, M.; Rayns, F.; Longhurst, P. Leveraging Resource Management and Duality Theories to Strengthen Circular Economy Practices in the Waste-to-Energy Industry. Bus. Strat. Environ. 2025, 34, 4642–4660. [Google Scholar] [CrossRef]
- European Parliament and Council of the European Union. Regulation (EC) No 1069/2009 of the European Parliament and of the Council of 21 October 2009 Laying Down Health Rules as Regards Animal by-Products and Derived Products Not Intended for Human Consumption and Repealing Regulation (EC) No 1774/2002 Regulation). 2009. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32009R1069 (accessed on 29 October 2025).
- European Commission. Legislative Framework—Industrial Carbon Management. Available online: https://climate.ec.europa.eu/eu-action/industrial-carbon-management/legislative-framework_en (accessed on 29 October 2025).
- Štrubelj, L. Waste, Fertilising Product, or Something Else? EU Regulation of Biochar. J. Environ. Law 2022, 34, 529–540. [Google Scholar] [CrossRef]
- Mertzanakis, C.; Vlachokostas, C.; Toufexis, C.; Michailidou, A.V. Closing the Loop between Waste-to-Energy Technologies: A Holistic Assessment Based on Multiple Criteria. Energies 2024, 17, 2971. [Google Scholar] [CrossRef]
- Eftaxias, A.; Kolokotroni, I.; Michailidis, C.; Charitidis, P.; Diamantis, V. Techno-Economic Assessment of Anaerobic Digestion Technology for Small- and Medium-Sized Animal Husbandry Enterprises. Appl. Sci. 2024, 14, 4957. [Google Scholar] [CrossRef]
- Wang, H.; Zeng, S.; Pan, X.; Liu, L.; Chen, Y.; Tang, J.; Luo, F. Bioelectrochemically assisting anaerobic digestion enhanced methane production under low-temperature. Renew. Energy 2022, 194, 1071–1083. [Google Scholar] [CrossRef]
- Tartakovsky, B.; Mehta, P.; Bourque, J.S.; Guiot, S.R. Electrolysis-enhanced anaerobic digestion of wastewater. Bioresour. Technol. 2011, 102, 5685–5691. [Google Scholar] [CrossRef]
- Jiang, X.; Chen, D.; Mu, Y.; Pant, D.; Cheng, H.; Shen, J. Electricity-stimulated anaerobic system (ESAS) for enhanced energy recovery and pollutant removal: A critical review. Chem. Eng. J. 2021, 411, 128548. [Google Scholar] [CrossRef]
- Kanellos, G.; Tremouli, A.; Arvanitakis, G.; Lyberatos, G. Boosting methane production and raw waste activated sludge treatment in a microbial electrolysis cell-anaerobic digestion (MEC-AD) system: The effect of organic loading rate. Bioelectrochemistry 2024, 155, 108555. [Google Scholar] [CrossRef] [PubMed]
- El-Sayad, R.M.; Khalil, A.S. Wastewater treatment and hydrogen production via Microbial electrolysis cells (MECs) and fermentation methods: A comparative review. J. Contemp. Technol. Appl. Eng. 2023, 2, 29–41. [Google Scholar] [CrossRef]
- Noori, M.T.; Rossi, R.; Logan, B.E.; Min, B. Hydrogen production in microbial electrolysis cells with biocathodes. Trends Biotechnol. 2024, 42, 815–828. [Google Scholar] [CrossRef]
- Jadhav, D.A.; Yu, Z.; Hussien, M.; Kim, J.H.; Liu, W.; Eisa, T.; Sharma, M.; Vinayak, V.; Jang, J.K.; Wilberforce Awotwe, T.; et al. Paradigm shift in Nutrient-Energy-Water centered sustainable wastewater treatment system through synergy of bioelectrochemical system and anaerobic digestion. Bioresour. Technol. 2024, 396, 130404. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, R.; Etcheverry, L.; Roubaud, E.; Basséguy, R.; Délia, M.L.; Bergel, A. Microbial electrolysis cell (MEC): Strengths, weaknesses and research needs from electrochemical engineering standpoint. Appl. Energy 2020, 257, 113938. [Google Scholar] [CrossRef]
- Nguyen, D.; Khanal, S.K. A little breath of fresh air into an anaerobic system: How microaeration facilitates anaerobic digestion process. Biotechnol. Adv. 2018, 36, 1971–1983. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wu, W.; Qi, D.; Ding, Y.; Zhao, Z. Review on microaeration-based anaerobic digestion: State of the art, challenges, and prospectives. Sci. Total. Environ. 2020, 710, 136388. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Banks, C.; Zhang, Y.; Heaven, S.; Longhurst, P. Quantifying the percentage of methane formation via acetoclastic and syntrophic acetate oxidation pathways in anaerobic digesters. Waste Manag. 2018, 71, 749–756. [Google Scholar] [CrossRef]
- Wang, H.; Fotidis, I.A.; Angelidaki, I. Ammonia effect on hydrogenotrophic methanogens and syntrophic acetate-oxidizing bacteria. FEMS Microbiol. Ecol. 2015, 91, fiv130. [Google Scholar] [CrossRef]
- Huang, Q.; Liu, Y.; Dhar, B.R. A critical review of microbial electrolysis cells coupled with anaerobic digester for enhanced biomethane recovery from high-strength feedstocks. Crit. Rev. Environ. Sci. Technol. 2022, 52, 50–89. [Google Scholar] [CrossRef]
- Zheng, T.; Bian, C.; Xiao, B.; Chen, X.; Wang, J.; Li, L. Performance enhancement of integrating microbial electrolysis cell on two-stage anaerobic digestion of food waste: Electro-methanogenic stage versus electro-two stages. Bioresour. Technol. 2023, 386, 129562. [Google Scholar] [CrossRef]
- Lim, J.W.; Chiam, J.A.; Wang, J.Y. Microbial community structure reveals how microaeration improves fermentation during anaerobic co-digestion of brown water and food waste. Bioresour. Technol. 2014, 171, 132–138. [Google Scholar] [CrossRef]
- Zakaria, B.S.; Dhar, B.R. Progress towards catalyzing electro-methanogenesis in anaerobic digestion process: Fundamentals, process optimization, design and scale-up considerations. Bioresour. Technol. 2019, 289, 121738. [Google Scholar] [CrossRef]
- Issahaku, M.; Derkyi, N.S.A.; Kemausuor, F. A systematic review of the design considerations for the operation and maintenance of small-scale biogas digesters. Heliyon 2024, 10, e24019. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.; Harris, P.; Lee, S.; McCabe, B.K. Investigating the impact of seasonal temperature variation on biogas production from covered anaerobic lagoons treating slaughterhouse wastewater using lab scale studies. J. Environ. Chem. Eng. 2019, 7, 103077. [Google Scholar] [CrossRef]
- Younus Bhuiyan Sabbir, A.S.M.; Saha, C.K.; Nandi, R.; Zaman, M.F.U.; Alam, M.M.; Sarker, S. Effects of seasonal temperature variation on slurry temperature and biogas composition of a commercial fixed-dome anaerobic digester used in bangladesh. Sustainability 2021, 13, 11096. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, S.I.; Kim, I.H. Effect of dietary Spirulina (Arthrospira) platensis on the growth performance, antioxidant enzyme activity, nutrient digestibility, cecal microflora, excreta noxious gas emission, and breast meat quality of broiler chickens. Poult. Sci. 2018, 97, 2451–2459. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, L.; Chen, S.; Buisman, C.; Ter Heijne, A. Bioelectrochemical enhancement of methane production in low temperature anaerobic digestion at 10 °C. Water Res. 2016, 99, 281–287. [Google Scholar] [CrossRef]
- Sun, J.; Pan, F.; Zhu, H.; Wu, Q.; Pan, C.; Lu, F. Enhancing low-temperature anaerobic digestion of low-strength organic wastewater through bio-electrochemical technology. Int. J. Hydrogen Energy 2024, 58, 1062–1074. [Google Scholar] [CrossRef]
- Commault, A.S.; Lear, G.; Packer, M.A.; Weld, R.J. Influence of anode potentials on selection of Geobacter strains in microbial electrolysis cells. Bioresour. Technol. 2013, 139, 226–234. [Google Scholar] [CrossRef]
- Call, D.F.; Wagner, R.C.; Logan, B.E. Hydrogen production by Geobacter species and a mixed consortium in a microbial electrolysis cell. Appl. Environ. Microbiol. 2009, 75, 7579–7587. [Google Scholar] [CrossRef]
- Lu, L.; Xing, D.; Ren, N. Pyrosequencing reveals highly diverse microbial communities in microbial electrolysis cells involved in enhanced H2 production from waste activated sludge. Water Res. 2012, 46, 2425–2434. [Google Scholar] [CrossRef] [PubMed]
- Rotaru, A.E.; Shrestha, P.M.; Liu, F.; Markovaite, B.; Chen, S.; Nevin, K.P.; Lovley, D.R. Direct interspecies electron transfer between Geobacter metallireducens and Methanosarcina barkeri. Appl. Environ. Microbiol. 2014, 80, 4599–4605. [Google Scholar] [CrossRef]
- Liu, T.; Ou, H.; Su, K.; Hu, Z.; He, C.; Wang, W. Promoting direct interspecies electron transfer and acetoclastic methanogenesis for enhancing anaerobic digestion of butanol octanol wastewater by coupling granular activated carbon and exogenous hydrogen. Bioresour. Technol. 2021, 337, 125417. [Google Scholar] [CrossRef]
- Hari, A.R.; Katuri, K.P.; Logan, B.E.; Saikaly, P.E. Set anode potentials affect the electron fluxes and microbial community structure in propionate-fed microbial electrolysis cells. Sci. Rep. 2016, 6, 38690. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Liu, Y.; Zhao, Z.; Zhao, Z.; Liu, S.; Zhao, H.; Quan, X. Enhancement of anaerobic methanogenesis at a short hydraulic retention time via bioelectrochemical enrichment of hydrogenotrophic methanogens. Bioresour. Technol. 2016, 218, 505–511. [Google Scholar] [CrossRef]
- Yang, Z.; Shi, X.; Wang, C.; Wang, L.; Guo, R. Magnetite nanoparticles facilitate methane production from ethanol via acting as electron acceptors. Sci. Rep. 2015, 5, 16118. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Leng, X.; Zhao, S.; Ji, J.; Zhou, T.; Khan, A.; Kakde, A.; Liu, P.; Li, X. A review on the applications of microbial electrolysis cells in anaerobic digestion. Bioresour. Technol. 2018, 255, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Demirel, B.; Scherer, P. The roles of acetotrophic and hydrogenotrophic methanogens during anaerobic conversion of biomass to methane: A review. Rev. Environ. Sci. Bio/Technol. 2008, 7, 173–190. [Google Scholar] [CrossRef]
- Hua, T.; Li, S.; Li, F.; Zhou, Q.; Ondon, B.S. Microbial electrolysis cell as an emerging versatile technology: A review on its potential application, advance and challenge. J. Chem. Technol. Biotechnol. 2019, 94, 1697–1711. [Google Scholar] [CrossRef]
- Wang, J.; Wang, K.; Li, W.; Wang, H.; Wang, Y. Enhancing bioelectrochemical processes in anaerobic membrane bioreactors for municipal wastewater treatment: A comprehensive review. Chem. Eng. J. 2024, 484, 149420. [Google Scholar] [CrossRef]
- Barbosa, S.G.; Rodrigues, T.; Peixoto, L.; Kuntke, P.; Alves, M.M.; Pereira, M.A.; Ter Heijne, A. Anaerobic biological fermentation of urine as a strategy to enhance the performance of a microbial electrolysis cell (MEC). Renew. Energy 2019, 139, 936–943. [Google Scholar] [CrossRef]
- Zeppilli, M.; Pavesi, D.; Gottardo, M.; Micolucci, F.; Villano, M.; Majone, M. Using effluents from two-phase anaerobic digestion to feed a methane-producing microbial electrolysis. Chem. Eng. J. 2017, 328, 428–433. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, L.; Chen, T.; Huang, Y.; Qian, X.; He, J.; Li, Z.; Ding, C.; Wang, A. Simultaneous recovery of bio-sulfur and bio-methane from sulfate-rich wastewater by a bioelectrocatalysis coupled two-phase anaerobic reactor. Bioresour. Technol. 2022, 363, 127883. [Google Scholar] [CrossRef]
- Kaur, M.; Srikanth, S.; Kumar, M.; Sachdeva, S.; Puri, S.K. An integrated approach for efficient conversion of Lemna minor to biogas. Energy Convers. Manag. 2019, 180, 25–35. [Google Scholar] [CrossRef]
- Baek, G.; Kim, J.; Kim, J.; Lee, C. Individual and combined effects of magnetite addition and external voltage application on anaerobic digestion of dairy wastewater. Bioresour. Technol. 2020, 297, 122443. [Google Scholar] [CrossRef]
- Zhang, S.; Guan, W.; Sun, H.; Zhao, P.; Wang, W.; Gao, M.; Sun, X.; Wang, Q. Intermittent energization improves microbial electrolysis cell-assisted thermophilic anaerobic co-digestion of food waste and spent mushroom substance. Bioresour. Technol. 2023, 370, 128577. [Google Scholar] [CrossRef]
- Zhang, Q.; Peng, C.; Pu, J.; Feng, Y.; Zhu, H.; Yang, M.; Xu, Z.; Zhang, Y.; Yang, L.; Luo, D.; et al. Intermittent energization improves anaerobic digestion of microbial electrolysis cell-assisted nitrogen-rich sludge under mesophilic and thermophilic conditions. J. Environ. Chem. Eng. 2024, 12, 111630. [Google Scholar] [CrossRef]
- Beer, D.D.E.; Stoodley, P. Liquid Flow and Mass Transport in Heterogeneous Biofilms. Water Res. 1996, 30, 2761–2765. [Google Scholar] [CrossRef]
- Kim, K.Y.; Yang, W.; Logan, B.E. Impact of electrode configurations on retention time and domestic wastewater treatment efficiency using microbial fuel cells. Water Res. 2015, 80, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Thangavel, S.; Guo, Z.C.; Cui, M.H.; Wang, L.; Wang, A.J.; Liu, W.-Z. Supplementary material Hydrodynamics analysis for an up-flow integrated anaerobic digestion reactor with microbial electrolysis under different HRTs: Effect of bioelectrode spatial distribution on functional communities involved in methane production and organic removal. ACS Sustain. Chem. Eng. 2019, 8, 190–199. [Google Scholar]
- Day, J.R.; Heidrich, E.S.; Wood, T.S. A scalable model of fluid flow, substrate removal and current production in microbial fuel cells. Chemosphere 2022, 291, 132686. [Google Scholar] [CrossRef] [PubMed]
- Cheon, A.; Sung, J.; Jun, H.; Jang, H.; Kim, M.; Park, J. Application of Various Machine Learning Models for Process Stability of Bio-Electrochemical Anaerobic Digestion. Processes 2022, 10, 158. [Google Scholar] [CrossRef]
- Zhang, Z.; Song, Y.; Zheng, S.; Zhen, G.; Lu, X.; Kobayashi, T.; Xu, K.; Bakonyi, P. Electro-conversion of carbon dioxide (CO2) to low-carbon methane by bioelectromethanogenesis process in microbial electrolysis cells: The current status and future perspective. Bioresour. Technol. 2019, 279, 339–349. [Google Scholar] [CrossRef]
- Price, C.A.H.; Reina, T.R.; Liu, J. Engineering heterogenous catalysts for chemical CO2 utilization: Lessons from thermal catalysis and advantages of yolk@shell structured nanoreactors. J. Energy Chem. 2021, 57, 304–324. [Google Scholar] [CrossRef]
- Song, C. Global challenges and strategies for control, conversion and utilization of CO2 for sustainable development involving energy, catalysis, adsorption and chemical processing. Catal. Today 2006, 115, 2–32. [Google Scholar] [CrossRef]
- Chatzis, A.; Orellana, E.; Gaspari, M.; Kontogiannopoulos, K.; Treu, L.; Zouboulis, A.; Kougias, P.G. Comparative study on packing materials for improved biological methanation in trickle Bed reactors. Bioresour. Technol. 2023, 385, 129456. [Google Scholar] [CrossRef]
- Kougias, P.G.; Treu, L.; Benavente, D.P.; Boe, K.; Campanaro, S.; Angelidaki, I. Ex-situ biogas upgrading and enhancement in different reactor systems. Bioresour. Technol. 2017, 225, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Kakuk, B.; Wirth, R.; Maróti, G.; Szuhaj, M.; Rakhely, G.; Laczi, K.; Kovacs, K.; Kornel, L.; Bagi, Z. Early response of methanogenic archaea to H2 as evaluated by metagenomics and metatranscriptomics. Microb. Cell Factories 2021, 20, 127. [Google Scholar] [CrossRef]
- Al-Fatesh, A.; Singh, S.K.; Kanade, G.S.; Atia, H.; Fakeeha, A.H.; Ibrahim, A.A.; El-Toni, A.M.; Labhasetwar, N.K. Rh promoted and ZrO2/Al2O3 supported Ni/Co based catalysts: High activity for CO2 reforming, steam–CO2 reforming and oxy–CO2 reforming of CH4. Int. J. Hydrogen Energy 2018, 43, 12069–12080. [Google Scholar] [CrossRef]
- Calbry-Muzyka, A.S.; Schildhauer, T.J. Direct Methanation of Biogas—Technical Challenges and Recent Progress. Front. Energy Res. 2020, 8, 570887. [Google Scholar] [CrossRef]
- Ghaib, K.; Ben-Fares, F.Z. Power-to-Methane: A state-of-the-art review. Renew. Sustain. Energy Rev. 2018, 81, 433–446. [Google Scholar] [CrossRef]
- Varvoutis, G.; Lampropoulos, A.; Mandela, E.; Konsolakis, M.; Marnellos, G.E. Recent Advances on CO2 Mitigation Technologies: On the Role of Hydrogenation Route via Green H2. Energies 2022, 15, 4790. [Google Scholar] [CrossRef]
- Dincer, I.; Zamfirescu, C. Sustainable Energy Systems and Applications. 2011. Available online: https://books.google.com/books?hl=el&lr=&id=nHqNDkUaYaoC&oi=fnd&pg=PR7&ots=wAqNsOmIp8&sig=_fA7oLfpOVgj31K0ql0WeO7ldlA (accessed on 27 March 2024).
- Chaudhary, K.; Bhardvaj, K.; Chaudhary, A. A qualitative assessment of hydrogen generation techniques for fuel cell applications. Fuel 2024, 358, 130090. [Google Scholar] [CrossRef]
- Chisholm, G.; Zhao, T.; Cronin, L. Hydrogen from water electrolysis. In Storing Energy: With Special Reference to Renewable Energy Sources; Elsevier: Amsterdam, The Netherlands, 2022; pp. 559–591. [Google Scholar]
- García-Salaberri, P.A. Proton exchange membranes for polymer electrolyte fuel cells: An analysis of perfluorosulfonic acid and aromatic hydrocarbon ionomers. Sustain. Mater. Technol. 2023, 38, e00727. [Google Scholar] [CrossRef]
- Schmidt, O.; Gambhir, A.; Staffell, I.; Hawkes, A.; Nelson, J.; Few, S. Future cost and performance of water electrolysis: An expert elicitation study. Int. J. Hydrogen Energy 2017, 42, 30470–30492. [Google Scholar] [CrossRef]
- Ebrahimian, F.; De Bernardini, N.; Tsapekos, P.; Treu, L.; Zhu, X.; Campanaro, S.; Karimi, K.; Angelidaki, I. Effect of pressure on biomethanation process and spatial stratification of microbial communities in trickle bed reactors under decreasing gas retention time. Bioresour. Technol. 2022, 361, 127701. [Google Scholar] [CrossRef]
- Treu, L.; Campanaro, S.; Kougias, P.G.; Sartori, C.; Bassani, I.; Angelidaki, I. Hydrogen-Fueled Microbial Pathways in Biogas Upgrading Systems Revealed by Genome-Centric Metagenomics. Front. Microbiol. 2018, 9, 1079. Available online: www.frontiersin.org (accessed on 15 April 2024). [CrossRef]
- Ghofrani-Isfahani, P.; Tsapekos, P.; Peprah, M.; Kougias, P.; Zhu, X.; Kovalovszki, A.; Zervas, A.; Zha, X.; Jacobsen, C.S.; Angelidaki, I. Ex-situ biogas upgrading in thermophilic up-flow reactors: The effect of different gas diffusers and gas retention times. Bioresour. Technol. 2021, 340, 125694. [Google Scholar] [CrossRef]
- De Bernardini, N.; Basile, A.; Zampieri, G.; Kovalovszki, A.; De Diego Diaz, B.; Offer, E.; Wongfaed, N.; Angelidaki, I.; Kougias, P.G.; Campanaro, S.; et al. Integrating metagenomic binning with flux balance analysis to unravel syntrophies in anaerobic CO2 methanation. Microbiome 2022, 10, 117. [Google Scholar] [CrossRef]
- Maegaard, K.; Garcia-Robledo, E.; Kofoed, M.V.W.; Agneessens, L.M.; de Jonge, N.; Nielsen, J.L.; Ottosen, L.D.M.; Nielsen, L.P.; Revsbech, N.P. Biogas upgrading with hydrogenotrophic methanogenic biofilms. Bioresour. Technol. 2019, 287, 121422. [Google Scholar] [CrossRef]
- Tao, B.; Alessi, A.M.; Zhang, Y.; Chong, J.P.J.; Heaven, S.; Banks, C.J. Simultaneous biomethanisation of endogenous and imported CO2 in organically loaded anaerobic digesters. Appl. Energy 2019, 247, 670–681. [Google Scholar] [CrossRef]
- Tsapekos, P.; Treu, L.; Campanaro, S.; Centurion, V.B.; Zhu, X.; Peprah, M.; Zhang, Z.; Kougias, P.G.; Angelidaki, I. Pilot-scale biomethanation in a trickle bed reactor: Process performance and microbiome functional reconstruction. Energy Convers. Manag. 2021, 244, 114491. [Google Scholar] [CrossRef]
- Khesali Aghtaei, H.; Püttker, S.; Maus, I.; Heyer, R.; Huang, L.; Sczyrba, A.; Reichl, U.; Benndorf, D. Adaptation of a microbial community to demand-oriented biological methanation. Biotechnol. Biofuels Bioprod. 2022, 15, 125. [Google Scholar] [CrossRef] [PubMed]
- Fontana, A.; Kougias, P.G.; Treu, L.; Kovalovszki, A.; Valle, G.; Cappa, F.; Morelli, L.; Angelidaki, I.; Campanaro, S. Microbial activity response to hydrogen injection in thermophilic anaerobic digesters revealed by genome-centric metatranscriptomics. Microbiome 2018, 6, 194. [Google Scholar] [CrossRef]
- Oliveira, J.M.S.; Ottosen, L.D.M.; Kofoed, M.V.W. Continuous biomethanation of flue gas-carbon dioxide using bio-integrated carbon capture and utilization. Bioresour. Technol. 2024, 399, 130506. [Google Scholar] [CrossRef]
- Spyridonidis, A.; Vasiliadou, I.A.; Stathopoulou, P.; Tsiamis, A.; Tsiamis, G.; Stamatelatou, K. Enrichment of Microbial Consortium with Hydrogenotrophic Methanogens for Biological Biogas Upgrade to Biomethane in a Bubble Reactor under Mesophilic Conditions. Sustainability 2023, 15, 15247. [Google Scholar] [CrossRef]
- Ghiotto, G.; Zampieri, G.; Campanaro, S.; Treu, L. Strain-resolved metagenomics approaches applied to biogas upgrading. Environ. Res. 2023, 240, 117414. [Google Scholar] [CrossRef] [PubMed]
- Ghiotto, G.; De Bernardini, N.; Giangeri, G.; Tsapekos, P.; Gaspari, M.; Kougias, P.G.; Campanaro, S.; Angelidaki, I.; Treu, L. From microbial heterogeneity to evolutionary insights: A strain-resolved metagenomic study of H2S-induced changes in anaerobic biofilms. Chem. Eng. J. 2024, 485, 149824. [Google Scholar] [CrossRef]
- Zhu, X.; Campanaro, S.; Treu, L.; Seshadri, R.; Ivanova, N.; Kougias, P.G.; Kyrpides, N.; Angelidaki, I. Metabolic dependencies govern microbial syntrophies during methanogenesis in an anaerobic digestion ecosystem. Microbiome 2020, 8, 22. [Google Scholar] [CrossRef]
- Kaster, A.K.; Goenrich, M.; Seedorf, H.; Liesegang, H.; Wollherr, A.; Gottschalk, G.; Thauer, R.K. More than 200 genes required for methane formation from H2 and CO2 and energy conservation are present in methanothermobacter marburgensis and methanothermobacter thermautotrophicus. Archaea 2011, 2011, 973848. [Google Scholar] [CrossRef]
- Bucci, L.; Ghiotto, G.; Zampieri, G.; Raga, R.; Favaro, L.; Treu, L.; Campanaro, S. Adaptation of Anaerobic Digestion Microbial Communities to High Ammonium Levels: Insights from Strain-Resolved Metagenomics. Environ. Sci. Technol. 2024, 58, 580–590. [Google Scholar] [CrossRef]
- Kougias, P.G.; Campanaro, S.; Treu, L.; Zhu, X.; Angelidaki, I. A novel archaeal species belonging to Methanoculleus genus identified via de-novo assembly and metagenomic binning process in biogas reactors. Anaerobe 2017, 46, 23–32. [Google Scholar] [CrossRef]
- Maus, I.; Wibberg, D.; Stantscheff, R.; Eikmeyer, F.G.; Seffner, A.; Boelter, J.; Szczepanowski, R.; Blom, J.; Jaenicke, S.; König, H.; et al. Complete Genome Sequence of the Hydrogenotrophic, Methanogenic Archaeon Methanoculleus bourgensis Strain MS2T, Isolated from a Sewage Sludge Digester. J. Bacteriol. 2012, 194, 5487–5488. [Google Scholar] [CrossRef]
- Lim, J.W.; Park, T.; Tong, Y.W.; Yu, Z. The microbiome driving anaerobic digestion and microbial analysis. In Advances in Bioenergy; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–61. [Google Scholar]
- Wahid, R.; Mulat, D.G.; Gaby, J.C.; Horn, S.J. Effects of H2:CO2 ratio and H2 supply fluctuation on methane content and microbial community composition during in-situ biological biogas upgrading. Biotechnol. Biofuels 2019, 12, 104. [Google Scholar] [CrossRef]
- Fu, S.; Angelidaki, I.; Zhang, Y. In situ Biogas Upgrading by CO2-to-CH4 Bioconversion. Trends Biotechnol. 2021, 39, 336–347. [Google Scholar] [CrossRef]
- Deschamps, L.; Imatoukene, N.; Lemaire, J.; Mounkaila, M.; Filali, R.; Lopez, M.; Theoleyre, M.-A. In-situ biogas upgrading by bio-methanation with an innovative membrane bioreactor combining sludge filtration and H2 injection. Bioresour. Technol. 2021, 337, 125444. [Google Scholar] [CrossRef]
- Nomura, M.; Oshita, K.; Takaoka, M. Influence of hydrogen supply factors on in-situ biological biogas upgrading in thermophilic anaerobic digestion of sewage sludge. J. Mater. Cycles Waste Manag. 2025, 27, 2899–2909. [Google Scholar] [CrossRef]
- Díaz, I.; Fdz-Polanco, F.; Mutsvene, B.; Fdz-Polanco, M. Effect of operating pressure on direct biomethane production from carbon dioxide and exogenous hydrogen in the anaerobic digestion of sewage sludge. Appl. Energy 2020, 280, 115915. [Google Scholar] [CrossRef]
- Zhang, L.; Kuroki, A.; Tong, Y.W. A Mini-Review on In situ Biogas Upgrading Technologies via Enhanced Hydrogenotrophic Methanogenesis to Improve the Quality of Biogas From Anaerobic Digesters. Front. Energy Res. 2020, 8, 69. [Google Scholar] [CrossRef]
- Zabranska, J.; Pokorna, D. Bioconversion of carbon dioxide to methane using hydrogen and hydrogenotrophic methanogens. Biotechnol. Adv. 2018, 36, 707–720. [Google Scholar] [CrossRef] [PubMed]
- Xirostylidou, A.; Gaspari, M.; Kontogiannopoulos, K.N.; Ghiotto, G.; Treu, L.; Campanaro, S.; Anastasios, I.; Zouboulis, A.I.; Kougias, P.G. Biomethanation on demand: Continuous and intermittent hydrogen supply on biological CO2 methanation. Chem. Eng. J. 2024, 495, 153677. [Google Scholar] [CrossRef]
- Spyridonidis, A.; Stamatelatou, K. Comparative Study of Mesophilic Biomethane Production in Ex Situ Trickling Bed and Bubble Reactors. Fermentation 2024, 10, 554. [Google Scholar] [CrossRef]
- Jiang, B.; Zhang, D.; Hu, X.; Söderlind, U.; Paladino, G.; Gamage, S.; Hedenström, E.; Zhang, W.; Arrigoni, J.; Lundgren, A.; et al. Low-Grade Syngas Biomethanation in Continuous Reactors with Respect to Gas–Liquid Mass Transfer and Reactor Start-Up Strategy. Fermentation 2022, 9, 38. [Google Scholar] [CrossRef]
- Wise, D.L.; Cooney, C.L.; Augenstein, D.C. Biomethanation: Anaerobic fermentation of CO2, H2 and CO to methane. Biotechnol. Bioeng. 1978, 20, 1153–1172. [Google Scholar] [CrossRef]
- Palù, M.; Peprah, M.; Tsapekos, P.; Kougias, P.; Campanaro, S.; Angelidaki, I.; Treu, L. In-situ biogas upgrading assisted by bioaugmentation with hydrogenotrophic methanogens during mesophilic and thermophilic co-digestion. Bioresour. Technol. 2022, 348, 126754. [Google Scholar] [CrossRef]
- Luo, G.; Angelidaki, I. Integrated Biogas Upgrading and Hydrogen Utilization in an Anaerobic Reactor Containing Enriched Hydrogenotrophic Methanogenic Culture. Biotechnol. Bioeng. 2012, 109, 2729–2736. [Google Scholar] [CrossRef]
- Anggraini, I.D.; Keryanti Kresnowati, M.T.A.P.; Purwadi, R.; Noda, R.; Watanabe, T.; Setiadi, T. Bioethanol production via syngas fermentation of clostridium ljungdahlii in a hollow fiber membrane supported bioreactor. Int. J. Technol. 2019, 10, 481–490. [Google Scholar] [CrossRef]
- Ghofrani-Isfahani, P.; Tsapekos, P.; Peprah, M.; Kougias, P.; Zervas, A.; Zhu, X.; Yang, Ζ.; Jacobsen, C.S.; Angelidaki, I. Ex-situ biogas upgrading in thermophilic trickle bed reactors packed with micro-porous packing materials. Chemosphere 2022, 296, 133987. [Google Scholar] [CrossRef] [PubMed]
- Dahl Jønson, B.; Ujarak Sieborg, M.; Tahir Ashraf, M.; Yde, L.; Shin, J.; Shin, S.G.; Triolo, J.M. Direct inoculation of a biotrickling filter for hydrogenotrophic methanogenesis. Bioresour. Technol. 2020, 318, 124098. [Google Scholar] [CrossRef] [PubMed]
- Goonesekera, E.M.; Grimalt-Alemany, A.; Thanasoula, E.; Yousif, H.F.; Krarup, S.L.; Valerin, M.C.; Angelidaki, I. Biofilm mass transfer and thermodynamic constraints shape biofilm in trickle bed reactor syngas biomethanation. Chem. Eng. J. 2024, 500, 156629. [Google Scholar] [CrossRef]
- Asimakopoulos, K.; Kaufmann-Elfang, M.; Lundholm-Høffner, C.; Rasmussen, N.B.K.; Grimalt-Alemany, A.; Gavala, H.N.; Skiadas, I.V. Scale up study of a thermophilic trickle bed reactor performing syngas biomethanation. Appl. Energy 2021, 290, 116771. [Google Scholar] [CrossRef]
- Asimakopoulos, K.; Łężyk, M.; Grimalt-Alemany, A.; Melas, A.; Wen, Z.; Gavala, H.N.; Skiadas, I.V. Temperature effects on syngas biomethanation performed in a trickle bed reactor. Chem. Eng. J. 2020, 393, 124739. [Google Scholar] [CrossRef]
- Voelklein, M.A.; Rusmanis, D.; Murphy, J.D. Biological methanation: Strategies for in-situ and ex-situ upgrading in anaerobic digestion. Appl. Energy 2019, 235, 1061–1071. [Google Scholar] [CrossRef]
- Berden, M.Z.; Reinhardt, R.; Rossi, S.; Ficara, E.; Rodero Mdel, R.; Muñoz, R. Algae and Biogas Plants: Digestate Remediation and Nutrient Recycling with Algal Systems. Algae Mediat. Bioremediat. Ind. Prospect. 2024, 1, 246–270. [Google Scholar]
- Berden Zrimec, M.; Sforza, E.; Pattaro, L.; Carecci, D.; Ficara, E.; Idà, A.; Ferrer-Ledo, Ν.; Canziani, S.; Mangini, S.; Lazar, B.; et al. Advances in Spirulina Cultivation: Techniques, Challenges, and Applications. In New Insights into Cyanobacteria—Fundamentals, Culture Techniques, Tools and Biotechnological Uses; IntechOpen: London, UK, 2024. [Google Scholar]
- Fal, S.; Smouni, A.; El Arroussi, H. Integrated microalgae-based biorefinery for wastewater treatment, industrial CO2 sequestration and microalgal biomass valorization: A circular bioeconomy approach. Environ. Adv. 2023, 12, 100365. [Google Scholar] [CrossRef]
- Yu, X.; Catanescu, C.O.; Bird, R.E.; Satagopan, S.; Baum, Z.J.; Lotti Diaz, L.M.; Zhou, Q.A. Trends in Research and Development for CO2 Capture and Sequestration. ACS Omega 2023, 8, 11643–11664. [Google Scholar] [CrossRef] [PubMed]
- Ochedi, F.O.; Yu, J.; Yu, H.; Liu, Y.; Hussain, A. Carbon dioxide capture using liquid absorption methods: A review. Environ. Chem. Lett. 2020, 19, 77–109. [Google Scholar] [CrossRef]
- Kunze, A.K.; Dojchinov, G.; Haritos, V.S.; Lutze, P. Reactive absorption of CO2 into enzyme accelerated solvents: From laboratory to pilot scale. Appl. Energy 2015, 156, 676–685. [Google Scholar] [CrossRef]
- Kammerer, S.; Borho, I.; Jung, J.; Schmidt, M.S. Review: CO2 capturing methods of the last two decades. Int. J. Environ. Sci. Technol. 2022, 20, 8087–8104. [Google Scholar] [CrossRef]
- Rodero Mdel, R.; Carvajal, A.; Arbib, Z.; Lara, E.; de Prada, C.; Lebrero, R.; Muñoz, R. Performance evaluation of a control strategy for photosynthetic biogas upgrading in a semi-industrial scale photobioreactor. Bioresour. Technol. 2020, 307, 123207. [Google Scholar] [CrossRef]
- Savile, C.K.; Lalonde, J.J. Biotechnology for the acceleration of carbon dioxide capture and sequestration. Curr. Opin. Biotechnol. 2011, 22, 818–823. [Google Scholar] [CrossRef]
- Fradette, L.; Lefebvre, S.; Carley, J. Demonstration Results of Enzyme-Accelerated CO2 Capture. In Energy Procedia; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1100–1109. [Google Scholar]
- Shi, J.; Jiang, Y.; Jiang, Z.; Wang, X.; Wang, X.; Zhang, S.; Han, P.; Yang, C. Enzymatic conversion of carbon dioxide. Chem. Soc. Rev. 2015, 44, 5981–6000. [Google Scholar] [CrossRef]
- Xu, X.; Kentish, S.E.; Martin, G.J.O. Direct Air Capture of CO2 by Microalgae with Buoyant Beads Encapsulating Carbonic Anhydrase. ACS Sustain. Chem. Eng. 2021, 9, 9698–9706. [Google Scholar] [CrossRef]
- Russo, M.E.; Olivieri, G.; Marzocchella, A.; Salatino, P.; Caramuscio, P.; Cavaleiro, C. Post-combustion carbon capture mediated by carbonic anhydrase. Sep. Purif. Technol. 2013, 107, 331–339. [Google Scholar] [CrossRef]
- McKenna, R.; Supuran, C.T. Carbonic Anhydrase Inhibitors Drug Design. In Carbonic Anhydrase: Mechanism, Regulation, Links to Disease, and Industrial Applications; McKenna, R., Frost, S.C., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; Volume 75, pp. 291–323. [Google Scholar] [CrossRef]
- Li, L.; Fu, M.L.; Zhao, Y.H.; Zhu, Y.T. Characterization of carbonic anhydrase II from Chlorella vulgaris in bio-CO2 capture. Environ. Sci. Pollut. Res. 2012, 19, 4227–4232. [Google Scholar] [CrossRef]
- Bloch, M.R.; Sasson, J.L.; Ginzburg, M.E.; Goldman, Z.; Ginzburg, B.Z.; Garti, N.; Porath, A. Oil Products from Algae. U.S. Patent 4,341,038, 26 July 1982. [Google Scholar]
- Effendi, S.S.W.; Ng, I.S. The prospective and potential of carbonic anhydrase for carbon dioxide sequestration: A critical review. Process. Biochem. 2019, 87, 55–65. [Google Scholar] [CrossRef]
- Badger, M.R.; Dean Price, G. The Role of Carbonic Anhydrase in Photosynthesis. Annu. Rev. Plam Physioi. Plam Mol. Biol 1994, 45, 369–392. Available online: www.annualreviews.org (accessed on 15 September 2025). [CrossRef]
- DiMario, R.J.; Machingura, M.C.; Waldrop, G.L.; Moroney, J.V. The many types of carbonic anhydrases in photosynthetic organisms. Plant Sci. 2018, 268, 11–17. [Google Scholar] [CrossRef]
- Kassim, M.A.; Adnan, M.F.I.M.; Tan, K.M.; Bakar, M.H.A.; Lalung, J.; Mohamed, M.S. Carbonic anhydrase (CA) activity by Chlorella sp. in immobilised matrix under carbon dioxide rich cultivation condition. IOP Conf. Ser. 2020, 716, 012015. [Google Scholar] [CrossRef]
- Basu, S.; Roy, A.S.; Mohanty, K.; Ghoshal, A.K. CO2 biofixation and carbonic anhydrase activity in Scenedesmus obliquus SA1 cultivated in large scale open system. Bioresour. Technol. 2014, 164, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Ores Jda, C.; de Amarante, M.C.A.; Fernandes, S.S.; Kalil, S.J. Production of carbonic anhydrase by marine and freshwater microalgae. Biocatal. Biotransform. 2016, 34, 57–65. [Google Scholar] [CrossRef]
- Craveiro, R.; Dusschooten, F.; Nabais, A.R.; Boboescu, I.; Lo, C.; Neves, L.A.; Sá, M. Deep eutectic systems for carbonic anhydrase extraction from microalgae biomass to improve carbon dioxide solubilization. J. CO2 Util. 2022, 65, 102225. [Google Scholar] [CrossRef]
- Pierre, A.C. Enzymatic Carbon Dioxide Capture. ISRN Chem. Eng. 2012, 2012, 1–22. [Google Scholar] [CrossRef]
- Leimbrink, M.; Tlatlik, S.; Salmon, S.; Kunze, A.K.; Limberg, T.; Spitzer, R.; Gottschalk, A.; Górak, A.; Skiborowski, M. Pilot scale testing and modeling of enzymatic reactive absorption in packed columns for CO2 capture. Int. J. Greenh. Gas Control. 2017, 62, 100–112. [Google Scholar] [CrossRef]
- Frost, S.; McKenna, R. Carbonic Anhydrase: Mechanism, Regulation, Links to Disease and Industrial Applications; Science & Business Media: Berlin/Heidelberg, Germany, 2014; Volume 75. [Google Scholar]
- Bond, G.M.; Stringer, J.; Brandvold, D.K.; Simsek, F.A.; Medina, M.G.; Egeland, G. Development of Integrated System for Biomimetic CO2 Sequestration Using the Enzyme Carbonic Anhydrase. Energy Fuels 2001, 15, 309–316. [Google Scholar] [CrossRef]
- de Oliveira Maciel, A.; Christakopoulos, P.; Rova, U.; Antonopoulou, I. Carbonic anhydrase to boost CO2 sequestration: Improving carbon capture utilization and storage (CCUS). Chemosphere 2022, 299, 134419. [Google Scholar] [CrossRef]
- Unsworth, L.D.; van der Oost, J.; Koutsopoulos, S. Hyperthermophilic enzymesstability, activity and implementation strategies for high temperature applications. FEBS J. 2007, 274, 4044–4056. [Google Scholar] [CrossRef]
- Neves, L.A.; Afonso, C.; Coelhoso, I.M.; Crespo, J.G. Integrated CO2 capture and enzymatic bioconversion in supported ionic liquid membranes. Sep. Purif. Technol. 2012, 97, 34–41. [Google Scholar] [CrossRef]
- Mukherjee, S.; Sen, R.; Ralph, P.J.; Poddar, N. The catalytic role of carbonic anhydrase in optimizing carbon fixation in microalgal cultures. J. Clean. Prod. 2025, 512, 145461. [Google Scholar] [CrossRef]
- Yoshimoto, M.; Walde, P. Immobilized carbonic anhydrase: Preparation, characteristics and biotechnological applications. World J. Microbiol. Biotechnol. 2018, 34, 151. [Google Scholar] [CrossRef] [PubMed]
- Molitor, H.R.; Schnoor, J.L. Using simulated flue gas to rapidly grow nutritious microalgae with enhanced settleability. ACS Omega 2020, 5, 27269–27277. [Google Scholar] [CrossRef]
- Chunzhuk, E.A.; Grigorenko, A.V.; Kiseleva, S.V.; Chernova, N.I.; Volkov, D.A.; Nurgaliev, R.G.; Leng, L.; Kumar, V.; Vlaskin, M.S. Features of the Microalgae and Cyanobacteria Growth in the Flue Gas Atmosphere with Different CO2 Concentrations. Sustainability 2024, 16, 7075. [Google Scholar] [CrossRef]
- Abdelfatah-Aldayyat, E.; González-Rojo, S.; Gómez, X. Reviewing Digestate Thermal Valorization: Focusing on the Energy Demand and the Treatment of Process Water. Environments 2024, 11, 239. [Google Scholar] [CrossRef]
- Wang, W.; Chang, J.S.; Lee, D.J. Digestate-derived carbonized char and activated carbon: Application perspective. Bioresour. Technol. 2023, 381, 129135. [Google Scholar] [CrossRef]
- Kovačić, Đ.; Lončarić, Z.; Jović, J.; Samac, D.; Popović, B.; Tišma, M. Digestate Management and Processing Practices: A Review. Appl. Sci. 2022, 12, 9216. [Google Scholar] [CrossRef]
- Basinas, P.; Rusín, J.; Chamrádová, K.; Kaldis, S.P. Pyrolysis of the anaerobic digestion solid by-product: Characterization of digestate decomposition and screening of the biochar use as soil amendment and as additive in anaerobic digestion. Energy Convers. Manag. 2023, 277, 116658. [Google Scholar] [CrossRef]
- Paniagua, S.; Lebrero, R.; Muñoz, R. Syngas biomethanation: Current state and future perspectives. Bioresour. Technol. 2022, 358, 127436. [Google Scholar] [CrossRef] [PubMed]
- Grimalt-Alemany, A.; Skiadas, I.V.; Gavala, H.N. Syngas biomethanation: State-of-the-art review and perspectives. Biofuels Bioprod. Biorefining 2018, 12, 139–158. [Google Scholar] [CrossRef]
- Rönsch, S.; Schneider, J.; Matthischke, S.; Schlüter, M.; Götz, M.; Lefebvre, J.; Götz, M.; Lefebvre, J.; Prabhakaran, P.; Bajohr, S. Review on methanation—From fundamentals to current projects. Fuel 2016, 166, 276–296. [Google Scholar] [CrossRef]
- Ren, J.; Liu, Y.L.; Zhao, X.Y.; Cao, J.P. Methanation of syngas from biomass gasification: An overview. Int. J. Hydrogen Energy 2020, 45, 4223–4243. [Google Scholar] [CrossRef]
- Xu, D.; Tree, D.R.; Lewis, R.S. The effects of syngas impurities on syngas fermentation to liquid fuels. Biomass Bioenergy 2011, 35, 2690–2696. [Google Scholar] [CrossRef]
- Ahmed, A.; Cateni, B.G.; Huhnke, R.L.; Lewis, R.S. Effects of biomass-generated producer gas constituents on cell growth, product distribution and hydrogenase activity of Clostridium carboxidivorans P7T. Biomass Bioenergy 2006, 30, 665–672. [Google Scholar] [CrossRef]
- Xu, D.; Lewis, R.S. Syngas fermentation to biofuels: Effects of ammonia impurity in raw syngas on hydrogenase activity. Biomass Bioenergy 2012, 45, 303–310. [Google Scholar] [CrossRef]
- Vega, J.L.; Klasson, K.T.; Kimmel, D.E.; Clausen, E.C.; Gaddy, J.L. Sulfur gas tolerance and toxicity of co-utilizing and methanogenic bacteria. Appl. Biochem. Biotechnol. 1990, 24, 329–340. [Google Scholar] [CrossRef]
- Guiot, S.; Cimpoia, R.; Sancho Navarro, S.; Prudhomme, A.; Filiatrault, M. Anaerobic digestion for bio-upgrading syngas into renewable natural gas (methane). In Proceedings of the 13th World Congress on Anaerobic Digestion, Santiago de Compostela, Spain, 25–28 June 2013; pp. 1–5. [Google Scholar]
- Sun, H.; Yang, Z.; Liu, G.; Zhang, Y.; Tong, Y.W.; Wang, W. Double-edged effect of tar on anaerobic digestion: Equivalent method and modeling investigation. Energy 2023, 277, 127738. [Google Scholar] [CrossRef]
- Santos RGdos Alencar, A.C. Biomass-derived syngas production via gasification process and its catalytic conversion into fuels by Fischer Tropsch synthesis: A review. Int. J. Hydrogen Energy 2020, 45, 18114–18132. [Google Scholar] [CrossRef]
- Oelgeschläger, E.; Rother, M. Carbon monoxide-dependent energy metabolism in anaerobic bacteria and archaea. Arch. Microbiol. 2008, 190, 257–269. [Google Scholar] [CrossRef]
- Diender, M.; Pereira, R.; Wessels, H.J.C.T.; Stams, A.J.M.; Sousa, D.Z. Proteomic Analysis of the Hydrogen and Carbon Monoxide Metabolism of Methanothermobacter marburgensis. Front. Microbiol. 2016, 7, 1049. [Google Scholar] [CrossRef]
- Daniels, L.; Fuchs, G.; Thauer, R.K.; Zeikus, J.G. Carbon Monoxide Oxidation by Methanogenic Bacteria. J. Bacteriol. 1977, 132, 118–126. [Google Scholar] [CrossRef]
- Rother, M.; Metcalf, W.W. Anaerobic growth of Methanosarcina acetivorans C2A on carbon monoxide: An unusual way of life for a methanogenic archaeon. Proc. Natl. Acad. Sci. USA 2004, 101, 16929–16934. [Google Scholar] [CrossRef]
- O’Brien, J.M.; Wolkin, R.H.; Moench, T.T.; Morgan, J.B.; Zeikus, J.G. Association of hydrogen metabolism with unitrophic or mixotrophic growth of Methanosarcina barkeri on carbon monoxide. J. Bacteriol. 1984, 158, 373–375. [Google Scholar] [CrossRef] [PubMed]
- Grimalt-Alemany, A.; Łężyk, M.; Kennes-Veiga, D.M.; Skiadas, I.V.; Gavala, H.N. Enrichment of Mesophilic and Thermophilic Mixed Microbial Consortia for Syngas Biomethanation: The Role of Kinetic and Thermodynamic Competition. Waste Biomass Valorization 2019, 11, 465–481. [Google Scholar] [CrossRef]
- Fournier, G.P.; Gogarten, J.P. Evolution of Acetoclastic Methanogenesis in Methanosarcina via Horizontal Gene Transfer from Cellulolytic Clostridia. J. Bacteriol. 2008, 190, 1124–1127. [Google Scholar] [CrossRef] [PubMed]
- Asimakopoulos, K.; Gavala, H.N.; Skiadas, I.V. Biomethanation of Syngas by Enriched Mixed Anaerobic Consortia in Trickle Bed Reactors. Waste Biomass Valorization 2020, 11, 495–512. [Google Scholar] [CrossRef]
- Grimalt-Alemany, A.; Asimakopoulos, K.; Skiadas, I.V.; Gavala, H.N. Modeling of syngas biomethanation and catabolic route control in mesophilic and thermophilic mixed microbial consortia. Appl. Energy 2020, 262, 114502. [Google Scholar] [CrossRef]
- Postacchini, P.; Menin, L.; Piazzi, S.; Grimalt-Alemany, A.; Patuzzi, F.; Baratieri, M. Syngas biomethanation by co-digestion with brewery spent yeast in a lab-scale reactor. Biochem. Eng. J. 2023, 193, 108863. [Google Scholar] [CrossRef]
- Li, C.; Bao, R.; Sun, Y.; Quan, J.; Angelidaki, I.; Yuan, Z. Microbial dynamics and CO consumption enhancement via co-digestion with carbohydrate-rich synthetic wastewater. Sci. Total. Environ. 2025, 968, 178887. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, M.; Younesi, H.; Najafpour, G.; Mohamed, A.R. Sustainable ethanol fermentation from synthesis gas by Clostridium ljungdahlii in a continuous stirred tank bioreactor. J. Chem. Technol. Biotechnol. 2012, 87, 837–843. [Google Scholar] [CrossRef]
- Bredwell, M.D.; Srivastava, P.; Worden, R.M. Reactor design issues for synthesis-gas fermentations. Biotechnol. Prog. 1999, 15, 834–844. [Google Scholar] [CrossRef]
- Klasson, K.T.; Ackerson, M.D.; Clausen, E.C.; Gaddy, J.L. Bioconversion of synthesis gas into liquid or gaseous fuels. Enzym. Microb. Technol. 1992, 14, 602–608. [Google Scholar] [CrossRef]
- Munasinghe, P.C.; Khanal, S.K. Biomass-derived syngas fermentation into biofuels: Opportunities and challenges. Bioresour. Technol. 2010, 101, 5013–5022. [Google Scholar] [CrossRef]
- Feickert Fenske, C.; Md, Y.; Strübing, D.; Koch, K. Preliminary gas flow experiments identify improved gas flow conditions in a pilot-scale trickle bed reactor for H2 and CO2 biological methanation. Bioresour. Technol. 2023, 371, 128648. [Google Scholar] [CrossRef]
- Porté, H.; Kougias, P.G.; Alfaro, N.; Treu, L.; Campanaro, S.; Angelidaki, I. Process performance and microbial community structure in thermophilic trickling biofilter reactors for biogas upgrading. Sci. Total Environ. 2019, 655, 529–538. [Google Scholar] [CrossRef]
- Figueras, J.; Benbelkacem, H.; Dumas, C.; Buffiere, P. Inhibitions from Syngas Impurities: Impact of Common Tar Components on a Consortium Adapted for Syngas Biomethanation. Waste Biomass Valorization 2023, 15, 2427–2437. [Google Scholar] [CrossRef]
- Postacchini, P.; Grimalt-Alemany, A.; Ghofrani-Isfahani, P.; Treu, L.; Campanaro, S.; Menin, L. Carbon monoxide inhibition on acidogenic glucose fermentation and aceticlastic methanogenesis. Bioresour. Technol. 2024, 407, 131076. [Google Scholar] [CrossRef] [PubMed]
- EBA Statistical Report-Abridged Version; European Biogas Association: Etterbeek, Belgium, 2020.
- Shrivastava, P.; Kumar, A.; Tekasakul, P.; Lam, S.S.; Palamanit, A. Comparative investigation of yield and quality of bio-oil and biochar from pyrolysis of woody and non-woody biomasses. Energies 2021, 14, 1092. [Google Scholar] [CrossRef]
- Zaman, C.Z.; Pal, K.; Yehye, W.A.; Sagadevan, S.; Shah, S.T.; Adebisi, G.A.; Marliana, E.; Rafique, R.F.; Johan, R.B. Pyrolysis: A Sustainable Way to Generate Energy from Waste. In Pyrolysis; InTech: London, UK, 2017. [Google Scholar]
- Altıkat, A.; Alma, M.H.; Altıkat, A.; Bilgili, M.E.; Altıkat, S. A Comprehensive Study of Biochar Yield and Quality Concerning Pyrolysis Conditions: A Multifaceted Approach. Sustainability 2024, 16, 937. [Google Scholar] [CrossRef]
- Pahnila, M.; Koskela, A.; Sulasalmi, P.; Fabritius, T. A Review of Pyrolysis Technologies and the Effect of Process Parameters on Biocarbon Properties. Energies 2023, 16, 6936. [Google Scholar] [CrossRef]
- National Energy Technology Laboratory. Syngas Composition. 2020. Available online: https://www.netl.doe.gov/research/coal/energy-systems/gasification/gasifipedia/syngas-composition (accessed on 16 March 2025).
- Benevenuti, C.; Amaral, P.; Ferreira, T.; Seidl, P. Impacts of Syngas Composition on Anaerobic Fermentation. Reactions 2021, 2, 391–407. [Google Scholar] [CrossRef]
- Couto, N.; Rouboa, A.; Silva, V.; Monteiro, E.; Bouziane, K. Influence of the biomass gasification processes on the final composition of syngas. Energy Procedia 2013, 36, 596–606. [Google Scholar] [CrossRef]
- Elsayed, A.; Laqa Kakar, F.; Mustafa Abdelrahman, A.; Ahmed, N.; AlSayed, A.; Sherif Zagloul, M.; Muller, C.; Bell, K.Y.; Santoro, D.; Norton, J.; et al. Enhancing anaerobic digestion Efficiency: A comprehensive review on innovative intensification technologies. Energy Convers. Manag. 2024, 320, 118979. [Google Scholar] [CrossRef]
- Hakawati, R.; Smyth, B.M.; McCullough, G.; De Rosa, F.; Rooney, D. What is the most energy efficient route for biogas utilization: Heat, electricity or transport? Appl. Energy 2017, 206, 1076–1087. [Google Scholar] [CrossRef]
- Masebinu, S.O.; Akinlabi, E.T.; Muzenda, E.; Aboyade, A.O. A review of biochar properties and their roles in mitigating challenges with anaerobic digestion. Renew. Sustain. Energy Rev. 2019, 103, 291–307. [Google Scholar] [CrossRef]
- Balli, O.; Aras, H. Energetic Analyses of the Combined Heat and Power (CHP) System. Energy Explor. Exploit. 2007, 25, 39–62. [Google Scholar] [CrossRef]
- Chai, S.Y.W.; Ngu, L.H.; How, B.S. Review of carbon capture absorbents for CO2 utilization. Greenh. Gases 2022, 12, 394–427. [Google Scholar] [CrossRef]
- Asadollahzadeh, M.J.; Ardjmand, M.; Seafkordi, A.A.; Heydarian, S.M. Efficient storage and utilization of CO2 in open raceway ponds for cultivation of microalgae. Korean J. Chem. Eng. 2014, 31, 1425–1432. [Google Scholar] [CrossRef]
- De Baere, L.; Mattheeuws, B. Anaerobic digestion of the organic fraction of municipal solid waste in Europe-Status, Experience and Prospects Anaerobic Digestion of the Organic Fraction of Municipal Solid Waste in Europe Anaerobic Digestion of the Organic Fraction of Municipal Solid Waste in Europe-Status, Experience and Prospects. Available online: https://www.researchgate.net/publication/284229577 (accessed on 17 September 2025).
- Sulewski, P.; Ignaciuk, W.; Szymańska, M.; Wąs, A. Development of the Biomethane Market in Europe. Energies 2023, 16, 2001. [Google Scholar] [CrossRef]
- Tiago, G.A.O.; Rodrigo, N.P.B.; Lourinho, G.; Lopes, T.F.; Gírio, F. Systematic and Bibliometric Review of Biomethane Production from Biomass-Based Residues: Technologies, Economics and Environmental Impact. Fuels 2025, 6, 8. [Google Scholar] [CrossRef]
- Koul, Y.; Devda, V.; Varjani, S.; Guo, W.; Ngo, H.H.; Taherzadeh, M.J.; Change, J.-S.; Wong, J.W.C.; Bilal, M.; Kim, S.-H.; et al. Microbial electrolysis: A promising approach for treatment and resource recovery from industrial wastewater. Bioengineered 2022, 13, 8115–8134. [Google Scholar] [CrossRef] [PubMed]
- Kadier, A.; Kalil, M.S.; Abdeshahian, P.; Chandrasekhar, K.; Mohamed, A.; Azman, N.F.; Logroño, W.; Yibadatihan Simayi, Y.; Hamid, A.A. Recent advances and emerging challenges in microbial electrolysis cells (MECs) for microbial production of hydrogen and value-added chemicals. Renew. Sustain. Energy Rev. 2016, 61, 501–525. [Google Scholar] [CrossRef]
- Devrajani, S.K. Enhancing anaerobic digestion through the integration of microbial electrolysis cells: A comprehensive review. Pure Appl. Biol. 2024, 13, 141–155. [Google Scholar] [CrossRef]
- Mehmeti, A.; Angelis-Dimakis, A.; Arampatzis, G.; McPhail, S.J.; Ulgiati, S. Life cycle assessment and water footprint of hydrogen production methods: From conventional to emerging technologies. Environments 2018, 5, 24. [Google Scholar] [CrossRef]
- Nguyen, K.; Iliuta, I.; Pasquier, L.C.; Bougie, F.; Iliuta, M.C. Enzymatic CO2 Capture in intensified packed-bed column bioreactors: A techno-economic assessment. Appl. Energy 2024, 376, 124207. [Google Scholar] [CrossRef]
- Bhatt, A.; Khanchandani, M.; Rana, M.S.; Prajapati, S.K. Techno-economic analysis of microalgae cultivation for commercial sustainability: A state-of-the-art review. J. Clean. Prod. 2022, 370, 133456. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Ahmad, A. Microbial Electrolysis Cell Technology: Fundamental to Sustainable Applications; Springer Nature: Berlin/Heidelberg, Germany, 2024. [Google Scholar]
- Hosseinzadeh, A.; Zhou, J.L.; Li, X.; Afsari, M.; Altaee, A. Techno-economic and environmental impact assessment of hydrogen production processes using bio-waste as renewable energy resource. Renew. Sustain. Energy Rev. 2022, 156, 111991. [Google Scholar] [CrossRef]
- Yang, C.; Mao, L.; Chen, Y.; Zhou, Y.; Zhang, R.; Yi, Z.; Zhang, D.; Zhang, G. Ancestral carbonic anhydrase with significantly enhanced stability and activity for CO2 capture and utilization. Bioresour. Technol. 2025, 419, 132054. [Google Scholar] [CrossRef]
- Yong, J.K.J.; Stevens, G.W.; Caruso, F.; Kentish, S.E. The use of carbonic anhydrase to accelerate carbon dioxide capture processes. J. Chem. Technol. Biotechnol. 2015, 90, 3–10. [Google Scholar] [CrossRef]
- Rasouli, H.; Nguyen, K.; Iliuta, M.C. Recent advancements in carbonic anhydrase immobilization and its implementation in CO2 capture technologies: A review. Sep. Purif. Technol. 2022, 296, 121299. [Google Scholar] [CrossRef]
- Michailos, S.; Walker, M.; Moody, A.; Poggio, D.; Pourkashanian, M. Biomethane production using an integrated anaerobic digestion, gasification and CO2 biomethanation process in a real waste water treatment plant: A techno-economic assessment. Energy Convers. Manag. 2020, 209, 112663. [Google Scholar] [CrossRef]
- Abdoulmoumine, N.; Adhikari, S.; Kulkarni, A.; Chattanathan, S. A review on biomass gasification syngas cleanup. Appl. Energy 2015, 155, 294–307. [Google Scholar] [CrossRef]
- Tripathi, A.; Kumar, S.; Jadhav, G.S.; Jadhav, D.A.; Ghankrekar, M.M.; Surampalli, R.Y. Electromethanogenic reactor for biogas production using agricultural and livestock waste and its comparative analysis with biogas plant: A mini-review. Biomass Bioenergy 2024, 185, 107246. [Google Scholar] [CrossRef]
- Novoveská, L.; Nielsen, S.L.; Eroldoğan, O.T.; Haznedaroglu, B.Z.; Rinkevich, B.; Fazi, S.; Robbens, J.; Vasquez, M.; Einarsson, H. Overview and Challenges of Large-Scale Cultivation of Photosynthetic Microalgae and Cyanobacteria. Mar. Drugs 2023, 21, 445. [Google Scholar] [CrossRef]
- European Court of Auditors. The EU’s industrial policy on renewable hydrogen. In European Court of Auditors Special Report; EU: Luxembourg, 2024. [Google Scholar]
- Chettri, D.; Boro, D.; Chirania, M.; Verma, A.K. Integrating biochar production in biorefineries: Towards a sustainable future and circular economy. Biofuels Bioprod. Biorefining 2024, 18, 2156–2176. [Google Scholar] [CrossRef]
- Kadier, A.; Jain, P.; Lai, B.; Kalil, M.S.; Kondaveeti, S.; Alabbosh, K.F.S.; Abu-Reesh, I.M.; Mohanakrishna, G. Biorefinery perspectives of microbial electrolysis cells (MECs) for hydrogen and valuable chemicals production through wastewater treatment. Biofuel Res. J. 2020, 7, 1128–1142. [Google Scholar] [CrossRef]
- Tan, J.; Ji, Y.N.; Deng, W.S.; Su, Y.F. Process intensification in gas/liquid/solid reaction in trickle bed reactors: A review. Pet. Sci. 2021, 18, 1203–1218. [Google Scholar] [CrossRef]
- Sposob, M.; Wahid, R.; Fischer, K. Ex-situ biological CO2 methanation using trickle bed reactor: Review and recent advances. Rev. Environ. Sci. Bio/Technol. 2021, 20, 1087–1102. [Google Scholar] [CrossRef]
- González, R.; Cabeza, I.O.; Casallas-Ojeda, M.; Gómez, X. Biological Hydrogen Methanation with Carbon Dioxide Utilization: Methanation Acting as Mediator in the Hydrogen Economy. Environments 2023, 10, 82. [Google Scholar] [CrossRef]
- Menin, L.; Asimakopoulos, K.; Sukumara, S.; Rasmussen, N.B.K.; Patuzzi, F.; Baratieri, M.; Gavala, H.N.; Skiadas, I.V. Competitiveness of syngas biomethanation integrated with carbon capture and storage, power-to-gas and biomethane liquefaction services: Techno-economic modeling of process scenarios and evaluation of subsidization requirements. Biomass Bioenergy 2022, 161, 106475. [Google Scholar] [CrossRef]
- Lodato, C.; Hamelin, L.; Tonini, D.; Astrup, T.F. Towards sustainable methane supply from local bioresources: Anaerobic digestion, gasification, and gas upgrading. Appl. Energy 2022, 323, 119568. [Google Scholar] [CrossRef]
- Ugarte-Lucas, P.; Jacobsen, J.B. Public perception of bioenergy with carbon capture and storage in Denmark: Support or reluctant acceptance? Int. J. Greenh. Gas Control. 2024, 136, 104187. [Google Scholar] [CrossRef]
- Bellamy, R.; Lezaun, J.; Palmer, J. Perceptions of bioenergy with carbon capture and storage in different policy scenarios. Nat. Commun. 2019, 10, 743. [Google Scholar] [CrossRef]
- Kadier, A.; Simayi, Y.; Kalil, M.S.; Abdeshahian, P.; Hamid, A.A. A review of the substrates used in microbial electrolysis cells (MECs) for producing sustainable and clean hydrogen gas. Renew. Energy 2014, 71, 466–472. [Google Scholar] [CrossRef]
- Freguia, S.; Rabaey, K.; Yuan, Z.; Keller, J. Non-catalyzed cathodic oxygen reduction at graphite granules in microbial fuel cells. Electrochimica Acta 2007, 53, 598–603. [Google Scholar] [CrossRef]
- Wei, X.; Sharma, S.; Waeber, A.; Wen, D.; Sampathkumar, S.N.; Margni, M.; Maréchal, F.; Van herle, J. Comparative life cycle analysis of electrolyzer technologies for hydrogen production: Manufacturing and operations. Joule 2024, 8, 3347–3372. [Google Scholar] [CrossRef]
- Götz, M.; Lefebvre, J.; Mörs, F.; McDaniel Koch, A.; Graf, F.; Bajohr, S.; Reimert, R.; Kolb, T. Renewable Power-to-Gas: A technological and economic review. Renew. Energy 2016, 85, 1371–1390. [Google Scholar] [CrossRef]
- Talebi, S.; Edalatpour, A.; Tavakoli, O. Algal biorefinery: A potential solution to the food-energy-water-environment nexus. Sustain. Energy Fuels 2022, 6, 2623–2664. [Google Scholar] [CrossRef]
- Pelagalli, V.; Matassa, S.; Race, M.; Langone, M.; Papirio, S.; Lens, P.N.L.; Lazzazzara, M.; Frugis, A.; Petta, L.; Esposito, G. Syngas-driven sewage sludge conversion to microbial protein through H2S-and CO-tolerant hydrogen-oxidizing bacteria. Water Res. 2023, 248, 120698. [Google Scholar] [CrossRef]
- Ardolino, F.; Arena, U. Biowaste-to-Biomethane: An LCA study on biogas and syngas roads. Waste Manag. 2019, 87, 441–453. [Google Scholar] [CrossRef]
- Monlau, F.; Francavilla, M.; Sambusiti, C.; Antoniou, N.; Solhy, A.; Libutti, A.; Zabaniotou, A.; Barakat, A.; Monteleone, M. Toward a functional integration of anaerobic digestion and pyrolysis for a sustainable resource management. Comparison between solid-digestate and its derived pyrochar as soil amendment. Appl. Energy 2016, 169, 652–662. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).