Abstract
The yield and quality have long been constraining factors for the sustainable cultivation of Glycyrrhiza uralensis. This study evaluated the effects of foliar applications of forchlorfenuron (CPPU) at different concentrations (0, 5, 10, 20, and 40 mg·L−1) on plant growth and secondary metabolism through comprehensive analyses of photosynthesis, endogenous phytohormones, root biomass, and medicinal components. To ensure consumer safety, CPPU residue dynamics and associated health risks were also assessed. The 10 mg·L−1 treatment yielded the most pronounced improvements, increasing root biomass by 46%, glycyrrhizic acid content by 92%, and liquiritin content by 98.7%. It also enhanced the activity of ribulose-1,5-bisphosphate carboxylase, thereby improving overall photosynthetic gas exchange capacity, and significantly stimulated the synthesis of zeatin, abscisic acid, and salicylic acid. Residue analysis showed that by the 56th day after treatment, the CPPU level in roots was merely 5.44 × 10−4 mg·kg−1, with a half-life of 11.74 days. The resulting risk quotient (RQ) was below 0.01%, well under the safety threshold of 1, indicating negligible health risk to consumers. Our results demonstrate that the targeted application of CPPU offers a highly effective and safe strategy for enhancing both the productivity and commercial quality of G. uralensis.
1. Introduction
Glycyrrhiza uralensis Fisch. is a perennial herb belonging to the Leguminosae family. Its dry root is rich in glycyrrhizic acid, liquiritin and other flavonoids. These compounds are associated with a spectrum of pharmacological activities, including anti-inflammatory [1], antitussive [2], neuroprotective [3], antiviral [4] and anticancer [5] effects. Moreover, glycyrrhizic acid is approximately 150 times sweeter than sucrose [6], which underpins its widespread use as a sweetener and flavor enhancer in various products, including candy [7], beverages [8], beer [9], and tobacco [10]. Consequently, licorice root is widely recognized and utilized as a functional food [11]. However, high demand has led to overharvesting, resulting in the extinction of numerous natural populations and a significant reduction in the sizes of surviving ones. Consequently, the cultivation of G. uralensis has been initiated as a sustainable alternative to wild harvesting [12,13]. Although cultivated licorice offers advantages in terms of supply stability, its medicinal quality is generally inferior to that of wild licorice [14]; in some cases, the content of medicinal ingredients even fails to meet the standards set by the Pharmacopoeia of the People’s Republic of China [15]. This quality gap arises primarily from physiological responses triggered by differences in growing environments. Wild licorice, exposed to natural stressors, activates secondary metabolic defense mechanisms that promote the synthesis and accumulation of medicinal compounds such as glycyrrhizic acid and flavonoids. In contrast, cultivated licorice, grown under managed agricultural conditions with significantly reduced environmental stress, tends to prioritize vegetative growth, resulting in insufficient accumulation of secondary metabolites. Therefore, there is an urgent need to develop novel agricultural technologies that can simultaneously enhance both the yield and quality of licorice.
Plant growth regulators—a class of synthetic exogenous substances that mimic plant hormones—are widely used in agricultural and horticultural production [16]. They play a role in promoting the development of underground storage organs and the biosynthesis of secondary metabolites, mainly by regulating cell division and activating metabolic pathways. Forchlorfenuron (CPPU), chemically known as N-(2-Chloro-4-pyridyl)-N′-phenylurea, is one such regulator with potent cytokinin activity and offering the advantage of high efficacy at low concentrations [17]. Studies have demonstrated that CPPU significantly promotes root development [18], organ enlargement [19], and yield enhancement [20,21]. Currently, the application of CPPU has been authorized for use on crops in many countries and regions [22]. However, current research on CPPU predominantly focuses on its effects on fruit yield, whereas its impact on medicinal plants, particularly concerning root biomass and secondary metabolite accumulation, has seldom been systematically investigated.
In recent years, the excessive use of plant growth regulators in agriculture has raised public concern about food safety [23]. Although studies have shown that CPPU exhibits low acute toxicity in mammals [24,25] and is regarded as a safe plant growth regulator, comprehensive assessments of its potential safety risks in medicinal plant production remain limited. Furthermore, research aimed at enhancing the yield and quality of medicinal plants also lacks systematic approaches. Therefore, there is a clear imperative to explore the potential applications of CPPU in medicinal plants and evaluate its safety through residue dissipation dynamics and dietary risk assessment.
This study examines how foliar application of different forchlorfenuron (CPPU) concentrations affects hormone profiles, photosynthetic characteristics, growth behavior, root biomass, and secondary metabolite accumulation in cultivated G. uralensis. The objectives are to identify the optimal CPPU dosage for improving licorice yield and medicinal quality, elucidate its regulatory effects on photosynthesis and hormones, and evaluate its safety by residue quantification and dissipation dynamics in licorice roots. The results are anticipated to establish a theoretical basis for improving G. uralensis cultivation and to deliver actionable insights for the secure and efficacious application of plant growth regulators in medicinal plant production systems.
2. Materials and Methods
2.1. Materials
The seeds of G. uralensis used in this study were collected from Changji, Xinjiang, China. A voucher specimen (Gu Junjun 20230328001) was re-examined and identified by Professor Ma Miao and it has been deposited in the herbarium of Shihezi University (SHI). Seeds of uniform size were selected and soaked in sulfuric acid with a concentration of 98% for 30 min, followed by washing with water to remove any residual sulfuric acid from their surfaces. Standards of CPPU, zeatin, salicylic acid, and abscisic acid (purity > 98%) were purchased from Solarbio Technology Co., Ltd. (Beijing, China), while glycyrrhizic acid and liquiritin standards were obtained from McLean Biochemical Technology Co., Ltd. (Shanghai, China).
2.2. Experimental Design
On 10 April 2023, seeds of G. uralensis were sown in a seedling tray with sterile sandy soil. After 30 days of growth, upon reaching the four-true-leaf stage, seedlings of comparable vigor were selected. These seedlings were transplanted together into a single plastic pot (23 cm height × 20 cm diameter) containing a sterilized substrate mixture of river sand and loam (3:7, v/v). The substrate had the following properties: pH 7.8; total nitrogen, phosphorus, and potassium concentrations were 0.315, 0.131 and 5.47 g·kg−1, respectively; available nitrogen, phosphorus, and potassium levels were 52.59, 5.23 and 50.04 mg·kg−1, respectively; and the organic matter content was 6.64 g·kg−1. The pots were maintained in an open area on the campus of Shihezi University under the following conditions: day/night temperatures of 25–36 °C/18–23 °C, relative humidity of 50–60%, and natural light. To ensure uniform light exposure, we rotated the positions of all pots weekly. Foliar applications began after an additional 20 days of growth (50 days after sowing), when the seedlings had developed eight true leaves. Seedlings were sprayed with 100 mL per plant of either distilled water (control) or CPPU solution at concentrations of 2.5, 5, 10, 20, and 40 mg·L−1, respectively. Spraying was conducted weekly for a total of four applications, with the spray timing set at 21:00 (Beijing time) in the evening to minimize the evaporation rate of the CPPU solution. Each application ensured that the surface of the licorice leaves was thoroughly moistened. The experiment followed a completely randomized design. Each treatment included four independent biological replicates (n = 4), with each replicate consisting of one pot containing four plants. Therefore, a total of 16 plants were used per treatment. Chlorophyll content, photosynthetic parameters, ribulose-1,5-bisphosphate carboxylase (RuBPCase) activity, and endogenous hormone content were measured on the seventh day after the final treatment application.
2.3. Determination of Photosynthetic Indexes
Relative chlorophyll content (expressed as SPAD values) was first measured on the first fully expanded leaves using a SPAD-502 chlorophyll meter. Immediately thereafter, the same set of leaves was used to determine photosynthetic gas exchange parameters—namely net photosynthetic rate (Pn), transpiration rate (Tr), stomatal conductance (Gs), and intercellular CO2 concentration (Ci) using a portable photosynthesis system (LI-6400; LI-COR Biosciences, Lincoln, NE, USA). The system was equipped with a red-blue LED light source (Model LI-6400-02B) and a CO2 injector. Six plants per treatment were randomly selected for these sequential measurements, which were conducted under controlled conditions: a CO2 concentration of 400 ppm and a light intensity of 1800 μmol·m−2·s−1. To assay ribulose-1,5-bisphosphate carboxylase (RuBPCase) activity, the second fully expanded leaf was excised from randomly selected seedlings, rapidly frozen in liquid nitrogen, and analyzed using a commercial RuBPCase assay kit (Jiangsu Jingmei Biotechnology Co., Ltd., Yancheng, China). Each sample was analyzed in triplicate, and the results are presented as mean values.
2.4. Determination of Endogenous Hormones
Standard stock solutions of salicylic acid, abscisic acid, and zeatin were prepared by separately weighing 5.0 mg of each standard, dissolving them in methanol, and making up to volume in 5 mL volumetric flasks. These stocks were then serially diluted with methanol to generate working standard solutions at concentrations of 1, 5, 10, 50, and 100 ng·mL−1. For sample analysis, the second fully expanded leaves from the top of seedlings were randomly collected, immediately frozen in liquid nitrogen, and lyophilized. Approximately 0.100 g of the freeze-dried powder was homogenized with a mortar and pestle after adding 5 mL of an 80% methanol solution. The mixture was extracted at 4 °C for 10 h, followed by centrifugation at 12,000 rpm for 15 min (4 °C) to collect the supernatant. The residue was re-extracted with 2 mL of 80% methanol under identical conditions for 2 h. The supernatants from both extraction steps were pooled for ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS; ACQUITY UPLC system coupled with a XEVO TQ-S mass spectrometer, Waters, Milford, MA, USA). Calibration curves were constructed by plotting peak area (y) against mass concentration (x), yielding the following regression equations: for salicylic acid, y = 1782.18x − 402.849 (R2 = 0.998); abscisic acid, y = 3566.98x + 434.445 (R2 = 0.999); and zeatin, y = 19,707.8x + 2295.31 (R2 = 0.998), as summarized in Table 1. Hormone quantification was performed using the fully validated UPLC-MS/MS method described by Xiang [26], which reported detection limits of 0.005–4.38 ng/mL, quantification limits of 0.02–8.75 ng/mL, and mean recoveries ranging from 80.33% to 114.99%. The mass spectrometry conditions are provided in Table 1. Each treatment was analyzed with three replicates, and data are presented as mean values.

Table 1.
Optimized mass spectrometry conditions, regression equations and correlation coefficients for 6 compounds.
2.5. Determination of Growth Index
The seedlings were harvested on the 60th day following CPPU treatment. From each of the four replicate pots per treatment, two plants were randomly selected. Their height, crown width, main root length, and root diameter were measured. Subsequently, the sandy soil adhering to the root surface was gently washed off with water. The clean root systems were carefully spread in a transparent acrylic tray (25 cm × 15 cm) for image acquisition using a WinRHIZO LA 2400 root scanner (WinRHIZO LA 2400, Epson, Suwa, Japan). Morphological parameters, including total root surface area and total root volume, were quantified using the WinRHIZO root analysis system. Finally, the plants were separated into stems, leaves, and roots. These parts were then oven-dried at 60 °C until a constant weight was achieved, after which their dry weights were determined.
2.6. Determination of Main Medicinal Components
The concentration of total flavonoids was determined using an ultraviolet spectrophotometer (UV-1900 spectrophotometer, Shimadzu, Kyoto, Japan) [27]. The dried roots within the same treatment group were pooled, pulverized, and sieved through a 40-mesh screen. Precisely 0.100 g of the powdered sample was weighed into a 10 mL test tube. Then, 7 mL of 60% ethanol was added, and the mixture was subjected to ultrasonic extraction for 2 h using a 300 W ultrasonic apparatus (KQ-300E, Kunshan Ultrasonic Instrument Co., Ltd., Kunshan City, China). The extract was then centrifuged at 12,000 rpm for 30 min. The supernatant was collected and filtered through a 0.45 μm micropore membrane. Thereafter, a 2 mL aliquot of this filtrate was mixed with 2 mL of 10% potassium hydroxide aqueous solution. After standing at room temperature for 5 min, the solution was diluted to 10 mL with 70% ethanol. The absorbance of the final solution was measured at 400 nm using an ultraviolet spectrophotometer (UV-1900, Shimadzu, Kyoto, Japan). A standard stock solution of liquiritin was used to establish a calibration curve over the concentration range of 0–26.7 μg·mL−1, yielding the regression equation y = 0.0487435x + 0.167866 (R2 = 0.995). Each treatment was measured 3 times, and the average total flavonoid content was calculated.
Standard stock solutions of glycyrrhizic acid and liquiritin were prepared and then serially diluted with methanol to produce a series of working standards at concentrations of 1, 10, 50, 100, and 500 ng·mL−1. The standard curves were generated based on the mass concentration (x) and peak area (y). The calibration curves were: y = 87.2x − 3.76, R2 = 0.999 and y = 750.9x + 356.9, R2 = 0.999. For sample analysis, 1.000 g of the licorice root powder was weighed into a test tube. Then, 10 mL of methanol was added, and the mixture was extracted by ultrasonication for 1 h. The extract was then centrifuged at 12,000 rpm for 30 min. The resulting supernatant was collected and filtered through a 0.45 μm microporous membrane prior to UPLC-MS/MS analysis. The concentrations of glycyrrhizic acid and liquiritin were quantified according to the fully validated method reported by Wang [28]. As cited therein, the method’s detection limits were 50 and 0.5 ng·mL−1 for glycyrrhizic acid and liquiritin, respectively, while the quantification limits were 150 and 1.5 ng·mL−1. The mean analytical recovery rates were 98.52% and 98.15%, respectively. Detailed mass spectrometry conditions are listed in Table 1. Each treatment was analyzed in triplicate, and the mean values were calculated.
2.7. Determination of CPPU Residue
The leaves of G. uralensis seedlings were sprayed with a 10 mg·L−1 CPPU solution. Root samples were then collected at multiple time points: 2 h, and 1, 3, 5, 7, 14, 21, 28, and 56 days post-treatment. Three biological replicates (n = 3) were obtained for each time point. The harvested roots were oven-dried at 60 °C until a constant weight was reached, then ground into a fine powder. A standard stock solution was prepared by dissolving 5 mg of CPPU in acetonitrile and diluting to 5 mL in a brown volumetric flask, yielding a concentration of 1 mg·mL−1. This stock was then serially diluted with acetonitrile to generate a calibration series at 0.01, 0.1, 1, 10, and 100 μg·mL−1. The calibration curve, constructed by plotting peak area (y) against mass concentration (x), was defined by the equation y = 1957.35x + 3915.69 (R2 = 0.991), as detailed in Table 1. 10.0 g of root powder was placed in a centrifuge tube. Then, 10 mL of acetonitrile was added, and the mixture was vortexed for 3 min. Subsequently, 4.0 g of MgSO4 and 1.0 g of NaCl were added, followed by another 3 min of vortexing and centrifugation at 6000 rpm for 10 min. The supernatant (1.5 mL) was added with 150 mg of MgSO4 and 50 mg of NaCl, vortexed for 3 min, followed by a centrifugation at 6000 rpm for 10 min. The resulting supernatant was filtered through a membrane with a pore size of 0.45 μm to obtain a filtrate for testing. The residual CPPU in the filtrate was determined using UPLC-MS/MS method validated by Jiang [29]. As stated in the article, the detection limits for CPPU was 0.2 μg·kg−1 the quantification limits was 0.5 μg·kg−1. The average analytical recovery rate was 100.13%. The mass spectrometry conditions are detailed in Table 1. Each treatment was repeated three times, and the average value was calculated.
Mass spectrometry conditions: The ion source was an electrospray ionization source (ESI), and the multi-reaction detection mode (MRM) was used for content determination. The desolvation temperature −450 °C, source temperature −150 °C, desolvation gas flow rate was 800 L·h−1, cone gas flow −150 L·h−1, capillary voltage −3000 V. The product ions of the six compounds were optimized based on the collision-induced dissociation of their parent ions. The quantitative analysis of ion pairs, fragmentation voltage and collision energy are shown in Table 1. Chromatographic conditions: Waters ACQUITY UPLC BEH C18 column (50 mm × 2.1 mm, 1.7 μm); flow rate: 0.3 mL·min−1; injection volume: 1 μL; column temperature: 30 °C. The elution procedure is shown in Table 2.

Table 2.
Gradient elution program of mobile phase A (0.1% formic acid-water) and B (acetonitrile).
The degradation kinetic of CPPU in licorice root was determined by plotting the curve of residual concentration over time. The residual concentration and half-life of CPPU were calculated. The equations used are as follows:
Ct = C0e−kt
t1/2 = ln(2)·k−1
In these formulas, t represents the time after CPPU application (in days), Ct is the residual concentration of CPPU at time t, C0 is the initial concentration of CPPU immediately after application (t = 0), k is the dissipation coefficient, and t1/2 is defined as the time required for the residual level of CPPU to decrease to half of its initial value following application.
The risk quotient (RQ) was employed to assess the chronic and acute dietary risks associated with CPPU [30]. An RQ value less than 1 indicates a level of risk that is acceptable to human health, whereas an RQ value exceeding 1 signifies a higher level of risk for consumers. The equations used are as follows:
EDI = (F × STMR)/(bw)
RQ = (EDI/ADI) × 100%
In these equations, EDI denotes to the estimated daily intake (mg·kg−1); F refers the vegetable intake (kg·d−1) from various countries and regions, including China [31], the United States, the European Union [32], Australia [33], and Japan [34]; bw represents the average weight of Chinese adults (63.7 kg) [35]; STMR is the average concentration (mg·kg−1) of residues on the 56th day after the treatment; RQ is the risk coefficient; and ADI indicates the acceptable daily intake (mg·kg−1·bw−1) established in different countries.
2.8. Statistical Analysis
SPSS 20.0 software was utilized for statistical analysis. One-way ANOVA was employed to compare the differences in various indicators among different treatments, while Duncan’s multiple comparison test was conducted for post hoc analysis. Origin Pro 2017 was used to create the histogram, where the column height represents the average value of each parameter, and the error bar indicates the standard deviation of the average value.
3. Results
3.1. Effects of CPPU on Photosynthetic Indexes
Net Photosynthetic rate (Pn; F(5,30) = 7.785, p < 0.001), Transpiration rate (Tr; F(5,30) = 9.646, p < 0.001), stomatal conductance (GS; F(5,30) = 9.694, p < 0.001) and the activity of ribulose-1,5-bisphosphate carboxylase activity (RuBPCase; F(5,30) = 53.899, p < 0.001) exhibited a hormetic response to CPPU concentration, These parameters increased to an optimum at 10 mg·L−1 before declining at higher concentrations. Intercellular CO2 concentration (Ci; F = 20.885, p < 0.001) was the exception to this trend, showing a declining pattern. CPPU at 5 and 10 mg·L−1 significantly enhanced the activity of RuBPCase, Pn and Tr in G. uralensis leaves. RuBPCase activity reached 110.48% (p < 0.001) and 161.29% (p < 0.001) of the control, respectively. Similarly, Pn increased by 24.26% (p = 0.016) and 38.99% (p < 0.001), while Tr increased by 40.99% (p = 0.005) and 53.30% (p < 0.001), relative to the control (Figure 1).
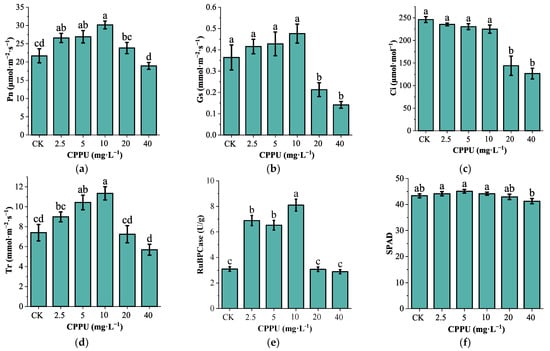
Figure 1.
Effect of CPPU treatment on photosynthetic potential of G. uralensis leaves. (a): Net photosynthetic rate, (b): Stomatal conductivity; (c): Intercellular CO2 concentration; (d): Transpiration rate; (e): RuBPCase; (f): Relative chlorophyll content. Note: Different lowercase letters represent significant differences at the level of p < 0.05.
3.2. Effect of CPPU on Concentration of Endogenous Hormones
The levels of salicylic acid (SA; F(5,12) = 28.339, p < 0.001), abscisic acid (ABA; F(5,12) = 21.837, p < 0.001) and Zeatin (F(5,12) = 25.414, p < 0.001) in the leaves increased significantly with rising CPPU concentrations. Compared to the control, the concentrations of SA in the 10, 20, and 40 mg·L−1 CPPU treatment increased by 48.15% (p = 0.001), 72.22% (p < 0.001), and 114.81% (p < 0.001), respectively. Zeatin levels in these three treatments increased by 87.21%, 74.42%, and 76.74% (all p < 0.001). Meanwhile, ABA levels increased by 46.32% (p = 0.001), and 87.89% (p < 0.001) under the 20 and 40 mg·L−1 CPPU treatments, respectively (Figure 2).
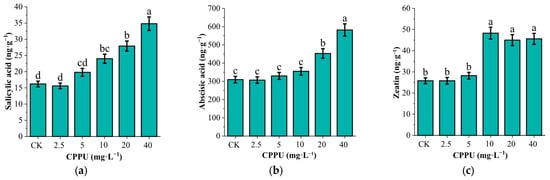
Figure 2.
Effects of CPPU treatment on hormone content in leaves of G. uralensis. (a) Salicylic acid; (b) Abscisic acid; (c) Zeatin. Note: Different lowercase letters represent significant differences at the level of p < 0.05.
3.3. EFFECT of CPPU on Growth Index
Visual assessment and quantitative measurements confirmed that CPPU treatments significantly influenced the growth architecture of G. uralensis plants (Figure 3). The plant height (F(5,42) = 15.256, p < 0.001), crown width (F(5,42) = 11.204, p < 0.001), taproot length (F(5,42) = 7.178, p < 0.001), taproot diameter (F(5,42) = 3.849, p = 0.006), total root surface area (F(5,42) = 2.495, p = 0.046) and total root volume (F(5,42) = 1.282, p = 0.29) of G. uralensis initially increased and then decreased, With the increasing CPPU concentration. Compared to the CK, only the 10 mg·L−1 CPPU treatment significantly promoted plant height (p < 0.001), taproot length (p < 0.001), taproot diameter (p = 0.05) and total root surface area (p = 0.016) of the seedlings, with increase of 49.44%, 17.07%, 11.35%, and 58.07%, respectively (Figure 4).

Figure 3.
Effects of CPPU treatment on the growth of G. uralensis seedlings.
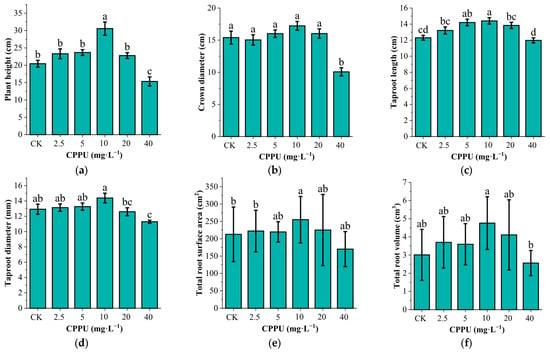
Figure 4.
Effects of CPPU treatment on development parameters of G. uralensis seedlings. (a) Plant height; (b) crown width; (c) Taproot length; (d) Taproot diameter; (e) Total root surface area; (f) Total root volume. Different lowercase letters represent significant differences at the level of p < 0.05.
3.4. Effects of CPPU on the Yield and Quality of G. uralensis
With the increasing concentration of CPPU, the biomass of the roots (F(5,42) = 3.688, p = 0.007), glycyrrhizic acid (F(5,12) = 22.102, p < 0.001), liquiritin (F(5,12) = 25.986, p < 0.001) and the total flavonoids contents(F(5,12) = 6.547, p = 0.004) initially increased before subsequently decreasing. The treatment with 10 mg·L−1 CPPU significantly enhanced root biomass to a value 1.46 times greater than that of the control (p = 0.014). CPPU at concentrations ranging from 5 to 10 mg·L−1 significantly enhanced the accumulation of glycyrrhizin, liquiritin, and total flavonoids. Treatment with 5 mg·L−1 CPPU increased their levels by 77.8% (p < 0.001), 92.4% (p < 0.001), and 9.7% (p = 0.003), respectively. Application of 10 mg·L−1 CPPU resulted in increases of 92% (p < 0.001), 98.7% (p < 0.001), and 15.4% (p = 0.041), respectively (Table 3).

Table 3.
Effects of CPPU on Root Biomass and Secondary Metabolites in G. uralensis.
3.5. Degradation Dynamics of CPPU in Roots of G. uralensis
In the roots of G. uralensis, the peak concentration of CPPU residue was observed on the first day following the spraying treatment, reaching a maximum of 6.75 × 10−3 mg·kg−1. By the 56th day, the residue in the roots had decreased to 5.44 × 10−4 mg·kg−1, which accounted for only 8% of the maximum residue (Figure 5). The dissipation process of CPPU residues followed a first-order kinetic reaction. Based on the formula Ct = C0e−kt, the half-life of CPPU dissipation in the roots of G. uralensis was determined to be11.74 days (Figure 5).
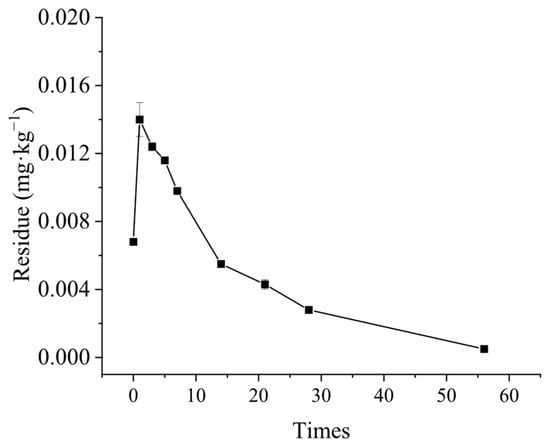
Figure 5.
Degradation dynamics of CPPU in G. uralensis plants.
In comparison to the safety standards for CPPU across five types of fruits and vegetables in various countries and regions (Table 4), the residual levels of CPPU in the roots after 56 days of treatment, complied with the safety standards established by China, the United States, the European Union, Australia, and Japan.

Table 4.
Residue limits for forchlorfenuron in 5 kinds of fruit and vegetable products in different region and countries (mg.kg−1).
On the 56th day following the application of CPPU, the residual concentrations in the roots of G. uralensis was measured at only 5.44 ×10−4 mg·kg−1. Based on the average weight of adults and the maximum per capita vegetable intake across various countries and regions, the estimated daily intake (EDI) of CPPU from the roots of G. uralensis was calculated to be 2.39 × 10−6, 4.71 × 10−6, 5.26 × 10−6, 1.75 × 10−6, and 2.97 × 10−6, respectively (Table 5). According to the acceptable daily intake (ADI) of CPPU established by China, the European Union, the United States, Australia, and Japan, the risk percentages associated with root intake in these countries or regions were 0.00%, 0.01%, 0.01%, 0.00%, and 0.00%, respectively. All risk quotient (RQ) values for the licorice roots were significantly below 1.

Table 5.
Risk assessment of CPPU long-term intake.
4. Discussion
Various agronomic strategies—such as optimized irrigation [36], adjusted planting density [37], precision fertilization [38], and germplasm improvement [39]—have been investigated in licorice cultivation to enhance yield and quality. However, the application of plant growth regulators, specifically CPPU, remains relatively unexplored in this context. Previous studies have demonstrated CPPU’s efficacy in improving crop productivity. For instance, it increased grape [40] and jatropha fruit [41] yield at 5 mg·L−1, and improved cucumber quality [42] at 10 mg·L−1. Beyond yield traits, CPPU can modulate plant metabolic pathways; it has been shown to reduce anthocyanin content in grapes [43] while stimulating phenolic compound synthesis in kiwifruit [44]. Our findings indicate that CPPU treatments (5–20 mg·L−1) significantly promoted the growth of G. uralensis, increasing plant height, taproot length, root diameter, total root volume, total root surface area, and biomass. The most beneficial effects were observed at 10 mg·L−1. Moreover, the contents of glycyrrhizic acid, liquiritin, and total flavonoids in the roots were also markedly elevated, with the greatest improvement likewise occurring at 10 mg·L−1. The concurrent increase in root biomass and key bioactive compounds demonstrates that CPPU can not only enhance the yield of fruit crops, but also significantly enhance the biomass accumulation of medicinal plants’ underground organs and improve their quality.
Our findings indicate that CPPU application induces a series of interconnected physiological responses in licorice. At the optimal concentration of 10 mg·L−1, leaf photosynthetic capacity was substantially enhanced, accompanied by a marked increase in RuBPCase activity to 161.29% of the control level. Unlike its documented role in enhancing chlorophyll content in Dioscorea opposita Thunb. [45] or delaying chlorophyll degradation in banana [46], the positive effect of CPPU on the photosynthetic system of licorice appears to be mediated principally through the upregulation of RuBPCase. The synchronized increase in both parameters suggests that RuBPCase activation plays a central mediating role in the CPPU-induced stimulation of photosynthetic carbon assimilation in this species.
The role of CPPU extends far beyond merely promoting growth; it recalibrates the hormonal network within the plant, thereby directing the flow of metabolic processes [47]. This study directly documents three core observations: (1) a significant increase in endogenous zeatin levels; (2) rise in salicylic acid (SA) content; and (3) under high-CPPU treatment, a substantial accumulation of abscisic acid (ABA) accompanied by severe suppression of photosynthesis. First, the marked elevation in endogenous zeatin provides direct evidence that the plant perceives the external CPPU signal. Given that zeatin is well established to stimulate cell division and expansion [48], we propose that its upregulated levels offer a plausible hormonal explanation for the observed increase in root biomass. Second, the rapid increase in SA coincided with the accumulation of glycyrrhizin and flavonoids. Based on the consensus that SA is a key regulator of the phenylpropanoid pathway [49], we hypothesize that CPPU-induced SA acts as a central signaling molecule. Functioning as a precise “metabolic switch,” it activates key enzymes in this pathway, transducing the upstream signal into downstream biosynthetic routes that enhance crop quality. Moreover, the growth inhibition and photosynthetic decline induced by high CPPU levels strongly correlated with the explosive increase in ABA. Extensive studies have demonstrated that ABA induces stomatal closure, thereby impairing photosynthesis [50]. We therefore conclude that high CPPU concentrations likely trigger excessive ABA synthesis, forcing the plant into a stressed state that ultimately restrains growth. In summary, these interrelated hormonal shifts collectively form an integrated regulatory framework. Through this framework, CPPU artfully coordinates structural growth with the accumulation of specialized metabolites. It should also be emphasized that high CPPU levels significantly reduced RuBPCase activity, implying that supraoptimal CPPU may exert phytotoxic effects on licorice plants.
The assessment of residue risks for medicinal materials is particularly critical to ensure that the safety of the final products meets international standards [51]. The dissipation rate of residues in the licorice root was relatively rapid initially, subsequently slowing over time, which was consistent with the dissipation patterns observed in grapes, watermelons, and citrus fruits. The degradation dynamics of CPPU in the root of G. uralensis followed first-order kinetics, with a half-life of 11.7 days. Reports indicate that the dissipation half-life of CPPU in melons ranges from 1.20 to 1.33 days [52], in watermelons from 1.16 to 1.66 days [53], in grapes at 5.0 days [54], and in citrus fruits from 15.8 to 23.0 days [55]. The longer dissipation half-life observed in licorice roots compared to fruits is likely due to the root being a storage organ with lower metabolic activity and different vascular connectivity compared to rapidly developing fruits, leading to slower compound degradation. After 56 days of CPPU application to the leaves of G. uralensis, the concentration of CPPU in its roots decreased to an extremely low level, significantly below the maximum residue limits established by countries such as China, the United States, the European Union, Australia, and Japan. Therefore, in terms of medicinal roots, the application of CPPU on G. uralensis meets safety standards. Given the absence of established residue limits for CPPU in medicinal plants, we assessed the long-term dietary exposure risk of CPPU to human health using data on the highest average vegetable consumption per person across various countries and regions, as well as the mean adult body weight in Asia. The research results showed that after two months of spraying treatment with 10 mg L−1 CPPU on G. uralensis leaves, the health risk associated with consuming the roots approached zero. In fact, the intake of licorice in food is significantly lower than that of vegetables. Therefore, applying CPPU to G. uralensis foliage presents no significant safety concern regarding root contamination.
In summary, this paper investigates the effects of spraying CPPU on the leaves of the medicinal plant G. uralensis, focusing on its growth, yield, quality, and safety risk. The results demonstrate that a 10 mg·L−1 CPPU solution not only markedly enhanced the photosynthetic capacity of G. uralensis but also significantly promoted its growth, yield, and quality of its medicinal materials. Furthermore, it notably impacted the content of endogenous hormones including zeatin, abscisic acid, and salicylic acid. The dissipation half-lives of CPPU in the roots of G. uralensis were found to be 11.74 days, with the residual amount of CPPU nearing zero after 56 days of the treatment, which is well below international safety standards. Thus, there is a negligible health risk to consumers associated with CPPU intake based on vegetable consumption levels. Our study provides a valuable reference for sustainable agriculture and medicinal plant cultivation.
Author Contributions
These authors contributed equally: J.G., H.L. and M.M.: funding acquisition, investigation, project Management; J.G.: writing—original, writing—Review and Editing; S.Y.: data Management; H.L.: supervision, pictures drawing. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by Grants from the Tianshan Talents Teaching Masters Project in Xinjiang, China (20240428).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
We thank the reviewers and editors for their work.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Song, L.; Wang, J.; Gong, M.; Duan, Y.; Zhang, Y.; Li, Y.; Qin, L.; He, Q.; Ji, L.; Zhang, T.; et al. Investigation of the principle of concoction by using the processing excipient Glycyrrhiza uralensis Fisch. juice to reduce the main toxicity of Dioscorea bulbifera L. and enhance its main efficacy as expectorant and cough suppressant. J. Ethnopharmacol. 2024, 319, 117372. [Google Scholar] [CrossRef]
- Barati, S.; Feizabadi, F.; Khalaj, H.; Sheikhzadeh, H.; Jamaati, H.R.; Farajidavar, H.; Dastan, F. Evaluation of noscapine-licorice combination effects on cough relieving in COVID-19 outpatients: A randomized controlled trial. Front. Pharmacol. 2023, 14, 1663–9812. [Google Scholar] [CrossRef]
- Sarkar, S.; Shaw, P.; Singh, P.; Chowdhury, A.A. Emerging neuroprotective potential of Liquorice: Mechanistic insights for neurological disorders. S. Afr. J. Bot. 2023, 154, 149–158. [Google Scholar] [CrossRef]
- Zuo, J.; Meng, T.; Wang, Y.; Tang, W. A review of the antiviral activities of glycyrrhizic acid, glycyrrhetinic acid and glycyrrhetinic acid monoglucuronide. Pharmaceuticals 2023, 16, 641. [Google Scholar] [CrossRef]
- Hajirahimkhan, A.; Mbachu, O.; Simmler, C.; Ellis, S.G.; Dong, H.; Nikolic, D.; Lankin, D.C.; van Breemen, R.B.; Chen, S.-N.; Pauli, G.F.; et al. Estrogen receptor (ER) subtype selectivity identifies 8-Prenylapigenin as an ERβ agonist from Glycyrrhiza inflata and highlights the importance of chemical and biological authentication. J. Nat. Prod. 2018, 81, 966–975. [Google Scholar] [CrossRef]
- Ruchi, S.; Singla, R.K.; Subhadip, B.; Rohit, S. Revisiting Licorice as a functional food in the management of neurological disorders: Bench to trend. Neurosci. Biobehav. Rev. 2023, 155, 105452. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chen, L.; Xu, C.; Shi, J.; Chen, S.; Tan, M.; Chen, J.; Zou, L.; Chen, C.; Liu, Z.; et al. A comprehensive review for phytochemical, pharmacological, and biosynthesis studies on Glycyrrhiza spp. Am. J. Chinese Med. 2020, 48, 17–45. [Google Scholar] [CrossRef] [PubMed]
- Fatemeh, A.M.; Erfan, A.; Reza, M.; Mohammad, Y.A.; Jalal, P. Effect of chewing gum containing Glycyrrhiza glabra, honey, and Vitamin E on oral Health. J. Herb. Med. 2024, 43, 2210–8033. [Google Scholar] [CrossRef]
- Tayebe, A.; Mehrdad, N.; Seyed, M.; Bagher, H.; Luisa, T. A three-step sensory-based approach to maximize consumer acceptability for new low-sugar licorice-chocolate-flavored milk drink. LWT-Food Sci. Technol. 2018, 91, 375–381. [Google Scholar] [CrossRef]
- Nardini, M.; Foddai, M.S. Phenolics profile and antioxidant activity of special beers. Molecules 2020, 25, 2466. [Google Scholar] [CrossRef]
- Carmines, E.L.; Lemus, R.; Gaworski, C.L. Toxicologic evaluation of licorice extract as a cigarette ingredient. Food Chem. Toxicol. 2005, 43, 1303–1322. [Google Scholar] [CrossRef]
- Wang, H.; Song, W.; Tao, W.; Zhang, J.; Zhang, X.; Zhao, J.; Yong, J.; Gao, X.; Guo, L. Identification wild and cultivated licorice by multidimensional analysis. Food Chem. 2021, 339, 128111. [Google Scholar] [CrossRef] [PubMed]
- AL-Hmadi, H.B.; Romdhani, A.; Majdoub, S.; Dhaouadi, H.; Zengin, G.; Hammami, S. Chemical composition, antioxidant and multi-enzymatic inhibitory potential of licorice harvested from wild populations in Iraq. S. Afr. J. Bot. 2023, 158, 56–62. [Google Scholar] [CrossRef]
- Wang, C.; Cai, H.; Zhao, H.; Yan, Y.; Shi, J.; Chen, S.; Tan, M.; Chen, J.; Zou, L.; Chen, C.; et al. Distribution patterns for metabolites in medicinal parts of wild and cultivated licorice. J. Pharm. Biomed. 2018, 161, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Lang, D.; Li, X.; Yang, L.; Xiao, X.; Zhang, X.; Long, W. Comparison of flavonoids contents in cultivated Glycyrrhiza uralensis of different producing areas and the correlation with soil factors. J. Chin. Med. Mater. 2022, 45, 1531–1537. (In Chinese) [Google Scholar] [CrossRef]
- Valverde, A.; Aguilera, A.; Ferrer, C.; Camacho, F.; Cammarano, A. Analysis of forchlorfenuron in vegetables by LC/TOF-MS after extraction with the buffered quechers method. J. Agric. Food Chem. 2010, 58, 2818–2823. [Google Scholar] [CrossRef]
- Ainalidou, A.; Tanou, G.; Belghazi, M.; Samiotaki, M.; Diamantidis, G.; Molassiotis, A.; Karamanoli, K. Integrated analysis of metabolites and proteins reveal aspects of the tissue-specific function of synthetic cytokinin in kiwifruit development and ripening. J. Proteom. 2016, 143, 318–333. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, X.; Guo, L.; Zeng, D.; Xiao, D.; He, L.; Wang, A. Effects of forchlorfenuron on yield and quality of kudzu root. J. South. Agric. 2017, 48, 1581–1586. (In Chinese) [Google Scholar]
- Yin, N.; Ma, X.; Zhang, W.; Feng, D.; Wang, H.; Kong, L.; Tian, J. Analysis of differential proteins induced by forchlorfenuron in wheat. Plant Mol. Biol. Rep. 2012, 30, 949–956. [Google Scholar] [CrossRef]
- Roussos, P.A.; Ntanos, E.; Denaxa, N.K.; Tsafouros, A.; Bouali, I.; Nikolakakos, V.; Assimakopoulou, A. Auxin (triclopyr) and cytokinin (forchlorfenuron) differentially affect fruit physiological, organoleptic and phytochemical properties of two apricot cultivars. Acta Physiol. Plant 2021, 43, 25. [Google Scholar] [CrossRef]
- Wang, J.; Cai, X.; Zeng, S.; Zhang, Z.; Chi, Q.; Guo, W. Effect of forchlorfenuron and thidiazuron on kiwifruits’ internal qualities, optical properties and their relationship during growth. Spectrochim. Acta A 2024, 308, 123749. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Tang, T.; Xu, H.; Li, Z.; Yang, G.; Wang, Q. Dietary intake risk assessment of chlorpyriprole residues in fruits and vegetables. Chin. J. Agric. Sci. 2012, 45, 1982–1991. (In Chinese) [Google Scholar]
- Chen, B.; Tan, R.; Hu, Y.; Li, G. Chemiluminescence method based on the KIO4−K2CO3−Mn2+ reaction for rapid and sensitive determination of forchlorfenuron in dried fruit. Luminescence 2023, 38, 1639. [Google Scholar] [CrossRef]
- Wang, Q.; Li, X.; Wang, H.; Li, S.; Zhang, C.; Chen, X.; Dong, J.; Shao, H.; Wang, J.; Jin, F. Spatial Distribution and Migration Characteristic of Forchlorfenuron in Oriental Melon Fruit by Matrix-Assisted Laser Desorption/Ionization Mass Spectrometry Imaging. Foods 2023, 12, 2858. [Google Scholar] [CrossRef]
- Shan, T.; Zhang, X.; Guo, C.; Guo, S.; Zhao, X.; Yuan, Y.; Yue, T. Identity, synthesis, and cytotoxicity of forchlorfenuron metabolites in kiwifruit. J. Agr. Food Chem. 2021, 69, 9529–9535. [Google Scholar] [CrossRef]
- Xiang, Y.; Song, X.; Qiao, J.; Zang, Y.; Li, Y.; Liu, Y.; Liu, C. An ultrahigh-performance liquid chromatography method with electrospray ionization tandem mass spectrometry for simultaneous quantification of five phytohormones in medicinal plant Glycyrrhiza uralensis under abscisic acid stress. J. Nat. Med. 2015, 69, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Mare, R.; Pujia, R.; Maurotti, S.; Greco, S.; Cardamone, A.; Coppoletta, A.R.; Bonacci, S.; Procopio, A.; Pujia, A. Assessment of Mediterranean Citrus Peel Flavonoids and Their Antioxidant Capacity Using an Innovative UV-Vis Spectrophotometric Approach. Plants 2023, 12, 4046. [Google Scholar] [CrossRef]
- Wang, J.; Dong, W.; He, Z.; Dong, L.; Zhao, L. Simultaneous determination of eight active components in Astragali Radix and Glycyrrhizae Radix by UPLC-MS/MS. J. Harbin Univ. Comm. 2020, 36, 665–670. (In Chinese) [Google Scholar]
- Jiang, Y.; Wang, C.; Wang, G.; Liu, X. Determination of forchlorfenuron residue in eggplant by ultra performance liquid chromatography-tandem mass spectrometry. J. Zhejiang Agric. Sci. 2015, 56, 1736–1737+1746. (In Chinese) [Google Scholar]
- Zhou, L.; Jiang, Y.; Lin, Q.; Wang, X.; Zhang, X.; Xu, J.; Chen, Z. Residue transfer and risk assessment of carbendazim in tea. J. Sci. Food Agr. 2018, 98, 5329–5334. [Google Scholar] [CrossRef]
- Lv, M.; Xiang, C.; Su, C.; Huang, F.; Wang, H.; Jia, X.; Du, W. Analysis of trends and factors of vegetable consumption of adult in China from 1991 to 2018. Chin. J. Food Hyg. 2018, 36, 955–961. (In Chinese) [Google Scholar] [CrossRef]
- Gregori, D.; French, M.; Gallipoli, S.; Lorenzoni, G.; Ghidina, M. Consumption of fruit and vegetables: The ROUND (world map of cnsumption of fruit and vegetables and Nutrient Deficits) project. Proc. Nutr. Soc. 2020, 79, E698. [Google Scholar] [CrossRef]
- Fayet-Moore, F.; McConnell, A.; Cassettari, T.; Tuck, K.; Petocz, P.; Kim, J. Vegetable intake in Australian children and adolescents: The importance of consumption frequency, eating occasion and its association with dietary and sociodemographic factors. Public Health Nutr. 2020, 23, 474–487. [Google Scholar] [CrossRef]
- Yuan, X.; Tajima, R.; Matsumoto, M.; Fujiwara, A.; Aoyama, T.; Okada, C.; Okada, E.; Takimoto, H. Analysing food groups and nutrient intake in adults who met and did not meet the daily recommended vegetable intake of 350g: The 2016 National Health and Nutrition Survey in Japan. J. Nutr. Sci. 2024, 13, e12. [Google Scholar] [CrossRef]
- Peng, C.; Cai, H.; Zhu, X.; Li, D.; Yang, Y.; Hou, R.; Wan, X. Analysis of naturally occurring fluoride in commercial teas and estimation of its daily intake through tea consumption. J. Food Sci. 2016, 81, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Marjan, S.H.; Morteza, E.; Javier, A.; Saeid, K.; Rasoul, A. Growth, phytochemical parameters and glycyrrhizin production in licorice (Glycyrrhiza glabra L.) grown in the field with saline water irrigation. Ind. Crop Prod. 2022, 177, 114444. [Google Scholar] [CrossRef]
- Jia, T.; Chen, B.; Ma, M. Effects of planting density on the growth, taproots yield and quality of Glycyrrhiza uralensis. Legume Res. 2023, 46, 62–68. [Google Scholar] [CrossRef]
- Tahereh, G.; Leila, T.; Vahideh, N.; Mohammad, E. Nutrient distribution in various tissues of licorice (Glycyrrhiza glabra L.) and the influence of soil fertility on the levels of its bioactive compounds. Ind. Crop Prod. 2024, 209, 118073. [Google Scholar] [CrossRef]
- Liu, Q.; Guo, S.; Zheng, X.; Shen, X.; Zhang, T.; Liao, B.; He, W.; Hu, H.; Cheng, R.; Xu, J. Licorice germplasm resources identification using DNA barcodes inner-variants. Plants 2021, 10, 2036. [Google Scholar] [CrossRef] [PubMed]
- Peppi, M.C.; Fidelibus, M.W. Effects of forchlorfenuron and abscisic acid on the quality of ‘Flame Seedless’ grapes. HortScience 2008, 43, 173–176. [Google Scholar] [CrossRef]
- Fröschle, M.; Horn, H.; Spring, O. Effects of the cytokinins 6-benzyladenine and forchlorfenuron on fruit-, seed- and yield parameters according to developmental stages of flowers of the biofuel plant Jatropha curcas L. (Euphorbiaceae). Plant Growth Regul. 2017, 81, 293–303. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, C.; Liu, J. The effects of chlorpyriprole and sodium chlorophenoxyacetate on cucumber fruit growth. North. Hortic. 2016, 20, 37–40. (In Chinese) [Google Scholar]
- Marie, A.; María-Rosa, G.; Rafael, M.; Pedro, M. Effects of the application of forchlorfenuron (CPPU) on the composition of verdejo grapes. BIO Web Conf. 2023, 56, 01022. [Google Scholar] [CrossRef]
- Bi, Y.; Qiao, C.; Han, L.; Xie, H.; Xu, Y.; Wu, D.; Zhuang, M.; Lv, X.; Cao, M. Key metabolites and mechanistic insights in forchlorfenuron controlling kiwifruit development. Food Res. Int. 2022, 164, 112412. [Google Scholar] [CrossRef]
- Li, C.; Wang, J.; Xu, J.; He, L.; He, H. Effects of forchlorfenuron and paclobutrazol combination on the physiological characteristics of yam leaves. J. South. Agric. 2012, 43, 1952–1957. (In Chinese) [Google Scholar]
- Huang, H.; He, W. Application of exogenous cytokinin regulates cytokinin oxidase and antioxidant activity to maintain chlorophyll pigment during ripening of banana fruit. Food Biosci. 2023, 55, 102998. [Google Scholar] [CrossRef]
- Dong, Q.; Yan, Y.; Guo, S.; Ling, H.; Yang, X.; Gao, J.; Shen, J.; Zu, C. Influence of exogenous forchlorfenuron on quality of upper leaves of flue-cured tobacco and its mechanism. Tob. Sci. Technol. 2024, 57, 26–35. (In Chinese) [Google Scholar] [CrossRef]
- Ahmad, I.; Kamran, M.; Meng, X.; Ali, S.; Bilegjargal, B.; Cai, T.; Liu, T.; Han, Q.F. Effects of plant growth regulators on seed filling, endogenous hormone contents and maize production in semiarid regions. J. Plant Growth Regul. 2019, 38, 1467–1480. [Google Scholar] [CrossRef]
- La, V.H.; Tran, D.H.; Han, V.; Nguyen, T.D.; Duong, V.C.; Nguyen, V.H.; Tran, A.T.; Nguyen, T.H.G.; Ngo, X.B. Drought stress-responsive abscisic acid and salicylic acid crosstalk with the phenylpropanoid pathway in soybean seeds. Physiol. Plant 2023, 175, e14050. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.R.; Bin Wang, Y.; Bin He, S.; Hao, F.S. Mechanisms for abscisic acid inhibition of primary root growth. Plant Signal Behav. 2018, 13, e1500069. [Google Scholar] [CrossRef] [PubMed]
- Arena, M.; Auteri, D.; Barmaz, S.; Bellisai, G.; Brancato, A.; Brocca, D.; Bura, L.; Byers, H.; Chiusolo, A.; Marques, D.C.; et al. Peer review of the pesticide risk assessment of the active substance forchlorfenuron. Efsa J. 2017, 15, 4874. [Google Scholar] [CrossRef]
- Wang, Q.; Su, H.; Yue, N.; Li, M.; Li, C.; Wang, J.; Jin, F. Dissipation and risk assessment of forchlorfenuron and its major metabolites in oriental melon under greenhouse cultivation. Ecotox Environ. Safe 2021, 225, 112700. [Google Scholar] [CrossRef] [PubMed]
- Wu, T. Solid-phase extraction-gas chromatography was used to determine the degradation dynamics and residual amount of forchlorfenuron in watermelon. Shanghai Agric. Sci. Technol. 2019, 2, 43–45. (In Chinese) [Google Scholar]
- Ugare, B.; Banerjee, K.; Ramteke, S.D.; Pradhan, S.; Oulkar, D.P.; Utture, S.C.; Pandurang, G.A.; Bharat, U.; Kaushik, B.; Ramteke, S.D.; et al. Dissipation kinetics of forchlorfenuron, 6-benzyl aminopurine, gibberellic acid and ethephon residues in table grapes (Vitis vinifera). Food Chem. 2013, 141, 4208–4214. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Jiao, B.; Su, X.; Zhao, Q.; Qin, D.; Wang, C. Dissipation and residue of forchlorfenuron in citrus fruits. B Environ. Contam. Tox. 2013, 90, 756–760. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).