Abstract
The ordinary Portland cement (OPC) manufacturing process is highly resource-intensive and contributes to over 5% of global CO2 emissions, thereby contributing to global warming. In this context, researchers are increasingly adopting geopolymers concrete due to their environmentally friendly production process. For decades, industrial byproducts such as fly ash, ground-granulated blast-furnace slag, and silica fume have been used as the primary binders for geopolymer concrete (GPC). However, due to uneven distribution and the decline of coal-fired power stations to meet carbon-neutrality targets, these binders may not be able to meet future demand. The UK intends to shut down coal power stations by 2025, while the EU projects an 83% drop in coal-generated electricity by 2030, resulting in a significant decrease in fly ash supply. Like fly ash, slag, and silica fume, natural clays are also abundant sources of silica, alumina, and other essential chemicals for geopolymer binders. Hence, natural clays possess good potential to replace these industrial byproducts. Recent research indicates that locally available clay has strong potential as a pozzolanic material when treated appropriately. This review article represents a comprehensive overview of the various treatment methods for different types of clays, their impacts on the fresh and hardened properties of geopolymer concrete by analysing the experimental datasets, including 1:1 clays, such as Kaolin and Halloysite, and 2:1 clays, such as Illite, Bentonite, Palygorskite, and Sepiolite. Furthermore, this review article summarises the most recent geopolymer-based prediction models for strength properties and their accuracy in overcoming the expense and time required for laboratory-based tests. This review article shows that the inclusion of clay reduces concrete workability because it increases water demand. However, workability can be maintained by incorporating a superplasticiser. Calcination and mechanical grinding of clay significantly enhance its pozzolanic reactivity, thereby improving its mechanical performance. Current research indicates that replacing 20% of calcined Kaolin with fly ash increases compressive strength by up to 18%. Additionally, up to 20% replacement of calcined or mechanically activated clay improved the durability and microstructural performance. The prediction-based models, such as Artificial Neural Network (ANN), Multi Expression Programming (MEP), Extreme Gradient Boosting (XGB), and Bagging Regressor (BR), showed good accuracy in predicting the compressive strength, tensile strength and elastic modulus. The incorporation of clay in geopolymer concrete reduces reliance on industrial byproducts and fosters more sustainable production practices, thereby contributing to the development of a more sustainable built environment.
1. Introduction
It is widely acknowledged that the manufacturing and incorporation of OPC in the construction industry are catalysts not only for emitting staggering amounts of Carbon dioxide (CO2) into the environment, but also for being energy-intensive [1,2,3]. Approximately, producing 1 ton of OPC requires 3.4 gigajoules (GJ) of thermal energy, 110 kilowatt-hours (kWh) of electrical energy and emits substantial amounts of CO2, ranging from 802 to 855 kg [4,5]. The atmosphere’s CO2 content surpassed 417 parts per million (ppm) in 2020, the highest level observed in the past 2.5 million years [6]. The introduction of geopolymer concrete (GPC) into the construction sector presents a promising prospect for sustainability in future built engineering projects by addressing environmental concerns and ensuring the responsible management of natural resources [7,8].
GPC is an eco-friendly inorganic composite derived from rich alumina- and silica-based supplementary cementitious materials (SCMs) with alkaline activators, which form a hardened binder through a chemical reaction called geopolymerisation—a polycondensation reaction that occurs under alkaline or acidic conditions [9,10]. In alkaline systems, aluminate and silicate species dissolve from their precursors and subsequently recombine to form sialate (-Si-O-Al-O-) and polysialate (-Si-O-Al-O–Si-O-) linkages, which are stabilised by alkali cations such as Na+ and K+ [11]. This process yields highly durable, chemically resistant binders that possess mechanical strengths exceeding those of many conventional cement-based systems [12]. Acidic activation remains less extensively studied; however, it is increasingly recognised for its role in forming aluminosilicate-phosphate networks (-Si-O-Al-O-P-), which enhance thermal stability and dielectric properties [13]. For decades, industrial byproducts and industrially processed aluminosilicate-based pozzolanic materials, such as fly ash [14], Ground Granulated Blast Furnace Slag (GGBFS) [15], silica fume [16], Metakaolin (MK) [17], glass powder [18], etc, have been used as SCMs for not only reducing carbon footprint, but also lowering the construction costs. These materials have been utilised as SCMs to partially replace OPC and to produce geopolymer concrete, with complete OPC replacement [19,20].
Research indicates that geopolymer concrete exhibits mechanical strength comparable to that of traditional concrete. Rahman and Al-Ameri [21] developed self-compacting geopolymer concrete using 100% fly ash, a single alkali activator, with no superplasticiser. This study achieved a compressive strength of 40 MPa after 28 days of ambient curing, comparable to that of conventional M40-grade concrete. Mayhoub et al. [22] investigated the development of geopolymer reactive powder concrete under varying curing conditions, including hot-air, heating, and microwave heating, by replacing 35%, 50%, and 100% of the OPC with slag. The results showed that 100% slag-containing samples achieved a compressive strength that was almost 9% higher than that of 50% slag-containing samples in microwave curing with a 4-min constant power level of 720 W. Srivastava et al. [23] also reported that the compressive strength of geopolymer concrete is identical or even higher than that of conventional concrete. Geopolymer concrete exhibited superior durability performance relative to traditional concrete. Generally, geopolymer concrete displays greater resistance to high temperatures and fire resistance due to its ceramic-like properties, in comparison to OPC concrete [23].
Nevertheless, the durability of geopolymer concrete primarily depends on the proportions of OPC and precursors, such as fly ash, slag, and other SCMs. Mehta and Siddique [24] reported that replacing up to 20% of OPC with fly ash significantly improved the mechanical strength, while porosity, water absorption, chloride permeability, and sorptivity decreased substantially. This reduction transpires due to the presence of both N-A-S-H and C-A-S-H, which contribute to the densification and compaction of the geopolymer matrix’s microstructure upon the addition of OPC [24].
The yearly production of fly ash in the US, Canada, UK, Germany, Australia, France, Denmark, Italy, the Netherlands, India, and China is 75, 6, 15, 40, 10, 3, 2, 2, 2, 112, and 100 million tons, respectively and among them only 20% is being used in the construction sector, worldwide [25]. However, the supply of traditional SCMs, such as fly ash, is expected to decline due to rising demand for carbon neutrality across industries, ongoing construction, and the decline of coal-fired power stations [26,27]. Given the global distribution disparities, it’s uncertain whether blast furnace slag, silica fume, and other industrial byproducts will be available in sufficient quantities to meet rising demand [28,29]. Therefore, current research should focus on converting locally available natural clay, waste, or contaminated soil, mud, and sewage sludge into pozzolanic SCMs. Among them, natural clay can be a potent option due to its worldwide availability, cost-effectiveness, and eco-friendliness, as it is an earth-based material with various mineral contents [30]. Being an abundant source of silica and alumina, clay can be effectively used as a pozzolanic material through proper surface activation modification [31,32]. However, there are some criteria to choose a clay as an SCM. According to ASTM, the total amount of SiO2, Al2O3, and Fe2O3 should be at least 70% by weight, ≤34% should be retained on a 45 µm sieve, the moisture content should be ≤3.0%, and the Strength Activity Index (SAI) should be at least ≥75% than that of the control mix [33]. Although clays have significant potential as SCMs, there appears to be limited effort among researchers to investigate specific clay types and their suitability for these applications. Figure 1 illustrates the research trend about different natural clays over the past 25 years, as collected from the Scopus database.
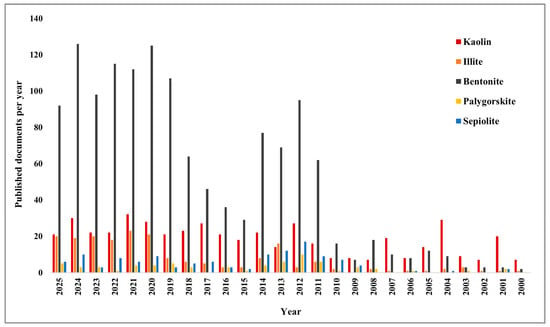
Figure 1.
Research trend of various natural clays over the past 25 years.
Although naturally available clays may represent a prime alternative for substituting existing supplementary cementitious materials (SCMs), limited research has been conducted to date. Most of this research primarily centres on clay activation techniques for various raw clays and their mechanical properties [34,35]. Furthermore, an appropriate treatment or modification method for converting raw clay into pozzolanic clay has not been conspicuously identified. The various types of clay, different activation methods, untreated clay, and their impact on the workability and mechanical performance of geopolymer concrete have not been comprehensively understood due to the absence of a systematic review. Therefore, this review summarises recent research conducted by various researchers, including available clay treatment methods, workability, and strength properties of clay-based geopolymers composites.
Furthermore, various prediction-based models and their accuracy in forecasting the mechanical performance of geopolymer concrete have been summarised. Additionally, the existing research gap and the recommended areas for future investigation have been included. The authors believe that, after thoroughly reading this review, researchers in this field will have a clear understanding of the existing research and the type of research that needs to be conducted in the future to optimise clay-geopolymer composites for the safe and sustainable use of concrete.
Potential Challenges in Utilising Clay as an SCM
Clay-based SCMs offer promising sustainability benefits; however, their practical implementation encounters various challenges. A primary challenge lies in the variability of mineral composition, as natural clays exhibit considerable differences in reactive minerals such as kaolinite, illite, and montmorillonite. This variability influences pozzolanic reactivity and complicates quality control processes, primarily when clays originate from diverse geological sources [36]. Furthermore, the complexity of activation presents a significant challenge: clays generally require thermal treatment (calcination) at precisely controlled temperatures—typically between 600 and 950 °C—to achieve proper reactivity. Incorrect calcination, whether too high or too low, can negatively impact performance. Moreover, the energy-intensive nature of this process raises concerns about sustainability [37,38]. Furthermore, impurities such as quartz, carbonates, or organic matter can disrupt hydration reactions and diminish the effectiveness of the SCM process [39]. These factors underscore the need for rigorous characterisation and tailored activation protocols when incorporating clay into cementitious systems.
2. Clays and Their Chemical Compositions
Clay is widely available on Earth’s surface and results from the erosion of precursor minerals, such as quartz, feldspar, and other clays in small quantities [40]. Illite, kaolinite, and montmorillonite (MMT) are three common clay minerals. In recent years, Kaolinite-based clay has been reported as an auspicious pozzolanic material [41]. However, in recent years, there has been a growing interest in exploring the potential of other types of clays as SCMs, such as Illite [32], Bentonite/Montmorillonite (MMT) [33], Halloysite [34], Palygorskite [35], and Sepiolite [36], among others. The following section outlines the properties and chemical constituents of the clays currently under exploration.
2.1. Kaolinite Clay
Both natural minerals and industrial by-products (secondary clays) can be used to make kaolinitic clay-based pozzolans [42]. Structurally, kaolinite consists of a single tetrahedral sheet made up of disilicate anion (Si2O5)2− and a single octahedral sheet composed of dihydroxy aluminium cation (Al2(OH)4)2+, which is called a 1:1 layered structure [43,44]. Under ambient conditions, kaolinite is incredibly stable. The mineral loses its crystallinity upon sufficient heating through dehydroxylation, releasing active silica and alumina [45,46]. Therefore, this dehydroxylation process forms highly reactive metakaolin (MK), widely used as a pozzolanic material [47].
Chemically, silicon oxide (SiO2), aluminium oxide (Al2O3) and iron oxide (Fe2O3) are the major components of this clay. To maintain high levels of pozzolanic reaction, these three major components should comprise above 70% of the total components [48]. In OPC and geopolymer composites, these three components play a crucial role. For OPC, SiO2, combined with CaO, forms two primary silicates, dicalcium silicate (C2S) and tricalcium silicate (C3S). These silicates further react with water to form calcium silicate hydrate (C-S-H) gel, which is the primary strength-providing compound in concrete [49]. Whereas Al2O3 forms tricalcium aluminate (C3A), which further forms ettringite and monosulfate. These compounds help improve workability and strength properties [50]. In the case of geopolymer, under an alkaline environment, Al and Si dissociate from the MK, forming an aluminosilicate network that enhances workability and strength [51]. Minor chemical oxides present in kaolin, such as iron oxide (Fe2O3), calcium oxide (CaO), magnesium oxide (MgO), potassium oxide (K2O), titanium dioxide (TiO2), and phosphorus pentoxide (P2O5), are instrumental in the geopolymerisation process and its fundamental mechanisms. Fe2O3 can substitute for Al3+ within the kaolinite structure, resulting in lattice distortion that improves the mechanical strength and densification of the geopolymer matrix [52]. CaO and MgO affect secondary gel phases such as C-A-S-H and M-A-S-H, altering setting time and strength development. At the same time, K2O influences thermal properties and phase stability [52,53]. TiO2 and P2O5 can function as nucleation sites or alter the polymeric network through Ti-O-Si or P-O-Al bonds, thereby enhancing the chemical resistance and structural integrity of the final geopolymer [54].
Challenges involved in surface modification and activation: One major issue is the limited reactivity of raw kaolin, which necessitates high temperature (600 °C to 800 °C). This process is energy-intensive [55]. Furthermore, high-speed ball grinding of clay—a mechanical activation method—increases surface area and induces structural disorder. Nonetheless, it necessitates meticulous regulation to avert excessive energy consumption and particle agglomeration [56].
2.2. Illite Clay
Illite clay, primarily used in brick production, is abundant and relatively inexpensive. However, they are not widely used as pozzolanic materials for SCMs due to their lower pozzolanic activity compared to kaolinitic clay [57]. Structurally, Illite is a 2:1 layered mineral clay, which means that it contains two sheets of silicon tetrahedra () entrapped one single octahedral aluminium (Al2(OH)4) [40]. Raw Illite clay is not reactive. For them to be pozzolanic, they need to have amorphous crystal structures. This process involves removing or vaporising the hydroxyl group from the structure through high heating, a process known as dehydroxylation [58]. After dehydroxylation, Illite also produces MK as a pozzolanic material, but it is less reactive than kaolinitic clay, despite having the same energy consumption. It is due to the structure of Illite, which is 2:1, requiring more energy to break the ionic bond than that of the 1:1 layer kaolin. SiO2, Al2O3, K2O and Fe2O3 are the major components of this clay [59]. SiO2 and Al2O3 serve as the fundamental components of the aluminosilicate framework [60]. Fe2O3 can replace Al3+ within the tetrahedral or octahedral layers, forming Fe–O–Si or Fe–O–Al bonds that enhance the mechanical strength and durability of the geopolymer. K2O, primarily found in illite, acts as a natural alkali source, facilitating the dissolution of aluminosilicate phases and promoting the development of geopolymeric gels [61,62]. Minor oxides, including CaO and MgO, facilitate the formation of secondary phases such as C-A-S-H and M-A-S-H gels, thereby supporting early strength development and modifying setting behaviour. Concurrently, TiO2 and P2O5 enhance chemical resistance and improve microstructural integrity [60,62]. Challenges involved in surface modification and activation: The process of modifying and activating illite clay for geopolymerisation presents several challenges owing to its complex mineral composition and comparatively lower reactivity relative to kaolinite. Its layered structure and elevated potassium content diminish its responsiveness to conventional thermal activation, making complete dehydroxylation difficult and impeding its transformation into a reactive amorphous phase. Mechanical activation may increase surface area and induce structural disorder; however, this method alone generally does not significantly improve reactivity [63]. Furthermore, the surface modification of illite via surfactants or microwave-assisted treatments can alter its electrical conductivity and morphology. Nevertheless, these techniques necessitate meticulous control and may not be appropriate for large-scale industrial applications due to their expense and complexity [64].
2.3. Bentonite/Montmorillonite (MMT) Clay
Bentonite, a type of clay formed from weathered volcanic ash, is primarily composed of montmorillonite (MMT). Bentonite is a 2:1 layered mineral clay, consisting of two sheets of silicon tetrahedra enclosing a single octahedral aluminium or magnesium (AlO6 or MgO6) [65]. Surface modification is required to make bentonite clay pozzolanic. At standard conditions, bentonite is extremely non-reactive due to its strong Si-O and Al-O bonds. The dehydroxylation process may enhance its pozzolanic activity, but a strongly alkaline medium is more favourable for its full-fledged amorphisation [66]. SiO2, Al2O3, MgO and Fe2O3 are the significant components of this clay. In an alkaline environment, soluble silica produces a geopolymer gel.
Additionally, aluminates form alumino-silicate bonds during the geopolymerization process, which affects early strength. CaO and MgO facilitate the formation of secondary gel phases such as C-A-S-H and M-A-S-H, thereby enhancing early strength and influencing setting behaviour. Simultaneously, K2O and Na2O serve as alkali sources, assisting in the dissolution of aluminosilicate phases and promoting gel formation. Furthermore, TiO2 and P2O5 act as nucleation sites or modify the polymeric network, thereby improving the chemical resistance and microstructural integrity of the final geopolymer product [67,68].
Challenges involved in surface modification and activation: Surface modification and activation of bentonite clay for geopolymerisation pose challenges due to its complex mineral composition and high water absorption capacity. Natural bentonite, predominantly composed of montmorillonite, exhibits low surface activity and limited sorption capabilities in its unmodified state, necessitating chemical or thermal treatment to enhance its efficacy [69]. Furthermore, surfactant modification may reduce surface area and impede interlayer expansion, thereby potentially limiting its effectiveness within geopolymer matrices [69].
2.4. Halloysite Clay
Halloysite, a naturally occurring, harmless clay belonging to the kaolinite subgroup, has a unique hollow, nanotubular structure. Although it is classified in the kaolin subclass, its structural layer is 1:1, like kaolin [70]. Halloysite consists of a single sheet of silicon tetrahedra (SiO4, with four oxygen atoms) that entraps a single octahedral unit of aluminium or magnesium (AlO6 or MgO6). Its outer layer consists of silicon-oxygen (Si-O-Si) bonds, and the inner layer consists of aluminium or magnesium surrounded by a hydroxyl group [70,71]. Halloysite can be utilised as a pozzolanic material in conjunction with OPC and highly reactive geopolymer composites, such as MK, after undergoing surface modification or calcination. The particle size of this clay is finer, with a larger surface area and improved adsorption capabilities [72,73].
Major and minor chemical oxides in Halloysite, such as Fe2O3, CaO, MgO, K2O, and Na2O, along with trace oxides such as TiO2 and P2O5, are essential to the geopolymerisation process and its underlying mechanism. The primary oxides, SiO2 and Al2O3, constitute the framework of the aluminosilicate network, which is pivotal for the formation of geopolymer gel. Furthermore, Fe2O3 can replace Al3+ in octahedral sheets, thereby enhancing mechanical strength and facilitating the formation of Fe-O-Si or Fe-O-Al bonds [74]. K2O and Na2O, which are commonly present in halloysite, function as natural alkali sources that enable the dissolution of aluminosilicate phases and promote gel formation. CaO and MgO contribute to the formation of secondary phases such as C-A-S-H and M-A-S-H gels, thereby enhancing early strength and influencing setting behaviour [75]. Furthermore, trace oxides, including TiO2 and P2O5, can serve as nucleation sites or modify the polymeric network, thus improving chemical resistance and the microstructure of the final geopolymer [76].
Challenges involved in surface modification and activation: The process of modifying and activating Halloysite clay for geopolymerisation presents several difficulties, arising from its nanotubular morphology, hydrated state, and heterogeneous surface chemistry. In contrast to Kaolinite, Halloysite incorporates interlayer water molecules, rendering thermal activation more intricate. Heating within the range of 500–900 °C induces dehydroxylation and structural disruption; however, exposures exceeding 1000 °C may compromise or decompose the tubular architecture, thereby diminishing reactivity and surface area [77]. Furthermore, the heterogeneous surface charge and hydrogen bonding of Halloysite contribute to aggregation and inadequate dispersion, impeding its interaction with alkali activators and consequently reducing its efficacy within geopolymer matrices [78].
2.5. Palygorskite Clay
With its distinct 2:1 layer structure (two sheets of silicon tetrahedra () and one single octahedral aluminium or magnesium (AlO6 or MgO6), Palygorskites are a fibrous clay mineral that belongs to the phyllosilicate group [79]. Palygorskite has a fibrous or needle-like morphology in contrast to common clays with a flat or lamellar structure, such as montmorillonite or kaolinite. Its unique structure contributes to its large surface area and adsorptive qualities, making it useful for a range of industrial operations, including geopolymer technologies, adsorption, and catalysis [80,81]. SiO2, Al2O3, MgO and Fe2O3 are the significant components of this clay [79]. In geopolymer composites, the presence of silica facilitates geopolymerization. Additionally, substituting calcium ions with alumina and magnesium makes the geopolymer composites thermally stable and less susceptible to chemical degradation. Minor oxides such as K2O and N2O facilitate the dissolution of aluminosilicate phases. Trace oxides, such as TiO2, enhance microstructural integrity, chemical resistance, and catalytic activity [79,82].
Challenges involved in surface modification and activation: Surface modification and activation of palygorskite clay for geopolymerisation present considerable challenges due to its fibrous morphology, channel-filled structure, and heterogeneous chemical composition. The principal issue is that naturally occurring palygorskite often contains impurities and aggregated particles, which reduces reactive surface area [83]. Furthermore, the formation of silica–palygorskite heterostructures through acid activation and surfactant-assisted assembly enhances porosity and adsorption capacity; however, it also introduces additional complexity into the synthesis process and scalability [84].
2.6. Sepiolite Clay
Sepiolite is unique among 2:1 clays, as it has a fibrous structure similar to that of palygorskite. Its distinct 2:1 layer structure contains two sheets of silicon tetrahedra () and one single octahedral magnesium (MgO6) [85]. Sepiolite has a higher surface area due to its fibrous structure, which enhances adsorption capacity and makes it a significant material for the extraction of heavy metals or pollutants [85,86]. SiO2, MgO, and Al2O3 are the significant components of this clay [85]. Sepiolite can be used as an SCM after activation by calcination or an alkaline solution [87].
The primary oxides, including MgO, SiO2, and Al2O3, constitute the fundamental structure of sepiolite. MgO contributes to the formation of the trioctahedral sheet and facilitates the development of magnesium-aluminosilicate hydrate (M-A-S-H) gels during alkali activation [85]. Minor oxides, such as Fe2O3, CaO, K2O, Na2O, along with trace elements like TiO2 and P2O5, also influence the geopolymerisation process by affecting the rates of dissolution, ionic equilibrium, and gel formation. Fe2O3 substitutes for Al3+ within the octahedral sheets, resulting in the formation of Fe-O-Si or Fe-O-Al bonds that enhance mechanical strength and durability [88]. CaO and Na2O promote the development of secondary gel phases such as C-A-S-H, thereby improving early strength characteristics. Furthermore, TiO2 and P2O5 may act as nucleation sites or modify the polymeric network, thereby enhancing chemical resistance and the microstructure of the final geopolymer [89].
Challenges involved in surface modification and activation: Surface modification and activation of sepiolite clay for geopolymerisation pose several challenges due to its fibrous morphology, low cation-exchange capacity, and complex channel structure. Sepiolite’s natural structure resists swelling and dispersion in aqueous media, making mechanical activation difficult without inducing aggregation or fibre damage and requires precise control [90]. Again, acid activation increases surface area and porosity by eliminating impurities such as dolomite and calcite. Nevertheless, excessive acid concentrations may induce structural collapse and lead to the formation of amorphous silica [91]. Furthermore, surface modification using coupling agents, such as silanes or surfactants, can enhance compatibility with polymer matrices and improve dispersion. However, these techniques are frequently costly and pose challenges in scaling up for industrial application [92]. Table 1 summarises the chemical constituents of different types of clay.

Table 1.
Chemical compositions of different types of clay (average values from references).
Raw clay must undergo various treatments to become reactive and pozzolanic, making it suitable for use as an SCM. However, clay materials must meet specific, crucial chemical and physical parameters to be considered a potential pozzolanic material. In clay minerals, SiO2 and Al2O3 are the two prerequisite components that control pozzolanic activity [111]. The European standard EN 197-1 stipulates that the chemical composition of a pozzolanic material must contain at least 25% active SiO2 by weight [112]. According to the French standard NF P18 513, the total quantity of SiO2 and Al2O3 should be0% for MK [113]. According to ASTM C 618, a material can be considered pozzolanic if the sum of SiO2, Al2O3, and Fe2O3 is at least 70% by weight [33].
Moreover, the standards mentioned above specify additional physical requirements, including types of materials, ignition loss, fineness of the pozzolanic material, and strength activity index. Table 2 summarises and compares the standards, describing the chemical and physical requirements of pozzolanic materials used as SCMs.

Table 2.
Chemical and physical requirements of pozzolanic materials following ASTM C 618, EN 197-1 and NF P18 513.
3. Clay Activation/Treatment Methods
Raw clay’s crystalline structure is inert and exhibits minimal pozzolanic activity; therefore, it must undergo processing before utilisation as an SCM. The procedure entails amorphisation, which involves disintegrating the crystalline structure to enhance reactivity upon transformation into an amorphous state [114]. By increasing the amount of reactive silica and alumina available, this structural alteration enables the material to interact efficiently with calcium hydroxide in cement, generating more calcium silicate hydrate (C–S–H) and thereby enhancing strength and durability [115]. Similarly, in geopolymer composites, reactive silica and alumina react with alkali activators to form sodium aluminosilicate hydrate (N-A-S-H) gel, similar to S-H in traditional cement [116].
The most popular clay treatment method is thermal/calcination, which has been employed in numerous studies [59,117,118,119,120]. However, considering the high energy requirements and high cost of the calcination process, researchers are diverting their interest to alternative treatment methods, including mechanical [121,122] and chemical [123,124,125,126]. Therefore, the following section contains various treatment methods for natural clay.
3.1. Thermal Activation
In thermal treatment, the clay material is heated to a specific temperature, which causes the octahedral layer to dehydroxylate-that is, the hydroxyl group (OH¯) is removed by water vapour. The crystalline structure of the clay is destroyed by this dehydroxylation process, which also turns it into an amorphous, disordered state [127,128,129]. This dehydration process may occur at temperatures exceeding 500 °C, which varies from clay to clay and is influenced by factors such as heating rate and duration, atmospheric conditions, and cooling rate [130]. However, excessive heat, typically exceeding 1000 °C, may alter recrystallisation, thereby decreasing the pozzolanic activity of calcined clay [38]. Table 3 shows various transformations of clay when exposed to high temperatures.

Table 3.
Effect of varying temperature ranges and changes in clay properties [37,38,131].
Earlier research concentrated on thermally activating natural clays at different temperatures, observing increased rates and durations. The following sections and Table 4 present the optimal calcination temperatures and durations for various types of clays.
For Kaolin, in most cases, the optimal calcination temperature to make MK ranges from 650 °C to 800 °C. However, according to Morat and Comel [132], Calcination temperatures between 700 °C and 800 °C are thought to provide the optimum thermal activation. In contrast, temperatures above 850 °C are thought to crystallise the MK and reduce its reactivity thereafter. The study by Gutiérrez et al. [133] reported that the optimal heating temperature for kaolin was between 700 °C and 800 °C. Recent studies by Elimbi et al. [134] and Adufu et al. [135] achieved the ideal temperature for Kaolin to convert highly pozzolanic MK at 700 °C for 10 h and 3 h, respectively. Many recent studies have determined that the optimal calcination temperature for kaolin is 750 °C, with varying calcination times. Salau et al. [117] and Albidah et al. [118] reported that the optimal calcination temperature for Kaolin was 750 °C. On the other hand, Zuhua et al. [136] calcined Kaolin-Based geopolymer and reported an optimal calcination temperature of 900 °C for 6 h.
Previous research has reported a marginally higher optimal calcination temperature for Illite, ranging from 850 °C to 950 °C, compared to Kaolin. Some researchers have identified the optimal calcination temperature for Illite used as a pozzolanic material as between 850 °C and 875 °C, with a calcination time of 1 to 3 h. For instance, Luzu et al. [59] and Hu et al. [137] reported optimal calcination temperatures of 850 °C for geopolymer and Illite-based composites, with calcination times of 2 h and 1 h, respectively. The study by Dietel et al. [127] demonstrated that the optimal calcination temperature for Illite was 875 °C, with a 3-h calcination period. Buchwald et al. [138] thermally treat illite at 550–950 °C for 1 h. This study observed a dramatic decrease in pozzolanic activity, accompanied by a lower Si-Al dissolution rate, at temperatures between 550 °C and 650 °C. By contrast, a trend of increased dissolution was observed from 750 °C to 950 °C. Similarly, Bonavetti et al. [120] and Seiffarth et al. [139] reported that the optimal calcination temperature for Illite-OPC-based composites was 950 °C for 1 h and 30 min, respectively.
It is interesting to note that most previous research has determined the optimal calcination temperature for Bentonite to be 800 °C, with calcination periods ranging from 3 to 4 h. Waqas et al. [140] calcined Bentonite at 800 °C for 3 h and reported sufficient geopolymerisation for a fly ash-based geopolymer with polypropylene fibres (PPF). Laidani et al. [141] reported that superior pozzolanic reactivity was observed when Bentonite was calcined at 800 °C for 4 h, and that up to 15% replacement of OPC with calcined Bentonite resulted in higher mechanical performance. Fode et al. [142], Reddy et al. [143] and Garg et al. [144] obtained the highest pozzolanic reactivity when OPC-based Bentonite was calcined at 800 °C. However, Ahmad et al. [102] achieved the highest pozzolanic reactivity for Bentonite calcined at 500 °C. For Halloysite, the optimal calcination temperature has been reported to be between 750 °C and 800 °C, with a calcination period of 2 to 3 h. Zhang et al. [145] demonstrated that 750 °C was the optimal temperature for achieving extensive dehydroxylation for Halloysite. Another study by Zhang et al. [146], Yu et al. [147] and MacKenzie et al. [148] also recommended that the optimal calcination temperature for Halloysite to achieve extensive geopolymerisation was 750 °C for 2 h. In contrast, Haw et al. [105] and Ranjbar et al. [149] reported optimal calcination temperatures of 800 °C for 3 h and 2 h, respectively.
Previous research indicates that the ideal calcination temperature for Palygorskite is 700–800 °C, with a duration of 1–3 h. Poussardin et al. [82] reported that Palygorskite calcined at 800 °C for 1 h exhibited the highest pozzolanic reactivity. Whereas Georgopoulos [150] and Hamid et al. [151] reported, at 750 °C, Palygorskite exhibited the highest pozzolanic reactivity in both OPC and geopolymer composites. Limited research has shown that the optimal calcination temperature for Sepiolite is between 800 °C and 900 °C. The study by Wu et al. [152] attributed the highest pozzolanic activity to the Sepiolite samples calcined at 800 °C. Another study by Saka et al. [153] reported that Sepiolite calcined at 900 °C, and replaced with 10% OPC, exhibited superior mechanical properties in the presence of 3% glass fibre.
To summarise the above discussion, it is evident that calcination activates all types of clays at varying temperatures and durations. This temperature variation might be related to the clay’s crystal structure. It is worth noting that 1:1 clays, such as Kaolin and Halloysite, require an optimal temperature range of 700 °C to 800 °C. Conversely, 2:1 clays, such as Illite, Bentonite, Palygorskite, and Sepiolite, require temperatures of 850 °C to 950 °C for complete dehydroxylation. For 1:1 clays, a relatively lower temperature for complete dehydroxylation can be attributed to the single sheet of silicon tetrahedra that encloses a single octahedral aluminium unit. 2:1 clays have two sheets of silicon tetrahedra and a single octahedral magnesium or aluminium, which requires more energy to remove the internal bound water and break the Si-Al bond than 1:1 clays do.

Table 4.
Optimal calcination temperature, duration, and increased rate of temperature for different types of clays.
Table 4.
Optimal calcination temperature, duration, and increased rate of temperature for different types of clays.
| Type of Clay | Optimal Temperature (°C) | Duration of Calcination (Hours)/Rate of Heat Increment | Remarks | Reference |
|---|---|---|---|---|
| Kaolinite | 650–850 | 3 | Kaolin with low alunite content was optimal at 650 °C, whereas kaolin with high alunite content required 850 °C. | [154] |
| 700–800 | 1 (2 °C/min) | Due to its high pozzolanic index, kaolin was transformed into metakaolin. | [133] | |
| 750 | 1 | Kaolin clay heated to this temperature and for this duration showed the highest strength when replaced with 10% cement. | [117] | |
| 750 | 3 | Metakaolin-based geopolymer concrete exhibits superior mechanical performance when heated to this specified temperature for the specified duration. | [118] | |
| 750 | 10 (2 °C/min) | Raw kaolin was heated to the specified temperature for the specified duration, converting it into a highly pozzolanic material. This pozzolanic clay was replaced with fly ash to make geopolymer composites. | [155] | |
| 700 | 10 (5 °C/min) | Above 700 °C, calcined kaolin began to lose its pozzolanic reactivity in geopolymer cement. | [134] | |
| 700 | 3 (10 °C/min) | To make geopolymer concrete, a variety of calcium-rich additives, including calcium carbide residue, lime, and OPC, were partially replaced with calcined kaolin. | [135] | |
| 600 | 1 | Kaolin clay heated to this temperature and for this duration showed mechanical performance when cured at 60 °C for 24 h. | [156] | |
| 750 | 1 | Kaolin heated at this temperature exhibited optimal pozzolanic activity when partially replaced with OPC. When heated at 850 °C, it displayed reduced pozzolanic activity due to recrystallisation. | [157] | |
| 650 | 1 (25 °C/min) | At this temperature, kaolin exhibited the highest dehydroxylation rate (>85%). | [158] | |
| 900 | 6 | At this temperature, kaolin exhibited the highest reactivity, and above it, reactivity sharply declined. | [136] | |
| Illite | 950 | 1 | Illite calcined at this temperature became a highly reactive geopolymer binder. | [138] |
| 950 | 1 | Calcined illite and limestone fillers were partially replaced with OPC. | [120] | |
| 950 | 0.5 | A higher soluble Si/Al ratio after treatment at 950 °C could improve the mechanical properties of the geopolymer binders. | [139] | |
| 850 | 2 | After treatment at this temperature, the illite clay became sufficiently pozzolanic for use in geopolymer composites. | [59] | |
| 875 | 3 | Achieved sufficient geopolymerisation and enhanced mechanical performance. | [119] | |
| 850 | 1 | An optimal quantity of a reactive and amorphous aluminosilicate precursor material has been observed at 850 °C. | [137] | |
| Bentonite | 800 | 3 | Bentonite thermally treated at this temperature demonstrated adequate pozzolanic performance, enabling it to replace 10% of the fly ash in geopolymer concrete. | [140] |
| 800 | 4 | Thermally activated bentonite partially substituted with OPC. A replacement rate of up to 15% was optimal for self-compacting concrete. | [141] | |
| 500 | 3 | Up to 30% replacement with OPC showed standard pozzolanic activity. | [102] | |
| 800 | 3 | A 20% replacement of OPC with calcined bentonite at 800 °C showed the highest pozzolanic activity compared to the replacements at 400 °C and 600 °C. | [142] | |
| 800 | - | A 20% replacement of OPC with calcined bentonite at 800 °C showed the highest pozzolanic activity compared to calcined bentonite at 700 °C. | [143] | |
| 800 | - | When calcined at this temperature, the maximum pozzolanic reactivity was observed with 30% replacement of OPC. | [144] | |
| Halloysite | 800 | 3 | Optimal pozzolanic activity was observed when the material was calcined at this temperature, with a 10% OPC replacement. | [105] |
| 750–850 | 2 | The highest geopolymerisation was observed at calcination temperatures between 750 °C and 850 °C, compared to 450 °C, 650 °C, and 1000 °C. | [145] | |
| 750 | 2 | Halloysite calcined at this temperature was able to participate in geopolymerisation along with fly ash and blast furnace slag. | [147] | |
| 750 | 2 | The highest level of geopolymerisation was observed at 750 °C, while the lowest was at 1000 °C. | [146] | |
| 800 | 2 | Halloysite calcined at this temperature exhibited the best pozzolanic activity in 3D-printed geopolymer composites. | [149] | |
| 750 | 1.6 | The highest pozzolanic reactivity was achieved when halloysite was calcined at 750 °C, and it sharply declined at 800 °C. | [148] | |
| Palygorskite | 800 | 1 (300 °C/h) | Enhanced pozzolanic reactivity was exhibited when 10% of the OPC was replaced. | [79] |
| 800 | 1 (5 °C/min) | Complete amorphisation and optimal pozzolanic activity were achieved when 20% of calcined palygorskite was replaced with OPC. | [82] | |
| 750 | 3 | The highest pozzolanic reactivity was observed when OPC replaced 20% of calcined palygorskite. | [150] | |
| 700 | 10 °C/min | Palygorskite calcined at 700 °C exhibited optimal pozzolanic reactivity in geopolymer. | [151] | |
| Sepiolite | 800 | 0.5 | Sepiolite calcined at this temperature exhibited optimal pozzolanic reactivity when used as a 20% replacement of OPC. | [152] |
| 900 | - | Optimal pozzolanic activity was observed with a 5% OPC replacement. | [153] | |
| 830 | - | Significant pozzolanic activity was observed when sepiolite was calcined at 830 °C, while sepiolite calcined at 370 °C and 570 °C showed no pozzolanic activity. | [120] |
3.2. Mechanical Activation
Mechanical activation of clay minerals through high-speed grinding, also known as mechanochemical activation, reduces the crystallinity of certain clays by increasing their specific surface area. Through dihydroxylation, mechanochemical milling causes the clay to fully delaminate, increasing the amount of water absorbed on its surface [159]. The chemical and physical alterations induced by the mechanochemical activation process enhance the pozzolanic reactivity of the treated clay, potentially surpassing that achieved through thermal treatment at equivalent cement replacement levels [160]. This mechanochemical activation results in a finer particle size, increasing surface area and facilitating the faster dissolution of Si and Al ions and their participation in the pozzolanic reaction [161]. However, the current research data reveal significant limitations of this activation method, whereas most previous studies have focused on thermal treatment. Among this limited knowledge, it is notable that mechanical activation proved effective for the 1:1 structured Kaolin clay, while other 1:1 clays, such as Halloysite, have not been extensively investigated. Moreover, using 2:1 clays, such as Illite, Bentonite, Palygorskite, and Sepiolite, may offer advantages over kaolinite (a 1:1 mineral) due to the additional silica layer, which could promote the formation of more calcium silicate hydrate [162]. The following sections and Table 5 describe the mechanical activation method used in previous research to activate different types of clays.
Clay activation through mechanical grinding depends on several factors, including grinding speed, the ratio of grinding body to clay powder, and the duration of the grinding process. To activate the clay, most previous research has employed ball grinding, maintaining a ball-to-powder ratio of 10–25, with grinding speeds of 300–600 rpm and varying grinding durations, depending on the ball diameter and grinding speed. Balczár et al. [163] demonstrated that early 100% amorphisation and the highest pozzolanic reactivity were observed with a ball-to-powder ratio of 11, a grind speed of 400 rpm, and a grinding duration of 240 min. Tole et al. [34] achieve optimal pozzolanic reactivity in kaolin clay by maintaining a ball-to-powder ratio of 25, using 3 mm stainless steel balls, and grinding for 20 min at 500 rpm. A higher grinding duration caused agglomeration.
In contrast, another study [164] reduced the grinding speed from 500 rpm to 300 rpm, increased the grinding duration from 20 min to 60 min, and decreased the ball-to-powder ratio from 25 to 10 mm diameter balls, to achieve optimal pozzolanic activity. However, Hounsi et al. [121] further reduced the grinding speed to 250 rpm and the ball-to-powder ratio to 1.56 by using balls of different diameters (19 mm and 10 mm) and achieved the highest amorphisation and pozzolanic reactivity of Kaolin clay. On the other hand, Ilić et al. [165] used the lowest grinding speed of 46 rpm and reported partial amorphisation.
Luzu et al. [59] achieved the optimal pozzolanic reactivity of Illite clay, maintaining a ball-to-powder ratio of 15, using 30 mm stainless balls, and grinding for 240 min at 400 rpm. In contrast, Zhao et al. [122] increased the grinding speed from 400 rpm to 500 rpm, decreased the grinding time from 240 min to 30 min, and raised the ball-to-powder ratio from 15 to 20 using 10 mm diameter balls. This study reported the highest dehydroxylation and pozzolanic reactivity of Illite up to 40% replacement of cement.
For Bentonite clay, Baki et al. [166] achieved optimal pozzolanic reactivity at a grinding speed of 500 rpm, using 10 mm zirconia balls, maintaining a ball-to-powder ratio of 10, and grinding for 60 min. Whereas Kovalchuk [167] reported the highest amorphisation of Bentonite using a grinding speed of 300 rpm, employing 20 mm Si3N4 balls, maintaining a ball-to-powder ratio of 10, and a grinding duration of 120 min.
MacKenzie et al. [148] employed two mechanical treatments for Halloysite: ball grinding and vibratory ring milling. For ball grinding, the grinding speed was 400 rpm, and the grinding duration was 1200 min. This study found a significant reduction in pozzolanic reactivity during ball grinding compared to vibratory ring milling. For Palygorskite clay, Georgopoulos et al. [168] reported the highest pozzolanic reactivity when ground for 15 min at 600 rpm. Limited research indicates that Sepiolite clay can also be pozzolanic upon mechanochemical activation. Tunç et al. [169] reported a 2.4% increase in pozzolanic reactivity after 60 min of grinding compared to 30 min, with a grinding speed of 600 rpm.
In conclusion, the documentation above demonstrates that mechanical ball grinding is an effective treatment method for converting crystal clay into highly pozzolanic amorphous clay. However, this activation method does not depend on the clay’s crystal structure (e.g., 1:1 or 2:1). Nevertheless, its efficiency depends on specific parameters, including grinding speed, duration, and the ball-to-clay ratio. It is advisable to optimise these parameters to obtain a highly pozzolanic clay. For instance, if the grinding speed and ball-to-clay ratio are high, the grinding duration should be decreased. Otherwise, the amorphous clay will agglomerate, reducing its specific surface area.

Table 5.
Clay activation through mechanical grinding (optimal condition).
Table 5.
Clay activation through mechanical grinding (optimal condition).
| Type of Clay | Grinding Body | Ball/Powder | Grinding Speed (rpm) and Time (min) | Result | Reference |
|---|---|---|---|---|---|
| Kaolin | Zirconia ball of 10 mm in diameter | 11 | 400 rpm and 240 min | A nearly 100% degree of amorphisation and the highest strength were obtained. | [163] |
| Stainless steel ball of 3 mm in diameter | 25 | 500 rpm and 20 min | The highest amorphisation and strength were exhibited. | [34] | |
| Zirconia ball of 10 mm in diameter | 20 | 300 rpm and 60 min | Most of the hydroxyl groups were removed, and the kaolin was highly amorphous. | [164] | |
| 10 balls of 19 mm and five balls of 10 mm in diameter | 1.56 | 250 rpm and 60 min | Optimal activation was attained for geopolymer-based mortar. | [121] | |
| Zirconia ball of 10 mm in diameter | 20 | 350 rpm and 120 min | Exhibited optimal amorphisation and pozzolanic reactivity | [170] | |
| Steel balls ranging from 20 to 60 mm in diameter | 10 | 46 rpm and 1200 min | Kaolin was partially amorphised, and the specific surface area increased up to 31% at 20 h of grinding compared to 10 h. | [165] | |
| Illite | 30 mm diameter ball | 15 | 400 rpm and 240 min | The highest pozzolanic reactivity and strength were obtained. | [59] |
| Balls of 10 mm diameter | 20 | 500 rpm and 30 min | Optimal pozzolanic reactivity and strength increased by up to 40% with OPC replacement. | [122] | |
| Bentonite | Zirconia ball of 10 mm in diameter | 10 | 500 rpm and 60 min | Optimal hydroxylation and amorphisation occurred. A 20% replacement of OPC with activated bentonite resulted in an over 90% strength activity index. | [166] |
| Si3N4 balls of 20 mm in diameter | 10 | 300 rpm and 120 min | The particle size was considerably decreased. Optimal crystal structures were converted to amorphous states. | [167] | |
| Halloysite | Two types of grinding machines were used: (1) a planetary ball mill with 3 mm Zirconia balls, and (2) an energetic vibratory ring mill equipped with a tungsten carbide bowl and rings. | 50 (for planetary ball grinding) | 400 rpm and 1200 min (for planetary ball grinding), 15 min (vibratory ring mill) | Halloysite ground in a planetary grinder showed significantly lower pozzolanic reactivity than that ground in a vibratory ring mill grinder. | [148] |
| Palygorskite | Zirconia balls of 4–5 mm in diameter | 60 and 64 | 600 rpm and 15 min | The highest pozzolanic reactivity was achieved when palygorskite was ground at 600 rpm for 15 min. Whereas at 30 min, 45 min, and 60 min, pozzolanic reactivity sharply declined at the same grinding speed. | [168] |
| Sepiolite | Tungsten carbide balls of 10 mm in diameter | 25 | 600 rpm and 60 min | The highest degree of amorphisation occurred at 60 min, reaching 92.3%. In comparison, at 30 min, it was 89.9% with the same grinding speed. | [169] |
3.3. Chemical Activation
Limited Research has employed chemical activation in addition to calcination and mechanical activation [123,124,125,126]. For clays containing 73% montmorillonite that were obtained from an area near London, Ayati et al. [124] used acid activation. This study employed 5 M hydrochloric acid at 20 °C and 90 °C, with treatment times ranging from 1 h to 21 days. The authors obtained the best pozzolanic activity and 93% amorphous clays (25% cement replacement with clays) by treating clays with 5 M hydrochloric acid for 8 h at 90 °C. Additionally, they found that smectite clays exhibit superior pozzolanic reactivity upon acid activation compared to calcination. Noor-ul-Amin et al. [126] used aqueous calcium chloride in varying concentrations (1–6%) as a chemical activator for Pakistani mine-derived clays. The study did not provide a detailed description of the clay’s mineral composition. However, the authors found that natural clays could be successfully activated using a 4% aqueous calcium chloride solution, replacing 20% of the cement. However, it was observed that a high concentration of free Cl− existed in the pore solution. Therefore, due to the possibility of corrosion, the authors noted that this activation technique would not be suitable for concrete applications with embedded steel [126] Mwiti et al. [125] activated pre-calcined natural clays from Kenya using 0.5 M sodium sulphate. Additionally, the study’s clay mineral composition was unavailable. The authors produced a blended cement with 50% cement replacement and enhanced its mechanical performance by activating it with 0.5 M sodium sulphate.
3.4. Importance of PSD and SSD in Clay Treatment and Concrete Performance
Particle size distribution (PSD) and specific surface area (SSA) are critical parameters influencing the efficacy of clay treatment and the performance of clay-based geopolymer concrete. Calcination during processing alters the clay’s mineral structure, thereby enhancing its pozzolanic reactivity. Additionally, ball milling increases SSA by reducing particle size and dispersing agglomerates. Bai et al. [171] demonstrated that extended grinding durations yield a greater abundance of ultrafine particles, thereby enhancing pozzolanic activity and mechanical strength. Elevated calcination temperatures augment reactivity; however, they may concurrently diminish fluidity due to particle agglomeration. SSA directly affects water demand and workability; an increase in SSA enlarges the reactive surface area but necessitates additional water, which can adversely impact workability if not adequately controlled. Muzenda et al. [172] noted that SSA exerted a greater influence on early hydration and compressive strength than metakaolin content, with SSA values ranging from 14 to 45 m2/g depending on clay mineralogy and treatment methods. Calcination, especially at optimal temperatures, alters the mineral phase and may induce agglomeration, thereby reducing the SSA unless counteracted through grinding. Clays such as kaolin and halloysite respond effectively to thermal activation, whereas bentonite, illite, palygorskite, and sepiolite may benefit more from mechanical or chemical activation [173]. Again, PSD influences packing density and rheology; finer particles enhance strength but diminish fluidity, whereas a broader PSD improves workability [174]. Therefore, it is essential to adjust PSD and SSA through appropriate treatment methods to optimise the reactivity and mechanical properties of clay-GPC systems.
4. Effect of Clay on Concrete Workability
The workability of concrete is severely affected by the presence of clay particles. The high specific surface areas and finer particle sizes of clay minerals increase water demand and reduce workability [175]. The following sections and Table 6 show the impact of various types of clays on concrete.
Ghais et al. [176] conducted a comparative study to evaluate the impact of fly ash and raw Kaolin clay on the replacement of 10% to 30% of OPC. For the fly ash-replaced specimen, a significant increase in the slump value has been observed. On the other hand, a dramatic decrease in slump has been observed for clay (Figure 2). The increase in the slump of fly ash can be attributed to its spherical shape, which improves workability. Whereas clay particles increase the water demand and, due to their plastic nature, significantly reduce the workability. The study by Salau et al. [117] replaced 10%, 20%, and 30% of calcined clay with OPC, maintaining a water-to-binder ratio (w/b) of 0.4. This study demonstrated high workability with a compacting factor of 0.95 for a 10% clay replacement, and medium workability for 20% and 30% clay replacements, with compacting factors of 0.92. The decrease in workability for the 20% and 30% clay inclusion was due to the high water demand created by the clay particles. However, this study suggested that a w/b of 0.5 might be suitable for achieving high workability with 30% replacement of OPC with calcined kaolin clay.
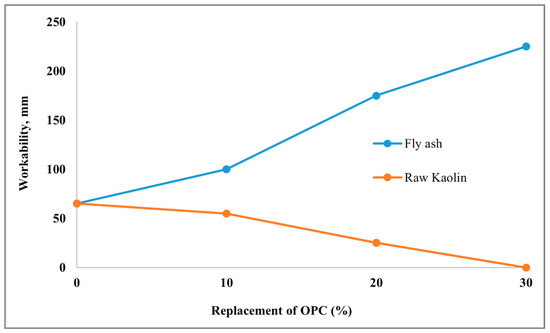
Figure 2.
Effect of fly ash and raw Kaolin on the workability [177].
To investigate the flowability of Illite-based mortar, Bonavetti et al. [120] calcined the Illite clay at 750 °C for 1 h, and 35% and 20of the limestone filler was replaced with OPC. The results showed that flowability decreased by 8% and 5% for the 35% clay and 20% filler, respectively. A study by Zhao et al. [122] investigated the flowability of Illite-rich waste shalusing calcination and mechanical activation methods to compare and replace 20%, 30%, and 40% of the waste with OPC, while maintaining a water-to-binder ratio of 0.485. The obtained results showed that, compared to mechanically activated Illite-rich samples, thermally activated samples had a more negative impact on flowability, resulting in a 30.8% reduction in flowability for 40% OPC replacement compared to mechanical activation. This greater reduction in flow for thermally activated samples can be linked to the increased water demand resulting from the porous structures caused by calcination [178].
The study by Waqas et al. [140] investigated the combined effects of raw and calcined Bentonite (800 °C), with and without polypropylene fibres (PPF), on the workability of fly ash-based geopolymer concrete. The results demonstrated that raw clay exhibited an almost 10% reduction in slump compared to calcined clay. This reduction in slump can be attributed to the higher specific surface area of the fly ash and its higher water demand. However, a slight increase in the slump of the calcined clay may be due to a decrease in surface area during calcination. This similar trend was also mentioned in the other research [179]. However, the addition of PPF further reduced the slump by up to 37% due to internal friction [180]. This phenomenon is consistent with typical findings in clay activation, in which thermal treatment can cause interlayer spaces to collapse and reduce the number of active sites, resulting in a less absorptive or workable material in fresh concrete mixes [63].
In contrast, Reddy et al. [143] compared Bentonite at room temperature (RT) and calcined at 700 °C and 800 °C, replacing OPC by 5%, 10%, 15%, 20%, 25%, and 30% by weight. The results obtained showed a decreasing trend in the slump value with increasing Bentonite content, and this decrease became more pronounced with higher calcination temperatures. This study indicated that higher calcination temperatures increased water demand, exceeding that of the RT Bentonite. This finding contrasts with that of Waqas et al. [140], who reported that raw Bentonite concrete had a lower slump than calcined concrete. The difference in slump behaviour between calcined bentonite observed by Waqas et al. [140] and Reddy et al. [143] can be explained by the nature and extent of calcination and its impact on bentonite’s physical and chemical properties. Moderate calcination, as reported by Waqas et al. [140], may reduce surface area due to partial dehydroxylation and collapse of interlayer spaces, thereby lowering water demand and improving workability, resulting in a slight increase in slump. In contrast, intense calcination at higher temperatures (700–800 °C), as studied by Reddy et al. [143] can cause structural disruption and increased internal porosity, creating more reactive sites that absorb water more aggressively, thereby reducing slump. Furthermore, the replacement level of Bentonite and the interaction with other mix components, such as fly ash and polypropylene fibres, significantly influence the rheological behaviour of the concrete, making direct comparisons between studies complex and context-dependent.
To examine the impact of raw Halloysite on the fresh properties of cement mortar, Dungca et al. [181] replaced 2.5% Halloysite nano clay (HNCL) with OPC by weight, maintaining a w/b ratio of 0.58. This study reported a 33.33% reduction in slump for 2.5% HNCL compared to the control sample. This decrease in workability is associated with the effect of clay particles on the water distribution within the cement mortar.
The study by Georgopoulos et al. examined the slump of mortar made with OPC and calcined Palygorskite (750 °C for 3 h) at a w/b ratio of 0.485. Replacing 20% of the calcined clay with OPC reduced the slump value, primarily due to its high water absorption capacity. The authors suggested that the required slump value can be achieved by increasing the superplasticiser dose while maintaining a constant water-to-binder ratio.
Jemina et al. [88] replaced OPC with 5%, 10%, 15%, 20%, and 25 sepiolite by weight, and replaced 10%, 20%, 30%, 40%, and 50% of the coarse aggregate with recycled aggregate. This study reported that clay negatively impacts the fresh properties by increasing water demand. This study maintained the target fresh properties by expanding the superplasticiser dosage by 32.7%, from 5% to 25% of sepiolite inclusion, while keeping the water-to-binder ratio constant at 0.47.
From the above discussion, it is recognised that including any type of clay, whether raw or treated, adversely affects workability due to its high water demand. Figure 3 illustrates the relationship between the reduced workability trend and clay inclusion. Workability started to decline as the clay inclusion rate increased. An almost 80% reduction in workability has been reported for a 50% replacement of Bentonite clay, and other clays exhibited a similar trend. However, up to 30% inclusion of Bentonite, either raw or calcined, showed minimal reduction in workability. By comparing the negative impacts of calcinated clay and mechanically activated clay, limited research suggests that calcinated clay generates a higher water demand than mechanically activated clay, due to its porous structure resulting from calcination. However, this statement is not sufficiently established due to insufficient evidence. On the other hand, some research statements [140] contradict each other regarding the workability assessment of raw and calcined Bentonite. Therefore, a more thorough investigation should be conducted in the future.
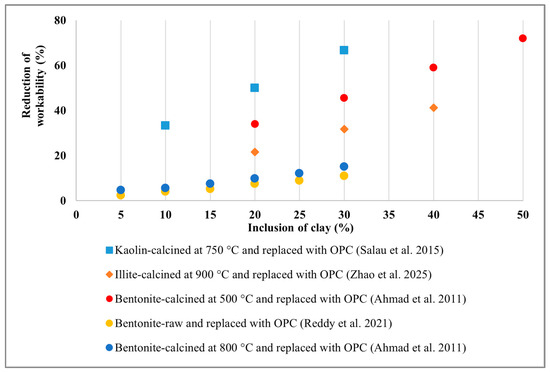
Figure 3.
Relationship between concrete workability and clay inclusion [102,117,122,143].

Table 6.
Effect of different types of clays on the workability of concrete.
Table 6.
Effect of different types of clays on the workability of concrete.
| Type of Clay | Pre-Treatment or Raw Condition of the Clay | Type of Composites | % of Clay Inclusion | Alkali/Binder (a/b) & Water/Binder (w/b) | Types of Workability Tests Performed | Remarks | Reference |
|---|---|---|---|---|---|---|---|
| Kaolin | Raw | OPC with FA, OPC with kaolin (concrete) | 0%, 10%,20% and 30% OPC replaced by FA and kaolin | w/b 0.4 | Slump test | Inclusion of up to 30% fly ash significantly increased the slump value. The inclusion of kaolin from 10% to 30% dramatically reduced workability due to higher water demand and plastic behaviour. | [176] |
| Calcined | OPC with clay (concrete) | 0%, 10%,20% and 30% OPC replaced by clay | w/b 0.4 | Slump test | From 0% to 30%, kaolin calcined at 750 °C for 1 h, exhibited a medium slump value. The author suggested an appropriate w/b of 0.5 for higher workability. | [117] | |
| Illite | Calcined | OPC with calcined clay (CC) and limestone filler (LF) (mortar) | 0%, 20%, LF and 35% CC with OPC | w/b 0.5 | flow | Mortar with 35% CC and 20% LF, reduced slump flow by 8% and 5%, respectively, compared to the reference sample. | [120] |
| Calcined and mechanically activated | OPC with illite-rich waste shale (mortar) | 0%, 20%, 30% and 40% OPC replaced by clay | w/b 0.485 | flow | For calcined clay, the mortar’s flow decreased from 21.6% to 41.2% when 20% to 40% of the clay was replaced with cement, compared to the control mix, due to the high-water demand. On the other hand, the mechanically activated clay exhibited a reduced reduction in flow, which is 8.5% to 10.4% compared to the reference sample. | [122] | |
| Bentonite | Raw and calcined | Fly ash (FA) with raw and calcined clay, polypropylene fibre (PPF) (geopolymer concrete) | 0%, 10% FA replaced with raw and calcined clay. 0.5%, 0.75% and 1% PPF. | a/b 0.4 | slump | A reduced trend of slump value was observed for raw clay compared to calcined clay. Additionally, the inclusion of PPF negatively impacted workability due to internal friction. | [140] |
| Raw | OPC, bentonite, recycled aggregate (RAC) and natural aggregate (NAC) (concrete) | 0%, 5%, 10%, 15% and 20% clay replaced by OPC. 100% NAC and RAC used | w/b 0.5, 0.53, 0.56, 0.59, 0.63 | Slump | The inclusion of bentonite negatively affected the workability of both RAC and NAC concrete, due to bentonite’s higher surface area compared to OPC. However, the addition of superplasticiser improved workability. | [182] | |
| Calcined | OPC replaced by calcined clay (self-compacting concrete) | 0%, 5%, 10%, 15%, 20%, 25%, and 30% OPC replaced by clay | w/b 0.4 | Slump flow, V-funnel and T-500 | The slump value decreased with increasing clay content in the concrete due to enhanced viscosity; therefore, the superplasticiser (SP) dose needed to be increased to achieve the required slump. For 25% and 30% of clay, SP doses were 1.2% and 1.67%, respectively. | [141] | |
| Calcined | OPC replaced by calcined clay (mortar) | 0%, 20%, 25%, 30%, 40% and 50% OPC replaced by clay | w/b 0.55 | Slump | The slump value was significantly reduced by almost 31.76% to 70.50% for 20% to 50% clay inclusion compared to the reference sample. This decrease in slump value is attributed to the higher water demand generated by the clay. | [102] | |
| Room temperature and calcined | OPC replaced by calcined clay (mortar) | 0%, 5%, 10%, 15%, 20%, 25%, and 30% OPC replaced by clay | - | Slump | A reduced trend of workability was observed with the inclusion of both types of clays. However, calcined clay showed a greater reduction in workability than room-temperature clay due to its higher water absorption capacity. | [143] | |
| Raw | OPC replaced raw clay (concrete) | 0%, 3%, 6%, 9%, 12%, 18%, and 21% OPC replaced by clay | w/b 0.58 | Slump | The slump values steadily decreased as the clay inclusion increased. This downward trend is due to the clay’s larger surface area. | [103] | |
| Halloysite | Raw | OPC replaced raw clay (mortar) | 0%, 2.5% OPC replaced by clay | w/b 0.58 | Slump | A 33.33% reduction in the slump value was observed for the 2.5% halloysite clay sample compared to the control sample. | [181] |
| Palygorskite | Calcined | OPC replaced calcined clay (mortar) | 0% and 20% OPC replaced by clay | w/b 0.485 | Slump | A reduced trend in slump value was observed with the inclusion of calcined clay, due to its high water-absorption capacity. The required slump value can be maintained by increasing the superplasticiser dose while maintaining a constant water-to-binder (w/b) ratio. | [150] |
| Sepiolite | - | OPC replaced clay, and coarse aggregate (CA) was replaced by recycled coarse aggregate (RCA) (self-compacting concrete) | 0%, 5%, 10%, 15%, 20%, and 25% OPC replaced by clay. 0%, 10%, 20%, 30%, 40%, and 50% CA replaced by RCA. | w/b 0.47 | Slump flow, T50, J-ring height, V-funnel flow, L-box | A combination of 41.78% fly ash (by weight of OPC) and sepiolite powder maintained workability within the required limits for all replacement levels by adjusting plasticiser dose. The highest flow value was achieved with a 25% clay and 50% RCA mixture. | [88] |
5. Effect of Clay on the Mechanical Properties of Concrete
The effect of raw and treated (primarily calcined) clays on the performance of cement-based materials has been evidenced by pioneering studies. The optimally calcined [155] and mechanically ground [163] clays improve the mechanical strength of concrete, whereas raw clay reduces the strength [34]. Since raw clays are crystalline, a dramatic reduction in strength has been observed when OPC replaces them. Although some studies reported an increase in strength up to a specific limit, thereafter, strength reduced. This increase may have resulted from the filling effect of fine clay particles [142]. However, some studies, especially those on Bentonite [183], have shown that a high amount of raw clay inclusion can increase strength due to the pozzolanic reactivity of Bentonite clay. These controversial results for clay must be highlighted in future research. However, most previous research has achieved increased mechanical performance by employing optimal calcination. During calcination, the clay’s crystalline structure is destroyed through dehydroxylation, transforming it into an amorphous, disordered state that renders it highly reactive in the cement and alkaline environment [127,128,129]. While limited research also indicated a similar increase in strength during mechanical activation. Mechanochemical activation involves reducing the crystallinity of certain clays by increasing their specific surface area through high-speed mechanical grinding [159]. The following sections illustrates the impact of raw and treated clays on the mechanical performance of concrete.
5.1. Compressive Strength
Most previous research incorporated calcination treatment to activate Kaolin clay. The study by Salau et al. [117] reports a greater decrease in strength when Kaolin was calcined above 750 °C. This decline in strength was due to the recrystallisation of the clay and the reduction of pozzolanic reactivity. In comparison, Boakye and Khorami [155] calcined clay at 750 °C for 2 h. Results showed that the highest compressive strength was achieved at a 20% replacement level, and that from 40% to 100% all samples demonstrated improved strength, indicating that the calcination process enhanced geopolymerisation. Again, the addition of calcium-rich additives also enhances geopolymerisation as shown by the study of Adufu et al. [135]. This study revealed that adding calcium-rich additives, especially quick lime (QLM), significantly enhanced geopolymerisation, and replacing up to 10% of calcined Kaolin with QLM improved compressive strength by 77.9% compared to control samples. However, limited research has examined the effect of mechanical activation on the compressive strength of Kaolin. For instance, Balczár et al. [163] demonstrated that the highest compressive strength (55.6 MPa) of mechanochemically activated samples surpassed that of the best thermally activated sample (43.0 MPa). Another study by Tole et al. [34] showed that raw Kaolin did not exhibit any compressive strength due to its lack of pozzolanic reactivity. Mechanically activated Kaolin exhibited high compressive strength.
Most previous research on Illite employed thermal activation to enhance its pozzolanic properties. Bonavetti et al. [120] calcined Illite clay at 750 °C for 1 h, with 35% of the OPC replaced by clay. The obtained results showed a reduction in strength. However, the addition of limestone filler improved strength. Fernández et al. [40] calcined the Illite clay at 600 °C for 1 h and obtained a reduction in strength of up to 36% for a 30% replacement of OPC. Another study by Eliche-Quesada et al. [183] revealed that up to 50% replacement of MK by Illite clay significantly enhanced the compressive strength by 66% and 62% at 28 days and 90 days of ambient curing, respectively. This increase in strength may be attributed to both the altered properties of the formed gel and the marginal increase in bulk density. The Si-O-Al bonds in the geopolymer gel diminished as the Si/Al ratio increased, whereas the Si-O-Si connections became more prominent. This phenomenon contributed to the gel’s enhanced mechanical strength, as the Si-O bond is inherently stronger than the Al-O bond. Moreover, the C-A-S-H gel was formed in the presence of calcium within the clay matrix [184]. A further investigation by Zhao et al. [122] revealed that mechanical activation significantly enhanced the compressive strength of Illite by altering its surface morphology, resulting in a synergistic effect due to high-speed grinding.
The study by Waqas et al. [140] demonstrated that the compressive strength increased by 10% for raw bentonite-GPC and by 18% for the calcined mixtures compared to the control samples. Adding PPF further enhanced the compressive strength. Another study conducted by Karthikeyan [185] further examined the mechanical performance of raw Bentonite by substituting 25%, 30%, and 35% by weight with OPC. The results indicated a 14.10% reduction in compressive strength for a 25% inclusion. Conversely, an increase of 19.3% was observed at 30% inclusion, followed by a further decrease of 61.72% at 35% inclusion, relative to the control mix. Although this study recommended a 20% to 30% replacement of raw Bentonite as sustainable, the strength reduction observed with a 25% inclusion of Bentonite is controversial. Another study by Ahmad et al. [177] reported a 7.14% reduction in strength with a 20% replacement of raw Bentonite with OPC and suggested that a 15% replacement might be optimal. At the same time, Fode et al. [142] obtained the opposite result in comparison to Ahmad et al. [177]. This study observed a slight increase in compressive strength with 5% raw Bentonite replacement, then a continuous decrease from 10% to 20% replacement. Conversely, calcined clay showed an increasing trend in compressive strength with increasing OPC replacement from 5% to 20%. Figure 4 illustrates the opposite trend in compressive strength when incorporating raw Bentonite, as observed by Ahmad et al. [177] and Fode et al. [142]. This research found that raw Bentonite does not exhibit pozzolanic properties unless calcined. These findings are inconsistent with those of prior research conducted by Karthikeyan et al. [185], Ahmad et al. [177] and Liu et al. [186]. These variations in compressive strength across studies using raw and calcined bentonite are attributable to several factors. Waqas et al. [140] documented enhancements in strength with both variants, particularly with the inclusion of PPF, likely owing to improved crack resistance and matrix densification. Conversely, Karthikeyan [185] observed inconsistent outcomes with raw Bentonite, noting a reduction in strength at a 25% replacement level and improvements at 30%, potentially due to optimal particle packing or filler effects. Ahmad et al. [177] and Fode et al. [142] demonstrated conflicting trends: Ahmad et al. [177] observed a 7.14% decrease at 20% replacement and recommended 15% as optimal, whereas Fode et al. [142] identified initial strength improvements at 5%, followed by declines, suggesting that raw bentonite may lack pozzolanic activity unless subjected to calcination. These discrepancies may arise from differences in Bentonite source, calcination conditions, mix design, and testing methodologies, thereby highlighting the necessity for standardised evaluation procedures.
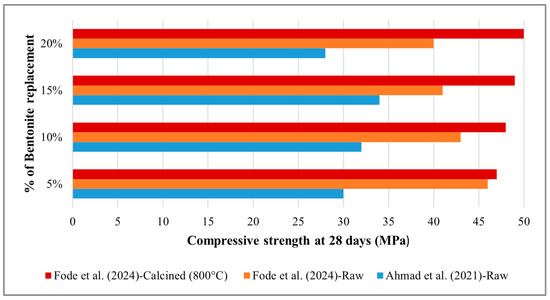
Figure 4.
Opposite trend of compressive strength of concrete containing raw Bentonite [142,177].
The study by Yu et al. [147] examined the mechanical performance of geopolymer mortar containing fly ash and GGBFS, with 1%, 2%, 4%, 6%, and 8% of calcined Halloysite (heated to 750 °C for 2 h) replacing fly ash and GGBFS. In the 2% replacement group, this study achieved the most significant increase in compressive strength after 28 days. A further investigation conducted by Zhang et al. [145] reported that Halloysite calcined at 750 °C exhibited the highest compressive strength with optimal geopolymerisation. The lowest compressive strength was observed at 1000 °C, which was attributed to recrystallisation.
Limited research has shown that the optimal calcination temperature for Palygorskite is between 750 °C and 800 °C. Poussardin et al. [79] reported that the compressive strength decreased slightly by approximately 2.5% with a 20% inclusion of calcined Palygorskite (750 °C) in OPC, which was considered negligible. Georgopoulos et al. [150] demonstrated that replacing 20% of OPC with Mg-rich calcined Palygorskite (heated to 800 °C for 1 h) resulted in a 0.96% increase in compressive strength compared to the control sample.
The study by Pu et al. [187] used raw Sepiolite clay to replace MK and fly ash in geopolymer paste. The results showed that including Sepiolite positively affected compressive strength, with the highest strength at 10% replacement; beyond that level, compressive strength began to decline due to the lack of pozzolanic material. Another study by Wu et al. [152] indicated that the optimal calcination temperature for Sepiolite was 800 °C for 30 min. This research replaced 20% of OPC with calcined Sepiolite. After 28 days, the compressive strength was nearly identical to that of the reference sample. In another investigation, Wu et al. [87] employed acid treatment to activate the Sepiolite. This study indicated that a strong acid at a higher concentration (HCl, 1 M) enhanced the strength by partially dissolving Si and Al. Nonetheless, this treatment was not as effective as calcination.
From the available literature, it is evident that the inclusion of different types of clays, either raw or treated, enhances the compressive strength up to a specific limit, beyond which the compressive strength starts to decline, as shown in Figure 5. It is recognised that calcination enhances the compressive strength of all types of clays, up to a replacement level of 20% to 40%. Limited research has shown that mechanically activated Illite can increase compressive strength by up to 50% with up to 50% replacement. It is interesting to note that raw clays, such as Sepiolite and Bentonite, also increased the compressive strength. For Sepiolite, 10% inclusion showed identical strength compared to calcined Kaolin.
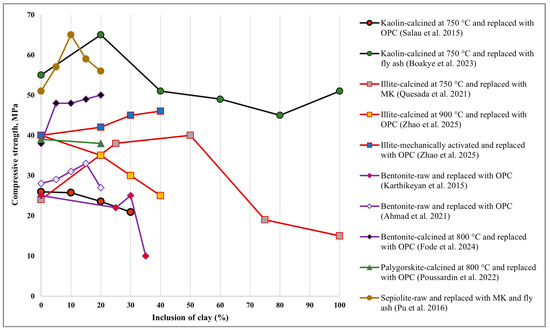
Figure 5.
Effect of clays on the compressive strength of concrete [79,117,122,142,155,177,183,185,187].
On the other hand, inclusion of raw Bentonite at 15–30% increased strength. However, a 20% inclusion of calcined Bentonite showed an upward trend in compressive strength, suggesting that higher inclusions beyond 20% may be achievable with calcination. The current state of research does not clearly establish the impact of mechanical activation on different types of clay and their replacement levels. Therefore, further research is needed.
5.2. Split Tensile Strength
Split tensile strength is a crucial parameter for assessing the mechanical performance of concrete. However, limited research has been conducted on the tensile strength of various clay types. Few studies have investigated the split tensile strength of concrete with Bentonite clay. The study by Waqas et al. [140] demonstrated that the split tensile strength increased by 4% for raw bentonite-GPC and 10% for treated mixtures compared to the control without bentonite. Adding PPF further enhanced the split tensile strength. Karthikeyan et al. [185] further examined the mechanical performance of raw Bentonite by substituting 25%, 30%, and 35% by weight with OPC. The results indicated an increasing trend in split tensile strength from 4% to 22.2% for a 25% to 30% replacement ratio. However, beyond 30% replacement, the strength declined by 6.8%, compared to the reference sample. Another study by Ahmad et al. [177] observed an increased trend in split tensile strength of 6.1%, 9.1%, and 21.3% for 5%, 10%, and 15% Bentonite replacement, respectively. However, the strength reduced by 3.03% when 20% Bentonite was replaced with OPC. This study indicated that a 15% replacement of raw Bentonite might be optimal, and the enhanced strength was attributed to the pozzolanic reaction of SiO2. Collectively, the studies suggest that Bentonite clay can enhance the split tensile strength of concrete when used in moderate proportions (typically 15–30%). The improvements are attributed to the pozzolanic activity of silica and the filler effect. However, excessive replacement reduces strength, likely due to lower cement content and poor particle packing. Future research should focus on microstructural analysis, long-term durability, and the synergistic effects of Bentonite with other additives, such as PPF, to optimise performance.
The literature review indicates that limited research has been conducted to analyse the split tensile strength of concrete containing clay. A limited study suggests that the inclusion of Bentonite, whether in its raw or calcined form, in standard or geopolymer concrete positively influences the split tensile strength up to a threshold; beyond this limit, the strength starts to diminish, as illustrated in Figure 6. The majority of prior research demonstrated peak strength with a 10% to 15% replacement of raw Bentonite, except Karthikeyan et al. [185]. This investigation achieved maximum strength at a substitution level of up to 30% raw Bentonite, whereas comparable studies identified an optimal replacement threshold of approximately 15%. Therefore, the current state of research on the effect of raw Bentonite on the split tensile strength of concrete is unclear. It is to be noted that raw Bentonite should remain structurally inert. The question arises as to why raw Bentonite is substituted with OPC rather than with sand. Further research must be conducted on raw Bentonite, regardless of whether it occurs naturally as a pozzolanic material. Simultaneously, the influence of other 1:1 and 2:1 clays on the split tensile strength of concrete should be examined. Furthermore, the effect of fibre should be thoroughly investigated. As documented in the literature, the incorporation of 1% PPF enhanced the strength by nearly 39% compared to samples without PPF.
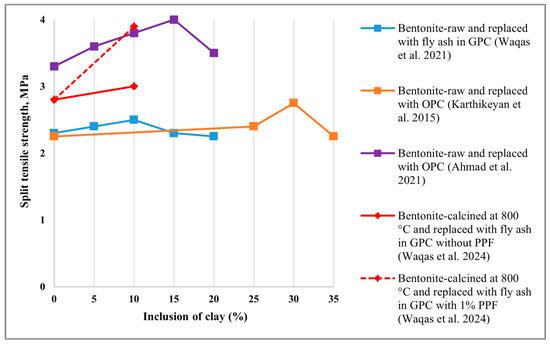
Figure 6.
Effect of clays on the split tensile strength of concrete [140,177,185].
5.3. Flexural Strength
Concrete flexural strength is a crucial factor in structural engineering, particularly for components such as beams, slabs, and pavements that undergo bending stresses. It indicates how well the material withstands tensile stresses at the ends, which is essential for ensuring structural integrity and longevity [188]. However, current research has not adequately explored the flexural strength of clay-based concrete. The limited research on the flexural strength of clay-based concrete has been summarised in the following sections.
The addition of calcium-rich additives to calcined Kaolin also enhances geopolymerisation. The study by Adufu et al. [135] revealed that replacing up to 10% of calcined Kaolin with QLM improved flexural strength by 700% compared to control samples, promoting geopolymerisation. Bonavetti et al. [120] demonstrated that when calcined Illite (750 °C for 1 h) and filler were combined, calcium hemi or mono-carboaluminate was more likely to form in the composite cements at later ages (90 days), which gradually improved the strength.
The study by Waqas et al. [140] showed that calcined Bentonite samples outperformed raw Bentonite samples, and adding 1% PPF further enhanced flexural strength by up to 23% for calcined Bentonite compared to raw Bentonite. Again, the study by Karthikeyan et al. [185] showed that beyond a 30% replacement with OPC, the strength declined by 16% compared to the reference sample. Conversely, Fode et al. [142] compared raw and calcined Bentonite (800 °C, 4 h) and replaced the same amount with 5%, 10%, 15%, and 20% OPC by weight. This study observed a reduced trend in flexural strength for raw Bentonite beyond 5% replacement, whereas calcined Bentonite exhibited an increased trend up to 20% replacement.
The study by Yu et al. [147] examined the mechanical performance of geopolymer mortar based on fly ash and GGBFS, replacing up to 8% of calcined Halloysite (heated to 750 °C for 2 h) with fly ash and GGBFS. In a 2% replacement study, the most significant increase in flexural strength was observed; beyond that level, strength decreased continuously. Again, the study by Pu et al. [187] used raw Sepiolite clay to replace MK and fly ash in geopolymer paste. It can be observed that the geopolymer containing more than 10% sepiolite exhibits a decline in flexural strength.
Figure 7 summarises the findings of the literature previously discussed. Based on limited available research, it is evident that calcined clay enhances the flexural strength of concrete when used as a replacement for up to 10% of the clay. Beyond this threshold, the strength begins to decrease. For calcined Bentonite, a replacement of up to 10% demonstrated an upward trend; however, for raw Bentonite, exceeding 5% resulted in a decrease in flexural strength. Conversely, raw Sepiolite exhibited the highest flexural strength with up to 10% replacement incorporating fly ash GPC.
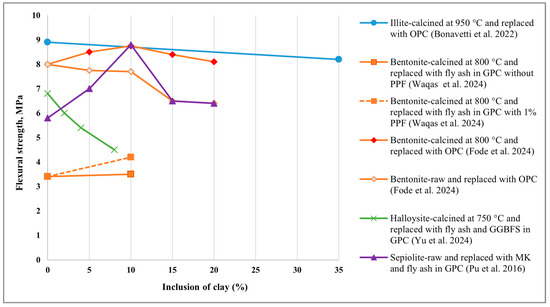
Figure 7.
Effect of clays on the flexural strength of concrete [120,140,142,147,187].
By analysing the above sections on the mechanical performance of clay-based geopolymer or OPC-based composites, proper treatment of clay significantly improves the mechanical performance, compared to untreated or raw clay. However, many previous studies have focused on calcination treatment of various types of clay to achieve high pozzolanic properties, whereas mechanical treatment has not been widely explored. Limited research suggests that mechanically activated clays exhibit greater pozzolanicity due to their larger surface area, which facilitates the quicker dissolution of Al and Si. Few researchers employed raw clay, particularly Bentonite [177], and achieved the highest compressive strength at a 15% replacement rate, attributing the increase to the pozzolanic reaction of SiO2. On the other hand, another study [142] reported a sharp decline in compressive strength with raw Bentonite at levels above 5%. This study argued that raw Bentonite cannot be pozzolanic unless it is treated, either by calcination or mechanical activation. Raw Bentonite is more likely to serve as a filler. Hence, there is a need for further research in this area. Table 7 summarises the impact of raw and treated clays on the mechanical performance of concrete.

Table 7.
Influence of different clay types and treatments on the mechanical performance of concrete.
6. Prediction-Based Modelling of GPC and OPC Concrete
Various factors affect the mechanical properties of GPC, including the concentrations of sodium hydroxide (NaOH) and sodium silicate (Na2SiO3), the alkaline liquid-to-binder ratio (L/B), the amount and composition of the binder, the buffering capacity of the alkaline liquids, and temperature [189]. Oderji et al. [190] illustrated that compressive strength (C-S) increases with escalating CaO content when CaO-rich precursors such as GGBS are utilised as partial replacements of FA. Chindaprasirt et al. [191] observed that increased SiO2 content results in microstructures with lower porosity, thereby improving the strength of GPC. Prasanphan et al. [192] found that 10M NH produces the highest strength in calcined kaolin processing waste-based GPC. Another study by Hasan et al. [193] observed that higher initial curing temperatures and extended durations, typically 24 h, contributed to improved C-S. Phoongernkham et al. [194] observed that the C-S value declined as the l/b ratio increased from 0.4 to 0.9. However, these laboratory tests were often expensive, time-consuming, and not always feasible for testing all mix proportions with varying ingredient proportions. In recent decades, researchers have predominantly supported data-driven methods, particularly artificial neural networks (ANN) [195]. Several recent studies ([196,197,198]) demonstrate the high accuracy of the ANN model in predicting the C-S of geopolymer concrete. In addition to ANN, researchers have also employed various models, including linear regression (LR) [196], Support Vector Regression (SVR) [199], and Multi-Expression Programming (MEP) [200], among others. The subsequent sections and Table 8 provide a summary of the most recent prediction-based models for forecasting the C-S and other properties of GPC- and OPC-based concrete.
Most previous research has employed various data-driven models to predict fly ash and GGBFS-based GPC. For instance, Ahmad et al. [201] employed various machine learning techniques, such as Bagging Regressor (BR) and AdaBoost Regressor (AR), to predict the C-S of fly ash-based GPC. By using a total of 154 data points, this study found that the BR model was more effective in predicting the C-S with the highest value of Coefficient of Determination (R2) of 0.97 and the lowest values of errors named Root Mean Square Error (RMSE) and Mean Absolute Error (MAE) of 1.94 MPa and 1.51 MPa, respectively. Another study by Dao et al. [197] also uses an ANN and an Adaptive Neuro-Fuzzy Inference System (ANFIS) to predict the C-S of fly ash-based GPC. This study used four input variables—fly ash, NaOH, Na2SiO3, and water—and concluded that both models were effective at predicting C-S. Additionally, Ahmed et al. [202] employed ANN, MEP, LR and Full Quadratic (FQ) to predict the C-S of fly ash-based GPC. Using 207 data points, this study found that the ANN model was more accurate at predicting C-S.
Limited research demonstrates the effectiveness of some prediction-based models for MK and Bentonite clay. For instance, Khan et al. [203] employed Gene Expression Programming (GEP), Extreme Gradient Boosting (XGB), and Bagging Regressor (BR) to predict the C-S of OPC and MK-based mortars. Using 249 data points, this study found that the XGB model was more accurate at predicting C-S. Amlashi et al. [204] employed a hybrid learning model optimising Forensic-Based Investigation Optimisation (FIBO) to predict the workability and mechanical properties of OPC- and Bentonite-based plastic concrete. The applied models were Random Forest (RF), Adaptive Boosting (ADB), Extreme Gradient Boosting (XGB), and Gradient Boosting Regression Tree (GBRT)-optimised with FIBO. This study used seven input variables, including the contents of gravel, Bentonite, silty clay, curing time, sand, cement and water. The obtained results showed that XGB-FIBO achieved the highest accuracy in predicting the slump and tensile strength. Whereas, for elastic modulus, GBRT-FIBO showed the highest accuracy. Another study by Ghanizadeh et al. [198] found that the ANN mode achieved higher accuracy in predicting the C-S of OPC and Bentonite-based plastic concrete than the Support Vector Machine (SVM). Khan et al. [205] employed the MEP to predict the slump, compressive strength, and elastic modulus of OPC and Bentonite-based plastic concrete, reporting high accuracy. Kumar et al. [206] also predicted the C-S of OPC and Bentonite-based plastic concrete by employing the Particle Swarm Optimisation (PSO) model.
To develop reliable predictive models for clay-based geopolymer concrete (clay-GPC), it is essential to consider key input variables such as the chemical composition of the clay (including SiO2 and Al2O3), the nature and treatment of the clay (whether raw or calcined), calcination temperature and duration, the type and concentration of the alkali activator, the liquid-to-solid ratio, curing conditions, and the proportion of clay substitution [207]. Research conducted by Abdullah et al. [208] and Gupta et al. [207] demonstrated that factors such as slag content, Na/Al and Si/Al ratios, and curing temperature are critical determinants of compressive strength. To effectively capture the complexities of clay treatment, models should encode treatment parameters as separate features and employ techniques such as feature selection, normalisation, and SHAP analysis to identify nonlinear interactions. Advanced models, including Random Forest, Gradient Boosting, and neural networks, have demonstrated high accuracy in predicting strength outcomes when these factors are properly incorporated [207].
In the context of clay-based geopolymer concrete systems, machine learning models have shown significant promise in predicting mechanical properties. However, there are some limitations too. This section summarises the advantages and shortcomings of the models discussed above. Machine learning models, particularly ANN, effectively forecast properties such as compressive strength. ANN surpasses alternative models in accuracy and demonstrates proficiency in managing complex relationships between mix components and curing factors, as exemplified by its successful application to nano-silica modifications.
Nevertheless, ANN requires extensive datasets and is frequently regarded as a “black box’ owing to its limited interpretability [209]. MEP offers explicit mathematical formulations, enhancing interpretability and facilitating integration within engineering contexts. However, it may lack the accuracy of more sophisticated models such as XGB or ANN. XGB is proficient at handling high-dimensional data, capturing intricate patterns, and often achieving superior accuracy compared to other ensemble techniques. Nevertheless, XGB models are computationally intensive and less transparent, which may limit their use in engineering applications that require interpretability [210]. BR provides probabilistic forecasts that are valuable for uncertainty quantification; however, it may encounter challenges when applied to nonlinear data in geopolymer systems. LSTM networks, primarily designed for time-series analysis, can model dependencies in the curing process but are limited in predicting static properties such as compressive strength and necessitate extensive data [210,211]. Support Vector Regression (SVR) demonstrates strong performance, particularly with limited datasets, and exhibits effective generalisation. A study indicated that SVR marginally surpassed ANN in predicting the compressive strength of geopolymer fibre-reinforced concrete. Nevertheless, the outcomes of SVR depend on kernel selection and parameter tuning, which can pose challenges. Each model possesses distinct advantages, and the optimal choice is influenced by dataset characteristics, interpretability requirements, and resource availability [212].
In conclusion, based on the literature and research findings discussed above, it is evident that prediction-based models are feasible and provide a rapid means of forecasting the strength and characteristics of materials. Several prediction-based models have already been developed to accurately estimate the strength of OPC concrete, fly ash, and GGBFS-based GPC. However, research on non-traditional clays, such as halloysite and palygorskite, in geopolymer concrete is notably scarce, indicating a significant gap in the existing literature. These clays, characterised by their distinctive tubular and fibrous morphologies, possess the potential to enhance mechanical and durability properties. However, their intricate structures and diverse compositions pose challenges for modelling efforts. Consequently, there are limited applications of machine learning techniques, such as predicting properties like compressive strength and durability, for geopolymers based on halloysite and palygorskite. This paucity of data and modelling frameworks underscores the necessity for further experimental and computational investigations to harness their potential and develop dependable predictive tools for these understudied materials.

Table 8.
Different prediction-based modelling of geopolymer and OPC-based concrete for predicting desired outcomes.
Table 8.
Different prediction-based modelling of geopolymer and OPC-based concrete for predicting desired outcomes.
| Applied Model | Type of Binder | Prediction Properties | R2 | RMSE | MAE | Remarks | Ref. |
|---|---|---|---|---|---|---|---|
| ANN LR LaR RR XGR | Amorphous or semicrystalline waste-based geopolymer | Compressive strength | 0.93 0.46 0.42 0.46 0.74 | 4.13 11.94 12.39 11.94 8.25 | 2.58 9.63 10.28 9.63 6.35 | The ANN model demonstrated the best accuracy, with an R2 of 0.93 and an RMSE of 4.13. | [196] |
| LR SVR ANN LSTM | Alkali-activated treated clay soil | Unconfined Compressive strength | 0.690 0.830 0.598 0.958 | 0.821 0.720 0.665 0.283 | 0.542 0.314 0.598 0.194 | The LSTM model showed the highest accuracy in predicting the strength. | [199] |
| GPR SVR | Expansive clay soil treated with hydrated-lime-activated rice husk ash | Unconfined Compressive strength | 0.9998 0.9998 | 0.4455 0.1408 | 0.3430 0.0514 | Both GPR and SVR models exhibited superior accuracy in predicting the strength. | [213] |
| MEP NLR ANN | OPC+Fly ash-based mortar | Compressive strength | 0.897 0.81 0.88 | 4.72 6.35 5.07 | 3.35 4.95 3.76 | The MEP model outperformed other models. | [200] |
| GEP XGB BR | OPC+ metakaolin (Mk)-based mortar | Compressive strength | 0.846 0.998 0.946 | 3.832 0.347 2.59 | 2.876 0.257 1.73 | XGB achieved the highest accuracy for strength determination, with a test R2 of 0.998, followed by a BR of 0.946. | [203] |
| BR AR | Fly ash-based geopolymer concrete | Compressive strength | 0.97 0.94 | 1.94 2.62 | 1.51 2.16 | The highest R2 value and the lowest error values (RMSE and MAE) indicated that the BR model was best suited for predicting strength. | [201] |
| ANFIS ANN | Fly ash-based geopolymer concrete | Compressive strength | 0.879 0.851 | 2.265 2.423 | 1.655 1.989 | Both models performed well in predicting compressive strength, but the ANFIS model was marginally better than the ANN. | [197] |
| RF-FIBO ADB-FIBO XGB-FIBO GBRT-FIBO | OPC+Bentonite-based concrete | Slump (S) Tensile strength (TS) Elastic modulus (E) | 0.943 (S) 0.962 (S) 0.966 (S) 0.966 (S) 0.949 (TS) 0.968 (TS) 0.977 (TS) 0.974 (TS) 0.943 (E) 0.962 (E) 0.966 (E) 0.966 (E) | 1.607 (S) 1.126 (S) 1.076 (S) 1.147 (S) 0.069 (TS) 0.053 (TS) 0.041 (TS) 0.049 (TS) 1.607 (E) 1.126 (E) 1.076 (E) 1.147 (E) | 1.149 (S) 0.747 (S) 0.797 (S) 0.835 (S) 0.052 (TS) 0.043 (TS) 0.030 (TS) 0.039 (TS) 1.149 (E) 0.747 (E) 0.797 (E) 0.835 (E | Among all models, XGB-FIBO achieved the highest accuracy for S and TS. In comparison, GBRT-FIBO excelled in predicting E. | [204] |
| ANN SVM | Bentonite+ OPC-based plastic concrete | Compressive strength | 0.9886 0.9648 | 0.3085 0.6744 | - - | The ANN model demonstrated the highest accuracy, with superior R2 and RMSE values, compared to the SVM. | [198] |
| MEP | OPC+Bentonite-based Plastic concrete | Slump Compressive strength Elastic modulus | 0.9998 0.9120 0.8299 | 0.5018 0.1897 258.9647 | 0.4272 0.1574 359.6862 | The MEP model demonstrated high accuracy in predicting the slump, compressive strength, and elastic modulus of bentonite-based concrete. | [205] |
| XGR-PSO XGR-GA XGR-DO | OPC+Bentonite-based concrete | Compressive strength | 0.974 0.968 0.969 | 0.038 0.041 0.040 | - - - | XGR-PSO exhibited the highest accuracy, with R2 = 0.974 and RMSE = 0.038, followed by XGR-DO and XGR-GA. | [206] |
| ANN MEP FQ LR | Fly ash-based geopolymer concrete | Compressive strength | 0.968 0.945 0.95 0.893 | 4.69 5.676 5.667 7.77 | - - - - | The ANN model achieved the highest accuracy, showcasing superior R2 and RMSE values compared to all other models. | [202] |
Abbreviations: ANN: Artificial Neural Network; LR: Linear Regression; LaR: Lasso Regression; RR: Ridge Regression; XGR: XGBoost Regressor; SVR: Support Vector Regression; LSTM: Long Short-Term Memory; GPR: Gaussian Process Regression; MEP: Multi Expression Programming; NLR: Nonlinear Regression; GEP: Gene Expression Programming; XGB: Extreme Gradient Boosting; BR: Bagging Regressor; AR: AdaBoost Regressor; ANFIS: Adaptive Neuro Fuzzy Inference; FIBO: Forensic-Based Investigation Optimisation; RF: Random Forest; ADB: Adaptive Boosting; GBRT: Gradient Boosting Regression Tree; SVM: Support Vector Machine; PSO: Particle Swarm Optimisation; GA: Genetic Algorithm; DO: Dragonfly Optimisation; FQ: Full Quadratic; R2: Coefficient of Determination; RMSE: Root Mean Square Error; MAE: Mean Absolute Error.
7. Durability and Microstructural Performance of Clay-Based GPC
7.1. Durability Performance
Research on the durability of clay-based geopolymer concrete (GPC), including Kaolin, Illite, Bentonite, Halloysite, Palygorskite, and Sepiolite, has yielded promising results. Kaolin-based GPC exhibited robust acid resistance, low water absorption (typically below 5%), and stable thermal conductivity (~0.45–0.54 W/m·K), especially after calcination and when combined with calcium-rich additives [214,215]. Experimental studies indicate that geopolymer formulations employing calcined clays and metakaolin attain compressive strengths exceeding 40 MPa. Additionally, they exhibit freeze-thaw resistance of over 75 cycles and possess sorptivity coefficients that are markedly lower than those observed in OPC mixtures [216]. Illite and low-grade kaolin formulations exhibited reduced chloride penetration and water sorptivity, with carbonation depths below 2 mm after six months, indicating strong long-term durability [217]. Bentonite-based GPC, when chemically or thermally activated, demonstrates good chemical resistance but may require additional grinding to reduce permeability and improve microstructural density [218].
Clays such as Halloysite, Palygorskite, and Sepiolite, recognised for their tubular and fibrous configurations, augment thermal insulation and chemical stability. For example, GPC produced with Palygorskite exhibited superior heat resistance and reduced thermal conductivity owing to its mineral structure. Water absorption and permeability were substantially influenced by particle size and porosity; smaller particles and optimised curing techniques, such as steam or autoclaving, diminish capillary absorption and improve overall performance and durability. Clay-GPC consistently exhibited greater resistance to acids and sulphates than OPC concrete, demonstrating minimal degradation even under aggressive conditions such as sulphic acid and chloride ions [219]. Halloysite-based GPC has been shown to possess a thermal conductivity as low as 0.38 W/m·K, rendering it suitable for insulation applications.
Additionally, it exhibits acid resistance, losing less than 5% of its mass after 28 days of [220]. Sepiolite’s high surface area and fibrous structure enhance sulphate resistance and reduce permeability, thereby improving long-term durability. Research indicates water absorption levels below 6% and chloride penetration depths less than 10 mm, confirming its robust resistance to aggressive environments [88].
Overall, clay-GPC systems exhibit superior durability compared to OPC concrete, providing enhanced resistance to acid and sulphate attacks, reduced permeability, and consistent thermal performance. The clay’s mineralogical composition predominantly influences these attributes, the activation procedures employed, and the specific curing conditions, underscoring the importance of tailored treatment methodologies for diverse clay varieties.
7.2. Microstructural Performance
The mineral composition, morphology, and activation method of the employed clay significantly influence the microstructural performance of clay-based GPC. Calcined kaolin, referred to as metakaolin, tends to generate a dense and uniform matrix due to its plate-shaped morphology and high reactivity. SEM and XRD analyses confirm the formation of dense aluminosilicate gels with low porosity [221]. Although it exhibits lower reactivity, illite can still contribute to the development of a compact microstructure when combined with other reactive materials or subjected to thermal activation [59]. Bentonite, characterised by its layered structure and high water-absorption capacity, typically produces porous matrices unless finely ground and chemically treated. Nevertheless, it enhances internal curing and crack resistance due to its swelling properties [140].
Halloysite, owing to its nanotubular morphology, exhibits superior microstructural compactness and early strength compared to kaolinite. Studies suggest that halloysite releases higher quantities of reactive silica and alumina during alkali activation, resulting in a more homogeneous and dense matrix [222]. Palygorskite and sepiolite, both fibrous clays, enhance microstructural integrity and thermal stability. Their high surface area and distinctive morphology aid in reducing porosity and fortifying interfacial bonding within the geopolymer matrix [223].
Research comparing mechanical activation and calcination indicates that their combined application significantly enhances microstructural properties. For example, mechanochemical activation coupled with calcination can increase SSA by up to 158% and improve reactivity by 127%, leading to denser gels and increased strength. Although calcination alone, especially flash calcination, may reduce the specific surface area due to particle agglomeration, it nonetheless improves reactivity when optimally executed [223].
Research indicates that substituting 15–30% of OPC with treated clay, depending on the specific type and activation technique, can sustain or improve microstructural integrity. For example, a 20% substitution with calcined clay offers an optimal balance of strength and microstructure. In contrast, higher substitution ratios could increase porosity unless mitigated by grinding or the use of blended activators.
8. Future Research Trends and Recommendations
The subsequent section delineates the research gap and proposes recommendations for further investigation to assess the feasibility of clay-based GPC within the construction industry and to advance pioneering research in this field, based on existing research data and literature analysis:
8.1. Research Trends
Treatment method: Most current research has employed calcination to treat the clay, while other established techniques, such as mechanical grinding, have not been extensively explored. Calcination of clay requires temperatures of almost 750 °C to 950 °C, which requires a significant amount of energy. On the other hand, mechanical grinding costs low energy and is more in line with sustainability. Additionally, the chemical activation methods require further exploration.
Raw and treated Bentonite: Recent research has claimed that raw Bentonite exhibits pozzolanic reactivity, while others dispute this. Therefore, further investigation is necessary to resolve this debate. If raw Bentonite is inherently pozzolanic, it can be used directly without any energy-intensive treatment.
Fracture mechanism: No research has yet been conducted to analyse the fracture mechanisms of different clay-based GPC. Therefore, further in-depth research is required to understand these mechanisms.
Durability analysis: Although this research did not include durability properties of clay-based GPC, the current state of research lacks access to these properties. Therefore, an in-depth systematic review and further research are needed.
Autogenous healing: No such contributions focus on the self-healing efficiency of various types of clay-based GPC for healing micro-cracks.
Prediction-based modelling: Limited data and modelling efforts, especially for halloysite, palygorskite, and sepiolite, highlight the need for further research to optimise their use in geopolymer concrete systems.
8.2. Recommendations for Real-World Applications
Different clay minerals possess distinct properties that can be utilised in various construction applications. For example, kaolin, with its high purity and reactivity, is well-suited for the manufacturing of high-strength structural components and precast elements. Illite, with moderate reactivity, is suitable for general-purpose concrete used in low-load-bearing structures. Bentonite, recognised for its swelling and sealing properties, is advantageous in underground construction and serves as a self-healing agent in water-retaining structures. Halloysite, characterised by its nanotubular structure, demonstrates potential for use in lightweight panels and high-performance composites due to its capacity to enhance mechanical strength and durability. Palygorskite, characterised by its fibrous structure and thermal stability, is ideally suitable for fire-resistant panels and infrastructure in demanding environments. Sepiolite, characterised by its high porosity and water-retention capabilities, can be utilised in permeable concrete and in environmentally sustainable construction materials.
9. Conclusions
This review demonstrates that integrating natural clay into geopolymer formulations can effectively mitigate environmental damage caused by widespread OPC production. Furthermore, it offers an alternative approach to reducing reliance on fly ash and GGBFS in the synthesis of geopolymers, thereby contributing to the development of a more sustainable built environment. However, optimal treatment and inclusion of clay within the geopolymer composites are mandatory to avoid the negative impact on the overall performance of the clay-based concrete. Analysing current research trends enabled the following conclusions.
The optimal calcination temperature varies among different types of clay, and this variation may be attributed to differences in chemical composition and crystalline structure. For 1:1 clays, such as Kaolin and Halloysite, an optimal temperature range of 700 °C to 800 °C is required. Conversely, 2:1 clays, such as Illite, Bentonite, Palygorskite, and Sepiolite, require temperatures of 850 °C to 950 °C for complete dehydroxylation. For 1:1 clays, a lower dehydroxylation temperature is due to a single silicon tetrahedral sheet enclosing a single octahedral aluminium unit. In contrast, 2:1 clays have two silicon tetrahedral sheets and one octahedral magnesium or aluminium, requiring more energy to remove bound water and break Si-Al bonds.
Mechanical ball grinding is an effective treatment method for converting crystal clay into highly pozzolanic amorphous clay. However, this activation method does not depend on the clay’s crystal structure (e.g., 1:1 or 2:1). Nevertheless, its efficiency depends on specific parameters, including grinding speed, duration, and the ball-to-clay ratio. It is advisable to optimise these parameters to obtain a highly pozzolanic clay. Most of the clay became pozzolanic at grinding speeds between 300 rpm and 600 rpm, with a grinding time of between 15 min and 120 min, and a ball-to-clay ratio of 20–25.
The activation of clay through chemicals has not been thoroughly investigated. Some limited research suggests that high concentrations of acid and activators may enhance the pozzolanic reactivity of clay. Further detailed investigation is necessary. However, based on the available literature, the following summary Table 9 has been drawn to summarise the ideal treatment methods and performance across various clay types:

Table 9.
Ideal treatment method and performance results across various clay types.
All types of clays, whether raw or treated, adversely affect workability due to their high water demand. The reduction in workability is more evident with the raw clay than with the treated clay. By comparing the negative impacts of calcinated clay and mechanically activated clay, limited research suggests that calcinated clay generates a higher water demand compared to mechanically activated clay, due to its porous structure resulting from calcination. However, this statement is not sufficiently established due to insufficient evidence. To achieve a standard slump, the raw clay should not exceed 10%, and the calcined and mechanically activated clay should be within 20% to 25% of this value.
The mechanical performance of clay-based geopolymer or OPC composites improves with proper treatment of the clay. Many studies focus on calcination to produce high-quality pozzolanic clays, while mechanical treatment has been overlooked. Limited research suggests that mechanically activated clays exhibit greater pozzolanicity due to their larger surface area. For improved mechanical performance, the raw clay content should be between 5% and 10%. For calcined and mechanically activated clay, 20% to 30% can be replaced with fly ash. However, greater replacement with fly ash remains possible to produce geopolymer concrete with an appropriate alkali activator if the clay is highly pozzolanic.
Prediction-based models, such as ANN, MEP, XGB, and BR, showed good accuracy in predicting the compressive strength of fly ash, MK, and Bentonite-based geopolymer. Furthermore, although applying AI techniques to geopolymer concrete production offers significant potential, challenges remain to be addressed. To effectively utilise AI in modelling clay-based geopolymer concrete, targeted research needs to overcome existing challenges. An important issue is the scarcity of high-quality, diverse datasets, particularly for clays, which limits the training and validation of machine learning models. Future research should focus on creating hybrid models that combine the high accuracy of deep learning with the interpretability of symbolic or rule-based systems. These efforts will help bridge the gap between AI capabilities and engineering requirements, leading to more reliable and explainable predictive tools for sustainable construction.
Clay-based GPCs show outstanding durability, outperforming OPC concrete in resisting acids and sulphates. Kaolin-based GPC has water absorption below 5% and a thermal conductivity of 0.45–0.54 W/m·K. Meanwhile, metakaolin mixes reach strengths over 40 MPa and endure more than 75 freeze–thaw cycles. Combinations of Illite and low-grade kaolin restrict chloride penetration to less than 10 mm and carbonation to less than 2 mm. Halloysite has a low thermal conductivity of 0.38 W/m·K and retains over 95% of its mass after sulfuric acid treatment. Sepiolite and Palygorskite reduce permeability and water absorption to less than 6%, thereby enhancing long-term durability.
The microstructure of clay-based geopolymer composites varies considerably depending on the mineral composition, shape, and activation process of the clay. Metakaolin produces dense and uniform matrices due to its high reactivity, whereas Halloysite’s nanotubular structure enhances early strength and compactness. Fibrous clays such as Palygorskite and Sepiolite improve thermal stability and interfacial bonding. When mechanochemical activation is combined with calcination, it significantly increases surface area and reactivity, resulting in denser gels. Substituting approximately 20% of OPC with treated clays consistently maintains or even improves microstructural integrity without increasing porosity.
Author Contributions
Conceptualisation, M.T.I., B.K. and R.A.-A.; methodology, M.T.I., B.K. and R.A.-A.; validation, M.T.I., B.K. and R.A.-A.; formal analysis, M.T.I.; writing—original draft preparation, M.T.I.; writing—review and editing, B.K. and R.A.-A.; visualisation, M.T.I.; supervision, R.A.-A. and B.K.; project administration, R.A.-A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Al-Tam, S.M.; Riad, A.; Mohammed, N.; Al-Otaibi, A.; Youssf, O. Advancement of eco-friendly slag-based high-strength geopolymer concrete. J. Mater. Res. Technol. 2024, 34, 1636–1653. [Google Scholar] [CrossRef]
- Helal, K.A.; Tahwia, A.M.; Youssf, O. Performance of eco-friendly ECC made of pre-treated crumb rubber and waste quarry dust. J. Build. Eng. 2024, 97, 110820. [Google Scholar] [CrossRef]
- Tahwia, A.M.; Aldulaimi, D.S.; Abdellatief, M.; Youssf, O. Physical, Mechanical and Durability Properties of Eco-Friendly Engineered Geopolymer Composites. Infrastructures 2024, 9, 191. [Google Scholar] [CrossRef]
- Mokhtar, A.; Nasooti, M. A decision support tool for the cement industry to select energy efficiency measures. Energy Strat. Rev. 2020, 28, 100458. [Google Scholar] [CrossRef]
- Prakasan, S.; Palaniappan, S.; Gettu, R. Study of Energy Use and CO2 Emissions in the Manufacturing of Clinker and Cement. J. Inst. Eng. (India) Ser. A 2020, 101, 221–232. [Google Scholar] [CrossRef]
- Islam, M.T.; Apurba, M.S.H. Effect of Inclusion of Recycled Waste Glass Powder in Geopolymer Concrete: A Review on Workability, Mechanical, Durability, and Micro-Structural Performance. Iran. J. Sci. Technol. Trans. Civ. Eng. 2025. [Google Scholar] [CrossRef]
- Farooq, F.; Jin, X.; Javed, M.F.; Akbar, A.; Shah, M.I.; Aslam, F.; Alyousef, R. Alyousef, Geopolymer concrete as sustainable material: A state of the art review. Constr. Build. Mater. 2021, 306, 124762. [Google Scholar] [CrossRef]
- Ramesh, V.; Muthramu, B.; Rebekhal, D. A review of sustainability assessment of geopolymer concrete through AI-based life cycle analysis. AI Civ. Eng. 2025, 4, 3. [Google Scholar] [CrossRef]
- Vijai, K.; Kumutha, R.; Vishnuram, B.G. Effect of types of curing on strength of geopolymer concrete. Int. J. Phys. Sci. 2010, 5, 1419–1423. [Google Scholar]
- Davidovits, J. Geopolymers: Inorganic polymeric new materials. J. Therm. Anal. Calorim. 1991, 37, 1633–1656. [Google Scholar]
- Xu, H.; Van Deventer, J.S.J. The geopolymerisation of alumino-silicate minerals. Int. J. Miner. Process. 2000, 59, 247–266. [Google Scholar] [CrossRef]
- Duxson, P.; Fernández-Jiménez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; van Deventer, J.S.J. Geopolymer technology: The current state of the art. J. Mater. Sci. 2007, 42, 2917–2933. [Google Scholar] [CrossRef]
- Zribi, M.; Baklouti, S. Investigation of Phosphate-based geopolymers formation mechanism. J. Non-Cryst. Solids 2021, 562, 120777. [Google Scholar] [CrossRef]
- Al-Mansour, A.; Chow, C.L.; Feo, L.; Penna, R.; Lau, D. Green concrete: By-products utilization and advanced approaches. Sustainability 2019, 11, 5145. [Google Scholar] [CrossRef]
- Thakur, G.; Singh, Y.; Singh, R.; Prakash, C.; Saxena, K.K.; Pramanik, A.; Basak, A.; Subramaniam, S. Development of GGBS-Based Geopolymer Concrete Incorporated with Polypropylene Fibers as Sustainable Materials. Sustainability 2022, 14, 10639. [Google Scholar] [CrossRef]
- Khater, H.M. Effect of silica fume on the characterization of the geopolymer materials. Int. J. Adv. Struct. Eng. 2013, 5, 12. [Google Scholar] [CrossRef]
- Beiraghi, H.; Doostmohammadi, M.R. Effect of metakaolin and microsilica on the mechanical behavior of slag-based geopolymer concrete containing PP fibers. Innov. Infrastruct. Solut. 2025, 10, 1–14. [Google Scholar] [CrossRef]
- Torres-Carrasco, M.; Puertas, F. Waste glass in the geopolymer preparation. Mechanical and microstructural characterisation. J. Clean. Prod. 2015, 90, 397–408. [Google Scholar] [CrossRef]
- Turner, L.K.; Collins, F.G. Carbon dioxide equivalent (CO2-e) emissions: A comparison between geopolymer and OPC cement concrete. Constr. Build. Mater. 2013, 43, 125–130. [Google Scholar] [CrossRef]
- Sharma, P.K.; Singh, J.P.; Kumar, A. Effect of Particle Size on Physical and Mechanical Properties of Fly Ash Based Geopolymers. Trans. Indian Inst. Met. 2019, 72, 1323–1337. [Google Scholar] [CrossRef]
- Rahman, S.K.; Al-Ameri, R. A newly developed self-compacting geopolymer concrete under ambient condition. Constr. Build. Mater. 2021, 267, 121822. [Google Scholar] [CrossRef]
- Mayhoub, O.A.; Nasr, E.-S.A.; Ali, Y.; Kohail, M. Properties of slag based geopolymer reactive powder concrete. Ain Shams Eng. J. 2021, 12, 99–105. [Google Scholar] [CrossRef]
- Srivastava, P.; Saahir, M.; Garg, R.; Prajapat, U. Comparison of Mechanical Properties of Geopolymer Concrete vs Cement Concrete (Conventional Concrete). Int. J. Trend Sci. Res. Dev. 2024, 8, 15–26. [Google Scholar]
- Mehta, A.; Siddique, R. Properties of low-calcium fly ash based geopolymer concrete incorporating OPC as partial replacement of fly ash. Constr. Build. Mater. 2017, 150, 792–807. [Google Scholar] [CrossRef]
- Pandey, V.C.; Singh, N. Impact of fly ash incorporation in soil systems. Agric. Ecosyst. Environ. 2010, 136, 16–27. [Google Scholar] [CrossRef]
- Sharma, P.K.; MacLeod, A.J.; Aldridge, L.P.; Collins, F.; Gates, W.P. Pozzolanic behaviour and environmental efficiency of heat-treated fines as a partial cement replacement in mortar mixes. J. Build. Eng. 2025, 99, 111656. [Google Scholar] [CrossRef]
- Fořt, J.; Šál, J.; Ševčík, R.; Doleželová, M.; Keppert, M.; Jerman, M.; Záleská, M.; Stehel, V.; Černý, R. Biomass fly ash as an alternative to coal fly ash in blended cements: Functional aspects. Constr. Build. Mater. 2021, 271, 121544. [Google Scholar] [CrossRef]
- Gupta, S.; Chaudhary, S. State of the art review on supplementary cementitious materials in India–II: Characteristics of SCMs, effect on concrete and environmental impact. J. Clean. Prod. 2022, 357, 131945. [Google Scholar] [CrossRef]
- Snellings, R. Assessing, understanding and unlocking supplementary cementitious materials. RILEM Technol. Lett. 2016, 1, 50–55. [Google Scholar] [CrossRef]
- Migunthanna, J.; Rajeev, P.; Sanjayan, J. Waste Clay Brick as a Part Binder for Pavement Grade Geopolymer Concrete. Int. J. Pavement Res. Technol. 2023, 17, 1450–1467. [Google Scholar] [CrossRef]
- Alujas, A.; Fernández, R.; Quintana, R.; Scrivener, K.L.; Martirena, F. Pozzolanic reactivity of low-grade kaolinitic clays: Influence of calcination temperature and impact of calcination products on OPC hydration. Appl. Clay Sci. 2015, 108, 94–101. [Google Scholar] [CrossRef]
- Teklay, A.; Yin, C.; Rosendahl, L. Flash calcination of kaolinite rich clay and impact of process conditions on the quality of the calcines: A way to reduce CO2 footprint from cement industry. Appl. Energy 2016, 162, 1218–1224. [Google Scholar] [CrossRef]
- American Society for Testing and Materials; Committee C-9 on Concrete and Concrete Aggregates. Standard Specification for Coal Fly Ash and Raw or Calcined Natural Pozzolan for Use in Concrete; ASTM International: West Conshohocken, PA, USA, 2008. [Google Scholar] [CrossRef]
- Tole, I.; Habermehl-Cwirzen, K.; Rajczakowska, M.; Cwirzen, A. Activation of a raw clay by mechanochemical process: Effects of various parameters on the process efficiency and cementitious properties. Materials 2018, 11, 1860. [Google Scholar] [CrossRef] [PubMed]
- Baki, V.A.; Ke, X.; Heath, A.; Calabria-Holley, J.; Terzi, C. Improving the pozzolanic reactivity of clay, marl and obsidian through mechanochemical or thermal activation. Mater. Struct. 2024, 57, 9. [Google Scholar] [CrossRef]
- Scrivener, K.; Martirena, F.; Bishnoi, S.; Maity, S. Calcined clay limestone cements (LC3). Cem. Concr. Res. 2018, 114, 49–56. [Google Scholar] [CrossRef]
- Hojamberdiev, M.; Eminov, A.; Xu, Y. Utilization of muscovite granite waste in the manufacture of ceramic tiles. Ceram. Int. 2011, 37, 871–876. [Google Scholar] [CrossRef]
- Snellings, R.; Mertens, G.; Elsen, J. Supplementary cementitious materials. Rev. Miner. Geochem. 2012, 74, 211–278. [Google Scholar] [CrossRef]
- Dhandapani, Y.; Marsh, A.T.M.; Rahmon, S.; Kanavaris, F.; Papakosta, A.; Bernal, S.A. Suitability of excavated London clay as a supplementary cementitious material: Mineralogy and reactivity. Mater. Struct. 2023, 56, 174. [Google Scholar] [CrossRef]
- Fernandez, R.; Martirena, F.; Scrivener, K.L. The origin of the pozzolanic activity of calcined clay minerals: A comparison between kaolinite, illite and montmorillonite. Cem. Concr. Res. 2011, 41, 113–122. [Google Scholar] [CrossRef]
- Yuan, J.; Yang, J.; Ma, H.; Su, S.; Chang, Q.; Komarneni, S. Hydrothermal synthesis of nano-kaolinite from K-feldspar. Ceram. Int. 2018, 44, 15611–15617. [Google Scholar] [CrossRef]
- Schankoski, R.A.; Pilar, R.; Prudêncio, L.R.; Ferron, R.D. Evaluation of fresh cement pastes containing quarry by-product powders. Constr. Build. Mater. 2017, 133, 234–242. [Google Scholar] [CrossRef]
- Ghorbel, H.; Samet, B. Effect of iron on pozzolanic activity of kaolin. Constr. Build. Mater. 2013, 44, 185–191. [Google Scholar] [CrossRef]
- Zhou, C.H.; Keeling, J. Fundamental and applied research on clay minerals: From climate and environment to nanotechnology. Appl. Clay Sci. 2013, 74, 3–9. [Google Scholar] [CrossRef]
- Souri, A.; Kazemi-Kamyab, H.; Snellings, R.; Naghizadeh, R.; Golestani-Fard, F.; Scrivener, K. Pozzolanic activity of mechanochemically and thermally activated kaolins in cement. Cem. Concr. Res. 2015, 77, 47–59. [Google Scholar] [CrossRef]
- Tironi, A.; Trezza, M.; Irassar, E.; Scian, A. Thermal Treatment of Kaolin: Effect on the Pozzolanic Activity. Procedia Mater. Sci. 2012, 1, 343–350. [Google Scholar] [CrossRef]
- Fitos, M.; Badogiannis, E.G.; Tsivilis, S.G.; Perraki, M. Pozzolanic activity of thermally and mechanically treated kaolins of hydrothermal origin. Appl. Clay Sci. 2015, 116–117, 182–192. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Kodur, V.; Qi, S.L.; Cao, L.; Wu, B. Development of metakaolin–fly ash based geopolymers for fire resistance applications. Constr. Build. Mater. 2014, 55, 38–45. [Google Scholar] [CrossRef]
- Kolovos, K.; Tsivilis, S.; Kakali, G. The effect of foreign ions on the reactivity of the CaO-SiO2-Al2O3-Fe2O3 system: Part II: Cations. Cem. Concr. Res. 2002, 32, 463–469. [Google Scholar] [CrossRef]
- Xin, R.F.; Du, Y.; Guo, X.M. Effect of Alumina on Crystallization Behavior of Calcium Ferrite in Fe2O3-CaO-SiO2-Al2O3 System. Materials 2022, 15, 5257. [Google Scholar] [CrossRef]
- Davidovits, J.; Davidovits, R. Geopolymer Institute Library Ferro-Sialate Geopolymers (-Fe-O-Si-O-Al-O-); Geopolymer Institute Library: Saint-Quentin, France, 2020. [Google Scholar] [CrossRef]
- Liu, Z.; Lian, W.; Liu, Y.; Zhu, J.; Xue, C.; Yang, Z.; Lin, X. Phase formation, microstructure development, and mechanical properties of kaolin-based mullite ceramics added with Fe2O3. Int. J. Appl. Ceram. Technol. 2021, 18, 1074–1081. [Google Scholar] [CrossRef]
- Hernández-Chávez, M.; Vargas-Ramírez, M.; Herrera-González, A.M.; García-Serrano, J.; Cruz-Ramírez, A.; Romero-Serrano, J.A.; Sánchez-Alvarado, R.G. Thermodynamic analysis of the influence of potassium on the thermal behavior of kaolin raw material. Physicochem. Probl. Miner. Process. 2020, 57, 39–52. [Google Scholar] [CrossRef]
- Usman Jamo, H. Structural Analysis and Surface Morphology of Kaolin. Sci. World J. 2014, 9, 33–37. [Google Scholar]
- Abdalqadir, M.; Gomari, S.R.; Pak, T.; Hughes, D.; Shwan, D. A comparative study of acid-activated non-expandable kaolinite and expandable montmorillonite for their CO2 sequestration capacity. React. Kinet. Catal. Lett. 2024, 137, 375–398. [Google Scholar] [CrossRef]
- Heah, C.Y.; Kamarudin, H.; Abdullah, M.M.A.B.; Binhussain, M.; Musa, L.; Nizar, I.K.; Ghazali, C.M.R.; Liew, Y. Effect of mechanical activation on kaolin-based geopolymers. Adv. Mater. Res. 2012, 479–481, 357–361. [Google Scholar] [CrossRef]
- Snellings, R.; Reyes, R.A.; Hanein, T.; Irassar, E.F.; Kanavaris, F.; Maier, M.; Marsh, A.T.; Valentini, L.; Zunino, F.; Diaz, A.A. Paper of RILEM TC 282-CCL: Mineralogical characterization methods for clay resources intended for use as supplementary cementitious material. Mater. Struct. 2022, 55, 149. [Google Scholar] [CrossRef]
- Jindal, B.B.; Alomayri, T.; Hasan, A.; Kaze, C.R. Geopolymer concrete with metakaolin for sustainability: A comprehensive review on raw material’s properties, synthesis, performance, and potential application. Environ. Sci. Pollut. Res. 2022, 30, 25299–25324. [Google Scholar] [CrossRef]
- Luzu, B.; Duc, M.; Djerbi, A.; Gautron, L. High performance illitic clay-based geopolymer: Influence of thermal/mechanical activation on strength development. Appl. Clay Sci. 2024, 258, 107445. [Google Scholar] [CrossRef]
- Jia, J.; Wu, D.; Ren, Y.; Lin, J. Nanoarchitectonics of Illite-Based Materials: Effect of Metal Oxides Intercalation on the Mechanical Properties. Nanomaterials 2022, 12, 997. [Google Scholar] [CrossRef]
- Siyal, A.A.; Radin Mohamed, R.M.S.; Shamsuddin, R.; Ridzuan, M.B. A comprehensive review of synthesis kinetics and formation mechanism of geopolymers. RSC Adv. 2024, 14, 446–462. [Google Scholar] [CrossRef] [PubMed]
- Hoc Thang, N. Geopolymerization: A Review on Physico-chemical Factors Influencing the Reaction Process. J. Polym. Compos. 2020, 8, 01–09. [Google Scholar] [CrossRef]
- Hazarika, A.; Huang, L.; Babaahmadi, A. Characterisation, activation, and reactivity of heterogenous natural clays. Mater. Struct. 2024, 57, 68. [Google Scholar] [CrossRef]
- Lu, H. Changes in conductivity of illite modified with cationic surfactant. J. Biotech. Res. 2024, 17, 324–332. [Google Scholar]
- Vallina, D.; Rodríguez-Ruiz, M.D.; Santacruz, I.; Cuesta, A.; Aranda, M.A.; De la Torre, A.G. Supplementary cementitious material based on calcined montmorillonite standards. Constr. Build. Mater. 2024, 426, 136193. [Google Scholar] [CrossRef]
- Chen, Y.G.; Li, Z.Y.; Ye, W.M.; Wang, Q. Swelling characteristics of montmorillonite mineral particles in Gaomiaozi bentonite. Constr. Build. Mater. 2024, 439, 137335. [Google Scholar] [CrossRef]
- Turhan, Ş.; Metin, O.; Kurnaz, A. Determination of Major and Minor Oxides in Bentonite Samples From Quarries in Turkey, International Journal of Scientific Research, Research Paper. Chem. Sci. 2022, 9, 12–16. [Google Scholar]
- Noskov, A.V.; Alekseeva, O.V.; Yashkova, D.N.; Agafonov, A.V.; Shipko, M.N.; Stepovich, M.A.; Savchenko, E.S. Modification of Bentonite Properties with Iron Oxide Nanoparticles. J. Surf. Investig. 2025, 19, 296–303. [Google Scholar] [CrossRef]
- Andrunik, M.; Bajda, T. Modification of bentonite with cationic and nonionic surfactants: Structural and textural features. Materials 2019, 12, 3772. [Google Scholar] [CrossRef]
- Fahimizadeh, M.; Wong, L.W.; Baifa, Z.; Sadjadi, S.; Auckloo, S.A.B.; Palaniandy, K.; Pasbakhsh, P.; Tan, J.B.L.; Singh, R.R.; Yuan, P. Halloysite clay nanotubes: Innovative applications by smart systems. Appl. Clay Sci. 2024, 251, 107319. [Google Scholar] [CrossRef]
- Al-Rubaie, N.; Al-Ameeri, A.; Mattar, S. The effects of using halloysite nano-clay in concrete on human health and environments: An overview study. Mater. Today Proc. 2023; in press. [Google Scholar] [CrossRef]
- Yuan, P.; Tan, D.; Annabi-Bergaya, F. Properties and applications of halloysite nanotubes: Recent research advances and future prospects. Appl. Clay Sci. 2015, 112–113, 75–93. [Google Scholar] [CrossRef]
- Lázaro, B.B. Halloysite and kaolinite: Two clay minerals with geological and technological importance. Rev. Acad. Cienc. Zaragoza 2015, 70, 7–38. [Google Scholar]
- Vašutová, V.; Bezdička, P.; Lang, K.; Hradil, D. Mineralogy of Halloysites and Their Interaction with Porphyrine. Ceramics–Silikáty 2013, 57, 243–250. [Google Scholar]
- Joussein, E.; Petit, S.; Churchman, J.; Theng, B.; Righi, D.; Delvaux, B. Halloysite clay minerals—A review. Clay Miner. 2005, 40, 383–426. [Google Scholar] [CrossRef]
- Massaro, M.; Noto, R.; Riela, S. Past, present and future perspectives on halloysite clay minerals. Molecules 2020, 25, 4863. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, Y.; Leng, F.; Huang, L.; Wang, Z.; Tian, W. Recent advances on surface modification of halloysite nanotubes for multifunctional applications. Appl. Sci. 2017, 7, 1215. [Google Scholar] [CrossRef]
- Zhang, H. Selective modification of inner surface of halloysite nanotubes: A review. Nanotechnol. Rev. 2017, 6, 573–581. [Google Scholar] [CrossRef]
- Poussardin, V.; Paris, M.; Wilson, W.; Tagnit-Hamou, A.; Deneele, D. Calcined palygorskite and smectite bearing marlstones as supplementary cementitious materials. Mater. Struct. 2022, 55, 224. [Google Scholar] [CrossRef]
- Wang, W.; Wang, A. Recent progress in dispersion of palygorskite crystal bundles for nanocomposites. Appl. Clay Sci. 2016, 119, 18–30. [Google Scholar] [CrossRef]
- Biswas, B.; Sarkar, B.; Naidu, R. Influence of thermally modified palygorskite on the viability of polycyclic aromatic hydrocarbon-degrading bacteria. Appl. Clay Sci. 2016, 134, 153–160. [Google Scholar] [CrossRef]
- Poussardin, V.; Roux, V.; Wilson, W.; Paris, M.; Tagnit-Hamou, A.; Deneele, D. Calcined Palygorskites as Supplementary Cementitious Materials. Clays Clay Miner. 2022, 70, 903–915. [Google Scholar] [CrossRef]
- Xavier, K.C.M.; dos Santos, M.D.S.F.; Santos, M.R.M.C.; Oliveira, M.E.R.; Carvalho, M.W.N.C.; Osajima, J.A.; Filho, E.C.d.S. Effects of acid treatment on the clay palygorskite: XRD, surface area, morphological and chemical composition. Mater. Res. 2014, 17, 3–8. [Google Scholar] [CrossRef]
- Benkacher, N.; Iggui, K.; Mekhania, Z.A.; Benhamida, A.; Djermoune, A.; Merzeg, F.A.; Satha, H.; Layachi, A. Influence of surface modification of Algerian palygorskite with triethoxyoctylsilane as a hydrophobic agent for enhanced performance. J. Coatings Technol. Res. 2025. [Google Scholar] [CrossRef]
- Tian, G.; Han, G.; Wang, F.; Liang, J. Sepiolite nanomaterials: Structure, properties and functional applications. In Nanomaterials from Clay Minerals: A New Approach to Green Functional Materials; Elsevier: Amsterdam, The Netherlands, 2019; pp. 135–201. [Google Scholar] [CrossRef]
- Ruiz-Hitzky, E.; Darder, M.; Fernandes, F.M.; Wicklein, B.; Alcântara, A.C.S.; Aranda, P. Fibrous clays based bionanocomposites. Prog. Polym. Sci. 2013, 38, 1392–1414. [Google Scholar] [CrossRef]
- Wu, C.R.; Hong, Z.Q.; Zhan, B.J.; Tang, W.; Cui, S.C.; Kou, S.C. Effect of acid treatment on the reactivity of natural sepiolite used as a supplementary cementitious material. Constr. Build. Mater. 2022, 316, 125860. [Google Scholar] [CrossRef]
- Angeline Jemina, J.Y.; Sophia, M.; Talluri, R.; Muthuraman, U. Durability Studies on Self-Compacting Concrete Containing Sepiolite Powder and Recycled Coarse Aggregates. In Lecture Notes in Civil Engineering; Springer Science and Business Media Deutschland GmbH: Berlin, Germany, 2024; pp. 493–505. [Google Scholar] [CrossRef]
- Ruiz, A.I.; Ruiz-García, C.; Ruiz-Hitzky, E. From old to new inorganic materials for advanced applications: The paradigmatic example of the sepiolite clay mineral. Appl. Clay Sci. 2023, 235, 106874. [Google Scholar] [CrossRef]
- Bilir, K.; Çağlar, U. Effects of mechanical activation parameters on the rheological behavior of acid-treated sepiolite. Physicochem. Probl. Miner. Process. 2025, 61, 1. [Google Scholar] [CrossRef]
- Kalaiselvi, C.; Sivakumar, M.; Subadevi, R. Activation of Sepiolite by Various Acid Treatments. Int. Res. J. Eng. Technol. 2017, 4, 19–22. [Google Scholar]
- Shi, Y.; Liu, J.; Yang, P.; Wang, H.; Guan, S.; Cui, J. Surface modification of sepiolite and its application in one-component silicone potting adhesive. e-Polymers 2024, 24, 20240051. [Google Scholar] [CrossRef]
- Güneyisi, E.; Mermerdaş, K. Comparative study on strength, sorptivity, and chloride ingress characteristics of air-cured and water-cured concretes modified with metakaolin. Mater. Struct. 2007, 40, 1161–1171. [Google Scholar] [CrossRef]
- Vejmelková, E.; Pavlíková, M.; Keppert, M.; Keršner, Z.; Rovnaníková, P.; Ondráček, M.; Sedlmajer, M.; Černý, R. High performance concrete with Czech metakaolin: Experimental analysis of strength, toughness and durability characteristics. Constr. Build. Mater. 2010, 24, 1404–1411. [Google Scholar] [CrossRef]
- Duan, P.; Shui, Z.; Chen, W.; Shen, C. Effects of metakaolin, silica fume and slag on pore structure, interfacial transition zone and compressive strength of concrete. Constr. Build. Mater. 2013, 44, 1–6. [Google Scholar] [CrossRef]
- Khatib, J.M.; Hibbert, J.J. Selected engineering properties of concrete incorporating slag and metakaolin. Constr. Build. Mater. 2005, 19, 460–472. [Google Scholar] [CrossRef]
- Al-Akhras, N.M. Durability of metakaolin concrete to sulfate attack. Cem. Concr. Res. 2006, 36, 1727–1734. [Google Scholar] [CrossRef]
- Yin, J.; Deng, C.; Yu, Z.; Wang, X.; Xu, G. Effective removal of lead ions from aqueous solution using nano illite/smectite clay: Isotherm, kinetic, and thermodynamic modeling of adsorption. Water 2018, 10, 210. [Google Scholar] [CrossRef]
- Hamza, A.; Hussein, I.A.; Mahmoud, M. Introduction to reservoir fluids and rock properties. In Developments in Petroleum Science; Elsevier B.V.: Amsterdam, The Netherlands, 2023; pp. 1–19. [Google Scholar] [CrossRef]
- Alaneme, G.U.; Mbadike, E.M. optimisation of strength development of bentonite and palm bunch ash concrete using fuzzy logic. Int. J. Sustain. Eng. 2021, 14, 835–851. [Google Scholar] [CrossRef]
- Khushnood, R.A.; Rizwan, S.A.; Memon, S.A.; Tulliani, J.-M.; Ferro, G.A. Experimental Investigation on Use of Wheat Straw Ash and Bentonite in Self-Compacting Cementitious System. Adv. Mater. Sci. Eng. 2014, 2014, 832508. [Google Scholar] [CrossRef]
- Ahmad, S.; Barbhuiya, S.A.; Elahi, A.; Iqbal, J. Effect of Pakistani bentonite on properties of mortar and concrete. Clay Miner. 2011, 46, 85–92. [Google Scholar] [CrossRef]
- Memon, S.A.; Arsalan, R.; Khan, S.; Lo, T.Y. Utilization of Pakistani bentonite as partial replacement of cement in concrete. Constr. Build. Mater. 2012, 30, 237–242. [Google Scholar] [CrossRef]
- Mirza, J.; Riaz, M.; Naseer, A.; Rehman, F.; Khan, A.; Ali, Q. Pakistani bentonite in mortars and concrete as low cost construction material. Appl. Clay Sci. 2009, 45, 220–226. [Google Scholar] [CrossRef]
- Haw, T.T.; Hart, F.; Rashidi, A.; Pasbakhsh, P. Sustainable cementitious composites reinforced with metakaolin and halloysite nanotubes for construction and building applications. Appl. Clay Sci. 2020, 188, 105533. [Google Scholar] [CrossRef]
- Aydın, M.T.A. A spectroscopic study on the effect of acid concentration on the physicochemical properties of calcined halloysite nanotubes. J. Aust. Ceram. Soc. 2024, 60, 629–642. [Google Scholar] [CrossRef]
- Huang, R.; Wu, L.; Wang, X.; Zhao, M.; Tian, W.; Tang, N.; Gao, L. Effect of isomorphic replacement of palygorskite on its heavy metal adsorption performance. AIP Adv. 2023, 13, 085218. [Google Scholar] [CrossRef]
- Allouche, F.; Ammous, A.; Tlili, A.; Kallel, N. Geological setting, geochemical, textural, and genesis of palygorskite in Eocene carbonate deposits from Central Tunisia. Carbonates Evaporites 2024, 39, 31. [Google Scholar] [CrossRef]
- Kolathayar, S.; Sreekeshava, K.S.; Vinod, N.; Menon, C. Recent Advances in Building Materials and Technologies: Select Proceedings of IACESD 2023; Springer: Singapore, 2024. [Google Scholar]
- Walczyk, A.; Michalik, A.; Napruszewska, B.; Kryściak-Czerwenka, J.; Karcz, R.; Duraczyńska, D.; Socha, R.; Olejniczak, Z.; Gaweł, A.; Klimek, A.; et al. New insight into the phase transformation of sepiolite upon alkali activation: Impact on composition, structure, texture, and catalytic/sorptive properties. Appl. Clay Sci. 2020, 195, 105740. [Google Scholar] [CrossRef]
- de Brito, J.; Kurda, R. The past and future of sustainable concrete: A critical review and new strategies on cement-based materials. J. Clean. Prod. 2021, 281, 123558. [Google Scholar] [CrossRef]
- SRPS EN 196-3:2017; Methods of Testing Cement—Part 3: Determination of Setting Times and Soundness. iTeh, Inc.: Newark, DE, USA, 2017.
- Mohamed, A.M.; Tayeh, B.A. Metakaolin in ultra-high-performance concrete: A critical review of its effectiveness as a green and sustainable admixture. Case Stud. Constr. Mater. 2024, 21, e03967. [Google Scholar] [CrossRef]
- Teklay, A.; Yin, C.; Rosendahl, L.; Bøjer, M. Calcination of kaolinite clay particles for cement production: A modeling study. Cem. Concr. Res. 2014, 61–62, 11–19. [Google Scholar] [CrossRef]
- Weng, L.; Sagoe-Crentsil, K. Dissolution processes, hydrolysis and condensation reactions during geopolymer synthesis: Part I-Low Si/Al ratio systems. J. Mater. Sci. 2007, 42, 2997–3006. [Google Scholar] [CrossRef]
- Kumar, K.; Kota, P.; Geopolymer, S. Elements of Geopolymer Concrete. In Geopolymer Concrete; Springer Briefs in Applied Sciences and Technology; Springer: Singapore, 2025. [Google Scholar] [CrossRef]
- Salau, M.; Osemeke, O. Effects of Temperature on the Pozzolanic Characteristics of Metakaolin-Concrete. Phys. Sci. Int. J. 2015, 6, 131–143. [Google Scholar] [CrossRef]
- Albidah, A.; Altheeb, A.; Alrshoudi, F.; Abadel, A.; Abbas, H.; Al-Salloum, Y. Bond performance of GFRP and steel rebars embedded in metakaolin-based geopolymer concrete. Structures 2020, 27, 1582–1593. [Google Scholar] [CrossRef]
- Dietel, J.; Warr, L.N.; Bertmer, M.; Steudel, A.; Grathoff, G.H.; Emmerich, K. The importance of specific surface area in the geopolymerization of heated illitic clay. Appl. Clay Sci. 2017, 139, 99–107. [Google Scholar] [CrossRef]
- Bonavetti, V.L.; Castellano, C.C.; Irassar, E.F. Designing general use cement with calcined illite and limestone filler. Appl. Clay Sci. 2022, 230, 106700. [Google Scholar] [CrossRef]
- Hounsi, A.D.; Lecomte-Nana, G.L.; Djétéli, G.; Blanchart, P. Kaolin-based geopolymers: Effect of mechanical activation and curing process. Constr. Build. Mater. 2013, 42, 105–113. [Google Scholar] [CrossRef]
- Zhao, P.; Ozersky, A.; Khomyakov, A.; Peterson, K. Comparison of thermal and mechanochemical activation for enhancing pozzolanic reactivity of illite-rich shale. Cem. Concr. Compos. 2025, 160, 106034. [Google Scholar] [CrossRef]
- Sarfo-Ansah, J.; Atiemo, E.; Boakye, K.A.; Momade, Z. Comparative Study of Chemically and Mechanically Activated Clay Pozzolana. Mater. Sci. Appl. 2014, 05, 86–94. [Google Scholar] [CrossRef]
- Ayati, B.; Newport, D.; Wong, H.; Cheeseman, C. Acid activated smectite clay as pozzolanic supplementary cementitious material. Cem. Concr. Res. 2022, 162, 106969. [Google Scholar] [CrossRef]
- Mwiti, M.J.; Karanja, J.; Muthengia, W.J. Properties of activated blended cement containing high content of calcined clay. Heliyon 2018, 4, 742. [Google Scholar] [CrossRef]
- Amin, N.U.; Alam, S.; Gul, S.; Muhammad, K. Chemical activation of clay in cement mortar, using calcium chloride. Adv. Cem. Res. 2013, 25, 164–170. [Google Scholar] [CrossRef]
- Sperinck, S.; Raiteri, P.; Marks, N.; Wright, K. Dehydroxylation of kaolinite to metakaolin—A molecular dynamics study. J. Mater. Chem. 2010, 21, 2118–2125. [Google Scholar] [CrossRef]
- White, C.E.; Provis, J.L.; Proffen, T.; Riley, D.P.; van Deventer, J.S.J. Combining density functional theory (DFT) and pair distribution function (PDF) analysis to solve the structure of metastable materials: The case of metakaolin. Phys. Chem. Chem. Phys. 2010, 12, 3239–3245. [Google Scholar] [CrossRef] [PubMed]
- Kassa, A.E.; Shibeshi, N.T.; Tizazu, B.Z. Kinetic analysis of dehydroxylation of Ethiopian kaolinite during calcination. J. Therm. Anal. Calorim. 2022, 147, 12837–12853. [Google Scholar] [CrossRef]
- Khalifa, A.Z.; Pontikes, Y.; Elsen, J.; Cizer, Ö. Comparing the reactivity of different natural clays under thermal and alkali activation. RILEM Technol. Lett. 2019, 4, 74–80. [Google Scholar] [CrossRef]
- Gridi-Bennadji, F.; Beneu, B.; Laval, J.; Blanchart, P. Structural transformations of Muscovite at high temperature by X-ray and neutron diffraction. Appl. Clay Sci. 2008, 38, 259–267. [Google Scholar] [CrossRef]
- Murat, M.; Comel, C. Hydration reaction and hardening of calcined clays and related minerals III. Influence of calcination process of kaolinite on mechanical strengths of hardened metakaolinite. Cem. Concr. Res. 1983, 13, 631–637. [Google Scholar] [CrossRef]
- Mejía De Gutiérrez, R.; Torres, J.; Vizcayno, C.; Castello, R. Influence of the calcination temperature of kaolin on the mechanical properties of mortars and concretes containing metakaolin. Clay Miner. 2008, 43, 177–183. [Google Scholar] [CrossRef]
- Elimbi, A.; Tchakoute, H.K.; Njopwouo, D. Effects of calcination temperature of kaolinite clays on the properties of geopolymer cements. Constr. Build. Mater. 2011, 25, 2805–2812. [Google Scholar] [CrossRef]
- Adufu, Y.D.; Sore, S.O.; Nshimiyimana, P.; Ahouandjinou, K.D.; Messan, A.; Escadeillas, G. Durability behaviors of calcined kaolin clay-based geopolymer concrete containing different calcium compounds and cured in ambient sub-Saharan climate. Constr. Build. Mater. 2025, 465, 140195. [Google Scholar] [CrossRef]
- Zuhua, Z.; Xiao, Y.; Huajun, Z.; Yue, C. Role of water in the synthesis of calcined kaolin-based geopolymer. Appl. Clay Sci. 2009, 43, 218–223. [Google Scholar] [CrossRef]
- Hu, N.; Bernsmeier, D.; Grathoff, G.H.; Warr, L.N. The influence of alkali activator type, curing temperature and gibbsite on the geopolymerization of an interstratified illite-smectite rich clay from Friedland. Appl. Clay Sci. 2017, 135, 386–393. [Google Scholar] [CrossRef]
- Buchwald, A.; Hohmann, M.; Posern, K.; Brendler, E. The suitability of thermally activated illite/smectite clay as raw material for geopolymer binders. Appl. Clay Sci. 2009, 46, 300–304. [Google Scholar] [CrossRef]
- Seiffarth, T.; Hohmann, M.; Posern, K.; Kaps, C. Effect of thermal pre-treatment conditions of common clays on the performance of clay-based geopolymeric binders. Appl. Clay Sci. 2013, 73, 35–41. [Google Scholar] [CrossRef]
- Waqas, R.M.; Zaman, S.; Alkharisi, M.K.; Butt, F.; Alsuhaibani, E. Influence of Bentonite and Polypropylene Fibers on Geopolymer Concrete. Sustainability 2024, 16, 789. [Google Scholar] [CrossRef]
- Laidani, Z.E.A.; Benabed, B.; Abousnina, R.; Gueddouda, M.K.; Kadri, E.H. Experimental investigation on effects of calcined bentonite on fresh, strength and durability properties of sustainable self-compacting concrete. Constr. Build. Mater. 2020, 230, 117062. [Google Scholar] [CrossRef]
- Fode, T.A.; Jande, Y.A.C.; Kivevele, T. Effects of raw and different calcined bentonite on durability and mechanical properties of cement composite material. Case Stud. Constr. Mater. 2024, 20, e03012. [Google Scholar] [CrossRef]
- Reddy, S.S.; Reddy, M.A.K. Optimization of Calcined Bentonite Caly Utilization in Cement Mortar using Response Surface Methodology. Int. J. Eng. 2021, 34, 1623–1631. [Google Scholar] [CrossRef]
- Garg, N.; Skibsted, J. Thermal activation of a pure montmorillonite clay and its reactivity in cementitious systems. J. Phys. Chem. C 2014, 118, 11464–11477. [Google Scholar] [CrossRef]
- Zhang, B.; Guo, H.; Yuan, P.; Li, Y.; Wang, Q.; Deng, L.; Liu, D. Geopolymerization of halloysite via alkali-activation: Dependence of microstructures on precalcination. Appl. Clay Sci. 2020, 185, 105375. [Google Scholar] [CrossRef]
- Zhang, B.; Guo, H.; Yuan, P.; Deng, L.; Zhong, X.; Li, Y.; Wang, Q.; Liu, D. Novel acid-based geopolymer synthesized from nanosized tubular halloysite: The role of precalcination temperature and phosphoric acid concentration. Cem. Concr. Compos. 2020, 110, 103601. [Google Scholar] [CrossRef]
- Yu, T.; Zhang, B.; Chen, J.; Fahimizadeh, M.; Pantongsuk, T.; Zhou, X.; Yuan, P. Ternary geopolymer with calcined halloysite: Impact on mechanical properties and microstructure. Clay Miner. 2024, 59, 310–324. [Google Scholar] [CrossRef]
- MacKenzie, K.J.D.; Brew, D.R.M.; Fletcher, R.A.; Vagana, R. Formation of aluminosilicate geopolymers from 1:1 layer-lattice minerals pre-treated by various methods: A comparative study. J. Mater. Sci. 2007, 42, 4667–4674. [Google Scholar] [CrossRef]
- Ranjbar, N.; Kuenzel, C.; Gundlach, C.; Kempen, P.; Mehrali, M. Halloysite reinforced 3D-printable geopolymers. Cem. Concr. Compos. 2022, 136, 104894. [Google Scholar] [CrossRef]
- Georgopoulos, G.; Aspiotis, K.; Badogiannis, E.; Tsivilis, S.; Perraki, M. Influence of mineralogy and calcination temperature on the behavior of palygorskite clay as a pozzolanic supplementary cementitious material. Appl. Clay Sci. 2022, 232, 106797. [Google Scholar] [CrossRef]
- Hamid, S.; Johari, I.; Othman, N. Optimization and characterization of sustainable geopolymer mortars based on palygorskite clay, water glass, and sodium hydroxide. Open Eng. 2024, 14, 20220546. [Google Scholar] [CrossRef]
- Wu, C.R.; Hong, Z.Q.; Zhan, B.J.; Cui, S.C.; Kou, S.C. Pozzolanic activity of calcinated low-grade natural sepiolite and its influence on the hydration of cement. Constr. Build. Mater. 2021, 309, 125076. [Google Scholar] [CrossRef]
- Saka, R.C.; Subasi, S.; Marasli, M. The effect of crude and calcined sepiolite on some physical and mechanical properties of glass fiber reinforced Concrete. Rom. J. Mater. 2023, 53, 33–42. [Google Scholar]
- Badogiannis, E.; Kakali, G.; Tsivilis, S. Metakaolin as supplementary cementitious material. J. Therm. Anal. Calorim. 2005, 81, 457–462. [Google Scholar] [CrossRef]
- Boakye, K.; Khorami, M. Impact of Low-Reactivity Calcined Clay on the Performance of Fly Ash-Based Geopolymer Mortar. Sustainability 2023, 15, 13556. [Google Scholar] [CrossRef]
- Khaled, Z.; Mohsen, A.; Soltan, A.M.; Kohail, M. Optimization of kaolin into Metakaolin: Calcination Conditions, mix design and curing temperature to develop alkali activated binder. Ain Shams Eng. J. 2023, 14, 102142. [Google Scholar] [CrossRef]
- Potgieter-Vermaak, S.S.; Potgieter, J.H. Metakaolin as an Extender in South African Cement. J. Mater. Civ. Eng. 2006, 18, 619–623. [Google Scholar] [CrossRef]
- Taylor-Lange, S.C.; Riding, K.A.; Juenger, M.C.G. Increasing the reactivity of metakaolin-cement blends using zinc oxide. Cem. Concr. Compos. 2012, 34, 835–847. [Google Scholar] [CrossRef]
- Vizcayno, C.; de Gutiérrez, R.; Castello, R.; Rodriguez, E.; Guerrero, C. Pozzolan obtained by mechanochemical and thermal treatments of kaolin. Appl. Clay Sci. 2010, 49, 405–413. [Google Scholar] [CrossRef]
- Yao, G.; Cui, T.; Jia, Z.; Sun, S.; Anning, C.; Qiu, J.; Lyu, X. Effect of anhydrite on hydration properties of mechanically activated muscovite in the presence of calcium oxide. Appl. Clay Sci. 2020, 196, 105742. [Google Scholar] [CrossRef]
- Yao, G.; Zang, H.; Wang, J.; Wu, P.; Qiu, J.; Lyu, X. Effect of Mechanical Activation on the Pozzolanic Activity of Muscovite. Clays Clay Miner. 2019, 67, 209–216. [Google Scholar] [CrossRef]
- Hollanders, S.; Adriaens, R.; Skibsted, J.; Cizer, Ö.; Elsen, J. Pozzolanic reactivity of pure calcined clays. Appl. Clay Sci. 2016, 132–133, 552–560. [Google Scholar] [CrossRef]
- Balczár, I.; Korim, T.; Kovács, A.; Makó, É. Mechanochemical and thermal activation of kaolin for manufacturing geopolymer mortars–Comparative study. Ceram. Int. 2016, 42, 15367–15375. [Google Scholar] [CrossRef]
- Mañosa, J.; de la Rosa, J.C.; Silvello, A.; Maldonado-Alameda, A.; Chimenos, J.M. Kaolinite structural modifications induced by mechanical activation. Appl. Clay Sci. 2023, 238, 106918. [Google Scholar] [CrossRef]
- Ilić, B.; Radonjanin, V.; Malešev, M.; Zdujić, M.; Mitrović, A. Effects of mechanical and thermal activation on pozzolanic activity of kaolin containing mica. Appl. Clay Sci. 2016, 123, 173–181. [Google Scholar] [CrossRef]
- Baki, V.A.; Ke, X.; Heath, A.; Calabria-Holley, J.; Terzi, C.; Sirin, M. The impact of mechanochemical activation on the physicochemical properties and pozzolanic reactivity of kaolinite, muscovite and montmorillonite. Cem. Concr. Res. 2022, 162, 106962. [Google Scholar] [CrossRef]
- Kovalchuk, I. Performance of Thermal-, Acid-, and Mechanochemical-Activated Montmorillonite for Environmental Protection from Radionuclides U(VI) and Sr(II). Eng 2023, 4, 2141–2152. [Google Scholar] [CrossRef]
- Georgopoulos, G.; Badogiannis, E.; Tsivilis, S.; Perraki, M. Thermally and mechanically treated Greek palygorskite clay as a pozzolanic material. Appl. Clay Sci. 2021, 215, 106306. [Google Scholar] [CrossRef]
- Tunç, T.; Özkan Toplan, H.; Yıldız, K. Thermal behavior of mechanically activated sepiolite. TOJSAT Online J. Sci. Technol. 2013, 3, 187–193. [Google Scholar]
- Mañosa, J.; Alvarez-Coscojuela, A.; Maldonado-Alameda, A.; Chimenos, J.M. A Lab-Scale Evaluation of Parameters Influencing the Mechanical Activation of Kaolin Using the Design of Experiments. Materials 2024, 17, 4651. [Google Scholar] [CrossRef]
- Bai, H.; Wang, K.; Zhang, X.; Jiang, Y.; Zhang, S. Potential Utilization of Loess in Grouting Materials: Effects of Grinding Time and Calcination Temperature. Minerals 2024, 14, 490. [Google Scholar] [CrossRef]
- Muzenda, T.R.; Georget, F.; Matschei, T. The effect of the physico-chemical properties of (calcined) clays on the rheological properties and early hydration of calcined clay-limestone cement. Constr. Build. Mater. 2025, 492, 142864. [Google Scholar] [CrossRef]
- Hanein, T.; Thienel, K.C.; Zunino, F.; Marsh, A.T.M.; Maier, M.; Wang, B.; Canut, M.; Juenger, M.C.G.; Ben Haha, M.; Avet, F.; et al. Clay calcination technology: State-of-the-art review by the RILEM TC 282-CCL. Mater. Struct. 2021, 55, 3. [Google Scholar] [CrossRef]
- Mehdipour, I.; Khayat, K.H. Effect of particle-size distribution and specific surface area of different binder systems on packing density and flow characteristics of cement paste. Cem. Concr. Compos. 2017, 78, 120–131. [Google Scholar] [CrossRef]
- Nehdi, M.L. Clay in cement-based materials: Critical overview of state-of-the-art. Constr. Build. Mater. 2014, 51, 372–382. [Google Scholar] [CrossRef]
- Ghais, A.; Ahmed, D.; Siddig, E.; Elsadig, I.; Albager, S. Performance of Concrete with Fly Ash and Kaolin Inclusion. Int. J. Geosci. 2014, 5, 1445–1450. [Google Scholar] [CrossRef]
- Ahmad, J.; Tufail, R.F.; Aslam, F.; Mosavi, A.; Alyousef, R.; Javed, M.F.; Zaid, O.; Khan Niazi, M.S. A step towards sustainable self-compacting concrete by using partial substitution of wheat straw ash and bentonite clay instead of cement. Sustainability 2021, 13, 824. [Google Scholar] [CrossRef]
- Schulze, S.E.; Rickert, J. Suitability of natural calcined clays as supplementary cementitious material. Cem. Concr. Compos. 2019, 95, 92–97. [Google Scholar] [CrossRef]
- Noyan, H.; Önal, M.; Sarikaya, Y. The Effect of Heating on the Surface Area, Porosity and Surface Acidity of a Bentonite. Clays Clay Miner. 2006, 54, 375–381. [Google Scholar] [CrossRef]
- Porkodi, R.; Dharmar, S.; Nagan, S. Experimental Study on Fiber Reinforced Self-Compacting Geopolymer Mortar. Int. J. Adv. Res. Sci. Eng. 2015, 4, 968–977. [Google Scholar]
- Dungca, J.R.; Edrada, J.E.B.; Eugenio, V.A.; Fugado, R.A.S.; Li, E.S.C. The combined effects of nano-montmorillonite and halloysite nanoclay on the workability and compressive strength of concrete. Int. J. GEOMATE 2019, 17, 173–180. [Google Scholar] [CrossRef]
- Masood, B.; Elahi, A.; Barbhuiya, S.; Ali, B. Mechanical and durability performance of recycled aggregate concrete incorporating low calcium bentonite. Constr. Build. Mater. 2020, 237, 117760. [Google Scholar] [CrossRef]
- Eliche-Quesada, D.; Bonet-Martínez, E.; Pérez-Villarejo, L.; Castro, E.; Sánchez-Soto, P.J. Effects of an Illite Clay Substitution on Geopolymer Synthesis as an Alternative to Metakaolin. J. Mater. Civ. Eng. 2021, 33, 04021072. [Google Scholar] [CrossRef]
- García-Lodeiro, I.; Fernández-Jiménez, A.; Palomo, A.; Macphee, D.E. Effect of calcium additions on N-A-S-H cementitious gels. J. Am. Ceram. Soc. 2010, 93, 1934–1940. [Google Scholar] [CrossRef]
- Karthikeyan, M.; Ramachandran, P.R.; Nandhini, A.; Vinodha, R. Application on partial substitute of cement by bentonite in concrete. Int. J. Chem. Technol. Res. 2015, 8, 384–388. [Google Scholar]
- Liu, M.; Hu, Y.; Lai, Z.; Yan, T.; He, X.; Wu, J.; Lu, Z.; Lv, S. Influence of various bentonites on the mechanical properties and impermeability of cement mortars. Constr. Build. Mater. 2020, 241, 118015. [Google Scholar] [CrossRef]
- Pu, S.; Duan, P.; Yan, C.; Ren, D. Influence of sepiolite addition on mechanical strength and microstructure of fly ash-metakaolin geopolymer paste. Adv. Powder Technol. 2016, 27, 2470–2477. [Google Scholar] [CrossRef]
- Campos, R.; Larrain, M.M.M.; Zaman, M.; Pozadas, V. Relationships between compressive and flexural strengths of concrete based on fresh field properties. Int. J. Pavement Res. Technol. 2021, 14, 161–167. [Google Scholar] [CrossRef]
- Ma, C.K.; Awang, A.Z.; Omar, W. Structural and material performance of geopolymer concrete: A review. Constr. Build. Mater. 2018, 186, 90–102. [Google Scholar] [CrossRef]
- Oderji, S.Y.; Chen, B.; Ahmad, M.R.; Shah, S.F.A. Fresh and hardened properties of one-part fly ash-based geopolymer binders cured at room temperature: Effect of slag and alkali activators. J. Clean. Prod. 2019, 225, 1–10. [Google Scholar] [CrossRef]
- Chindaprasirt, P.; De Silva, P.; Sagoe-Crentsil, K.; Hanjitsuwan, S. Effect of SiO2 and Al2O3 on the setting and hardening of high calcium fly ash-based geopolymer systems. J. Mater. Sci. 2012, 47, 4876–4883. [Google Scholar] [CrossRef]
- Prasanphan, S.; Wannagon, A.; Kobayashi, T.; Jiemsirilers, S. Reaction mechanisms of calcined kaolin processing waste-based geopolymers in the presence of low alkali activator solution. Constr. Build. Mater. 2019, 221, 409–420. [Google Scholar] [CrossRef]
- Hassan, A.; Arif, M.; Shariq, M. Effect of curing condition on the mechanical properties of fly ash-based geopolymer concrete. SN Appl. Sci. 2019, 1, 1694. [Google Scholar] [CrossRef]
- Phoo-Ngernkham, T.; Phiangphimai, C.; Damrongwiriyanupap, N.; Hanjitsuwan, S.; Thumrongvut, J.; Chindaprasirt, P. A Mix Design Procedure for Alkali-Activated High-Calcium Fly Ash Concrete Cured at Ambient Temperature. Adv. Mater. Sci. Eng. 2018, 2018, 2460403. [Google Scholar] [CrossRef]
- Jiang, G.; Keller, J.; Bond, P.L.; Yuan, Z. Predicting concrete corrosion of sewers using artificial neural network. Water Res. 2016, 92, 52–60. [Google Scholar] [CrossRef]
- Islam, M.; Provath, A.M.; Islam, G.M.S.; Islam, T. Strength Prediction of Geopolymer Concrete With Wide-Ranged Binders and Properties Using Artificial Neural Network. Adv. Civ. Eng. 2024, 2024, 8534390. [Google Scholar] [CrossRef]
- Dao, D.V.; Ly, H.B.; Trinh, S.H.; Le, T.T.; Pham, B.T. Artificial intelligence approaches for prediction of compressive strength of geopolymer concrete. Materials 2019, 12, 983. [Google Scholar] [CrossRef] [PubMed]
- Ghanizadeh, A.R.; Abbaslou, H.; Amlashi, A.T.; Alidoust, P. Modeling of bentonite/sepiolite plastic concrete compressive strength using artificial neural network and support vector machine. Front. Struct. Civ. Eng. 2019, 13, 215–239. [Google Scholar] [CrossRef]
- Arab, M.G.; Maged, A.; Rammal, R.; Haridy, S. LSTM-based deep learning model for alkali activated binder mix design of clay soils. Innov. Infrastruct. Solut. 2024, 9, 471. [Google Scholar] [CrossRef]
- Abdalla, A.; Mohammed, A.S. Surrogate Models to Predict the Long-Term Compressive Strength of Cement-Based Mortar Modified with Fly Ash. Arch. Comput. Methods Eng. 2022, 29, 4187–4212. [Google Scholar] [CrossRef]
- Ahmad, A.; Ahmad, W.; Aslam, F.; Joyklad, P. Compressive strength prediction of fly ash-based geopolymer concrete via advanced machine learning techniques. Case Stud. Constr. Mater. 2022, 16, e00840. [Google Scholar] [CrossRef]
- Ahmed, H.U.; Mohammed, A.S.; Faraj, R.H.; Abdalla, A.A.; Qaidi, S.M.A.; Sor, N.H.; Mohammed, A.A. Innovative modeling techniques including MEP, ANN and FQ to forecast the compressive strength of geopolymer concrete modified with nanoparticles. Neural Comput. Appl. 2023, 35, 12453–12479. [Google Scholar] [CrossRef]
- Khan, N.M.; Ma, L.; Bin Inqiad, W.; Khan, M.S.; Iqbal, I.; Emad, M.Z.; Alarifi, S.S. Interpretable machine learning approaches to assess the compressive strength of metakaolin blended sustainable cement mortar. Sci. Rep. 2025, 15, 19414. [Google Scholar] [CrossRef] [PubMed]
- Amlashi, A.T.; Ghanizadeh, A.R.; Firouzranjbar, S.; Moghaddam, H.M.; Navazani, M.; Isleem, H.F.; Dessouky, S.; Khishe, M. Predicting workability and mechanical properties of bentonite plastic concrete using hybrid ensemble learning. Sci. Rep. 2025, 15, 7686. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Ali, M.; Najeh, T.; Gamil, Y. Computational prediction of workability and mechanical properties of bentonite plastic concrete using multi-expression programming. Sci. Rep. 2024, 14, 6105. [Google Scholar] [CrossRef]
- Kumar, P.; Kamal, S.S.; Kumar, A.; Kumar, N.; Kumar, S. Compressive strength of bentonite concrete using state-of-the-art optimised XGBoost models. Nondestruct. Test. Eval. 2024, 40, 4868–4891. [Google Scholar] [CrossRef]
- Gupta, P.; Gupta, N.; Goyal, S. Predicting Compressive Strength of Calcined Clay, Fly Ash-Based Geopolymer Composite Using Supervised Learning Algorithm. Adv. Appl. Math. Sci. 2022, 21, 4151–4161. [Google Scholar]
- Abdullah, G.M.S.; Ahmad, M.; Babur, M.; Badshah, M.U.; Al-Mansob, R.A.; Gamil, Y.; Fawad, M. Boosting-based ensemble machine learning models for predicting unconfined compressive strength of geopolymer stabilized clayey soil. Sci. Rep. 2024, 14, 2323. [Google Scholar] [CrossRef]
- Ahmed, H.U.; Mohammed, A.S.; Mohammed, A.A. Proposing several model techniques including ANN and M5P-tree to predict the compressive strength of geopolymer concretes incorporated with nano-silica. Environ. Sci. Pollut. Res. 2022, 29, 71232–71256. [Google Scholar] [CrossRef]
- Cihan, M.T.; Cihan, P. Bayesian-Optimized Ensemble Models for Geopolymer Concrete Compressive Strength Prediction with Interpretability Analysis. Buildings 2025, 15, 3667. [Google Scholar] [CrossRef]
- Shi, X.; Chen, S.; Wang, Q.; Lu, Y.; Ren, S.; Huang, J. Mechanical Framework for Geopolymer Gels Construction: An Optimized LSTM Technique to Predict Compressive Strength of Fly Ash-Based Geopolymer Gels Concrete. Gels 2024, 10, 148. [Google Scholar] [CrossRef]
- Yaswanth, K.K.; Kumar, V.S.; Revathy, J.; Murali, G.; Pavithra, C. Compressive strength prediction of ternary blended geopolymer concrete using artificial neural networks and support vector regression. Innov. Infrastruct. Solut. 2024, 9, 32. [Google Scholar] [CrossRef]
- Ahmad, M.; Al-Mansob, R.A.; Bin Ramli, A.B.; Ahmad, F.; Khan, B.J. Unconfined compressive strength prediction of stabilized expansive clay soil using machine learning techniques. Multiscale Multidiscip. Model. Exp. Des. 2023, 7, 217–231. [Google Scholar] [CrossRef]
- Alahmari, T.S.; Abdalla, T.A.; Rihan, M.A.M. Review of Recent Developments Regarding the Durability Performance of Eco-Friendly Geopolymer Concrete. Buildings 2023, 13, 3033. [Google Scholar] [CrossRef]
- Esievo, O.P.; Awodiji, C.T.G.; Sule, S. Optimising Durability Performance of Dehydroxylated Kaolin Geopolymer Concrete Using Central Composite Design. J. Eng. Res. Rep. 2025, 27, 328–346. [Google Scholar] [CrossRef]
- Alloul, A.; Amar, M.; Benzerzour, M.; Abriak, N.E. High strength, durability, and microstructure of geopolymer concrete with flash-calcined soils cured under ambient conditions. J. Mater. Cycles Waste Manag. 2025, 27, 1854–1872. [Google Scholar] [CrossRef]
- Cordoba, G.P.; Zito, S.; Tironi, A.; Rahhal, V.F.; Irassar, E.F. Calcined Clays for Sustainable Concrete Proceedings of the 3rd International Conference on Calcined Clays for Sustainable Concrete; RILEM Bookseries; Springer: Singapore, 2020; Available online: http://www.springer.com/series/8781 (accessed on 30 October 2025).
- Yang, Z.; Zhang, Y.; Zhu, Y.; Li, Y.; Fu, R. The Strength, Permeability, and Microstructure of Cement–Bentonite Cut-Off Walls Enhanced by Polypropylene Fiber. Sustainability 2025, 17, 3656. [Google Scholar] [CrossRef]
- Przybek, A.; Romańska, P.; Korniejenko, K.; Krajniak, K.; Hebdowska-Krupa, M.; Łach, M. Thermal Properties of Geopolymer Concretes with Lightweight Aggregates. Materials 2025, 18, 3150. [Google Scholar] [CrossRef]
- Mostazid, M.I. Acid Resistance of Geopolymer Concrete–Literature Review, Knowledge Gaps, and Future Development. J. Brilliant Eng. 2024, 4, 4875. [Google Scholar] [CrossRef]
- Abdel-Maksoud, Y.G.; Abu-Zeid, M.M.; El-Raoof, F.A.; Soltan, A.M.; Hazem, M.M. Characterization and Potentiality of Calcined Kaolins for Metakaolin Production. Egypt. J. Chem. 2025, 68, 493–509. [Google Scholar] [CrossRef]
- Zhang, B.; Yu, T.; Guo, H.; Chen, J.; Liu, Y.; Yuan, P. Effect of the SiO2/Al2O3 Molar Ratio on the Microstructure and Properties of Clay-based Geopolymers: A Comparative Study of Kaolinite-based and Halloysite-based Geopolymers. Clays Clay Miner. 2022, 70, 882–902. [Google Scholar] [CrossRef]
- Tchadjie, L.N.; Ekolu, S.O. Enhancing the reactivity of aluminosilicate materials toward geopolymer synthesis. J. Mater. Sci. 2018, 53, 4709–4733. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).