Rice Bran and American Ginseng Residue as Media for Black Truffle Solid-State Fermentation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Strain Preservation and Pre-Activation
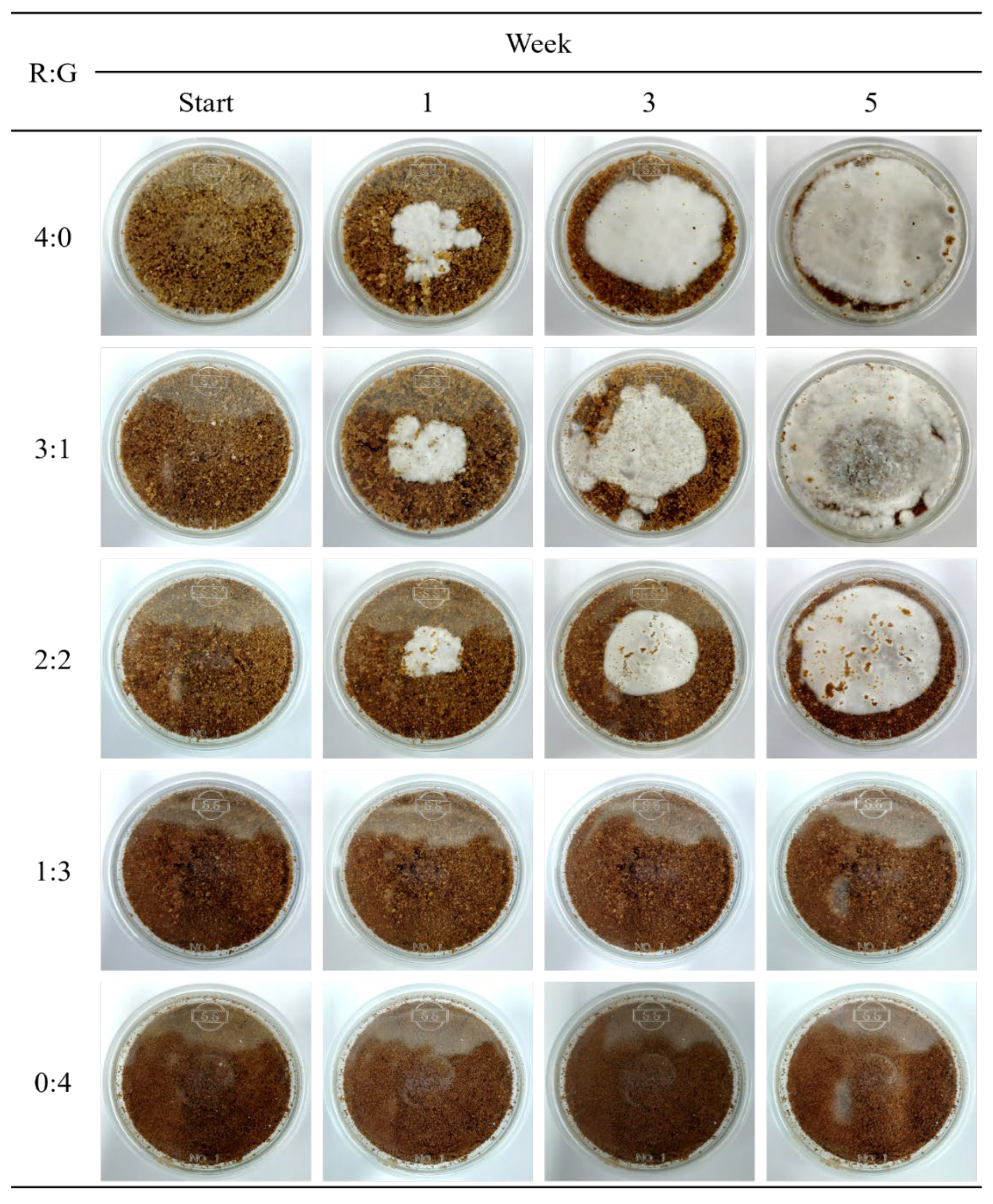
2.3. Petri-Dish Preparation and Black Truffle Cultivation
2.4. Bioactive Compounds Analysis of Black Truffle Fermented Products
2.4.1. Microwave Extraction
2.4.2. Crude Polysaccharide Analysis
2.4.3. Crude Triterpenoid Analysis
2.4.4. Total Phenol Analysis
2.4.5. Total Flavonoid Analysis
2.4.6. Ginsenoside Rg3 Analysis
2.5. Whitening Experiment
2.5.1. Tyrosinase Inhibitory Activity
2.5.2. Image Analysis of Zebrafish Embryos
2.6. Different Drying Methods for Rice Bran and American Ginseng Residue Medium Treatment
2.6.1. Hot Air-Assisted Radio Frequency Drying (HARF)
2.6.2. Radio Frequency Vacuum Drying (RFV)
2.6.3. Microwave Drying (MW) and Hot Air Drying (HA)
2.6.4. Calculation of Energy Consumption and Greenhouse Gas Emissions During Drying
2.7. Statistical Analysis
3. Results and Discussion
3.1. Cultivation Conditions and Bioactive Components of Black Truffle
3.2. Whitening Effect of Black Truffle Solid-State Fermented Product
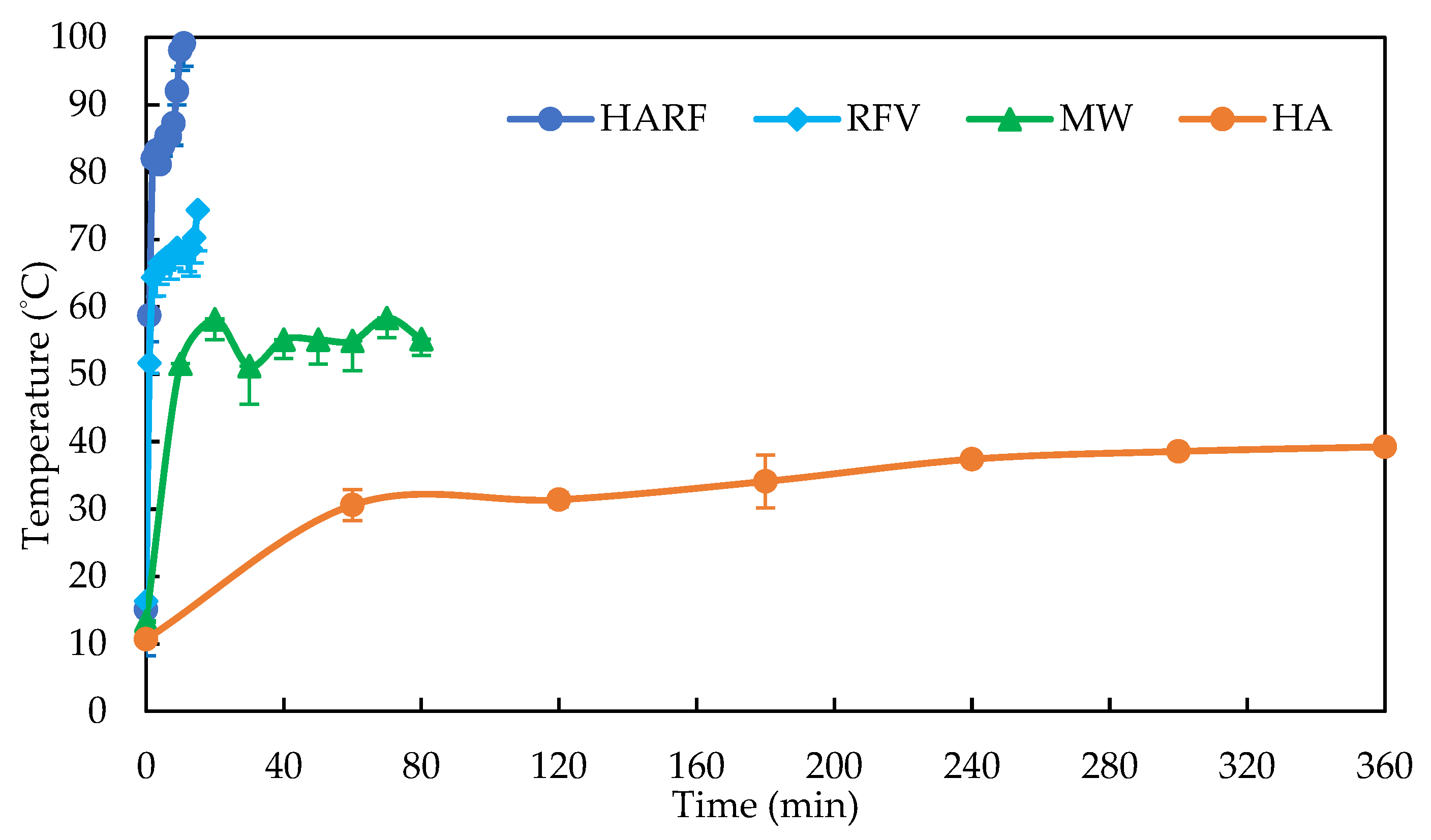
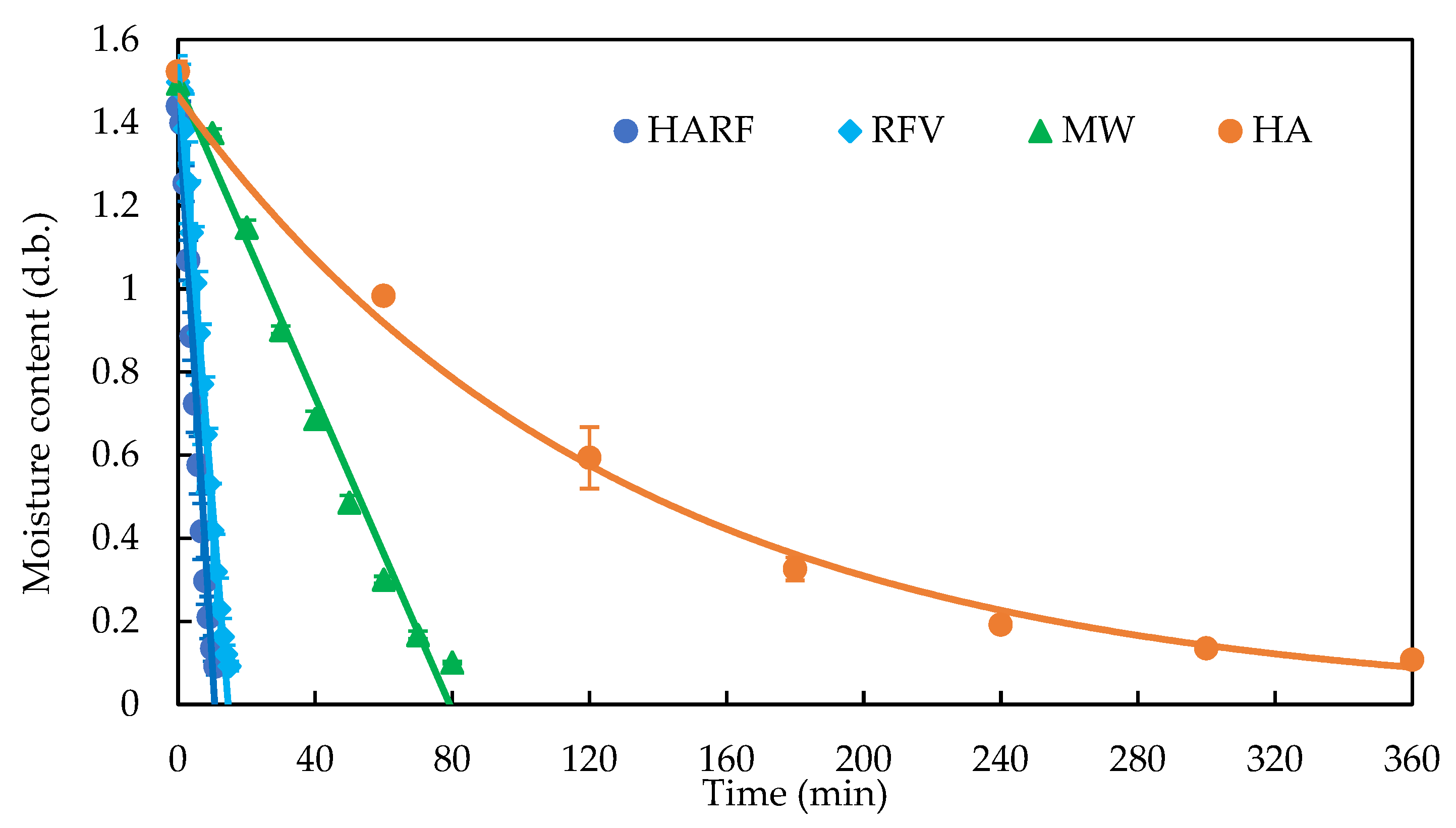
3.3. Scale-Up Production of the Medium for Black Truffle Solid-State Fermentation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chu, L.L.; Bae, H. Bacterial endophytes from ginseng and their biotechnological application. J. Ginseng Res. 2022, 46, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Baeg, I.H.; So, S.H. The world ginseng market and the ginseng (Korea). J. Ginseng Res. 2013, 37, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.D.; Do, J.H.; Lee, K.S. Effect of ginseng residue on the growth of Ganoderma lucidum. Microbiol. Biotechnol. Lett. 1986, 14, 279–283. [Google Scholar]
- Hu, Y.; Li, Y.; Cao, Y.; Shen, Y.; Zou, X.; Liu, J.; Zhao, J. Advancements in enzymatic biotransformation and bioactivities of rare ginsenosides: A review. J. Biotechnol. 2024, 392, 78–89. [Google Scholar] [CrossRef]
- Zheng, M.M.; Xu, F.X.; Li, Y.J.; Xi, X.Z.; Cui, X.W.; Han, C.C.; Zhang, X.L. Study on transformation of ginsenosides in different methods. Biomed. Res. Int. 2017, 2017, 8601027. [Google Scholar] [CrossRef]
- Hsu, B.Y.; Chen, C.H.; Lu, T.J.; Hwang, L.S. Bioconversion of ginsenosides in the American ginseng (Xī Yáng Shēn) extraction residue by fermentation with Lingzhi (Líng Zhī, Ganoderma Lucidum). J. Tradit. Complement. Med. 2013, 3, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Galanakis, C.M. Emerging technologies for the production of nutraceuticals from agricultural by-products: A viewpoint of opportunities and challenges. Food Bioprod. Process 2013, 91, 575–579. [Google Scholar] [CrossRef]
- Zhang, Y.; Pandiselvam, R.; Zhu, H.; Su, D.; Wang, H.; Ai, Z.; Kothakota, A.; Khaneghah, A.M.; Liu, Y. Impact of radio frequency treatment on textural properties of food products: An updated review. Trends Food Sci. Technol. 2022, 124, 154–166. [Google Scholar] [CrossRef]
- Zahoor, I.; Mir, T.A.; Ayoub, W.S.; Farooq, S.; Ganaie, T.A. Recent applications of microwave technology as novel drying of food-review. Food Humanit. 2023, 1, 92–103. [Google Scholar] [CrossRef]
- Huang, C.Y.; Cheng, Y.H.; Chen, S.D. Hot air assisted radio frequency (HARF) drying on wild bitter gourd extract. Foods 2022, 11, 1173. [Google Scholar] [CrossRef]
- Gul, K.; Yousuf, B.; Singh, A.K.; Singh, P.; Wani, A.A. Rice bran: Nutritional values and its emerging potential for development of functional food—A review. Bioact. Carbohydr. Diet. Fibre 2015, 6, 24–30. [Google Scholar] [CrossRef]
- Liou, T.N.; Chen, S.D. Study of active components and tyrosinase inhibition of Poria cocos solid-state fermented rice bran products. Taiwan. J. Agric. Chem. Food Sci. 2021, 59, 77–86. [Google Scholar]
- Lee, H.; Nam, K.; Zahra, Z.; Farooqi, M.Q.U. Potentials of truffles in nutritional and medicinal applications: A review. Fungal Biol. Biotechnol. 2020, 7, 9. [Google Scholar] [CrossRef]
- Coquet, C.; Chabert, R. Inventors; ISP Investments LLC., Assignee. Cosmetic Preparation Containing White Truffle Extract and Cosmetic Method Thereof. European Patent EP 3,609,469, 2021. Available online: https://patentimages.storage.googleapis.com/a7/0d/83/b54f0ab2b4b768/EP3609469B1.pdf (accessed on 17 February 2021).
- Beara, I.N.; Lesjak, M.M.; Četojević-Simin, D.D.; Marjanović, Ž.S.; Ristić, J.D.; Mrkonjić, Z.O.; Mimica-Dukić, N.M. Phenolic profile, antioxidant, anti-inflammatory and cytotoxic activities of black (Tuber aestivum Vittad.) and white (Tuber magnatum Pico) truffles. Food Chem. 2014, 165, 460–466. [Google Scholar] [CrossRef]
- Khalifa, S.A.; Farag, M.A.; Yosri, N.; Sabir, J.S.; Saeed, A.; Al-Mousawi, S.M.; Taha, W.; Musharraf, S.G.; Patel, S.; El-Seedi, H.R. Truffles: From Islamic culture to chemistry, pharmacology, and food trends in recent times. Trends Food Sci. Technol. 2019, 91, 193–218. [Google Scholar] [CrossRef]
- Zambonelli, A.; Iotti, M.; Hall, I. Current status of truffle cultivation: Recent results and future perspectives. Italian J. Mycol. 2015, 44, 31–40. [Google Scholar] [CrossRef]
- Strong, P.J.; Self, R.; Allikian, K.; Szewczyk, E.; Speight, R.; O’Hara, I.; Harrison, M.D. Filamentous fungi for future functional food and feed. Curr. Opin. Biotechnol. 2022, 76, 102729. [Google Scholar] [CrossRef]
- Sun, Q.; Zhang, Z.; Xu, L.; Shi, W.; Liu, X.; Wang, F. Increasing production of truffle polysaccharides in the solid-state fermentation of Tuber melanosporum by diosgenin based on orthogonal matrix and nonlinear regression analysis. Food Sci. Technol. Res. 2020, 26, 487–494. [Google Scholar] [CrossRef]
- Hsu, J.Y.; Chen, M.H.; Lai, Y.S.; Chen, S.D. Antioxidant profile and biosafety of white truffle mycelial products obtained by solid-state fermentation. Molecules 2022, 27, 109. [Google Scholar] [CrossRef] [PubMed]
- Splivallo, R.; Ottonello, S.; Mello, A.; Karlovsky, P. Truffle volatiles: From chemical ecology to aroma biosynthesis. New Phytol. 2010, 189, 688–699. [Google Scholar] [CrossRef] [PubMed]
- Roméro-Graillet, C.; Aberdam, E.; Clément, M.; Ortonne, J.P.; Ballotti, R. Nitric oxide produced by ultraviolet-irradiated keratinocytes stimulates melanogenesis. J. Clin. Invest. 1997, 99, 635–642. [Google Scholar] [CrossRef]
- Pillaiyar, T.; Manickam, M.; Namasivayam, V. Skin whitening agents: Medicinal chemistry perspective of tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2017, 32, 403–425. [Google Scholar] [CrossRef]
- Ferreira, A.M.; de-Souza, A.A.; Koga, R.D.C.R.; Sena, I.D.S.; Matos, M.D.J.S.; Tomazi, R.; Ferreira, I.M.; Carvalho, J.C.T. Anti-melanogenic potential of natural and synthetic substances: Application in zebrafish model. Molecules 2023, 28, 1053. [Google Scholar] [CrossRef]
- The European Parliament and the Council of the European Union (EU). The Protection of Animals Used for Scientific Purposes (Text with EEA Relevance). Final Rules. The Treaty on the Functioning of the EU. 2010. Available online: https://eur-lex.europa.eu/eli/dir/2010/63/oj/eng (accessed on 22 September 2010).
- Naomi, R.; Bahari, H.; Yazid, M.D.; Embong, H.; Othman, F. Zebrafish as a model system to study the mechanism of cutaneous wound healing and drug discovery: Advantages and challenges. Pharmaceuticals 2021, 14, 1058. [Google Scholar] [CrossRef]
- Lee, D.Y.; Lee, J.; Jeong, Y.T.; Byun, G.H.; Kim, J.H. Melanogenesis inhibition activity of floralginsenoside A from Panax ginseng berry. J. Ginseng Res. 2017, 41, 602–607. [Google Scholar] [CrossRef]
- Lee, D.Y.; Kim, H.G.; Lee, Y.G.; Kim, J.H.; Lee, J.W.; Choi, B.R.; Jang, I.B.; Kim, G.S.; Baek, N.I. Isolation and quantification of ginsenoside Rh23, a new anti-melanogenic compound from the leaves of Panax ginseng. Molecules 2018, 23, 267. [Google Scholar] [CrossRef]
- Chen, Y.H.; Yen, Y.F.; Chen, S.D. Effects of radio frequency heating on the stability and antioxidant properties of rice bran. Foods 2021, 10, 810. [Google Scholar] [CrossRef]
- Chen, B.H.; Chen, S.D. Microwave extraction of polysaccharides and triterpenoids from solid-state fermented products of Poria cocos. Taiwan. J. Agric. Chem. Food Sci. 2013, 51, 188–194. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.T.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Sun, Z.; Liu, H.; Huang, Y.; Ju, J. Determination of total triterpenoid and oleanolic acid contents in Tibetan medicine Indian Swertia. China J. Ethnomed. Ethnophamacy 2021, 1, 21. [Google Scholar]
- Lin, J.Y.; Tang, C.Y. Determination of total phenolic and flavonoid contents in selected fruits and vegetables, as well as their stimulatory effects on mouse splenocyte proliferation. Food Chem. 2007, 101, 140–147. [Google Scholar] [CrossRef]
- Masuda, T.; Yamashita, D.; Takeda, Y.; Yonemori, S. Screening for tyrosinase inhibitors among extracts of seashore plants and identification of potent inhibitors from Garcinia subelliptica. Biosci. Biotechnol. Biochem. 2005, 69, 197–201. [Google Scholar] [CrossRef]
- The Greenhouse Gas Emission Coefficient Management List, 6.0.4th ed.; Climate Change Administration, Ministry of Environment: Taipei City, Taiwan, 2019. Available online: https://reurl.cc/dQXeay (accessed on 22 January 2024).
- Kumar, A.; Sharma, M.P.; Yang, T. Estimation of carbon stock for greenhouse gas emissions from hydropower reservoirs. Stoch. Environ. Res. Risk Assess. 2018, 32, 3183–3193. [Google Scholar] [CrossRef]
- Peng, J.T.; Lee, C.M.; Tsai, Y.F. Effect of rice bran on the production of different king oyster mushroom strains during bottle cultivation. J. Agric. Res. China 2000, 49, 60–67. [Google Scholar]
- Hua, M.; Lu, J.; Qu, D.I.; Liu, C.; Zhang, L.; Li, S.; Chen, J.; Sun, Y. Structure, physicochemical properties and adsorption function of insoluble dietary fiber from ginseng residue: A potential functional ingredient. Food Chem. 2019, 286, 522–529. [Google Scholar] [CrossRef]
- Chen, W.; Balan, P.; Popovich, D.G. Comparison of ginsenoside components of various tissues of New Zealand forest-grown Asian ginseng (Panax ginseng) and American ginseng (Panax quinquefolium L.). Biomolecules 2020, 10, 372. [Google Scholar] [CrossRef]
- Tang, Y.J.; Liu, R.S.; Li, H.M. Current progress on truffle submerged fermentation: A promising alternative to its fruiting bodies. Appl. Microbiol. Biotechnol. 2015, 99, 2041–2053. [Google Scholar] [CrossRef]
- Chia, L.Y.; Chen, Y.T.; Chen, S.D. Study on radio frequency treating American ginseng residue and rice bran as solid-state fermented media of Hericium erinaceus. Ilan Univ. J. Bioresour. 2023, 19, 1–17. [Google Scholar] [CrossRef]
- Du, X.W.; Wills, R.B.H.; Stuart, D.L. Changes in neutral and malonyl ginsenosides in American ginseng (Panax quinquefolium) during drying, storage and ethanolic extraction. Food Chem. 2004, 86, 155–159. [Google Scholar] [CrossRef][Green Version]
- Park, S.J. Antioxidant activities and whitening effects of ethanol extract from Panax ginseng sprout powder. J. Korean Soc. Food Sci. Nutr. 2019, 48, 276–281. [Google Scholar] [CrossRef]
- Liu, X.Y.; Xiao, Y.K.; Hwang, E.; Haeng, J.J.; Yi, T.H. Antiphotoaging and antimelanogenesis properties of ginsenoside C-Y, a ginsenoside Rb2 metabolite from American ginseng PDD-ginsenoside. Photochem. Photobiol. 2019, 95, 1412–1423. [Google Scholar] [CrossRef] [PubMed]
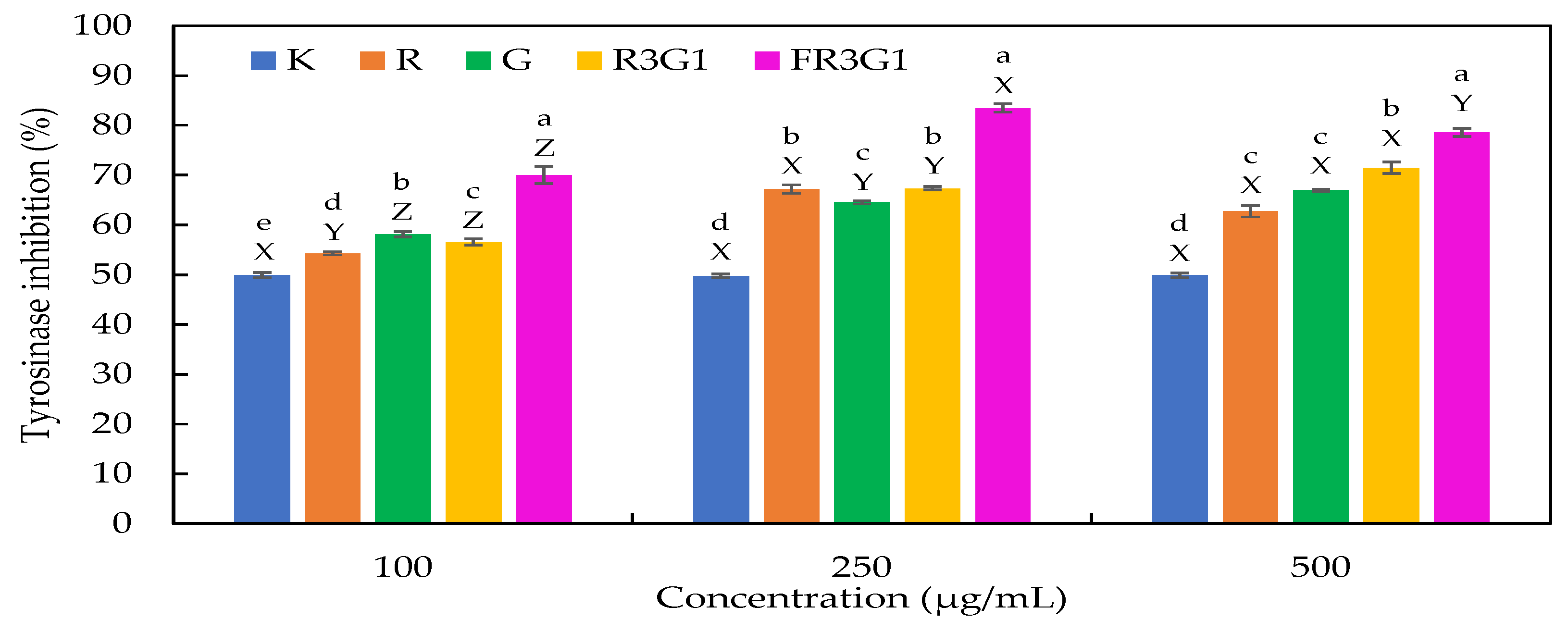
| Extracts | Polysaccharides (mg DE/g DW) | Triterpenoids (mg OE/g DW) | Polyphenols (mg GE/g DW) | Flavonoids (μg QE/g DW) | Ginsenoside (mg Rg3/g DW) |
|---|---|---|---|---|---|
| R | 15.45 ± 1.03 d | 32.10 ± 3.96 a | 4.91 ± 0.01 b | 52.33 ± 0.32 d | ND |
| G | 80.29 ± 0.79 b | 30.80 ± 2.91 a | 2.55 ± 0.08 d | 203.66 ± 3.21 b | 2.84 ± 0.28 b |
| R3G1 | 38.96 ± 0.55 c | 31.63 ± 3.68 a | 4.52 ± 0.08 c | 101.53 ± 4.25 c | 0.33 ± 0.02 c |
| FR3G1 | 114.97 ± 1.36 a | 32.02 ± 3.95 a | 5.16 ± 0.07 a | 272.84 ± 6.49 a | 4.04 ± 0.12 a |
| Sample | Relative Area (%) | Picture |
|---|---|---|
| Control | 100.00 ± 0.00 a |  |
| K | 64.34 ± 2.78 c,d |  |
| R | 81.17 ± 9.38 b | 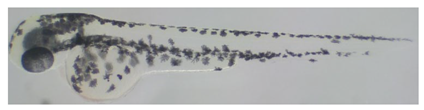 |
| G | 71.61 ± 4.32 c | 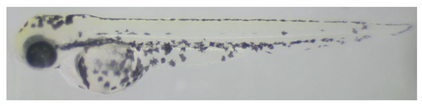 |
| R3G1 | 78.98 ± 3.82 b |  |
| FR3G1 | 57.26 ± 6.08 d | 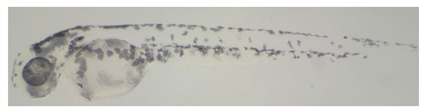 |
| Methods | Drying Curve | R2 | Rate (g/min) | Time (min) | Energy (kWh/kg) | GHG (kg CO2e/kg) |
|---|---|---|---|---|---|---|
| HARF | W = −29.380 t + 508.5 | 0.988 | 29.38 | 9.56 | 2.27 | 1.12 |
| RFV | W = −22.325 t + 513.0 | 0.988 | 20.97 | 13.03 | 3.80 | 1.88 |
| MW | W = −3.778 t + 499.9 | 0.986 | 3.79 | 75.53 | 2.18 | 1.08 |
| HA | W = −0.904 t + 457.8 | 0.903 | 0.90 | 260.63 | 15.72 | 7.78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Z.-Y.; Lin, Z.-J.; Chen, S.-D. Rice Bran and American Ginseng Residue as Media for Black Truffle Solid-State Fermentation. Sustainability 2025, 17, 5562. https://doi.org/10.3390/su17125562
Lin Z-Y, Lin Z-J, Chen S-D. Rice Bran and American Ginseng Residue as Media for Black Truffle Solid-State Fermentation. Sustainability. 2025; 17(12):5562. https://doi.org/10.3390/su17125562
Chicago/Turabian StyleLin, Zih-Yang, Zi-Jun Lin, and Su-Der Chen. 2025. "Rice Bran and American Ginseng Residue as Media for Black Truffle Solid-State Fermentation" Sustainability 17, no. 12: 5562. https://doi.org/10.3390/su17125562
APA StyleLin, Z.-Y., Lin, Z.-J., & Chen, S.-D. (2025). Rice Bran and American Ginseng Residue as Media for Black Truffle Solid-State Fermentation. Sustainability, 17(12), 5562. https://doi.org/10.3390/su17125562








