Abstract
In most cases, plant containers used in the horticulture industry are not reusable, have many negative impacts on the environment, and take a long time to degrade. To reduce the use of non-biodegradable plant containers, many bio-degradable plant containers have been developed for the horticulture industry. However, the full potential of the sustainability of plant containers is yet to be completely explored. Therefore, two novel biodegradable plant containers are developed in this research to effectively contribute to sustainability’s environmental, social, and economic dimensions. The two biocomposite formulations are developed by mixing soy hull and soy protein isolate (SPI) with polylactic acid (PLA) matrix for plant containers. In the first formulation, the proportion of PLA and soy hulls are 70 wt% and 30 wt%, respectively; in the second formulation, 65 wt% PLA is blended with 30 wt% soy hulls and 5 wt% SPI. The injection molding process is used to manufacture the plant containers from the two formulations. In a field trial, four plant species are grown in the novel plant containers along with polyethylene and commercial PLA/DDGS (dried distiller’s grains with solubles and PLA-based container) containers to investigate biodegradability and plant growth. The results show that the containers from the new formulations outperform existing biodegradable PLA/DDGS containers in terms of certain plant growth and biodegradability.
1. Introduction
Petroleum-based plastics are used to manufacture a wide range of products due to their versatility and low cost [1]. In 2021, the yearly global production of petroleum-based plastics reached 390.7 million tons, and the trend is continuously growing [2]. As of 2015, approximately 6300 million tons of plastic waste had been generated, around 9% of which had been recycled, 12% was incinerated, and 79% was accumulated in landfills or the natural environment [3]. Most plastic materials are not biodegradable, and the massive deposition of plastic waste in the natural environment creates serious environmental problems by increasing greenhouse gas, reducing soil porosity, decreasing air circulation, affecting microbial populations, potentially decreasing farmland fertility, and polluting rivers and oceans [4,5,6]. One of the significant uses of plastic materials is in producing plant containers used to grow horticultural crops. Studies showed that every year, in the United States alone, over 4 billion plastic containers are used by the horticulture industry and 98% of these containers are disposed of in landfills [7]. Using plastic containers presents sustainability concerns for the finite petroleum reserves and environmental problems because of the accumulation of nondegradable solid wastages [8]. Therefore, it is crucial to identify proper alternatives to plastic containers that are environmentally friendly and, more importantly, sustainable.
A feasible alternative to petroleum-based plant containers is biodegradable plant containers, also known as biocontainers or biopots. Biocontainers consist of plant and animal-based fillers and degrade by naturally occurring microorganisms in inorganic products and biomass, such as carbon dioxide and water, when planted into the soil or placed into a compost pile [4,9]. Biocontainers are further categorized as being plantable or compostable [9]. The plantable containers are left intact on the root ball and planted into the field, landscape bed, or final container, allowing plant roots to grow through the container walls. On the contrary, compostable containers are not directly planted into the soil as the plant roots cannot physically break through container walls because of their considerably slow degradation rates. Compostable containers are designed to be removed before final planting, broken apart, and composted [10].
Over the years, numerous types of biocontainers have been developed, typically composed of peat, paper, coconut fiber, rice hulls, poultry feather fiber, rice straw, dairy manure, or other organic components [4,9]. Peat containers consist of peat and paper fiber [10,11]; paper containers are produced from paper pulp with a binder [12]; coconut fiber containers are made from the medium and long fibers extracted from coconut husks and a binder [10]; rice hull containers are made from ground rice hulls and a binder [9]; rice straw containers are made from 80% rice straw, 20% coconut fiber, and a binder [10]; dairy manure containers are made from composted dairy manure with a binder [9]; and wood fiber containers are composed of 80% cedar fibers and 20% peat and lime [10]. Some biocontainers, made from plant fibers, may be smelly, costly, and unsuitable in terms of mechanical performance [13].
Biocontainer researchers have paid attention to the area of biocontainer development from biodegradable plastic or industrial waste [13,14,15,16], patent development [17], comparative analyses of mechanical properties of biocontainers [9,18,19], consumer willingness to pay (WTP) for biocontainers [20,21,22], comparative analyses of the performance of biocontainers in terms of water use efficiency, plant growth parameters, yields, and root circling [10,13,23,24,25]. Biocontainers are expensive compared to petroleum-based plastic containers, but consumers are willing to pay a premium to use them. With the increase in biodegradation rates, biocontainers’ water use efficiency and mechanical performance deteriorate, but the root circling situation of plant roots improves [26]. Plant growth and yield depend on the mechanical and chemical properties of biocontainers [27]. Thus, finding a sustainable development of biocontainer that is eco-friendly, socially impactful, and economically viable is challenging.
Bio-based polymers and their composites are biodegradable, biocompatible, and widely used in agricultural, industrial, and medical applications [28]. Plant containers made from bio-based polymers and their composites satisfy the functional requirements and biodegrade in soil under natural conditions. Poly(3-hydroxybutyrate-co-3-hydroxyvalerate), PHBV, is a biocompatible, biodegradable, non-toxic, and polyhydroxyalkanoate-type polymer [29]. The excellent properties of PHBV, such as its absorption capacity, biological origin, low cytotoxicity, piezoelectricity, and thermoplasticity, make it a potential candidate for substituting petroleum-derived polymers [30]. However, the application of PHBV is limited because it lacks mechanical strength, water sorption and diffusion, electrical and/or thermal properties, antimicrobial activity, wettability, biological properties, and porosity [30]. Many research efforts have been directed at the improvement of the mechanical properties, processability, and quality of the molded parts. In the endeavors, many biocomposite materials are developed and tested using a matrix of PHBV and reinforcement of plant fibers, such as flax, hemp, wood [31], coconut fibers [32], bamboo [33], agave [34], spent coffee ground [35], and vine shoots [36]. Biocomposites developed using PHBV matrix and natural fillers show improved mechanical properties and better quality of the molded pieces [31]. Nevertheless, the use of PHBV and its composites in producing plant containers is hardly expected because of their poor mechanical properties and high production cost.
Polylactic acid (PLA) is a bio-based, biodegradable, renewable, aliphatic thermoplastic polyester that is considered an eco-friendly and sustainable alternative to petroleum-based polymers [37]. PLA shows excellent biocompatibility and bio-absorbability, which make it attractive for bio-disposable plastic products such as plant containers [38]. Although PLA is biocompatible, it suffers from a slow degradation rate, poor toughness, hydrophobicity, and higher price than petroleum-based plastics [39]. PLA can be blended with biodegradable polymers, non-biodegradable polymers, and natural fibers to improve different properties [37,40]. In some early research, PLA was mixed with native starch to reduce the cost of PLA-based materials and enhance biodegradability [41,42]. Protein-based materials, such as soy protein isolate (SPI) and soy protein concentrate (SPC), were blended with PLA to increase biodegradability [26,43,44]. Soybean by-products are rich in nitrogen contents and act as fertilizer when added to plants’ growth medium [45,46]. Therefore, it is intuitive that plant containers made from soybean by-products would be eco-friendly, sustainable, and more economically affordable. Additionally, adding these natural fillers made of soybean by-products can be an inherent fertilizer source [47].
This research develops two novel bioplastic plant containers using PLA as a polymer matrix and two natural fillers, soy hulls, and soy protein isolate. Two novel formulations for plant containers are developed and compared with two other existing plant containers: petroleum-based plastic (polyethylene) container; and dried distiller’s grains with solubles (DDGS) and PLA-based (PLA/DDGS) container. For experimental comparison, four species of plants are grown in the four types of plant containers, and the performance of the plant containers is evaluated in terms of plant height, plant width, shoot fresh weight, fresh root weight, shoot dry weight, root dry weight, and root circling. In addition, the biodegradability of the bio-based plant containers is analyzed in this research.
Overall, the objectives of this research are to (1) develop two novel plant containers using PLA, soy hulls, and SPI, (2) cultivate four species of horticultural plants in the plant containers, (3) conduct performance analyses of the containers in terms of plant growth, yield, and root circling, and (4) assess degradation rates of the novel plant containers. The rest of the paper is organized as follows. The materials and method section includes new container formulations, production, and an experimental study. The result section describes the output of the experimental analysis. The next section introduces a discussion of the experimental investigations and findings. Finally, concluding remarks are made in the conclusion section.
2. Materials and Methods
2.1. Materials for the Biocomposite Formulations
In this research, two novel composite material formulations, named Formulation 1 (F1) and Formulation 2 (F2), are prepared using PLA, soy hulls, and SPI in different proportions. In F1, PLA is a polymer matrix and soy hull is a filler, but in F2, soy hulls and SPI are blended with a PLA matrix. F1 consists of 70 wt% PLA and 30 wt% soy hulls, and F2 consists of 65 wt% PLA, 30 wt% soy hulls, and 5 wt% SPI. PLA (3001D) is supplied by Nature Works, LLC, Minnetonka, MN, USA; Soy hull pellets are collected from NCI (Northern Crops Institute) Feed Production Center, Fargo, ND, USA; and SPI are procured from Honeyville.com. Two existing containers, commercial PLA/DDGS biodegradable containers (produced by a US-based company with the brand excluded for confidentiality purposes) and regular polyethylene containers, are collected for comparison with the two new formulations.
By considering various palleting and molding process constraints, several smaller- pre-experiment batches were conducted to maximize the proportion of filler (s) with PLA. F1 and F2 ratios, instead of other mass ratios, were obtained. The soy hulls are lightweight compared to PLA or SPI. Soy hulls of more than 30% wt resulted in very brittle formulations, which cannot be molded without cracking as there was insufficient PLA in the formulation. Thus, F1 and F2 formulations were selected for the rest of the experiment.
It is also worth mentioning that the second filler, SPI, was used in F2 in order to study the impact of soy hulls and SPI on plant health and containers’ biodegradability, even though SPI is expensive compared to existing other fillers. This is because SPI contains 85–90% protein on a dry basis. When SPI is deposited into the soil, the constituent proteins, such as β-conglycinin, glycinin, and lipophilic, are degraded to peptides and amino acids by soil microorganisms, initiating the nitrogen cycle in the soil. During nitrogen fixation in the nitrogen cycle, plant-absorbable nitrogen compounds are generated, which can otherwise be generated by applying nitrogen fertilizer. Nitrogen enables a plant to capture sunlight energy by photosynthesis, driving plant growth and grain yield. Therefore, SPI was used in this study as a potential fertilizer despite the higher cost of this substance.
2.2. Methods for the Plant Container Production
The steps for plant container manufacture from the two newly developed formulations are demonstrated in Figure 1. The soy hull particles were broken down to a granular size in the first step using the Retsch SK 100-mill machine. The size of granular soy hulls is less than 100 μm. PLA and soy hulls were then dried at 176 °F in an oven for 12 h to remove any trace of moisture. After that, PLA and soy hulls were mixed thoroughly and uniformly by a mixer for around 10 min to develop F1. Similarly, PLA, soy hulls, and SPI were mixed using the same mixer. The same process was followed for F2.
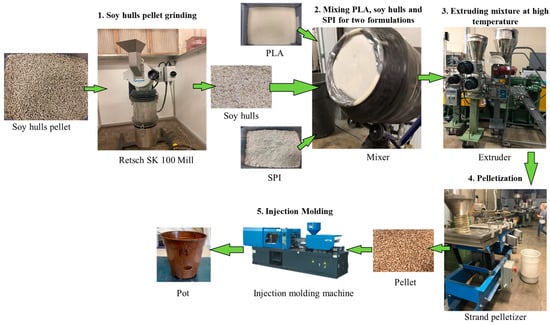
Figure 1.
The main steps in the production process of plant container.
In the next step, the mixed constituents of each formulation were poured into the barrel of the extruder. The barrel has three heating zones: feed zone, transition zone, and metering zone [39]. For extruding the formulations, a co-rotating twin screw extruder, of model SHJ-43 and manufactured by Nanjing Giant Machinery Company Limited, Nanjing, China, was used. The extrusion temperature was varied from 290 °F to 315 °F. The rotational speed and die temperature for the twin screw extruder were set to 180 RPM and 315 °F, respectively. The temperature at different zones of the barrel and rotational speed were varied to set an optimum combination of parameters for good-quality pellets. The outcome of the extrusion process was biocomposite strands, which passed through a water channel after extrusion through the die for cooling and solidification. In the following step, a pelletizer was used to cut the solidified strands into pellets of approximately 3 mm in length. After extrusion and pelleting, the amounts of pellets obtained for Formulation 1 and Formulation 2 were 26.05 lbs and 33.6 lbs, respectively.
After that, the pellets were dried for 6 h at 122 °F to remove moisture content absorbed during water cooling. All the steps mentioned above, from drying raw material to pelleting for each formulation, were carried out at the North Dakota State University Department of Industrial and Manufacturing Engineering and the C2renew Inc. facilities in Fargo (Fargo, ND, USA).
After the pelleting process, pellets of both formulations were delivered to the commercial PLA/DDGS facility for the injection molding of the bioplastic pellets into plant containers. An Arburg injection molding machine was used to produce the plant containers. The produced containers from Formulations 1 and 2 are shown in Figure 2 along with the two control pots.

Figure 2.
Four types of plant containers used in the study.
2.3. Material and Methods for the Greenhouse Field Experiment
Four horticultural plants: Tacitus Lettuce, Sheyenne Tomato, Zinnia, and French Marigold, were selected for a field trial with the plant containers. Pro-Mix BX medium (with microbial fungicide) by Sun Gro® Horticulture was used as a growing medium for all plant types. A water-soluble fertilizer, Jacks 20-20-20 General Purpose, was used with water to prepare a 200 ppm (parts per million) solution for providing plant nutrient elements. The fertilizer-mixed water was applied in the growing medium regularly until plant harvesting. The greenhouse field experiment involves many steps, including plant cultivation, data collection, and data analysis. The major steps are broken down in chronological order, as shown in Figure 3.

Figure 3.
The activities involved in the field experiment.
Seedlings. The field trial started with the seeding process. Seeds for all four plant species were planted in 4-cell plastic plant seed trays in the North Dakota State University Plant Science Horticulture Greenhouse (Fargo, ND, USA) on 10 August 2021. Pro-Mix BX medium was used as the growing medium for seeds. A 200 ppm solution was prepared by mixing 20-20-20 General Purpose fertilizer with water as a source of plant nutrients [48]. The solution was then used regularly to make the growing medium fertile and wet. The conditions of the growing medium and seedlings’ growth were routinely monitored.
Transplantation: After approximately four weeks of seedling growth in plastic trays, the seedlings were transplanted into four types of three-inch plant containers on 6 September 2021. Four seedlings were transplanted for each plant and container type. Therefore, a sum of sixteen (16) seedlings was transplanted for Tacitus Lettuce, Sheyenne Tomato, Zinnia, and French Marigold. A total of 64 plants were grown in the greenhouse using the same growing medium previously used for planting seeds.
Growth Inspection: The plant growth, the growing medium, and water requirements were observed regularly. The fertilizer-mixed water was routinely applied to keep the growing medium fertile and wet. After three weeks of the transplantation, the summary of the visual inspection results were (1) all four plant species grew well, (2) no visual difference in plant growth in different containers for each plant species, and (3) no sign of plant diseases. More detail on the visual inspection results for all four pots comparison will be further elaborated in Section 3.1.
Stringing: Stringing is generally used to keep tall plants from falling over due to the plants’ weight and the small pot size. After four weeks of the transplantation, two plant species (Sheyenne Tomato and Zinnia) had grown too high in three-inch containers. To provide structural support for climbing and carrying the weight of the stalks and stems, the plants of Sheyenne Tomatoes and Zinnia were stringed on 23 September 2021. The stringing was not necessary for the other two plant species. Plant growths, health, and growing medium were inspected regularly until plant harvesting.
Harvesting and Plant Growth Measurement: After around 6 weeks of plant species growth, all 64 plants were harvested for plant health and root circling analyses. Plant growth measurement was carried out by removing the plants from containers, separating root and shoot, and washing away the soil from the roots, as shown in Figure 4. After the plant was removed from the pots, comparison photographs of the same plant for all four pots were collected for visual inspection or qualitative analysis of the plant growth. The four parameters: plant height, plant width, fresh weight, and root weight; were recorded immediately after harvesting. Additional steps were carried out before measuring shoot dry weight, fresh root weight, and root dry weight. The collected data were later processed for quantitative and statistical analysis. The plant growth measurement results will be detailed in Section 3.2.
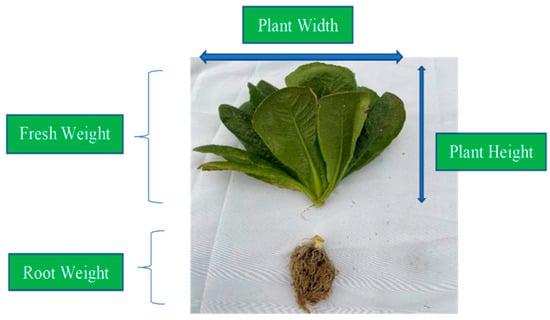
Figure 4.
Measurement parameters for the plant growth assessment.
Observation of Root Circling: Before separating the root and shoot of each plant, close-up photographs were taken for root circling observations. After separating the root and shoot of each plant, the root was washed to separate the growing medium from the root and then dried before the fresh root weight was taken. The root shown in Figure 4 is after washing and drying.
Drying and Dry Conditions Plant Growth Measurement: After harvesting and data collection of the fresh plant measurement had been completed, the root and shoot of all plants were put in paper bags for the dry condition plant health parameters measurement. The paper bags containing different plant parts were kept in a dryer at 176 °F for seven days before the dry condition parameter was measured.
2.4. Methods for Statistical Analysis
After all the data collection of the plant growth measurements had been completed, statistical analysis was applied to better infer the collected data and to determine whether the plant health measurement parameters were significantly different for different plant containers. The single-factor (one-way) analysis of variance (ANOVA) test was performed to identify the significance of plant container types on the plant growth parameters.
The single factor of the ANOVA test is the plant container type, which has four levels: F1, F2, PLA/DDGS, and polyethylene container. Plant growth parameters (height, width, shoot fresh weight, fresh root weight, shoot dry weight, and root dry weight) are defined as response variables. An ANOVA test was performed for each response variable of a plant species. According to the field experiment design, each level of a response variable has four data (observations), and whether the averages of the data are deemed the same across the levels is evaluated statistically using the single-factor ANOVA. Formally, the ANOVA test hypotheses are defined as follows:
Hypothesis 1 (H1).
.
Hypothesis 2 (H2).
where are the data means (average) of a response variable in levels F1, F2, PLA/DDGS, and plastic container, respectively. The null hypothesis (H1) refers to the condition where the means of the response data are the same for all the levels, and the alternative hypothesis (H2) denotes a condition with at least one pair of levels whose data means are different. for at least one pair ( ) of levels.
A probability value, p-value, quantifies how likely the collected data have been obtained by random chance or quantifies whether the null hypothesis is true. This statistical significance level is often expressed with a p-value between 0 and 1. For this study, the significance level for the ANOVA test is taken at 5% (0.05). The smaller the p-value indicates, the stronger the evidence that the null hypothesis should be rejected. In other words, if the p-value obtained from the ANOVA test is less than the significance level, the null hypothesis is rejected; otherwise, there is a lack of evidence to reject the null hypothesis. A statistical software, Minitab, was used to perform the ANOVA tests.
Additionally, in the case of null hypothesis rejection, a post-hoc analysis, named Tukey pairwise comparison, was conducted to identify the exact pair of plant container types that possessed significantly different mean response data from the rest of the factor levels. Tukey’s method was further carried out to avoid possible contradictory results obtained from small sample sizes during the analysis. Tukey’s test creates confidence intervals for all possible pairwise combination differences between their factor level means. The statistical analysis results will be detailed in Section 3.4.
2.5. Methods for Biodegradation Analysis
Monitoring weight reduction is one of the convenient methods of evaluating the biodegradability of the pots. By measuring a plant container’s dry weight before plantation and after harvesting, the degradation or weight loss percentage (%) of each plant container can be determined by using Equation (1) given in Ref. [49]. This weight loss method is a common approach in horticulture research [50,51].
where Wi is the dry weight of a plant container before starting the experiment and Wf is the dry weight of the same container at the end of the harvesting. The final weight, Wf, for each container, is measured at room temperature after removing moisture content from it at 212 °F for 24 h. The biodegradable analysis results will be detailed in Section 3.5.
3. Results
3.1. Results from Visual Inspection
Before the harvesting activities and obtaining the numerical plant growth data, as detailed in Section 2.3, photographs were taken during the same data collection period for visual inspection during all data collection steps. The four different container types were placed side-by-side for better growth comparison and understanding. For a visual comparison of the growth parameters in the four types of containers, Tacitus Lettuce, Sheyenne Tomato, Zinnia, and French Marigold plants are shown in Figure 5, Figure 6, Figure 7 and Figure 8, respectively.

Figure 5.
Tacitus Lettuce plants after removing the containers during harvesting.

Figure 6.
Sheyenne Tomato plants after removing the containers during harvesting.

Figure 7.
Zinnia plants after removing the containers during harvesting.

Figure 8.
French Marigold plants after removing the containers during harvesting.
3.2. Results from Plant Growth Measurement
In the field trial, a total of 64 plants of four species were grown in four types of plant containers. There were, however, some plants that died during the field experiment. The total number of dead plants was four, resulting in a 6.25% failed growth in the trial. The details of the dead plants are two Sheyenne Tomato from F1 containers, one Shayenne Tomate from a PLA/DDGS container, and one French Marigold from an F1 container. The dead plants are not included in the study. This incident reduces the sample size of the field experiment; thus, the average measurement is taken only from the remaining sample number available.
The plant growth parameters for Tacitus Lettuce were measured for both fresh and dry conditions. The average measure for each parameter with an error bar, which represents one standard deviation from the average, is shown in Figure 9. It is seen in the figure that, except for the shoot fresh weight parameter, the average response in all other parameters obtained from different container types is similar. In the case of shoot fresh weight, the highest and lowest average responses are obtained from the polyethylene and F1 containers, respectively. Even though average shoot fresh weight apparently varies across the container types, further statistical analysis is required to reach an objective conclusion, which will be covered in Section 3.4.
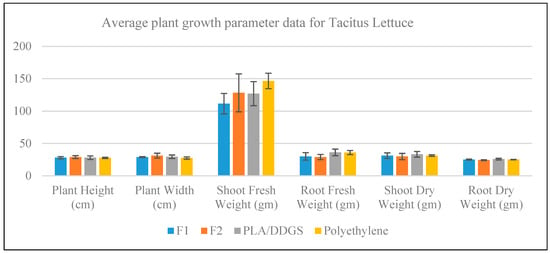
Figure 9.
Growth measurement data for Tacitus Lettuce.
For the case of the Sheyenne Tomato, the average measure for each plant growth parameter with an error bar of one standard deviation from the average is shown in Figure 10. The bar chart in the figure shows that the performance of polyethylene containers is apparently better than all other container types. Except for shoot fresh weight and root fresh weight parameters, the average response for each of the other parameters is similar across the container types. In the case of shoot fresh weight, the average response obtained from polyethylene containers is better than others. The biodegradable containers (F1, F2, and PLA/DDGS) apparently only outperform polyethylene containers in the case of root fresh weight.
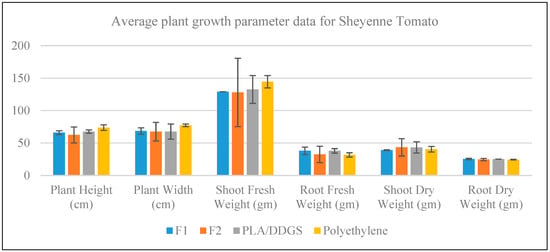
Figure 10.
Growth measurement data for Sheyenne Tomato.
Fresh and dry condition plant growth parameters of Zinnia plants are measured and shown as a bar chart with error bars in Figure 11. It is evident from the figure that neither of the container types emerged as better than the others in terms of providing an average response. For the case of plant width, shoot dry weight, and root dry weight, the average responses from all container types are very close. However, in the case of plant height, F1 and F2 containers render higher average responses than the PLA/DDGS and polyethylene containers; in the case of shoot fresh weight, the average response of PLA/DDGS container is the lowest, while the other containers responses are very close; in the case of root fresh weight, F2 container gives better average response than the other container types.
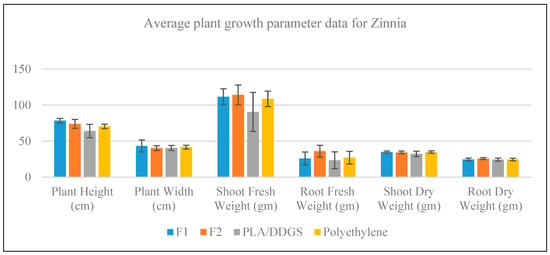
Figure 11.
Growth measurement data for Zinnia.
A bar chart with error bars for the measurement of plant growth parameters of French Marigold plants is shown in Figure 12. It is noticeable from the figure that in terms of average response, F1 and polyethylene containers are better than or at least competitive with the F2 and PLA/DDGS containers. In the case of shoot fresh weight, the F1 and polyethylene containers provide better average responses than the F2 and PLA/DDGS containers. Moreover, in the case of root fresh weight, the F1, F2, and polyethylene render similar average responses, while the lowest average response is obtained from the PLA/DDGS container. On the contrary, for the case of plant height, plant width, shoot dry weight, and root dry weight, the plant containers provide very similar average responses.
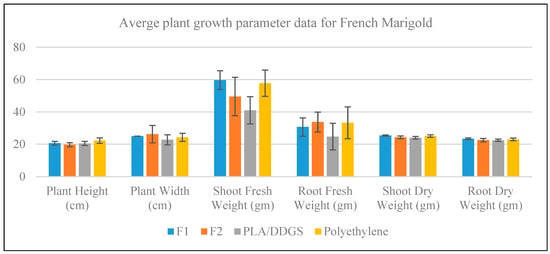
Figure 12.
Growth measurement data for French Marigold.
When evaluating the different container type performances, the direct plant growth measurement data obtained vary from parameter to parameter for each plant type. This could be due to the small sample size included in the study. Although the polyethylene containers show a better growth performance in many cases, the direct measurement results also indicate that F1 and F2 containers are competitive with the PLA/DDGS containers, with F2 showing a superior plant growth measurement when compared to F1. Due to the variability of growth performance from different samples, the statistical analysis was conducted to ensure that the quantitative measurement results for the new formulation containers F1 and F2 can be deemed competitive with the PLA/DDGS and polyethylene containers.
3.3. Results from Root Circling Observation
Horticultural plants are exposed to a limited amount of growing medium when grown in three-inch containers. If a root-bound plant is planted in a small container, the root hairs of the plant circle around the inner surface of the container, and this condition of the root hairs are called root circling [52]. Root circling often creates root girdling problems, where one or more roots completely or partially encircle the stem. This may eventually cause bark and wood compression. In this experiment, the root circling condition is observed for all plant species and all container types. Particularly for the case of Sheyenne Tomato and French Marigold, irrespective of the container type used for plantation, the root circling condition is observed. From the visual analysis of plant roots, it can be summarized that root circling condition is prevalent for root-bound plants.
The visual observation of root circling in new formulation containers for French Marigold is shown in Figure 13. The new formulations, F1 and F2 containers, exhibit fewer root circles for the root circling analysis than the existing PLA/DDGS and polyethylene containers. Thus, it can also be deemed that the roots taken from bioplastic containers tend to show less root circling than those taken from polyethylene containers.
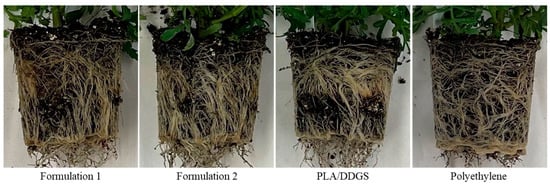
Figure 13.
Root circling of French Marigold plants caused in different container types.
3.4. Results from Statistical Analysis
Statistical analyses are conducted on the data collected from the field experiment, which are shown in Figure 9, Figure 10, Figure 11 and Figure 12, to identify the performance of the plant containers on the plant growth parameters. A one-way ANOVA test was conducted to verify if the effect of plant containers on the performance of a growth parameter of a plant species is significant among the samples or not. If the test result shows that the plant container significantly contributes to the plant growth performance, then the Tukey pairwise comparison method was further applied to identify the exact plant container types. The one-way ANOVA analysis results of the plant growth data of the Tacitus Lettuce, Sheyenne Tomato, Zinnia, and French Marigold plant species are shown in Table 1.

Table 1.
One-way ANOVA analyses results.
According to statistical analysis of the collected data, new plant containers F1 and F2 significantly outperform the existing biodegradable PLA/DDGS containers in terms of the certain plant health and growth parameters. Specifically, the plant height of Zinnia and shoot fresh weight of French Marigold are significantly better if they are grown in the F1 containers rather than PLA/DDGS containers. In support of this statement, Tukey pairwise comparison test results for plant height data of Zinnia and shoot fresh weight data of French Marigold are shown in Table 2.

Table 2.
Tukey pairwise comparison results for plant height of Zinnia and shoot fresh weight of French Marigold.
In Table 2, the factor indicates the type of containers, N is the sample size of four pots (S1–S4), mean is the average plant measurement obtained from the four sample sizes sorted from largest to smallest, and there are two groupings, A or B, for the pairwise comparison. If the factor only has one grouping, either A or B, it belongs only to that particular group. If the factor has two groupings, it means that it can belong to groups A and B. It can be seen that for the plant height of Zinnia, F1 is superior compared to PLA/DDGS, which indicates that they belong to two different groups. A similar interpretation can be drawn for the shoot fresh weight of the French Marigold Tukey pairwise comparison results.
3.5. Results from Biodegradation Analysis
As polyethylene containers are not biodegradable, they are not considered for biodegradability analysis. All of the weights for each individual pot were measured. Since the pots were all produced using the injection molding process with the same mold, which required relatively same amounts of materials for each pot type, the initial weight for each pot type is consistent across all the sample for the same pot type. The initial weights, Wi, of F1, F2, and PLA/DDGS containers are about 30.81 gm, 30.87 gm, and 31.04 gm, respectively, as shown in Figure 14. The biodegradation results of the biobased containers (F1, F2, PLA/DDGS) showing the average initial dry weight, final dry weight, and percentage of weight loss with error bars are shown in Figure 15. The error bars show very little spread for initial dry weight, final dry weight, and weight loss (%) for all the biobased containers. The result shows that the average weight loss (%) of F1, F2, and PLA/DDGS containers is 3.10%, 5.64%, and 2.80%, respectively. Among the three biodegradable containers, the F2 container had the highest decomposition rate during the study period and the PLA/DDGS container had the lowest decomposition rate throughout cultivation. The degradation rate of the F2 container is almost double the degradation rate of the PLA/DDGS container. In addition, the degradation rate of the F1 container is 10% more than the degradation rate of the PLA/DDGS container. Based on these results, comparing F1 and F2 formulations, it can be concluded that SPI in the F2 pot formulation may enhance the biodegradation process. However, more advanced material property research should be conducted to verify further the claim that SPI aids in the degradation process.

Figure 14.
Initial dry weight of the three types of biodegradable plant containers.
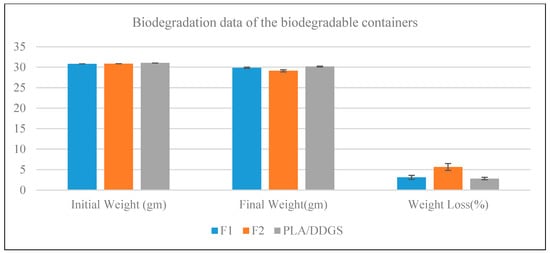
Figure 15.
Biodegradation results of Formulation 1, Formulation 2, and PLA/DDGS containers.
4. Discussion
The research results demonstrate that the novel formulations for biodegradable plant containers, F1 and F2 containers, made from soy hull, soy protein isolate (SPI), and PLA, satisfy the functional requirements of plant containers and the social, economic, and environmental dimensions of sustainable development. From the social factor of sustainable development, if the new formulations of this plastic container are optimized and can be marketed to replace plastic pots, the use of soybean production by-products in the plant containers will increase the demand for soybeans in the agricultural industry and farmers would benefit from producing more soybeans. This would eventually create more jobs and businesses in various stages in development process of the biodegradable pots [53,54].
From the economic factor of sustainable development, the proposed soy-based biodegradable pots may reduce fertilizer dependency during the growth process and eventually reduce the overall operation cost. The soybean by-products are in high concentrations of nitrogen, which act as an inherent source of fertilizer for horticultural crops [46]. The experimental results also demonstrate the knowledge regarding plant growth parameter values. These novel containers still need to be optimized and studied further to perform better than the PLA/DDGS container, which does not have higher nitrogen concentrations, for all plant growth parameters. Comparing the proposed soy-based plant containers with the traditional plastic containers, they are comparable in terms of plant growth parameters and yields. However, soy-based biodegradable pots still lack the price-to-performance ratio due to the relatively more involved production process. Although there are abundant soybean by-products, developing these soy-based biodegradable pots can be more expensive as it requires additional pre-processing steps to obtain the bioplastic formulation before it can be molded into pots [39]. The efforts of treating and processing the soybean by-products are different than processing other bio-fillers, such as DDGS, in the bioplastic matrix. The production cost of the pots may further influence the consumer’s willingness to pay a premium price tag to employ biodegradable plant containers, especially for commercial purposes. Thus, additional future studies on mass production efficiency to alleviate this high price tag would be beneficial to increase the market share for biodegradable pots.
From the environmental factor of sustainable development, biodegradable pots’ benefits are apparent in their biodegradability [55]. The novel plant containers show a significantly better biodegradation rate than the PLA/DDGS containers, which makes the novel containers possibly more eco-friendly than the PLA/DDGS containers. Additional efforts should be directed to compare whether the extra energy used in the production can be offset from the biodegradation rate, which may eventually result in better carbon footprints for the proposed soy-based pot formulations.
In short, from the experimental outcomes, the proposed biobased containers improved several plant growth parameters, reduced root circling conditions, and showed better biodegradability. These preliminary results positively contribute to the environment, society, and economy, covering the main dimensions of sustainability. Therefore, the proposed soy-based plant containers may be a potentially sustainable development alternative to petroleum-based plastic containers. Before this can be realized, many follow-up efforts need to be conducted.
Future research efforts will be directed towards optimizing the formulation in the biodegradable plant container to have more nitrogen content and biodegradation rate, while satisfying the functional requirements. In addition to analyzing the pots’ properties, more details will be given to exploring the detailed properties of the soy-based bioplastic composition in terms of uniformity dispersion analysis, moldability, and biodegradation process. Regarding data analysis, in addition to ANOVA, other statistical methods, for example, Taguchi methods, will be considered. Additionally, larger-scale field experimentation (~100 pots type per plant species) will be designed with the experimental methods approach and conducted to verify the preliminary results presented in this paper.
5. Conclusions
The summary of the study conducted is as follows. The primary objectives of the research are to (1) fabricate two novel bioplastic-based plant containers that are degradable, eco-friendly, and act as an inherent source of fertilizer, (2) cultivate four plant species in the containers for observing plant growth and root circling conditions, and (3) assess biodegradation rate of the biobased plant containers. For this purpose, two novel formulations for plantation containers are developed from PLA, soy hulls, and SPI. Then, the formulations, F1 and F2, are taken through several processes to fabricate two plant containers by the injection molding process. The performance of the new plant containers is compared with the PLA/DDGS and regular polyethylene containers in terms of plant growth, root circling conditions, and biodegradability. In the study, four horticultural plants are cultivated in the four types of plant containers for plant growth and root circling assessment. The statistical analyses indicate that in Zinnia plants, the plant height is significantly higher if planted in the F1 containers instead of the PLA/DDGS container. The shoot fresh weight is considerably heavier for the F1 containers than the PLA/DDGS containers for French Marigold. From the visual inspections of the plant roots, after removal of the containers, it is seen that the root circling problem is rarely present if the plant is taken from newly developed bioplastic containers. In addition, the newly developed soy-based containers showed better biodegradation than the existing PLA/DDGS containers. In the future, the proposed formulation needs to be optimized to ensure they are price-to-ratio competitive against traditional plastic containers.
Author Contributions
Conceptualization, M.M.R., A.D., N.Y., C.W.L. and D.G.; methodology, M.M.R., A.D., N.Y., C.W.L. and D.G.; data collection M.M.R., A.D., N.Y. and C.W.L.; software, M.M.R. and A.D.; validation, M.M.R., A.D., N.Y., C.W.L. and D.G.; formal analysis, M.M.R., A.D. and C.W.L.; investigation, M.M.R., A.D. and C.W.L.; resources, N.Y., C.W.L. and D.G.; data curation, M.M.R., A.D. and C.W.L.; writing—original draft preparation M.M.R., A.D., and N.Y.; writing—review and editing, M.M.R., A.D., N.Y., C.W.L. and D.G.; visualization, M.M.R., A.D. and N.Y.; supervision, N.Y., C.W.L. and D.G.; project administration, N.Y., C.W.L. and D.G.; funding acquisition, N.Y., C.W.L. and D.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially funded by the North Dakota Soybean Council (NDSC) under FAR0032186 and FAR0033623 grants.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors would like to acknowledge the help, guidance, and support from the following individuals. Nichols Kendall (North Dakota Soybean Council) for the research support. Jad Randalls (NatureWorks) for donating a portion of the PLA 3001D. Danny Mishek (SelfEco) for molding our experimental pots. Brian Neville (Carrington Research Extension Center) and Kurt Johnsen (Northern Crops Institute) for supplying soy hulls for the experiments. Helen Song for assisting with designing the greenhouse experiment. Anunay Gupta for conducting a pilot study and supporting the initial stage of the project. Raihan Quader for helping with the pelleting process. Labiba Asha and Evan Scully for helping with field data collection.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mekonnen, T.; Mussone, P.; Khalil, H.; Bressler, D. Progress in bio-based plastics and plasticizing modifications. J. Mater. Chem. A 2013, 1, 13379–13398. [Google Scholar] [CrossRef]
- Tiseo, I. Global Plastic Production 1950–2021. 2022. Available online: https://www.statista.com/statistics/282732/global-production-of-plastics-since-1950/ (accessed on 20 January 2023).
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed]
- Tomadoni, B.; Merino, D.; Alvarez, V.A. Biodegradable materials for planting pots. Adv. Appl. Bio-Degrad. Green Compos. 2020, 68, 85. [Google Scholar]
- Wang, J.; Lv, S.; Zhang, M.; Chen, G.; Zhu, T.; Zhang, S.; Teng, Y.; Christie, P.; Luo, Y. Effects of plastic film residues on occurrence of phthalates and microbial activity in soils. Chemosphere 2016, 151, 171–177. [Google Scholar] [CrossRef]
- Scalenghe, R. Resource or waste? A perspective of plastics degradation in soil with a focus on end-of-life options. Heliyon 2018, 4, e00941. [Google Scholar] [CrossRef] [PubMed]
- Schrader, J.A. Report on the Annual Consumption of Plastics for Specialty-Crop Containers in the United States; Project: Bioplastic Container Cropping Systems; Iowa State University: Ames, IA, USA, 2013. [Google Scholar] [CrossRef]
- Dey, A.; Eagle, I.R.; Yodo, N. A Review on Filament Materials for Fused Filament Fabrication. J. Manuf. Mater. Process. 2021, 5, 69. [Google Scholar] [CrossRef]
- Evans, M.R.; Taylor, M.; Kuehny, J. Physical Properties of Biocontainers for Greenhouse Crops Production. Horttechnology 2010, 20, 549–555. [Google Scholar] [CrossRef]
- Evans, M.R.; Koeser, A.K.; Bi, G.; Nambuthiri, S.; Geneve, R.; Lovell, S.T.; Stewart, J.R. Impact of Biocontainers With and Without Shuttle Trays on Water Use in the Production of a Containerized Ornamental Greenhouse Crop. Horttechnology 2015, 25, 35–41. [Google Scholar] [CrossRef]
- Group, J. Jiffy Pots. Available online: https://jiffygroup.com/solutions/jiffy-pots/ (accessed on 27 January 2023).
- Company, W.P.P. Sustainability. Available online: https://www.westernpulp.com/sustainability (accessed on 27 January 2023).
- Schettini, E.; Santagata, G.; Malinconico, M.; Immirzi, B.; Mugnozza, G.S.; Vox, G. Recycled wastes of tomato and hemp fibres for biodegradable pots: Physico-chemical characterization and field performance. Resour. Conserv. Recycl. 2013, 70, 9–19. [Google Scholar] [CrossRef]
- Grazuleviciene, V.; Augulis, L.; Grazulevicius, J.V.; Kapitanovas, P.; Vedegyte, J. Biodegradable starch, PVA, and peat composites for agricultural use. Russ. J. Appl. Chem. 2007, 80, 1928–1930. [Google Scholar] [CrossRef]
- Santos, C.; Mateus, A.; Mendes, A.; Malça, C. Processing and Characterization of Thin Wall and Biodegradable Injected Pots. Procedia Manuf. 2017, 12, 96–105. [Google Scholar] [CrossRef]
- Sartore, L.; Schettini, E.; Bignotti, F.; Pandini, S.; Vox, G. Biodegradable plant nursery containers from leather industry wastes. Polym. Compos. 2016, 39, 2743–2750. [Google Scholar] [CrossRef]
- Trabka, G. Bottomless Plant Container. U.S. Patent 2009/0025290 A1, 29 January 2009. Available online: https://patents.google.com/patent/US20090025290 (accessed on 20 January 2023).
- Castronuovo, D.; Picuno, P.; Manera, C.; Scopa, A.; Sofo, A.; Candido, V. Biodegradable pots for Poinsettia cultivation: Agronomic and technical traits. Sci. Hortic. 2015, 197, 150–156. [Google Scholar] [CrossRef]
- Lopez, R.G.; Camberato, D.M. Growth and Development of ‘Eckespoint Classic Red’ Poinsettia in Biodegradable and Compostable Containers. Horttechnology 2011, 21, 419–423. [Google Scholar] [CrossRef]
- Morone, P.; Caferra, R.; D’Adamo, I.; Falcone, P.M.; Imbert, E.; Morone, A. Consumer willingness to pay for bio-based products: Do certifications matter? Int. J. Prod. Econ. 2021, 240, 108248. [Google Scholar] [CrossRef]
- Salazar, H.A.; Oerlemans, L. Do We Follow the Leader or the Masses? Antecedents of the Willingness to Pay Extra for Eco-Products. J. Consum. Aff. 2015, 50, 286–314. [Google Scholar] [CrossRef]
- Notaro, S.; Lovera, E.; Paletto, A. Consumers’ preferences for bioplastic products: A discrete choice experiment with a focus on purchase drivers. J. Clean. Prod. 2021, 330, 129870. [Google Scholar] [CrossRef]
- Sun, Y.; Niu, G.; Koeser, A.K.; Bi, G.; Anderson, V.; Jacobsen, K.; Conneway, R.; Verlinden, S.; Stewart, R.; Lovell, S.T. Impact of Biocontainers on Plant Performance and Container Decomposition in the Landscape. Horttechnology 2015, 25, 63–70. [Google Scholar] [CrossRef]
- Wang, X.; Fernandez, R.T.; Cregg, B.M.; Auras, R.; Fulcher, A.; Cochran, D.R.; Niu, G.; Sun, Y.; Bi, G.; Nambuthiri, S.; et al. Multistate Evaluation of Plant Growth and Water Use in Plastic and Alternative Nursery Containers. Horttechnology 2015, 25, 42–49. [Google Scholar] [CrossRef]
- Nambuthiri, S.S.; Ingram, D.L. Evaluation of Plantable Containers for Groundcover Plant Production and Their Establishment in a Landscape. Horttechnology 2014, 24, 48–52. [Google Scholar] [CrossRef]
- Yang, S.; Madbouly, S.A.; Schrader, J.A.; Srinivasan, G.; Grewell, D.; McCabe, K.G.; Kessler, M.R.; Graves, W.R. Characterization and biodegradation behavior of bio-based poly (lactic acid) and soy protein blends for sustainable horticultural applications. Green Chem. 2015, 17, 380–393. [Google Scholar] [CrossRef]
- Flax, N.J.; Currey, C.J.; Schrader, J.A.; Grewell, D.; Graves, W.R. Herbaceous Perennial Producers Can Grow High-quality Blanket Flower in Bioplastic-based Plant Containers. Horttechnology 2018, 28, 212–217. [Google Scholar] [CrossRef]
- Al-Oqla, F.M.; Sapuan, S.M. Advanced Processing, Properties, and Applications of Starch and Other Bio-Based Polymers; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Fei, B.; Chen, C.; Wu, H.; Peng, S.; Wang, X.; Dong, L.; Xin, J.H. Modified poly(3-hydroxybutyrate-co-3-hydroxyvalerate) using hydrogen bonding monomers. Polymer 2004, 45, 6275–6284. [Google Scholar] [CrossRef]
- Rivera-Briso, A.L.; Serrano-Aroca, Á. Poly(3-Hydroxybutyrate-co-3-Hydroxyvalerate): Enhancement Strategies for Advanced Applications. Polymers 2018, 10, 732. [Google Scholar] [CrossRef] [PubMed]
- Frącz, W.; Janowski, G.; Smusz, R.; Szumski, M. The Influence of Chosen Plant Fillers in PHBV Composites on the Processing Conditions, Mechanical Properties and Quality of Molded Pieces. Polymers 2021, 13, 3934. [Google Scholar] [CrossRef]
- Torres-Giner, S.; Hilliou, L.; Melendez-Rodríguez, B.; Figueroa-Lopez, K.J.; Madalena, D.; Cabedo, L.; Covas, J.A.; Vicente, A.A.; Lagaron, J.M. Melt processability, characterization, and antibacterial activity of compression-molded green composite sheets made of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) reinforced with coconut fibers impregnated with oregano essential oil. Food Packag. Shelf Life 2018, 17, 39–49. [Google Scholar] [CrossRef]
- Zhuo, G.; Zhang, X.; Jin, X.; Wang, M.; Yang, X.; Li, S. Effect of different enzymatic treatment on mechanical, water absorption and thermal properties of bamboo fibers reinforced poly (Hydroxybutyrate-co-Valerate) biocomposites. J. Polym. Environ. 2020, 28, 2377–2385. [Google Scholar] [CrossRef]
- Gallardo-Cervantes, M.; González-García, Y.; Pérez-Fonseca, A.A.; González-López, M.E.; Manríquez-González, R.; Rodrigue, D.; Robledo-Ortíz, J.R. Biodegradability and improved mechanical performance of polyhydroxyalkanoates/agave fiber biocomposites compatibilized by different strategies. J. Appl. Polym. Sci. 2021, 138, 50182. [Google Scholar] [CrossRef]
- Presnell, K.R. Impact of Adding Spent Coffee Grounds (SCG) Fiber Composites to (3-hydroxybutyrate-co-3-hydroxyvalerate)(PHBV). Doctoral dissertation, The Ohio State University, Columbus, OH, USA, 2022. [Google Scholar]
- David, G.; Heux, L.; Pradeau, S.; Gontard, N.; Angellier-Coussy, H. Upcycling of Vine Shoots: Production of Fillers for PHBV-Based Biocomposite Applications. J. Polym. Environ. 2020, 29, 404–417. [Google Scholar] [CrossRef]
- Hamad, K.; Kaseem, M.; Ayyoob, M.; Joo, J.; Deri, F. Polylactic acid blends: The future of green, light and tough. Prog. Polym. Sci. 2018, 85, 83–127. [Google Scholar] [CrossRef]
- Dey, A.; Yodo, N. A Systematic Survey of FDM Process Parameter Optimization and Their Influence on Part Characteristics. J. Manuf. Mater. Process. 2019, 3, 64. [Google Scholar] [CrossRef]
- Dey, A.; Rahman, M.M.; Yodo, N. Soy-based Biocomposite Filament Development for Fused Filament Fabrication Process. In Proceedings of the IIE Annual Conference, Seattle, WA, USA, 21–24 May 2022. [Google Scholar]
- Jiang, L.; Wolcott, M.P.; Zhang, J. Study of Biodegradable Polylactide/Poly(butylene adipate-co-terephthalate) Blends. Biomacromolecules 2005, 7, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Sun, X.; Seib, P. Strengthening blends of poly (lactic acid) and starch with methylenediphenyl diisocyanate. J. Appl. Polym. Sci. 2001, 82, 1761–1767. [Google Scholar] [CrossRef]
- Zhang, J.-F.; Sun, X. Mechanical properties of poly (lactic acid)/starch composites compatibilized by maleic anhydride. Biomacromolecules 2004, 5, 1446–1451. [Google Scholar] [CrossRef] [PubMed]
- González, A.; Igarzabal, C.I.A. Soy protein—Poly (lactic acid) bilayer films as biodegradable material for active food packaging. Food Hydrocoll. 2013, 33, 289–296. [Google Scholar] [CrossRef]
- Zhu, R.; Liu, H.; Zhang, J. Compatibilizing Effects of Maleated Poly(lactic acid) (PLA) on Properties of PLA/Soy Protein Composites. Ind. Eng. Chem. Res. 2012, 51, 7786–7792. [Google Scholar] [CrossRef]
- Matsumiya, Y.; Kubo, Y.M.A.M. Soybean Peptide: Novel Plant Growth Promoting Peptide from Soybean. In Soybean and Nutrition; El-Shemy, H., Ed.; InTechOpen: London, UK, 2011. [Google Scholar] [CrossRef]
- Yoshiki, M.; Sachie, H.; Toshihide, M.; Motoki, K. Soybean as a nitrogen supplier. Chapter 2013, 3, 49–60. [Google Scholar]
- Schrader, J.A.; Srinivasan, G.; Grewell, D.; McCabe, K.G.; Graves, W.R. Fertilizer Effects of Soy-plastic Containers during Crop Production and Transplant Establishment. Hortscience 2013, 48, 724–731. [Google Scholar] [CrossRef]
- Choi, J.M.; Lee, C.W.; Chun, J.-P. Optimization of substrate formulation and mineral nutrition during the production of vegetable seedling grafts. Hortic. Environ. Biotechnol. 2012, 53, 212–221. [Google Scholar] [CrossRef]
- Rafee, S.N.A.M.; Lee, Y.L.; Jamalludin, M.R.; Razak, N.A.; Makhtar, N.L.; Ismail, R.I. Effect of Different Ratios of Biomaterials to Banana Peels on the Weight Loss of Biodegradable Pots. Acta Technol. Agric. 2019, 22, 1–4. [Google Scholar] [CrossRef]
- Lu, H.; Madbouly, S.A.; Schrader, J.A.; Srinivasan, G.; McCabe, K.G.; Grewell, D.; Kessler, M.R.; Graves, W.R. Biodegradation behavior of poly (lactic acid)(PLA)/distiller’s dried grains with solubles (DDGS) composites. ACS Sustain. Chem. Eng. 2014, 2, 2699–2706. [Google Scholar] [CrossRef]
- Madbouly, S.A.; Schrader, J.A.; Srinivasan, G.; Liu, K.; McCabe, K.G.; Grewell, D.; Graves, W.R.; Kessler, M.R. Biodegradation behavior of bacterial-based polyhydroxyalkanoate (PHA) and DDGS composites. Green Chem. 2013, 16, 1911–1920. [Google Scholar] [CrossRef]
- Warren, S.L.; Blazich, F.A. Influence of Container Design on Root Circling, Top Growth, and Post-Transplant Root Growth of Selected Landscape Species. J. Environ. Hortic. 1991, 9, 141–144. [Google Scholar] [CrossRef]
- Toloi, M.N.V.; Bonilla, S.H.; Toloi, R.C.; Silva, H.R.O.; Nääs, I.D.A. Development Indicators and Soybean Production in Brazil. Agriculture 2021, 11, 1164. [Google Scholar] [CrossRef]
- Jia, F.; Peng, S.; Green, J.; Koh, L.; Chen, X. Soybean supply chain management and sustainability: A systematic literature review. J. Clean. Prod. 2020, 255, 120254. [Google Scholar] [CrossRef]
- Rai, P.; Mehrotra, S.; Priya, S.; Gnansounou, E.; Sharma, S.K. Recent advances in the sustainable design and applications of biodegradable polymers. Bioresour. Technol. 2021, 325, 124739. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).