Abstract
The nutrient concentration of fruits and vegetables in the U.S.A. has declined in the past 50–70 years. Crop management practices utilizing on-farm inputs are thought to increase crop nutritional quality, but few studies have evaluated this under long-term side-by-side trials. An experiment was conducted from 2004 to 2005 at Rodale Institute’s long-term Farming Systems Trial to investigate the nutritional quality of vegetables under organic manure (MNR) and conventional (CNV) farming systems, with or without arbuscular mycorrhizal fungi (AMF) treatment. AMF reduced the vitamin C content in carrots in both systems in 2004, but the reduction was 87% in CNV and 28% in MNR. AMF also reduced antioxidants in carrots in both CNV and MNR. This trend was likely due to the suppression of native AMF colonization by the non-native AMF inoculum used. Between 2004 and 2005, MNR increased the vitamin C in green peppers by 50% while CNV decreased the vitamin C in red peppers by 48%. Tomatoes under MNR had a 40% greater vitamin C content compared to CNV in 2005. The vegetable yield declined between 2004 and 2005, except for tomato, where the yield increased by 51% and 44% under CNV and MNR, respectively. In general, MNR tended to increase the nutrient concentration of vegetables compared with CNV, while the AMF effects were inconclusive.
1. Introduction
Organic farming practices have been shown to have significant impacts on soil quality. Studies have shown that organically managed soils usually have higher contents of soil organic matter (SOM), soil organic carbon (SOC), soil organic nitrogen (SON), macro and micro elements, and biological components and a lower bulk density (BD) compared to conventionally managed soils [1,2]. A 21-year study in central Europe found that organic farming not only improved key soil fertility components, but also reduced the fertilizer, energy, and pesticide input by 34%, 53%, and 97%, respectively, when compared to conventional farming systems [3]. A long-term study of organically and conventionally managed farming practices in a Naff silt loam soil in Washington indicated that organically farmed soil had a significantly higher SOM by up to 1%, a higher cation exchange capacity (CEC) by up to 11%, a higher polysaccharide content by up to 13%, a higher total N by up to 11%, a higher exchangeable K by up to 71%, as well as a higher water content and aggregate structure compared to conventionally managed adjacent soil [4].
However, studies have also shown that, in general, the global crop yield from organically managed farms is 80–81% of the yield from conventionally grown crops, suggesting a 20% yield gap on average [5,6,7]. Lower organic yields have been attributed to challenges associated with (i) weed pressure, (ii) nutrient management without synthetic fertilizer input, (iii) systems’ inability to adapt to the transition [5,8,9], and (iv) climate change [10]. However, while the extensive use of agro-chemicals in conventional farming has increased crop yields and enhanced food security around the globe [11], advances in modern agricultural technology with a sole focus on crop yields has resulted in a decline, over the past 70 years, in the crop nutrient quality from the baseline values to which food have traditionally been compared [12]. The nutrient decline in crop produce has been attributed to several factors, such as crop variety, geographical location, ripeness, as well as the degradation of the soil in which crops are grown [13]. For example, the lower mineral nutrient concentrations in crops were suggested to be related to changes in cultivars selected for higher growth, as different cultivars extract, transport, and synthesize proteins, vitamins, and other nutrients differently [14]. While that study revealed that the lower mineral concentrations in varieties bred for higher yields with increased carbohydrate were not accompanied by proportional increases in minerals, implying a “dilution effect” [14], a selective dilution effect was also observed, suggesting that not all soil nutrients can be decreased at the same rate [15]. Conventional farming systems are also associated with declines in soil quality, declines in soil microbial diversity and abundance, decreases in water infiltration, increases in nitrogen leaching and ground water contamination, and the depletion of soil nutrients [16,17,18,19]. These dynamics call for side-by-side field trials to understand the relationships between soil quality and nutrient density in crop produce.
In addition to significantly improving soil quality, organic farming has also been shown to increase crop yield under weather-challenged conditions such as droughts when compared to conventional farming [20]. The greater yields in organic farming under drought conditions have been attributed to improved soil health indicators such as SOM and soil aggregate stability, leading to a greater water holding capacity [4,21,22]. In a summarized review collected from 88 research studies conducted in the tropics and sub-tropics, a 26% higher yield under organic compared to conventional management practices was attributed to soil fertility improvement as well as the better response of organic compared to conventional systems to input- or resource-poor conditions [23]. Additionally, organic farming improves the environmental impacts of agriculture when compared to traditional conventional farming [24,25,26]. A recent meta-analysis based on 107 studies and 360 observations published from 1977 to 2012 revealed that significant improvements in energy efficiency and reductions in greenhouse gas (GHG) emissions were associated with organic rather than conventional farming [26]. Thus, while there may be global evidence of crop yield gap in favor of conventional management practices, there is a strong case to be made for organic farming, as reported in a recent in-depth comparative system analysis [27]. There are also concerted research efforts to close or reduce the yield gap between the two systems [8,28].
Various studies have reported the impacts of organic farming practices on the nutritional quality of produce. In one study, organically grown tomatoes (Solanum lycopersicum) were more enriched with higher human health-promoting nutrients, phytochemical content, and antioxidant activity than conventionally grown tomatoes [29]. Similarly, several studies found that a range of organically grown produce such as orange (Citrus x sinensis), apple (Malus spp.), potato (Solanum tuberosum), tomato, and papaya (Carica papaya) had higher levels of vitamin C, phenolic compounds, total sugars, and flavonoids than those grown conventionally [30,31,32,33].
Although a number of studies have documented the quantity and quality of nutrients in organic produce, most of those analyses have been based on crop produce obtained from grocery stores or from separate organic and conventional farms [34,35]. A few studies have assessed the nutritional quality of vegetables and fruits in side by side comparisons of organic and conventional crop management practices, but the results from these studies have often been contradictory. For example, organically produced jujube fruit (Ziziphus spp.) had a significantly higher content of pigments, chlorophyll, carotenoid, sugars, organic acids, and total volatile compounds but a significantly lower size of fruit and lower protein and flavonoid contents than conventional jujubes in a 2-year study in Spain [36]. In another study, different cultivars of organically produced jambu (Acmella oleracea) fruit had higher contents of total phenolics and carotenoids than conventionally grown fruit [37]. However, the vitamin C in the fruit and the total organic N in the leaves and flowers were higher under the conventional than the organic system. Maggio et al. [38] reported that the marketable yields of cauliflower (Brassica oleracea L. var. botrytis) and zucchini (Cucurbita pepo L.) were significantly higher under the conventional than the organic system, and although the protein content was not significantly affected, the K content and hydrophilic antioxidant activities in the zucchini were significantly increased under the organic compared to the conventional system. A recent comprehensive review suggests that, due to inconsistencies in the vegetable nutritional quality under organic systems, it is difficult to make systematic general conclusions on the higher health-promoting value of organic vegetables compared to conventionally grown produce [39], and hence there is need for more field research on this aspect.
Arbuscular mycorrhizal fungi (AMF) are soilborne fungi that form mutualistic symbiotic associations with a majority of crop plants and benefit host plants through the enhanced mineral nutrient uptake of immobile soil nutrients such as P, improved water relations and disease resistance, as well as improving the soil food web and soil structure by external hyphae [40,41,42]. Several studies have shown that AMF can improve soil health and crop yield [43,44]. Agricultural operations can also affect AMF populations [45,46]. A recent meta-analysis conducted using 54 field studies found that low-intensity tillage increased AMF colonization by 30% [47]. Farmers can enhance their utilization of AMF symbiosis through the adoption of farm management practices that enhance the functioning of the AMF community indigenous to the soil [48]—for example, through the use of overwintering cover crops [49,50], reduced tillage [51], and diverse crop rotations [52]. Organic farming practices have been reported to enhance AMF colonization due to diverse crop rotations, the use of summer and winter cover crops, enhanced SOM, and the absence of synthetic chemical usage compared to conventionally managed fields [53]. A comprehensive study involving 32 farm fields in Korea revealed that AMF inoculum significantly improved red peppers (Capsicum annum L.) grown under organic rather than conventional farming practices, and the AMF abundance and diversity were higher under organic compared to conventional management practices [54]. Although agricultural benefits of AMF have been widely reported, we are not aware of studies that have evaluated the effects of side-by-side organic and conventional management practices on the concentration of phytochemicals (such as vitamins, antioxidants, and plant pigments) in vegetables over two-year period. The objective of this study was to evaluate the effects of different agricultural cropping systems and AMF inoculation on the yield and nutritional quality of vegetables, measured as vitamins, antioxidants, and other phytochemicals such as fruit pigmentation under side-by-side organic and conventional management systems over two successive growing seasons. The parameters evaluated included disease incidence, total antioxidants, vitamin C, lycopene, alpha and beta carotenoids, mineral composition, vegetable quality assessment, and yield.
2. Materials and Methods
2.1. Study Site and Weather
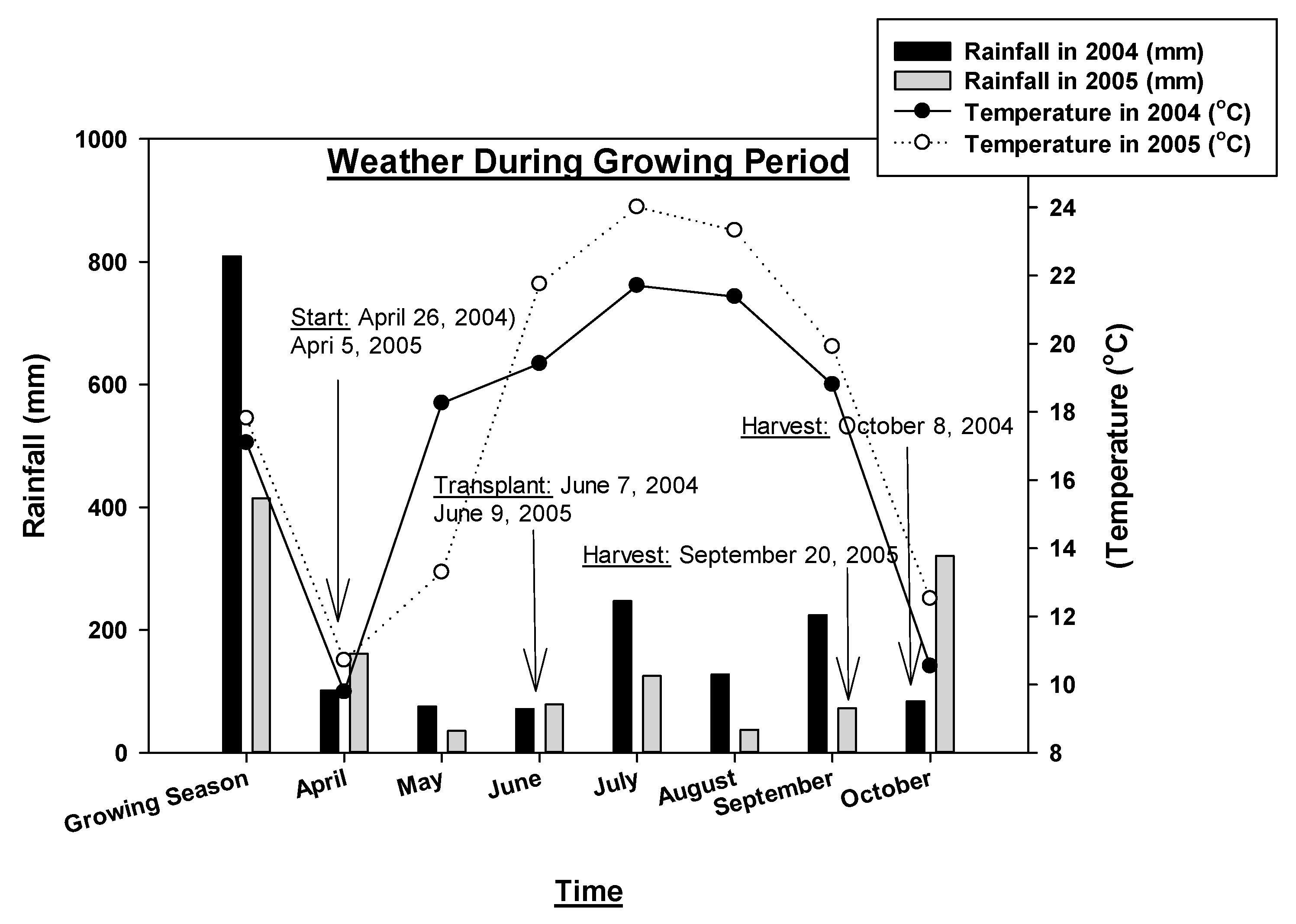
Between 2004 and 2005, a two-year experiment was superimposed within the Farming Systems Trial (FST) at Rodale Institute Experimental Farm, Kutztown, Pennsylvania, to evaluate the nutritional differences in select vegetables between the organic and conventional crop management systems. The goal of the project was to assess the long-term (24 years) organic and conventional management systems’ effects on the nutrient quality of those vegetables. The FST was established in 1981 at Rodale Institute in Berks County, south-eastern Pennsylvania (40°37′97′′ N and 40°75′98′′ W), and is the longest running side-by-side comparison of organic and conventional grain cropping systems in North America. Nested within a 135 ha research farm that has been managed organically since 1975, the FST field occupies an area of 6.1 ha and had moderately well-drained Comly silt loam soil with a neutral average pH of 6.8. The weather during the experimental period was variable, and the temperature and precipitation data are presented in Figure 1 (monthly summary), and Supplementary Figure S1 (daily data). The rainfall during the growing season of 2004 was 95% higher than in 2005. This was a 100-year high summer rainfall (Figure 1, Supplementary Figure S1).

Figure 1.
Total growing season, monthly rainfall, and temperature in 2004 and 2005.
2.2. Experimental Design
The experimental design was a split-plot randomized complete block with eight replications. The main plots consisted of one conventional (CNV) and one organic (MNR) system. The main plots measured 18 × 92 m and were separated by 1.5 m grass strips to minimize the transport of soil, fertilizers, and pesticides among the cropping system plots. Three 6 × 92 m subplot treatments (crop rotation sequence entry points) were located within each main plot. Entry point subplots ensured that multiple crops from the rotations were present each year. The management practices conducted were similar to those described in Ryan et al. [55] and are summarized in Table 1. The CNV system utilized synthetic fertilizers and herbicides according to the Pennsylvania State University (Penn State) Cooperative Extensive Service recommendations.

Table 1.
Management cropping systems differences between organic manure (MNR) and conventional (CNV) systems in the Farming Systems Trial.
The organic manure system (MNR) used composted manure applied at 40 Mg ha−1 (wet weight) for grain corn and 60 Mg ha−1 (wet weight) for silage corn. The characteristics of the compost used are listed in Supplementary Table S1. Cultural and mechanical weed management strategies were utilized in the MNR system. These included delayed planting, cover crops, and the crop rotation of summer-grown row crops with winter cover crops. Depending on the weed density and ability to cultivate, as determined by the soil moisture and crop height, mechanical weed management included one to two passes with a rotary hoe or spring tine cultivator prior to crop emergence, one to two passes with a rotary hoe or spring tine cultivator after crop emergence, and one to three passes with an inter-row cultivator. Additional field site and experiment details can be found in Liebhardt et al. [56]; Lotter et al. [57]; Pimentel, Hepperly, Hanson, Douds, and Seidel [20]; and Ryan, Smith, Mortensen, Teasdale, Curran, and Seidel [55].
2.2.1. Establishment of Vegetables
Vegetable strip plots measuring 6.1 × 6.1 m were established in one of the three entry points in organic manure (MNR) and CNV main plots that had soybean (Glycine max) in 2004 and 2005. Tomatoes, peppers (Capsicum annuum “Jalapeño”), and carrots (Daucus carota) were planted in those strip plots in three out of the eight replications (Rep) (Rep 1, 3, and 4 in 2004; Rep 3, 5, and 8 in 2005). Soybeans were planted with 76.2 cm row spacing using a Monosem precision vacuum planter (Monosem Inc./North America) in the sub-plot entry points before establishing the strip plots. Soybeans in the strip plots were then uprooted after germination. One-month-old tomato and pepper seedling plugs, previously established in the greenhouse, were transplanted into previous soybean rows at a 76.2 cm row spacing (to facilitate cultivation) and 45.7 cm apart within the row (intra-row spacing), as recommended by the Campbell Soup Company production practices. Carrots were directly seeded in the fields. The Campbell Soup Company had previously provided the Rodale Institute with seeds of the “Malinta” cultivar tomato, “SOC1374” commercial carrot, and “PX109” sweet jalapeno pepper in 2004, and “CXD253” tomato, “SDC1374” carrot, and “PX109” pepper in 2005. The Malinta variety of tomato was replaced by CXD253 in 2005 as a disease-resistant variety due to crop failure in 2004. Tomatoes and peppers were started in the greenhouse in the month of April in each year of the study (on 26 April 2004 and 5 April 2005, Figure 1).
2.2.2. Mycorrhizal Culture and Inoculation
Mycorrhizal inoculum was cultivated on-farm the season before each use, as previously described (Douds et al. 2010). Briefly, individual AMF species: Glomus mosseae (Nicol. and Gerd.), Gerdemann and Trappe; Glomus claroideum, Schenck and Smith; Glomus geosporum (Nicol. and Gerd.), Walker; Glomus etunicatum, Becker and Gerdemann; and Glomus intraradices, Schenck and Smith (DAOM 181602) were inoculated onto bahiagrass (Paspalum notatum Flugge) seedlings and grown for 3 months in a greenhouse prior to out planting in 28-liter grow bags filled with a 1:4 mixture of compost:vermiculite. The bags were watered and weeded as needed to support the plants, then overwintered outdoors to winterkill the bahiagrass. The media containing AMF spores were then combined and homogenized prior to use in the seedling germination pots for tomatoes and peppers or applied to the furrow in the case of directly seeded carrots.
Mycorrhizae inoculum was placed directly with the seeds in half of the pots. In the 20-foot-wide plot, 16 tomato plants (four rows by four plants) with mycorrhizae and 16 plants without mycorrhizae were planted in each of the three reps (3 × 32 = 96 plants per system). Data were collected from the four center plants of each section. At least 192 tomato plants (96 × 2 systems) and 192 pepper plants were initially planned to be planted in the greenhouse. Peppers were set up the same way as tomatoes. Carrots were directly seeded in 4 rows with mycorrhizae and 4 rows without mycorrhizae. A total of 216 pots of both tomatoes and peppers (with two seeds per pot) were planted. In each system, the transplanted strip plots were split into plants that were inoculated with mycorrhizae fungi (Myc) and plants without mycorrhizae (Non-Myc). Carrots were directly seeded in the month of June (on 7 June 2004 and 9 June 2005, respectively) and later thinned to 6 plants per 30.5 cm of row, with 4 rows with mycorrhizae and 4 rows without mycorrhizae (Supplementary Figure S2).
2.2.3. Vegetable Management, Harvesting, and Sample Collection
Plants in the conventional system received mineral fertilizer and herbicide spray regiment based on the Penn State Extension recommendations. Plants in the organic system received dairy manure-leaf compost at planting time and several applications of compost tea during plant development as supplementary fertilizer. Weeds were controlled with black plastic in both the organic and conventional plots. Any additional weeds were removed by hand hoeing and hand weeding in both the organic and conventional systems, as there are few selective herbicide options for conventional vegetables and the preplant and pre-emergent herbicides recommended for vegetables have a short residual activity. Watering took place as necessary by hand as well.
Sound, undamaged fresh fruit and carrot roots were harvested and shipped fresh to Campbell Soup Company, R&D, Davis, California. Upon arrival, the samples were prepared for analysis, analyzed, and the reference samples stored. Tomato fruits were harvested from the center of the plants when 90% of the fruit was red. Peppers were harvested twice—once “early” when all the fruit was green and a second time for red-only fruits. Vegetables were harvested by 180 days to maturity either in September or October of the studied years. Carrots were harvested as late as October. The center two rows of plots were harvested for yield determination and sampled for quality tests (Supplementary Figure S2). Note that the tomato yield and, consequently, the nutritional quality were not monitored in the initial year of 2004 due to crop failure. As protocol, any fruit that was either too small, not firm any more, or in any other way diseased was rejected. Green pepper rejects were usually too small, red pepper rejects were too soft or had blossom end rot. Various diseases affecting the studied vegetables in 2004 and 2005 are listed in Supplementary Table S2. Marketable yields were determined by separating the harvested vegetables based on the visual appearances of the disease-impacted produce along with the size of the vegetables. The harvested vegetables that were not counted as “marketable” were canker-sore carrots; virus-affected peppers; and any small or undeveloped, too small, too soft, misshapen, split/scarred, damaged, diseased, or rotten vegetables.
2.2.4. Determination of Phytochemicals and Vegetable Nutrient Quality
For the vegetable nutritional quality, the vitamin C and total antioxidants were determined for all vegetables: tomatoes, peppers, and carrots. In addition to these, α- and β-carotenes were also determined in carrots, and lycopene determined in tomatoes. Further, peppers were sub-divided into two groups–green and red for most analyses. For the determination of the ascorbic and total antioxidant, the Campbell Soup, Food Analytical Laboratory in Camden, New Jersey, was used.
Water-soluble and fat-soluble antioxidants from vegetables were extracted using a method described by Roberts and Gordon, [35]. Specifically, 100–200 g of chopped up vegetables was homogenized under argon with an industrial blender. A 5% metaphosphoric acid (MPA): methanol mixture was immediately added to the homogenate in a flask, flushed with argon for 20 s, and sealed. This was then shaken for ten minutes on a mechanical shaker, after which the extract solution was filtered under vacuum with a Whatman no. 1 filter paper. The residue was washed with 10 mL of MPA-methanol mixture, after which an evaporator was used to remove the methanol from the filtrate in a water bath set at 40 °C. Pulp and filter paper from the original extraction were then extracted using 10 mL of a 50:50 solution of water:methanol. Water-soluble extract was then obtained by mixing the two aqueous fractions obtained after the evaporation of methanol and made up to 75 mL with water. To obtain a lipid-soluble extract, the remaining residue and filter paper were mixed with 15 g of anhydrous sodium sulfate, extracted twice with 50 mL of acetone, and then the acetone extracts were combined and evaporated to dryness on an evaporator in a water bath at 40°C. Re-dissolving the dry solid in 75 mL of methanol produced the lipid-soluble extract.
After filtering the water-soluble and lipid-soluble extract through a Whatman 0.1 µm polycarbonate cyclopore membrane, High Performance Liquid Chromatography (HPLC) was used to assess the antioxidant activity of the extract using the liposome peroxidation assay [35].
The HPLC analysis was also used to determine the ascorbic acid in plant extracts diluted with 1:1 mobile phase (ammonium dihydrogen phosphate buffer containing 0.15% (w/v) MPA with detection at 245 nm), and 20 µL of the solution injected onto a 5 µm Nucleosil (250 mm × 4.6 mm I.D) C18 analytical column protected by a C18 guard column (Hichrom, Reading, UK) [35].
Liposomes were prepared by an extrusion method described by Hope et al. (1985) and extracted using a method described in detail by Roberts and Gordon [35].
The mineral nutrient contents in the dried produce samples were determined by atomic spectral analysis at Pennsylvania State Agricultural Analytical Services Laboratory using ICP and presented in Supplementary Table S2.
2.3. Statistical Analysis
All values in the tables or figures are presented as means and standard deviations. A 3-way analysis of variance (ANOVA) was run to analyze response variables. Three independent variables, systems (organic manure and conventional), mycorrhizal treatment [AMF (mycorrhizae or no-mycorrhizae)], time (years), and their interactions were tested. Significant differences between the classification variables were analyzed using Tukey’s adjusted multiple comparison procedure by the Tukey–Kramer grouping in PROC GLIMMIX in SAS version 9.4 [58]. Treatment differences were considered significant at p < 0.05. The figures were plotted using Sigmaplot version 14.0 [59]. The systems and AMF are abbreviated in the figures and tables as the following: organic manure (MNR), conventional (CNV), mycorrhizae (Myc), and without mycorrhizae (Non-Myc).
There is no universal agreement whether the post-hoc mean separation should be reported as valid in cases where the overall ANOVA was not statistically significant. However, while ANOVA compares the entire (nested) model, Tukey–Kramer grouping compares individual levels, thereby responding to two different questions. In other words, while ANOVA gives a more general pattern of the overall data, especially when several levels (three levels in the present study) are present in the model, Tukey’s multiple comparison test is considered to be much stronger than ANOVA to find differences in means between the individual groups.
3. Results
3.1. Crop Diseases
Record-breaking summer rainfall in 2004 (Figure 1, Supplementary Figure S1) resulted in a high disease incidence observed in all vegetables (Supplementary Table S3). On tomatoes, late blight (Phytophthora infestans) was particularly damaging, eliminating the marketable yield and antioxidant analysis, thus rendering tomatoes in 2004 a crop failure. On carrots and peppers, however, a good differentiation of antioxidants and vitamins was achieved between treatment combinations, with or without AMF inoculation in either organically managed or conventionally managed soil. Disease was also a major factor in carrots, with root canker being a key quality issue.
Other diseases were green pepper virus complex in 2004 and alternaria leaf blight (Alternaria dauci) of carrot in 2004 and 2005 (Supplementary Table S3). In general, plots under organic management showed a lower disease incidence and severity than those under conventional management. In 2005, 25% of the carrots in the conventional system were cankered compared to 5% to 7% in organic plots. In 2004, no virus was observed in green jalapeno peppers grown in the organic system, while 67% of conventionally grown green peppers had visible symptoms of pepper virus complex (Supplementary Table S3). Mycorrhizal inoculation had a less observable impact on these diseases, as no statistically significant differences were noted between inoculated and non-inoculated plants within each system (data not shown). There was a tendency for a non-significant increase in diseases in mycorrhizal-inoculated vegetables in both systems.
3.2. Analysis of Variance
Analysis of variance for this study did not reveal a statistically significant three-way interaction between year, cropping systems, and AMF for any of the vegetable nutrients (Table 2A–C). There was also no statistically significant cropping system by AMF interaction for any of the nutrients. However, year had variously statistically significant two-way interaction effects with cropping systems or AMF for antioxidants, vitamin C, and lycopene in several of the vegetables. Data were therefore analyzed separately by year and AMF or year and cropping system where the interaction effects were statistically significant.

Table 2.
Analyses of variance (ANOVA) to test the effects of management, Arbuscular mycorrhiza fungal (AMF) treatment, and years on the produce yield (A) and vegetable nutrient concentrations ((B) and (C)); underlined values are statistically significant at the 0.05 level, and italic ones are significant at the 0.1 level.
3.2.1. Mycorrhizal Treatment Effects
1. Vitamin C
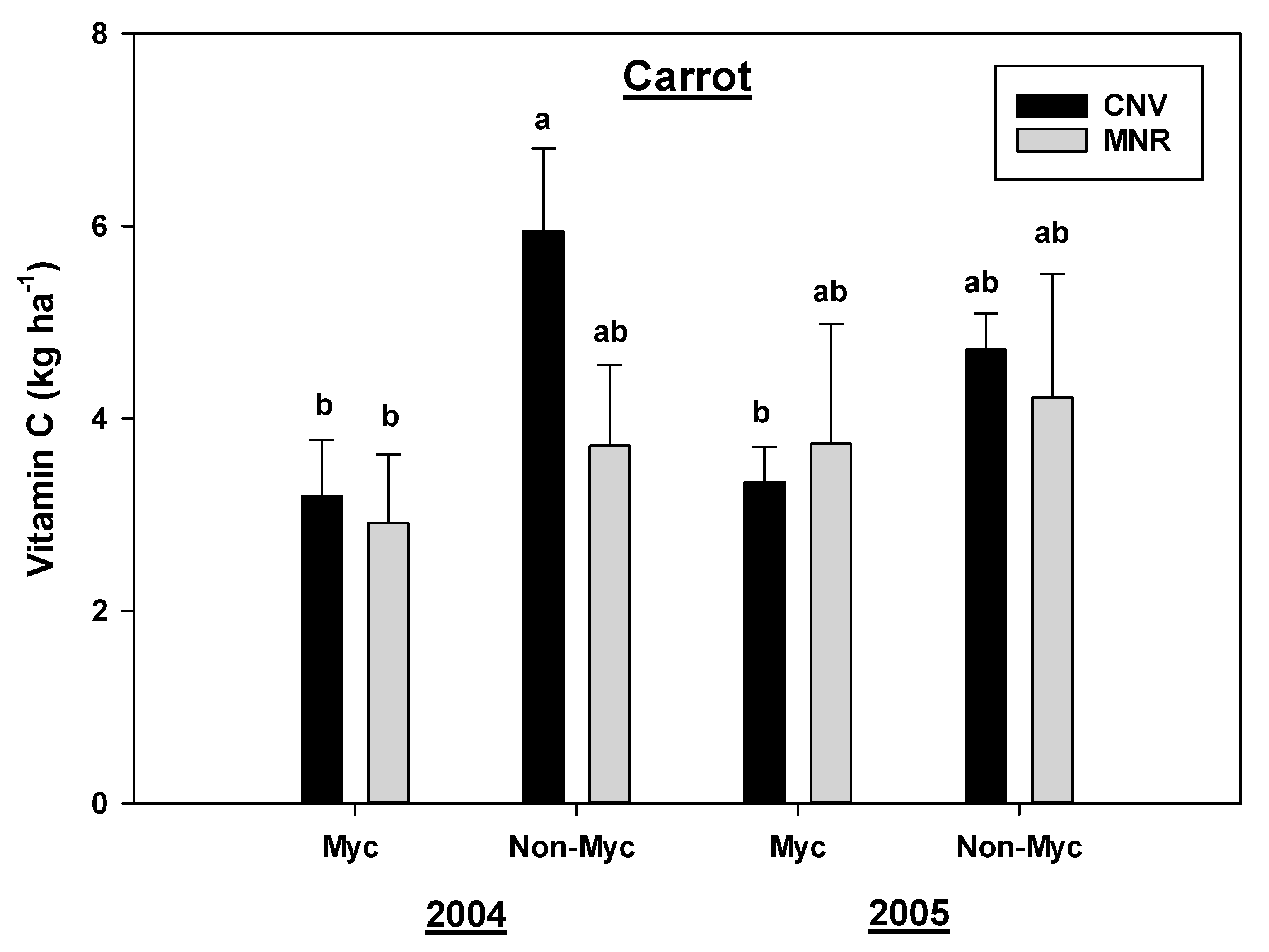
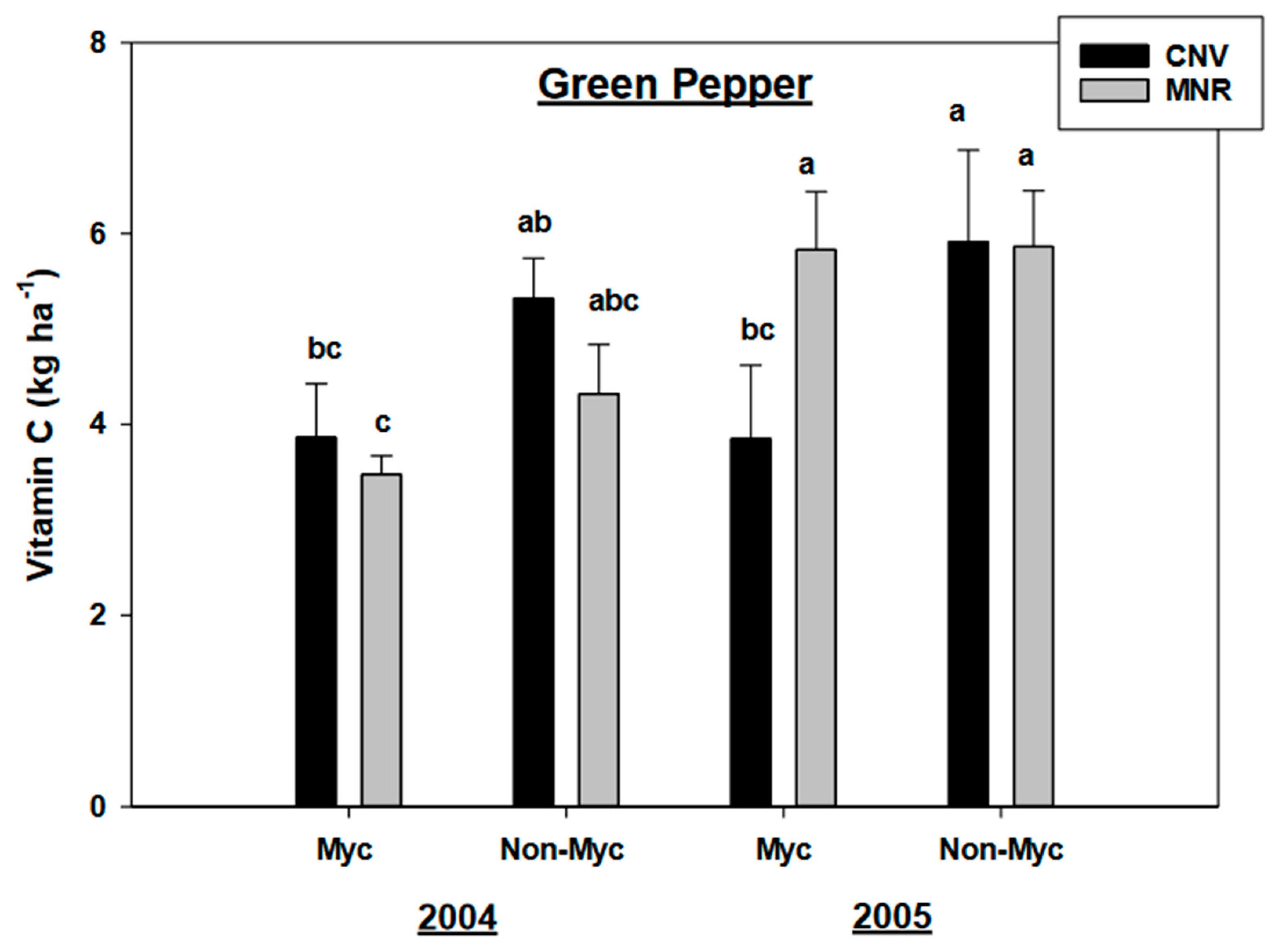
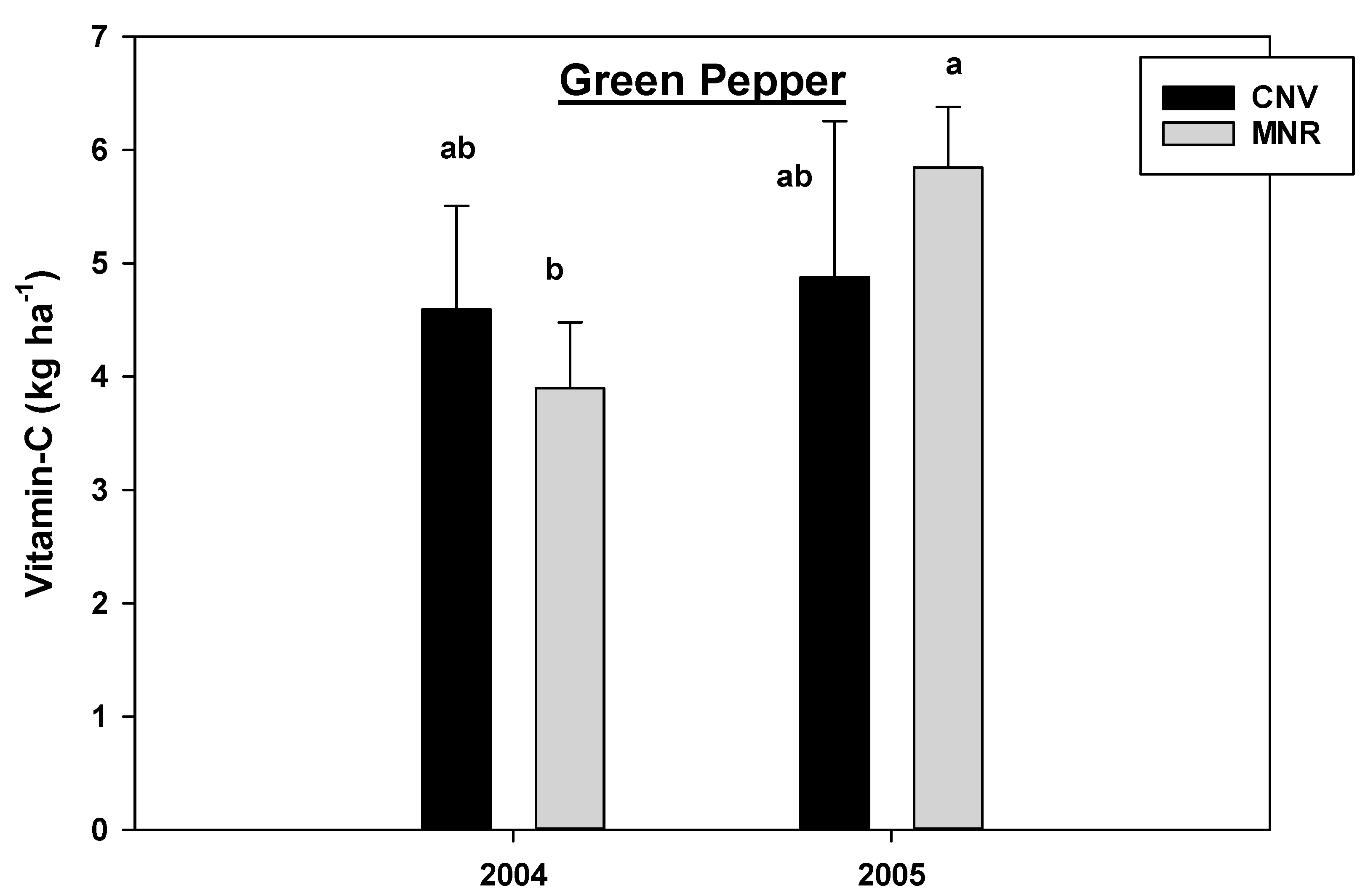
Analysis of variance for this study found, respectively, a marginally significant and statistically significant year by mycorrhizal fungal inoculation interaction for vitamin C in carrots and green peppers (p = 0.0560 and 0.0181, respectively, Table 2); hence, data were presented separately by these variables (Figure 2 and Figure 3). Mycorrhizal treatment significantly reduced the vitamin C content in carrots grown in both systems in 2004, but the reduction in CNV was 87% compared to 28% in MNR (Figure 2). There was a similar reduction in 2005, but the change was not statistically significant. However, there was no statistical difference in the vitamin C content between the two systems in 2004 and 2005 (Figure 2). Similarly, mycorrhizal treatment reduced the vitamin C content in green peppers in both systems in each of the two years, but the decline was only statistically significant in the CNV system in 2005 (Figure 3). Vitamin C was also significantly greater in AMF-treated green peppers grown in the MNR system in 2005. There was no statistical difference in the vitamin C content of treated and non-treated green peppers between CNV and MNR in 2004 or non-treated green peppers in 2005 (Figure 3).

Figure 2.
Effects of management and AMF on vitamin C in carrot over a two-year period. Means on a bar followed by the same letter are not statistically different (alpha 0.05). Key: Myc (mycorrhizal treatment), MNR (organic), CNV (conventional).

Figure 3.
Effects of management and AMF on vitamin C in green pepper over a two-year period. Means on a bar followed by the same letter are not statistically different (alpha 0.05). Key: Myc (mycorrhizal treatment), MNR (organic), CNV (conventional).
2. Antioxidants
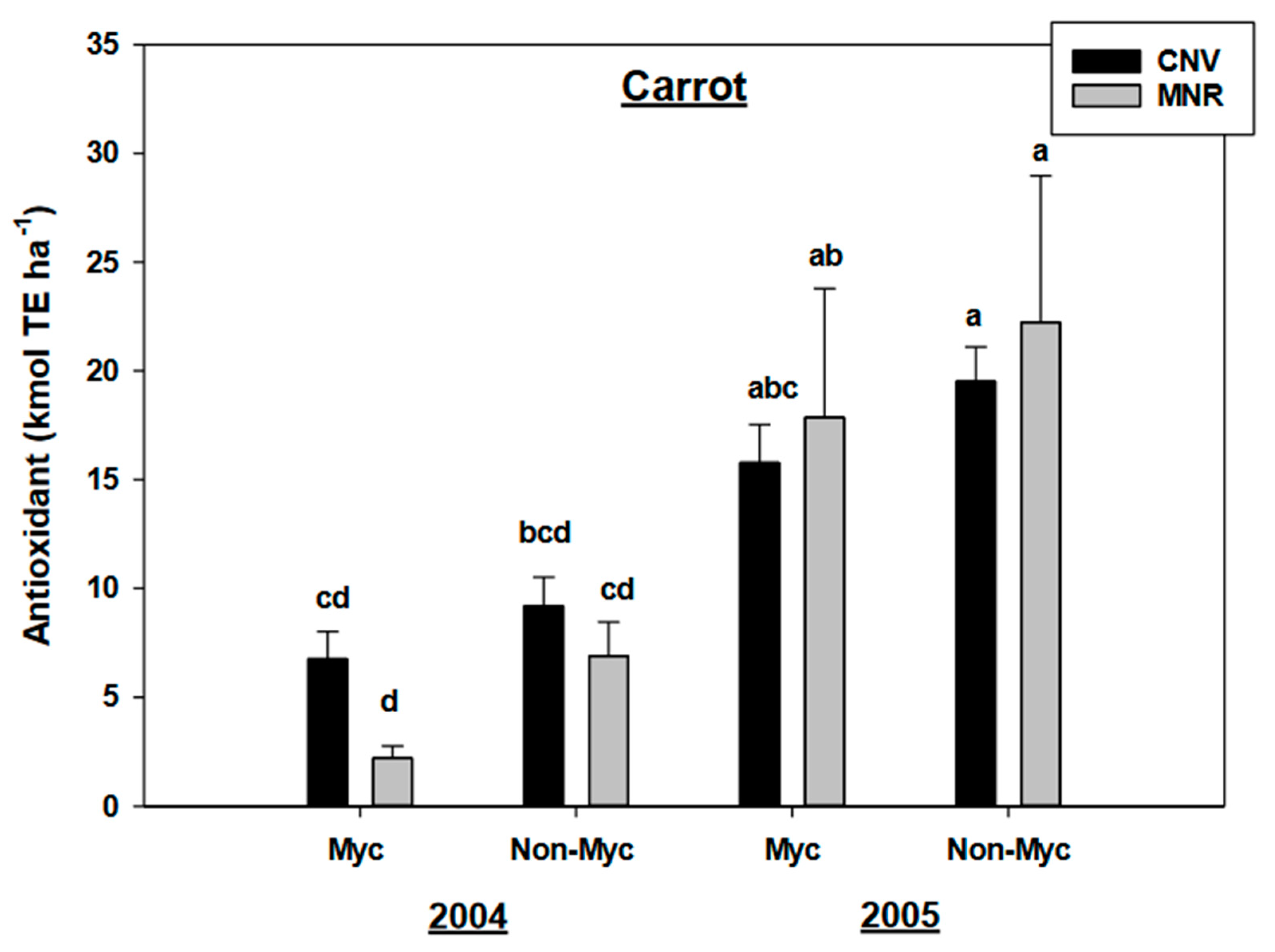
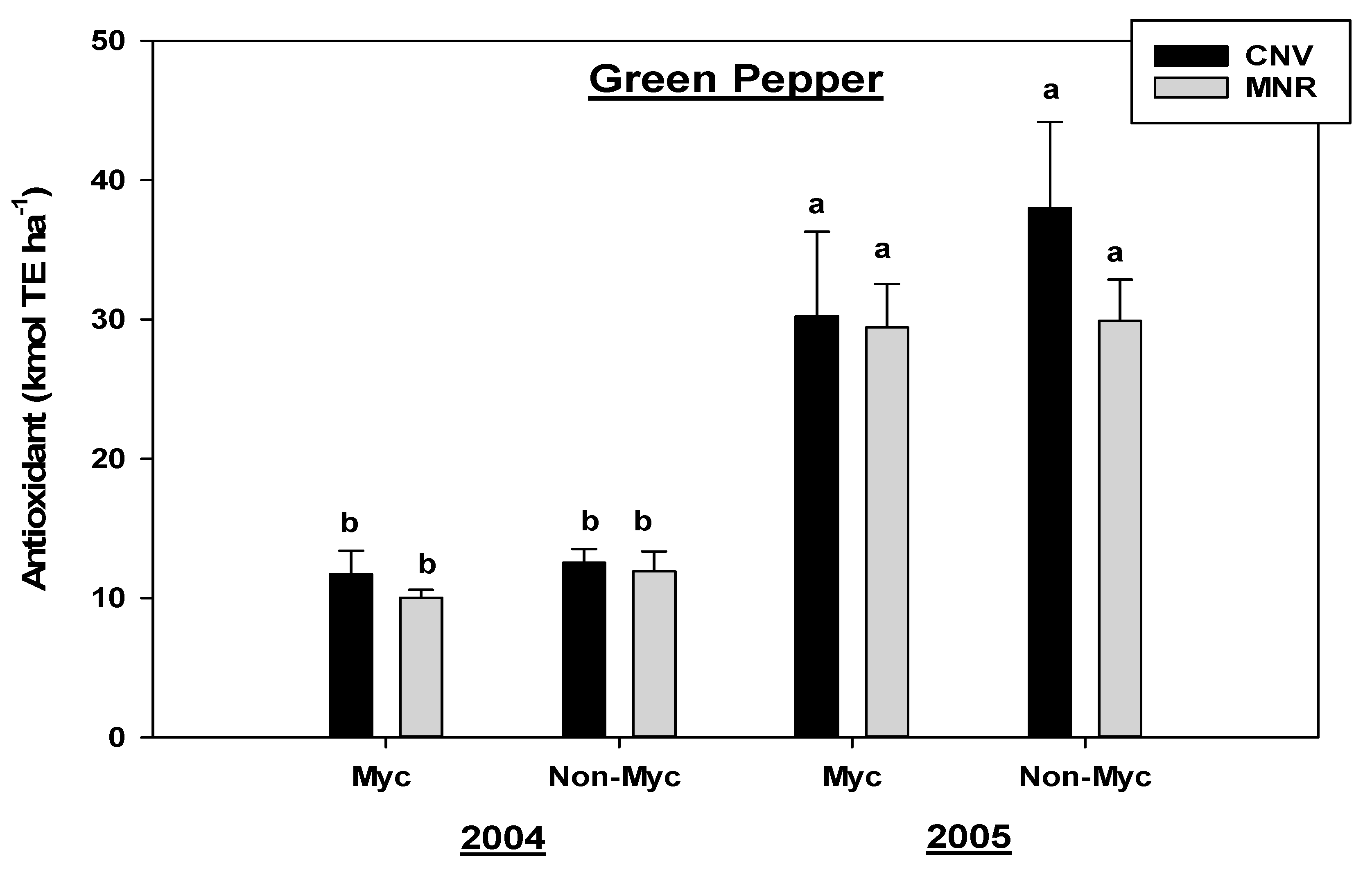
There was no statistically significant year by mycorrhizal treatment interaction for antioxidants in all the vegetables. However, the mycorrhizal treatment had a statistically significant effect (p = 0.0144) and marginally significant effect (p = 0.0767) on antioxidants in carrots and green peppers, respectively (Table 2B). Time (years of study) had a highly significant effect (p < 0.0001) on both vegetables (Table 2). Mycorrhizal treatment reduced the antioxidants in carrots in both CNV and MNR in both 2004 and 2005, even though the reduction was not statistically significant, but surprisingly there was a 68% reduction in the antioxidant concentration in MNR compared to only 26% in CNV in 2004 (Figure 4). However, both the treated and non-treated carrots significantly increased their antioxidant level from 2004 to 2005, except for the treated carrots in the CNV system (Figure 4).

Figure 4.
Effects of management and AMF on the antioxidants in carrot over a two-year period. The Trolox equivalent antioxidant capacity assay was used to measure the antioxidant capacity in the vegetable compared to the standard. Means on a bar followed by the same letter are not statistically different (alpha 0.05). Key: Myc (mycorrhizal treatment), MNR (organic), CNV (conventional).
Similar results as above were observed for antioxidants in green peppers. There was no statistical difference in antioxidant concentration in both the AMF-treated and untreated green peppers between the CNV and MNR systems within each year (Figure 5). However, there was a statistically significant increase in the antioxidants in green peppers under both CNV and MNR between 2004 and 2005 (Figure 5).

Figure 5.
Effects of management and AMF on the antioxidants in green pepper over a two-year period. Means on a bar followed by the same letter are not statistically different (alpha 0.05). Key: Myc (mycorrhizal treatment), MNR (organic), CNV (conventional).
3. Pigments
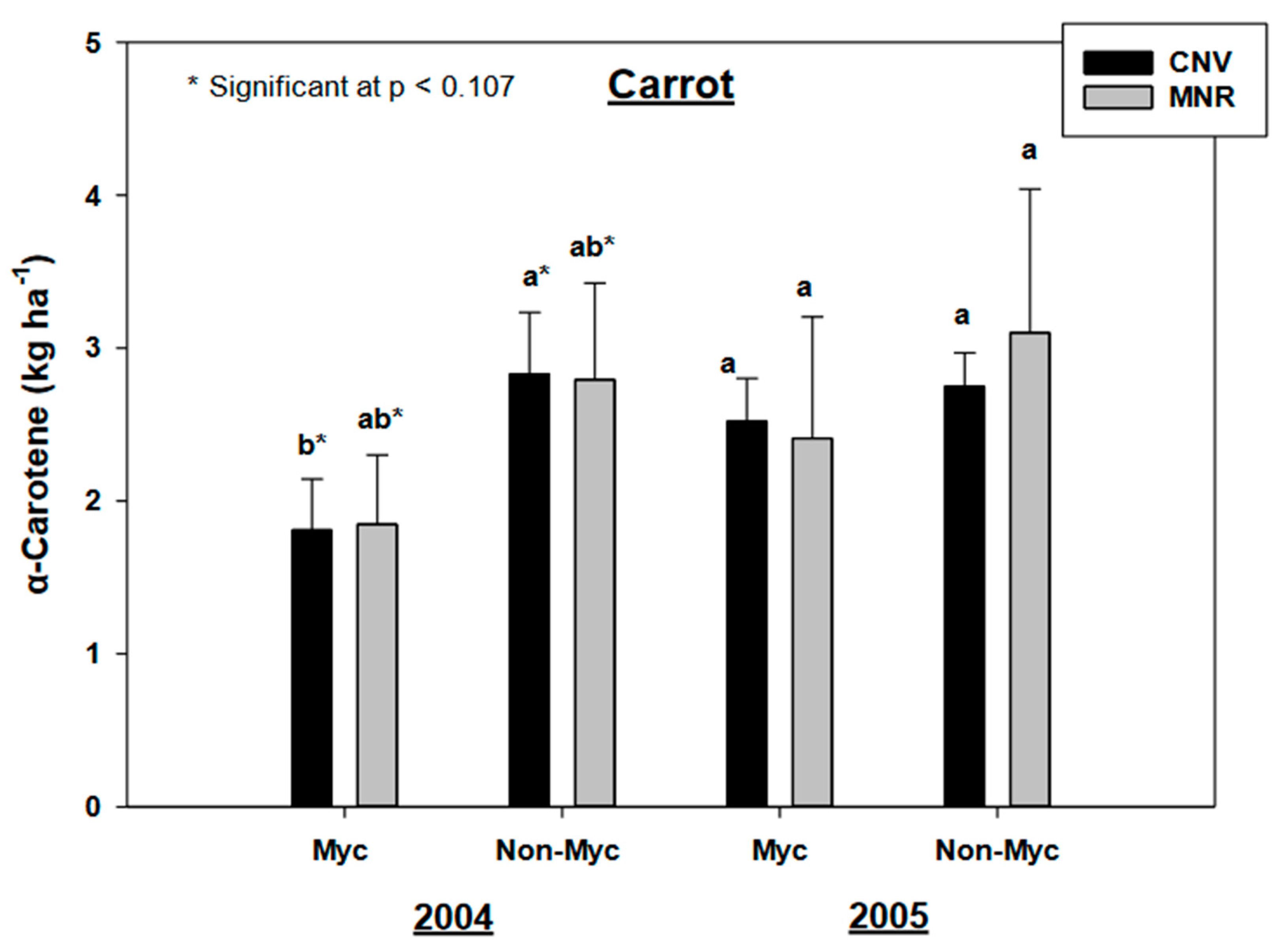
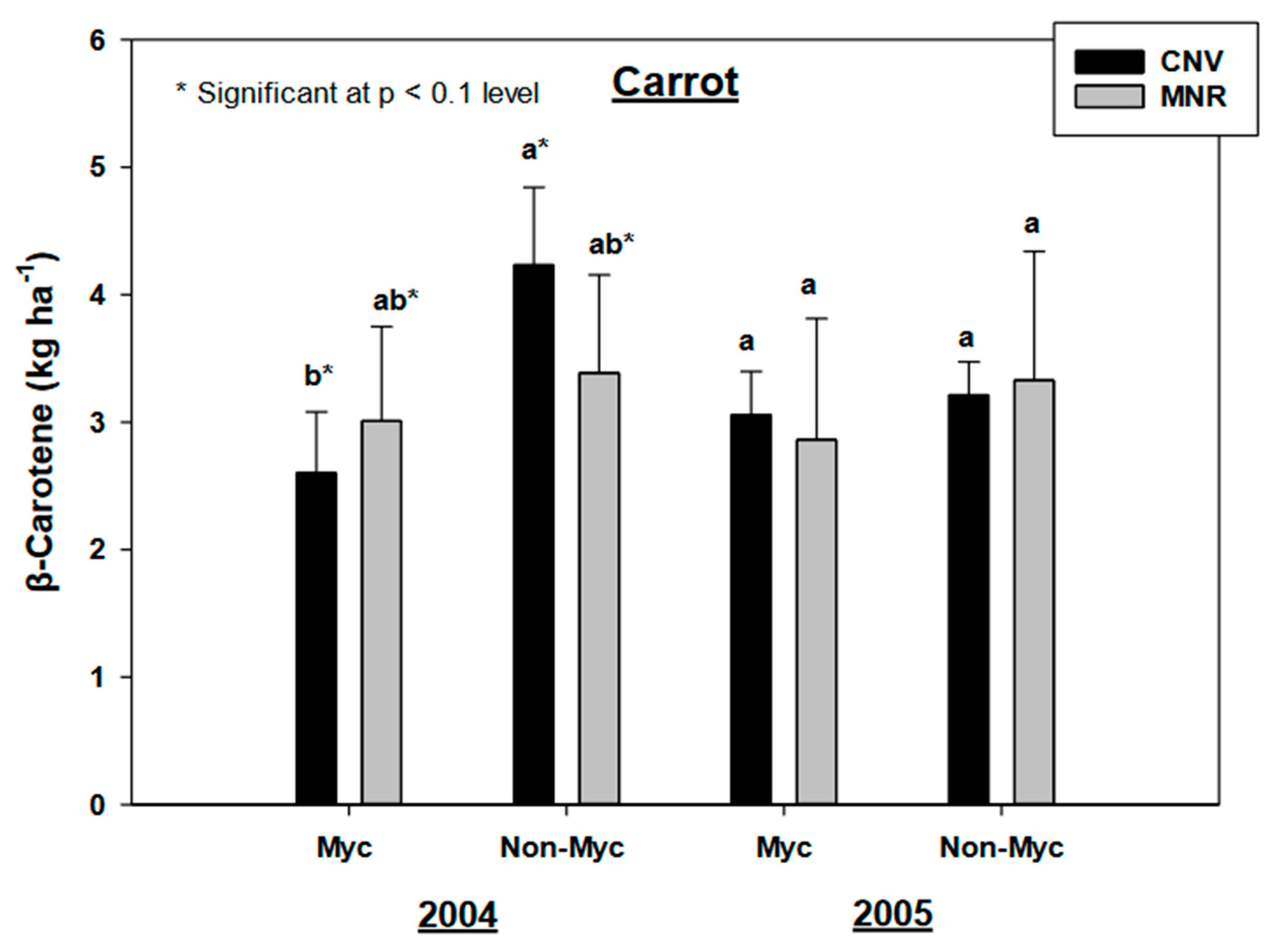
Our results found no statistically significant year by AMF inoculation effects for alpha- and beta carotenes in carrots (C in Table 2). However, AMF inoculation had a statistically significant effect on the alpha (p = 0.0062) and beta carotenes (p = 0.0337) in carrots, but as both year and system had no statistically significant impacts on the pigments many of the observed trends were non-significant at p < 0.05. Mycorrhizal treatment marginally decreased both the alpha- and beta carotenes in carrots under MNR and CNV systems in 2004, but the decline in beta carotenes was marginally significant only in CNV-managed carrots in both years (p < 0.107 and p < 0.1, respectively) (Figure 6 and Figure 7). A pair-wise analysis by year did not reveal any significance between treatments under management practices over the two-year period.

Figure 6.
Effects of management and AMF on the α-carotene in carrot over a two-year period. Means on a bar followed by the same letter are not statistically different (alpha 0.05). The asterisks define marginally significant (p = 0.1) of mycorrhizal treatment reduction in beta carotene in CNV managed carrots compared to MNR. Key: Myc (mycorrhizal treatment), MNR (organic), CNV (conventional).

Figure 7.
Effects of management and AMF on the β-carotene in carrot over a two-year period. Means on a bar followed by the same letter are not statistically different (alpha 0.05). Key: Myc (mycorrhizal treatment), MNR (organic), CNV (conventional).
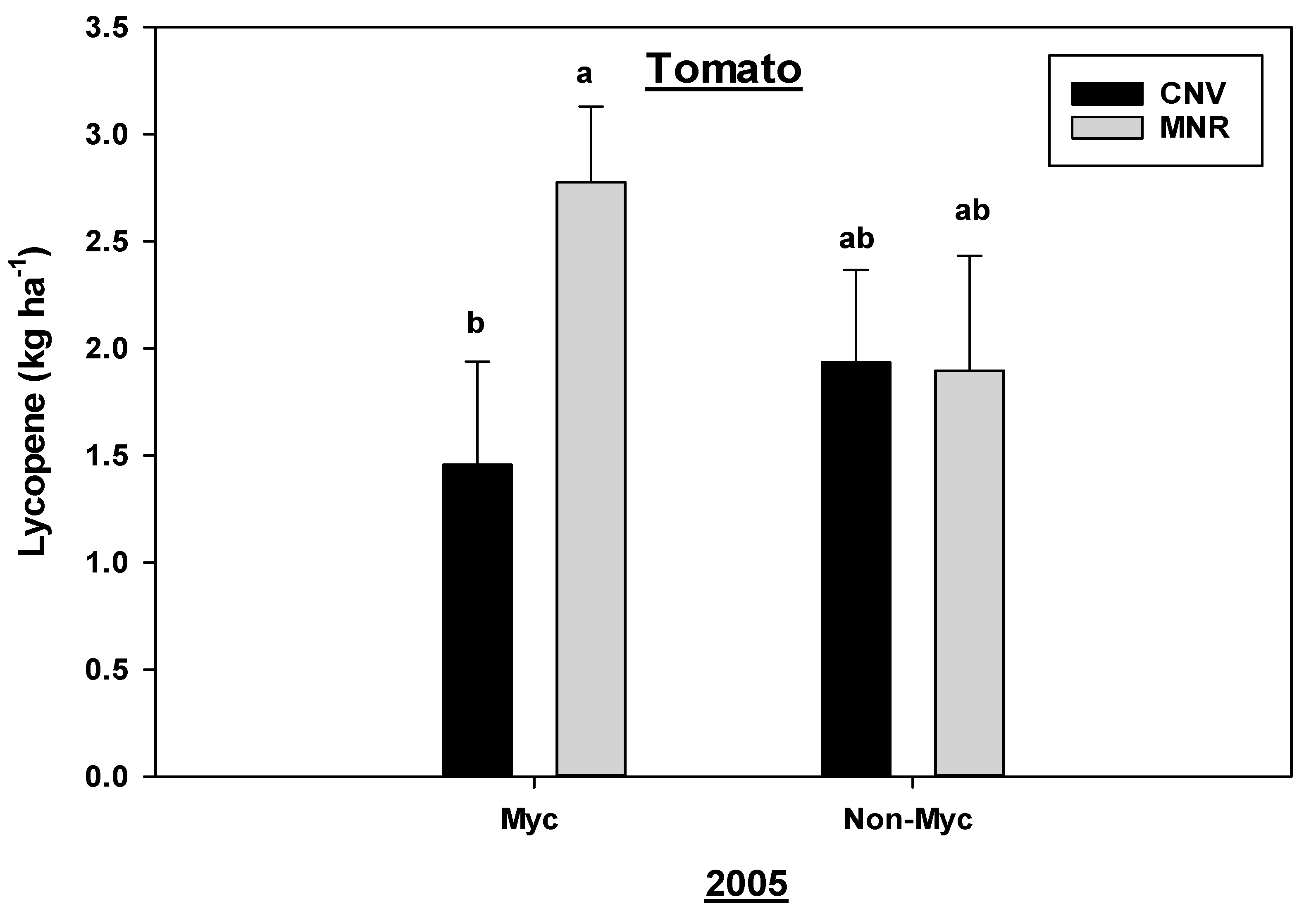
Cropping systems had no statistically significant effects on lycopene in tomatoes. While there was a statistically significant (p = 0.0408) year by mycorrhizal treatment interaction for lycopene in tomatoes, only data for 2005 are reported due to a crop failure of tomatoes in 2004. Mycorrhizal treatment did not have a statistically significant effect on the lycopene concentration in tomatoes under both systems (Figure 8). However, treated tomatoes under the MNR system had a significantly greater lycopene concentration than under the CNV system. Even though our results indicate that mycorrhizal treatment tended to reduce the lycopene in tomatoes under CNV systems, the effect of year could not be accounted for due to crop failure in 2004, and hence these results should be interpreted carefully.

Figure 8.
Effects of management and AMF on the lycopene in tomato in 2005. Means on a bar followed by the same letter are not statistically different (alpha 0.05). Key: Myc (mycorrhizal treatment), MNR (organic), CNV (conventional).
4. Yields
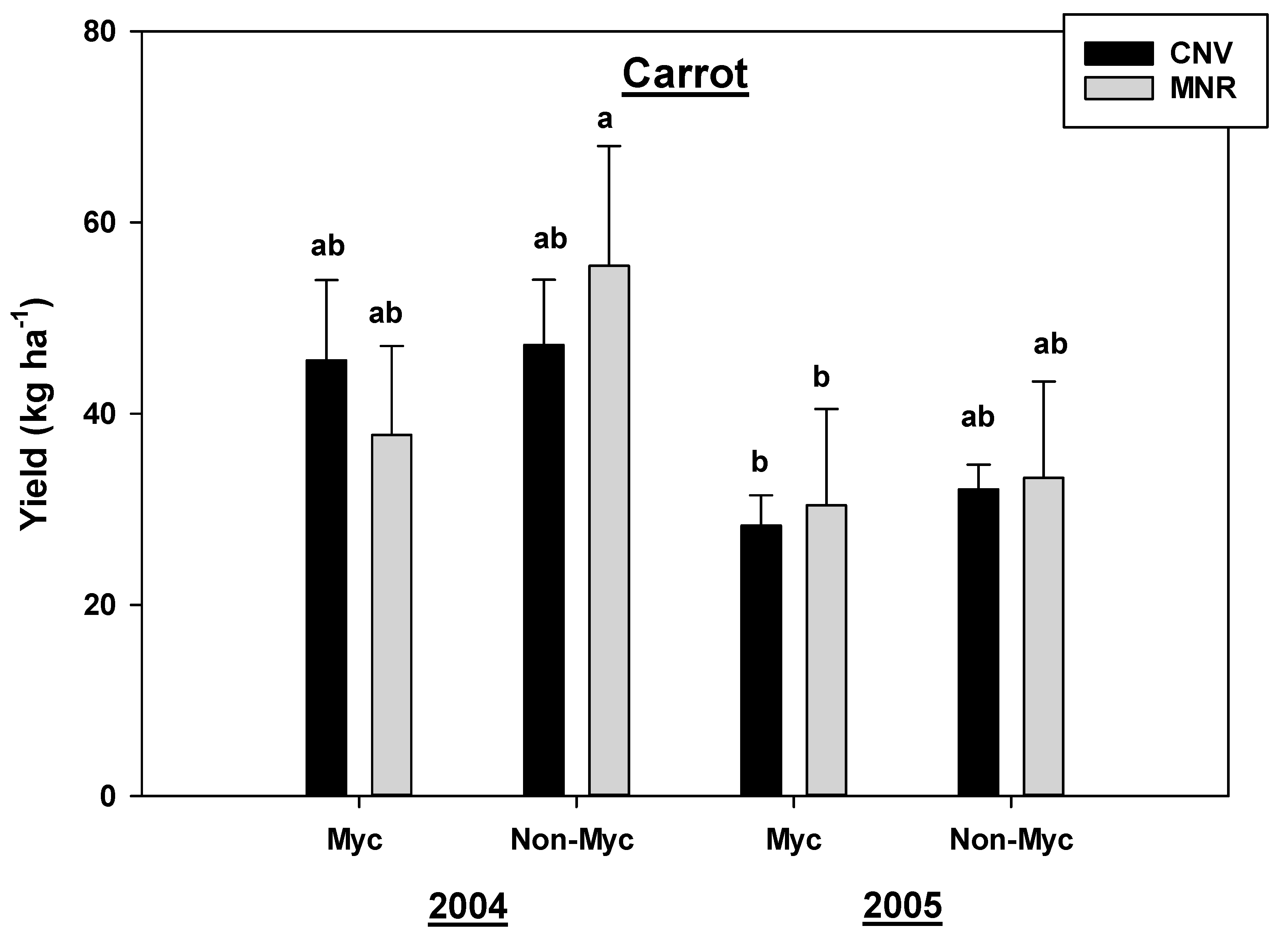
There was a statistically significant year by cropping systems by AMF treatment effect for the total yield of carrots (p = 0.0441; Table 1A), hence the data were analyzed separately by those three independent variables. As observed in the nutrients described above, mycorrhizal treatment marginally reduced the total carrot yields in 2004. Although the yields in both the AMF-treated and non-treated carrots significantly declined in 2005 compared to 2004, there was no statistical total yield difference between the CNV and MNR in both years (Figure 9).

Figure 9.
Effects of management and AMF on the yield in carrot over a two-year period. Means on a bar followed by the same letter are not statistically different (alpha 0.05). Key: Myc (mycorrhizal treatment), MNR (organic), CNV (conventional).
While there was no statistically significant year by cropping systems by AMF treatment interaction for the total yield in pepper, there was a marginally significant cropping systems effect (p = 0.0641) (Table 2A). However, there was no statistical yield difference between CNV and MNR in peppers in both years.
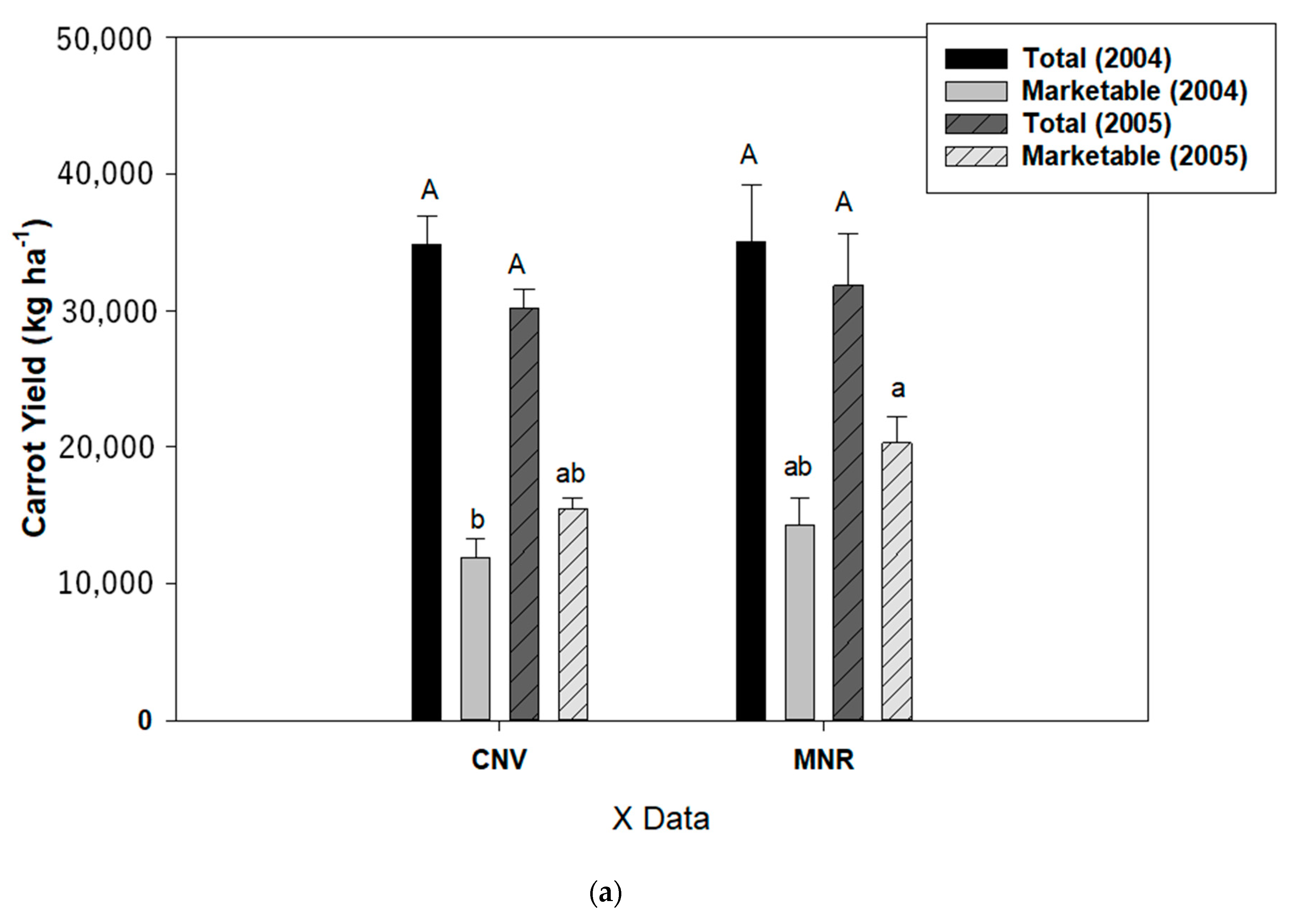
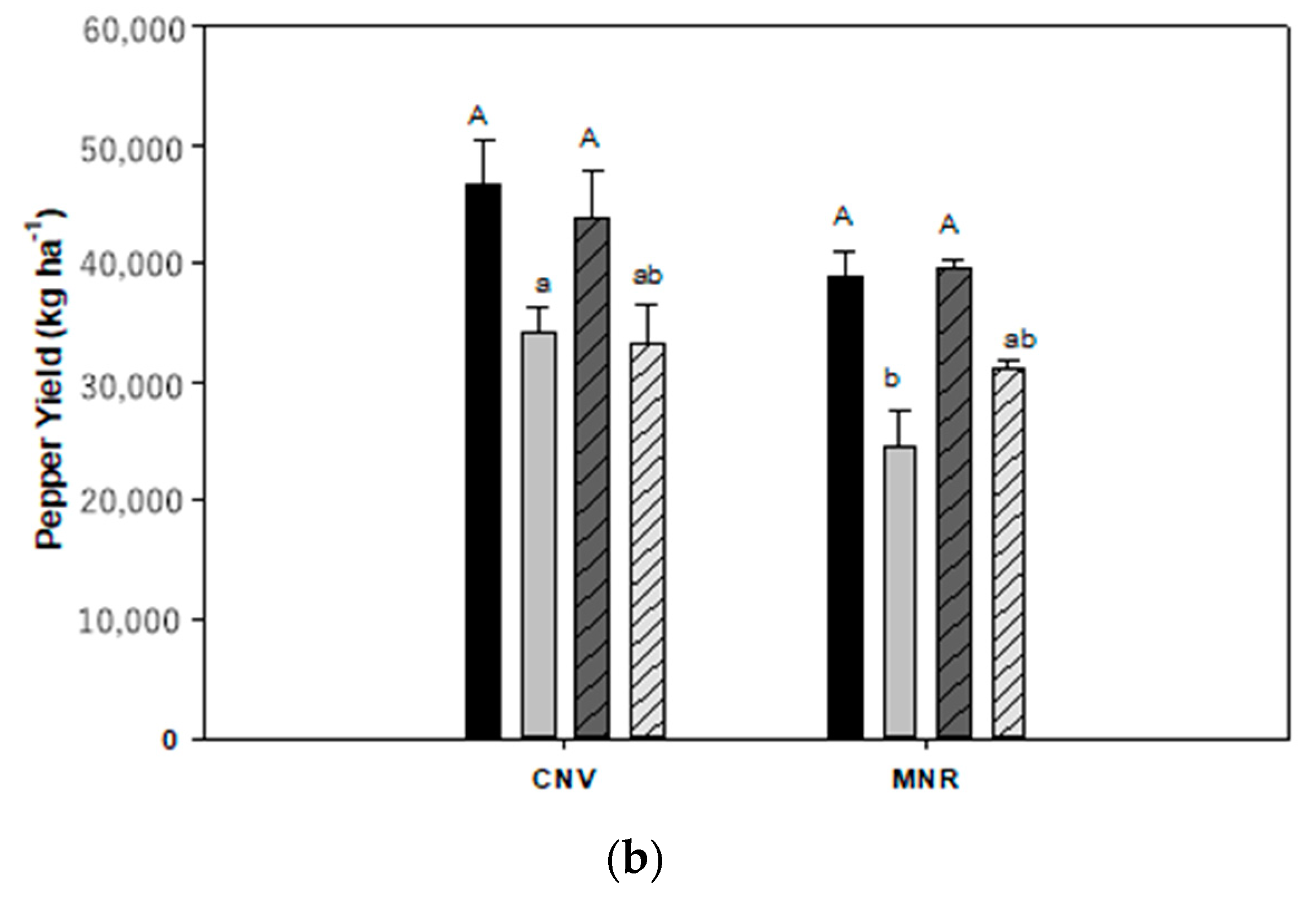
There was, however, a highly significant year by cropping systems by AMF treatment interaction for the marketable yield of pepper (p < 0.0001) and carrot (p = 0.0101) (Table 2A), and thus data were analyzed separately by those independent variables for carrot and pepper (Figure 10a,b). Mycorrhizal treatment had no statistically significant effect on the marketable yield of the two vegetables, but cropping systems had a statistically significant effect on the marketable yield of both vegetables (p = 0.0414 and 0.0250, respectively, for carrot and pepper), while year had a statistically significant effect on the marketable yield of carrot (p = 0.0085). While MNR tended to have a higher marketable yield of carrot than CNV in both years, the difference was not statistically significant (Figure 10a). However, CNV had a statistically significantly greater marketable yield of pepper than MNR in 2004 (Figure 10b).


Figure 10.
Total and marketable yields of (a) carrot and (b) pepper in 2004 and 2005. Means on a bar followed by the same upper- or lower-case letter are not statistically different (alpha 0.05). The upper-case letters compare the means of the total yield while the lower-case letters compare the means of the marketable yield. Key: MNR (organic), CNV (conventional).
3.2.2. Cropping Systems Effects
1. Vitamin C
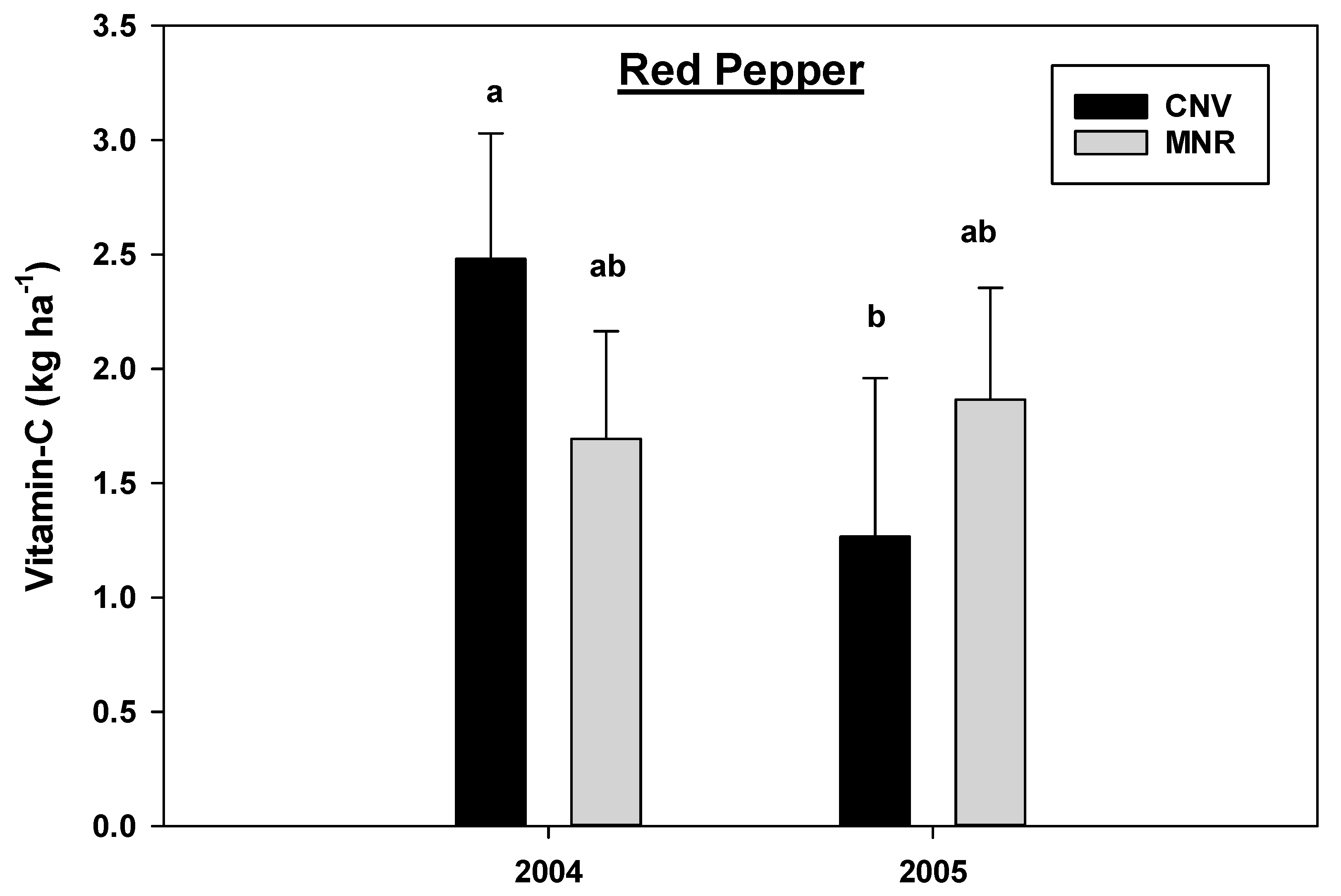
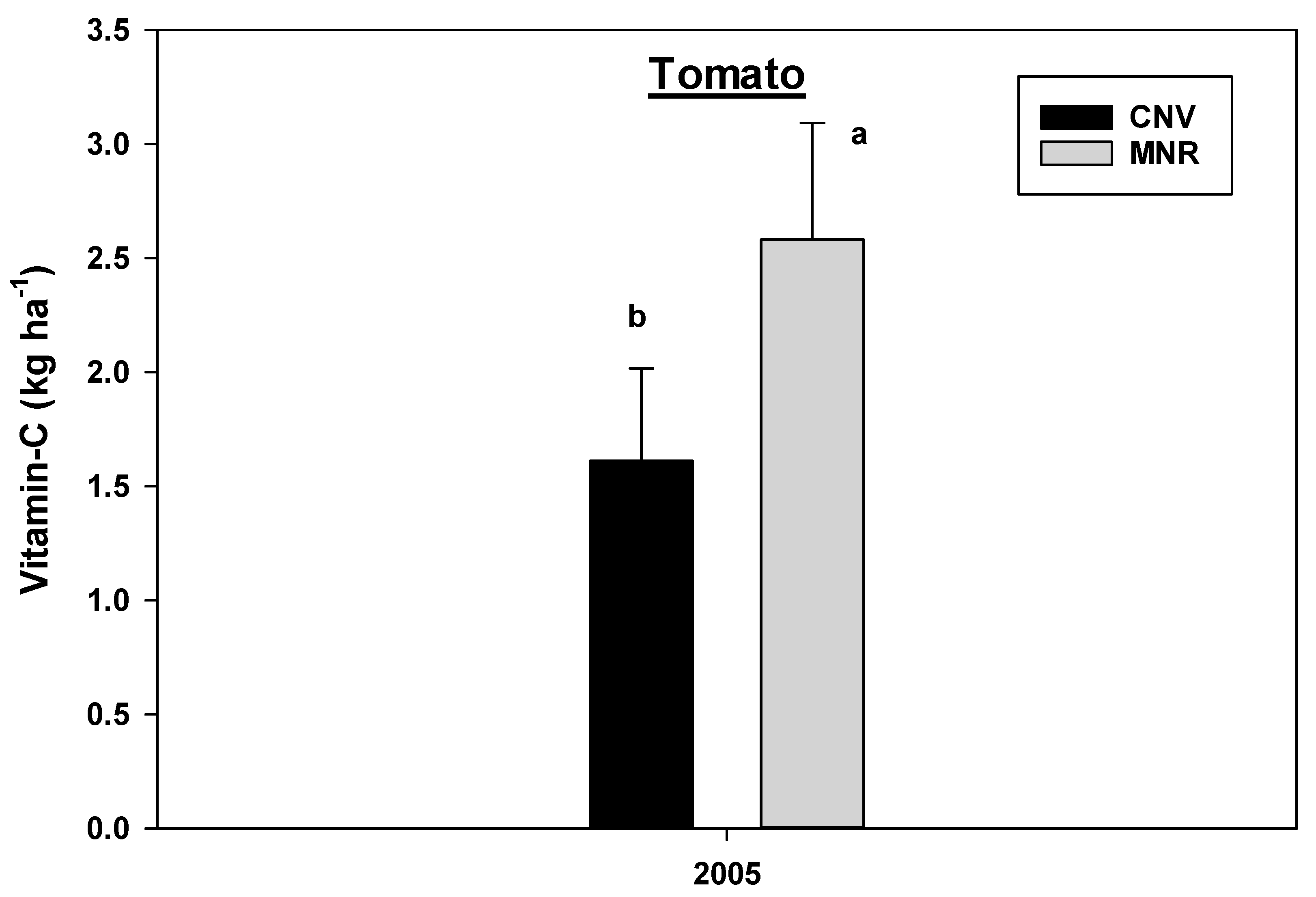
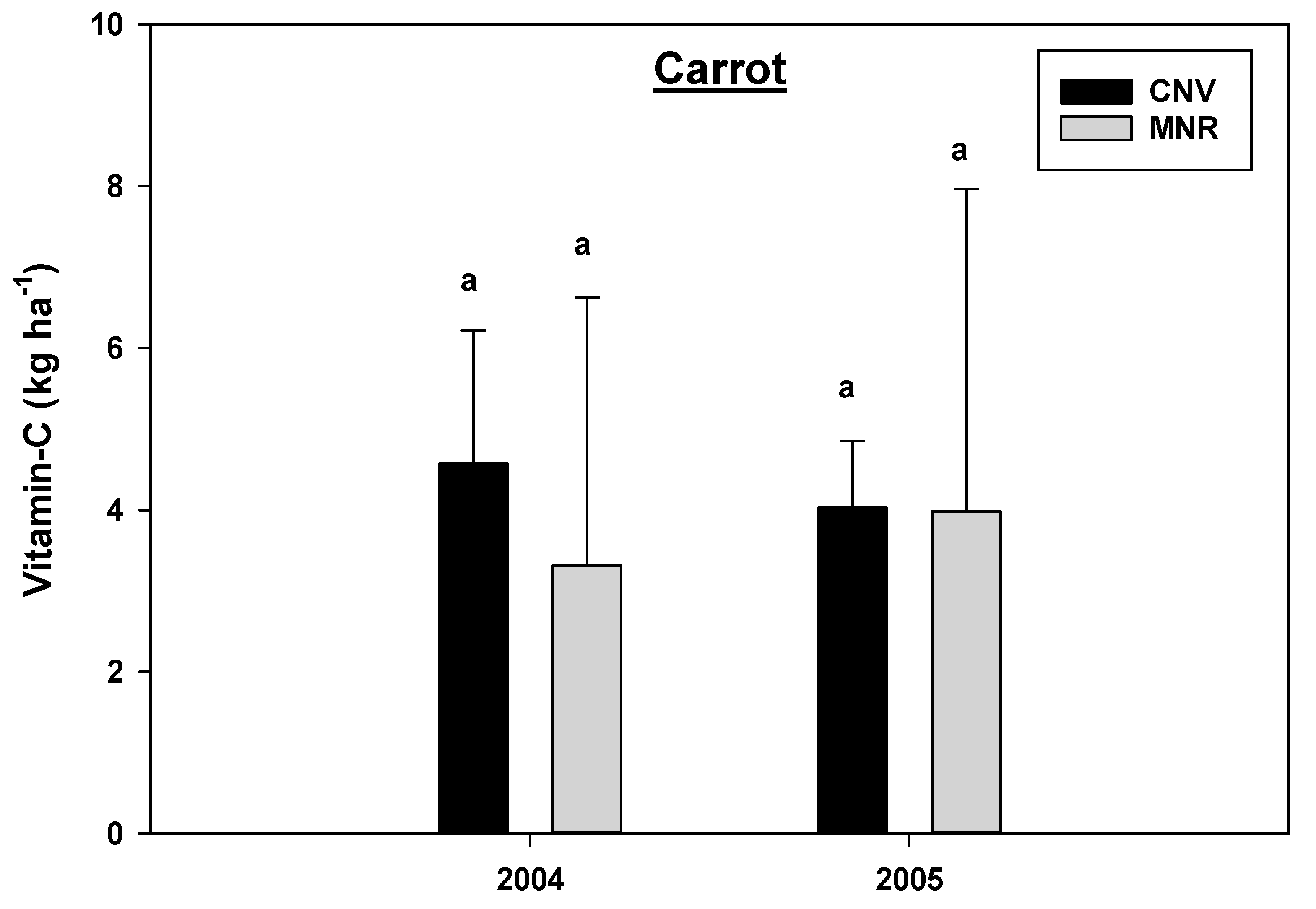
The analysis of variance revealed a statistically significant year by cropping systems interaction effects for vitamin C concentration in green peppers (p = 0.0044) and red peppers (p = 0.0126) and a marginally significant interaction for carrots (p = 0.0997) (Table 2B), hence the data were analyzed separately by year and cropping systems. Organic MNR system significantly increased vitamin C concentration in green peppers in 2005 compared to 2004 (Figure 11), while there was a statistically significant decline in the vitamin C concentration in red peppers under CNV systems in the same year period (Figure 10). However, there was no statistical difference in the vitamin C concentration between the two cropping systems in the two vegetables within each year of the study (Figure 12 and Figure 13). As mentioned earlier, there was a crop failure in tomatoes in 2004, and hence data from that year were not reported for tomato. However, tomatoes under organic MNR had a significantly greater (p = 0.0102) vitamin C concentration compared to CNV in 2005 (Table 2B, Figure 13). There was no statistically significant difference in the vitamin C concentration in carrots between the two cropping systems in 2004 and 2005 (Figure 14). Changes in the vitamin C concentration in carrots between 2004 and 2005 were also not statistically significant (data not shown).

Figure 11.
Effects of management on the vitamin C in green pepper in 2004 and 2005. Means on a bar followed by the same letter are not statistically different (alpha 0.05). Key: MNR (organic), CNV (conventional).

Figure 12.
Effects of management on the vitamin C in red pepper in 2004 and 2005. Means on a bar followed by the same letter are not statistically different (alpha 0.05). Key: MNR (organic), CNV (conventional).

Figure 13.
Effects of management on the vitamin C in tomato in 2004 and 2005. Means on a bar followed by the same letter are not statistically different (alpha 0.05). Key: MNR (organic), CNV (conventional).

Figure 14.
Effects of management on the vitamin C in carrot in 2004 and 2005. Means on a bar followed by the same letter are not statistically different (alpha 0.05). Key: MNR (organic), CNV (conventional).
2. Antioxidants
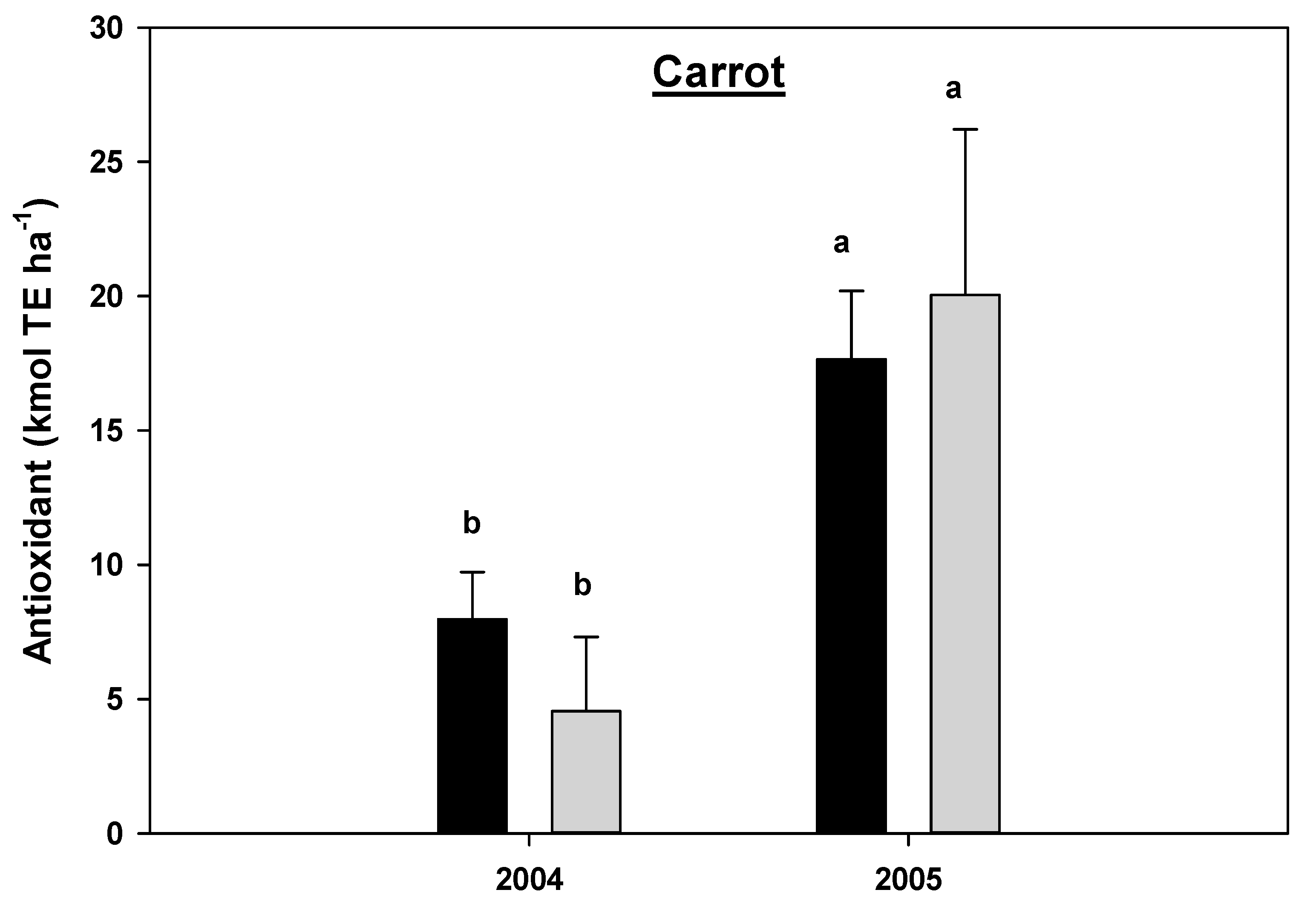
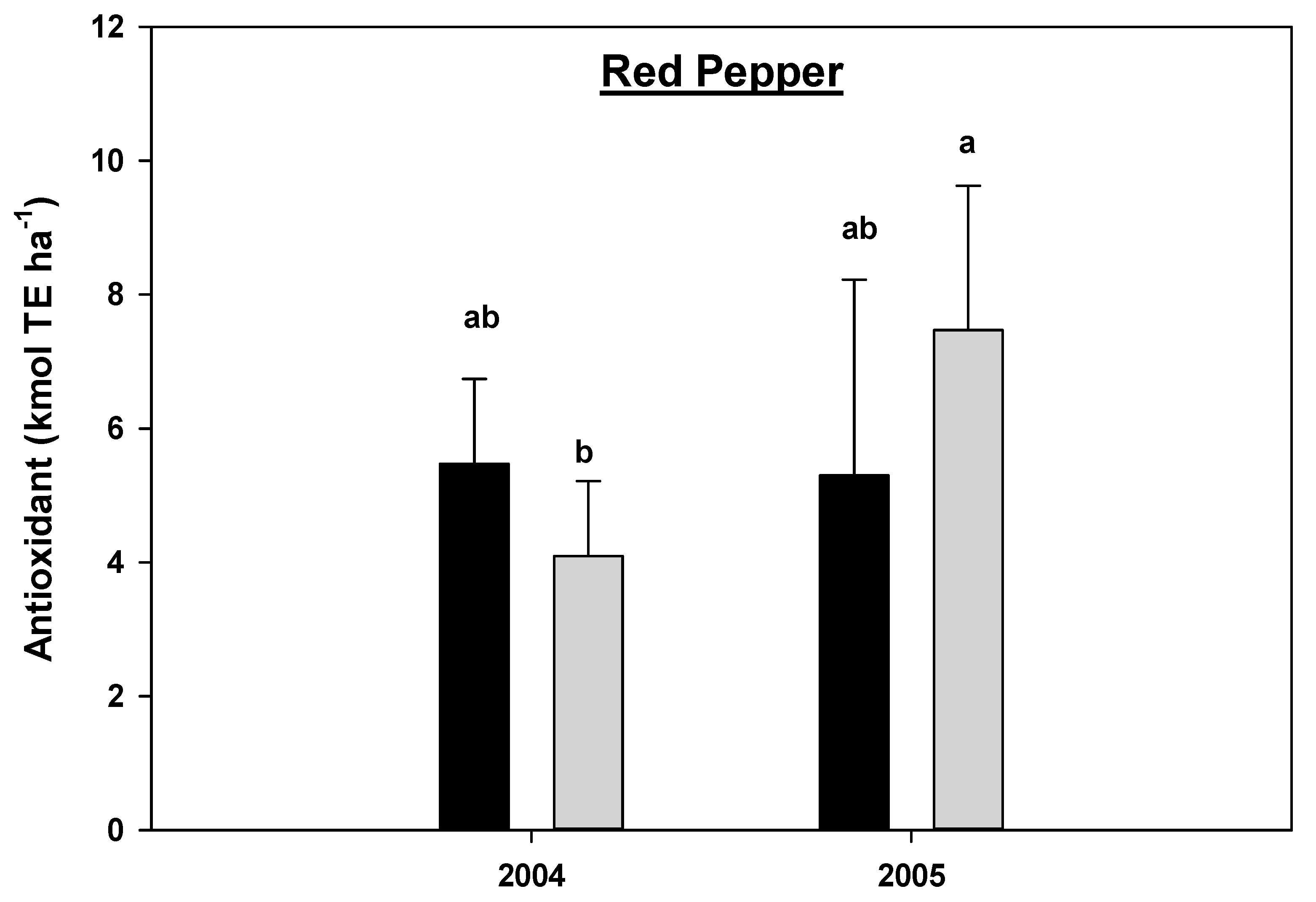
There was a statistically significant year by cropping systems interaction effects for the antioxidant concentration in carrots (p = 0.0523) and marginally significant interaction for red peppers (p = 0.0639) (Table 2B), hence the data for antioxidant concentration for carrots and red peppers were analyzed separately by year and cropping systems. There was no statistical difference in the antioxidant concentration between the CNV and MNR systems in carrots and red peppers within a single year (Figure 15 and Figure 16). The antioxidant concentration significantly increased in carrots under both CNV and MNR in 2005 compared to 2004 (Figure 15). However, the increase was only statistically significant in MNR in red peppers (Figure 16).

Figure 15.
Effects of management on the antioxidants in carrot in 2004 and 2005. Means on a bar followed by the same letter are not statistically different (alpha 0.05). Key: MNR (organic), CNV (conventional).

Figure 16.
Effects of management on the antioxidants in red pepper in 2004 and 2005. Means on a bar followed by the same letter are not statistically different (alpha 0.05). Key: MNR (organic), CNV (conventional).
3. Mineral nutrients
Like organic nutrients, all the mineral nutrients from vegetative tissue analyses were not affected by treatments across the systems and year regime (ANOVA not shown). Thus, all the mineral nutrients data were presented by management practices over a two-year period (Supplementary Table S2). Although various macro- and micro-nutrients were either significantly increased or decreased from 2004 to 2005 in 15 cases, only seven cases were significantly impacted by management practices, with five of those in 2005 (Supplementary Table S2). The organic MNR system significantly increased the Na (in carrot and pepper), Fe, Mg, and S (in pepper) and decreased the Zn in tomato compared to the CNV system. However, except in one case (S in pepper), all the statistically significant changes were observed only in the first year (2004) (Supplementary Table S2).
4. Discussion
Diseases as a consequence of weather: A plant physiological stress mechanism for antioxidant production explains the observed results from this study. Extreme weather conditions and changes, from a historically wet 2004 to a relatively dry 2005 (Figure 1, Supplementary Figure S1), may have caused statistically significant stress to the vegetables in this project. In 2005, an early and periodic drought stress differentiated the growing season conclusively from the wet 2004. The interpretation of our results is based on the hypothesis that significant drought stress conditions induce plant antioxidants as plant-defensive reactions [60,61]. Water stress was the bigger problem in 2005, while excessive water challenges were prevalent in 2004; both extremes resulting in marked differences in the order of antioxidant production, which explains the statistically significant treatment by year interaction for antioxidant production. In general, the highest levels of ascorbic acid (vitamin C) and lycopene were found in the mycorrhizal treated vegetables in the organic system and the lowest levels in the conventional mycorrhizal treatment. A range from about 35% to 60% more antioxidant vitamins were found under the organic system with mycorrhizal inoculation. However, the major influencing factor appears to be the system rather than mycorrhizal inoculation. Our results suggest that improved soil (under long-term organically managed plots) and mycorrhizal inoculation may work together to mitigate stress under dry (2005) but generally favorable agronomic environments. Prior studies indicate that both organic soil improvement and mycorrhizae expand the ability of plants to absorb water from the soil [40,41,42], supporting this hypothesis. Our results suggest that the antioxidant response was more flexible under improved organically managed soil than under conventional soils. The higher antioxidant production in 2005 compared to 2004 in carrots and peppers suggest that those vegetables responded to water stress by increasing antioxidant production (drought tolerance) (Figure 4 and Figure 5). Since vegetables were grown under plastic, excessive rains in 2004 did not cause flooding, hence the substantially lower physiological drought stress and lower antioxidant production in 2004. Disease tolerance was also consistently improved under organic management compared to conventional management. On the other hand, the total crop yields were generally not different between the organic and conventional soils. However, through the improved plant stress reaction, the quality and marketable yield were favored in the organic system under stress environments (Table 2A, Figure 10). We propose that the biggest advantages for organic production in this study were tolerance to water and disease stress. At low stress levels, improved organic soil conditions avoided the stress reaction and thereby caused a lower antioxidant production. At a higher water stress level, however, as observed in 2005, it activated a higher defense reaction measured in a specific antioxidant reaction, including vitamin C and lycopene production. These observations suggest that organic systems with improved soil are more flexible in their plant stress reaction compared to conventional systems.
This study did not find statistically significant total yield differences for the organic, conventional, mycorrhizal, and non-mycorrhizal treatment combinations. However, while the conventional system had a greater marketable yield of pepper in the wetter, non-water stressed 2004, the organic system tended to yield a greater, but non-significant marketable carrot yield in the dryer 2005 (Figure 10). These results are in conformity with multiple studies that have shown that conventional systems tend to yields that are about 20% higher than organic yields under non-water stress conditions [5,6,7], while organic systems generally produce greater crop yields than conventional systems under water-stressed conditions [4,20,21,22]. These results coupled with the lower disease incidence observed in all vegetables under the organic system in both years compared to the conventional system (Supplementary Table S3) suggest that organic management has the potential to stabilize yields and better manage crop diseases. However, there is a need to carry out similar studies over a much longer period than two years to ascertain this potential.
Effects of cropping systems: Our results show that, in general, organic systems performed better than conventional systems; for example, vitamin C significantly increased in green peppers under MNR (Figure 11) and the same nutrient significantly decreased in red peppers under CNV in 2005 (Figure 12). While the differences were not statistically significant (except in tomatoes under MNR, which had significantly greater vitamin C than CNV in 2005 (Figure 13)), conventional systems tended to perform better in 2004 but the reverse was the case in 2005 (Figure 11, Figure 12, Figure 14, Figure 15 and Figure 16). The better performance of the CNV in 2004, which was a wet year compared to 2005, may partly be attributed to more options for providing synthetic mineral nutrients to vegetables, including foliar application under soil water saturation conditions, as well as pesticide control of diseases that were more prevalent in 2004. The better performance of the organic system in 2005 may be attributed to an improvement in our crop management practices with time, increased colonization and mycelial network of functional mycorrhizal fungi under drier conditions, and enhancement of soil biology in organic systems. A number of recent studies found that vitamin C and the antioxidant levels of fruits, vegetables, and crops grown under organic farming system were significantly enhanced when compared to the same under conventional farming practices [62]. For example, various studies found significantly higher vitamin C under organically grown (i) tomato [29,63], (ii) different fruits [31,64,65], and (iii) spinach [66] compared to conventional farming. Similarly, higher antioxidant activities were found in tomatoes and tomato-juice under organic than conventional management practices [63,67,68]. A meta-analysis indicated that organically produced fruits significantly increased the overall carotenoid content by 25% compared to a decrease of 38% in the same under conventionally grown fruits [62]. However, further and longer-term studies are required to measure the effect of management practices; microbial community structure; mechanisms for nutrient synthesis and/or uptake; and changes in soil physical, chemical, and biological properties with time on influencing the nutrient concentration in vegetables. Environmental factors such as excessive precipitation or droughts, the availability of N, or disease intensity might also be responsible for the changes in vitamin C and antioxidant levels in vegetables. The availability of N has been attributed to synthesis of high antioxidant and vitamin C in crop produce [69,70]. However, specific effects of N content, disease severity, and abiotic stresses on vegetable nutrients were not statistically quantified in the current study and are researchable priorities. Our results also indicate that the organic system increased most of the minerals that had significantly greater concentration in two of the three studied vegetables, including sodium in carrot and pepper and iron, magnesium, and sulfur in pepper. However, given that the majority of the mineral results were inconsistent and/or insignificant, there is need for further studies over a longer period of time that will focus on mineral nutrient dynamics in vegetable production under the long-term side by side comparison of organic and conventional management systems. Such a study will also examine the potential interactions between minerals that might influence their availability, deficiency, or plant uptake.
While cropping systems did not have a statistically significant effect on the total pepper yields (Figure 10b), CNV tended to have greater yields than MNR in the wetter 2004. As already mentioned, this is in conformity with multiple studies that have revealed that yields of organically managed crops tend to be lower than those of conventionally managed crops under stress-free conditions [5,8,9,71]. Te Pas [23] reported a 26% higher yield under organic than conventional farming in a summarized review collected from 88 research studies conducted in the tropics and sub-tropics; the higher organic yields were attributed to soil fertility improvement as well as the better response of organic than conventional systems to inputs under resource-poor conditions. The benefits of organic management practices often require a “whole systems approach” that includes long and diverse crop rotations, the use of cover crops between cash crops, the tactical use of inputs such as composted and green manures, and substantial time [72,73]. While our study was superimposed upon a long-term FST study, the inclusion of mycorrhizal treatment and the fact that the FST is a grain-based study (with different management needs, rotations, and input systems than vegetables) may have required more time than two years to realize more consistent yield effects. However, the fact that, even within a short amount of time spanning just two years, organic systems generally performed better than conventional system with regard to vitamin C, antioxidants, and other phytonutrients, and produced vegetable yields that were generally not significantly different from the conventional yields, but of better marketable quality than the conventional produce, suggests that organic systems can play a more prominent role in facilitating food security, both quantitatively and qualitatively. Ongoing concerted efforts to close or reduce the yield gap between organic and conventional systems [8,28] are encouraging.
5. Conclusions
The data from this study reveal how the concentration of organic nutrients in carrot, pepper, and tomato can be impacted not only by different farming systems and AMF inoculation, but also by extremes of weather. Our research suggests that antioxidants are turned on and off by environmental stress cues. The flexibility of this response appears enhanced under organic legacy with improved soil. This implies that stress management will be an effective strategy for increasing antioxidants, vitamins, and other phytonutrients. While water stress is erratic under sub-humid rainfall, it is much more manageable under semi-arid and arid conditions, whereby the programmed and precise management of water applications can be used to apply and withdraw stress to crop plants. Studies such as the current research can provide additional tools needed to generate defined stress levels than can enhance the antioxidant quality of vegetables and fruits. Given that limited soil moisture was identified as a key factor for antioxidant production, the major findings of this study, thus, were that (1) antioxidant responses were more flexible under organically improved soil, given the lower concentration of antioxidants produced in organically grown produce under no-stress environments and the higher concentrations under drought conditions compared to conventionally grown produce; (2) disease tolerance was consistently improved under organic management compared to conventional management; and (3) the total crop yields were not different between the organic and conventional soils. However, through improved plant stress reaction, the quality and marketable yield was favored in the organic system under a drought stress environment. We can thus surmise that the biggest advantages for organic production were identified as tolerance to water and disease stress.
Contrary to expectations, AMF inoculation reduced the vitamin C, antioxidants, and phytonutrients in vegetables during the first year of the study, which was partly attributed to the wet conditions in 2004. Because of potential antagonistic and synergistic interactions between native and introduced fungal species, a longer-term study with more vegetable species is required to establish these relationships and effects over time. Management systems effects on pests and diseases and the secondary effect of those on nutrients were not quantified by this research. Future research should combine disease identification with quantifying key soil nutrients (N, P, K) in a long-term study to further investigate if these trends persist.
Our research gives scientific support to contention held by organic agriculture pioneers that organic soil management has a significant general beneficial effect on lowering disease damage compared to conventional management. This is related to the better stress tolerance in organically managed soils. Sustainable and conventional farmers can utilize this soil buffering capacity by concentrating more effort on soil improvement rather than employing strategies that require heavy fertilization.
Supplementary Materials
The following are available online at https://www.mdpi.com/2071-1050/12/21/8965/s1: Figure S1: Daily rainfall, and temperature during the growing seasons in 2004, and 2005; Figure S2: Map, and plot layout for both years; Table S1: Characteristics of compost (dairy manure and leaf) used (date sampled: 13 April 2016); units are given in parenthesis; Table S2: Impacts of management practices of different vegetative mineral (macro- and micro-) nutrients over two years period; Nutrients concentrations are expressed in ppm. Means within a row within a nutrient followed by the same letter are not statistically different (alpha 0.05). Key: MNR (organic); CNV (conventional); Table S3: Disease epidemic, and environmental factors during 2004, and 2005.
Author Contributions
The followings are the specific contributions from the authors of this manuscript: Conceptualization, P.R.H.; methodology, P.R.H., R.S.; software, A.M. and E.C.O.; validation, A.M., E.C.O., R.S., P.R.H. and W.P.H.; formal analysis, A.M. and E.C.O.; investigation, P.R.H., R.S., A.M., E.C.O. and W.P.H.; resources, P.R.H., A.M. and E.C.O.; data curation, A.M. and E.C.O.; writing—original draft preparation, A.M., P.H. and E.C.O.; writing—review and editing, A.M., E.C.O., P.R.H., R.S. and W.P.H.; visualization, A.M., E.C.O.; supervision, E.C.O.; project administration, P.R.H.; funding acquisition, P.R.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Campbell Soup Company, R&D, Davis, California.
Acknowledgments
We want to extend gratitude to the Campbell Soup Company for providing material (such as seed) and funding support towards this project. We also want to thank David Douds, formerly of USDA, ARS NEA, Eastern Regional Research Center, Pennsylvania, for culturing and providing the arbuscular mycorrhizal inoculum used for this study as well as the detailed consultancy on the AMF component of this research.
Conflicts of Interest
The funders of this study provided additional support in analyzing vegetables for the phytonutrients in their well-equipped laboratory. As is the common practice, sample identities were not required or provided to the laboratory for these analyses. The authors declare no other conflict of interest.
References
- Carr, P.M.; Delate, K.; Zhao, X.; Cambardella, C.A.; Carr, P.L.; Heckman, J.R. Impacts on Soil, Food, and Human Health. In Soils and Human Health; CRC Press: Boca Raton, FL, USA, 2012; Volume 241. [Google Scholar]
- Reeve, J.; Hoagland, L.; Villalba, J.; Carr, P.; Atucha, A.; Cambardella, C.; Davis, D.; Delate, K. Organic farming, soil health, and food quality: Considering possible links. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 2016; Volume 137, pp. 319–367. [Google Scholar]
- Maeder, P.; Fliessbach, A.; Dubois, D.; Gunst, L.; Fried, P.; Niggli, U. Soil Fertility and Biodiversity in Organic Farming. Science 2002, 296, 1694. [Google Scholar] [CrossRef] [PubMed]
- Reganold, J.P. Comparison of soil properties as influenced by organic and conventional farming systems. Am. J. Altern. Agric. 2009, 3, 144–155. [Google Scholar] [CrossRef]
- de Ponti, T.; Rijk, B.; van Ittersum, M.K. The crop yield gap between organic and conventional agriculture. Agric. Syst. 2012, 108, 1–9. [Google Scholar] [CrossRef]
- Ponisio, L.C.; Ehrlich, P.R. Diversification, Yield and a New Agricultural Revolution: Problems and Prospects. Sustainability 2016, 8, 1118. [Google Scholar] [CrossRef]
- Seufert, V.; Ramankutty, N. Many shades of gray—The context-dependent performance of organic agriculture. Sci. Adv. 2017, 3, e1602638. [Google Scholar] [CrossRef]
- Roos, E.; Mie, A.; Wivstad, M.; Salomon, E.; Johansson, B.; Gunnarsson, S.; Wallenbeck, A.; Hoffmann, R.; Nilsson, U.; Sundberg, C.; et al. Risks and opportunities of increasing yields in organic farming. A review. Agron. Sustain. Dev. 2018, 38, 21. [Google Scholar] [CrossRef]
- Weyers, S.L.; Archer, D.W.; Forcella, F.; Gesch, R.; Johnson, J.M.F. Strip-tillage reduces productivity in organically managed grain and forage cropping systems in the Upper Midwest, USA. Renew. Agric. Food Syst. 2018, 33, 309–321. [Google Scholar] [CrossRef]
- Smith, M.R.; Myers, S.S. Impact of anthropogenic CO2 emissions on global human nutrition. Nat. Clim. Chang. 2018, 8, 834–839. [Google Scholar] [CrossRef]
- Pang, X.P.; Letey, J. Organic farming: Challenge of timing nitrogen availability to crop nitrogen requirements. Soil Sci. Soc. Am. J. 2000, 64, 863–885. [Google Scholar] [CrossRef]
- World watch. Crop Yields Expand, but Nutrition Is Left Behind. In Vision for a Sustainable World; World watch: Washington, DC, USA, 2016; Volume 2016. [Google Scholar]
- Marles, R.J. Mineral nutrient composition of vegetables, fruits and grains: The context of reports of apparent historical declines. J. Food Compos. Anal. 2017, 56, 93–103. [Google Scholar] [CrossRef]
- Davis, D.R.; Epp, M.D.; Riordan, H.D. Changes in USDA food composition data for 43 garden crops, 1950 to 1999. J. Am. Coll. Nutr. 2004, 23, 669–682. [Google Scholar] [CrossRef] [PubMed]
- Ficco, D.; Riefolo, C.; Nicastro, G.; De Simone, V.; Di Gesu, A.; Beleggia, R.; Platani, C.; Cattivelli, L.; De Vita, P. Phytate and mineral elements concentration in a collection of Italian durum wheat cultivars. Field Crop. Res. 2009, 111, 235–242. [Google Scholar] [CrossRef]
- Ikemura, Y.; Shukla, M.K. Soil quality in organic and conventional farms of New Mexico, USA. J. Org. Syst. 2009, 4, 34–47. [Google Scholar]
- McGarry, D.; Bridge, B.J.; Radford, B.J. Contrasting soil physical properties after zero and traditional tillage of an alluvial soil in the semi-arid subtropics. Soil Tillage Res. 2000, 53, 105–115. [Google Scholar] [CrossRef]
- Araújo, A.S.; Leite, L.F.; Santos, V.B.; Carneiro, R.F. Soil microbial activity in conventional and organic agricultural systems. Sustainability 2009, 1, 268–276. [Google Scholar] [CrossRef]
- Lori, M.; Symnaczik, S.; Mäder, P.; De Deyn, G.; Gattinger, A. Organic farming enhances soil microbial abundance and activity—A meta-analysis and meta-regression. PLoS ONE 2017, 12, e0180442. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, D.; Hepperly, P.; Hanson, J.; Douds, D.; Seidel, R. Environmental, energetic, and economic comparisons of organic and conventional farming systems. BioScience 2005, 55, 573–582. [Google Scholar] [CrossRef]
- Johnson, J.M.-F.; Reicosky, D.C.; Allmaras, R.R.; Sauer, T.J.; Venterea, R.T.; Dell, C.J. Greenhouse gas contributions and mitigation potential of agriculture in the central USA. Soil Tillage Res. 2005, 83, 73–94. [Google Scholar] [CrossRef]
- Hudson, B.D. Soil organic matter and available water capacity. J. Soil Water Conserv. 1994, 49, 189–194. [Google Scholar]
- Te Pas, C.M.; Rees, R.M. Analysis of Differences in Productivity, Profitability and Soil Fertility between Organic and Conventional Cropping Systems in the Tropics and Sub-tropics. J. Integr. Agric. 2014, 13, 2299–2310. [Google Scholar] [CrossRef]
- Tuomisto, H.L.; Hodge, I.D.; Riordan, P.; Macdonald, D.W. Does organic farming reduce environmental impacts?—A meta-analysis of European research. J. Environ. Manag. 2012, 112, 309–320. [Google Scholar] [CrossRef]
- Puech, C.; Baudry, J.; Joannon, A.; Poggi, S.; Aviron, S. Organic vs. conventional farming dichotomy: Does it make sense for natural enemies? Agric. Ecosyst. Environ. 2014, 194, 48–57. [Google Scholar] [CrossRef]
- Lee, K.S.; Choe, Y.C.; Park, S.H. Measuring the environmental effects of organic farming: A meta-analysis of structural variables in empirical research. J. Environ. Manag. 2015, 162, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Fess, T.; Benedito, V. Organic versus Conventional Cropping Sustainability: A Comparative System Analysis. Sustainability 2018, 10, 272. [Google Scholar] [CrossRef]
- Cordoa, E.M.; Chirinda, N.; Li, F.; Olesen, J.E. Contributions from carbon and nitrogen in roots to closing the yield gap between conventional and organic cropping systems. Soil Use Manag. 2018, 34, 335–342. [Google Scholar] [CrossRef]
- Vinha, A.F.; Barreira, S.V.; Costa, A.S.; Alves, R.C.; Oliveira, M.B. Organic versus conventional tomatoes: Influence on physicochemical parameters, bioactive compounds and sensorial attributes. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2014, 67, 139–144. [Google Scholar] [CrossRef]
- Magkos, F.; Arvaniti, F.; Zampelas, A. Organic food: Nutritious food or food for thought? A review of the evidence. Int. J. Food Sci. Nutr. 2003, 54, 357–371. [Google Scholar] [CrossRef]
- Tarozzi, A.; Hrelia, S.; Angeloni, C.; Morroni, F.; Biagi, P.; Guardigli, M.; Cantelli-Forti, G.; Hrelia, P. Antioxidant effectiveness of organically and non-organically grown red oranges in cell culture systems. Eur. J. Nutr. 2006, 45, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Petkovsek, M.M.; Slatnar, A.; Stampar, F.; Veberic, R. The influence of organic/integrated production on the content of phenolic compounds in apple leaves and fruits in four different varieties over a 2-year period. J. Sci. Food Agric. 2010, 90, 2366–2378. [Google Scholar] [CrossRef]
- Hallmann, E. The influence of organic and conventional cultivation systems on the nutritional value and content of bioactive compounds in selected tomato types. J. Sci. Food Agric. 2012, 92, 2840–2848. [Google Scholar] [CrossRef] [PubMed]
- Al-Sayyed, H.; Refa’t Al-Kurd, M.M.; Qader, S.A. Determination of Antioxidant Content and Activity in Eight Jordanian Fresh Green Leafy Vegetables. Agric. Res. Technol. Open Access J. 2019, 19, 556102. [Google Scholar] [CrossRef]
- Roberts, W.G.; Gordon, M.H. Determination of the total antioxidant activity of fruits and vegetables by a liposome assay. J. Agric. Food Chem. 2003, 51, 1486–1493. [Google Scholar] [CrossRef]
- Reche, J.; Hernández, F.; Almansa, M.; Carbonell-Barrachina, Á.; Legua, P.; Amorós, A. Effects of organic and conventional farming on the physicochemical and functional properties of jujube fruit. LWT 2019, 99, 438–444. [Google Scholar] [CrossRef]
- da Silva Borges, L.; de Souza Vieira, M.C.; Vianello, F.; Goto, R.; Lima, G.P.P. Antioxidant compounds of organically and conventionally fertilized jambu (Acmella oleracea). Biol. Agric. Hortic. 2016, 32, 149–158. [Google Scholar] [CrossRef]
- Maggio, A.; De Pascale, S.; Paradiso, R.; Barbieri, G. Quality and nutritional value of vegetables from organic and conventional farming. Sci. Hortic. 2013, 164, 532–539. [Google Scholar] [CrossRef]
- Sobieralski, K.; Siwulski, M.; Sas-Golak, I. Nutritive and health-promoting value of organic vegetables. Acta Sci. Pol. Technol. Aliment. 2013, 12, 113–123. [Google Scholar]
- Chen, M.; Arato, M.; Borghi, L.; Nouri, E.; Reinhardt, D. Beneficial Services of Arbuscular Mycorrhizal Fungi—From Ecology to Application. Front. Plant Sci. 2018, 9, 14. [Google Scholar] [CrossRef]
- Douds, D.; Nagahashi, G.; Hepperly, P. Production of inoculum of indigenous AM fungi and options for diluents of compost for on-farm production of AM fungi. Bioresour. Technol. 2010, 101, 2326–2330. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis; Academic Press: Cambridge, MA, USA, 2010. [Google Scholar]
- Pellegrino, E.; Öpik, M.; Bonari, E.; Ercoli, L. Responses of wheat to arbuscular mycorrhizal fungi: A meta-analysis of field studies from 1975 to 2013. Soil Biol. Biochem. 2015, 84, 210–217. [Google Scholar] [CrossRef]
- Lekberg, Y.; Koide, R.T. Is plant performance limited by abundance of arbuscular mycorrhizal fungi? A meta-analysis of studies published between 1988 and 2003. New Phytol. 2005, 168, 189–204. [Google Scholar] [CrossRef]
- Kabir, Z. Tillage or no-tillage: Impact on mycorrhizae. Can. J. Plant Sci. 2005, 85, 23–29. [Google Scholar] [CrossRef]
- Ryan, M.H.; Graham, J.H. Is there a role for arbuscular mycorrhizal fungi in production agriculture? Plant Soil 2002, 244, 263–271. [Google Scholar] [CrossRef]
- Bowles, T.M.; Jackson, L.E.; Loeher, M.; Cavagnaro, T.R. Data from: Ecological intensification and arbuscular mycorrhizas: A meta-analysis of tillage and cover crop effects. J. Appl. Ecol. 2017, 54, 1785–1793. [Google Scholar] [CrossRef]
- Douds, D.; Seidel, R. The contribution of arbusclar mycorrhizal fungi to the success or failure of agricultural practices. In Microbial Ecology; Taylor Francis Group: Boca Raton, FL, USA, 2012; pp. 133–152. [Google Scholar]
- Boswell, E.; Koide, R.; Shumway, D.; Addy, H. Winter wheat cover cropping, VA mycorrhizal fungi and maize growth and yield. Agric. Ecosyst. Environ. 1998, 67, 55–65. [Google Scholar] [CrossRef]
- Galvez, L.; Douds, D.; Wagoner, P.; Longnecker, L.; Drinkwater, L.; Janke, R. An overwintering cover crop increases inoculum of VAM fungi in agricultural soil. Am. J. Altern. Agric. 1995, 10, 152–156. [Google Scholar] [CrossRef]
- Castillo, C.G.; Rubio, R.; Rouanet, J.L.; Borie, F. Early effects of tillage and crop rotation on arbuscular mycorrhizal fungal propagules in an Ultisol. Biol. Fertil. Soils 2006, 43, 83–92. [Google Scholar] [CrossRef]
- Johnson, N.C.; Copeland, P.J.; Crookston, R.K.; Pfleger, F. Mycorrhizae: Possible explanation for yield decline with continuous corn and soybean. Agron. J. 1992, 84, 387–390. [Google Scholar] [CrossRef]
- Gosling, P.; Hodge, A.; Goodlass, G.; Bending, G.D. Arbuscular mycorrhizal fungi and organic farming. Agric. Ecosyst. Environ. 2006, 113, 17–35. [Google Scholar] [CrossRef]
- Lee, S.W.; Lee, E.H.; Eom, A.H. Effects of organic farming on communities of arbuscular mycorrhizal fungi. Mycobiology 2008, 36, 19–23. [Google Scholar] [CrossRef]
- Ryan, M.R.; Smith, R.G.; Mortensen, D.A.; Teasdale, J.R.; Curran, W.S.; Seidel, R. Weed–crop competition relationships differ between organic and conventional cropping systems. Weed Res. 2009, 49, 572–580. [Google Scholar] [CrossRef]
- Liebhardt, W.; Andrews, R.; Culik, M.; Harwood, R.; Janke, R.; Radke, J.; Reiger-Schwartz, S. Crop production during conversion from conventional to low-input methods. Agron. J. 1989, 81, 150–159. [Google Scholar] [CrossRef]
- Lotter, D.W.; Seidel, R.; Liebhardt, W. The performance of organic and conventional cropping systems in an extreme climate year. Am. J. Altern. Agric. 2003, 18, 146–154. [Google Scholar] [CrossRef]
- SAS. SAS 9.4 Language Reference: Concepts; SAS Institute Inc.: Cary, NC, USA, 2014; p. 828. [Google Scholar]
- SigmaPlot. SigmaPlot Version 14.0; Systat Software Inc.: San Jose, CA, USA, 2018. [Google Scholar]
- Ahmad, R.; Hussain, S.; Anjum, M.A.; Khalid, M.F.; Saqib, M.; Zakir, I.; Hassan, A.; Fahad, S.; Ahmad, S. Oxidative stress and antioxidant defense mechanisms in plants under salt stress. In Plant Abiotic Stress Tolerance; Springer: Berlin/Heidelberg, Germany, 2019; pp. 191–205. [Google Scholar]
- Laxa, M.; Liebthal, M.; Telman, W.; Chibani, K.; Dietz, K.-J. The role of the plant antioxidant system in drought tolerance. Antioxidants 2019, 8, 94. [Google Scholar] [CrossRef]
- Mditshwa, A.; Magwaza, L.S.; Tesfay, S.Z.; Mbili, N. Postharvest quality and composition of organically and conventionally produced fruits: A review. Sci. Hortic. 2017, 216, 148–159. [Google Scholar] [CrossRef]
- Oliveira, A.B.; Moura, C.F.H.; Gomes-Filho, E.; Marco, C.A.; Urban, L.; Miranda, M.R.A. The Impact of Organic Farming on Quality of Tomatoes Is Associated to Increased Oxidative Stress during Fruit Development. PLoS ONE 2013, 8, e56354. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, P.C.; Tomazini, A.P.B.; Stringheta, P.C.; Ribeiro, S.M.R.; Pinheiro-Sant’Ana, H.M. Vitamin C and carotenoids in organic and conventional fruits grown in Brazil. Food Chem. 2011, 126, 411–416. [Google Scholar] [CrossRef]
- Janzantti, N.S.; Macoris, M.S.; Garruti, D.S.; Monteiro, M. Influence of the cultivation system in the aroma of the volatile compounds and total antioxidant activity of passion fruit. LWT-Food Sci. Technol. 2012, 46, 511–518. [Google Scholar] [CrossRef]
- Koh, E.; Charoenprasert, S.; Mitchell, A.E. Effect of Organic and Conventional Cropping Systems on Ascorbic Acid, Vitamin C, Flavonoids, Nitrate, and Oxalate in 27 Varieties of Spinach (Spinacia oleracea L.). J. Agric. Food Chem. 2012, 60, 3144–3150. [Google Scholar] [CrossRef]
- de Oliveira, A.B.; Lopes, M.M.D.; Moura, C.F.H.; Oliveira, L.D.; de Souza, K.O.; Gomes, E.; Urban, L.; de Miranda, M.R.A. Effects of organic vs. conventional farming systems on quality and antioxidant metabolism of passion fruit during maturation. Sci. Hortic. 2017, 222, 84–89. [Google Scholar] [CrossRef]
- Vallverdú-Queralt, A.; Medina-Remón, A.; Casals-Ribes, I.; Lamuela-Raventos, R.M. Is there any difference between the phenolic content of organic and conventional tomato juices? Food Chem. 2012, 130, 222–227. [Google Scholar] [CrossRef]
- Berry, P.M.; Sylvester-Bradley, R.; Philipps, L.; Hatch, D.J.; Cuttle, S.P.; Rayns, F.W.; Gosling, P. Is the productivity of organic farms restricted by the supply of available nitrogen? Soil Use Manag. 2002, 18, 248–255. [Google Scholar] [CrossRef]
- Seufert, V.; Ramankutty, N.; Foley, J.A. Comparing the yields of organic and conventional agriculture. Nature 2012, 485, 229. [Google Scholar] [CrossRef] [PubMed]
- Brandt, K.; Leifert, C.; Sanderson, R.; Seal, C. Agroecosystem management and nutritional quality of plant foods: The case of organic fruits and vegetables. Crit. Rev. Plant Sci. 2011, 30, 177–197. [Google Scholar] [CrossRef]
- Simmons, B.L.; Coleman, D.C. Microbial community response to transition from conventional to conservation tillage in cotton fields. Appl. Soil Ecol. 2008, 40, 518–528. [Google Scholar] [CrossRef]
- Jonason, D.; Andersson, G.K.; Öckinger, E.; Rundlöf, M.; Smith, H.G.; Bengtsson, J. Assessing the effect of the time since transition to organic farming on plants and butterflies. J. Appl. Ecol. 2011, 48, 543–550. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).